Patents
Literature
80 results about "Tetrabutylammonium iodide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
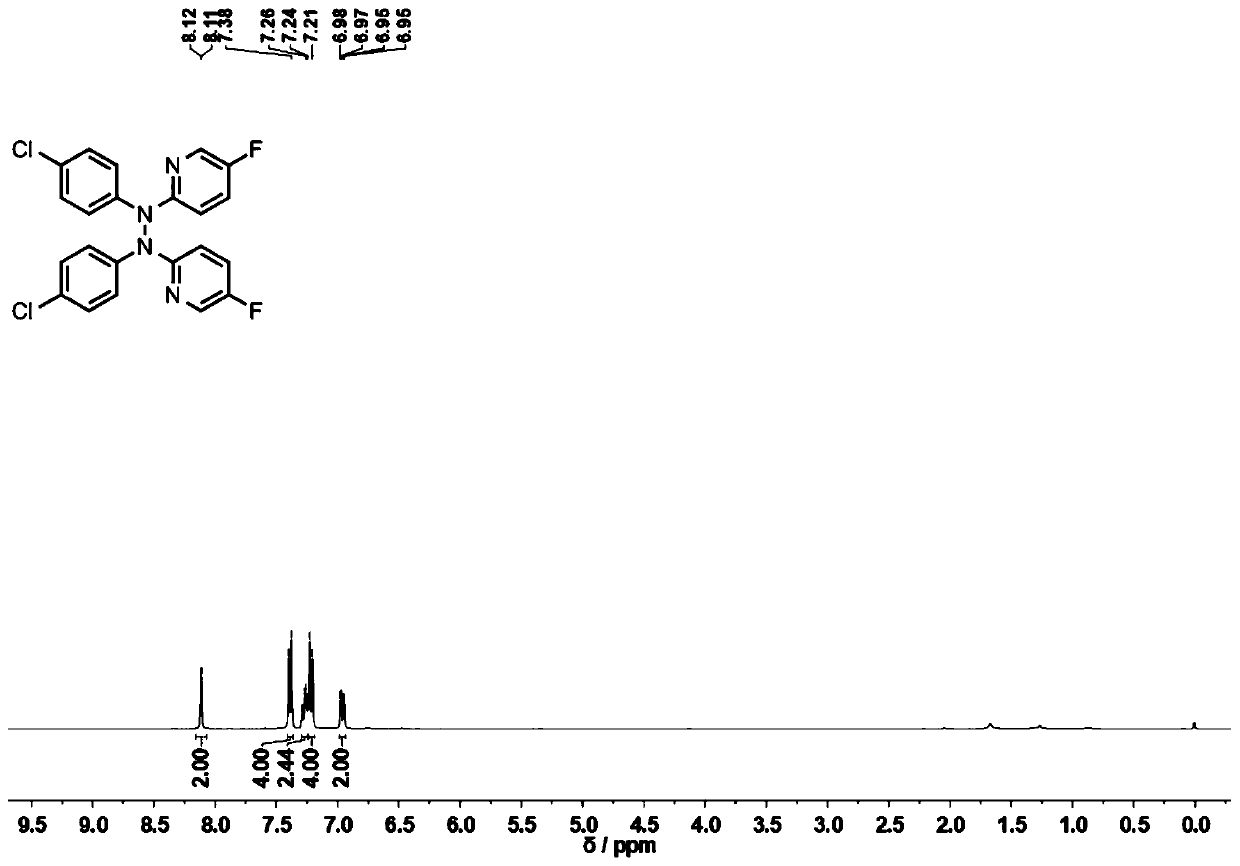
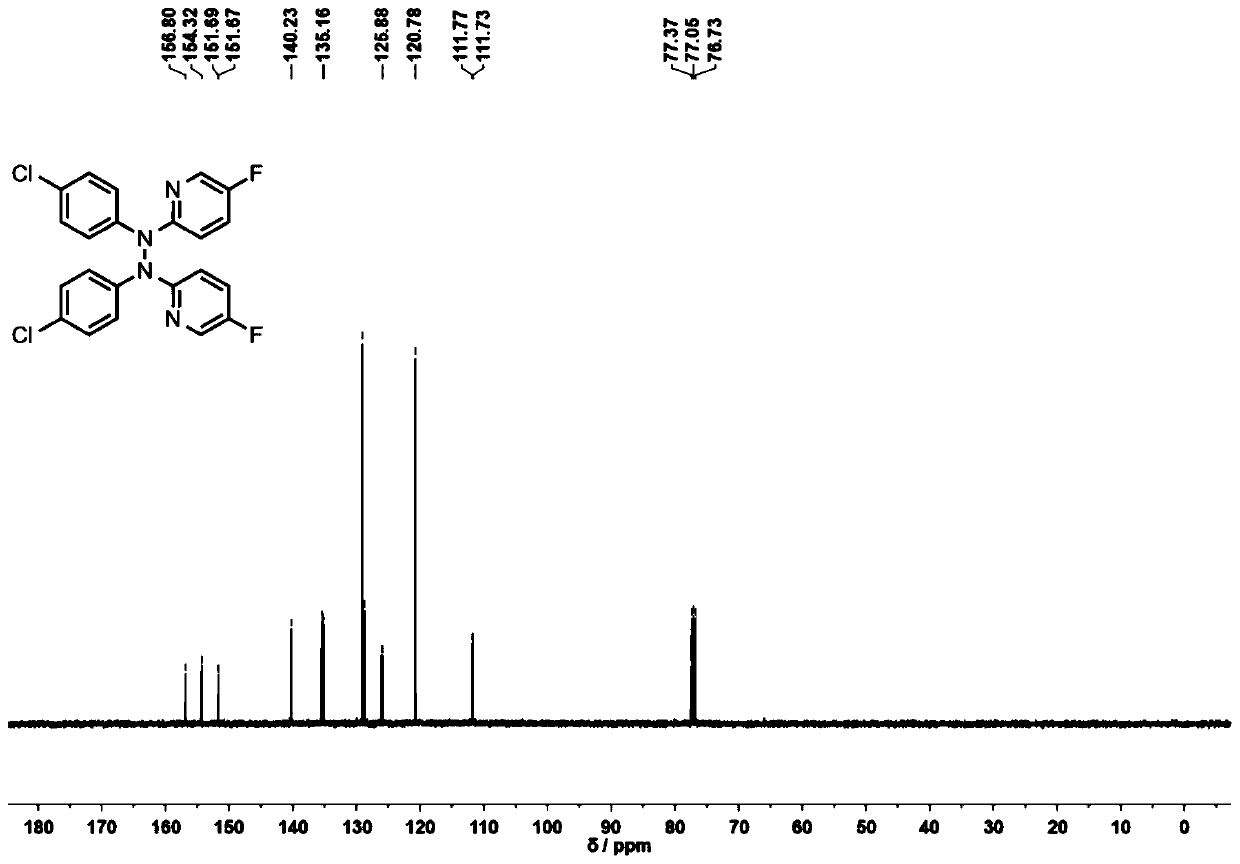
Tetrabutylammonium iodide (TBAI) has been used as a catalyst in the following reactions: • Synthesis of O -benzyl- N -Boc-L-tyrosine benzyl ester from N -Boc- L -tyrosine. • Conversion of 8-fluoro-1-aminonaphthale ne into 1-(8-fluoro-naphthalen-1- yl)piperazine hydrochloride.
Method for preparing cyclic carbonate
InactiveCN103641811AClear structureThe synthesis method is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsQuaternary ammonium cationRare earth metal compounds
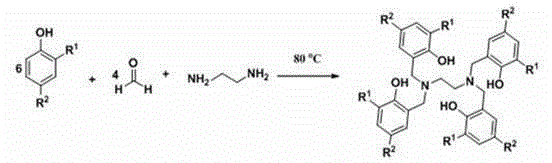
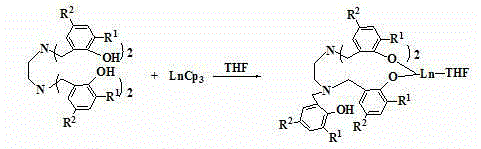
The invention discloses a method for preparing cyclic carbonate. The method specifically comprises the following step: with a quadri-aryloxy bridged rare earth metal compound as a catalyst, catalyzing carbon dioxide and alkylene oxide to react in the present of quaternary ammonium salt, wherein the general formula of the quadriaryloxy bridged rare earth metal compound is LLn(THF), wherein L refers to ethanediamine group bridged quadri-aryloxy, Ln refers to rare earth metal ions, and the quaternary ammonium salt is one of tetrabutylammonium iodide, tetrabutylammonium bromide, tetrabutylammonium chloride, tetraoctyl ammonium bromide, bis(triphenylphosphine) ammonium chloride and benzyl butyl ammonium bromide. The rare earth catalyst in the catalysis system is clear in structure, easy to synthesize, high in catalysis activity, less in using amount, mild in reaction conditions and wide in universality to alkylene oxide. According to the preparation method disclosed by the invention, raw materials are easily available, the reaction conditions are wild, a reaction substrate is wide in universality, the reaction time is short, the yield of the target product, namely the cyclic carbonate is high, and the reaction operation and the posttreatment process are simple.
Owner:SUZHOU UNIV
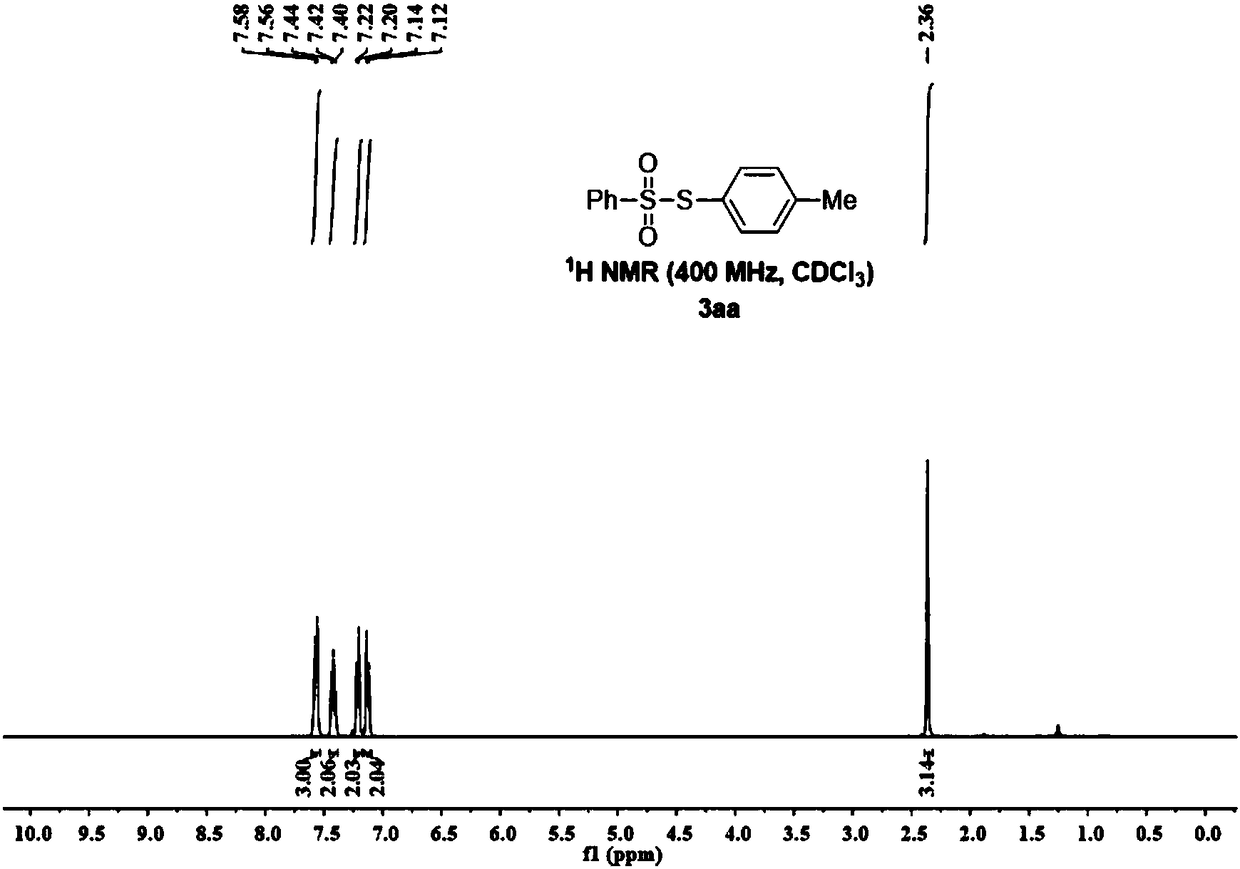
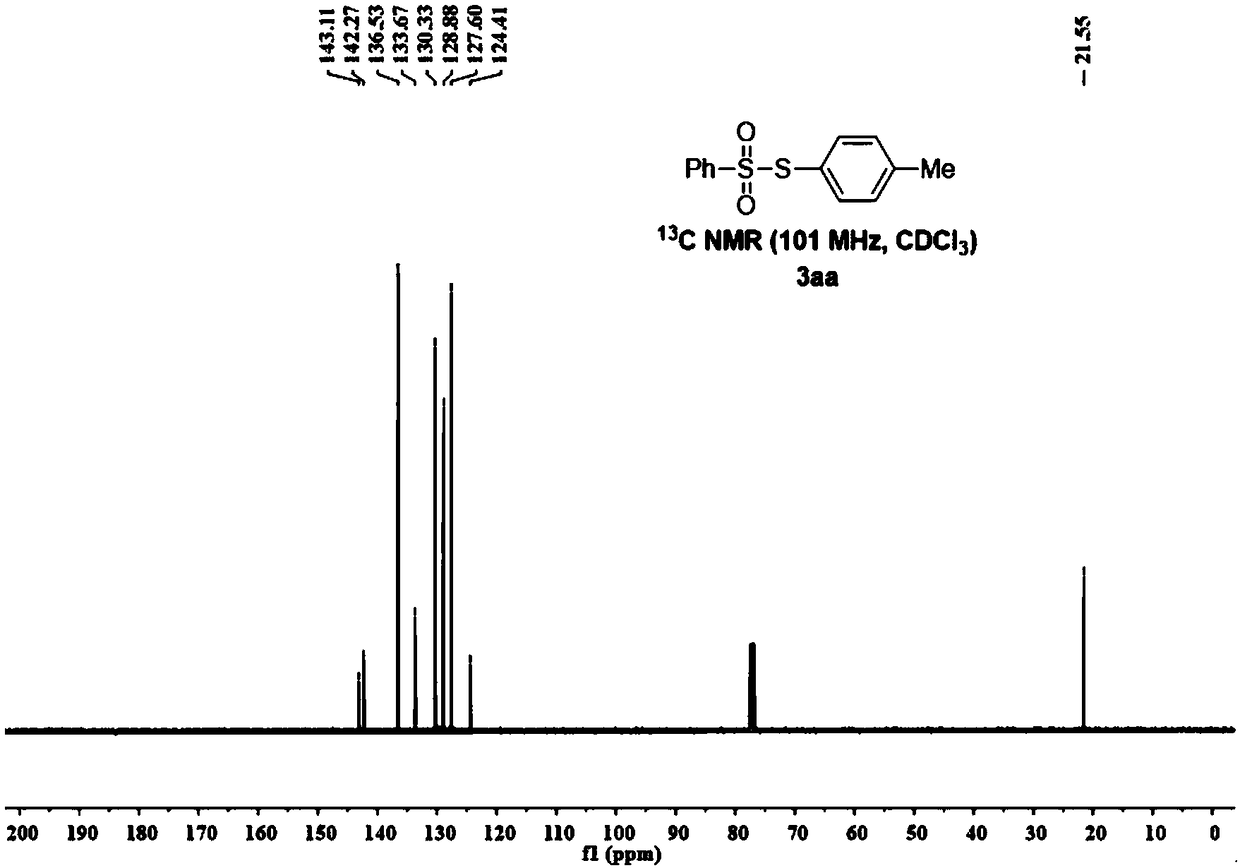
Synthesis method of thiosulfonate compound
ActiveCN108191729AImprove conversion rateNo pollutionOrganic chemistryOrganic synthesisThiosulfonic Acids
The invention belongs to the field of organic synthesis, and particularly relates to a synthesis method of a thiosulfonate compound. The invention provides the synthesis method of the thiosulfonate compound. The method comprises the following steps that methanesulfonohydrazide compounds, thiophenol compounds, oxidizing agents and catalysts are dissolved into a solvent for reaction; purification isperformed to obtain the thiosulfonate compound; oxidizing agents are any one kind or several kinds of materials from tertiary butanol peroxide and catalysts are sodium iodide, potassium iodide, ammonium iodide, iodine element and tetrabutylammonium iodide. Through experiment testing, the thiosulfonate compound prepared by the technical scheme provided by the invention has the advantages that no side reaction occurs; the yield can reach 84 to 100 percent; the conversion rate is high; meanwhile, the used catalysts and catalysts are environment-friendly compounds; the environment pollution cannot be caused; the reaction temperature is low; the method is suitable for industrial mass popularization. The defects that in the prior art, the damage to the environment in the asymmetric thiosulfonates synthesis process is great, and the application to industrial production cannot be realized are overcome.
Owner:GUANGDONG UNIV OF TECH
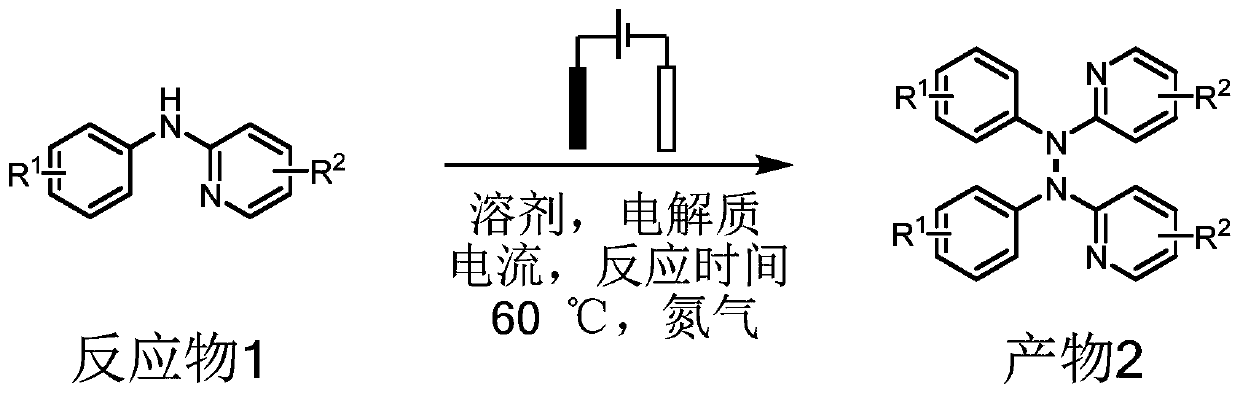
Method for electrochemical synthesis of tetraarylhydrazine compound
ActiveCN111235599AReduce usageSimple reaction systemElectrolysis componentsElectrolytic organic productionArylPtru catalyst
The invention relates to a method for electrochemical synthesis of a tetraarylhydrazine compound, and belongs to the technical field of electrochemical organic synthesis. The method comprises the following steps: sequentially adding a diarylamine compound, a solvent and an electrolyte into an unseparated electrolytic tank, inserting an anode and a cathode, stirring, electrifying, reacting under the conditions of constant current and nitrogen, and after the reaction is finished, separating and purifying to obtain the product tetraarylhydrazine compound. According to the method, tetrabutylammonium iodide is used as an electrolyte for a reaction, and a metal catalyst, an oxidizing agent or acid-base does not need to be additionally added, so that toxic substances are effectively prevented from being used, the reaction is performed under non-toxic and harmless conditions, and a reaction system is simple, efficient and environment-friendly.
Owner:QILU UNIV OF TECH
Method for preparing cyclic carbonate
InactiveCN103641811BClear structureThe synthesis method is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsRare earth metal compoundsIon
Owner:SUZHOU UNIV
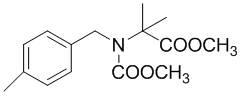
Method for synthesizing 2-(N-4-methyl benzyl) methoxy-acetamido methyl isobutyrate
InactiveCN102660753AEfficient use ofEfficient conversionElectrolysis componentsCarbamic acid derivatives preparationN dimethylformamideMethyl acetate
