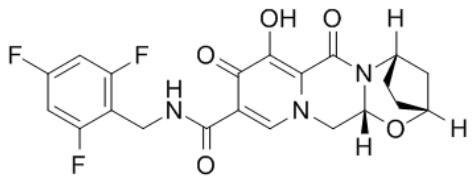
Patents
Literature
49 results about "Diphenylphosphoryl azide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
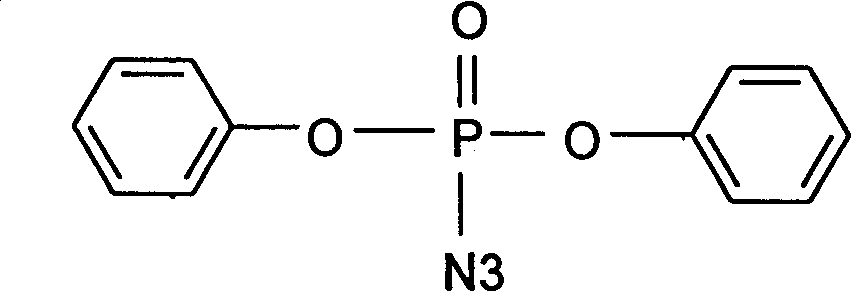
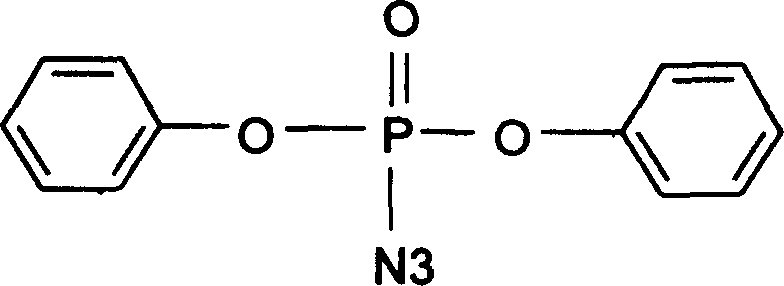
Diphenylphosphoryl azide (DPPA) is an organic compound. It is widely used as a reagent in the synthesis of other organic compounds.
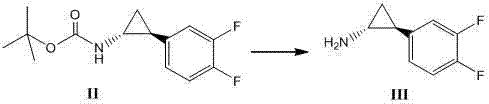
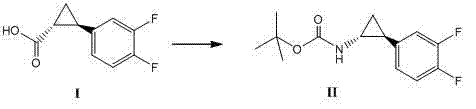
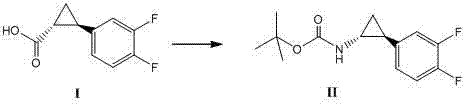
Method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
InactiveCN102249929AReduce difficulty of reactionHigh reaction yieldOrganic compound preparationAmino compound preparationBiochemical engineeringTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine which is an intermediate for preparing an anticoagulation medicine Ticagrelor. The method provided by the invention mainly comprises the following steps of: synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by tertiarybutoxy carbonyl through carrying out a rearrangement reaction of DPPA (Diphenylphosphoryl Azide); then removing the protective group of the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by the tertiarybutoxy carbonyl and then alkalifying to obtain the product. The whole reaction can be finished through a one-pot boiling synthetic method so that synthesizing steps and synthesizing time are greatly saved, the cost is effectively reduced and the yield is improved; and the method for synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine has the very active meaning in the industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
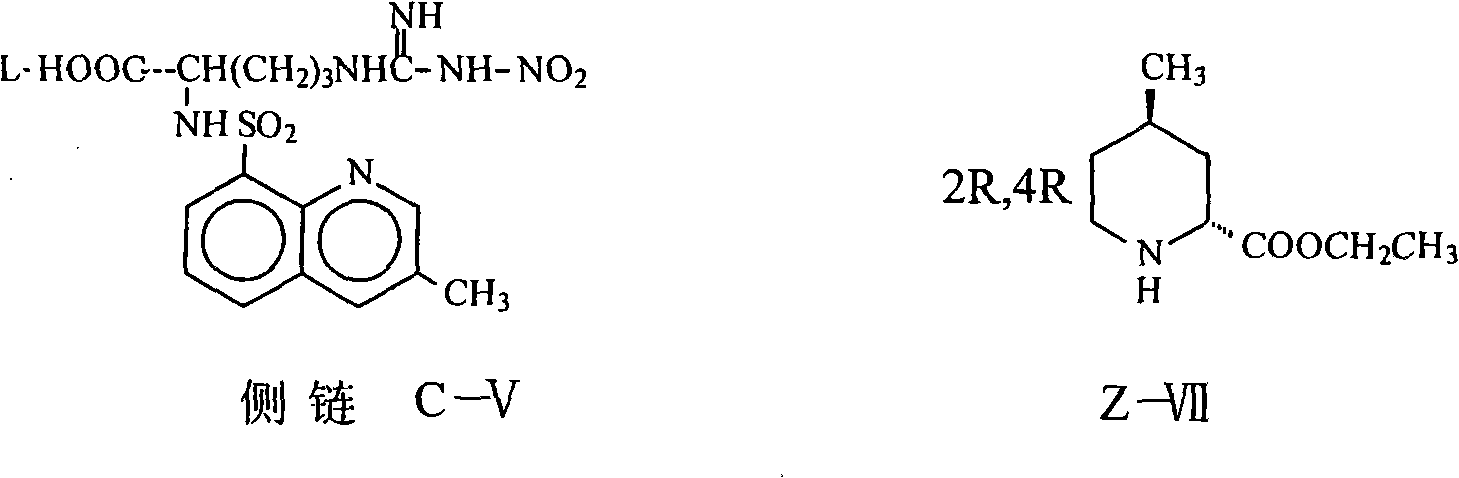
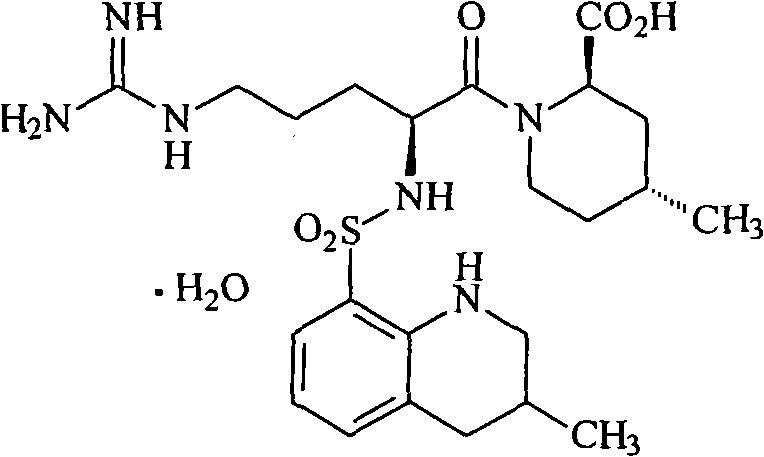
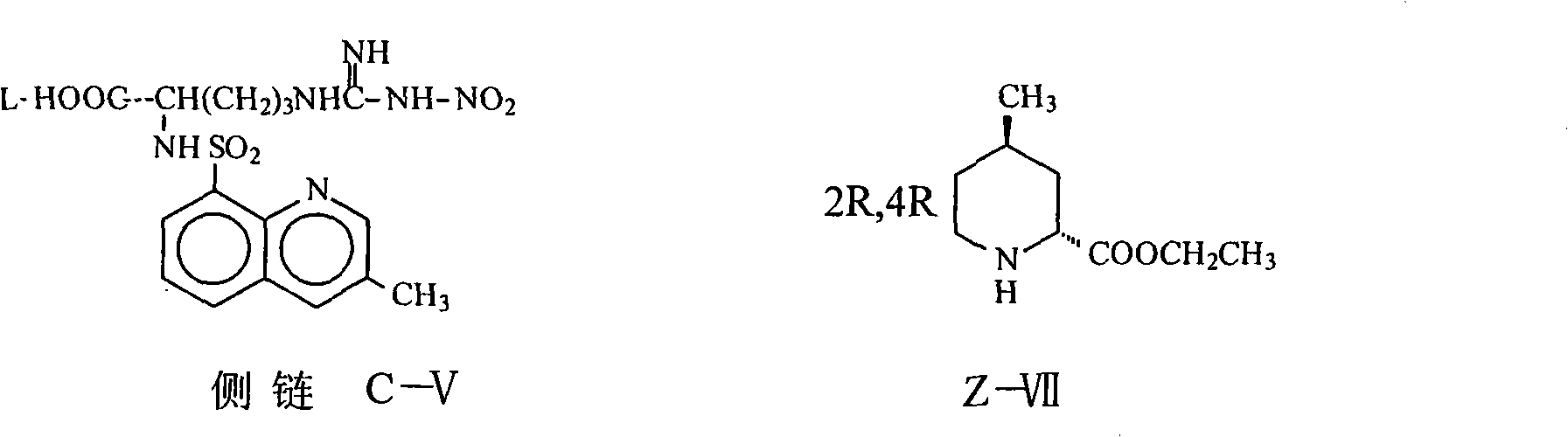
Argatroban and preparation thereof
InactiveCN101348481AThe synthesis process is simpleEasy to operateOrganic chemistryDiethyl phosphateOrganic solvent
The invention relates to a method for synthesizing argatroban. The method comprises the following steps that nitryl L-arginie and quinoline sulfonchloride are condensed, and undergo amidation with piperidine ethyl formate, followed by hydrolysis and hydrogenation to obtain argatroban; the amidation is to make carboxylate (c-v) and (2R, 4R) 4MPE (Z-VII) react in an organic solvent in the presence of condensing agent, or the presence of both condensing agent and dehydration promoter, in which the molecular ratio of carboxylate (c-v): (2R, 4R) 4MPE (Z-VII): condensing agent: dehydration promoter is 1: 0.8-1.2: 0.8-1.2: 0-1.2. The condensing agent adopted by the invention is diphenylphosphoryl azide, diethylthiophosphoryl, chlorophosphoric acid diethyl or bromophosphoric acid diethyl. The invention has simplified operation, lowered cost, decreased pollution, increased yielding rate, and is suitable for large-scale industrialized production of argatroban.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV +1
Water-based paint for furniture
The invention discloses a water-based paint for furniture. The water-based paint comprises an aqueous polyurethane dispersion, deionized water, a film-forming agent, an antifoaming agent, a leveling agent, a wetting agent, a dispersant, carbon black, calcium carbonate, nanometer aluminum oxide, a thickener, a cross-linking agent, a buffer agent, cerium 2-ethylhexanoate, 2-[1-(ethylsulfonyl)azetidin-3-ylidene]acetonitrile, propylene glycol ceteth-3 acetate, diphenyl azidophosphate and diphenyl carbonate. The aqueous polyurethane dispersion and the water are adopted as the main body of the wholepaint, so the whole paint system is aqueous; the synergistic effects of above materials greatly improve the hardness and the wear resistance of the system, so the hardness and the wear resistance canreach the hardness and the wear resistance of normal oily paints, and the paint in the invention also has good heat resistance; and the paint still has good hardness and wear resistance at a high temperatures.
