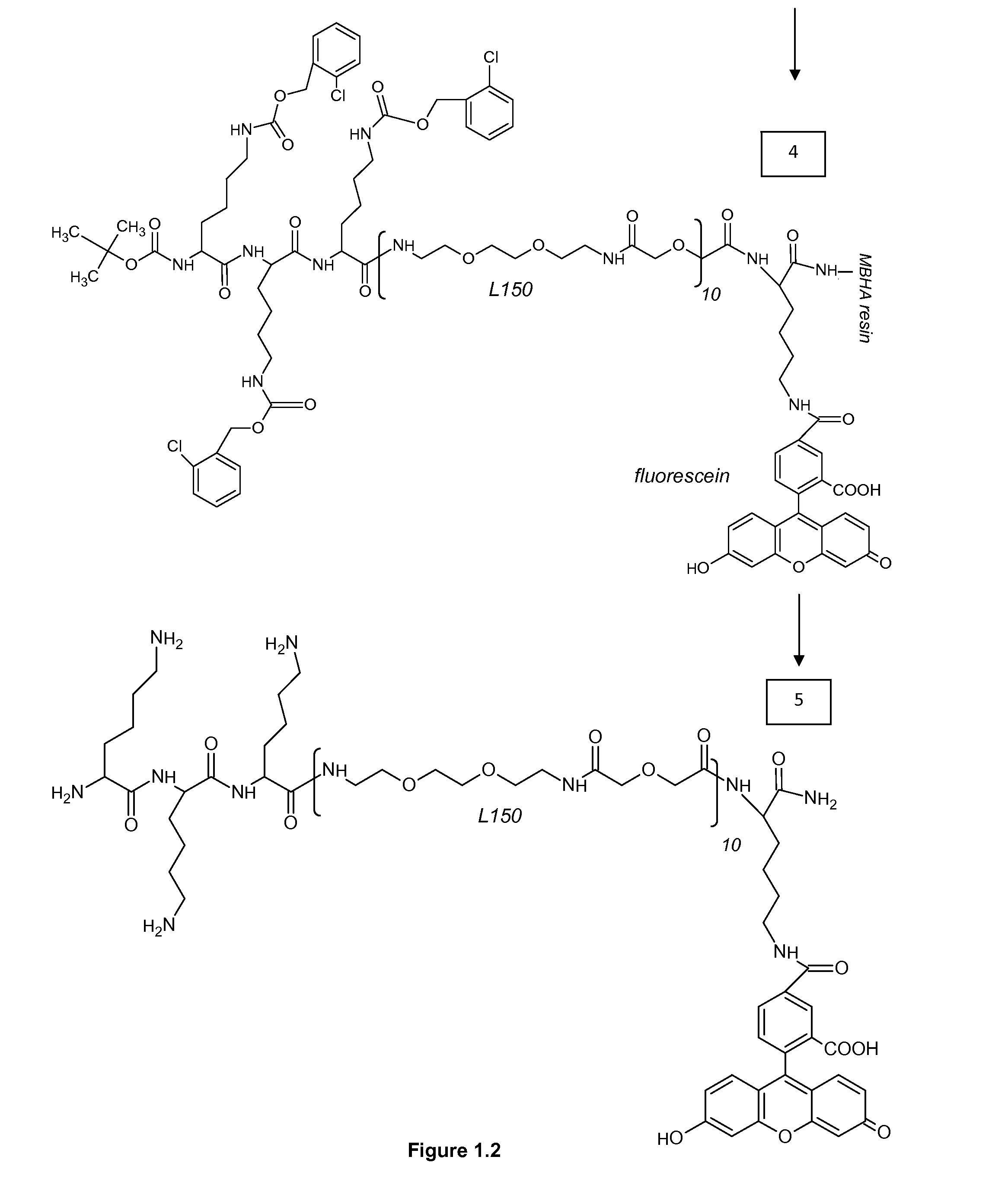
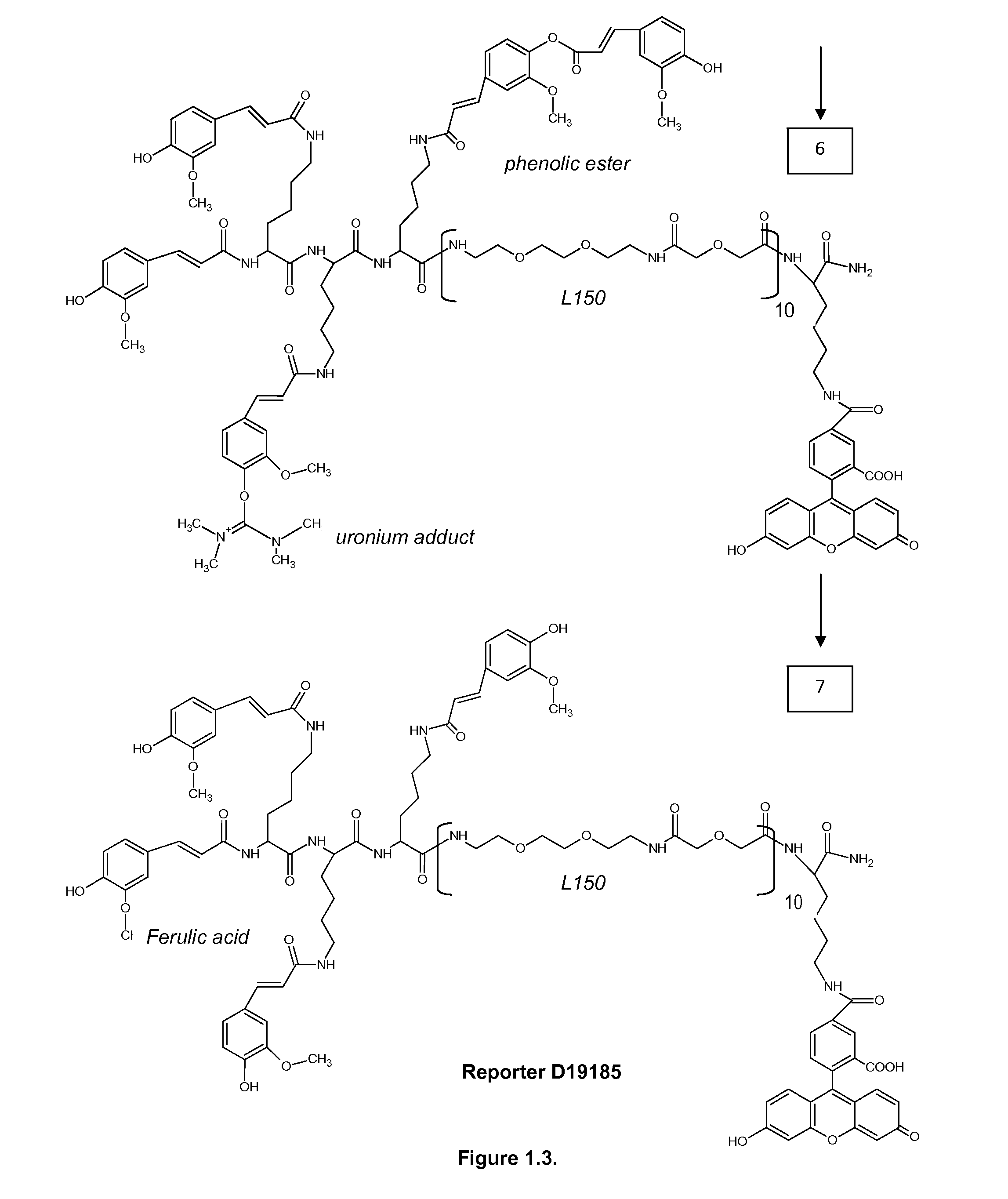
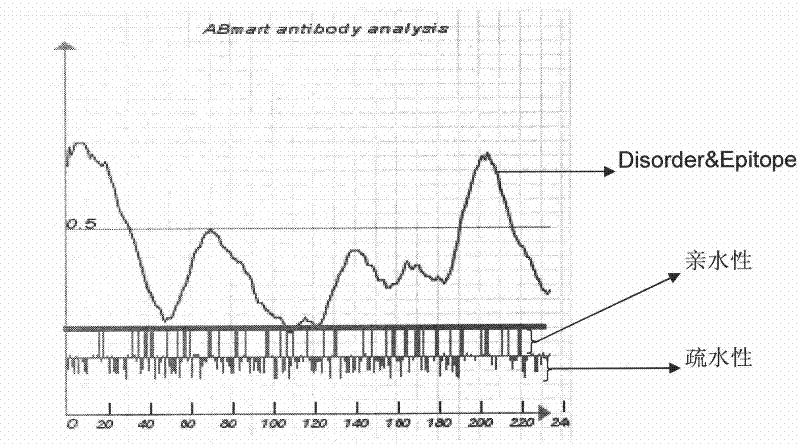
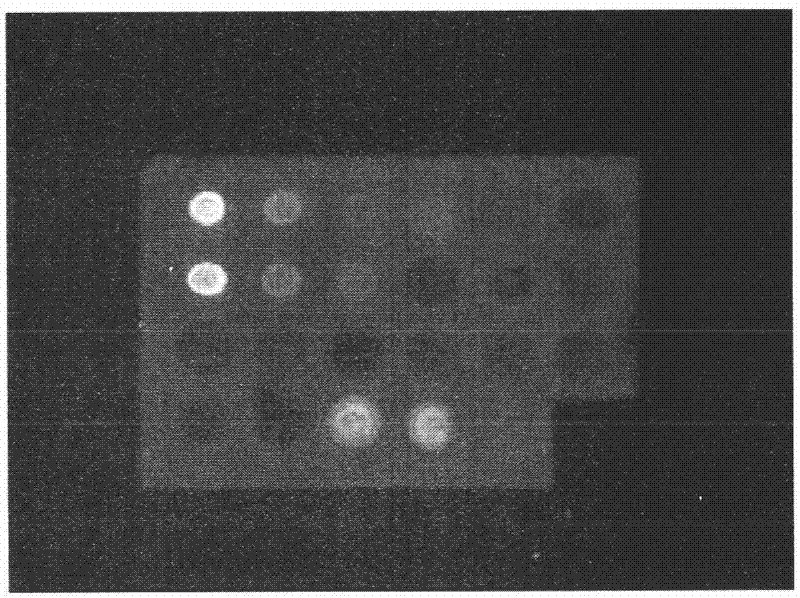
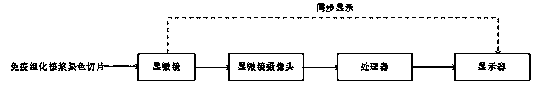
Patents
Literature
577 results about "Immunome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
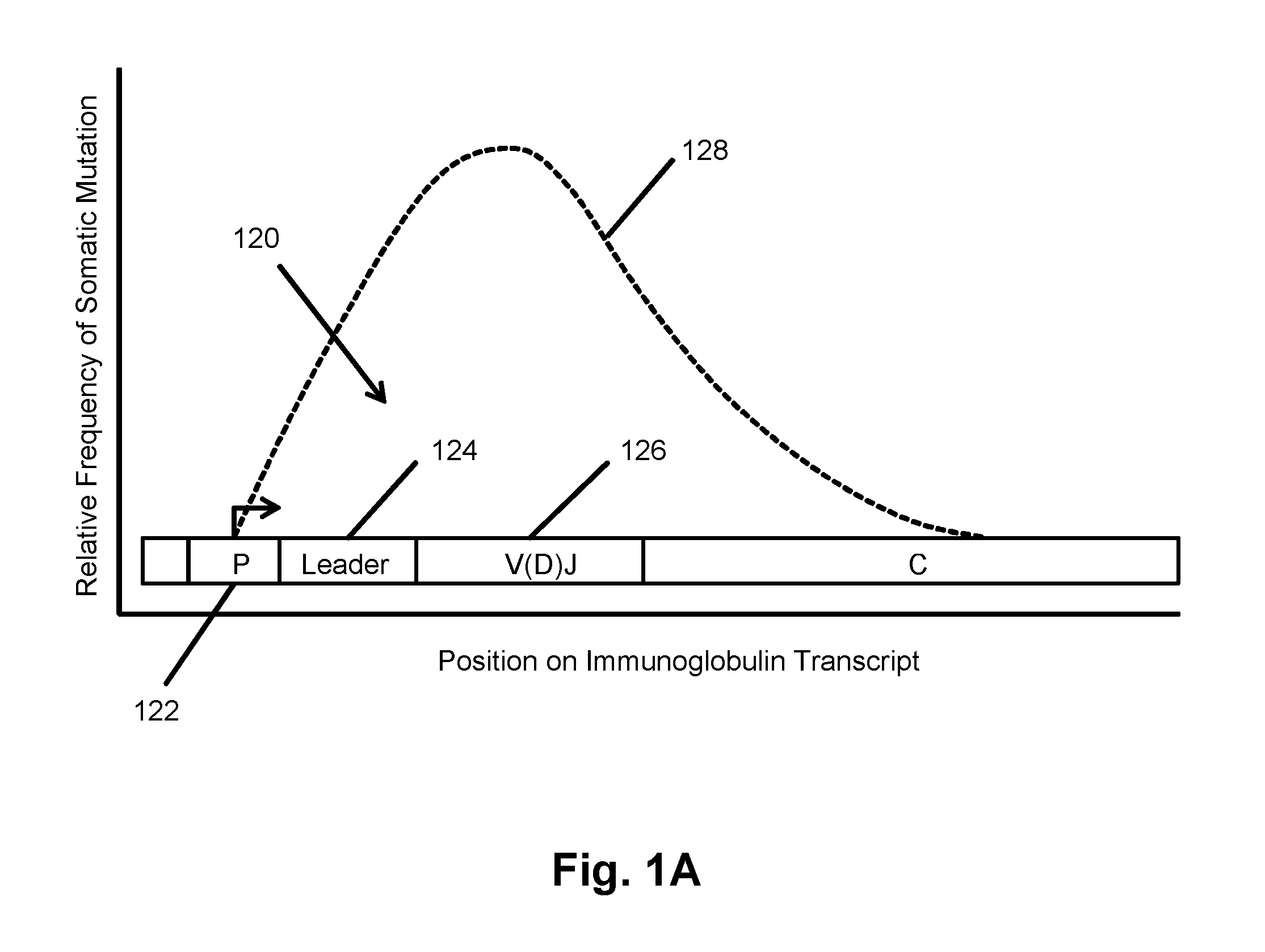
The Immunome is the set of the genes and proteins that constitute the immune system. Typically, immunomes are studied using immunofluorescence microscopy to determine the presence and activity of immune-related enzymes and pathways.
Single cell bar-coding for antibody discovery
Provided herein are methods and composition for immune repertoire sequencing and single cell barcoding for heavy and IgL pairing if antibodies.
Owner:ABVITRO LLC
Ex vivo human lung/immune system model using tissue engineering for studying microbial pathogens with lung tropism
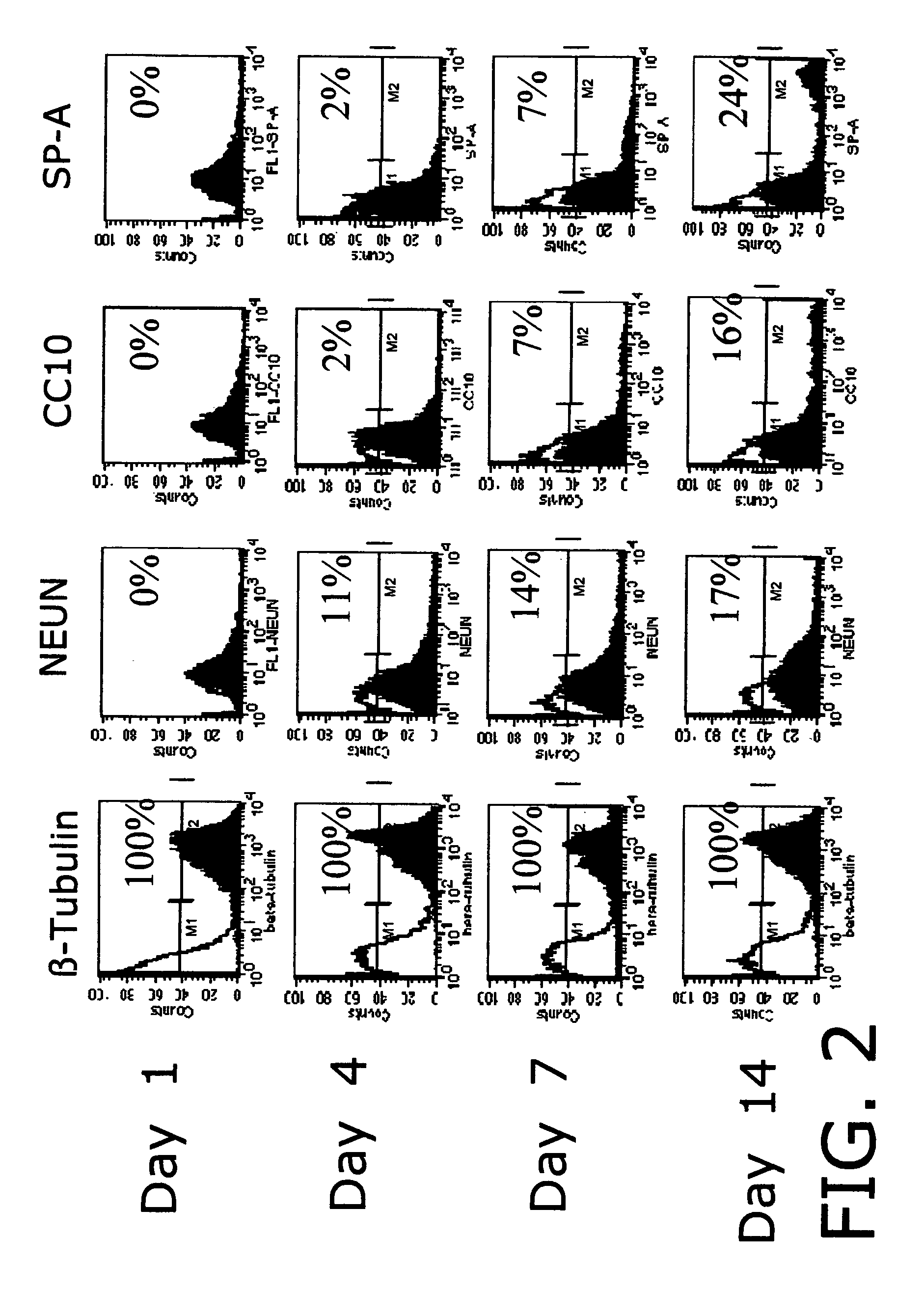
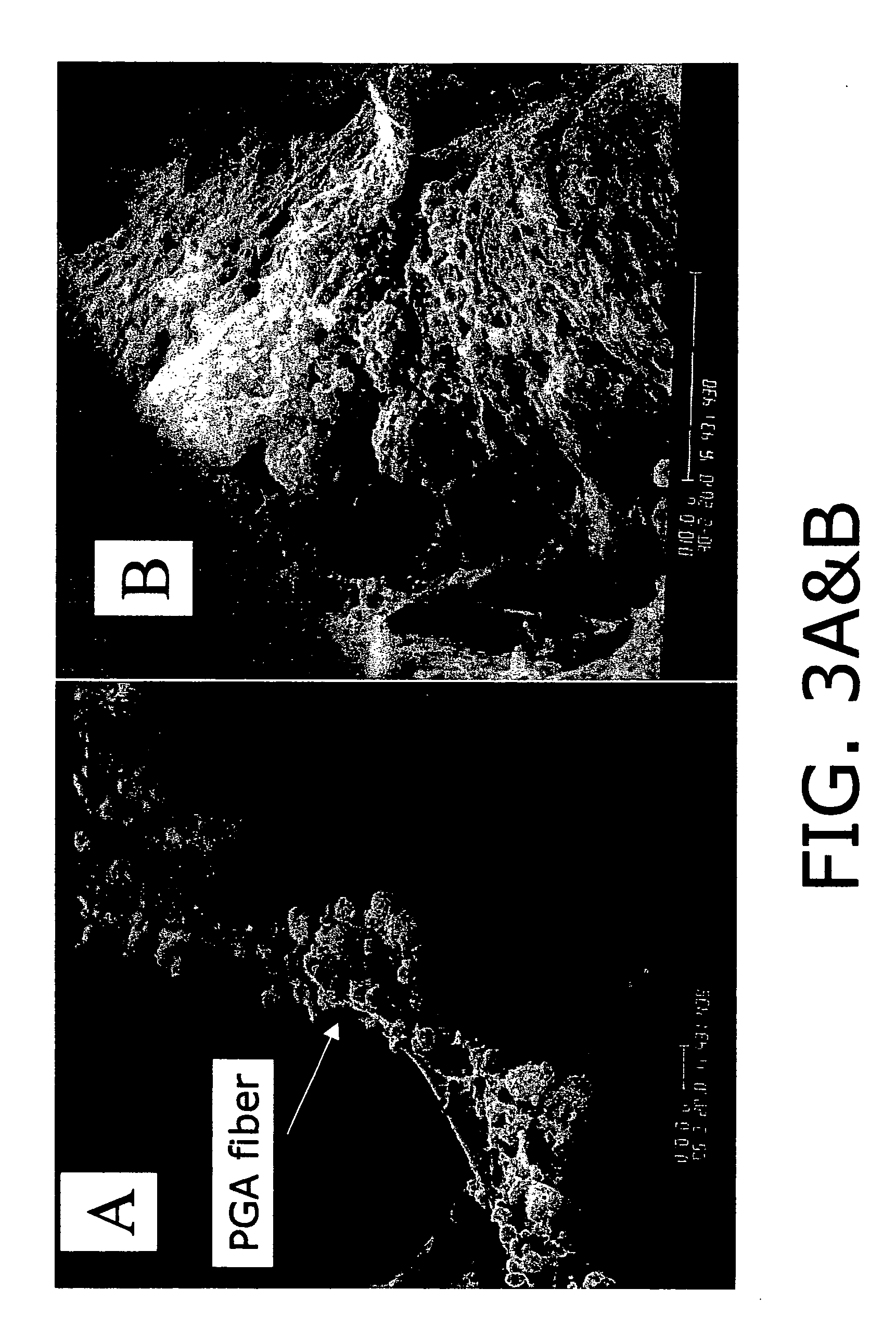
A method for studying scaffold-based tissue engineering approaches in combination with the use of progenitor or stem cells to generate new lung tissue in an in vitro system. The engineered tissue system of this invention is used to monitor lung and immune system exposure of pathogen and / or toxins. The method involves growing engineered lung / immune tissue from progenitor cells in a bioreactor and then exposing the engineered lung / immune tissue to a pathogen and / or toxin. Once exposed, response of the engineered tissue is monitored to determine the effects of exposure to the immune component of the tissue and to lung component of the tissue. This invention also involves development of mixed engineered tissues including a first fully functional engineered tissue such as lung tissue and a second fully functional engineered tissued such as immune tissue from a single animal donor. The mixed systems can include more than two engineered tissues.
