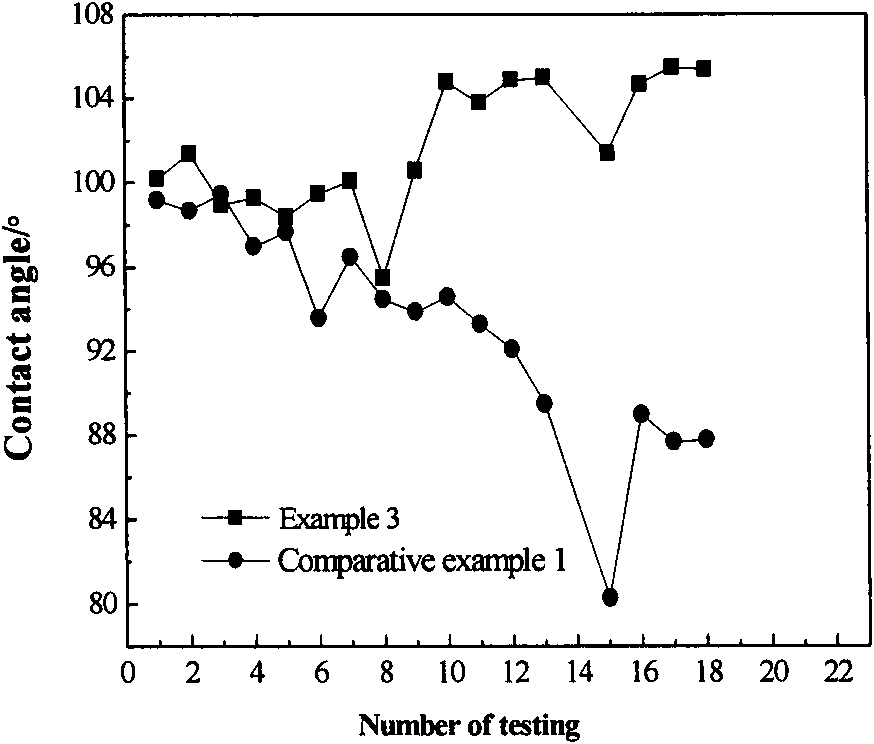
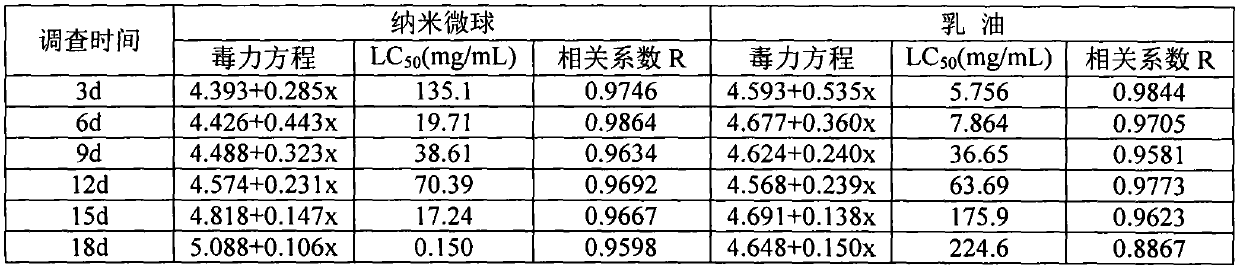
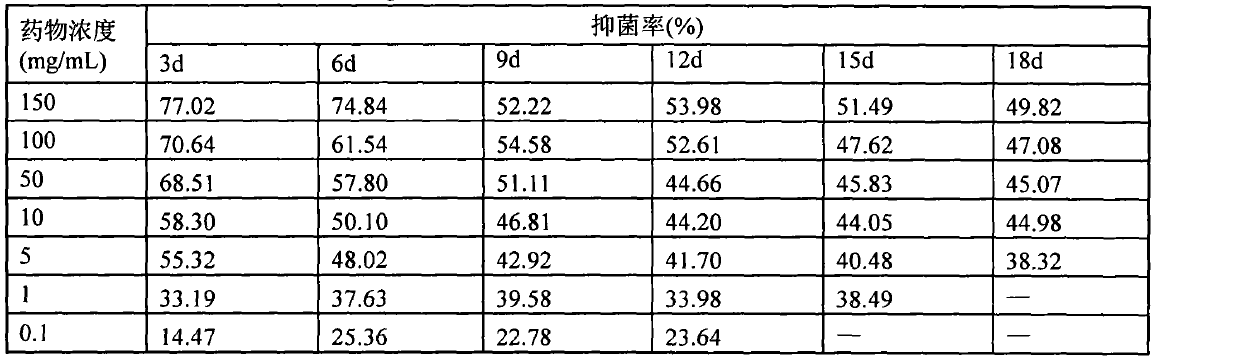
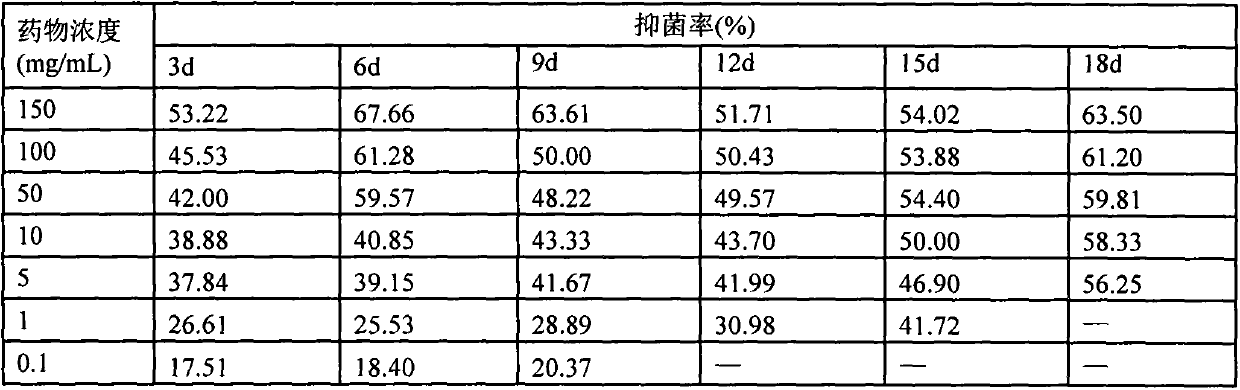
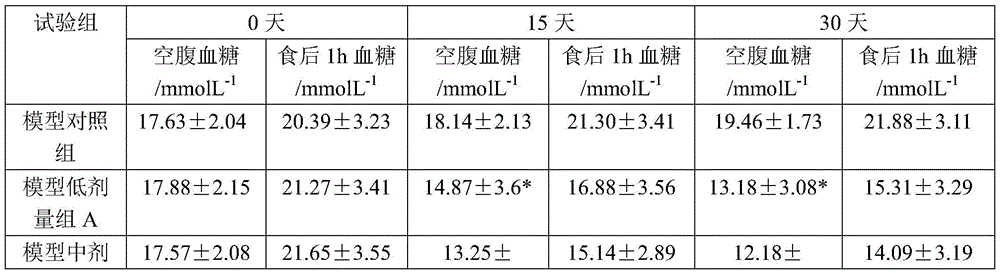
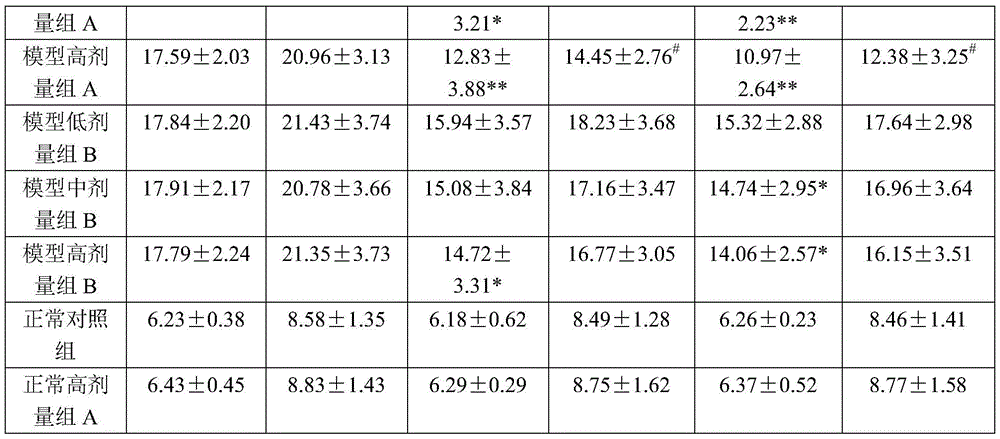
Patents
Literature
464 results about "Ultrasonic emulsification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
System and method for controlling a transverse phacoemulsification system with a footpedal
A method and system for controlling an ultrasonically driven handpiece employable in an ocular surgical procedure is provided. The method includes operating the ultrasonically driven handpiece in a first tip displacement mode, such as a longitudinal mode according to a first set of operational parameters, and enabling a user to alter the ultrasonically driven handpiece to employ a second tip displacement mode, such as a transversal or torsional mode, using a second set of operational parameters. Enabling the user to alter performance of the handpiece comprises the user being enabled to dynamically select operational parameters for the first tip displacement mode relative to the second tip displacement mode by using, for example, a switching apparatus such as a footpedal.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Pesticide emulsion in water and preparation method thereof
ActiveCN101790978ASmall sizeNarrow size distributionBiocideAnimal repellantsUltrasonic emulsificationSmall particle
The invention discloses a method for preparing pesticide emulsion in water by ultrasonic emulsification and a pesticide emulsion in water prepared by the method. Compared with the prior art, the pesticide emulsion in water, which is prepared by the method of the invention, has smaller particle size range, thus the pesticide emulsion in water has the advantages of favourable stability, high pesticide effect and the like; meanwhile, compared with the currently universal high-shear and homogenizing emulsification, the emulsion in water, which is prepared by ultrasonic emulsification, has the advantages of simple preparation, high production efficiency, low cost, high emulsification quality and the like.
Owner:QINGDAO STAR CROPSCI
ASA emulsification with ultrasound
The invention provides a method and apparatus for optimally feeding an ASA emulsion into a papermaking process. An intense transmission of ultrasonic energy rapidly and efficiently forms an ASA emulsion. Nearly simultaneous to forming the emulsion, the emulsion is fed into a high-speed dual conduit feeding apparatus, which quickly mixes the ASA emulsion with other chemicals and feeds the mixture directly into a papermaking process. The invention operates so fast that virtually no hydrolysis of the ASA occurs. This both prevents damage to the papermaking machinery and improves the quality of the produced paper. The invention operates 10-20 times faster than mechanical based emulsion methods and requires 1 / 7 as much energy to operate. The invention also self-monitors the process and can automatically correct any detected problems with the fed ASA emulsion.
Owner:ECOLAB USA INC
Preparation method of water-borne long-chain acrylate separant
InactiveCN102675527ARaw materials are easy to getSimple processCoatingsUltrasonic emulsificationSilicon
The invention discloses a preparation method of a water-borne long-chain acrylate separant. The method comprises the following steps: (1) adding a monomer, an emulsifying agent and the initiator into deionized water, pre-emulsifying at a constant temperature between 40 and 50 DEG C for 20 to 40 minutes to obtain a pre-emulsion; (2) performing ultrasonic emulsification on the pre-emulsion obtained in the step (1) to obtain a fine emulsion; and (3) pouring the fine emulsion obtained in the step (2) into a reactor, and reacting at a constant temperature between 75 and 85 DEG C for 6 to 7 hours to obtain the water-borne long-chain acrylate separant. According to the method, the crylic acid long-chain alkyl ester has strong hydrophobicity and the function of assisting emulsion, and other assistant emulsions are not added. The water-borne separant prepared by using the method has no silicon, low transfer possibility, good stability, high transformation rate and excellent separation effect. Furthermore, the water-borne separant has the advantages that raw materials are easy to prepare, the preparation process is simple, VOC (volatile organic compounds) emission amount is extremely low and environmental-friendliness effect is achieved.