Owner:浙江顶丰家具有限公司
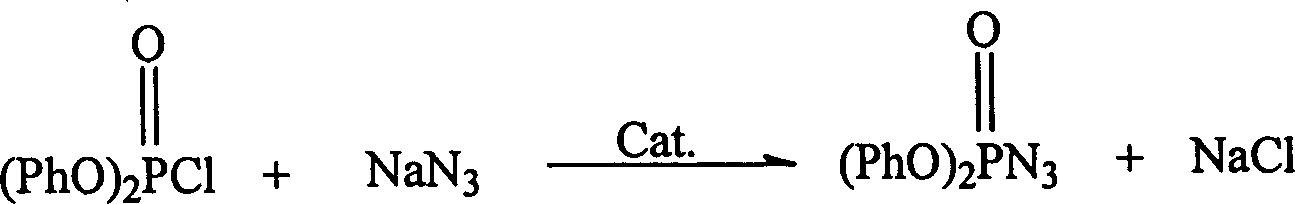
Catalystic preparation of nitrine diphenyl posphate
InactiveCN1552719AShorten the timeEasy to operatePhosphorus organic compoundsDiphenyl phosphateQuaternary ammonium cation
A process for preparing diphenyl azidophosphate by catalytic method features the reaction between its raw materials (diphenyl chlorophosphate and sodium azide) and the phase-transfer catalyst chosen from quaternary ammonium salt, inorganic tetraamine salt, strong-alkaline alamine and crown ether at 15-30 deg.c for 10-15 hr. Its advantages are simple process and high purity of product.
Owner:GUILIN GUIKAI BIOLOGICAL SCI & TECH
Application of diphenyl phosphoryl azide (DPPA) in aspect of treating wax coat on leaf surfaces of plants and surface of fruit
ActiveCN104186462AReduce hindrancePromote absorptionBiocideSpecial ornamental structuresWaxGrowth plant
The invention provides the application of diphenyl phosphoryl azide (DPPA) in the aspect of treating the wax coat on the leaf surfaces of plants and the surface of fruit. The invention provides a new use of the DPPA; when the DPPA is used for treating the wax coat on the leaf surfaces of plants and the surface of fruit, the speed is rapid, and the effect is good; the leaves and the fruit are not damaged by the DPPA within a wider concentration range, and the wax coat on the leaf surfaces of plants and the surface of fruit can be effectively destroyed by the DPPA, so that the obstruction function of the wax coat on the leaf surfaces of plants and the surface of fruit for fertilization and other effective components can be greatly reduced, the absorption is improved, and the fertilizer and other effective components are better absorbed and utilized. Furthermore, a new raw material capable of bringing a remarkable effect is provided for the design of formula of a leaf fertilizer and a plant growth regulator used for the leaf surfaces. Furthermore, based on the research of the invention, a new processing reagent and an effective processing method are provided for smoothly, rapidly and completely removing the wax coat on the leaf surfaces when a leaf artwork is made.
Owner:GUILIN GUIKAI BIOLOGICAL SCI & TECH
Oil well cement high-temperature retarder microcapsule and preparation method thereof
ActiveCN108570313AAvoid hyperretardingGood thickening lineDrilling compositionPolyesterMaterials science
The invention discloses an oil well cement high-temperature retarder microcapsule. Raw materials of the microcapsule include, by weight, 50-60 parts of a high-temperature retarder, 5-10 parts of a reactive diluent, 20-30 parts of a water-insoluble oligomer wall material, 2-3 parts of a photoinitiator and 1-3 parts of an emulsifier. The water-insoluble oligomer wall material is polyurethane acrylicresin or polyester acrylic resin. The reactive diluent is at least one of alkyl acrylate, hydroxy acrylate, methacrylate, ethylene glycol diacrylate, propylene glycol diacrylate, trimethylolpropane triacrylate, dimethyl dithiophosphate, pentaerythritol triacrylate and diphenylphosphoryl azide. The microcapsule is prepared from the abovementioned raw materials through a W / O / W composite emulsion photopolymerization method. The microcapsule ensures that the cement slurry has similar thickening time and compressive strength under the condition of a large temperature difference, that the comprehensive performance of the cement slurry system satisfies the requirements of cementing construction of thermal production wells, and the process is simple and is favorable for industrialization. .
Owner:SOUTHWEST PETROLEUM UNIV
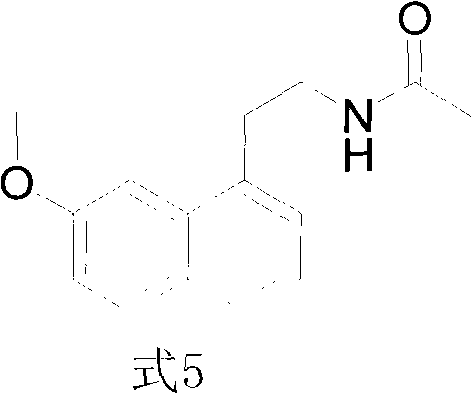
Synthesis method of valdoxan intermediate 2-(7-methoxy-1-naphthyl)ethylamine
ActiveCN101973897ALow costShort routeOrganic compound preparationAmino-hyroxy compound preparationPropanoic acidSynthesis methods
The invention provides a preparation method of a valdoxan intermediate 2-(7-methoxy-1-naphthyl)ethylamine, which has the advantages of simple and convenient operation, moderate conditions, short reaction time, high yield and high purity. In the method, 3-(7-methoxy-1-naphthyl)propionic acid reacts with diphenylphosphoryl azide to generate an acyl azide, and basic hydrolysis is carried out on the acyl azide to obtain the target product without separation and purification. The method is applicable to industrial production.
Owner:NANTONG BOTAO CHEM
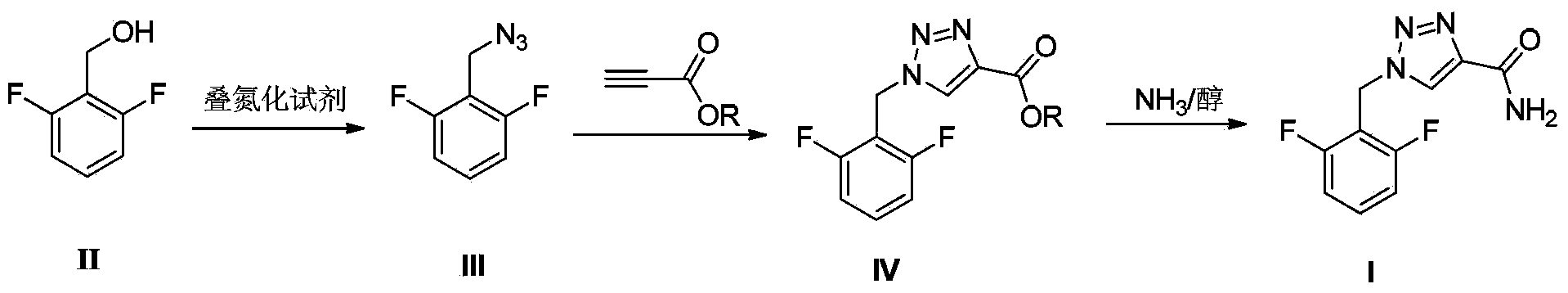
A rufinamide preparing method
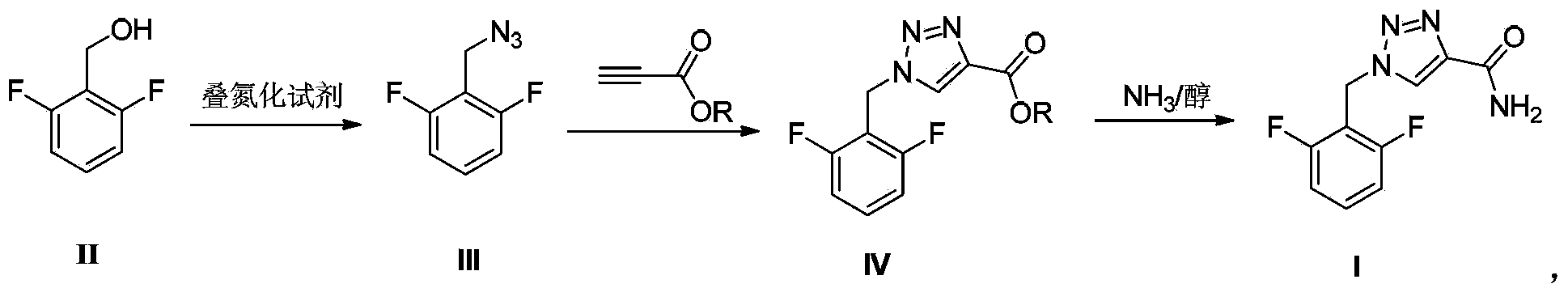
The invention belongs to the field of medicine synthesis and provides a rufinamide preparing method. 2,6-difluorobenzylalcohol is adopted as an initial raw material, and is reacted with an azidation agent diphenylphosphoryl azide to produce a critical intermediate 2,6-difluorobenzylazide, the 2,6-difluorobenzylazide is reacted with propargyl ester, and then the rufinamide is obtained by an ammonolysis reaction. The method has advantages of low toxicity, low pollution, high safety, short production period, simple and convenient operation, and the like, and is suitable for industrial production.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
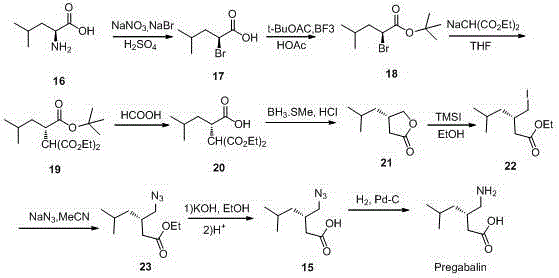
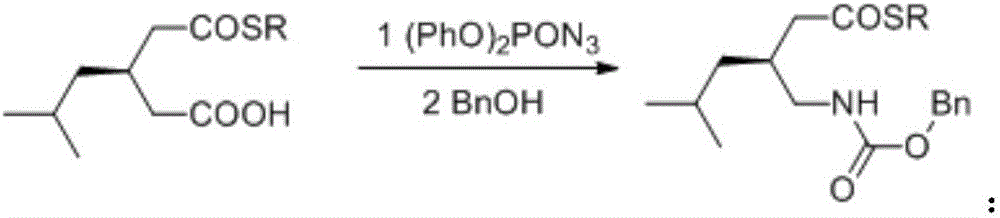
Preparation method for asymmetrical synthesis of pregabalin
ActiveCN105753726ANovel synthetic routeFew stepsCarbamic acid derivatives preparationOrganic compound preparationThiolThiourea
The invention discloses a preparation method for asymmetric synthesis of pregabalin.The preparation method includes: using 3-isobutylglutaric anhydride as a starting raw material; under catalytic action of chiral thiourea ammonium salt, enabling the starting raw material to be in asymmetric alcoholysis with mercaptan to generate mercaptide; enabling mercaptide to react with diphenylphosphoryl azide and benzyl alcohol sequentially to generate benzyloxy carbonyl methyl mercaptide; obtaining pregabalin through hydrolysis and hydrogenation reduction.The synthetic method is adopted to synthesize pregabalin, asymmetric catalysis is adopted to synthesize a key intermediate, a chemical resolution reagent is not used, total yield is greater than 50%, and ee value is greater than 94%.The preparation method has the advantages of novel synthesis path, few steps, mild reaction condition and the like.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Insulated highly-fire-retardant cable sheath material and preparation method thereof
InactiveCN105504429AImprove flame retardant performanceShort burning distancePlastic/resin/waxes insulatorsInsulated cablesDiphenylphosphoryl azidePolytetrafluoroethylene
The invention discloses an insulated highly-fire-retardant cable sheath material. The insulated highly-fire-retardant cable sheath material comprises, by weight, 50-70 parts of polyolefin elastomers, 20-35 parts of polytetrafluoroethylene, 10-15 parts of dioctyl phthalate, 8-14 parts of dibutyl phthalate, 12-18 parts of prednisolone sodium phosphate, 15-20 parts of diphenylphosphoryl azide, 5-13 parts of 3-pyridinecarboxylic acid magnesium salt, 3-9 parts of aluminum naphthenate, 20-30 parts of flyash, 15-25 parts of guaifenesin, 3-10 parts of trichlorovinylsilane, 9-13 parts of methylaluminoxane and 4-9 parts of 2-camphanone. The insulated highly-fire-retardant cable sheath material is excellent in fire-retardant effect and capable of better guaranteeing production and living safety.
