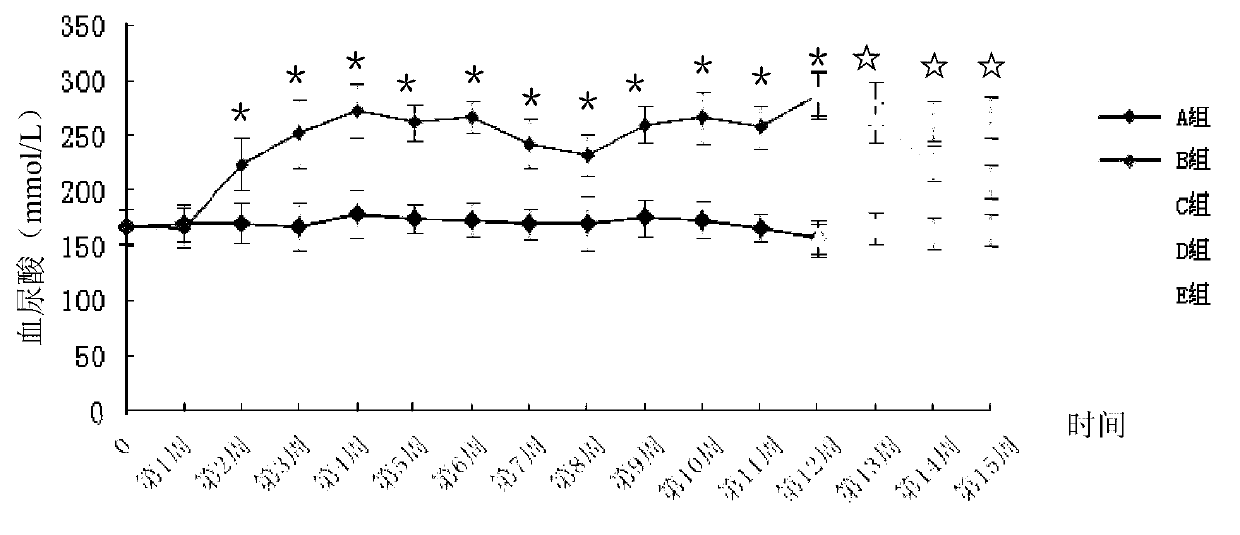
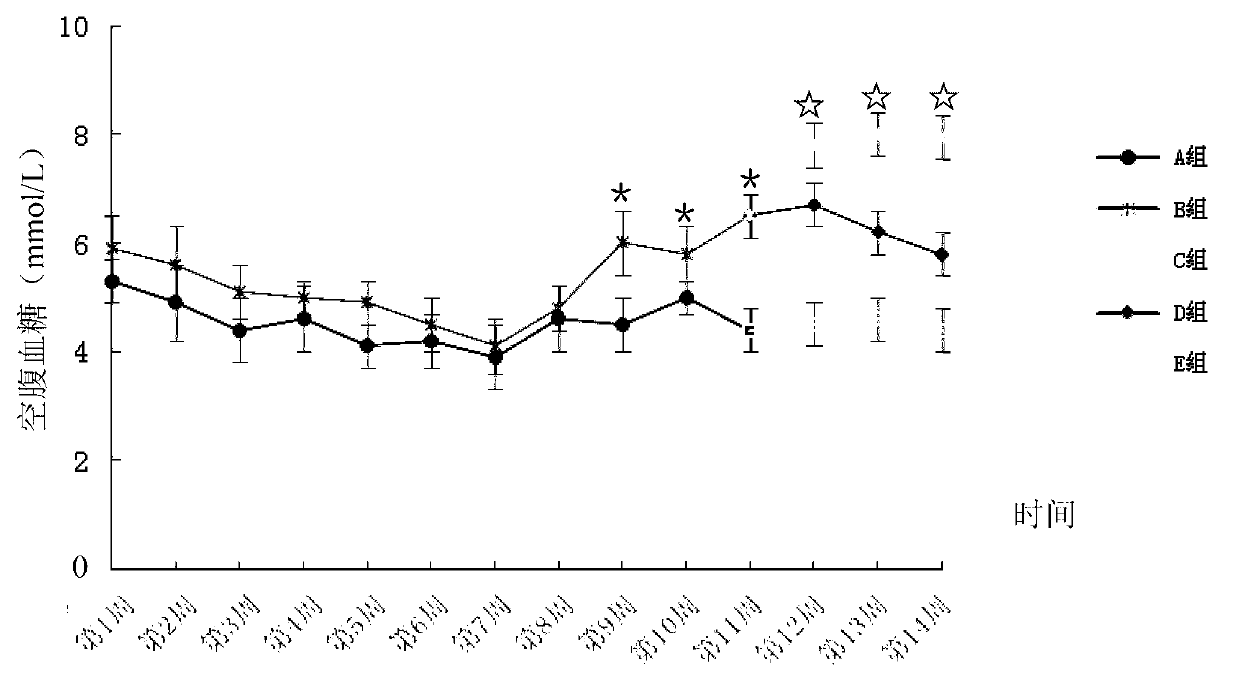
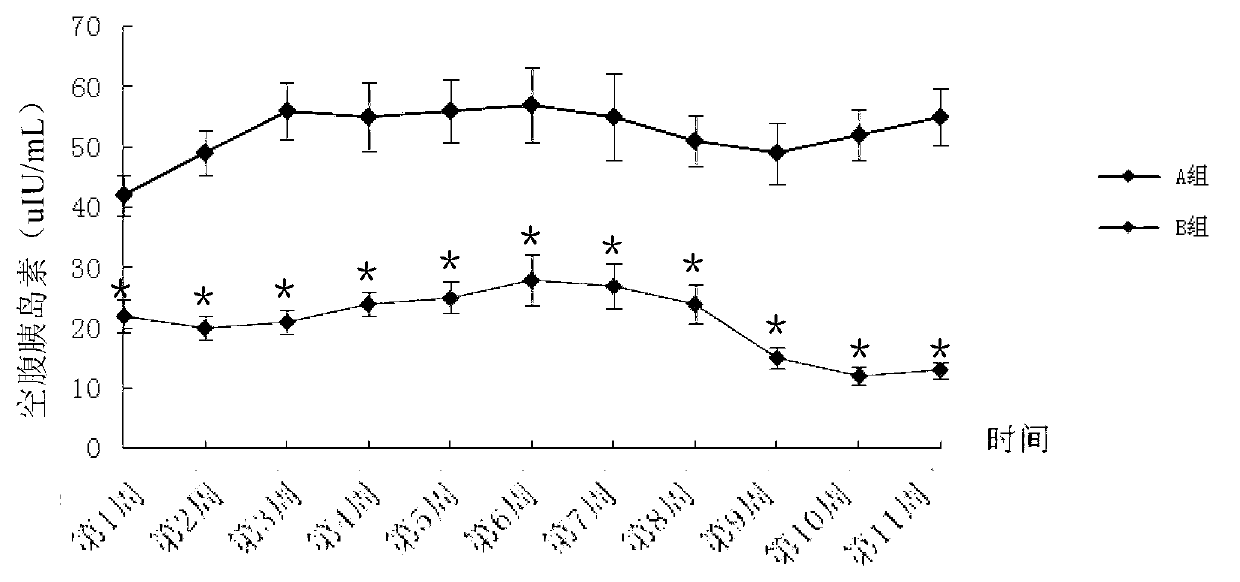
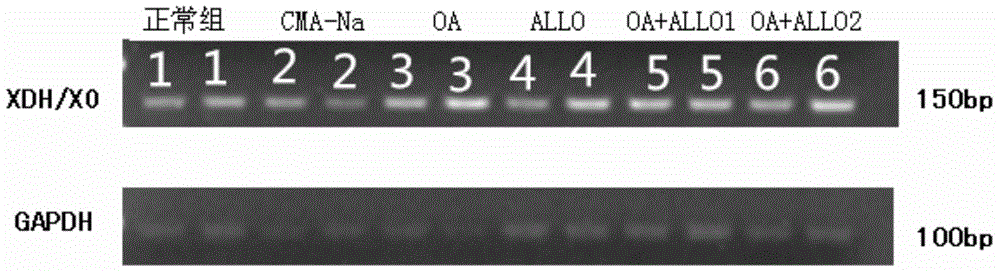
