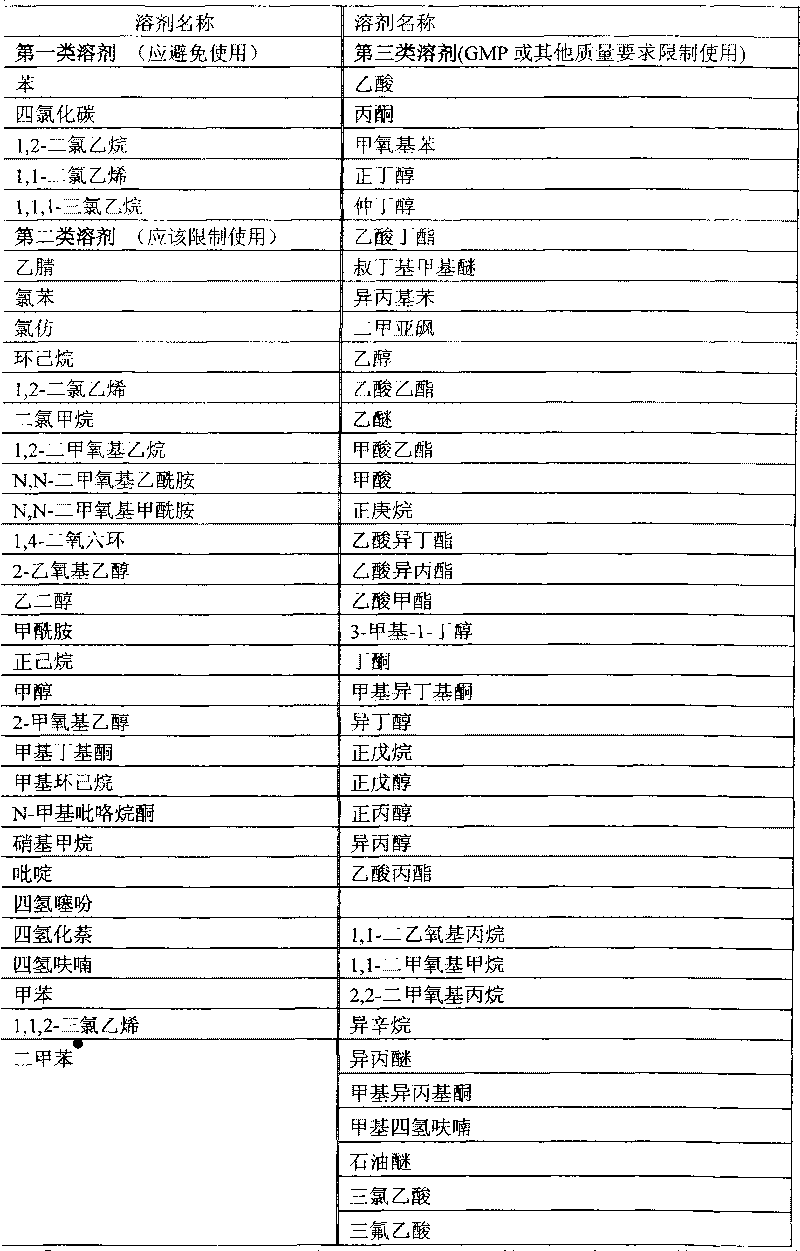
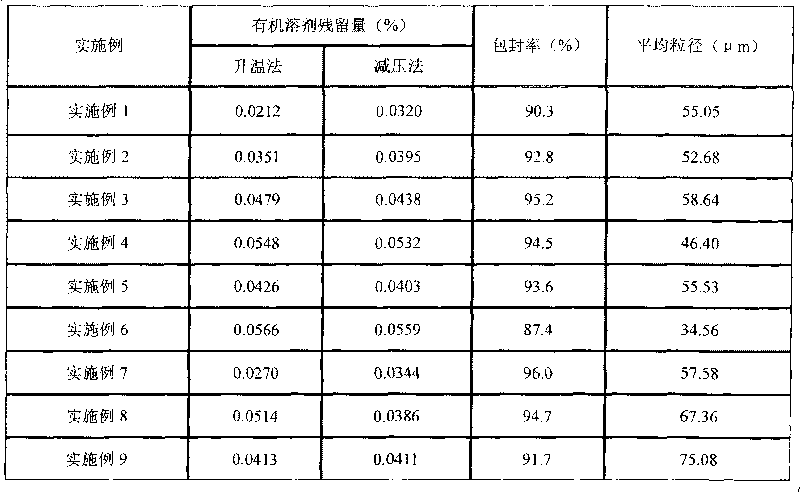
Method for controlling residue of organic solvent in thymic peptide alpha1 microballoon
An organic solvent, thymosin technology, applied in the field of medicine, can solve the problems of fusion or rupture, microsphere adhesion, leakage or outflow of thymosin α1, etc., to achieve the effect of maintaining a complete shape and a stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] 1. Preparation of inspection samples
[0039] Prescription 1 (release time 4 weeks)
[0040] Ta1 1.0g
[0041] PLGA(0.15~0.25dl / g)(50 / 50) 36.0g
[0042] Selected organic solvent: dichloromethane
[0043] Preparation process: add the prescribed amount of Ta1 into 30ml of pH 7.4 phosphate buffer solution at room temperature, dissolve it completely, and obtain the aqueous phase for later use;
[0044] Add the prescribed amount of PLGA into 150ml of dichloromethane at room temperature to dissolve it completely to obtain an oil phase for subsequent use;
[0045] At 10-25°C, adjust the speed of the emulsifier to 2000-2800 rpm, inject the water phase into the oil phase, and emulsify for 5-10 minutes to obtain colostrum (O / W) for later use;
[0046] Cool 800ml of aqueous solution containing 3% PVA and 5% NaCl to below 10°C, adjust the speed of the mixer to 500-1000 rpm, add colostrum, stir for 2 minutes, then add 3% PVA, 5% Add 200ml of NaCl aqueous solution to obtain double...
Embodiment 1
[0090] Embodiment 1 The present invention controls the method for residual organic solvent in thymosin α1PLGA microspheres
[0091] Ta1 1.0g
[0092] PLGA(0.15~0.25dl / g)(50 / 50) 38.0g
[0093] Preparation Process
