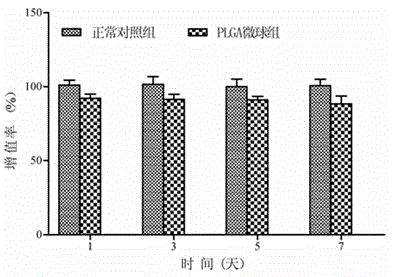
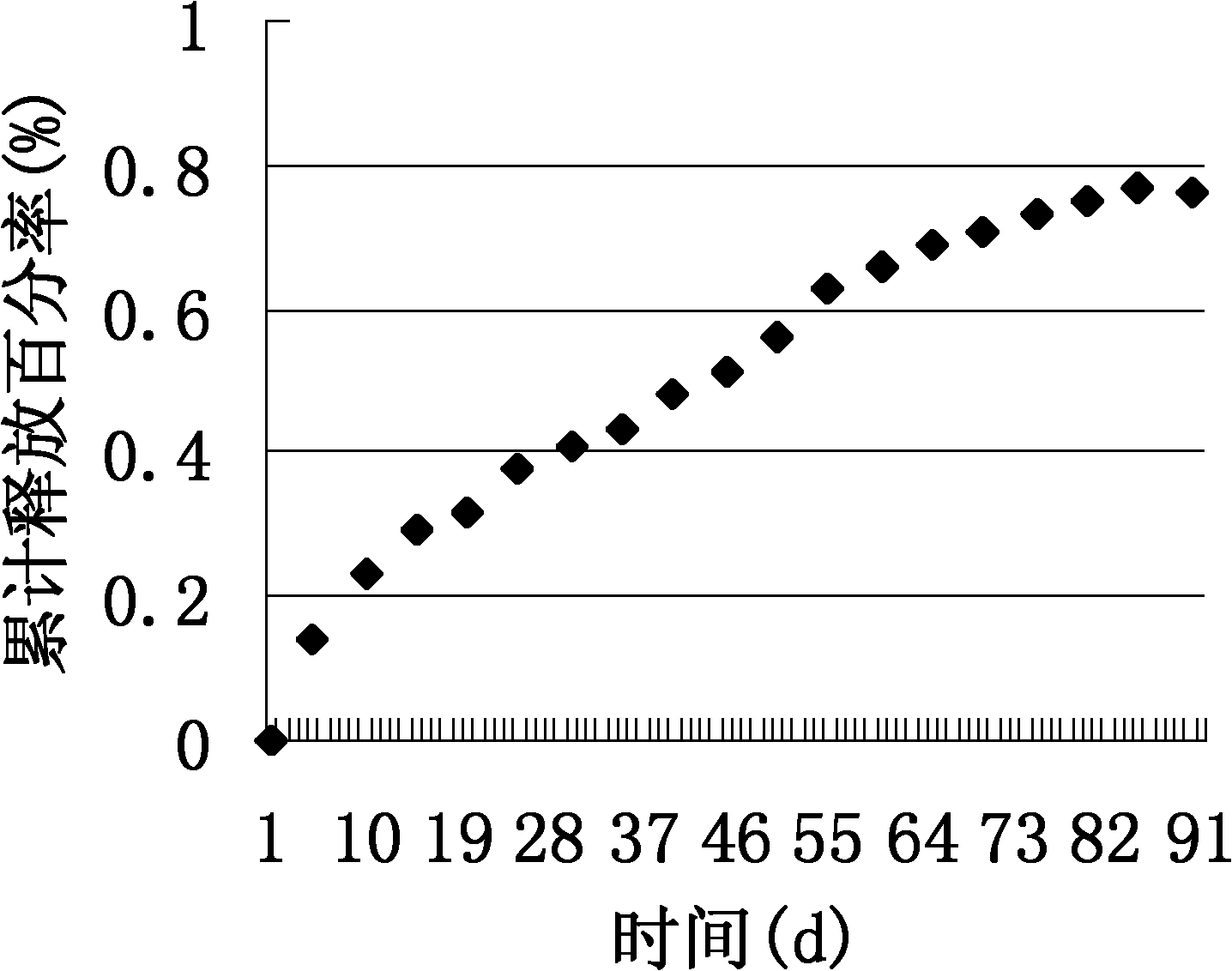
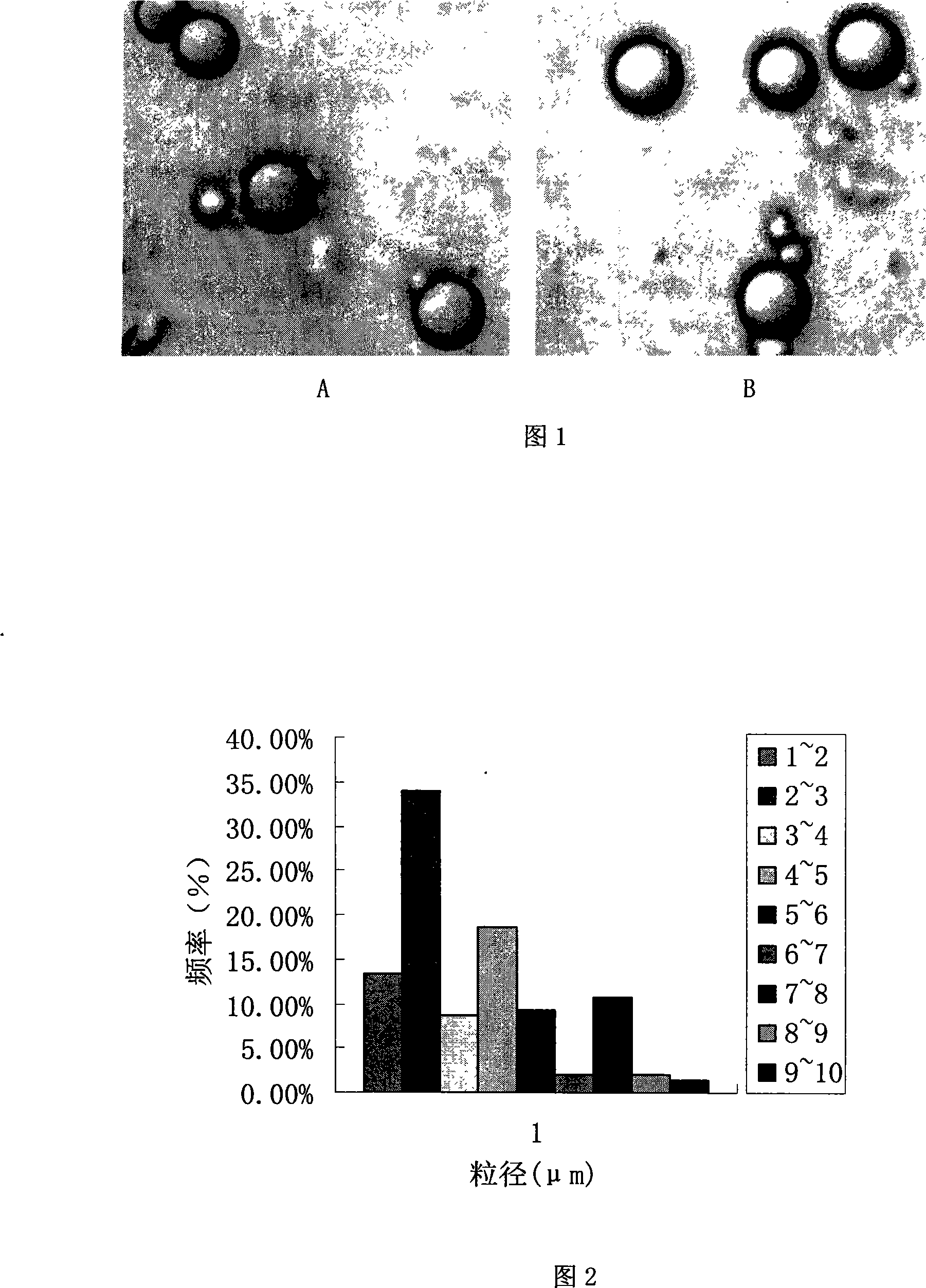
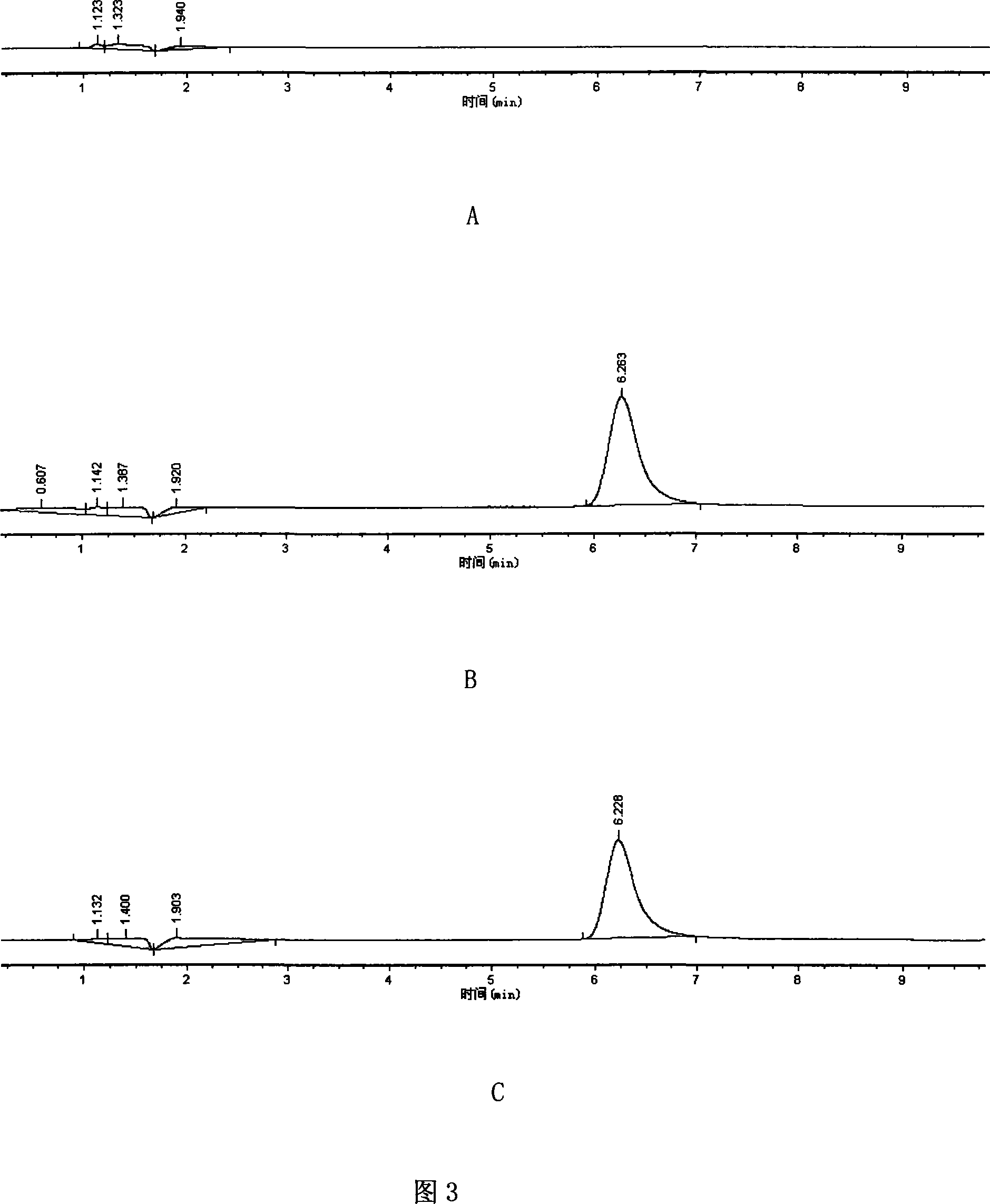
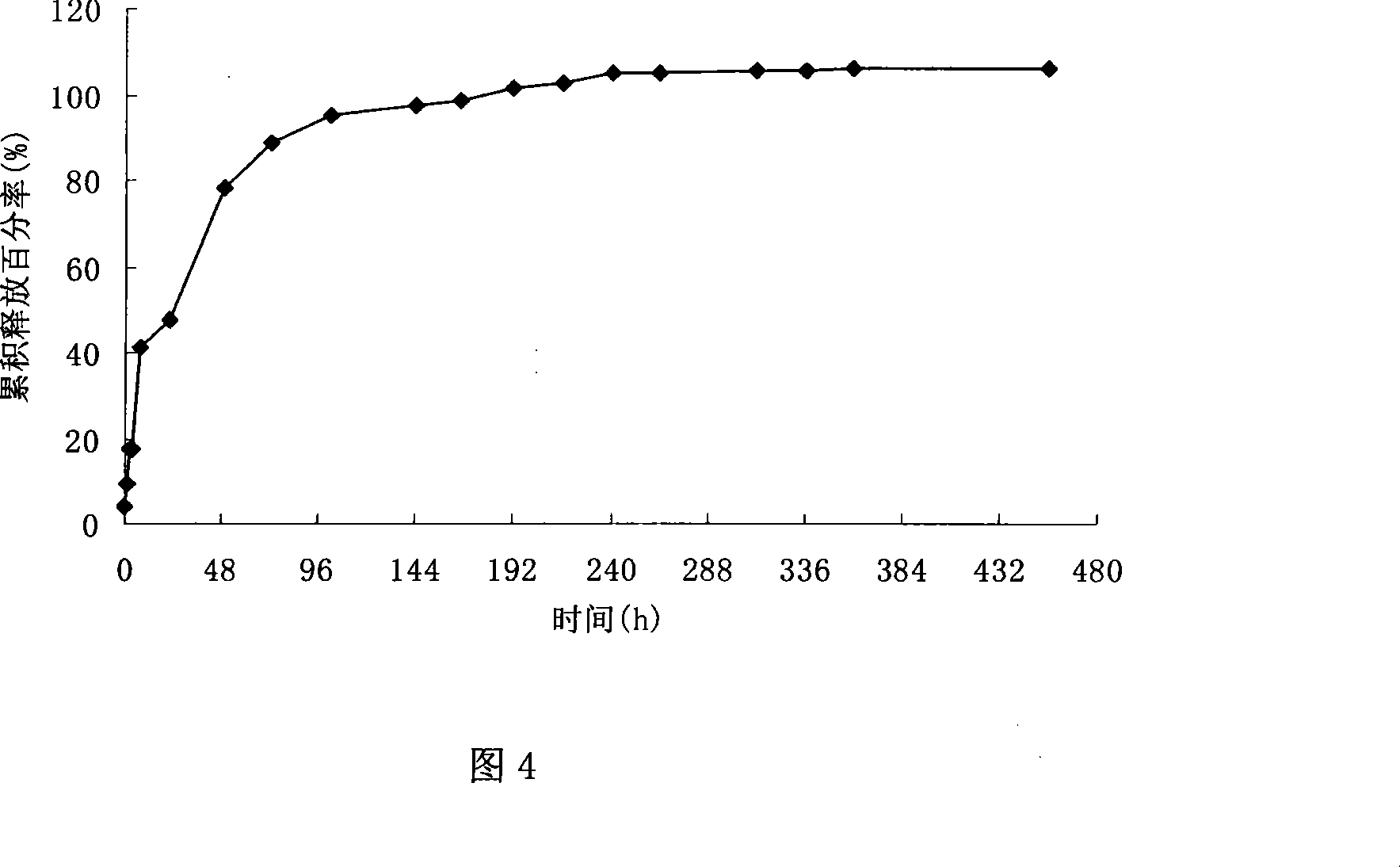
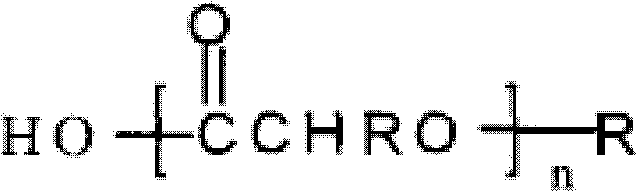Patents
Literature
110 results about "Plga microspheres" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method and use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors
InactiveCN102485274AGood monodispersityImprove stabilityAntibacterial agentsGenetic material ingredientsMicrosphereGluconic acid
The invention discloses a preparation method and a use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors. A result of an animal immunization experiment shows that the PLGA microspheres can be utilized as gene vaccine vectors. Principles of the PLGA microspheres comprise that 1, the PLGA microspheres have core-shell structures; surface polymers comprise polymine, PLGA, glucose, chitosan, polylysine, FeCl3 and FeCl2; and through static electricity, dewatering interaction and hydrogen bond-nucleic acid vaccine interaction, a nucleic acid vaccine is concentrated to form a compact nucleic acid vaccine so that nucleic acid vaccine degradation is reduced in vivo; 2, the PLGA microspheres have magnetism and thus after immunization injection, in a strong magnetic field, the distribution of the PLGA microspheres in muscular tissue is improved and the defect of limited contact between the PLGA microspheres and target cells is overcome; and 3, through long-term strong magnetic field induction, a magnetic PLGA microsphere / nucleic acid vaccine complex can enter into the skin; and because of rich antigen presenting cells in the skin, a strong and fast immune response can be induced.
Owner:JILIN UNIV
Polylactic acid-hydroxyacetic acid copolymer nano-drug carrier as well as preparation method and application thereof
ActiveCN104001178AImprove intake capacityEliminate side effectsNanomedicinePharmaceutical non-active ingredientsCrosslinked chitosanMicrosphere