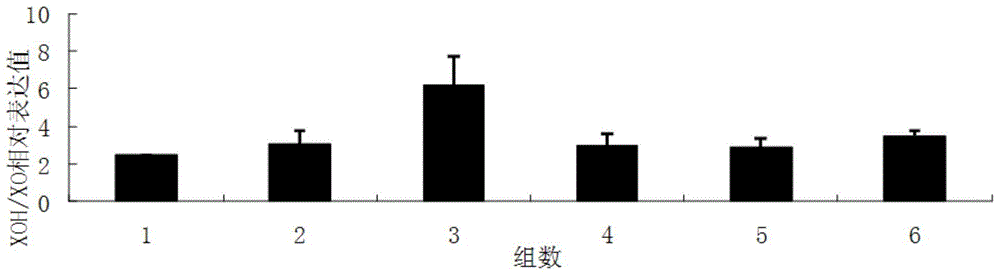
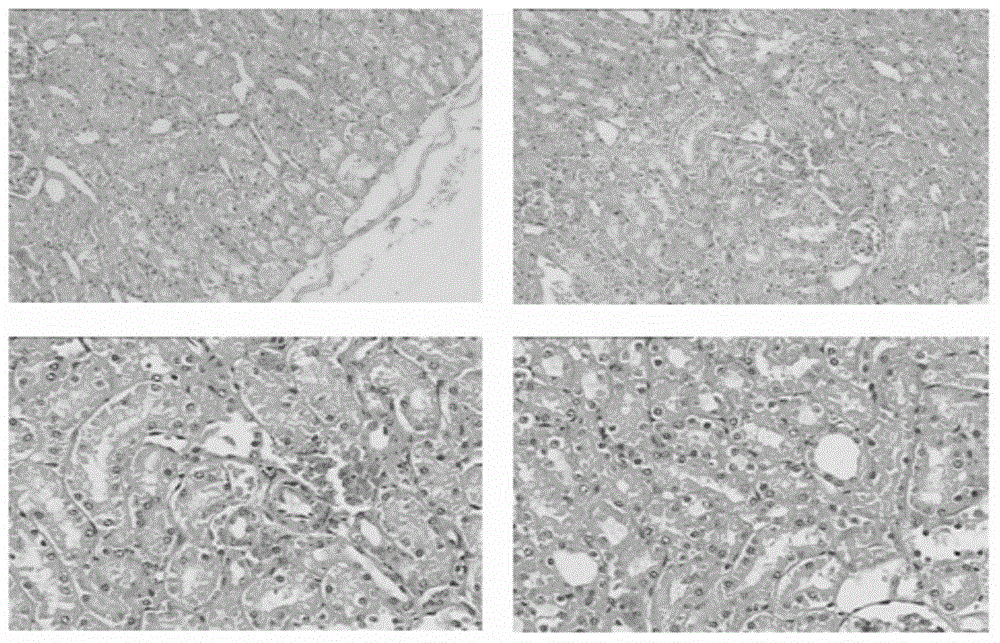
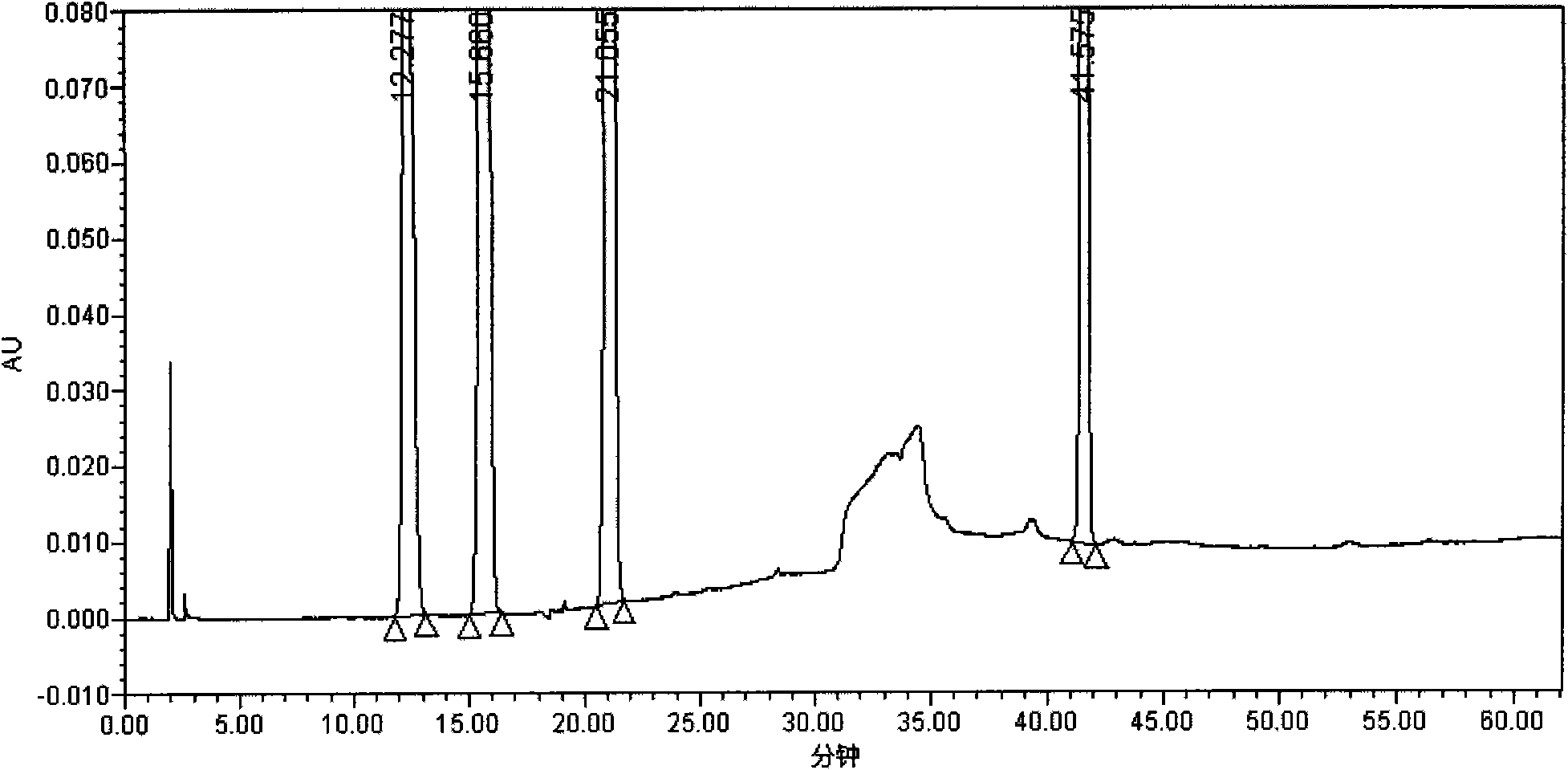
