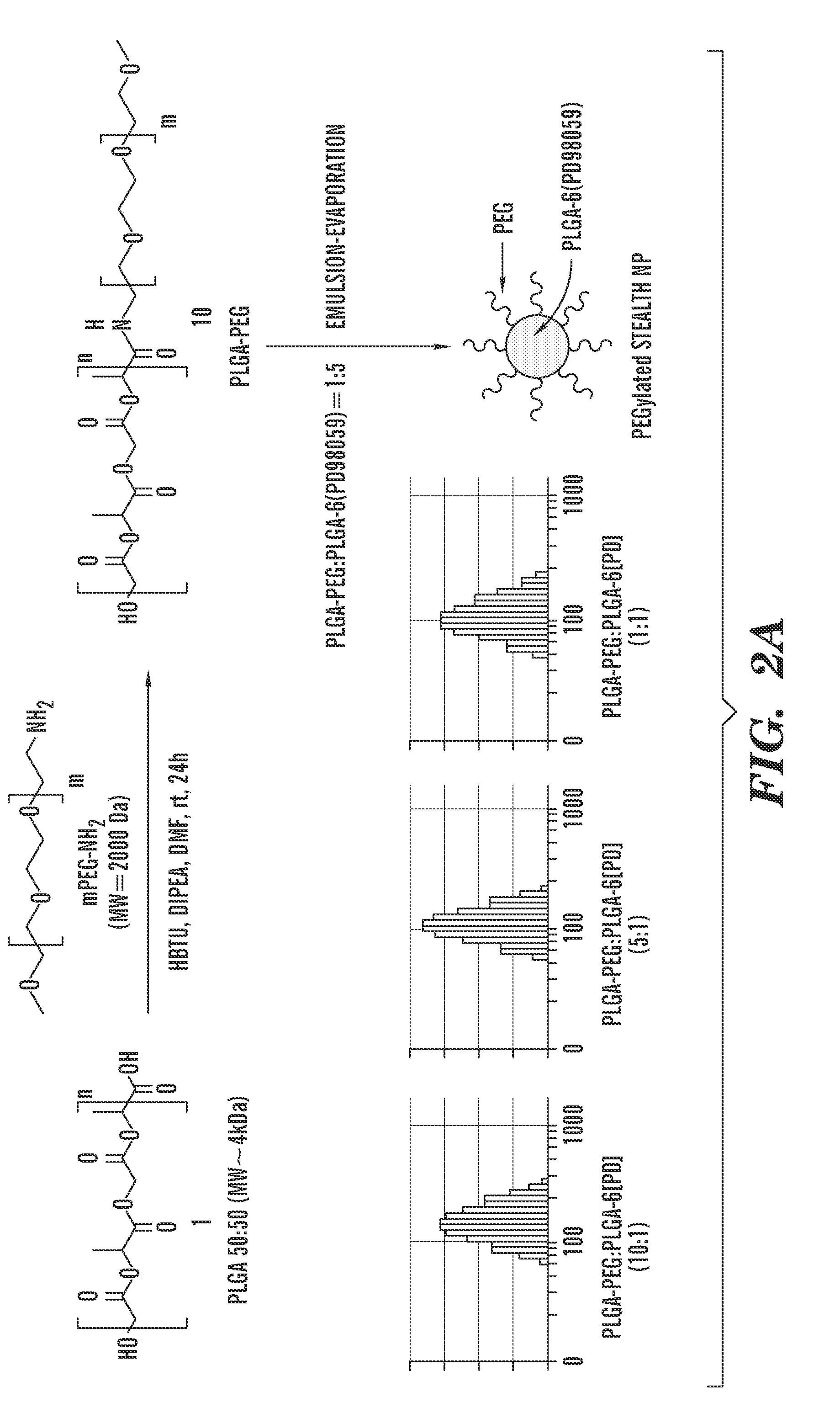
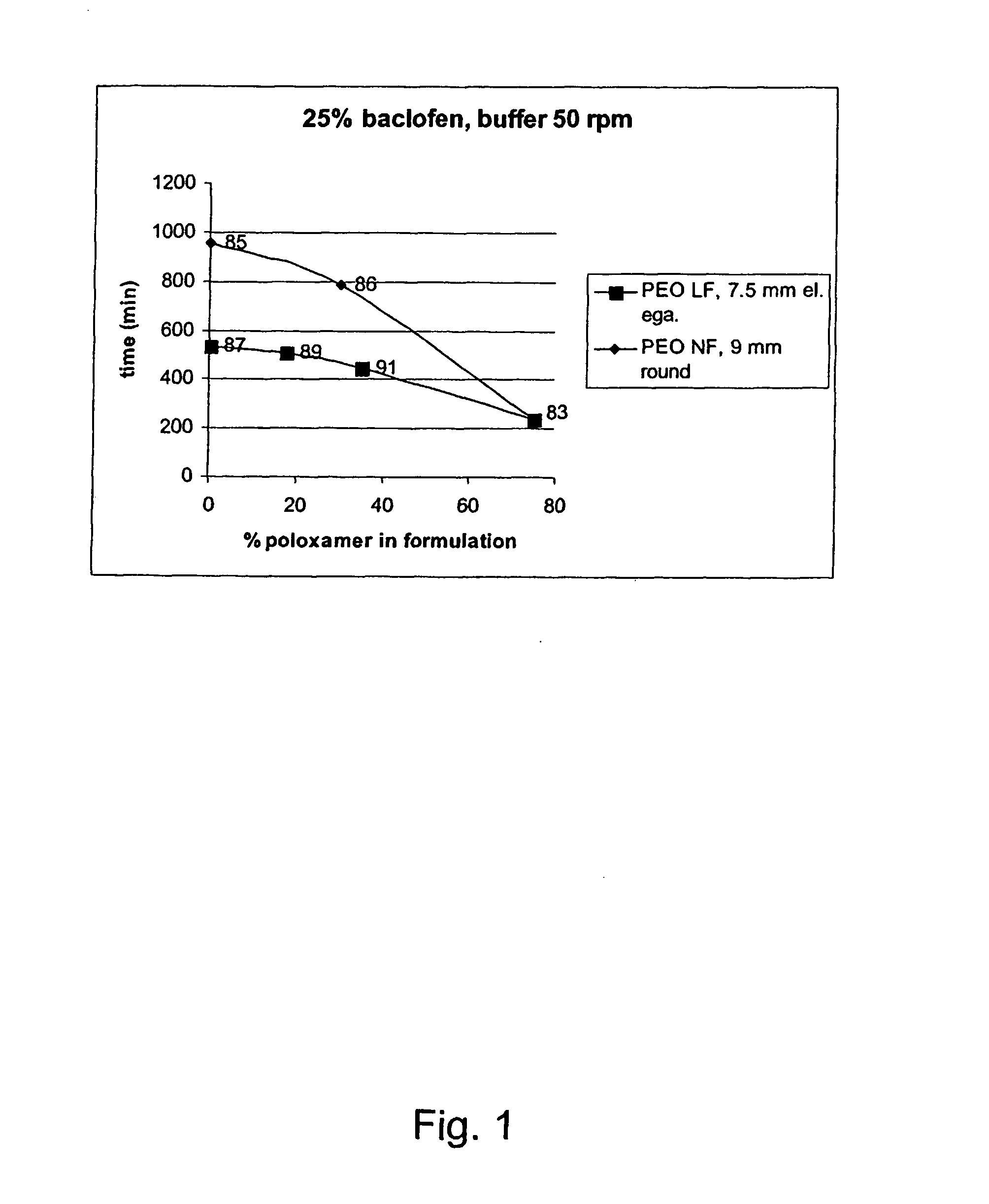
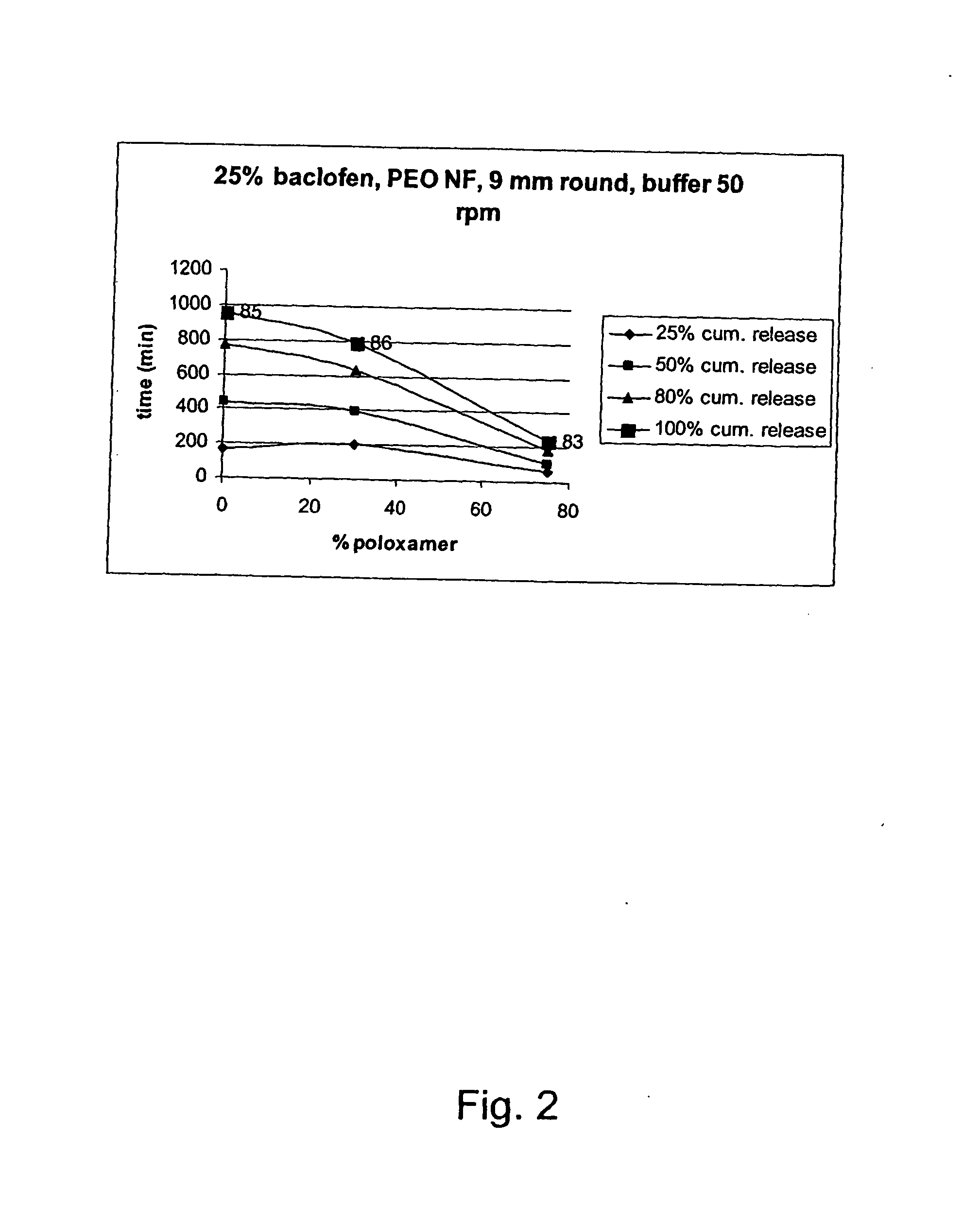
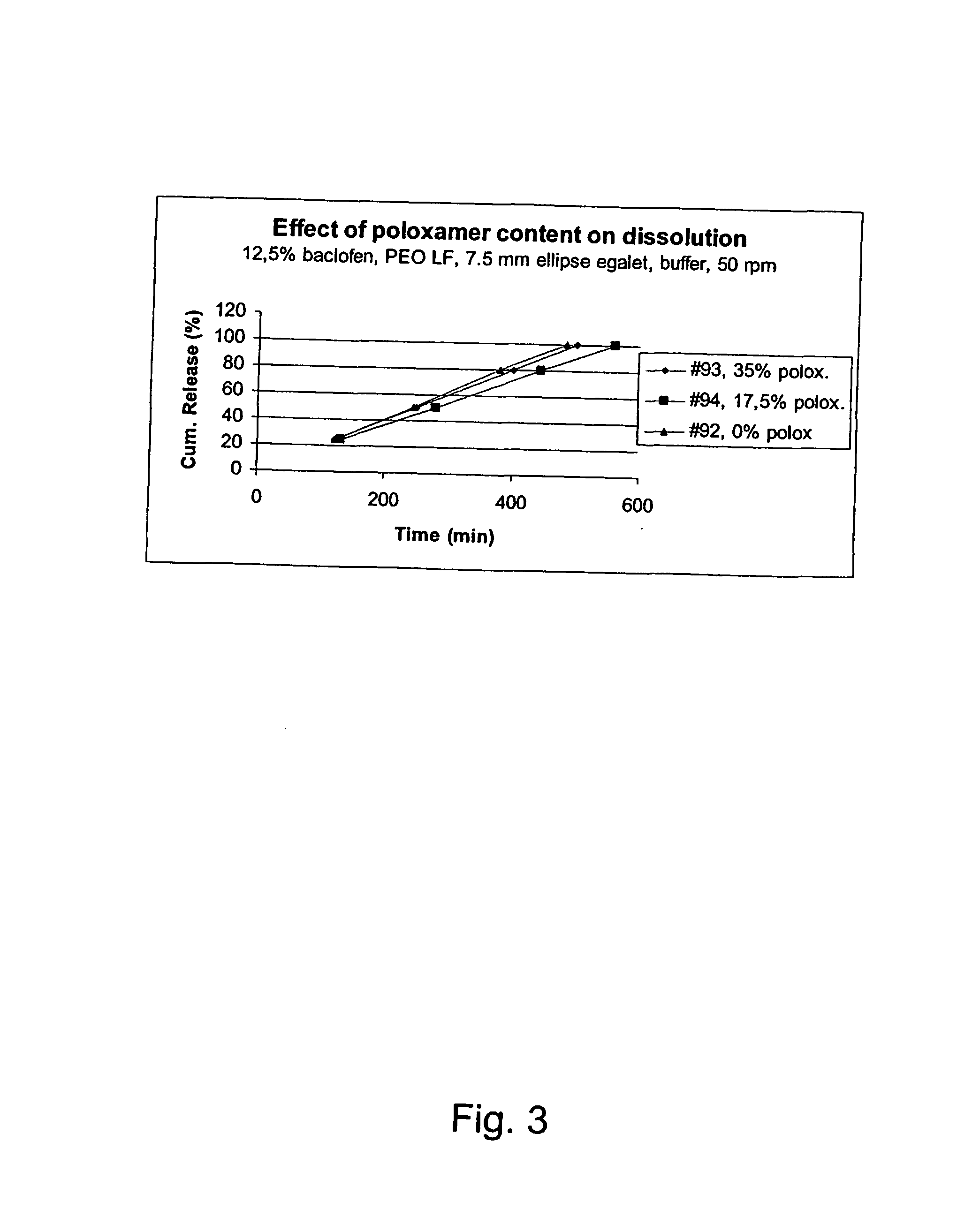
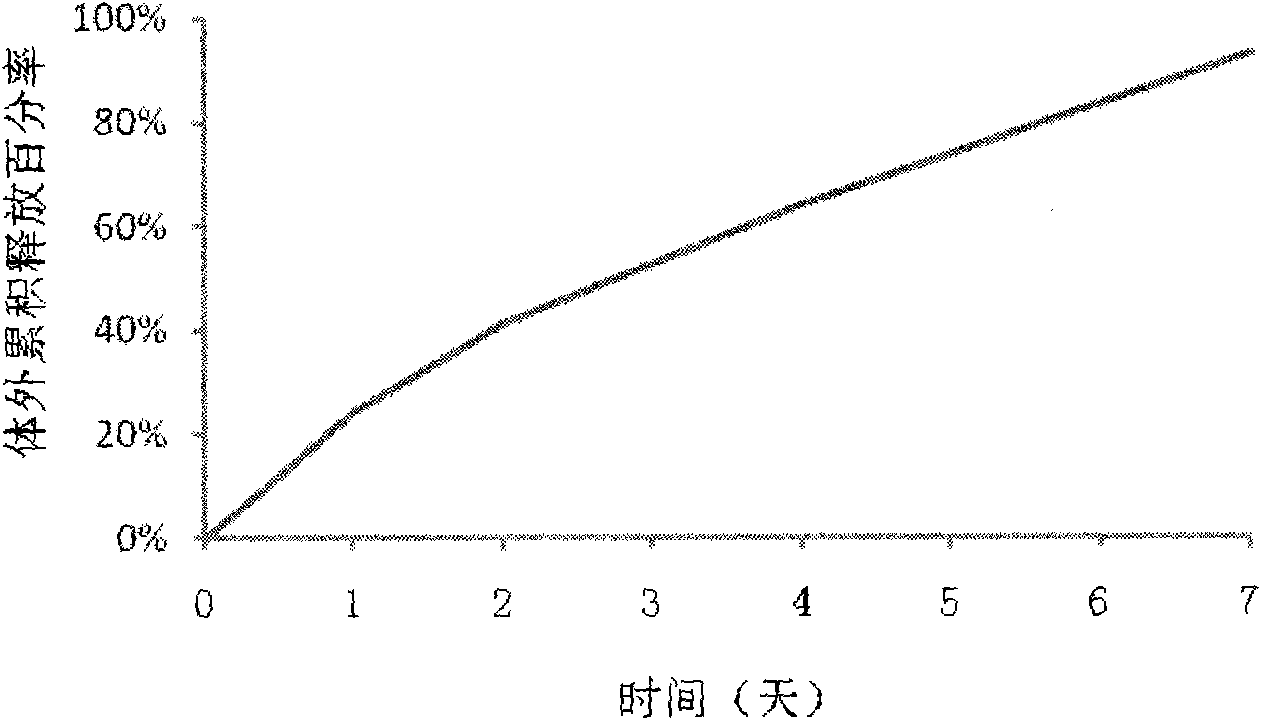
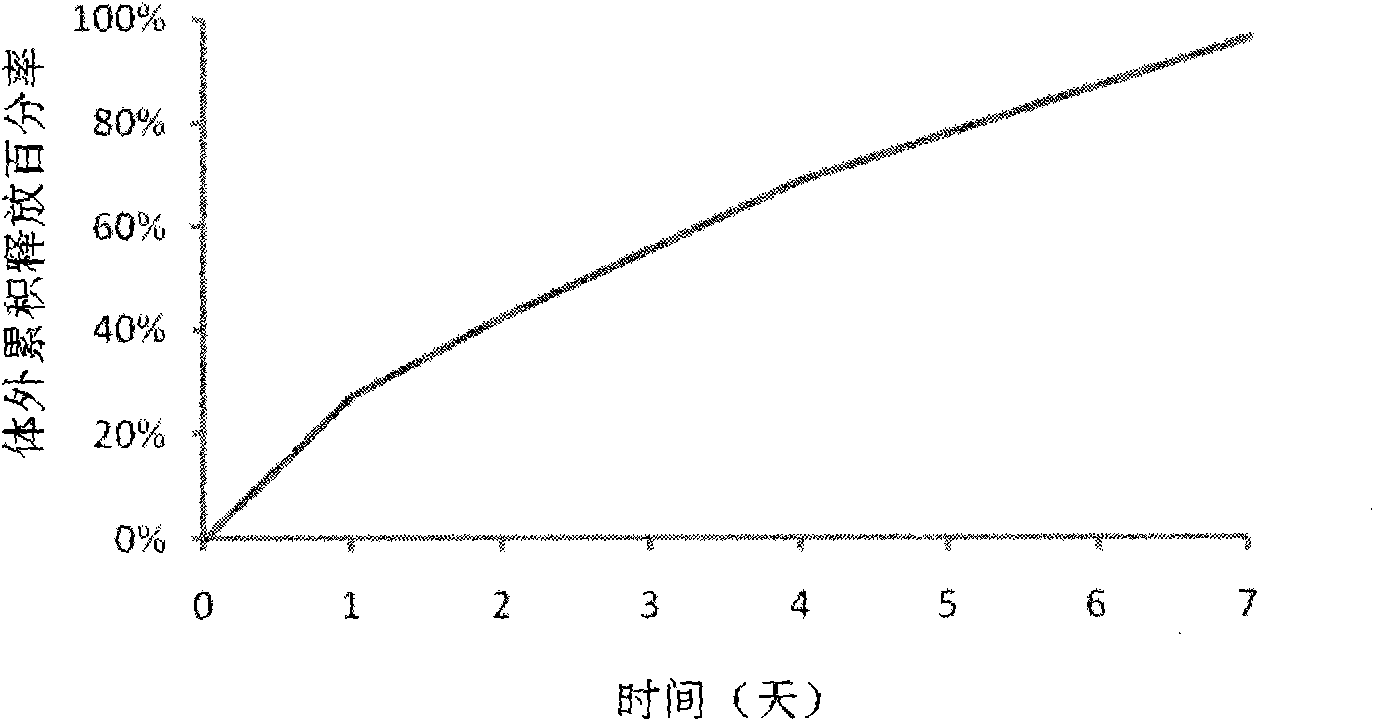
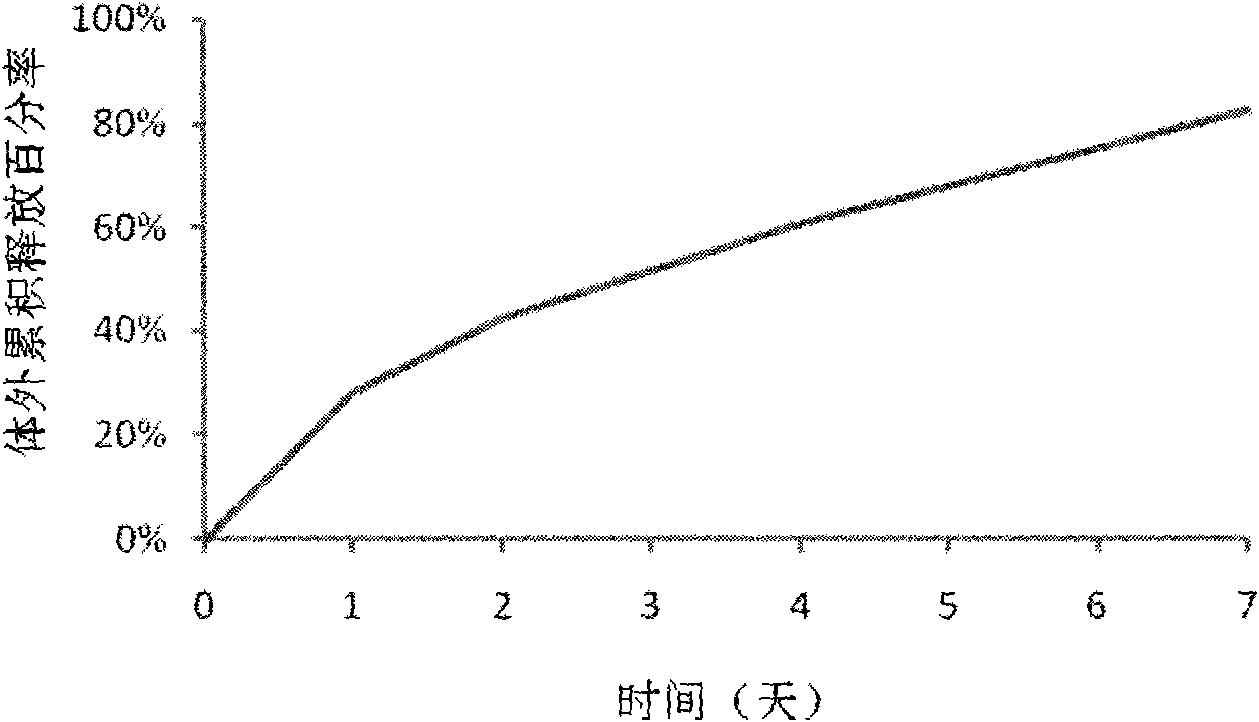
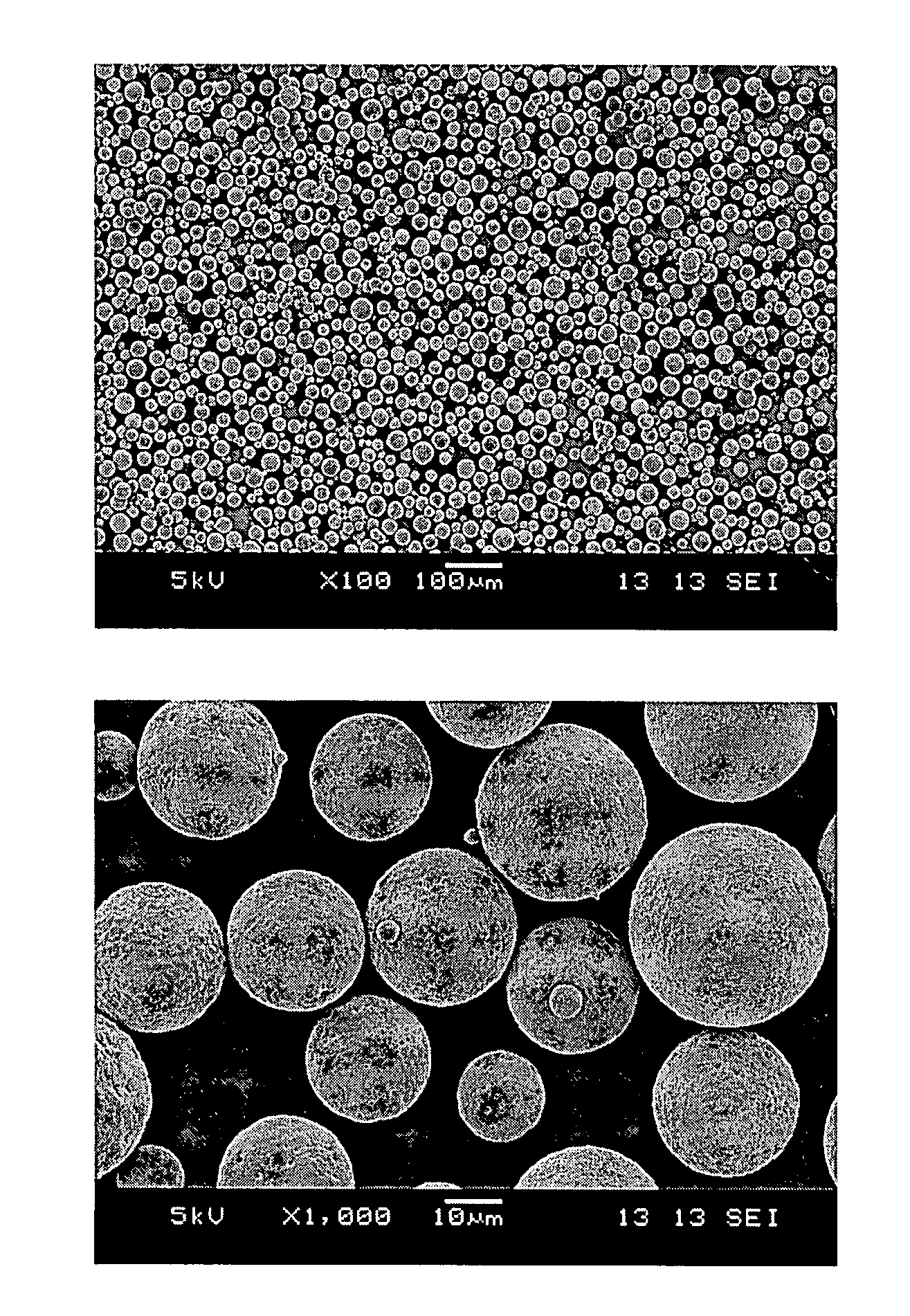
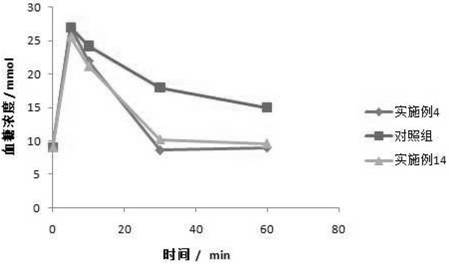
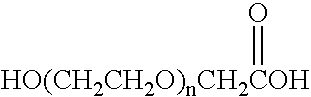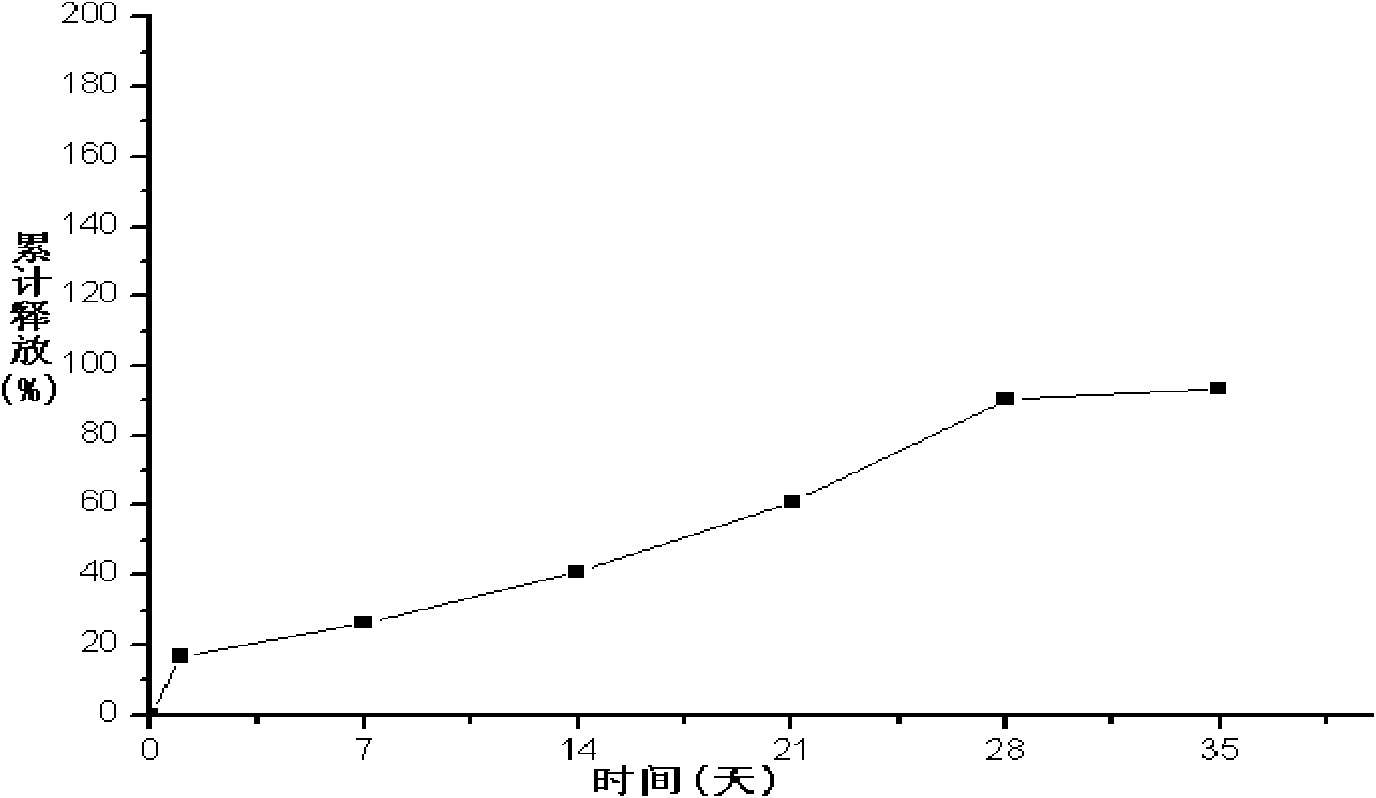