Owner:THE BOARD OF RGT TEXAS UNIV SYST
Monitoring transformation of follicular lymphoma to diffuse large b-cell lymphoma by immune repertoire analysis
InactiveUS20140349883A1Raise the possibilityMicrobiological testing/measurementLibrary screeningProgenitorSomatic cell
The invention is directed to a method of prognosing in an individual a transformation from follicular lymphoma to diffuse large B-cell lymphoma (DLBCL) by measuring changes and / or lack of changes in certain groups of related clonotypes, referred to herein as “clans,” in successive clonotype profiles of the individual. A clan may arise from a single lymphocyte progenitor that gives rise to many related lymphocyte progeny, each possessing and / or expressing a slightly different immunoglobulin receptor due to somatic mutation(s), such as base substitutions, inversions, related rearrangements resulting in common V(D)J gene segment usage, or the like. A higher likelihood of transformation from follicular lymphoma to DLBCL is correlated with the persistence of clans in successive clonotype profiles whose clonotype membership fails to undergo diversification over time.
Owner:ADAPTIVE BIOTECH
Synthetic immunogenic but non-deposit-forming polypeptides and peptides homologous to amyloid beta, prion protein, amylin, alpha-synuclein, or polyglutamine repeats for induction of an immune response thereto
InactiveUS7479482B2Reduce formationAvoid formingHormone peptidesNervous disorderPassive ImmunizationsAmyloid beta
The present invention relates to immunogenic but non-depositing-forming polypeptides or peptides homologous to amyloid β, prion, amylin or α-synuclein which can be used alone or conjugated to an immunostimulatory molecule in an immunizing composition for inducing an immune response to amyloid β peptides and amyloid deposits, to prion protein and prion deposits, to amylin and amylin deposits, to α-synuclein and deposits containing α-synuclein, or to polyglutamine repeats and deposits of proteins containing polyglutamine repeats. Described are also antibodies directed against such peptides, their generation, and their use in methods of passive immunization to such peptides and deposits.
Owner:NEW YORK UNIV
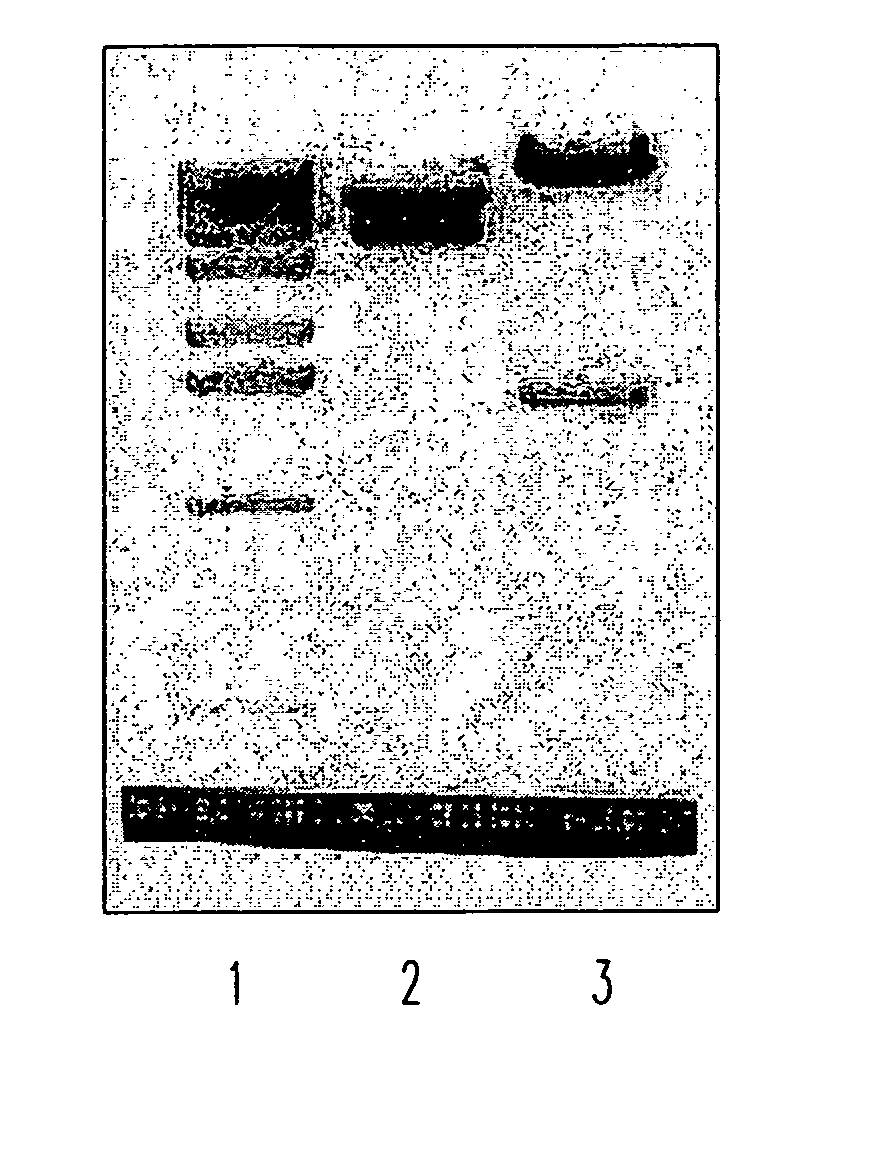
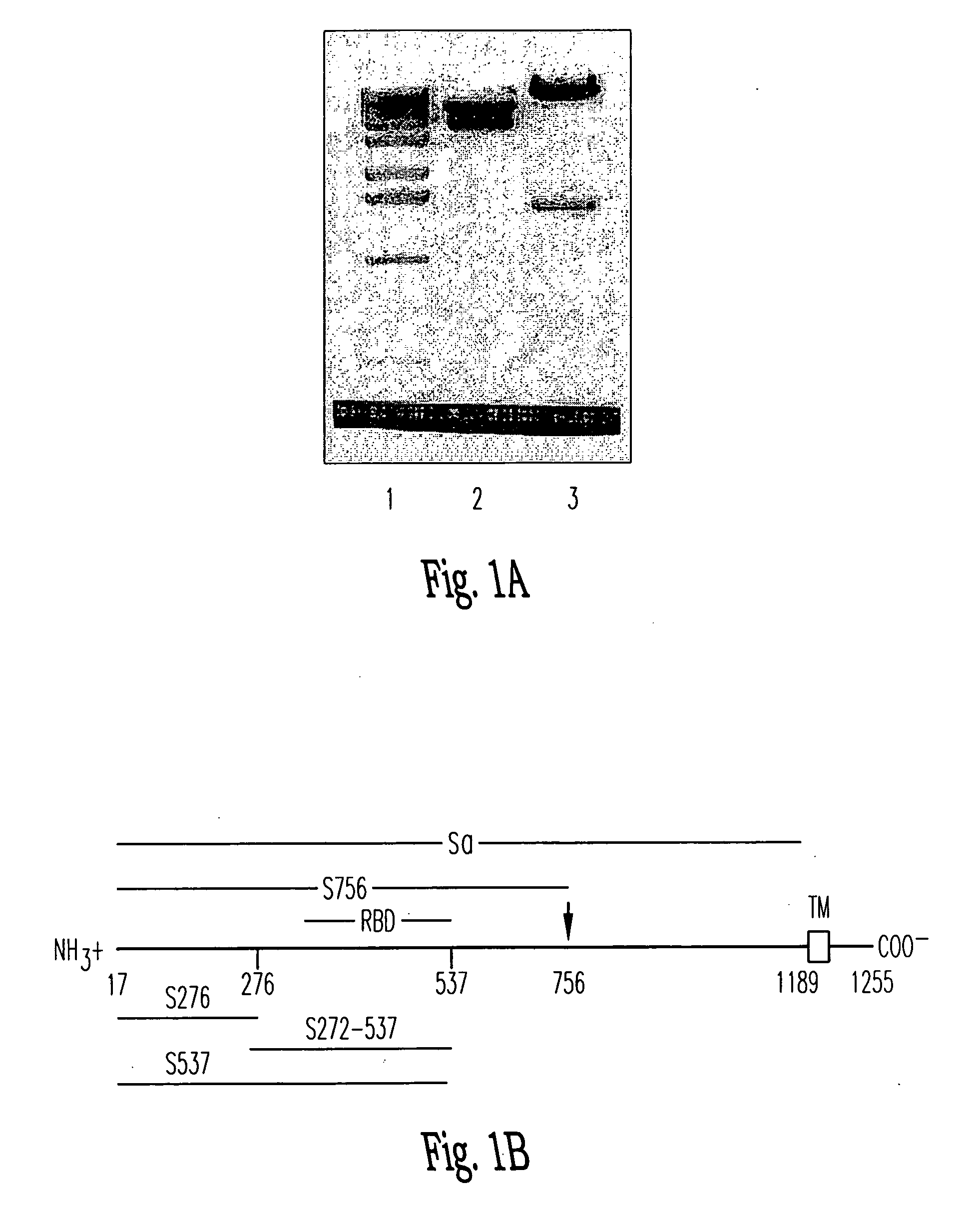
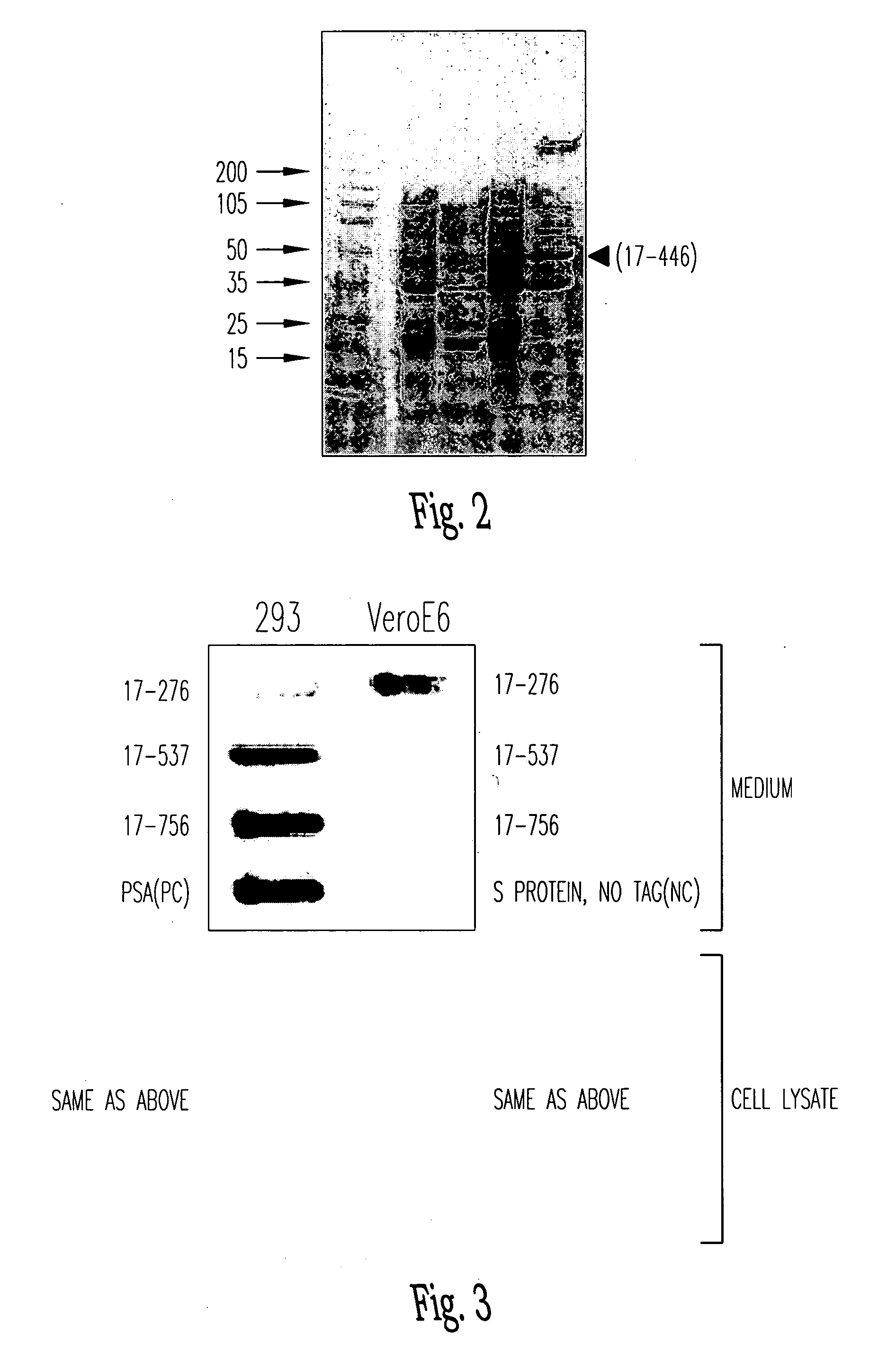
Soluble fragments of the SARS-CoV spike glycoprotein
InactiveUS20060240515A1Easy to produceImprove resistance to degradationSsRNA viruses positive-senseAntibody mimetics/scaffoldsAptamerVaccination
The invention relates to the spike protein from the virus (SARS-CoV) that is etiologically linked to severe acute respiratory syndrome (SARS); polypeptides and peptide fragments of the spike protein; nucleic acid segments and constructs that encode the spike protein, polypeptides and peptide fragments of the spike protein, and coupled proteins that include the spike protein or a portion thereof; peptidomimetics; vaccines; methods for vaccination and treatment of severe acute respiratory syndrome; antibodies; aptamers; and kits containing immunological compositions, or antibodies (or aptamers) that bind to the spike protein.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF US SEC THE DEPT OF
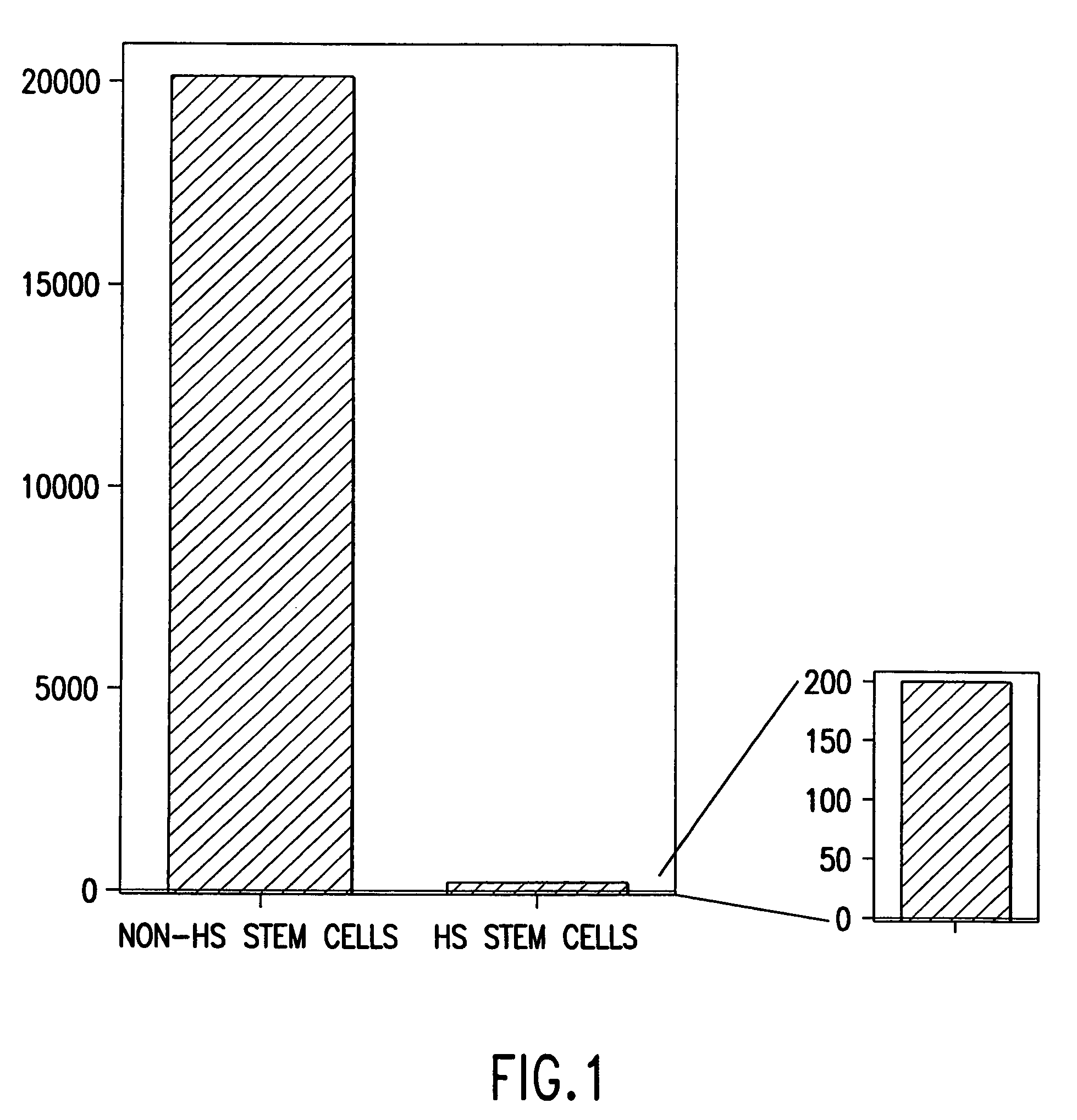
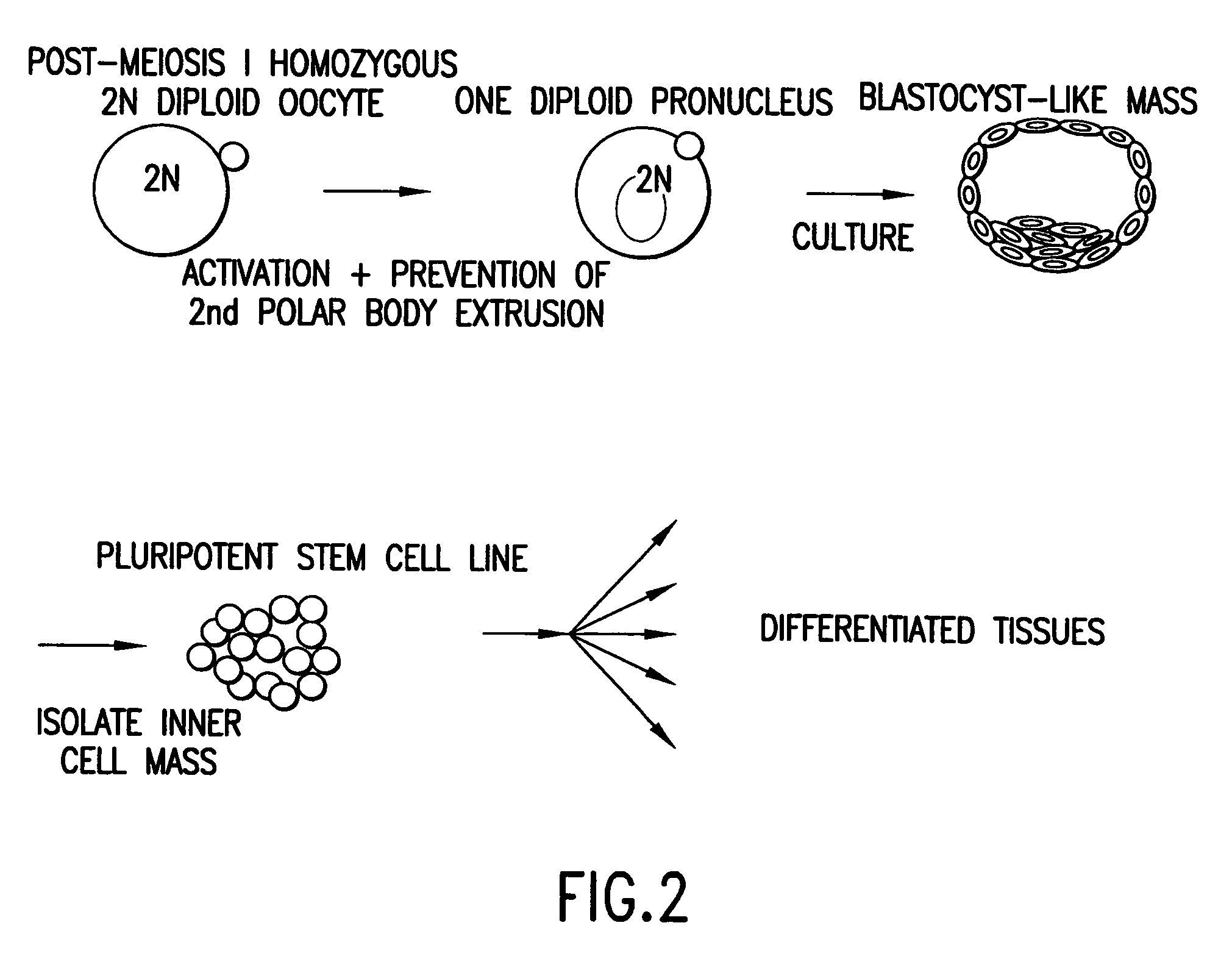
Method for producing a population of homozygous stem cells having a pre-selected immunotype and/or genotype, cells suitable for transplant derived therefrom, and materials and methods using same
InactiveUS7030292B2Reduce demandThe implementation process is simpleBiocideGenetic material ingredientsInjury causeHla haplotypes
A method of producing a homogenous population of homozygous stem (HS) cells pre-selected for immunotype and / or genotype from donor cells is described herein. The invention relates to methods of using immunohistocompatible HS cells for diagnosis, therapeutic and cosmetic transplantation, and the treatment of various genetic diseases, neurodegenerative diseases, traumatic injuries and cancer. The invention further relates to methods for using histocompatible HS stem cells pre-selected for a non-disease genotype for prophylactic and therapeutic intervention including, but not limited to, therapeutic and cosmetic transplantation, and the treatment of various genetic diseases, neurodegenerative diseases, and cancer. Furthermore, the invention relates to a catalogued transplant depository of HS cells derived from multiple donors, each of the HS cells being homozygous for a unique HLA haplotype, for the purpose of having a constant, reliable, comprehensive supply of immunohistocompatible cells for diagnosis, treatment and / or transplantation.