The invention provides a PLGA (polylactic acid-hydroxyacetic acid) nano-drug carrier which is composed of PLGA nano microsphere kernel and an anillic aldehyde crosslinked chitosan housing. A preparation method of the polylactic acid-hydroxyacetic acid copolymer nano-drug carrier is as follows: dispersing a PLGA organic phase which takes dichloromethane and alcohol as a mixed solvent in a water phase to prepare PLGA microspheres by taking PVA (polyvinyl acetate) as an emulsifier by virtue of an one-off emulsion process; then, adding the PLGA microspheres into chitosan liquor, so that the chitosan is adsorbed on the surfaces of the PLGA microspheres, and then adding the chitosan on the surfaces of anillic aldehyde crosslinked PLGA microspheres. The product has a certain pH environmental responsiveness, can realize controlled release of the drug according to in-vivo pH environmental changes, is high in stability, strong in up-taking capacity for microsphere-coated medicaments by the cells, and has very good application prospect in the drug carrier for treating tumors.
Owner:SUN YAT SEN UNIV
Sustained release hydrophobic bioactive PLGA microspheres
InactiveUS6855331B2Effective long-term releaseReduced bioavailabilityOrganic active ingredientsPeptide/protein ingredientsControlled releaseMicrosphere
A controlled release microcapsulate pharmaceutical formulation for burst-free, sustained, programmable release of hydrophobic bioactive agent over a duration from 24 hours to 100 days comprising: and a blend of end-capped uncapped biocompatible, biodegradable poly(lactide / glycolide).
Owner:UNITED STATES ARMY U S ARMY MEDICAL RES & MATERIEL COMMAND
Composite electrostatic spinning nanofiber film and preparation method and application thereof
ActiveCN105107012AGood biocompatibilityOvercoming the risk of wearing down organsConjugated cellulose/protein artificial filamentsAbsorbent padsFiberMicrosphere
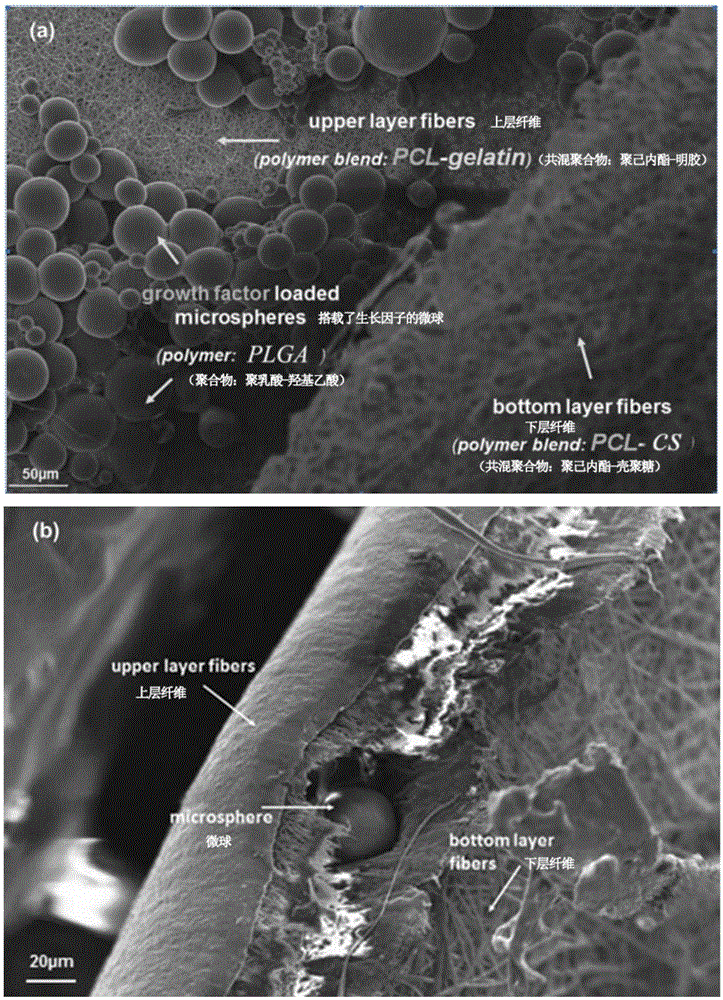
The invention discloses a composite electrostatic spinning nanofiber film and a preparation method and application thereof. The preparation method of the composite electrostatic spinning nanofiber film carrying drug controlled-release microspheres includes: (1), preparing polylactic acid-glycolic acid copolymer PLGA microspheres with basic fibroblast growth factors (bFGF) embedded; (2), respectively preparing polycaprolactone / chitosan electrostatic spinning liquid, polycaprolactone electrostatic spinning liquid and gelatin electrostatic spinning liquid; (3), using an electrospinning method to prepare a PCL / CS-PLGA microsphere-PCL / GE electrostatic spinning nanofiber film, namely the composite electrostatic spinning nanofiber film. The nanofiber film is utilized to improve bio-compatibility of PU sponge, promote tissue healing and reduce visceral wear; the microspheres are introduced to realize drug controlled-release, and many blanks of clinically-existing VAC can be filled.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