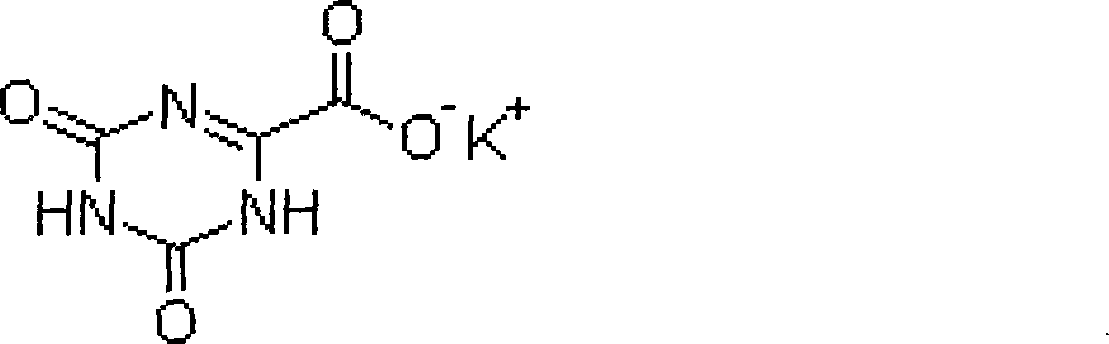Patents
Literature
85 results about "OTERACIL POTASSIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The combination drug tegafur/gimeracil/oteracil (trade name Teysuno, and TS-1 in Japan), ... Oteracil potassium References This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it ...
