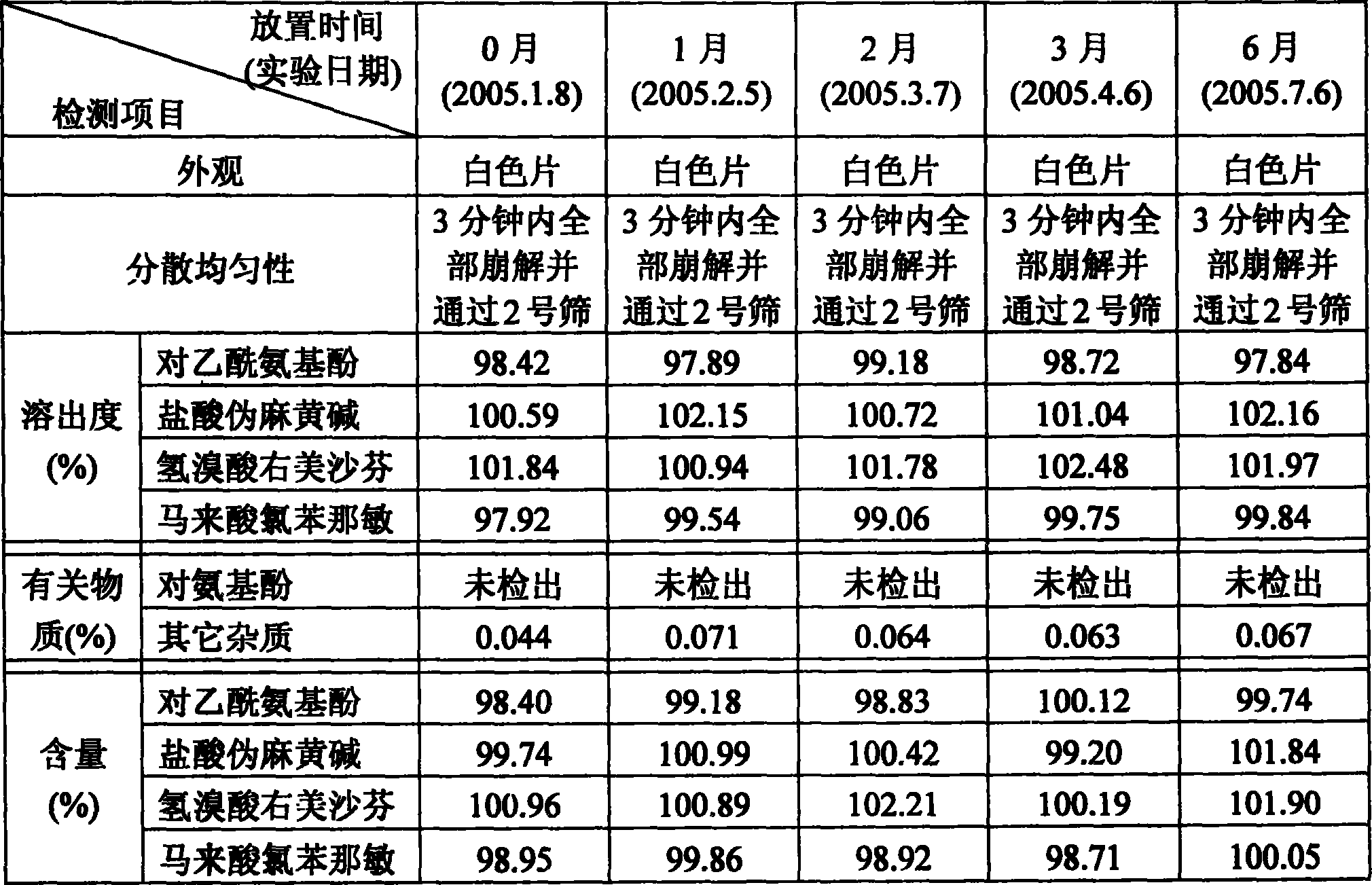
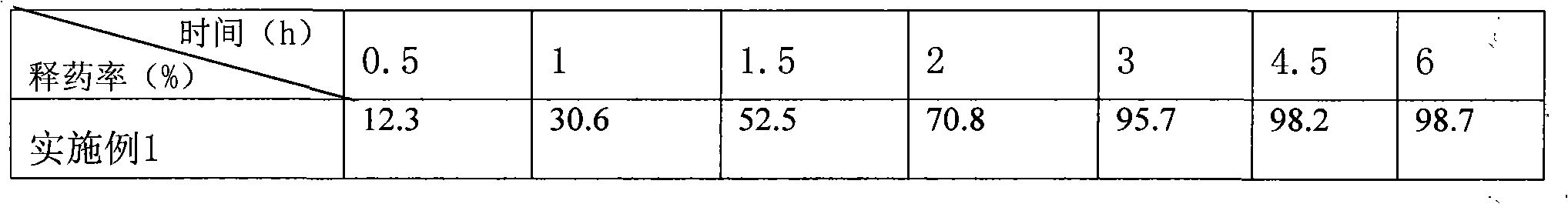
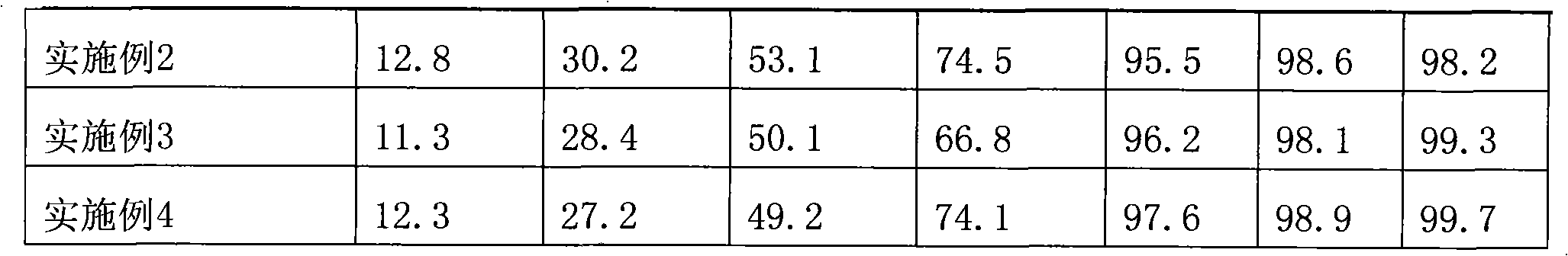
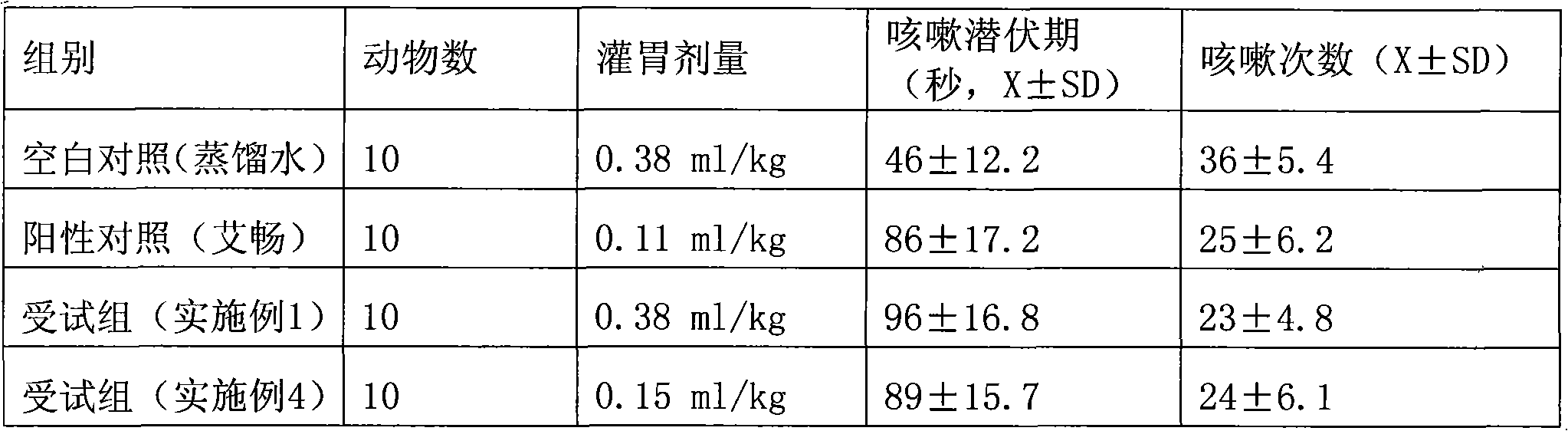
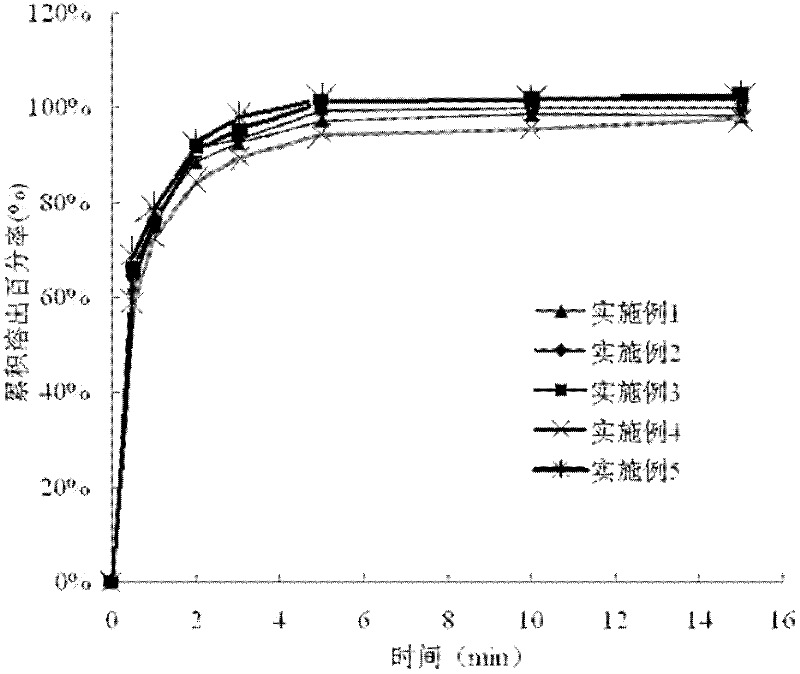
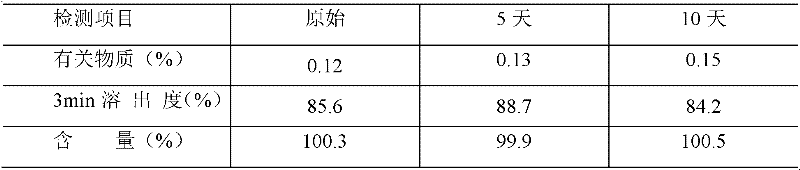
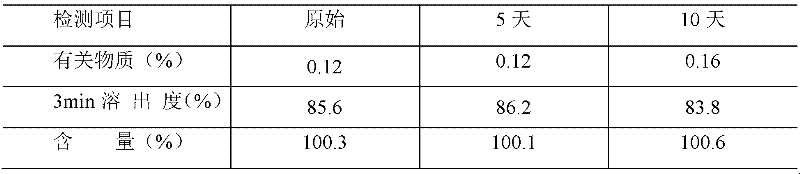
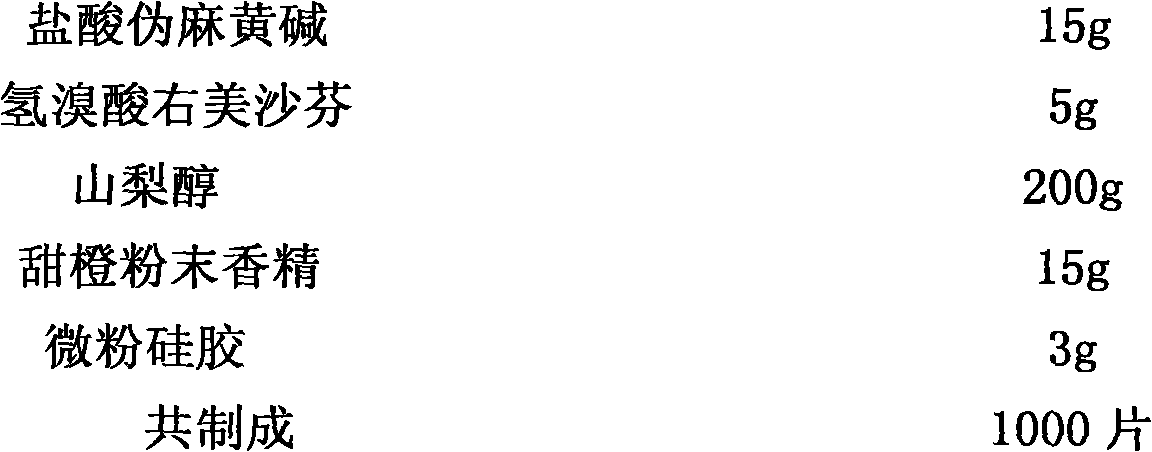Patents
Literature
152 results about "Dextromethorphan Hydrobromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrobromide salt form of dextromethorphan, a synthetic, methylated dextrorotary analogue of levorphanol, a substance related to codeine and a non-opioid derivate of morphine. Dextromethorphan exhibits antitussive activity and is devoid of analgesic or addictive property. This agent crosses the blood-brain-barrier and activates sigma opioid receptors on the cough center in the central nervous system, thereby suppressing the cough reflex.
