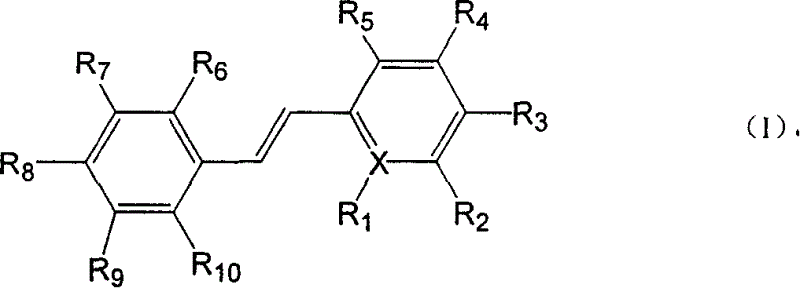
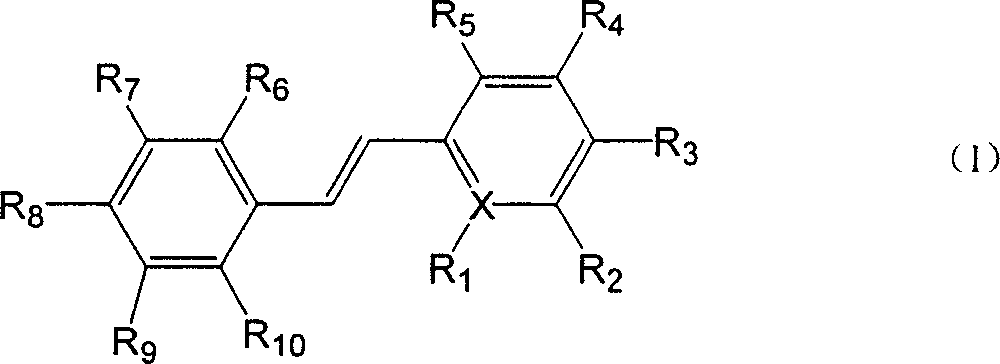
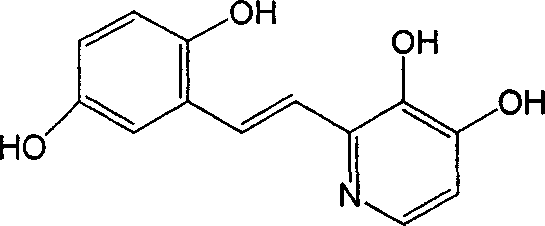
Polyhydroxy stilbenes compound preparation and uses as drugs for suppressing SARS
A polyhydroxy stilbene and compound technology, which is applied in the preparation of polyhydroxy stilbene compounds and the use of SARS virus-inhibiting drugs, can solve the problems of not detecting that SARS-CoV has an effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] (E)-2-[2-(3,5-dihydroxyphenyl)vinyl]-3,5-dimethyl-4-hydroxypyridine; (also known as: (E)-4,6-dimethyl base-3',5,5'-trihydroxystilbene-2-nitrogen)
[0110] Synthesis of 3,5-dimethyl-4-methoxypyridine-2-methylphosphonate: 2-chloromethyl-3,5-dimethyl-4-methoxypyridine was added to a 50mL three-neck flask (0.01mol), 2.5g (0.016mol) of triethyl phosphite, a small amount of tetrabutylammonium iodide, stir, heat in an oil bath to 100-110°C, and react for 3-6h. The reactant was distilled under reduced pressure below 90°C to remove excess triethyl phosphite, and the remaining light orange-red sticky matter in the bottle was directly used for the next reaction.
[0111] Synthesis of (E)-2-[2-(3,5-dimethoxyphenyl) vinyl]-3,5-dimethyl-4-methoxypyridine: the phosphonate solution obtained in the previous step reaction In 50 mL of dry tetrahydrofuran (THF), cool in an ice-salt bath to below 0°C, quickly add NaH powder (0.025 mol) under stirring, and stir for 30 min. With stirring, ...
Embodiment 2
[0114] (E)-2-[2-(4-hydroxyphenyl)vinyl]-3,5-dimethyl-4-hydroxypyridine (also known as: (E)-4,6-dimethyl-4, 5'-dihydroxystilbene-2-nitrogen)
[0115] Synthesis of 3,5-dimethyl-4-methoxypyridine-2-methylphosphonate: 2-chloromethyl-3,5-dimethyl-4-methoxypyridine was added to a 50mL three-neck flask (0.01mol), triethyl phosphite 2.5g (0.016mol), a small amount of tetrabutylammonium iodide, stirred, heated in an oil bath to 100-110°C, and reacted for 3-6h. The reactant was distilled under reduced pressure below 90°C to remove excess triethyl phosphite, and the remaining light orange-red sticky matter in the bottle was directly used for the next reaction.
[0116] Synthesis of (E)-2-[2-(4-methoxyphenyl)vinyl]-3,5-dimethyl-4-methoxypyridine: the phosphonate obtained in the previous step reaction was dissolved in 50 mL and dried In tetrahydrofuran (THF), cool in an ice-salt bath to below 0°C, add NaH powder (0.025mol) rapidly under stirring, and stir for 30min. With stirring, 30 mL...
Embodiment 3
[0119] (E)-2-[2-(2,5-dihydroxyphenyl)vinyl]-3,5-dimethyl-4-hydroxypyridine (also known as: (E)-4,6-dimethyl -2,5,5'-trihydroxystilbene-2-nitrogen)
[0120] Synthesis of 3,5-dimethyl-4-methoxypyridine-2-methylphosphonate: 2-chloromethyl-3,5-dimethyl-4-methoxypyridine was added to a 50mL three-neck flask (0.01mol), triethyl phosphite 2.5g (0.016mol), a small amount of tetrabutylammonium iodide, stirred, heated to 100-110°C in an oil bath, and reacted for 3-6h. The reactants were distilled under reduced pressure below 90°C to remove excess triethyl phosphite, and the remaining light orange-red viscous in the bottle was directly used for the next reaction.
[0121]Synthesis of (E)-2-[2-(2,5-dimethoxyphenyl) vinyl]-3,5-dimethyl-4-methoxypyridine: the phosphonate solution obtained in the previous step reaction In 50 mL of dry tetrahydrofuran (THF), cool in an ice-salt bath to below 0°C, quickly add NaH powder (0.025 mol) under stirring, and stir for 30 min. With stirring, 30 mL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com