Synthesis method of bictegravir intermediate
A synthesis method and intermediate technology, applied in the field of medicinal chemistry, can solve the problems of high equipment and personnel operation requirements, no large synthetic route, large amount of waste residue, etc., to avoid chemical splitting, short reaction route and less by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
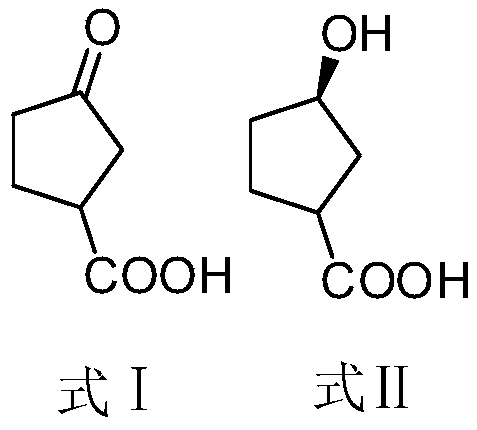
[0038] Embodiment 1: the preparation of (3R)-3-hydroxycyclopentane carboxylic acid (formula II)
[0039]
[0040] Add 500ml of 0.1mol / L phosphate buffer (pH=6.7), 50g of 3-oxocyclopentacarboxylic acid into a 1L reaction flask, and stir to dissolve completely. Then add 10 g of carbonyl reductase, 5 g of coenzyme NAD+, 25 g of glucose, and 5 g of glucose dehydrogenase. The system was reacted at 25°C and stirred for 24 hours. The system was filtered with 20 g of diatomaceous earth, and the aqueous phase was extracted three times with ethyl acetate, each time with 200 ml. The ethyl acetate phase was dried over anhydrous sodium sulfate and concentrated to obtain 52.2 g of a pale yellow oil. ee value ≥ 98.5%, yield 102.8%.
[0041] ESI-MS 129.1(M-1).
Embodiment 2
[0042] Example 2: Preparation of (1R,5S)-2-oxo-4-azabicyclo[3.2.1]octan-3-one
[0043]
[0044] Add 400ml of toluene, 50g of (3R)-3-hydroxycyclopentanecarboxylic acid, and 116.2g of diphenylphosphoryl azide into a 1L reaction flask, and heat up to 70-80°C for 10 hours. Cool the reaction system to below 30°C, add 300ml 10% sodium carbonate aqueous solution and stir for 30 minutes, separate the liquids, add 300ml 10% sodium carbonate aqueous solution and stir for 30 minutes, separate the liquids again and combine the water phases, and use 400ml toluene for the water phases After extraction, the toluene phases were combined, dried over anhydrous sodium sulfate, and the toluene was dried under reduced pressure to obtain 48.3 g of a tan solid. 48.3 g of tan solid was recrystallized from 150 ml of acetonitrile to obtain 31.6 g of pale yellow solid. ee value ≥ 97%, yield 64.8%. ESI-MS 128.1 (M+1).
