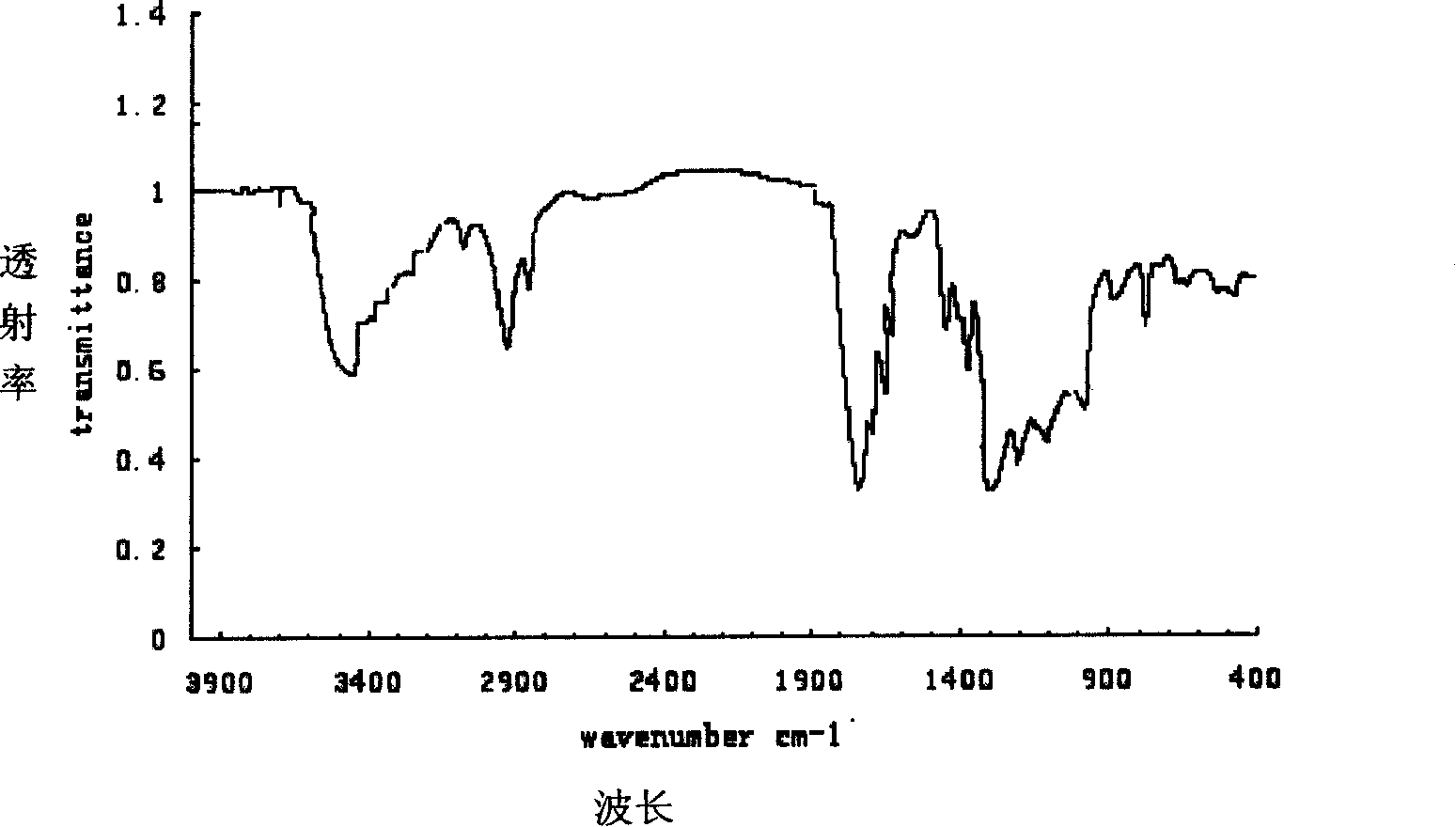
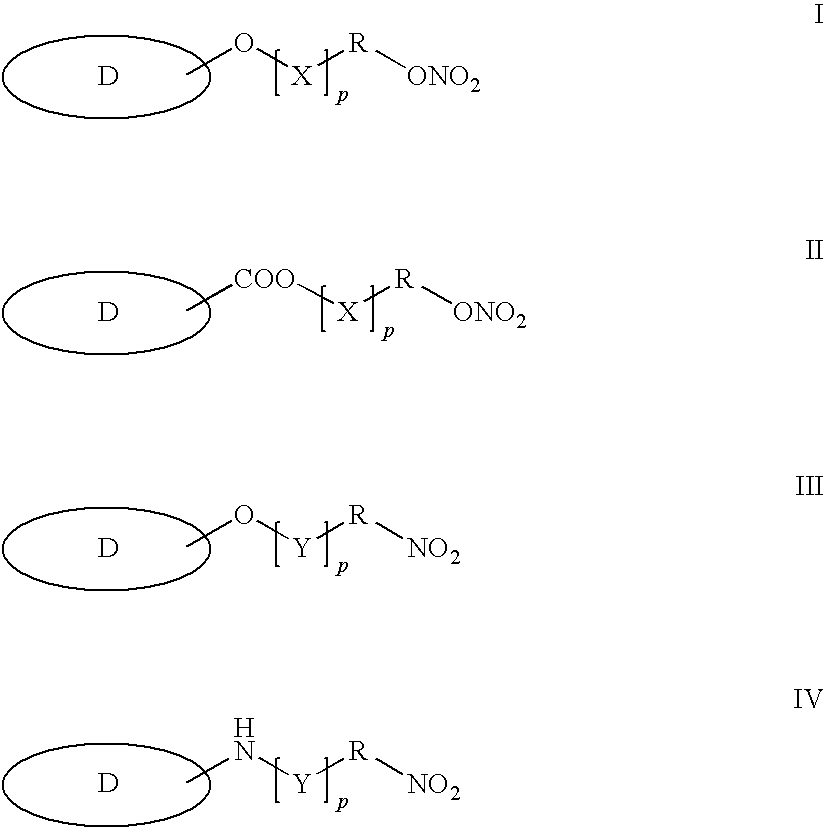
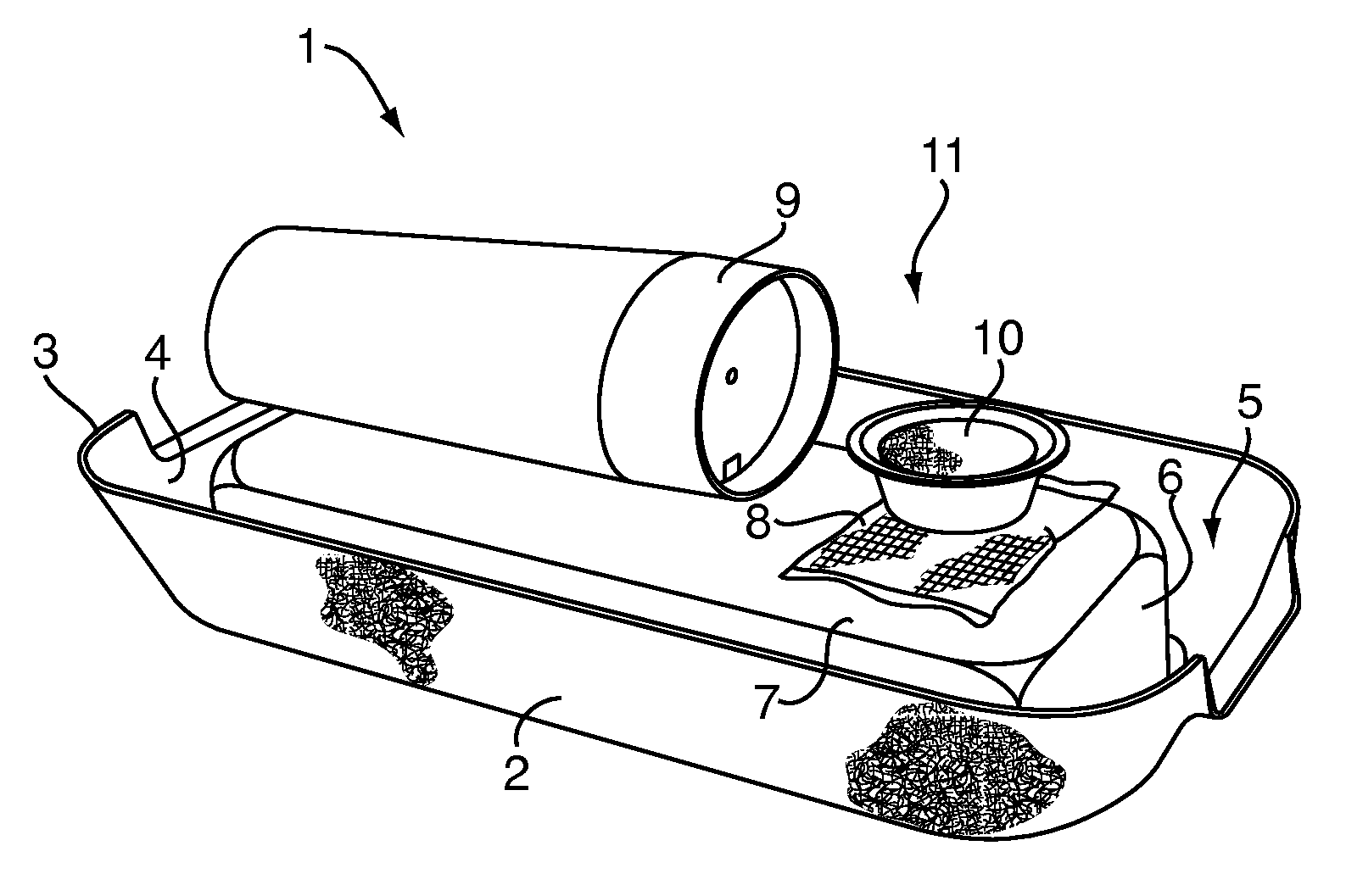
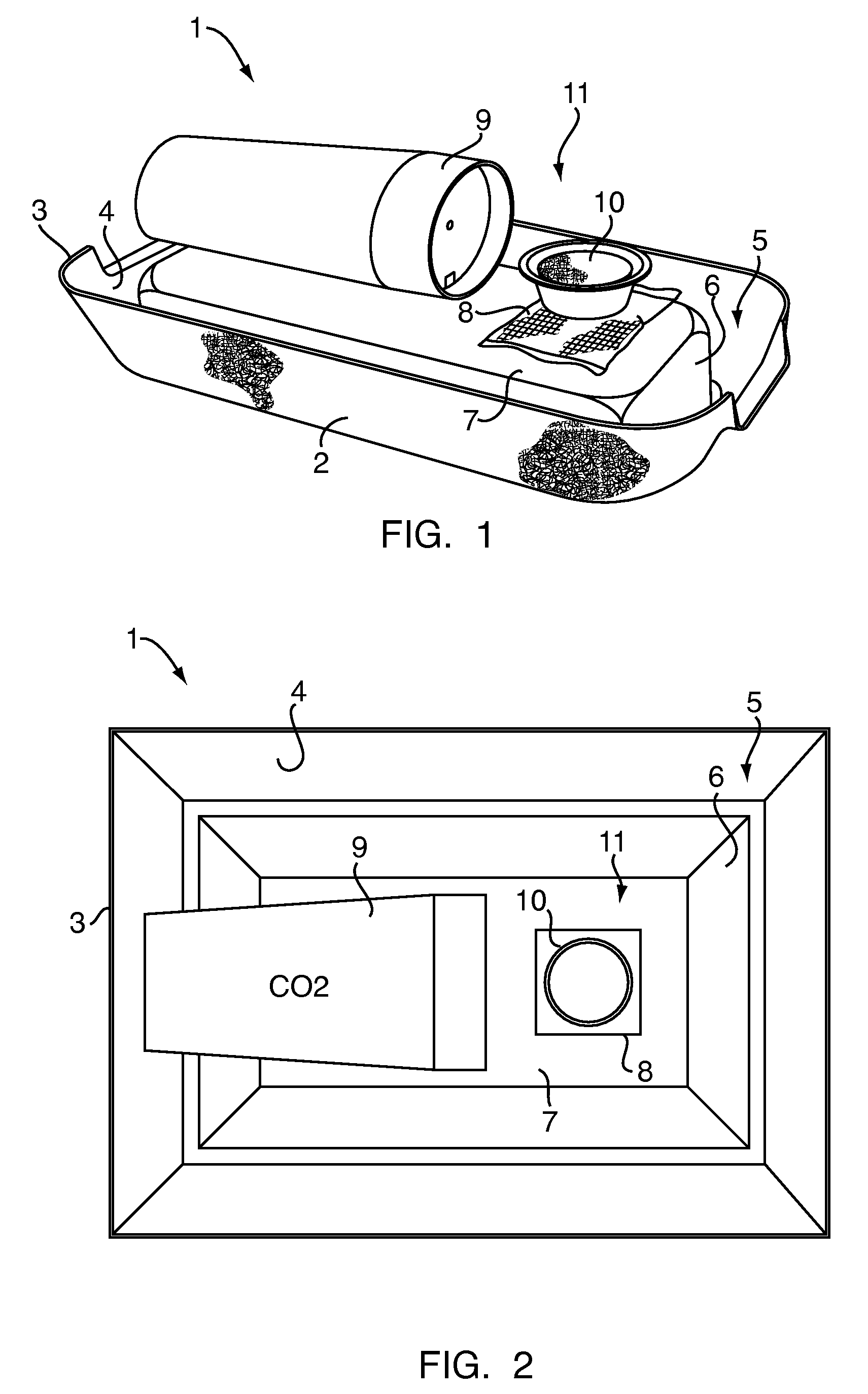
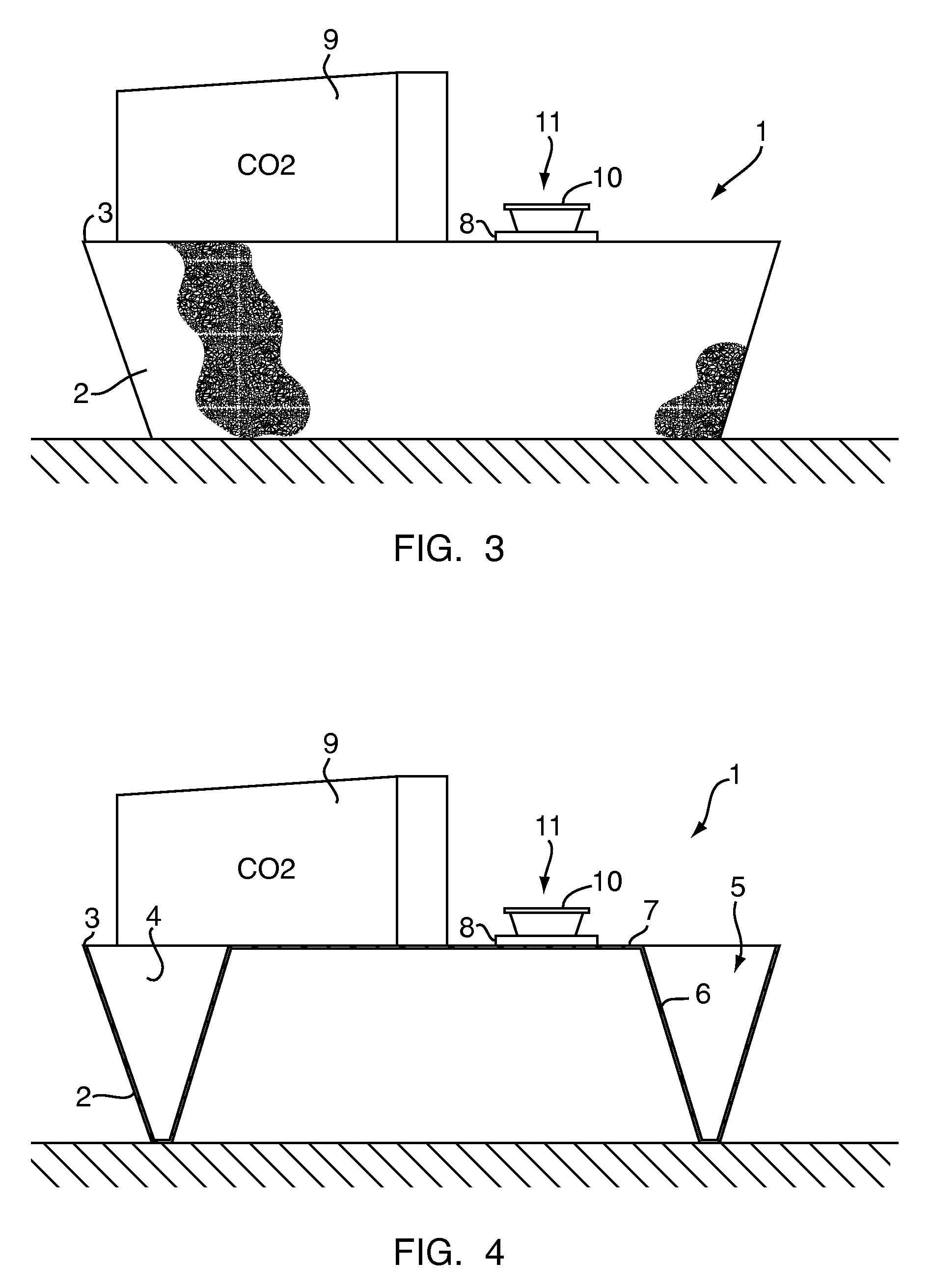
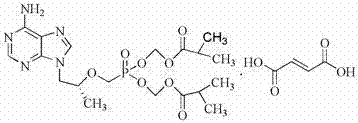
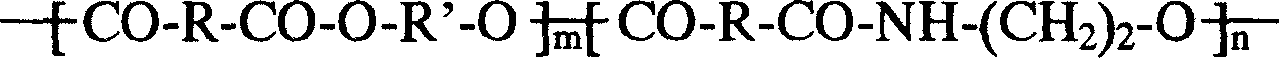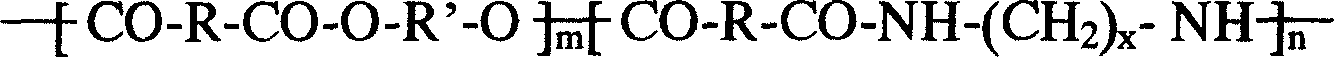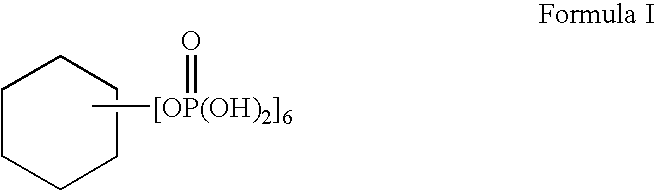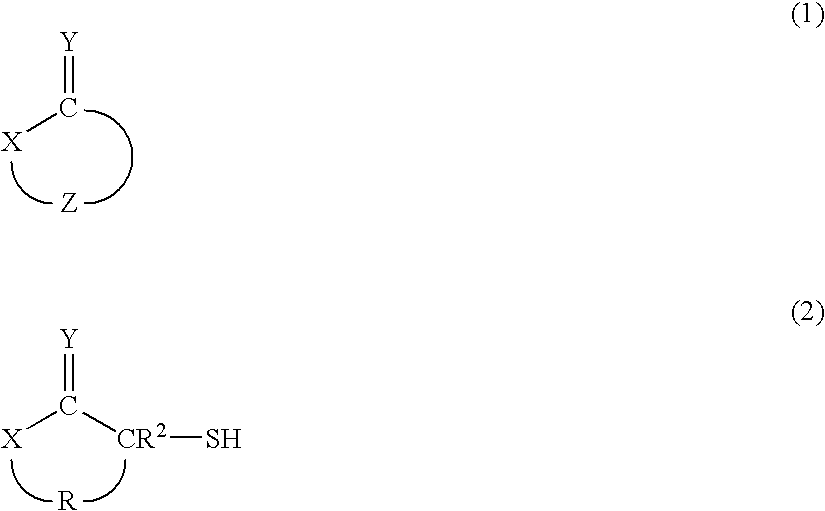Patents
Literature
782 results about "Lacceroic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lacceroic acid (or dotriacontanoic acid) is a saturated fatty acid.