The invention discloses a method for synthesizing 2-(N-4-methyl benzyl) methoxy-acetamido methyl isobutyrate. The method is characterized by comprising the following steps of: mixing p-methyl iminobenzyl methyl acetate, N,N-dimethylformamide and tetrabutylammonium iodide, saturating carbon dioxide under normal pressure, performing electrical carboxylation reaction under the condition of constant current, esterifying and purifying to obtain the 2-(N-4-methyl benzyl) methoxy-acetamido methyl isobutyrate. Compared with the prior art, the method has the advantages of simple process, convenience for operation, safety and readily available raw materials; and the carbon dioxide serving as greenhouse effect gas is utilized effectively, atmospheric pollution is reduced greatly, and the conversion of aromatic imine compounds and the effective synthesis of N-carboxylation products are realized simultaneously, so the method is a process route with industrial synthetic value.
Owner:EAST CHINA NORMAL UNIV
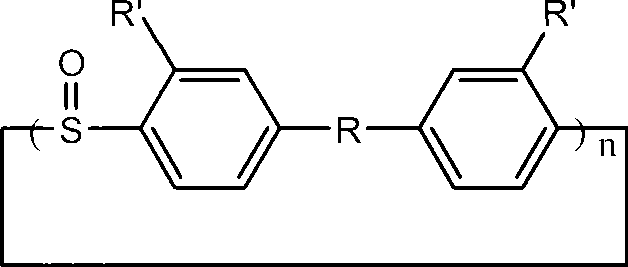
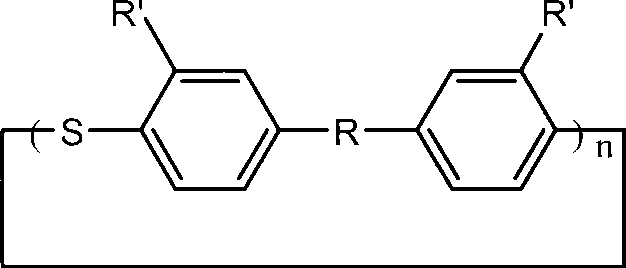
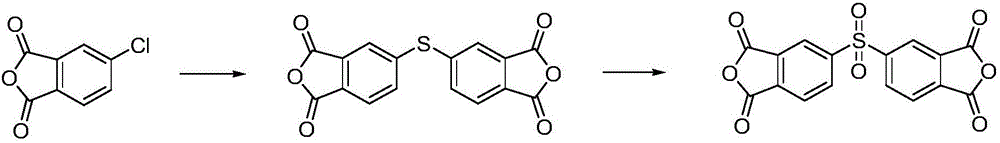
Macrocyclic aryl thioether ether sulfone oligomer and preparation method thereof
The invention relates to a macrocyclic aryl thioether ether sulfone oligomer and preparation method thereof. Under 'pseudo-high dilution' condition, Friedel-Crafts-Karrer reaction is utilized to open up a new route of taking thionyl chloride and substituted aromatic hydrocarbon monomers as basic raw materials to synthesize a macrocylic oligomer of high-performance resin, namely the macrocyclic aryl thioether ether sulfone oligomer with high yield. Under the action of tetrabutylammonium iodide and oxalyl chloride, sulfoxide groups of the macrocyclic aryl thioether ether sulfone oligomer can be reduced into thioether bonds to generate a cyclic polythioether oligomer. The cyclic polythioether oligomer can be melted and subjected to ring opening polymerization under the initiation of a free radical initiator to obtain a high-performance linear arylene sulfide by using a cyclic structure and low melt viscosity peculiar to the aromatic ring-shaped oligomer. Products subjected to ring opening polymerization do not contain ions and can be widely applied in the fields of micro-electronics, high temperature resisting viscose agents, and the like requiring excellent electrical insulating property.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Synthetic method of (E)-alkenyl sulfone compounds
InactiveCN109096157AHigh purityHigh yieldOrganic compound preparationOrganic chemistry methodsSulfohydrazideMetal catalyst
The invention discloses a synthetic method of (E)-alkenyl sulfone compounds. The method comprises the following steps: beta-nitroolefin compounds shown as formula I in the description, sulfohydrazidecompounds shown as formula II in the description, an iodine-containing nonmetal catalyst and a peroxide oxidizing agent are mixed for a reaction, and a mixture obtained after the reaction is subjectedto postprocessing, and the (E)-alkenyl sulfone compounds shown as formula III in the description are obtained. Beta-nitroolefin compounds, the sulfohydrazide compounds, the iodine-containing nonmetalcatalyst and the peroxide oxidizing agent are taken as main raw materials, the corresponding (E)-alkenyl sulfone compounds are synthesized through free radical reaction, the iodine-containing nonmetal catalyst iodine or tetrabutylammonium iodide is used, the raw materials are simple, easily available, cheap, non-toxic and pollution-free to the environment, the dosage of the catalyst is small, thecatalyst has higher specificity for the reaction, synthetic reaction time can be shortened, the problem that the metal catalyst is difficult to separate in the prior art can be well solved, postprocessing difficulty is reduced, yield of the final product is increased, and purity of the final product is improved.
Owner:HUNAN UNIV OF ARTS & SCI
Preparation method of 1-bromoalkyne and 1-iodoalkyne
ActiveCN108658724AEasy to operateSimple post-processingOrganic compound preparationHalogenated hydrocarbon preparationSodium iodideLithium bromide
The invention discloses a preparation method of 1-bromoalkyne and 1-iodoalkyne. The preparation method comprises the following steps that terminal alkyne is adopted as a raw material, a chloramine salt and an iodine salt or a bromine salt is adopted as a halogenation system, and reaction is carried out in a solvent to synthesize a series of 1-bromoalkyne and 1-iodoalkyne type compounds. In the formula shown in the description, R in terminal alkyne is selected from chain-like alkyne, ring-shaped alkyne, olefin, ester group, cyanogroup, substituted phenyl group and heterocyclic arene; the chloramine salt is selected from one of chloramine B, chloramine T or o-chloramine T; the iodine salt is selected from one of sodium iodide, potassium iodide, ammonium iodide, lithium iodide or tetrabutylammonium iodide; the bromine salt is selected from one of sodium bromide, potassium bromide, lithium bromide, magnesium bromide, ammonium bromide or tetrabutylammonium bromide; the solvent is selected from one or is mixed by two of water, benzene, methylbenzene, 1, 4-dioxane, ethyl acetate, dimethyl sulfoxide, methanol, tetrahydrofuran, alcohol, isopropyl alcohol, N, N-dimethyl formamide, n-pentane,dichloromethane, petroleum ether, methyl tert-butyl ether, chloroform, n-hexane, carbon tetrachloride, n-butyl alcohol, 1,2-dichloroethane or acetonitrile. The preparation method disclosed by the invention has the beneficial effect that the application is wide.