Owner:SUZHOU KEMAO ELECTRONICS MATERIALS TECH
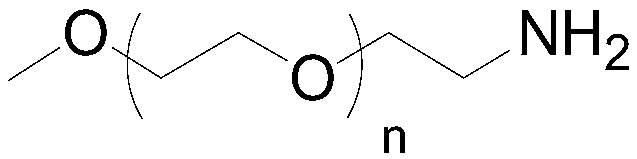
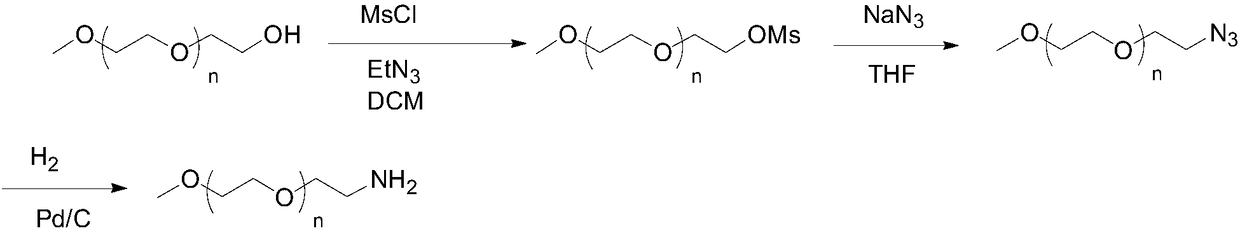
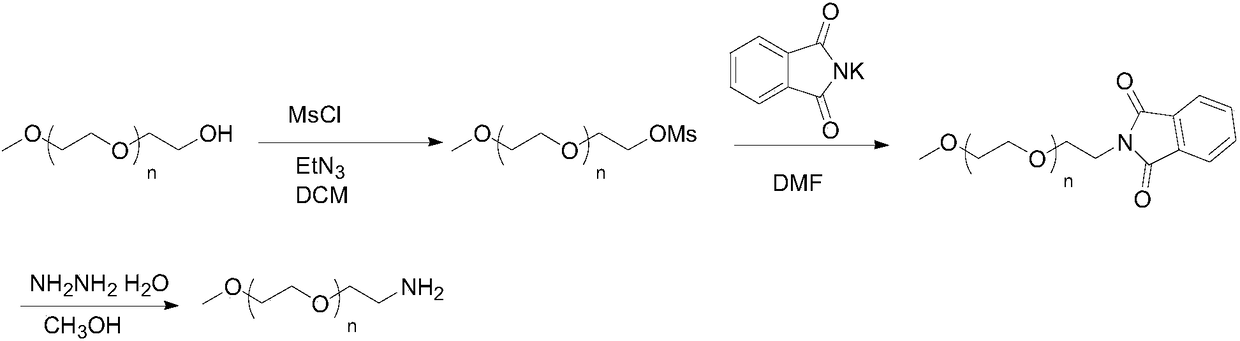
Preparation method of mPEG-amine
The invention discloses a preparation method of mPEG-amine and belongs to the field of chemical synthesis. A compound shown in the formula (1), namely, mPEG serving as an initial material and DPPA (diphenylphosphoryl azide) are subjected to a Mitsunobu substitution reaction in a first solvent under the action of triphenyl phosphine and a catalyst, a product and hydrogen are subjected to a reduction reaction, and the target compound mPEG-amine is obtained. The target compound mPEG-amine can be obtained through two-step synthesis with the method, and the method has controllable conditions, simple aftertreatment, few side reactions and high yield and meets the requirement of industrial production.
Owner:EAST CHINA NORMAL UNIV +1
Synthesis method of bictegravir intermediate
ActiveCN110092726AChiralityHigh yieldOrganic compound preparationCarboxylic compound preparationHydrogen pressureGrignard reagent
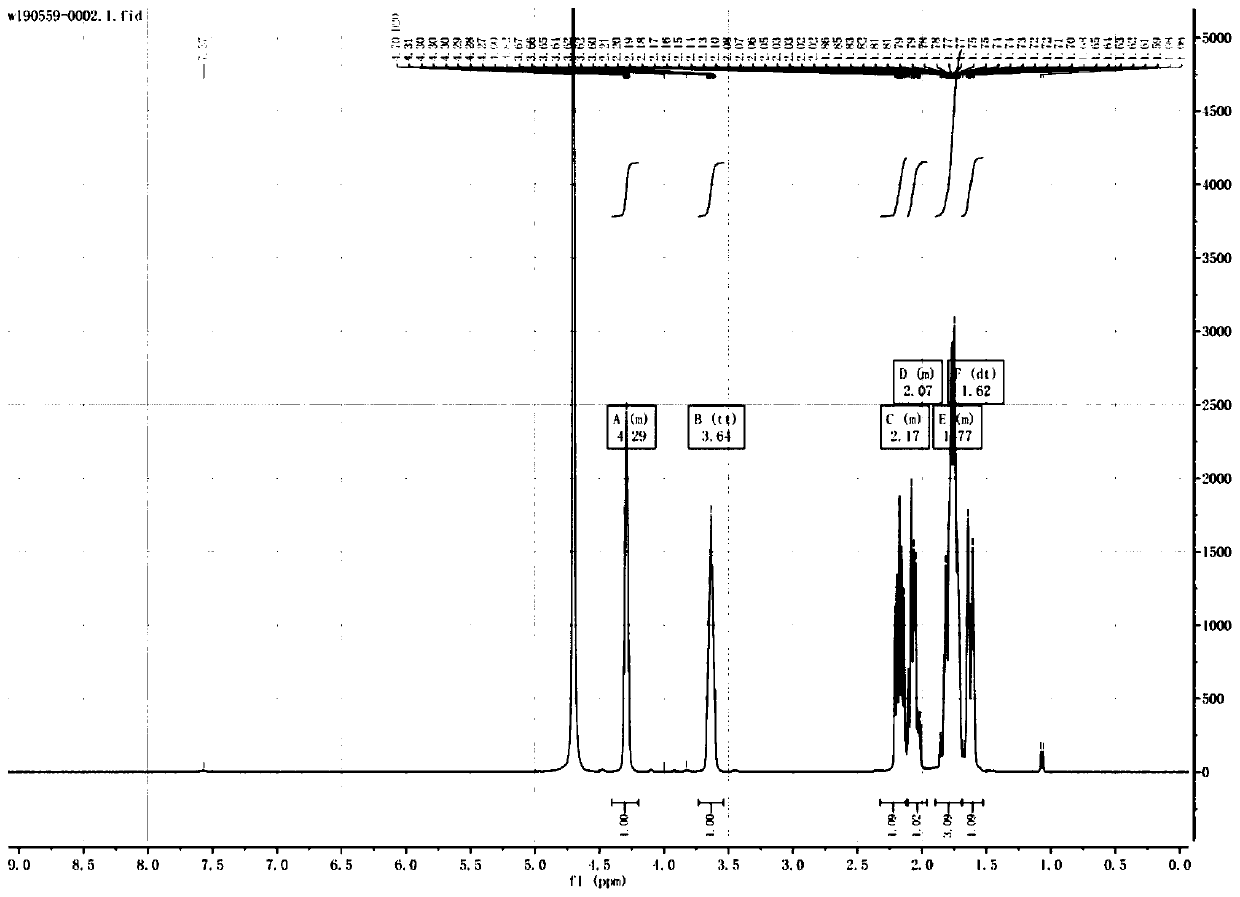
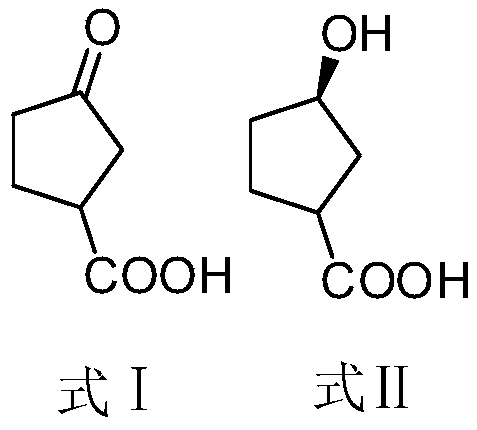
The invention discloses a synthesis method of a bictegravir intermediate. Starting material 3-carbonyl cyclopentacarboxylic acid (formula I) undergoes asymmetric reduction reaction under the conditionof an enzyme to generate (3R)-3-hydroxycyclopentane carboxylic acid (formula II); the (3R)-3-hydroxycyclopentane carboxylic acid (formula II) and diphenylphosphoryl azide (DPPA) undergoe rearrangement cyclization reaction to generate (1R, 5S)-2-oxy-4-azabicyclo [3.2.1] octane-3-one (formula III); the (1R, 5S)-2-oxy-4-azabicyclo [3.2.1] octane-3-one (formula III) is hydrolyzed in hydrochloric acidto directly obtain the bictegravir intermediate (1R, 3S)-3-aminocyclopentanol hydrochloride. The raw materials used in the method are cheap and easily available, and the cost is low; the reaction selectivity is high, by-products are few, the yield is high, and the total yield reaches 63.5%; the synthesis method has the advantages of short reaction route, shortened production period, reduced discharge of three wastes, avoidance of hydrogen pressure reduction and Grignard reagent reaction, safety and environmental protection, and suitability for industrial production.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
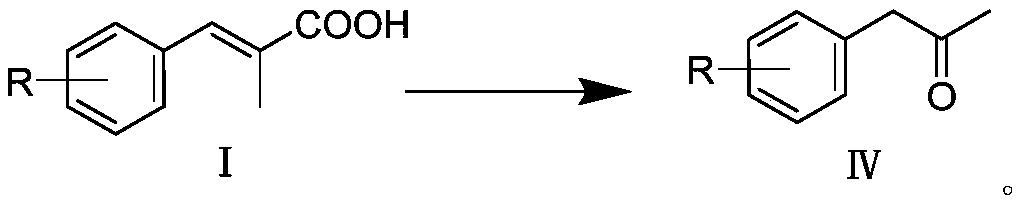
1-aryl-2-acetone compound preparation method
PendingCN110590529AFew reaction stepsImprove operational safetyOrganic compound preparationCarbonyl compound preparationArylStructural formula
The invention discloses a 1-aryl-2-acetone compound preparation method, which comprises: (1) carrying out a reaction on (E)-2-methyl-3-aryl acrylic acid (I) as a starting raw material and diphenyl azidophosphate (DPPPA) in the presence of an organic alkali; (2) carrying out a heating reaction; and (3) adding an acidic aqueous solution into the reaction solution, and carrying out a reaction to obtain the 1-aryl-2-acetone compound with a structural formula (IV). According to the present invention, the method can solve the technical problems of difficultly available, highly-corrosive, highly toxic and highly explosive reagents, long steps, low yield, tedious operation and the like in the 1-aryl-2-acetone synthesis in the prior art, has characteristics of easily available raw materials, safe and simple operation, mild condition and high yield, and is suitable for industrial production.