Owner:SOUTH CHINA UNIV OF TECH
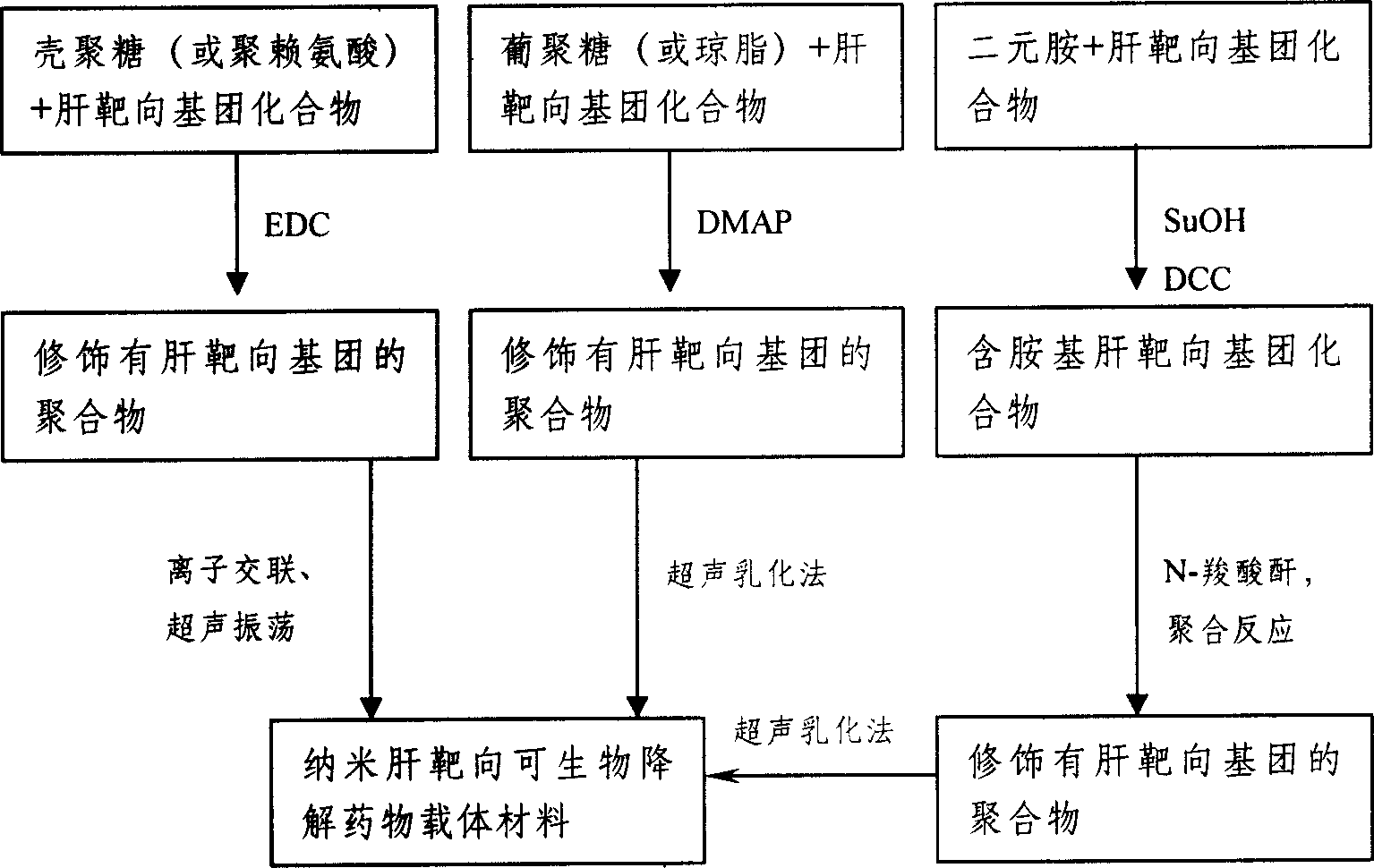
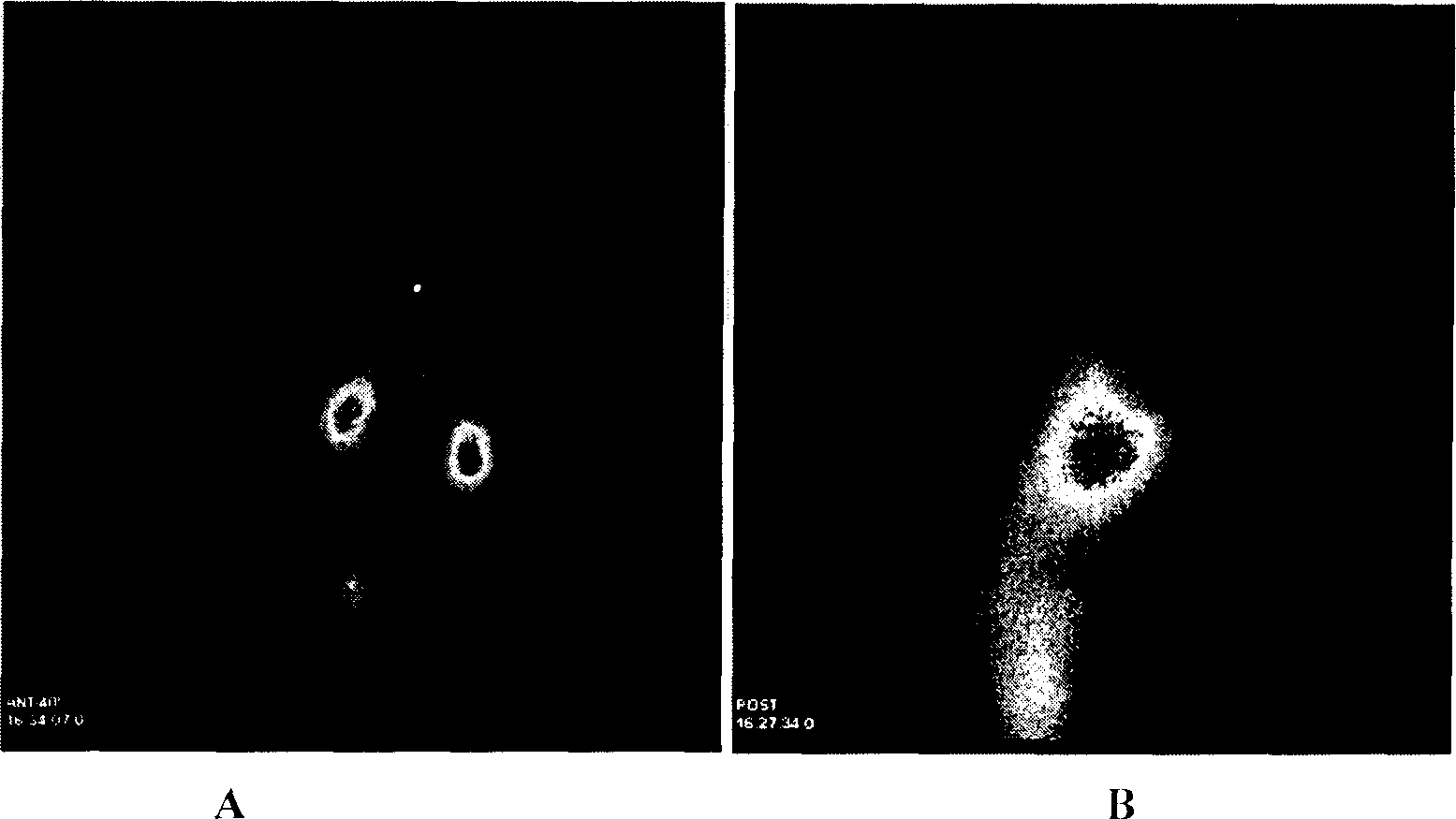
Method for preparing nano liver-target biodegradating medicine carrier material
InactiveCN1743008ATargeted sustained-release therapy achievedNo side effectsOrganic active ingredientsPharmaceutical non-active ingredientsUltrasonic emulsificationIon exchange
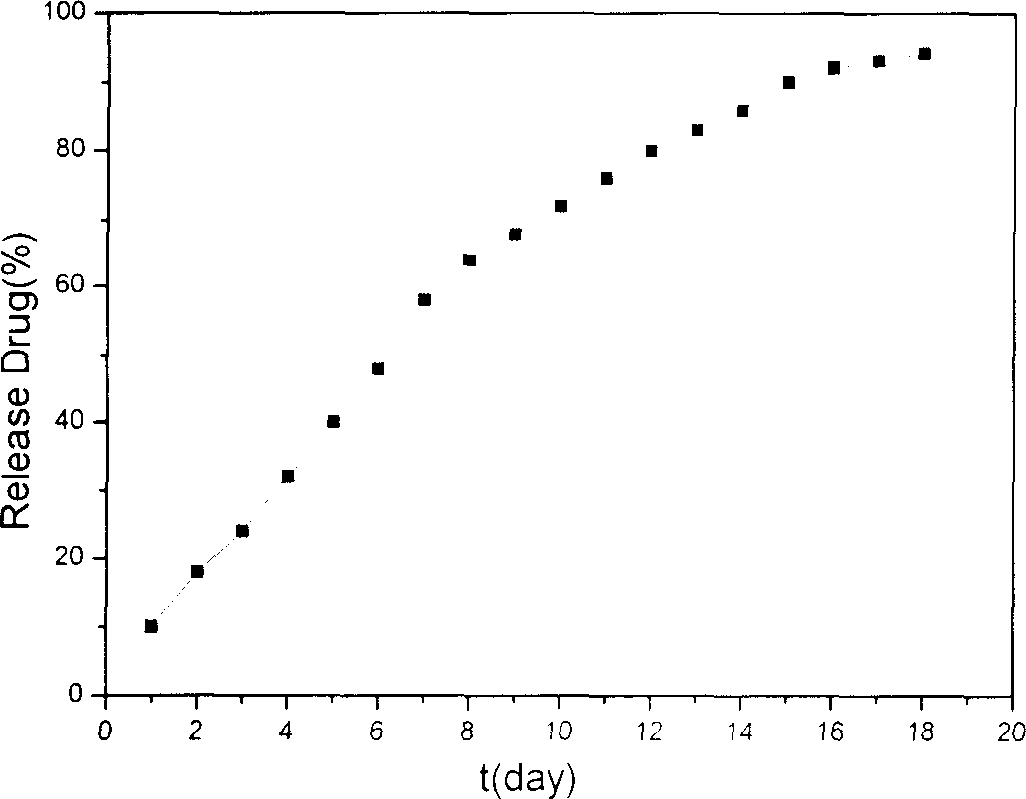
The preparation method includes the following steps: modifying hepatic target compound onto degradable polymer (chitosan, polylysine, glucosan, agar, polyglutamic acid-benzyl ester, polyalanine) with biological compatibility, and adopting ion exchange or ultrasonic emulsification process to obtain the invented nano hepatic target bio-degradable medicine carrier material. The hepatic target nano particle solution has good target performance for liver, the medicine enriched rate in the liver can be up to 75%, and its slowly-released administration can be up to above 15 days.