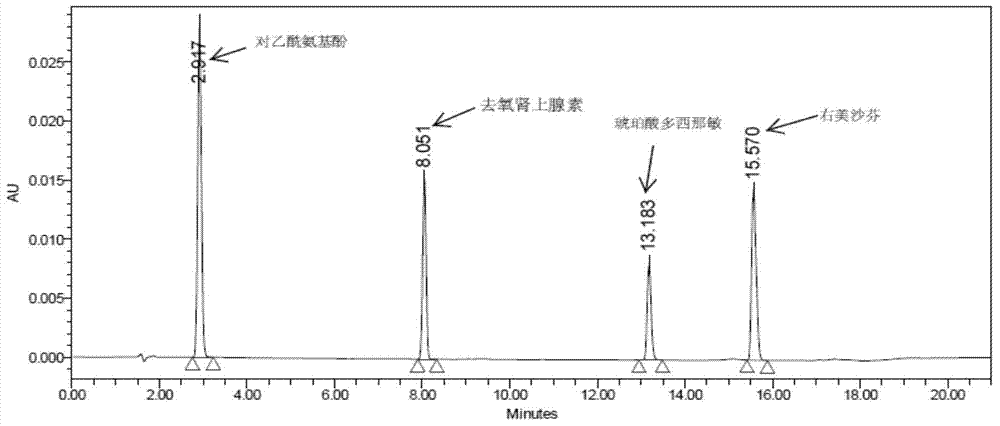
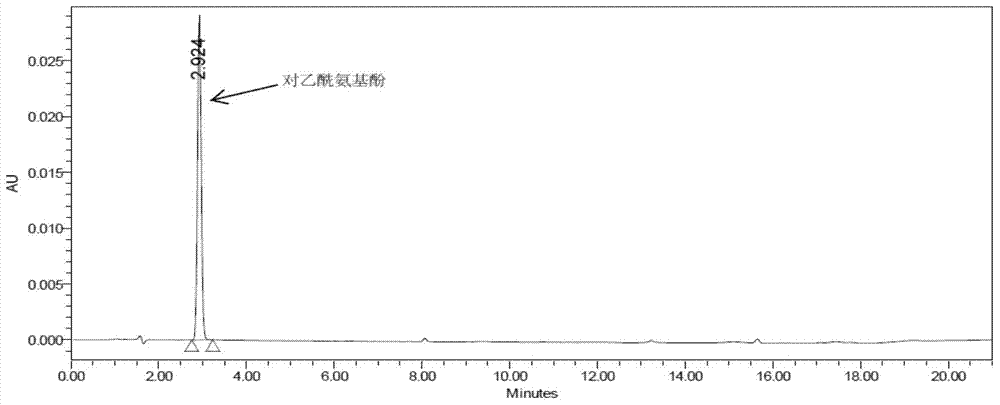
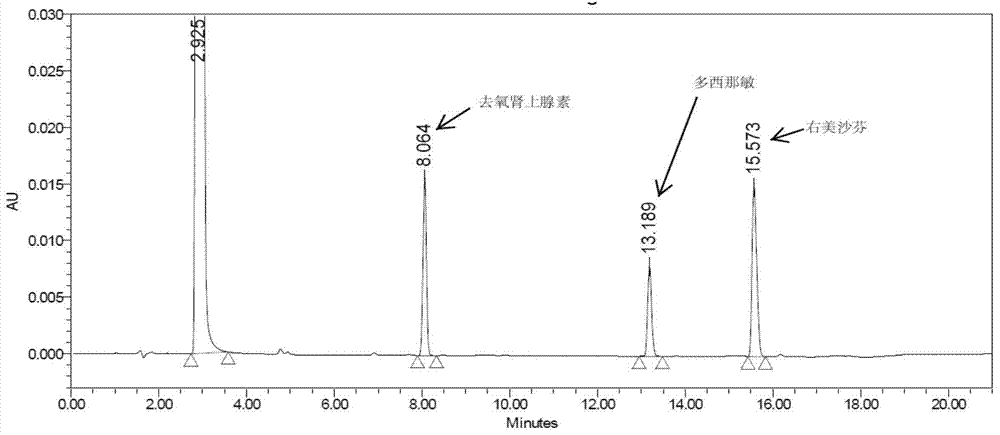
Method for analyzing night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography)
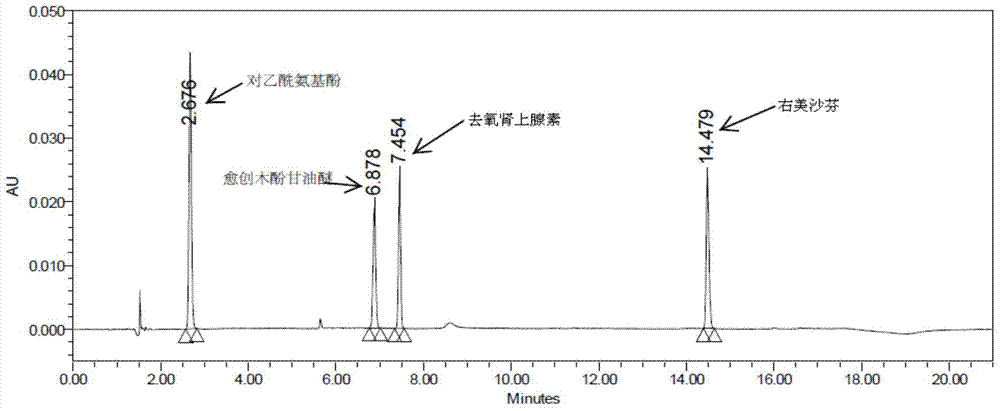
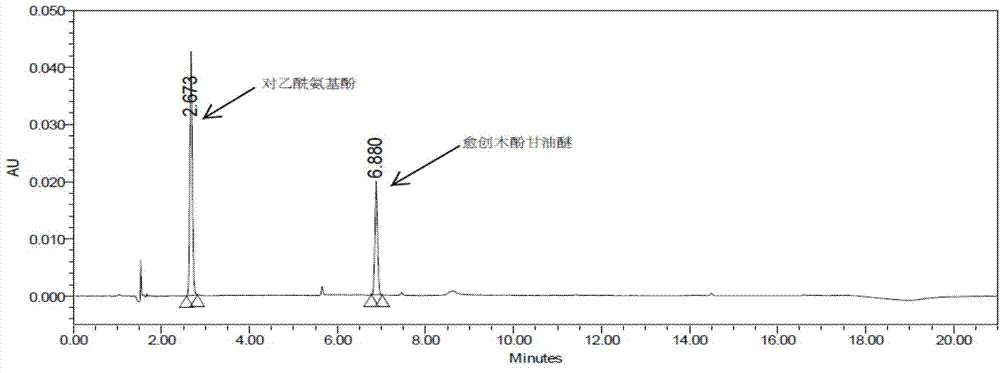
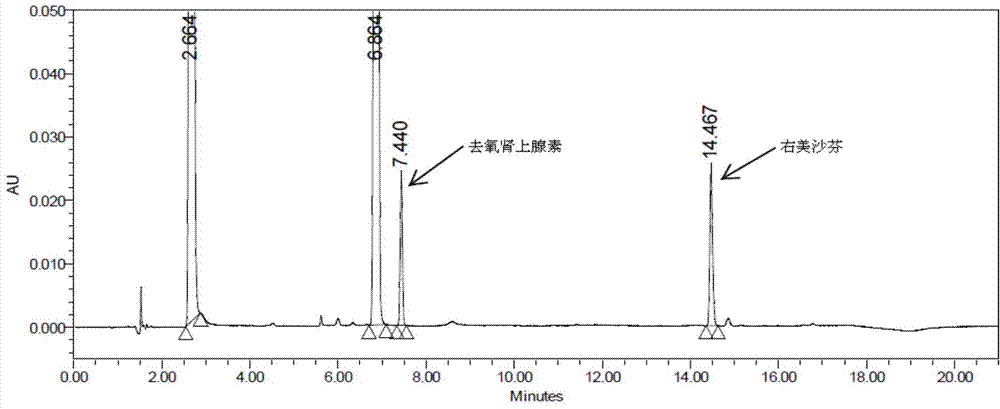
The invention discloses a method for analyzing a night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography). The night cold flu cough allergy capsule contains acetaminophen, phenylephrine hydrochloride, succinic acid doxylamine and dextromethorphan hydrobromide. In the HPLC analysis, an octadecyl silane bonded silica gel column is adopted as a chromatographic column; a sodium 1-octanesulfonate-phosphate buffer solution with the pH of 2.0-3.0 acts as a mobile phase A; acetonitrile and a mixed solution of acetonitrile and methyl alcohol act as a mobile phase B. The method can be simultaneously and effectively used for detecting four effective ingredients in the night cold flu cough allergy capsule, is simple to operate, analyzes rapidly, is good in repeatability, has favorable specificity, and can effectively and comprehensively control the product quality of the night cold flu cough allergy capsule.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Arbidol containing compound preparation
InactiveCN1572298AProlonged cough incubation periodLess frequent coughingOrganic active ingredientsAntiviralsAdjuvantNose
The present invention relates to a compound preparation containing antiviral drug arbidol, which is especially adapted to respiratory tract infection disease caused by virus. During the respiratory tract infection period, the patient has the discomfort symptoms of fever and nose stuff, therefore, the anti influenza compound preparation on the invention also includes one, two or three of the ibuprofen, alcaine pseudoephedrine, and dextromethorphan hydrobromide on the basis of including alcaine arbidol, and is then added with take orally preparation medicinal adjuvant to make take orally solid dosage forms. The medical effect experiment shows that the cooperation of the active components in the compound preparation is well, and the preparation is convenient in used by patient.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Anti-cold medicine soft capsule and its preparing method
ActiveCN1615866AThe appearance is clear and transparentReasonable compositionAntiinfectivesCapsule deliveryDispersed mediaDextromethorphan Hydrobromide
The present invention provides a soft anti-cold medicine capsule and its preparation process and belongs to the field of medicine technology. The content consists of active medicine component and stuffing, the active medicine component includes acetaminophen, pseudoephedrine hydrochloride and dextromethorphan hydrobromide, and the stuffing includes dispersing medium, surfactant, suspension assistant and microemulsifier. The present invention has fast acting and obvious curative effect on cold syndrome, and the soft capsule is clear and transparent.
