Patents
Literature
81 results about "Guaifenesin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
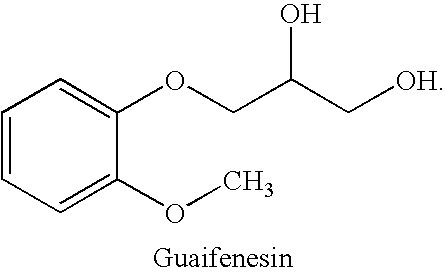
Guaifenesin is used to treat coughs and congestion caused by the common cold, bronchitis, and other breathing illnesses. This product is usually not used for ongoing cough from smoking or long-term breathing problems (such as chronic bronchitis, emphysema) unless directed by your doctor.
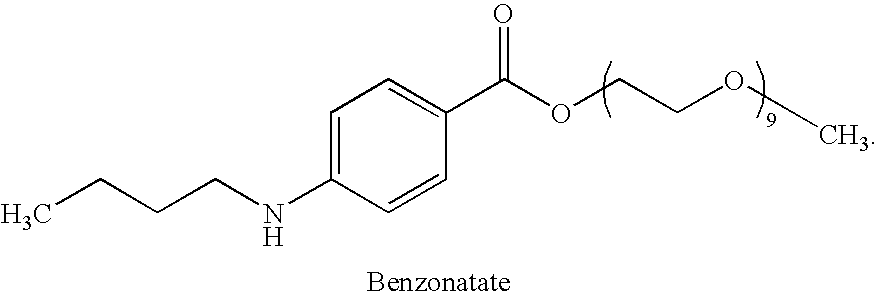
Combined administration of benzonatate and guaifenesin
Pharmaceutical compositions are provided containing a combination of benzonatate and guaifenesin, or pharmaceutically acceptable salts thereof. Methods of using compositions comprising benzonatate and guaifenesin, or pharmaceutically acceptable salts