Owner:NANKAI UNIV
Phase change energy storage microcapsule material and its preparation method
InactiveCN103509527AUniform particle sizeImprove stabilityHeat-exchange elementsMicroballoon preparationWater bathsUltrasonic emulsification
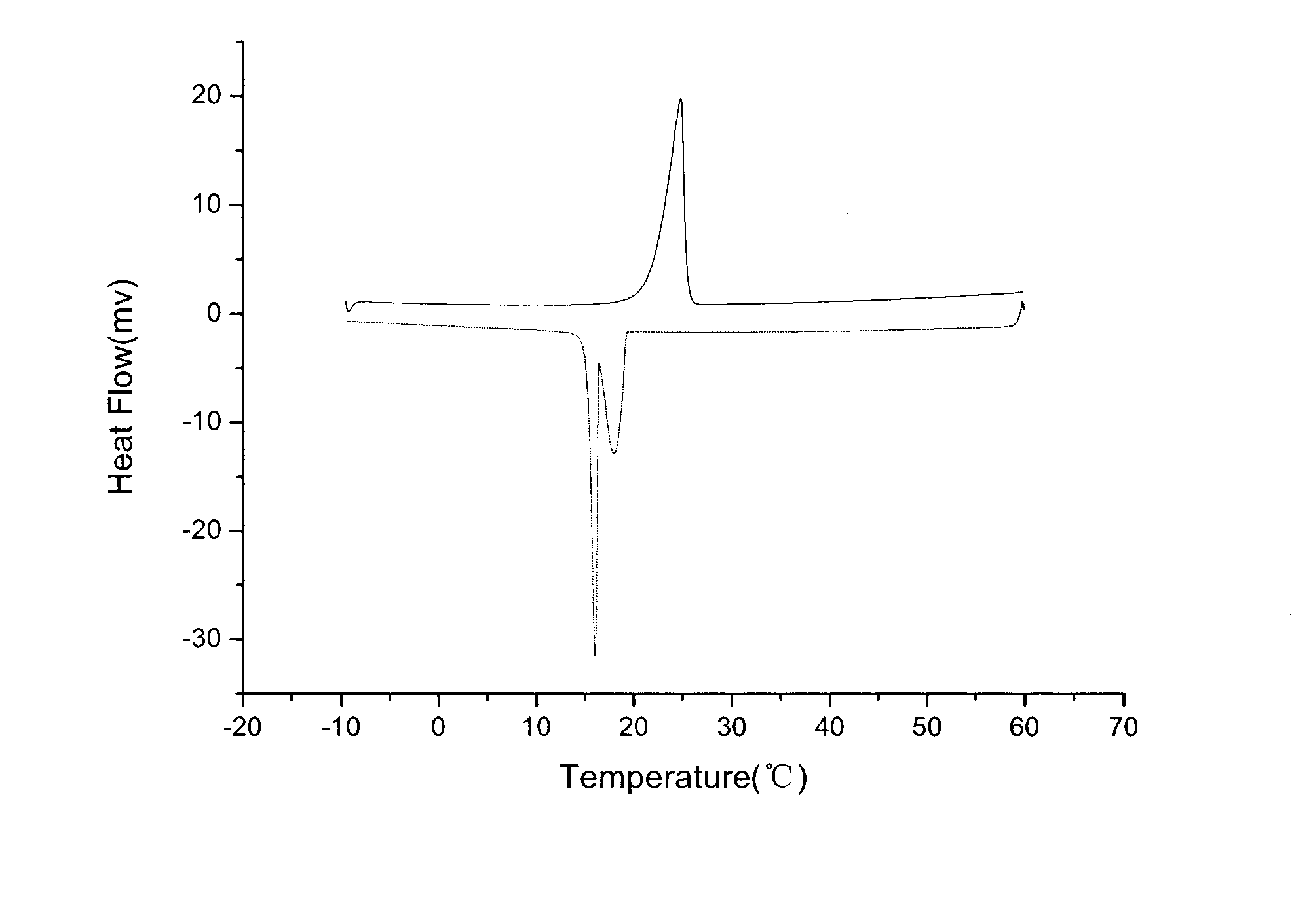
The invention relates to a phase change energy storage microcapsule, which takes n-dodecanol as a phase change material and adopts methyl methacrylate-acrylic acid copolymer as a capsule wall material. Ultrasonic-assisted emulsification is adopted, and interfacial polymerization is employed to perform microcapsule packaging. The preparation method of the phase change energy storage material includes: firstly mixing n-dodecanol with an emulsifier, performing ultrasonic emulsification, then conducting mechanical stirring, adding methyl methacrylate and an initiator under 80DEG C water bath to react for certain time, then adding acrylic acid to react, and completing polymerization; and carrying out cooling, filtering, and drying so as to obtain a sample. According to the invention, n-dodecanol is adopted as the phase change material, the phase change temperature can be controlled in the vicinity of room temperature. The methyl methacrylate-acrylic acid copolymer is taken as the capsule wall, which has very good chemical stability and strong adaptability to the environment. Ultrasonic assisted emulsification is employed to form a miniemulsion, and the reaction undergoes more easily. The phase change energy storage microcapsule material provided by invention has the advantages of simple process, low cost, and good stability, and can effectively improve the energy storage effect of a phase change material.
Owner:NANJING UNIV OF TECH
Silicone oil microcapsule with slow-release characteristics and preparation method thereof
InactiveCN102030990AStrong persistenceImprove waterproof performanceCoatingsUltrasonic emulsificationOil phase
The invention provides a silicone oil microcapsule with slow-release characteristics and a preparation method thereof, relating to the field of new chemical materials. In the invention, ethyl orthosilicate or methyl orthosilicate, non-functional linear silicone oil and silane coupling agents with long-chain alkyl are used as oil phases, silicone oil microcapsule dispersion liquid taking silicon dioxide as a shell is prepared by the following steps of: compounding anionic surface active agents with nonionic surface active agents into emulsifying agents; carrying out ultrasonic emulsification to obtain miniemulsion; stirring at room temperature for hydrolysis; and finally regulating pH value; wherein the silicone oil microcapsule dispersion liquid taking silicon dioxide has grain diameter of 140-260 nanometers and solid content of 13%-20%. The silicone oil microcapsule has high storage stability and good slow-release characteristics and can be directly used for the preparation of high-durability hydrophobic coating.
Owner:FUDAN UNIV
Method for preparing nano-microballons of Avermectins medicine and usage
InactiveCN1444946AImprove bioavailabilitySmall toxicityPowder deliveryOrganic active ingredientsUltrasonic emulsificationMicrosphere
A nanosphere of abamectin used as both medicine and agricultural chemical is prepared through preparing the polylactic acid-polyglycollic acid copolymer (PLGA), mixing abemectin with PLGa, ultrasonicemulsifying, volatilizing solvent, supersonic centrifugal treating, mixing, concentrating, leaching with distilled water, and supersonic centrifugal treating, mixing, concentrating again, and freeze drying at -106- -108 deg.C. Its advantages are slow release, low poison and low cost.
Owner:方淑昌 +1
Pyraclostrobin nanoparticle and preparation method of pyraclostrobin nanoparticle
The invention discloses a pyraclostrobin nanoparticle and a preparation method of the pyraclostrobin nanoparticle. The pyraclostrobin nanoparticle is prepared by adopting an ultrasonic emulsification-volatilization method; a solid spherical nanoparticle is prepared by taking polyester as a carrier and taking arabic gum as a dispersing agent; and the drug loading capacity of the prepared nanoparticle is 5.0-30%, the embedding rate is 65-90%, and the volume immediate diameter is about 0.6 micron. The pyraclostrobin nanoparticle disclosed by the invention has quick release and slow release functions, and the lasting period is lower than that of a conventional particle and is higher than that of missible oil. The nanoparticle prepared by the method is secondarily processed into a conventional dosage form, and can be applied to foliar spraying to prevent and treat various diseases of crops.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Method for preparing degradable dry beancurd preservation packaging film
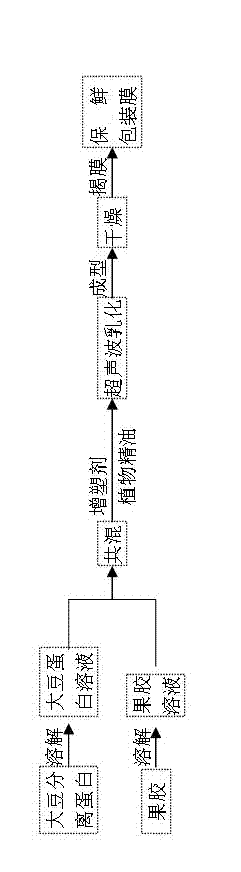
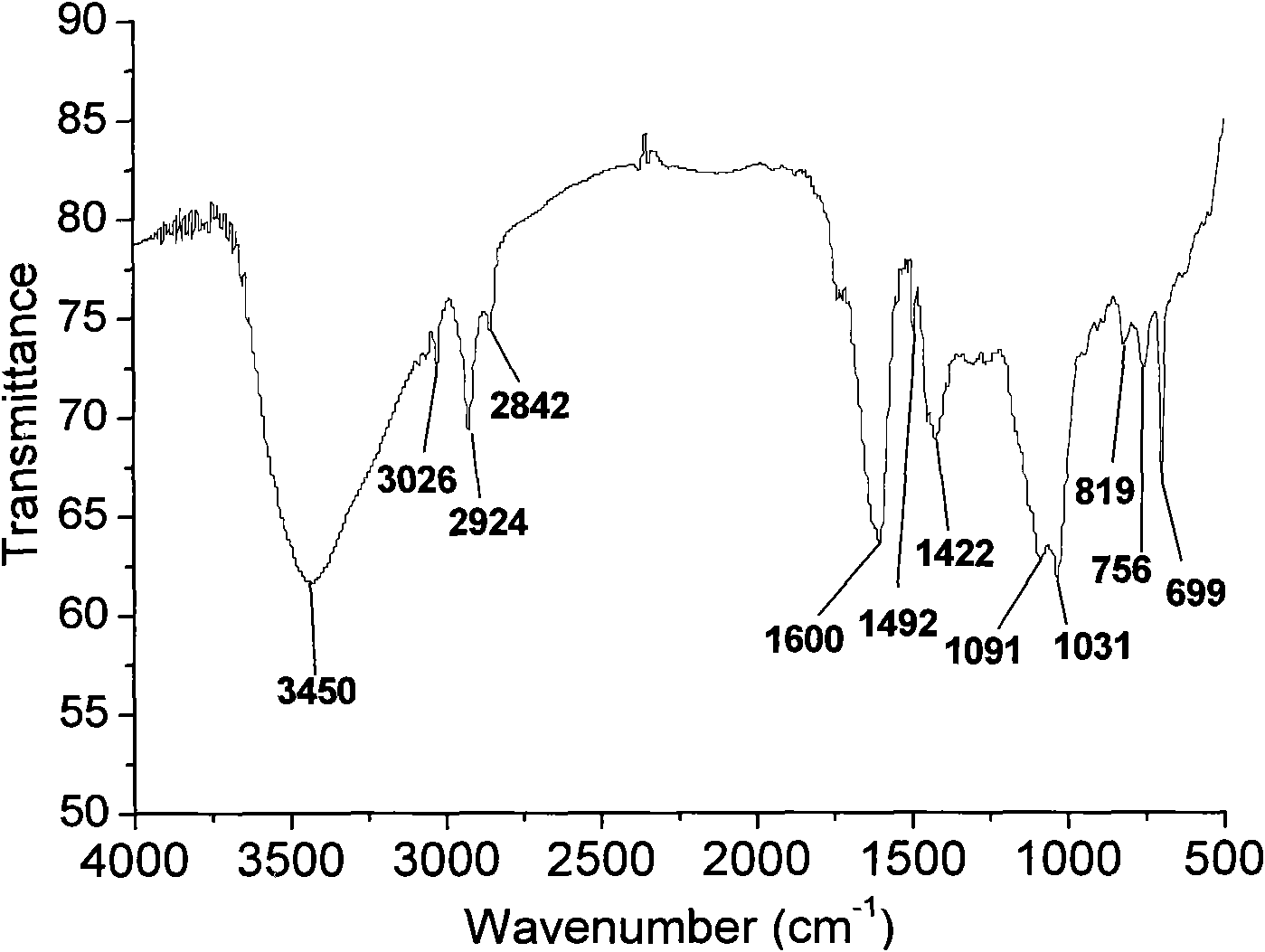
ActiveCN102731810AShorten the timeReduce energy consumptionFlexible coversWrappersUltrasonic emulsificationProtein molecules
The invention provides a method for preparing a degradable dry beancurd preservation packaging film. The method comprises the following steps: adding soybean protein isolates to deionized water, adjusting the pH value and stirring the materials to obtain soybean protein solution; adding pectin to deionized water and stirring the materials to obtain pectin solution; blending the soybean protein solution with the pectin solution, adding a plasticizer and essential oil and carrying out ultrasonic emulsification to obtain film liquid; and pouring the film liquid into an organic glass tank to be formed, drying the formed product in the hot air environment and tearing off the film, thus obtaining the preservation packaging film. The method has the following advantages that 1) interaction between polysaccharides and protein molecules is fused, thus enhancing the moisture resistance and mechanical performances of the preservation packaging film; 2) time is saved by ultrasonic emulsification, the energy consumption is lower than that in the high pressure homogenization method and the emulsion is stable; 3) the loaded essential oil can endow the film material with excellent bacteriostasis performance; and 4) the total number of colonies of the dry beancurd is effectively reduced by utilizing the preservation film to package the dry beancurd.
