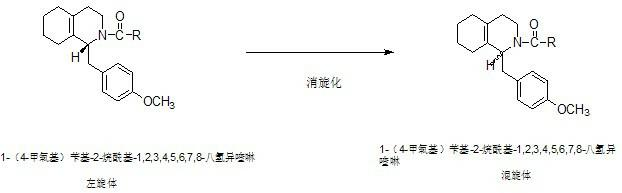
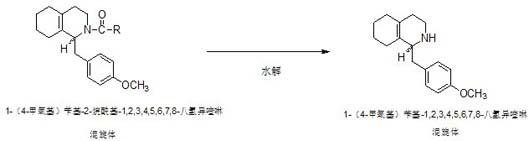
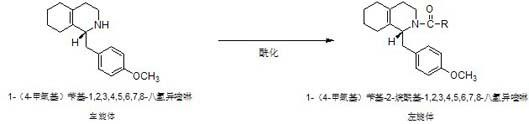
Preparation process for key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as cough relieving medicine
A technology of octahydroisoquinoline and medicinal hydrobromic acid, applied in the direction of organic chemistry and the like, can solve the problems of high cost and low utilization rate, and achieve the effect of reducing cost, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (L-isomer):
[0023] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 150g of 1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (L-isomer), 1.1 times molar amount of acetic anhydride, nitrogen protection, heating up to 80°C, heat preservation reaction for 3 hours, after heat preservation reaction is completed, vacuum distillation, after distillation is complete, add 2 mole amount of absolute ethanol Recrystallized, centrifuged, and dried to obtain light yellow solid 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline ( L-isomer) 142.8 g, the yield was 81.8%.
[0024] Step 2: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (heteromorph):
[0025] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 142.8g of 1-(4-methoxy)be...
Embodiment 2
[0029] Step 1: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (L-isomer).
[0030] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 150g of 1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (L-isomer), 1.1 times molar amount of acetic acid, nitrogen protection, heating to 80 ° C, heat preservation reaction for 4 hours, heat preservation reaction is completed, vacuum distillation, after distillation, add 2 mole weight of absolute ethanol Crystallized, centrifuged, and dried to obtain light yellow solid 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (Levorotatory body) 143.4 g, the yield was 82.1%.
[0031] Step 2: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (heteromorph):
[0032] In a 500 ml four-necked flask with a thermometer and a stirring device, start stirring, and add 143.4 g of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5...
Embodiment 3
[0036] Step 1: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (L-isomer):
[0037] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 150g of 1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (L-isomer), 1.1 times the molar amount of formic acid, pass through nitrogen protection, heat up to 80 ° C, heat preservation reaction for 3 hours, heat preservation reaction is completed, vacuum distillation, after distillation is completed, add 2 moles of anhydrous methanol Crystallized, centrifuged, and dried to give light yellow solid 1-(4-methoxy)benzyl-2-formyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (Levorotatory body) 134.4 g, the yield was 80.8%.
[0038]Step 2: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (heteromorph):
[0039] In a 500 ml four-necked flask with a thermometer and a stirring device, start stirring, and add 134.4 g of 1-(4-methoxy)be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com