thereof, provide relief from cough, pulmonary congestion or both. The compositions and methods provide relief to opiate-sensitive individuals, in particular, as well as to infants and other pediatric patients.
Owner:VICTORY PHARMA
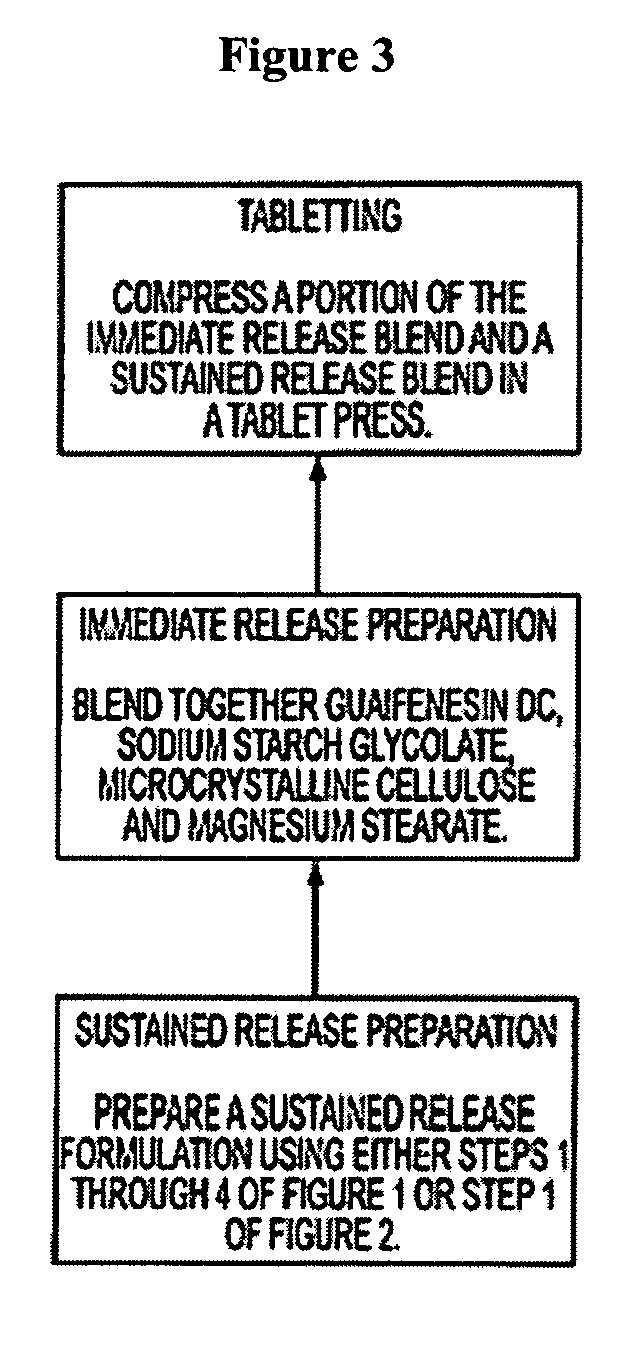
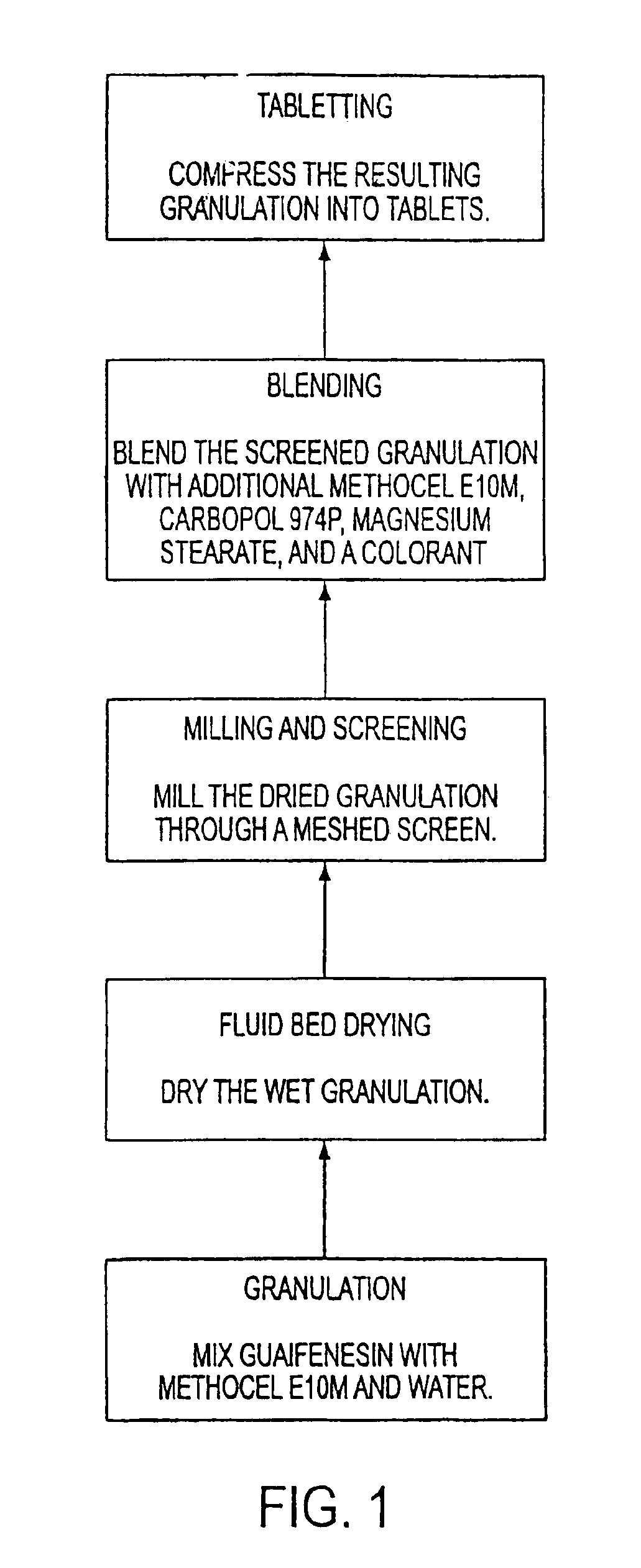
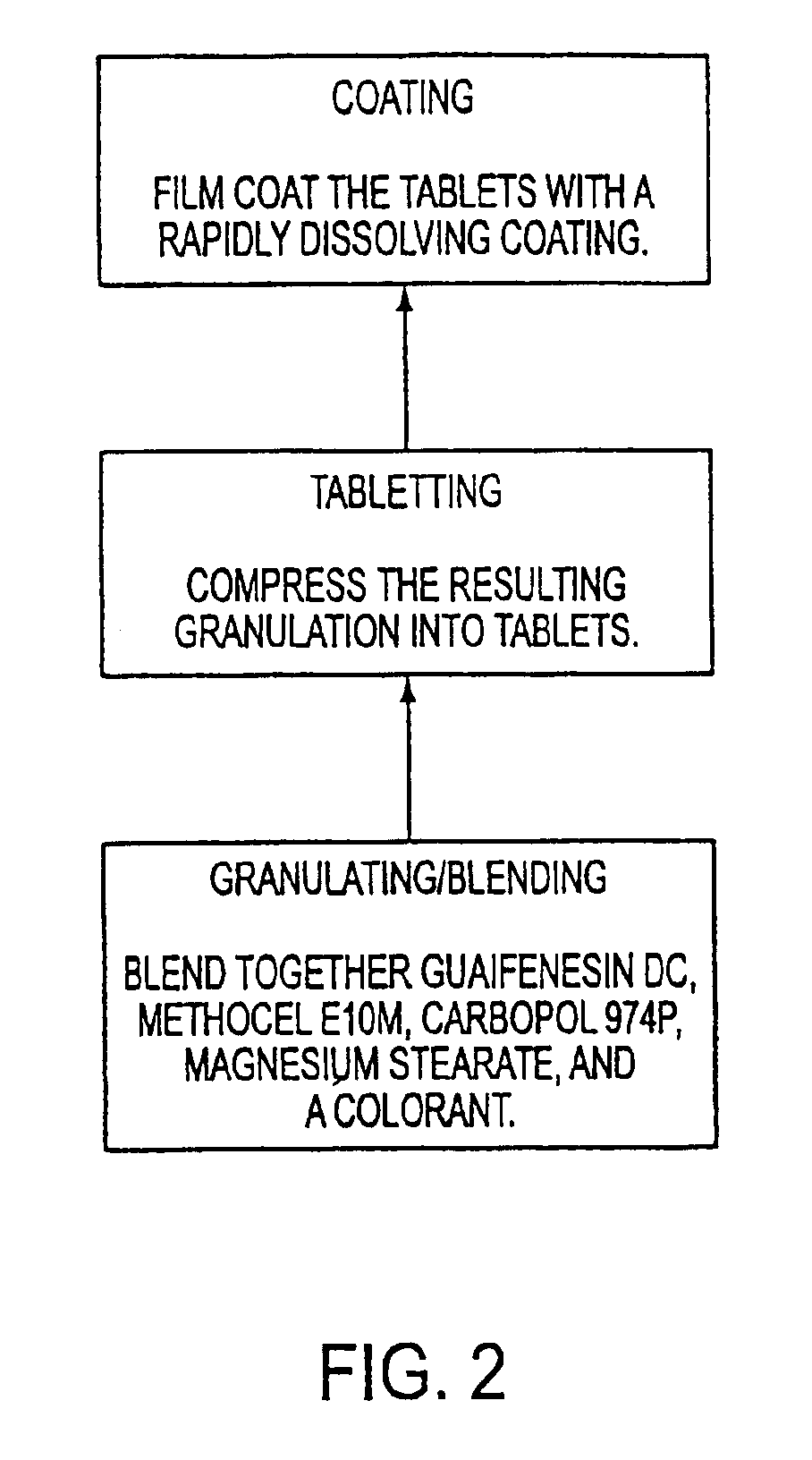
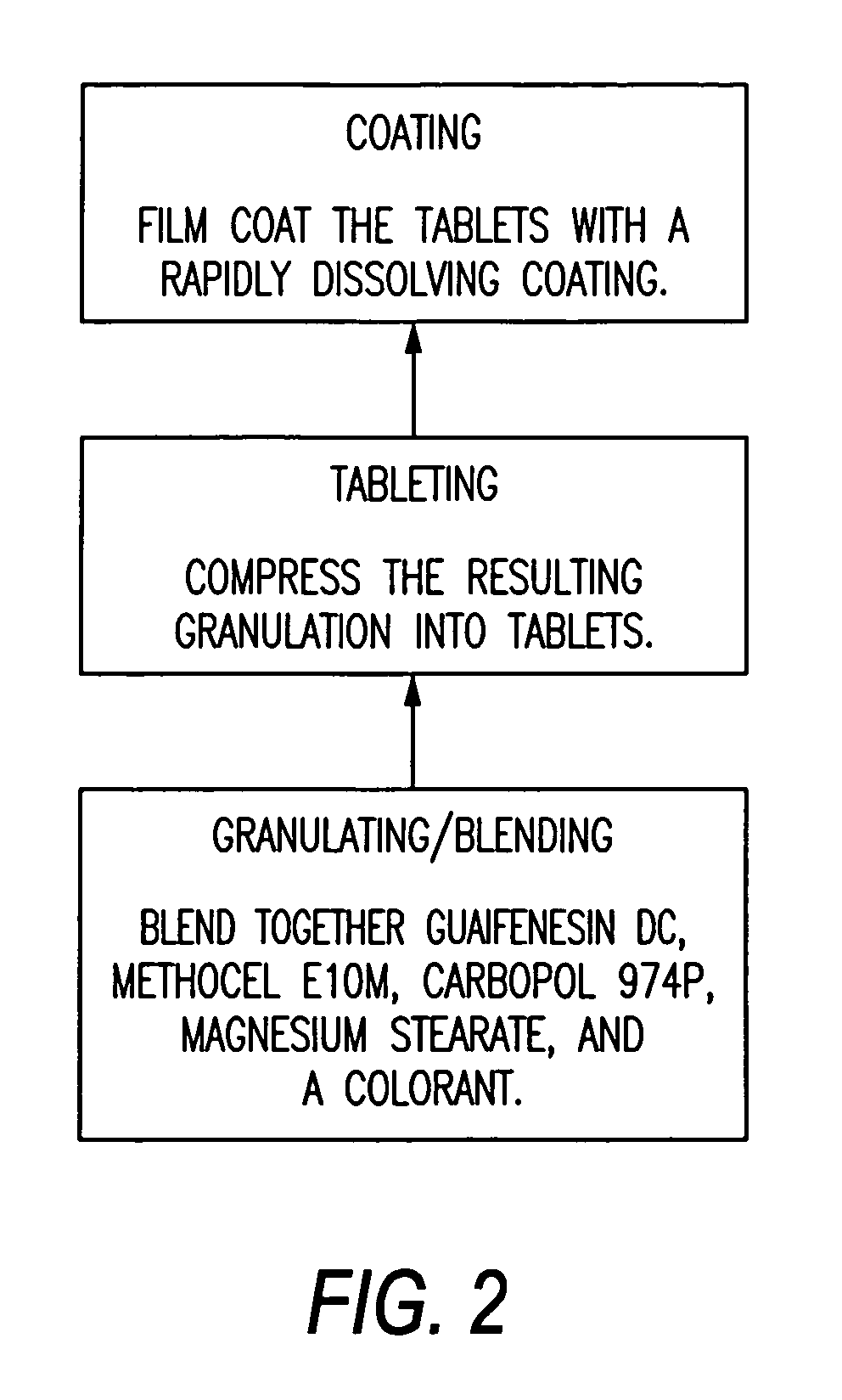
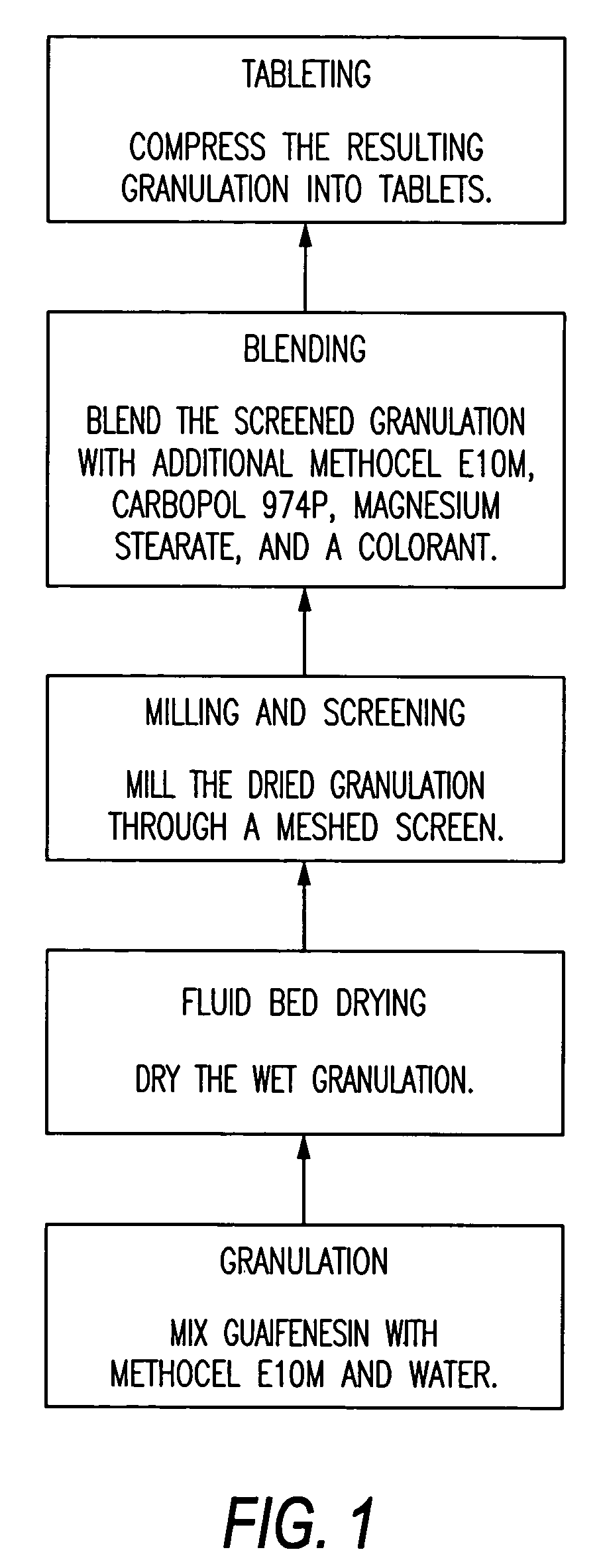
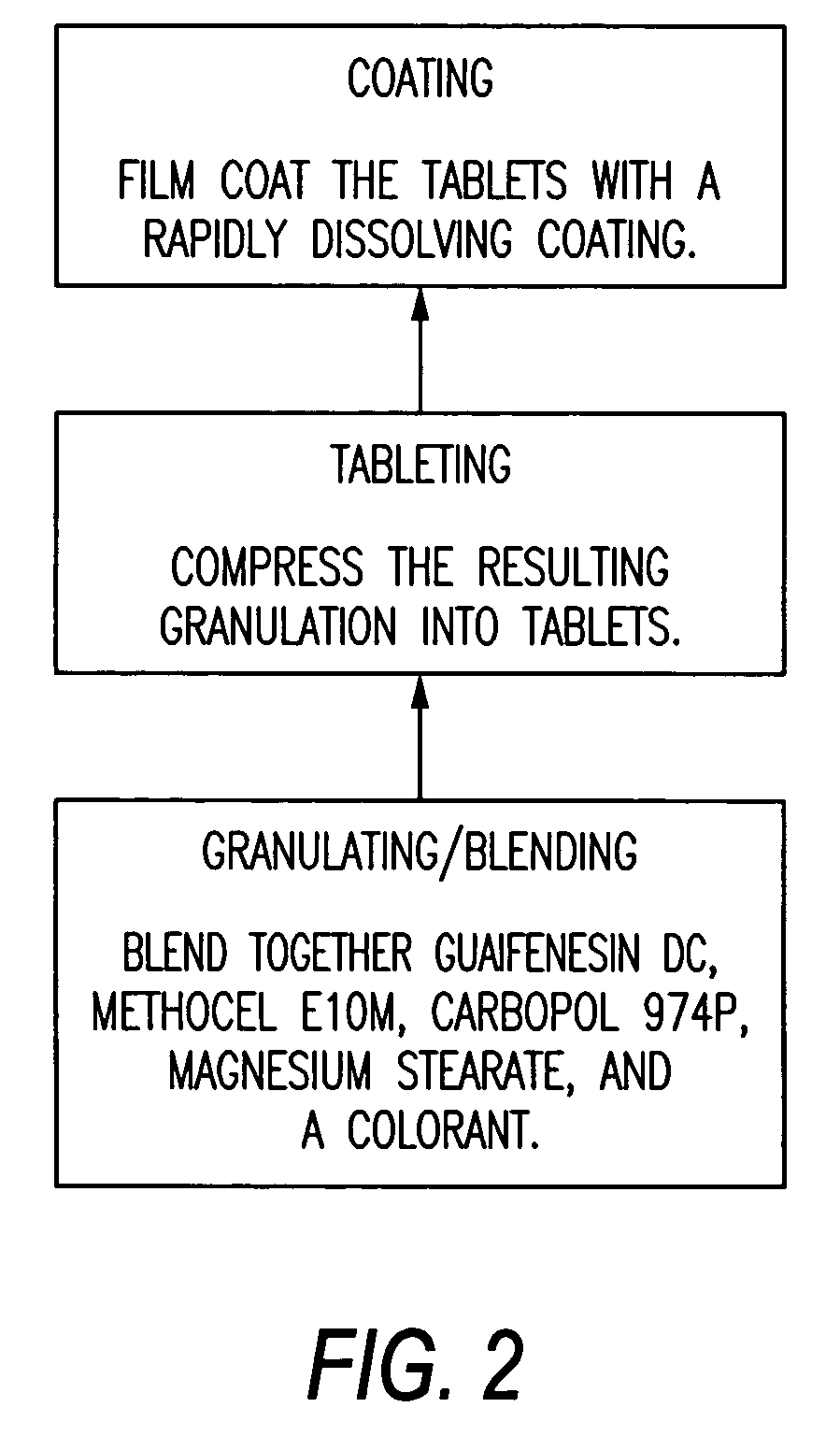
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS20050276852A1Increase in drug strengthSustained releaseOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationImmediate release
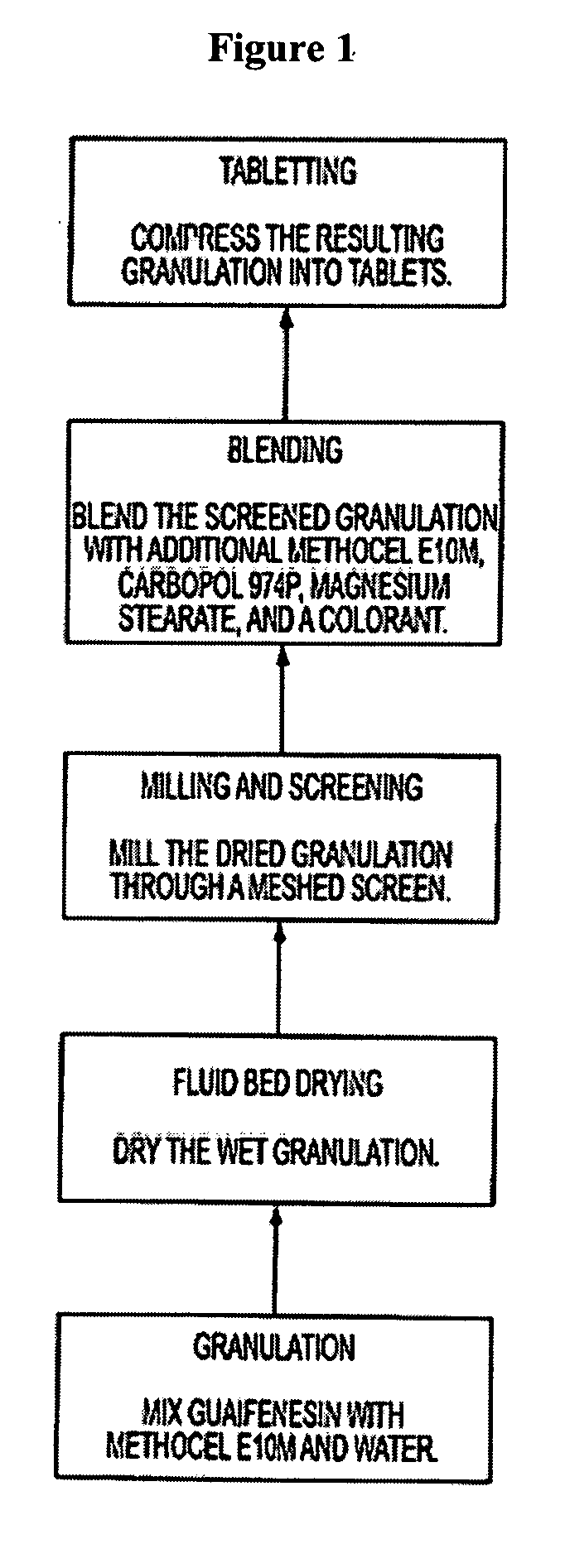
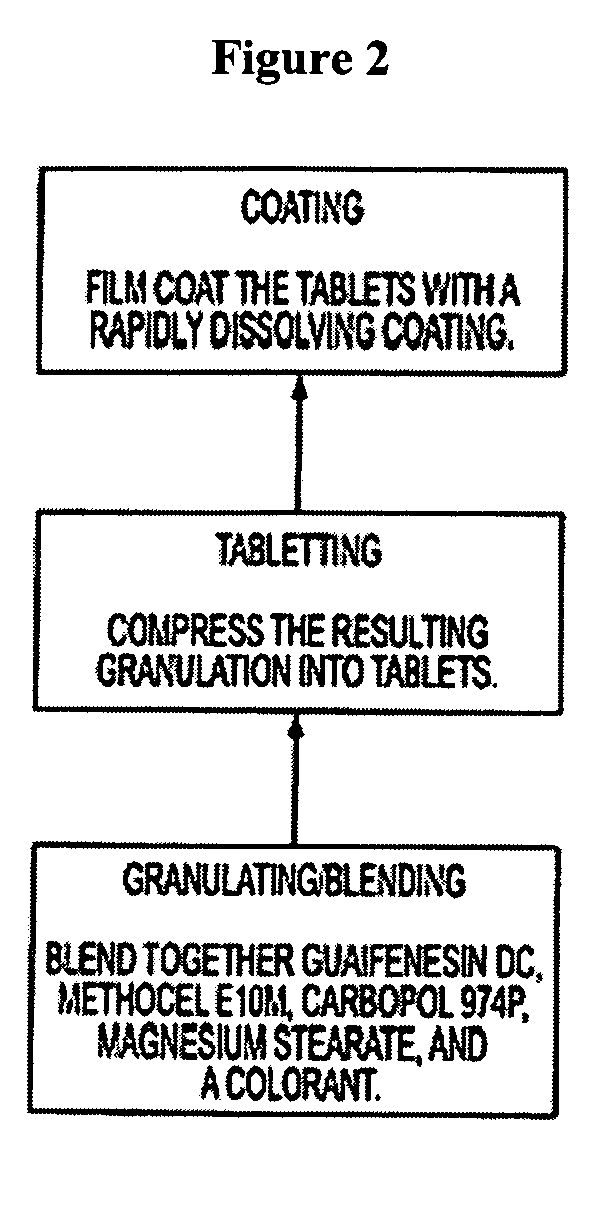
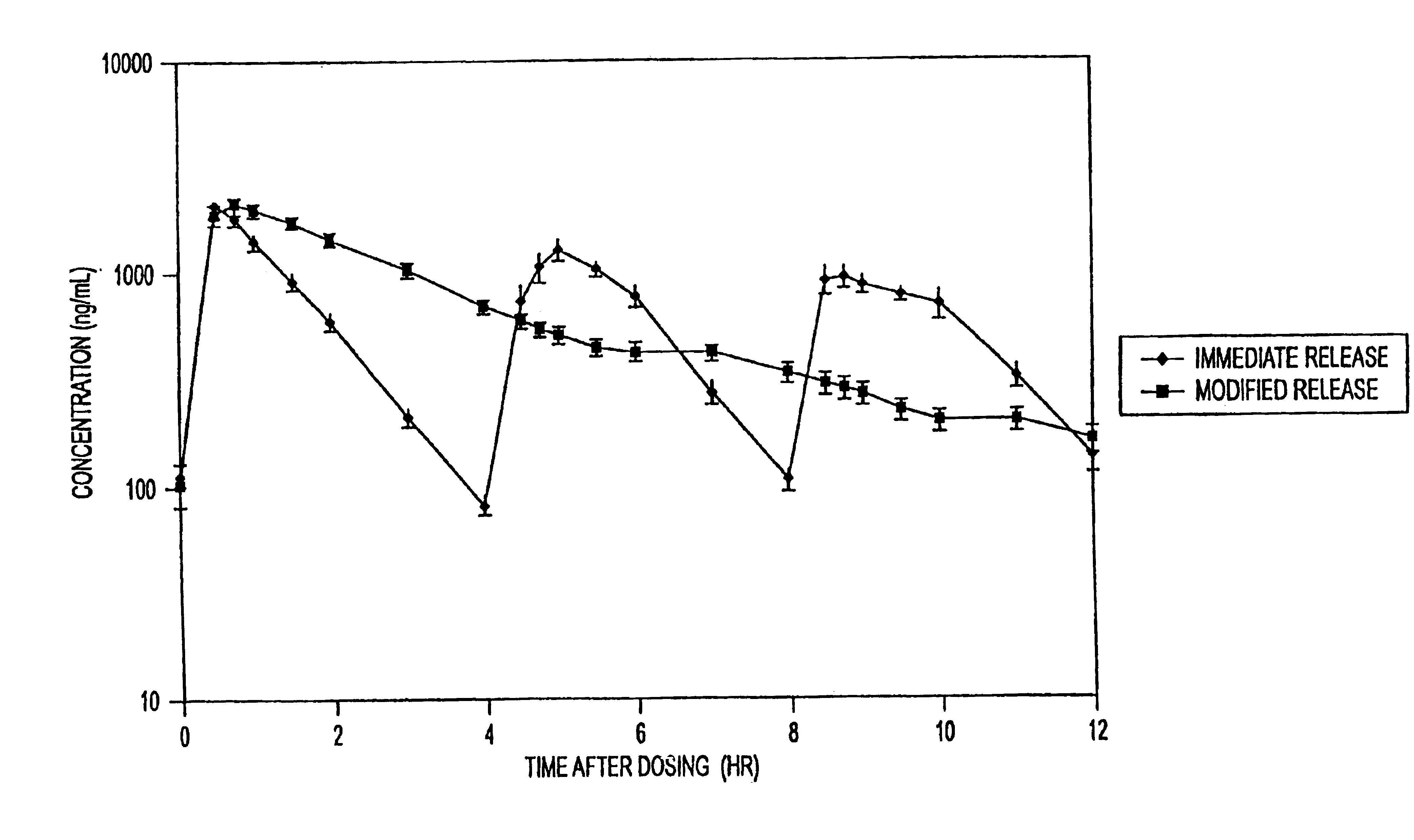
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS6955821B2Increase in drug strengthSustained releaseEther/acetal active ingredientsOrganic non-active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Topical compositions with long lasting effect
InactiveUS20080014252A1Lasting effectAvoid seizuresAntibacterial agentsOrganic active ingredientsAdrenergic DrugsActive agent
Topical pharmaceutical formulations and compositions for transdermal delivery of a variety of active agents are described. The formulations and compositions are formulated to provide a long lasting effect of the agent being delivered. The formulations and compositions of the present invention contain a transdermal vehicle and an adrenergic drug. The formulations and compositions may also contain guaifenesin.
