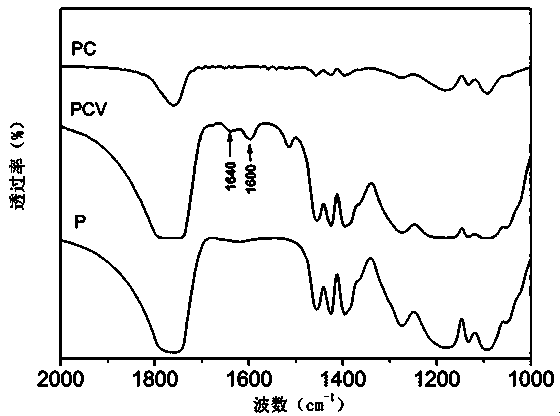
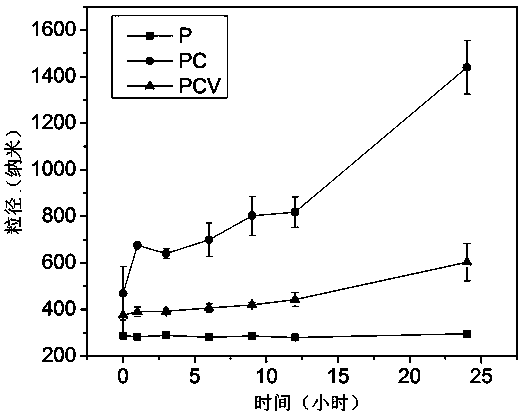
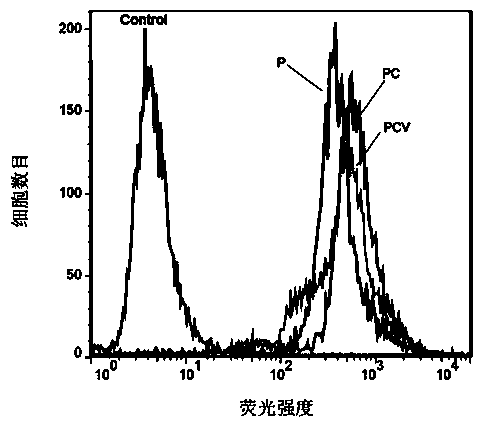
Polylactic acid-hydroxyacetic acid copolymer nano-drug carrier as well as preparation method and application thereof
A glycolic acid copolymer, nano-drug carrier technology, applied in nano-drugs, nano-technology, nano-technology and other directions, can solve the problems of poor drug absorption effect, weak cell uptake ability, lack of specific binding, etc., to achieve enhanced Drug treatment effect, enhancement of cellular uptake capacity, effect of enhanced uptake capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] PLGA was dissolved in a mixture of dichloromethane and ethanol at a volume ratio of 3:1 to prepare a PLGA solution with a concentration of 20 mg / mL. The above PLGA solution was dropped into a 0.75% mass percent PVA aqueous solution under the condition of vortex oscillation, and phacoemulsified with an ultrasonic cell pulverizer for 60 s under the condition of ultrasonic power of 12 W, and then an equal volume of the above The PVA aqueous solution was added to the emulsion, and the organic solvent was removed by continuous stirring for 3 hours to solidify the nanospheres. The nanospheres were centrifuged at 4°C and 13000 rpm for 10 min and washed twice to obtain product P. Using aqueous acetic acid as the solvent, chitosan solutions with concentration gradients of 1.25 mg / mL, 2.5 mg / mL, and 3.75 mg / mL were prepared and adjusted to pH=6.0. Disperse the collected microspheres in the above chitosan solutions of different concentrations, stir for 3 hours to make the chitosan ...
Embodiment 2
[0053] Dissolve PLGA in a mixed solvent of dichloromethane and ethanol with a volume ratio of 3:1 to prepare a PLGA solution with a concentration of 20 mg / mL; prepare an aqueous solution of chitosan with a concentration of 3.75 mg / mL and adjust the pH to 6.0. The above PLGA solution was dropped into a 0.75% mass percent PVA aqueous solution under the condition of vortex oscillation, and phacoemulsified with an ultrasonic cell pulverizer for 60 s under the condition of ultrasonic power of 12 W, and then an equal volume of the above The PVA aqueous solution was added to the emulsion, and the organic solvent was removed by continuous stirring for 3 hours to solidify the nano-microspheres. The nanospheres were centrifuged at 4 ℃ and 13000 rpm for 10 min and washed twice to obtain product P. The collected microspheres were dispersed in the above chitosan aqueous solution, stirred continuously for 3 h, so that the chitosan was adsorbed on the PLGA microspheres, and centrifuged at 4°C...
Embodiment 3
[0056] PLGA was dissolved in a mixture of dichloromethane and ethanol at a volume ratio of 3:1 to prepare a PLGA solution with a concentration of 20 mg / mL. Prepare a chitosan aqueous solution with a concentration of 3.75 mg / mL and adjust the pH to 6.0. The above PLGA solution was dropped into a 0.75% mass percent PVA aqueous solution under the condition of vortex oscillation, and phacoemulsified with an ultrasonic cell pulverizer for 60 s under the condition of ultrasonic power of 12 W, and then an equal volume of the above The PVA aqueous solution was added to the emulsion, and the organic solvent was removed by continuous stirring for 3 hours to solidify the nano-microspheres. The nanospheres were centrifuged at 4 ℃ and 13000 rpm for 10 min and washed twice to obtain product P. Disperse the collected microspheres in the above chitosan aqueous solution and stir continuously for 3 hours to make the chitosan adsorb to the PLGA microspheres. Centrifuge at 4 ℃ and 13000 rpm for 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com