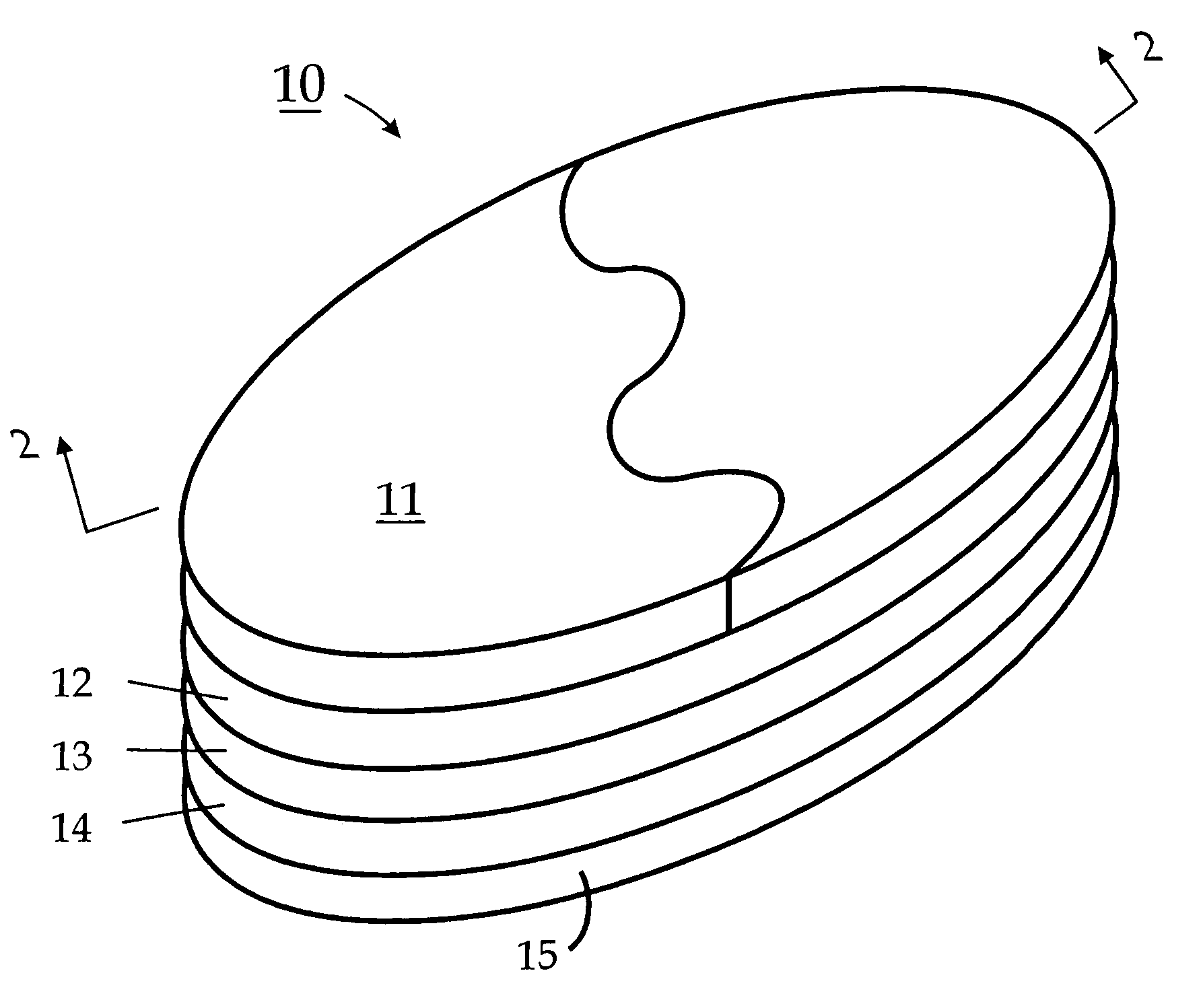
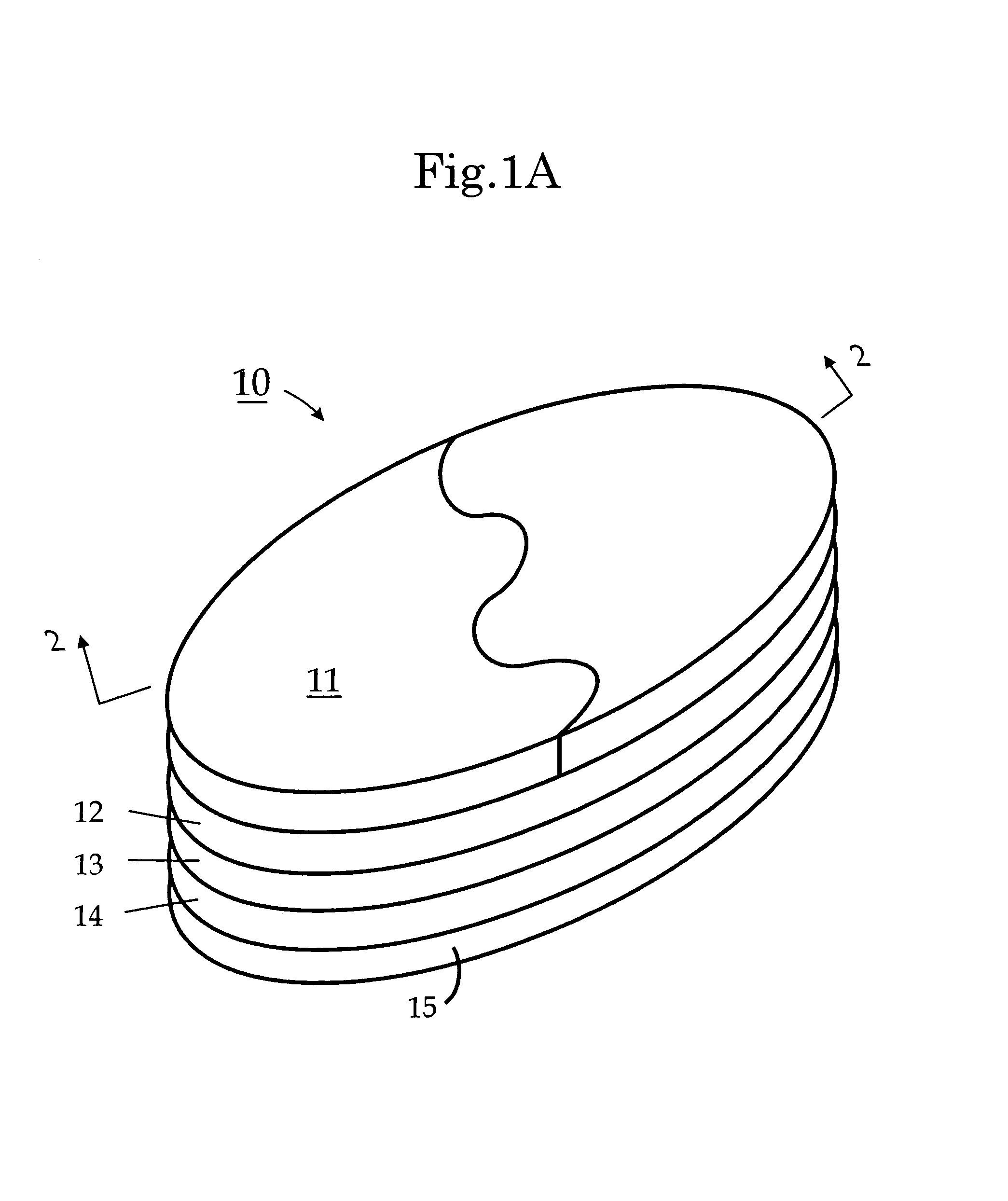
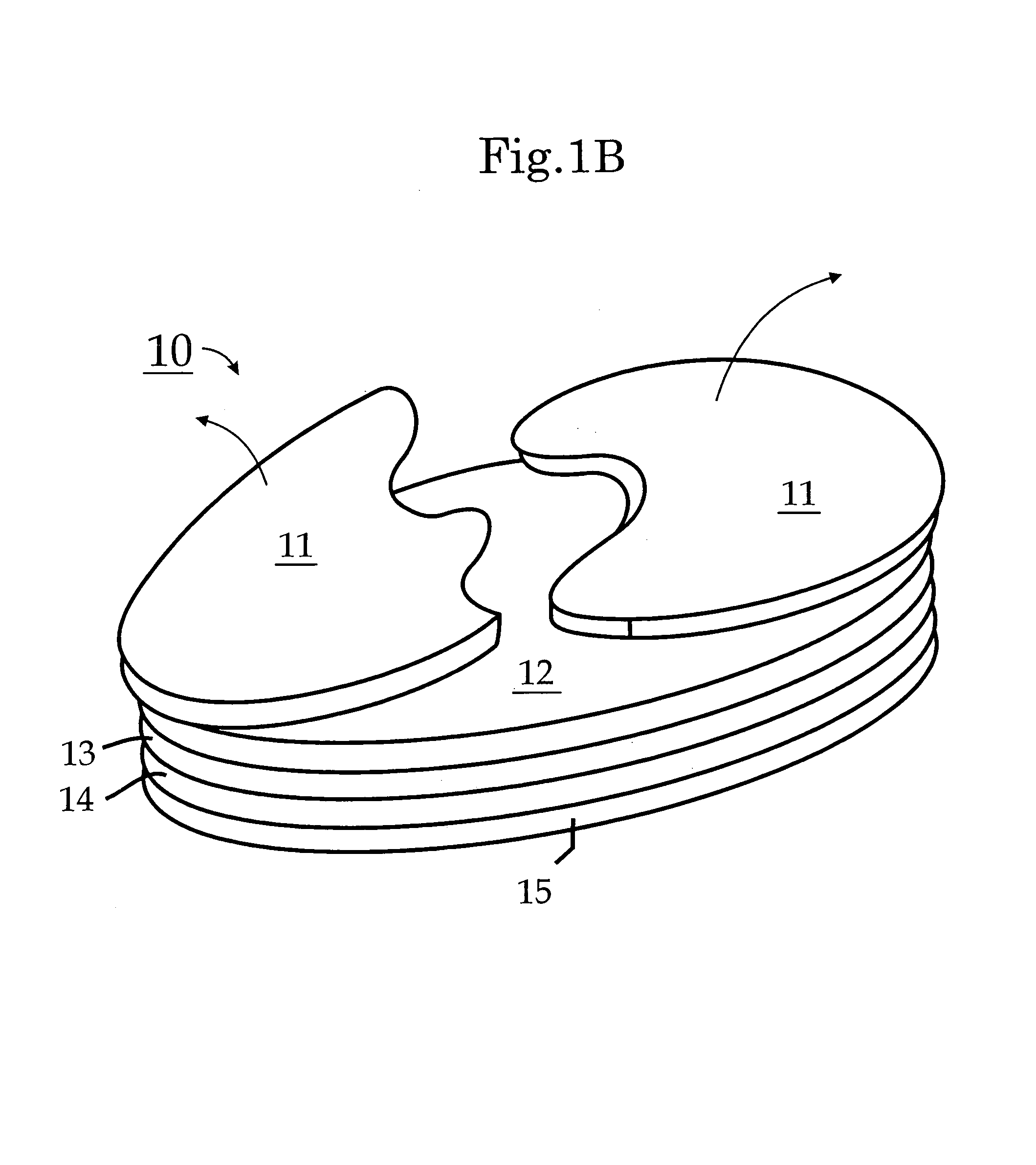
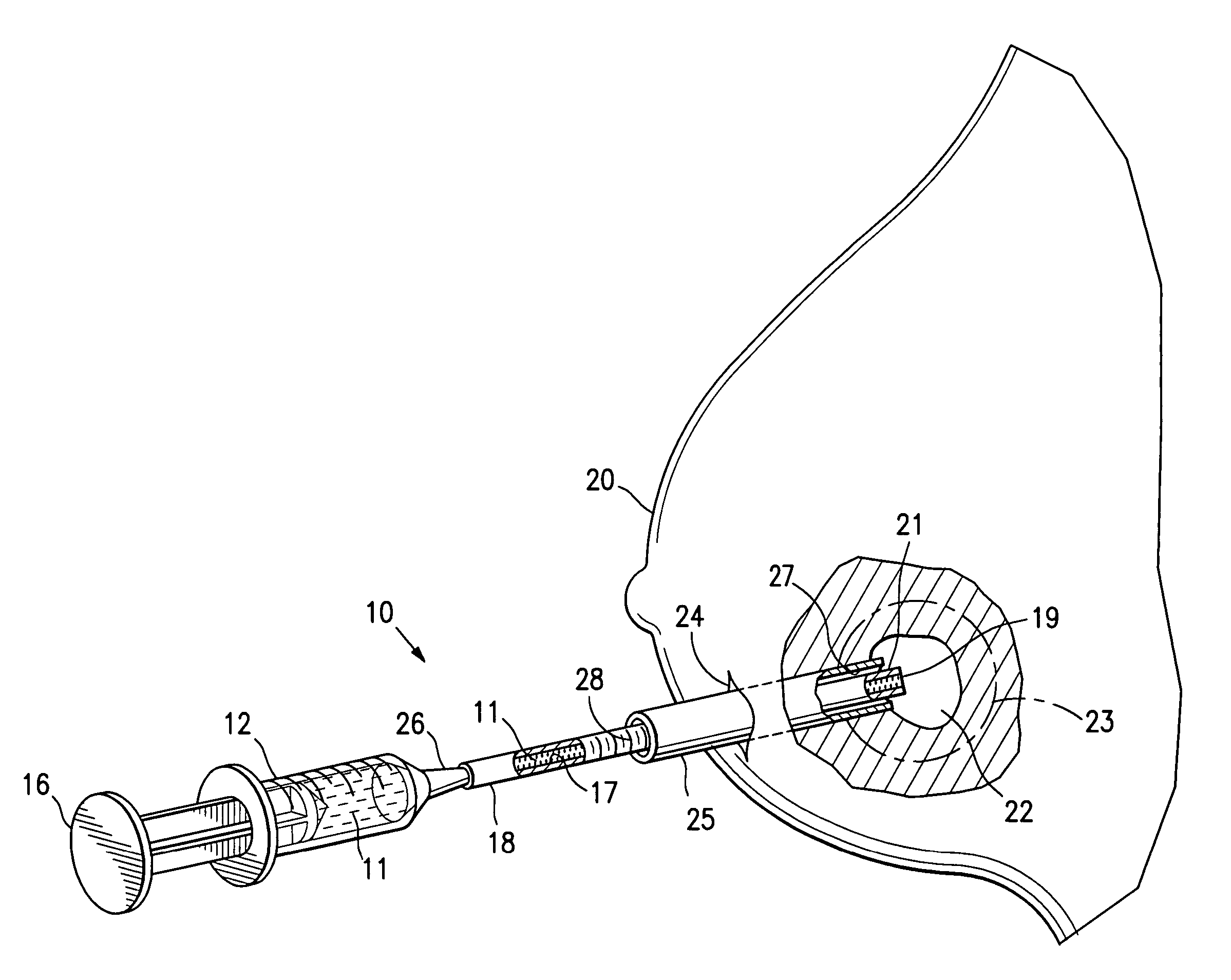
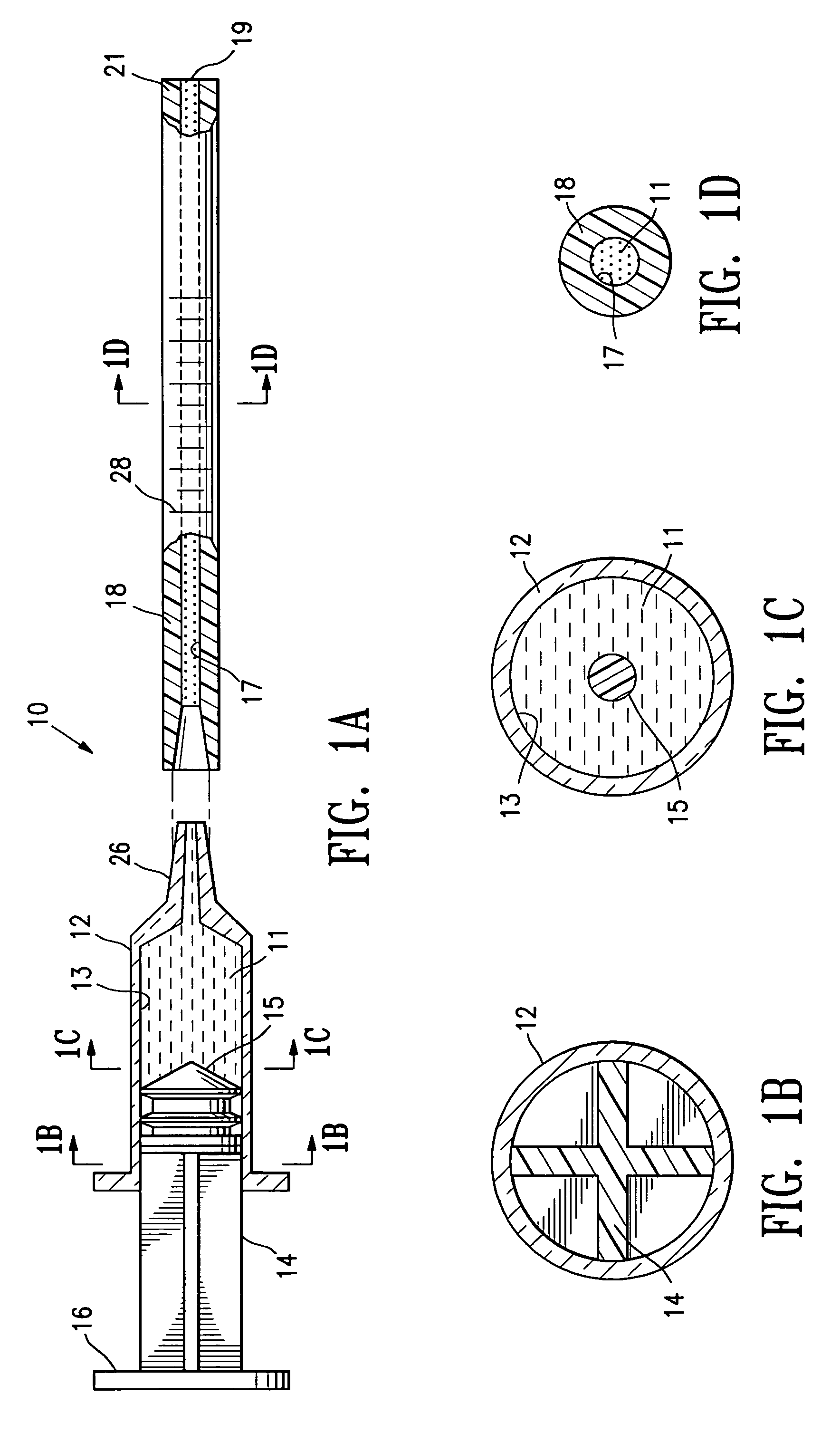
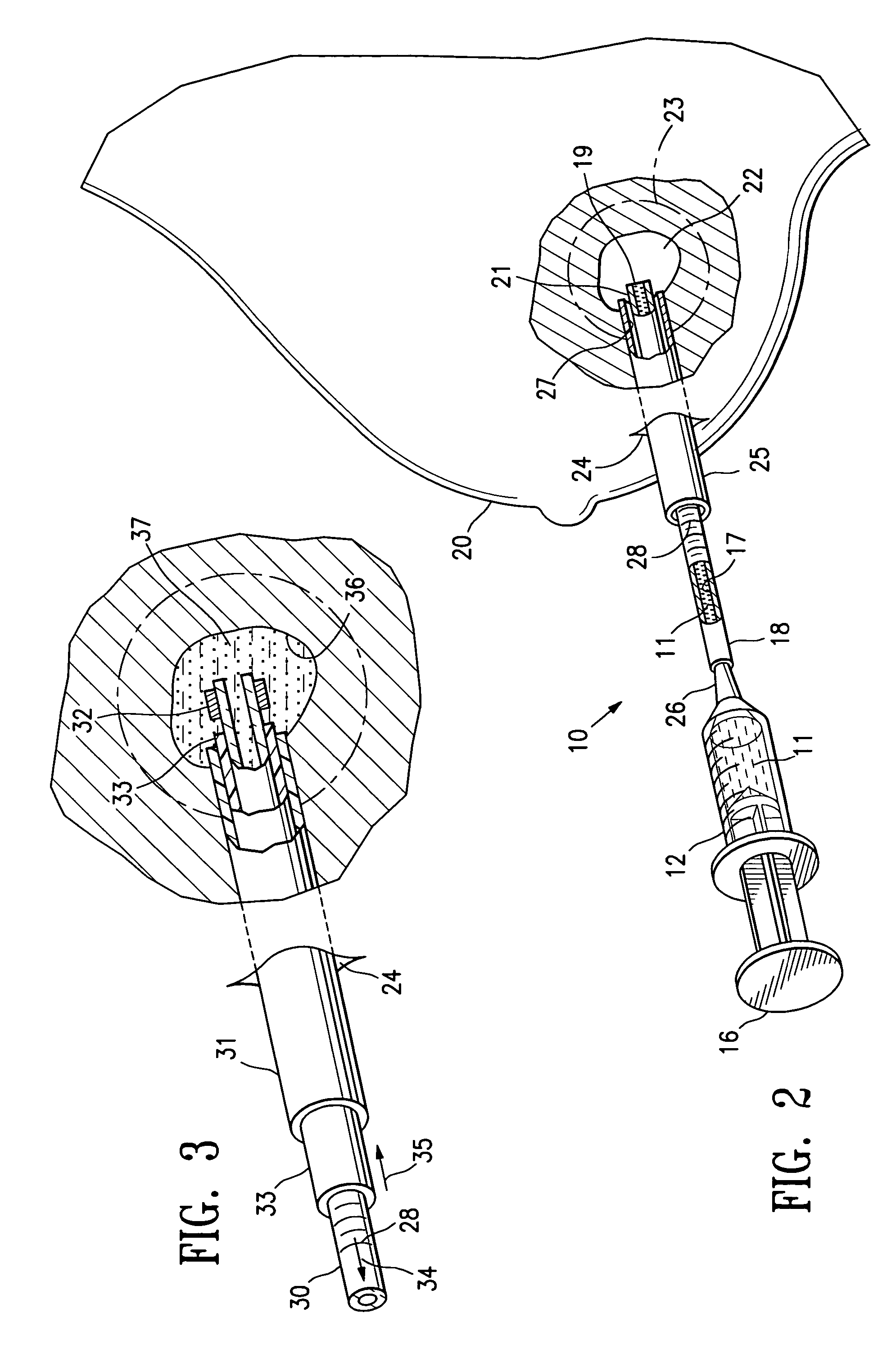
Biodegradable injectable implants and related methods of manufacture and use
InactiveUS20030093157A1Broaden applicationSolution deliveryPharmaceutical non-active ingredientsGlycolic acidImplant
This invention is directed to the field of medical implants, and more specifically to biodegradable injectable implants and their methods of manufacture and use. The injectable implants disclosed herein comprise glycolic acid and bio-compatible / bio-absorbable polymeric particles containing a polymer of lactic acid. The particles are small enough to be injected through a needle but large enough to avoid engulfment by macrophages. The injectables of this invention may be in a pre-activated solid form or an activated form (e.g., injectable suspension or emulsion).
Owner:MEDGRAFT MICROTECH
Proteinic drug delivery system using membrane mimetics
A mixed liposome pharmaceutical formulation with multilamellar vesicles, comprises a proteinic pharmaceutical agent, water, an alkali metal lauryl sulphate in a concentration of from 1 to 10 wt. / wt. %, at least one membrane-mimetic amphiphile and at least one phospholipid. The membrane-mimetic amphiphile is hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, lauramidopropyl betain, lauramide monoisopropanolamide, sodium