Owner:CSPC OUYI PHARM CO LTD
Formula of cough-relieving medicament and preparation method thereof
InactiveCN102579926AImprove immunityPromote absorptionOrganic active ingredientsRespiratory disorderFormularySucrose
The invention discloses a formula of a cough-relieving medicament and a preparation method thereof. The medicament is composition solution prepared by procedures such as decoction, filtering, boiling and mixing of folium eriobotryae, pericarpium papaveris, radix stemonae, cynanchum glaucescens, the root bark of white mulberry, platycodon grandiflorum, menthol crystal, cane sugar, citric acid, essence, sodium benzoate, dextromethorphan hydrobromide, promethazine hydrochloride, 75% of alcoholic solution, distilled water and the like. The cough-relieving medicament can be suitable for curing cough caused by various respiratory incentives. After patients orally take the cough-relieving medicament, no body function allergic reaction occurs. The cough-relieving medicament has no toxic or side effect and can be rapidly absorbed and utilized by bodies. The cure purpose of relieving cough and asthma can be achieved by tonifying kidneys, strengthening spleens and moistening lungs. The cough-relieving medicament is low in cost, and the preparation process is simple, convenient and easy to implement.
Owner:吴宜谦
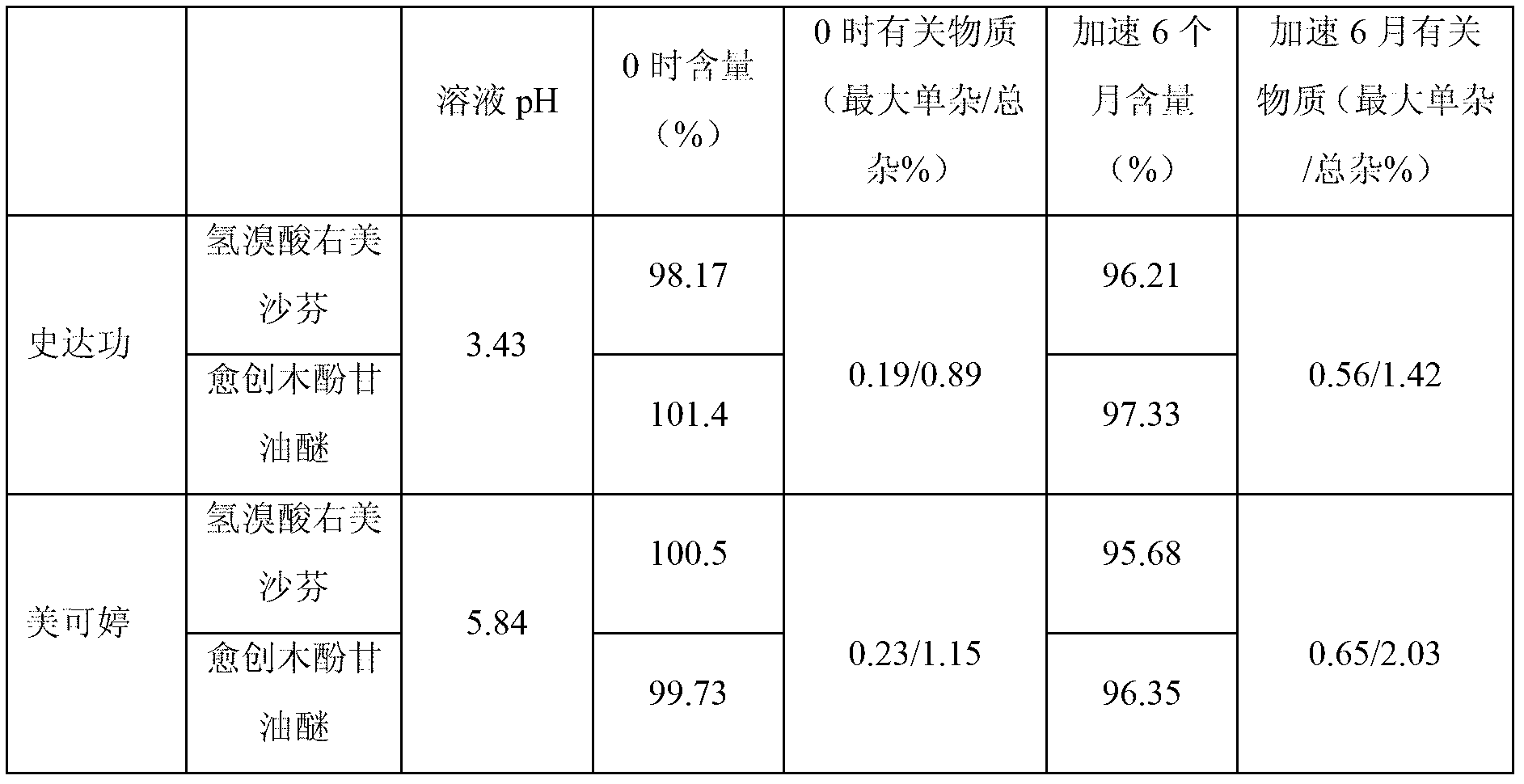
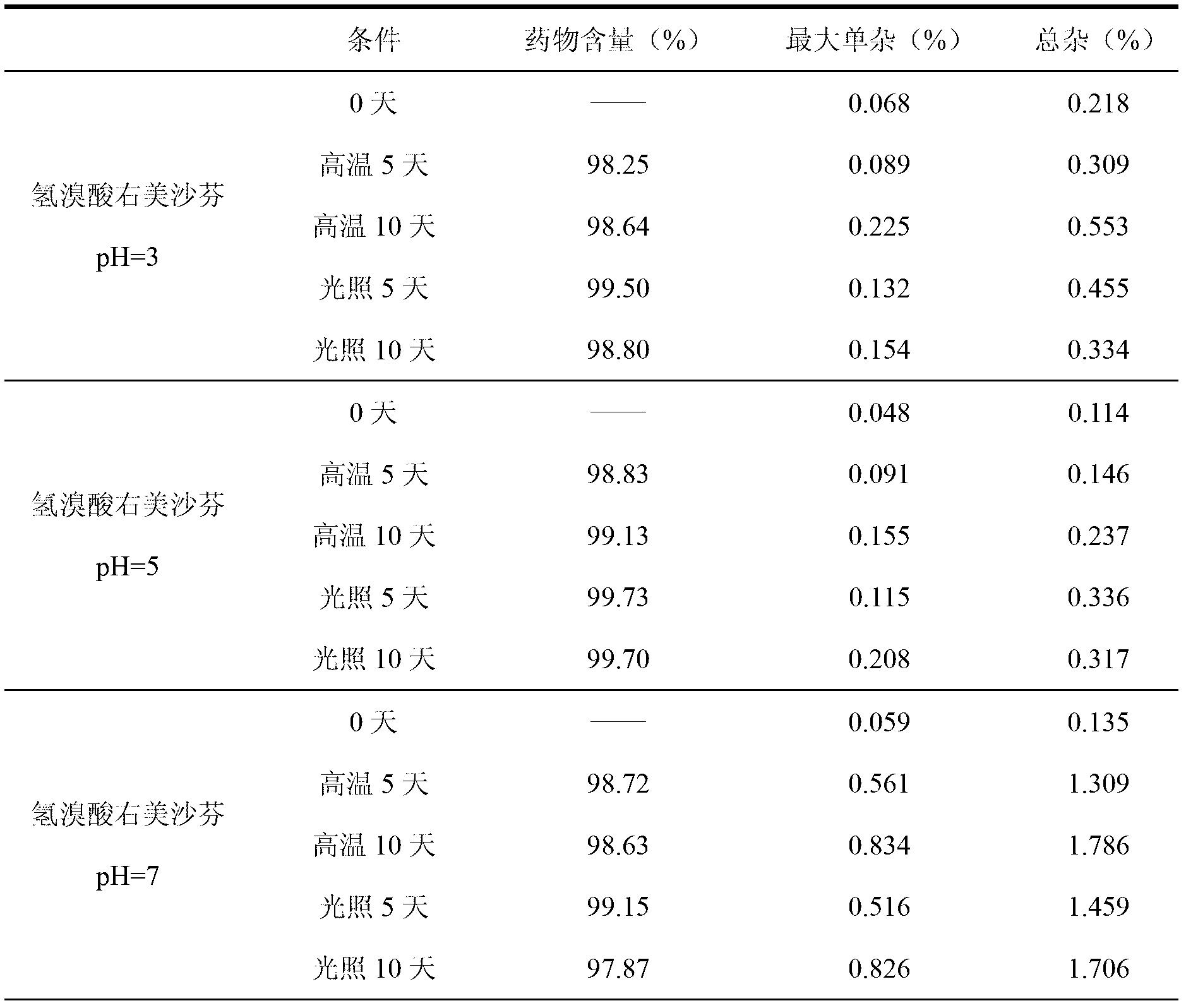
Dextromethorphan hydrobromide and guaiacol glycerin ether oral liquid and preparation method thereof
InactiveCN103191116ALess impuritiesQuality improvementPharmaceutical delivery mechanismEther/acetal active ingredientsDextromethorphan HydrobromideEther
The invention discloses a dextromethorphan hydrobromide and guaiacol glycerin ether oral liquid. The oral liquid contains dextromethorphan hydrobromide, guaiacol glycerin ether, acidifying and alkalizing agents and purified water, wherein the acidifying and alkalizing agents are used for regulating the pH value of the dextromethorphan hydrobromide and guaiacol glycerin ether oral liquid to be 4.0-5.5.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
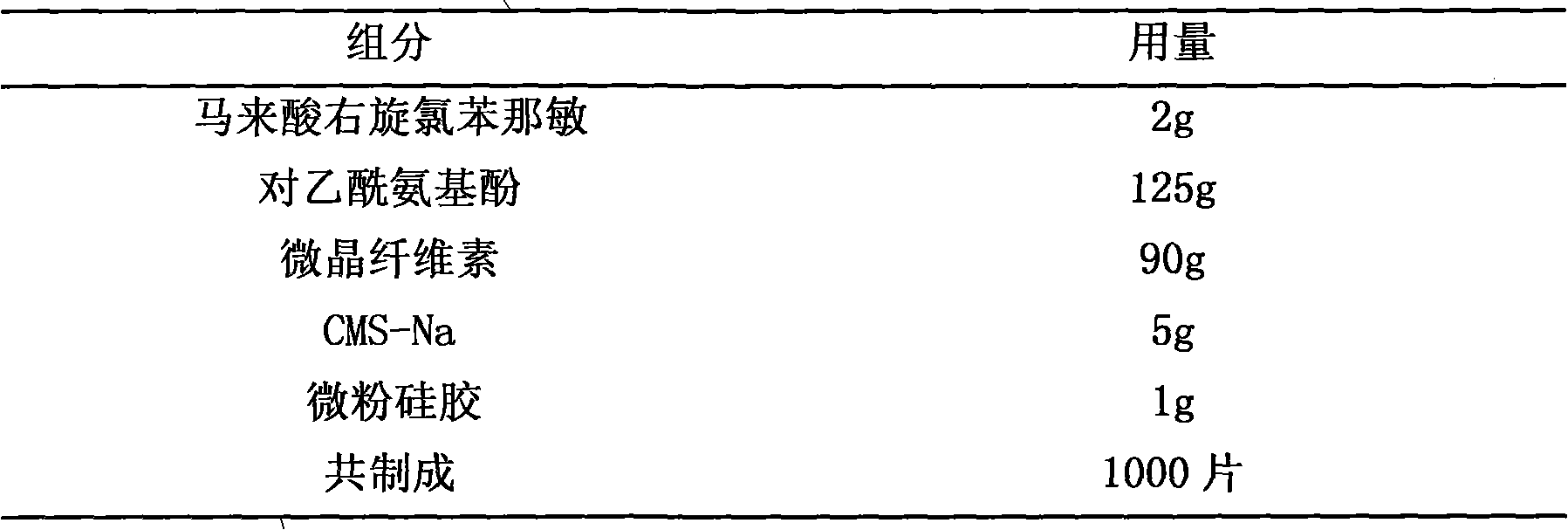
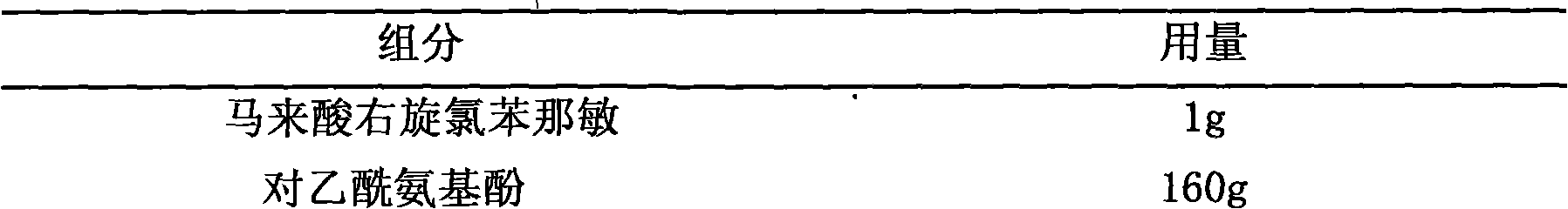
Pharmaceutical composition containing dexchlorpheniramine and preparation thereof
The invention discloses a combination containing r-chlorpheniramine, which is formed by the r-chlorpheniramine and the medicine salt and the other or a plurality of active ingredients or pharmaceutical carriers selected from non-steroidal anti-inflammatory drug, ephedrine alkaloids, coffeine, dextromethorphan hydrobromide, carbetapentane citrate, glyceryl guaiacolate, bromhexine hydrochloride, artificial cow-bezoar, amantadine hydrochloride, aminophylline and zinc gluconate. The orally taken preparation developed from the combination comprises granule, tablet, capsule, dispersible tablet, chewable tablet, effervescent tablet, orally disintegrating tablet, buccal tablet, dry suspension and certain solid formulation.
