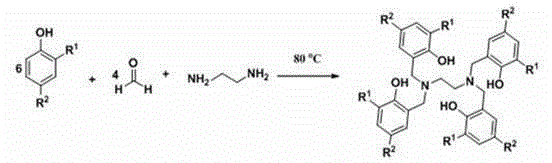
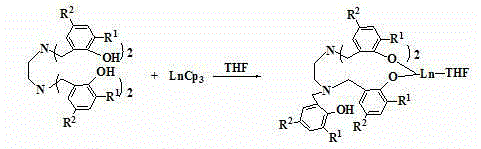
Method for preparing cyclic carbonate
A technology of cyclocarbonate and alkylene oxide, which is applied in the field of preparation of organic compounds, can solve the problems of low activity of disubstituted alkylene oxide, narrow scope of application of substrates, complex catalyst synthesis, etc., and achieve clear structure, excellent catalytic performance, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment one: preparation bridged tetraaryloxy rare earth metal compound LNd (THF) (R 1 =R 2 = Bu t )
[0040] (1) Add 2.80 g of LH 4 (3.00 mmol) dissolved in tetrahydrofuran, added to the solution containing 1.23 g NdCp 3 (THF) (3.00 mmol) in tetrahydrofuran solution, stirred at room temperature for 4 hours, and the system was a blue transparent solution;
[0041] (2) Remove the solvent, add 14 ml of toluene and 0.5 ml of tetrahydrofuran, heat to 60°C for extraction, and centrifuge. The supernatant was transferred and left at room temperature until blue crystals (2.65 g, 2.31 mmol) were precipitated, with a yield of 77%. Melting point: 182-184 °C. Elemental analysis: C, 68.80; H, 9.11; N, 2.54; Nd, 12.76. Infrared spectrum (KBr, cm -1 ): 3436(s), 2957(w), 2904(s), 2869(s), 1609(s), 1479(s), 1442(s), 1411(s), 1362(s), 1304(s) ), 1276(s), 1235(s), 1204(s), 1165(s), 1132(s), 1026(s), 994(s), 912(s), 877(s), 837(s) ), 806(s), 759(s), 741(s), 683(s), 529(s), ...
Embodiment 2
[0042] Embodiment two: preparation bridged tetraaryloxy rare earth metal compound LY (THF) (R 1 =R 2 = Bu t )
[0043] (1) Add 2.80 g of LH 4 (3.00 mmol) dissolved in tetrahydrofuran, added to the solution containing 1.07 g of YCp 3 (THF) (3.00 mmol) tetrahydrofuran solution, stirring reaction at room temperature for 4 hours, the system is a light yellow transparent solution;
[0044] (2) Remove the solvent, add 15 ml of toluene and 0.5 ml of tetrahydrofuran, heat to 60°C for extraction, and centrifuge. The supernatant was transferred and left at room temperature until colorless crystals (2.59 g, 2.37 mmol) were precipitated, with a yield of 79%. Melting point: 178-180 °C. Elemental analysis: C, 72.59; H, 9.65; N, 2.62; Y, 8.57. Infrared spectrum (KBr, cm -1 ): 3437(s), 2953(w), 2904(s), 2867(s), 1603(s), 1479(s), 1442(s), 1414(s), 1362(s), 1304(s) ), 1271(s), 1238(s), 1202(s), 1167(s), 1132(s), 1108(s), 974(s), 912(s), 875(s), 837(s) ), 805(s), 770(s), 744(s), 66...
Embodiment 3
[0045] Embodiment three: preparation bridged tetraaryloxy rare earth metal compound LSm (THF) (R 1 =R 2 = Bu t )
[0046] (1) Add 2.80 g of LH 4 (3.00 mmol) dissolved in tetrahydrofuran, added to the solution containing 1.25 g of SmCp 3 (THF) (3.00 mmol) in tetrahydrofuran solution, stirring reaction at room temperature for 4 hours, the system is a yellow transparent solution;
[0047] (2) Remove the solvent, add 14 ml of hexane and 0.5 ml of tetrahydrofuran, heat to 60°C for extraction, and centrifuge. The supernatant was transferred and left at room temperature until yellow crystals (2.50 g, 2.16 mmol) were precipitated, with a yield of 72%. Melting point: 199-201 °C. Elemental analysis: C, 68.52; H, 8.69; N, 2.53; Sm, 13.27. Infrared spectrum (KBr, cm -1 ): 3423(s), 2960(w), 2904(s), 2869(s), 1603(s), 1477(s), 1440(s), 1414(s), 1362(s), 1301(s) ), 1276(s), 1240(s), 1202(s), 1167(s), 997(s), 969(s), 959(s), 913(s), 875(s), 833(s) ), 808(s), 770(s), 741(s), 691(s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com