cocoamphopropionate, bishydroxypropyl dihydroxypropyl stearammonium chloride, polyoxyethylene dihydroxypropyl stearammonium chloride, dioctadecyldimethylammonium chloride, sulphosuccinates, stearamide DEA, gamma-linoleic acid, borage oil, evening of primrose oil, monoolein, sodium tauro dihydro fusidate, fusidic acid, alkali metal isostearyl lactylates, alkaline earth metal isostearyl lactylates, panthenyl triacetate, cocamidopropyl phosphatidyl PG-diammonium chloride, stearamidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidylcholine, polysiloxy pyrrolidone linoleyl phospholipid, trihydroxy-oxo-cholanylglycine and alkali metal salts thereof, and octylphenoxypolythoxyethanol, polydecanol X-lauryl ether, polydecanol X-oleyl ether, wherein X is from 9 to 20, or combinations thereof. The phospholipid is phospolipid GLA, phosphatidyl serine, phosphatidylethanolamine, inositolphosphatides, dioleoylphosphatidylethanolamine, sphingomyelin, ceramides, cephalin, triolein, lecithin, saturated lecithin and lysolecithin, or a combination thereof. The amount of each membrane mimetic amphiphile and phospholipid is present 1 to 10 wt. / wt. % of the total formulation, and the total concentration of membrane mimetic amphiphiles and phospholipids is less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
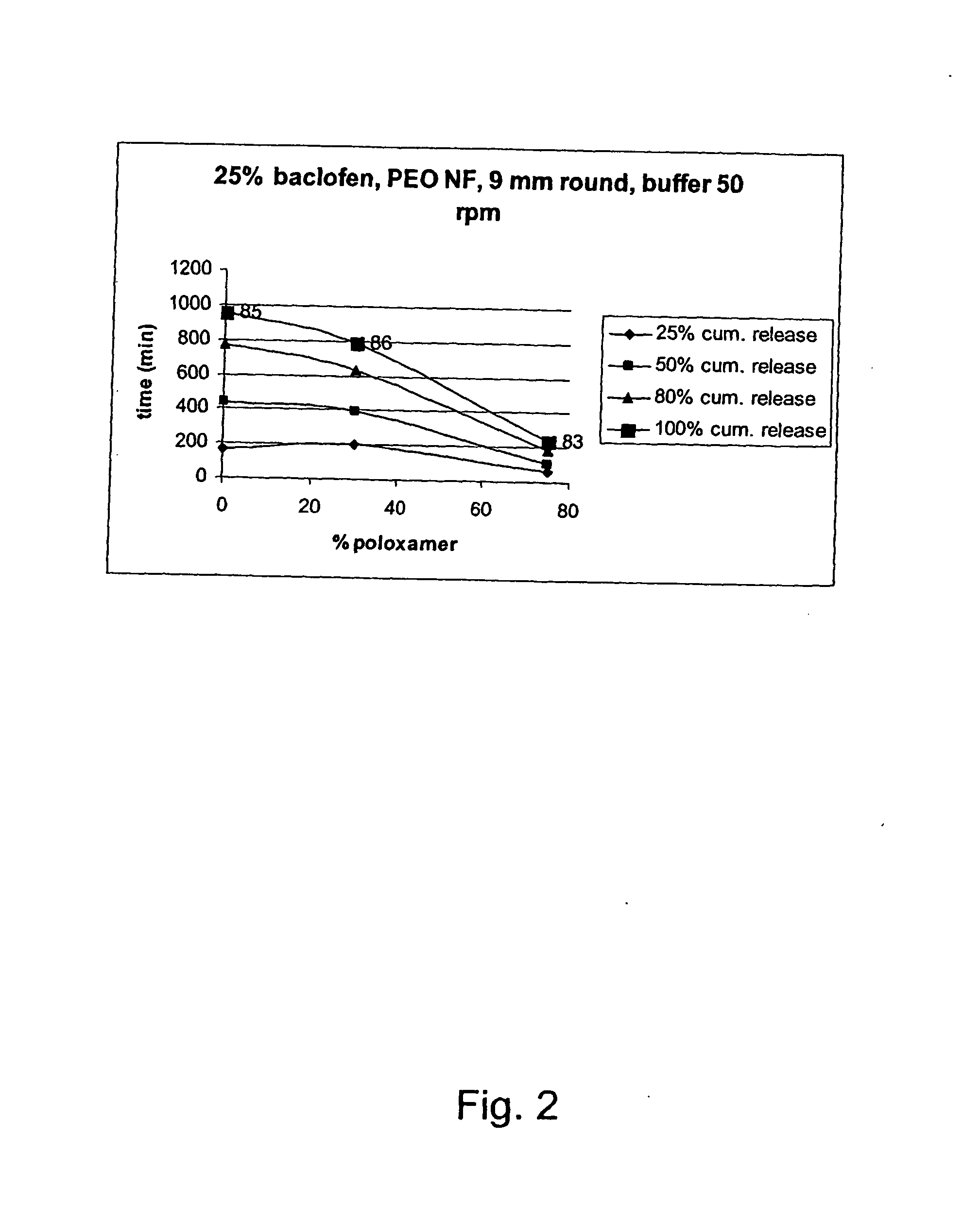
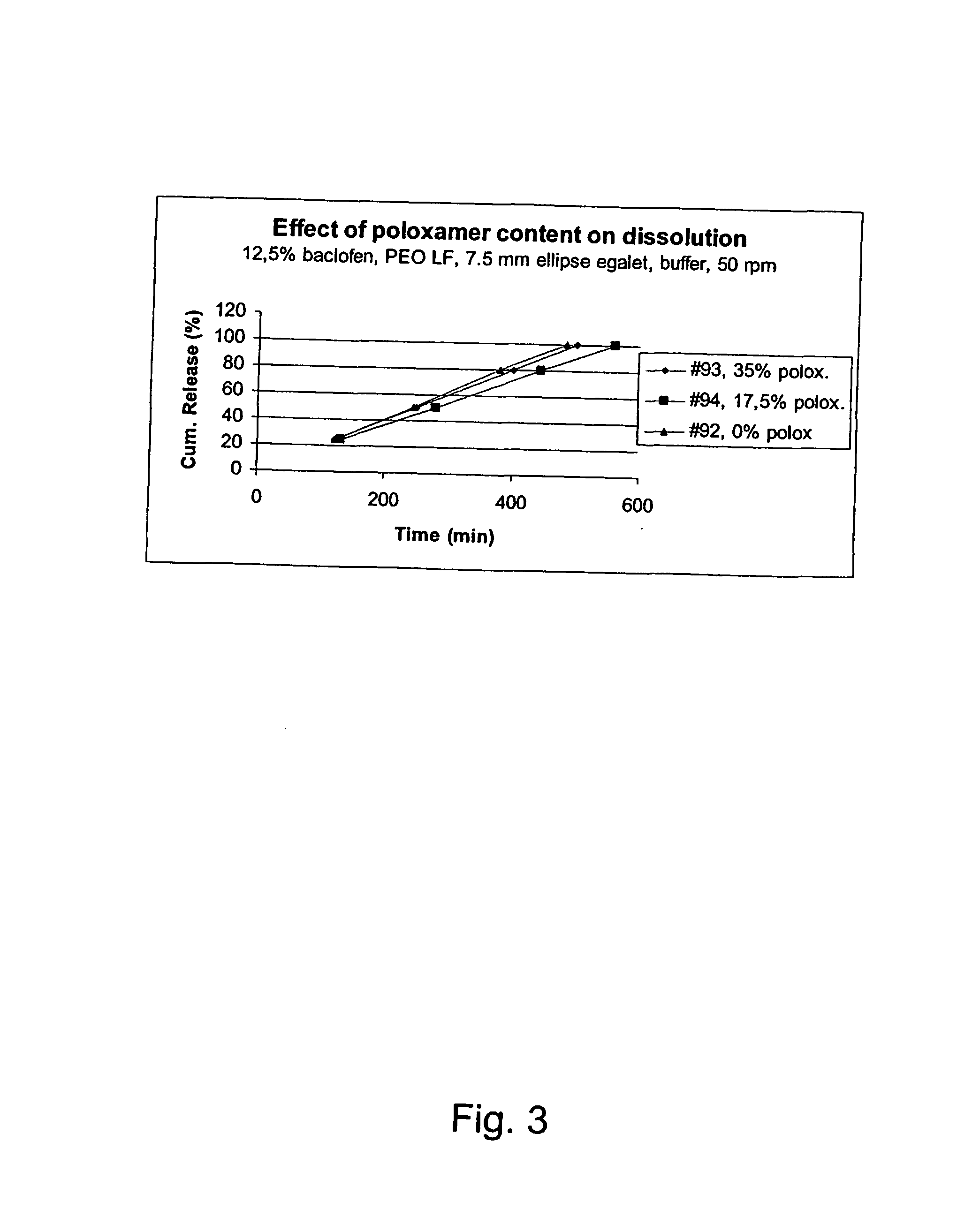
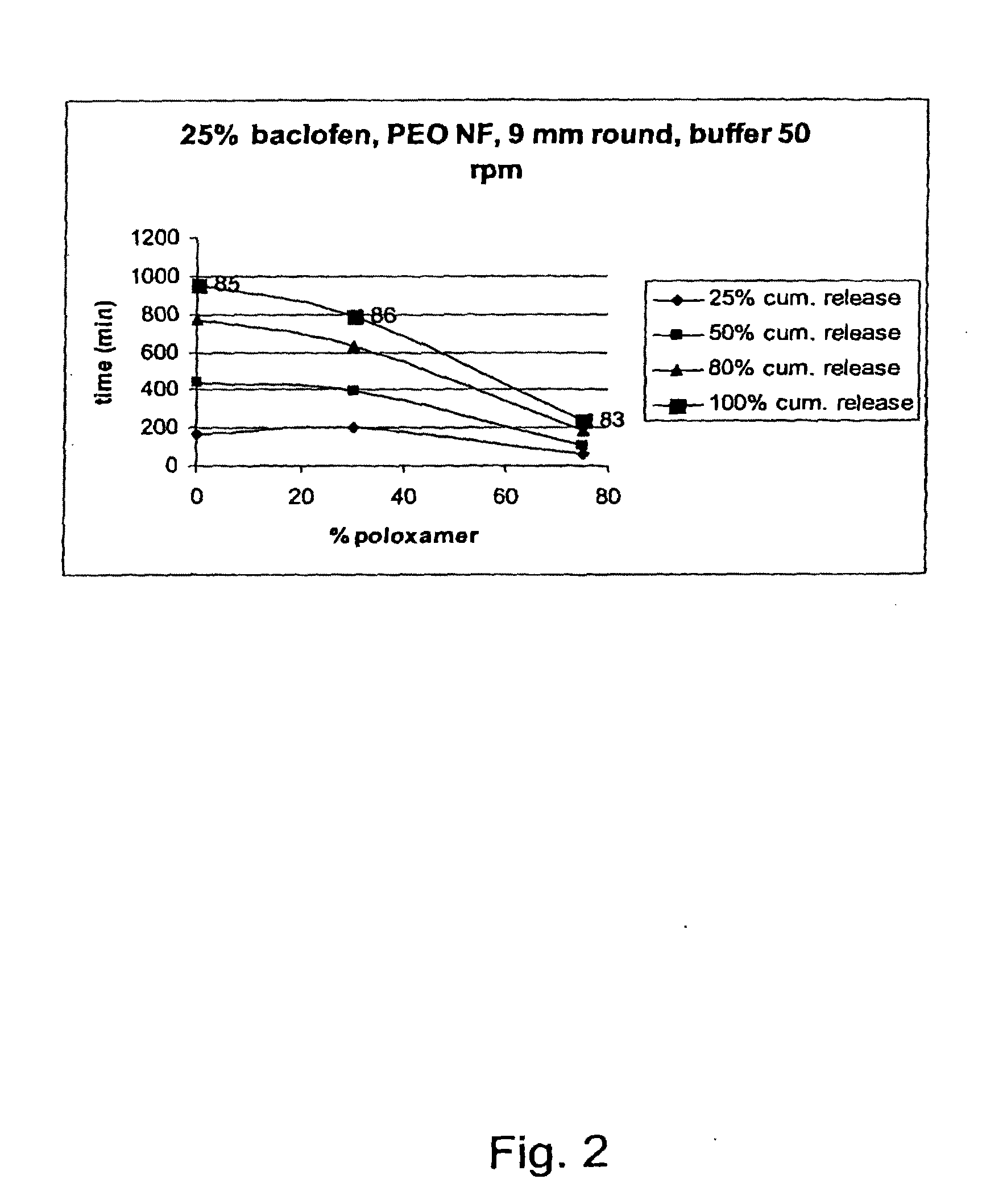
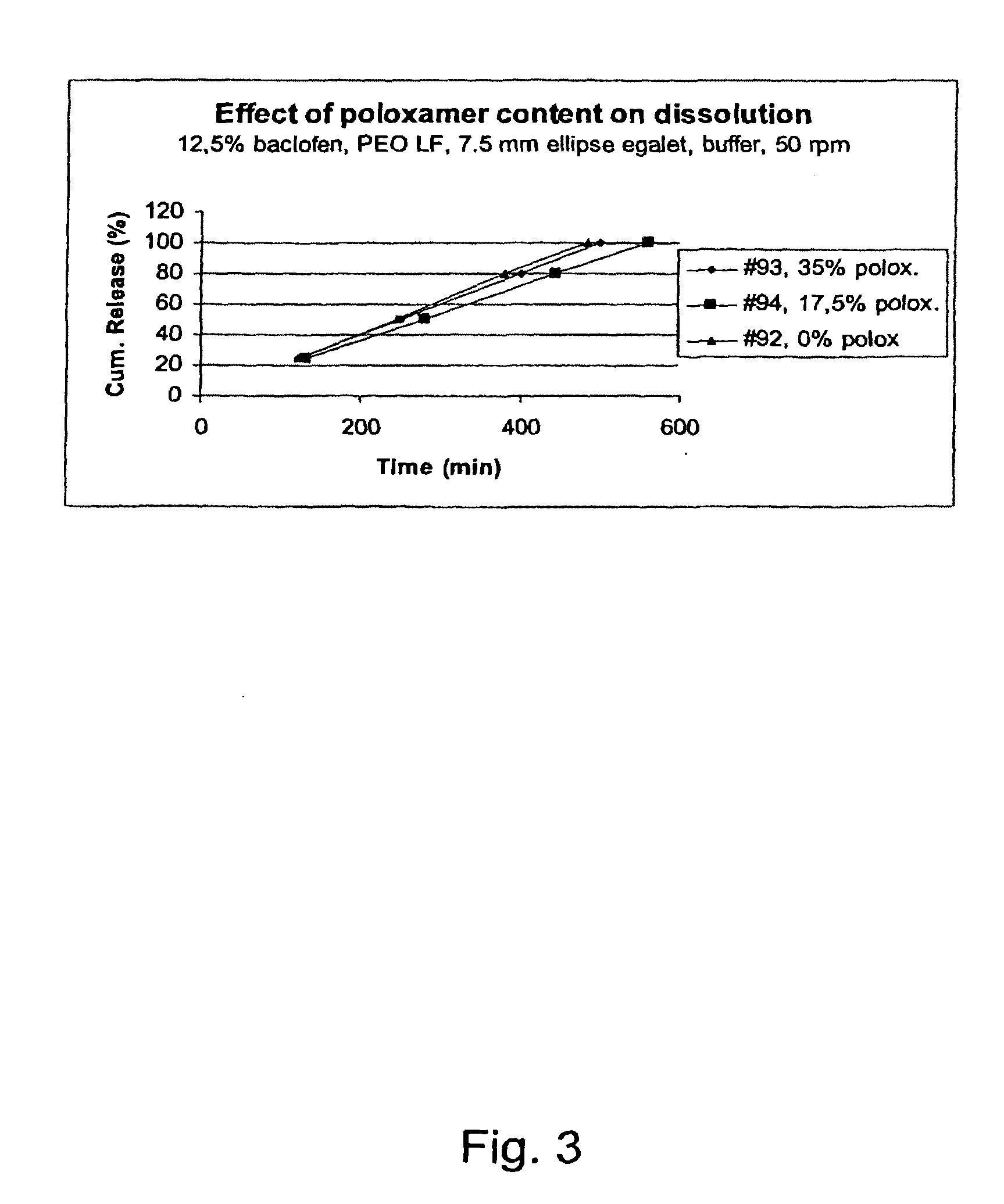