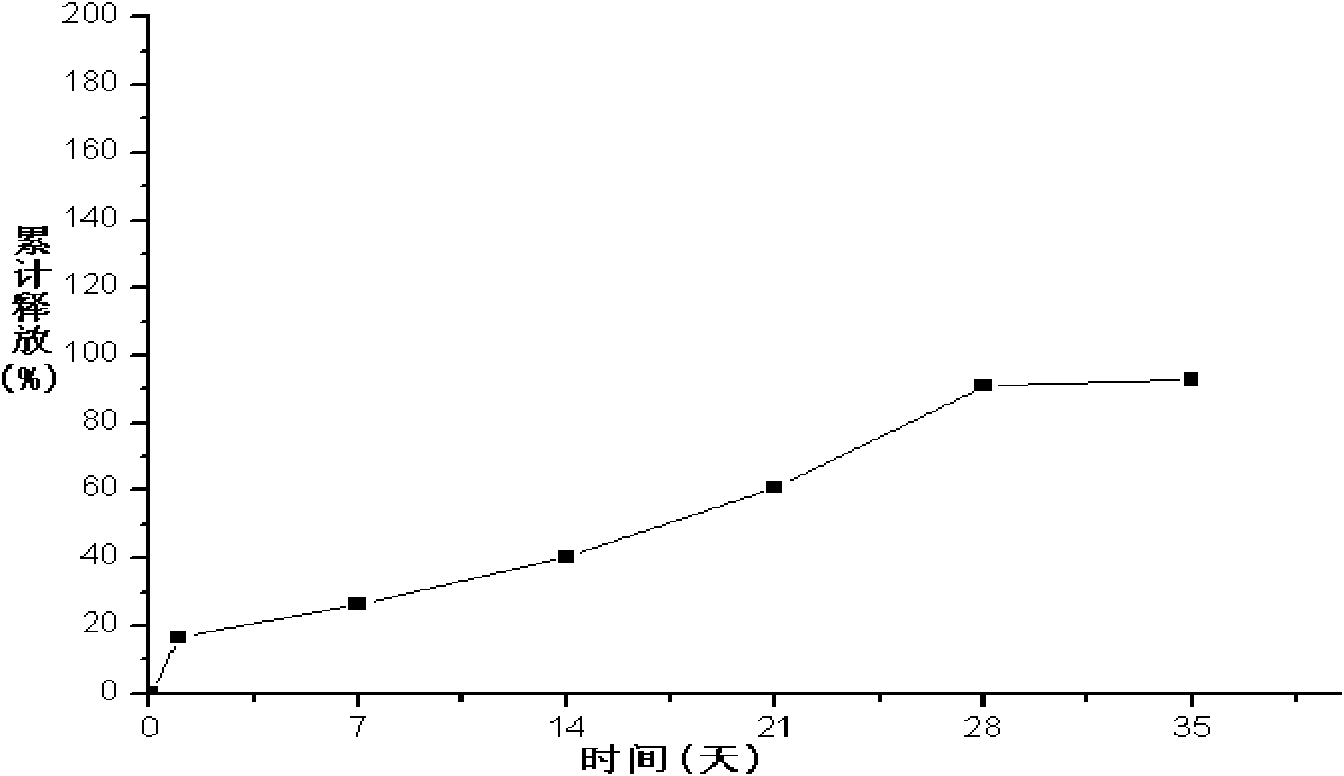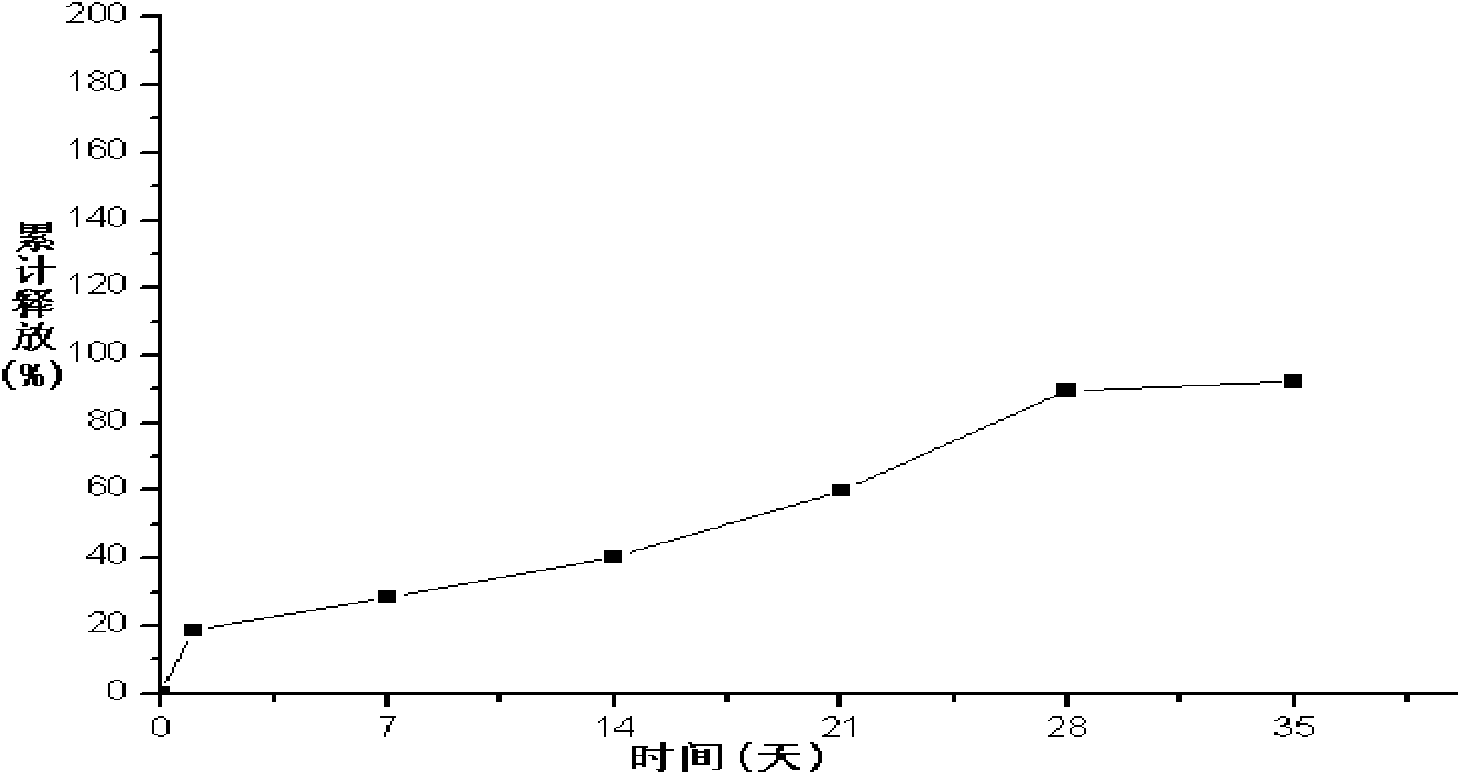Patents
Literature
1156 results about "Glycollic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycolic acid (hydroacetic acid or hydroxyacetic acid); chemical formula C2H4O3 (also written as HOCH2CO2H), is the smallest α-hydroxy acid (AHA). This colorless, odorless, and hygroscopic crystalline solid is highly soluble in water. It is used in various skin-care products.