Owner:STEMRON
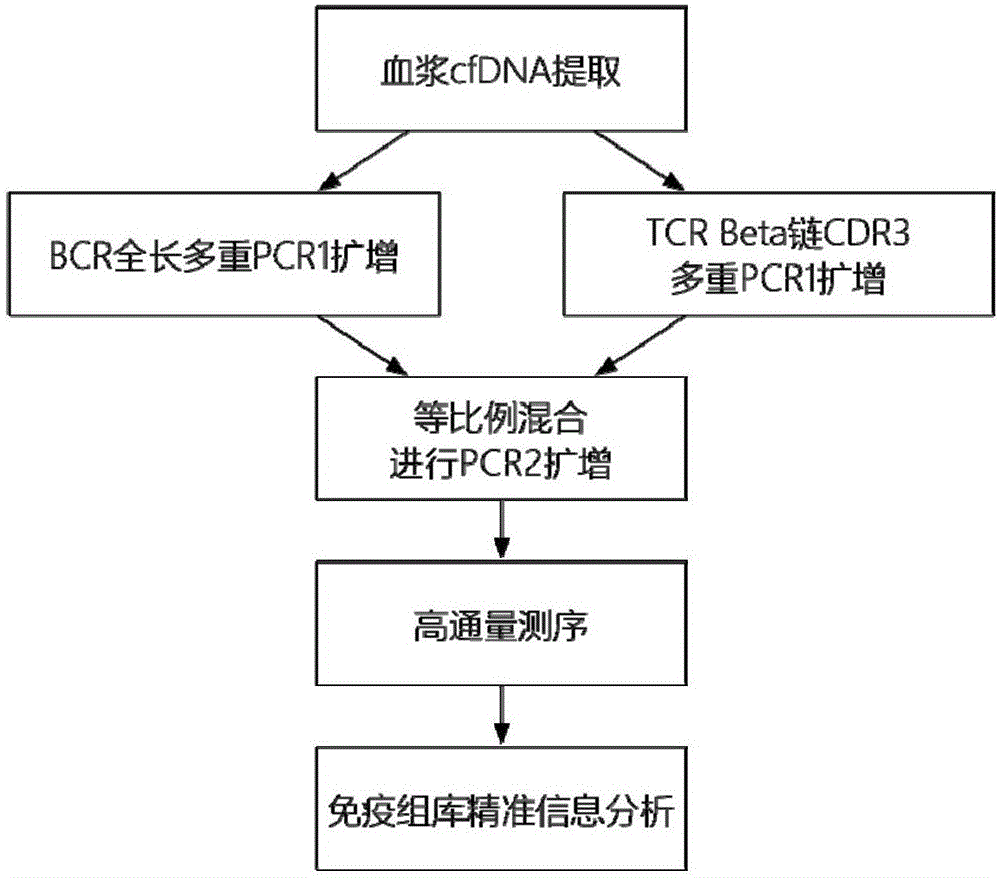
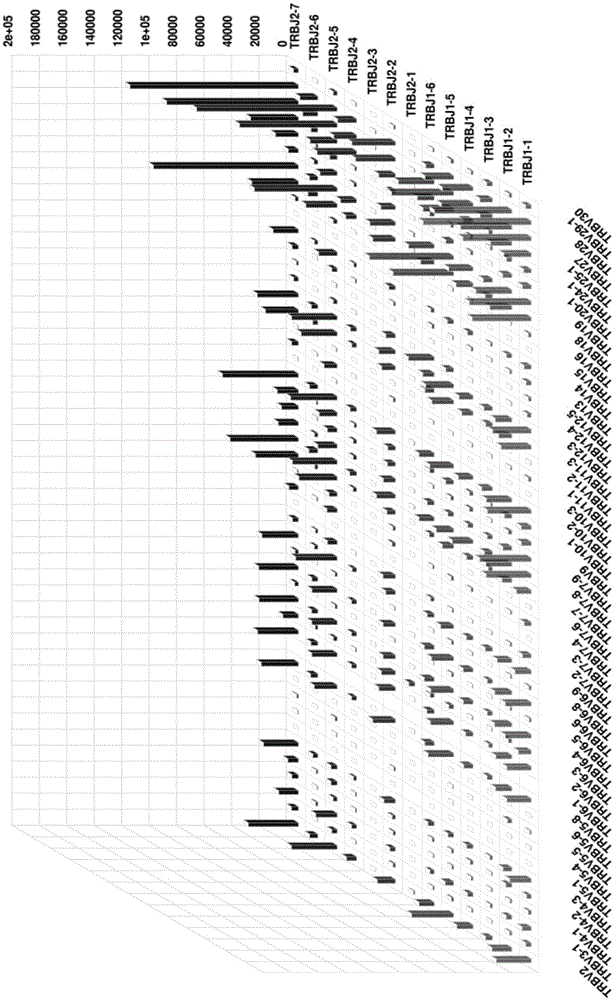
Method for detecting BCR and TCR immune repertoire in blood plasma cfDNA
ActiveCN105087789AEliminate errorsImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationInformation analysisSubcloning
The invention provides a method for detecting BCR and TCR immune repertoire in blood plasma cfDNA. The method includes: blood plasma cfDNA extraction, BCR H chain overall length multiple PCR amplification, TCR beta chain CDR3 multiple PCR amplification, PCR amplification after the products of the two previous amplifications are mixed in an equal-volume manner, high-throughput sequencing, and immune repertoire precise information analysis. The primers of the three amplifications are respectively shown in SEQ ID No. 1-28, SEQ ID No. 29-77 and SEQ ID No. 78-79. The invention further provides a kit, which contains the primers, for detecting the BCR and TCR in the blood plasma cfDNA. By the method and the kit which are promising in application prospect in the field of non-invasive detection, the BCR and TCR in the blood plasma cfDNA can be detected at the same time, precise information analysis and precise quantification of immune repertoire subcloning are achieved.
Owner:BEIJING GENEPLUS TECH +2
Anti-Golgi apparatus protein monoclonal antibody and use
InactiveCN101407544AHigh sensitivityQuantitatively accurateImmunoglobulins against animals/humansFermentationSerum igeSerum samples
The invention relates to an anti-Golgi protein antibody and an application thereof. The invention recombines human GP73 protein immunity animal to obtain an anti-GP73 polyclonal antibody and a monoclonal antibody which specifically aims at GP73 and builds a plurality of methods for detecting GP73 in clinical tissue sections and serum samples, such as immunohistochemical stain and double antibody sandwiched ELISA method and the like. Tests prove that the polyclonal and monoclonal antibodies can be used for preparing a plurality of GP73 detecting agents of different detecting methods.
Owner:曹伯良 +1
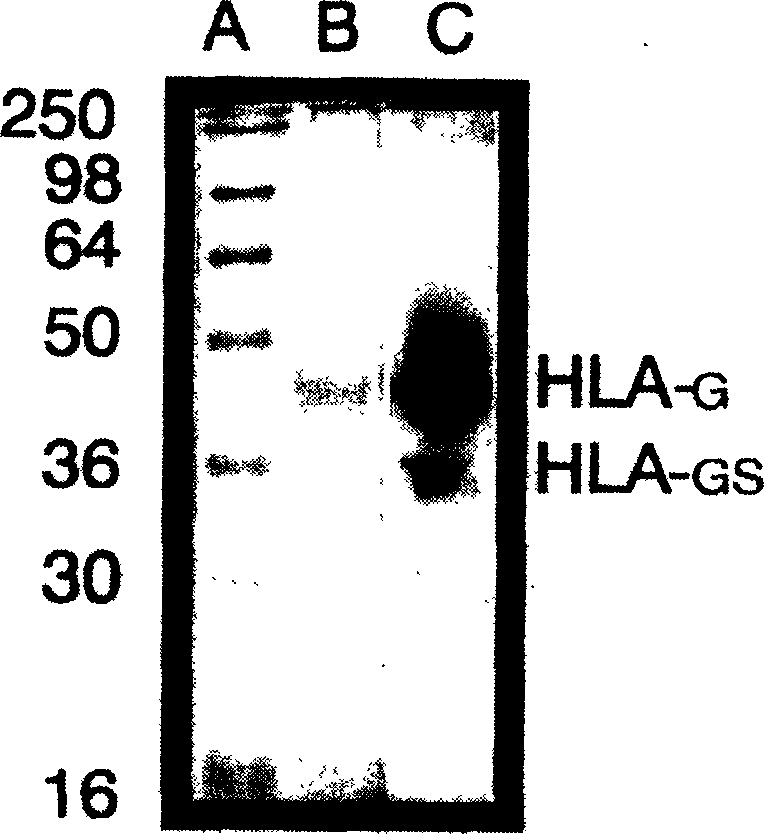
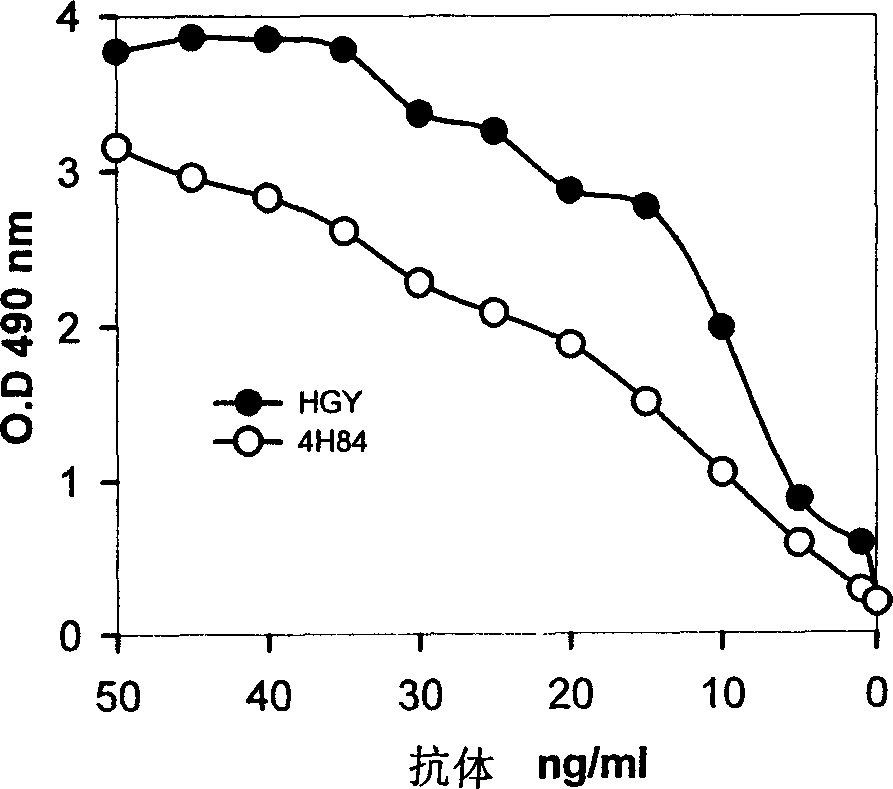
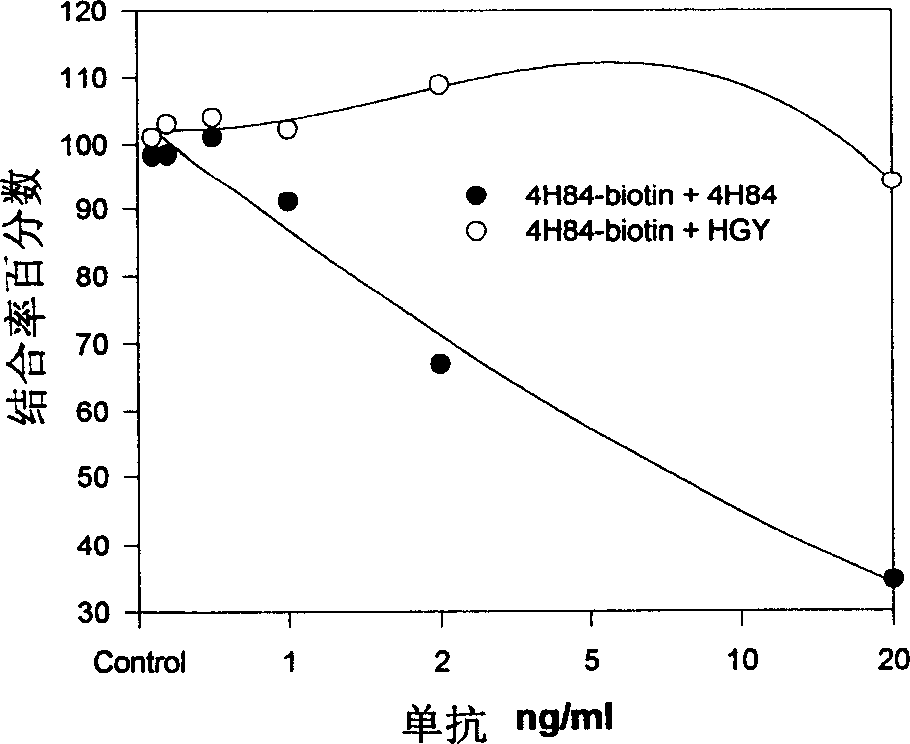
Monoclone antibody for anti HLA-G and hybrid tumour cell secreting same, cancer dignosis method, diagnosis reagent box and its application
ActiveCN1718588AIncreased sensitivityEasy to useImmunoglobulins against cell receptors/antigens/surface-determinantsGenetic engineeringCancerCell strain
A monoclonal antibody HGY for HLA-G, which can generate the antigen-antibody reaction on 7 HLA-G isomers, a hybridoma cell strain able to secrete it, a method with high sensitivity for diagnosing cancer with said antibody, and an immunohistochemical reagent kit prepared from said antibody and its application are disclosed.
Owner:四川首创生物制药有限公司
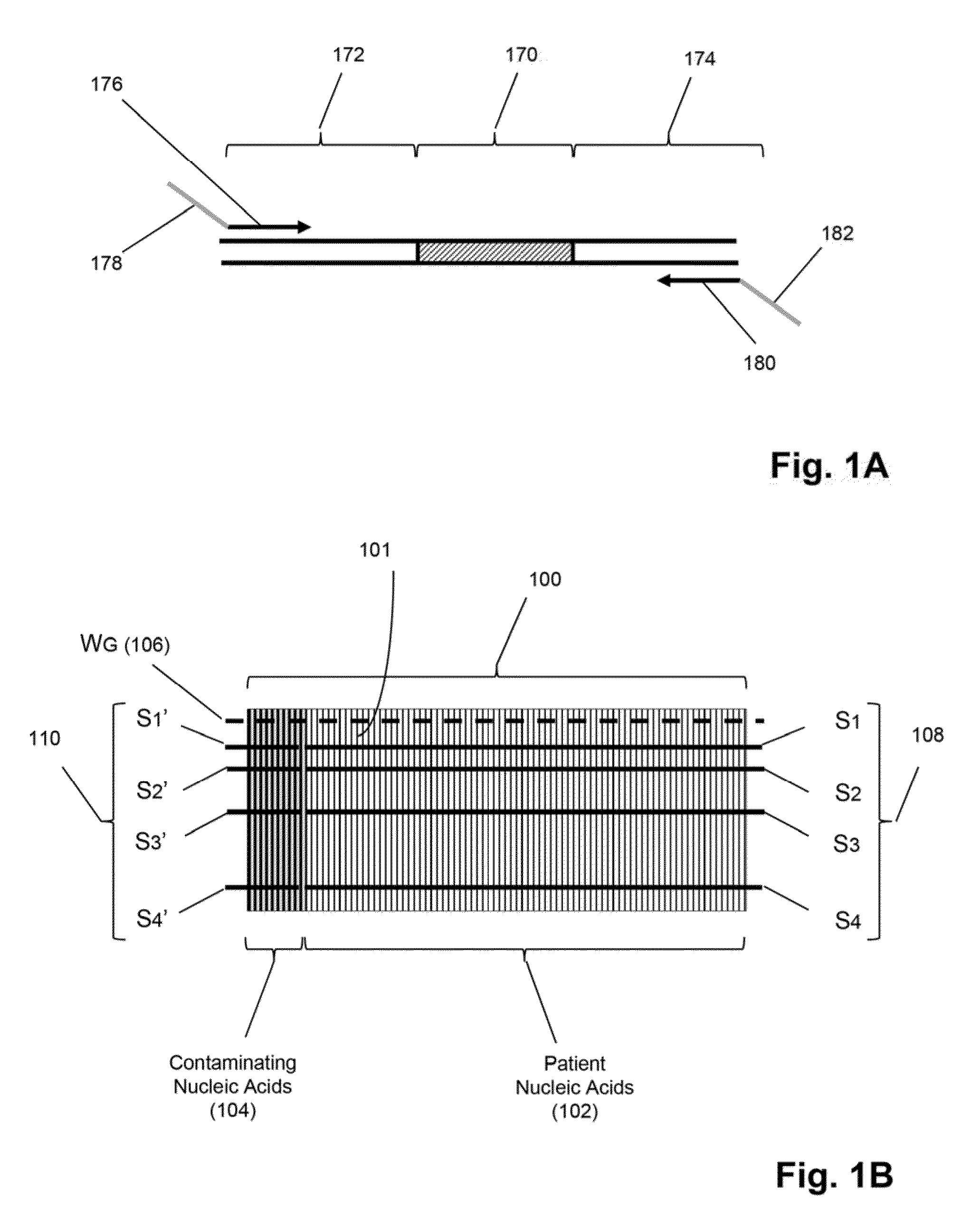
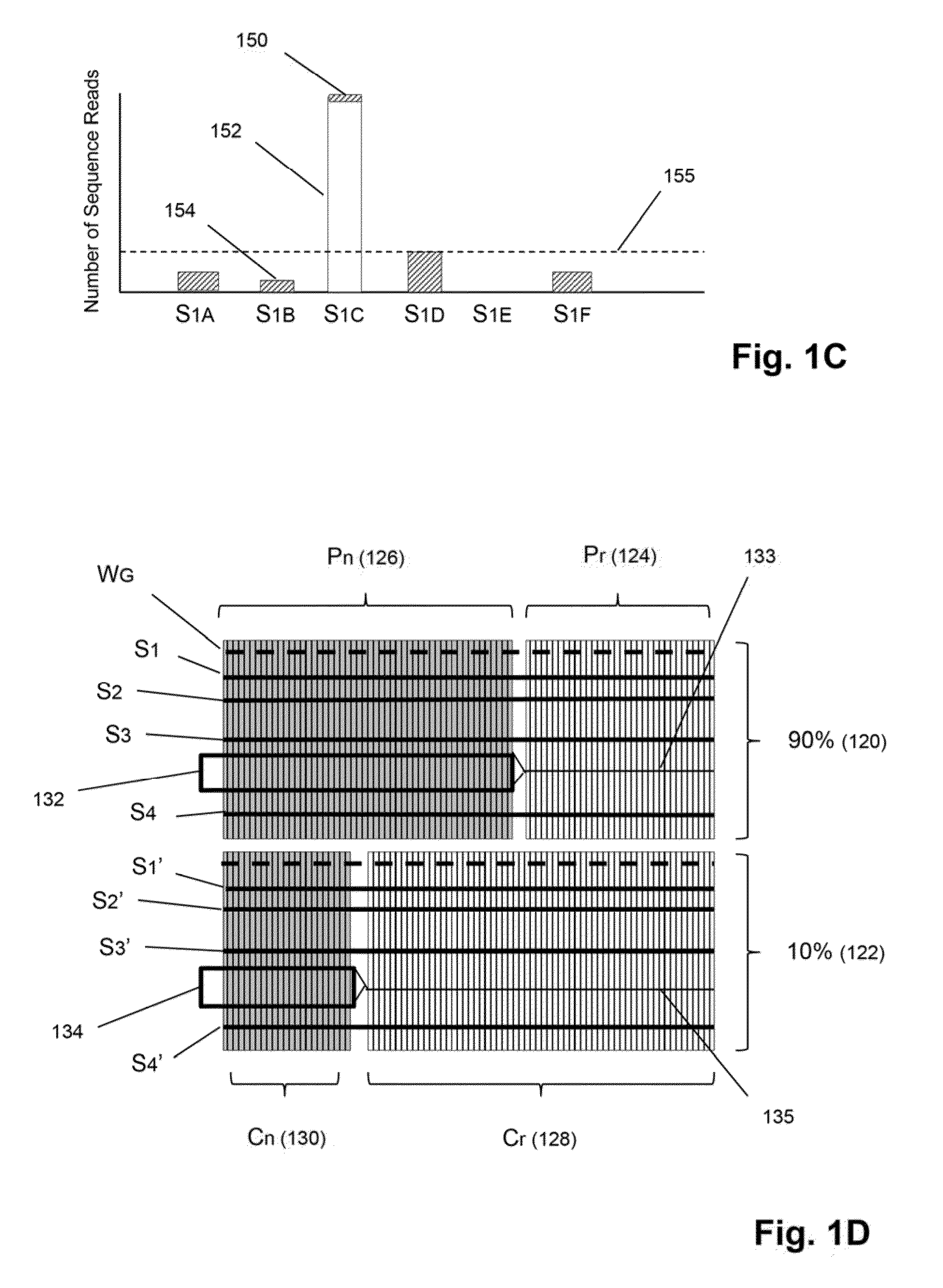
Detection and quantification of sample contamination in immune repertoire analysis
The invention is directed to methods for detecting and quantifying nucleic acid contamination in a tissue sample of an individual containing T cells and / or B cells, which is used for generating a sequence-based clonotype profile. In one aspect, the invention is implemented by measuring the presence and / or level of an endogenous or exogenous nucleic acid tag by which nucleic acid from an intended individual can be distinguished from that of unintended individuals. Endogenous tags include genetic identity markers, such as short tandem repeats, rare clonotypes or the like, and exogenous tags include sequence tags employed to determine clonotype sequences from sequence reads.