Owner:FUKANGREN BIO PHARMA
Rationed fenmameimin drops
InactiveCN1436530AReasonable passive medicationEasy to acceptAntiinfectivesHeterocyclic compound active ingredientsPEG 400Dextromethorphan Hydrobromide
The present invention is one kind of medicine named Fenmameimin, and the medicine is prepared with four kinds of medicine material including acetaminophenol, pseudoephedrine hydrochloride, dextromethorphan hydrobromide and Chlorpheniramine; four kinds of solvent including polyglycol 400, glycerin, propylene glycol and water; as well as corrective, preservative, coloring agent, etc. During the preparation, rationing pump is used for rationing pump out mechanically. The medicine Fenmameimin is one kind of liquid preparation and has the advantages of fast absorption, accurate administration, convenient administration especially for infant and good taste.
Owner:黄振华
Dispersible tablet for treating cold and its preparing process
InactiveCN1850083AOrganic active ingredientsAntiinfectivesCross-linkLow-substituted hydroxypropylcellulose
The present invention relates to a dispersion tablet for curing common cold and its preparation process. Said invention is formed from main medicine including paracetamol, pseudoephedrine hydrochloride, dextromethorphan and chlorpheniramine maleate and auxiliary medicine including avicel, low-substituted hydroxypropyl cellulose, povidone K30, cross-linked povidone, aspartame, aspartame, micropowder silica gel and magnesium stearate.
Owner:江西聚仁堂药业有限公司
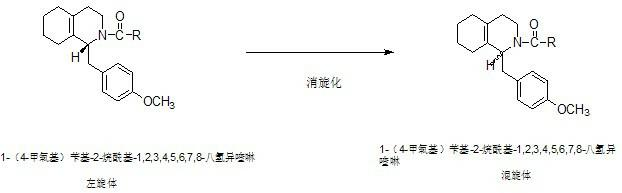
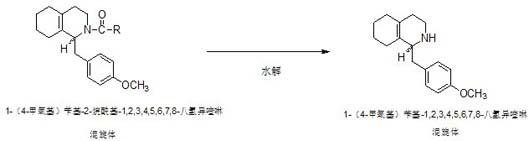
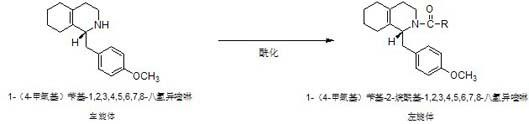
Preparation process for key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as cough relieving medicine
ActiveCN102219737ALow costSimple and safe operationOrganic chemistryIsoquinolineDextromethorphan Hydrobromide
The invention discloses a preparation process for a key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as a cough relieving medicine, which comprises the following steps of: performing acylation reaction to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (laevo isomer); and racemizing under the alkaline condition to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer); and hydrolyzing under the alkaline condition to obtain the 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer). The process aims to overcome the defect of the synthetic process and reduce cost, so that the process is simply and safely operated, the industrial production is qualified, and the yield of products is between 55 and 69 percent.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Soft capsule composition containing acetaminophen
A composite soft capsule contains proportionally the active component chosen from paracetranol and one or more of pseudoephedrine hydrochloride, chlorpheniramine maleate, dextromethorphen hydrobromate, bagodryl hydrochloride and coffin, polyethanediol, sodium (or potassium) acetate, and polyvidone.
Owner:ZHEJIANG WANLIAN PHARMA IND +1
Method for analyzing daytime severe cold and flu capsules by high performance liquid chromatography
The invention discloses a method for analyzing daytime severe cold and flu capsules by high performance liquid chromatography. The daytime severe cold and flu capsules contain acetaminophen, phenylephrine hydrochloride, dextromethorphan hydrobromide and guaifenesin; high performance liquid chromatography analysis conditions are as follows: a chromatographic column adopts an octadecyl silane bonded silica gel column, a sodium octanesulfonate containing 0.1v% triethylamine is used as a mobile phase A, and acetonitrile is used as a mobile phase B. The method can efficiently and simultaneously detect four effective components in the daytime severe cold and flu capsules; furthermore, the method is easy to operate, high in analysis speed, high in repetitiveness and extremely high in specificity; the product quality of a daytime severe cold and flu capsule preparation can be relatively efficiently and comprehensively controlled.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Preparation of hydrobromic acid dextro methaphen and beta cyclodeatrin compounding object
InactiveCN1589789AQuick cough reliefQuick resultsOrganic active ingredientsPill deliveryDextromethorphan HydrobromideBeta-Cyclodextrins
A medicine in the form of chewing tablet or buccal lozenge is the match of dextromethorphan hydrobromide and beta-cyclodextrin. It is a pectoral absorbing rate up to 98%.
Owner:HARBIN KEDI PHARMA
Preparation method for daytime-taken softgel for treating cold
ActiveCN103462927ANice appearanceStrong complianceOrganic active ingredientsAntipyreticMelting tankAlcohol
The invention discloses a preparation method for a daytime-taken softgel for treating a cold. The preparation method comprises the following steps: A, preparation of a gelatin liquor: dissolving plasticizer in water, adding into a gelatin melting tank, adding gelatin, stirring to dissolve, adding coloring agent into water, stirring uniformly to obtain a pigment solution, adding the pigment solution into an obtained gelatin solution, stirring and vacuumizing to exhaust air bubbles, so as to obtain the gelatin liquor; B, preparation of contents: adding mixed polyethylene glycol and polyhydric alcohol into polyvidone, stirring to dissolve, adding acetaminophen, stirring to obtain a suspension, adding into a proportioning tank, vacuumizing, filling with inert gas, heating to dissolve, cooling, adding dextromethorphan hydrobromide and phenylephrine hydrochloride, vacuumizing, and keeping the temperature to dissolve, so as to obtain a content solution; C preparation of the softgel: adding the gelatin liquor and the content solution into a pelleting machine respectively, pelleting, drying, pellet plastering and packaging. The invention aims to provide the preparation method for the daytime-taken softgel which is used for treating the cold, and is simple in process, convenient to manufacture and good in product stability.
Owner:安士制药(中山)有限公司
A kind of compound dextromethorphan hydrobromide syrup and preparation method thereof
ActiveCN102600210BBacteria material medical ingredientsEther/acetal active ingredientsFlavouring agentDextromethorphan Hydrobromide
The invention provides a compound dextromethorphan hydrobromide syrup and a preparation method for the same. The compound dextromethorphan hydrobromide syrup is used for eliminating phlegm in cough, and comprises dextromethorphan hydrobromide, guaifenesin and a bacillus natto extract. The syrup improves the safety of medication and is especially suitable for children, reduces bitter taste and the consumption of a flavouring agent, improves the stability, especially the colour of solution and the stability of the colour, and improves the curative effect on the cough caused by cold and bronchitis simultaneously.
Owner:湖北凤凰白云山药业有限公司 +1
Compound dextromethorphan hydrobromide syrup and preparation method for same
ActiveCN102600210ABacteria material medical ingredientsPharmaceutical delivery mechanismFlavouring agentDextromethorphan Hydrobromide
The invention provides a compound dextromethorphan hydrobromide syrup and a preparation method for the same. The compound dextromethorphan hydrobromide syrup is used for eliminating phlegm in cough, and comprises dextromethorphan hydrobromide, guaifenesin and a bacillus natto extract. The syrup improves the safety of medication and is especially suitable for children, reduces bitter taste and the consumption of a flavouring agent, improves the stability, especially the colour of solution and the stability of the colour, and improves the curative effect on the cough caused by cold and bronchitis simultaneously.
Owner:湖北凤凰白云山药业有限公司 +1
Pharmaceutical composition for treating cold in children and preparation method thereof
InactiveCN102038704AEasy to understandOrganic active ingredientsInorganic active ingredientsSolubilityAllergic reaction
The invention provides a pharmaceutical composition for treating cold in children and a preparation method thereof. The pharmaceutical composition comprises the following components and medicine prepared in ratio: dextromethorphan hydrobromide, chlorphenamine maleate, ammonium chloride, seasoning agent, antiseptic and water, wherein the dextromethorphan hydrobromide is coated by beta-cyclodextrin and is prepared into preparation; the clathrate compound improves the mouthfeel and the water solubility of the dextromethorphan, and realizes delayed release effect so that, after being administrated, the dextromethorphan continuously develops functions of relieving cough in 2-6h, and the ammonium chloride is rapidly released for dispelling phlegm within 1h, thereby effectively solving the problem of antagonism between the dextromethorphan and the ammonium chloride. The pharmaceutical composition can be prepared into syrup, oral solution and drop which are easily accepted by the children. The pharmaceutical composition is simple in preparation technique, easy in production and operation, good in producing stability, reliable in product quality, sweet in taste, good in compliance, and acceptable by children; the dose is accurately measured according to age of the children; and the pharmaceutical composition can safely and rapidly eliminate or relieve cold symptoms of the children, such as cough, excessive and thick phlegm, pharyngalgia, rhinobyon, rhinorrhoea, sternutation and the like caused by infection of upper respiratory tract and allergic reaction.
Owner:BEIJING YIYUE MEDICAL TECH
Taste masked suspension prescription for treating infant cold and method for preparing same
ActiveCN1969850APalatable tasteRapid drug releaseOrganic active ingredientsAntipyreticDextromethorphan HydrobromideGlycerol
The invention discloses a taste-masking typed children-influenza dry-mixing suspension and making method, which is characterized by the following: adopting acetamidophenol, ephedrine hydrochloride, hydrobromic acid dextromethorphan and auxiliary drug material as raw material; cladding drug through glyceride compound to mask taste.