Owner:DELPRETE KEITH
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise an expectorant, an extended release antitussive, and an extended release decongestant. Specifically, the compositions comprise guaifenesin, phenylephrine tannate, and dextromethorphan tannate. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
Owner:EVERETT LAB
Non-narcotic biphasic release compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise immediate release and extended release guaifenesin, extended release phenylephrine and immediate and extended release dextromethorphan. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Method for six active components in drug sample
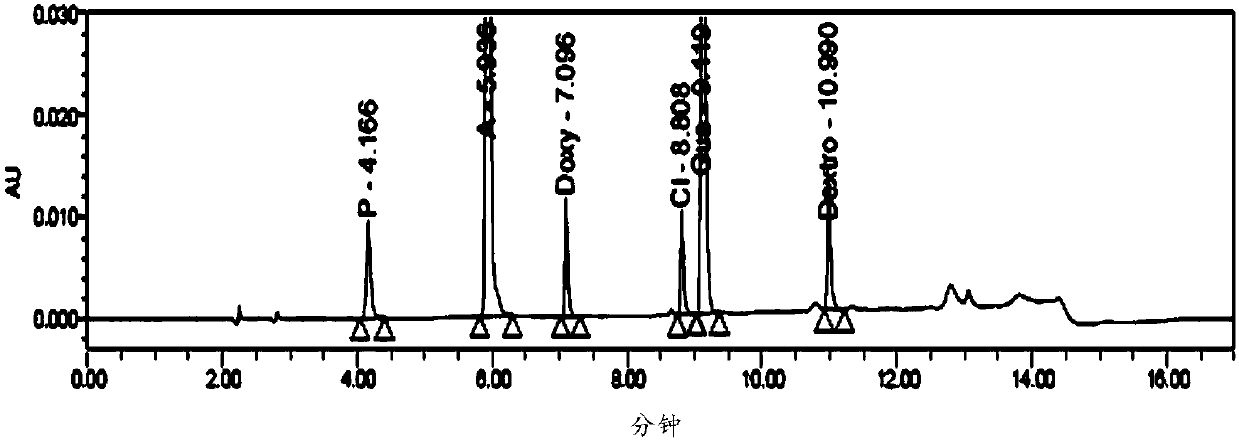
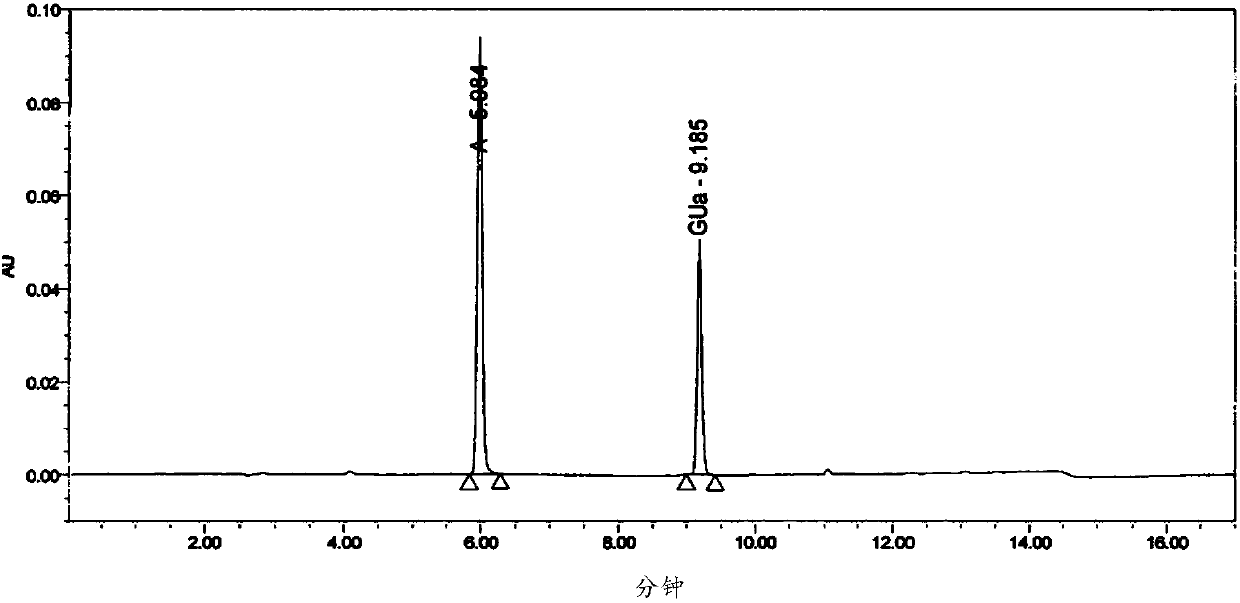
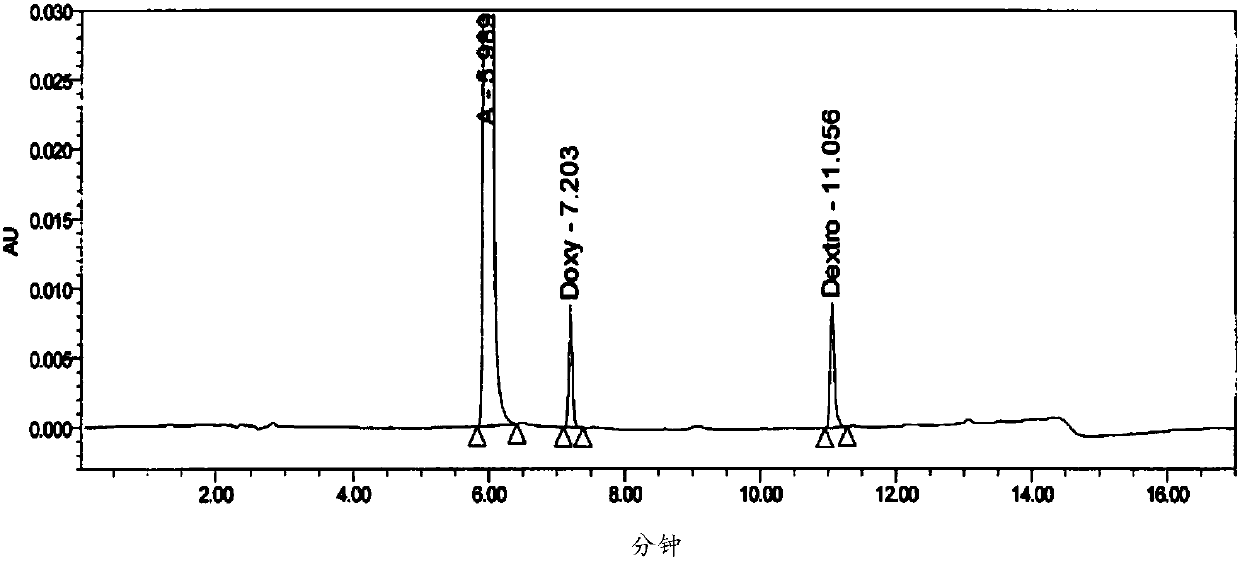
The invention provides a method for six active components in a drug sample. In the method, the six active components comprise paracetamol, phenylephrine hydrochloride, doxylamine succinate, dextromethorphan hydrobromide, guaifenesin and chlorpheniramine maleate; the method uses high performance liquid chromatography (HPLC) to detect the drug sample, wherein the mobile phase of HPLC comprises a mobile phase A which is an aqueous solution containing trifluoroacetic acid of which the concentration is 0.1v / v% and a mobile phase B which is a mixed solution of acetonitrile and methanol in a volume ratio of 60: 40. The detection method provided by the invention can simply and quickly detect the six active components in the drug sample simultaneously, and the six active components can be separatedeffectively, and the detection method is simply operated, and quickly analyzed, suitable for detection of most cold medicines and wide in detection application range.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Sustained release micro-pellet of guaifenesin and preparation process thereof
InactiveCN1994285AMedication convenienceQuick treatmentEther/acetal active ingredientsGranular deliveryActive componentLignan
The invention relates to a method for producing lignan slow-release micro drop and relative preparation, wherein it comprises active component, and carrier element, slow-release package and quick-release drug layer. The invention uses round technique to prepare high-carrier drug element to be packed and coated with quick-release layer; the slow-release layer uses cellulose ethyl ether; package is processed at fluid condition. The invention can release drug for 12h.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Sustained release of guaifenesin
InactiveUS7838032B2Reduce the possibilityOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationWater insoluble
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Medicine composition for treating productive cough and its use
InactiveCN1857241AEasy dischargePromote secretionOrganic active ingredientsAerosol deliveryDiseaseLower respiratory infection
The present invention relates to a kind of medicine composition for treating productive cough, and the medicine composition has ambroxol hydrochloride, guaifenesin and levosalbutamol in certain weight proportion and pharmaceutically acceptable supplementary material. The medicine composition may be prepared into pharmaceutically acceptable preparation forms for treating productive cough caused by chronic bronchitis, asthma, repeatable lower respiratory infection and other respiratory system diseases.