Owner:ZUNYI MEDICAL UNIVERSITY
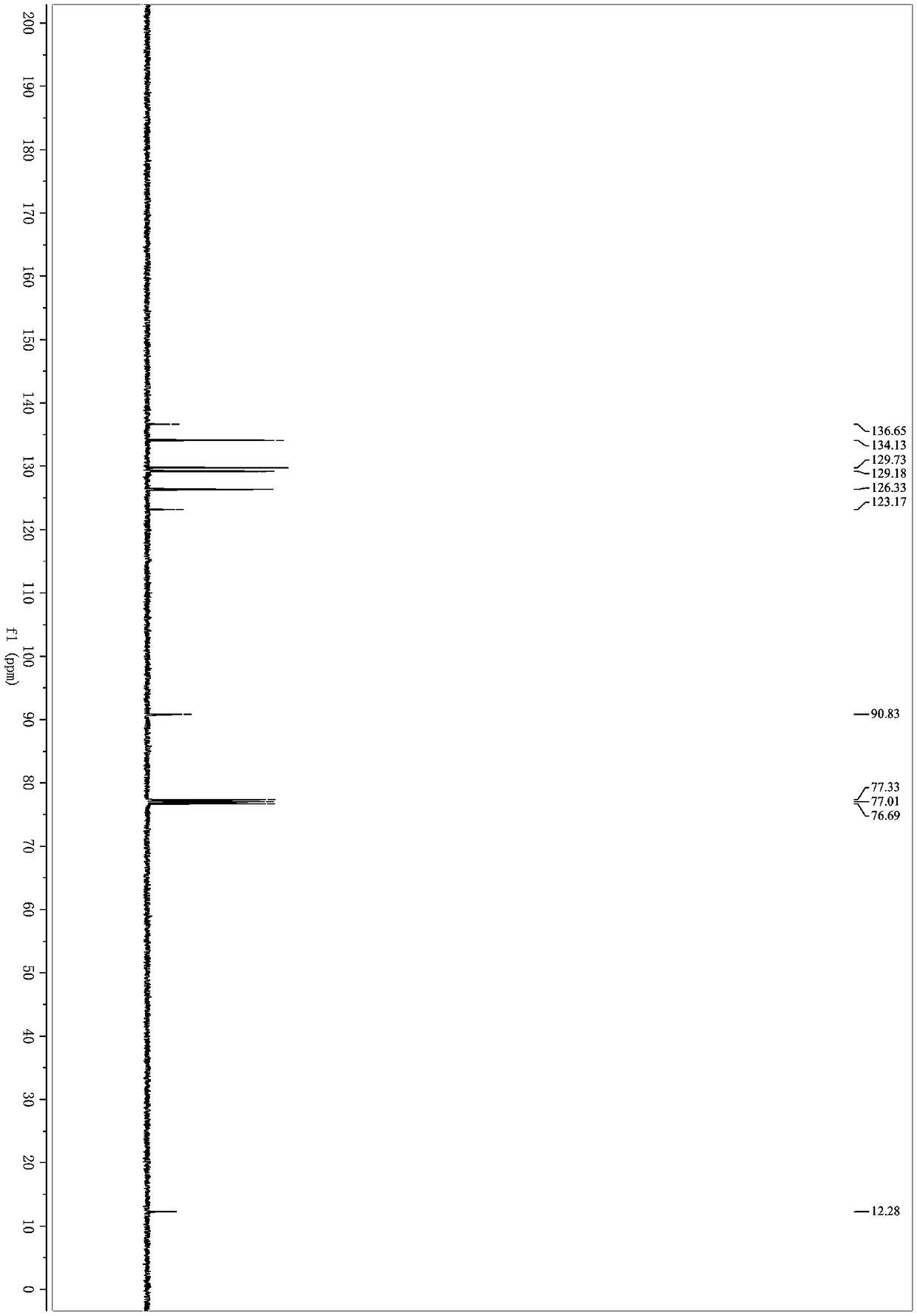
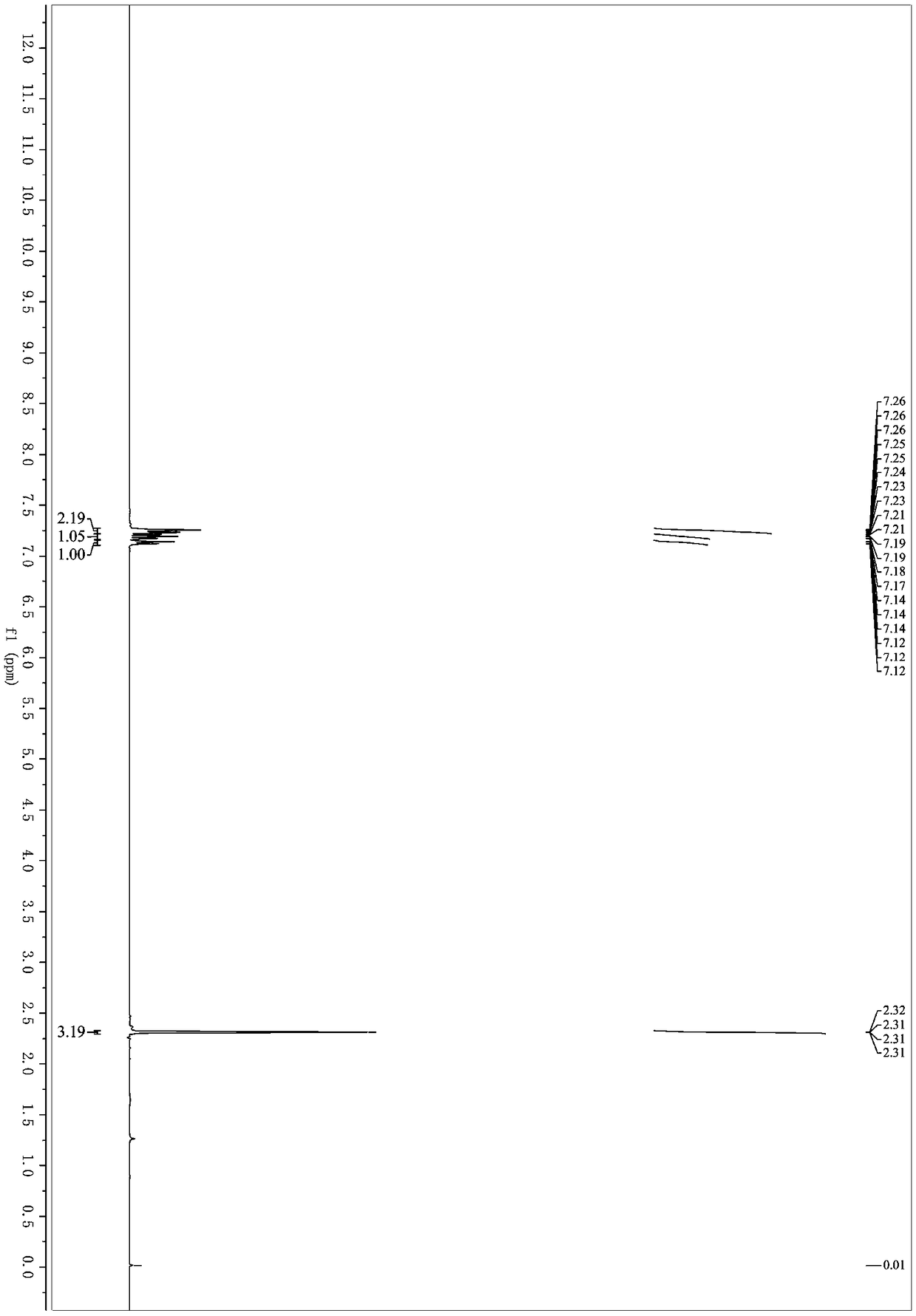
Preparation method of 3-quinolyl-5-trifluoromethyl substituted 1, 2, 4-triazole compound
ActiveCN113307790AStrong designabilityWide range of toleranceOrganic chemistryTrifluoromethylTetrabutylammonium iodide
The invention discloses a preparation method of a 3-quinolyl-5-trifluoromethyl substituted 1, 2, 4-triazole compound, which comprises the following steps: adding tetrabutylammonium iodide, a tert-butyl hydroperoxide aqueous solution, diphenyl phosphoric acid, trifluoroethyl imine hydrazide and 2-methylquinoline into an organic solvent, heating to 80-100 DEG C, reacting for 8-14 hours completely, and performing post-treatment to obtain the 3-quinolyl-5-trifluoromethyl substituted 1, 2, 4-triazole compound. The preparation method is simple to operate, the initial raw materials are cheap and easy to obtain, the reaction does not need to be operated under anhydrous and anaerobic conditions, a heavy metal does not need to be used as a catalyst, and the 1, 2, 4-triazole compound which is diverse in substitution at different positions and simultaneously contains quinolyl and trifluoromethyl can be synthesized through substrate design. And the applicability of the method is widened while the operation is convenient.
Owner:HANGZHOU VOCATIONAL & TECHN COLLEGE
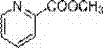
Synthesis method of methyl picolinate
InactiveCN107400898AEfficient use ofReduce pollutionElectrolysis componentsElectrolytic organic productionTetramethylammonium bromideTetrafluoroborate
The invention discloses a synthesis method of methyl picolinate. The method comprises the following steps that 2-bromopyridine, N,N-dimethylformamide or acetonitrile and tetraethylammonium chloride or tetraethylammonium bromide or tetraethylammonium iodide or tetraethylammonium tetrafluoroborate or tetrabutylammonium chloride or tetrabutylammonium bromide or tetrabutylammonium iodide are mixed into an electrolyte; carbon dioxide is introduced for 30min at normal pressure; then, electric carboxylation reaction is performed by using constant current (the carbon dioxide is continuously introduced in the electrolysis process until the electrolysis is completed); then, the methyl picolinate is obtained through esterification and post treatment. A reaction system is simple; the control is easy; in addition, rich Cl resources and CO2 are used as one of raw materials; the raw materials are cheap and can be easily obtained; the cost is low; a novel path is developed for the study on challenging organic matters of green synthetic heterocyclic ring carboxylic acid derivatives and the like.
Owner:LIAOCHENG UNIV
Preparation method of alpha-acyloxy ether
InactiveCN102010281AMild reaction conditionsImprove compatibilityOrganic compound preparationCarboxylic acid esters preparationSodium iodideOrganic synthesis
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method of alpha-acyloxy ether. The method comprises the following steps: (1) configuring a reaction system, wherein the reaction system comprises reaction substrates carboxylic acid, ether, a catalyst and an oxidant; the formula of the carboxylic acid is shown in the specification, wherein R is selected from one of aryl of C6-C12, alkyl of C1-C15, alkenyl of C2-C8 or unsubstituted five / six-membered heterocyclic radical, the ether is selected from one of unsubstituted saturated open-chain ether or cyclic ether, the catalyst is selected from one of potassium iodide, iodine, amine tetrabutylammonium iodide, benzyl trimethyl ammonium iodide or sodium iodide, and the oxidant is tert-butyl hydroper oxide or hydrogen peroxide; and (2) mixing the above reaction system at a temperature ranging from room temperature to 100 DEG C for 2-24hours, thus obtaining the alpha-acyloxy ether. The method is green and moderate, and has high selectivity and wide application.
Owner:SUZHOU UNIV
Synthetic method of methyl-2,3,4-trioxy-benzyl-beta-D-pyran riboside
ActiveCN104119407AHigh yieldHigh activitySugar derivativesSugar derivatives preparationPotassium hydroxideBenzyl chloride
The invention provides a synthetic method of methyl-2,3,4-trioxy-benzyl-beta-D-pyran riboside. The method comprises the following step: enabling 1-methyl-beta-D-pyran riboside and benzyl chloride to react under the actions of potassium hydroxide and tetrabutylammonium iodide or under the actions of potassium hydroxide and tetrabutylammonium bromide to prepare methyl-2,3,4-trioxy-benzyl-beta-D-pyran riboside. According to the synthetic method provided by the invention, potassium hydroxide is used for replacing sodium hydride as alkali, and tetrabutylammonium bromide or tetrabutylammonium iodide is added at the same time to successfully prepare methyl-2,3,4-trioxy-benzyl-beta-D-pyran riboside; the synthetic method can be used for avoiding risk factors caused by the application of sodium hydride and avoiding harsh requirements for the environment humidity, and ensures that the reaction has relatively high yield.
