A kind of n2s2 bromobenzyl ether derivative, preparation method and application
A technology of bromobenzyl ether and derivatives, which is applied in the fields of radiopharmaceutical chemistry and clinical nuclear medicine, can solve the problems of unmet PD-L1 expression level monitoring requirements, achieve strong cell uptake ability, and overcome the effect of immunohistochemical methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of N2S2 bromobenzyl ether derivatives comprises the following steps:
[0029]
[0030] S1, preparation of 2-bromo-3-phenyltoluene
[0031] Under nitrogen, add 100-5000mg of 2-bromo-3-iodotoluene into a 100mL three-necked flask, add dioxane (60%-90%) in 30-70mL of aqueous solution to dissolve it, and stir evenly Then add 50-2500mg of phenylboronic acid, 100-8000mg of cesium carbonate and 10-300mg of triphenylphosphine palladium in sequence, stir at 70-90°C for 12-24h, stop the reaction, cool down to room temperature, and remove the solvent by rotary evaporation , adding water and ethyl acetate to extract 3 times, combining the organic phases, drying with anhydrous sodium sulfate, evaporating the organic phases, and passing through a silica gel column to obtain 2-bromo-3-phenyltoluene as a colorless oil;
[0032] S2, preparation of 2-bromo-3-phenylbenzyl bromide
[0033]Under nitrogen, dissolve 100-2000mg of 2-bromo-3-phenyltoluene and anhydrou...
Embodiment 1
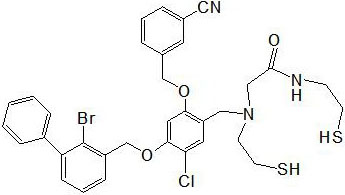
[0047] Such as Figure 1-2 As shown, a kind of N2S2 bromobenzyl ether derivatives have the following structural formula (I):
[0048]
[0049] A preparation method of N2S2 bromobenzyl ether derivatives, comprising the steps of:
[0050] Preparation of S1, 2-bromo-3-phenyltoluene:
[0051] Under nitrogen, add 1400mg of 2-bromo-3-iodotoluene into a 100mL three-necked flask, add 50mL of dioxane / water (volume ratio 5 / 1) to dissolve it, stir for 10min, then add 700mg of benzene Boric acid, 3600mg cesium carbonate and 160mg triphenylphosphine palladium were stirred at 75°C for 18h, the reaction was stopped, and the temperature was lowered to room temperature. Dry over sodium sulfate, evaporate the organic phase to dryness, and pass through a silica gel column to obtain 1015 mg of a colorless oil, with a yield of 87.2%. 1 H NMR (400MHz, DMSO-d 6 )δ7.42-7.31(m, 5H, Ar-H), 7.25-7.20(d, 2H, Ar-H), 7.12-7.08(m, 1H, Ar-H), 2.45(s, 3H, Ar- CH 3 ).
[0052] Preparation of S2, 2-br...
Embodiment 2
[0063] A sort of 99m Tc-labeled N2S2 bromobenzyl ether derivatives ( 99m Tc-N2S2-CBMBC), prepared by N2S2 bromobenzyl ether derivatives, comprising the following steps:
[0064] Dissolve 100 μg of the labeling precursor (N2S2 bromobenzyl ether derivative) in 100 μL of ethanol, add 120 μL of 50 mg / mL sodium glucoheptonate, 50 μL of 20 mg / mL disodium edetate, 10 μL of 10 mg / mL of stannous chloride and 0.5mL pertechnetic acid solution (1mCi / mL), shake well, heat at 90-100°C for 30min, then take it out and cool it down to obtain the desired 99m Tc-N2S2-CBMBC.