Acid coated sand for gravel pack and filter cake clean-up
InactiveUS20040055747A1Enhances filter cake removalSpeed up the flowCleaning apparatusFluid removalCarboxylic acidGlycollic acid
A method of the preparation and utilization of polymerized alpha-hydroxycarboxylic-acid-coated proppants for gravel pack and removal of filter cake that was deposited by reservoir drilling fluid. In a preferred example, polyglycolic-acid-coated sand is used to replace conventional gravel pack sand typically used for gravel packing. Under downhole conditions, the acidic by-product generated from the hydration of polyglycolic-acid-coated sand can break down acid-soluble and / or acid-breakable components embedded in the filter cake. This reaction enhances the filter cake removal and the flow of hydrocarbon from the producing formation. The polyglycolic-acid-coated sand may be produced by polymerizing a glycolic acid with a natural or synthetic proppant like 20-40 mesh commercial sand, at temperatures of about 210° F. or higher.
Owner:MI
Wound healing polymer compositions and methods for use thereof
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
Bioabsorbable polymeric implants and a method of using the same to create occlusions
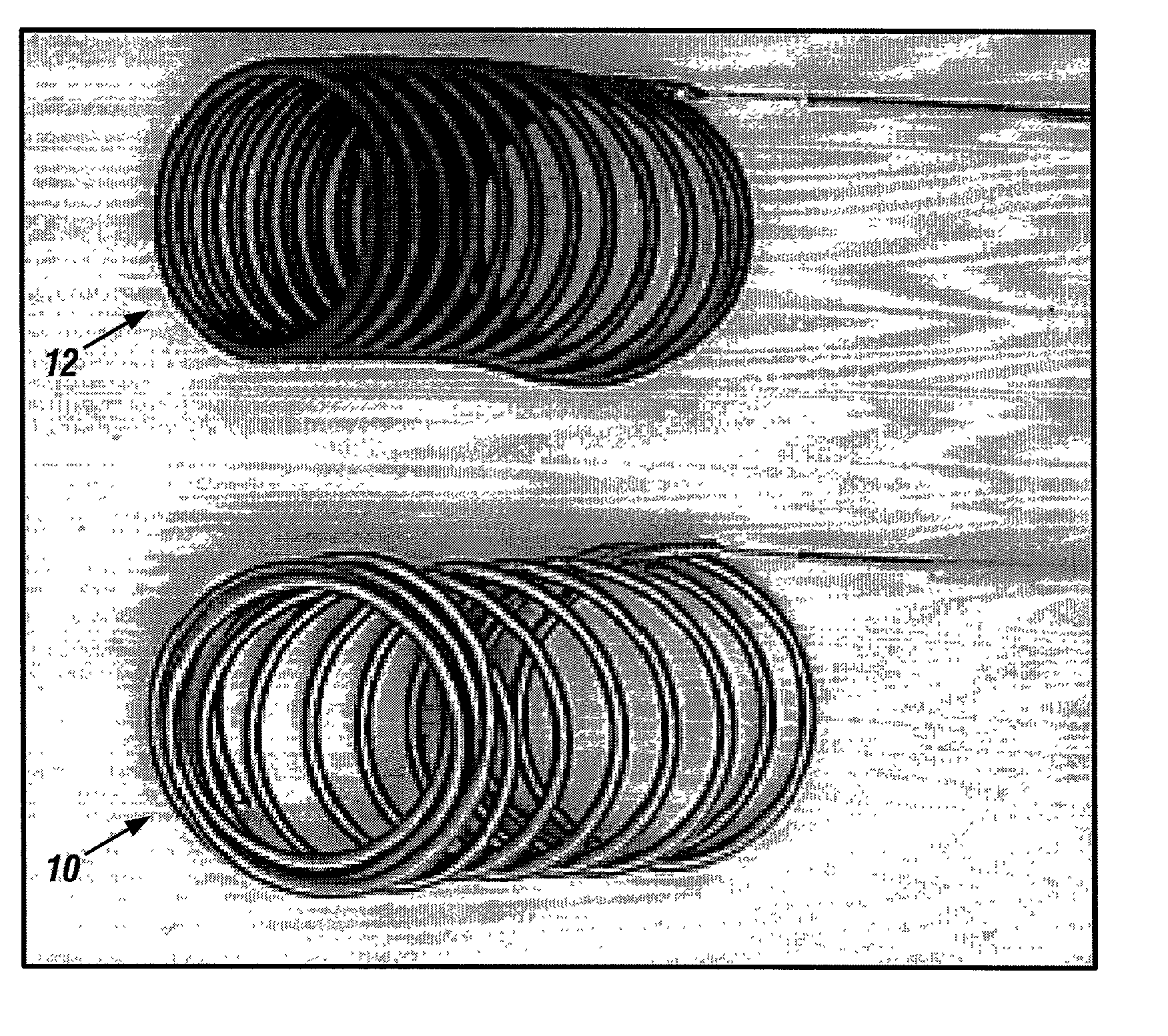
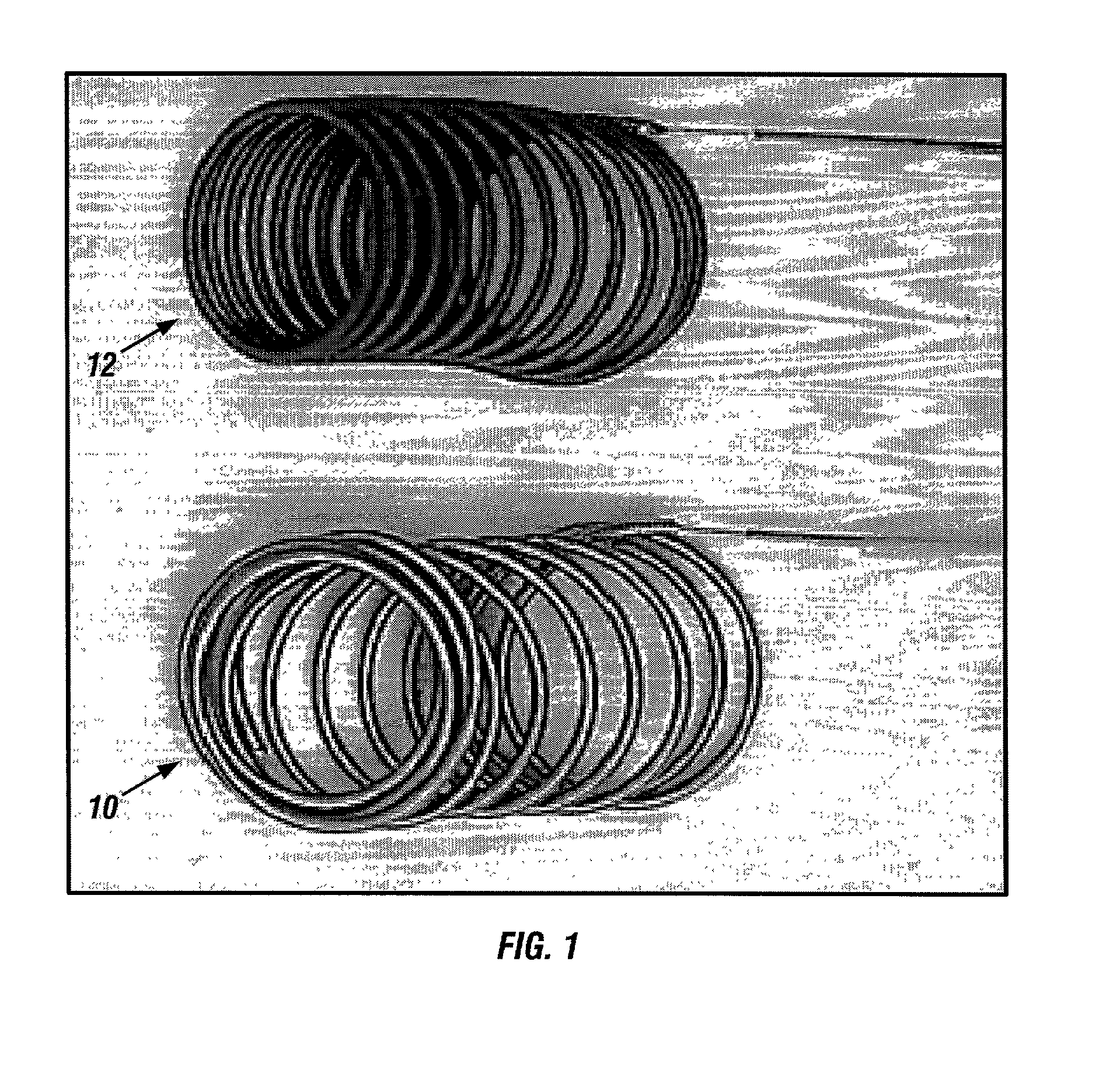
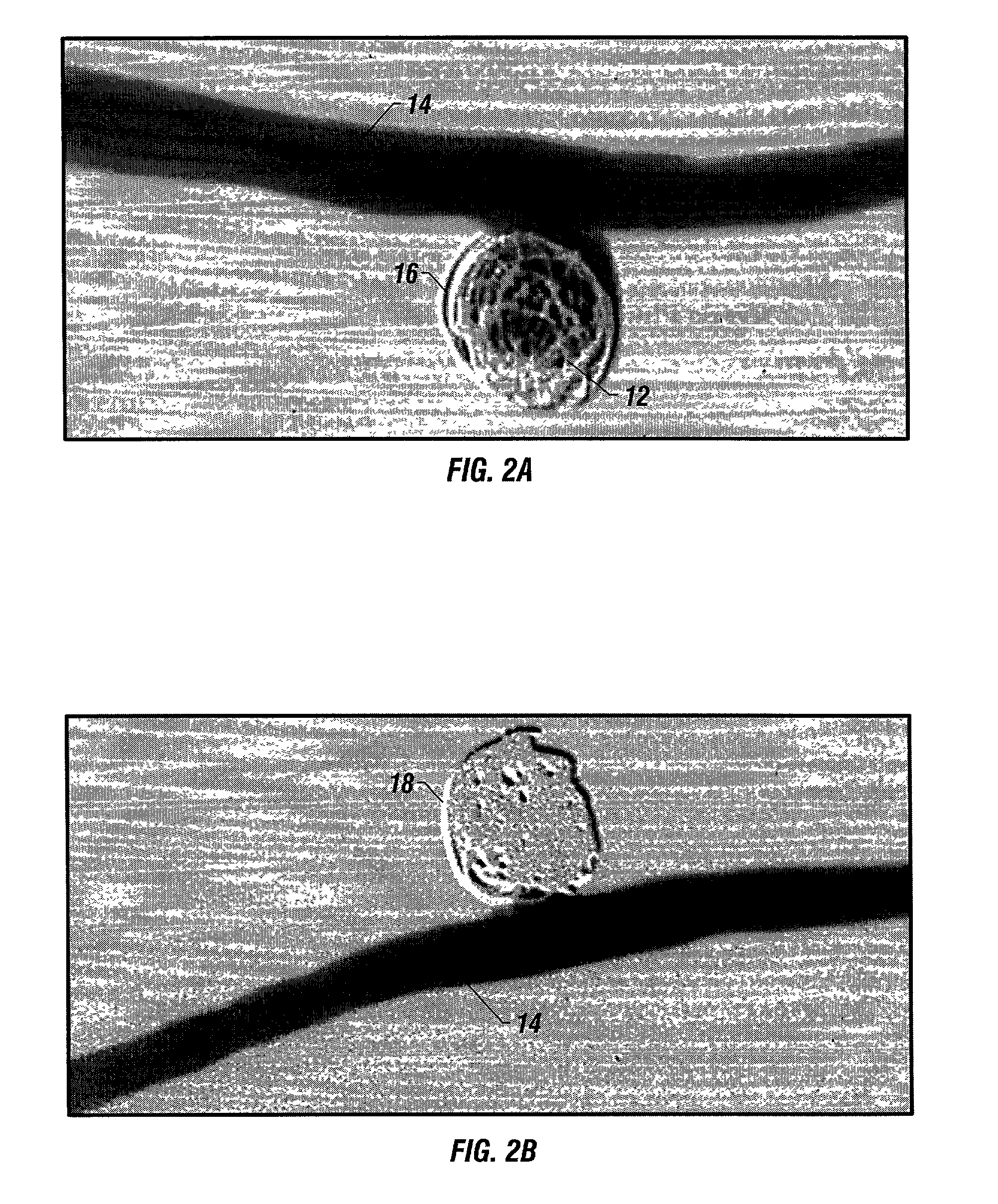
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly˜glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Acid coated sand for gravel pack and filter cake clean-up
InactiveUS6817414B2Speed up the flowGood removal effectCleaning apparatusFluid removalGlycollic acidCarboxylic acid
A method of the preparation and utilization of polymerized alpha-hydroxycarboxylic-acid-coated proppants for gravel pack and removal of filter cake that was deposited by reservoir drilling fluid. In a preferred example, polyglycolic-acid-coated sand is used to replace conventional gravel pack sand typically used for gravel packing. Under downhole conditions, the acidic by-product generated from the hydration of polyglycolic-acid-coated sand can break down acid-soluble and / or acid-breakable components embedded in the filter cake. This reaction enhances the filter cake removal and the flow of hydrocarbon from the producing formation. The polyglycolic-acid-coated sand may be produced by polymerizing a glycolic acid with a natural or synthetic proppant like 20-40 mesh commercial sand, at temperatures of about 210° F. or higher.