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Absorbent article comprising a synthetic polymer derived from a renewable resource and methods of producing said article
An element of an absorbent article is provided. The element has a bio-based content of at least about 50% based on the total weight of the element, and comprises a synthetic polymer derived from a renewable resource via a first intermediate compound selected from the group consisting of crotonic acid, propiolactone, ethylene oxide, i-propanol, butanol, butyric acid, propionic acid, 2-acetoxypropanoic acid, methyl 2-acetoxypropanoate, methyl lactate, ethyl lactate, polyhydroxybutyrate, and a polyhydroxyalkanoate comprising 3-hydroxypropionate monomers. An absorbent article comprising the element and a method of making an element for an absorbent article also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
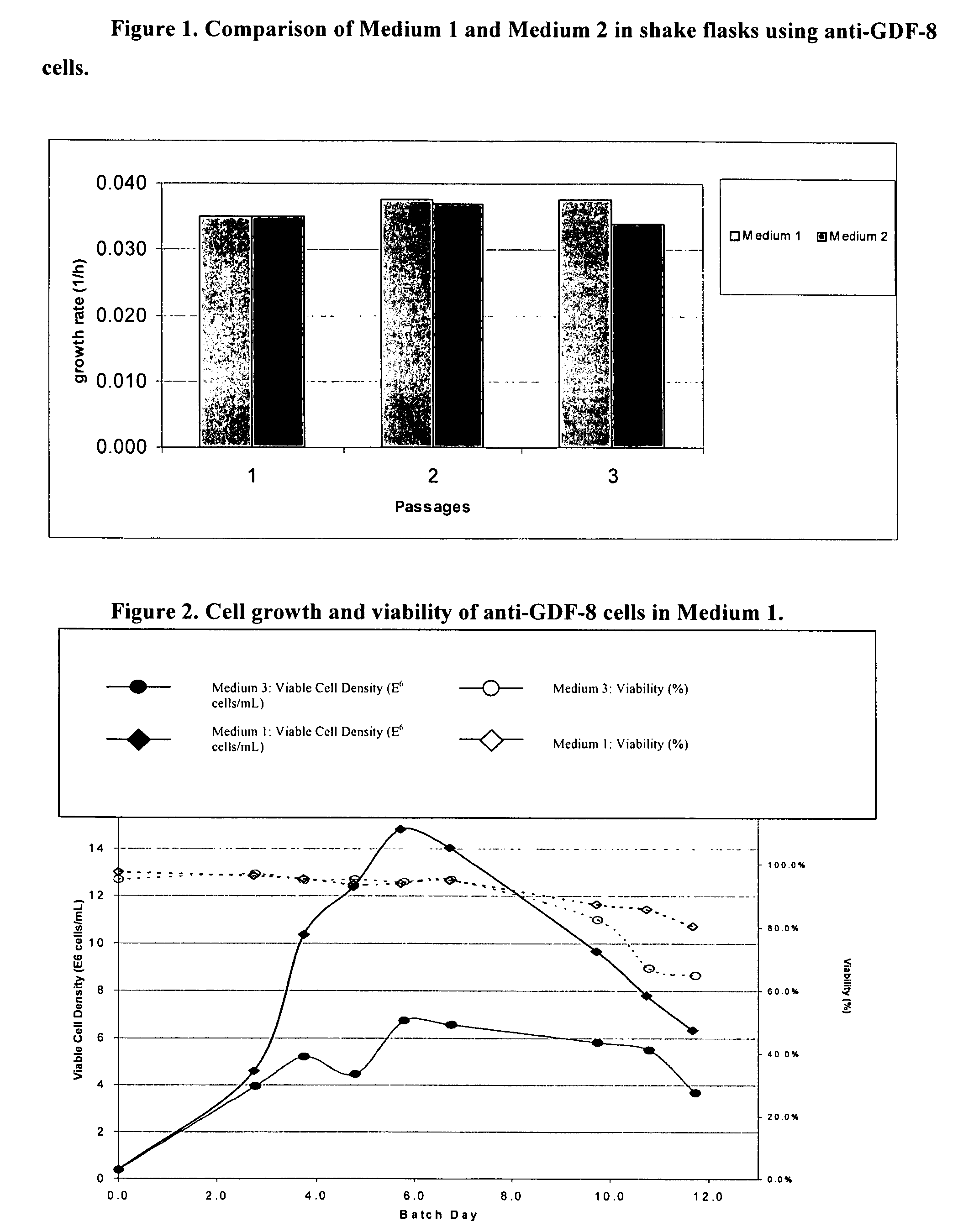
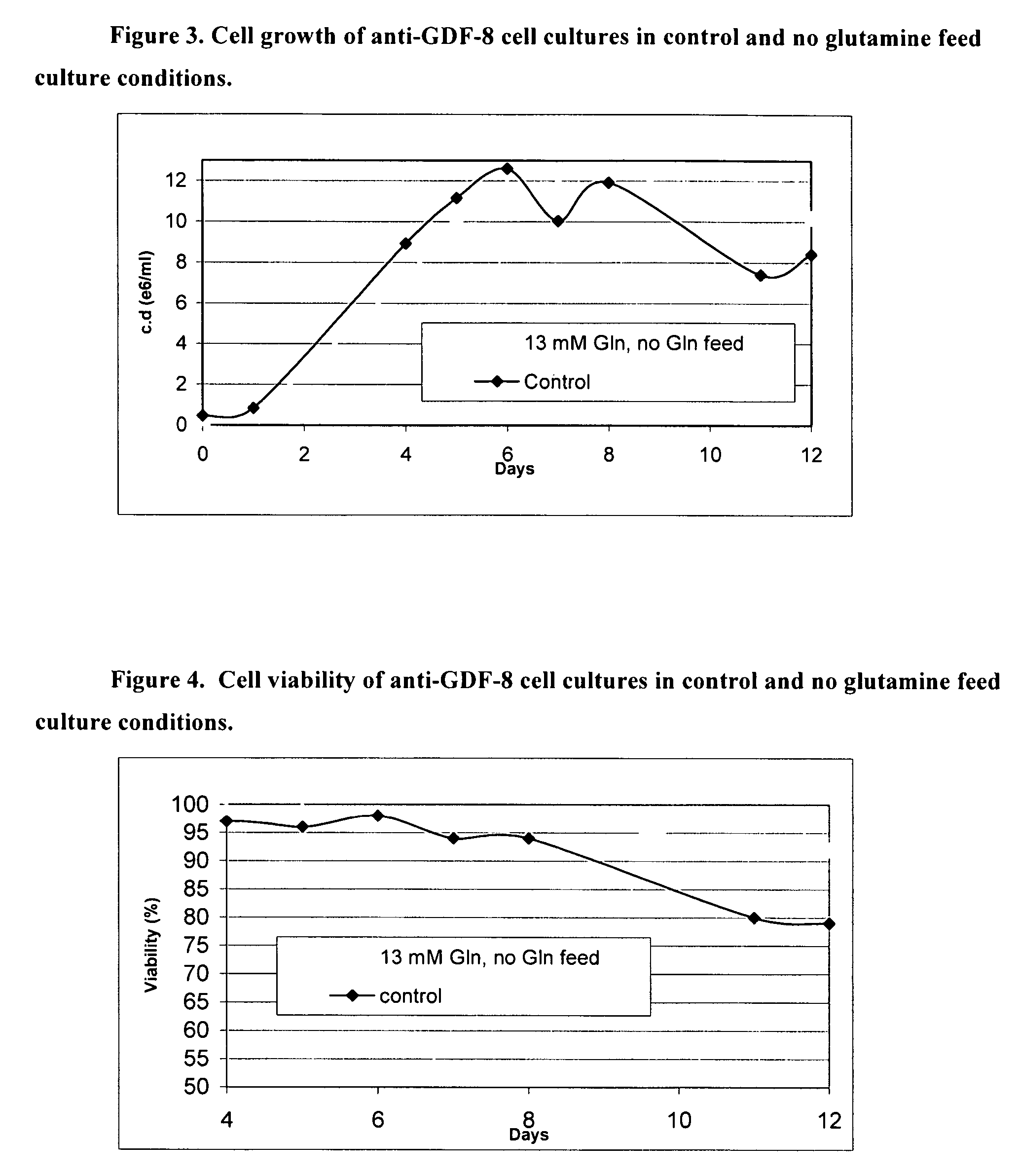
Production of polypeptides
ActiveUS20060121568A1Cell receptors/surface-antigens/surface-determinantsGenetically modified cellsTotal amino acidsInorganic ions
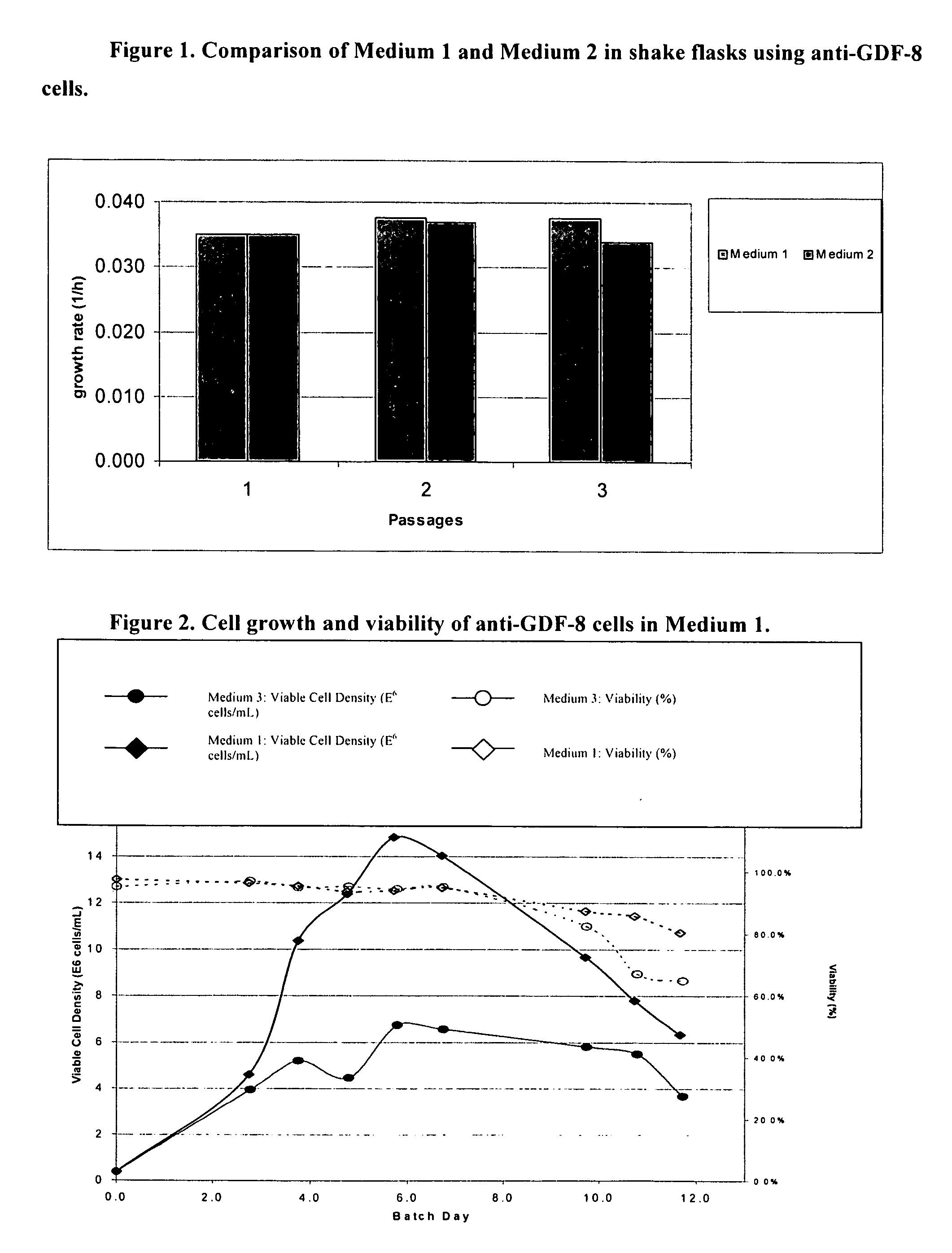
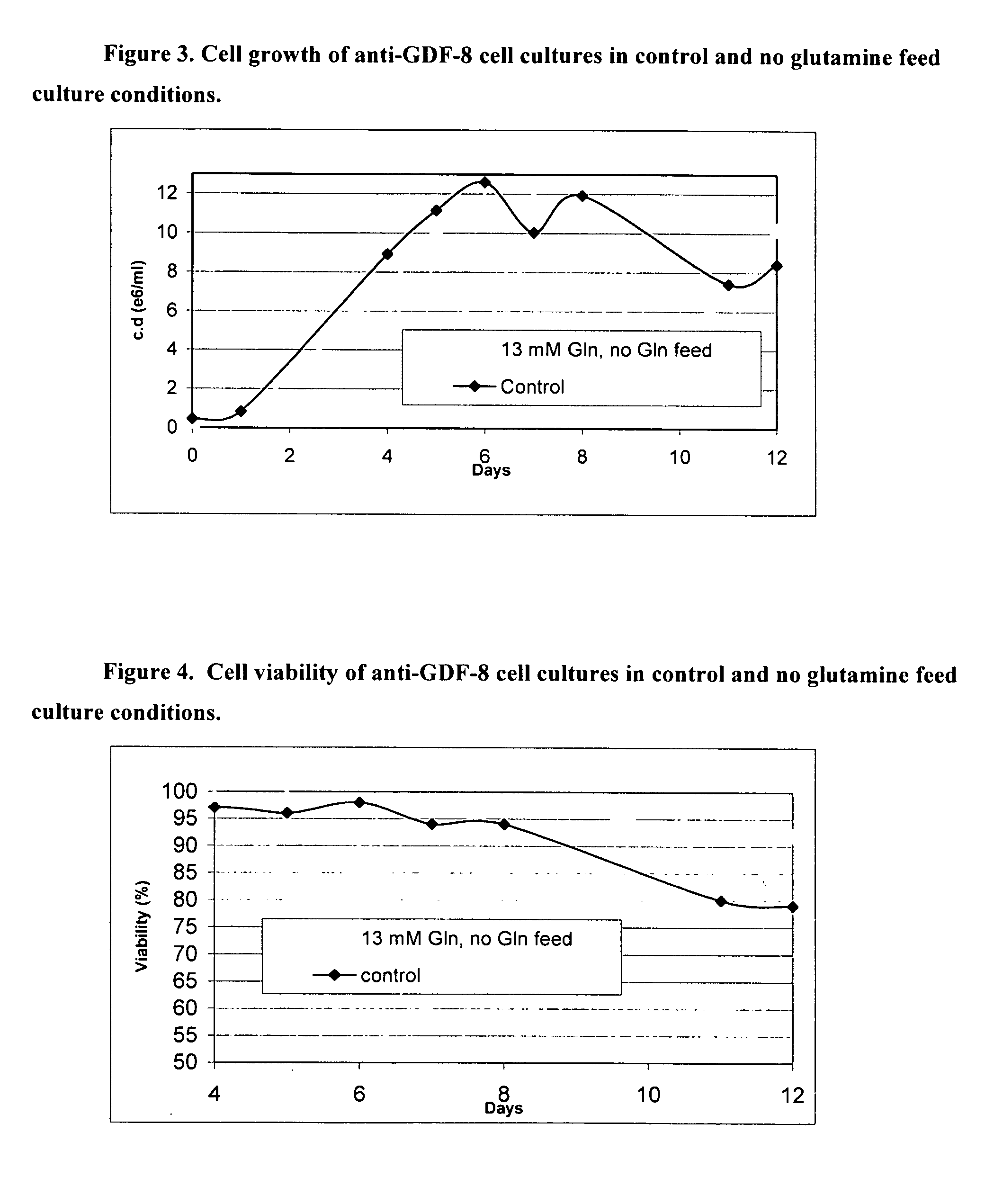
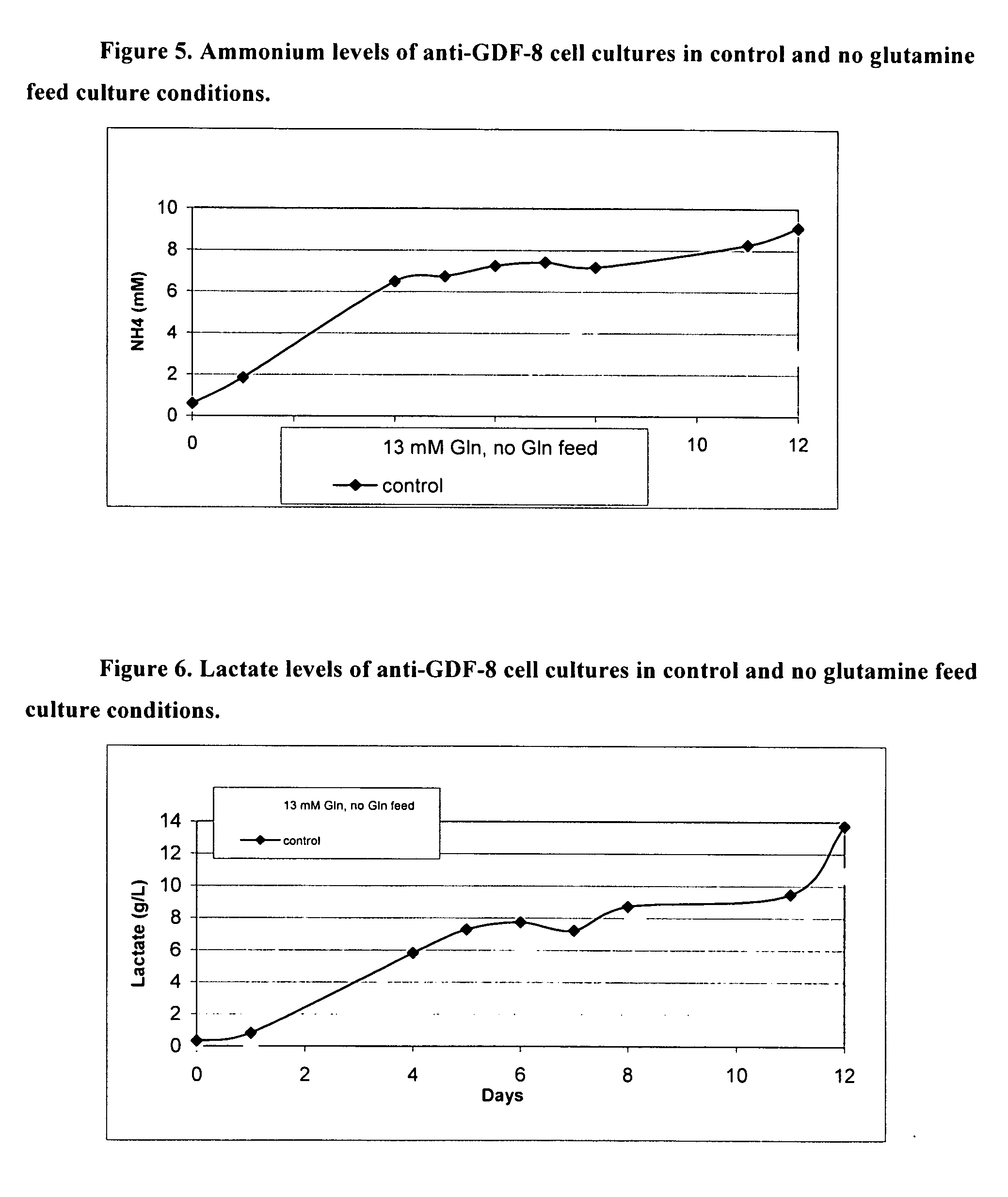
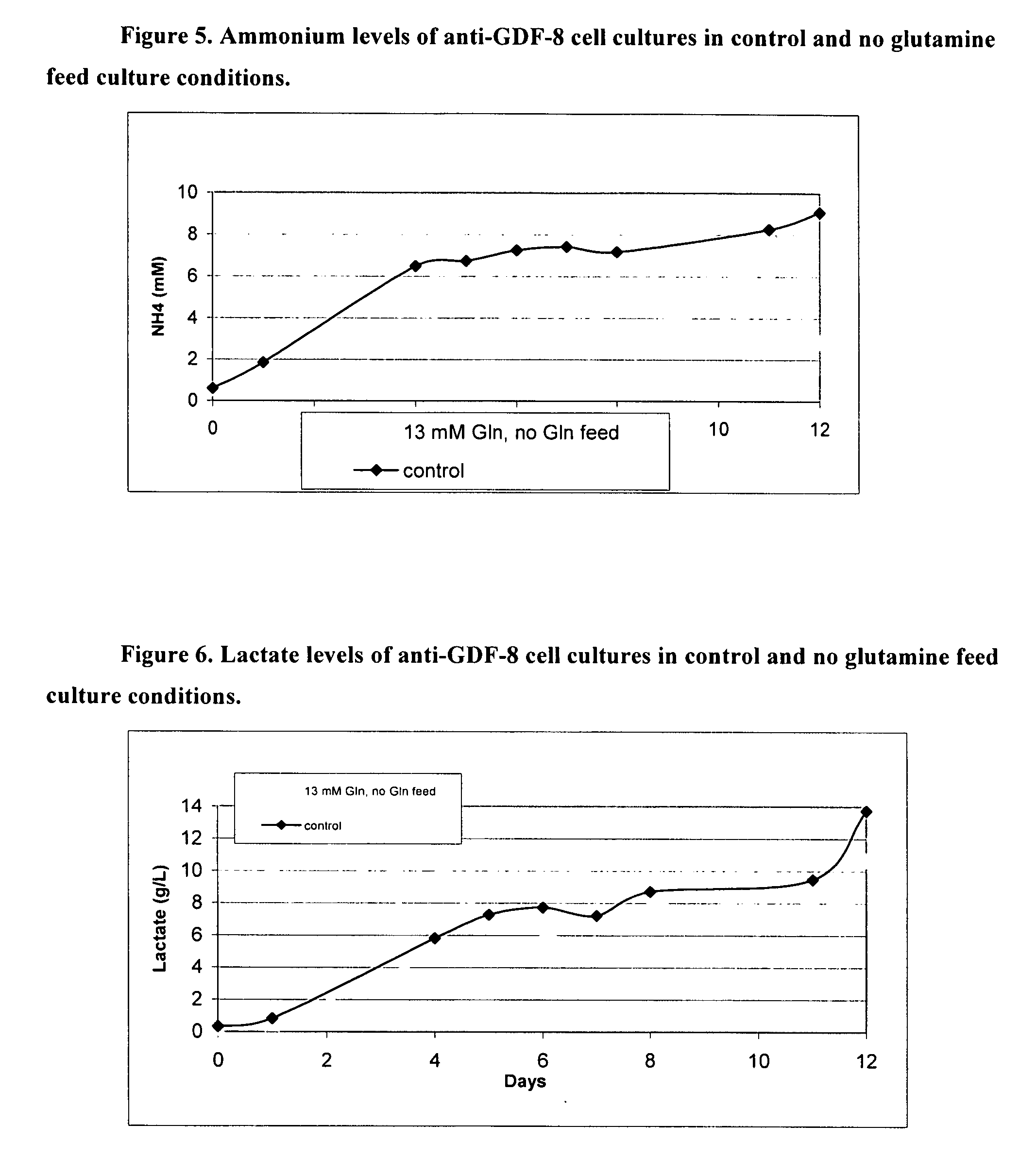
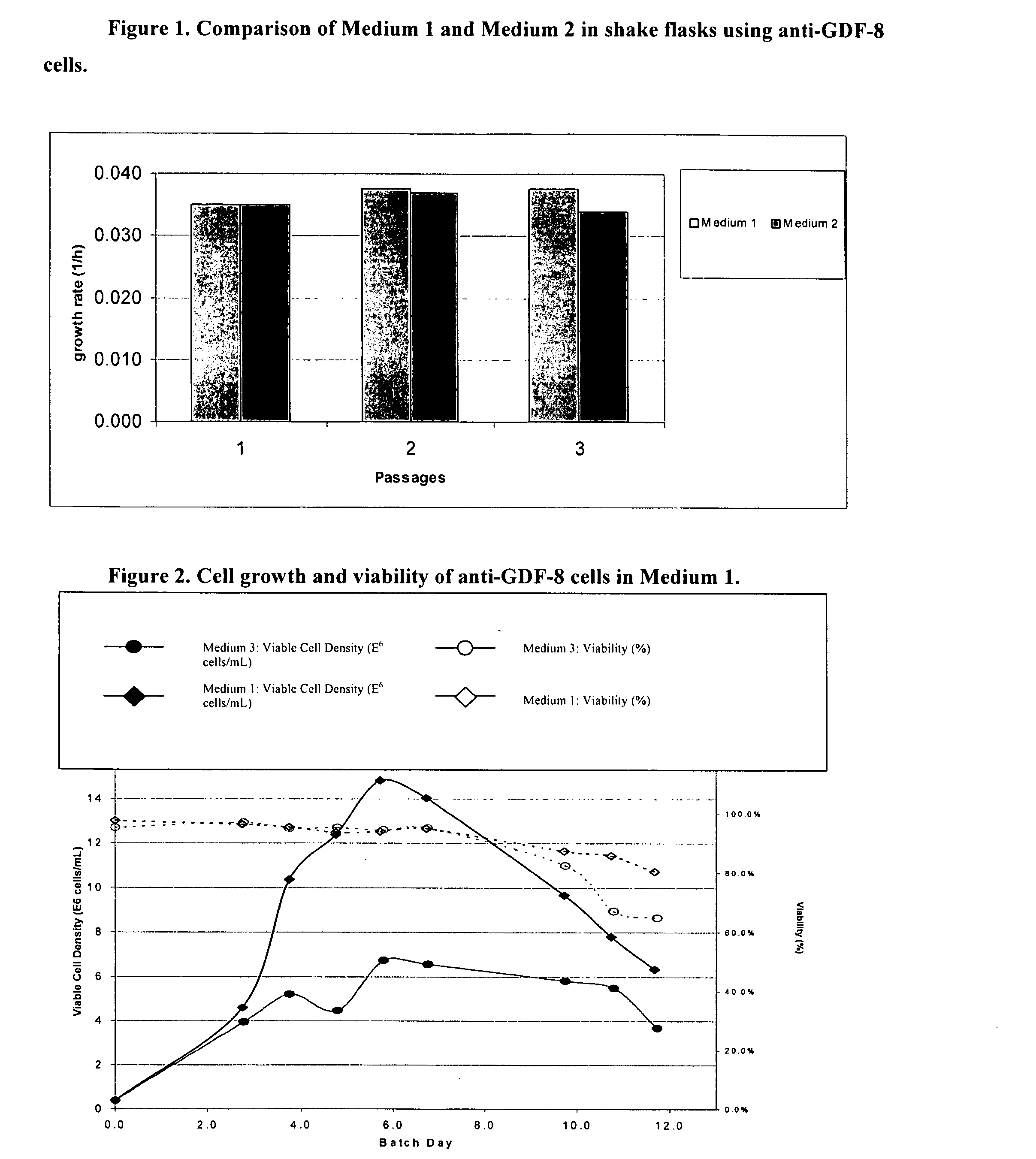
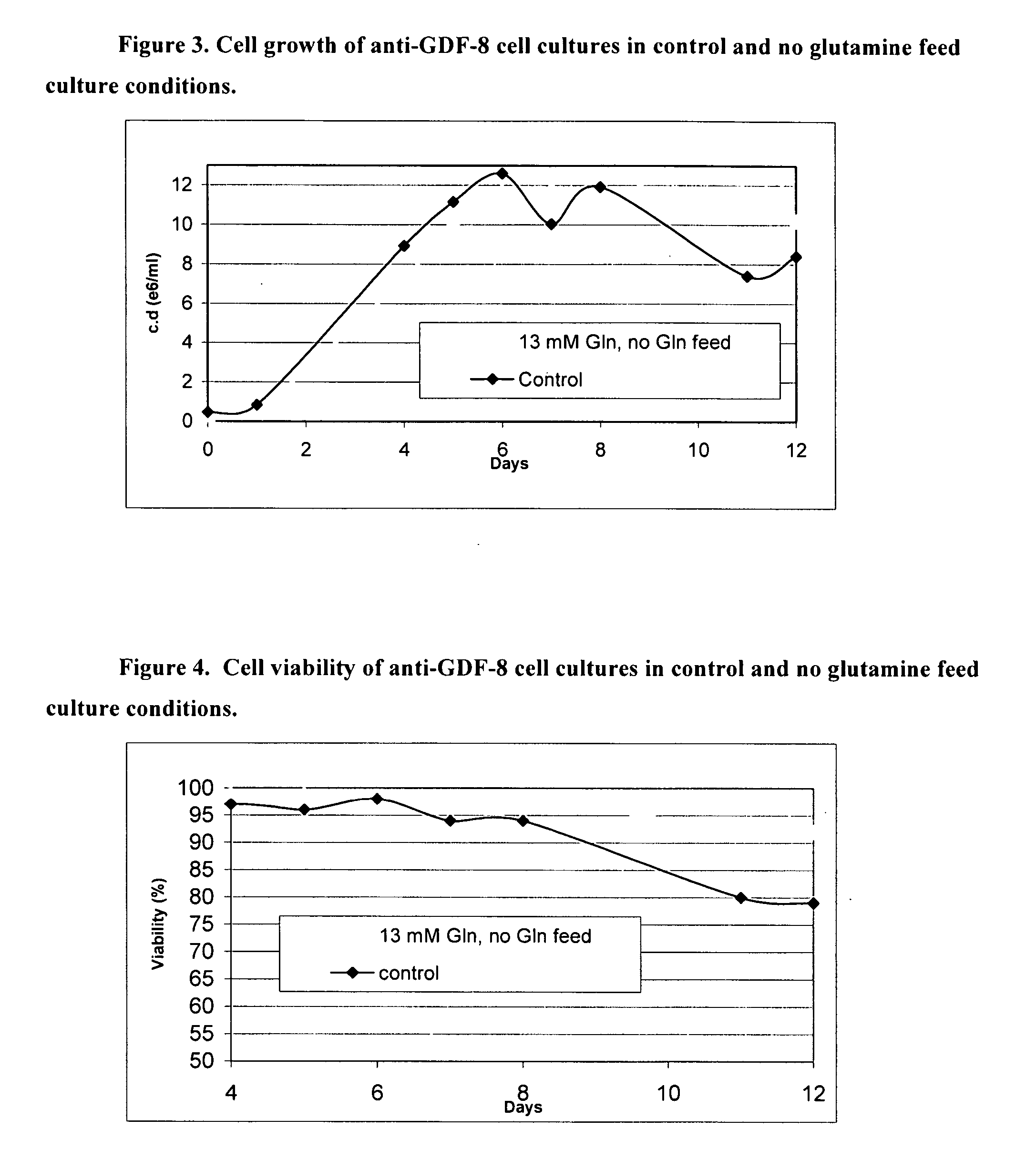
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Transdermal method and apparatus
Owner:ALDRED KATHERINE M
Marker or filler forming fluid
ActiveUS7877133B2Easy to detectEasy to distinguishCosmetic preparationsImpression capsWater insolubleGlycolic acid