Owner:济南尚博医药股份有限公司
Synthetic method of isonicotinic acid
InactiveCN107354477AEfficient use ofEfficient synthesisElectrolysis componentsElectrolytic organic productionChemical industryTetramethylammonium bromide
The invention discloses a synthetic method of isonicotinic acid. The method comprises the following steps: mixing 4-bromopyridine with N, N-dimethylformamide or acetonitrile and tetraethylammonium chloride or tetraethylammonium bromide or tetraethylammonium iodide or tetraethylammonium tetrafluoroborate or tetrabutylammonium chloride or tetrabutylammonium bromide or tetrabutylammonium iodide to form an electrolyte; introducing carbon dioxide at normal pressure for 30 minutes; and carrying out electrolysis at a constant current, keeping introducing the carbon dioxide during the electrolysis process until the electrolysis is ended, and obtaining the isonicotinic acid after carrying out post-treatments. According to the synthetic method of the isonicotinic acid, the reaction system is simple and easy to control, the rich C1 resource, namely the carbon dioxide is used as one of the raw materials, is cheap and easy to obtain, is low in cost and does not pollute the environment, and therefore a new way is developed for the study of the green isonicotinic acid, an extremely good application prospect of the synthetic method is displayed in the medicine industry, the food industry, the chemical industry and the electronic industry, and the synthetic method of the isonicotinic acid is a process route with high industrial synthetic value.
Owner:LIAOCHENG UNIV
Preparation method of 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride
The invention discloses a preparation method of 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride. The preparation method comprises the following steps that 1, 4-chlorophthalic anhydride is dissolved into dimethyl sulfoxide, then sulfur and sodium tert-butoxide are added, carbon disulfide is dropwise added when the temperature is increased to 40 DEG C, after the raw materials completely react, part of the solvent is recycled under reduced pressure, a remaining product is added into water, and filtering, water washing and drying are conducted to obtain 3,3',4,4'-diphenyl thioether tetracarboxylicdianhydride; 2, 3,3',4,4'-diphenyl thioether tetracarboxylicdianhydride is dissolved into acetonitrile, ceric ammonium nitrate, tetrabutylammonium iodide and water are added, potassium persulfate is added in batches under the heating condition, after 3,3',4,4'-diphenyl thioether tetracarboxylicdianhydride completely reacts, water is added into reaction liquid, the temperature is lowered to 5 DEG C, filtering, water washing and drying are conducted, and 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride is obtained. The preparation method of 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride has the following advantages that the raw materials are easy to purchase, the reaction solvent can be recycled, aftertreatment operation is easy, the yield is high, the product quality is good, and environmental pollution is low.
Owner:天津众泰材料科技有限公司
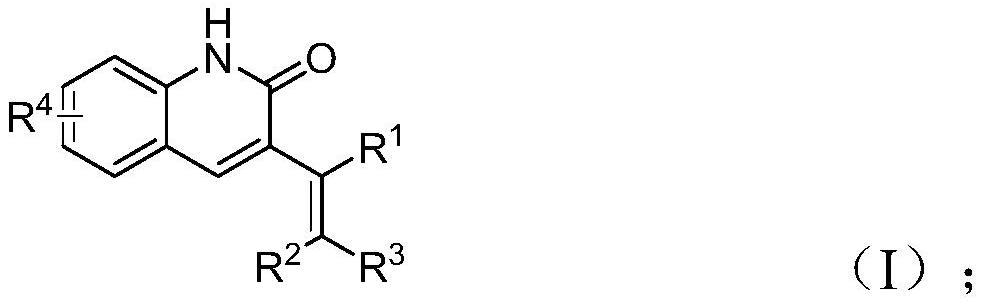
Preparation method of 3-alkenyl quinoline-2 (1H) ketone derivative
ActiveCN114478375AWide range of toleranceEasy to prepareOrganic chemistryAgainst vector-borne diseasesQuinolineEthylic acid
The invention discloses a preparation method of a 3-alkenyl quinoline-2 (1H) ketone derivative, which comprises the following steps: carrying out reaction on palladium acetate, tris (3-methoxyphenyl) phosphine, carbonyl molybdenum, cesium carbonate, tetrabutylammonium iodide, o-nitrobenzaldehyde and allyl aryl ether at 100 DEG C for 30 hours, and after the reaction is completed, carrying out post-treatment to obtain the 3-alkenyl quinoline-2 (1H) ketone derivative. According to the preparation method, o-nitrobenzaldehyde is used as a nitrogen source and a formyl source, the operation is simple, the reaction initial raw materials are cheap and easy to obtain, the tolerance range of substrate functional groups is wide, and the reaction efficiency is high. Various 3-alkenyl quinoline-2 (1H) ketone derivatives can be synthesized according to actual needs, and the practicability of the method is widened while the operation is convenient.
Owner:ZHEJIANG SCI-TECH UNIV
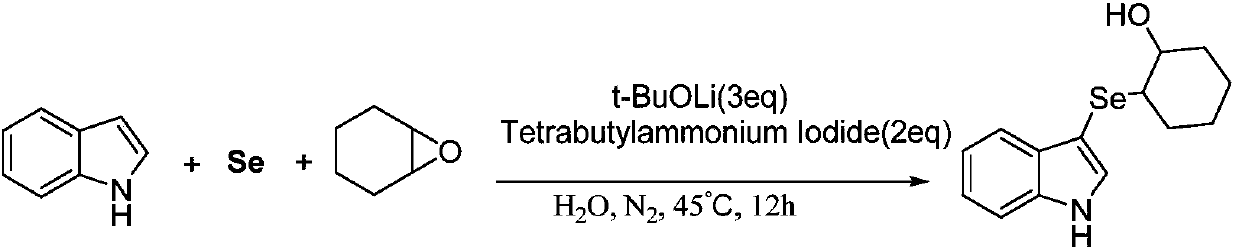
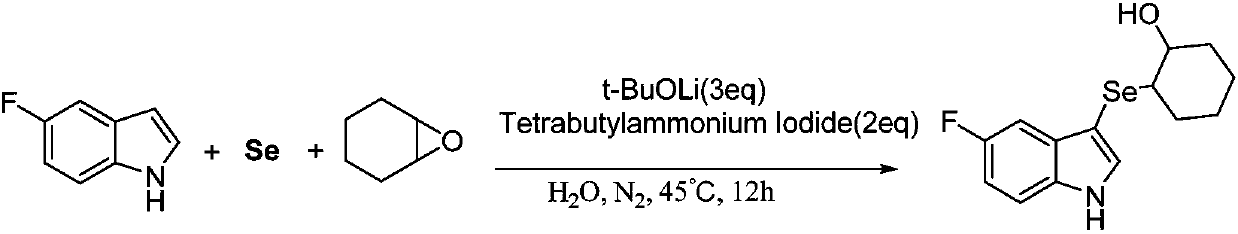
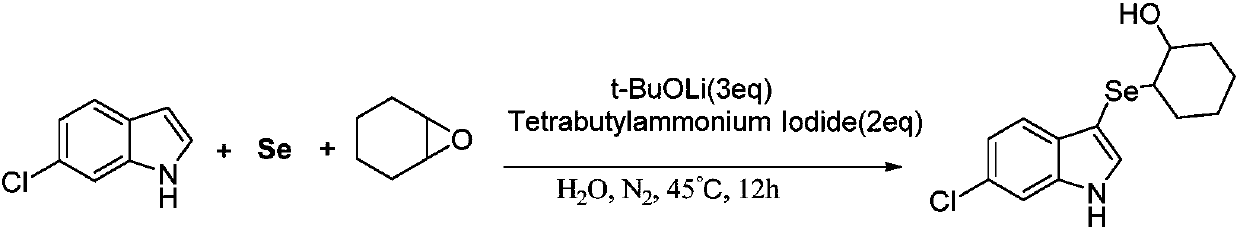
Method for synthesizing 3-indole selenide alcohol organic compound
ActiveCN108047118AImprove toleranceMild reaction conditionsOrganic chemistryCyclopenteneNitrogen gas
The invention discloses a method for synthesizing a 3-indole selenide alcohol organic compound. The method is characterized by comprising the following steps: taking an indole compound and cyclohexeneoxide / cyclopentene oxide / 1,2-epoxybutane / isobutylene oxide / 3,3-dimethyl epoxybutane / 2-furfuryl glycidyl ether as reaction substrates, taking elemental selenium as a selenium source, taking water or an organic solvent as a reaction solvent, adding a phase transfer catalyst tetrabutylammonium iodide into the water when the water serves as the reaction solvent, and performing transition-metal-free catalysis in a nitrogen atmosphere in the presence of an inorganic base, thereby obtaining the 3-indole selenide alcohol organic compound. The method disclosed by the invention has the beneficial effects that the 3-indole selenide alcohol organic compound can be synthesized at high yield and high purity by adopting the method disclosed by the invention, and the method is mild in reaction conditions, wide in reaction substrate range, excellent in functional group tolerance, simple in after-treatment, simple and convenient to operate and suitable for large-scale industrialized production and provides a brand new synthetic route for high-efficiency and rapid synthesis of the compounds.
Owner:温州大学苍南研究院
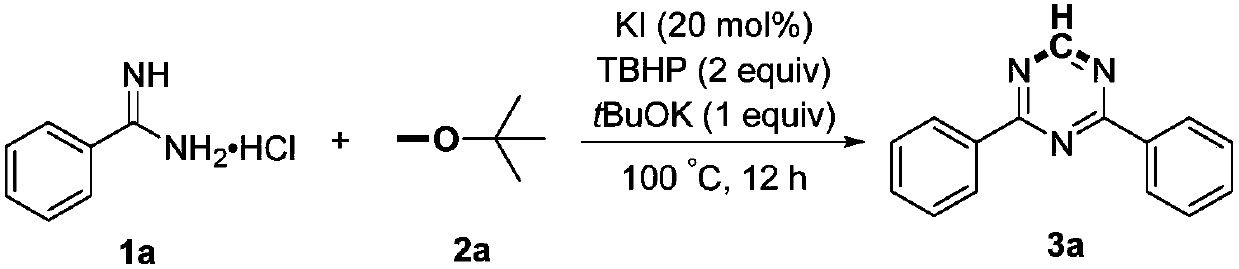
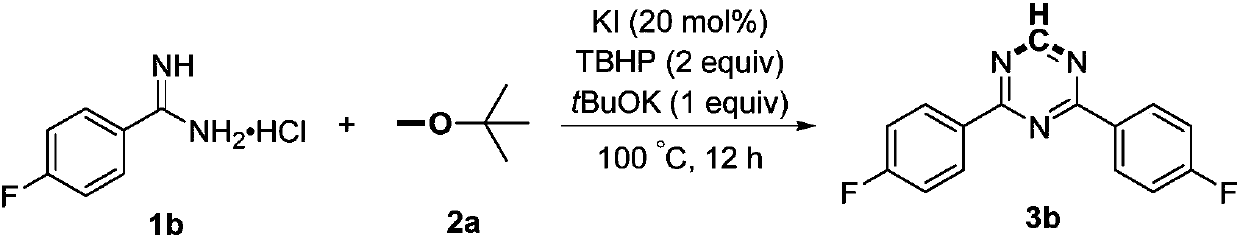
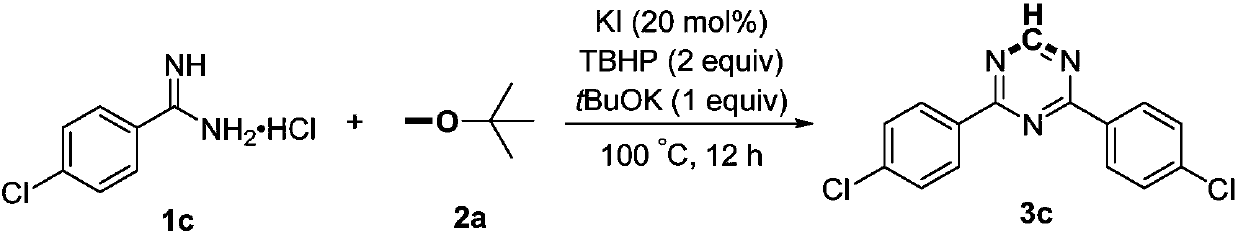
Preparation method of 2,4-di-substituted-1,3,5-triazine
ActiveCN107759530AWide variety of sourcesEasy to getOrganic chemistryPotassium iodinePotassium carbonate
The invention discloses a method for preparing 2,4-di-substituted-1,3,5-triazine. The method comprises the specific step: with substituted formamidine hydrochloride as a reaction substrate an iodine-containing compound as a catalyst, tert-butyl hydroperoxide as an oxidant, inorganic base as an acid binding agent and aliphatic ether as an organic solvent (also used as a carbon source), carrying outcarbon-hydrogen and carbon-oxygen bond deletion, nucleophilic addition, deaminizing condensation and oxidative aromatization reaction to obtain a 2,4-di-substituted-1,3,5-triazine compound, wherein achemical structural general formula of the substituted formamidine hydrochloride is shown in the description; the iodine-containing compound is selected from one of potassium iodide (KI), tetrabutylammonium iodide (TBAI), elemental iodine (I2) and N-iodosuccinimide (NIS); the inorganic base is selected from one of anhydrous potassium carbonate, anhydrous sodium carbonate, cesium carbonate, potassium hydroxide and potassium tert-butoxide; and the aliphatic ether is selected from one of methyl tert-butyl ether, ethyl ether and ethylene glycol dimethyl ether. The preparation method disclosed bythe invention has the characteristics of easily-obtained raw materials, low price and low toxicity of a catalyst, wide range of the substrate, simplicity and convenience in operation, greenness, environmental protection and the like.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
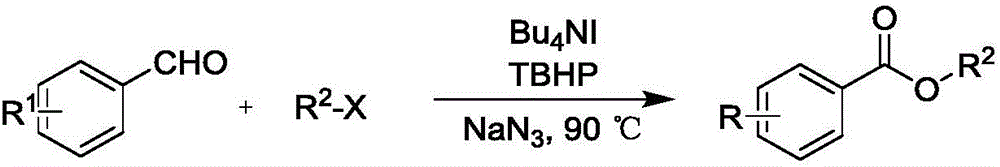
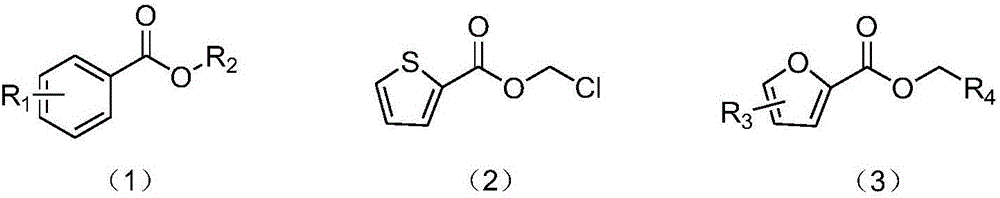
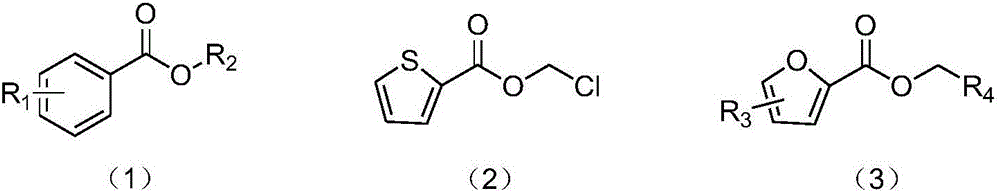
Synthesis method of carboxylic ester compound
ActiveCN106588644AHas antibacterial propertiesInsecticidalOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsStrong acids
The invention provides a synthesis method of an ester compound represented by the formula (1), the formula (2) or the formula (3), wherein the method comprises the steps: dissolving an aldehyde compound, sodium azide, tetrabutylammonium iodide and a halogenated compound in a solvent, or directly dissolving an aldehyde compound, sodium azide and tetrabutylammonium iodide in a halogenated compound, carrying out a reaction for 1-12 h at the temperature of 90-100 DEG C, and after the reaction is finished, postprocessing the reaction liquid to obtain the compound represented by the formula (1). The synthesis method has the advantages of mild reaction conditions, no participation of strong acids, strong alkalis or metals, simpliness, high efficiency, high yield, wide application range of substrates, amplified production potential, and relatively large potential economic value.
