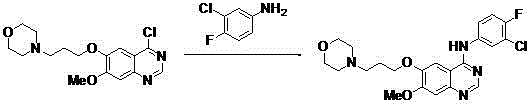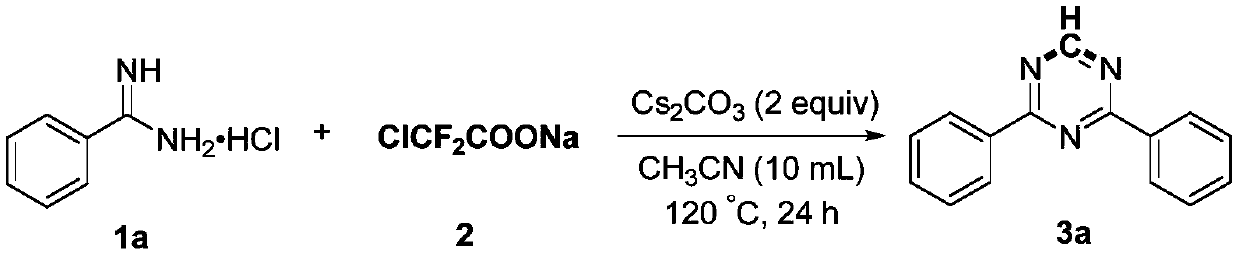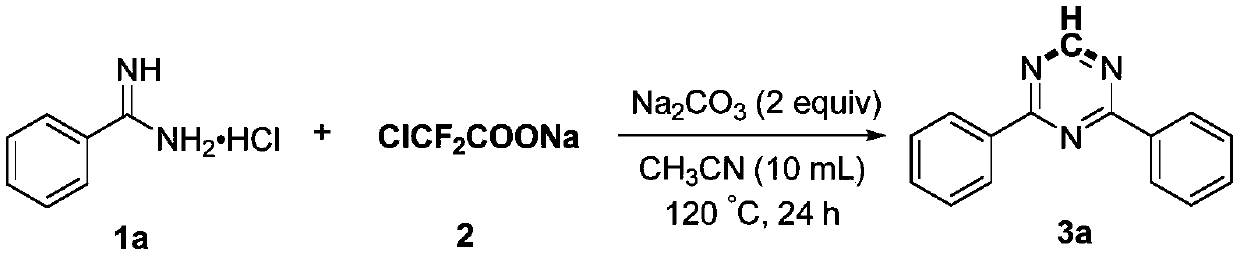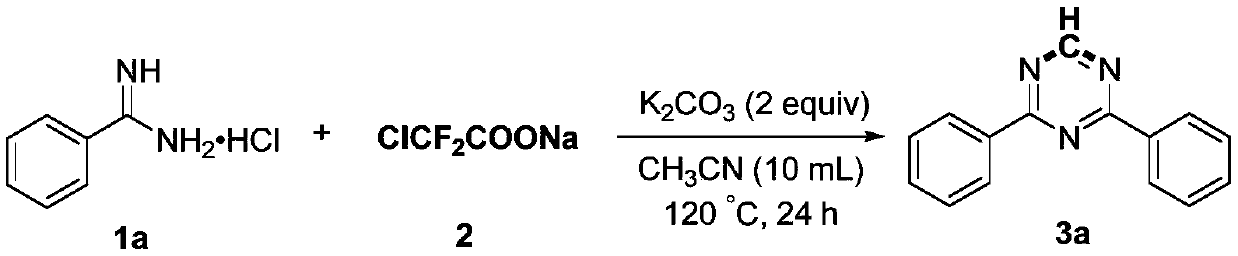Patents
Literature
34 results about "Formamidine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
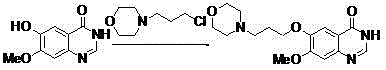
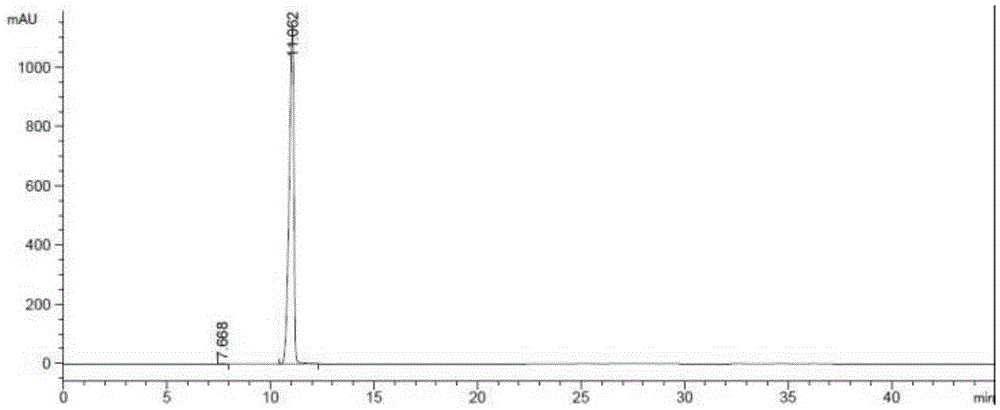
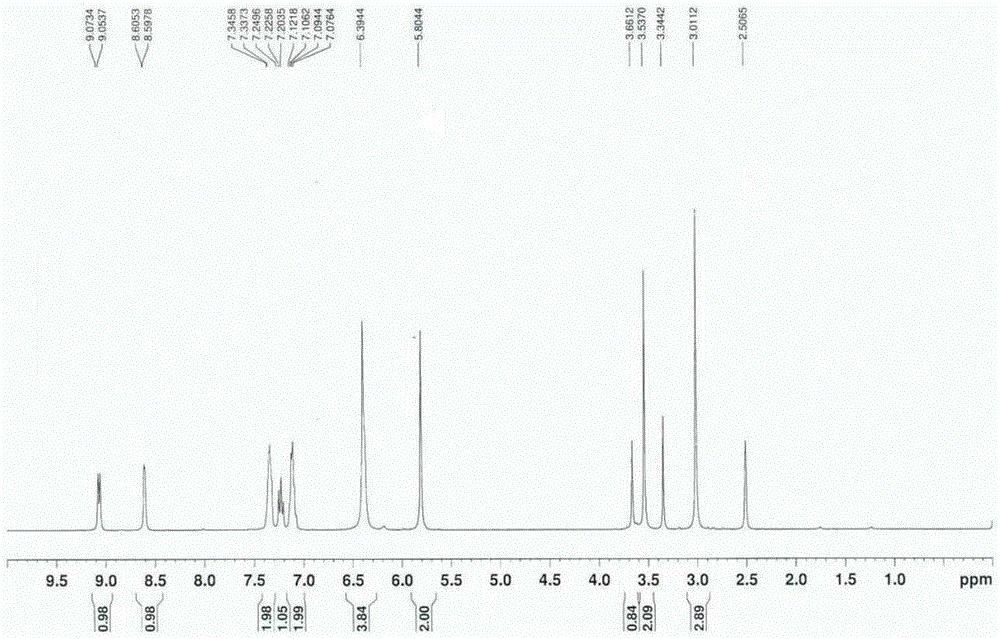
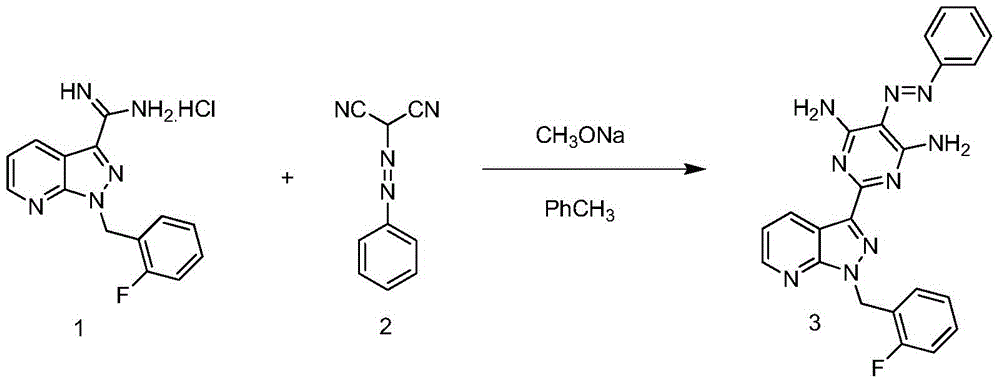
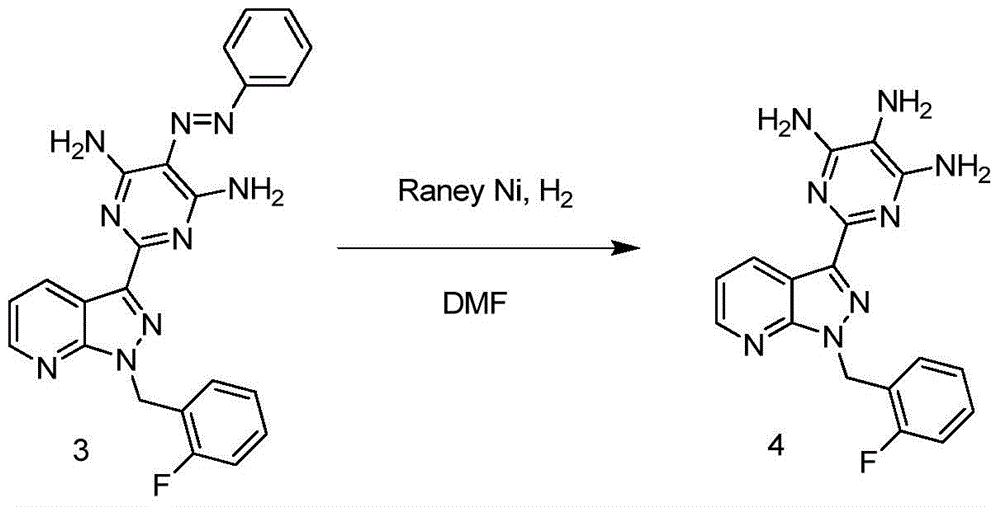
Method for synthesizing riociguat
ActiveCN104530044AImprove responseThorough responseOrganic chemistrySolventFormamidine hydrochloride
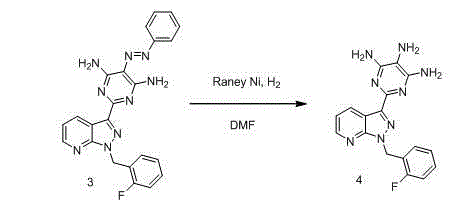
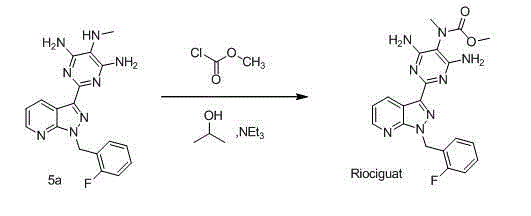
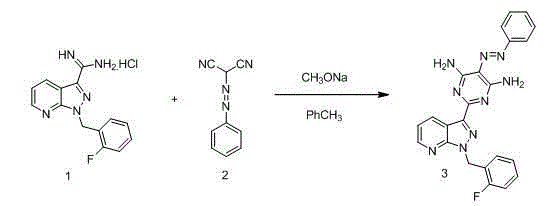
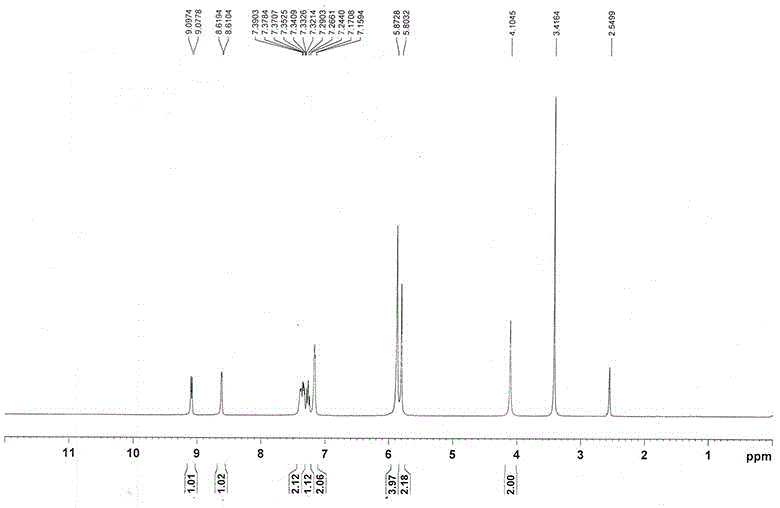
The invention relates to a method for synthesizing riociguat. The method includes the following steps: 1, a compound 1-(2-fluorobenzyl)-1H-[3,4-b] pyridine derivative-3-formamidine hydrochloride (1) and a compound benzeneazomalononitrile (2) are reacted in methylbenzene under the condition that sodium methylate exists to obtain a compound (3); 2, the compound (3) is dissolved into DMF, then raney nickel is added to serve as catalysts, and hydrogenation reduction is carried out to obtain a compound (4); 3, a formyl group is firstly synthesized into the compound (4), then reduction is carried out through boron hydride to obtain a single-methyl compound (5a); 4, isopropyl alcohol is used as solvents to be reacted with methylclhlorofonmate to obtain the product riociguat. The synthesizing method has the advantages of being easy and convenient to operate, gentle in condition, high in total yield and high in product purity, and is suitable for large-scale synthesizing of the high-purity riociguat.