Owner:MI
Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices
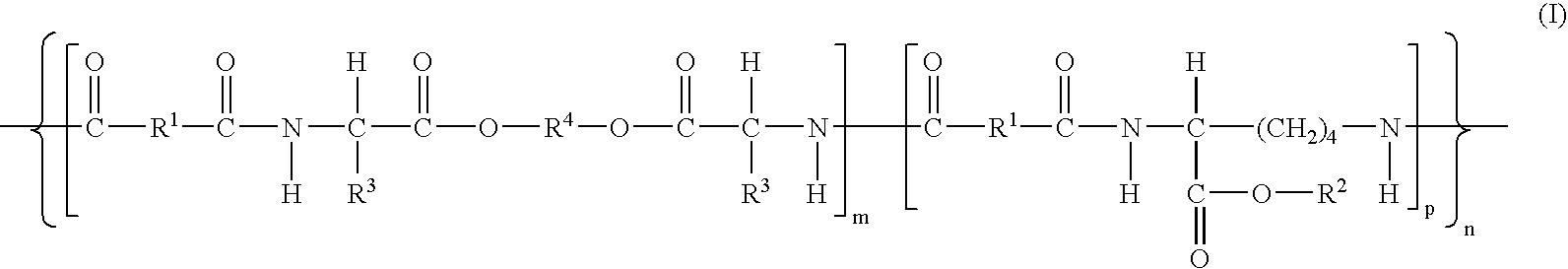
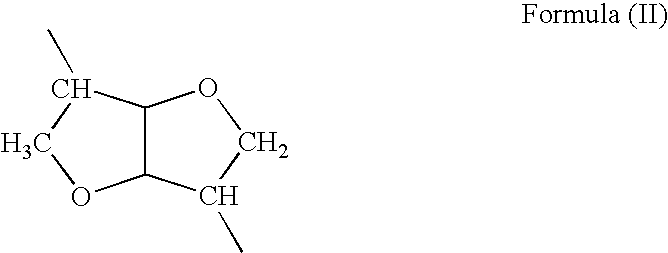
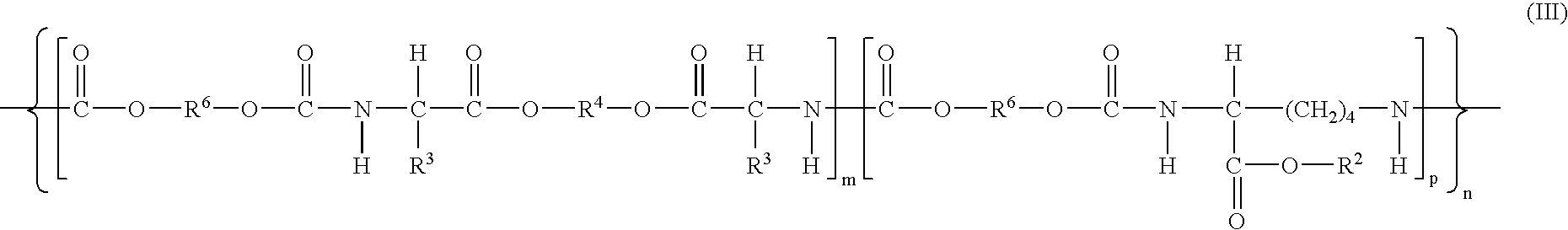
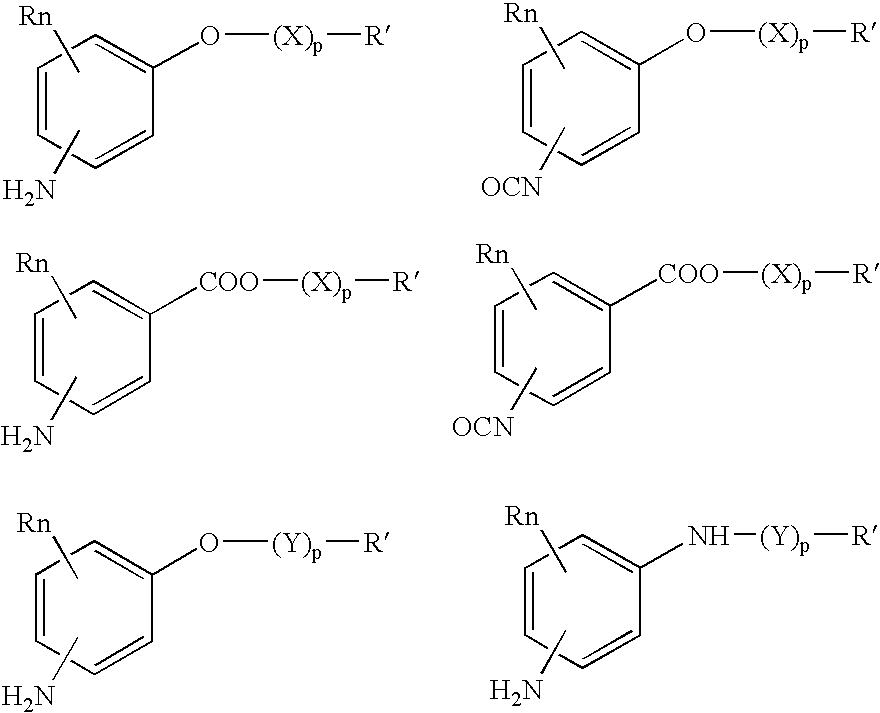
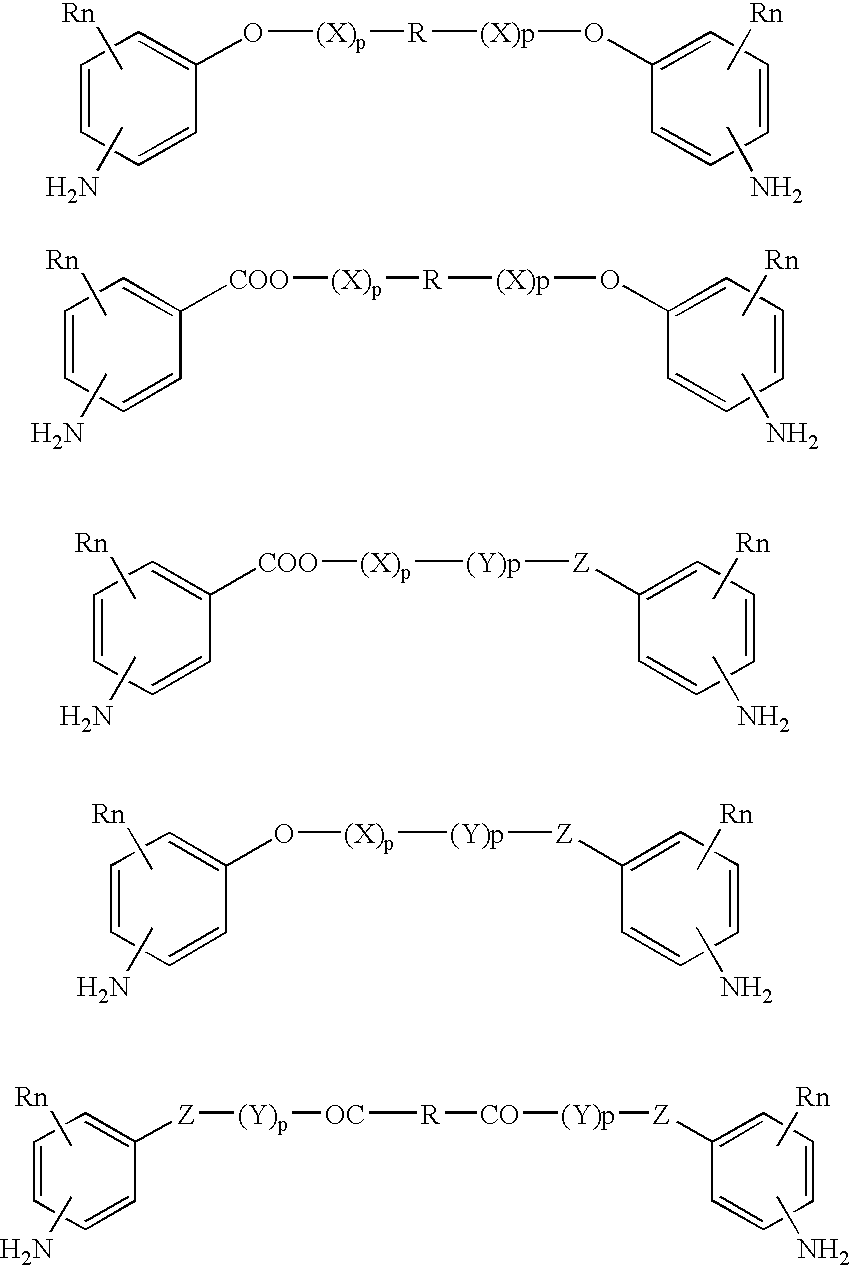
Absorbable polyurethanes, polyamides and polyester urethanes prepared from at least one compound selected from:or the diamines and diisocyanates thereof, wherein each X represents a member independently selected from —CH2COO— (glycolic acid moiety), —CH(CH3)COO— (lactic acid moiety), —CH2CH2OCH2COO— (dioxanone), —CH2CH2CH2CH2CH2COO— (caprolactone moiety), —(CH2)yCOO— where y is one of the numbers 2, 3, 4 or 6-24 inclusive, and —(CH2CH2O)z′CH2COO— where z′ is an integer between 2 and 24, inclusive; each Y represents a member independently selected from —COCH2O— (glycolic ester moiety), —COCH(CH3)O— (lactic ester moiety), —COCH2OCH2CH2O— (dioxanone ester), —COCH2CH2CH2CH2CH2O— (caprolactone ester), —CO(CH2)mO— where m is an integer between 2, 3, 4 or 6-24 inclusive, —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; R′ is hydrogen, benzyl or an alkyl group, the alkyl group being either straight-chained or branched; p is an integer between 1 and 4, inclusive; and Rn represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid and —NO2, which is attached directly to an aromatic ring or attached through an aliphatic chain. Absorbable polymers prepared from these compounds are useful for drug delivery, tissue engineering, tissue adhesives, adhesion prevention and other implantable medical devices.
Owner:BEZWADA BIOMEDICAL LLC
Polymeric nanoparticles with enhanced drug-loading and methods of use thereof
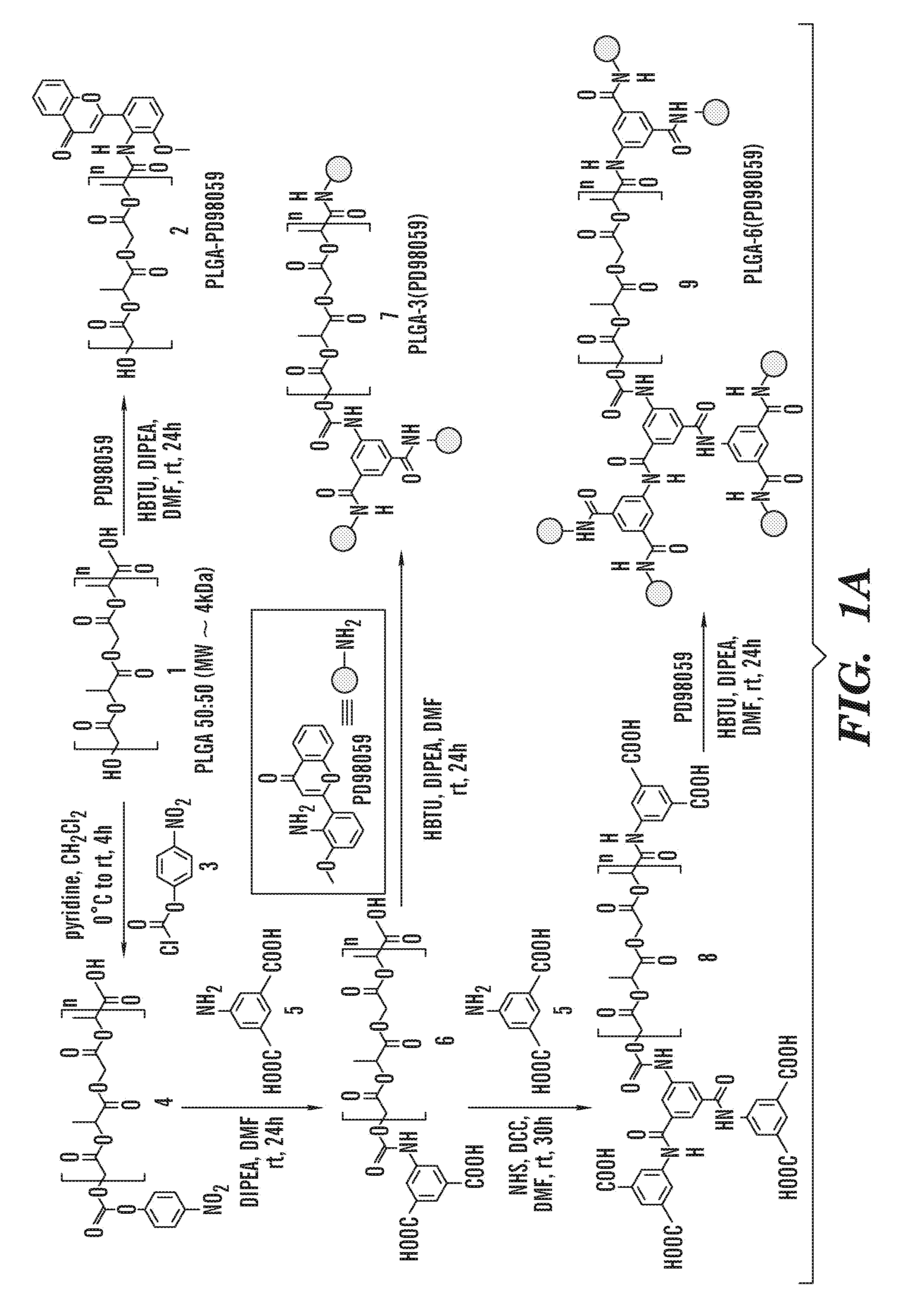
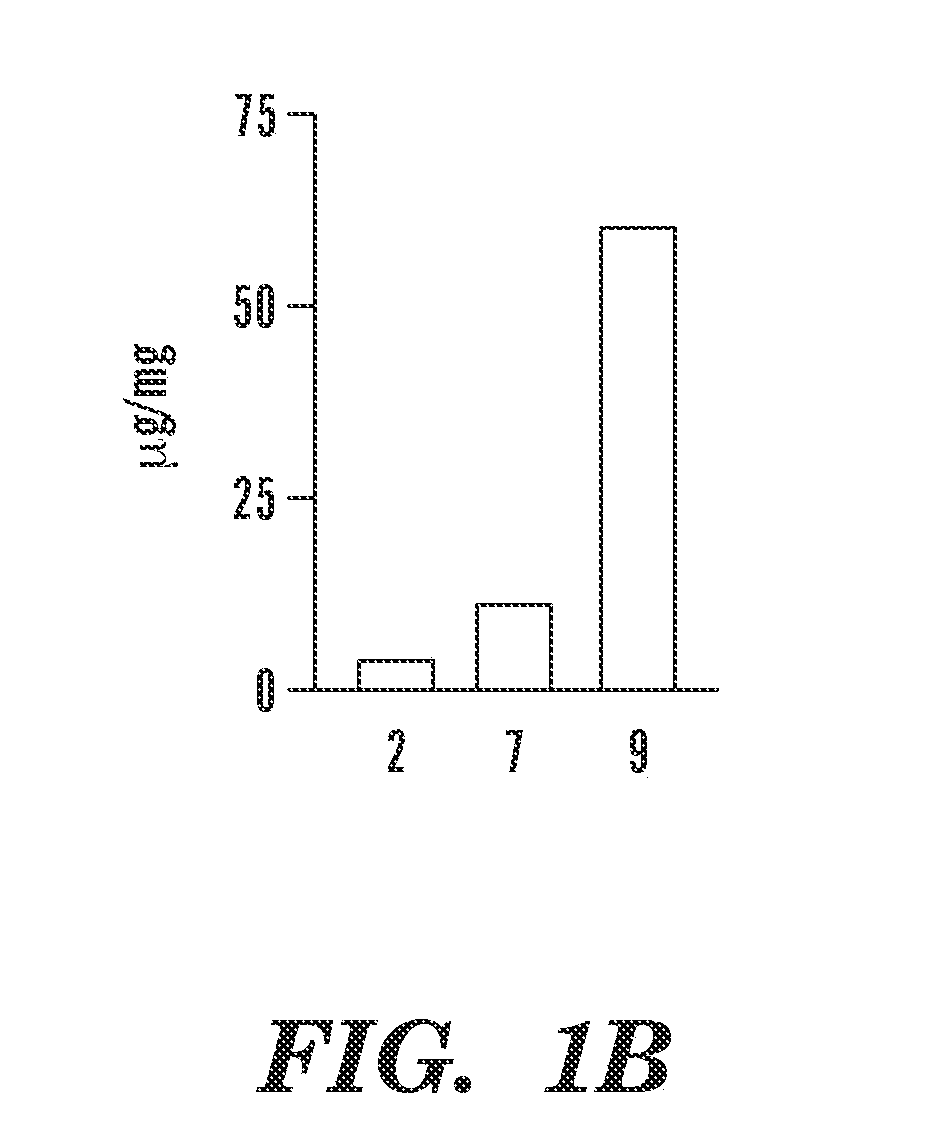
The invention is directed to modified polymers with increased drug-loading including compounds of formula (I): wherein Z is a poly(lactic-co-glycolic acid) (PLGA) polymer having molecular weight from 1-15 kDa and where the ratio of lactide to glycolide in the PLGA polymer is from 1:10 to 10:1; formula (II) R1 are independently H, R2, OH, O-alkyl, —O—R2, NH—R2, -linker-R2, or -and R2 are independently one or more therapeutic agents. The invention is also directed to nanoparticle drug delivery systems including a PLGA-b-PEG block copolymer; and a stabilizer and to drug delivery systems including PLGA-b-PEG block copolymer polyvinyl alcohol (PVA) nanoparticle; and the modified polymer substantially as described herein.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
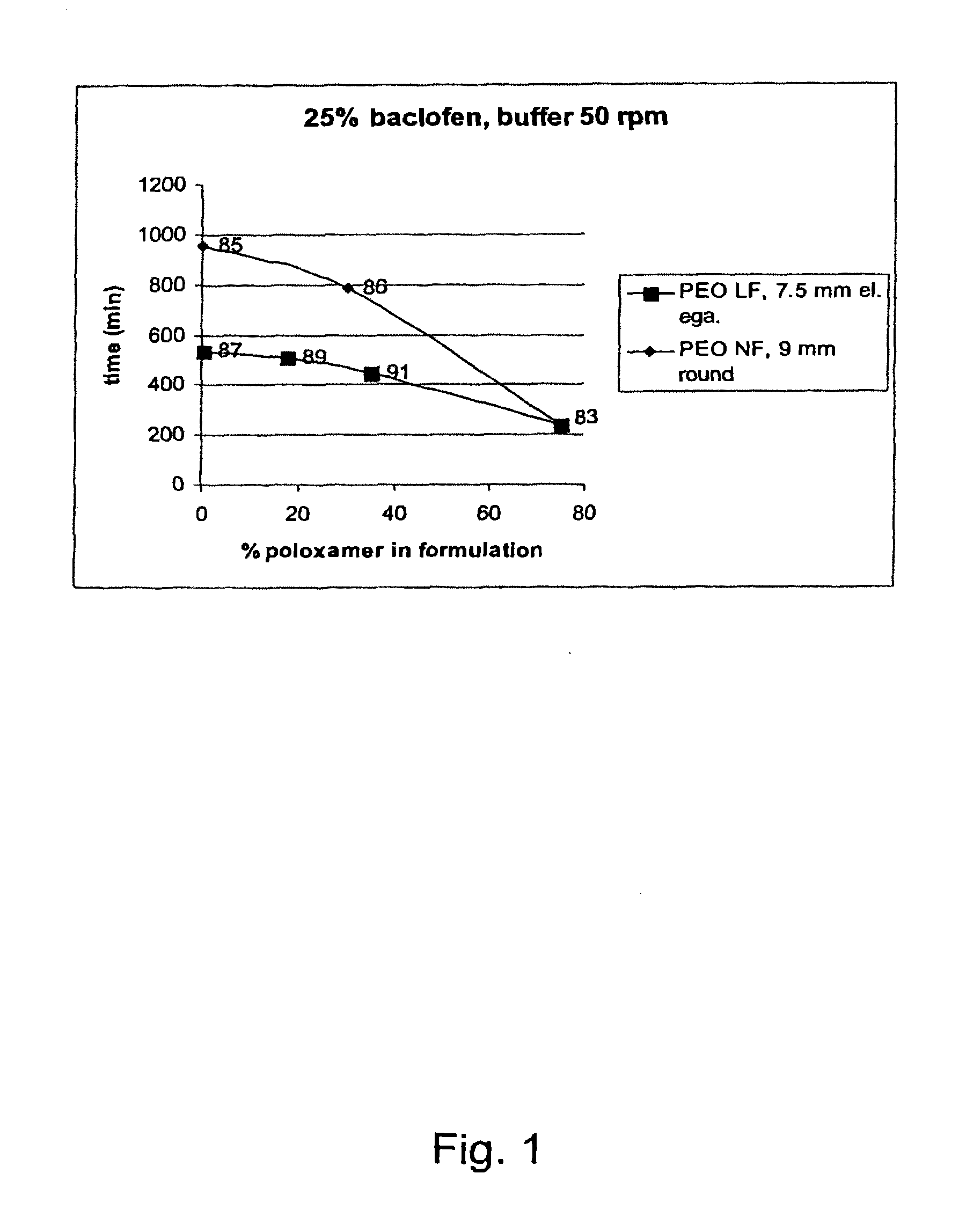
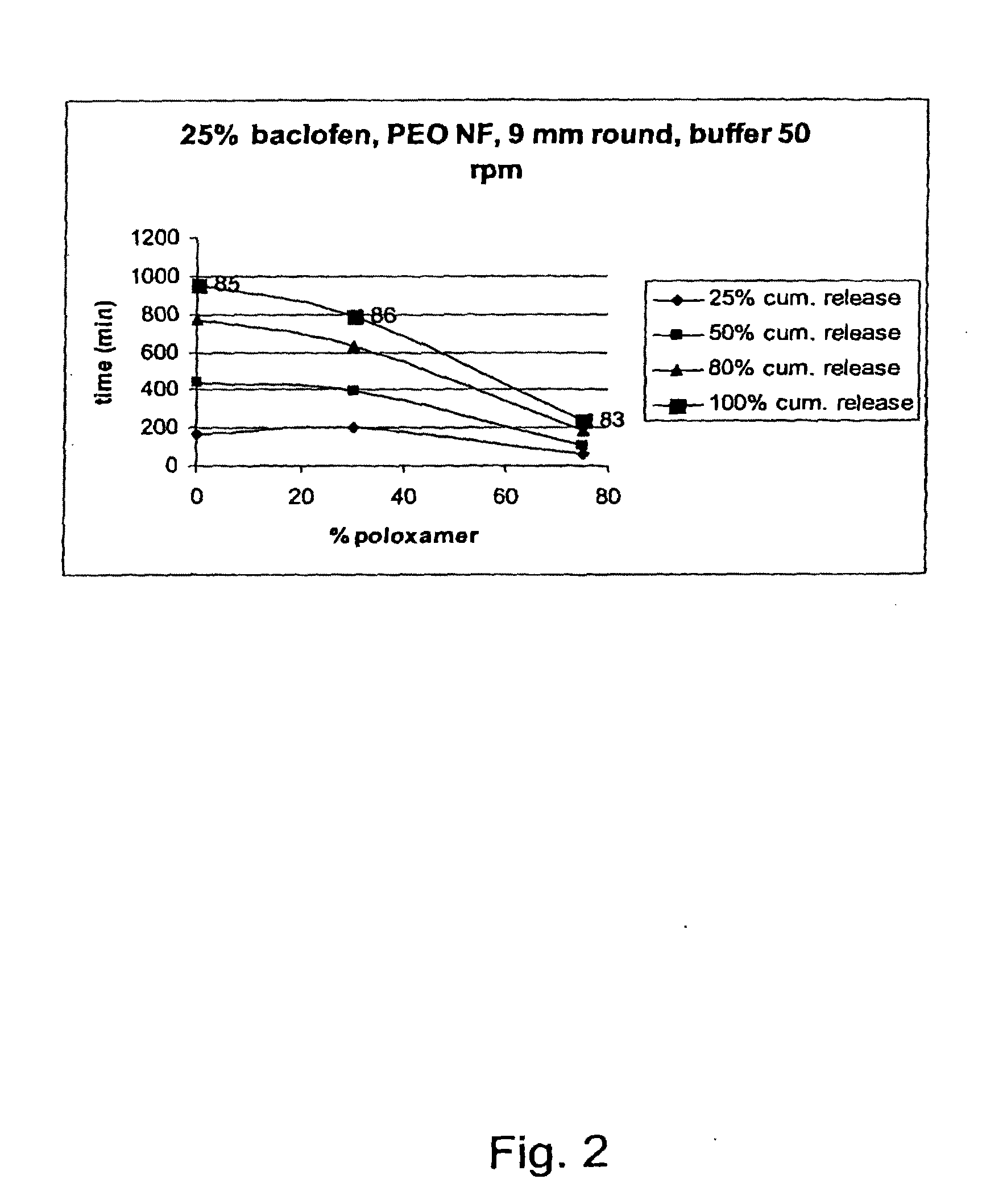