Owner:肖广常 +1
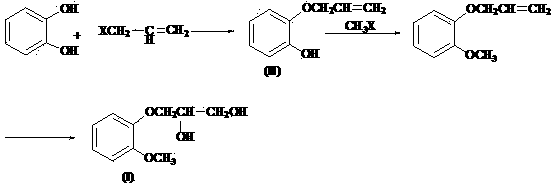
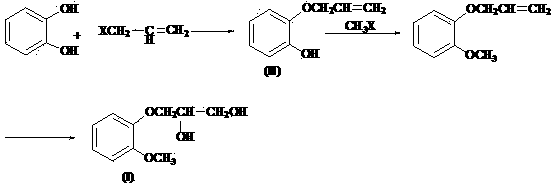
Solid base catalyst, preparation of solid base catalyst as well as application of solid base catalyst to preparation of guaifenesin
ActiveCN104138765APromote regenerationHigh activityPhysical/chemical process catalystsOrganic chemistryCellulosePtru catalyst
The invention discloses a composite solid base catalyst which is mainly prepared from the following components in parts by mass: 100 parts of gamma-Al2O3 as a carrier, 0.5-5 parts of a binding agent as a loaded component, 1-25 parts of a magnesium-containing compound, 1-25 parts of a calcium-containing compound, 0.5-5 parts of polyacrylamide and 0.1-2 parts of inorganic base, wherein the binding agent is selected from one of glycerol, starch, methylcellulose, polypropylene glycol, alumina sol and sodium silicate; the magnesium-containing compound is selected from one of MgO, Mg(OH)2, Mg(CO3)2, Mg(OAc)2 and MgCl2; the calcium-containing compound is selected from one of CaO, Ca(OH)2, Ca(CO3)2 and CaCl2; the inorganic base is selected from one of NaOH, KOH, Na2CO3 and K2CO3. The catalyst is used for preparing guaifenesin, is simple and convenient to operate, environmentally friendly, low in energy consumption and high in yield.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Oral solid preparation containing ambroxol hydrochloride and guaifenesin active components
InactiveCN101099730APlace stableEasy to carryEther/acetal active ingredientsPill deliveryDiseaseActive component
The present invention discloses an oral solid preparation containing ambroxal hydrochloride and guaifenesin active components. It is made up by using ambroxol hydrochloride and guaifenesin as active components and auxiliary material according to a certain ratio through a certain preparation process. Said oral solid preparation can be used for mainly curing the diseases of chronic bronchitis and periodic lower respiratory tract infection.
Owner:天津康鸿医药科技发展有限公司
Method for analyzing daytime severe cold and flu capsules by high performance liquid chromatography
The invention discloses a method for analyzing daytime severe cold and flu capsules by high performance liquid chromatography. The daytime severe cold and flu capsules contain acetaminophen, phenylephrine hydrochloride, dextromethorphan hydrobromide and guaifenesin; high performance liquid chromatography analysis conditions are as follows: a chromatographic column adopts an octadecyl silane bonded silica gel column, a sodium octanesulfonate containing 0.1v% triethylamine is used as a mobile phase A, and acetonitrile is used as a mobile phase B. The method can efficiently and simultaneously detect four effective components in the daytime severe cold and flu capsules; furthermore, the method is easy to operate, high in analysis speed, high in repetitiveness and extremely high in specificity; the product quality of a daytime severe cold and flu capsule preparation can be relatively efficiently and comprehensively controlled.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Topical agent for muscle treatment
A topical liposomal base delivery agent for physiologically enhancing muscle efficiency and relaxation, which combines the physiological effects of guaifenesin, magnesium, and methylsulfonylmethane. The resulting physiological reaction reduces muscle and joint soreness in the host. The present invention is an improvement over existing methods that deliver magnesium to muscle tissue.
Owner:HOLGATE ERIC
Extended release formulations of guaifenesin
InactiveUS20090202633A1BiocideEther/acetal active ingredientsExtended Release FormulationsGuaifenesin
The present invention relates to extended release formulations comprising expectorant. More particularly, the present invention relates to extended release formulations comprising guaifenesin. The present invention also relates to a process for the preparation of extended release formulations comprising guaifenesin.
Owner:AUROBINDO PHARMA LTD
A kind of compound dextromethorphan hydrobromide syrup and preparation method thereof
ActiveCN102600210BBacteria material medical ingredientsEther/acetal active ingredientsFlavouring agentDextromethorphan Hydrobromide