Owner:BOHAI UNIV
Polystyrene/calcium alginate composite gel microsphere in nuclear shell structure and preparation method thereof
InactiveCN101857698AAchieve adsorptionSynthetic conditions are mildPharmaceutical non-active ingredientsOn/in organic carrierUltrasonic emulsificationFiltration
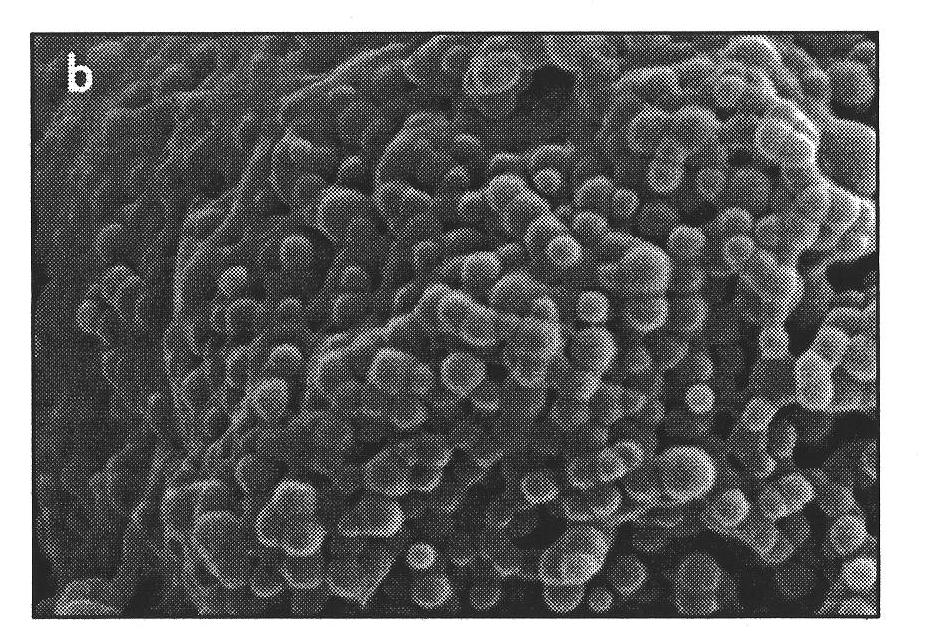
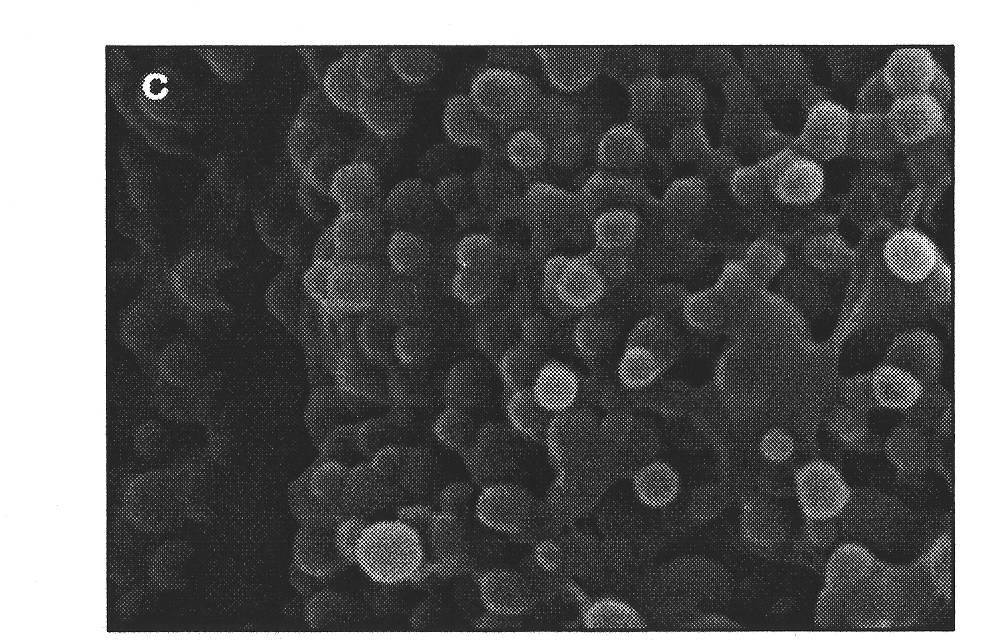
The invention provides a polystyrene / calcium alginate composite gel microsphere in a nuclear shell structure and a preparation method thereof, relating to biopolymer materials. The polystyrene / calcium alginate composite gel microsphere in the nuclear shell structure takes a polystyrene microsphere as a core and calcium alginate gel as a shell, wherein the grain size of the core is 1-100 microns, and the thickness of the shell layer is 50-800 nm. The preparation method comprises the following steps of: mixing polystyrene microsphere with aqueous solution containing a surface active agent I, and carrying out ultrasonic emulsification to obtain mixed solution A; adding sodium alga acid solution in the mixed solution A, and stirring to obtain mixed solution B; adding organic solution containing a surface active agent II into the mixed solution B, and stirring to obtain mixed solution C; adding calcium chloride solution which has the same volume with the sodium alga acid solution into the mixed solution C under stirring to react, and carrying out vacuum filtration, washing the solution until no calcium ion residue exists on the surface and drying to obtain a product D; and dispersing the product D into water, centrifuging the mixture, pouring out supernate and taking out precipitate to obtain the product.
Owner:XIAMEN UNIV
Polylactic acid-capsaicin nano-capsule and preparation method thereof
InactiveCN102019160ALarge particle sizeSimple preparation processBiocidePest repellentsUltrasonic emulsificationPolyvinyl alcohol
The invention discloses a polylactic acid-capsaicin nano-capsule and preparation method thereof. The method comprises the following steps: (1) preparing a dichloromethane solution of polylactic acid and capsaicin; (2) preparing a polyvinyl alcohol aqueous solution; (3) adding the solution in step (1) into the solution in step (2) and performing ultrasonic emulsification to obtain an emulsion; (4) heating the emulsion in step (3) to 40-50 DEG C to obtain a white emulsion and filtering the emulsion by a 100 nm microporous filter membrane to obtain a colloidal solution; (5) centrifuging the colloidal solution in step (4) to obtain a solid and vacuum drying the solid to obtain a polylactic acid nano-capsule. The polylactic acid nano-capsule of the present invention has a small particle size with a distribution of 30-90 nm; and the raw materials are biodegradable, is benefic to environment protection; the preparation process is simple, of low production cost, and easy to carry out. The prepared polylactic acid nano-capsule of capsaicin is applicable to adhesives, paints, electrical wires, cables, communication facilities, medicine, etc.
Owner:SHANGHAI UNIV OF ENG SCI
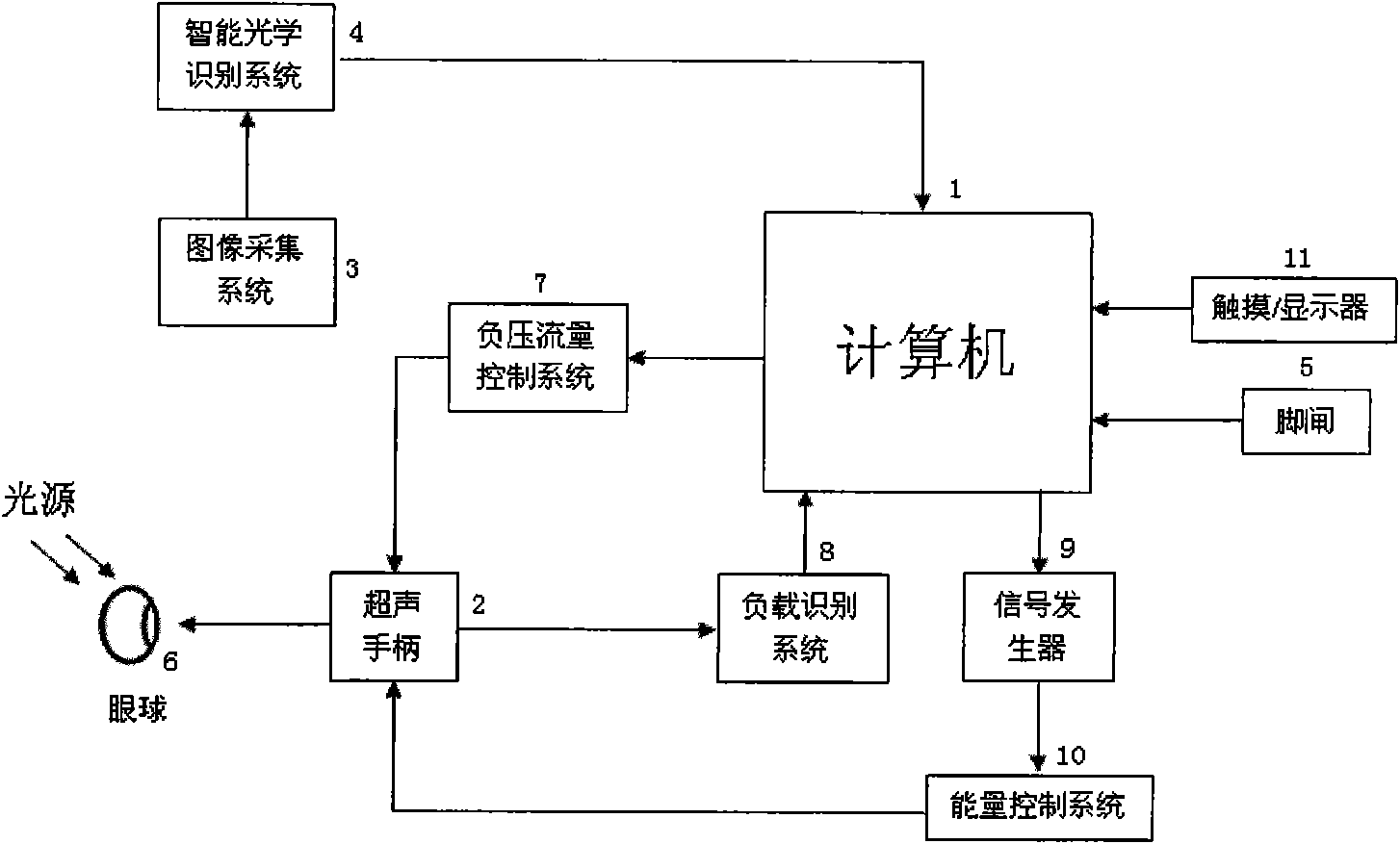
Intelligent ophthalmic ultrasonic emulsification apparatus
ActiveCN101632610AImprove real-time performanceReduce computationEye surgeryUltrasonic emulsificationSonification
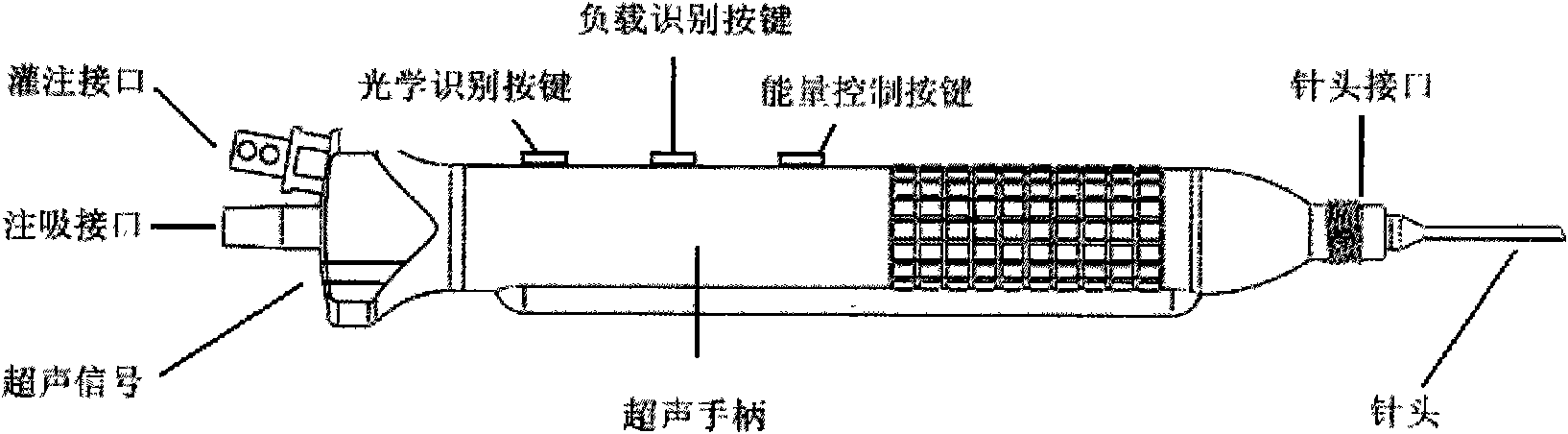
The invention relates to an intelligent ophthalmic ultrasonic emulsification apparatus, comprising a host machine of a surgical apparatus, an ultrasonic handle, an image collection system and a foot brake, wherein the host machine of the surgical apparatus comprises a signal generator, a process and affected-part state identification system, a negative-pressure flow control system, an energy control system and a touch / display device. The ultrasonic handle is provided with three keys including an optical identification key, a loading identification key and an energy control key. The intelligent ophthalmic ultrasonic emulsification apparatus can realize intelligent operation of ultrasonic emulsification apparatus and reduce the work intensity of doctors.
Owner:北京速迈医疗科技有限公司
Infrared phase-changing material and preparation method thereof
InactiveCN101845293AReduce thicknessDoes not involve effective associativity issuesHeat-exchange elementsParaffin waxUltrasonic emulsification
The invention relates to an infrared phase-changing material, which relates to an optoelectronic material. The infrared phase-changing material comprises an inner shell, an outer shell, and a core material, the inner shell comprises a copolymer containing styrene and methylacrylic acid, the outer shell comprises conductive polyaniline, and the core material comprises various kinds of paraffins. The preparation method comprises the following steps: uniformly mixing the paraffins, styrene, methylacrylic acid, dodecyl mercaptan and azobisisobutyronitrile according to a certain proportion; dissolving a certain amount of sodium dodecyl sulfate and TritonX-100 in de-ionized water; completely mixing the two solutions together, moving the solutions in a four-hole bottle after ultrasonic emulsification in ice-bath, filling nitrogen, heating and agitating; after six hours, obtaining a powder sample through cooling, emulsion breaking, filtration, washing and drying; uniformly mixing a certain proportion of powder sample, aniline monomer and dilute hydrochloric acid together, and slowly adding a certain amount of ammonium persulfate and hydrochloric acid solution after cooling down; and after six hours, obtaining the final product through filtration, washing and drying. The invention solves the problem that two materials are needed for the temperature control and the reduction of the infrared emissivity, increases the associative property of different materials, and reduces the coating thickness and the actual construction links.