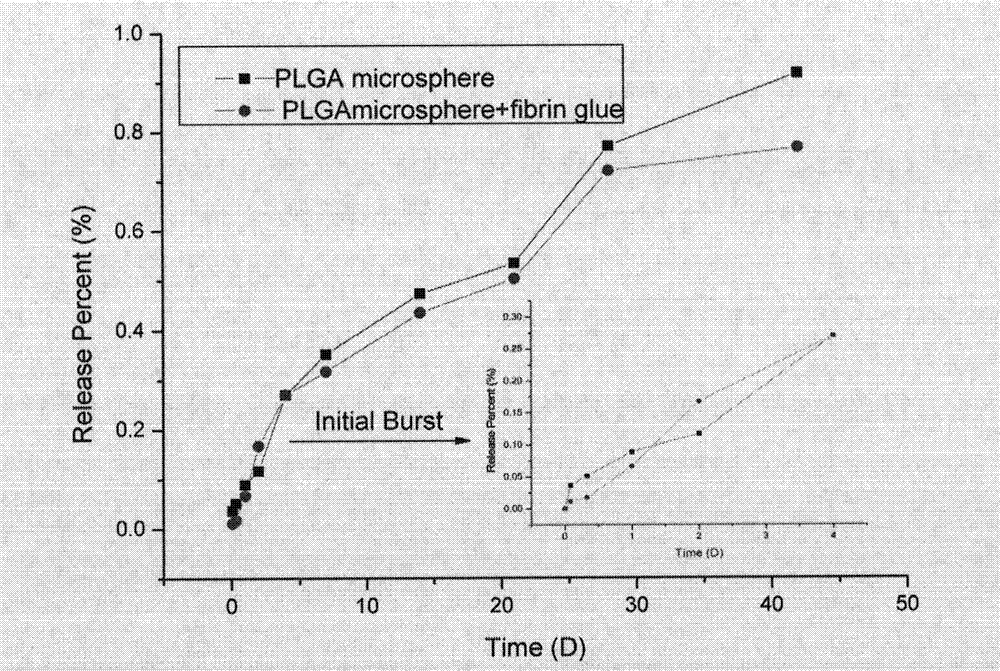
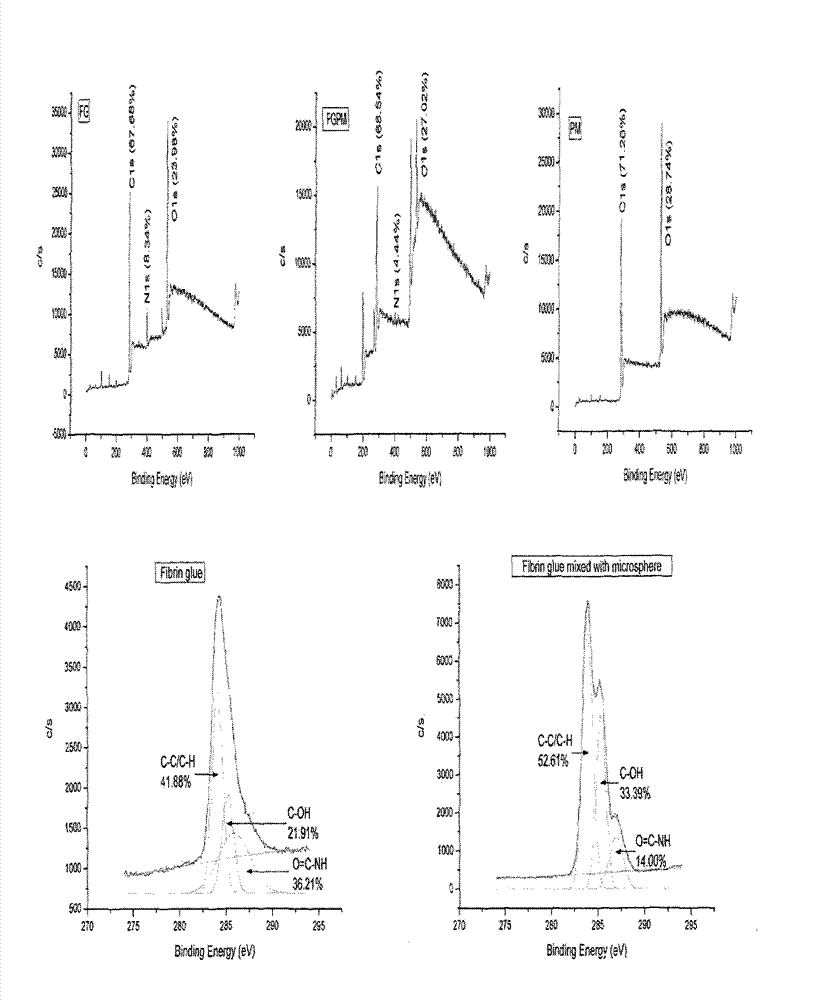
Preparation method and application of fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere
The invention relates to a preparation method and application of a fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere. The preparation method comprises the steps of: preparing a slow release microsphere with a proper particle diameter; and then constructing an rhBMP-2 / PLGA microsphere / fibrin glue composite material. The rhBMP-2 / PLGA microsphere fibrin glue composite material can be used through local injection, surgical trauma can be reduced, a healing process of bone fracture and nonunion is accelerated by continuously supplementing the local bone morphogenetic protein, thus the fibrin glue composite recombinant human bone morphogenetic protein-2 is a bone repair material with excellent degradability and osteogenic activity.
Owner:姚琦 +2
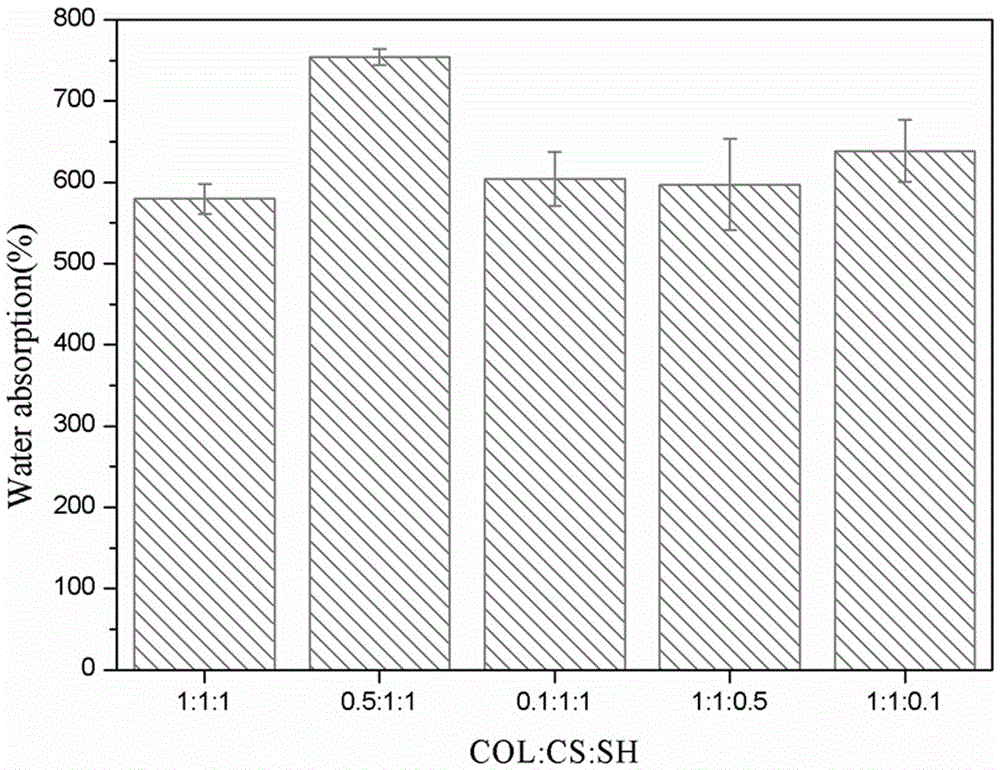
KGN-carried collagen/chitosan/sodium hyaluronate composite scaffold
InactiveCN106729987AImprove mechanical propertiesPromote degradationTissue regenerationProsthesisMicrosphereDefect repair
The invention discloses a KGN-carried collagen / chitosan / sodium hyaluronate composite scaffold. The scaffold is prepared from collagen, chitosan, sodium hyaluronate, KGN (kartogenin) and PLGA (poly(lactic-co-glycolic acid)). The preparation method comprises the following steps: preparing KGN-micromolecule-carried PLGA microspheres, mixing the PLGA microspheres, and preparing the collagen / chitosan / sodium hyaluronate composite scaffold. The composite scaffold has the characteristics of favorable biocompatibility, favorable biodegradability and the like, has the lubrication action of sodium hyaluronate and the marrow mesenchymal stem cell cartilage differentiation promotion action of KGN, is beneficial to cartilage defect repair of medium / early osteoarthritis, and is a favorable tissue regeneration material for osteoarthritis cartilage defects.