The invention provides a compound dextromethorphan hydrobromide syrup and a preparation method for the same. The compound dextromethorphan hydrobromide syrup is used for eliminating phlegm in cough, and comprises dextromethorphan hydrobromide, guaifenesin and a bacillus natto extract. The syrup improves the safety of medication and is especially suitable for children, reduces bitter taste and the consumption of a flavouring agent, improves the stability, especially the colour of solution and the stability of the colour, and improves the curative effect on the cough caused by cold and bronchitis simultaneously.
Owner:湖北凤凰白云山药业有限公司 +1
Compound dextromethorphan hydrobromide syrup and preparation method for same
ActiveCN102600210ABacteria material medical ingredientsPharmaceutical delivery mechanismFlavouring agentDextromethorphan Hydrobromide
The invention provides a compound dextromethorphan hydrobromide syrup and a preparation method for the same. The compound dextromethorphan hydrobromide syrup is used for eliminating phlegm in cough, and comprises dextromethorphan hydrobromide, guaifenesin and a bacillus natto extract. The syrup improves the safety of medication and is especially suitable for children, reduces bitter taste and the consumption of a flavouring agent, improves the stability, especially the colour of solution and the stability of the colour, and improves the curative effect on the cough caused by cold and bronchitis simultaneously.
Owner:湖北凤凰白云山药业有限公司 +1
Narcotic biphasic release compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
InactiveUS20080008772A1BiocideEther/acetal active ingredientsHYDROCODONE BITARTRATEImmediate release
The present invention relates to compositions that comprise immediate release and extended release guaifenesin, and extended release hydrocodone bitartrate. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Combined herbal and pharmaceutical composition and method
InactiveUS20170368124A1Short retention timeHigh resolutionHydroxy compound active ingredientsEther/acetal active ingredientsEthylenediaminePolyethylene glycol
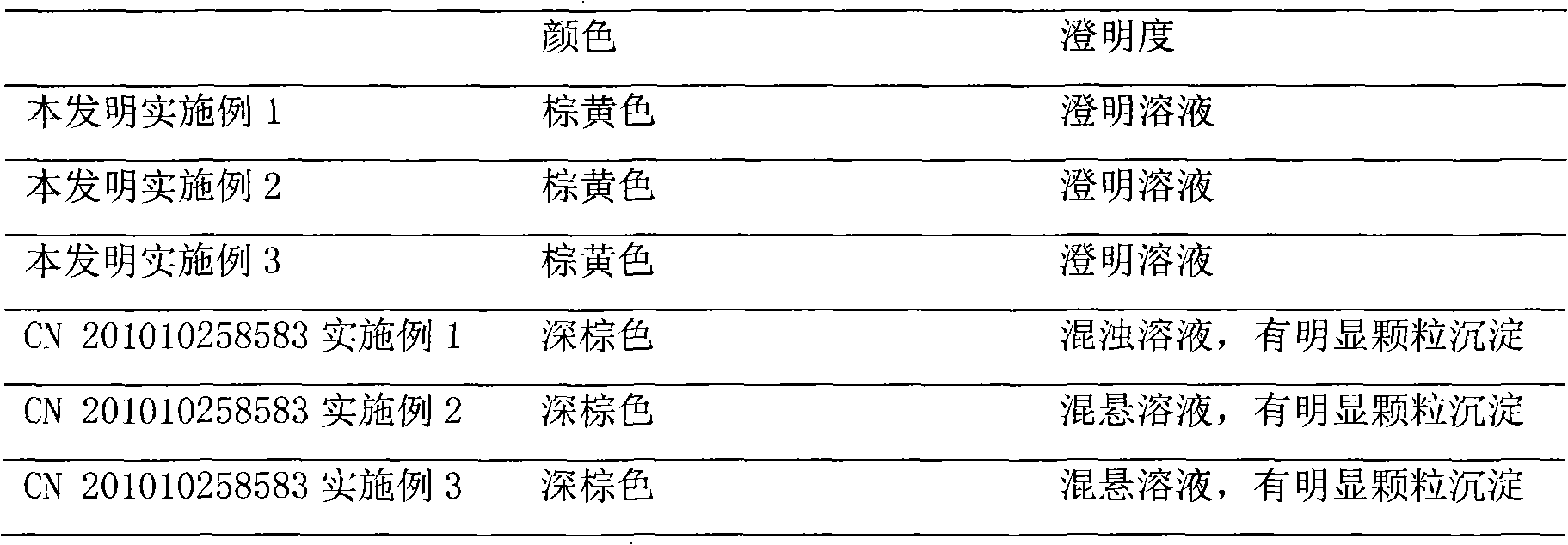
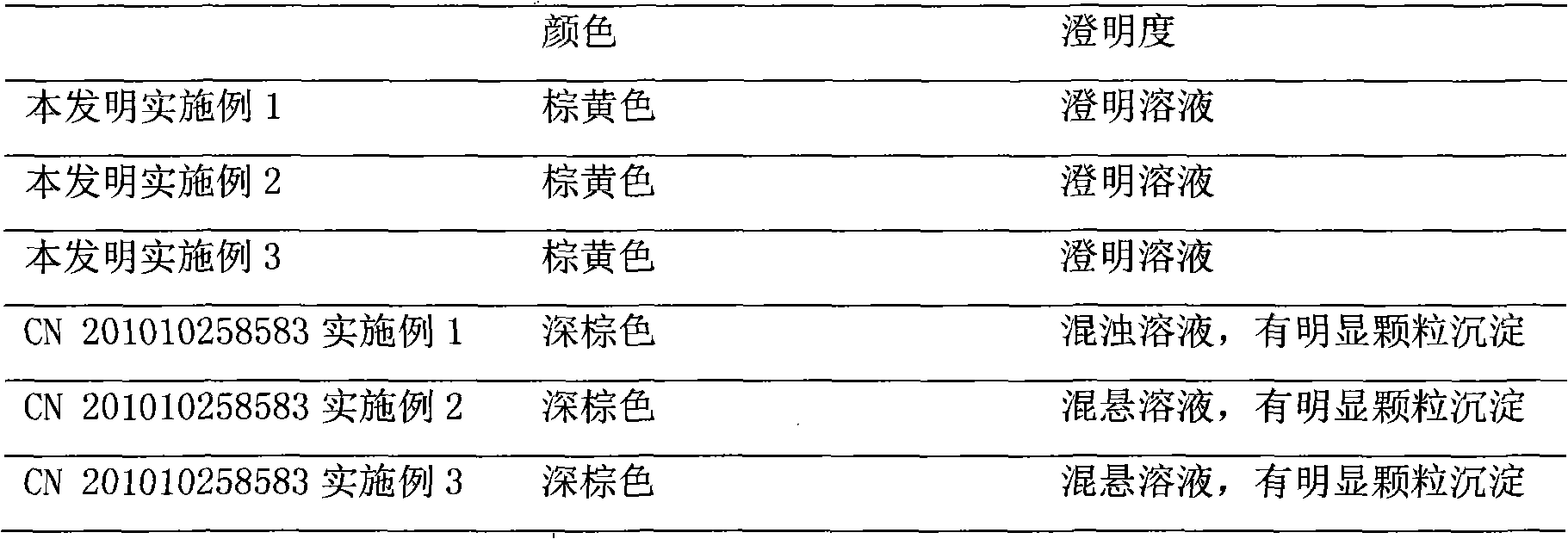
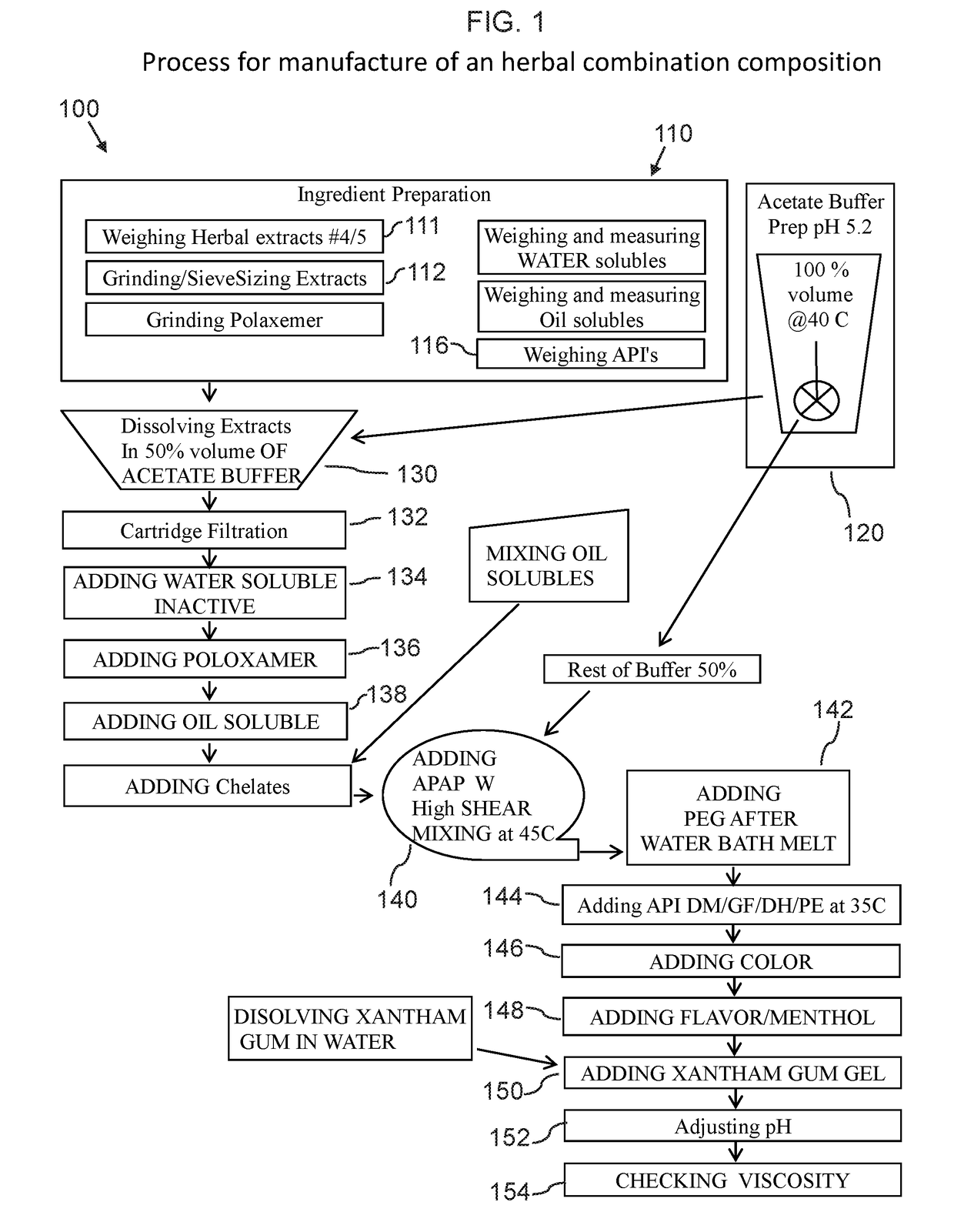
An herbal combination composition can include, herbal extracts, including combinations of: Hyssopus officinalis, Zingiber officinale, Viola odorata, Ziziphus jujuba, Chamomile, and Ocimum tenuiflorum; pharmaceutical compositions, including combinations of: dextromethorphan, guaifenesin, acetaminophen, phenylephrine, diphenhydramine; polyethylene glycol; propylene glycol; poloxamer 407; ethylenediaminetetraacetic acid; methyl paraben; potassium sorbate; propyl paraben; xanthan gum; citric acid; anhydrous citric; and purified acetate buffered water. Also disclosed is a method for manufacture of an herbal combination composition, including dissolving herbal extracts, adding poloxamer, adding pharmaceutical compositions, adding acetate buffer, adding xanthan gum gel, adding acetate buffer.
Owner:SYED UWAIS M
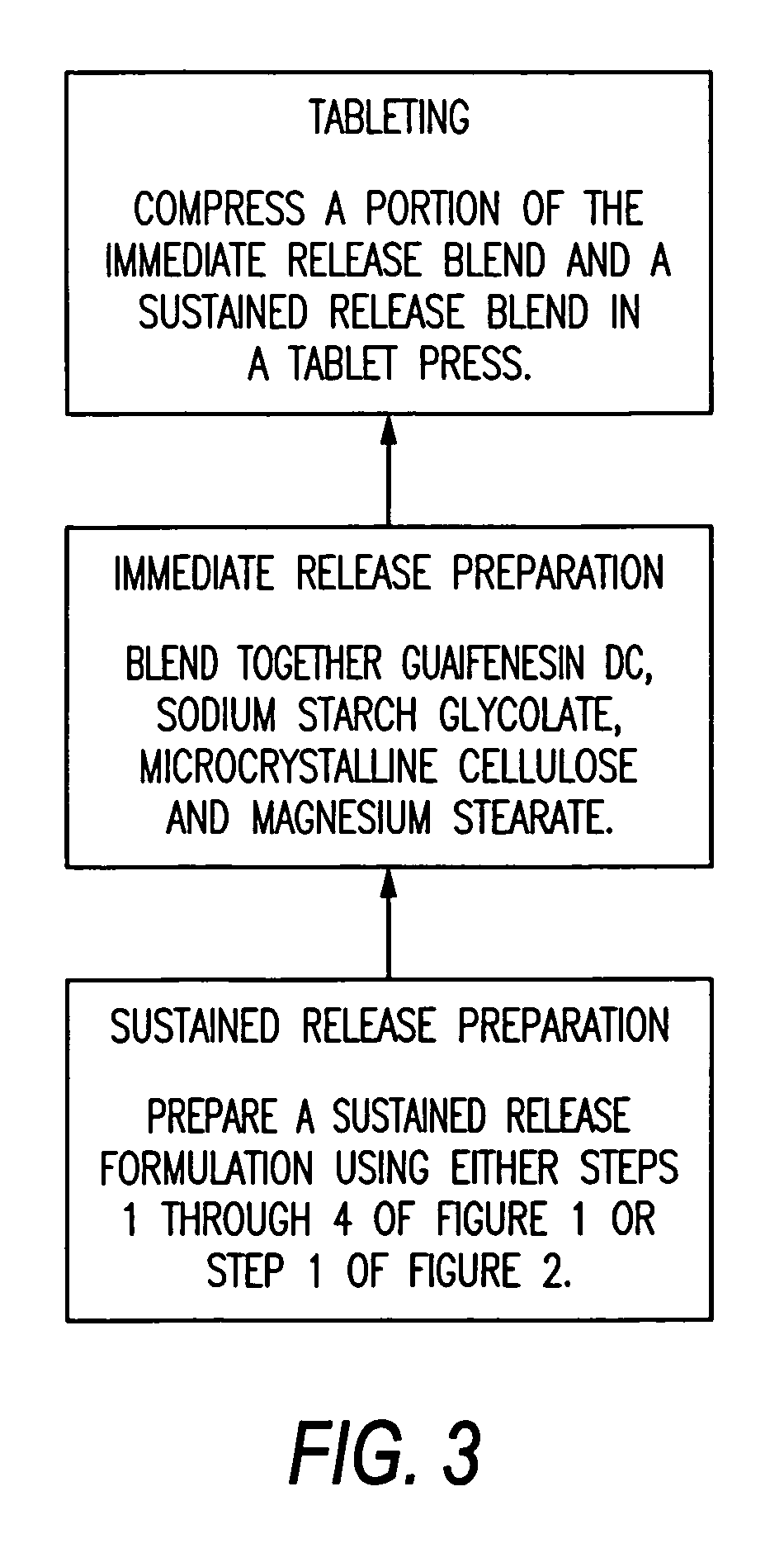
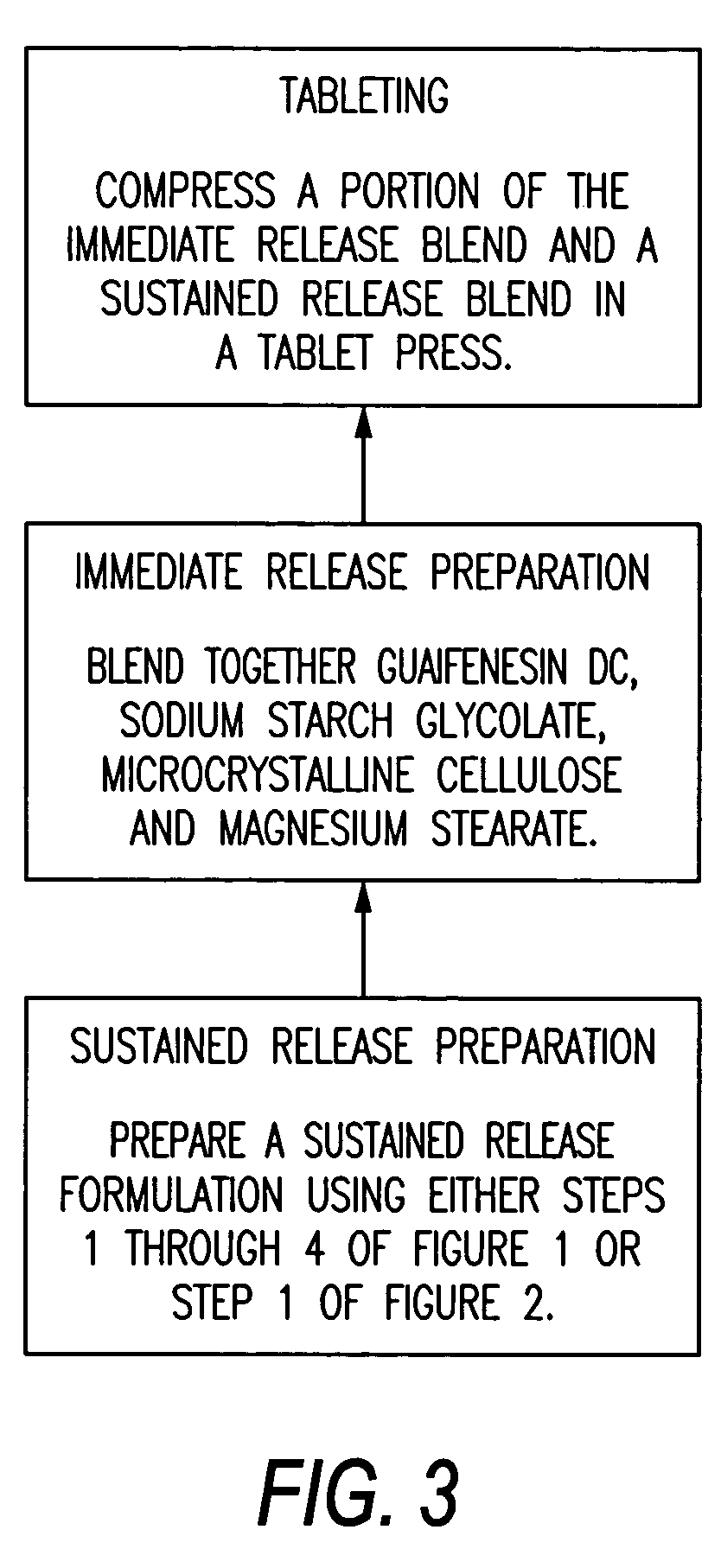
Pharmaceutical Formulation
InactiveUS20160228386A1Observed effectCompatibility concernEther/acetal active ingredientsPill deliveryImmediate releasePOLACRILIN POTASSIUM
A pharmaceutical composition in the form of a tablet including a first portion and a second portion, wherein the first portion includes guaifenesin having an immediate release profile and a second drug having a sustained release profile, and wherein the second portion includes guaifenesin having a sustained release profile. The second drug can be in the form of a drug-resin complex. The second drug can be either an anti-tussive or a decongestant. The drug-resin complex includes a drug complexed to an ion exchange resin. The ion exchange resin can be a polystyrene sulfonate resin, polacrilex resin, polacrilin potassium, cholestyramine resin, or a colestyramine resin. The drug-resin complex can be provided with a coating, the coating thickness being selected to obtain the desired release profile. The drug-resin complex can be provided with a coating level of from 5% to 50%. The coating level can be from 10% to 35%.