Owner:BEIJING JIAOTONG UNIV
Preparation method of multiplex-mode iodized oil nano-emulsion contrast medium
The invention discloses a preparation method of multiplex-mode iodized oil nano-emulsion contrast medium. Aiming at the problems of incomplete detection information of complex diseases and the like because of the shortages when CT (Computed Tomography), MRI (Magnetic Resonance Imaging) and the fluorescence imaging technology are independently used, the invention provides a preparation method of multiplex-mode iodized oil nano-emulsion contrast medium. The method is characterized in that an oil-soluble Fe3O4 nano particles are chosen as an MRI contrast medium part and an oil-soluble CdSe / ZnS quantum dots are chosen as an optical probe part on the basis that iodized oil injection is chosen as a CT contrast medium. Firstly, the oil-soluble Fe3O4 nano particles and / or oil-soluble CdSe / ZnS quantum dots are fully dissolved into the iodized oil injection to serve as an oil phase; the oil phase is added into an aqueous phase containing joint surfactant; stable oil-in-water nano-emulsion is formed with a stirring and ultrasonic emulsification method; and finally, the transparent and clear multiplex-mode iodized oil nano-emulsion contrast medium with proper size, good homogeneity, good stability and high biocompatibility is obtained.
Owner:SOUTHEAST UNIV
Preparation method of noble-metal-supported catalyst
ActiveCN103372430AEvenly dispersedEasy to makeMetal/metal-oxides/metal-hydroxide catalystsUltrasonic emulsificationWater in oil emulsion
The invention relates to the field of preparation of a catalyst and particularly relates to a preparation method of a noble-metal-supported catalyst. The preparation method of the noble-metal-supported catalyst, disclosed by the invention, comprises the following steps: 1) dissolving a metal oxide precursor in water to prepare a water phase; dissolving a noble metal precursor in an oil-phase solvent to prepare an oil phase; 2) mixing the water phase and the oil phase in the step 1 with a surfactant in a volume ratio within a range from (1 to 10) to (1 to 3); carrying out ultrasonic emulsification to form a water-in-oil emulsion, wherein the surfactant accounts for 2.0-20% of the total volume or the total mass of the water-in-oil emulsion; 3) carrying out steps of atomizing, igniting, combusting, cooling and clustering on the water-in-oil emulsion to prepare noble-metal-supported catalyst powder. According to the preparation method disclosed by the invention, a preparation process is simple, continuous and easy to control and has theoretical and practical application values.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Large-amplitude sandwich-type piezoelectric ultrasonic compound transducer
InactiveCN104014473AAmplify longitudinal excitation displacement amplitudeIncrease the ultrasonic radiation areaMechanical vibrations separationUltrasonic emulsificationTransformer
The invention relates to a piezoelectric ultrasonic compound transducer which can generate large displacement amplitude in the longitudinal direction and is particularly applicable to the technical field of liquid high-strength ultrasonic processing such as ultrasonic disintegration, ultrasonic emulsification and ultrasonic degradation. In order to achieve the effects, the large-amplitude sandwich-type piezoelectric ultrasonic compound transducer comprises a longitudinal vibration sandwich-type piezoelectric ultrasonic transducer body, a longitudinal vibration variable cross-section metal tube amplitude transformer and a bending vibration metal disk. By the adoption of the technical scheme, compared with a traditional piezoelectric ultrasonic transducer, the large-amplitude sandwich-type piezoelectric ultrasonic compound transducer has larger displacement amplitude and higher ultrasonic radiation intensity, impedance matching between the transducer and loaded liquid is improved, ultrasonic energy can be more effectively transmitted to a load through the ultrasonic transducer, and the ultrasonic radiation efficiency is improved.
Owner:CHINA JILIANG UNIV
Method for preparing mono-disperse polymer functional microspheres with photochemical method
The invention discloses a method for preparing mono-disperse polymer functional microspheres with a photochemical method. According to the invention, under the regulation of a reversible addition-fragmentation chain transfer (RAFT) reagent, polymer functional microspheres with various sizes such as nano-grade sizes and micron-grade sizes, and with uniform particle sizes are synthesized by using the photochemical method. First, mono-functional group carbon-carbon double-bond monomers, multifunctional group carbon-carbon double-bond cross-linking agents, monomers containing functional groups, a dispersing agent, a stabilizing agent, the RAFT reagent, a photoinitiator, a pore-forming agent, and the like are added into a mixed solvent; the mixture is subjected to ultrasonic emulsification, such that a uniform and stable dispersion liquid is produced; the dispersion liquid is transferred into a reaction bottle, and is stirred; vacuum pumping is carried upon the reaction bottle, and nitrogen is filled into the reaction bottle until constant pressure is achieved; the reaction bottle is irradiated by using an ultraviolet lamp under room temperature for approximately 8 hours, and the reaction is then stopped; an obtained reaction liquid is subjected to centrifugal sedimentation, washing, and drying, such that the mono-disperse polymer functional microspheres with uniform particle sizes are obtained. The preparation method provided by the invention is advantaged in high production efficiency, and is energy-saving and environment-friendly. According to different requirements, thousands of specifications of mono-disperse microspheres with particle sizes of 5 nanometers to 1000 micrometers can be prepared. The method is widely applied in fields related to paints, adhesives, biomedicines, and electronic information.
Owner:苏州异导光电材料科技有限公司
Castor oil-based aqueous lubricant used for medical instruments and preparation method thereof
InactiveCN102504923AHigh dilutionImprove stabilityLubricant compositionUltrasonic emulsificationProcess engineering
The invention relates to a castor oil-based aqueous lubricant used for medical instruments and a preparation method of the Castor oil-based aqueous lubricant, and solves the problems in the prior art that the lubricants have poor biodegradability, cause severe pollution and can not be recycled. The invention adopts a technical scheme as follows: the castor oil-based aqueous lubricant used for medical instruments is composed of the following components: 5 to 20% of industrial-grade castor oil, 5 to 30% of an emulsifier system, 0.5 to 5% of co-emulsifiers, 0.05 to 1% of antifoaming agents and the balance of water. The preparation method comprises the following steps of: heating the emulsifier system and water up to 50 DEG C to 90 DEG C under stirring conditions to obtain an emulsifier water solution; mixing industrial-grade castor oil, co-emulsifiers and antifoaming agents with the emulsifier water solution; ultrasonically emulsifying; and cooling down to room temperature to obtain the final product. The lubricant has good stability and dilution stability and no toxicity, is suitable for both manual operation and operation using an automatic cleaning machine, and can well meet the lubrication requirements of medical instruments.
Owner:SHAANXI UNIV OF SCI & TECH
Chitosan oligosaccharide composite powder with adjuvant hpyerglycemic function and preparation method thereof
InactiveCN105595308AImprove solubilityGood dispersionYeast food ingredientsNatural extract food ingredientsSolubilityUltrasonic emulsification
The invention relates to a chitosan oligosaccharide composite powder with adjuvant hpyerglycemic function. The chitosan oligosaccharide composite powder comprises, by weight, 40 to 60 parts of oat beta-glucan, 5 to 20 parts of chitosan oligosaccharide, 5 to 20 parts of sealwort extract, 3 to 8 parts of mulberry leaf extract, 2 to 6 parts of balsam pear extract, 0.05 to 0.5 part of chromium-rich yeast and 10 to 20 parts of a filler. The invention has the following advantages: the chitosan oligosaccharide composite powder is a chitosan oligosaccharide compound prepared from chitosan oligosaccharide and oat beta-glucan, used as main effective components, and a plurality of traditional Chinese medicinal active components, used as adjuvant materials, through refining in virtue of ultrasonic emulsification and spray drying technologies; the chitosan oligosaccharide composite powder has the advantages of good dissolvability, good dispersibility and high absorption and availability; all the components of the chitosan oligosaccharide composite powder cooperate with each other to exert substantial effect on promotion of blood glucose metabolism and maintenance and adjustment of the functions of the pancreas islet; moreover, a variety of active components included in the chitosan oligosaccharide composite powder has obvious synergism in improving the sensitivity of insulin and stabilizing postprandial blood sugar.
Owner:颐海产业控股有限公司
Method for preparing nano silicon dioxide-acrylate polymeric microball material
The invention relates to a preparation method of nanometer silicon dioxideí¬methacrylate macromolecule microballoon material. It includes the following steps that the first is that oil solubility silicon dioxide with 10-100 shares quality are added into the same share methactylate monomer; then mixed liquor is gained after ultra sonic dispersion for 1-30min; The second is that anion with 1-50 shares quality or non ionic surface active agent, and peroxide initiator with 1-30 shares quality or azo genus initiator are added into the mixed liquor to form reaction system. Lipophilicity stabilizer with 1-30 shares quality is added into the 300 shares quality water to churn to form mixing water solution; then it is do super-sound emulsification for 1-30min to gain fine emulsion with average particle diameter 50-500nm. The third is that warming up to 50-90 centigrade degree, reacting for 0.5-8h, then nanometer silicon dioxideí¬methacrylate macromolecule microballoon material with average particle diameter 50-500nm is gained. The advantages of invention are that the cost is low; the contamination is litter; and it can gain package structure nanometer silicon dioxideí¬methacrylate macromolecule microballoon material.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
LID-PEG-PLGA controlled-release nano microsphere and preparation method thereof
InactiveCN101961316AImprove hydrophobicityHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsWater bathsMatrix solution
The invention discloses an LID-PEG-PLGA controlled-release nano microsphere and a preparation method thereof. The microsphere is the controlled-release microsphere which contains medicinal lidocaine and a degradable carrier. The degradable carrier contains polylactic-glycolic acid and PEG-2000. The mass percentage of the lidocaine in the controlled-release microsphere is 30 to 35 percent. The preparation method comprises the following steps of: preparing the carrier into matrix solution; dispersing the lidocaine into the matrix solution and preparing the lidocaine into an oil phase; mixing the oil phase and the aqueous solution of polyvinyl alcohol, and performing ultrasonic emulsification on the mixture under a water bath condition to obtain W / O-type protogala; mixing the W / O-type protogala and the aqueous solution of polyvinyl alcohol again, and further emulsifying the mixture into W / O / W-type complex emulsion; volatilizing the emulsion by reducing pressure at the normal temperature to obtain cured lidocaine-carried nano microsphere; and scattering, blending, packaging, freezing, sterilizing and the like. The medicament loading rate of the controlled-release nano microsphere can be up to 15 to 22 percent; the entrapment rate can be up to 68 to 78 percent; and the half-life period can be prolonged to 3 to 4 days. Therefore, the microsphere has relatively good effect of burst in the first day after the microsphere is taken and good effect of slow release in later days.