Owner:FUZHOU UNIV
Enhanced calcium phosphate bone repair material and preparation method thereof
ActiveCN103007354AImprove mechanical propertiesImprove brittlenessMicroballoon preparationProsthesisMicrospherePhosphoric acid
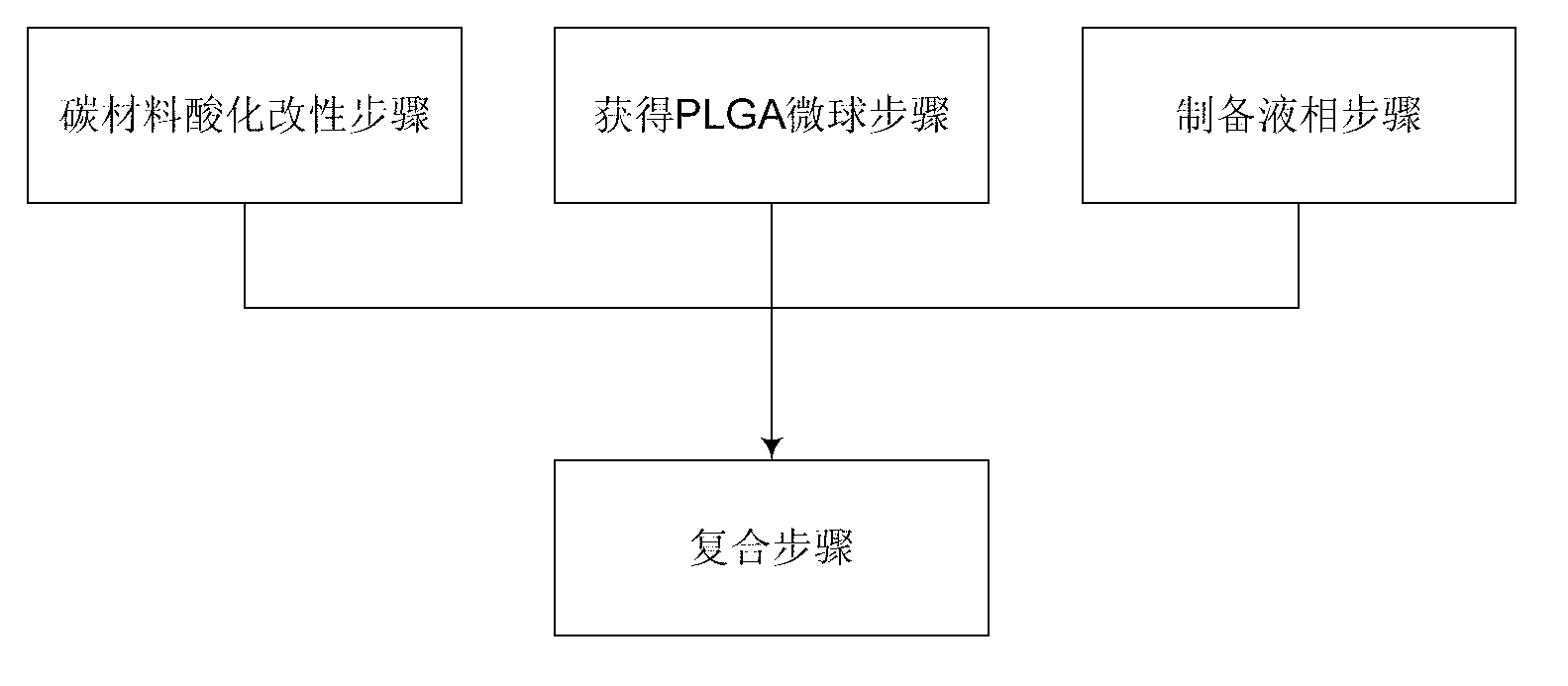
The invention relates to an enhanced calcium phosphate bone repair material and a preparation method thereof. The bone repair material is formed by mixing solid phase and liquid phase, wherein the solid phase comprises phosphoric acid calcium salt, a carbon material and PLGA (polylactic-co-glycolic acid) microspheres; and the liquid phase is any one of the following liquid materials: distilled water, normal saline, chitosan solution, phosphoric acid solution, sodium dihydrogen phosphate solution, dibasic sodium phosphate solution and citric acid solution. The preparation method comprises the following steps: acidifying and modifying the carbon material to obtain a chloracetylated carbon material; obtaining PLGA microspheres; preparing the liquid phase; and compositing, adding the chloracetylated carbon material into a liquid-phase solution for ultrasonic treatment, then quickly mixing the PLGA microspheres, the phosphoric acid calcium salt and the liquid-phase solution and uniformly stirring to obtain the enhanced calcium phosphate bone repair material. The enhanced calcium phosphate bone repair material has remarkably improved mechanical property, improved fragility and degradation property, favorable biocompatibility and syringeability and can be used for damage repair of osseous tissues.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Preparation method of taxol-loading polylactic acid-hydroxyacetic acid microspheres
InactiveCN107049984ARound shapeSmall particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSide effectPolyvinyl alcohol
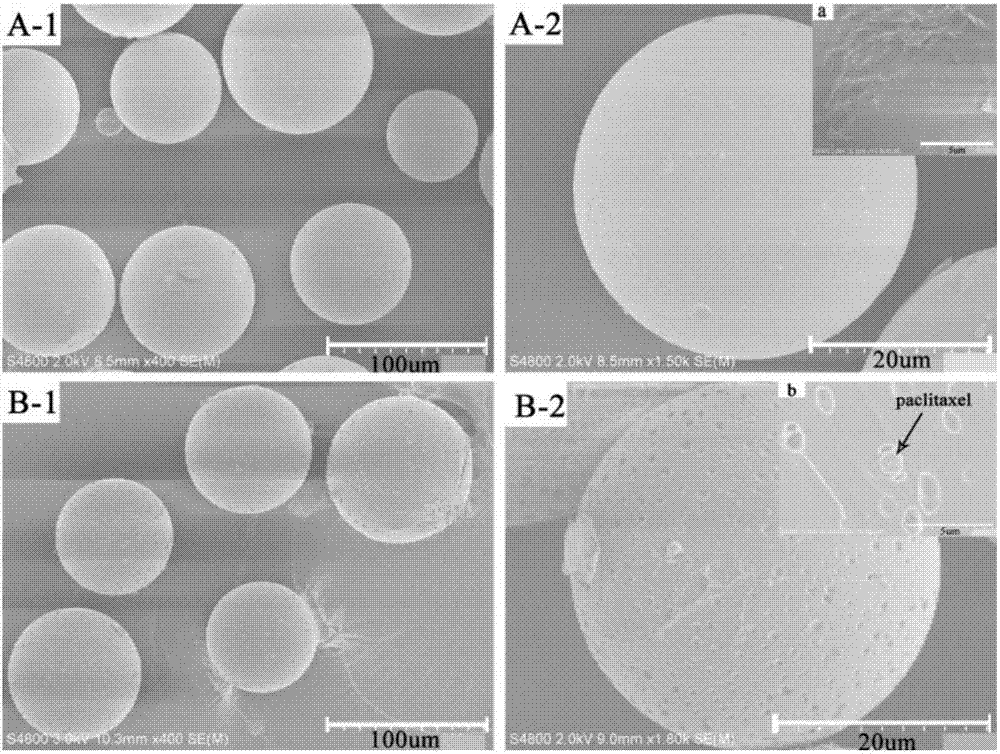
The invention belongs to the field of preparation of targeted therapeutic microspheres of slow release formulation and in particular relates to a preparation method of taxol-loading polylactic acid-hydroxyacetic acid (PLGA) microspheres. The preparation method comprises the following steps: dissolving a polylactic acid-hydroxyacetic acid copolymer in an organic solvent, and adding a taxol drug, wherein the polymer and the taxol drug are uniformly dissolved to obtain an organic phase; dropwise adding the organic phase into a fresh polyvinyl alcohol aqueous solution and curing and forming microspheres; and performing centrifugal separation on the cured drug-loading microspheres to obtain the uniformly dispersed taxol-loading polylactic acid-hydroxyacetic acid (PLGA) microspheres. The taxol-loading targeted therapeutic PLGA microspheres prepared by the preparation method have an ideal drug loading ratio and encapsulation efficiency while guaranteeing the drug stability of taxol, can reach an effect of slow release, maintain the optimum drug concentration in vivo, prolong the action time of the drug and reduce the side effects brought by burst release of the drug, and have important theoretical value and actual application prospect.
Owner:WUHAN UNIV OF TECH
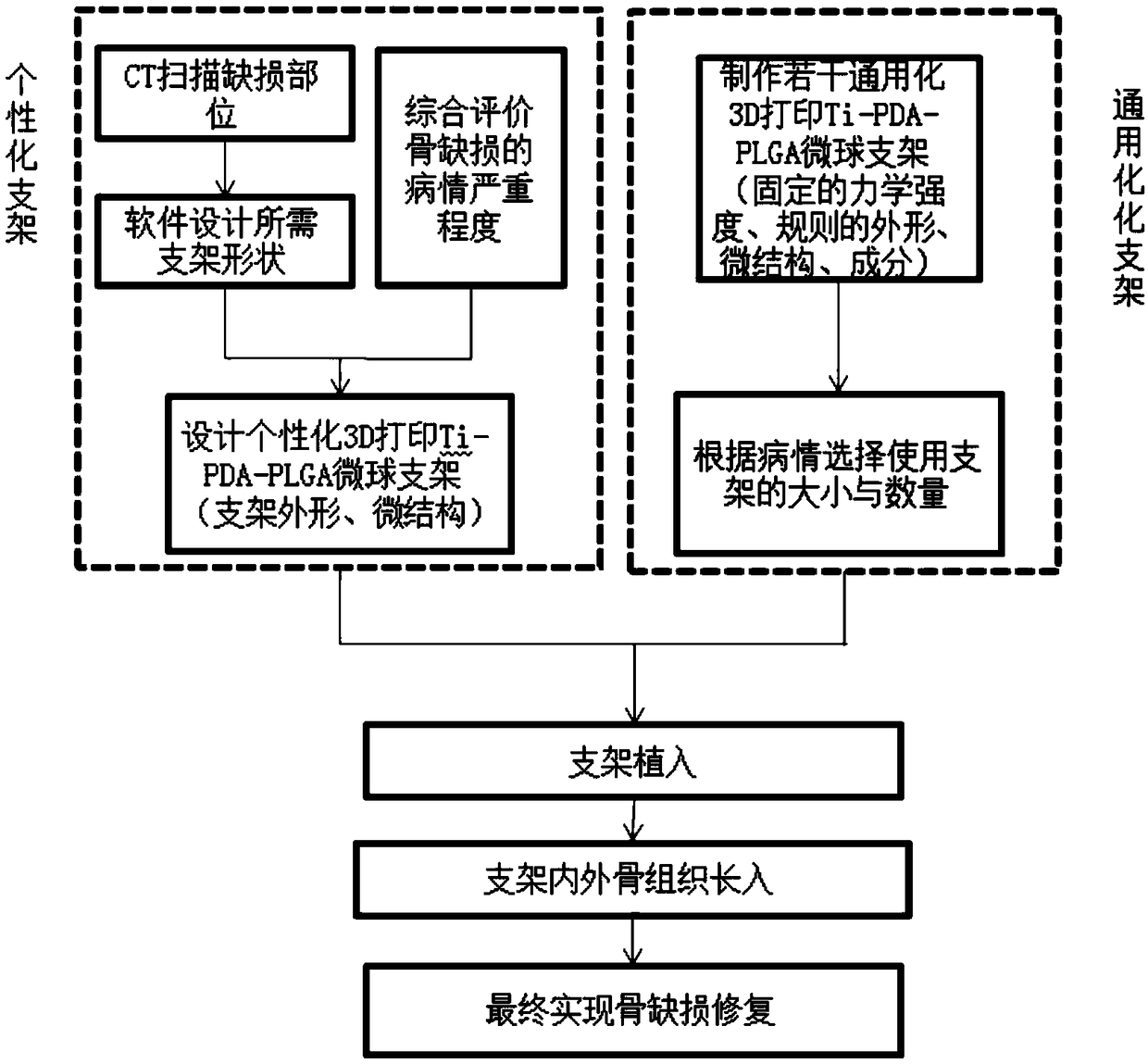
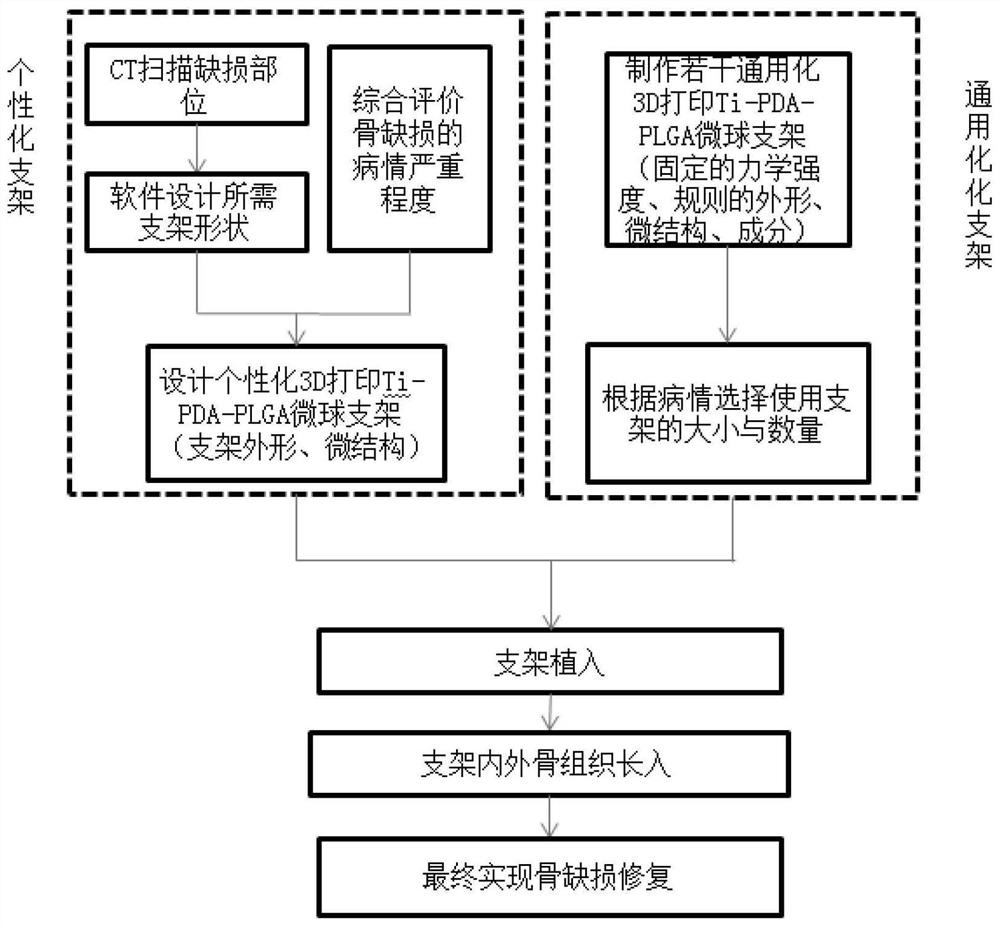
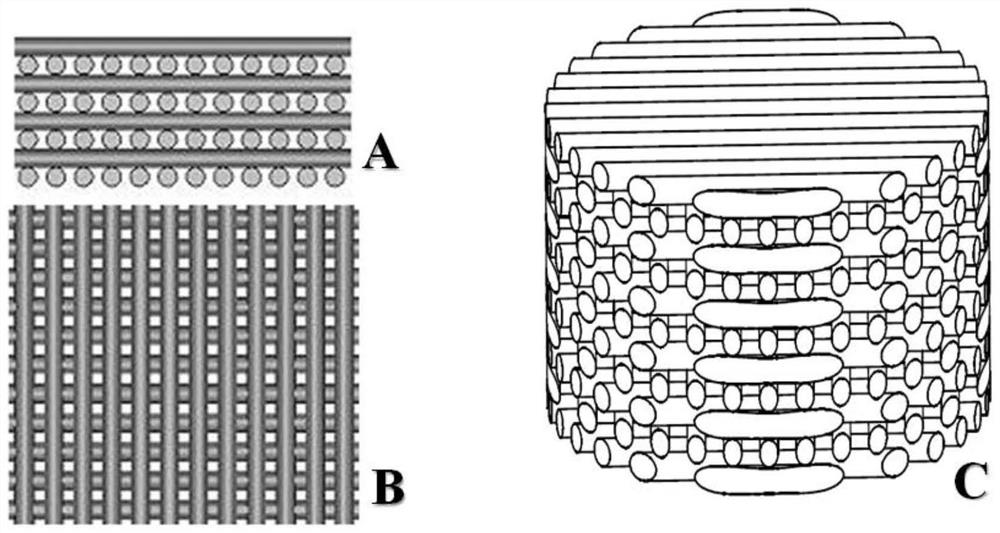
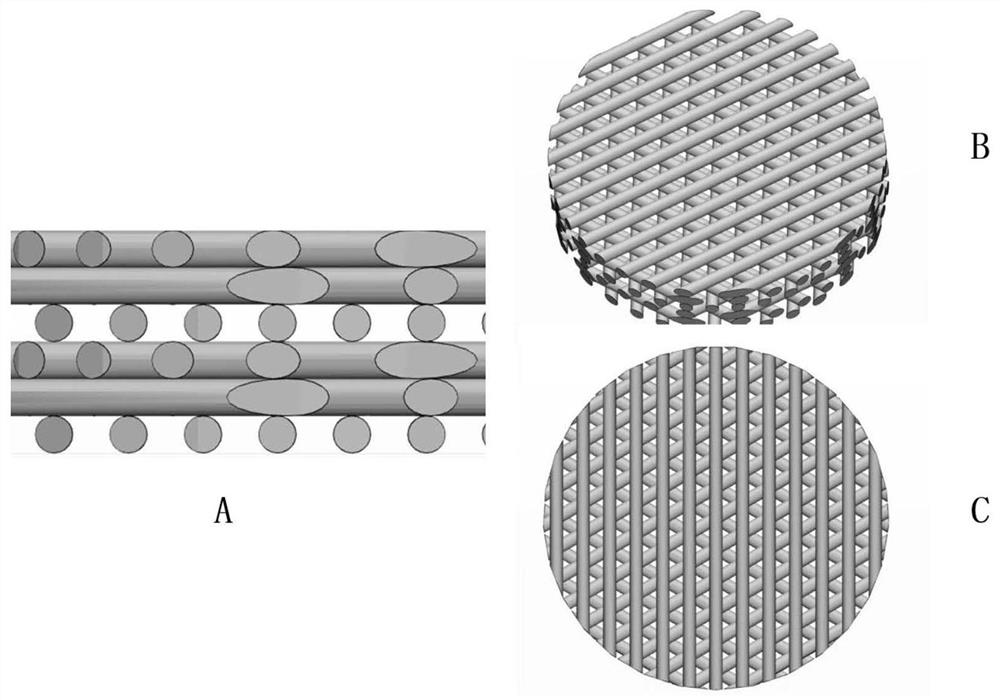
3D printed Ti-PDA-PLGA microsphere bone defect repair stent
InactiveCN108853577AAchieve fine controlAchieve sustained releaseAdditive manufacturing apparatusTissue regeneration3d printRepair tissue