Owner:RB HEALTH (US) LLC
Continuous chromatographic resolution method of guaifenesin racemate
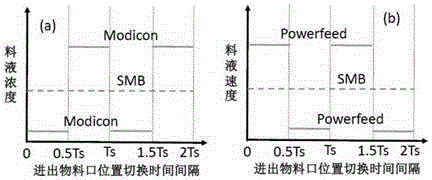
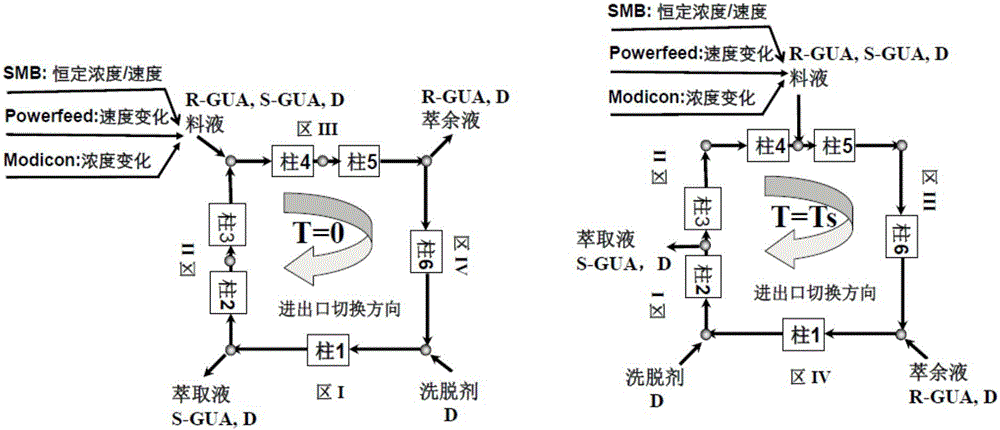
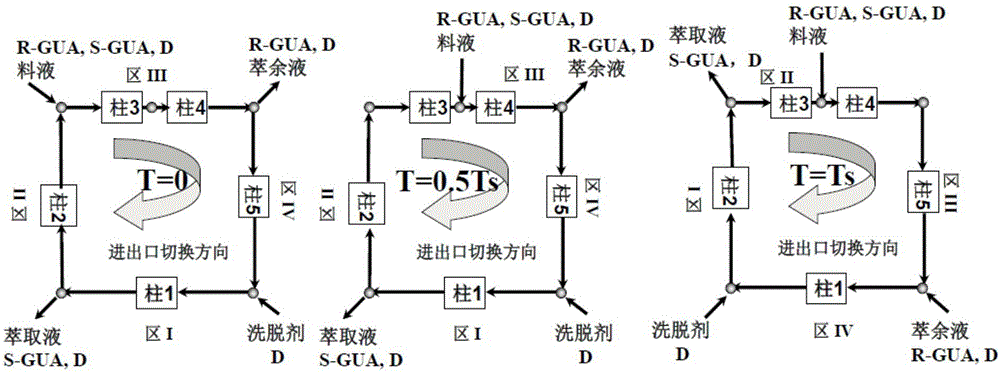
InactiveCN106495995AContinuous productionIncrease productivityEther separation/purificationOrganic chemistry methodsCelluloseSimulated moving bed
The invention relates to a countercurrent continuous chromatographic resolution method of a guaifenesin racemate. The method utilizes simulated moving bed chromatography to realize continuous separation and comprises one or more of a synchronous control process (SMB), an asynchronous control process (Varicol), a feed rate periodic change process (Powerfeed) and a feed concentration periodic change process (Modicon). When silica gel coated with cellulose-tris(3, 5-dimethylphenylcarbamate) is used as a chiral stationary phase and a mixture of n-hexane and ethanol is used as a mobile phase, the method can continuously and efficiently resolve a guaifenesin racemate, acquire a R-guaifenesin racemate enantiomer and a S-guaifenesin racemate enantiomer with high purity (greater than 99%) and meet the pharmaceutical market standards. The method has a high degree of production automation, high production efficiency and simple processes. In the whole process, the solvent can be recycled so that a cost is low and pollution is avoided.
Owner:EAST CHINA UNIV OF SCI & TECH
Drug composition for relieving cough and reducing phlegm
InactiveCN102038684AImprove securityGood effectEther/acetal active ingredientsRespiratory disorderDiseaseDextromethorphan Hydrobromide
The invention relates to a drug combination for relieving cough and reducing phlegm, which is composed of active constituents and a pharmaceutic adjuvant, wherein the active constituents comprise butamirate critrate, dextromethorphan hydrobromide and guaifenesin. The composition can be prepared into various kinds of oral preparations such as granules, troches, capsules, oral solution, syrup and the like and can be used for carrying out symptomatic treatment on related diseases with symptoms of cough, abundance of phlegm and the like.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Novel environment-friendly flame-retardant finishing agent and preparation method thereof
The invention discloses a novel environment-friendly flame-retardant finishing agent. The novel environment-friendly flame-retardant finishing agent is prepared from, by weight, 20-30 parts of glycerophosphate, 10-25 parts of ethylene glycol monostearate, 5-11 parts of ammonium acetate, 8-12 parts of hydroxymethyl cellulose, 3-7 parts of chitosan, 4-9 parts of potassium xylenesulphonate, 11-22 parts of guaifenesin, 6-12 parts of ethoxymethylenmalonic acid diethylester, 2-7 parts of 3-(2-aminoethylamino)propyl-dimethoxymethylsilane and 15-25 parts of sorbitol. The flame-retardant level and flame-retardant property of the finishing agent can be significantly improved, and the finishing agent is environmentally friendly, free of pollution and friendly to the environment.
Owner:湖州市千金丝织厂(普通合伙)
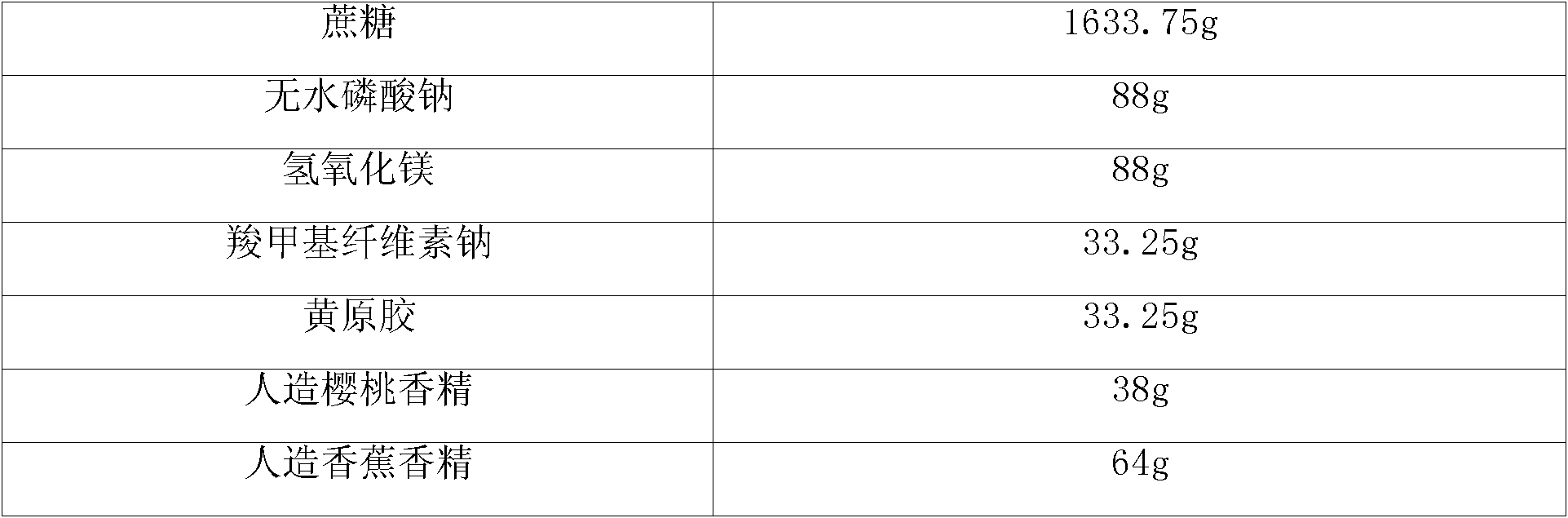
Compound preparation for preparing dextromethorphan or physiologically acceptable salts thereof and guaifenesin
The invention belongs to the field of pharmaceutical preparations and relates to a compound syrup preparation composition for preparing dextromethorphan or physiologically acceptable salts thereof and guaifenesin. The compound syrup preparation composition consists of cane sugar, sodium benzoate, ethylparaben, citric acid, sodium citrate, pigments and essence. The compound preparation can be used for effectively relieving cough-related symptoms caused by respiratory tract infection and bronchitis, has a synergistic effect of medicines and also can be used for effectively and conveniently controlling the administration amount of a patient, and greatly reducing the children administration difficulty. Therefore, the dosage form has the characteristics that the compound preparation is high in dispersity, fast in absorption and fast in effectiveness after being taken by the patient, and the bioavailability of the medicine is improved.