Owner:ADAPTIVE BIOTECH
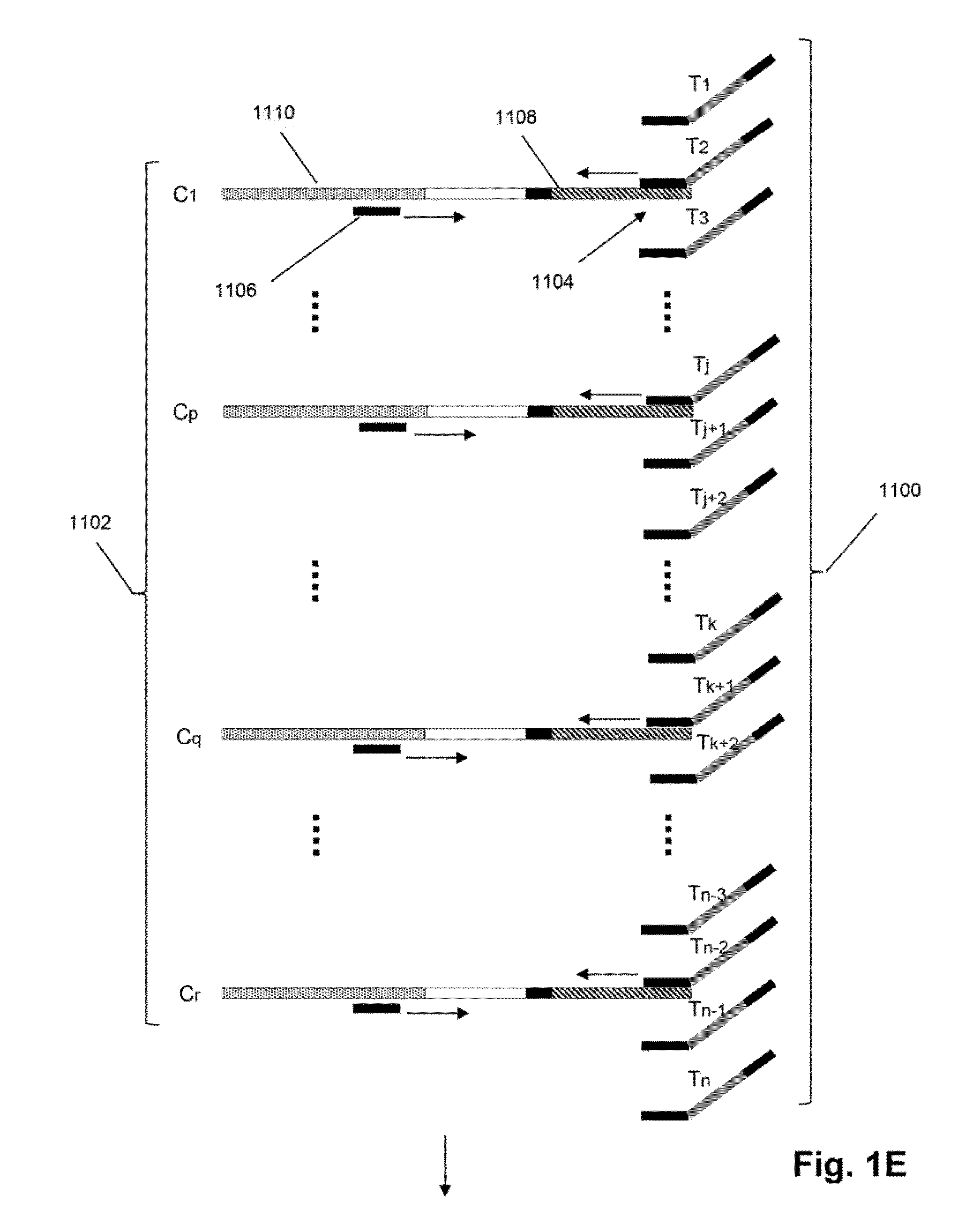
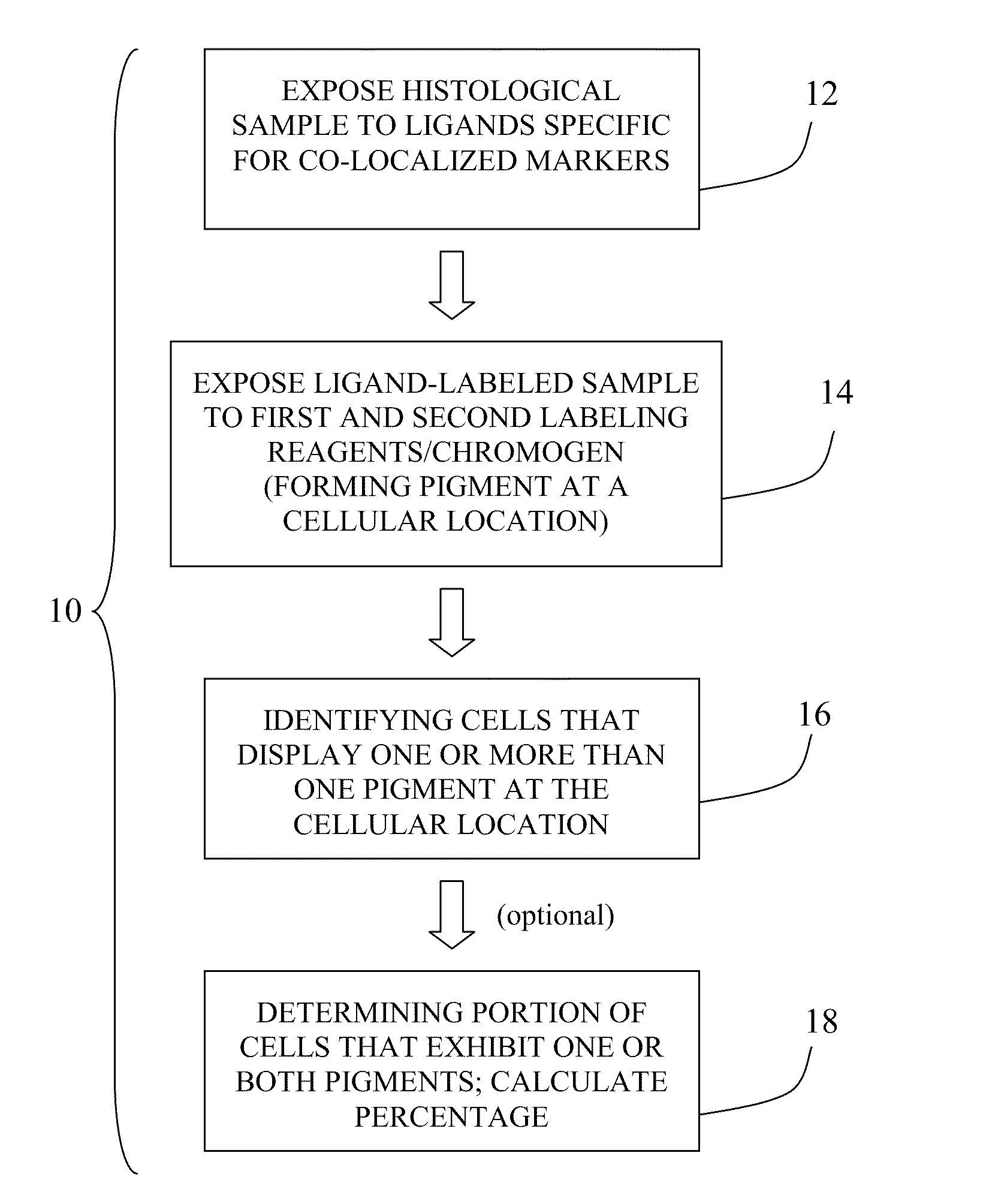
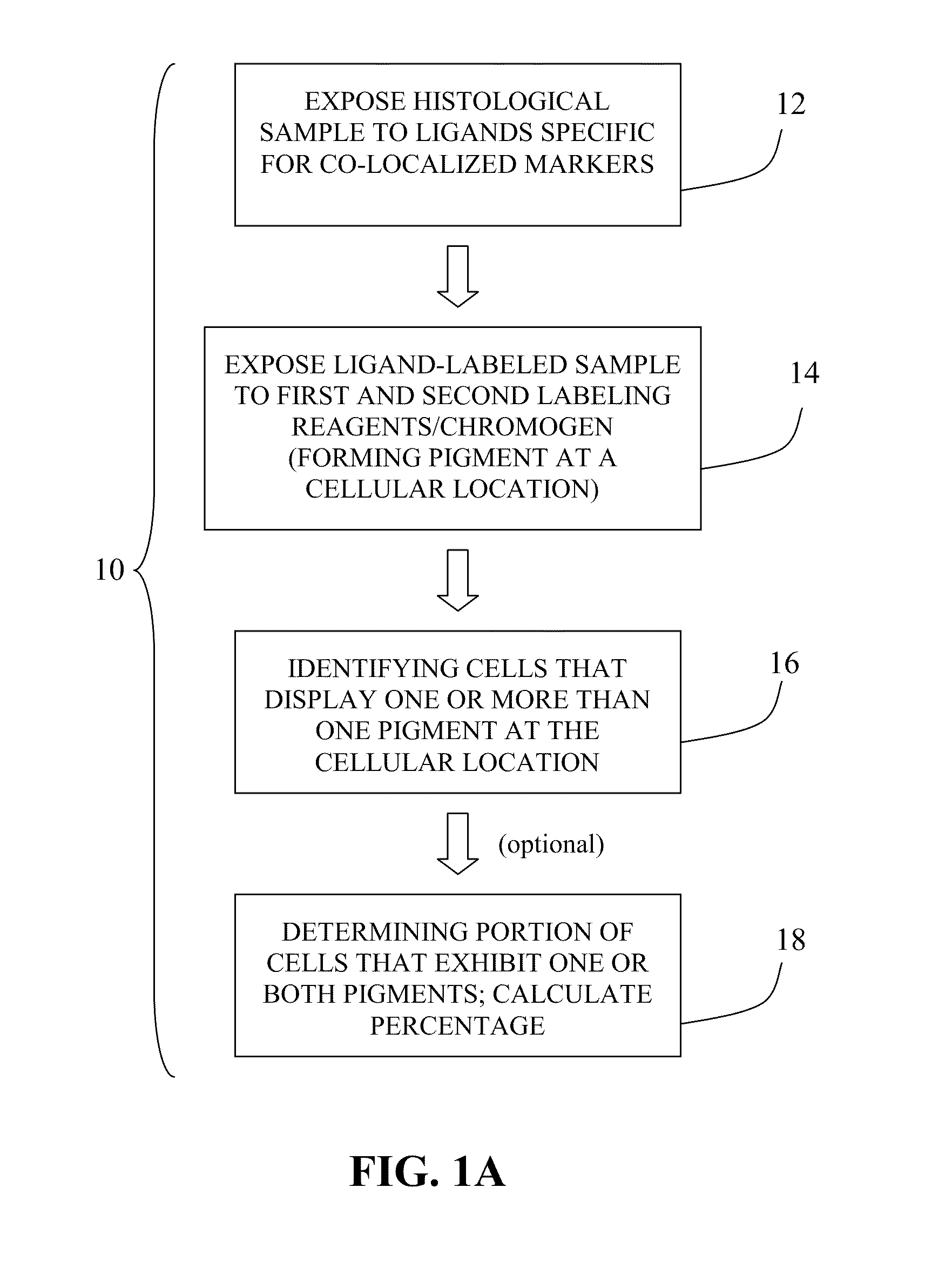
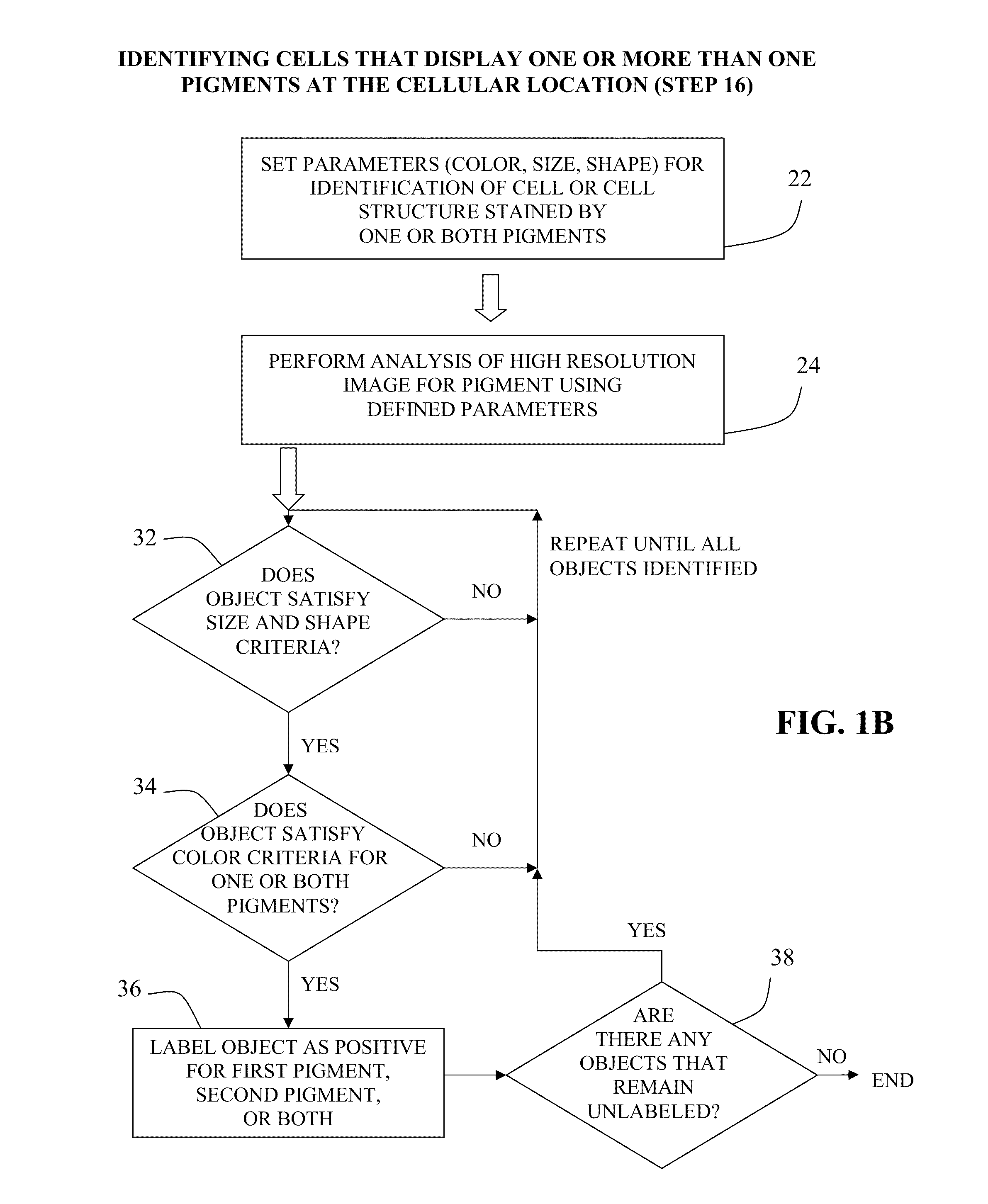
Method for double staining in immunohistochemistry
ActiveUS20100151447A1Measurably differentMicrobiological testing/measurementBiological testingTissue sampleCo localization
The present invention relates to kits and methods for performing dual-staining immunohistochemistry (IHC) for the detection of specific cell populations in tissue samples containing heterogeneous populations of cells, which can be observed by a light microscope for co-localization of distinct pigments. The method includes providing a tissue sample comprising fixed cells; exposing the sample to first and second ligands that recognize different marker proteins found at the same cellular location, thereby forming a ligand-labeled sample; exposing the ligand-labeled sample to first and second labeling reagents, the first labeling reagent binding to the first ligand and the second labeling reagent binding to the second ligand, the first and second labeling reagents each forming distinct pigments; and identifying the number of cells that display only one particular pigment, or more than one pigment, by the different coloration of the cellular location labeled by the distinct pigment.
Owner:CORNELL UNIVERSITY
Tissue chip used for tumour early stage diagnosis and preparation device
Three kinds of tissues including cancer tissue, precancerosis and corresponding normal tissue are sliced up, dyed, marked, and positioned. Receptor holes are prepared by leading designed lattice array mould paper to paste on surface of wax block of receptor. Wax block with tissue core bar is prepared by using perforating needle and puncture needle for tissue. Common cancer such as lung cancer, nasopharyngeal carcinoma, oesophagus cancer etc. and having integrated clinical data and pathology features are selected. Through in situ hybridization, testing mRNA of relevant gene and expression of protein on tissue chip, consistent result between the invented product and traditional test is validated. In the product, cellular morphology is clear and even, and there is no fallen off tissue point. The invention is applicable to filter cancers, early diagnosis and forecasting prognosis.