Owner:SENORX
Production of anti-abeta
InactiveUS7335491B2Antibody mimetics/scaffoldsGenetically modified cellsBiotechnologyTotal amino acids
Owner:PFIZER IRELAND PHARM CORP
Method and system for measuring lactate levels in vivo
InactiveUS20060234386A1Addressing slow performanceContinuous monitoringRadiation pyrometryRaman scatteringInfraredNear infrared raman spectroscopy
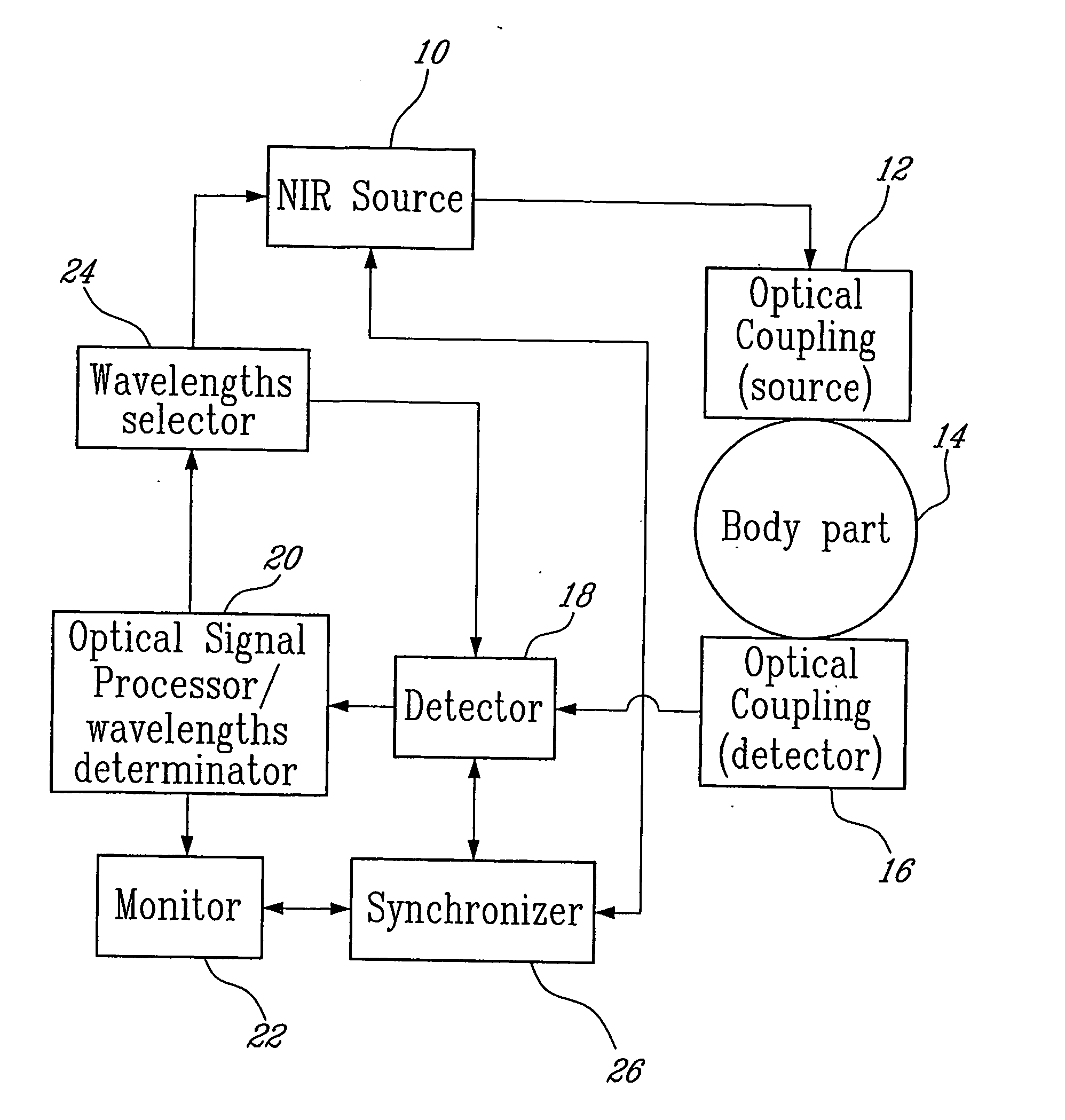
There is described a system and method for the in vivo determination of lactate levels in blood using Near-Infrared Spectroscopy (NIRS) and / or Near-infrared Raman Spectroscopy (NIR-RAMAN). The method teaches measuring lactate in vivo comprising: optically coupling a body part with a light source and a light detector the body part having tissues comprising blood vessels; injecting near-infrared (NIR) light at one or a plurality of wavelengths in the body part; detecting, as a function of blood volume variations in the body part, light exiting the body part at at least the plurality of wavelengths to generate an optical signal; and processing the optical signal as a function of the blood volume variations to obtain a lactate level in blood.
Owner:MCGILL UNIV
Emulsifiable concentrate (EC) formulations for pesticides
Pesticidal emulsifiable concentrate compositions containing at least one EPA exempt plant essential oil compound as a pesticidally active ingredient and a carrier containing an EPA exempt solvent, such as butyl lactate, and methods for using same are disclosed.
Owner:GIBRALTAR BUSINESS CAPITAL LLC
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
Preparation method and use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors
InactiveCN102485274AGood monodispersityImprove stabilityAntibacterial agentsGenetic material ingredientsMicrosphereGluconic acid
The invention discloses a preparation method and a use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors. A result of an animal immunization experiment shows that the PLGA microspheres can be utilized as gene vaccine vectors. Principles of the PLGA microspheres comprise that 1, the PLGA microspheres have core-shell structures; surface polymers comprise polymine, PLGA, glucose, chitosan, polylysine, FeCl3 and FeCl2; and through static electricity, dewatering interaction and hydrogen bond-nucleic acid vaccine interaction, a nucleic acid vaccine is concentrated to form a compact nucleic acid vaccine so that nucleic acid vaccine degradation is reduced in vivo; 2, the PLGA microspheres have magnetism and thus after immunization injection, in a strong magnetic field, the distribution of the PLGA microspheres in muscular tissue is improved and the defect of limited contact between the PLGA microspheres and target cells is overcome; and 3, through long-term strong magnetic field induction, a magnetic PLGA microsphere / nucleic acid vaccine complex can enter into the skin; and because of rich antigen presenting cells in the skin, a strong and fast immune response can be induced.