Owner:BEIJING VENTUREPHARM BIOTECH
Method and formulation for cold treatment in adults and children with increased safety
InactiveUS20110281892A1Effective symptom reliefEffective symptomBiocideAntipyreticCold treatmentChild and adolescent
Methods and dosage formulations for the safe treatment of cold, cough, flu, and sinus symptoms in children and adolescents by age and nature of symptoms, and adults and the elderly by age, nature of symptoms, and concomitant patient medical conditions. The method of the present invention comprises the formulation of a cold medication which consists essentially of combinations of a nonsedating or minimally sedating antihistamines with pain relievers and the expectorant, guaifenesin.
Owner:SILVERMAN BERNARD
Sustained release of guaifenesin combination drugs
InactiveUS7985420B2Reduce the possibilityOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationImmediate release
Owner:RB HEALTH US LLC
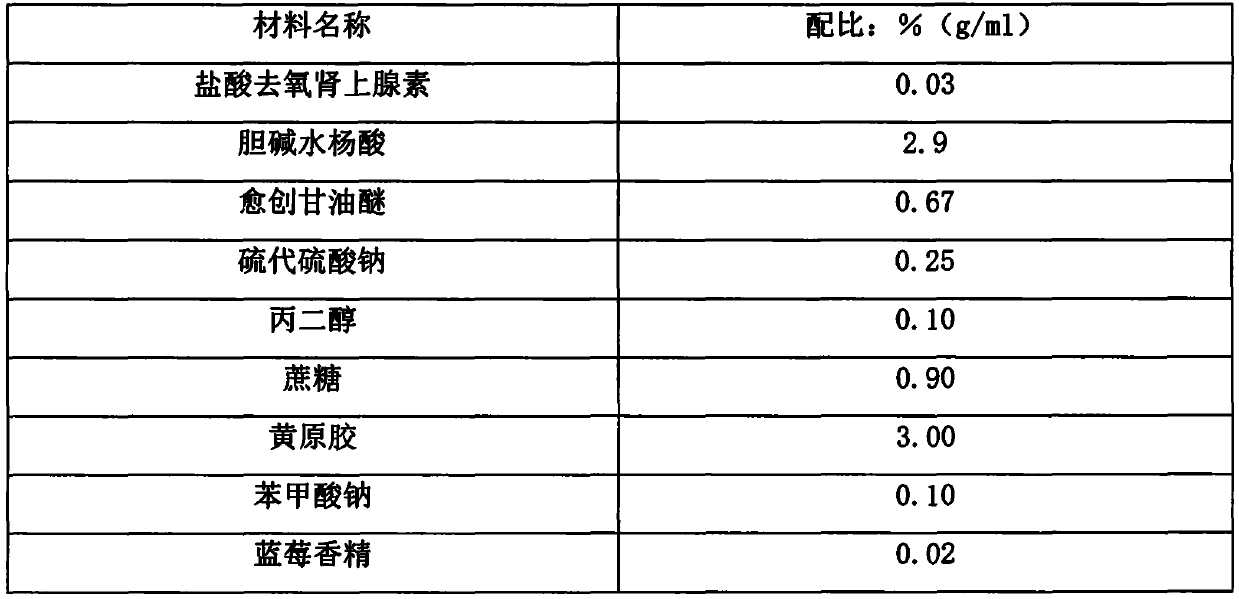
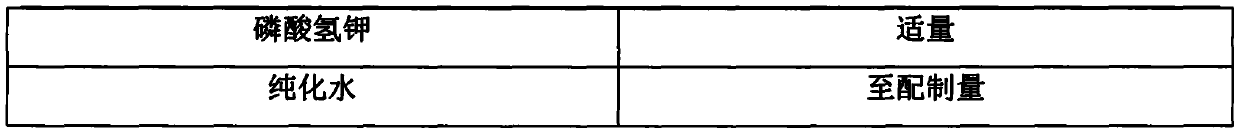
Liquid composition containing phenylephrine hydrochloride as well as preparation and application thereof
PendingCN111588694AImprove stabilityReduce solubilitySalicyclic acid active ingredientsAntipyreticSalicylic acidSuccinic acid
The invention relates to a liquid composition containing phenylephrine hydrochloride as well as a preparation and application thereof. The composition comprises three compositions: the first is a combination of the phenylephrine hydrochloride, choline salicylic acid and guaifenesin; the second is a combination of the phenylephrine hydrochloride, acetaminophen and pheniramine maleate; and the thirdis a combination of the phenylephrine hydrochloride, the acetaminophen, dextromethorphan hydrobromide and doxylamine succinate. The composition disclosed by the invention can be prepared into a liquid preparation, and a preferable dosage form is suspension.
Owner:北京博智绿洲医药科技有限公司
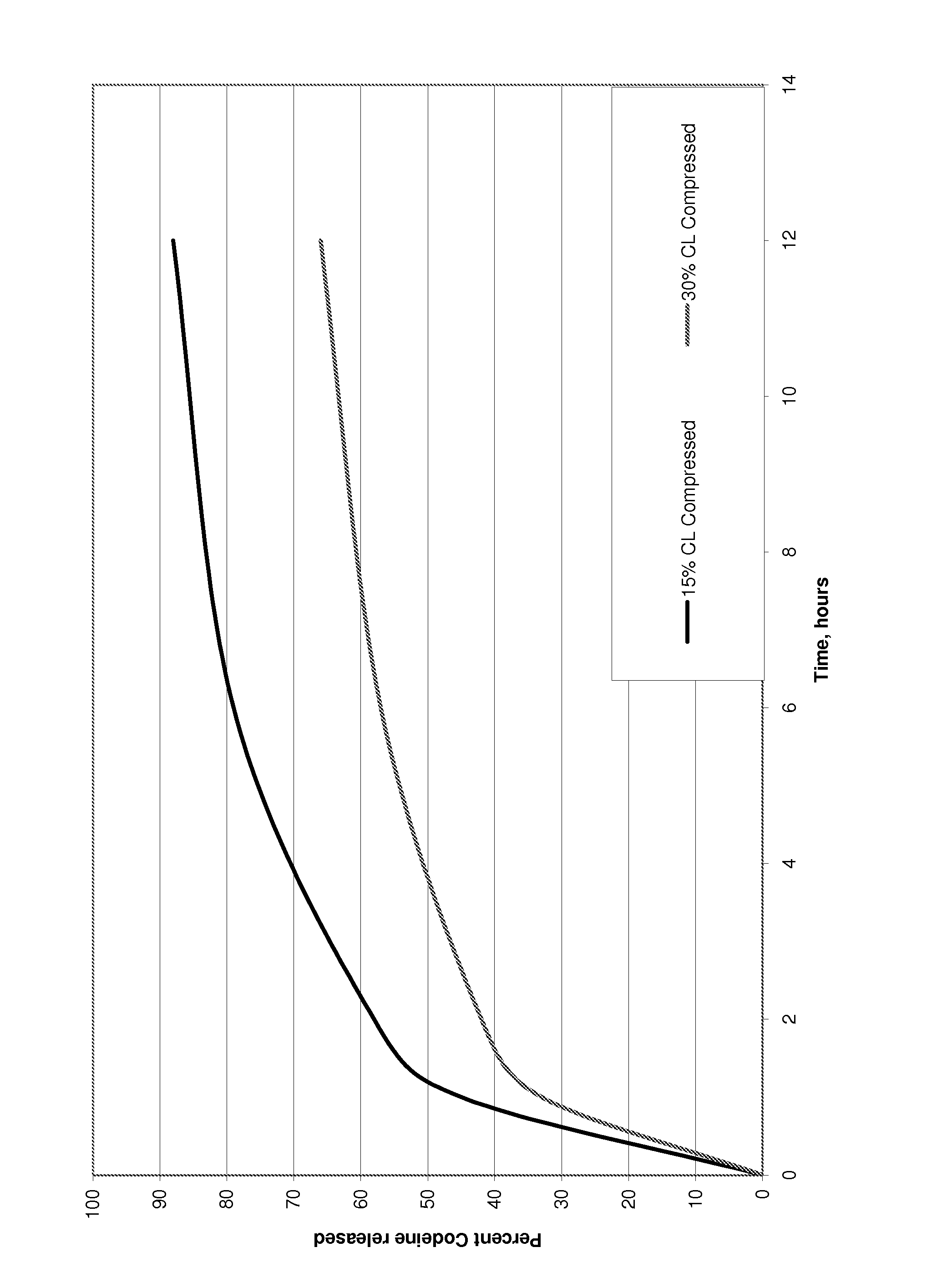
Guaifenesin and Dextromethorphan sustained release tablets and preparation method thereof
InactiveCN106727561APromote secretionImprove complianceEther/acetal active ingredientsPill deliveryTherapeutic effectAntitussive Agent
The invention discloses Guaifenesin and Dextromethorphan sustained release tablets and a preparation method thereof. The Guaifenesin and Dextromethorphan sustained release tablets are composed of dextromethorphan hydrobromide and guaifenesin. The guaifenesin is a phlegm-expelling agent, and is capable of reflexively promoting increase of respiratory tract gland secretion by stimulating gastric mucosa to cause slight nausea, phlegm is diluted and coughed up; dextromethorphan hydrobromide is a central antitussive agent, has detect effect on coughing center of medulla oblongata, can inhibit cough reaction, and generates no addition. The Guaifenesin and Dextromethorphan sustained release tablets are used for treating cough and expectoration due to upper respiratory infection and bronchitis; compared with various same kinds of the product, the Guaifenesin and Dextromethorphan sustained release tablets have dual efficacies of eliminating phlegm and relieving cough, has the advantages of definite therapeutic effect, stable quality, and convenient administration, and is an ideal selection for medicine capable of relieving cough and resolving phlegm.
Owner:HARBIN SHENGJI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com