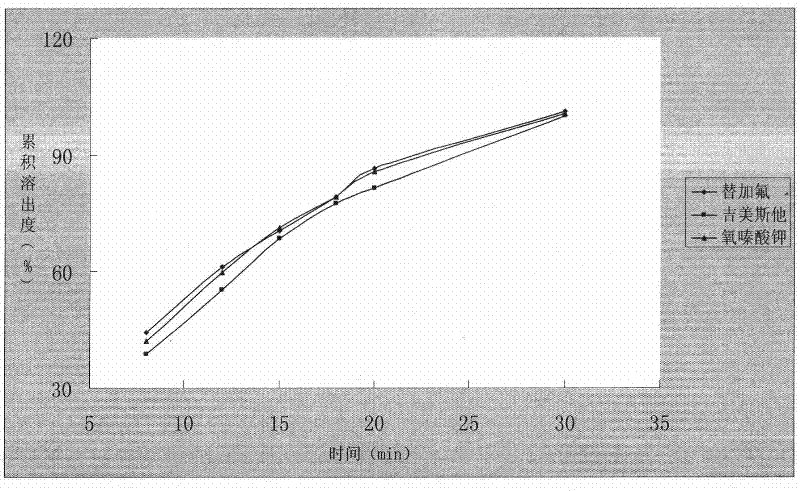
Disintegration piece taken through oral cavity containing Gimeracil and Oteracil Potassium with fluorine being added
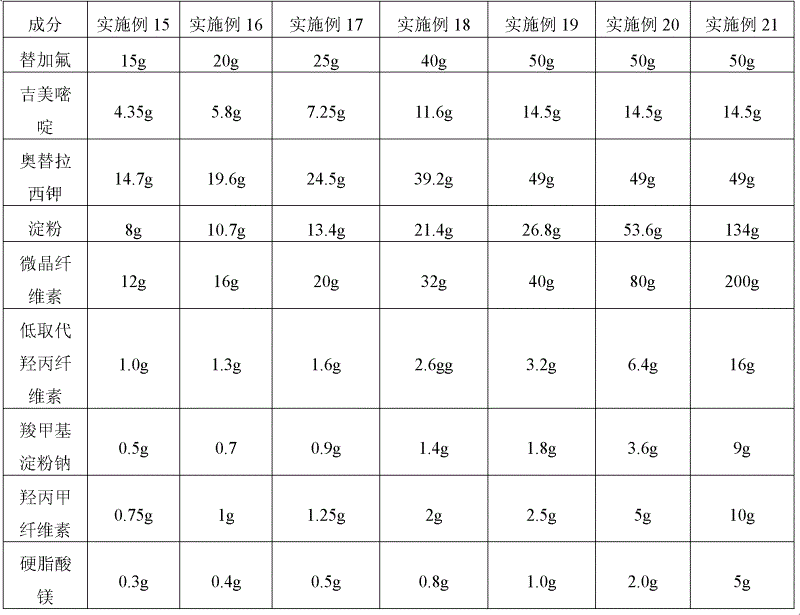
InactiveCN1660105AOvercome the disadvantage of strong bitter tasteImprove complianceOrganic active ingredientsPill deliveryOTERACIL POTASSIUMOrally disintegrating tablet
