Ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere and preparation method thereof
A technology of polylactic acid glycolic acid and ginsenoside, which is applied in the field of medicine, can solve the problems of low blood drug concentration, limited clinical drug efficacy, and reduced drug utilization rate, and achieve the effects of reducing side effects, good therapeutic effect, and reducing the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
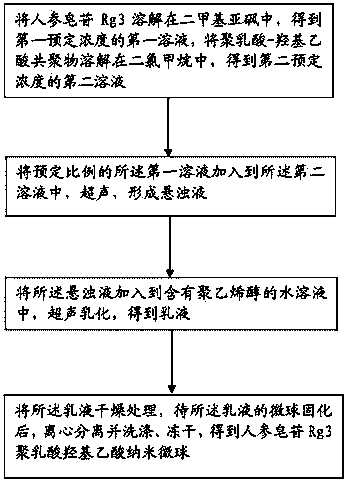
[0030] like figure 1 As shown, the preparation method of ginsenoside Rg3 polylactic-glycolic acid nano-microspheres according to an embodiment of the present invention comprises the following steps:
[0031] S1: Dissolve ginsenoside Rg3 in dimethyl sulfoxide (DMSO) to obtain the first solution of the first predetermined concentration, and dissolve polylactic acid-glycolic acid copolymer in dichloromethane (DCM) to obtain the second predetermined concentration. concentration of the second solution.
[0032] S2: adding a predetermined ratio of the first solution to the second solution, and ultrasonicating to form a suspension.
[0033] S3: Add the suspension into an aqueous solution containing polyvinyl alcohol (PVA), and perform ultrasonic emulsification to obtain an emulsion.
[0034] S4: drying the emulsion, and after the microspheres of the emulsion are solidified, centrifuge, wash, and freeze-dry to obtain ginsenoside Rg3 polylactic-glycolic acid nanospheres.
[0035] Ac...
Embodiment 1
[0044] Example 1: Firstly, ginsenoside Rg3 was dissolved in DMSO with a drug concentration of 40 mg / ml, and PLGA was dissolved in DCM with a concentration of 30 mg / ml. Precisely measure 1ml of PLGA solution, add 80μl of ginsenoside Rg3 solution to it, ultrasonic power 210w, supersonic for 5 seconds, pause for 5 seconds, and ultrasonic for 1 minute.
[0045] The suspension was quickly added to 10 ml of aqueous solution containing 1% PVA, and the aforementioned ultrasonic conditions were repeated, and the resulting emulsion was poured into 30 ml of aqueous solution containing 1% PVA, and after 3 hours of magnetic stirring at 1000 r, the microspheres were separated by high-speed freezing and centrifugation. Washed three times with deionized water, freeze-dried and stored at -20°C. Microsphere scanning electron microscope photo as figure 2 As shown in A.
Embodiment 2
[0046] Example 2: Firstly, ginsenoside Rg3 was dissolved in DMSO with a drug concentration of 40 mg / ml, and PLGA was dissolved in DCM with a concentration of 30 mg / ml. Precisely measure 1ml of PLGA solution, add 60μl of ginsenoside Rg3 solution to it, ultrasonic power 210w, supersonic for 5 seconds, stop for 5 seconds, and ultrasonic for 1 minute.
[0047] The suspension was quickly added to 10 ml of aqueous solution containing 1% PVA, and the aforementioned ultrasonic conditions were repeated, and the resulting emulsion was poured into 30 ml of aqueous solution containing 1% PVA, and after 3 hours of magnetic stirring at 1000 r, the microspheres were separated by high-speed freezing and centrifugation. Washed three times with deionized water, freeze-dried and stored at -20°C. Microsphere scanning electron microscope photo as figure 2 Shown in B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com