Owner:中南大学湘雅医学院肿瘤研究所
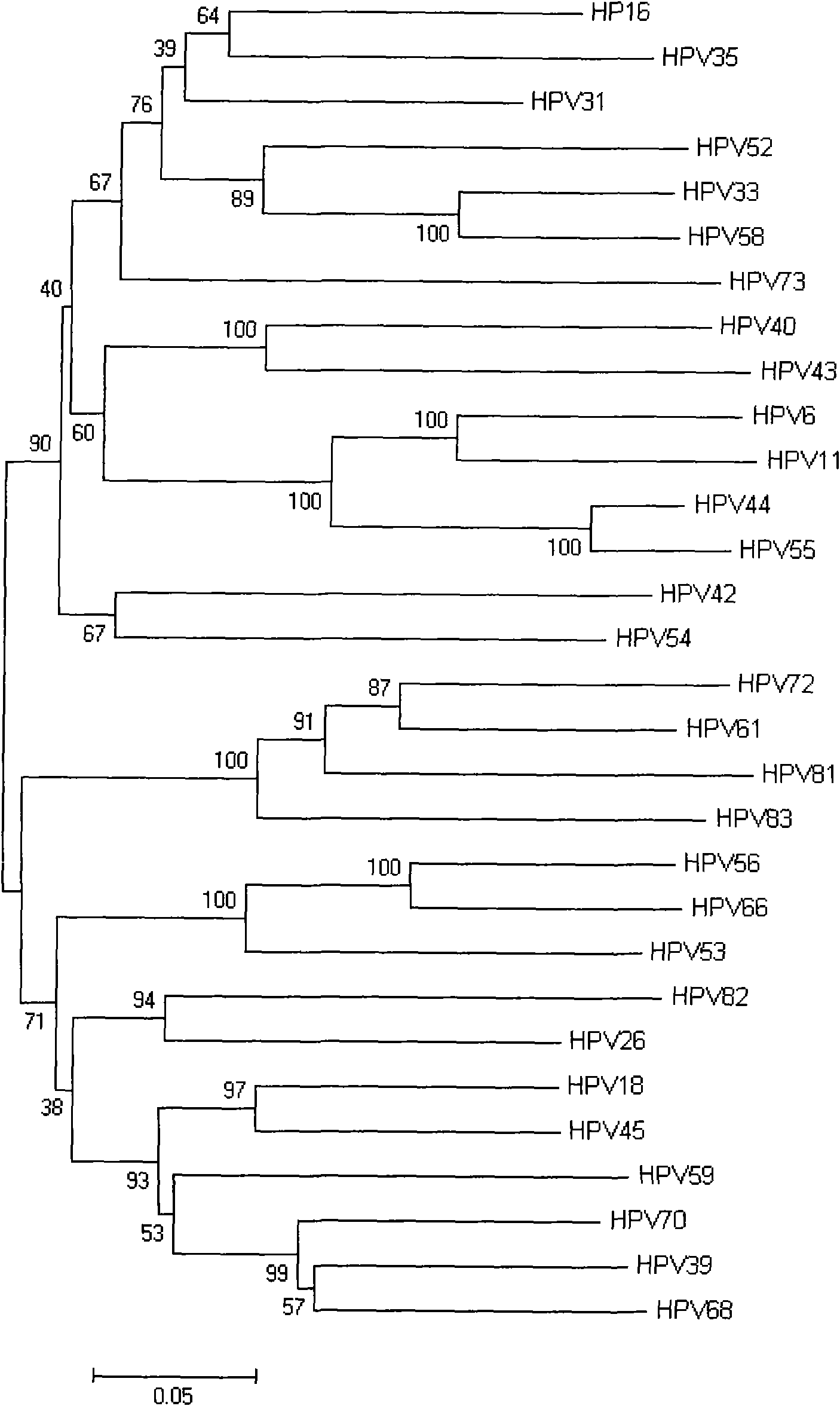
Human papillomavirus HPV DNA fragment, specific primer and application thereof
InactiveCN101851630ASimple methodMature technologyMicrobiological testing/measurementMicroorganism based processesDiseaseHuman papillomavirus DNA
The invention provides a human papillomavirus HPV DNA fragment, a mix primer for human papillomavirus HPV DNA detection and / or parting and application thereof. The mixed primer can specifically amplify to obtain one or a plurality of DNA sequences from HPV6, HPV 11, HPV 16, HPV 18, HPV 26, HPV 31, HPV 33, HPV 35, HPV 39, HPV 40, HPV 42, HPV 43, HPV 44, HPV45, HPV 51, HPV 52, HPV 53, HPV 54, HPV 55, HPV 56, HPV 58, HPV 59, HPV 61, HPV 66, HPV 68, HPV 70, HPV 72, HPV 73, HPV 81, HPV 82 and HPV 83, can be used for the human papillomavirus HPV DNA detection and / or parting and prepared into a PCR kit. The invention can detect common types of HPV at a time, greatly improve the HPV detection efficiency, overcome the defect of missed detection of latent infection by a serology and IHC detection method, realize early diagnosis of HPV diseases, and is suitable for clinical HPV gene detection and parting and guides prevention and cure of cervical carcinoma.
Owner:白向阳 +1
Methods and compounds for detection of molecular targets
ActiveUS8435735B2Faster and more sensitive and precise detectionEasy to detectSugar derivativesMicrobiological testing/measurementIn situ hybridisationChemical compound
The present invention relates to methods and compounds for detection of molecular targets, such as biological or chemical molecules, or molecular structures, in samples using a host of experimental schemes for detecting and visualizing such targets, e.g. immunohistochemistry (IHC), in situ hybridization (ISH), ELISA, Southern, Northern, and Western blotting, etc.
Owner:AGILENT TECH INC
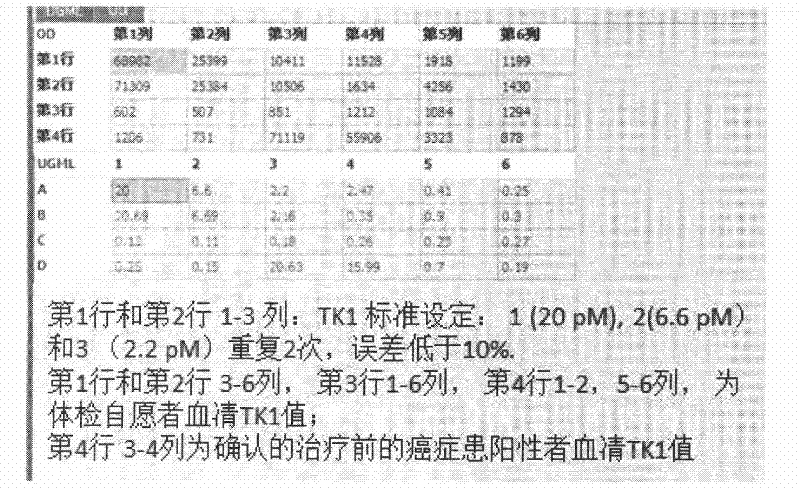
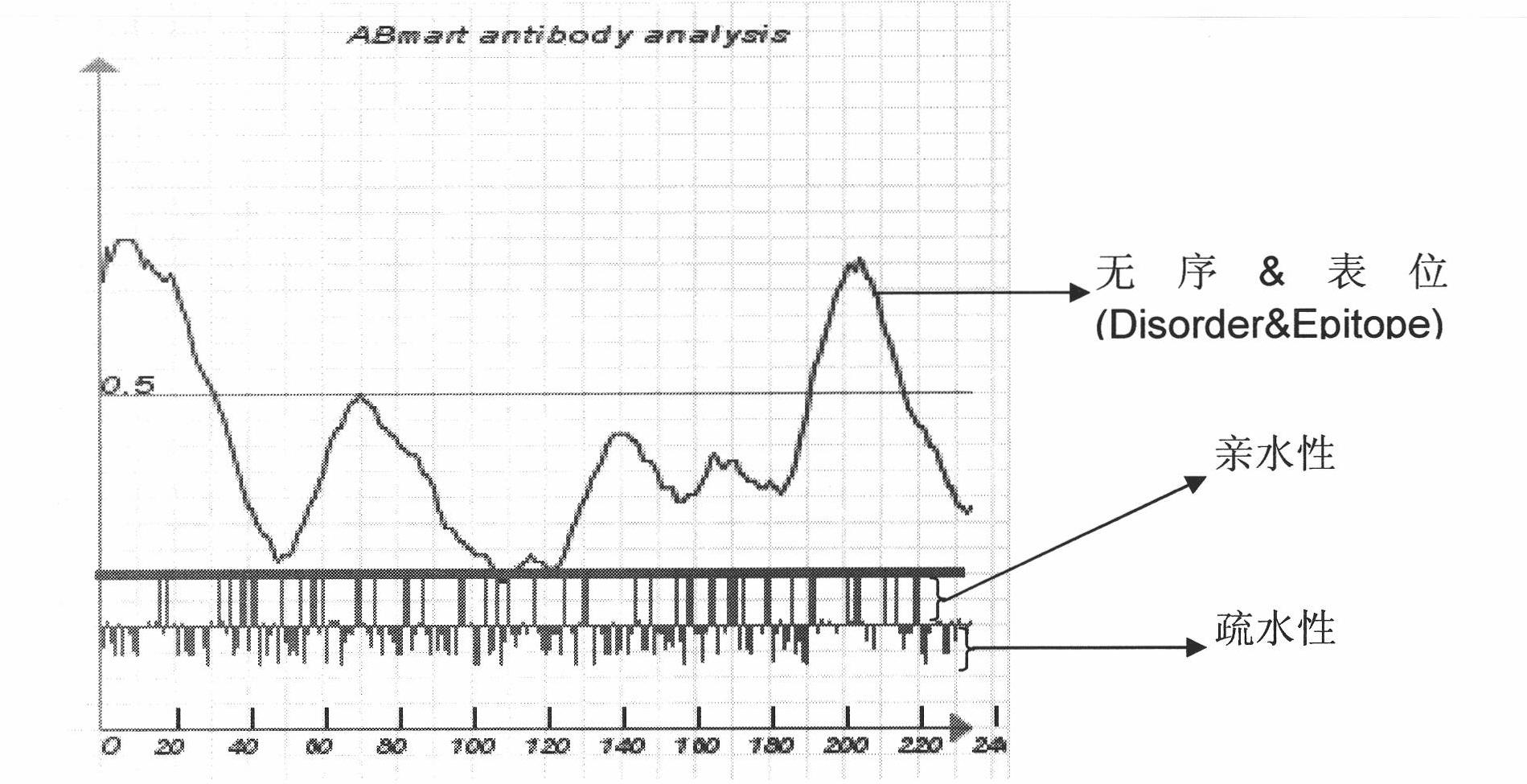
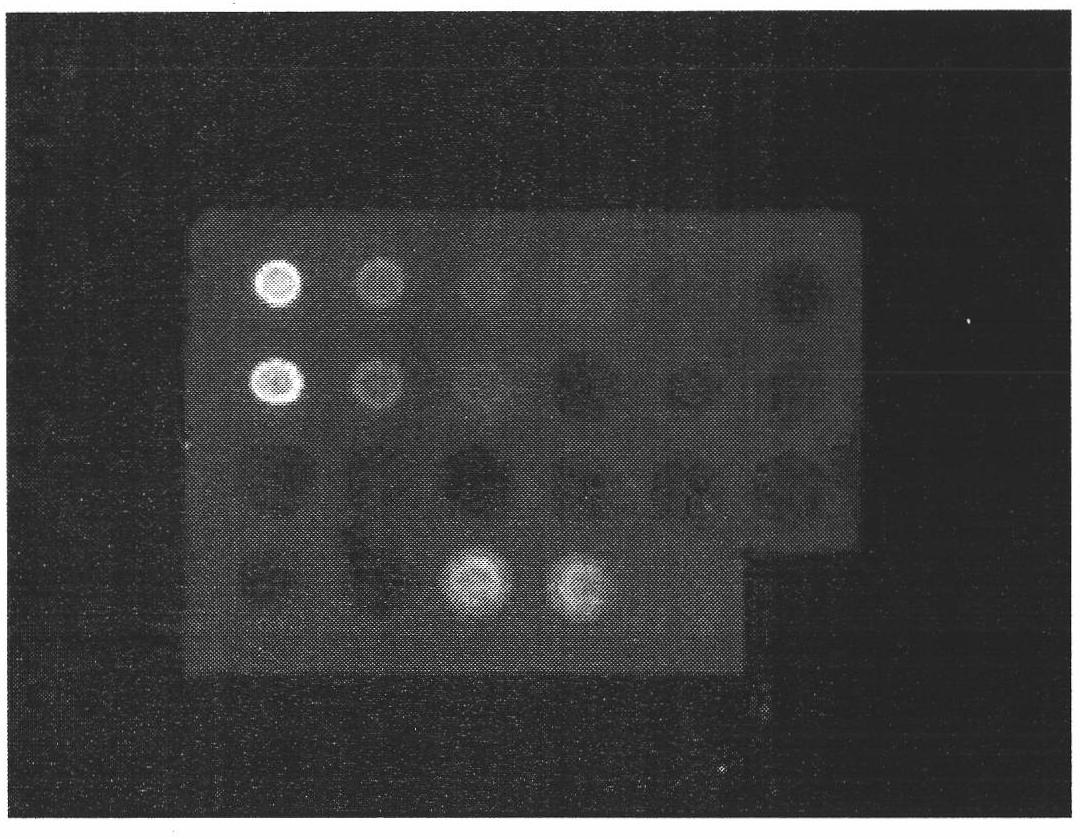
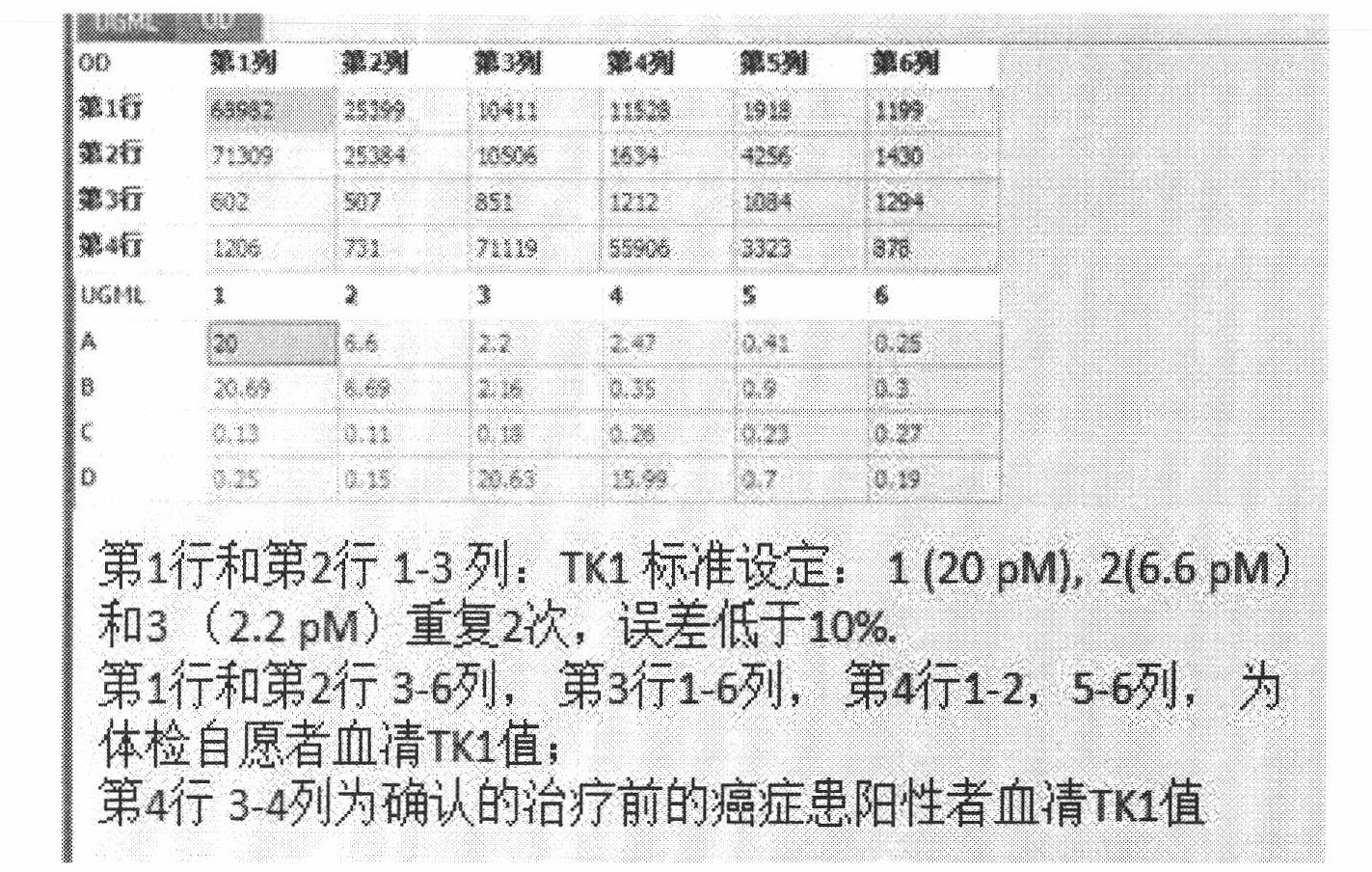
Preparation of multi-epitope TK-1 antibody, and application of multi-epitope TK-1 antibody in evaluating treatment effect on tumor patient
ActiveCN102516390AHigh purityIncrease productionEgg immunoglobulinsTransferasesAntigenTreatment effect
The invention provides a high-specificity high-sensitivity coordinated compound anti-human-TK1 antibody prepared from an antigenic determinant composed of human cervical cancer cell TK1 monomer N-terminal 23 peptide, C-terminal 20 peptide and C-terminal 28 peptide, and application thereof in tumor diagnosis. The antigenic determinant contains the following amino acid sequences: N-terminal 23 peptide (3-25): CINLPTVLPGSPSKTRGQIQVIL, C-terminal 20 peptide (206-225) CPVPGKPGEAVAARKLFAPQ, and C-terminal 28 peptide (198-225) AGPDNKENCPVPGKPGEAVAARKLFAPQ. The invention also relates to a method for preparing the antibody prepared from the antigen. The antibody kit provided by the invention has the characteristics of high sensitivity, high specificity, low cost and the like. The treatment effect on the tumor patient is evaluated by an enhanced chemiluminescent point blotting detection method, immunohistochemical detection and the detection kit.