Owner:安徽联创生物医药股份有限公司
Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate
The invention discloses a preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate. The method comprises the following steps: contacting liquid sodium alkoxide with formamidine hydrochloride and then contacting an obtained substance with a compound represented by a formula (1) so as to obtain 4-amino-5-formylaminomethylpyrimidine; contacting 4-amino-5-formylaminomethylpyrimidine with an alkaline aqueous solution and then contacting an obtained substance with carbon disulfide and gamma-chloroacetyl propanol so as to obtain a compound represented by a formula (2); contacting an acidic aqueous solution with the compound represented by the formula (2) so as to obtain a compound represented by a formula (3); and contacting the compound represented by the formula (3) with hydrogen peroxide, then contacting an obtained substance with nitrate, carrying out neutralization with alkali and then carrying out solid-liquid separation so as to obtain 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate. With 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate prepared in the invention, accurate qualitative and quantitative analysis of demethylated thiamine can be realized.
Owner:江西天新药业股份有限公司
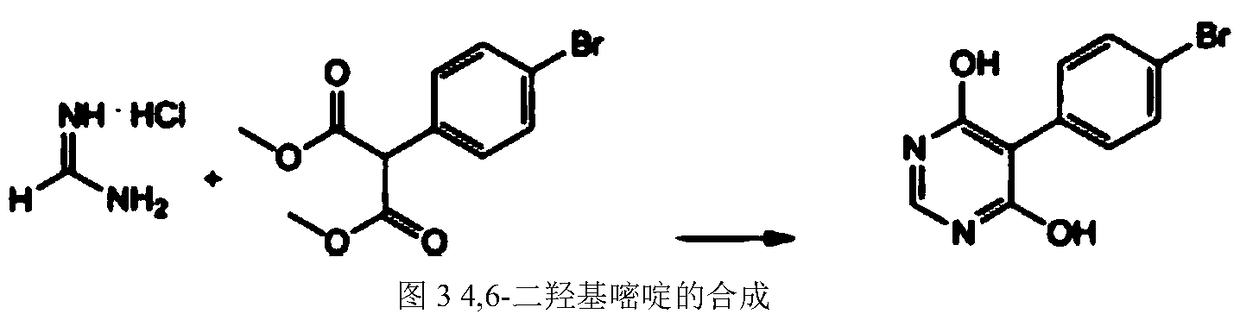
Method for preparing 4,6-dihydroxypyrimidine
The invention discloses a method for preparing 4,6-dihydroxypyrimidine, relates to the method for preparing the 4,6-dihydroxypyrimidine, and belongs to the technical field of a medicine. The method comprises the following steps: firstly, feeding formamidine hydrochloride, malonic ester and alkali to a solvent to reflux, agitate and react for 1-12 hours; washing by acid water until the solvent is neutral; adding water, cooling, agitating and filtering so as to obtain pale yellow crystal after recovering the solvent at reduced pressure; finally re-crystallizing the pale yellow crystal by acetone to obtain the white crystal 4,6-dihydroxypyrimidine. According to the method, the 4,6-dihydroxypyrimidine is prepared from formamidine hydrochloride and malonic ester as materials in a cyclization manner; the used materials are cheap; the reaction yield can be up to over 90%; the content of the 4,6-dihydroxypyrimidine after re-crystallization is greater than 98%.
Owner:YANGZHOU TIANHE PHARM CO LTD
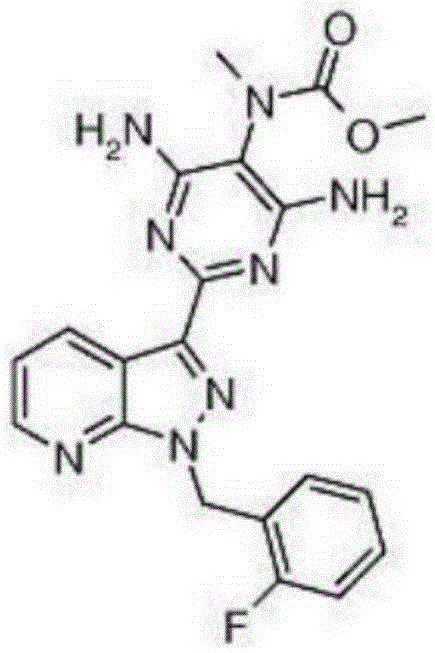
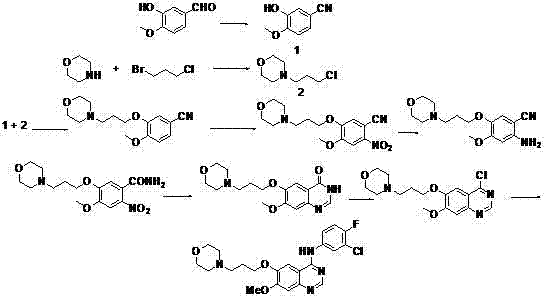
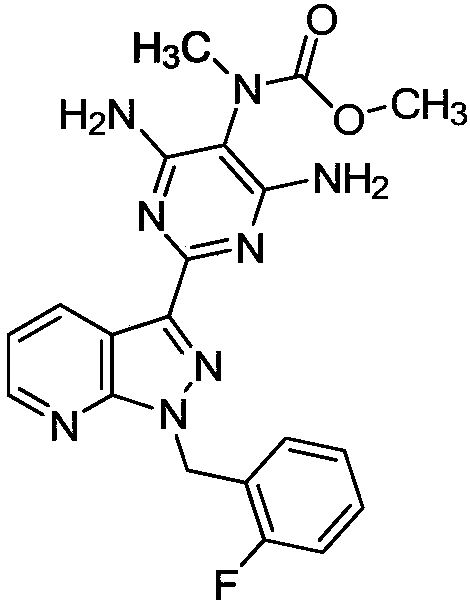
Preparation method of riociguat
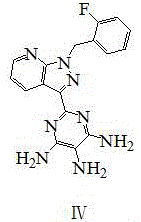
The invention discloses a preparation method of riociguat. The preparation method comprises the steps of firstly, taking 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride and 2-aminopropanediamide as basic raw materials, adding strong base and a solvent to perform a reflux reaction, so as to prepare 2-[1-(2-fluorobenzyl) -1H-pyrazolo[3,4-b]pyridine-3-yl]pyrimidine-4,5,6-triamine, namely a compound 3; then taking the compound 3 and dimethyl carbonate as basic raw materials, dissolving the same into methyl alcohol for reaction, so as to prepare 4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]-5-pyrimidinyl methyl carbamate, namely a compound 5, finally, dissolving the compound 5 and the strong base into the methyl alcohol, and adding methyl iodide for reacting, so as to prepare a riociguat crude product, and performing recrystallization on the riociguat crude product to obtain a riociguat refined product. The preparation method provided by the invention is relatively high in total recovery, simplified in operation and easy to industrialize.
Owner:郑州大明药物科技有限公司
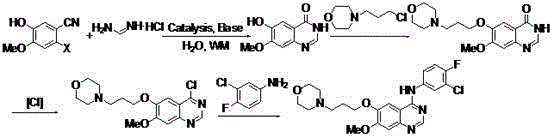
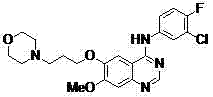
New method for performing microwave synthesis on gefitinib and derivative thereof
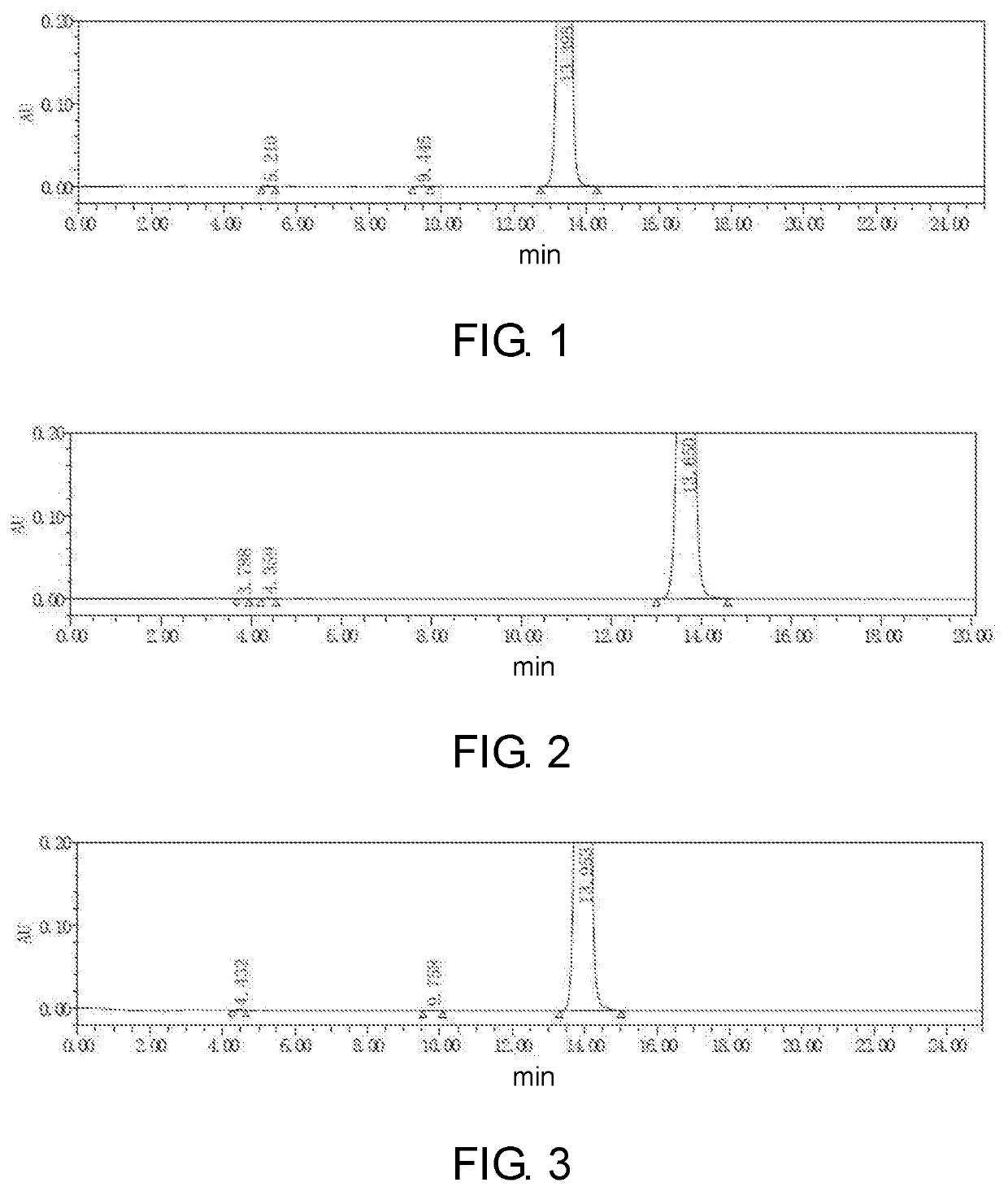
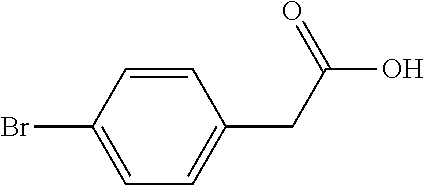
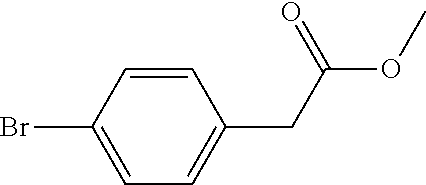
The invention discloses a new method for performing microwave synthesis on gefitinib and a derivative thereof. The method comprises the following steps of: at first, synthesizing 6-methoxyl-7-hydroxyl quinazoline-4-one from 2-iodo (bromo)-4-methoxyl-5-hydroxyl cyanophenyl used as an initial raw material and formamidine hydrochloride in a microwave mode; then performing reaction with 4-(3-bromo propyl) morpholine to introduce an alkyl side chain so as to obtain 7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline-4-ketone; in the presence of a catalyst, performing reaction on the obtained product and a chlorination reagent to obtain 4-chloro-7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline; finally, performing reaction on 4-chloro-7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline and 3-chloro-4-fluoroaniline to obtain a final product 4-(3-chloro-4-fluoroanilino)-7-methoxyl-6-(3-morpholinyl propoxy) quinazoline (gefitinib). The whole route steps are simplified into four steps, the yield is high, the method is convenient to carry out, the dangerousness is low, and the application of high-pollution reagents is reduced; moreover, the whole route is high in economy and suitable for industrial production, and the product cost is reduced. The reaction formula is as shown in the specification.