Owner:浙江康德药业集团股份有限公司
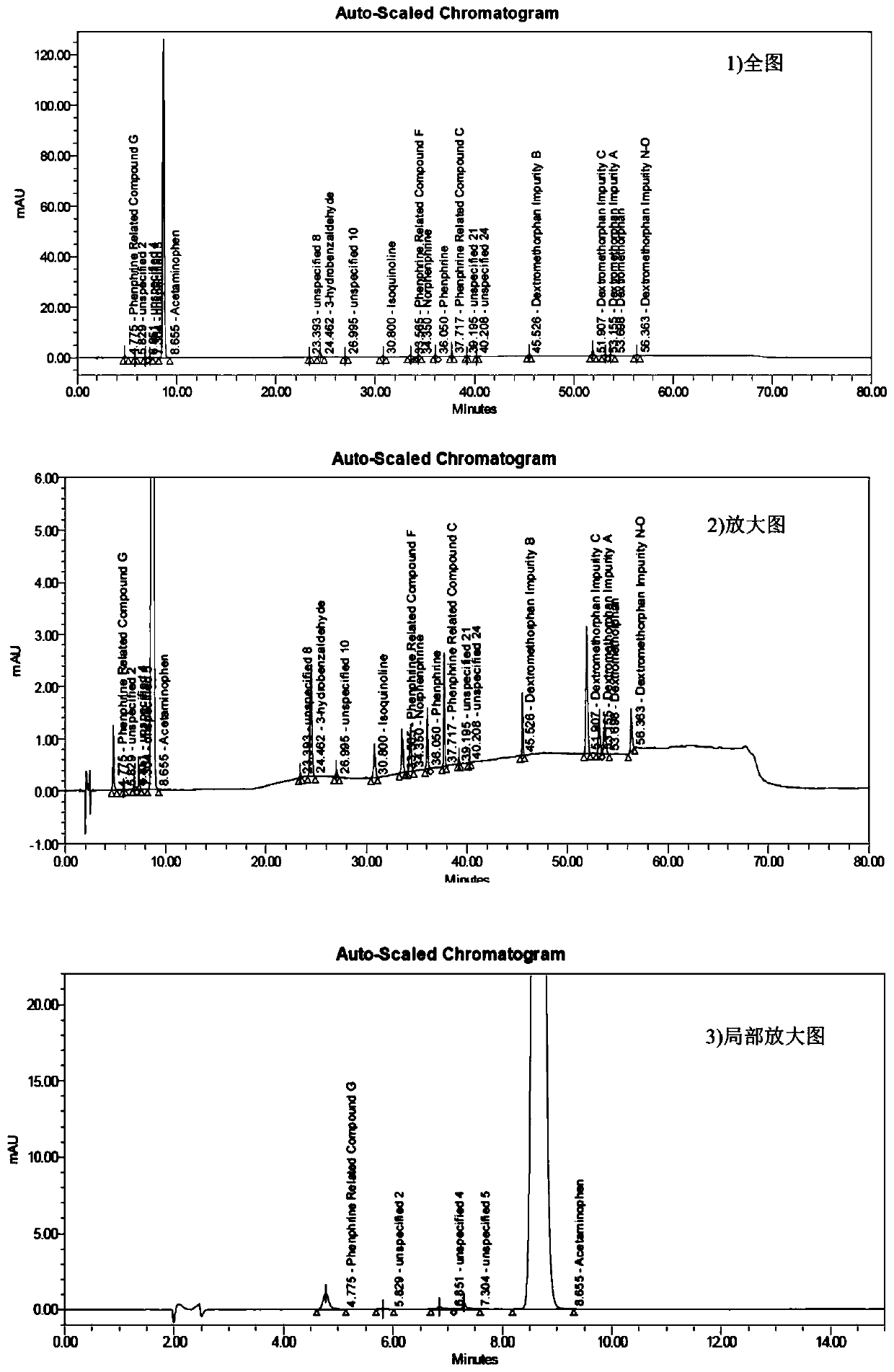
Method for determining related substances of pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride
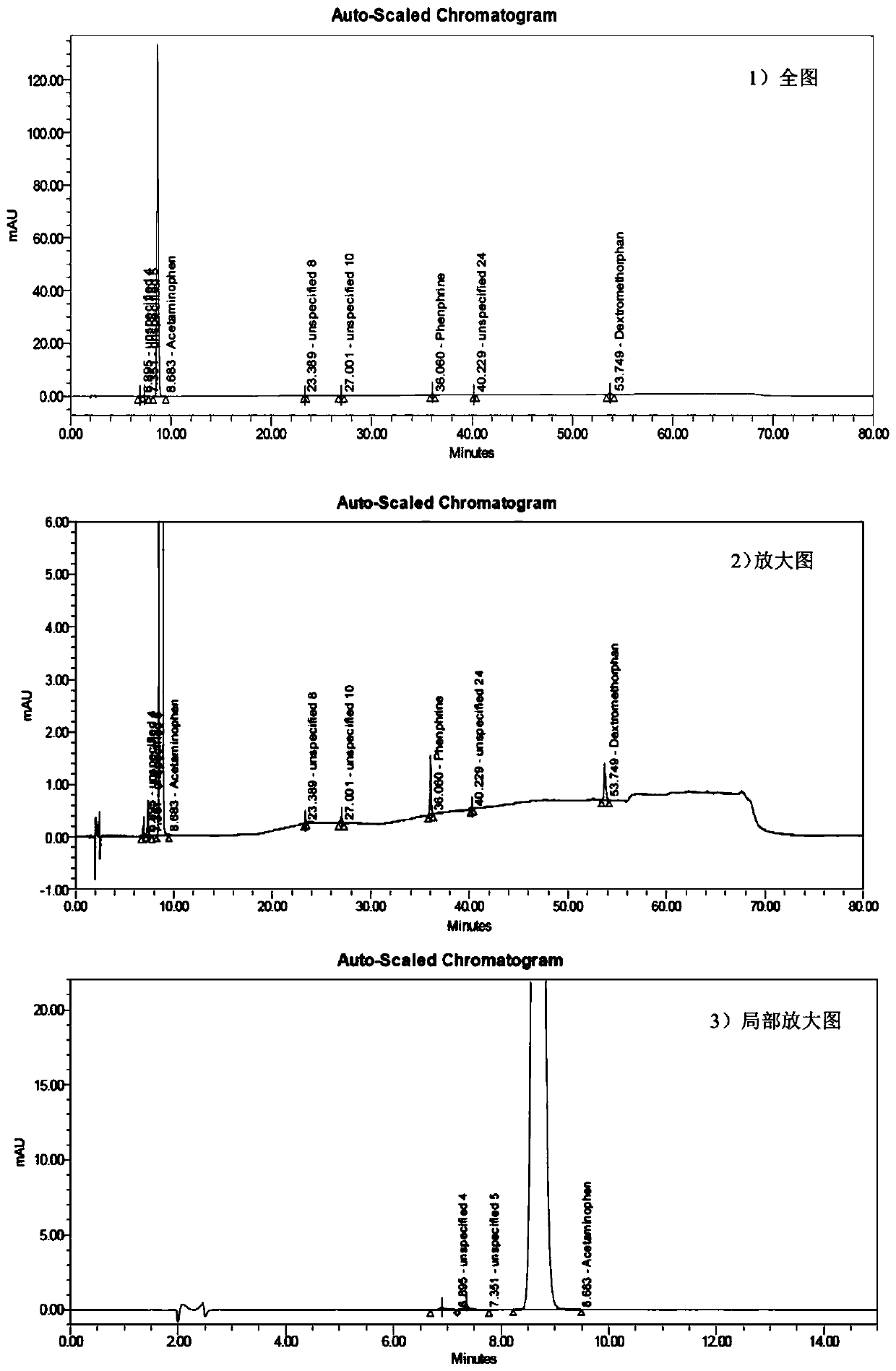
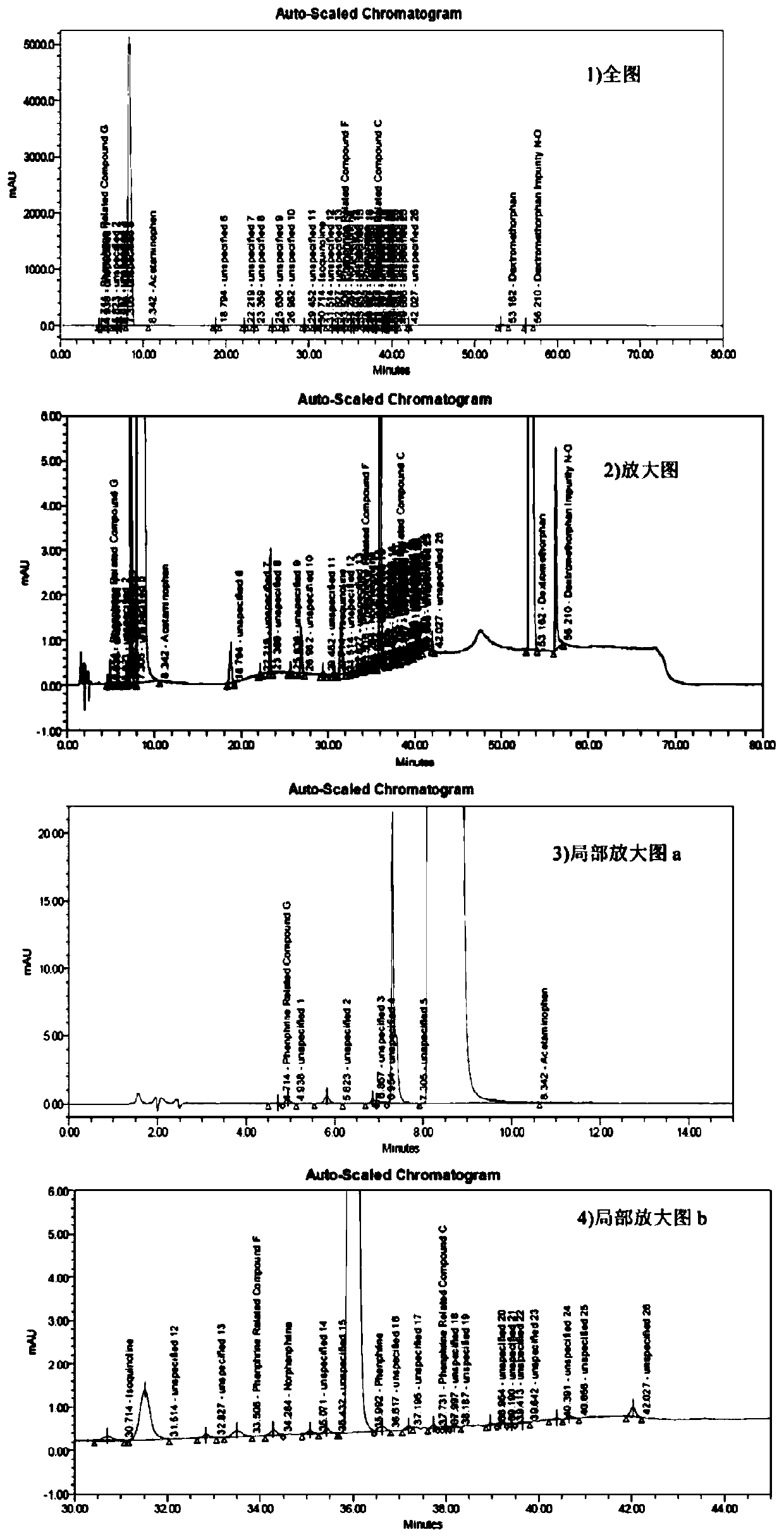
The invention discloses a method for determining related substances of a pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride. The determination method disclosed by the invention is performed by adopting a high performance liquid chromatography; proper HPLC chromatographic conditions are screened out; and 10 related substances of dextromethorphan impurities I, dextromethorphan impurities II, dextromethorphan impurities III, dextromethorphan impurities IV, norepinephrine, a phenylephrine related substance F, a 4,6 diol isoquinoline analogue, a phenylephrine related substance C, 3-hydroxybenzaldehyde and a phenylephrine related substance G in the pharmaceutical preparation of acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride can be simultaneously determined under the same condition. Effective separation of a variety of impurities can be achieved, and detection time and detection cost are greatly saved. The method can be used for quality research and quality control of pharmaceutical preparation products containing the acetaminophen, the dextromethorphan hydrobromide and the phenylephrine hydrochloride.