Owner:SHENZHEN HUARUI TONGKANG BIOTECHNOLOGICAL
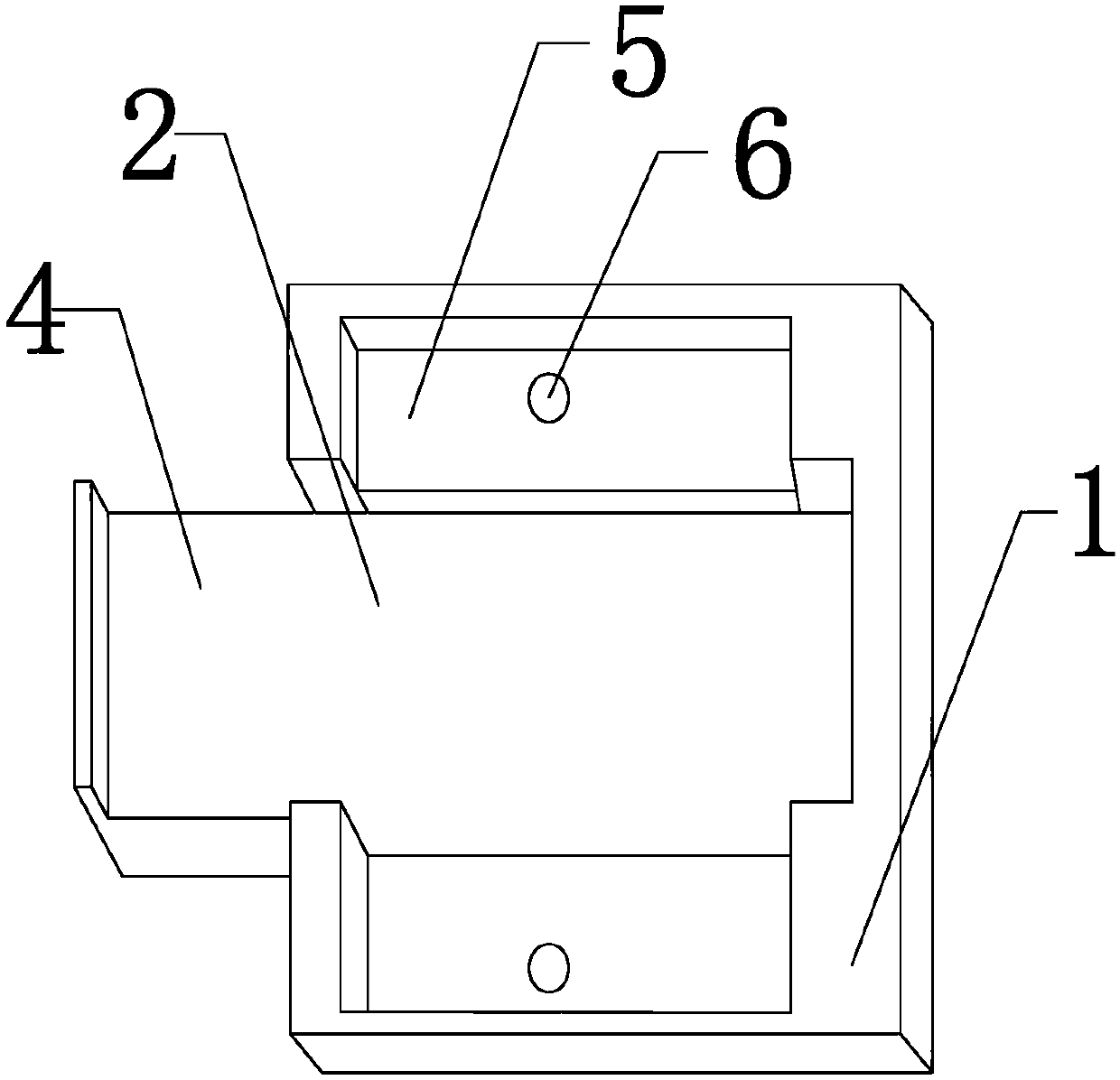
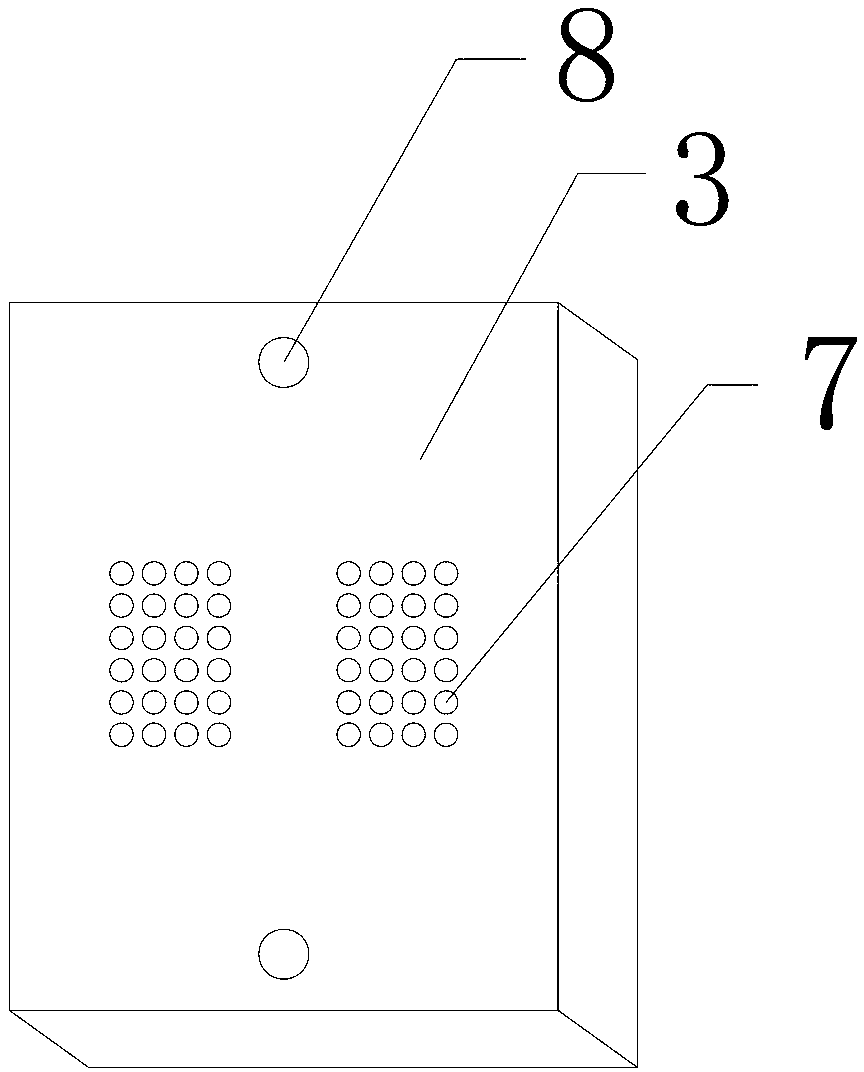
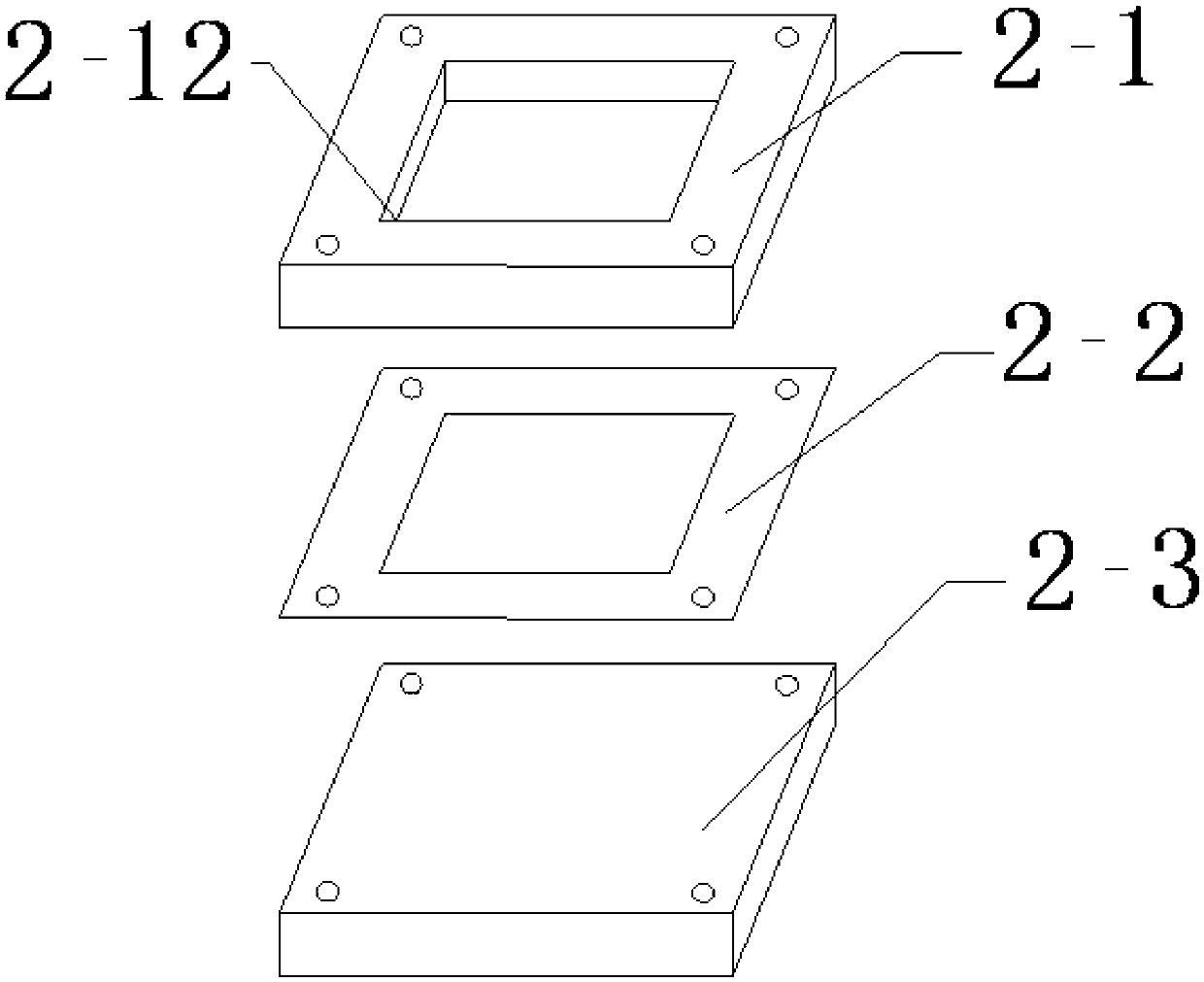
High-throughput immunohistochemical detection method and multi-sample immunohistochemical detection board
InactiveCN103344760AImprove the status quo of the extremely low R&D success rateMaterial analysisTransverse grooveHigh flux
The invention discloses a multi-sample immunohistochemical detection board, and also discloses a high-throughput immunohistochemical detection method with the detection board. The method comprises the following steps of: (1) preparing a tissue section to be detected; (2) flatly putting the tissue section to be detected in a step (1) in a transverse groove (2) after dewaxing, hydrating, carrying out antigen retrieval, and carrying out closed treatment; (3) putting a perforated plate (3) in a longitudinal groove (5) to be fixed, wherein one surface covered with a flexible waterproof lining puts down; (4) adding various antibody solutions to be detected to different through holes (7) for incubation; (5) carrying out color development on the tissue section to be detected, sealing the tissue section, and observing. The invention also discloses a mold for preparing a cell tissue block. The high-throughput immunohistochemical detection method can simultaneously carry out the detection on various antibodies on a same slide and has a good application prospect.
Owner:成都安铂奥金生物科技有限公司
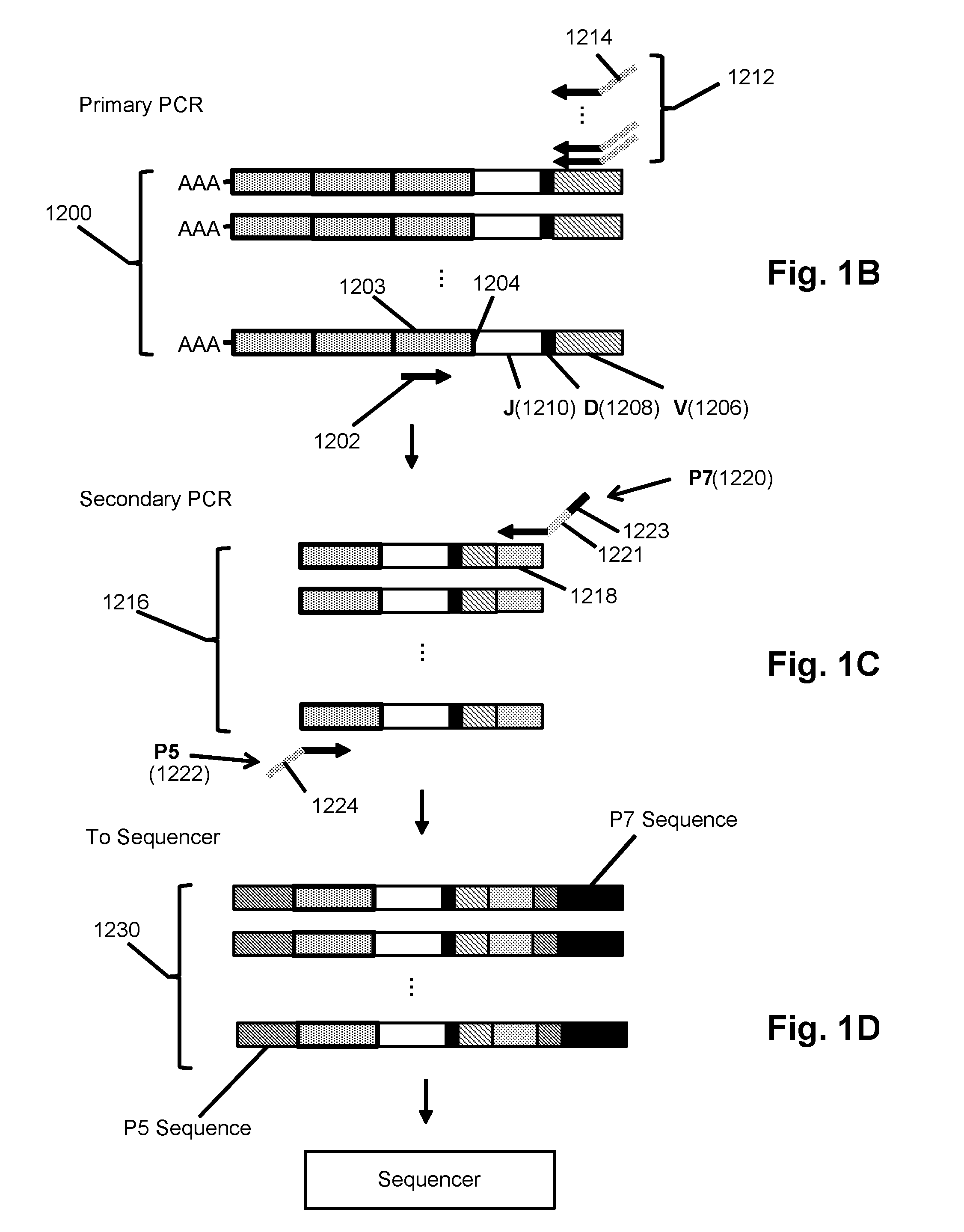
Primers for immune repertoire profiling
ActiveUS20210355484A1Immunoglobulin superfamilyMicrobiological testing/measurementSingle cell transcriptomeNucleotide
Disclosed herein include systems, methods, compositions, and kits for immune repertoire profiling. There are provided, in some embodiments, primer panels enabling the determination of the nucleotide sequence of the complete variable region of nucleic acids encoding mouse B cell receptor (BCR) and T cell receptor (TCR) polypeptides. In some embodiments, the method comprises single cell transcriptomic analysis.
Owner:BECTON DICKINSON & CO
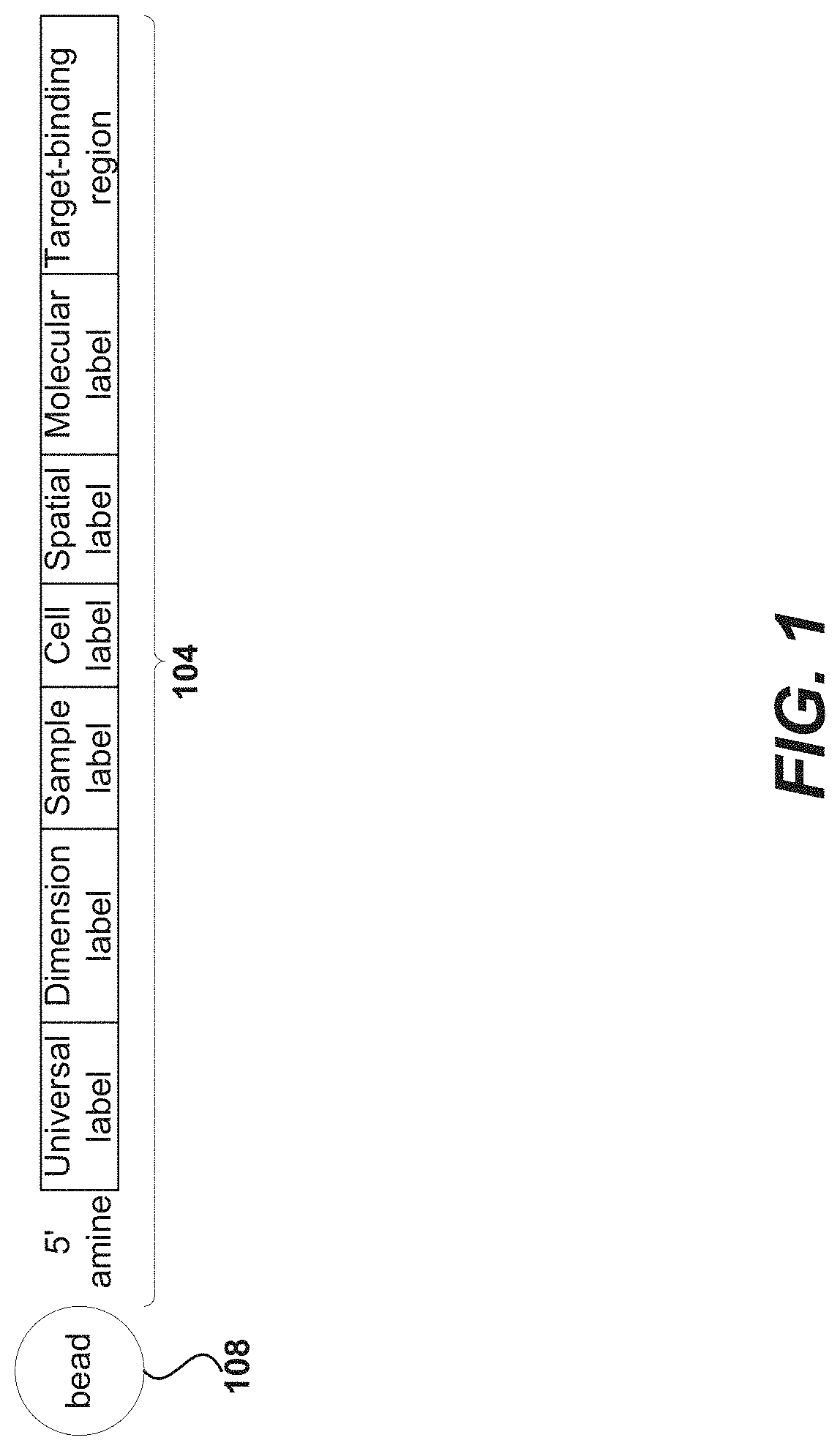
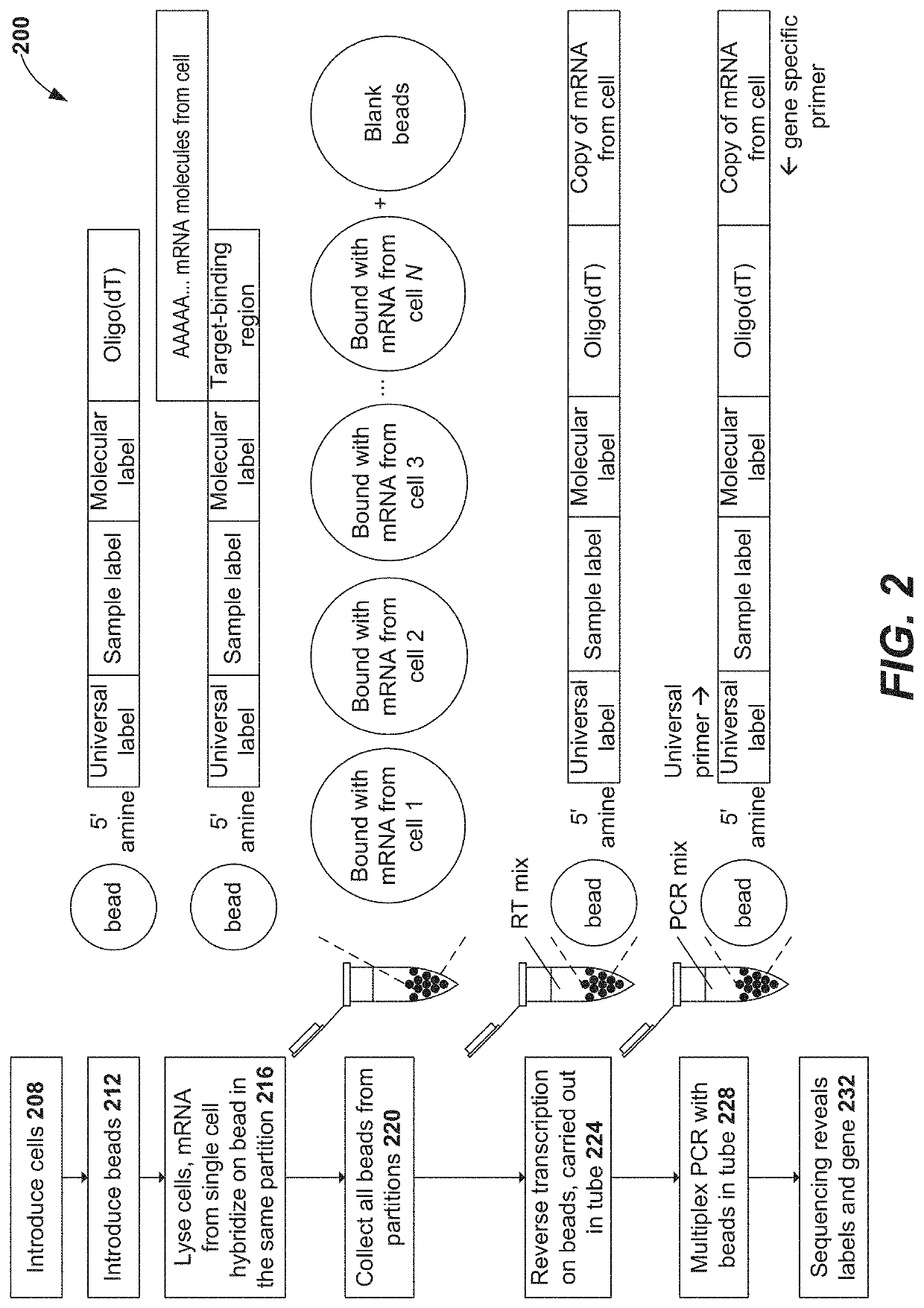
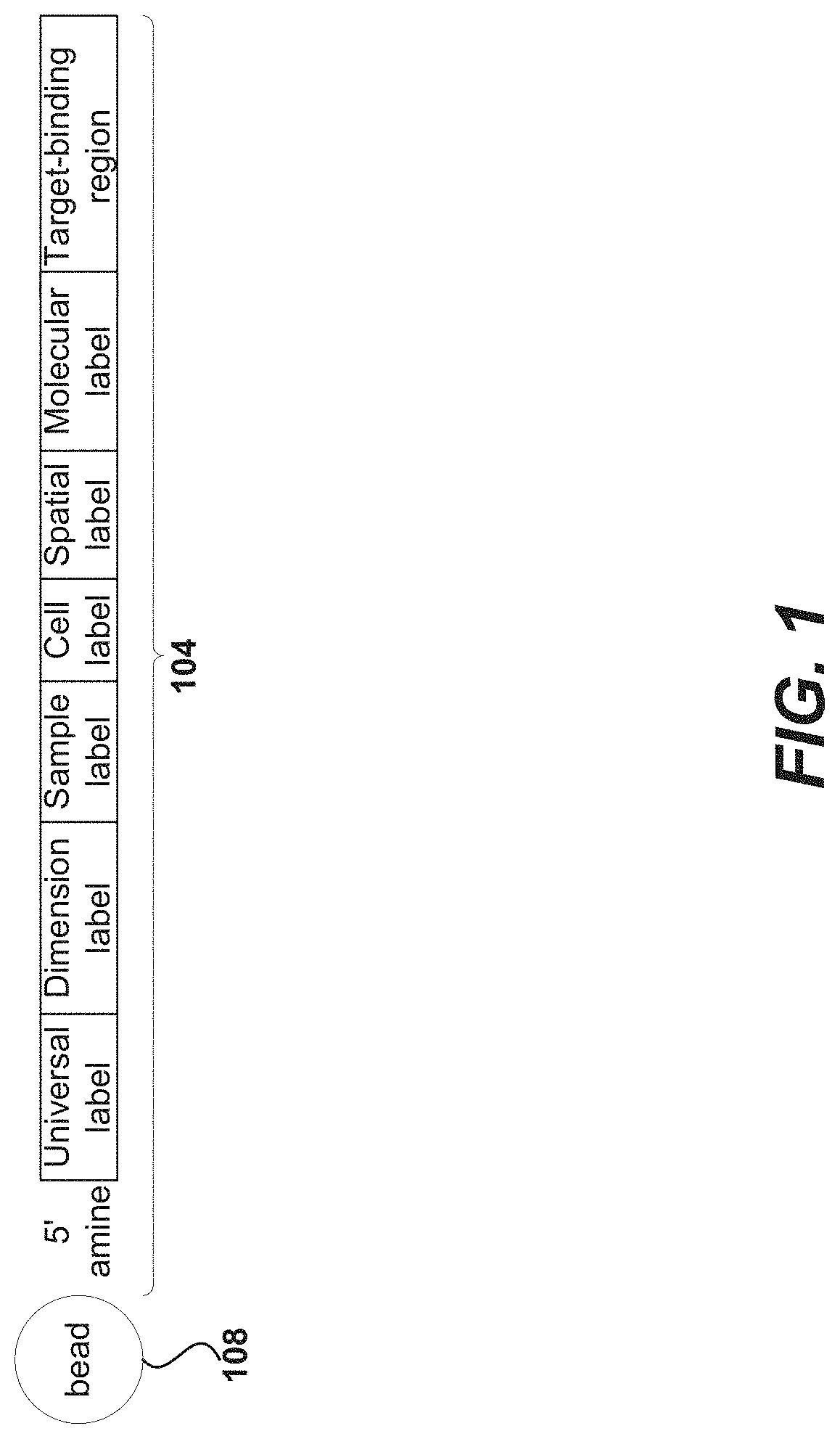
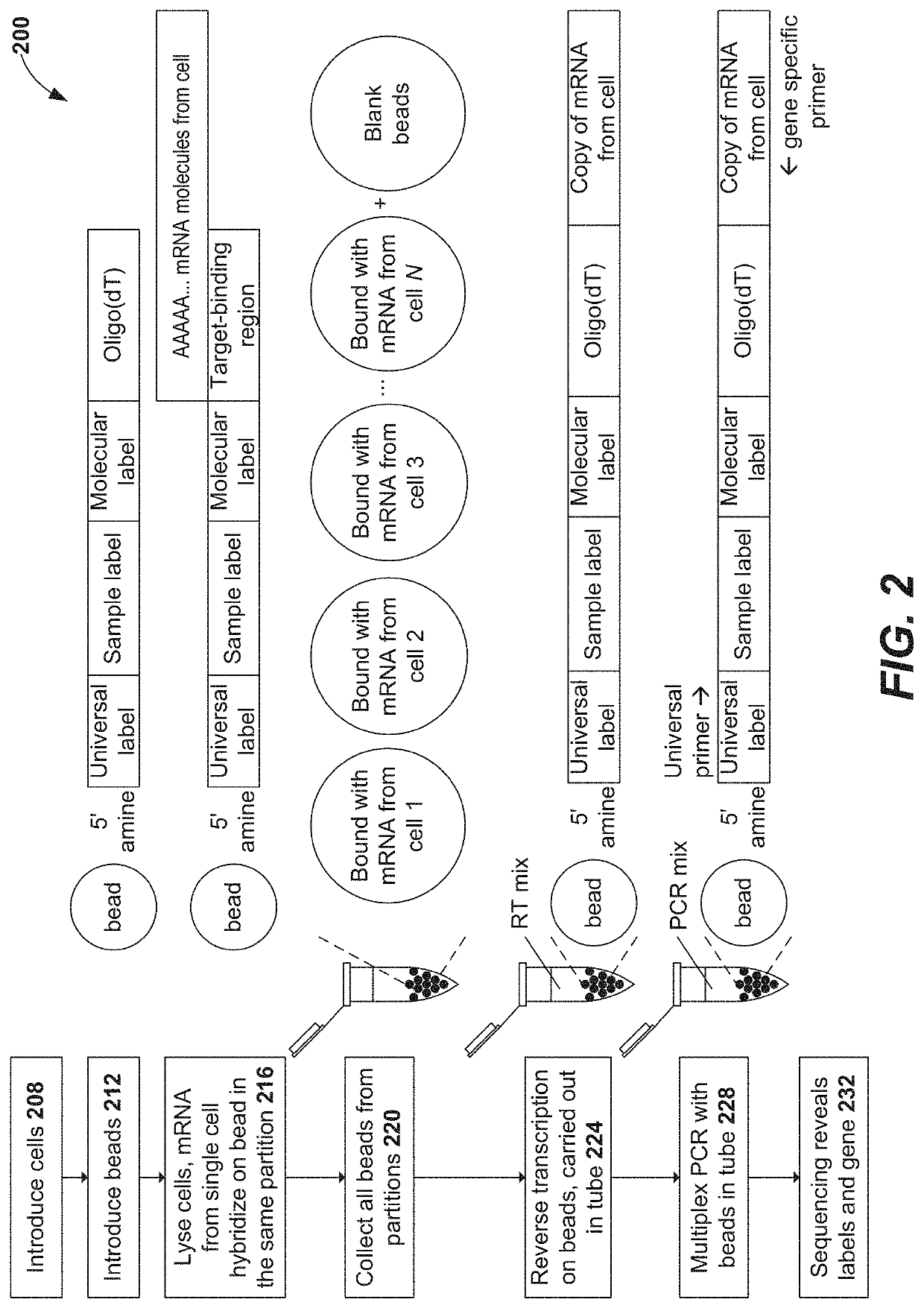
Oligonucleotides and beads for 5 prime gene expression assay
Disclosed herein include systems, methods, compositions, and kits for 5′-based gene expression profiling. Some embodiments provide synthetic particles (e.g., beads) associated with a first plurality of oligonucleotide barcodes and a second plurality of oligonucleotide barcodes. In some embodiments, nucleic acid targets (e.g., mRNAs) are initially barcoded on the 3′ end with the first plurality of oligonucleotide barcodes and subsequently barcoded on the 5′ end following a template switching reaction and intermolecular hybridization with the first plurality of oligonucleotide barcodes and extension. Immune repertoire profiling methods are also provided in some embodiments.