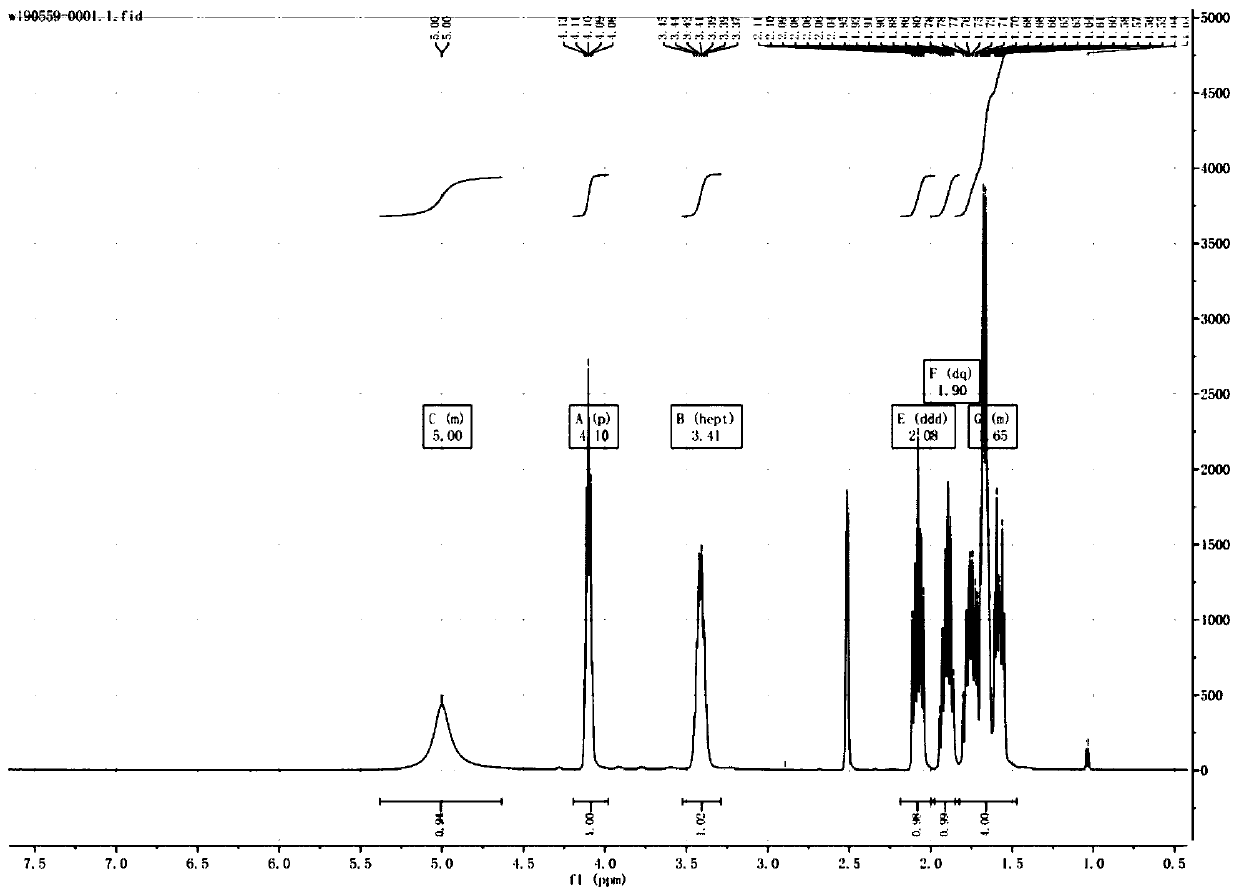
[0045]1H-NMR
[0046] (500MHz, DMSO) δ5.00(m, 1H), 4.10(p, 1H), 3.41(hept, 1H)...
Embodiment 3
[0048] Embodiment 3: Preparation of (1R,3S)-3-aminocyclopentanol hydrochloride
[0049]
[0050] Add 200ml of 4mol / L hydrochloride and 20g of (1R,5S)-2-oxo-4-azabicyclo[3.2.1]octan-3-one into a 500ml reaction bottle, heat up to reflux reaction, and react for 5 hours . After cooling down, concentrate under reduced pressure, add 200ml of pure water after basically shrinking to dryness, then add 3g of activated carbon and stir at room temperature for 2 hours to decolorize. Filter, evaporate the filtrate to dryness under reduced pressure, add 200ml of toluene to concentrate under reduced pressure, and take away the remaining water in the product. Add 100ml of acetonitrile to the finally obtained solid, stir well, filter and dry the solid to obtain 20.7g of white solid. ee value ≥ 97%, yield 95.4%. The overall yield of the synthetic route is 63.5%. ESI-MS 102.1 (M+1).