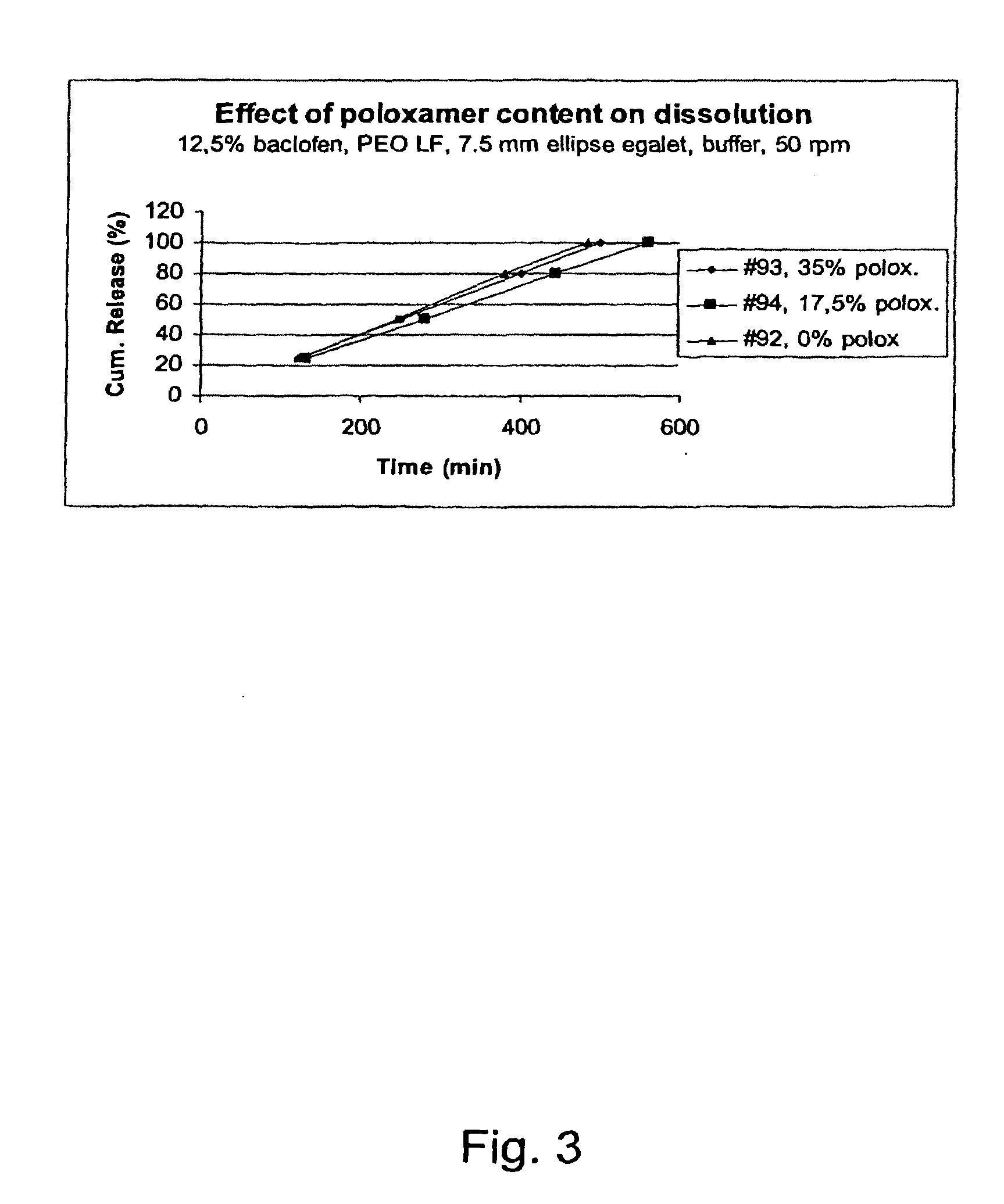
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Mixed micellar delivery system and method of preparation
InactiveUS6221378B1Antibacterial agentsOrganic active ingredientsOctylphenoxy PolyethoxyethanolChamomile extract
A mixed micellar pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an lkali metal C8 to C22 alkyl sulphate, alkali metal salicylate, a pharmaceutically acceptable edetate and at least one absorption enhancing compounds. The absorption enhancing compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, octylphenoxypolyethoxyethanol, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linolenic acid, borage oil, evening of primrose oil, trihydroxy oxo cholanylglycine, glycerin, polyglycerin, lysine, polylysine, triolein and mixtures thereof. The amount of each absorption enhancing compound is present in a concentration of from 1 to 10 wt. / wt. % of the total formulation, and the total concentration of absorption enhancing compounds are less than 50 wt. / wt. % of the formulation. Preferably, the formulation is administered, in combination with a propellant, to the buccal cavity, using a metered dose dispenser.
Owner:GENEREX PHARMA INC
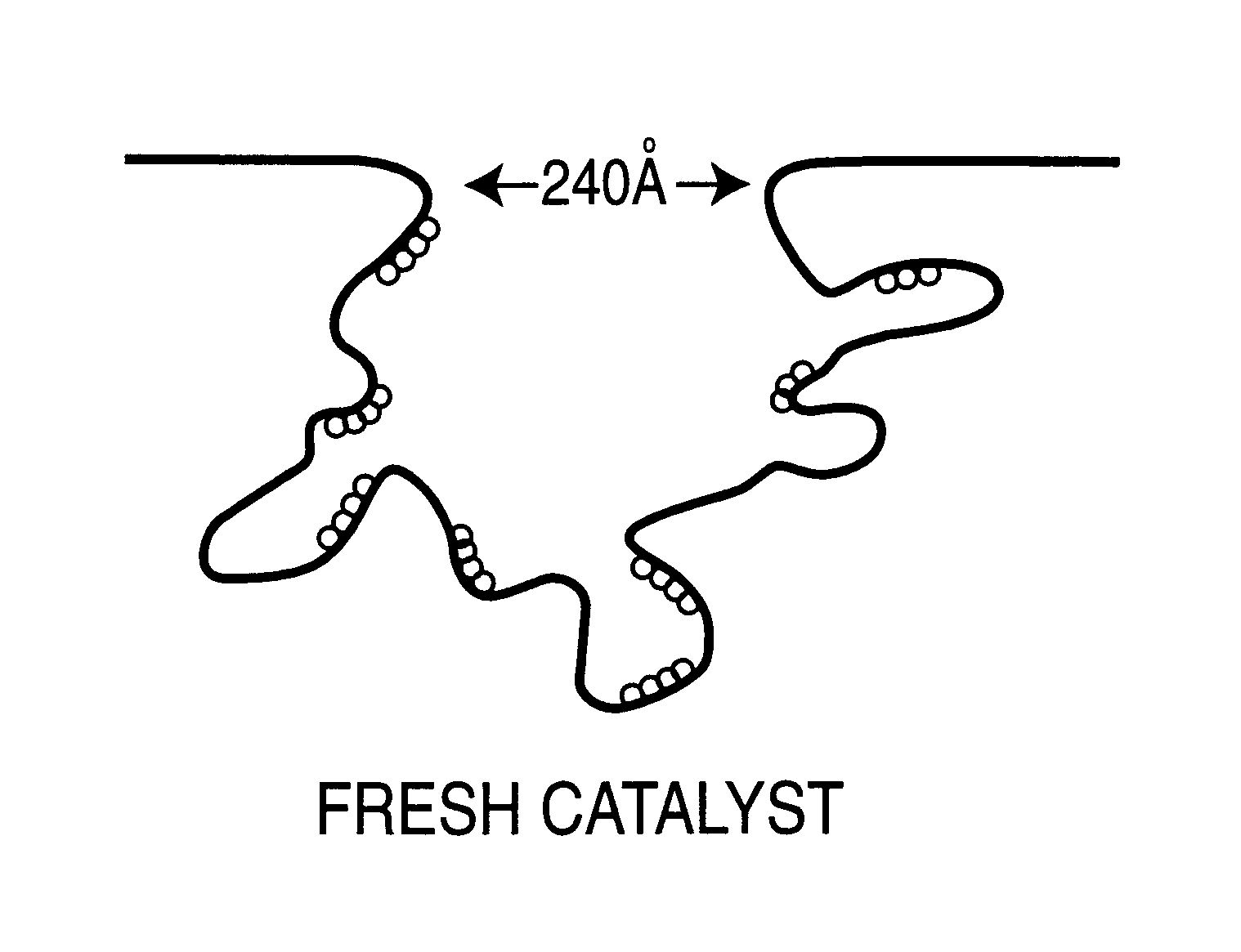
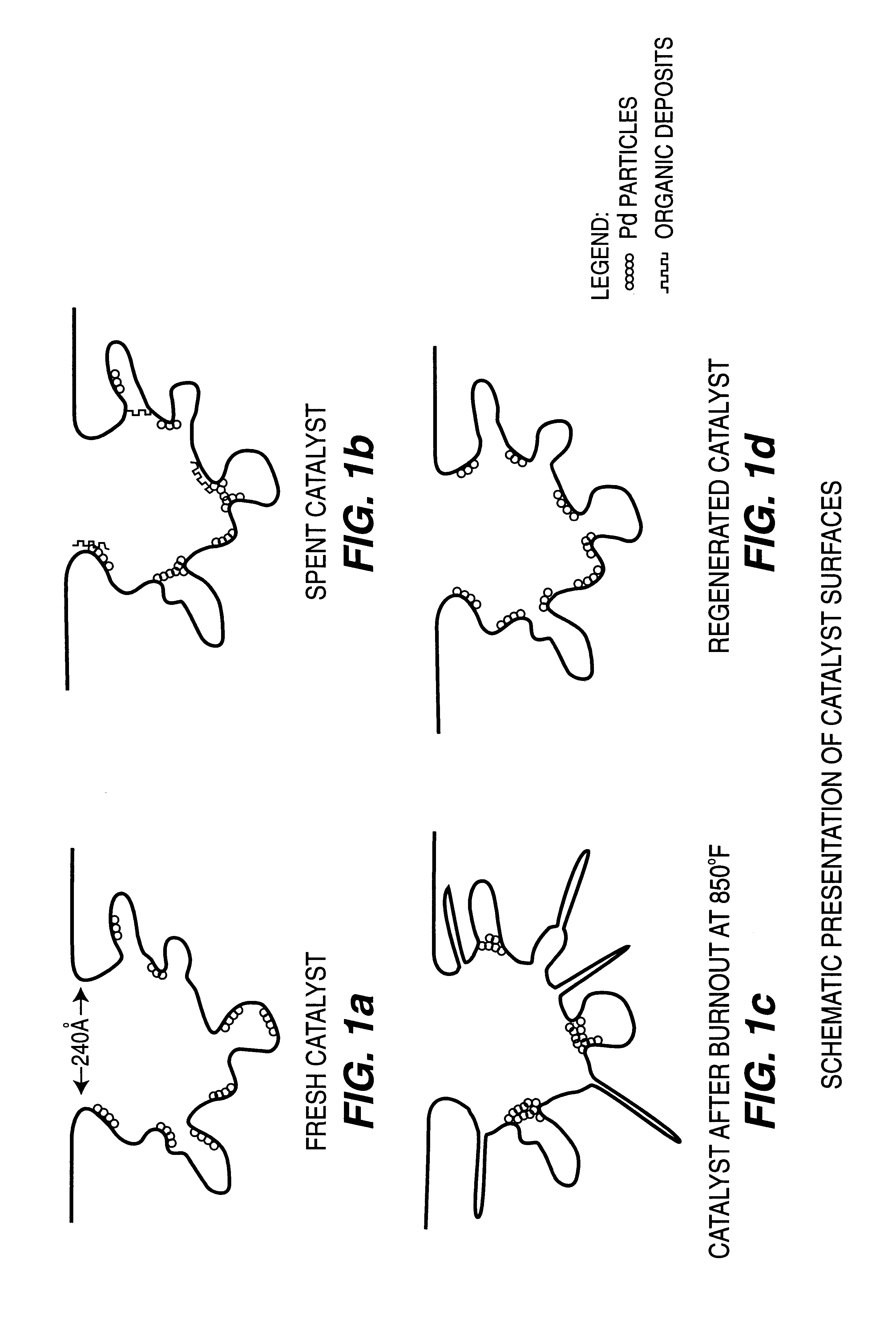
Regeneration of used supported noble metal catalysts
InactiveUS6740615B2Efficient removalImproves expositionHydrogen peroxideOther chemical processesPalladium catalystMetal particle
A method for regenerating used supported noble metal catalysts, which method includes solvent cleaning the used catalyst by contact with a suitable organic liquid cleaning solvent such as alcohols, ketones and such to remove organic deposits from the catalyst, followed by drying and calcining at elevated temperature to remove any remaining organic deposits from the catalyst, then treating the catalyst with an organo-metallic complex forming agent having ionization constant pK1 greater than about 2.5, such as glycolic acid and the like. The organic-metallic complex forming agent acts to break down large clusters of noble metal particles such as palladium (Pd) and redistributes the metal particles on the catalyst support such as alumina (Al2O3) in the same or other larger pores, so as to increase catalyst surface area and catalytic activity to provide a catalytic activity level at least 80% or even exceeding that of the fresh catalyst. This regeneration method is particularly useful for regenerating used supported palladium catalysts utilized for hydrogenation of ethyl anthraquinone (EAQ) for producing hydrogen peroxide (H2O2) product.