Owner:HANGZHOU NORMAL UNIVERSITY
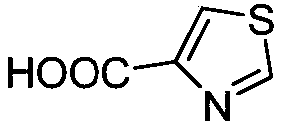
Synthetic method of thiazole-4-formic acid
ActiveCN108977839ASimple systemMild conditionsElectrolysis componentsComponent separationTetramethylammonium iodideTetramethylammonium bromide
The invention discloses a synthetic method of thiazole-4-formic acid. The method comprises the steps that 4-bromo-thiazole is added into a quaternary ammonium salt solution to prepare electrolyte, theconstant current is connected into the electrolyte to conduct an electric carboxylation reaction in the atmosphere of carbon dioxide, an acetonitrile water solution is adopted to conduct hydrolysis,and thiazole-4-formic acid can be obtained, wherein quaternary ammonium salt is selected from tetraethylammonium chloride hydrate, tetraethylammonium bromide, tetraethylammonium iodide, tetraethyl tetrafluoroborate, tetrabutylammonium chloride hydrate, tetrabutylammonium bromide and tetrabutylammonium iodide, the solvent of the electrolyte is N,N-dimethyl formamide or acetonitrile. The reaction system of the synthetic method is simple, the condition is moderate, the process is easy to control, carbon dioxide can be utilized, and a route is provided for solution of greenhouse effect gas.
Owner:LIAOCHENG UNIV
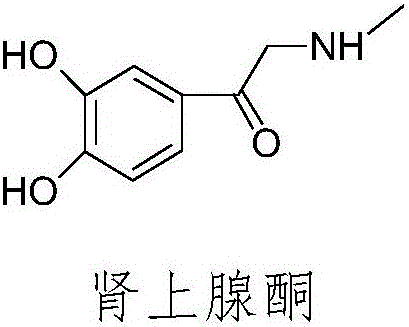
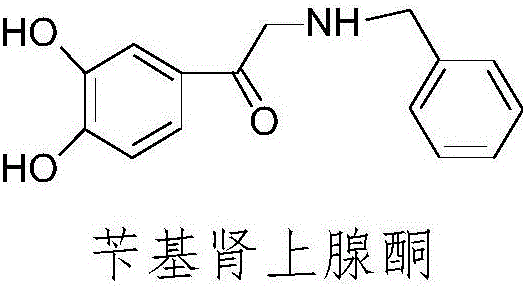
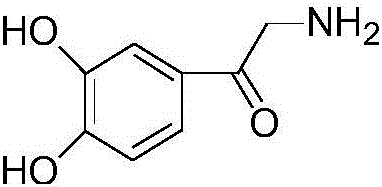
Preparation method of adrenalone hydrochloride
ActiveCN106748835AIncrease contact areaReduce generationOrganic chemistryOrganic compound preparationAlcoholReaction speed
The invention relates to a preparation method of adrenalone hydrochloride. In a process of preparing the adrenalone hydrochloride from chloracetyl catechol and methylamine, reaction is controlled to be carried out in an alcohol solution and tetrabutylammonium bromide or tetrabutylammonium iodide is selected as a phase transfer catalyst. According to the method provided by the invention, the reaction is controlled to be carried out in the alcohol solution and sufficient proceeding of the reaction is promoted; meanwhile, the tetrabutylammonium bromide or the tetrabutylammonium iodide is added to be used as the phase transfer catalyst, so that the reaction is accelerated, the reaction time is shortened and the accumulation of impurities is reduced. The adrenalone hydrochloride with high quality can be prepared at a single time without the need of refining, and a high-quality intermediate is provided for preparation of L-adrenaline.
Owner:BENGBU BBCA MEDICINE SCI DEV
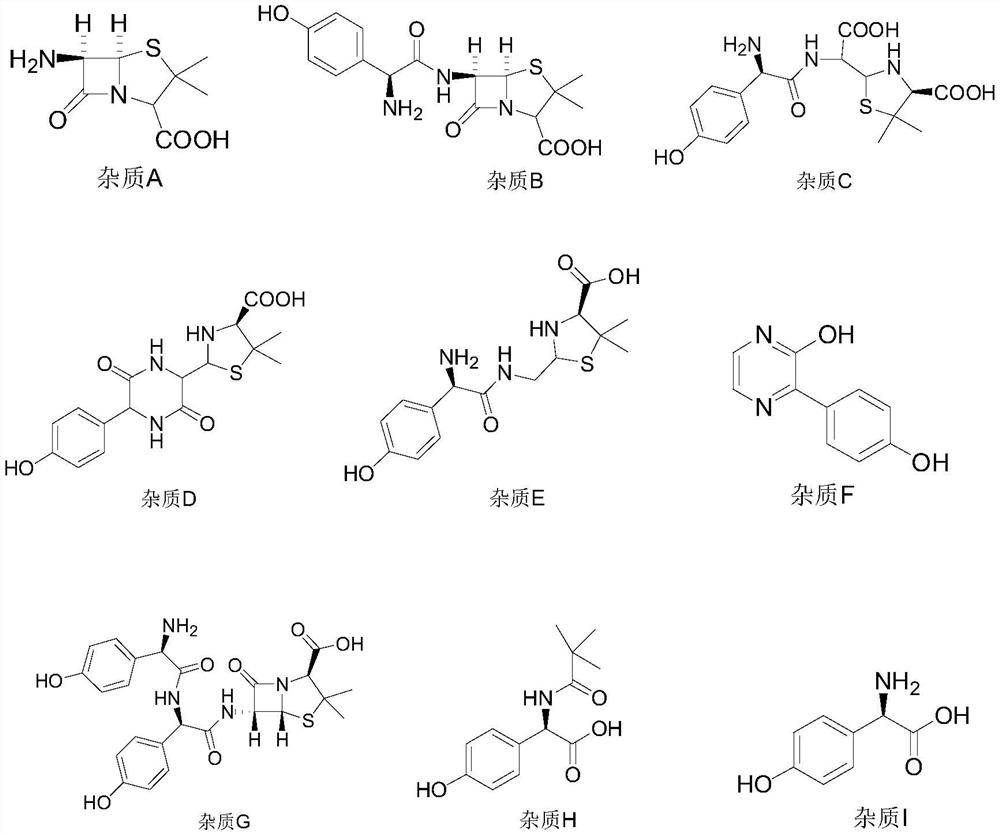
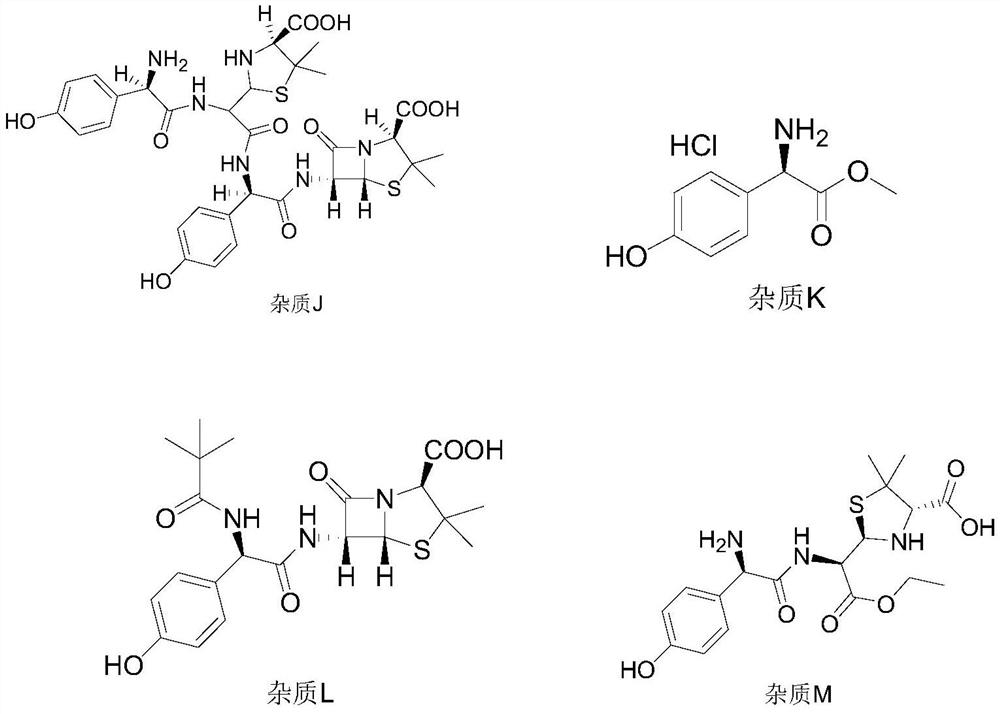
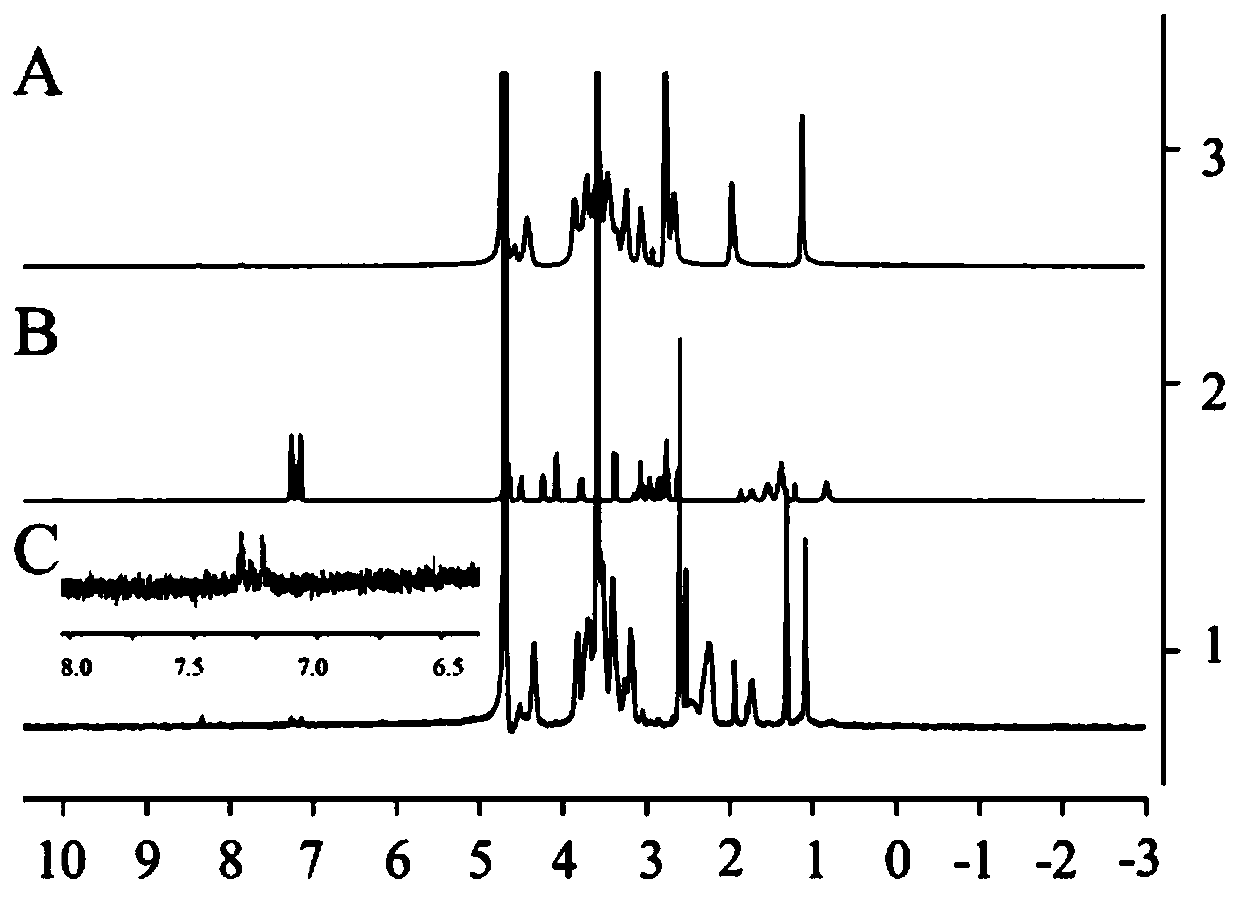
Impurity detection method of amoxicillin bulk drug
The invention belongs to the field of pharmaceutical analysis, and particularly relates to a method for separating impurities from an amoxicillin bulk drug by adopting a liquid chromatographic columnobtained by nano-silver hybrid silica gel filler. The method provided by the invention comprises the following steps of: preparing a liquid chromatographic column taking nano-silver hybrid silica gelas a filler, and then performing detection by adopting high performance liquid chromatography. The preparation method of the nano-silver hybrid silica gel comprises the following steps: mixing an acidified silica gel matrix and nano-silver sol, and enabling the mixture to react with octadecyl mercaptan to obtain the nano-silver hybrid silica gel. The chromatographic conditions are as follows: theused mobile phase consists of a mobile phase A and a mobile phase B, the mobile phase A is a solution prepared from tetrabutylammonium iodide, glacial acetic acid and water, the mobile phase B is a mixed solution of methanol and water, and the mobile phase A and the mobile phase B are mixed and then subjected to ultrasonic treatment for 5-30 minutes. Compared with a traditional detection method, the novel chromatographic column obtained by the invention is high in analysis effect, relatively high in column efficiency and separation degree and relatively short in analysis time, and is particularly suitable for quality monitoring of amoxicillin bulk drugs.