Owner:ZHEJIANG PHARMA COLLEGE
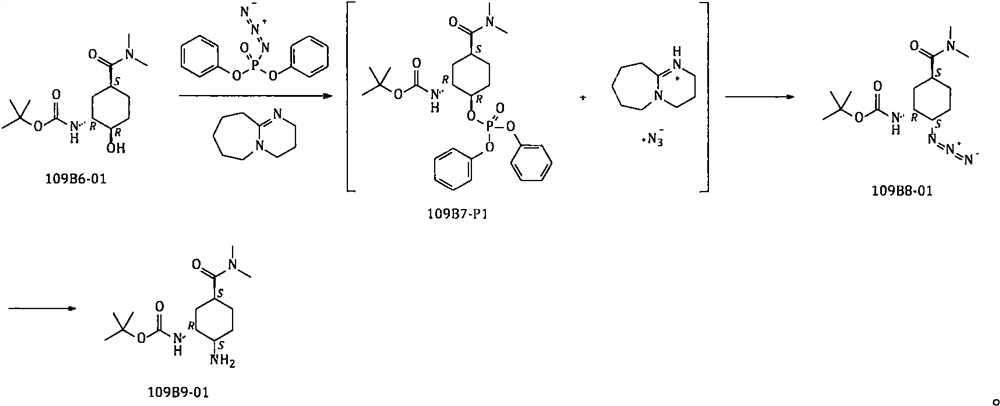
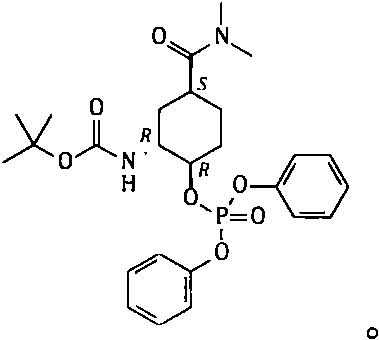
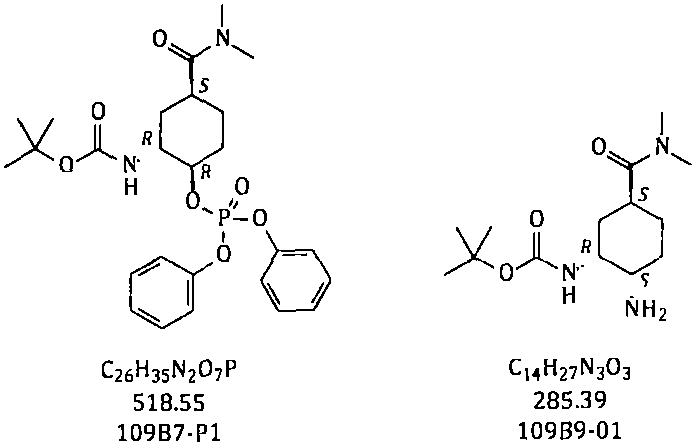
Method for preparing edoxaban chiral amine intermediate
ActiveCN111606827AReduce usageImprove securityCarbamic acid derivatives preparationOrganic compound preparationPhosphoric Acid EstersPhosphate
The invention provides a safe, simple and convenient method which is more suitable for industrial large-scale production and preparation of N-[(1R, 2S, 5S)-2-amino-5-[(dimethylamino) carbonyl] cyclohexyl] tert-butyl carbamate. A compound N-[(1R, 2R, 5S)-5-[(dimethylamino) carbonyl]-2-hydroxycyclohexyl] tert-butyl carbamate is used as a raw material; toluene, n-heptane and other hydrocarbons are used as reaction solvents; in presence of DBU, reacting with diphenylphosphoryl azide to obtain a mixture of N-[(1R, 2R, 5S)-5-[(dimethylamino) carbonyl]-2-[(diphenoxy phosphoryl) oxy] cyclohexyl] tert-butyl carbamate and DBU azide acid salt; adding a proper amount of an alkali, and replacing phosphate with azide generated in the system to obtain corresponding azide N-[(1R, 2S, 5S)-2-azide-5-[(dimethylamino) carbonyl] cyclohexyl] tert-butyl carbamate; and reducing the azido group to obtain the corresponding amino compound N-[(1R, 2S, 5S)-2-amino-5-[(dimethylamino) carbonyl] cyclohexyl] tert-butyl carbamate.
Owner:内蒙古京东药业有限公司
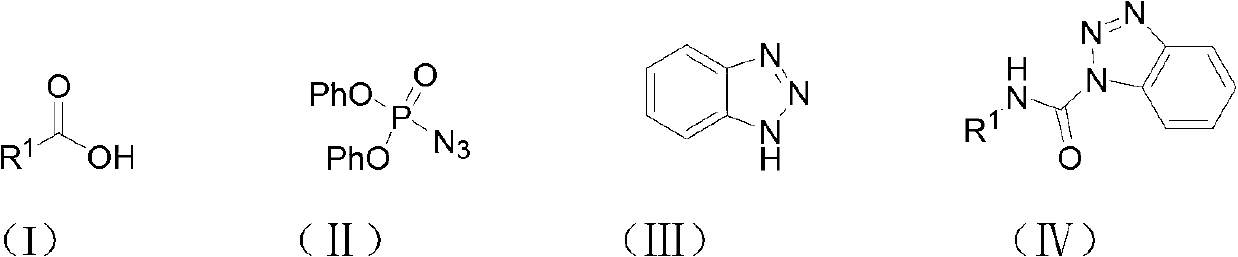
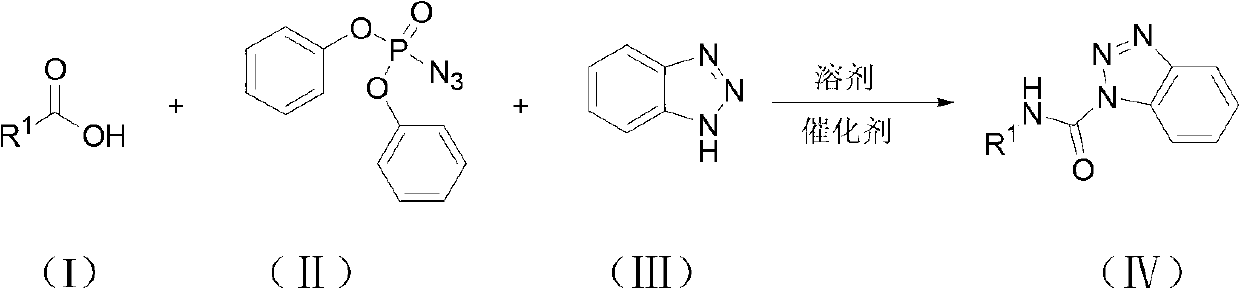
Method for synthesizing carbamyl benzotriazole by three-component one-pot method
InactiveCN102731419ASuitable for industrial productionEasy to operateOrganic chemistryAcetic acidBenzene
The invention discloses a method for synthesizing carbamyl benzotriazole by a three-component one-pot method. The method is characterized by taking organic carboxylic acid, diphenylphosphoryl azide and benzotriazole as raw materials and comprising the following steps of: under the effect of a catalyst, performing reflux reaction in toluene for 2-8 hours; performing after-treatment of the obtained reaction mixture; and recrystallizing in ethyl acetate to obtain carbamyl benzotriazole, wherein the substance amount ratio of the organic carboxylic acid to diphenylphosphoryl azide to benzotriazole to catalyst is 1.0:(1.0-3.0):(1.0-1.5):(1.5-3.0). The raw materials are simple and easily available, the price is low, the conditions are mild, the operation is simple and convenient, the yield is relatively high, the after-treatment is relatively convenient, and the method is suitable for industrial production.