Owner:POROCEL INT LLC +1
Aerosol formulations for buccal and pulmonary application
InactiveUS6436367B1Antibacterial agentsOrganic active ingredientsChamomile extractOleic Acid Triglyceride
A mixed micellar aerosol pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an alkali metal lauryl sulphate, at least three micelle forming compounds, a phenol and a propellant. The micelle forming compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linoleic acid, linolenic acid, monoolein, monooleates, monolaurates, borage oil, evening of primrose oil, menthol, trihydroxy oxo cholanyl glycine and pharmaceutically acceptable salts thereof, glycerin, polyglycerin, lysine, polylysine, triolein, polyoxyethylene ethers and analogues thereof, polidocanol alkyl ethers and analogues thereof. The amount of each micelle forming compound is present in a concentration of from 1 to 20 wt. / wt. % of the total formulation, and the total concentration of micelle forming compounds are less than 50 wt. / wt. % of the formulation. The propellant, e.g. a fluorocarbon propellant, provides enhanced absorption of the pharmaceutical agent.
Owner:GENEREX PHARMA
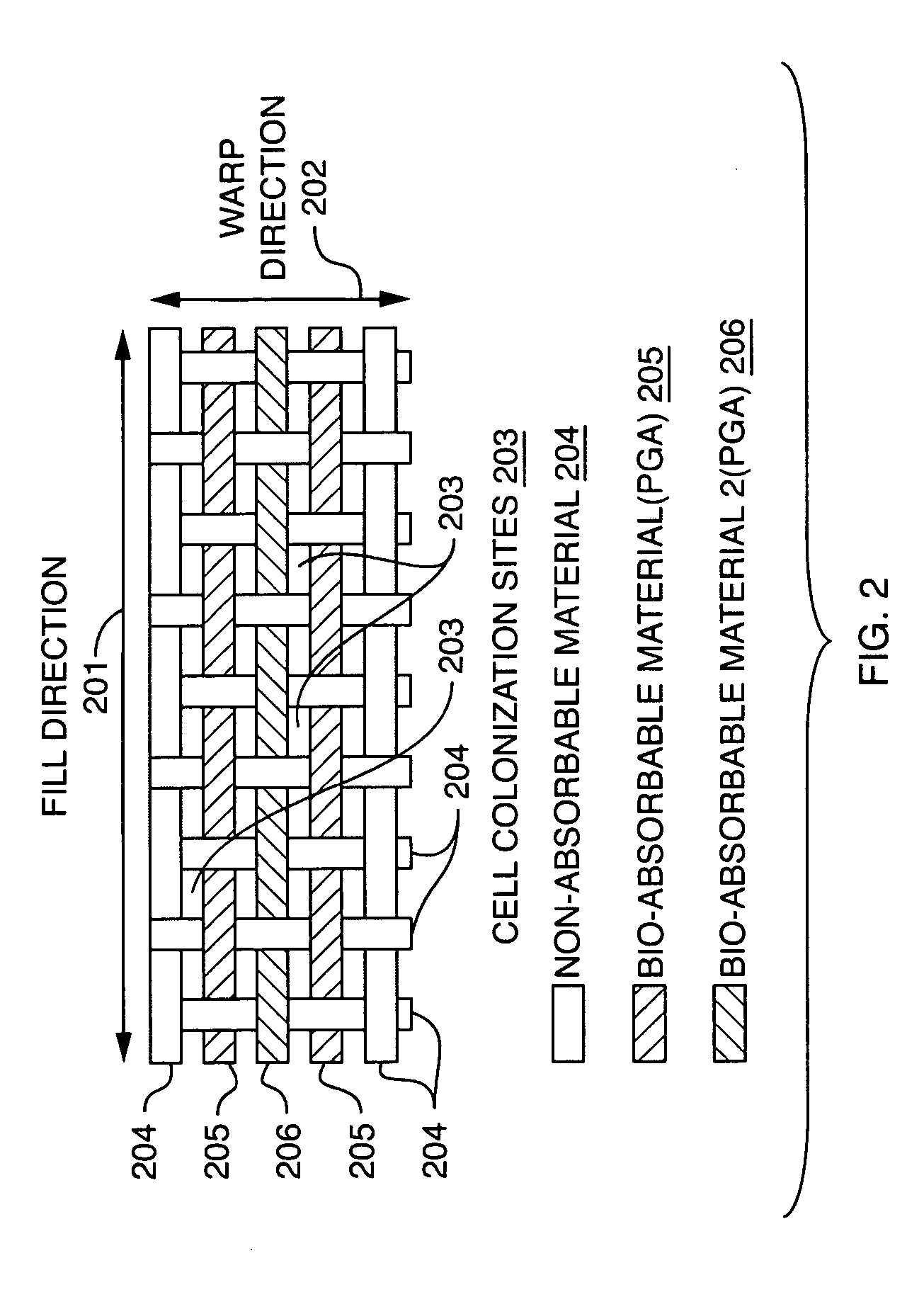
Controlled absorption biograft material for autologous tissue support
An implantable tissue grafting medical system and material using a combination of bio-absorbable and non bio-absorbable fibers and materials such as Poly Glycolic Acid (PGA) and polyester (PET), provides a permeable mesh or weave of fibers with an initial interstice size and permeability factor suitable to initial implant requirements, and a pre-engineered bio-absorption pattern and rate that controls the gradual expansion of interstice size within the mesh or weave in one or two dimensions up to a pre-engineered maximum interstice size, consistent with the anticipated rate of tissue regeneration on the implant, while retaining a primary grid or circumferential pattern of non-absorbable fibers at the maximum interstice size for supporting the new tissue for an extended period. Various means for combining materials to obtain initial interstice size, pattern and permeability, with the desired absorption pattern and rate, and the desired end point interstice size and spacing, are also disclosed.
Owner:WARWICK MILLS INC
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Stretch blow molded container and production process thereof
InactiveUS6159416AImprove barrier propertiesHigh mechanical strengthBottlesRefuse receptaclesShear rateVolumetric Mass Density
The invention provides a stretch blow molded container formed from a thermoplastic resin material which comprises polyglycolic acid having (a) a repeating unit represented by the following formula (1): (b) a melt viscosity, eta * [as measured at a temperature of (the melting point, Tm of the polymer+20 DEG C.) and a shear rate of 100 / sec] of 500-100,000 Pa.s; (c) a melting point, Tm of at least 180 DEG C.; (d) a melt enthalpy, DELTA Hm of at least 20 J / g; and (e) a density of at least 1.50 g / cm3 as measured in an unoriented, crystallized form, wherein the stretch blow molded container has tensile strength (in a circumferential direction) of at least 100 MPa and a carbon dioxide permeability (as measured at a temperature of 23 DEG C. and 80% relative humidity; in terms of the thickness of 50 mu m) of 300 cc / m2.day.atm or smaller at the body sidewall thereof, and a production process thereof.
Owner:KUREHA KAGAKU KOGYO KK
Degradable compound biomaterial membrane for medical purpose
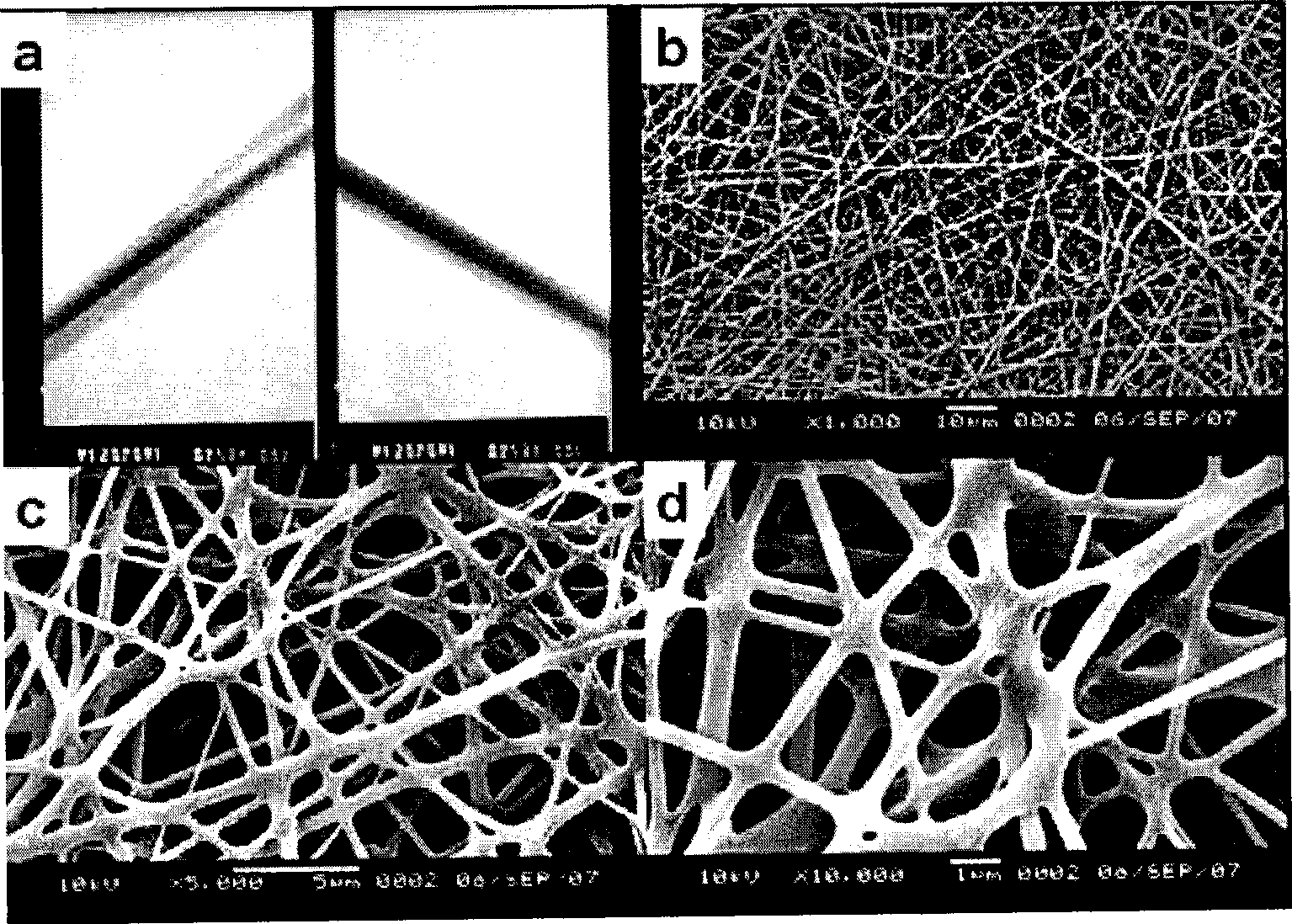
InactiveCN1994476AHigh porosityLarge specific surface areaAbsorbent padsBandagesFiberElectrospinning
The invention relates to a method for preparing composite biological medical material used to avoid adhering organisms, wherein said film is formed by two layers; the layer used as base material is formed by degradable composite macromolecule polylactic acid, glycolic acid, or their copolymer, treated by static spin technique, to make film fiber into nanometer level; then coats one layer of degradable natural macromolecule chitose or relative derivant on the base film, to be treated to obtain the degradable composite biological medical film; and the invention can add different amounts of medical elasticizer between two layers to adjust the degrade speed. The invention can improve immunity with haemostasis function, etc.
Owner:BEIJING HUASHI BENQUAN TECH
Nanoparticles containing water-soluble non-peptide low-molecular weight drug
InactiveUS20090317479A1Cause side effectReduce accumulationAntibacterial agentsHeavy metal active ingredientsNanoparticlePolyethylene glycol
Drug-containing nanoparticles are provided that enable effective targeting and sustained-release of a water-soluble, non-peptide, low-molecular weight drug and cause reduced accumulation of the drug in the liver. The nanoparticles containing a water-soluble, non-peptide, low-molecular weight drug are obtained by hydrophobicizing the water-soluble, non-peptide, low-molecular weight drug by a metal ion, and reacting the hydrophobicized drug with a poly(lactic acid)-polyethylene glycol block copolymer or a poly(lactic-co-glycolic acid)-polyethylene glycol block copolymer. The nanoparticles have favorable targeting and sustained-release properties and cause reduced accumulation of the drug in the liver.