The invention discloses a 3D printed Ti-PDA-PLGA microsphere bone defect repair stent. A 3D printed Ti stent is prepared by a laser sintering technology. Then, under certain conditions, dopamine is self-polymerized on the fiber surface of the 3D printed Ti stent to form a PDA coating, thereby preparing a 3D printed Ti-PDA stent; then PLGA microspheres carrying VEGF are prepared by a double emulsion-solvent evaporation method, and finally, BMP-2 and PLGA microspheres carrying VEGF are adsorbed and immobilized on the surface of the stent by an adsorption method, and finally the 3D printed Ti-PDA-PLGA microsphere bone defect repair stent is formed. The bone defect repair tissue engineering stent disclosed by the invention has the advantages of reliable mechanical property, high biological activity and safety, convenient implantation, small trauma and low cost, and can be used for the repair treatment of bone defect after bone traumas, bone tumors and bone infection.
Owner:南京冬尚生物科技有限公司
Preparation method of novel magnetic 5-fluorouracil carrying polylactic-co-glycolic acid (PLGA) material
InactiveCN103877029AWide variety of sourcesUniform particle sizeOrganic active ingredientsInorganic non-active ingredientsMicrosphereFreeze-drying
The invention relates to a preparation method of a novel magnetic 5-fluorouracil carrying polylactic-co-glycolic acid (PLGA) material. The preparation method of the novel magnetic 5-fluorouracil carrying PLGA material comprises the following steps: adding nano particles into a PLGA solution, dissolving 5-fluorouracil into dimethyl sulfoxide (DMSO), adding 5-Fu DMSO solution into the PLGA solution, and carrying out ultrasonic treatment, so that oil in oil (O / O) emulsion is obtained; then adding the O / O emulsion into PVA aqueous solution saturated by 5-Fu, stirring for evaporating a solvent, solidifying microspheres, washing with deionized water, and performing freeze drying, so that the magnetic 5-Fu carrying PLGA microspheres are obtained. The obtained drug carrying microspheres are uniform in particle size, and the particle size is 100-500 microns. Drug loading capacity of the microspheres is high and can be 10%. Drug releasing rate is regulated by regulating ratio of LA to GA in PLGA. As magnetic nano particles are introduced, under the control action of an external magnetic field, the drug carrying microspheres can be concentrated in a tumour region, and drug concentration in the tumour region is increased, so that apoptosis of tumour cells is quick, and harm of anti-tumour drug to normal cells is reduced to minimum. The preparation method of the novel magnetic 5-fluorouracil carrying PLGA material is simple and practicable, raw materials can be industrially produced, and the preparation method of the novel magnetic 5-fluorouracil carrying PLGA material has good popularization and application values.
Owner:TONGJI UNIV
Method for preparing PLGA microspheres
InactiveCN107714674AAdjust particle sizeNo input requiredOrganic active ingredientsPeptide/protein ingredientsMicrosphereOil water
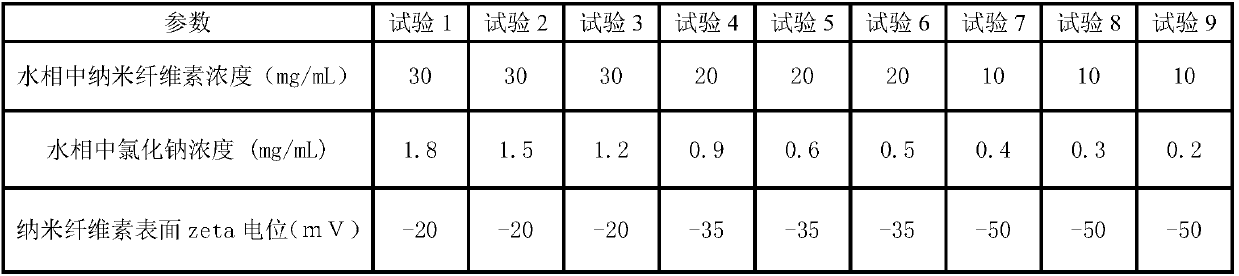
The invention relates to a method for preparing PLGA microspheres with hydrophilic nano-cellulose as an emulsifier. After the nano-cellulose is adsorbed to the surfaces of the PLGA microspheres, the nano-cellulose stabilizes an oil-water interface as a stabilizer. Meanwhile, because the nano-cellulose has certain zeta electric potentials, the nano-cellulose is easy to scatter and difficult to gather, after an oil phrase and a water phrase are mixed, there is no need to consume a large amount of energy, and the microspheres can be formed through simple ultrasound. Finally, due to the presence of the zeta electric potentials, in the formation process of the microspheres, the thickness of electrified layers of the surfaces of the microspheres can be further adjusted by adjusting the ion strength in the water phase, such as by adding sodium chloride, and the function of adjusting the particle size of the microspheres is achieved at last.