Owner:安士制药(中山)有限公司
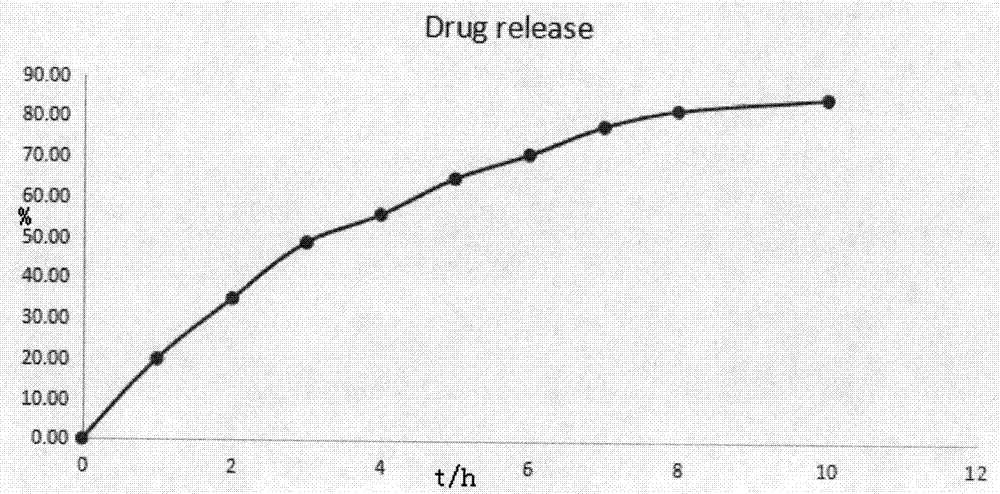
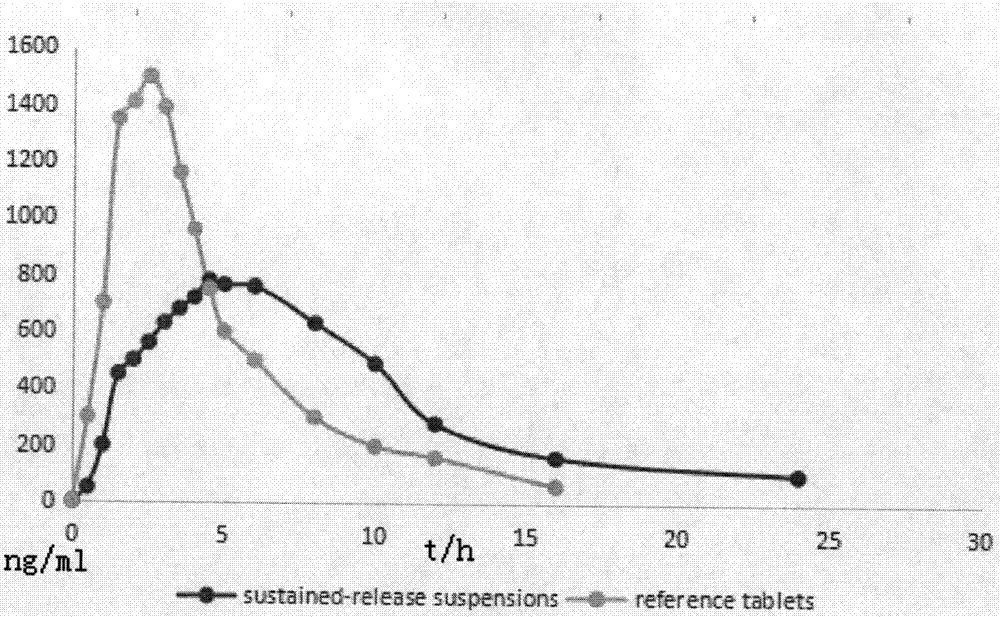
Dextromethorphan hydrobromide sustained-release suspension and preparation method thereof
InactiveCN109771372AGood sustained release effectLarge distribution areaOrganic active ingredientsSolution deliveryVegetable oilTherapeutic drug action
The invention discloses a dextromethorphan hydrobromide sustained-release suspension. The suspension is prepared from, by mass, 85-95% of a suspension matrix, 0.5-1.5% of coated resin, 0.1-0.5% of drug-loaded resin, 0.01-0.08% of EDTA, 0.1-0.3% of a surface active agent and the balance water, wherein the drug-loaded resin is prepared from a dextromethorphan hydrobromide crude drug, sodium polystyrenesulfonate type cation exchange resin and purified water; the coated resin is prepared from impregnating resin, dichloromethane, vegetable oil, ethyl cellulose and acetone, and the impregnating resin is prepared from polyethylene glycol, purified water and the prepared drug-loaded resin. The invention further discloses a preparation method of the dextromethorphan hydrobromide sustained-release suspension. The sustained-release effect is good, the drug treating effect is lasting, the treatment effect is good, the number of drug applying times can be obviously reduced, the problems that a solid sustained-release preparation is poor in taste, difficult to swallow and inaccurate in fractional dose are solved, and the dextromethorphan hydrobromide sustained-release suspension is convenient for children patients to take.
Owner:JIANGSU SIHUAN BIOENGINEERING PHARM CO LTD
Dextromethorphan hydrobromide slow-release dry suspension and preparation method thereof
The invention discloses a slow-release dry suspension of dextromethorphan hydrobromide which is slowly released in the gastrointestinal tract environment. The formulation contains dextromethorphan hydrobromide and a pharmaceutically acceptable polymer. According to weight percentage, the preparation contains 10-90% of dextromethorphan hydrobromide and 10-90% of auxiliary materials. The supplementary materials for sustained release are one or more of cation exchange resin, methyl cellulose, ethyl cellulose, acrylic resin and hydroxypropyl methyl cellulose. Compared with the immediate-release preparation, the controlled-release preparation of the present invention can maintain the effective blood drug concentration within 24 hours, improve the curative effect, have less toxic and side effects, be convenient to take and carry, and reduce the number of times of taking. The sustained-release preparation of the invention can maintain a more stable blood drug concentration within 24 hours, improve curative effect, and have less toxic and side effects. This preparation only needs to be administered once a day. The controlled-release preparation of the present invention will be clinically used as an antitussive.
Owner:刘宏飞
Spongy dextromethorphan hydrobromide film agent with micro-pore and preparation method thereof
ActiveCN102641258ADissolve fastGuaranteed physical strengthOrganic active ingredientsPharmaceutical non-active ingredientsCellulosePolyethylene oxide
The invention discloses a spongy dextromethorphan hydrobromide film agent with micro-pore and a preparation method of the film agent; the spongy dextromethorphan hydrobromide film agent with micro-pore comprises the following components: a dextromethorphan hydrobromide, a water-soluble polymer material, and a water-insoluble micro-powder dispersed in the water-soluble polymer material; the water-soluble polymer material is any two of HPMC (hydroxy propyl methyl cellulose), PVA (polyvinyl alcohol), HPC (hydroxy propyl cellulose), CMC-NA (sodium carboxyl methyl cellulose), PVP (polyvinyl pyrrolidone), sodium alginate or PEO (polyethylene oxide), and wherein one is a lower molecular weight water-soluble polymer material with molecular weight of 10, 000-200, 000 Dalton, the other one is a higher molecular weight water-soluble polymer material with molecular weight of 200, 000-10, 000, 000 Dalton. According to the invention, effects are very significant; films are dissolved fast through the lower molecular weight water-soluble polymer material, physical strength and toughness of films are ensured through the higher molecular weight water-soluble polymer material so as to realize the purpose of quick release and ensure strength of films.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
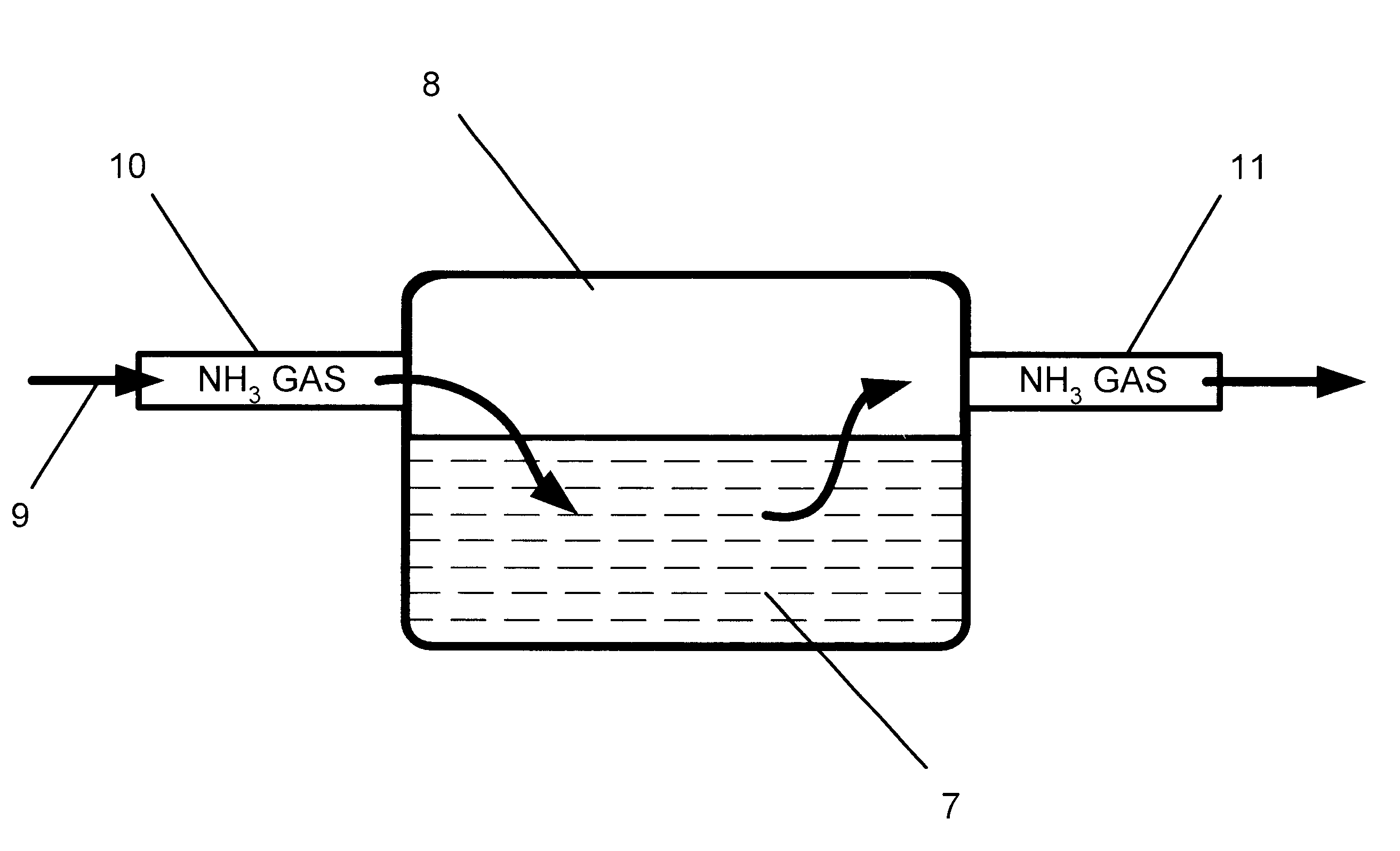
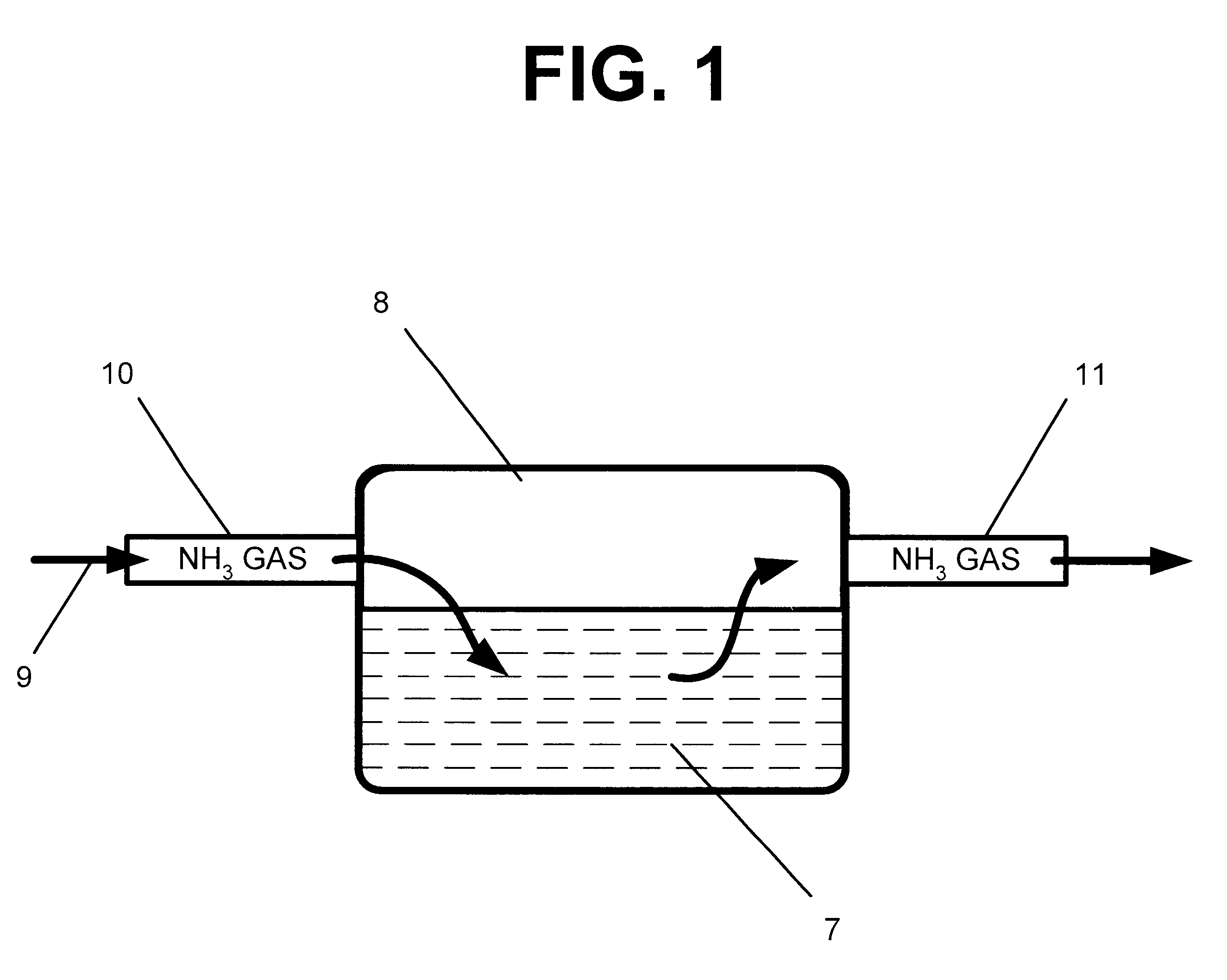
Method of treating commercial grade products to remove undesirable odors and flavors
A method of treating antimicrobial products, dairy products, pharmaceutical products and other products having offensive tastes or odors to remove the off-odors and off-tastes from the products. The method involves exposing a selected commercial grade product that contains a small amount of free acid impurities to an ammonia gas. The ammonia gas reacts with the free acid impurities to convert the free acids into ammonium salts, thereby reducing or eliminating the off-flavor and off-odor of the product. The products to be treated include antimicrobial products selected from the group consisting of sodium benzoate, calcium benzoate, potassium benzoate, sodium diacetate, paraben, niacin, calcium acetate, calcium diacetate, sodium sorbate, calcium sorbate, potassium sorbate, sodium propionate, calcium propionate, potassium propionate and mixtures thereof; dairy products selected from the group consisting of casein, whey, skim milk powder, and calostrum; pharmaceutical products selected from the group consisting of acetaminiphen, aspirin, ibuprophen, dextromethorphan hydrobromide, guaejenesin, paracetamol, and sodium erythorbate; and various other products selected from the group consisting of butylate hydroxy tolulene, polydextrose powder, sodium acid sulfate, and sodium diacetate. The common characteristic of the commercial grades of each of these products is that they contain a small amount of free acid impurities that react favorably with ammonia gas.