Owner:ARMY MEDICAL UNIV
Preparation method of copper-free click crosslinking polysaccharide microspheres
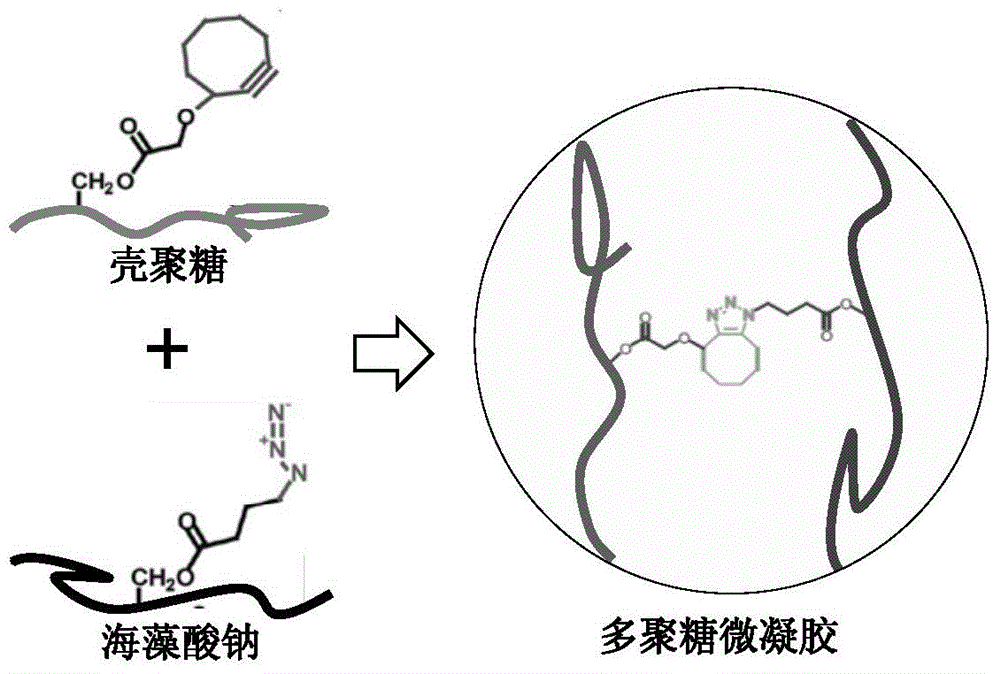
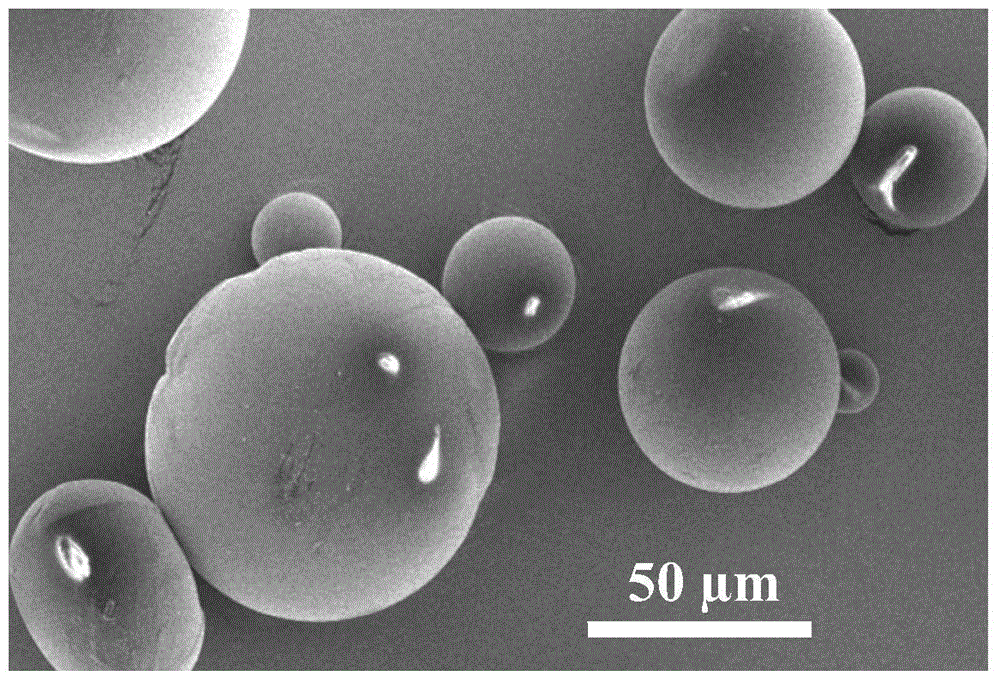
InactiveCN106140040AReduce usageGuaranteed CompatibilityMicroballoon preparationMicrocapsule preparationUltrasonic emulsificationFreeze-drying
The invention discloses a preparation method of copper-free click crosslinking polysaccharide microspheres. The preparation method comprises the following steps that chitosan and 1-hydroxyl benzotriazole are dissolved in water at room temperature, and cyclooctyne-3-glycolic acid is dissolved in a tetrahydrofuran / water mixed solvent; after the two solutions are mixed, diisopropylethylamine is added, stirring reaction is performed, and cyclooctyne chitosan is obtained through dialysis and freeze-drying; sodium alginate and carbodiimide are dissolved in water, 11-azido-3,6,9-triethyl ether-1-amine is added, stirring reaction is performed, and azido sodium alginate is obtained through dialysis and freeze-drying; water solutions of the cyclooctyne chitosan and the azido sodium alginate are respectively prepared, after mixing according to the volume ratio, emulsifier Span-80 containing paraffin oil is added, and an emulsification emulsion is obtained through ultrasonic emulsification; the emulsification emulsion volatilizes and stays overnight and is poured into isopropanol for precipitation of microspheres, and the crosslinking polysaccharide microspheres are obtained through freeze-drying after cleaning. The process is simple, makes products safe and non-toxic and is suitable for the medical fields of drug release, gene therapy, tissue engineering and the like.
Owner:NANJING UNIV OF SCI & TECH
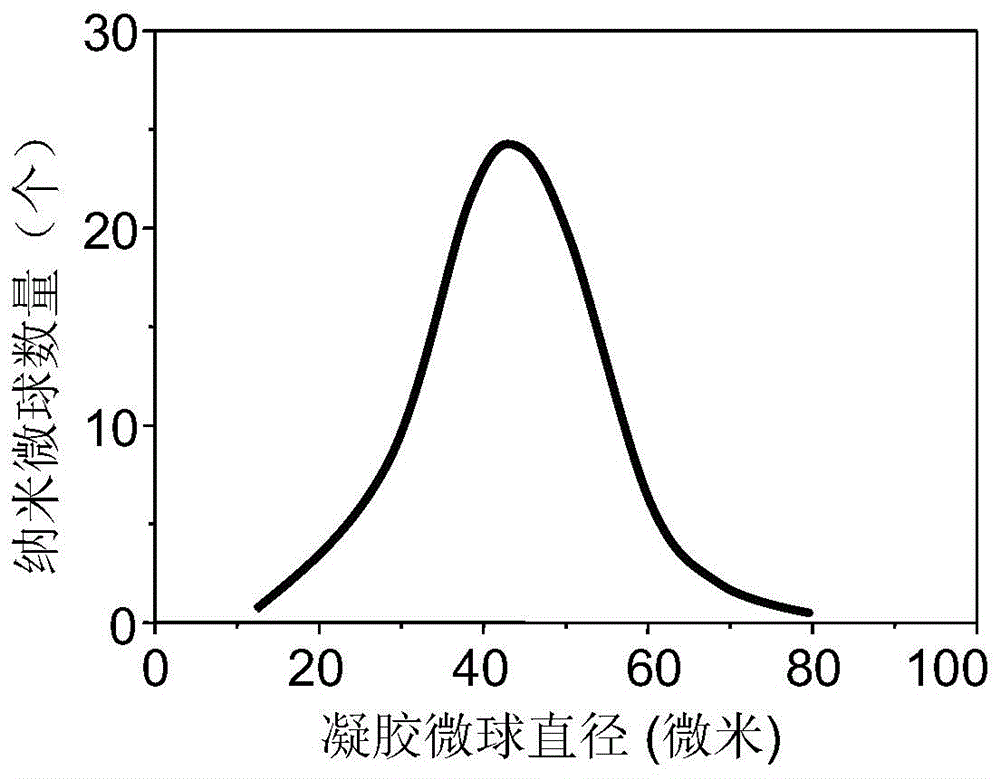
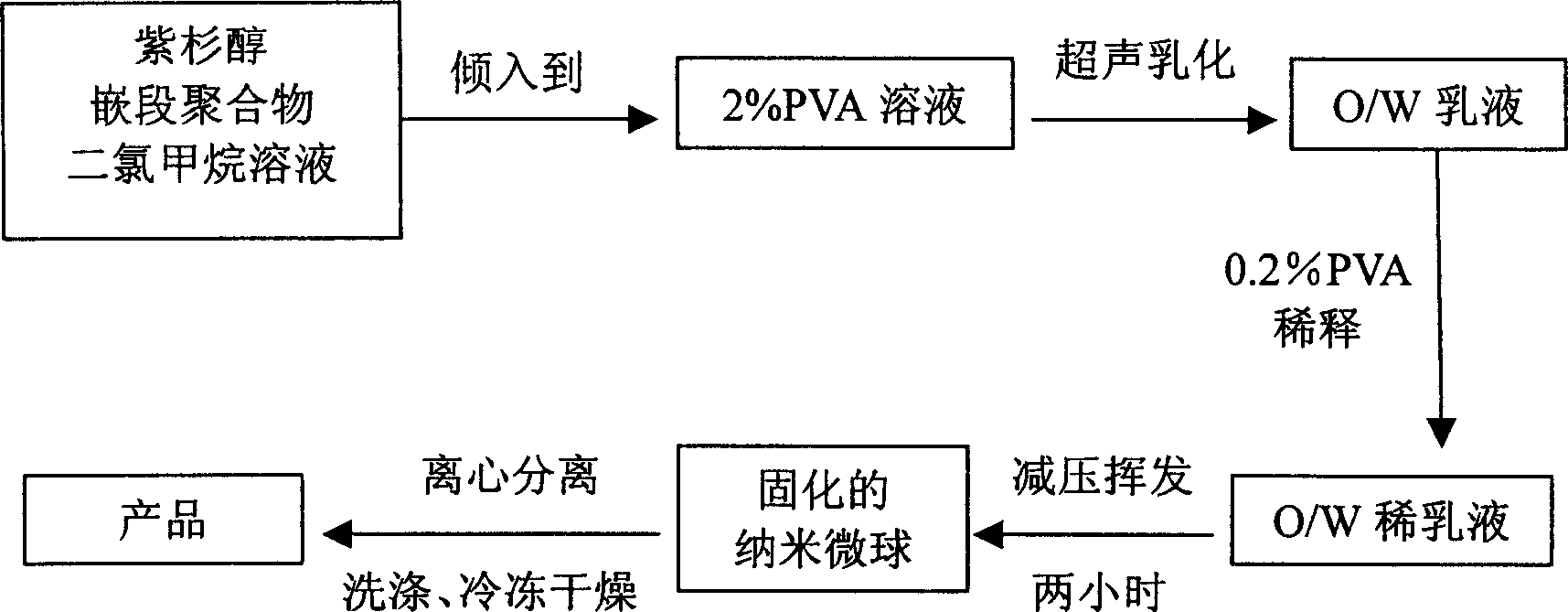
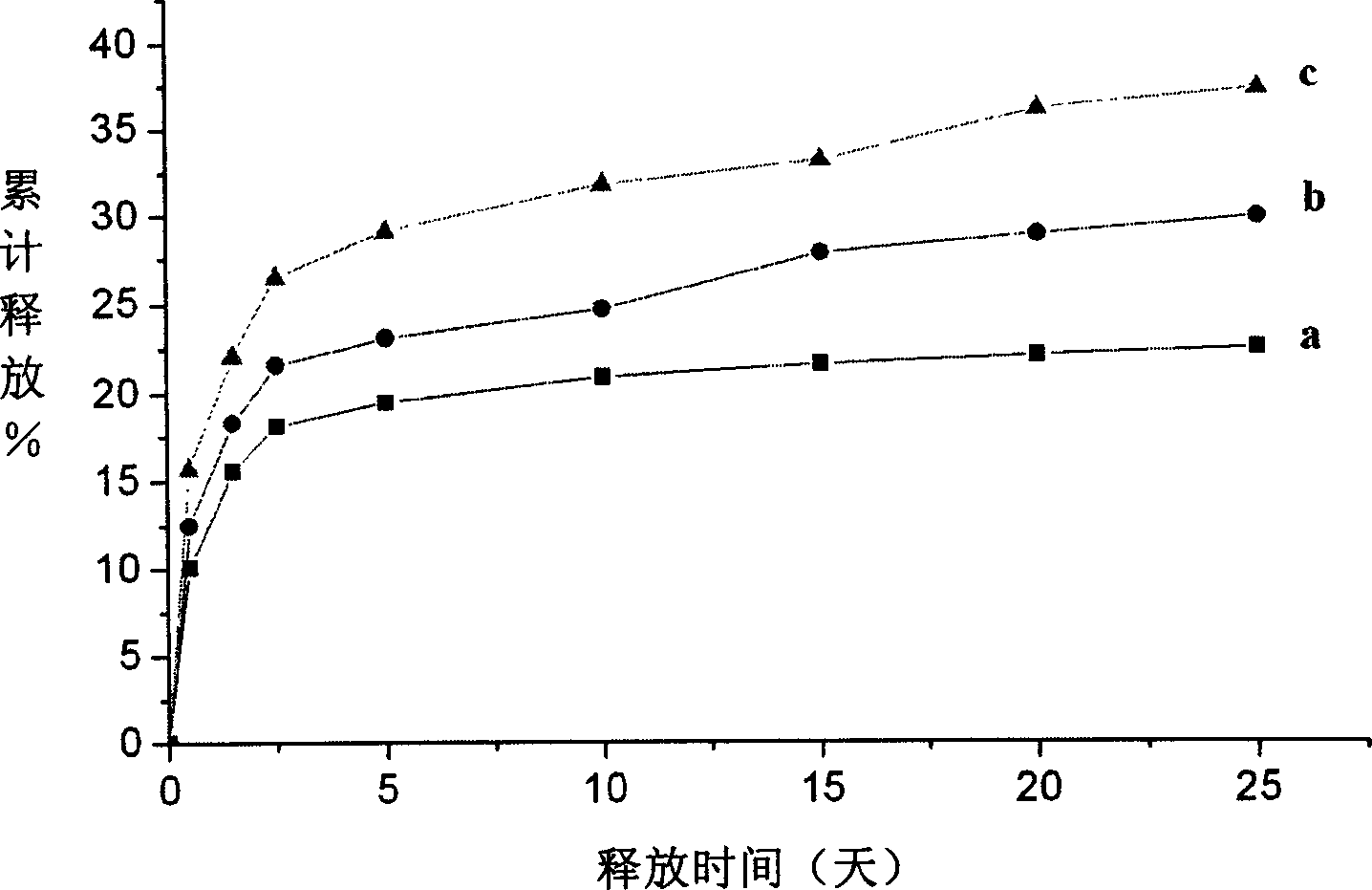
Nano micro ball with taxol capable of biologically degradating high molecule and its preparing method
InactiveCN1561987AEasy to useSmall doseOrganic active ingredientsPowder deliveryPolymer scienceUltrasonic emulsification
A biodegradable high-molecular nanoball is prepared from the block copolymer of polylactone and polyethanediol and taxusol through dissolving them in organic solvent, adding the solution to the aqueous solution of polyvinyl alcohol or gelatin, ultrasonic emulsifying, and vacuum volatilization of organic solvent.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
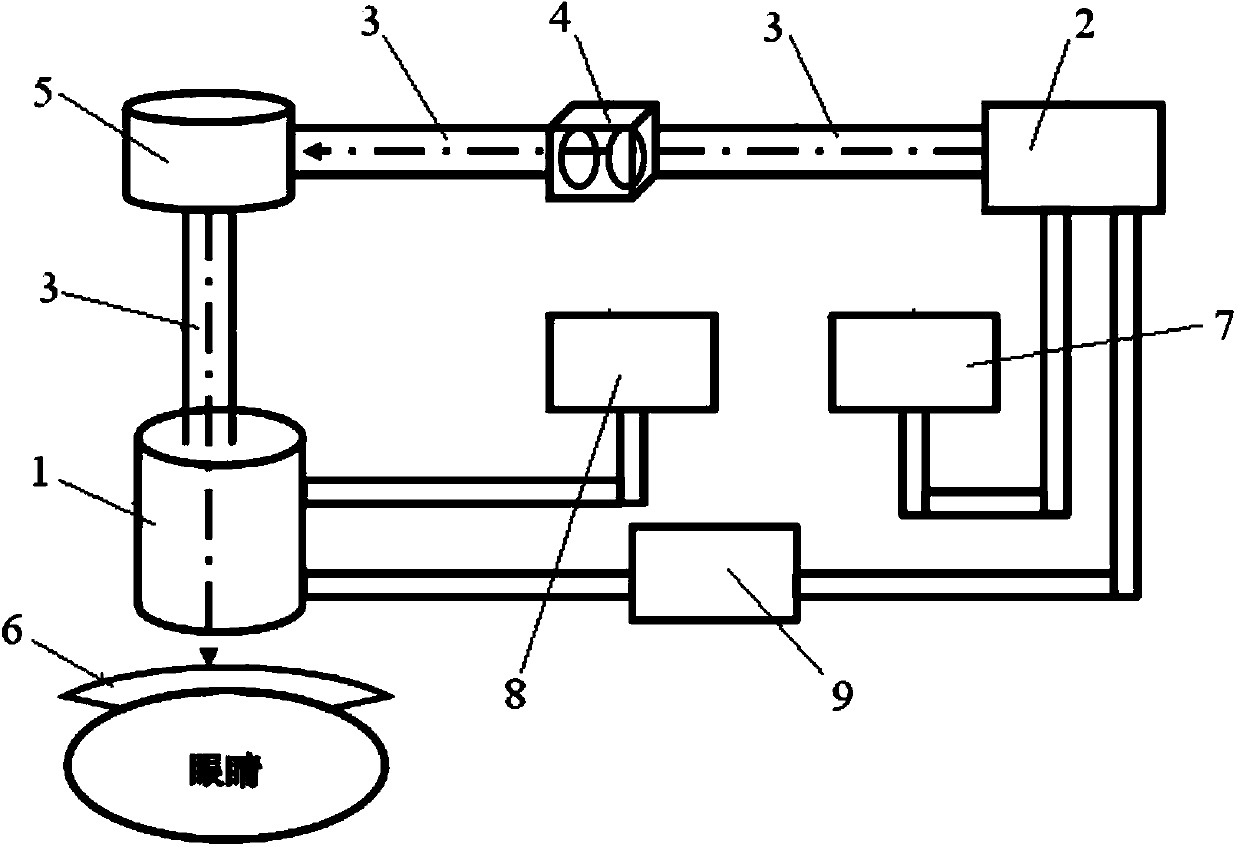
Femtosecond laser cataract surgery treatment device
ActiveCN104042403AAvoid postoperative astigmatismAvoid EndophthalmitisLaser surgeryUltrasonic emulsificationFemto second laser
The invention discloses a femtosecond laser cataract surgery treatment device which comprises an optical coherence tomography scanning imaging system, a data processing system, a laser light source system, a laser energy detection system, a laser scanning system and a press mirror. The laser light source system is provided with a femtosecond laser. The laser light source system and the laser energy detection system, the laser energy detection system and the laser scanning system, the laser scanning system and the optical coherence tomography scanning imaging system are respectively connected through a light beam transmission system. The femtosecond laser cataract surgery treatment device has the advantages that the device is precise, efficient and safe, and astigmatism and endophthalmitis after operation are avoided; envelope tearing and suspensory ligament injury are reduced, surrounding tissue injuries are small; complications produced during crystal nucleus emulsification and artificial lens implantation are decreased as far as possible; the operating difficulty is reduced; the required energy for ultrasonic emulsification is greatly decreased, and the corneal endothelium is effectively protected.
Owner:广东麦特维逊医学研究发展有限公司
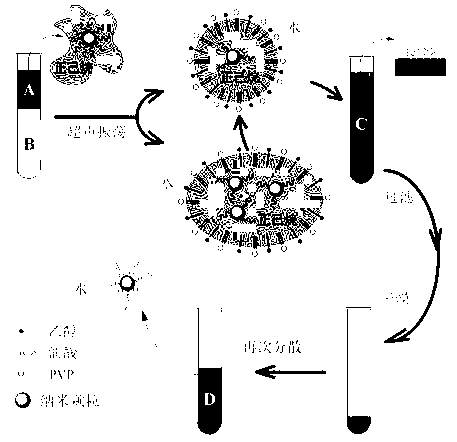