Owner:FUJIAN MEDICAL UNIV
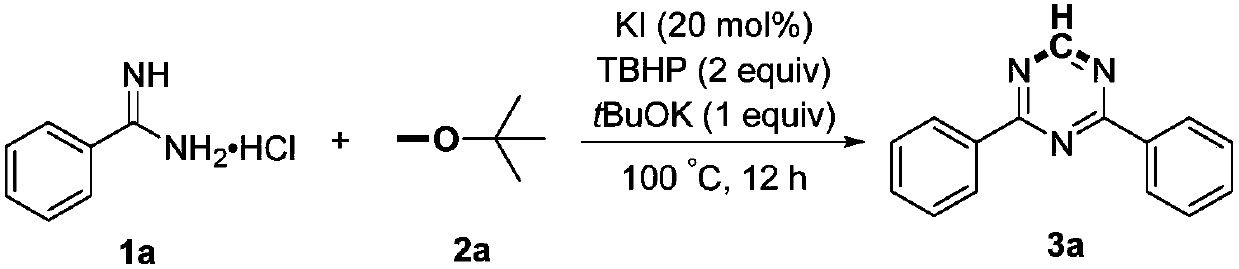
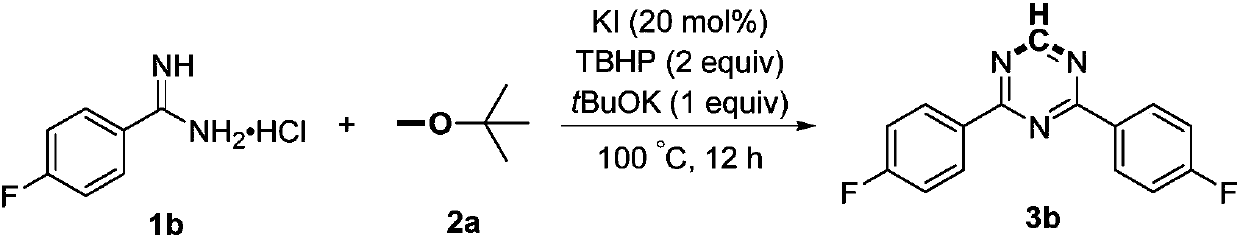
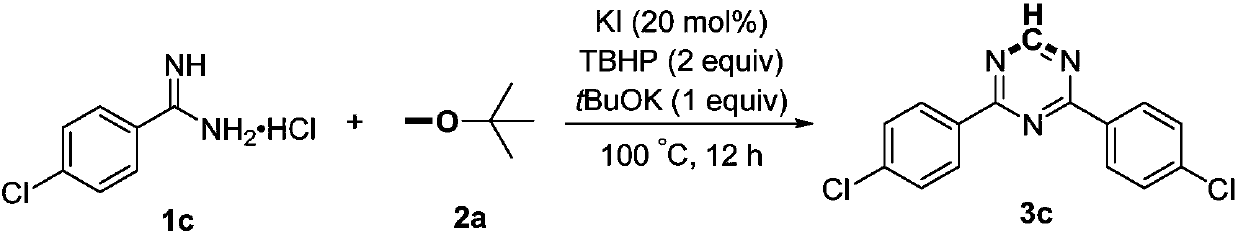
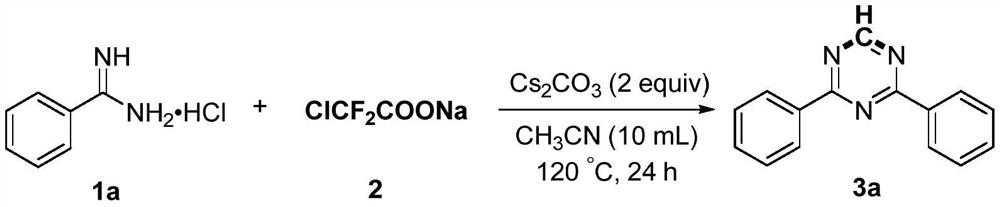
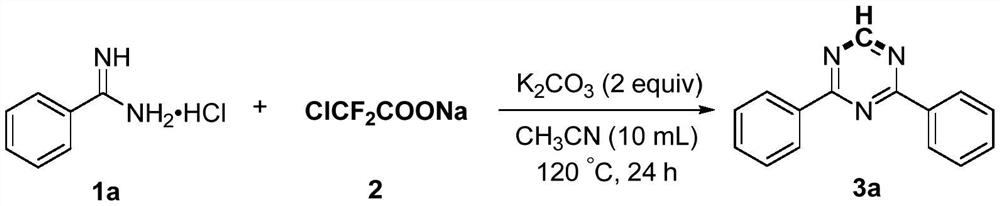
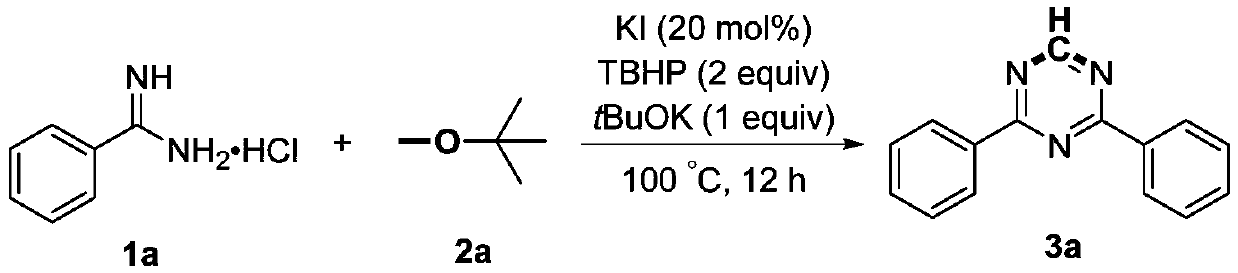
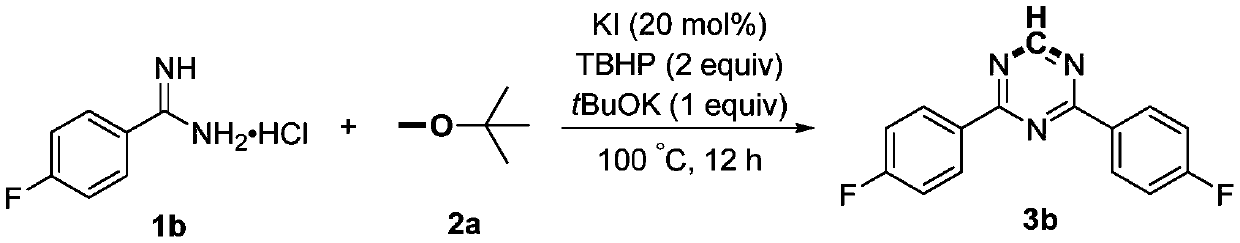
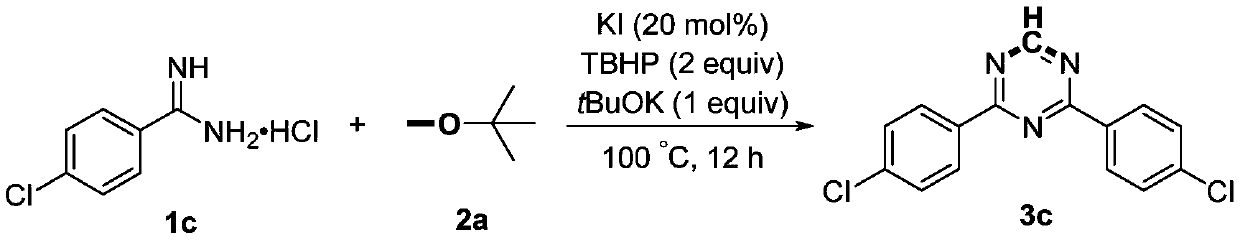
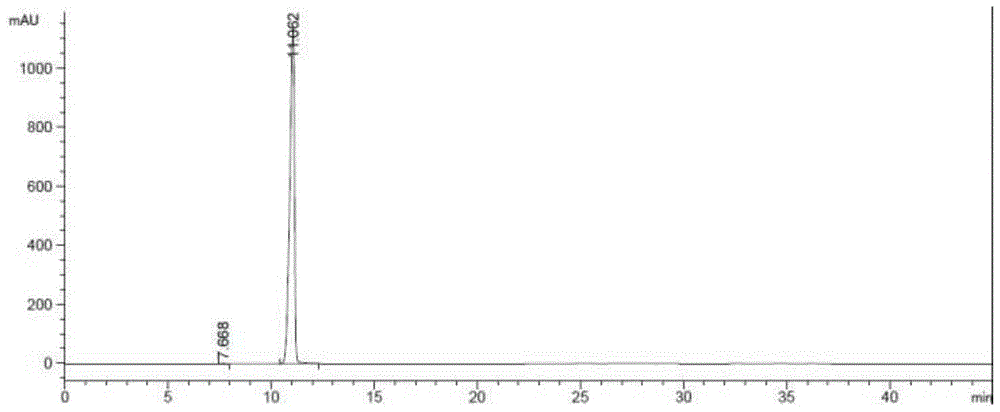
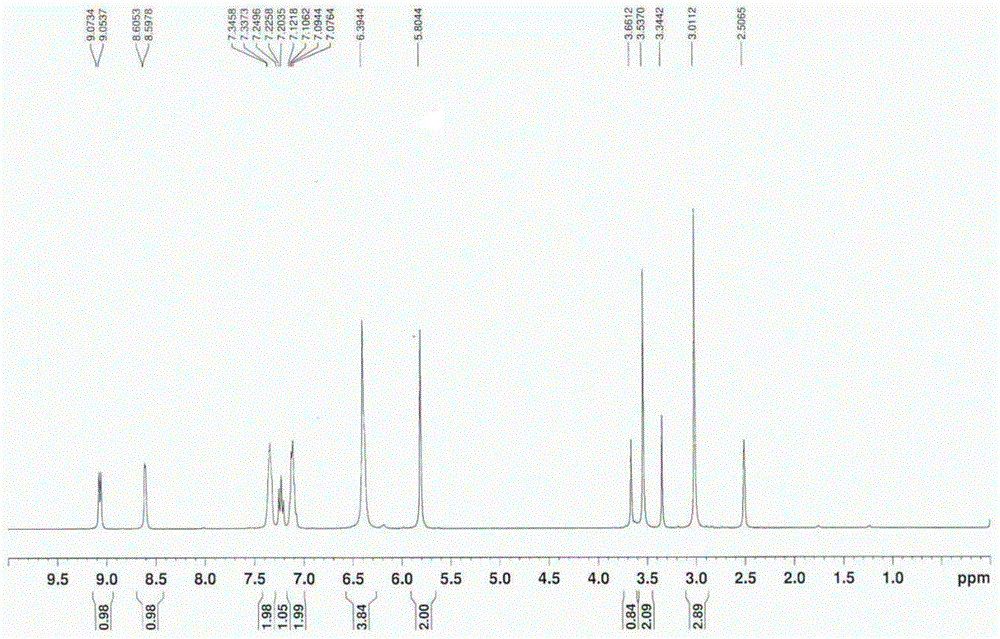
Preparation method of 2,4-di-substituted-1,3,5-triazine
ActiveCN107759530AWide variety of sourcesEasy to getOrganic chemistryPotassium iodinePotassium carbonate
The invention discloses a method for preparing 2,4-di-substituted-1,3,5-triazine. The method comprises the specific step: with substituted formamidine hydrochloride as a reaction substrate an iodine-containing compound as a catalyst, tert-butyl hydroperoxide as an oxidant, inorganic base as an acid binding agent and aliphatic ether as an organic solvent (also used as a carbon source), carrying outcarbon-hydrogen and carbon-oxygen bond deletion, nucleophilic addition, deaminizing condensation and oxidative aromatization reaction to obtain a 2,4-di-substituted-1,3,5-triazine compound, wherein achemical structural general formula of the substituted formamidine hydrochloride is shown in the description; the iodine-containing compound is selected from one of potassium iodide (KI), tetrabutylammonium iodide (TBAI), elemental iodine (I2) and N-iodosuccinimide (NIS); the inorganic base is selected from one of anhydrous potassium carbonate, anhydrous sodium carbonate, cesium carbonate, potassium hydroxide and potassium tert-butoxide; and the aliphatic ether is selected from one of methyl tert-butyl ether, ethyl ether and ethylene glycol dimethyl ether. The preparation method disclosed bythe invention has the characteristics of easily-obtained raw materials, low price and low toxicity of a catalyst, wide range of the substrate, simplicity and convenience in operation, greenness, environmental protection and the like.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Preparation method of riociguat intermediate
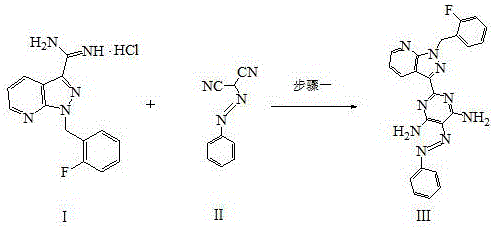
InactiveCN108069960ASolve the problem of many new impurities and difficult purificationReduce usageOrganic chemistryChemical synthesisPhenyldiazene
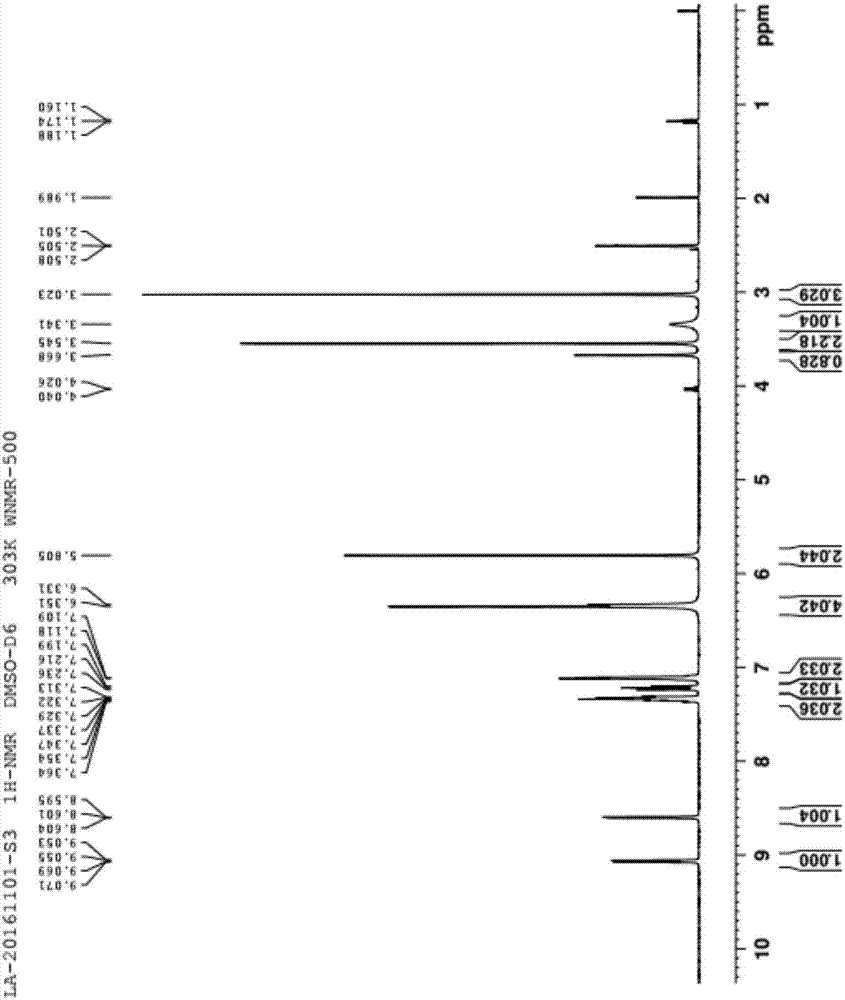
The invention relates to a preparation method of a riociguat intermediate, in particular to a chemical synthesis method of a riociguat intermediate 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]pyrimidine-4,5,6-triamine. 1-(2-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride and (phenylhydrazono)malononitrile are used as initial materials and subjected to a cyclization reaction, 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]-5-[(E)phenyldiazenyl-4,6-pyrimidinediamine is prepared and further subjected to a reduction reaction, and 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]pyrimidine-4,5,6-triamine is obtained. The preparation method has the advantages as follows: raw materials are cheap and easy to obtain, the total yield is high, the productpurity is high, the operation is convenient, high-temperature catalytic hydrogenation equipment is not used, and industrial production is facilitated.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for compounding riociguat intermediate
ActiveCN105461715AAvoid High Pressure Catalytic Hydrogenation ReactionsLower requirementOrganic chemistryHigh pressureFormamidine hydrochloride
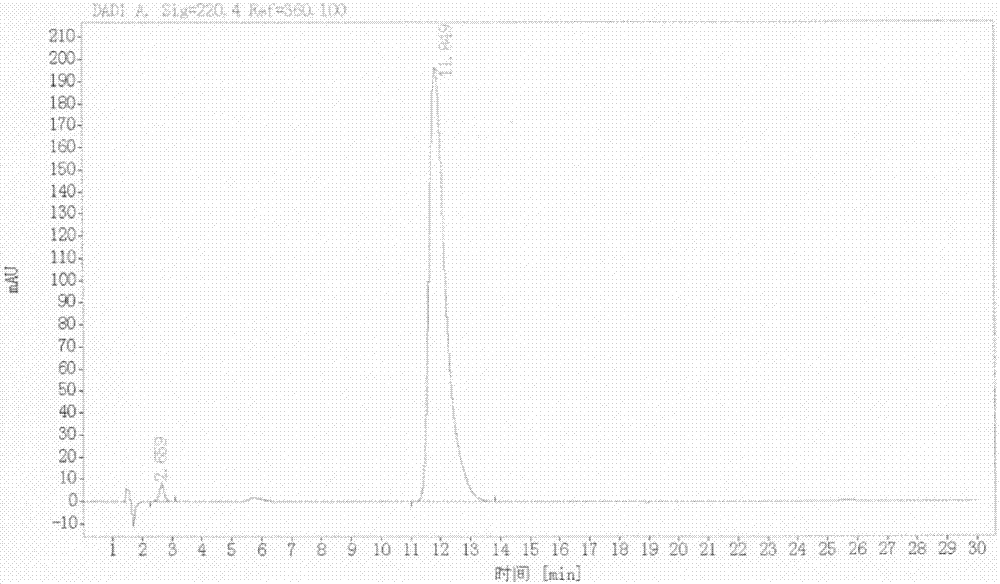