Owner:ZHEJIANG NORMAL UNIVERSITY
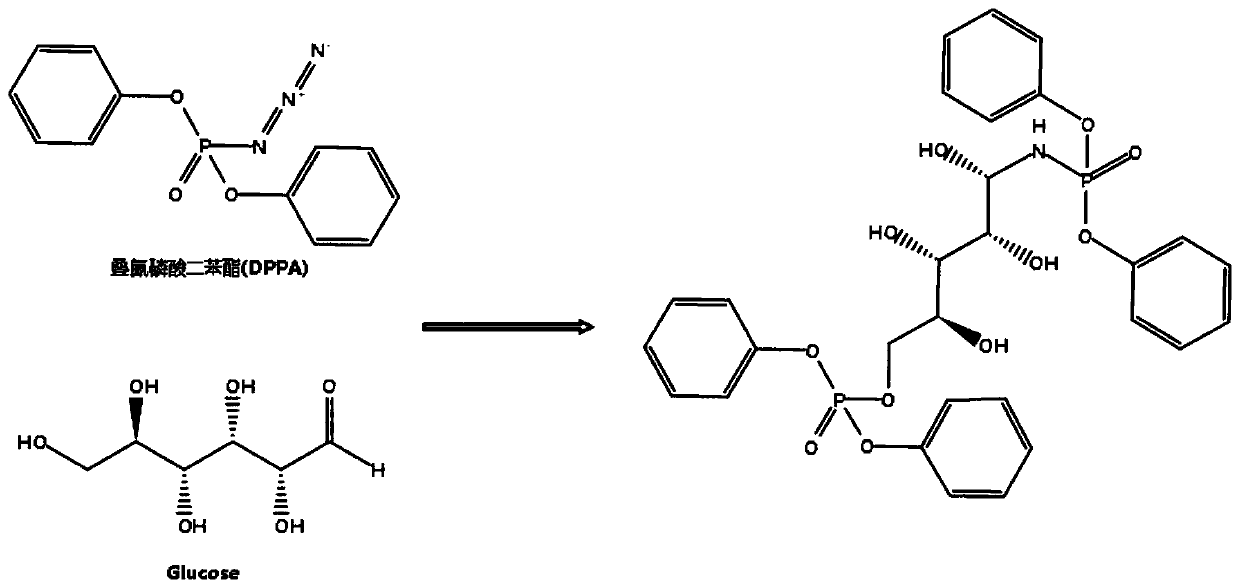
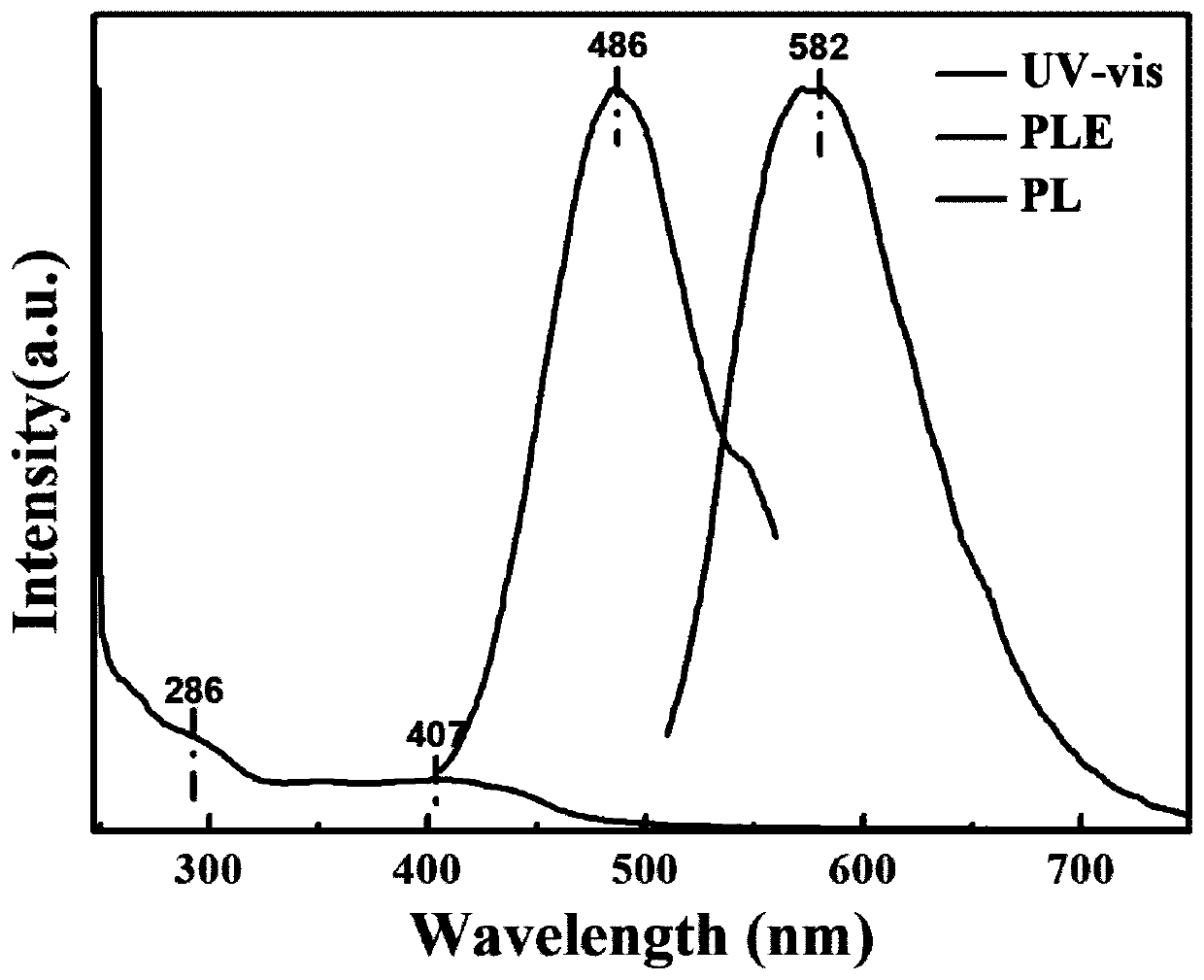
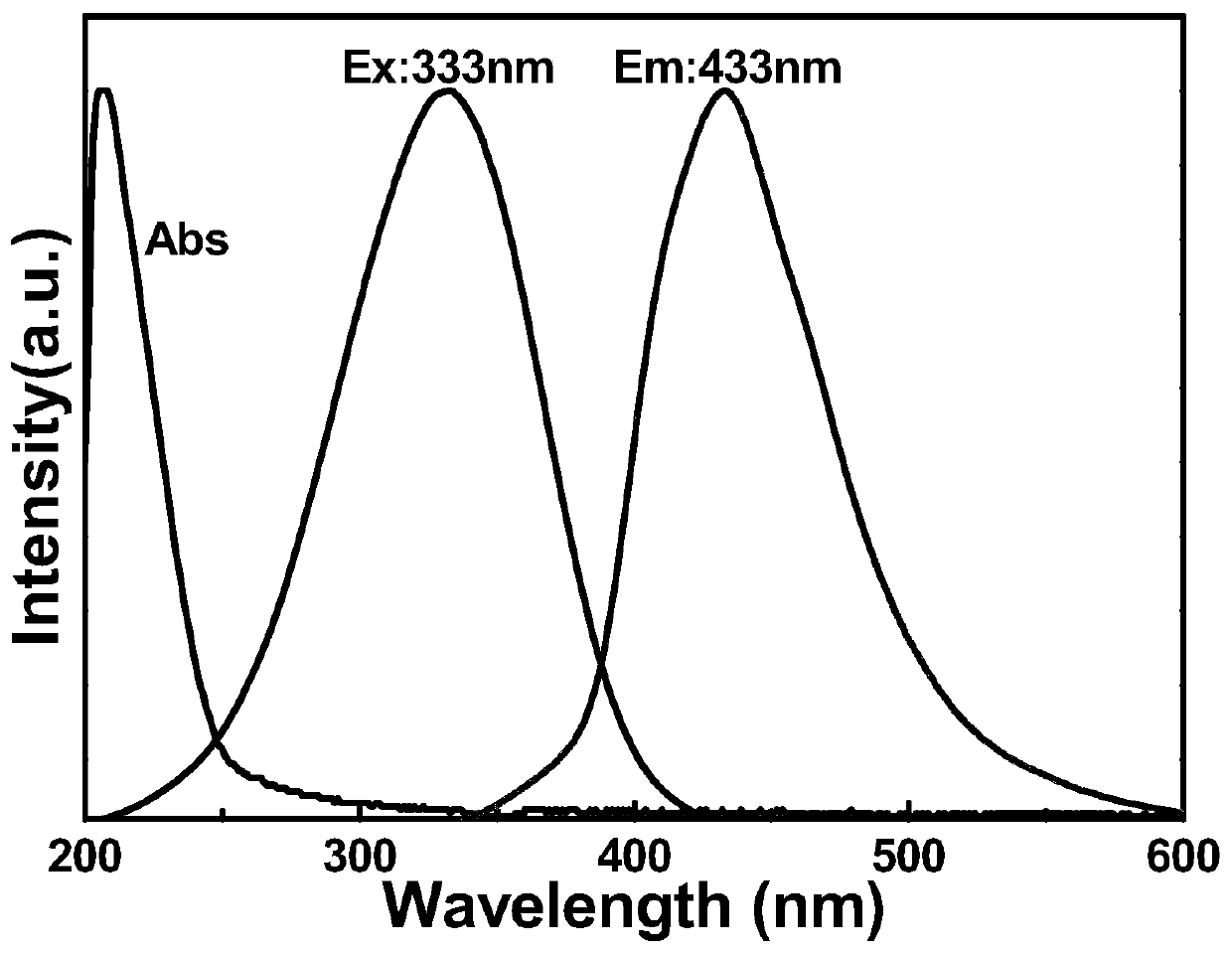
Preparation method of diphenyl azide phosphate modified graphene quantum dots
ActiveCN110951487AEfficient formationEfficient modificationGrapheneNanotechnologyPhosphatePhosphoric acid
The invention discloses a preparation method of diphenyl azide phosphate modified graphene quantum dots. The method comprises the following steps: dissolving diphenyl azide phosphate and glucose intoa solvent to form a first precursor solution, adjusting the pH value of the first precursor solution to be acidic to form a second precursor solution, carrying out hydrothermal reaction on the secondprecursor solution, filtering and dialyzing a hydrothermal product to obtain a filtrate, and drying the filtrate to form the azido-modified graphene quantum dots. Azido is a group which is easy to accept electrons and strong in negative charge bearing capacity; in the hydrothermal reaction process, the azido group can effectively enable diphenyl azide phosphate to react with glucose to form the diphenyl azide phosphate modified graphene quantum dots, meanwhile, formation of an intermediate product in the hydrothermal reaction can be inhibited in an acid environment, and the hydrothermal reaction temperature is controlled to adjust the particle size of the quantum dots. The obtained graphene quantum dots are good in stability, relatively high in quantum yield and relatively high in fluorescence intensity.
Owner:NINGBO UNIV
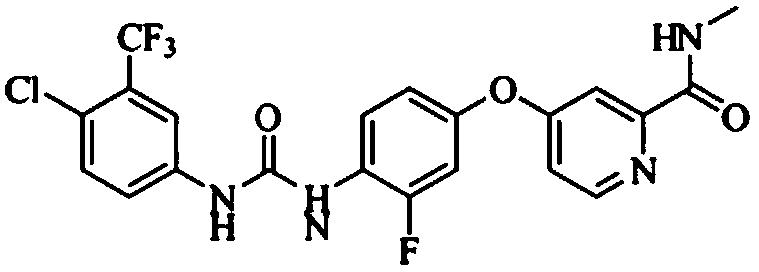
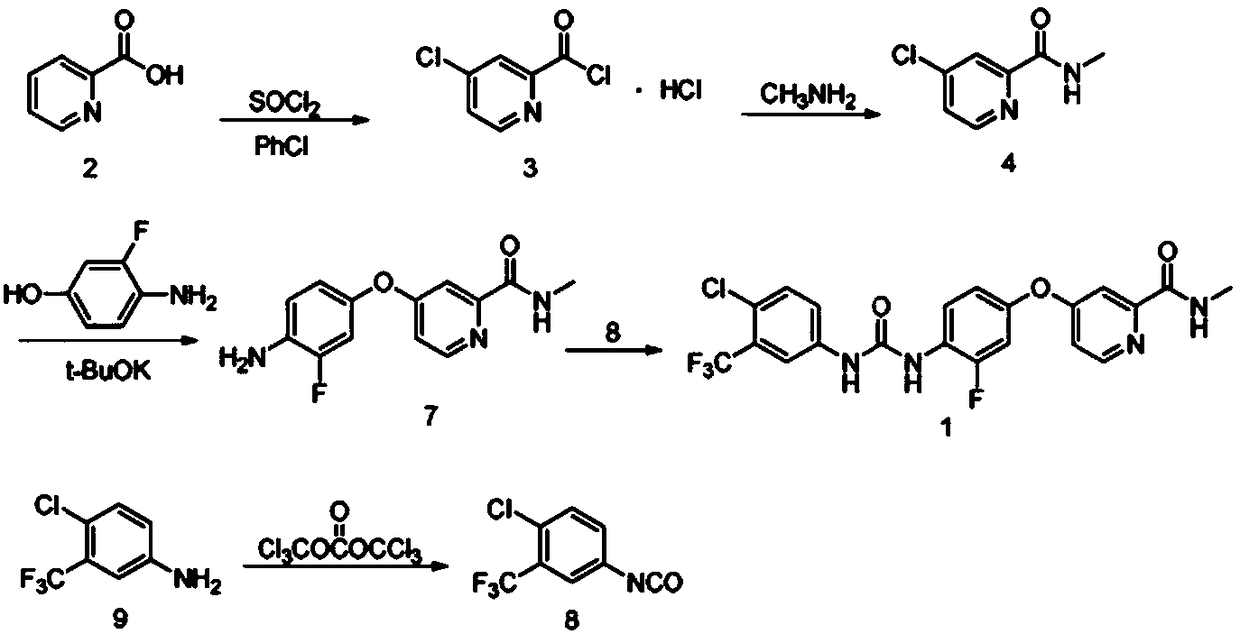
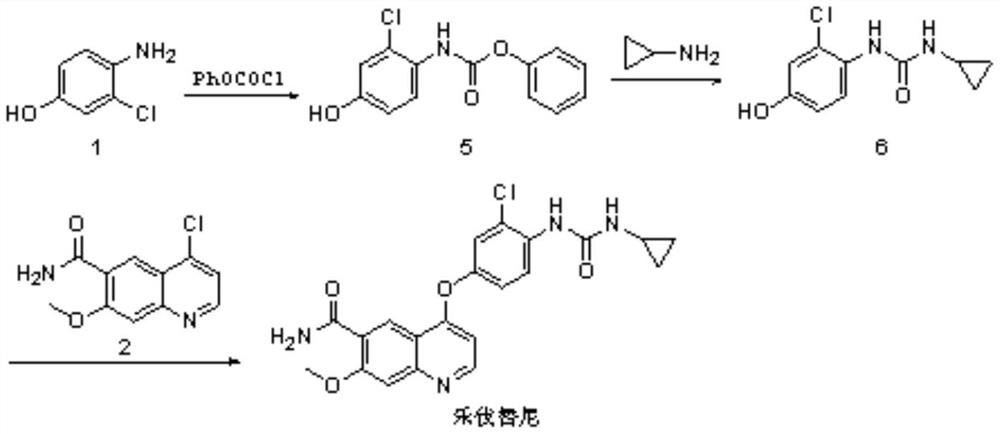
Preparation method of regorafenib
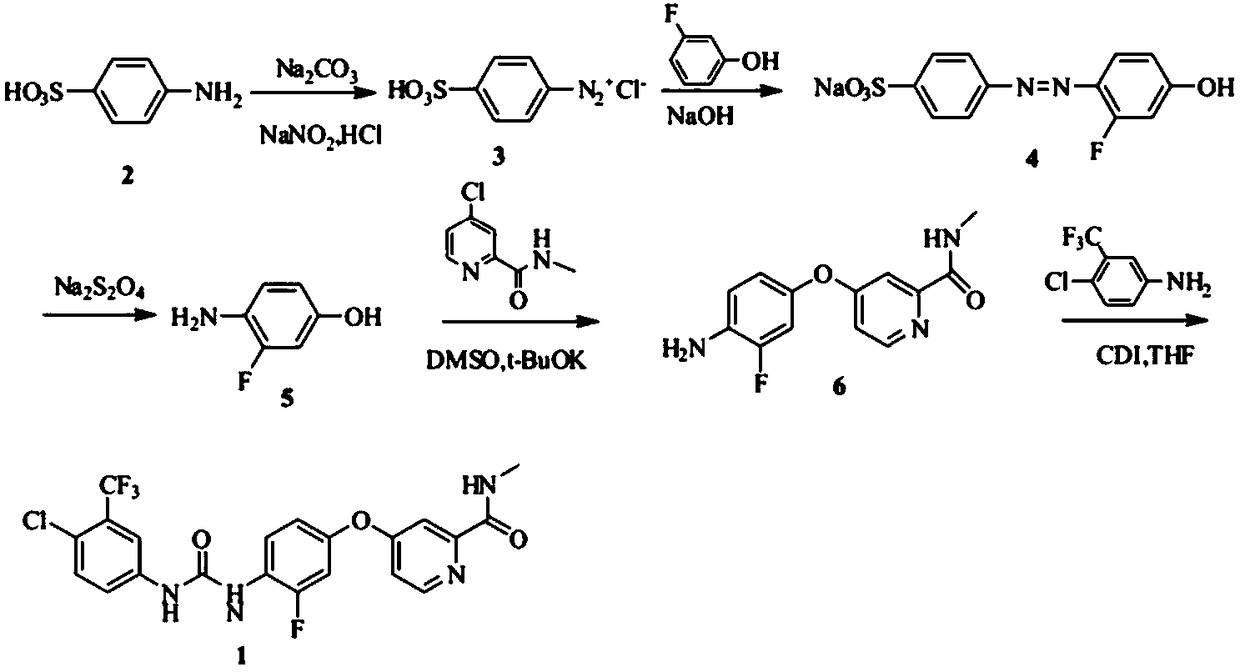
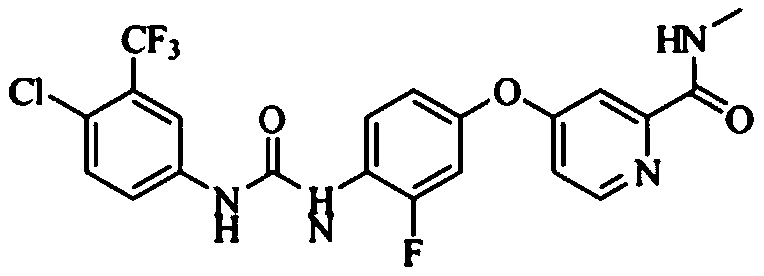
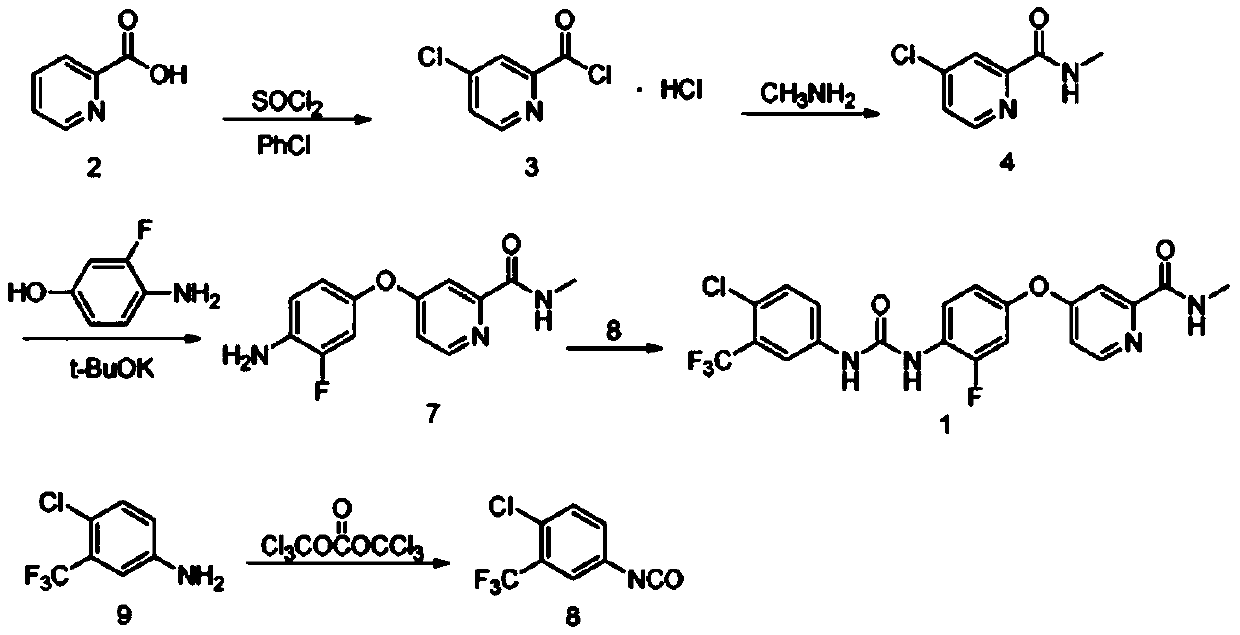
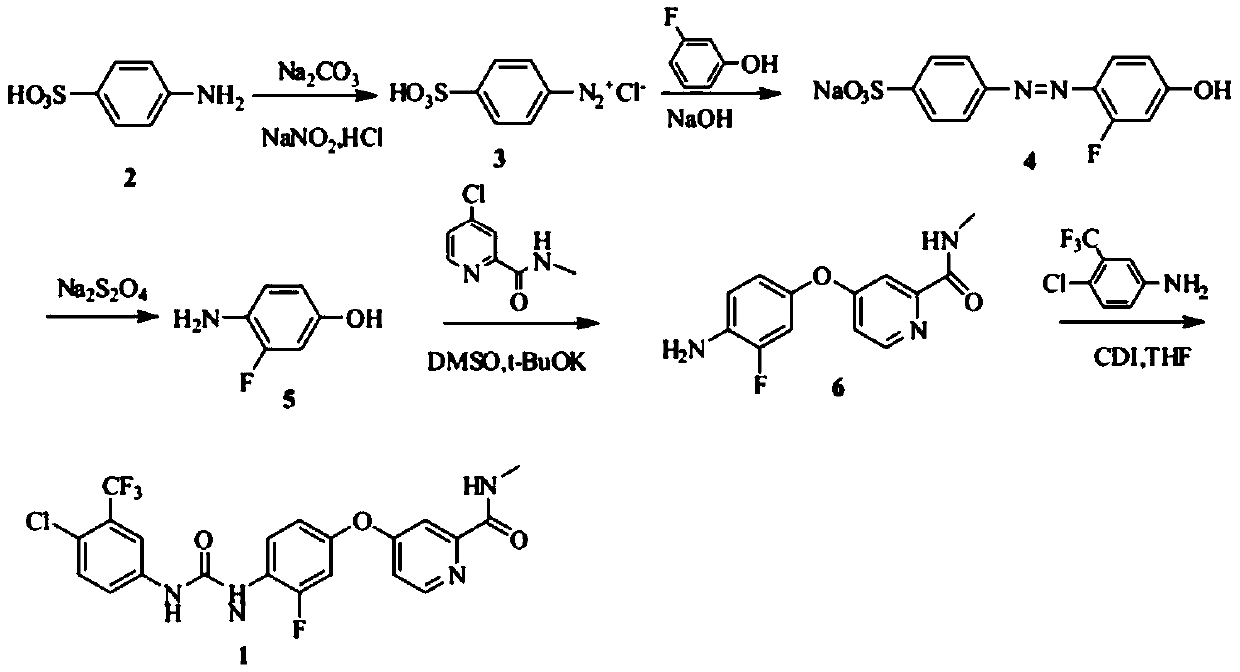