[0094] Add the prescribed amount of Ta1 to 30ml of pH 7.4 phosphate buffer at room temperature to dissolve completely to obtain the aqueous phase for later use;
[0095] Add the prescribed amount of PLGA into 150ml of dichloromethane at room temperature to dissolve it completely to obtain an oil phase for subsequent use;
[0096]At 10-25°C, adjust the speed of the emulsifier to 2000-2800 rpm, inject the water phase into the oil phase, and emulsify for 5-10 minutes to obtain colostrum (O / W) for later use;
[0097] Cool 800ml of aqueous solution containing 3% PVA and 5% NaCl to below 10°C, adjust the speed of the mixer to 500-1000 rpm, add colostrum, stir for 2 minutes, then add 3% PVA, 5% 200ml of NaCl aqueous solution to obtain double emulsion (O / W / O). Stir for ...
Embodiment 2
[0098] Embodiment 2 The present invention controls the method for residual organic solvent in thymosin α1PLGA microspheres
[0099] Ta1 1.0g
[0100] PLGA(0.24~0.56dl / g)(50 / 50) 32.0g
[0101] Preparation Process
[0102] Add the prescribed amount of Ta1 to 30ml of pH 7.4 phosphate buffer at room temperature to dissolve completely to obtain the aqueous phase for later use;
[0103] Add the prescribed amount of PLGA into 100ml of ethyl formate at room temperature to dissolve it completely to obtain an oil phase for later use;
[0104] At 10-25°C, adjust the speed of the emulsifier to 2500-3200 rpm, inject the water phase into the oil phase, and emulsify for 5-10 minutes to obtain colostrum (O / W) for later use;
[0105] Cool 1600ml of aqueous solution containing 2% PVA and 3% NaCl to below 10°C, adjust the speed of the mixer to 500-1000 rpm, add colostrum, stir for 2 minutes, then add 2% PVA, 3% 400ml of NaCl aqueous solution to obtain double emulsion (O / W / O). Stir for 3 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com