The invention discloses a method for compounding riociguat intermediate, firstly 1-(2-fluorine benzyl) 1H-pyrazolo [3, 4-b] pyridine-3-formamidine hydrochloride are used as starting materials, and 2-[1-(2-fluorine benzyl)-1 H- pyrazolo [3, 4-b] pyridine-3-yl]-4, 5, 6-pyrimidine triamine of the riociguat intermediate are prepared by catalyzing and hydrogenating in normal pressure through a catalyst. The method for compounding the riociguat intermediate is mild in reaction conditions, prevents using a high pressure reaction kettle, reduces process demands to equipment, enables product purity to be bigger than 99.0% at last, and is suitable for commercial process.
Owner:郑州大明药物科技有限公司
Riociguat preparation method
The invention discloses a riociguat preparation method. According to the preparation method, 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride is utilized as a raw material. The preparation method comprises the four steps of reaction of 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]-5-[(E)-phenyl diazenyl]-4,6-thonzylamine preparation, intermediate of 2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]-4,5,6-pyrimidine triamine preparation, intermediate of 4,6-diamido-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl]-5-pyrimidyl methyl carbamate isopropanol solvate preparation and riociguat preparation. A technological path of the riociguat preparation method disclosed by the invention has the advantages of having high selectivity and high yield, being simple and safe to operate, having low equipment requirement, and being suitable for industrial production and the like.
Owner:SPRINGPHARMA CO LTD
Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine
ActiveCN109651424AImprove economyLow costGroup 4/14 element organic compoundsBulk chemical productionHydrogenFormamidine hydrochloride
The invention discloses a simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine. The method comprises the following steps: preparing compound (IV) by a dehydrohalogenation reaction between cyanoacetoacetate and haloacetaldehyde glycol, and then preparing a compound (V) by condensation between the compound (V) and formamidine hydrochloride and alkali; preparing a compound (VIII) by amino group protection through a protecting group reagent, methylation of DMFDMA and hydrazine hydrate condensation; preparing a compound (IX) by reacting with a chlorination reagent; and carrying out catalytic hydrodechlorination to obtain 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine. The raw materials used in the invention are cheap and easily available; by using the ''one-pot method'' operation twice, the environmental protection property is high, and the process route is simple; the reaction operation is convenient, and the reaction selectivity is high; the obtained product has high purity, high yield and low cost; and the method is beneficial to green industrial production.
Owner:XINFA PHARMA
Bactericide for sulfate reducing bacteria in polymer-containing produced liquid as well as preparation method and application of bactericide
ActiveCN114149062AChange permeabilityViscosity does not affectWaste water treatment from quariesBiocideBiotechnologyAnionic polymers
The invention provides a bactericide for sulfate reducing bacteria in polymer-containing produced liquid as well as a preparation method and application of the bactericide. The bactericide is prepared by the following steps: reacting linear alkylamine with 1H-pyrazole-1-formamidine hydrochloride to obtain an intermediate, and reacting the intermediate with anhydride. The bactericide provided by the invention does not react with anionic polymers in the polymer-containing produced liquid, does not influence the viscosity of the polymer-containing produced liquid, has a good bactericidal effect, can realize an excellent bactericidal effect at a small concentration of the bactericide, has strong adaptability, does not cause the drug resistance of bacteria, and also has a certain corrosion inhibition effect.