The invention relates to a preparation method of regorafenib. The method comprises steps as follows: 4-amino-3-fluorophenol and 4-chloro-N-methylpyridine-2-formamide are subjected to ether condensation reaction under the action of anhydrous potassium carbonate and PEG-400, and an intermediate I is generated; then the intermediate I and 3-trifluoromethyl 4-chlorobenzoic acid are subjected to one-pot reaction with diphenylphosphoryl azide under the action of alkali, a crude regorafenib product is obtained and further purified, and a pure regorafenib product is obtained. The method is high in conversion rate, safe, harmless, pollution-free, high in yield and suitable for industrial production, the reaction conditions are mild, and the product purity is high.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
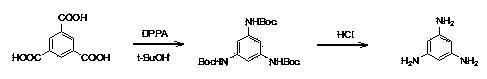
Synthesis process of 1, 3, 5-triaminobenzene
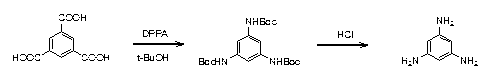
InactiveCN103214377AEasy post-processingRaw materials are easy to getOrganic compound preparationAmino compound preparationPhosphoric acidTrimesic acid
The invention relates to a synthesis process of 1, 3, 5-triaminobenzene. The process is characterized by: taking trimesic acid and diphenylphosphoryl azide as reaction raw materials, and adopting toluene and tert-butyl alcohol (in a volume ratio of 5:3) as solvents, letting them react at 70DEG C-90DEG C for 3-4h, then raising the temperature to 100DEG C-120DEG C and leaving them to react for 2h, thus obtaining 1, 3 5-tri-Boc aminobenzene; and placing the 1, 3 5-tri-Boc aminobenzene into a mixed solution of ethanol and concentrated hydrochloric acid (in a volume ratio of 1:1) to be stirred overnight, thus obtaining the 1, 3, 5-triaminobenzene. The process provided in the invention is simple, needs no flammable and explosive reagent, the reaction conditions are mild, and the product yield is high.
