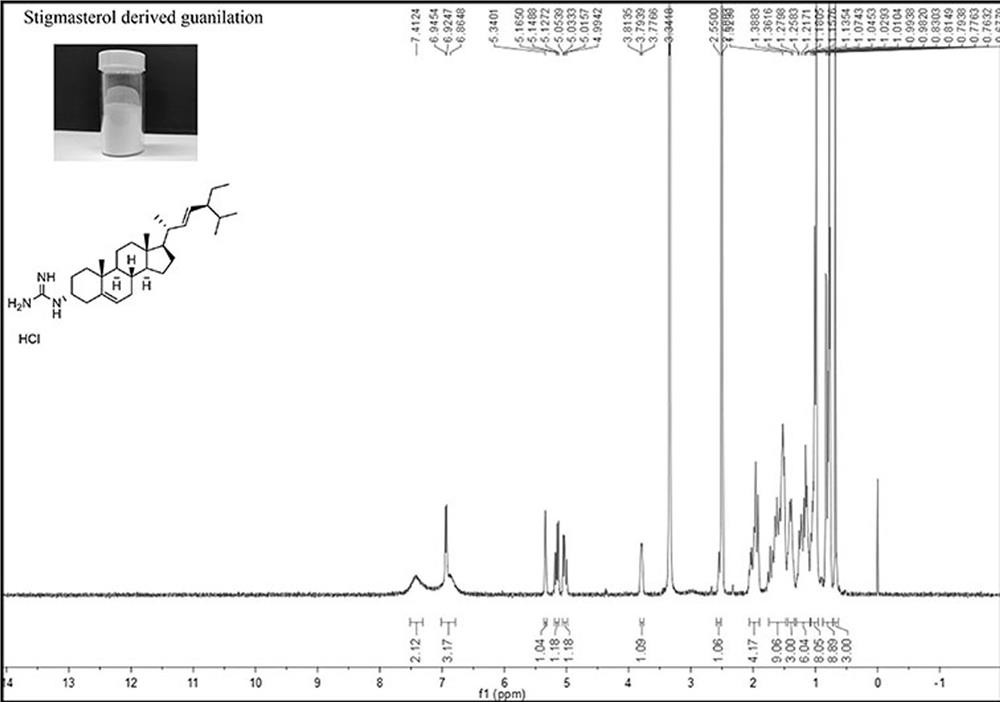
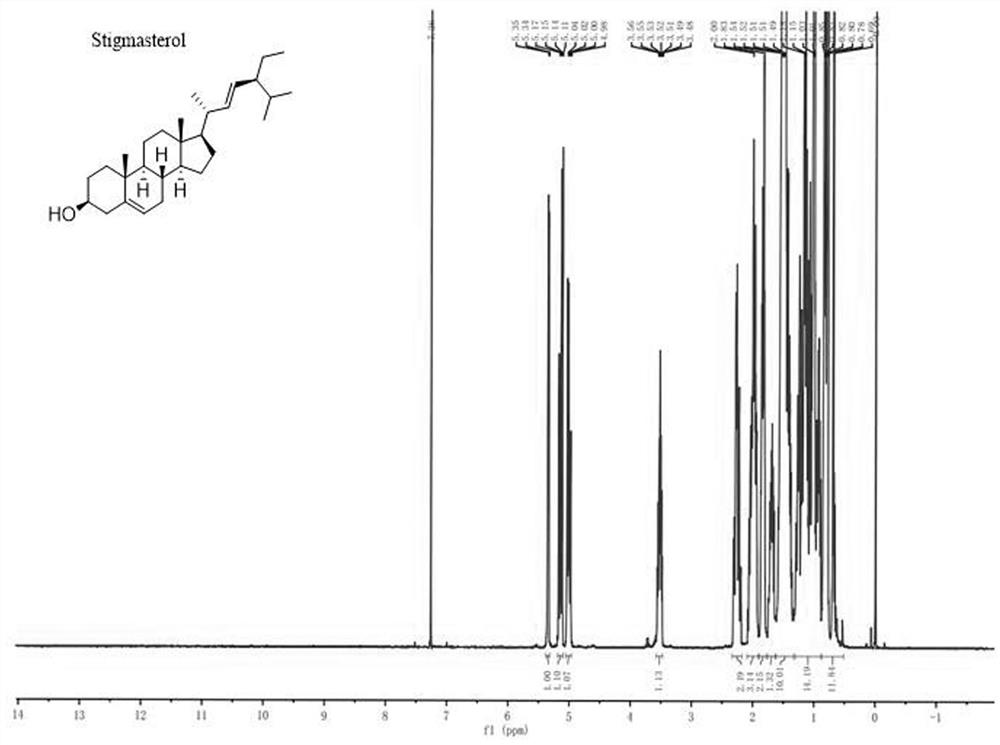
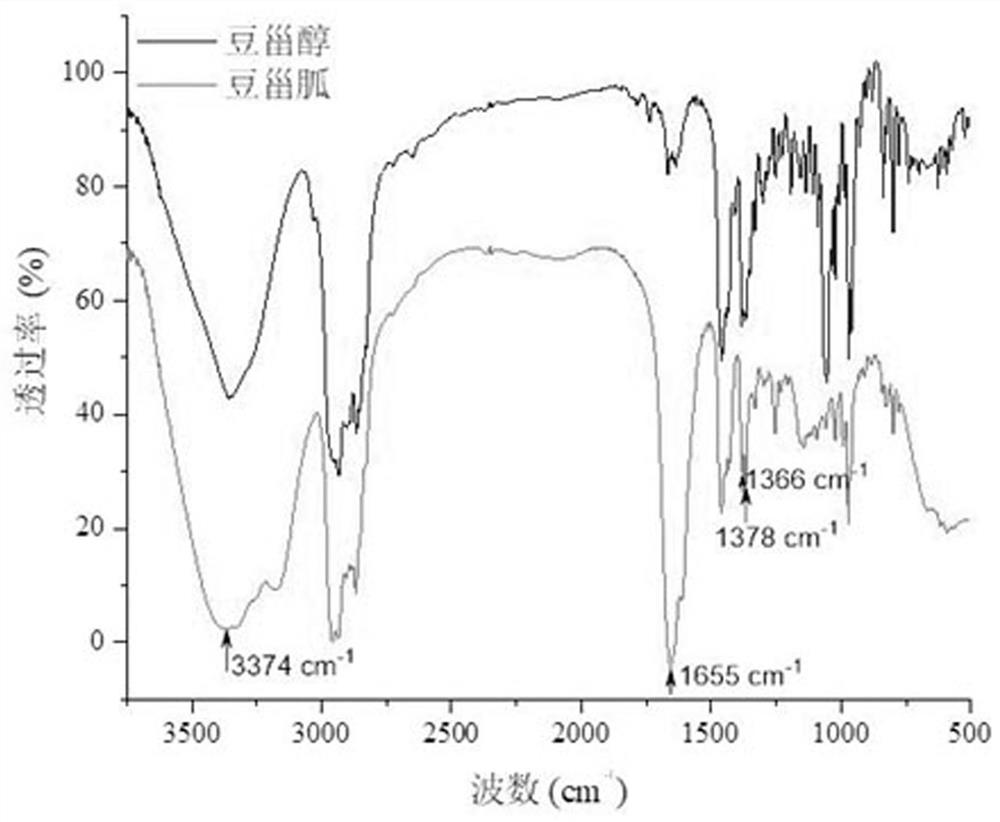
Novel cholic acid chelating agent modified by guanidinylation of biosterol as well as preparation method and application of cholic acid chelating agent
A technology of biosterol and guanidinylation, which is applied in the field of preparation of bile acid chelating agent, can solve problems such as the influence of thyroid function and gastrointestinal discomfort, and achieve the effect of simple production process, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of the bile acid chelating agent modified by stigmasterol guanidation, the steps are as follows:
[0050](1) Under anhydrous and anaerobic conditions, add 0.2 g stigmasterol and 0.18 g triphenylphosphine to 5 mL of ultra-dry tetrahydrofuran solvent, stir well, and cool the mixture to 0 °C, then quickly add 0.15 g diisopropyl azodicarboxylate, and then 0.18 g of diphenylphosphonium azide was added dropwise. After the dropwise addition, the temperature was raised to room temperature, and the reaction was carried out for 22 h, then stirred at 50 °C for 3 h, and then 0.20 g of triphenylphosphonium was added. , heated and stirred at 50 °C for 2 h, and stirred until no gas was formed. Cool to room temperature, add 0.006 g of water, continue to stir for 3 h, cool, spin dry, separate and purify by column chromatography, concentrate, and dry to obtain stigmasterol derivative A as a pale yellow solid with a yield of 55.8%.
[0051] (2) Add 0.050 g of biost...
Embodiment 2
[0053] The preparation method of the bile acid chelating agent modified by stigmasterol guanidation, the steps are as follows:
[0054] (1) Under anhydrous and anaerobic conditions, add 1.00 g of stigmasterol and 0.90 g of triphenylphosphonium to 20 mL of ultra-dry tetrahydrofuran solvent, stir evenly, and cool the mixture to 0 °C, then quickly add 0.75 g diisopropyl azodicarboxylate, followed by dropwise addition of 0.90 g of diphenylphosphoryl azide, after the dropwise addition, the temperature was raised to room temperature, the reaction was carried out for 22 h, and then stirred at 50 °C for 3 h, and then 1.00 g of triphenyl was added. Phosphorus was heated and stirred at 50 °C for 2 h, and stirred until no gas was formed. Cooled to room temperature, added 0.030 g of water, continued to stir for 3 h, cooled, spin-dried, separated and purified by column chromatography, concentrated, and dried to obtain stigmasterol derivative A as a pale yellow solid with a yield of 55.8%. ...
Embodiment 3
[0057] The preparation method of the bile acid chelating agent modified by stigmasterol guanidation, the steps are as follows:
[0058] (1) Under anhydrous and anaerobic conditions, add 5.0 g of stigmasterol and 4.0 g of triphenylphosphonium to 50 mL of ultra-dry tetrahydrofuran solvent, stir evenly, and cool the mixture to 0 °C, then quickly add 3.0 g diisopropyl azodicarboxylate, then 2.8 g diphenylphosphonium azide was added dropwise, after the dropwise addition, it was warmed to room temperature, reacted for 24 h, then stirred at 50 °C for 3 h, and then 4.0 g triphenylphosphonium was added , heated and stirred at 50 °C for 2 h, and stirred until no gas was formed. Cool to room temperature, add 0.1 g of water, continue stirring for 3 h, cool, spin dry, separate and purify by column chromatography, concentrate, and dry to obtain light yellow solid stigmasterol derivative A with a yield of 60.4%.
[0059] (2) 0.850 g of biosterol derivative A prepared in step (1), 0.300 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com