Owner:LTT BIO PHARMA
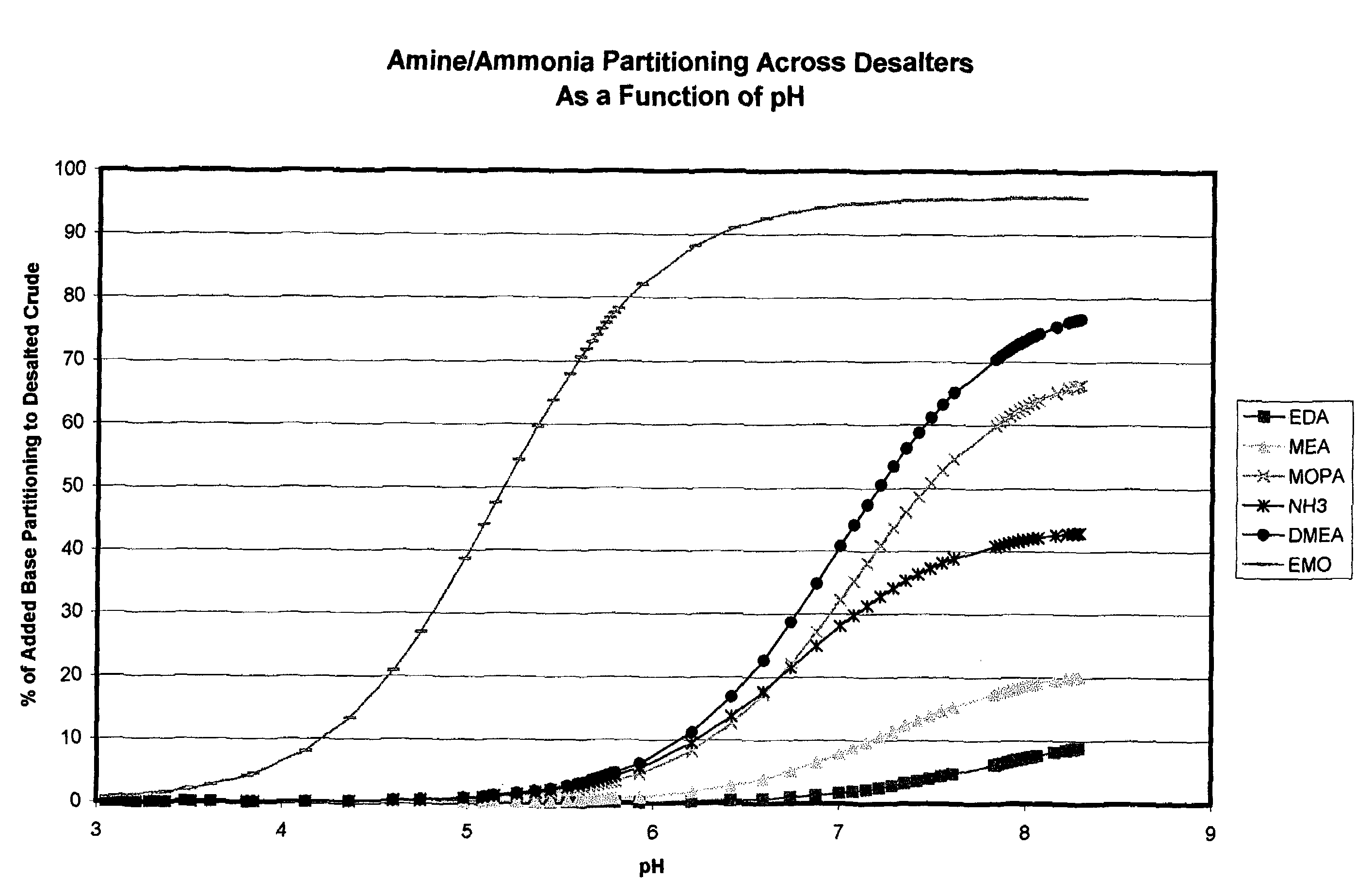
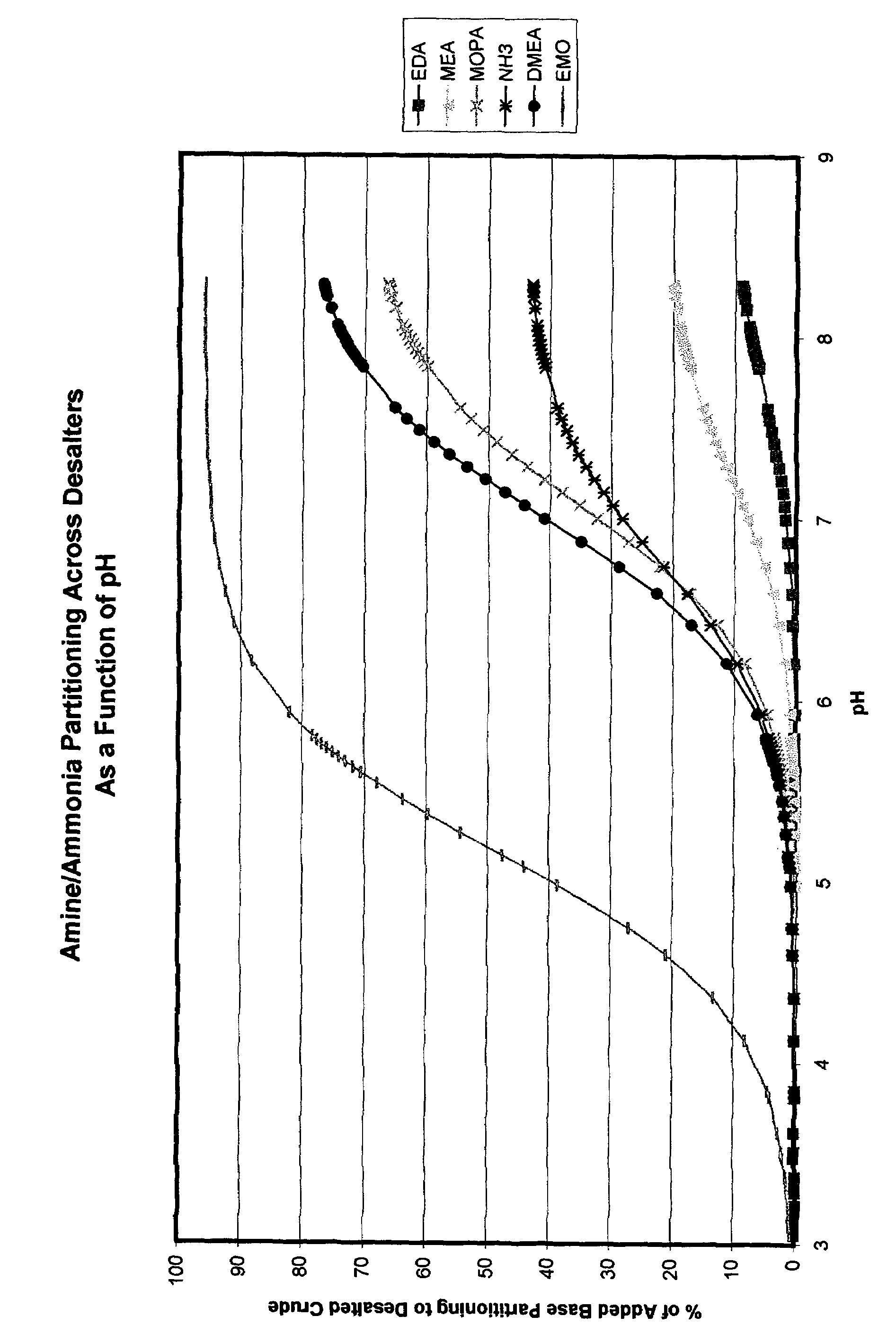
Additives to enhance metal and amine removal in refinery desalting processes
ActiveUS7497943B2Dewatering/demulsification with chemical meansDewatering/demulsification with electric/magnetic meansWash waterChloroacetic acids
It has been discovered that metals and / or amines can be removed or transferred from a hydrocarbon phase to a water phase in an emulsion breaking process by using a composition that contains water-soluble hydroxyacids. Suitable water-soluble hydroxyacids include, but are not necessarily limited to glycolic acid, gluconic acid, C2-C4 alpha-hydroxy acids, poly-hydroxy carboxylic acids, thioglycolic acid, chloroacetic acid, polymeric forms of the above hydroxyacids, poly-glycolic esters, glycolate ethers, and ammonium salt and alkali metal salts of these hydroxyacids, and mixtures thereof. The composition may also include at least one mineral acid to reduce the pH of the desalter wash water. A solvent may be optionally included in the composition. The invention permits transfer of metals and / or amines into the aqueous phase with little or no hydrocarbon phase undercarry into the aqueous phase. The composition is particularly useful in treating crude oil emulsions, and in removing calcium and other metals therefrom.
Owner:BAKER HUGHES INC
Nerve catheter as well as preparation method and application thereof
InactiveCN101439205APromote regenerationHigh porosityFilament/thread formingCatheterMedicineActive component
The invention discloses a nerve duct and a preparation method as well as use thereof. The nerve duct is composed of 'shell-core' structured nanofiber, a core layer of the 'shell-core' structured nanofiber contains a biological active component, and a shell layer of the 'shell-core' structured nanofiber contains a biodegradable material; the inside diameter of the nerve duct is 0.5-5.0mm, and the outside diameter thereof is 1.0-6.0mm; the biological active component is a neurotrophic factor; and the biodegradable material is selected from one or more than one of the following components: polylactic-co-glycolic acid (PLGA), poly(lactic acid-caprolactone copolymer) (P (LLA-CL)) and poly(3-hydroxybutyrate) (PHB) or polyphosphoester (PPE).
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
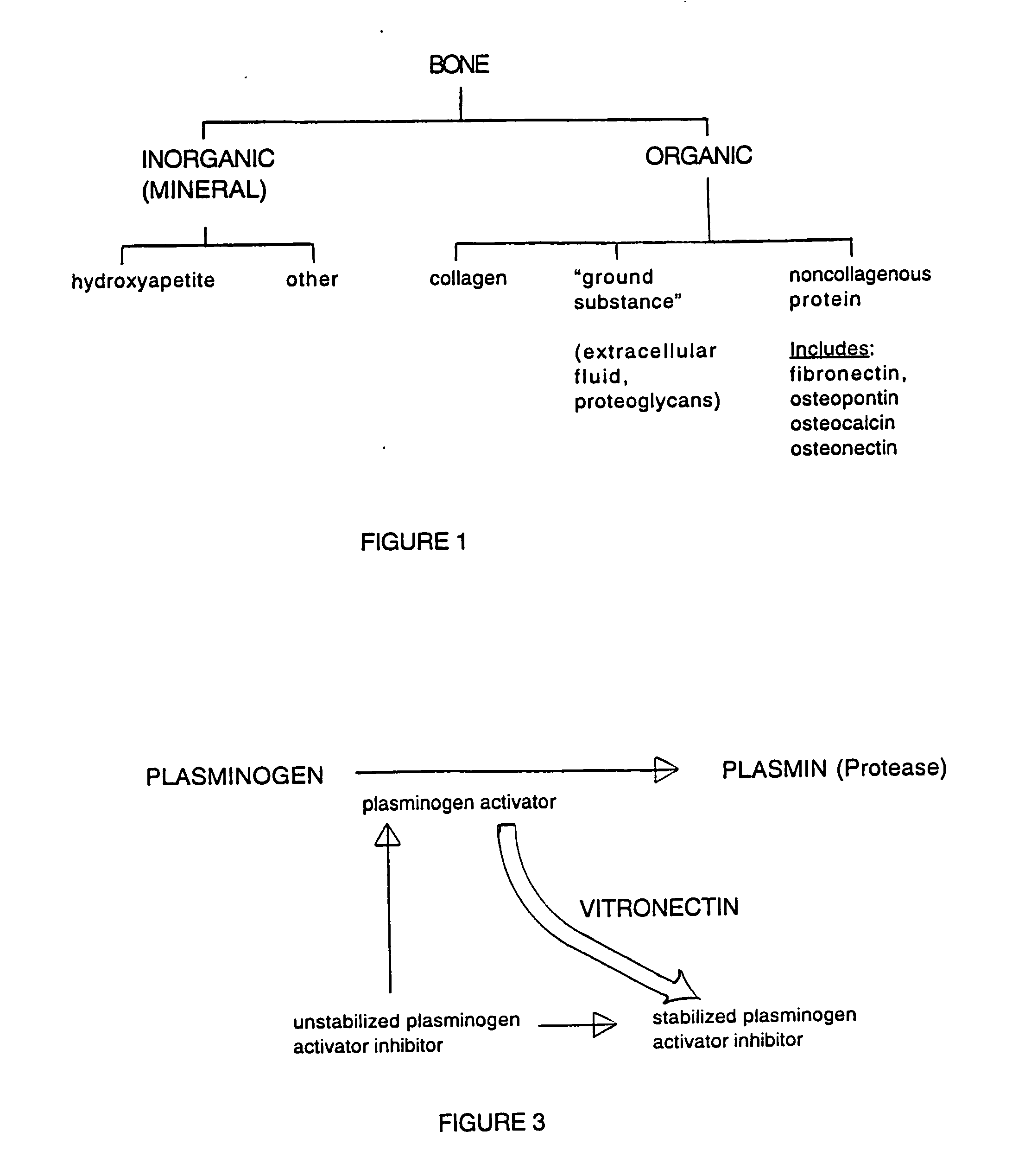
Composition and method for bone regeneration
InactiveUS20050147645A1Good effectDipeptide ingredientsTripeptide ingredientsOsteoblastBone formation
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:BUDNY JOHN ARNOLD
Tissue induction biomedical material
InactiveCN106267341AConjugated cellulose/protein artificial filamentsTissue regenerationAcetic acidCollagen i
The invention discloses a tissue induction biomedical material, and particularly relates to a degradable active biological membrane / powder. The tissue induction biomedical material is prepared from, by weight, 0.1-99% of degradable medical polyurethane, 0-99.9% of polyhydroxyalkanoate, 0-99.9% of polylactic acid and 1-99.9% of collagen, wherein polyhydroxyalkanoate is one of PHA, PHB, PHBV, PHBHHx and P3HB4HB, polylactic acid is one of polyglycolic acid and polylactic acid-glycolic acid copolymer, and collagen is one or more of collagen I, collagen II, collagen VI, collagen IX, collagen X, collagen XI and other various in-vivo collagen. The ratio of all the components is adjusted according to the requirements of the implanting positions of different in-vivo tissue for material performance, degradation products and PH values, so better clinical service is provided.
Owner:YUANRONG BIOLOGICAL PHARMA WUXI CO LTD
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
Exenatide release microsphere preparation, preparation method and application thereof
InactiveCN101658496AProlong the action timeEffective plasma concentrationPeptide/protein ingredientsMetabolism disorderAcetic acidTreatment effect
The invention discloses an Exenatide release microsphere preparation mainly comprising the following components in percentage by weight: 0.5-10 percent of Exenatide and 85-99 percent of polylactic acid-glycolic acid copolymer. The invention also discloses a preparation method and an application of the Exenatide release microsphere preparation. The Exenatide release microsphere preparation not onlycan prolong the action time of the Exenatide in the body and reduce the medicine application frequency, but also can maintain the effective blood-medicine concentration of the Exenatide and improve the treatment effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Controlled release microparticles
ActiveUS20070292475A1Extended durationReduce burstOrganic active ingredientsPowder deliveryControlled releaseMedicine
Formulations for controlled, sustained release of biologically active agents for the treatment of ocular disorders have been developed. These formulations are based on solid microparticles formed of the combination of biodegradable, synthetic polymers such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and copolymers thereof. The microparticles are characterized by low burst levels and efficient drug loading and sustained release.
Owner:EYETECH
Preparation method and use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors
InactiveCN102485274AGood monodispersityImprove stabilityAntibacterial agentsGenetic material ingredientsMicrosphereGluconic acid
The invention discloses a preparation method and a use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors. A result of an animal immunization experiment shows that the PLGA microspheres can be utilized as gene vaccine vectors. Principles of the PLGA microspheres comprise that 1, the PLGA microspheres have core-shell structures; surface polymers comprise polymine, PLGA, glucose, chitosan, polylysine, FeCl3 and FeCl2; and through static electricity, dewatering interaction and hydrogen bond-nucleic acid vaccine interaction, a nucleic acid vaccine is concentrated to form a compact nucleic acid vaccine so that nucleic acid vaccine degradation is reduced in vivo; 2, the PLGA microspheres have magnetism and thus after immunization injection, in a strong magnetic field, the distribution of the PLGA microspheres in muscular tissue is improved and the defect of limited contact between the PLGA microspheres and target cells is overcome; and 3, through long-term strong magnetic field induction, a magnetic PLGA microsphere / nucleic acid vaccine complex can enter into the skin; and because of rich antigen presenting cells in the skin, a strong and fast immune response can be induced.