Owner:SUZHOU KANGRUN PHARMA
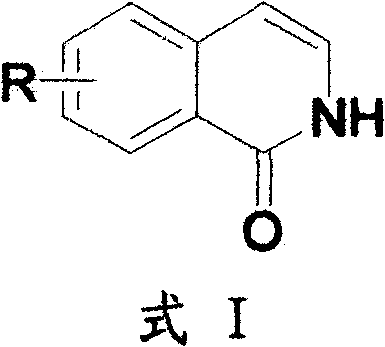
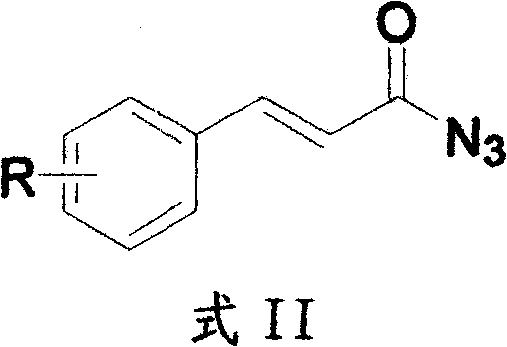
Method for preparing 2H-isoquinoline-1-ketones
InactiveCN102219738ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryIsoquinolineOrganic base
The invention discloses a method for preparing 2H-isoquinoline-1-ketones, which comprises the steps of taking corresponding cinnamic acid derivatives and diphenylphosphoryl azide (DPPA) as raw materials, synthesizing nitrine intermediates under the existence of organic base, and carrying out reaction under the conditions of the organic base and a proper solvent as well as proper temperature. Compared with other methods, the method for preparing the 2H-isoquinoline-1-ketones has the advantages of high yield, short reaction time, environment friendliness, simplicity in operation, low cost and the like, thus not only being suitable for small-scale synthesis in a laboratory, but also being suitable for industrial enlargement production. The prepared 2H-isoquinoline-1-ketones can be used for screening of bioactivity or research and development of new drugs.
Owner:SHANGHAI YISHI CHEM TECH
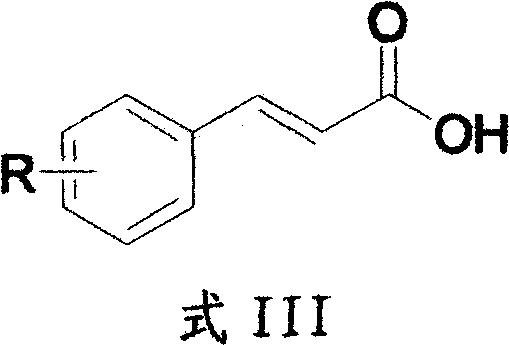
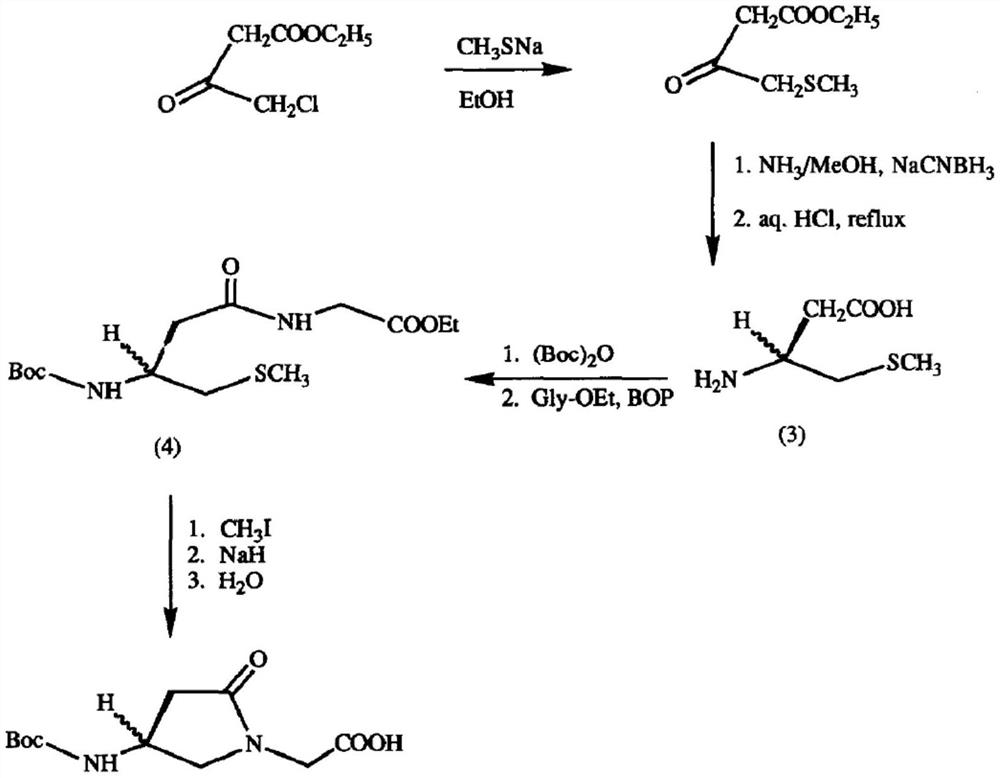
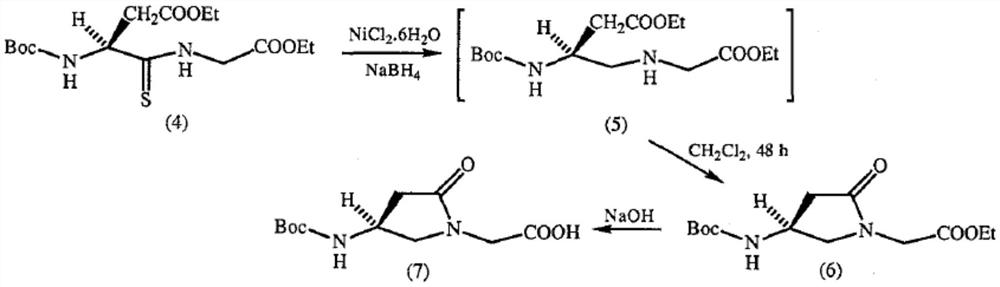
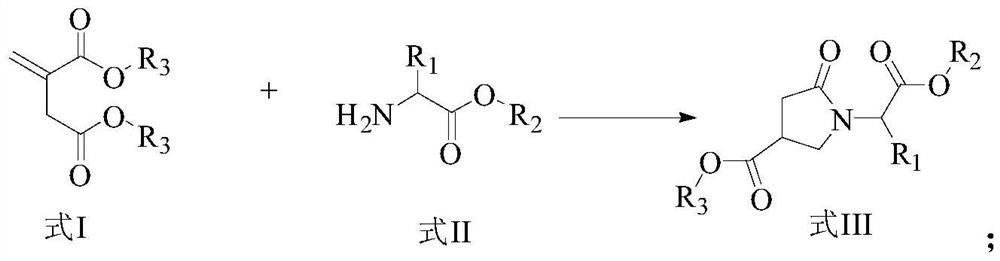
A kind of preparation method of γ-lactam bridged dipeptide compound
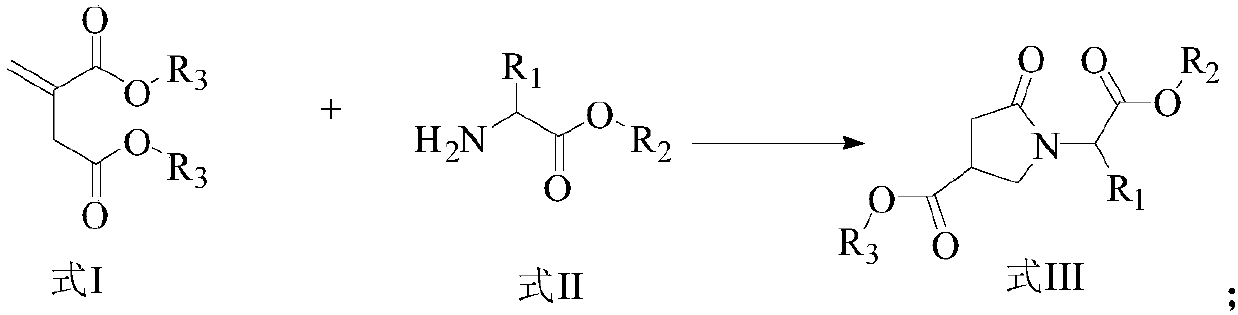
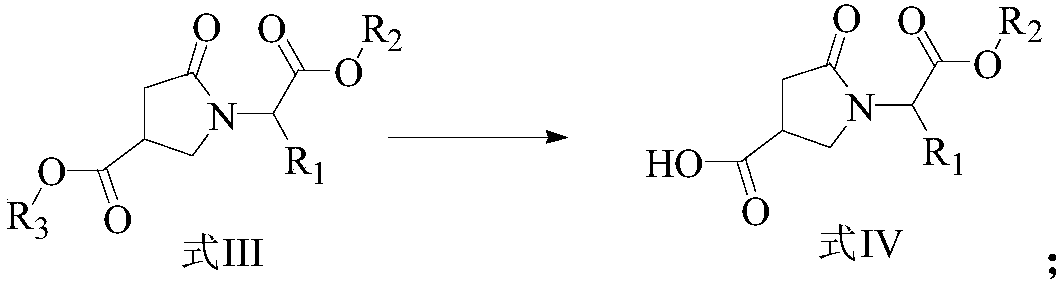
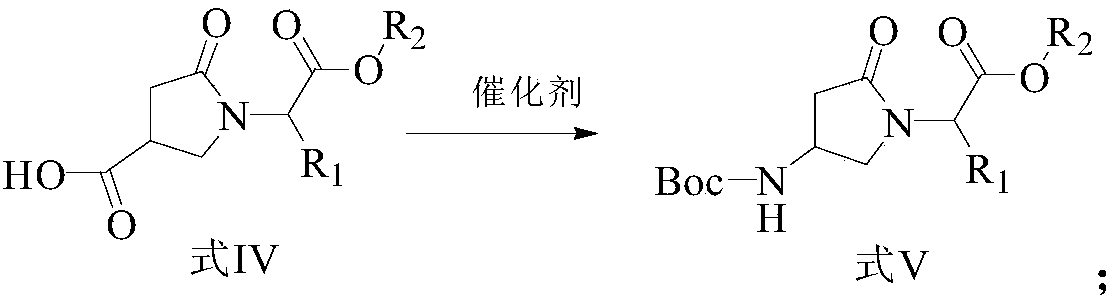
ActiveCN107903302BHigh yieldPromote environmental protectionPeptide preparation methodsDipeptideCarboxyl radical
The present invention provides a preparation method of γ-lactam bridged dipeptide compounds. The method is to react a dibasic acid ester shown in formula I with tert-butoxycarbonylethylamine shown in formula II to obtain 2 shown in formula III. ‑[4‑(alkoxycarbonyl)‑2‑oxopyrrolidinyl acetate; said 2‑[4‑(alkoxycarbonyl)‑2‑oxopyrrolidinyl acetate is Hydrolysis in the presence of 2-(4-carboxy-2-oxopyrrolidinyl) acetate shown in formula IV; Butanol reacts in the presence of diphenylphosphoryl azide to obtain the pyrrolidinyl acetate of Boc protection shown in formula V; then hydrolysis obtains 2-{4-[(tert-butoxy)carbonylamino] shown in formula VI ‑2‑Oxopyrrolidinyl}acetic acid. The preparation method of the invention does not use sulfide, is beneficial to environmental protection, has simple synthesis route and high yield.