Owner:TILLIN
Orally disintegrating tablet containing paracetamol, pseudoephedrine hydrochloride and dextromethorphan hydrobromide and preparation method thereof
InactiveCN101756918AImprove liquidityOrganic active ingredientsAntiviralsFluidized bedDextromethorphan Hydrobromide
The invention relates to a compound orally disintegrating tablet containing paracetamol, pseudoephedrine hydrochloride and dextromethorphan hydrobromide and a preparation method thereof. The preparation method is characterized by comprising the following steps of: uniformly mixing and dissolving the three kinds of active substances, uniformly mixing the three kinds of active components by adopting a spray drying technology of a fluidized bed to process so that mixed powder is obtained, and then pelletizing, coating and tabletting so that the orally disintegrating tablet is obtained. The orally disintegrating tablet has the advantages of simple process and uniform quality.
Owner:CHONGQING PHARMA RES INST
Slow-release type solid corrosion inhibitor and preparation method thereof
ActiveCN105295880AIn line with the development trendNo environmental problemsDrilling compositionBorehole/well accessoriesDiphenhydramine hydrochlorideStearic acid
The invention discloses a slow-release type solid corrosion inhibitor and a preparation method thereof. The solid corrosion inhibitor is prepared from the following materials: 60 percent to 70 percent of a main material and 30 percent to 40 percent of an auxiliary material; the main material is formed by compounding three components of amantadine hydrochloride, diphenhydramine hydrochloride and dextromethorphan hydrobromide according to a molar mass ratio of 2 to 2 to 1; the auxiliary material is formed by compounding carnauba wax, pregelatinized starches, polyvinylpyrrolidone, microcrystalline cellulose, carboxymethyl starch sodium and stearic acid according to a mass ratio of 3 to 3 to 2 to 2 to 2 to 1. The preparation method comprises the following steps: after mixing the auxiliary material evenly according to a formula ratio, stirring and heating to 80 DEG C to 90 DEG C, melting the auxiliary material, slowly adding the main material, which is mixed evenly according to a formula ratio, into the molten auxiliary material, continuously stirring and heating, after sufficiently mixing the main material and the auxiliary material evenly, putting into a mould for compression molding, and cooling to obtain a finished product of the solid corrosion inhibitor. The corrosion inhibitor is low in usage amount, long in release period, good in corrosion inhibiting effect, simple in preparation and putting process, and suitable for anticorrosion for an oil well and an underground tube and rod.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
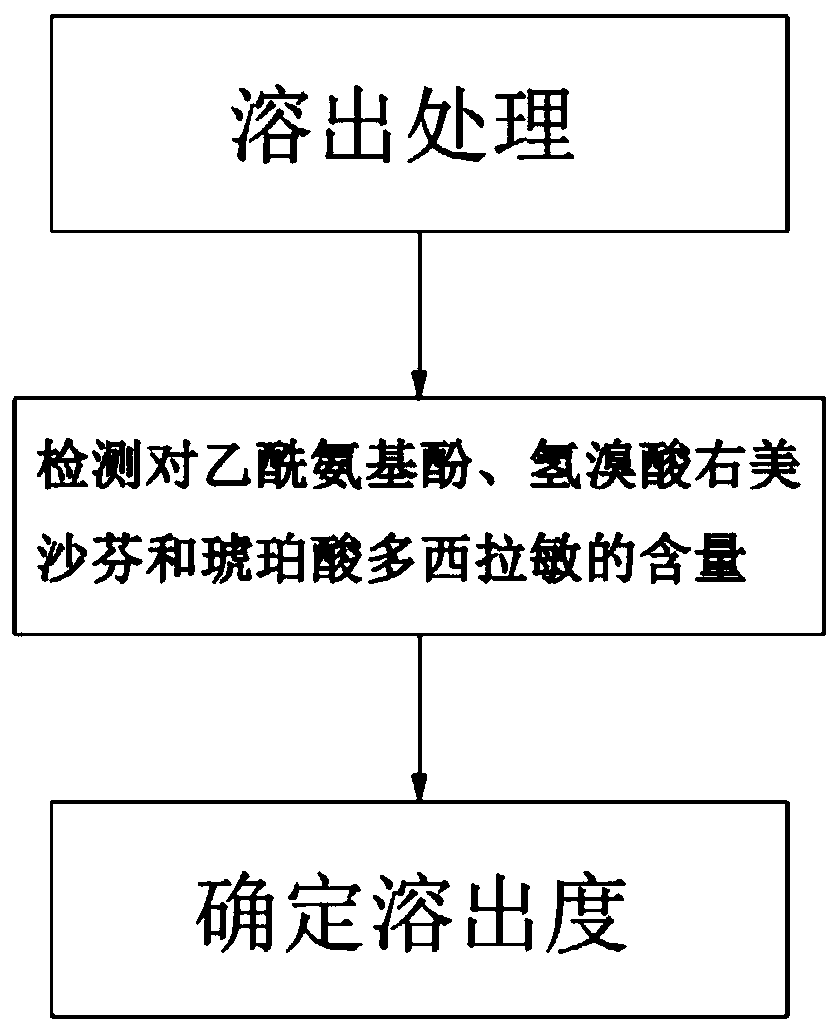
Method for determining dissolution rate of medicinal preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate
ActiveCN110286162ASave time and costSave testing costComponent separationDextromethorphan HydrobromideQuality control
The invention discloses a method for determining a dissolution rate of a medicinal preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate. The method comprises the following steps of: (1) adding medicinal preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate into a dissolution medium for dissolution treatment, performing sampling and filtering to obtain dissolution liquid; (2) detecting the contents of acetaminophen, dextromethorphan hydrobromide and doxylamine succinate in the dissolution liquid; and (3) and determining the dissolution rate of the medicinal preparation according to the detection result of the step (2). The method can effectively simulate the dissolution behavior in vivo in vitro, can determine the dissolution rate of the soft capsule containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate, and provides technical support for the research and development and quality control of medicaments. The method can simultaneously determine the dissolution results of the three substances under the same condition, greatly saves the detection time and the detection cost, and has good accuracy and reproducibility.
Owner:安士制药(中山)有限公司
Medicine composition for treating cold symptoms such as running noses and nasal obstruction and preparation method and application of medicine composition
ActiveCN110279695AStable blood concentrationGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismIrritationNose
The invention discloses a medicine composition for treating cold symptoms such as running noses and nasal obstruction and a preparation method and application of the medicine composition. The medicine composition is an oral liquid preparation consisting of brompheniramine maleate, dextromethorphan hydrobromide, phenylephrine hydrochloride as well as certain amounts of auxiliary materials such as a cosolvent, a preservative, a complexing agent, a pH value adjusting agent, a flavoring agent and an aromatic. The medicine composition has functions of alleviating and treating nasal obstruction caused by cold, cough, sore throats, headache, slight ache and pain and fever caused by slight irritation of throats and bronchi, and the like. The invention provides a cold medicine for children of 6-12 years old and adults and children greater than 12 years old.