Owner:SHANDONG UNIV
A kind of preparation method of multi-substituted 1,3,5-triazine
ActiveCN109810069BReduce generation costImprove securityOrganic chemistrySodium chloroacetatePtru catalyst
The invention discloses a preparation method of polysubstituent 1,3,5-triazine. Specifically, substituen formamidine hydrochloride is used as a reactive substrate, chlorodifluoroacetic acid sodium salt is used as a carbon synthon, and the symmetrical or asymmetrical polysubstituent 1,3,5-triazine is obtained through fracture of carbon-chlorine, carbon-carbon and carbon-fluorine bonds under the action of equivalent inorganic base. The chemical structural general formula of the substituen formamidine hydrochloride is as shown in specification, and the inorganic is one selected from potassium carbonate, sodium carbonate, cesium carbonate. The preparation method disclosed by the invention has the advantages of easily available raw materials, no need for catalyst or oxidant, a wide variety of substrates, simple and convenient operation and environmental friendliness.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
A kind of preparation method of 2,4-disubstituted-1,3,5-triazine
ActiveCN107759530BWide variety of sourcesEasy to getOrganic chemistryPotassium iodinePotassium carbonate
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
A kind of preparation method of riociguat
Owner:郑州大明药物科技有限公司
Synthetic method of adenine and its derivatives
ActiveCN106883233BReduce pollutionRaw materials are cheap and easy to getOrganic chemistryMalonateFormamidine hydrochloride
The invention discloses a method for synthesizing adenine and derivatives thereof. The method comprises the steps: firstly, subjecting diethyl malonate and dimethyl dioxirane to an oxidation reaction, so as to obtain 2-hydroxyldiethyl malonate; then, carrying out an ammonolysis reaction with ammonia water, so as to obtain 2-hydroxyl malonamide; then, carrying out a cyclization reaction with trimethyl orthoformate, so as to obtain 5-hydroxyl pyrimid-4,6-(1H,5H)-dione; then, carrying out a chlorination reaction with phosgene, so as to obtain 4,5,6-trichloropyrimidine; then, carrying out a cyclization reaction with formamidine hydrochloride, so as to obtain 4-chloro-1H-pyrazol[3, 4-d]pyrimidine; finally, carrying out an ammoniation reaction with ammonia water or an amine compound in the presence of triethylamine, thereby obtaining the adenine and derivatives thereof. According to the method, the raw materials are moderately-priced and readily available, the reaction conditions are mild, the reaction process is safe, the requirements on production equipment are low, particularly, the environmental pollution is light, and the yield is relatively high, so that the method is applicable to industrial large-scale production.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
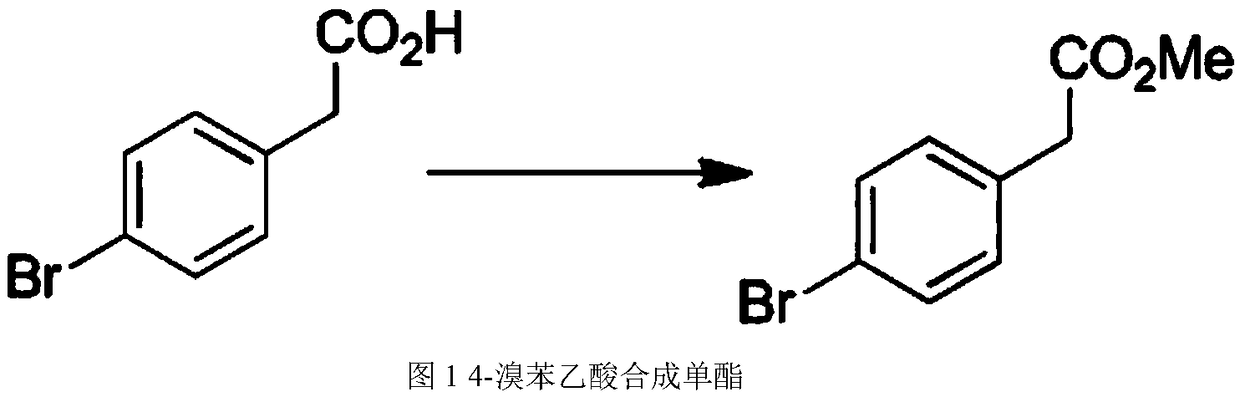
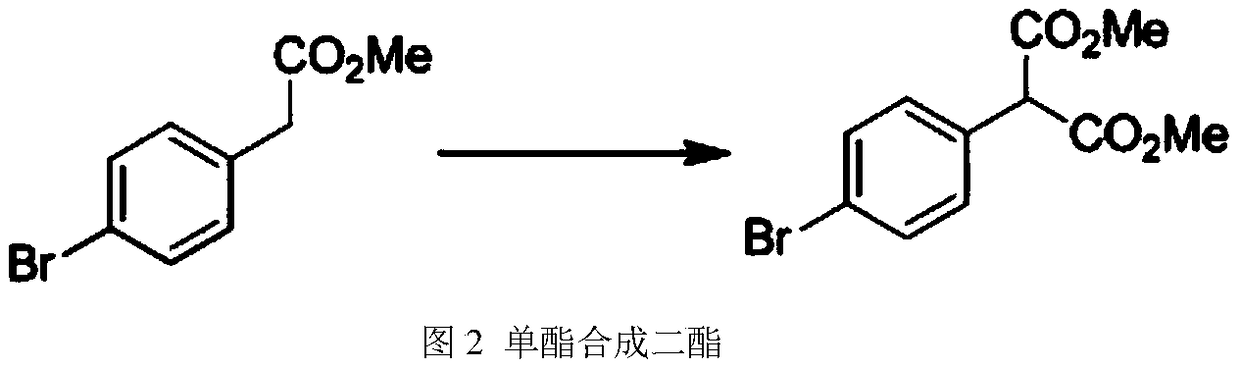
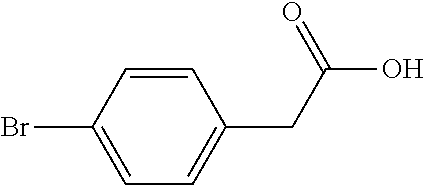
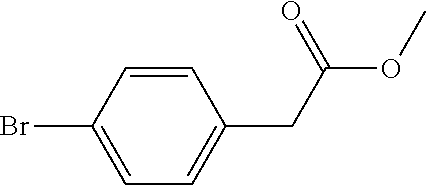
Method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine
ActiveUS10556871B1The synthesis process is simpleSimplifies post-treatment stepOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPtru catalystSolid acid
A method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine is provided. The method comprises the steps of: preparing methyl p-bromophenylacetate (Intermediate I) by catalytic esterification of p-bromophenylacetic acid, and then reacting with dimethyl carbonate to synthesize 2-(4-bromophenyl)-malonic acid-1,3-dimethyl ester (Intermediate 2), cyclizing with formamidine hydrochloride to obtain 5-(4-bromophenyl)-4,6-dihydroxypyrimidine (Intermediate 3), and then chlorinating to give the product 5-(4-bromophenyl)-4,6-dichloropyrimidine. In the process of preparing Intermediate 1 in the present invention, a solid acid is used as a catalyst. Moreover, in the process of preparing Intermediate 2, sodium methoxide is used as a base in place of sodium hydride or sodium amide used in the prior art. Furthermore, Intermediate 3 is prepared by a one-pot process.
Owner:ZHEJIANG XIANFENG TECH
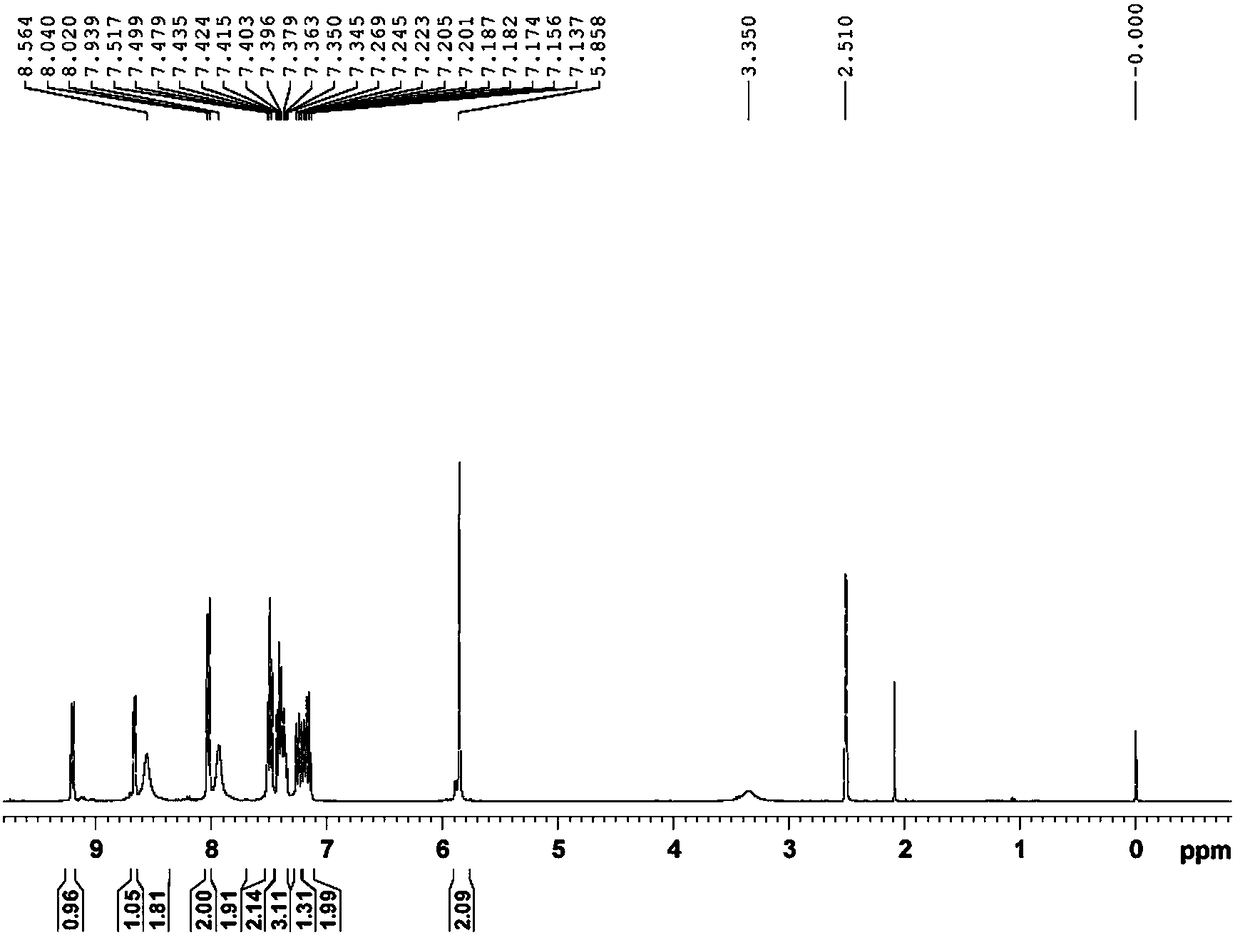
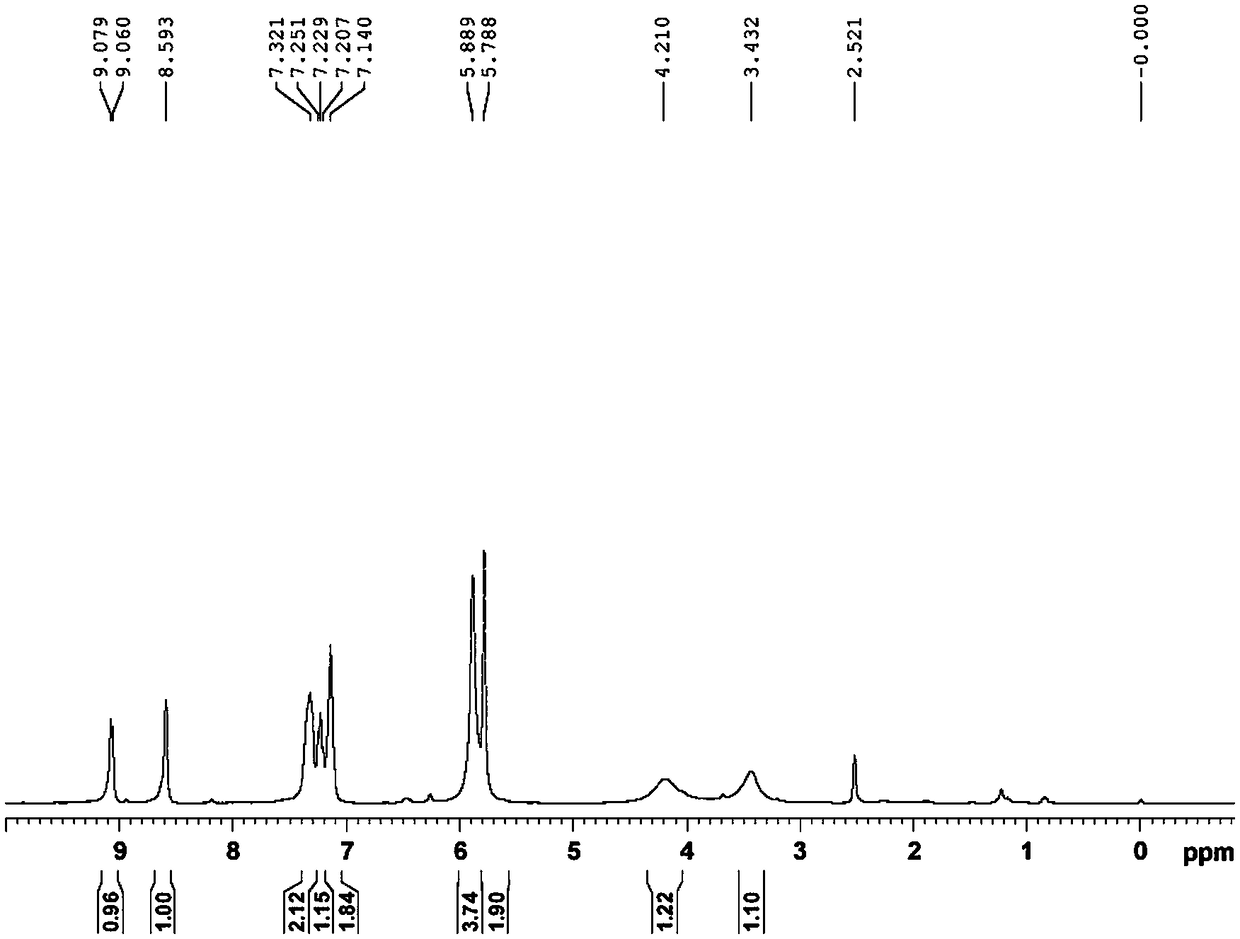
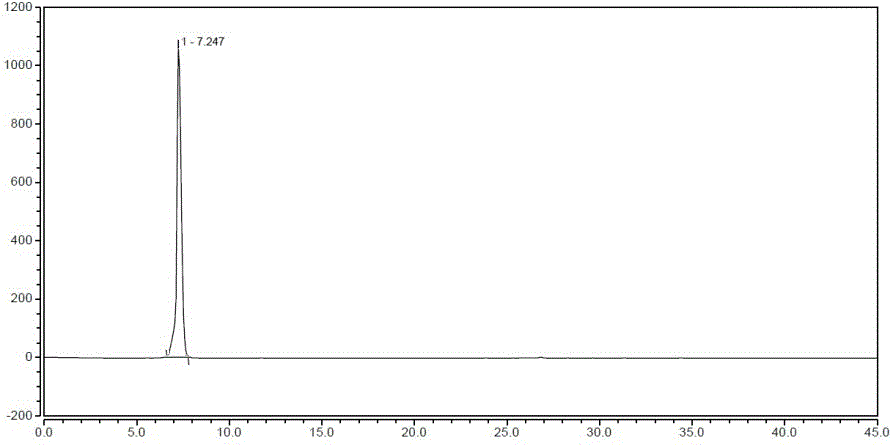
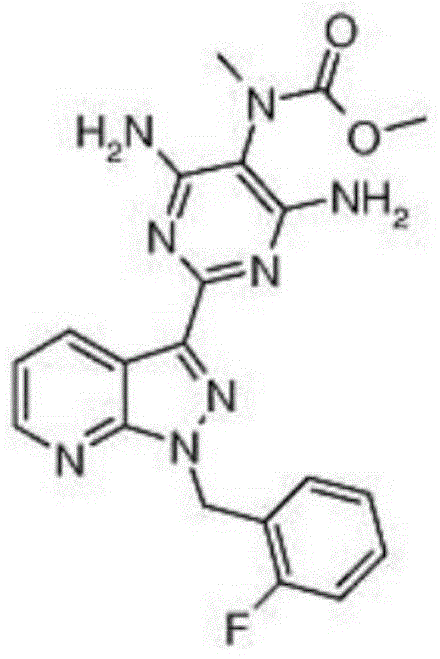
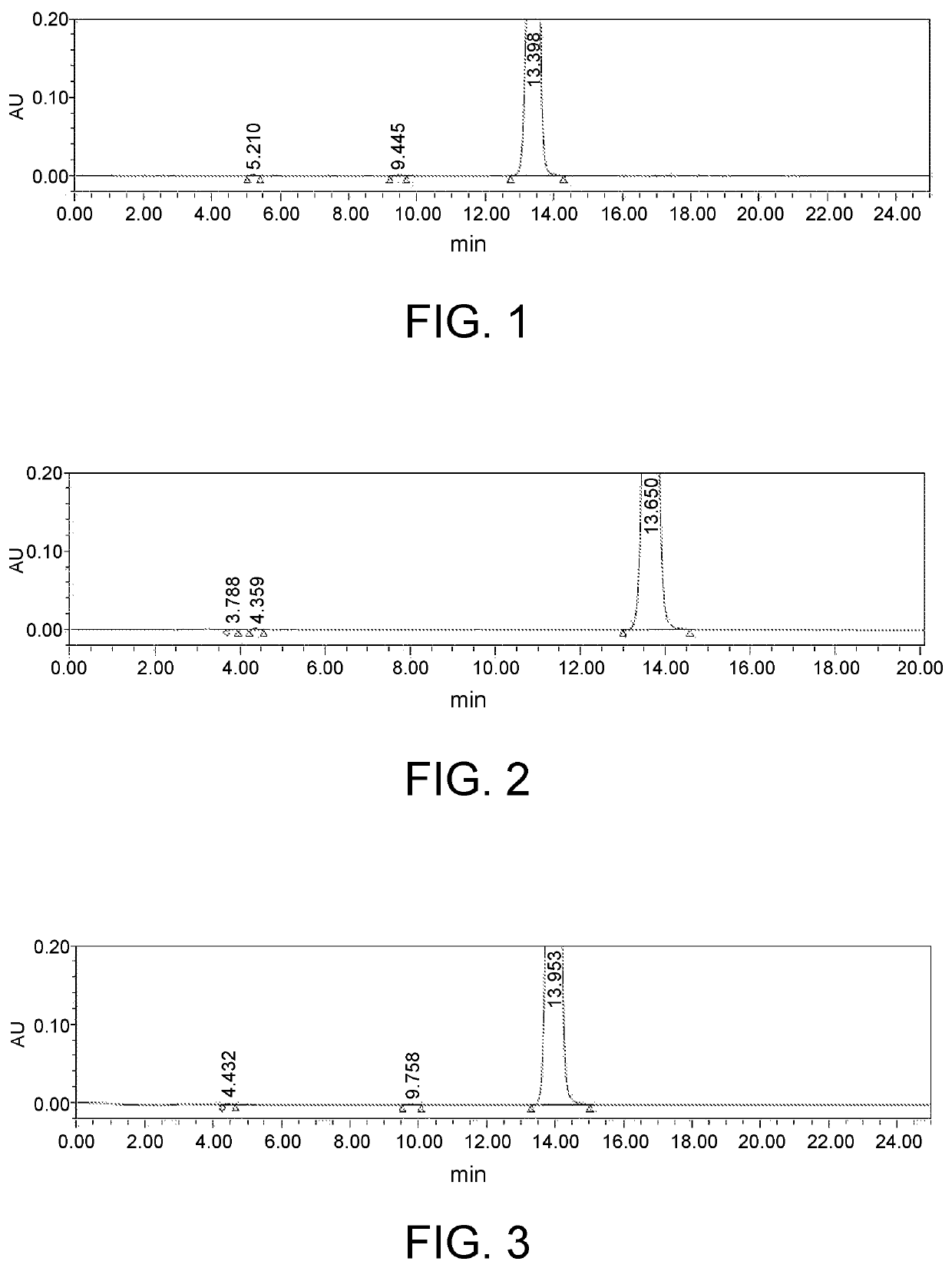
Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride
ActiveUS20140309425A1High yieldMild reaction conditionsOrganic chemistryChemical synthesisSynthesis methods
The present invention relates to the field of chemical synthesis, and in particular to a method for synthesizing 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-formamidine hydrochloride, which is an important intermediate of Riociguat that is an anti-thromboembolic-disease medicine. The method is characterized in that: 3-iodo-1H-pyrazolo[3,4-b]pyridine is used as a raw material; the raw material is reacted with fluorobenzyl bromide to form a compound (10); the compound (10) is reacted with zinc cyanide to form a compound (6); the compound (6) is reacted with sodium methoxide, ammonium chloride, acetic acid and methanol to form a compound (8); and the compound (8) is reacted with chlorine hydride gas to form 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-formamidine hydrochloride. The method has the characteristics of cheap and readily available raw materials, high yield, mild reaction conditions and the like, and is a synthesis method having a large-scale preparation value.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
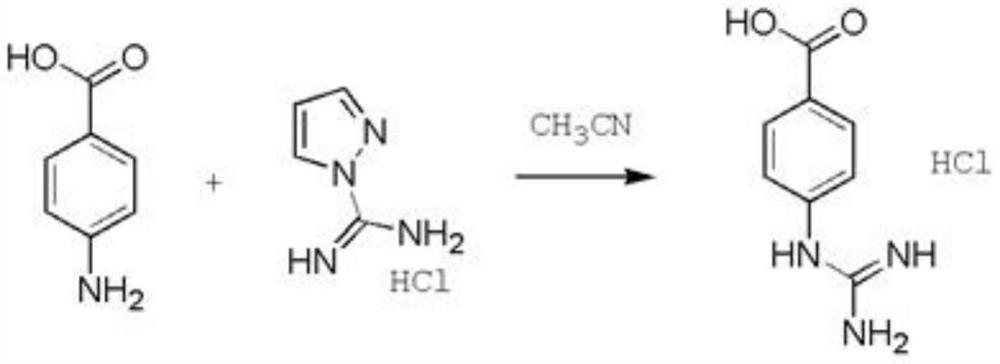
Preparation method of p-guanidinobenzoic acid hydrochloride
PendingCN114315651AImprove securityShort routeOrganic chemistryOrganic compound preparationP-guanidinobenzoic acidBenzoic acid
The invention provides a preparation method of p-guanidinobenzoic acid hydrochloride, which comprises the following step: carrying out reflux reaction on p-aminobenzoic acid and pyrazol formamidine hydrochloride to generate the p-guanidinobenzoic acid hydrochloride. The preparation method provided by the embodiment of the invention has the advantages of short route, high safety of raw materials, strong experimental operability and suitability for industrial production.