Owner:GUANGZHOU LIXIN PHARM CO LTD
Synthesis method of O-[2]-[[tert-Butoxycarbonyl]amino]ethyl]-N-[fluorene methoxycarbonyl]-L-tyrosine
ActiveCN110015978ASimple reaction conditionsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationSodium bicarbonateTert-Butyloxycarbonyl protecting group
The invention relates to a synthesis method of O-[2]-[[tert-Butoxycarbonyl]amino]ethyl]-N-[fluorene methoxycarbonyl]-L-tyrosine. The present invention mainly solves the technical problem of high scale-up production cost existing in the present synthesis method. The synthetic steps of that invention comprise: arranging L-tyrosine alanine benzyl ester p-toluenesulfonate in the mixture of 1,4-dioxaneand water, adding sodium bicarbonate and Cbz-Cl to obtain compound 1, wherein that product does not need to be purified; dissolving the compound 1 and N-Boc bromoethylamine in DMF, adding potassium carbonate and tetrabutylammonium iodide to react to obtain compound 2, which can be purified by beating in ethanol; dissolving the compound 2 in tetrahydrofuran, adding palladium-carbon, hydrogenatingat normal pressure, and then adding directly aqueous sodium bicarbonate and Fmoc-Osu into the mixture to react at room temperature, pulping the crude product in the mixed solvent, and filtering to obtain the target product.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Method for preparing luminescent plastic rattan
The invention discloses a method for preparing luminescent plastic rattan. The method includes operation steps of (1), uniformly mixing strontium carbonate, aluminum oxide, europium sesquioxide, basicbismuth carbonate, dysprosium oxide and boric acid with one another and carrying out high-temperature calcining to obtain long-afterglow luminescent materials; (2), mixing the long-afterglow luminescent materials and tetrabutylammonium iodide with one another, heating mixtures until the tetrabutylammonium iodide is completely dissolved, uniformly stirring the mixtures, and then cooling the mixtures until the temperatures of the mixtures reach the room temperature so as to obtain long-afterglow luminescent materials with coated films; (3), uniformly mixing polyethylene resin, the long-afterglow luminescent materials with the coated films, antioxidants and dispersing agents with one another to obtain mixtures, adding the mixtures into a screw extruder, melting and extruding the mixtures toobtain functional master batch and preparing the luminescent plastic rattan from the functional master batch. The method has the advantages that the luminescent plastic rattan prepared by the aid of the method is excellent in water resistance, and the luminescent materials can be prevented from being dissolved out in use procedures; the luminescent materials are free of influence on the original excellent performance of the polyethylene resin.
Owner:ANHUI JINYUAN HOUSEHOLD ARTS
Synthesis method of bis [(trifluoromethyl) sulfonyl] methane
PendingCN111349030AHigh yieldHigh purityOrganic chemistryOrganic compound preparationElectrolytic agentAcyl group
The invention discloses a synthesis method of bis [(trifluoromethyl) sulfonyl] methane. The invention belongs to the technical field of battery electrolyte. The method comprises the following steps: adding sodium trifluoromethanesulfinate, methyl magnesium bromide and tetrabutylammonium iodide into an organic solvent to obtain a mixed solution; under the stirring condition, subjecting the mixed solution to heating reflux reaction for 8 to 12h; and cooling the reaction solution to room temperature, filtering, adding diethyl ether, adding tert-butyl lithium and trifluoromethanesulfonic anhydrideat -20 to -40 DEG C, stirring for 20-30 minutes, heating to 30-40 DEG C, continuing stirring for 20-30 minutes, washing, drying, filtering, and concentrating to obtain bis [(trifluoromethyl) sulfonyl] methane. The synthesis method is simple, and the obtained bis [(trifluoromethyl) sulfonyl] methane is high in yield, high in purity and low in moisture content.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
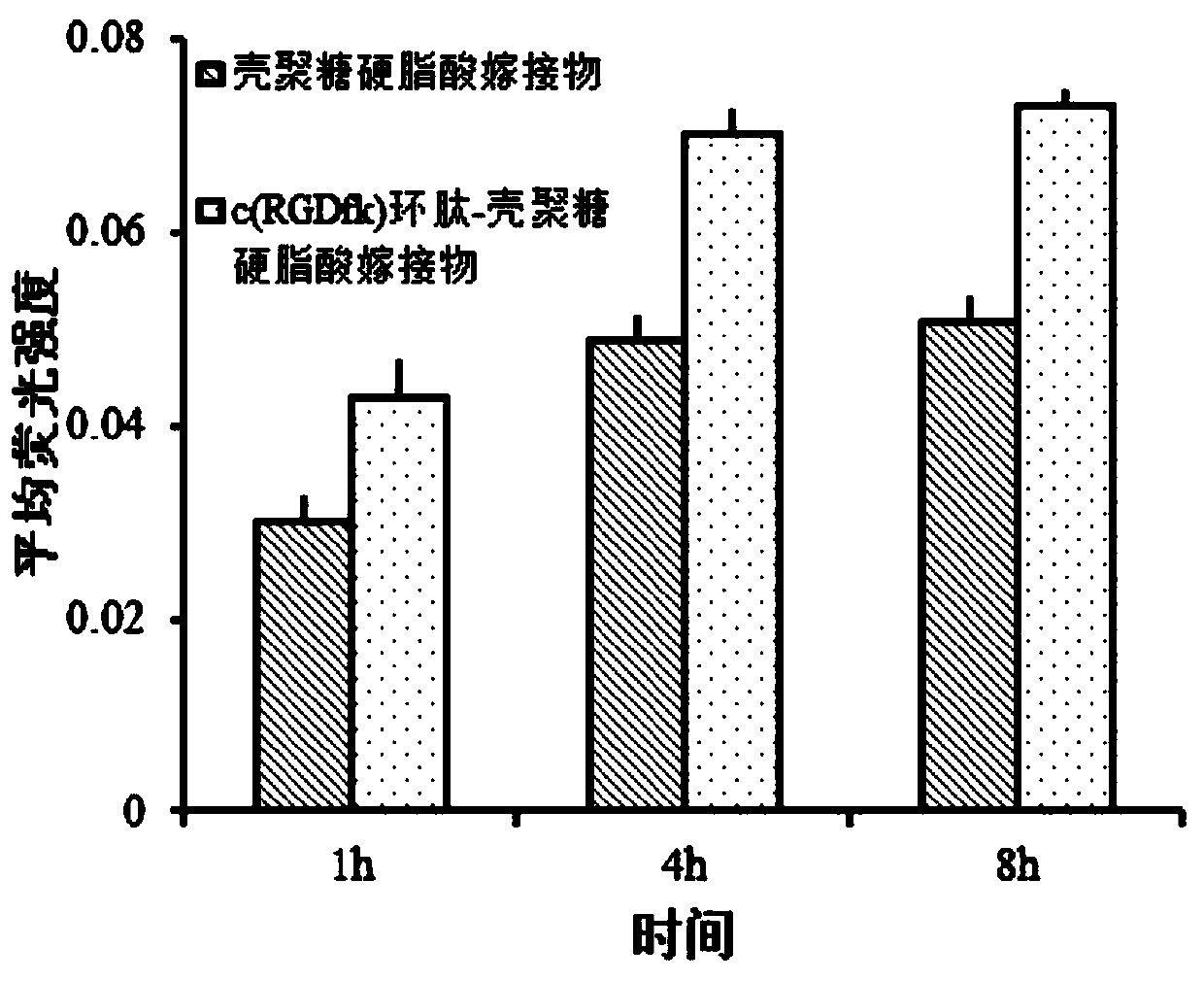
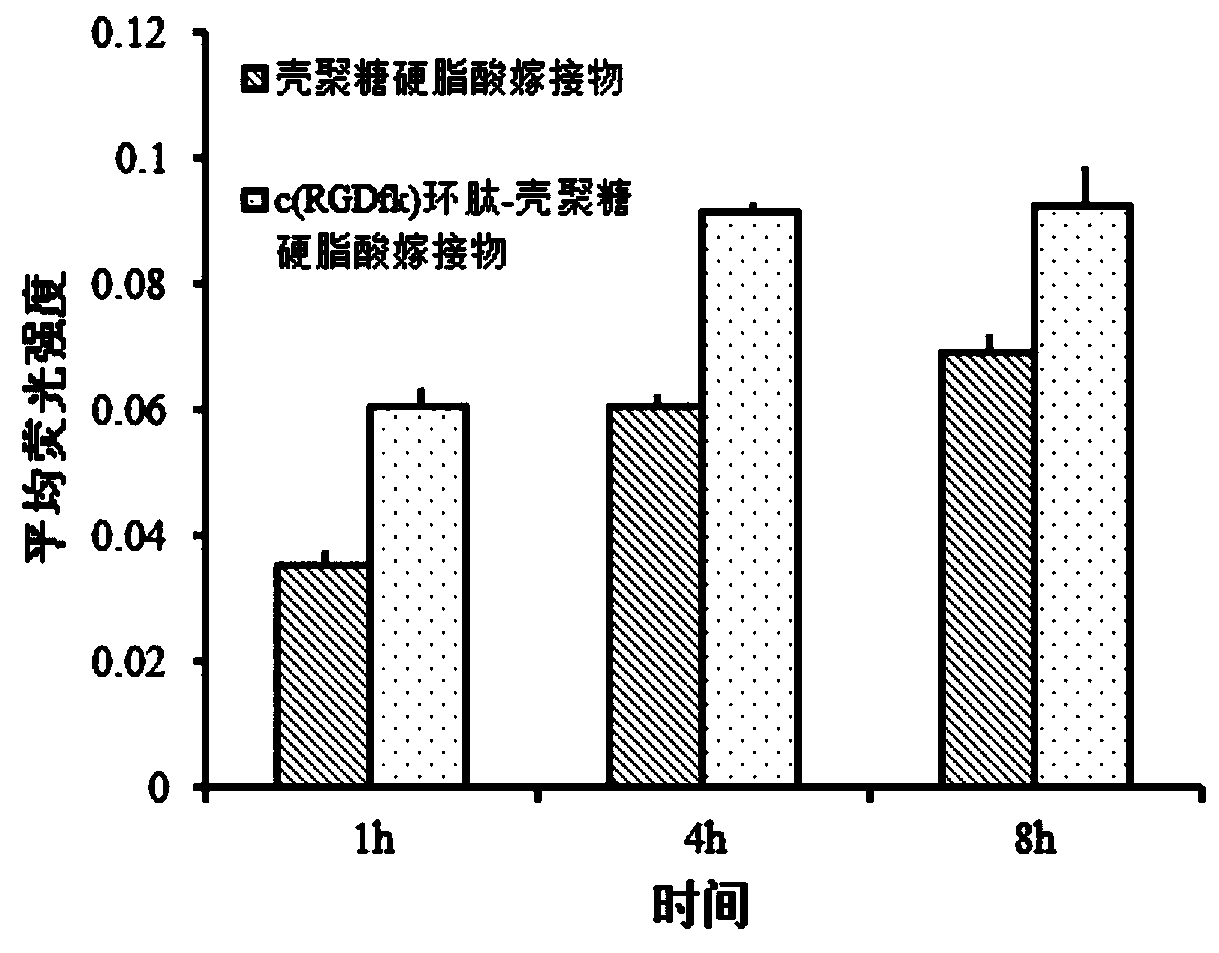
c(RGDfk) cyclic peptide-chitosan stearic acid graft drug-loaded micelles as well as preparation and application thereof
ActiveCN109851799AInduce apoptosisIncrease the concentration of photosensitizerEnergy modified materialsPharmaceutical non-active ingredientsCyclic peptideVascular endothelium
The invention provides c(RGDfk) cyclic peptide-chitosan stearic acid graft drug-loaded micelles. The micelles are obtained by the steps of grafting c(RGDfk) cyclic peptide to chitosan stearic acid toobtain a c(RGDfk) cyclic peptide-chitosan stearic acid graft, performing complexation on tetrabutylammonium iodide and photosensitizer indocyanine green through charge interaction to obtain photosensitizer hydrophobized indocyanine green, and performing encapsulation on the hydrophobized indocyanine green through a dialysis method to obtain the c(RGDfk) cyclic peptide-chitosan stearic acid graft drug-loaded micelles. The drug-loaded micelles provided by the invention have dual targeting functions of brain glioma cells and neovascular endothelial cells, and can selectively deliver the photosensitizer, improve a concentration of the photosensitizer of the brain glioma cells and the tumor neovascular endothelial cells and increase stability of the photosensitizer; and after near-infrared laser illumination, indocyanine green high-efficiency photothermal conversion and reactive oxygen generation are realized, apoptosis of the brain glioma cells is induced, tumor neovascularization is inhibited, and efficacy of phototherapy against brain glioma is improved.