Owner:JILIN UNIV
Methods for the Preparation of Injectable Depot Compositions
ActiveUS20130177603A1Simple methodConstant and effective plasma levelBiocideNervous disorderMedicineGlycolic acid
Injectable depot compositions, comprising a biocompatible polymer which is a polymer or copolymer based on lactic acid and / or lactic acid plus glycolic acid having a monomer ratio of lactic to glycolic acid in the range from 48:52 to 100:0, a water-miscible solvent having a dipole moment of about 3.7-4.5 D and a dielectric constant of between 30 and 50, and a drug, were found suitable for forming in-situ biodegradable implants which can evoke therapeutic drug plasma levels from the first day and for at least 14 days.
Owner:LAB FARM ROVI SA
Antifungal composition
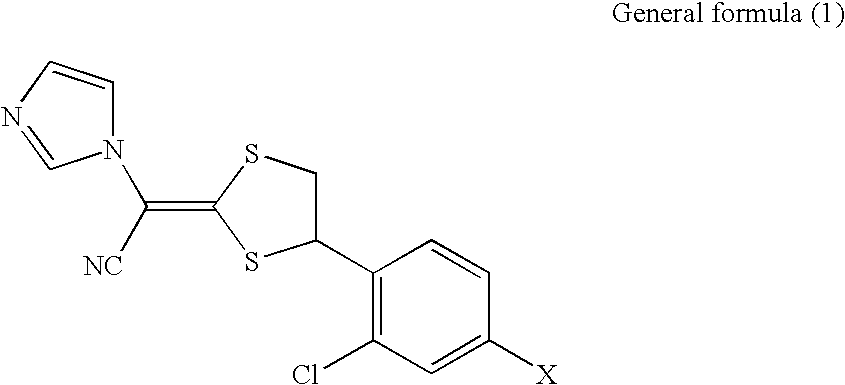
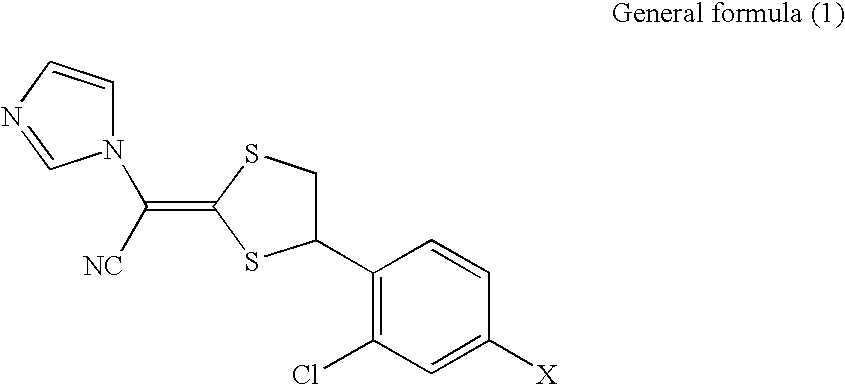
Disclosed is a pharmaceutical composition for antifungal use, comprising: 1) one or more compounds selected from compounds represented by the general formula (1) below and physiologically acceptable salts thereof; 2) one or more compounds selected from polypropylene glycol, diesters of dibasic acids, triacetin, 2-ethyl-1,3-hexanediol, lauromacrogol, and polyoxyethylene-polyoxypropylene glycol; and 3) one or more compounds selected from glucono-δ-lactone, propylene glycol, glycerin, and lactic acid. General formula (1) (In the formula, X represents a halogen or hydrogen).wherein X represents a halogen or hydrogen.
Owner:POLA PHARMA +1
Noninvasive Measurement and Identification of Biomarkers in Disease State
ActiveUS20090104596A1Quick measurementQuickly and reliably and inexpensively identify disease stateMicrobiological testing/measurementDisease diagnosisArginineTyrosine
The invention is methods and related kits for diagnosing a disease state of cachexia by measuring biomarker profiles from a biological sample. Rapid measurement of early onset or progression of the disease in a subject is determined by measuring biomarker levels from the subject and optionally comparing the biomarker levels to a standard biomarker profile or metabolome phase portrait for the disease. The biomarkers measured in the assay and related kit for cachexia progression include biomarkers selected from the group consisting of lactate, citrate, formate, acetoacetate, 3-hydroxy butrate, alanine, glutamine, glutamate, valine, isoleucine leucine, thrionine, lysine, arginine, tyrosine, phenyl alanine, histidine and tryptophan.
Owner:WISCONSIN ALUMNI RES FOUND
Testing system for determining hypoxia induced cellular damage
InactiveUS20130052675A1Quick testEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsLactate dehydrogenaseCell damage
The present invention relates to a testing system for assessing hypoxia induced cellular damage in a mammal including human, comprising a disposable device having a sample inlet and a collection chamber separated by a separation device wherein the collection chamber is connected to at least two, a first and a second, visible detection compartments, whereof at least one is arranged with chemical means for direct visual detection, said first detection compartment being arranged to determine whether level of hemoglobin (Hb) in a sample of body fluid taken from said mammal exceeds a predetermined threshold value, and said second detection compartment being arranged to evaluate level of total amount of lactate dehydrogenase (LDH) in said sample.
Owner:CALMARK SWEDEN AB
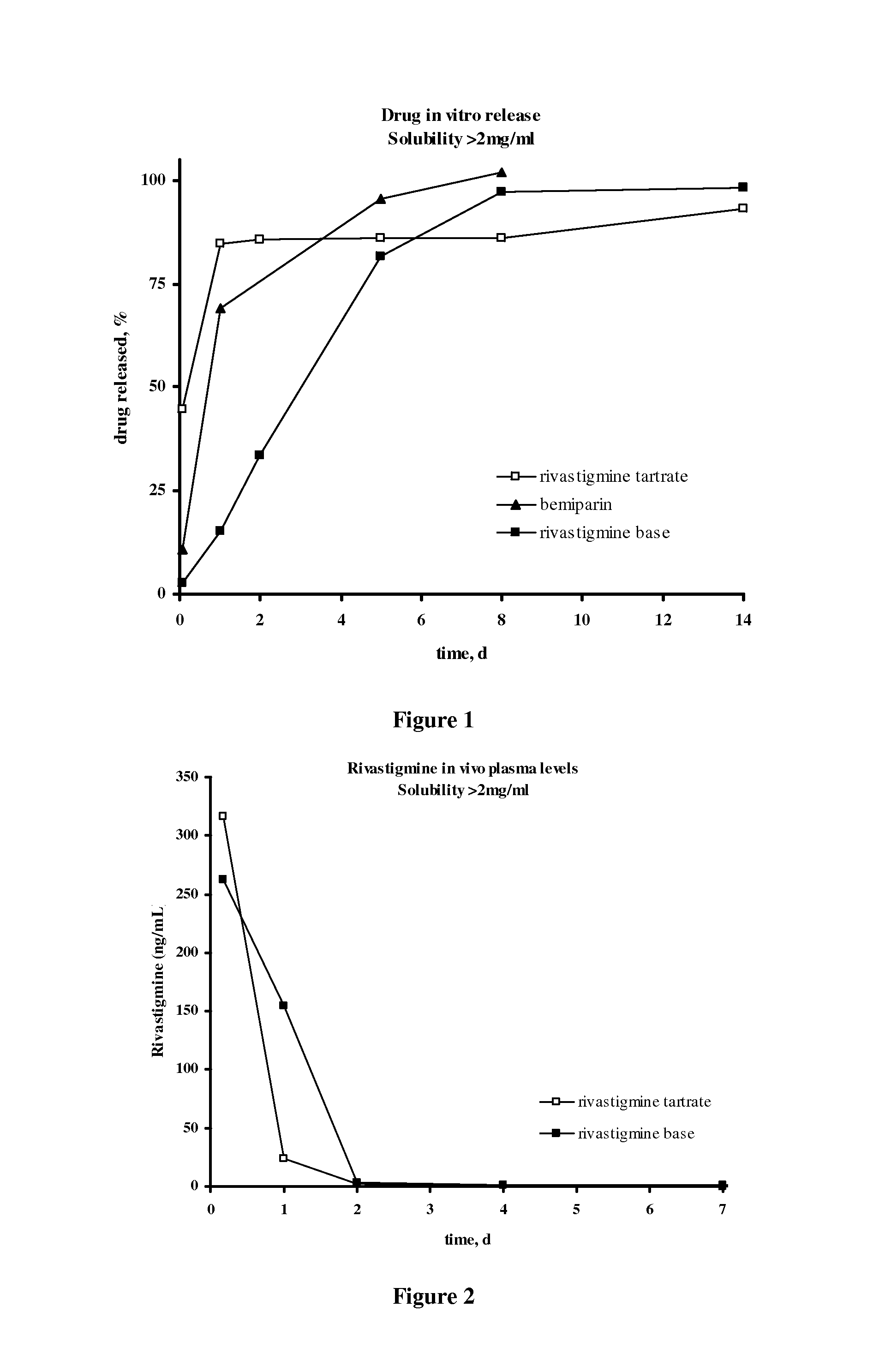
Rivastigmine slow-release microspheres and preparation method thereof
InactiveCN101708164AHigh drug loadingHigh encapsulation efficiencyNervous disorderPharmaceutical non-active ingredientsMicrosphereRivastigmine
The invention discloses slow-release microspheres coated with rivastigmine tartrate / rivastigmine for PLGA / PLA injection and a preparation method thereof. The slow-release microspheres mainly comprise rivastigmine tartrate / rivastigmine and PLGA and PLA serving as biodegradable polymer medicinal materials, and can be prepared by adopting a W1 / O / W2 composite emulsified solvent volatilization method, an O / W emulsified solvent volatilization method, an O1 / O2 emulsion drying method and a spray drying method. The grain diameter of the microspheres is less than 100 microns. The slow-release microspheres have high medicament loading amount, have no obvious burst release effect, can be continuously released for one week, one mouth or three mouths, are used for treating senile diseases such as Alzheimer's disease, Parkinson disease and the like, and can prolong the acting time of the medicament, reduce the application times and greatly improve the administration compliance of patients.
Owner:SUZHOU UNIV
Degradable unsaturated polyesteramide resin and synthesis method thereof
The invention adopts a melt polycondensation method. A few of monomers of C2-5 aliphatic dibasic alcohol, diethylene glycol, polyglycol, fumaric acid, maleic anhydride, lactic acid, glycolic acid, ethanolamine, C2 to C12 aliphatic diamine, glutamic acid, lysine, glycine, etc. are taken as basic materials to be synthesized to obtain non toxic unsaturated polyester-amide resin with adjustable degradation rate and lower cost; wherein, the partial fumaric acid or maleic anhydride can be replaced with phthalic anhydride, isophthalic acid, or adipic acid. The resin yearns for being used as matrix resin of medical bone internal fixation material, tissue engineering scaffold material, bone tissue temporary substitutes, environmental protection type bonding agent, environmental protection type fiberglass reinforced plastics, environmental protection type coating material, disposable tableware, packing material, shopping bags, disposable bags, drug coating or capsule, drug delivery (controlled-release) material, agricultural mulching films, etc., and can be recovered to be utilized.
Owner:HUNAN UNIV
Controlled Release of Nitric Oxide And Drugs From Functionalized Macromers And Oligomers
ActiveUS20100209469A1Rapid hydrolysisHighly controllable hydrolysis profileSuture equipmentsBiocidePolymer scienceDrug release
The present invention provides NO and, optionally, drug releasing macromers and oligomers wherein the drug molecule and NO releasing moiety are linked an absorbable macromer or oligomeric chain susceptible to hydrolytic degradation and wherein the macromer or oligomer comprises of repeat units derived from safe and biocompatible molecules such as glycolic acid, lactic acid, caprolactone and p-dioxanone. Furthermore, the present invention relates to controlled release of nitric oxide (NO) and / or drug molecule from a NO and drug releasing macromer or oligomer. Moreover, the present invention also relates to medical devices, medical device coatings and therapeutic formulations comprising of nitric oxide and drug releasing macromers and oligomers of the present invention.
Owner:BEZWADA BIOMEDICAL LLC
Neutral blockage removing agent composition used for oil recovery formation in oilfield and preparation method thereof
ActiveCN104194759AImprove cleanlinessNo pollutionDrilling compositionActivated attapulgitePolythylene glycol
The invention relates to a neutral blockage removing agent composition used for an oil recovery formation in an oilfield. The neutral blockage removing agent composition is prepared from the following raw materials in parts by weight: 30-36 parts of disodium polyepoxysuccinicate, 18-25 parts of sodium gluconate, 2-3 parts of polyethylene glycol, 2-4 parts of triethanolamine, 9-12 parts of ethyl lactate, 7-10 parts of fatty alcohol polyoxyethylene ether sodium sulfate, 8-12 parts of amino acid sodium, 10-15 parts of activated attapulgite, 0.1-0.2 part of vanadium pentoxide, 5-7 parts of lipase, 5-8 parts of sorbitan fatty acid ester, 15-18 parts of P-hydroxy sodium sulfonate, 3-7 parts of tert-butyl hydroperoxide and 0.1-0.3 part of diethylene glycol monolaurate. The neutral blockage removing agent composition has high blockage removing speed, is neutral, is free of corrosion and can quickly dissolve material scales, asphalt sediment scales, carbonate scales, silicate scales and the like generated by an ASP flooding system; waste liquid for blockage removal can be degraded and does not need to be discharged onto the ground to be subjected to sewage treatment, the formation in the oilfield is cleaned to remove the blockage, no corrosion, dead angle, precipitation or secondary well blockage is generated, and the blockage removal time does not exceed 24 hours.
Owner:GANSU HEIMA PETROCHEM ENG
Production of alpha-ABeta
InactiveUS20060160180A1Reduce accumulationHigh level of proteinAntibody mimetics/scaffoldsImmunoglobulins against growth factorsTotal amino acidsInorganic ions
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Trap for bed bugs and the like
Owner:J T EATON
Synthetic process of tenofovir
InactiveCN102295660AHigh yieldEasy post-processingGroup 5/15 element organic compoundsDiethyl phosphatePurine
The invention relates to a synthesis technique of tenofovir, and relates to the field of medicinal chemistry. Using natural (R)-lactate as the starting material, chiral R-1,2-propanediol is obtained through reduction, and then reacted with diethyl carbonate under the action of alkali to obtain the key reaction intermediate R-propylene carbonate ester. Use adenine and R-propylene carbonate to prepare R-9-(2-hydroxypropyl)adenine; Gained R-9-(2-hydroxypropyl)adenine and diethyl p-toluenesulfonyloxyphosphate Under the catalysis of magnesium tert-butoxide, the condensation reaction is carried out to obtain R-9[(diethylphosphorylmethoxy)propyl]purine; the obtained R-9[2-(diethylphosphorylmethoxy) Propyl]purine is hydrolyzed to obtain Tenofovir. The process has short reaction steps, short required reaction time, high quality yield and good product quality, and is suitable for industrialized production.
Owner:CHANGZHOU UNIV
Adsorbable internal fixing material for bone fracture and its preparing process
An absorbable internal fixing material for bone fracture is prepared from polylactic acid (50-90 wt.%), tricalcium phosphate (1-40 wt.%) and hydroxylapatitie (1-40 wt.%) through mixing then and then hot die pressing at 80-1230 deg.C and under 0.11-25 MPa or hot extruding at 80-230 deg.c. The granulality of hydroxylapatite and tricalcium phosphate is less than 100 microns. Its advantages are high strength, high strength, high X-ray visualization and high biocompatibility.