Owner:BECTON DICKINSON & CO
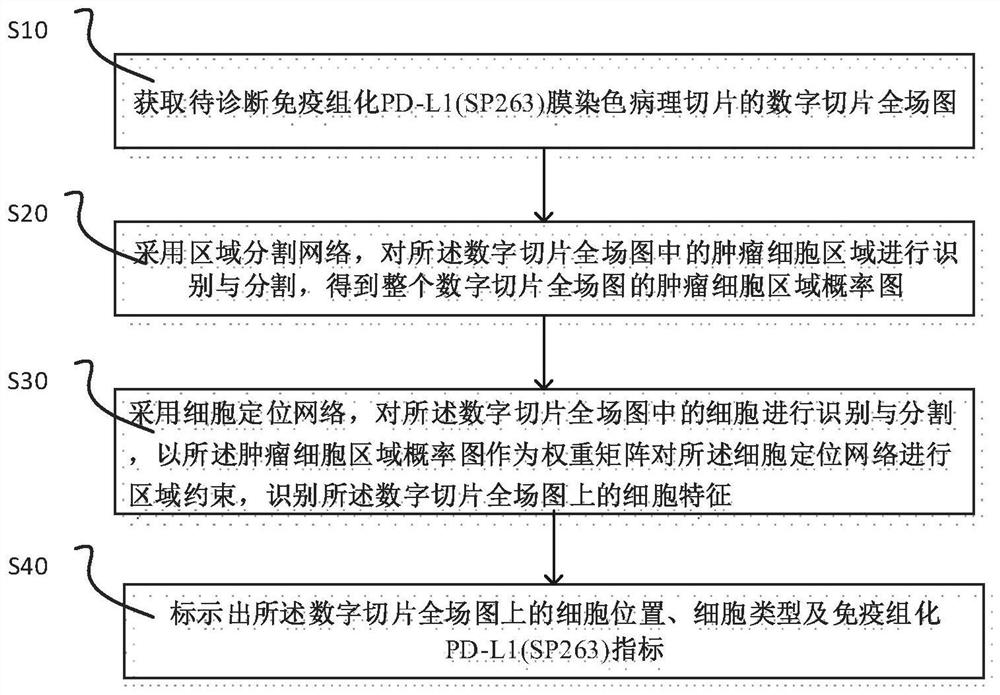
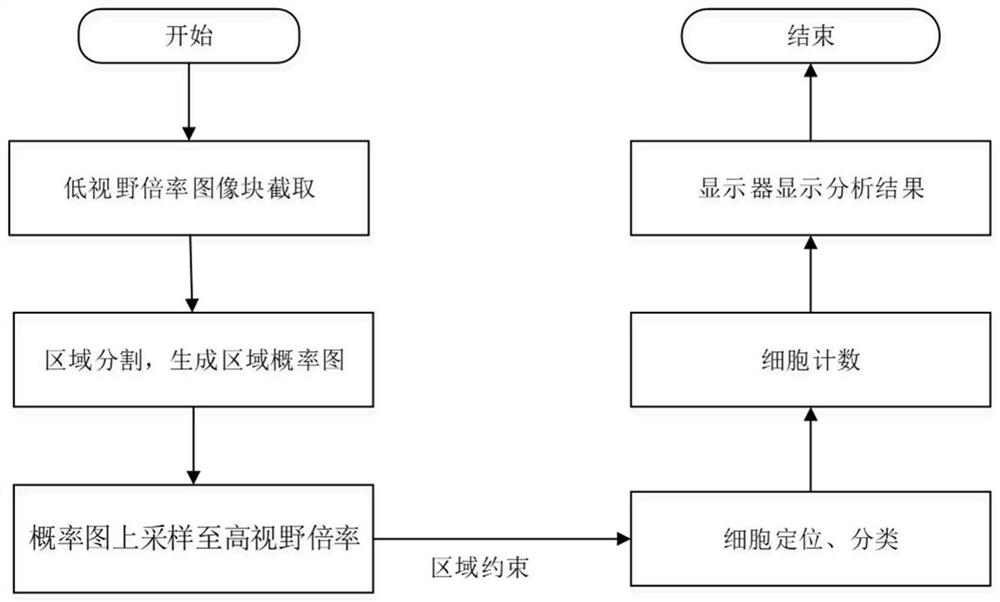
Immunohistochemical PD-L1 membrane staining pathological section image processing method, device and equipment
The invention relates to an immunohistochemical PD-L1 membrane staining pathological section image processing method, device and equipment. The image processing method comprises the following steps: acquiring a digital section full-field image of a to-be-diagnosed immunohistochemical PD-L1 (SP263) membrane staining pathological section; adopting a region segmentation network to identify and segment a tumor cell region in the digital slice full-field image under the first visual field multiplying power to obtain a tumor cell region probability graph of the whole digital slice full-field image;identifying and segmenting cells in each digital slice full-field graph, taking the tumor cell region probability graph as a weight matrix to carry out region constraint on the cell positioning network, identifying cell characteristics on the digital slice full-field graph, and positioning and classifying various cells on the digital slice full-field graph; and marking the cell position, the celltype and the immunohistochemical PD-L1 (SP263) index on the full-field diagram of the digital slice. By designing a multi-level feature collaborative diagnosis strategy, the tumor proportion score isaccurately evaluated in a mode of constraining cell features by using regional features.
Owner:杭州迪英加科技有限公司 +1
Anti-silk fibroin polyclonal antibody and preparation method thereof
InactiveCN104059131AStrong specificityHigh puritySerum immunoglobulinsImmunoglobulins against animals/humansPolyclonal antibodiesBioinformatics
The invention relates to the technical field of biology and in particular discloses an anti-silk fibroin polyclonal antibody and a preparation method thereof. The anti-silk fibroin polyclonal antibody is produced by an animal under the condtion that polypeptide with an amino acid sequence shown in SEQ ID NO:1 is taken as an immunogen. The anti-silk fibroin polyclonal antibody has the characteristics of good specificity and high purity, can be specifically conjugated with silk fibroin and is widely applicable to immunohistochemistry, immunoblotting and in vitro immunoassay. The preparation method of the anti-silk fibroin polyclonal antibody is simple in operation and is applicable to preparation of a large quantity of anti-silk fibroin polyclonal antibodies.
Owner:SILK PLUG BEIJING BIOMEDICINE TECH
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Immunohistochemical membrane staining section diagnosis method and device
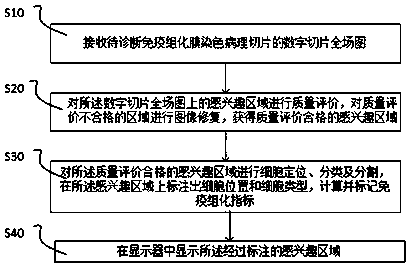
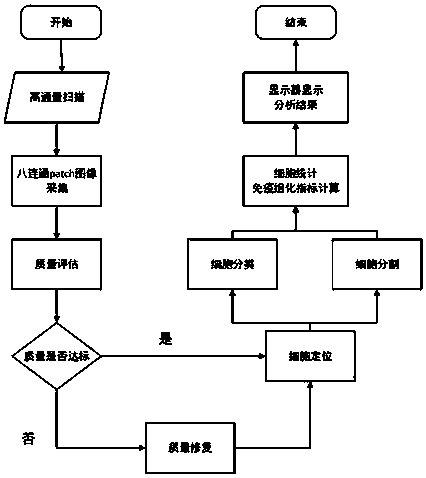
PendingCN110853005AGuaranteed accuracyImprove robustnessImage enhancementImage analysisFull fieldImage restoration
The invention relates to an immunohistochemical membrane staining section diagnosis method. The method comprises the following steps: S10, receiving a digital section full-field graph of a to-be-diagnosed immunohistochemical membrane staining pathological section; S20, performing quality evaluation on the region of interest on the digital slice full-field image, and performing image restoration onthe region with unqualified quality evaluation to obtain a region of interest with qualified quality evaluation; S30, carrying out cell positioning, classification and segmentation on the regions ofinterest with qualified quality evaluation, marking cell positions and cell types on the regions of interest, and calculating and marking immunohistochemical indexes; and S40, displaying the marked region of interest. The invention further relates to an immunohistochemical membrane staining section diagnosis device and a computer readable medium. Since the context information of the lesion area isof great significance to interpretation of the immunohistochemical membrane staining section, the accuracy of interpretation is ensured by collecting the eight-connected patch image of the immunohistochemical membrane staining pathological section with time sequence for training and testing.
Owner:杭州迪英加科技有限公司
Preparation of multi-epitope thymidine kinase 1 (TK1) antibody and use of multi-epitope TK1 antibody for early tumor detection and risk early warning in mass physical examination screening
The invention provides a high-specificity and high-sensitivity coordinated antibody against human thymidine kinase 1 (TK1) prepared from an antigenic determinant consisting of 23 peptides at an N terminal, 20 peptides at a C terminal and 28 peptides at the C terminal of the TK1 monomer from a human hela cell and the use of the detection and diagnosis system of the antibody in tumor diagnosis. The antigenic determinant comprises the following amino acid sequences: the sequence of the 23 peptides (3-25) at the N terminal:CINLPTVLPGSPSKTRGQIQVIL; the sequence of the 20 peptides(206-225) at the C terminal: CPVPGKPGEAVAARKLFAPQ; and the sequence of the 28 peptides (198-225) at the C terminal: AGPDNKENCPVPGKPGEAVAARKLFAPQ. The invention also provides a method for preparing the antibody by using the antigen. An antibody kit provided by the invention has the characteristics of high sensitivity, high specificity, low cost and the like; and the early tumor can be detected and pre-warned in mass physical examination screening by enhanced chemiluminescence dot blot assay, immuno-histochemistry and the detection kit.
Owner:SHENZHEN HUARUI TONGKANG BIOTECHNOLOGICAL
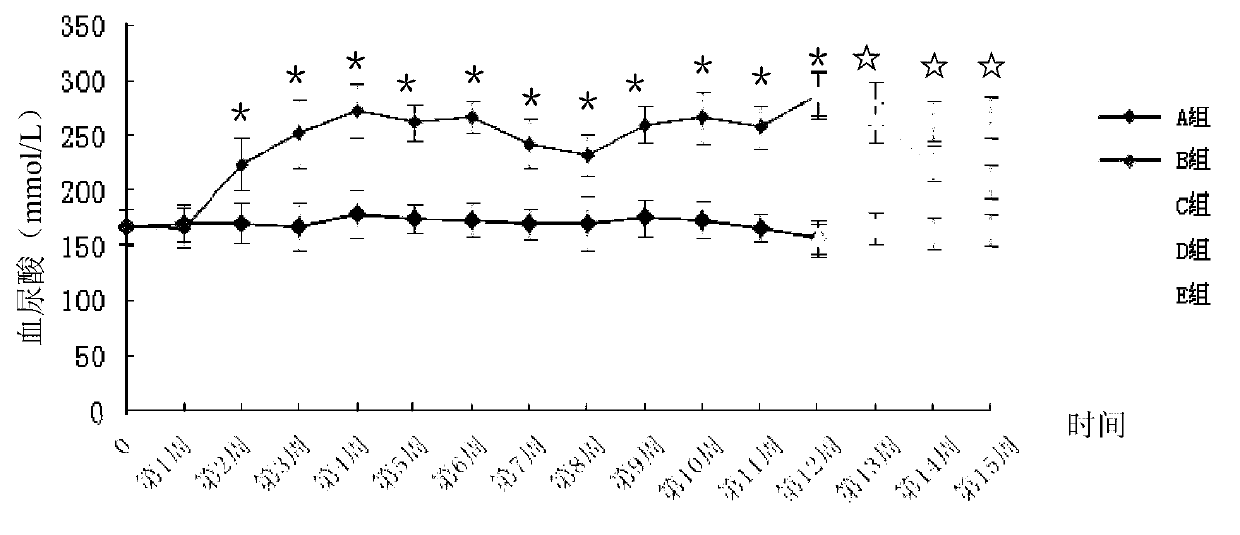
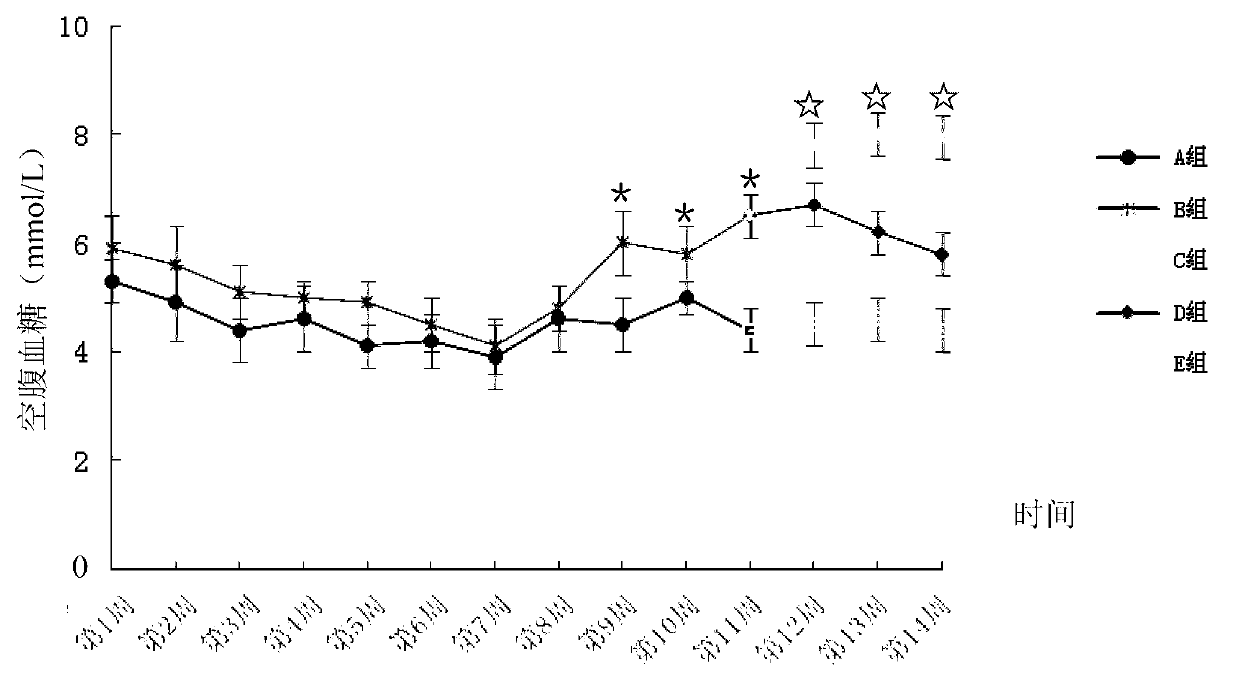
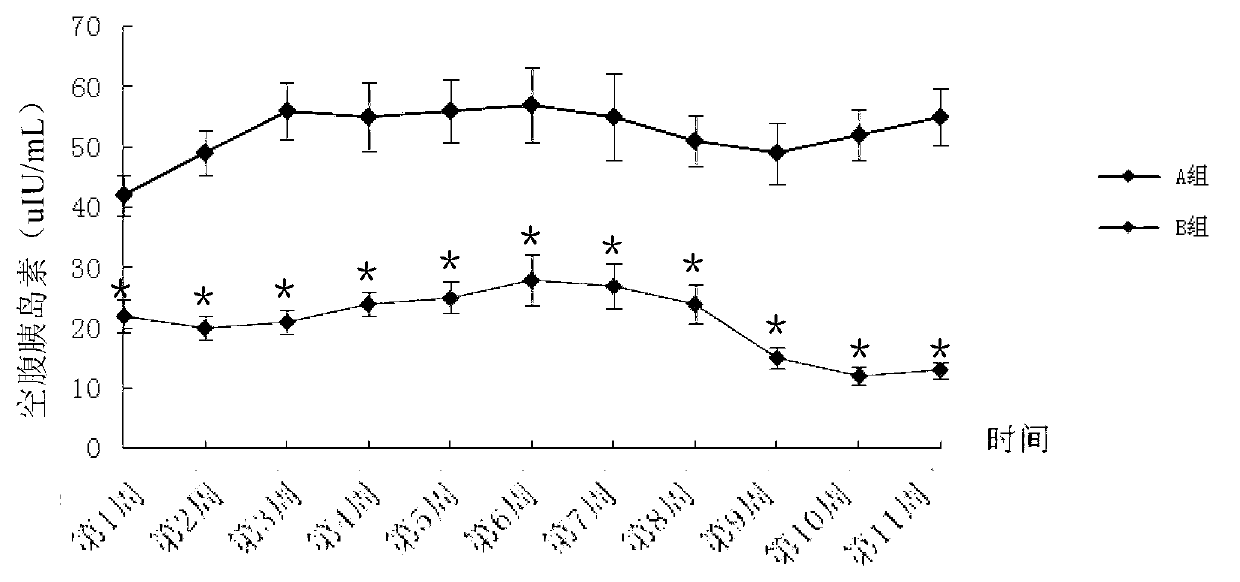
Method for building animal model with hyperuricemia-combined diabetes
The invention discloses a method for building an animal model with hyperuricemia-combined diabetes. The method comprises the following steps: 1) feeding an target animal with fodder containing 10% (by mass) of yeast powder; subjecting the target animal to a lavage with a 4% (by mass) adenine water solution according to the following dosage of 100 milligram of adenine per day per kilogram of body weight until the blood uric acid level of the target animal starts falling; 2) halving the dosage of the adenine water solution in step 1), subjecting the target animal to a subcutaneous injection with an oteracil potassium emulsion twice a day according to the following dosage of 100 milligram of oteracil potassium emulsion per day per kilogram of body weight until the target animal shows clinical symptoms of diabetes, and then obtaining the animal model with the hyperuricemia-combined diabetes. The method builds the animal model with the hyperuricemia-combined diabetes for the first time, the animal model has typical disease symptoms, the quantity index has statistical significance, and studies such as glucose clamp test and immunohistochemistry also prove that the modeling is successful. Therefore, the method for building the animal model with the hyperuricemia-combined diabetes is a reliable and practical technique.