Owner:ZHEJIANG UNIV
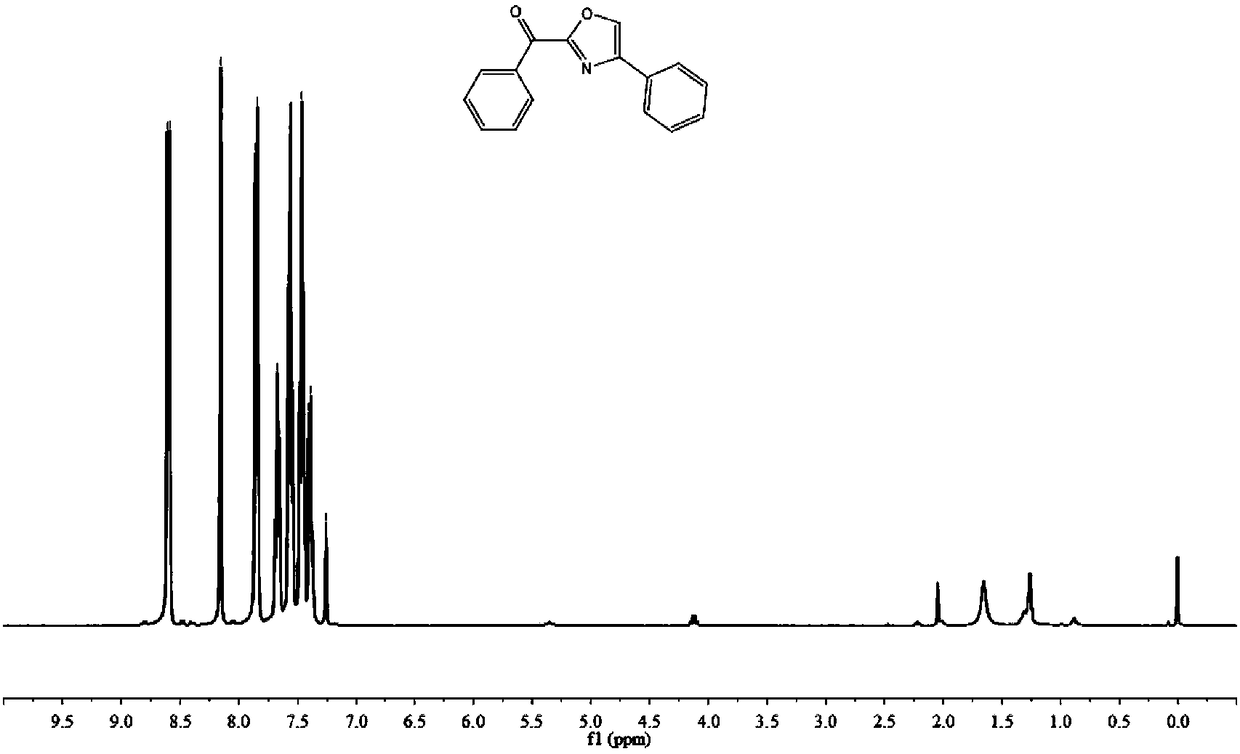
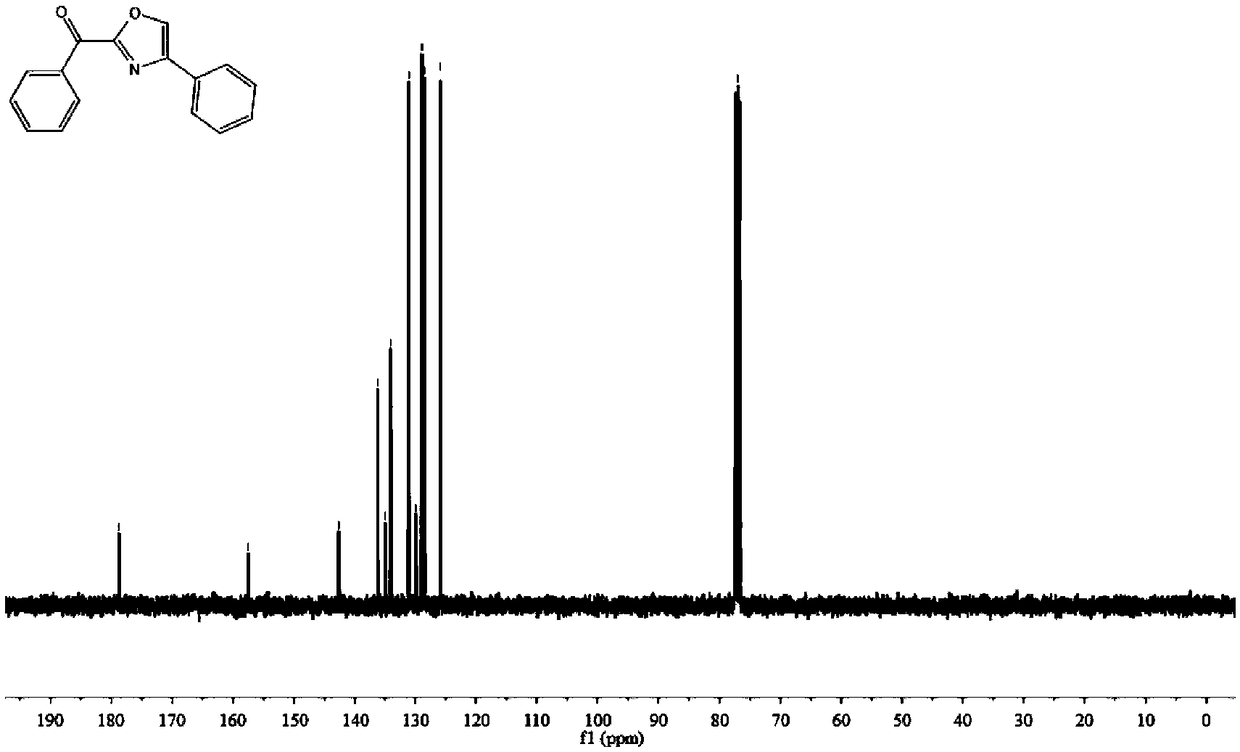
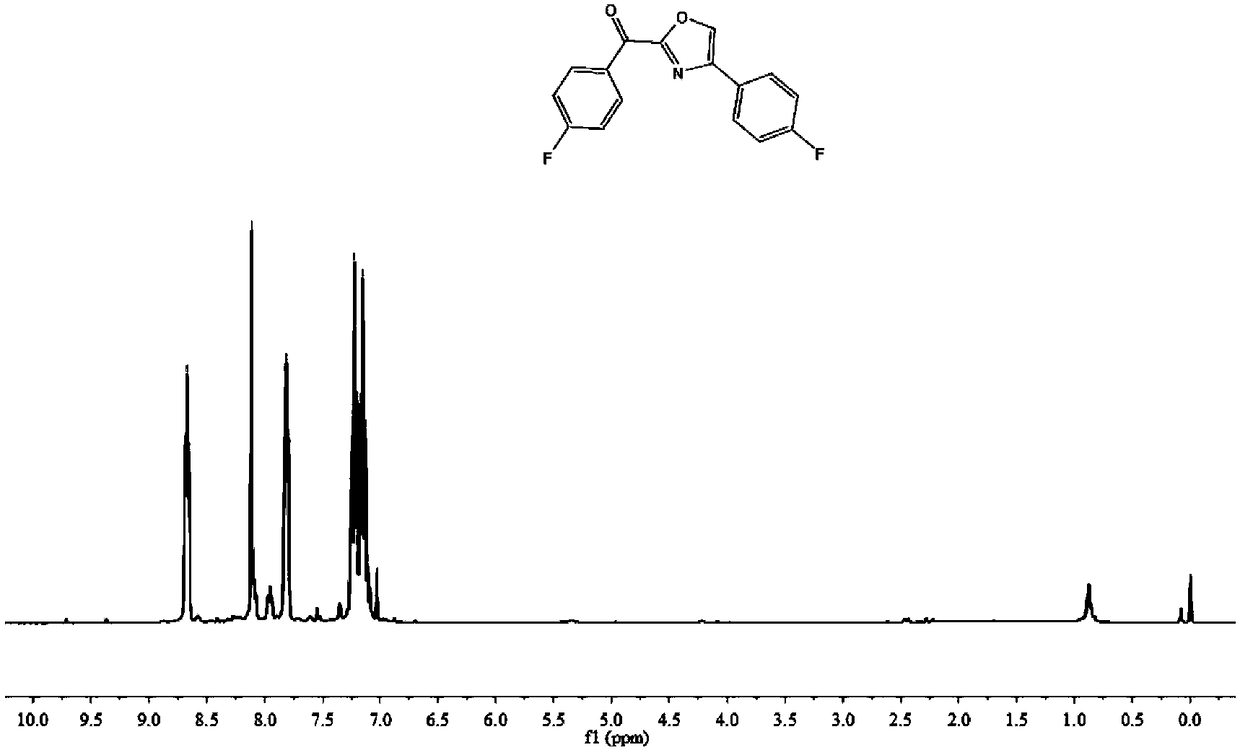
Method for constructing 2,4-diaryloxazole by acetophenone compound, ammonium persulfate and dimethylsulfoxide jointly
The invention discloses a method for constructing 2,4-diaryloxazole by acetophenone compound, ammonium persulfate and dimethylsulfoxide jointly. The method includes that the acetophenone compound is subjected to cyclization reaction in DMSO (dimethylsulfoxide) solution which contains ammonium persulfate and TBAI (tetrabutylammonium iodide) to obtain 2,4-diaryloxazole. According to the method, thecheap acetophenone compound, ammonium persulfate, dimethylsulfoxide and the like are used as raw materials for one-pot high-yield synthesis of 2,4-diaryloxazole compounds under mild reaction conditions without heavy metal or precious metal catalytic action, and industrial production is benefited.