Owner:JILIN UNIV
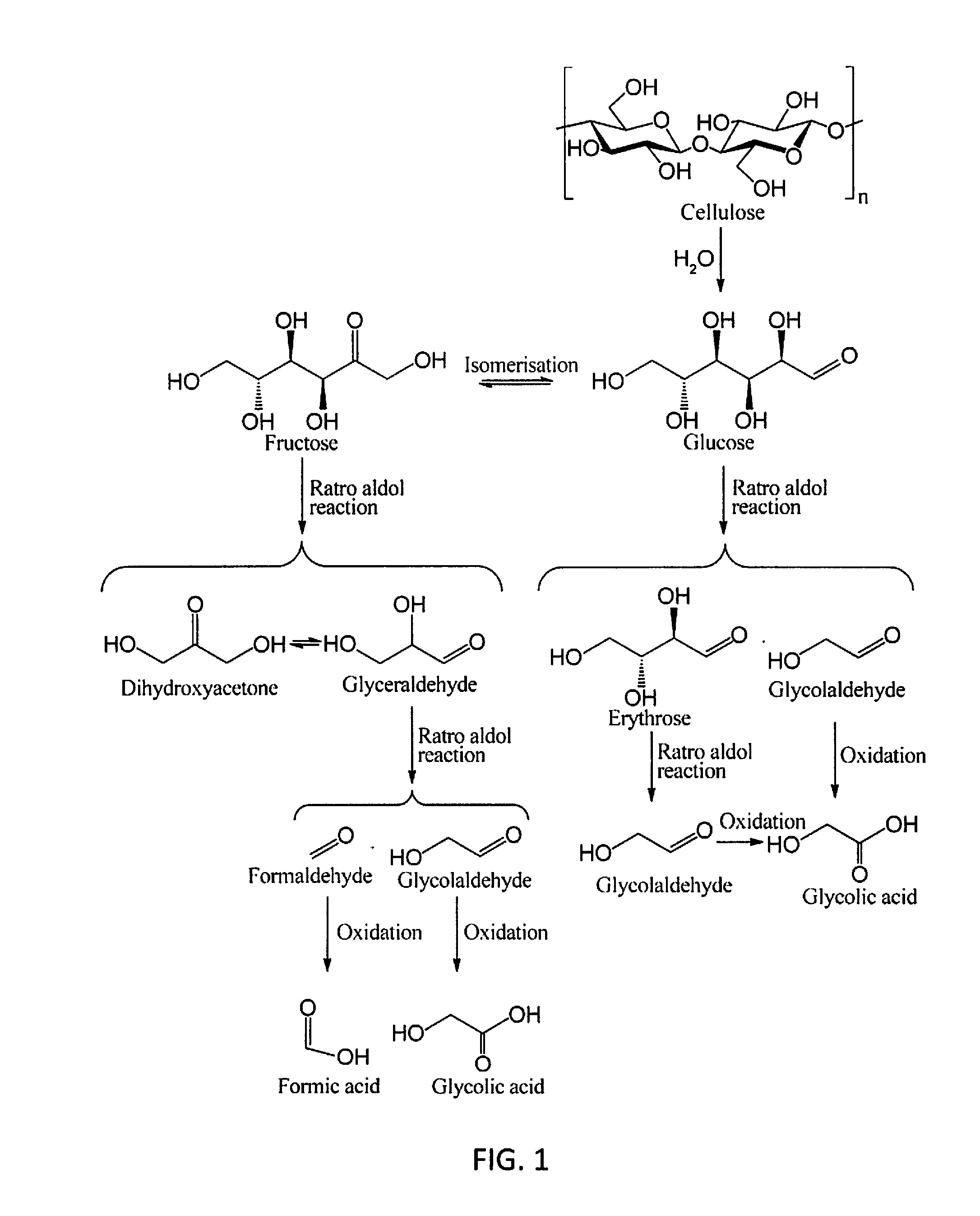
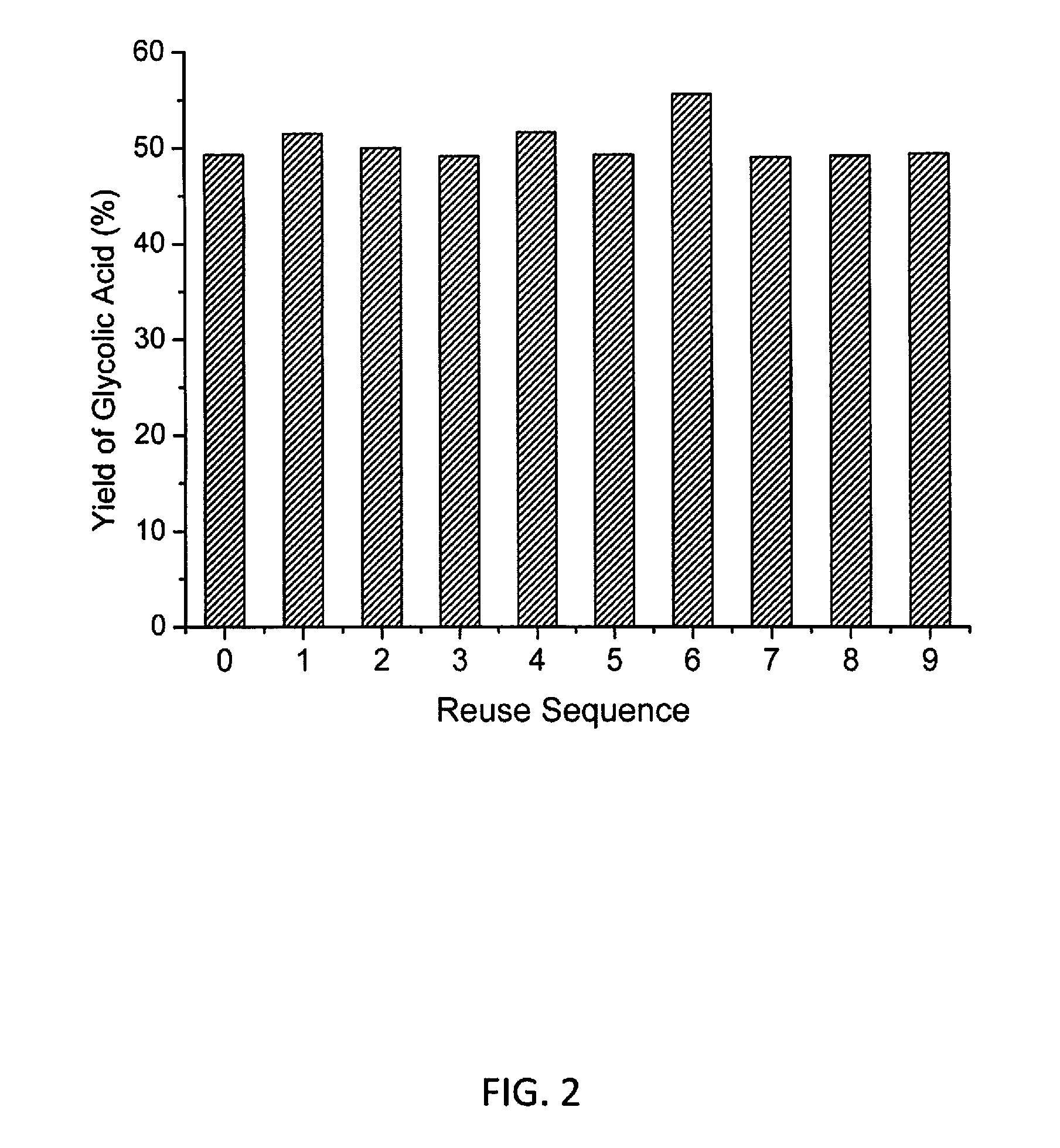
Molybdenum-containing Acidic Catalysts to Convert Cellulosic Biomass to Glycolic Acid
ActiveUS20130281733A1Greener and more cost-efficientOrganic compound preparationCarboxylic compound preparationCelluloseHeteropoly acid
Embodiments of the present invention include methods and compositions related to catabolic conversion of cellulosic biomass to glycolic acid using molybdenum-containing acidic catalysts. The invention includes the use of heteropoly and isopoly acids and salts as the molybdenum-containing multi-functional catalysts for biomass conversion. In embodiments of the invention, the reactions employ successive hydrolysis, retro-aldol fragmentation, and selective oxidation in a noble metal-free system.
Owner:KING ABDULLAH UNIV OF SCI & TECH
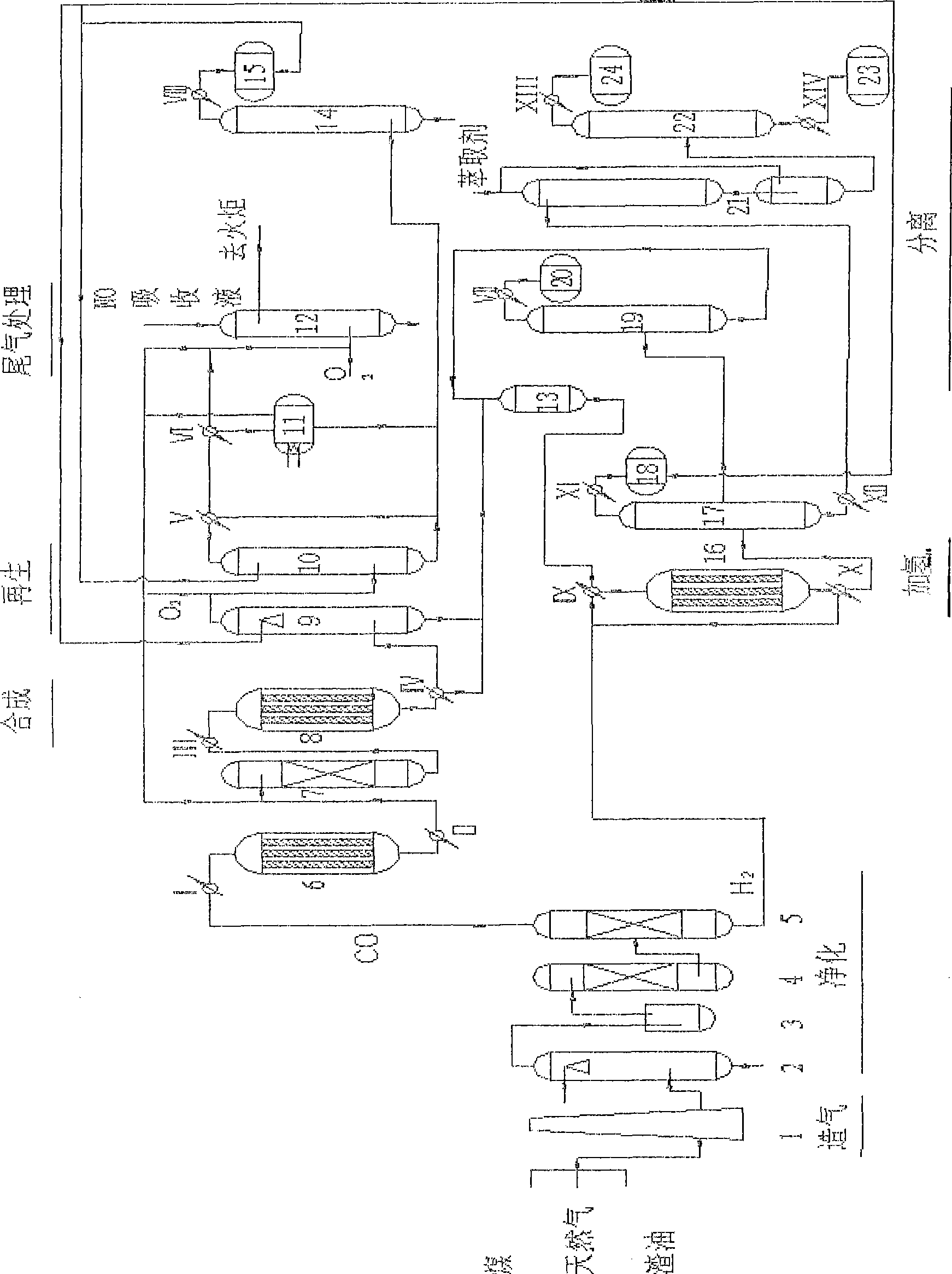
Method for producing polymer grade ethylene glycol and co-producing methyl glycolate
ActiveCN101544539AChange the ratioReduce energy consumptionOrganic compound preparationCarboxylic acid esters preparationNitric oxideOxygen
The invention discloses a method for producing polymer grade ethylene glycol and co-producing methyl glycolate, which comprises the following steps that: feed gas produced by taking coal, natural gas or residual oil as a raw material is subjected to purification treatment to obtain carbon monoxide and hydrogen through pressure varying absorption; the carbon monoxide, the hydrogen and auxiliary raw materials, namely industrial methanol, oxygen and nitrogen oxide are subjected to processes such as nitrosation reaction, carbonylation reaction, hydrogenation reaction, rectification separation, tail gas purification treatment and the like to obtain the ethylene glycol and the methyl glycolate; and the yield proportion of the ethylene glycol and the glycollic acid can be adjusted by controlling the hydrogenation reaction and the rectification process. In the method, the carbonylation reaction is performed on a Pd / alpha-Al2O3 catalyst added with two additives, and the carbonylation reaction is performed on a Cu-SiO2 or Cu-Cr2O3 or Cu-Zn-Al methanol catalyst, wherein the conversion ratio of the CO and the H2 is over 99.9 percent; and the selectivity is over 98 percent. Compared with the prior method for producing the ethylene glycol and the methyl glycolate, the method has the characteristics of low cost and high benefit.
Owner:HAISO TECH +2
Preparation method of biodegradable diaphragm for promoting regeneration of periodontal tissue
The invention relates to a preparation method of a biodegradable diaphragm for promoting regeneration of periodontal tissue, belonging to the field of biological regeneration medicine. In the invention, polylactic acid, polycaprolactone, polylactic acid-glycollic acid copolymer, collagen, modified chitosan and other biodegradable synthetic polyester polymers or natural biodegradable high molecular materials are used as raw materials to prepare a thin-film material with a nano structure through the electrostatic spinning process, and then, various growth factors are loaded on the thin-film material through the supermolecule loading technology. The diaphragm for reparative regeneration of the periodontal tissue, which is prepared by the method of the invention, has good biocompatibility and can realize the purposes of inhibiting inflammation and inducing regeneration of the periodontal tissue through the controlled release of the loaded growth factors.
Owner:TONGJI UNIV
Polypeptide drug sustained-release microsphere preparation and preparation method thereof
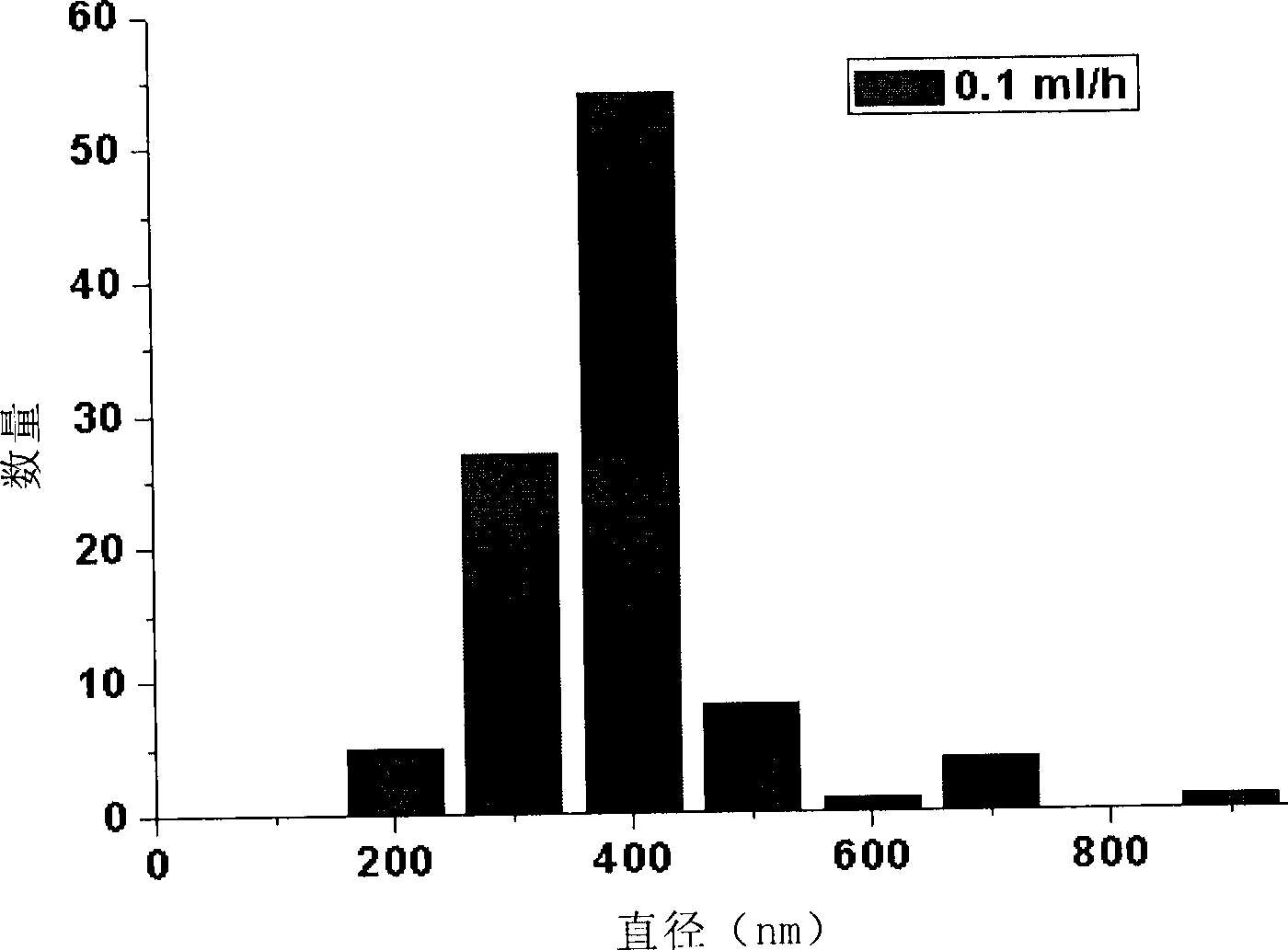
ActiveCN102688198AUniform particle sizeNarrow particle sizePeptide/protein ingredientsMetabolism disorderFreeze-dryingMicrosphere
The invention discloses a polypeptide drug sustained-release microsphere preparation and a preparation method thereof. The method comprises the following steps of: dissolving the polylactic acid-glycollic acid copolymer or polylactic acid, a protective agent and a polypeptide drug in an organic solvent to form a completely uniform mixed solution; adding the mixed solution into an oil phase to form emulsion; removing the organic solvent; and performing centrifugal washing and freeze drying to obtain the polypeptide drug sustained-release microsphere. In the invention, an O / O method is adopted, the problem that the drug spreads toward the outer aqueous phase in the multiple-emulsion preparation method is solved, and the drug encapsulation efficiency is improved to 60-95%. The biological active polypeptide drug is degraded in the body and slowly released with the polymer material of the microsphere through the pores on the microsphere surface; the release time can be as long as several weeks and even several months; and the in-vitro release test indicates that the release conforms to similar zero-order release.
Owner:AC PHARMA CO LTD
Liraglutide long-acting microsphere injection and preparation method thereof
ActiveCN102085355AReduce the burden of treatmentImprove Medication AdherencePowder deliveryPeptide/protein ingredientsTreatment burdenMicrosphere
The invention provides a long-acting microsphere injection as an antidiabetic medicament, the long-acting microsphere injection comprises liraglutide, PLGA (poly(lactic-co-glycolic acid)), excipients and a surfactant, and the invention simultaneously relates to a preparation method of the injection. Liraglutide long-acting sustained-release microspheres provided by the invention are designed to perform subcutaneous injection once every 28 days, thereby greatly reducing treatment burden on a patient, improving medication compliance and reducing treatment cost; simultaneously, results of in vitro release studies, animal experiments and the like prove that the obtained sustained-release microspheres can slowly release a medicament for a long time in vitro and in vivo.
Owner:蚌埠丰原涂山制药有限公司
Low cost bio-based full degradable film and preparation method thereof
The present invention discloses a low cost bio-based full degradable film and preparation method thereof, the ratio of each component in parts by mass of the film is as follows: 15-25 parts of a polyglycolic acid, 25-35 parts of corn starch, 35-55 parts of poly(butylene adipate-co-terephthalate), 5 parts of a compatilizer, 3.75-12.25 parts of a starch plasticizer, 0.5-0.7 part of citric acid, 0.75-1.25 parts of acetyl tributyl citrate, 0.3-0.5 part of maleic anhydride, 0.2 part of antioxidant 164, and 0.2 part of 2-(2″-hydroxyl-5″-methylphenyl)benzotriazole. The low cost bio-based full degradable film provided by the present invention has a bio-based content, which can reach 30% or more, a lower cost, and a tensile strength exceeding a traditional PE thin film, and has very important significance for solving the problem of “white pollution” and promoting the popularization and application of full biodegradable materials.
Owner:JIANGSU JINJU ALLOY MATERIAL
Polishing pad comprising biodegradable polymer
The invention is directed to a polishing pad for use in chemical-mechanical polishing comprising a biodegradable polymer. The biodegradable polymer comprises a repeat unit selected from the group consisting of glycolic acid, lactic acid, hydroxyalkanoic acids, hydroxybutyric acid, hydroxyvaleric acid, caprolactone, p-dioxanone, trimethylene carbonate, butylene succinate, butylene adipate, monosaccharides, dicarboxylic acid anhydrides, enantiomers thereof, and combinations thereof. The invention is further directed to methods of its use.
Owner:CABOT MICROELECTRONICS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com