Owner:ACCELA CHEMBIO CO LTD
A kind of preparation method of regorafenib
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
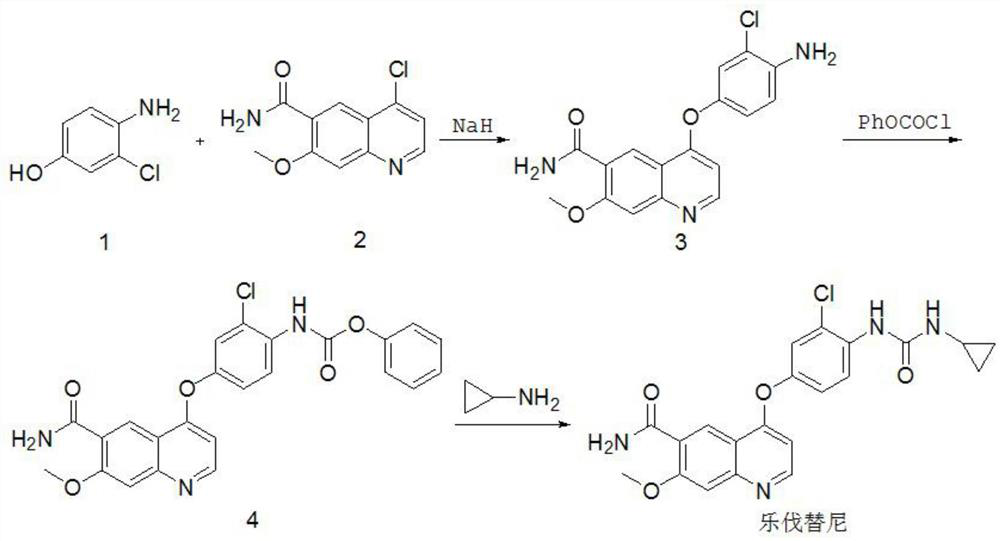
Synthesis method of lenvatinib
The invention relates to a systhesis method of lenvatinib. The method comprises the following steps: by taking 2-chloro-4-methyl hydroxybenzoate and 4-chloro-7-methoxyquinoline-6-amide as starting raw materials, carrying out substitution reaction to obtain 4-[3-chloro-4-methoxycarbonyl phenoxy]-7-methoxy-6-quinolinecarboxamide, carrying out an alkaline hydrolysis reaction to obtain 4-(3-chloro-4-carboxyphenoxy)-7-methoxy-6-quinolinecarboxamide, carrying out a Curtius rearrangement reaction on the 4-(3-chloro-4-carboxyphenoxy)-7-methoxy-6-quinolinecarboxamide and diphenyl azide phosphate (DPPA), and carrying out a reaction on the obtained product and cyclopropylamine to obtain lenvatinib through a one-pot method. The invention provides a novel method for synthesizing lenvatinib. The method has the advantages of simple reaction steps, simple and easily available raw materials, simple operation and low production cost.
Owner:SHANDONG HUIHAI PHARMA & CHEM
Catalystic preparation of nitrine diphenyl posphate
InactiveCN1275974CShorten the timeEasy to operatePhosphorus organic compoundsDiphenyl phosphateQuaternary ammonium cation
Owner:GUILIN GUIKAI BIOLOGICAL SCI & TECH
Building coating
The invention discloses a building coating. The building coating is prepared from, by weight, 2.5-6.3 parts of 3-ethyl-2-methyl-3-pentanol, 2.4-7.4 parts of borax, 10-16 parts of calcite powder, 5.4-8.6 parts of diphenylphosphoryl azide, 4.6-7.5 parts of aqueous silicone oil, 2.7-6.5 parts of phenylacetaldehyde, 25-30 parts of white latex adhesive, 5-7.5 parts of rosin-polythylene oxide ester and 2.5-6.3 parts of acrylate modified butadiene resin. Compared with present products, the building coating has the following advantages: the building coating has good water and alkali resistance due to addition of the calcite powder and the aqueous silicone oil; the building coating has strong penetration ability and adhesion due to addition of phenylacetaldehyde and the white latex adhesive; and the building coating can shield wall cracks due to addition of the rosin-polythylene oxide ester and the acrylate modified butadiene resin.
Owner:QINGDAO JINLIANXIN BUSINESS & TRADE
Asymmetric alcoholysis catalyzed by chiral thiourea amine salt
InactiveCN106008242ANovel synthetic routeFew stepsCarbamic acid derivatives preparationOrganic compound preparationThiolThiourea
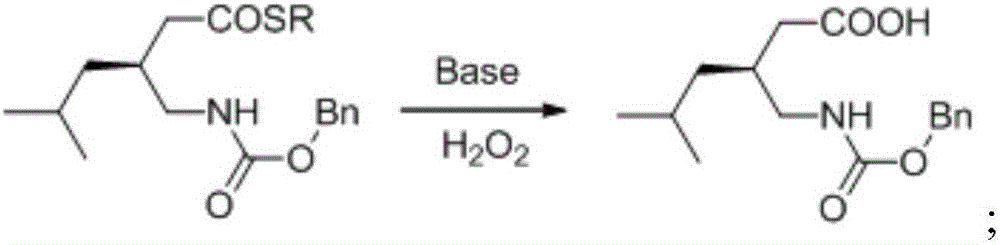
The invention provides asymmetric alcoholysis catalyzed by a chiral thiourea amine salt. The process includes the steps of: S1. subjecting 3-isobutylglutaric anhydride and mercaptan to asymmetric alcoholysis in the presence of a chiral thiourea amine salt catalyst to generate dextral mercaptide; S2. subjecting the dextral mercaptide obtained by asymmetric alcoholysis to reaction with diphenyl azidophosphate and benzyl alcohol in order to generate levo-benzyloxycarbonylaminomethyl mercaptide; and S3. hydrolyzing the levo-benzyloxycarbonylaminomethyl mercaptide under alkaline hydrogen peroxide condition to obtain levo-benzyloxycarbonylaminomethylhexanoic acid. The method provided by the invention does not use chemical resolution agent, the total yield is more than 50%, and the ee value is greater than 94%. The method has the advantages of novel synthesis route, few step, and mild reaction conditions.
Owner:RAFFLES PHAMRMATECH CO LTD
Preparation method of gamma-lactam bridging dipeptide compound
ActiveCN107903302AHigh yieldPromote environmental protectionPeptide preparation methodsDipeptidePhosphate
The invention provides a preparation method of a gamma-lactam bridging dipeptide compound. The preparation method comprises the following steps: performing reaction on dibasic acid ester as shown in aformula I and tert-butyl oxycarbonyl ethylamine as shown in a formula II to obtain 2-[4-(alkoxy carbonyl)-2-oxypyrrole alkyl acetate as shown in a formula III; hydrolyzing the 2-[4-(alkoxy carbonyl)-2-oxypyrrole alkyl acetate under the existence of an alkaline substance to obtain 2-(4-carboxyl-2-oxo-pyrrolidine)acetic acid ester; performing reaction on the 2-(4-carboxyl-2-oxo-pyrrolidine)acetic acid ester and tertiary butanol under the existence of diphenyl azide phosphate to obtain Boc-protected pyrrolityl acetate as shown in a formula V; and performing hydrolysis to obtain 20{4-[(tert-butoxy)carboyl amino]-2-oxo-pyrrolidine}acetic acid as shown in a formula VI. The preparation method provided by the invention does not use sulfide, and is favorable for environmental protection, simple insynthesis route and high in yield.
Owner:ACCELA CHEMBIO CO LTD
A kind of oil well cement high temperature retarder microcapsule and preparation method thereof
ActiveCN108570313BAvoid hyperretardingGood thickening lineDrilling compositionPolyesterPolymer science
The invention discloses an oil well cement high-temperature retarder microcapsule. Raw materials of the microcapsule include, by weight, 50-60 parts of a high-temperature retarder, 5-10 parts of a reactive diluent, 20-30 parts of a water-insoluble oligomer wall material, 2-3 parts of a photoinitiator and 1-3 parts of an emulsifier. The water-insoluble oligomer wall material is polyurethane acrylicresin or polyester acrylic resin. The reactive diluent is at least one of alkyl acrylate, hydroxy acrylate, methacrylate, ethylene glycol diacrylate, propylene glycol diacrylate, trimethylolpropane triacrylate, dimethyl dithiophosphate, pentaerythritol triacrylate and diphenylphosphoryl azide. The microcapsule is prepared from the abovementioned raw materials through a W / O / W composite emulsion photopolymerization method. The microcapsule ensures that the cement slurry has similar thickening time and compressive strength under the condition of a large temperature difference, that the comprehensive performance of the cement slurry system satisfies the requirements of cementing construction of thermal production wells, and the process is simple and is favorable for industrialization. .
Owner:SOUTHWEST PETROLEUM UNIV
A kind of synthetic method of bictegravir intermediate
ActiveCN110092726BChiralityHigh yieldOrganic compound preparationCarboxylic compound preparationGrignard reagentHydrogen pressure
The invention discloses a method for synthesizing a Bictegravir intermediate. The starting material 3-carbonyl cyclopentanecarboxylic acid (formula I) undergoes an asymmetric reduction reaction under the condition of an enzyme to generate (3R)-3-hydroxycyclopentane Carboxylic acid (Formula II); Formula II reacts with diphenylphosphoryl azide (DPPA) to generate (1R,5S)‑2‑Oxy‑4‑azabicyclo[3.2.1]octane -3-ketone (formula III); formula III is hydrolyzed in hydrochloric acid to directly obtain the Bictegravir intermediate (1R,3S)-3-aminocyclopentanol hydrochloride. The raw materials used in the present invention are cheap and easy to obtain, and the cost is low; the reaction selectivity is high, the by-products are few, the yield is high, and the total yield reaches 63.5%; Hydrogen pressurized reduction and Grignard reagent reaction are adopted, which is safe and environmentally friendly, and suitable for industrial production.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Synthesis process of 1, 3, 5-triaminobenzene
InactiveCN103214377BEasy post-processingRaw materials are easy to getOrganic compound preparationAmino compound preparationPhosphoric acidTrimesic acid
The invention relates to a synthesis process of 1, 3, 5-triaminobenzene. The process is characterized by: taking trimesic acid and diphenylphosphoryl azide as reaction raw materials, and adopting toluene and tert-butyl alcohol (in a volume ratio of 5:3) as solvents, letting them react at 70DEG C-90DEG C for 3-4h, then raising the temperature to 100DEG C-120DEG C and leaving them to react for 2h, thus obtaining 1, 3 5-tri-Boc aminobenzene; and placing the 1, 3 5-tri-Boc aminobenzene into a mixed solution of ethanol and concentrated hydrochloric acid (in a volume ratio of 1:1) to be stirred overnight, thus obtaining the 1, 3, 5-triaminobenzene. The process provided in the invention is simple, needs no flammable and explosive reagent, the reaction conditions are mild, and the product yield is high.
Owner:SUZHOU KANGRUN PHARMA
A kind of contrast agent and its preparation method
ActiveCN105833302BGood biocompatibilityGood contrast effectEchographic/ultrasound-imaging preparationsSide effectCholesterol
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com