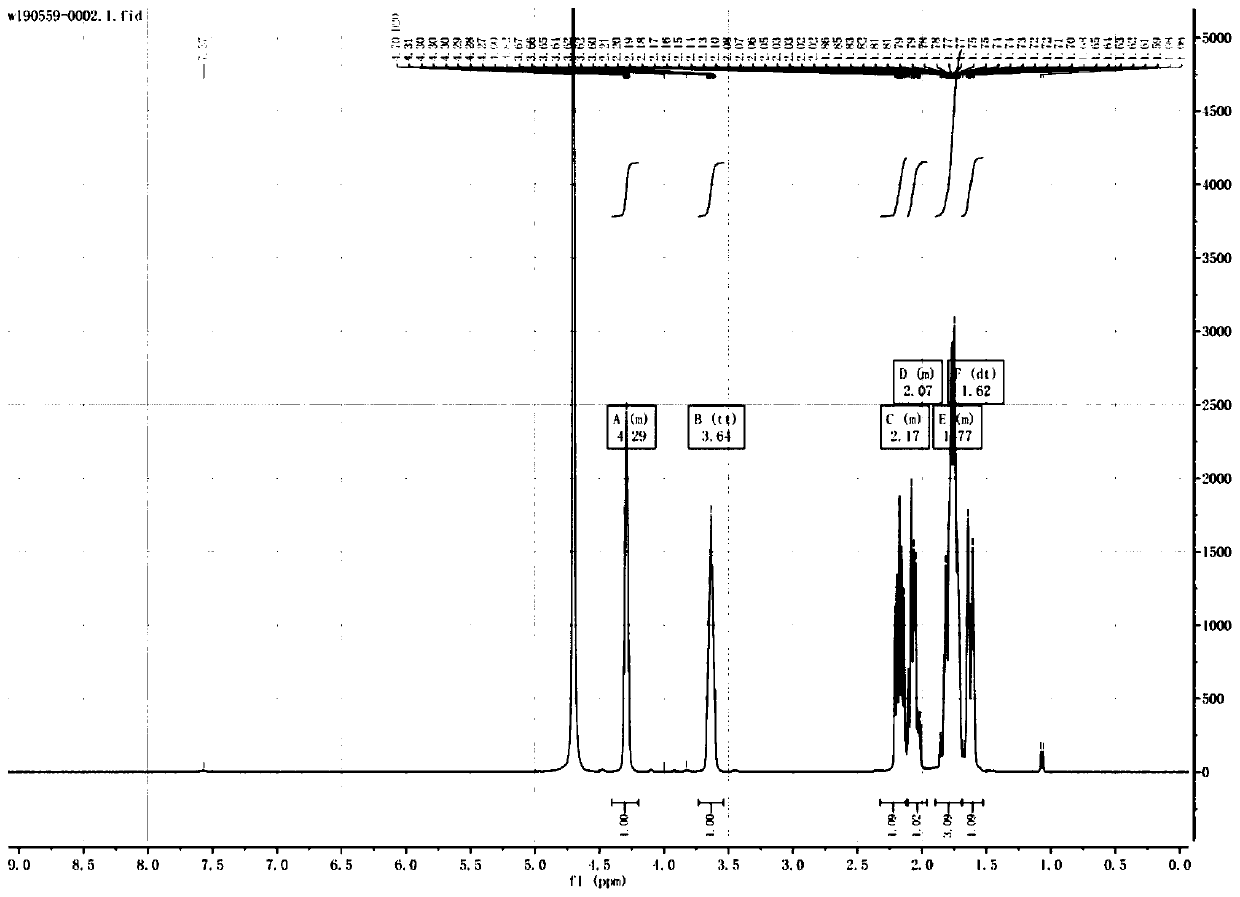
[0051] 1H-NMR (500MHz, D2O)
[0052] δ4.29 (m, 1H), 3.64 (tt, 1H), 2.17 (m, 1H), 2.07 (m, 1H), 1.77 (m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com