Medicine composition for treating cold symptoms such as running noses and nasal obstruction and preparation method and application of medicine composition
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, drug delivery, etc., can solve problems such as toxic side effects, adverse health, and damage to children's health, and achieve stable blood drug concentration, good therapeutic effect, and low toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
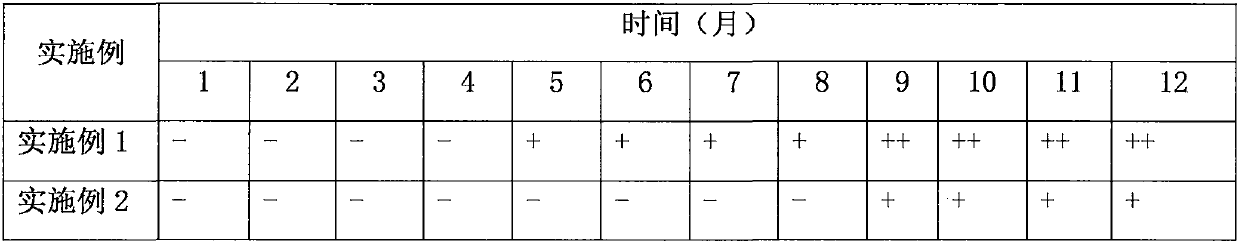
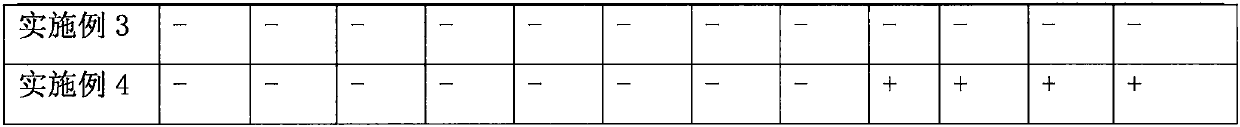
Examples
Embodiment 1
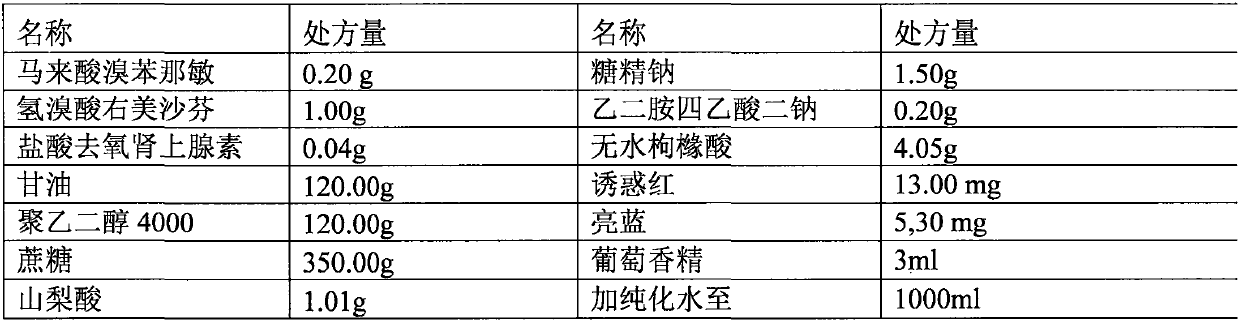
[0051] Embodiment 1 (taking preparation 1000ml as example)
[0052] prescription:
[0053]
[0054] Preparation:
[0055] 1. Weigh the prescribed amount of glycerin and polyethylene glycol 4000, put them in a certain container, stir and mix to obtain solution a;
[0056] 2. Take the above solution a, add the prescribed amount of sorbic acid, stir, and disperse to obtain solution b;
[0057] 3. Sequentially weigh disodium ethylenediaminetetraacetic acid, anhydrous citric acid, sodium saccharin, allura red, brilliant blue, and grape essence in the prescribed amount, and add them into solution b to obtain solution c;
[0058] 4. Measure a small amount of purified water, add it to solution c, stir, fully dissolve to obtain a transparent and uniform solution, and obtain solution d;
[0059] 5. Finally, add the prescription amount of brompheniramine maleate, phenylephrine hydrochloride, and dextromethorphan hydrobromide to the solution d in turn, stir, add water to 1000ml, stir ...
Embodiment 2
[0060] Embodiment 2 (taking preparation 1000ml as example)
[0061] prescription:
[0062] name Prescription amount name Prescription amount brompheniramine maleate 0.30g sodium saccharin 1.50g Dextromethorphan Hydrobromide 1.00g Hexametaphosphate 0.25g Phenylephrine Hydrochloride 0.50g Anhydrous citric acid 4.00g Propylene Glycol 120.00g allura red 12.00mg polyethylene glycol 4000 120.00g Bright blue 4,10mg Sorbitol 230.00g grape essence 3ml Ethylparaben 0.75g Add purified water to 1000ml
[0063] Preparation:
[0064] 1. Weigh the prescribed amount of propylene glycol and polyethylene glycol 4000, put them in a certain container, stir and mix to obtain solution a;
[0065] 2. Take the above solution 1, add the prescribed amount of sorbitol, stir, and disperse to obtain solution b;
[0066] 3. Add a small amount of prescribed amount of water to solution b, heat in a water bath at 60°C for 15 ...
Embodiment 3
[0069] Embodiment 3 (taking preparation 1000ml as example) (preferred)
[0070] name Prescription amount name Prescription amount brompheniramine maleate 0.20g sodium benzoate 1.04g Dextromethorphan Hydrobromide 1.00g sodium saccharin 1.80g Phenylephrine Hydrochloride 0.50g Edetate disodium 0.35g glycerin 123.0g Anhydrous citric acid 5.72g Propylene Glycol 120.0g allura red 14.0mg Sorbitol 377.0g Bright blue 5.30mg grape essence 4.0ml Add purified water to 1000ml
[0071] Preparation:
[0072] 1. Weigh the glycerin and propylene glycol of the above prescription amount, put them in a certain container, stir and mix well, and obtain solution a;
[0073] 2. Take the above solution a, add the prescribed amount of sorbitol, stir, and disperse to obtain solution b;
[0074] 3. Sequentially weigh the prescribed quantities of edetate disodium, anhydrous citric acid, sodium benzoate, sodium saccharin, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com