Method for transferring nano-particles from oil phase into aqueous phase
ActiveCN103127744AUniform size without changeImprove solubilityLiquid displacementCooking & bakingUltrasonic emulsification
The invention provides a method for transferring nano-particles from an oil phase into an aqueous phase. The method includes the steps of mixing nano-particle solution dispersed in the non-polar oil phase with water, a stabilizer and cosurfactant solution, enabling the mixture to be subjected to ultrasonic emulsification and made into oil-in-water microemulsion, carrying out evaporation and drying on the microemulsion in a baking oven to obtain stabilizer-nano-particle composite dry jelly, adding polar solvent capable of dissolving the stabilizer into the dry jelly, slightly shaking so as to enable the dry jelly to be dissolved and dispersed into the solvent, and finally achieving the purpose of dispersion of the nano-particles transferred from the non-polar oil phase to the polar solvent. The method is simple in process, simple, convenient and quick in operation, and capable of effectively transferring the nano-particles dispersed in the oil phase to the aqueous phase.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Online method for measuring oil density in oil containing sewage and device thereof
InactiveCN1687746AAvoid inhomogeneityEnsure stabilityPreparing sample for investigationColor/spectral properties measurementsUltrasonic emulsificationDisplay device
The invention discloses the online measuring the oil concentration of the sewage and its equipment. The method adopts the ultrasonic emulsification oil-water mixture and uses the optical nephelo method to test the oil concentration. The equipment comprises the measuring section vertically put on the sewage pipeline, the ultrasonic oscillator set at the input and the output ends of the measuring section; three pairs of white gem windows transmitting beams set at the outside pipe wall, and the windows arrange in 120 Deg angle mutually; three groups of photoelectric converters are set corresponding to the windows; connect the industrial controller with a displayer through the signal receiver and the amplifier. When the sewage flows through the test section, the ultrasonic oscillator emulsificates the sewage and mixes it evenly; the testing beam is the violet beam with fixed wavelength; the photoelectric converter converses the optical signal into the electric signal and the optical nephelometer calculates the oil concentration of the sewage.
Owner:XI AN JIAOTONG UNIV
Method for producing low fat peanut paste using degreased peanut powder
Disclosed is a method to produce low-fat peanut butter from defatted peanut flour. The production method includes the following steps: baking: baking the defatted peanut flour in a tunneling-based microwave dryer for 100-400 seconds and then cooling through strong wind until reaching room temperature; aromatizing: adding aromatizing agent water solution into the baked peanut flour based on weight ratio of 2.2:1 and mixing to be even; seasoning: adding pure peanut oil into aromatized peanut butter based on weight ration of 3-10:100 and mixing to be even; emulsifying: bathing the peanut butter in water with temperature of 60-80 EDG C to enable the interior and the exterior of the peanut butter to be heated evenly, inserting the probe of an ultrasonic cell disruptor into the peanut butter and conducting emulsification treatment for 20-30 minutes per 50kg under the conditions of harmony indicator output of 50, dutyfactor of 60, temperature of 60-80 DEG C; cooling: speedily stirring the emulsified butter and cooling to 20-50 DEG C through air to avoid the accumulation of the heat of the butter, which leads to the precipitation of the fat; store maturity: putting the cooled peanut butter into a packing container and keeping still for 48h to make the butter totally steady and fixed.