Owner:安徽昊帆生物有限公司
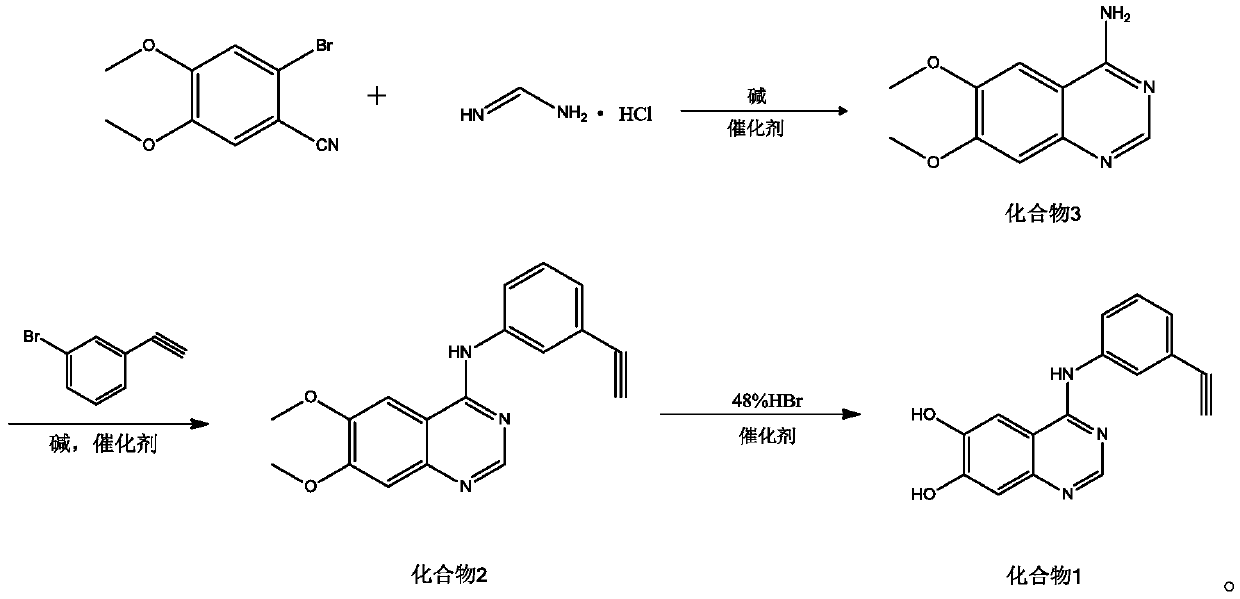
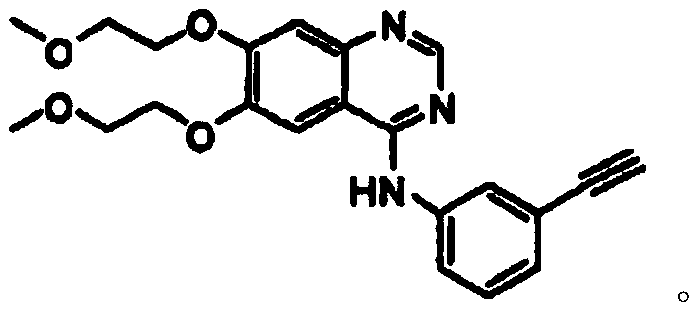
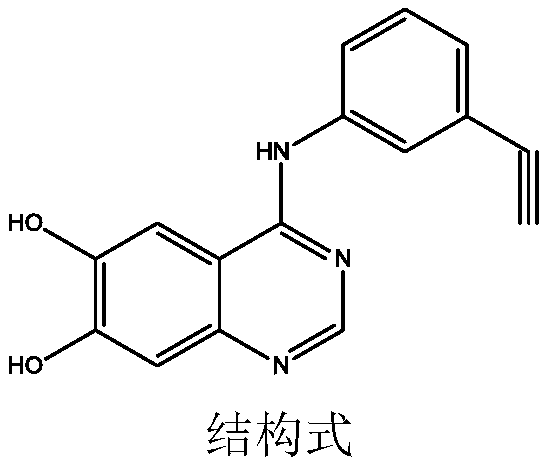
Synthetic method of erlotinib intermediate
InactiveCN111087351AOvercoming the Difficulties of Genotoxic ImpuritiesImprove securityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsErlotinibPtru catalyst
The invention relates to a synthesis method of an erlotinib intermediate, which comprises the following steps: reacting 2-bromo-4,5-dimethoxybenzonitrile with formamidine hydrochloride under the actions of alkali and a catalyst to generate a compound 3; reacting with 1-bromo-triethynylbenzene under the catalysis of alkali to generate a compound 2; carrying out a reaction on the compound 2 with 48%hydrobromic acid under the action of a catalyst to obtain a compound 1. The method is mild in condition, simple in step, safe, environmentally friendly and suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine
ActiveCN109651424BImprove economyLow costGroup 4/14 element organic compoundsBulk chemical productionAcetoacetatesHydrogen halide
The invention discloses a simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine. The method comprises the following steps: preparing compound (IV) by a dehydrohalogenation reaction between cyanoacetoacetate and haloacetaldehyde glycol, and then preparing a compound (V) by condensation between the compound (V) and formamidine hydrochloride and alkali; preparing a compound (VIII) by amino group protection through a protecting group reagent, methylation of DMFDMA and hydrazine hydrate condensation; preparing a compound (IX) by reacting with a chlorination reagent; and carrying out catalytic hydrodechlorination to obtain 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine. The raw materials used in the invention are cheap and easily available; by using the ''one-pot method'' operation twice, the environmental protection property is high, and the process route is simple; the reaction operation is convenient, and the reaction selectivity is high; the obtained product has high purity, high yield and low cost; and the method is beneficial to green industrial production.
Owner:XINFA PHARMA
A kind of synthetic method of riociguat
The invention relates to a method for synthesizing riociguat. The method includes the following steps: 1, a compound 1-(2-fluorobenzyl)-1H-[3,4-b] pyridine derivative-3-formamidine hydrochloride (1) and a compound benzeneazomalononitrile (2) are reacted in methylbenzene under the condition that sodium methylate exists to obtain a compound (3); 2, the compound (3) is dissolved into DMF, then raney nickel is added to serve as catalysts, and hydrogenation reduction is carried out to obtain a compound (4); 3, a formyl group is firstly synthesized into the compound (4), then reduction is carried out through boron hydride to obtain a single-methyl compound (5a); 4, isopropyl alcohol is used as solvents to be reacted with methylclhlorofonmate to obtain the product riociguat. The synthesizing method has the advantages of being easy and convenient to operate, gentle in condition, high in total yield and high in product purity, and is suitable for large-scale synthesizing of the high-purity riociguat.
Owner:安徽联创生物医药股份有限公司
The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate
Owner:江西天新药业股份有限公司
Method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine
ActiveUS20200048206A1Easy to separateSimple processOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPtru catalystSolid acid
Owner:ZHEJIANG XIANFENG TECH
A new method for microwave synthesis of gefitinib and its derivatives
The invention discloses a new method for performing microwave synthesis on gefitinib and a derivative thereof. The method comprises the following steps of: at first, synthesizing 6-methoxyl-7-hydroxyl quinazoline-4-one from 2-iodo (bromo)-4-methoxyl-5-hydroxyl cyanophenyl used as an initial raw material and formamidine hydrochloride in a microwave mode; then performing reaction with 4-(3-bromo propyl) morpholine to introduce an alkyl side chain so as to obtain 7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline-4-ketone; in the presence of a catalyst, performing reaction on the obtained product and a chlorination reagent to obtain 4-chloro-7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline; finally, performing reaction on 4-chloro-7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline and 3-chloro-4-fluoroaniline to obtain a final product 4-(3-chloro-4-fluoroanilino)-7-methoxyl-6-(3-morpholinyl propoxy) quinazoline (gefitinib). The whole route steps are simplified into four steps, the yield is high, the method is convenient to carry out, the dangerousness is low, and the application of high-pollution reagents is reduced; moreover, the whole route is high in economy and suitable for industrial production, and the product cost is reduced. The reaction formula is as shown in the specification.
Owner:FUJIAN MEDICAL UNIV
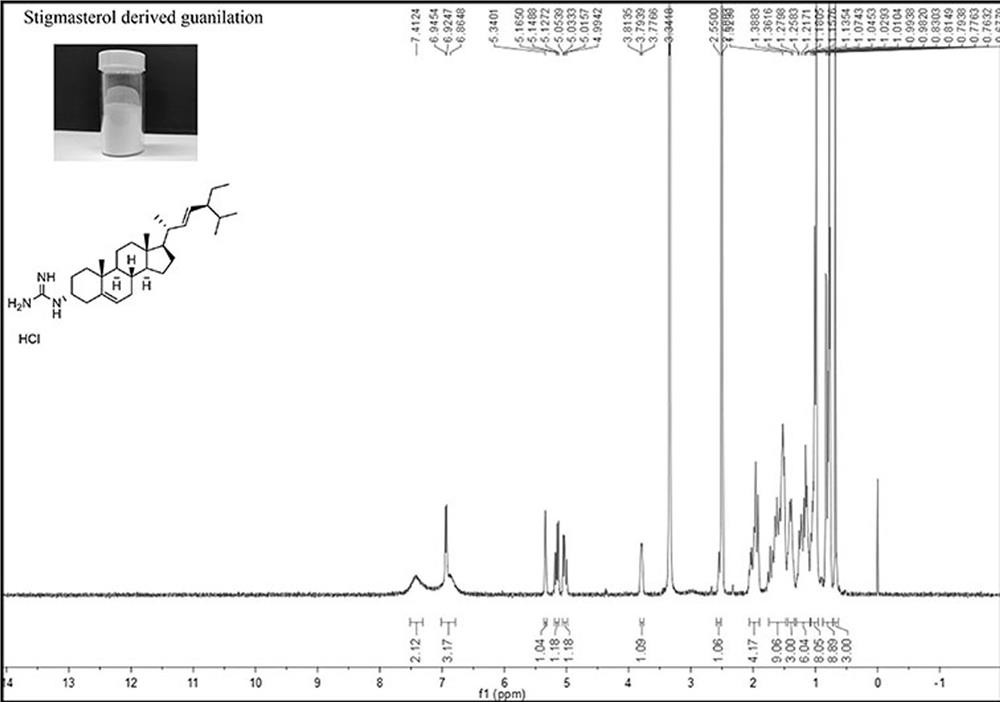
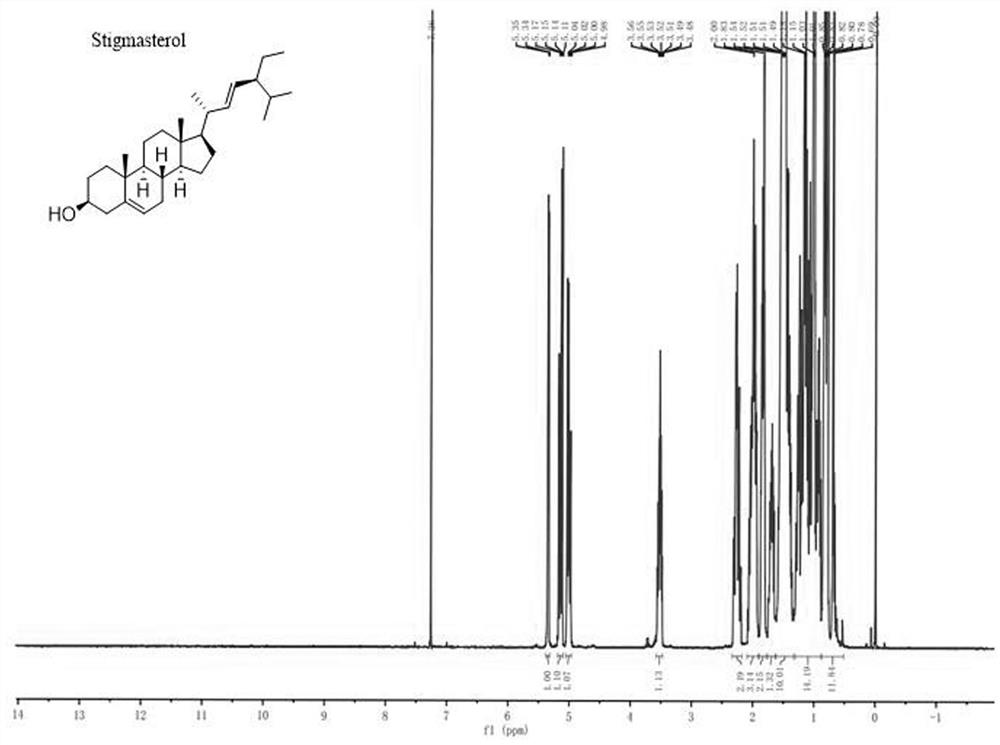
Novel cholic acid chelating agent modified by guanidinylation of biosterol as well as preparation method and application of cholic acid chelating agent
ActiveCN114716499AGood biocompatibilityHigh biosecurityOrganic active ingredientsMetabolism disorderCholic acidSterol
The invention relates to the technical field of medical small molecule synthesis, in particular to preparation and application of a novel biological sterol guanidinylation modified cholic acid chelating agent, prepared biological sterol derivatives react with pyrazole-1-formamidine hydrochloride and N, N-diisopropylethylamine, and then column chromatography separation and purification and concentration are performed to obtain the novel biological sterol guanidinylation modified cholic acid chelating agent. Compared with a traditional macromolecule resin type cholic acid chelating agent, the cholic acid chelating agent modified by biosterol guanidination has the advantages that the cholic acid binding capacity is high, the food-borne property is strong, the biocompatibility is good, the safety is good, the molecular weight is small, the yield is high, and the side effect is small.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Preparation method of polysubstituent 1,3,5-triazine
ActiveCN109810069AReduce generation costImprove securityOrganic chemistrySynthonCarbon–fluorine bond