Owner:天津双硕医药科技有限公司
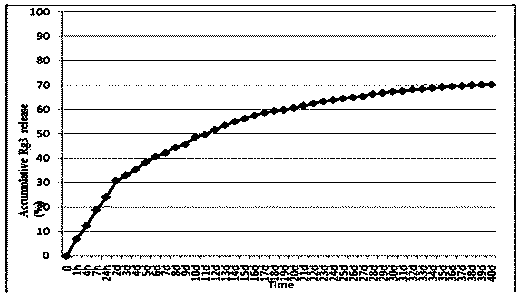
Ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere and preparation method thereof
InactiveCN104288111ASolve the shortcomings of poor water solubility and difficult absorption after oral administrationLittle side effectsPowder deliveryOrganic active ingredientsFreeze-dryingMicrosphere
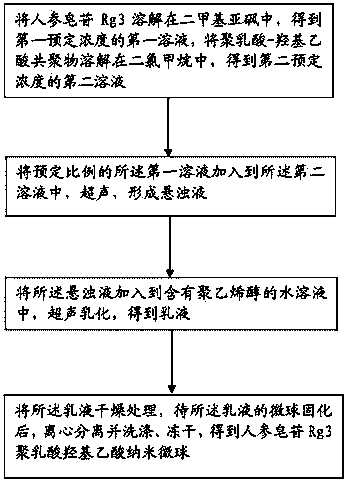
The invention discloses a ginsenoside Rg3 poly(lactic-co-glycolic acid) (PLGA) nano microsphere and a preparation method thereof. The preparation method of the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere comprises the following steps: S1, dissolving ginsenoside Rg3 in dimethyl sulfoxide to obtain a first liquid with a first predetermined concentration, and dissolving poly(lactic-co-glycolic acid) copolymer into dichloromethane to obtain a second liquid with a second predetermined concentration; S2, adding the first liquid in a predetermined proportion into the second liquid to perform ultrasonic treatment to form a turbid liquid; S3, adding the turbid liquid into an aqueous liquid containing polyvinyl alcohol to perform ultrasonic emulsion to obtain emulsion; and S4, drying the emulsion, centrifugally separating and washing, and freeze-drying the emulsion after curing the microspheres of the microsphere of the emulsion to obtain the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere. According to the preparation method of the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere of the embodiment of the invention, the prepared ginsenoside PLGA microsphere is good in pesticide effect.
Owner:BEIJING JIAOTONG UNIV
BMP loaded silk fibroin/collagen scaffold material and preparation method thereof
The invention discloses a BMP loaded silk fibroin / collagen scaffold material. The material is prepared with collagen, BMP, silk fibroin and PLGA as raw materials; a preparation method comprises the steps of preparation of a loose-layer silk fibroin / collagen membrane and a dense-layer silk fibroin / collagen membrane, preparation of BMP loaded PLGA microspheres, composite of the silk fibroin / collagen membranes and the microspheres and the like. A scaffold is a tissue engineering scaffold having no toxic or side effect on bodies and having good biocompatibility and tissue repair capacity. Based on combination of respective advantages of good biological compatibility, biological degradation and the like of two different natural biological macromolecules of silk fibroin and collagen, the BMP is introduced to the scaffold, a directional controlled-release slow-release technology is adopted, the BMP is allowed to be released persistently, the problems of relatively short in-vivo half-life period of the BMP, growth factor directional introduction local defect repairing and the like are solved effectively, and full playing of functions are prolonged.
Owner:FUZHOU UNIV
Blank PLGA microspheres and preparation method thereof
InactiveCN104448356ANo toxicityDoes not affect growth activityProsthesisBiologic scaffoldPolyvinyl alcohol
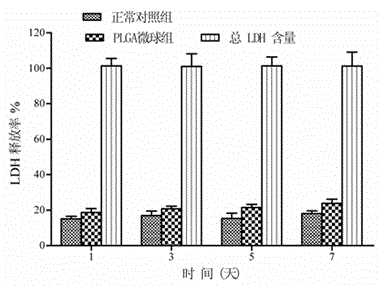
The invention provides blank polylactic acid-glycolic acid copolymer microspheres which are prepared by a water-oil-water emulsion process. The PLGA microspheres are prepared from PLGA powder by using excellent dissolving capacity of dichloromethane and emulsification of polyvinyl alcohol. Through representation observation and particle size determination of the microspheres, the diameter and the surface sign of a biological scaffold material are obtained; and the diameters of the PLGA blank microspheres accord with the injection size. Through CCK-8 determination and lactic dehydrogenase determination, the microspheres have no toxicity on attached mesenchymal stem cells, and do not affect the growth activity. Compared with chitosan particles, the microspheres are simple in preparation method, high in repetitive rate, clear structure, and easy to degrade in a living body, do not affect microenvironment metabolism, and can used as good cell carriers for different treatment targets in tissue regeneration engineering.
Owner:ZHEJIANG UNIV
3D printed Ti-PDA-PLGA microsphere bone defect repair stent and preparation method thereof
InactiveCN112295014AAchieve fine controlAchieve sustained releaseAdditive manufacturing apparatusTissue regenerationRepair tissueMicrosphere
The invention discloses a 3D printed Ti-PDA-PLGA microsphere bone defect repair stent and a preparation method thereof. A 3D printed Ti stent is prepared through a laser sintering technology; then, under a certain condition, dopamine is self-polymerized on the fiber surface of the 3D printed Ti stent to form a PDA coating, so that the 3D printed Ti-PDA stent is prepared; and then VEGF-carrying PLGA microspheres is prepared by a multiple emulsion solvent evaporation method, and finally the BMP2 and the VEGF-carrying PLGA microspheres are absorbed and fixed on the surface of the stent by an adsorption method, finally the 3D printed Ti-PDA-PLGA microsphere bone defect repair stent is prepared. The bone defect repair tissue engineering stent of the invention has the advantages of reliable mechanical property, high biological activity and safety, convenience in implantation, small trauma and low cost, which can be used for repairing treatment of bone trauma, bone tumor and bone defect afterbone infection.
Owner:NANJING DONGSHANG BIOTECHNOLOGY CO LTD
Hyaluronic acid-gelatin composite hydrogel carrying PLGA microspheres and preparation method thereof
InactiveCN111228565ALong-term antibacterialLong-term swellingPharmaceutical delivery mechanismBandagesAminoethyl methacrylateMicrosphere
The invention relates to hyaluronic acid-gelatin composite hydrogel carrying PLGA microspheres. The hydrogel includes, by mass percentage, 1-20% of aminoethyl methacrylate hyaluronic acid, 1-20% of methacrylate gelatin, 0.01-1.0% of PLGA@GS composite microspheres, and 0.01-0.5% of a photoinitiator, with the balance being water. The hydrogel simultaneously carries the PLGA@GS microspheres, HA-AEMAand GelMA, so that the hydrogel can show a three-dimensional microporous structure and has the abilities of slowly releasing drugs and long-term antibacterial properties; the hydrogel shows excellentswelling and mechanical properties and low cytotoxicity, and has the effects of rapid hemostasis; and the hydrogel is the potential materials of hemostatic and anti-infective materials in wound dressing application.
Owner:海南卓瑞生物医药有限公司
Preparation method of medicated slow-release degradable bone scaffold
InactiveCN102188756AAvoid connectionImprove antibacterial propertiesSurgeryProsthesisPolyesterMicrosphere
The invention discloses a preparation method of a medicated slow-release degradable bone scaffold, which comprises the following steps: preparing a biological degradable polyester material (such as PLGA (poly(lactic-co-glycolic acid)) and a loaded antitubercular medicament (such as rifampicin or isoniazide) into PLGA microspheres containing the rifampicin or isoniazide, respectively mixing the PLGA microspheres containing the rifampicin or isoniazide with a biological medical adhesive, and molding with a mold, thus obtaining the medicated slow-release degradable bone scaffold. Having a certain porosity, the medicated slow-release degradable bone scaffold prepared by the preparation method is beneficial to the transportation and exchange of body water, inorganic salt and other nutrient substances and cell metabolism products, thereby being more beneficial to the normal growth and physiologic metabolism of bone cells, and providing an ideal place for the growth of bone tissues; and accompanied by the degradation of the PLGA, the medicament can be continuously released in the focal position and can be kept at a certain concentration, thereby inhibiting the growth of tubercle bacillus, and ultimately degrading the PLGA into carbon dioxide and water which are removed from the body through body metabolism.
Owner:TIANJIN HAIHE HOSPITAL
Acetyl amino abamectin sustained-release microsphere and preparation method as well as sustained-release microsphere injection
InactiveCN102423300AConducive to intensive farmingReduce dosageOrganic active ingredientsPharmaceutical product form changeMicrosphereRelease time
The invention discloses an acetyl amino abamectin sustained-release microsphere and a preparation method as well as sustained-release microsphere injection. In the acetyl amino abamectin sustained-release microsphere, PLGA (polylactic-co-glycolic acid) is taken as a host material. The acetyl amino abamectin / PLGA microsphere is a long-acting constant-speed release preparation, and the medicine is controlled to release at zeroth order rate without burst release. By controlling the particle size of the microsphere, the PLGA molecular weight and copolymer proportioning, the microspheres at different medicine releasing time are obtained, and the microsphere releases the medicine for at least 50 days. Compared with the existing acetyl amino abamectin preparation formulation, the sustained-release microsphere injection has the advantages that the medicine quantity and the medicine utilization cost are remarkably reduced, the bioavailability is improved over 60%; and compared with the normal injection, the sustained-release microsphere injection has the advantages that the dosing times are reduced, and the intensification cultivation of animal husbandry is facilitated.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
RAPA-PLGA scaffold
InactiveCN106110390APromote regenerationReduce hindranceOrganic active ingredientsPharmaceutical non-active ingredientsSide effectMicrosphere
The invention relates to the field of medicine, in particular to an RAPA-PLGA scaffold. RAPA-loaded PLGA microspheres are prepared through a solvent evaporation method, then RAPA-PLGA slow-release microspheres can be constructed, and the microspheres are added into the scaffold in the construction process of the nerve scaffold. RAPA can be locally and continuously released after the RAPA-PLGA scaffold is implanted in vivo in the early injury stage, not only secondary injuries caused by an injured nerve immunological rejection reaction are relieved, but also obstruction of scar tissue on nerve regeneration is reduced, and meanwhile the side effects of systemic administration are avoided; the purposes of relieving the injured nerve secondary injuries and promoting regeneration of peripheral nerves are achieved.