Owner:BOHAI UNIV
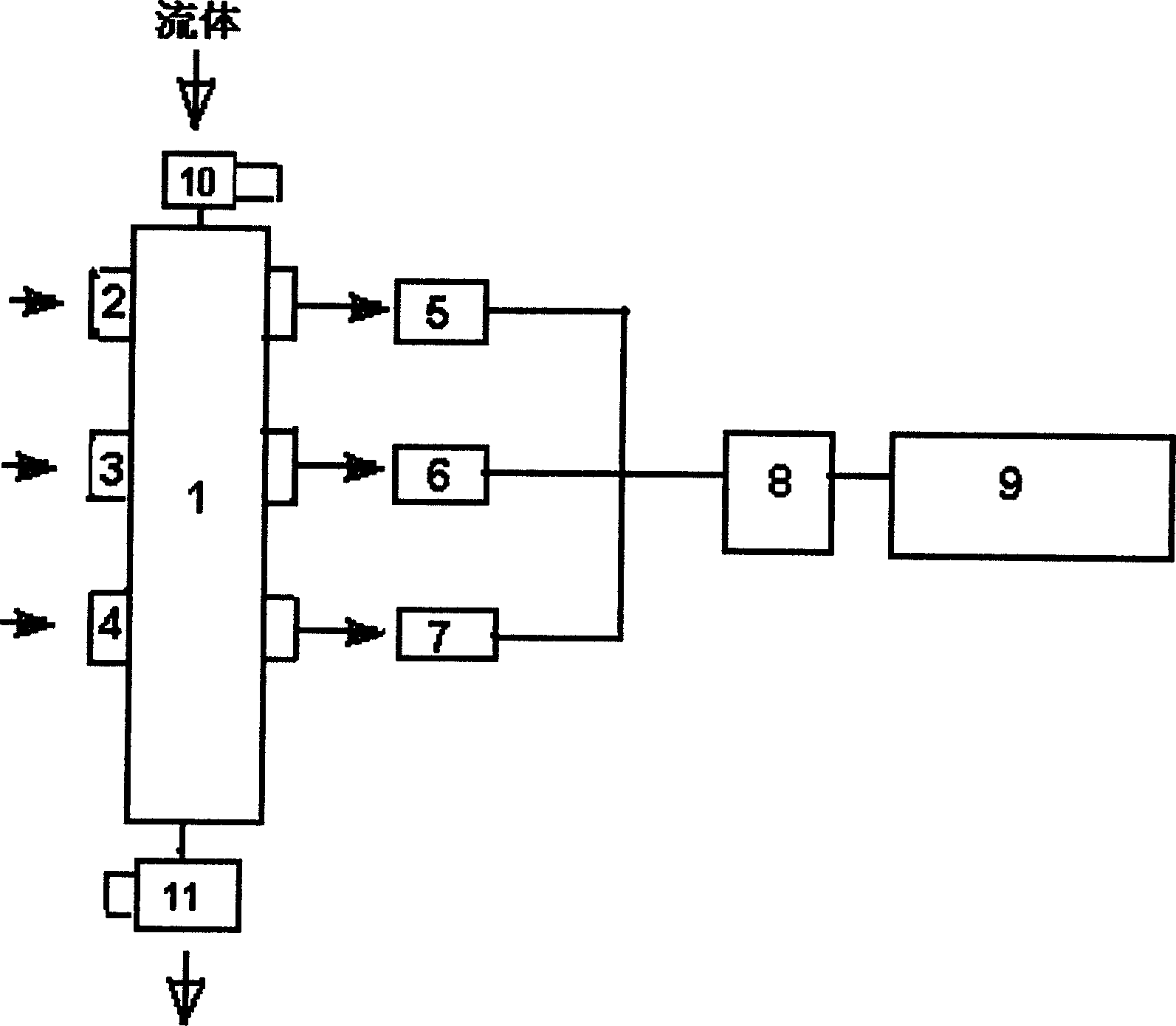
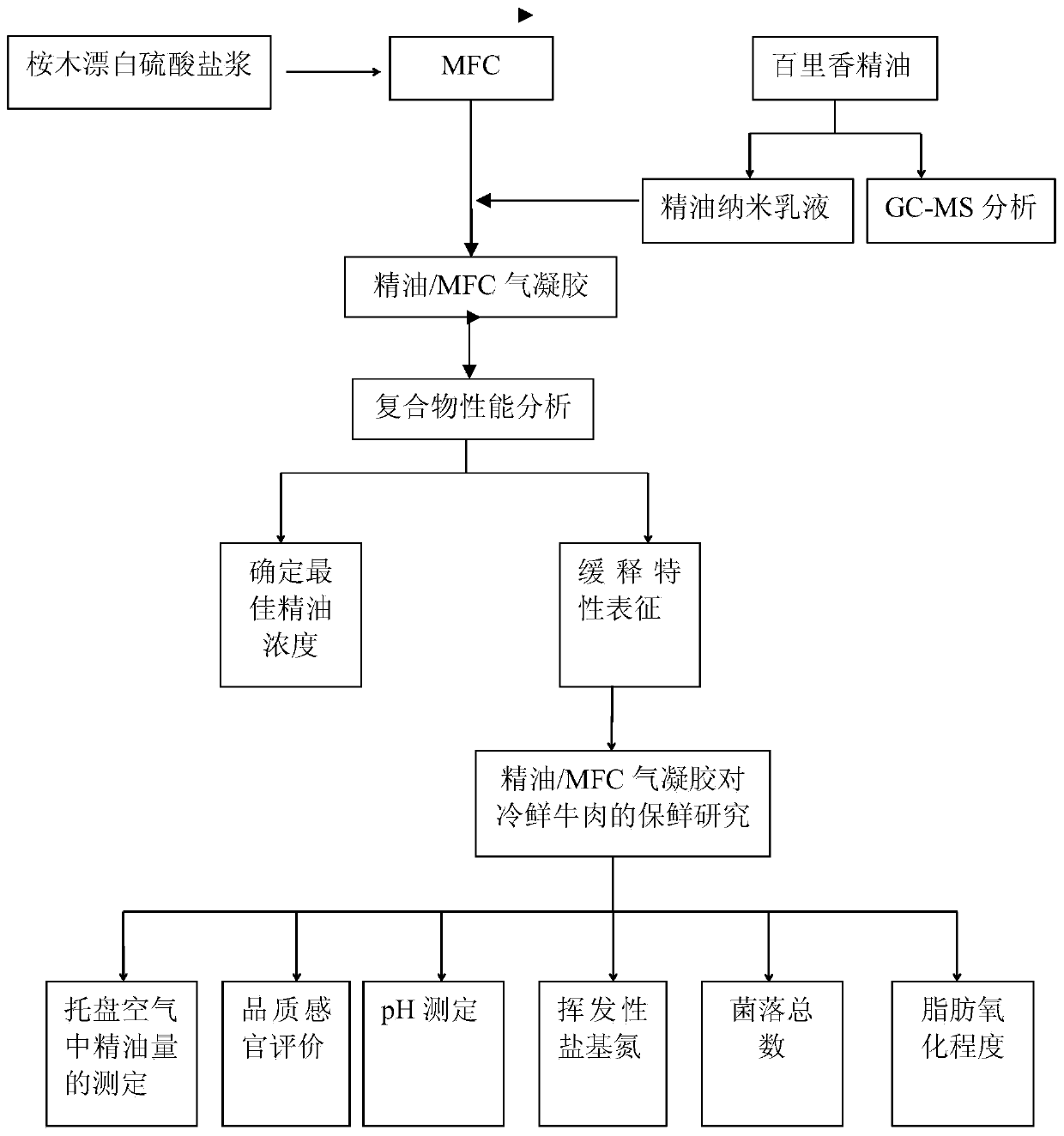
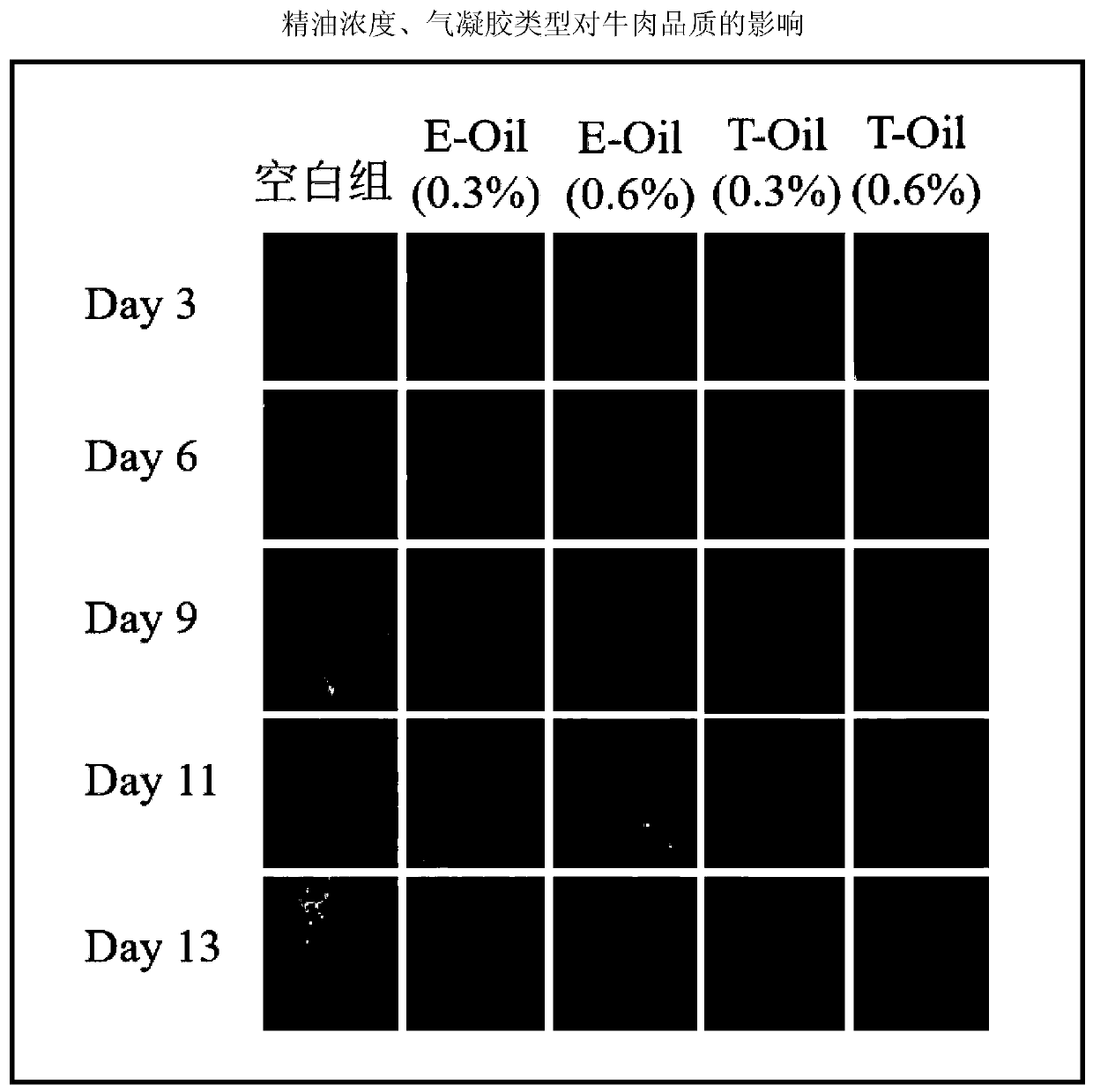
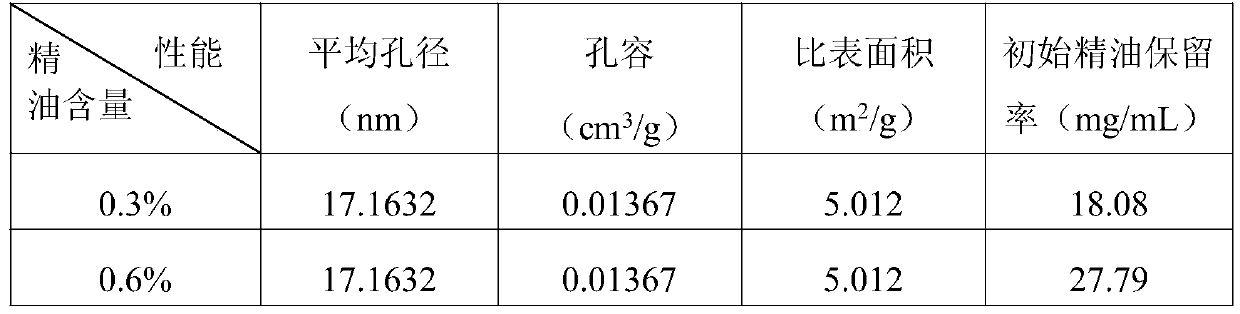
Preparation method of plant-source slow-release antibacterial aerogel
The invention relates to a preparation method of a plant-source slow-release antibacterial aerogel. The preparation method comprises following steps: an eucalyptus wood bleached sulfate pulp is takenas a raw material to prepare microfibrillated cellulose MFC, and ultrasonic emulsification technology is adopted to prepare thyme essential oil nanometer emulsion; a MFC water suspension and the thymeessential oil nanometer emulsion are combined and mixed to be uniform at a certain ratio, and freeze-drying is carried out so as to obtain the essential oil / MFC aerogel. The MFC aerogel is high in porosity, ultra low in density, and excellent in adsorption performance, so that absorption of the high-efficiency essential oil with broad-spectrum antibacterial performance by the internal part of theaerogel is realized, essential long term continuous release is realized, the concentration can be maintained at a certain range in a long time period, the plant-source slow-release antibacterial aerogel can be used in refrigerated food antibacterial package, the preparation method is simple and convenient, is economical, and is friendly to the environment, and refrigerated food service life can be prolonged effectively.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Medicament coating stent capable of preventing blood vessel restenosis and preparation method thereof
ActiveCN102657899AImprove adhesionImprove corrosion resistanceSurgeryCoatingsUltrasonic emulsificationPolyvinyl alcohol
The invention provides a medicament coating stent capable of preventing blood vessel restenosis and a preparation method thereof. The stent is coated with a PLGA (polylactic acid-glycollic acid) nano particle coating which comprises a medicament carrier and a medicament material; the medicament carrier is PLGA; and the medicament material is one or two of rapamycin or paclitaxel. The preparation method comprises the following steps of: dissolving PLGA with dichloromethane, adding the medicament material, dissolving fully, and performing ultrasonic emulsification by use of an ultrasonic cell disruptor under an ice bath condition so as to prepare a suspension; slowly adding a 2% PVA (Polyvinyl Alcohol) water solution into the suspension while performing magnetic stirring, and emulsifying the suspension again so as to prepare an emulsion; performing rotary evaporation on the emulsion under reduced pressure so as to enable dichloromethane to volatilize completely, so that a nano particular colloidal dispersion system is obtained; adding a 1% coupling agent into the system, and then soaking the stent into the system for 5 minutes, so as to prepare the coating stent; and freeze-drying the coating stent for 10 hours, and preserving the freeze-dried stent at the temperature of 4 DEG C.
Owner:DONGGUAN KEWEI MEDICAL INSTR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com