The invention discloses a preparation method of polysubstituent 1,3,5-triazine. Specifically, substituen formamidine hydrochloride is used as a reactive substrate, chlorodifluoroacetic acid sodium salt is used as a carbon synthon, and the symmetrical or asymmetrical polysubstituent 1,3,5-triazine is obtained through fracture of carbon-chlorine, carbon-carbon and carbon-fluorine bonds under the action of equivalent inorganic base. The chemical structural general formula of the substituen formamidine hydrochloride is as shown in specification, and the inorganic is one selected from potassium carbonate, sodium carbonate, cesium carbonate. The preparation method disclosed by the invention has the advantages of easily available raw materials, no need for catalyst or oxidant, a wide variety of substrates, simple and convenient operation and environmental friendliness.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Synthesis method of diafenthiuron impurities A and B
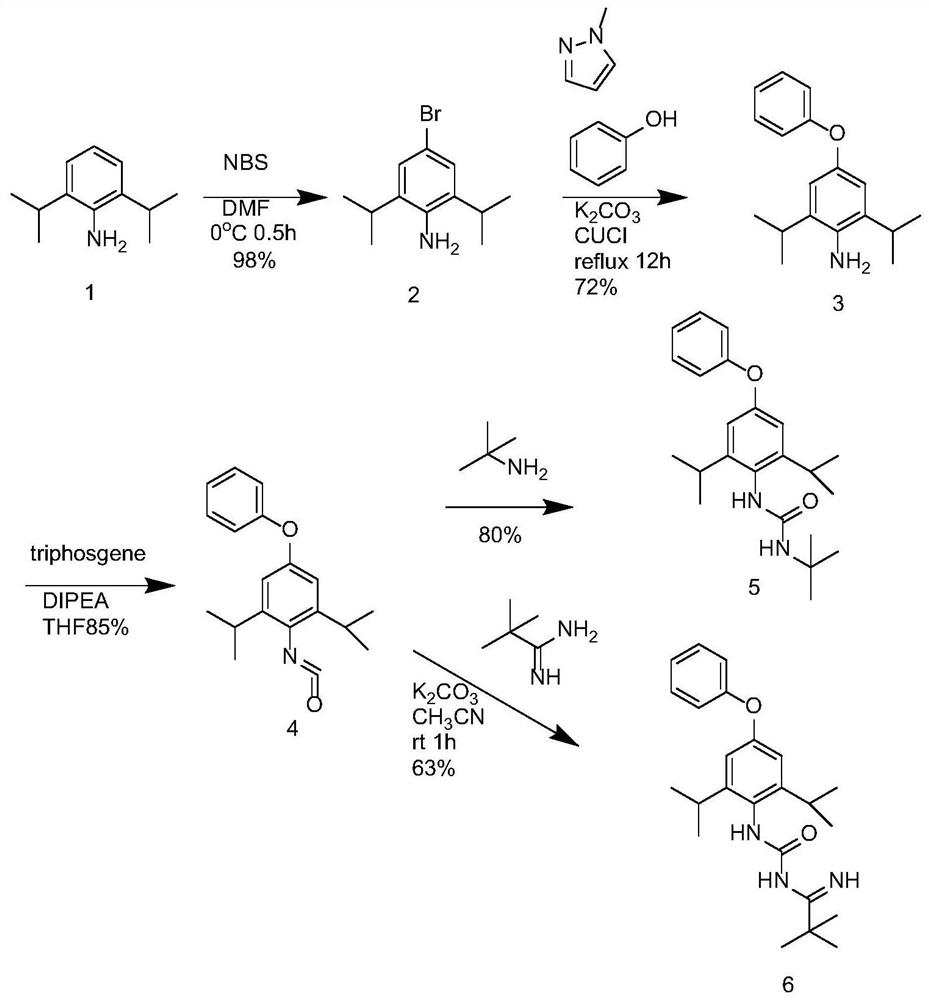
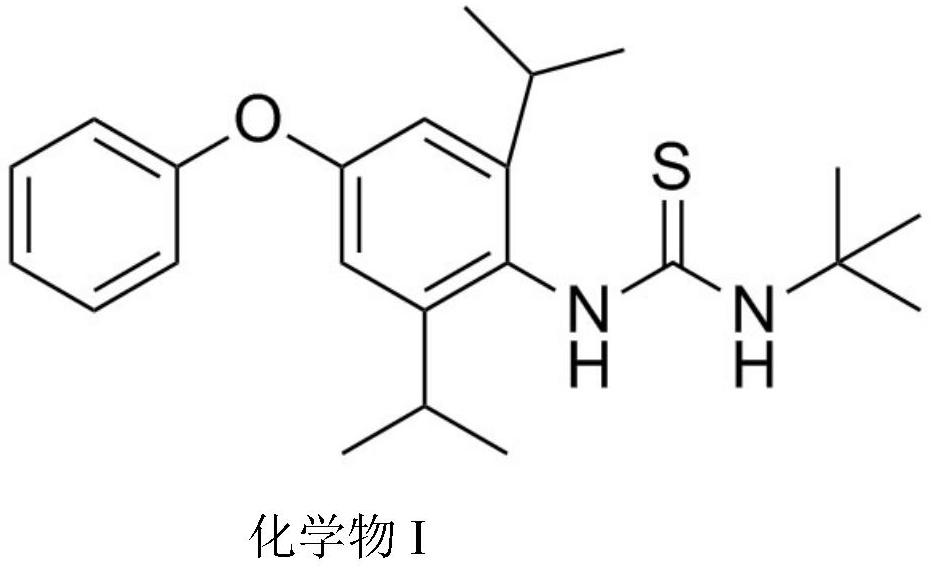
PendingCN113200865ARaise quality standardsUrea derivatives preparationIsocyanic acid derivatives preparationUse medicationTert-Butylamine
The invention provides a preparation method of the diafenthiuron impurities A and B. 2, 6-isopropylaniline, phenol, triphosgene, tert-butylamine and tert-butyl formamidine hydrochloride are used as raw materials, a material basis is provided for normatively researching the impurities, and the method can also be used for qualitative and quantitative analysis of the impurities in diafenthiuron production. And the impurities are controlled within a safe and reasonable limit range, so that the quality standard of the diafenthiuron can be improved, and important guiding significance is provided for safe medication of the masses.
Owner:LONGXINING SHANGHAI PHARMA TECH CO LTD +1
Synthetic method for macitentan drug intermediate
InactiveCN109081814AControl feed ratioTemperature controlOrganic chemistrySulfonyl chlorideDimethylaniline N-oxide
The invention discloses a synthetic method for a macitentan drug intermediate. The synthetic method for the macitentan drug intermediate comprises the following raw material components: 20-30 parts ofanhydrous methanol, 6-8 parts of 4-bromophenylacetic acid, 8-10 parts of sulfonyl chloride, 20-40 parts of sodium methoxide, 10-15 parts of dimethyl carbonate, 5-10 parts of formamidine hydrochloride, 60-80 parts of phosphorus oxychloride (newly evaporated) and 1-2 parts of N,N-dimethylaniline. According to the provided synthetic scheme, synthetic raw materials are simple, the purity of the obtained product is higher than that of like products, and the requirement for keeping synthesizing macitentan can be met.
Owner:嘉善中嘉化工有限公司
Pesticide containing mono formamidine hydrochloride soluble powder
InactiveCN1650714AGood synergyIncreased co-toxicity coefficientBiocideAnimal repellantsSide effectFormamidine hydrochloride
The pesticides which contains soluble powder of single-formamidine hydrochlorate belongs to the technic field of the composite pesticides, to resolve the drug resistance of the mite to the existing pesticides, the existing pesticides have a great poisonous character and have a side-effect to the environment. The pesticide which is made of abstracted single-formamidine hydrochlorate soluble powder compounded with pyrethroid species material, have a synergy effect and a stronger effect of destroying intectional worms.
Owner:阴太安
A kind of synthetic method of riociguat intermediate
ActiveCN105461715BAvoid High Pressure Catalytic Hydrogenation ReactionsLower requirementOrganic chemistryPyridineHigh pressure
The invention discloses a method for compounding riociguat intermediate, firstly 1-(2-fluorine benzyl) 1H-pyrazolo [3, 4-b] pyridine-3-formamidine hydrochloride are used as starting materials, and 2-[1-(2-fluorine benzyl)-1 H- pyrazolo [3, 4-b] pyridine-3-yl]-4, 5, 6-pyrimidine triamine of the riociguat intermediate are prepared by catalyzing and hydrogenating in normal pressure through a catalyst. The method for compounding the riociguat intermediate is mild in reaction conditions, prevents using a high pressure reaction kettle, reduces process demands to equipment, enables product purity to be bigger than 99.0% at last, and is suitable for commercial process.
Owner:郑州大明药物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka.patsnap.com/patent_img/0155700f-3441-4fcd-a8bd-98585c7e7a95/HDA00002661029300011.PNG)
![Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka.patsnap.com/patent_img/0155700f-3441-4fcd-a8bd-98585c7e7a95/HDA00002661029300021.PNG)
![Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka.patsnap.com/patent_img/0155700f-3441-4fcd-a8bd-98585c7e7a95/HDA00002661029300031.PNG)
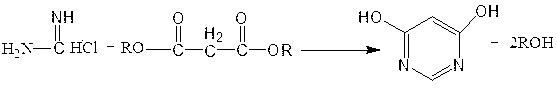

![Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/e6fcf0a3-942e-4adb-8315-5d31a2d877d7/BDA0001430817270000011.png)
![Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/e6fcf0a3-942e-4adb-8315-5d31a2d877d7/BDA0001430817270000021.png)
![Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine Simple synthetic method of 7-protecting group-4-(1-hydrogen-pyrazole-4-yl)pyrrole[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/e6fcf0a3-942e-4adb-8315-5d31a2d877d7/BDA0001430817270000031.png)
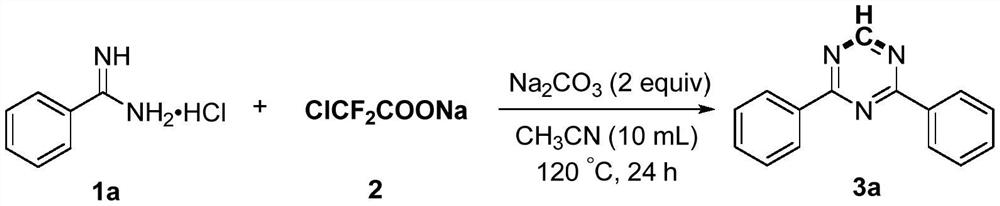
![Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride](https://images-eureka.patsnap.com/patent_img/5fa76047-38cf-4cba-93e0-3e1d8ba116ca/US20140309425A1-20141016-C00001.png)
![Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride](https://images-eureka.patsnap.com/patent_img/5fa76047-38cf-4cba-93e0-3e1d8ba116ca/US20140309425A1-20141016-C00002.png)
![Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride Method for synthesizing 1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridine-3-formamidine hydrochloride](https://images-eureka.patsnap.com/patent_img/5fa76047-38cf-4cba-93e0-3e1d8ba116ca/US20140309425A1-20141016-C00003.png)
![A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/82a03760-596b-4857-8b4c-d99ebe1095a0/FDA0002737010110000011.png)
![A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/82a03760-596b-4857-8b4c-d99ebe1095a0/GDA0002737010120000011.png)
![A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine A kind of synthetic method of 7-protecting group-4-(1-hydrogen-pyrazol-4-yl)pyrrole[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/82a03760-596b-4857-8b4c-d99ebe1095a0/GDA0002737010120000021.png)
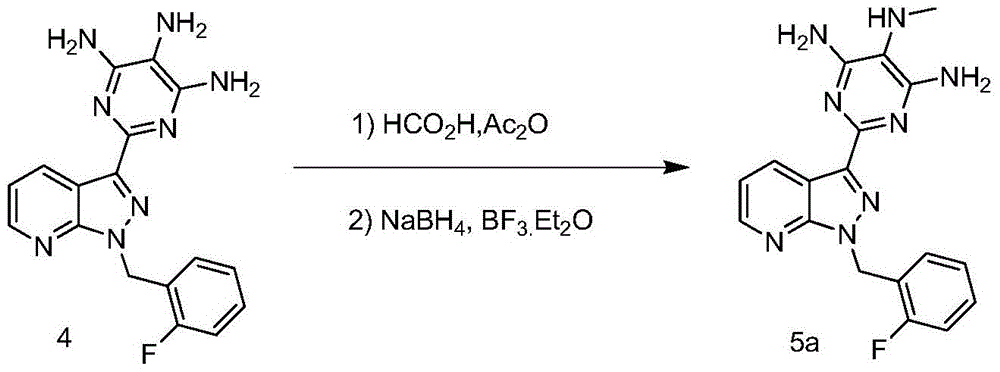
![The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka.patsnap.com/patent_img/8fd486a1-fc3d-4024-8b59-d90242ffe9d4/HDA00002661029300011.PNG)
![The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka.patsnap.com/patent_img/8fd486a1-fc3d-4024-8b59-d90242ffe9d4/HDA00002661029300021.PNG)
![The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate The preparation method of 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka.patsnap.com/patent_img/8fd486a1-fc3d-4024-8b59-d90242ffe9d4/HDA00002661029300031.PNG)