Owner:丁坦
Polypropylene mesh/electro-spinning nano-fiber membrane as well as preparation method and application thereof
The invention relates to a polypropylene mesh / electro-spinning nano-fiber membrane as well as a preparation method and an application thereof. The polypropylene mesh / electro-spinning nano-fiber membrane is prepared through the following steps: (1) preparing a chitosan-polyethylene glycol hydrogel; (2) preparing basic fibroblast growth factor (bFGF) embedded polylactic acid-poly lactic-co-glycolic acid (PLGA) microspheres; (3) preparing an electro-spinning solution; (4) preparing the polypropylene mesh / electro-spinning nano-fiber membrane by use of an electro-spinning method. According to the polypropylene mesh / electro-spinning nano-fiber membrane, the polypropylene mesh and the nano-fiber membrane are compounded creatively, and therefore, the biocompatibility of the polypropylene mesh is improved by use of the nano-fiber membrane and the tensile strength of the nano-fiber membrane is improved by use of the polypropylene mesh, and the polypropylene mesh and the nano-fiber membrane supplement each other; in addition, the microspheres are introduced to realize controlled drug release; as a result, lots of defects of the clinical existing polypropylene meshes can be overcome.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
Medicine for promoting pigling growth and improving pigling immunity and preparing method thereof
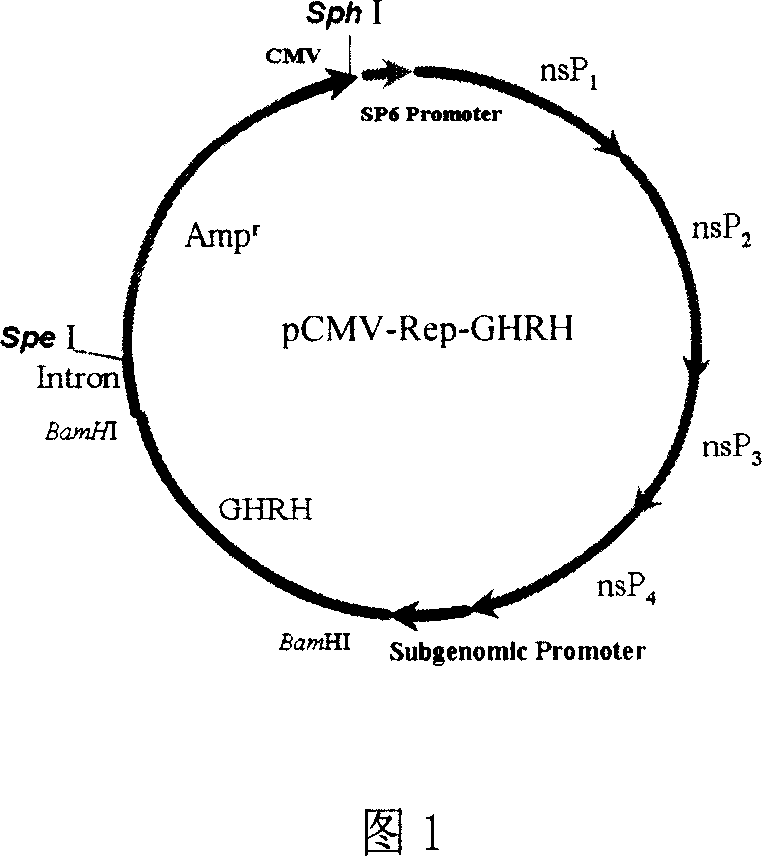
InactiveCN1927403APromote growthPromote growth and developmentGenetic material ingredientsPharmaceutical delivery mechanismMicrosphereGrowth hormone–releasing hormone
The invention relates to a drug used to accelerate the growth of pigling and improve its immunity, and relative production. Wherein, it is formed by PLGA microball that packing anterior pituitary growth hormone release hormone expression particle pCMV-Rep-GHRH; via ejecting said PLGA microball particles pCMV-Rep-GHRH into female pig, to express GHRH and improve the GH level, it can accelerate the growth of pigling and improve its immunity.
Owner:张永亮
Method for preparing PLGA microspheres with porous surfaces
InactiveCN105664242ANo side effectsUniform particle sizeProsthesisOrganic solventUltrasonic emulsification
The invention provides a method for preparing surface porous PLGA microspheres, comprising: adding PLGA to an organic solvent, stirring and dissolving, adding dropwise a pore-forming solution, and supersonic emulsification to form an emulsion; wherein, the LA of PLGA and the content of GA The molar ratio range is: 50:50~90:10; the emulsion is added dropwise to the stirred external water phase, and a preset volume of deionized water is added, and the speed is changed to continue stirring until the organic solvent is completely volatilized. Among them, The external water phase is a water-soluble surfactant added to deionized water; the solution after the organic solvent is completely volatilized is centrifuged, and washed with deionized water, and the supernatant is removed to obtain PLGA microspheres; NaOH solution is added to the PLGA microspheres. spheres, mixed evenly, and placed on a shaker to continue the reaction; the reacted PLGA microspheres were repeatedly centrifuged and washed with deionized water, and freeze-dried to obtain PLGA microspheres with porous surfaces. The invention has simple process and short period, and is suitable for industrial large-scale production.
Owner:CHONGQING UNIVERSITY OF SCIENCE AND TECHNOLOGY
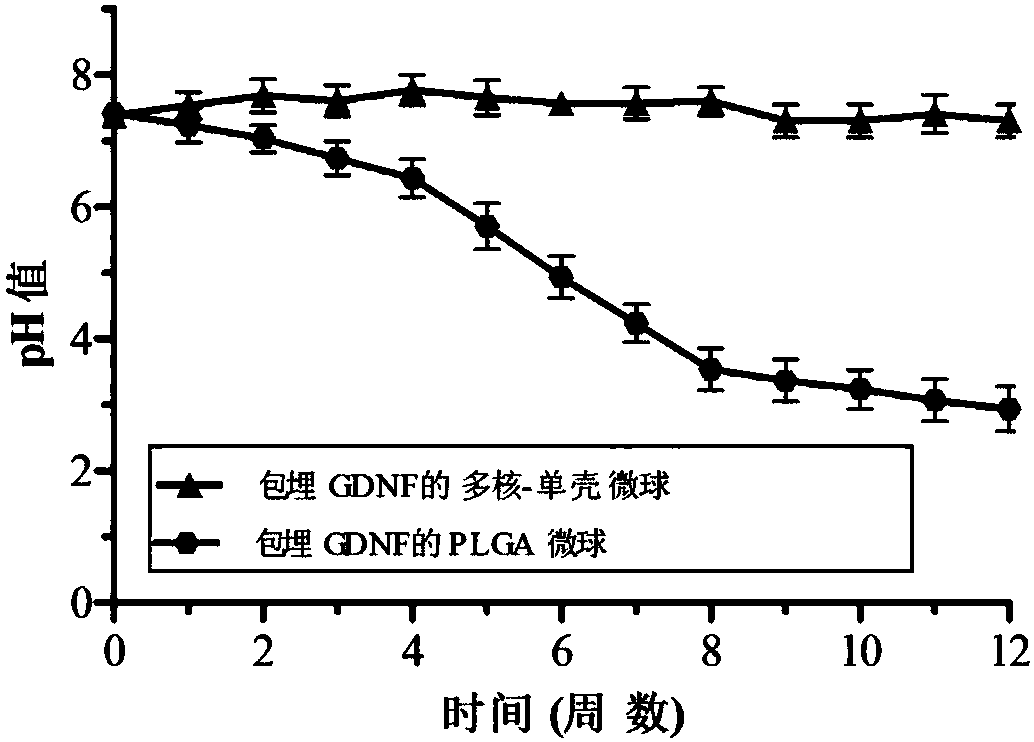
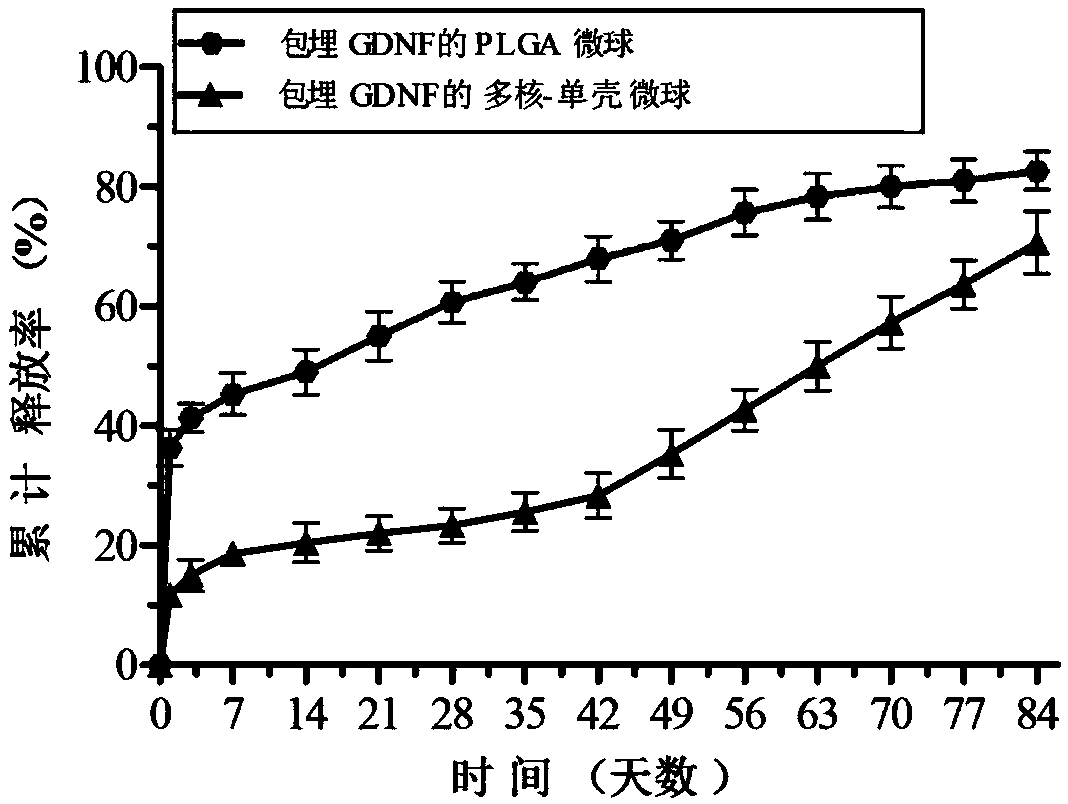
Preparation method for multicore-single shell microsphere sustained-release system with embedded GDNF
ActiveCN108042793AGood regeneration environmentImprove the acidic microenvironmentNervous disorderPeptide/protein ingredientsAcetic acidOil emulsion
The invention discloses a preparation method for a multicore-single shell microsphere sustained-release system with embedded GDNF. The preparation method comprises the following steps: preparing PLGAmicrospheres with embedded GDNF; subjecting the PLGA microspheres with embedded GDNF to flushing, centrifugation and freeze-drying successively; preparing a chitosan solution from chitosan powder andan aqueous solution of glacial acetic acid, dissolving the PLGA microspheres with the embedded GDNF in water to prepare an aqueous solution of the PLGA microspheres, adding the aqueous solution of thePLGA microspheres into the chitosan solution to prepare a water phase W, mixing liquefied petrolatum with a surfactant to prepare an oil phase O, mixing the water phase W with the oil phase O to prepare a water / oil emulsion W / O, and mixing a sodium tripolyphosphate solution with the water / oil emulsion W / O so as to prepare an emulsion; and cleaning the emulsion so as to obtain the multicore-singleshell microsphere sustained-release system with the embedded GDNF. The multicore-single shell microsphere sustained-release system prepared by using the method can neutralize acidic degradation products of the PLGA microspheres and provides a good local regeneration environment to damaged nerves.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
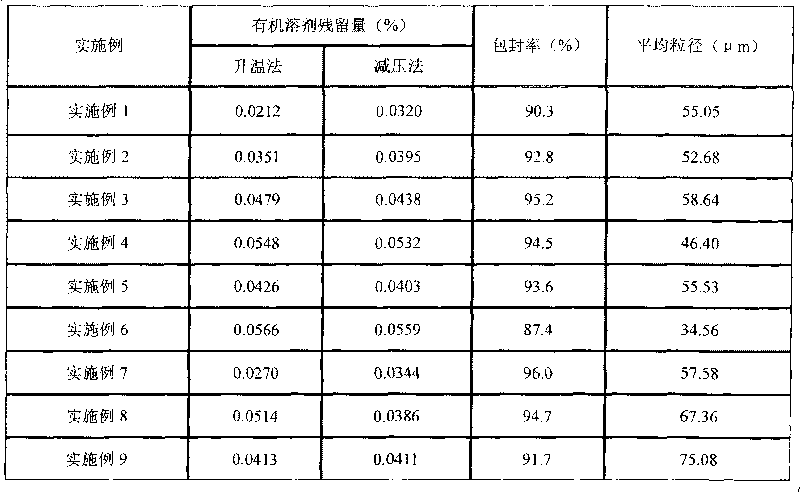
Method for controlling residue of organic solvent in thymic peptide alpha1 microballoon
ActiveCN101757609AControl residueKeep intactPeptide/protein ingredientsDigestive systemEmulsionOrganic solvent
The invention provides a method for controlling residue of organic solvent in thymic peptide alpha1 microvballoon, including that thymic peptide alpha1 PLGA microballoon emulsion or multiple emulsion obtained by preparation is stirred under the conditions that temperature is 15-42 DEG C and rotating speed is 20-3000r / min. The method for controlling residue of organic solvent in thymic peptide alpha1 microballoon of the invention is efficient and controllable, stable in technology and safe and reasonable, can effectively control the residue problem of organic solvent such as dichloromethane and meanwhile can maintain the form of microballoon to be complete.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
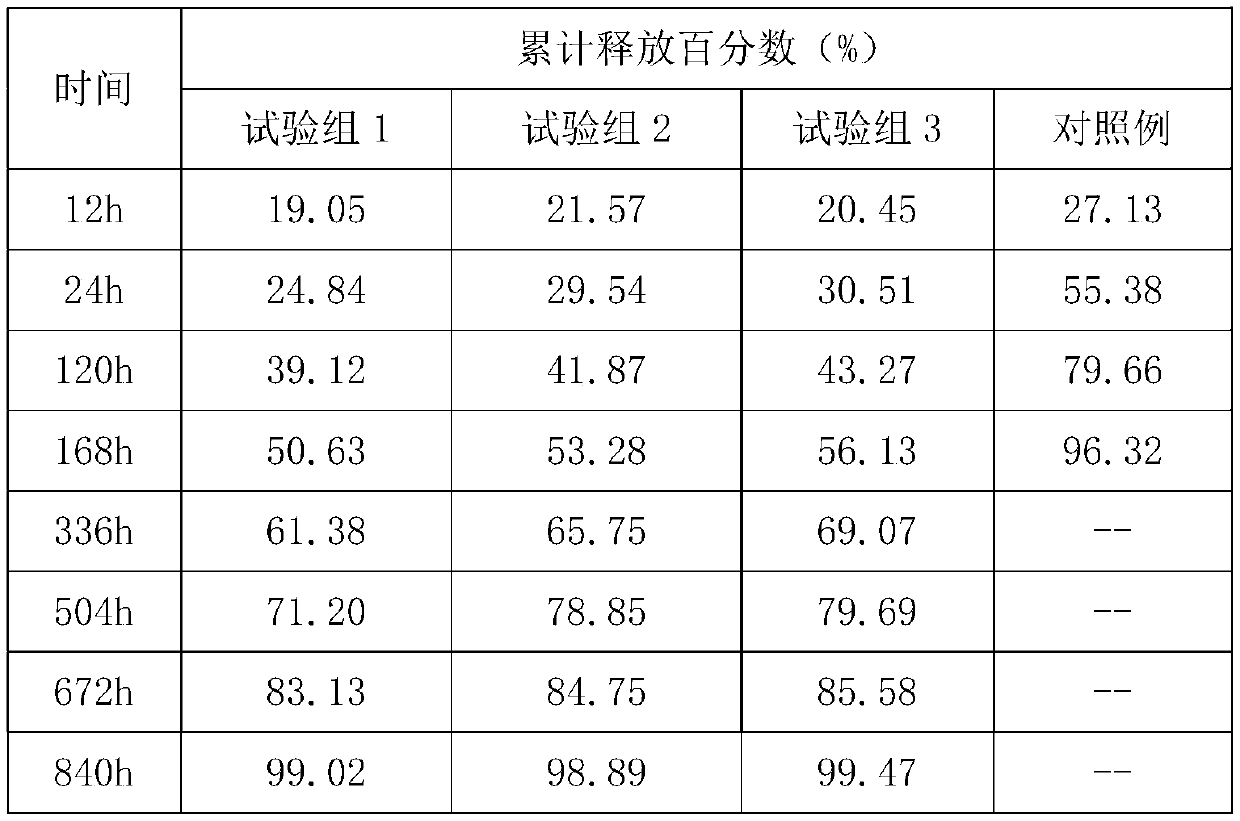
rhBMP-2-loaded bone repair material microsphere and preparation method thereof
ActiveCN110522946AGood biocompatibilityPromote growthTissue regenerationProsthesisMicrosphereRepair material
The invention belongs to the field of biomedical materials containing active proteins and the field of regenerative medicine. The invention provides a bone repair material, which comprises rhBMP-2 (recombinant bone morphogenetic protein-2) and PLGA microspheres, rhBMP-2 is wrapped in the PLGA microspheres, and the surfaces of the microspheres are modified with mussel mucoprotein.
Owner:ZHEJIANG RISING BIOTECH CO LTD
Encapsulated chelator