Owner:李长贵
Method And Apparatus For Automated Rapid Immunohistochemistry
InactiveUS20080194034A1Reduce manufacturing costEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsGuidelineMicroscopic scale
A sample processing system that may be configured to achieve parallel or coincidental sample processing such as histochemical processing may involve a plurality of samples arranged for coincidental movement perhaps by use of angular microscopic slide movements to cause processing activity that may include repeated elimination and reapplication of a fluidic substance perhaps through the action of capillary motion in order to refresh a microenvironment adjacent to a sample such as a biopsy or other such sample. Snap in antibody and other substances may be included to ease operator actions and to permit location specific substance applications perhaps by including single container multiple chamber multiple fluidic substance magazines, linearly disposed multiple substance source, and primary antibody cartridges. Through refreshing of a microenvironment, depletion of the microenvironment is avoided and the time necessary for slide processing may be dramatically shortened from a more common 60 to 120 minutes to perhaps less than 15 minutes so as to permit use of such a system in an intraoperative or surgical environment such as recommended by the College of American Pathologists intraoperative guidelines or the like. Patients may thus avoid a need to be subjected to an additional surgical procedure when lab results become available to see if tumors or the like were fully removed in a prior procedure.
Owner:CELERUS DIAGNOSTICS
Viral particles as immunogens against enterovirus infection and production thereof
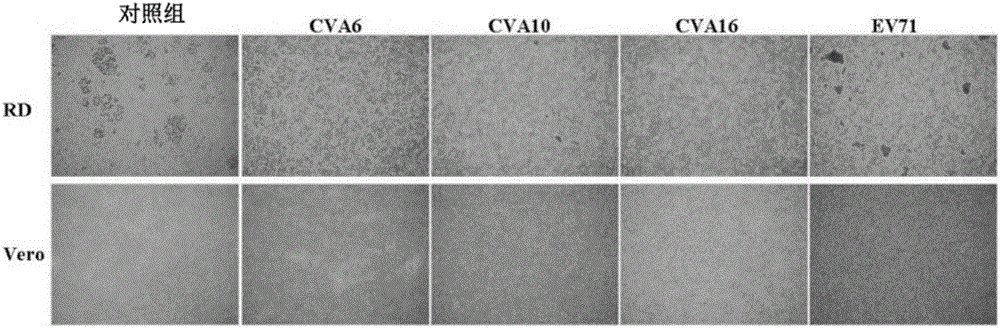
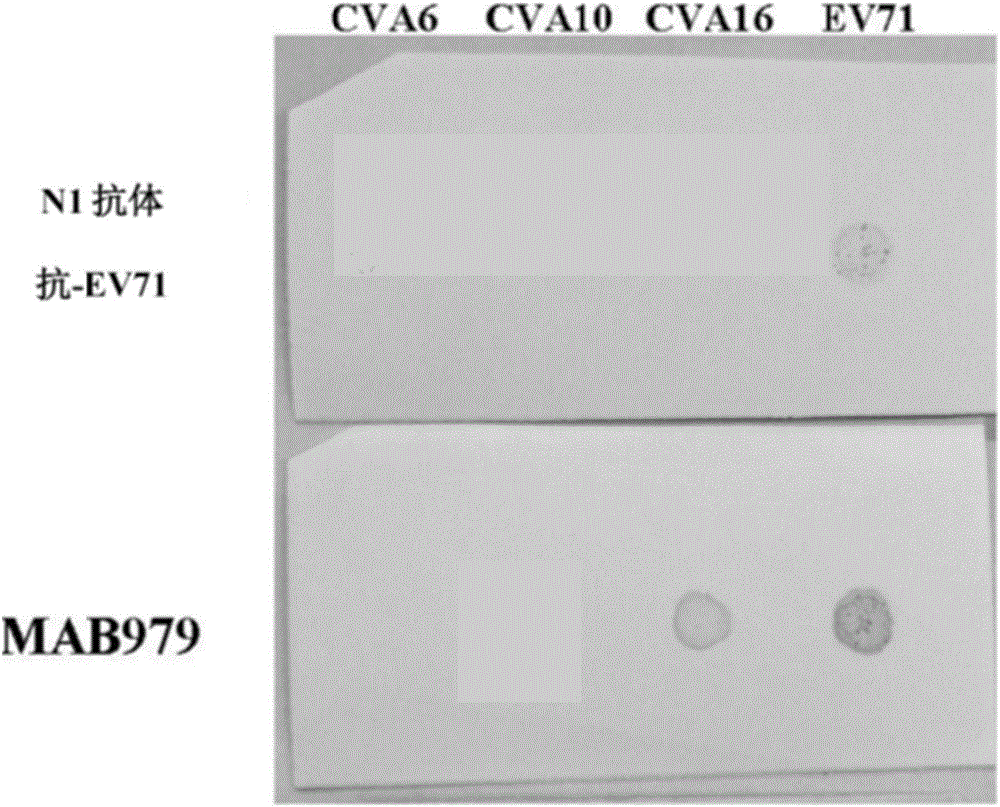
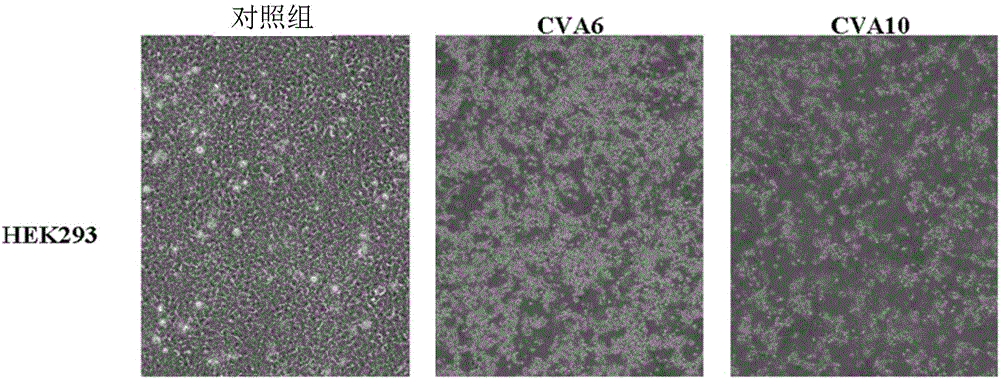
The present invention relates to viral particles as immunogens against enterovirus infection and a method of producing the same. Specifically, the present invention features that human embryo kidney 293 (HEK 293) cells are used to produce viral particles of Enterovirus A, particularly Coxsackievirus A6 (CVA6) particles or Coxsackievirus A10 (CVA10) particles or both and optionally additional viral particles of other Enterovirus A e.g. Coxsackievirus A16 (CVA16) and / or Enterovirus A71 (EV71). The yield of the viral particles in HEK 293 cells is unexpectedly high and effective to induce an immune response against enterovirus infection, especially CVA6 and CVA10. The present invention also relates to an immunogenic composition against enterovirus infection for human use comprising the viral particles as described herein and a method of preventing enterovirus infection or a disease as caused, particularly Hand-Foot-Mouth diseases (HFMD), by administering the immunogenic composition to a subject in need thereof.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
Compositions for immunising against Staphylococcus aureus
ActiveUS8632783B2Reduce and eliminate haemolytic activityAntibacterial agentsBacterial antigen ingredientsAntigenStaphylococcus aureus
Staphylococcus aureus Hla polypeptides having various deletions, insertions, and / or mutations which are useful for immunization are provided herein. Also provided herein are Hla heptamers which are non-haemolytic. Additionally, an effective Staphylococcus aureus vaccine may require several antigenic components, and so various combinations of S. aureus antigens, including Hla polypeptides having deletions, insertions, and / or mutations, are identified for use in immunization. These polypeptides may optionally be used in combination with S. aureus saccharides.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Immunohistochemical karyoplasm staining section diagnosis method and system
PendingCN110736748AReduce volumeEasy to carryImage enhancementImage analysisMicroscope cameraStaining
The invention relates to an immunohistochemical karyoplasm staining section diagnosis method. The method includes: S10, collecting a pathological image of the immunohistochemical karyoplasm staining section under the microscope by a microscope camera, and sending the same to a processor; S20, preprocessing the pathological image by the processor to enable HSV color space of the pathological imageto be consistent with set thresholds; S30, carrying out cell detection and cell classification on the pre-processed pathological image by the processor, labeling cell positions, cell types and corresponding immunohistochemical indexes on the pathological image, and outputting the labeled pathological image; and S40, receiving and displaying the labeled pathological images from the processor by a displayer. The application also provides an immunohistochemical karyoplasm staining section diagnosis system. By adopting the method, analysis can be realized without the need for storing a large number of GB-level digital pathological sections, and a storage overhead of the processor is saved.
Owner:杭州迪英加科技有限公司
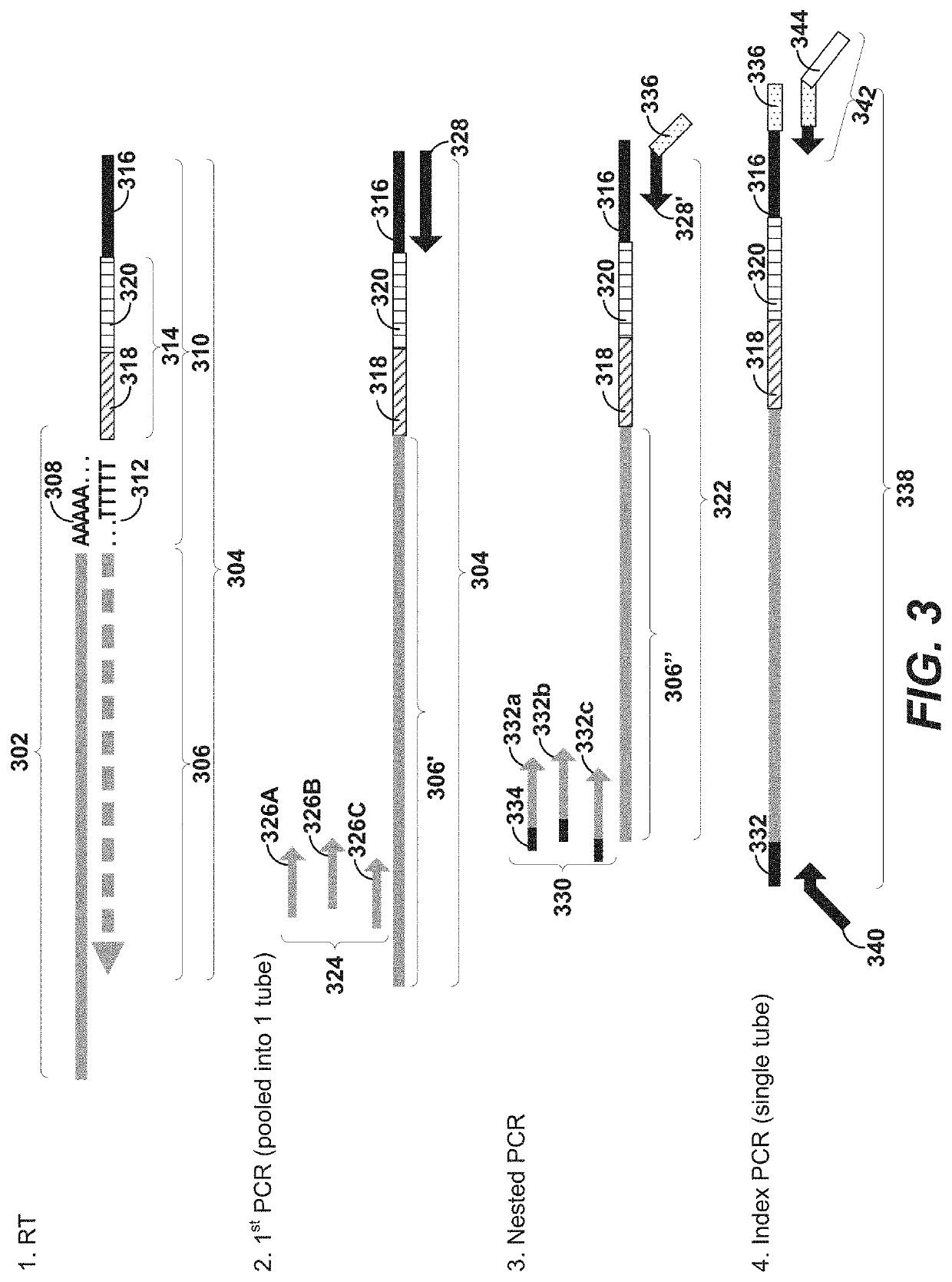
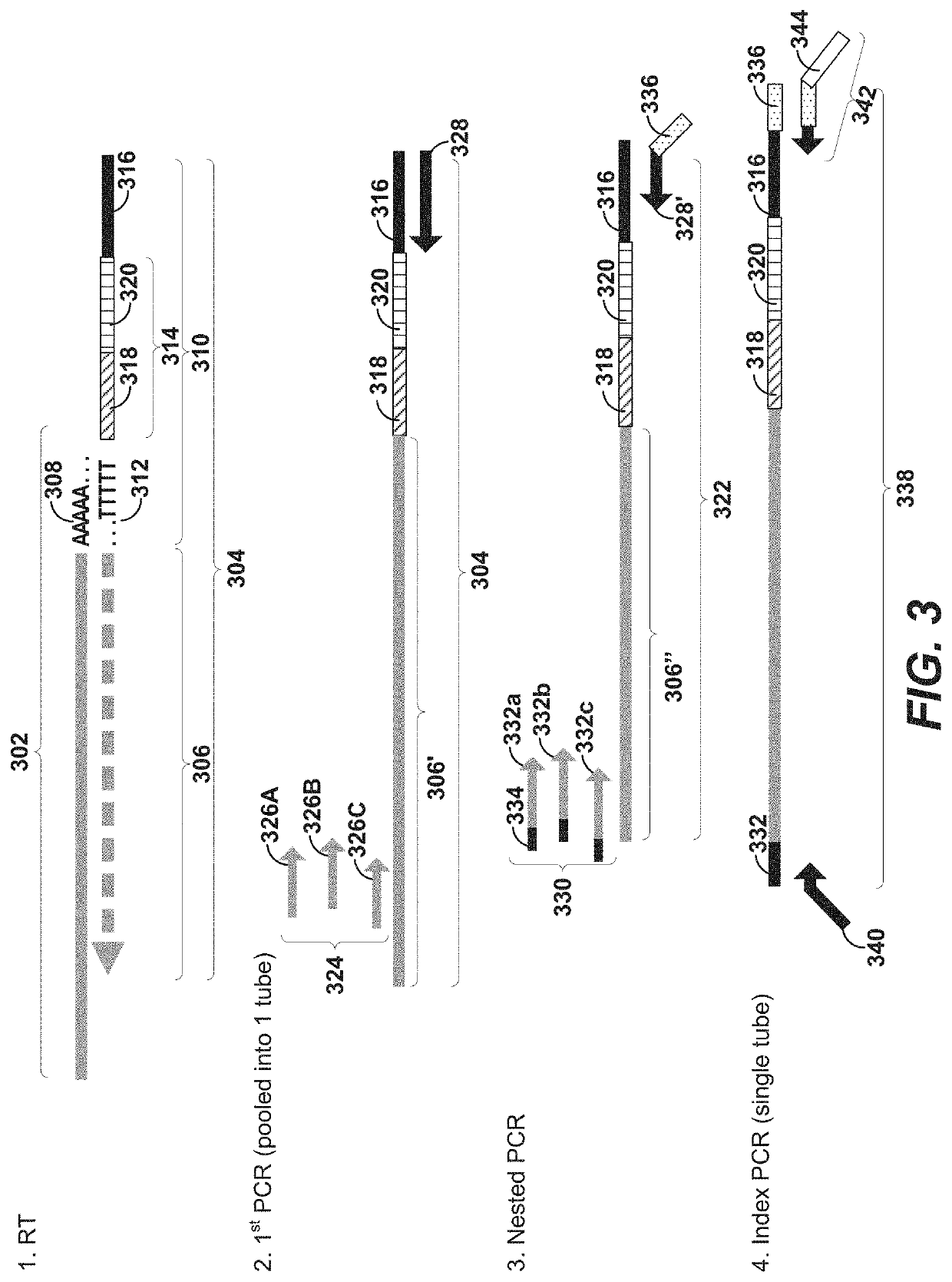
Using random priming to obtain full-length v(d)j information for immune repertoire sequencing
ActiveUS20210139970A1Microbiological testing/measurementDNA preparationGenomic cloneTemplate switching
Disclosed herein include systems, methods, compositions, and kits for labeling nucleic acid targets in a sample. In some embodiments, nucleic acid targets (e.g., mRNAs) are initially barcoded on the 3′ end and are subsequently barcoded on the 5′ end following a template switching reaction and intermolecular and / or intramolecular hybridization and extension. 5′- and / or 3′-barcoded nucleic acid targets can serve as templates for amplification reactions and / or random priming and extension reactions to generate a sequencing library. The method can comprise generating a full-length sequence of the nucleic acid target by aligning a plurality of sequencing reads. Immune repertoire profiling methods are also provided in some embodiments.
Owner:BECTON DICKINSON & CO
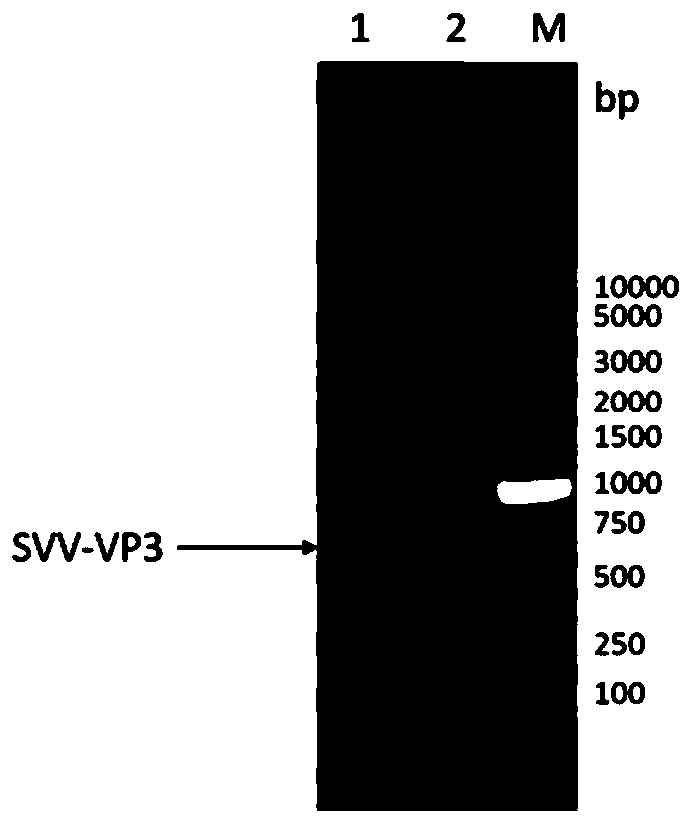
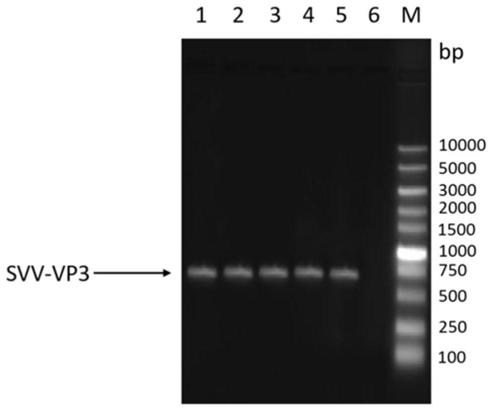
Novel genetic engineering vaccine of porcine Seneca virus as well as preparation method and application of novel genetic engineering vaccine
ActiveCN110279855AEasy to assembleGood antigenicitySsRNA viruses positive-senseVirus peptidesVaccine ProductionImmunogenicity
The invention discloses an immunological composition which comprises porcine Seneca virus structural protein VP3 and VP1 proteins, as well as porcine Seneca virus structural protein VP2 and / or VP4 protein. Further, the immunological composition can further comprise a porcine Seneca virus structural protein VP0. The immunological composition can be used for preparing a novel genetic engineering subunit vaccine of porcine Seneca virus, the antigenicity, immunogenicity and function of the vaccine are similar to those of natural proteins, the expression level is relatively high, the immunogenicity is strong, and no pathogenicity is caused to animals; the vaccine can be prepared by large-scale serum-free suspension culture in a bioreactor, thereby greatly reducing the cost of vaccine production.
Owner:苏州世诺生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com