Owner:YUANJIANG HUALONG CATALYST TECH
Green preparation method for vinyl sulfone derivative in aqueous phase
ActiveCN108623503ASuitable for industrial productionOrganic chemistryOrganic compound preparationAir atmosphereVinyl sulfone
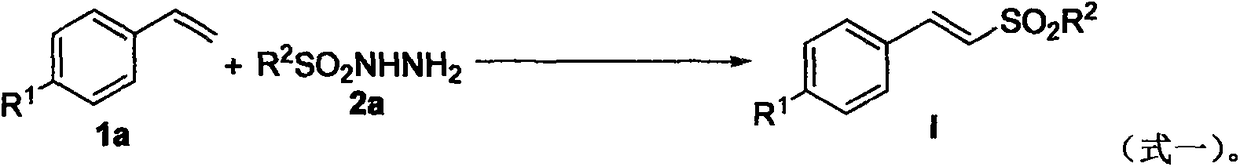
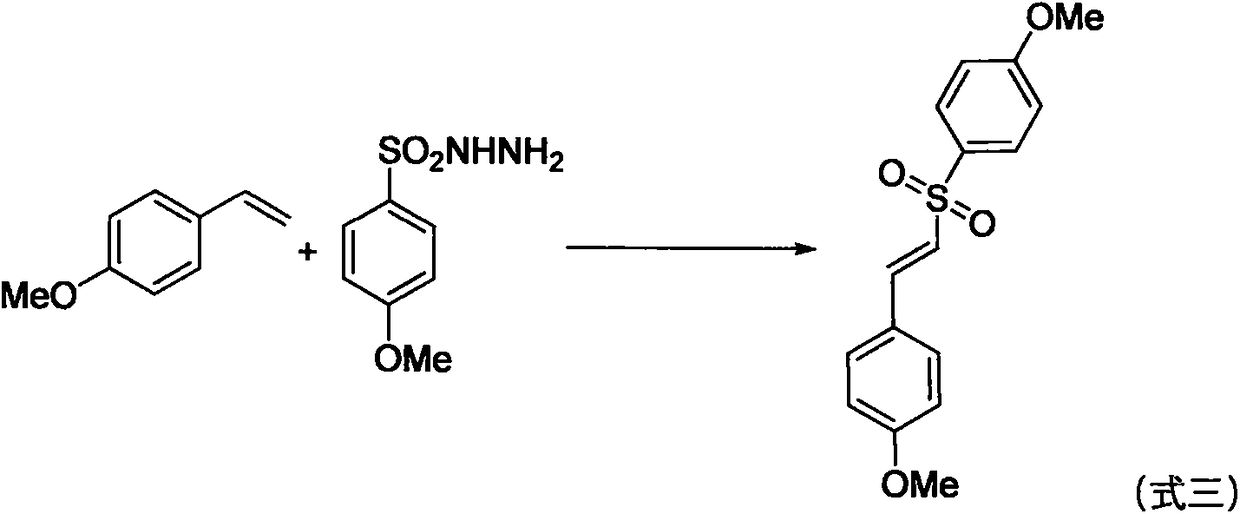
The invention relates to a green preparation method for a vinyl sulfone derivative in an aqueous phase. The method comprises the following steps: putting an olefin compound (1a), a sulfonyl hydrazidecompound of formula (2a) as shown in the specification, tetrabutylammonium iodide (TBAI), tert-butyl hydroperoxide (TBHP) and solvent water into a Schlenk reaction kettle, carrying out a stirring reaction on the reaction kettle at a certain temperature and an air atmosphere, monitoring the reaction process through TLC (Thin Layer Chromatography) or GC (Gas Chromatograph) till raw materials are completely reacted, and carrying out aftertreatment, thereby obtaining a target product, namely the vinyl sulfone derivative (I) as shown in the specification.
Owner:NINGBO UNIV
Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate
InactiveCN109651368AReasonable reaction process designLower synthesis costOrganic chemistryMonoethyl maleatePotassium fluoride
The invention relates to a preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate and mainly solves the technical problem that there is no method suitable for industrial synthesis so far. The preparation method comprises the following two steps: Step 1, a compound 1 and dimethyl maleate react under the action of tetrabutylammonium iodide and potassium fluoridewith dimethyl sulfoxide used as a solvent to generate a compound 2; and Step 2, the compound 2 is subjected to raney nickel / H2 catalytic hydrogenation in methanol used as a solvent to obtain a compound 3, namely 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate. The reaction equation is as shown in the specification.
Owner:武汉药明康德新药开发有限公司
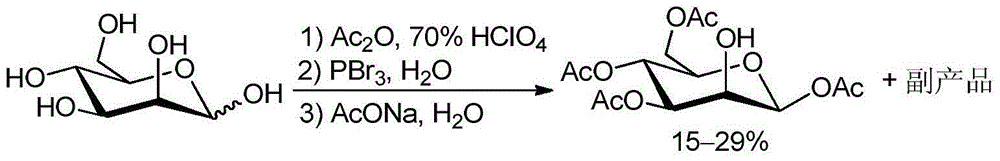
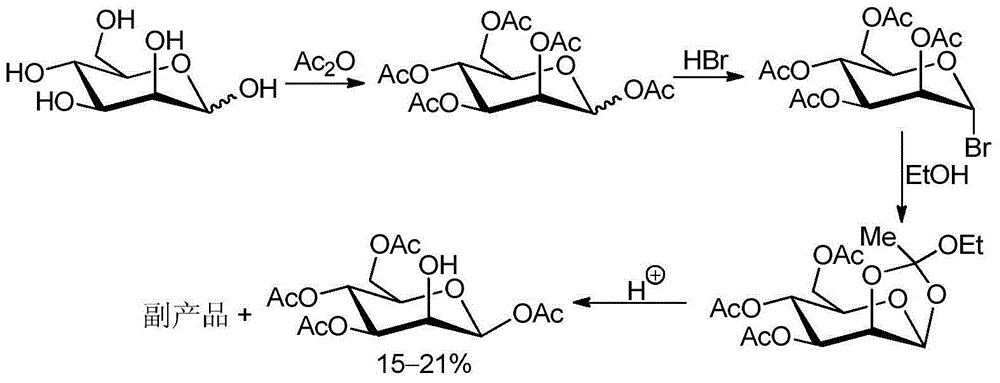
Method for recycling byproduct associated during preparation process of mannose triflate intermediate 1,3,4,6-tetraacetyl-beta-D-mannose
InactiveCN104558062AReduce pollutionHigh yieldEsterified saccharide compoundsSugar derivativesSodium acetateAcetic anhydride
The invention relates to a method for recycling for recycling a byproduct associated during preparation process of mannose triflate intermediate 1,3,4,6-tetraacetyl-beta-D-mannose. The method comprises the following steps: adding the byproduct associated during preparation process of the mannose triflate intermediate 1,3,4,6-tetraacetyl-beta-D-mannose while stirring into a acetic anhydride mixed solution containing perchloric acid and tetrabutylammonium iodide to obtain a mixture, adding a pre-cooled hydrogen bromide glacial acetic acid solution into the mixture, stirring at room temperature, adding dichloromethane again into a reaction flask, separating to obtain an organic phase and washing to obtain a yellow bromide 2,3,4,6-tetraacetyl-alpha-D-mannose crude product; dissolving the crude product in a mixed solvent of acetic acid and acetic anhydride, cooling, adding a pre-cooled aqueous solution of sodium acetate, stirring and reacting; extracting with methylene chloride, washing, drying and filtering to remove the solvent to obtain a white solid 1,3,4,6-tetraacetyl-beta-D-mannose. The average yield of the recycled byproduct reaches 16%, the purity of the product is not less than 99.5%, the yield is increased and the environmental pollution is decreased.
Owner:TIANJIN UNIV
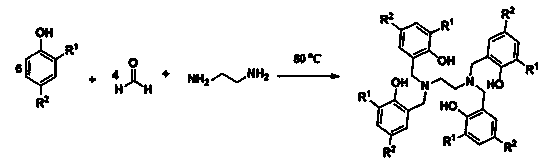
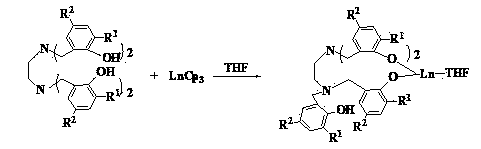
Method for preparing triblock non-ionic fluorine-containing short-chain surfactant by non-isocyanate route
ActiveCN111138659AImprove thermal stabilityGood chemical stabilityTransportation and packagingMixingPolymer sciencePolyethylene glycol
The invention discloses a method for preparing a triblock non-ionic fluorine-containing short-chain surfactant through a non-isocyanate route. The triblock non-ionic fluorine-containing short-chain surfactant is prepared by the following steps: firstly, carrying out a reaction between polyethylene glycol diglycidyl ether and carbon dioxide at an equimolar ratio under the co-catalysis of tetrabutylammonium iodide and high-fluorine tert-butyl alcohol to obtain a prepolymer with cyclic carbonates at two ends, and then performing a ring-opening reaction between the prepolymer and short-chain fluoroamine to obtain the triblock non-ionic fluorine-containing short-chain surfactant. The preparation method is characterized in that a cyclic carbonate route is used for replacing an isocyanate route to synthesize a polyurethane structure, so that the non-isocyanate route synthesis of the triblock non-ionic fluorine-containing short-chain surfactant containing the polyurethane structure is realized, and the use of toxic isocyanate monomers is avoided. Besides, the preparation method of the triblock non-ionic fluorine-containing short-chain surfactant is simple, the surface activity is excellent, and the triblock non-ionic fluorine-containing short-chain surfactant has very good biocompatibility and biodegradability and has a wide application prospect.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


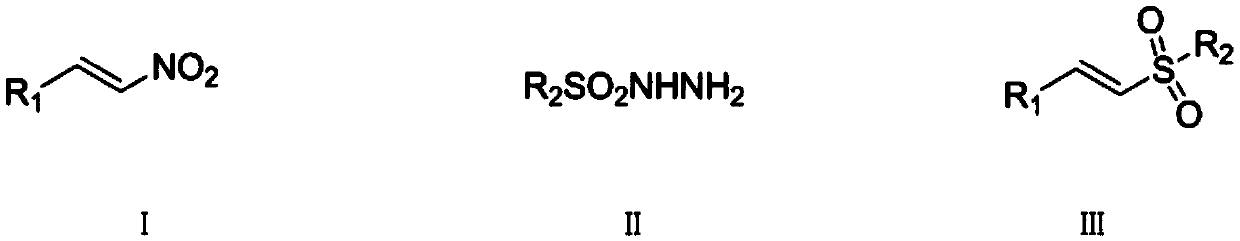
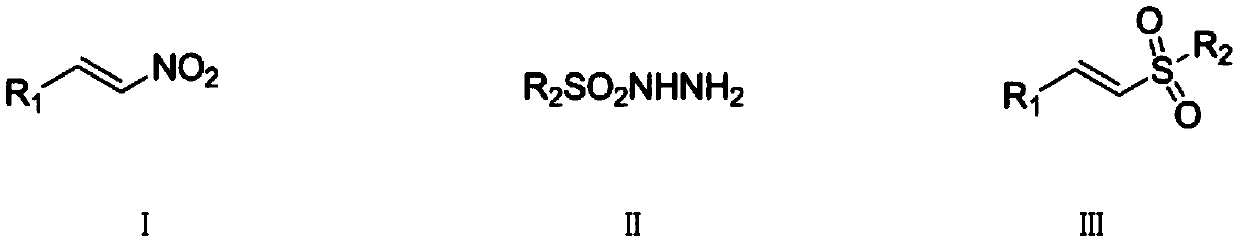


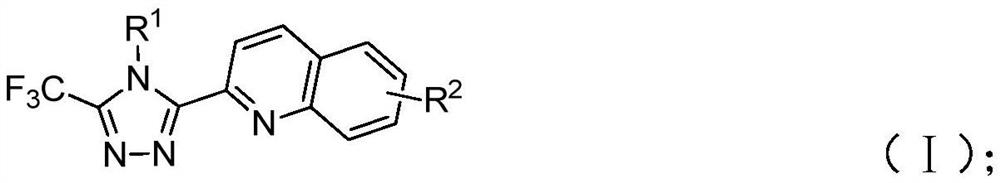
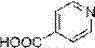


![Synthesis method of O-[2]-[[tert-Butoxycarbonyl]amino]ethyl]-N-[fluorene methoxycarbonyl]-L-tyrosine Synthesis method of O-[2]-[[tert-Butoxycarbonyl]amino]ethyl]-N-[fluorene methoxycarbonyl]-L-tyrosine](https://images-eureka.patsnap.com/patent_img/8150676c-68ca-442e-84c5-a5005bc4fcad/100002_DEST_PATH_IMAGE001.png)
![Synthesis method of bis [(trifluoromethyl) sulfonyl] methane Synthesis method of bis [(trifluoromethyl) sulfonyl] methane](https://images-eureka.patsnap.com/patent_img/a0450023-7b62-4ddb-a657-26c30c005f97/HDA0001916500730000011.png)
![Synthesis method of bis [(trifluoromethyl) sulfonyl] methane Synthesis method of bis [(trifluoromethyl) sulfonyl] methane](https://images-eureka.patsnap.com/patent_img/a0450023-7b62-4ddb-a657-26c30c005f97/BDA0001916500720000071.png)
![Synthesis method of bis [(trifluoromethyl) sulfonyl] methane Synthesis method of bis [(trifluoromethyl) sulfonyl] methane](https://images-eureka.patsnap.com/patent_img/a0450023-7b62-4ddb-a657-26c30c005f97/BDA0001916500720000072.png)

![Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate](https://images-eureka.patsnap.com/patent_img/036da539-82b9-4b8d-b27b-d6eb8b6e883b/607281DEST_PATH_IMAGE002.png)
![Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate](https://images-eureka.patsnap.com/patent_img/036da539-82b9-4b8d-b27b-d6eb8b6e883b/DEST_PATH_IMAGE001.png)