Owner:SOUTHEAST UNIV
Method for administering insulin to the buccal region
A mixed micellar pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an alkali metal C8 to C22 alkyl sulphate, alkali metal salicylate, a pharmaceutically acceptable edetate and at least one absorption enhancing compounds. The absorption enhancing compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, octylphenoxypolyethoxyethanol, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linolenic acid, borage oil, evening of primrose oil, trihydroxy oxo cholanyiglycine, glycerin, polyglycerin, lysine, polylysine, triolein and mixtures thereof. The amount of each absorption enhancing compound is present in a concentration of from 1 to 10 wt: / wt. % of the total formulation, and the total concentration of absorption enhancing compounds are less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA INC
Neutral blockage removing agent composition used for oilfield mechanical recovery well and preparation method thereof
ActiveCN104194757AGood compatibilityGood miscibilityDrilling compositionSodium acetateActivated attapulgite
The invention relates to a neutral blockage removing agent composition used for a mechanical recovery well in an oilfield. The neutral blockage removing agent composition is prepared from the following components in parts by weight: 16-20 parts of polyepoxysuccinic acid amine, 7-12 parts of hydroxyethylidene dipllosphate sodium, 21-25 parts of divinyl pentaacetic acid amine, 20-25 parts of hydroxyl ammonium acetate, 12-16 parts of activated attapulgite, 21-25 parts of ammonium persulfate, 9-12 parts ofdicyclohexyl sulfobutanedioate sodium, 7-9 parts of butyl lactate, 0.2-0.3 part of vanadium pentoxide, 5-8 parts of straight chain sodium dodecylbenzenesulfonate, 10-14 parts of nitrilotriacetic acid sodium salt, 4-7 parts of urotropin and 0.1-0.3 part of glycerin fatty acid ester. The neutral blockage removing agent composition is suitable for scale removal and blockage removal of an oil pumping unit well and a screw pump well, can effectively control the reaction speed of the blockage removing agent and the scale of the oil pumping unit well and the scaled screw pump well, and prevent the falling-off of big scale residues to result in the secondary blockage of the mechanical recovery well and an oil recovery pump thereof, has high blockage removal speed, is neutral and is free of corrosion; the waste liquid for blockage removal does not need to be discharged onto the ground to be subjected to sewage treatment, the blockage removal time does not exceed 24 hours, and the effect of safe and corrosion-free blockage removal is achieved.
Owner:GANSU HEIMA PETROCHEM ENG
Sacubitril intermediate and preparation method of sacubitril intermediate and sacubitril
ActiveCN105924355AImprove qualityHigh yieldOrganic compound preparationCarboxylic acid esters preparationChemical reactionOrganic synthesis
The invention discloses a sacubitril intermediate and a preparation method of the sacubitril intermediate and sacubitril, and belongs to the technical field of organic synthesis of drugs. On one hand, the invention discloses a novel compound-sacubitril intermediate compound B and a preparation method thereof, and on the other hand, the invention discloses a novel preparation method of the sacubitril. The sacubitril intermediate and the preparation method of the sacubitril intermediate and the sacubitril have the following advantages that the synthesizing route is short, the product can be prepared only through four steps of chemical reactions, and the steps of existing patent routes all exceed nine steps; the dicarbonyl compound is prepared through Reformasky condensation supplied by the method, and the cost is low; a first chiral center is introduced by fully utilizing a chiral compound L-(S)-ethyl lactate which is low in cost and easy to obtain in the natural world, and the cost is low; a second chiral center is constructed through a biological enzyme catalysis technique, the optical selectivity reaches up to 99.9%, the quality is good, and the cost is low. By means of the technological means, the total yield of the prepared sacubitril is increased, and the quality of the sacubitril is improved.
Owner:ZHEJIANG HONGYUAN PHARMA
Milrinone sodium chloride injection and production thereof
ActiveCN1679566AImprove toleranceSimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneSodium Chloride Injection
A milrinone-sodium chloride injection for treating congestive heart failure is proportionally prepared from milrinone, sodium chloride and lactic acid.
Owner:LUNAN BETTER PHARMA
Phytase-treated acid stable soy protein products
This invention is directed to an acidic beverage composition, comprising; (A) a hydrated protein material having a combination of an inositol-6-phosphate content, an inositol-5-phosphate content, an inositol-4-phosphate content and an inositol-3-phosphate content of less than 8.0 μmol / g, with (B) a hydrated protein stabilizing agent and (C) at least one acid comprising a fruit juice, a vegetable juice, citric acid, malic acid, tartaric acid, lactic acid, ascorbic acid, glucono delta lactone or phosphoric acid, wherein the acidic beverage composition has a pH of from 3.0 to 4.5.
Owner:SOLAE LLC
Hair Processing Agent And Method For Permanent Waving Hair
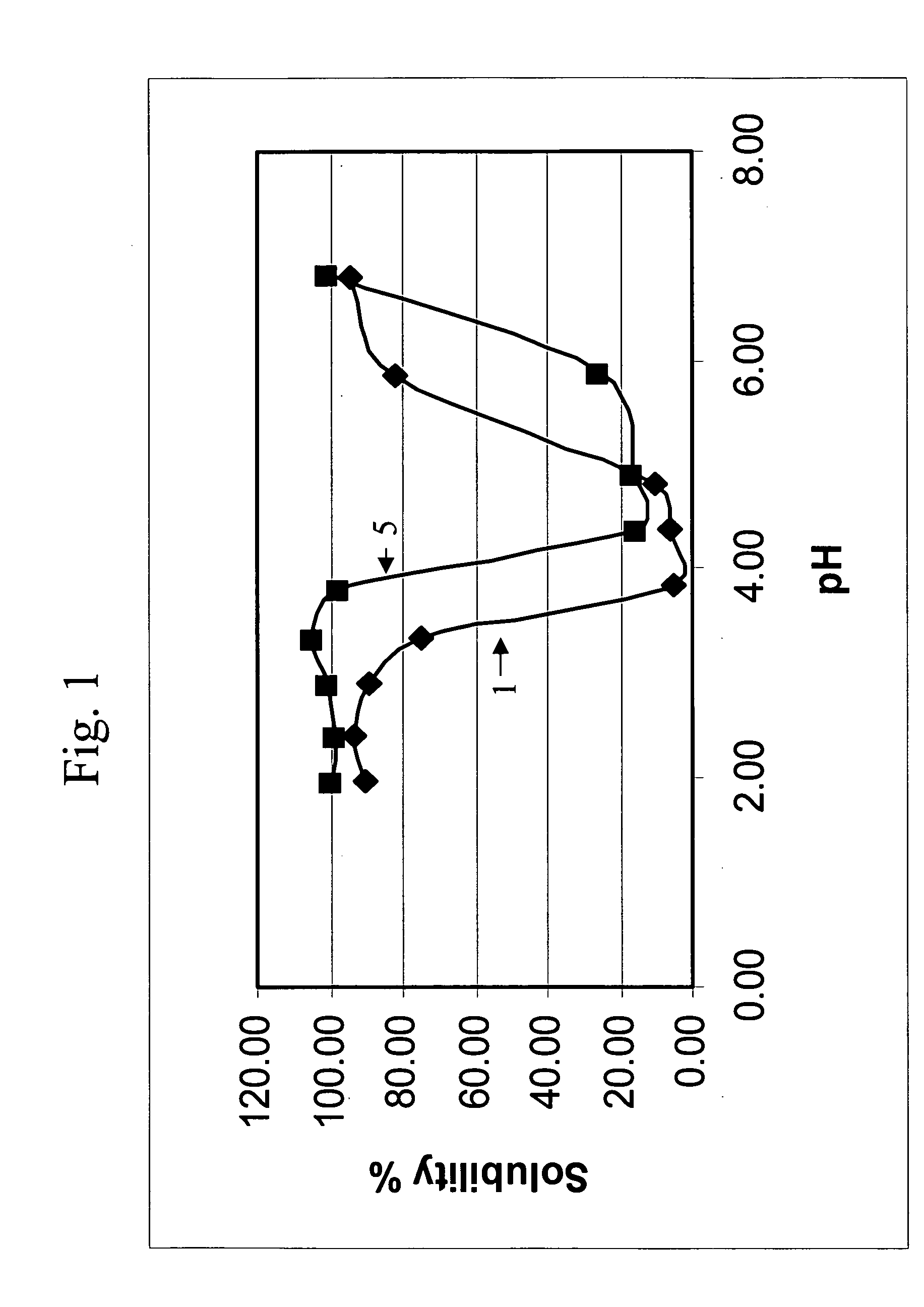
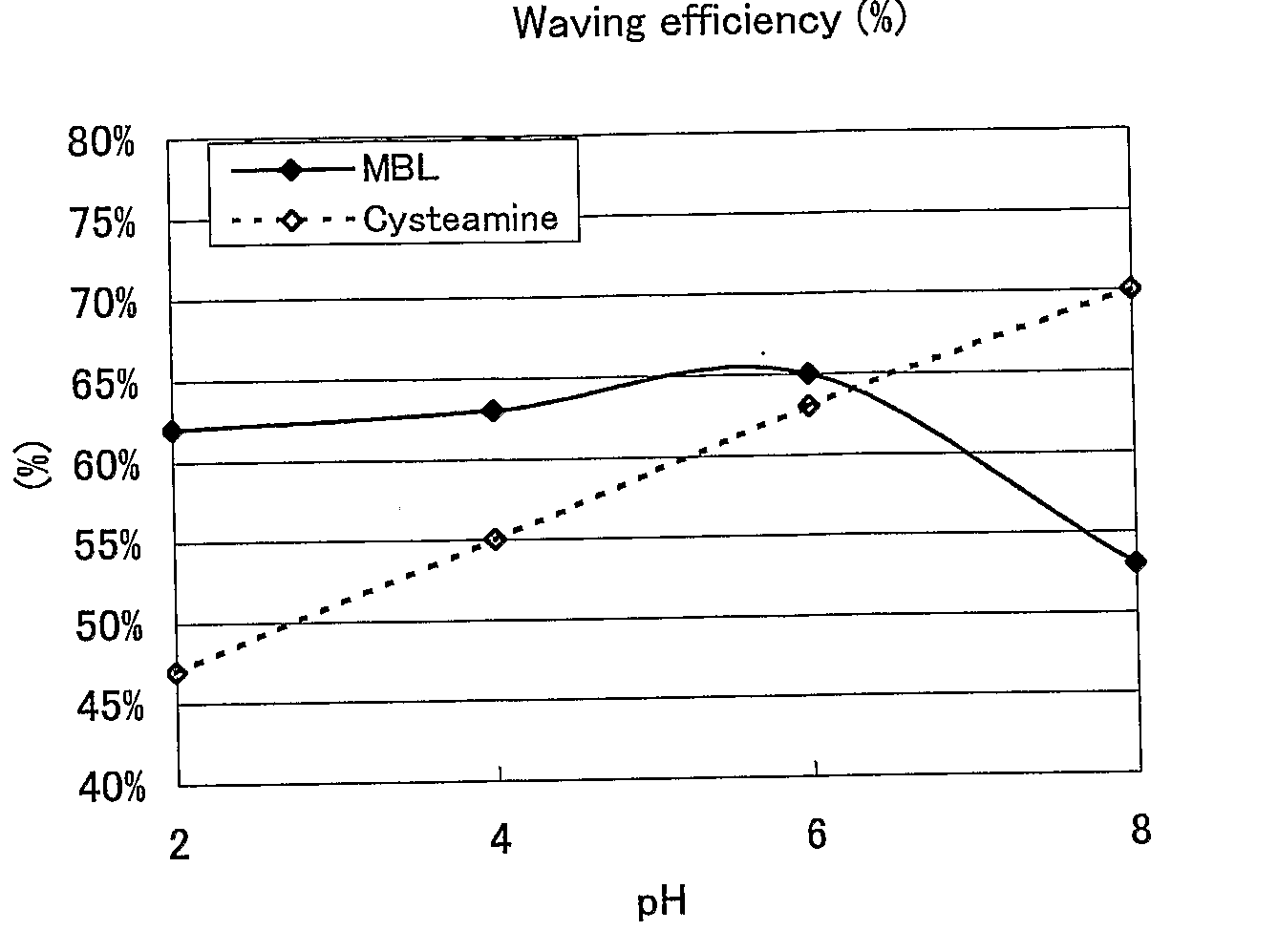
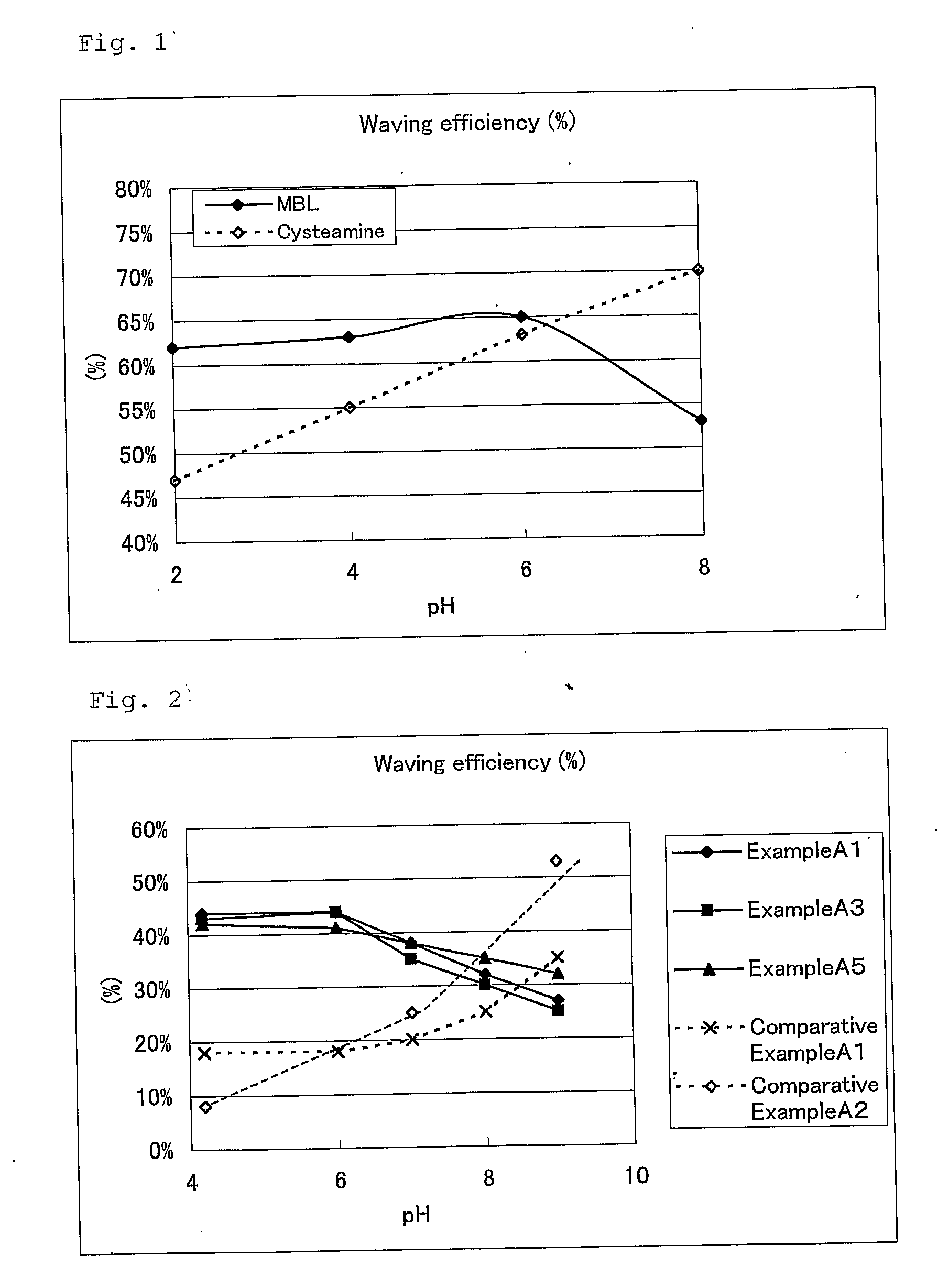
ActiveUS20080085251A1Improve performanceGood lookingCosmetic preparationsHair cosmeticsThiolactic acidCysteamine
Provided are hair processing agents capable of permanent waving hair even at a neutral to weakly acidic pH range that causes less irritation to the skin, and hair processing agents in which an unpleasant odor is masked. Hair processing agents contain at least one compound represented by the formula (2). Hair processing agents contain a compound of the formula (2) and at least one compound (ii) selected from thioglycolic acid, thiolactic acid, cysteine, acetylcysteine, cysteamine, acylcysteamine, salts thereof and ester derivatives thereof. Hair processing agents contain a compound of the formula (2), a surfactant and water, and are emulsified. Hair processing agents contain a compound of the formula (2) and a specific perfume. wherein X is a structure selected from —O—, —S—, —NH— and —NR1—; R1 is an alkyl group of 1 to 6 carbon atoms; Y is an oxygen atom or a sulfur atom; in the formula (1), Z is a divalent organic residue having at least one mercapto group; in the formula (2), R is a divalent organic residue optionally having a mercapto group; and R2 is a hydrogen atom or an alkyl group of 1 to 6 carbon atoms.
Owner:RESONAC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com