Owner:北京博智绿洲医药科技有限公司
Dextromethorphan hydrobromide drop pills and preparation process thereof
InactiveCN1449755AEasy to prepareLow costOrganic active ingredientsPill deliveryDextromethorphan HydrobromideMedicine
The present invention discloses a dextromethorphan hydrobromide dripping pills. It is made up by heating dextromethorphan hydrobromide, polyethylene glycol, glycerine and Tween-80, comelting them and retaining comelting state, drop-adding them into methyl silinon oil condensate and forming dripping pills. It is simple in preparation process and low in cost, at the same time the dissolution of said dripping pills can be reached to 90% of labelled amount within 15 min., and is higher than that of tablet. Said dripping pills can be dissolved inwater, can be substituted for solution preparation, and its stability is far better than that of solution preparation.
Owner:山东禹王制药有限公司
Preparation method and quality test method of pseudoephedrine hydrochloride and dextromethorphan hydrobromide chewable tablets
ActiveCN101810617AControllable bitternessGood content uniformityOrganic active ingredientsComponent separationDextromethorphan HydrobromidePseudoephedrine Hydrochloride
The invention discloses a preparation method and a quality test method of pseudoephedrine hydrochloride and dextromethorphan hydrobromide chewable tablets. The preparation method is characterized in that: medicaments are dispersed in sodium alga acid uniformly first; then auxiliary materials are added, and the mixture is granulated; and finally, the grains are coated with a coating material to isolate the bitterness of medicaments. The quality test method comprises thin layer chromatography and a content measurement method, has high specificity and ensures the preparation process of the invention has high controllability.
Owner:JIANGXI HERBI SKY CO LTD
Daily compound preparation for treating cold and preparation method thereof
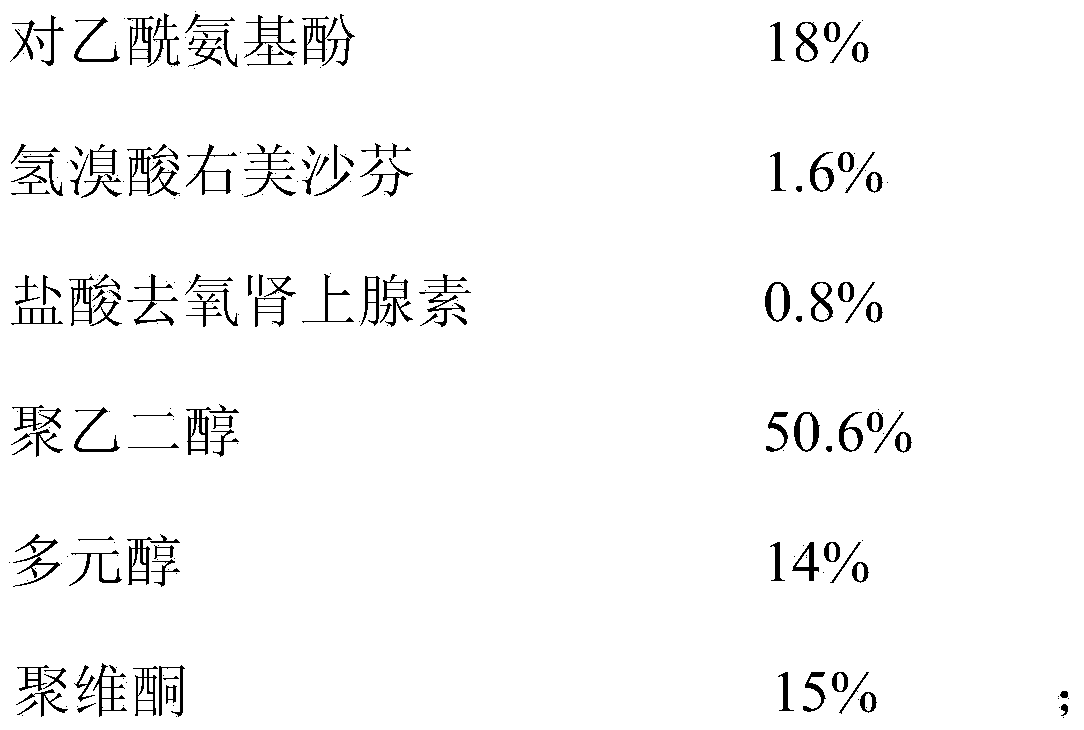
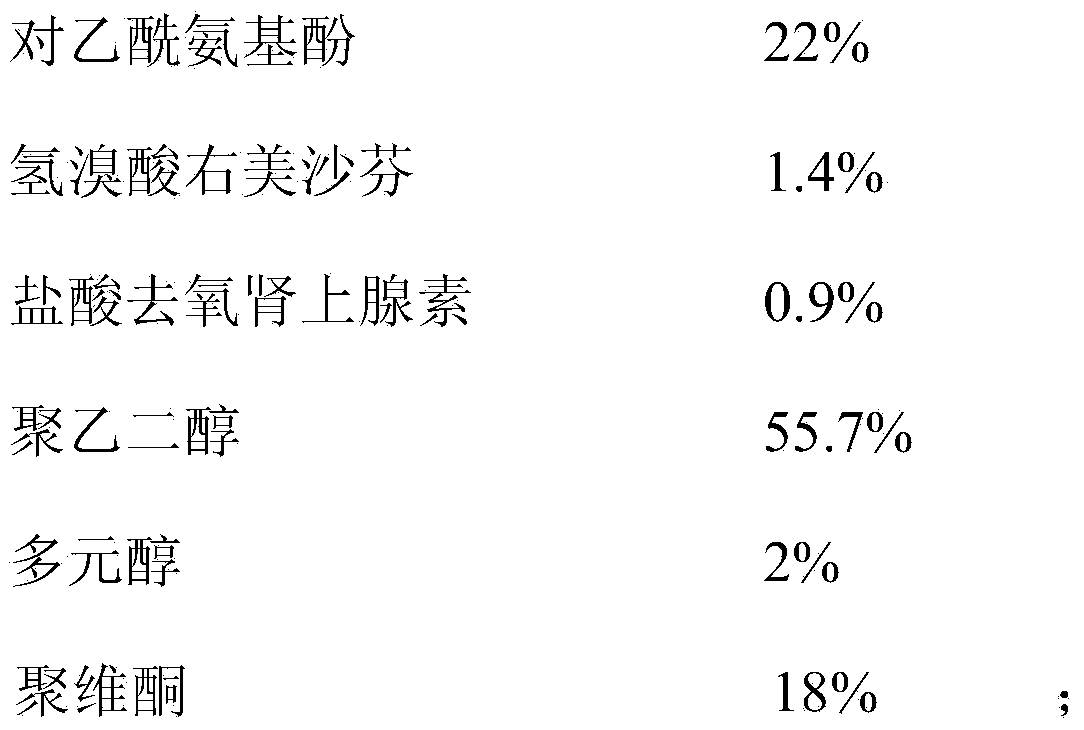
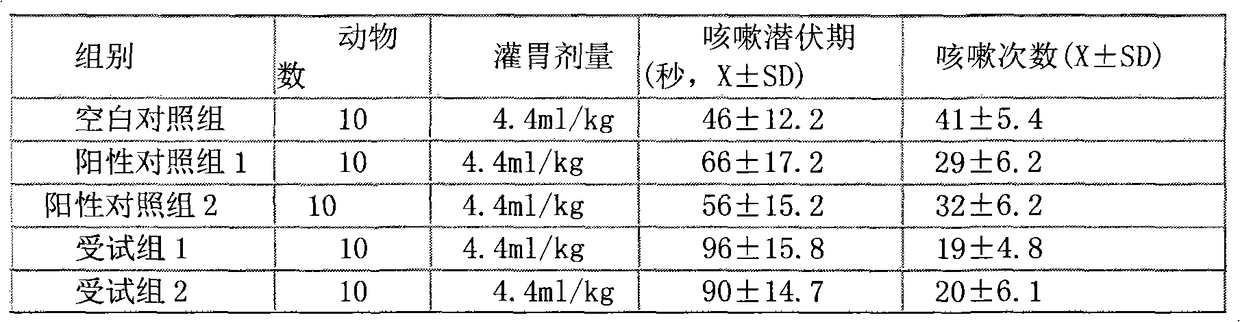
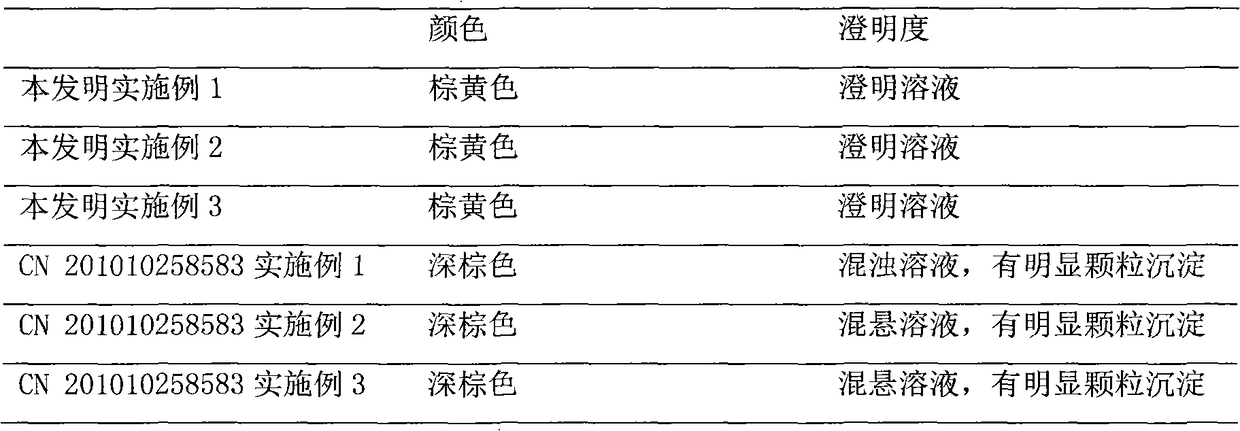
ActiveCN112716956APromote absorptionImprove efficacyOrganic active ingredientsAntiviralsPolyethylene glycolGlycerol
The invention discloses a daily compound preparation for treating cold, and particularly belongs to the technical field of pharmaceutics. The daily compound preparation is in a capsule form and comprises a capsule shell and a content in the capsule shell; the content comprises the following chemical raw materials: acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride, a solubilizer, purified water and one or a mixture of two or more of polyethylene glycol, propylene glycol or glycerol. The content is a clarified liquid composition, and the clarified liquid composition is good in stability, high in bioavailability, rapid in effect and capable of well treating clinical cold.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Dextromethorphan hydrobromide chewable tablet and preparation method thereof
The invention belongs to the technical field of medicinal preparations, and discloses dextromethorphan chewing gum tablets and an industrially applicable preparation method thereof. The preparation is delivered through a chewing process, so that the defects of the marketed products at present can be overcome, and the tablets have the advantages of no need of water for taking, convenience in taking, good mouthfeel, less adverse effects, quick response, capability of eliminating thirst and the like. The dextromethorphan chewing gum tablets prepared by the invention are mainly used for dry coughand are suitable for cold, acute or chronic bronchitis, bronchial asthma, faucitis, phthisis and cough during other upper respiratory tract infections. The dextromethorphan chewing gum tablets provided by the invention have the advantages of simple preparation process, low cost, easiness in control and easiness for industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com