An enhanced chelator includes a chelating agent and a volatile material encapsulated in a biologically benign microcapsule. The enhanced chelator possesses significantly improved shelf-life in aqueous biological buffer solutions because the chelating agent is encapsulated in the microcapsule and, therefore, separated from solution components with which the chelating agent would react. The enhanced chelator is activated at a predetermined elevated temperature defined by the boiling point of the volatile material. At this predetermined elevated temperature, the volatile material exerts a vapor pressure sufficient to rupture the microcapsule and thereby release the chelating agent from the microcapsule. In one embodiment, a manganese chelator such as ethylene glycol tetraacetic acid (EGTA) is solubilized in ethanol and encapsulated in a poly(lactic-co-glycolide) (PLGA) microsphere. Upon heating to 80° C., ethanol boils within the PLGA microsphere and undergoes several orders of magnitude volume change, thereby rupturing the PLGA microsphere and releasing EGTA.
Owner:IBM CORP
Method for coaxially preparing injectable PLGA drug-carrier microsphere by utilizing electrospinning machine
InactiveCN108078954AMitigation of sudden releaseGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereHigh pressure
The invention belongs to preparation of drug-carrier microspheres, and discloses a method for coaxially preparing an injectable PLGA (poly(lactic-co-glycolic acid)) drug-carrier microsphere by utilizing an electrospinning machine. The method comprises the following steps: dissolving PLGA in hexafluoro isopropyl alcohol to prepare a PLGA solution as a shell layer solution; dissolving PTX (Paclitaxel) and ETP (Etoposide) in a dichloromethane solution to prepare a solution as a nuclear layer solution; stirring the two solutions and respectively injecting the solutions into a disposable 1ml plastic syringe; performing high-pressure electrostatic spraying by utilizing the electrospinning machine; then performing lyophilization to prepare PLGA microspheres with embedded PTX and ETP. The coaxialdrug-carrier microspheres prepared by the invention can be subjected to sustained-release and controlled-release and can be used for injecting. According to the method disclosed by the invention, through characteristic observation on the microspheres and determination on particle sizes of the microspheres, the average particle size of the drug-carrier microspheres is 3 microns, and the surfaces ofthe drug-carrier microspheres are smooth without holes. The composite microspheres prepared by the method disclosed by the invention are safe and free of toxicity, and can be used for postoperative treatment of osteosarcoma; a specific sustained-release effect is beneficial for reducing the damage of anti-tumor drugs to a human body, the anti-tumor activity of the drugs is improved, and the microspheres have a broad research value and development prospects.
Owner:JILIN UNIV
Porous semi-degraded hydrogel material used for cartilage repair and preparation method thereof
InactiveCN103405808AGood biomechanical propertiesGood mechanical propertiesProsthesisMicrosphereCartilage repair
The invention discloses a porous semi-degraded hydrogel material used for cartilage repair and a preparation method thereof, belonging to the technical field of articular cartilage repair. The preparation method is specifically characterized by adding mixed liquor of degradable PLGA (poly(lactic-co-glycolic acid)) microspheres with biological activities and a compound pore-foaming agent to a hydrogel solution and finally obtaining the porous semi-degraded hydrogel material through several times of physical crosslinking of freezing and unfreezing after stirring the solution uniformly at high temperature. The hydrogel material has the obvious advantages that the advantage of biological activities of the PLGA microspheres and the advantage of excellent biomechanical properties of the hydrogel material are combined; the porous structure of the prepared porous hydrogel material containing the PLGA microspheres is suitable for cell seeding and migration; regeneration of cartilage tissues is induced by degradation of the PLGA microspheres, so that the problem of connection between repair materials and surrounding tissues can be effectively solved; and meanwhile, the excellent mechanical properties of the hydrogel material are conductive to ensuring the mechanical strength of implanting initial materials.
Owner:NANJING UNIV OF SCI & TECH
An alepsin slow-releasing gel for promoting paradontal part reborn as well as preparation method and application
InactiveCN101156849AHigh and long-lasting drug concentrationSave human effortOrganic active ingredientsDigestive systemSustained release drugMicrosphere
The invention discloses phenytoin sodium sustained-release gel which promotes periodontal regeneration, and the phenytoin sodium sustained-release gel is made of components of phenytoin sodium, PLGA sustained-release microspheres and poloxamer gel, wherein, the content of an active component, the phenytoin sodium is 2 to 5 mg / ml. The preparation method of the phenytoin sodium sustained-release gel which promotes the periodontal regeneration has the steps that firstly, the PLGA microspheres containing the phenytoin sodium is prepared in W / O / W type emulsified solvent volatilization method; secondly, the microspheres are mixed into 25 percent of the poloxamer gel, to produce microsphere gel containing 2 to 5 mg / ml phenytoin sodium. The invention provides a new safe, effective drug with acceptable price and easy promotion and application for local administration of periodontal disease therapy. The preparation method of the phenytoin sodium sustained-release gel provided by the invention is convenient, and has strong operability, the prepared sustained-release drug has stable performance, and the distribution of the microsphere graininess is more uniform.
Owner:SHANDONG UNIV
Preparation technique of swine influenza DNA vaccine PLGA microspheres
InactiveCN101422618AHigh encapsulation efficiencyIncrease concentrationGenetic material ingredientsAntiviralsEmulsionOrganic solvent
The invention relates to a preparation process of swine influenza DNA vaccine PLGA microspheres, which solves the problems that common DNA vaccines are short in function lasting time, easy to be degraded in bodies and cannot realize the aim of sustained release. The preparation process of the swine influenza DNA vaccine PLGA microspheres comprises the steps as follows: colostrum is taken to prepare multiple emulsion, and the multiple emulsion is transferred into a beaker and magnetically stirred for 5 hours, and then an organic solution is evaporated at the room temperature condition, and the centrifugal process is carried out for 10 minutes, and the microspheres are collected and washed by sterile water, and the swine influenza DNA vaccine PLGA microsphere can be obtained by the vacuum freezing and drying. The process can be used in the preparation of the swine influenza DNA vaccine PLGA microspheres.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com