[0065] 99m Detection of Tc-N2S2-CBMBC labeling rate
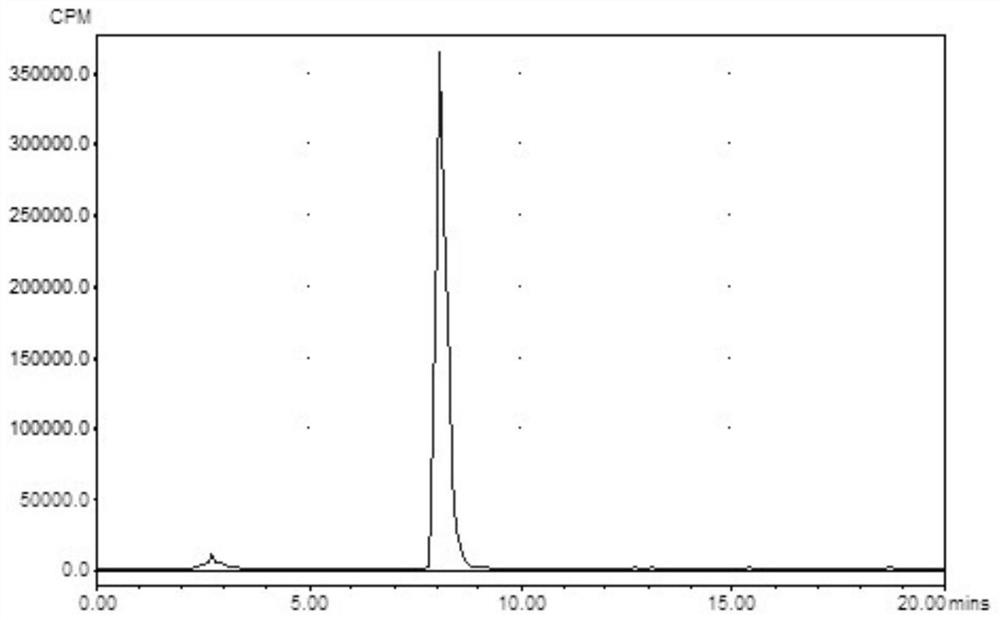
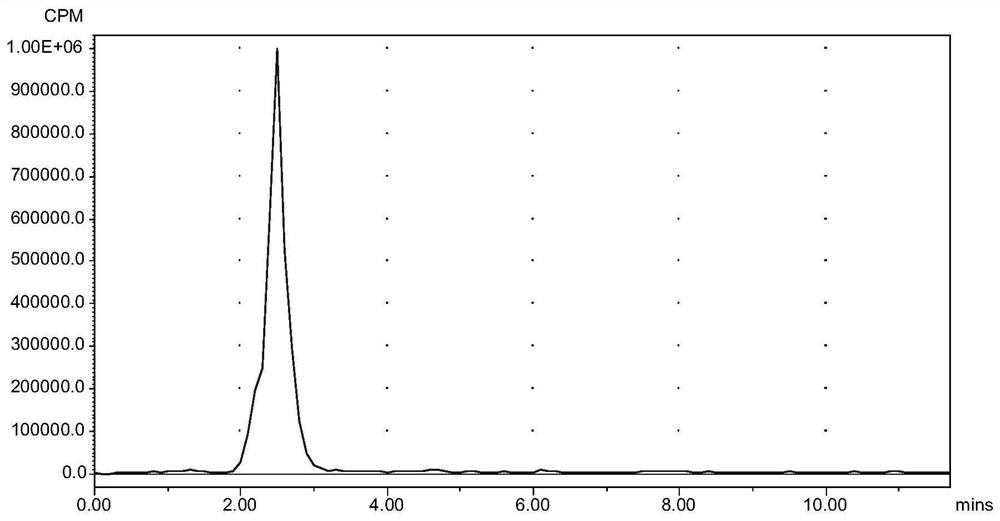
[0066] prepared by the above method 99m Tc-N2S2-CBMBC detects the labeling rate by isotope high-performance liquid phase, C18 reverse phase column, mobile phase is water / methanol=1 / 1 (v / v), flow rate is 1mL / min, labeling rate is greater than 94%, such as figure 1 shown. No tagged precursor, other conditions are the same as tagged 99m Tc-blank, as in figure 2 sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com