Owner:LUNAN PHARMA GROUP CORPORATION
Application of celery seed extract to preparation of medicine or health-care food for resisting to hyperuricemia and gout
InactiveCN105535048AEasy to solveGood treatment effectSkeletal disorderNatural extract food ingredientsSerum uric acidAdditive ingredient
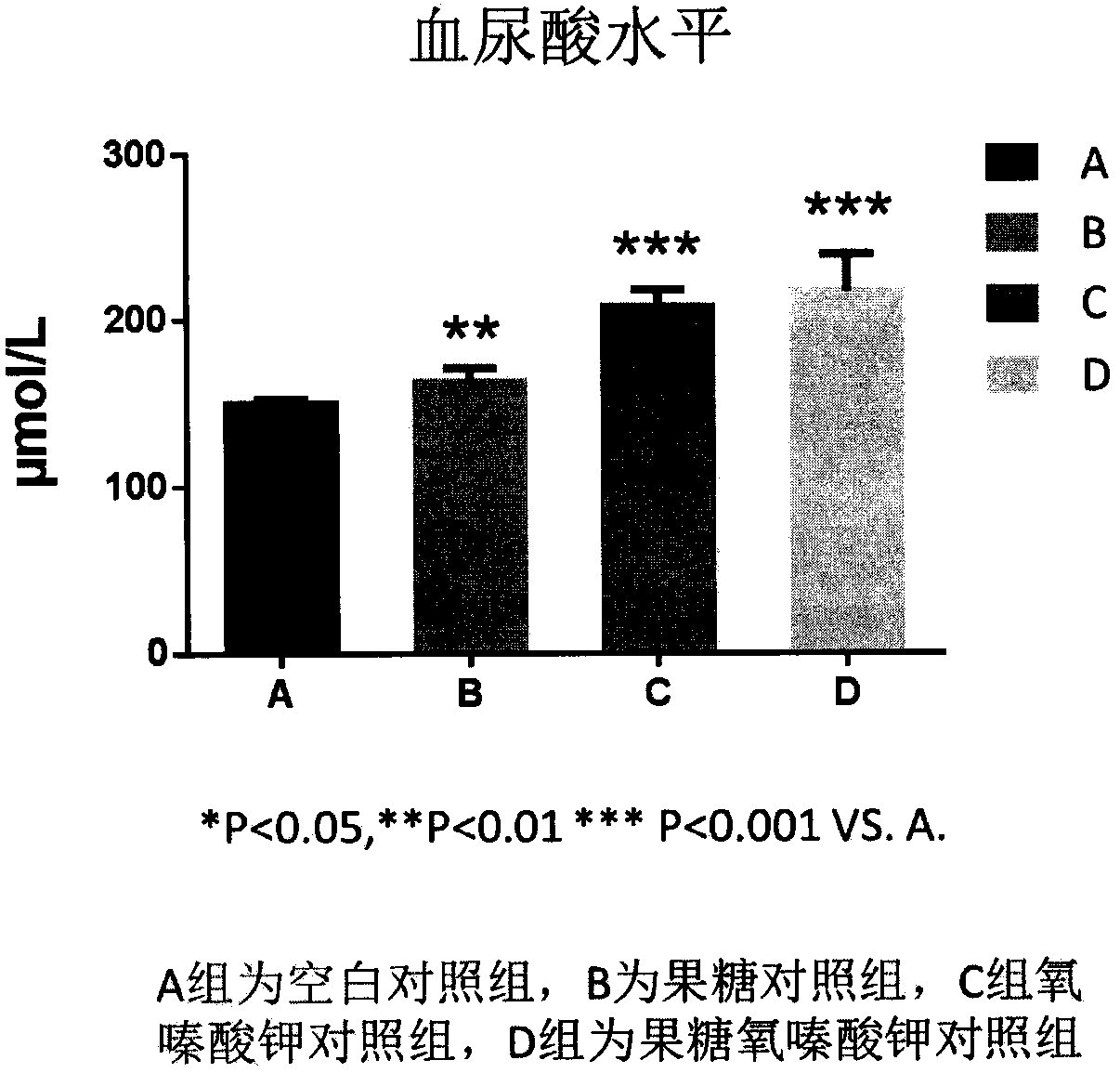
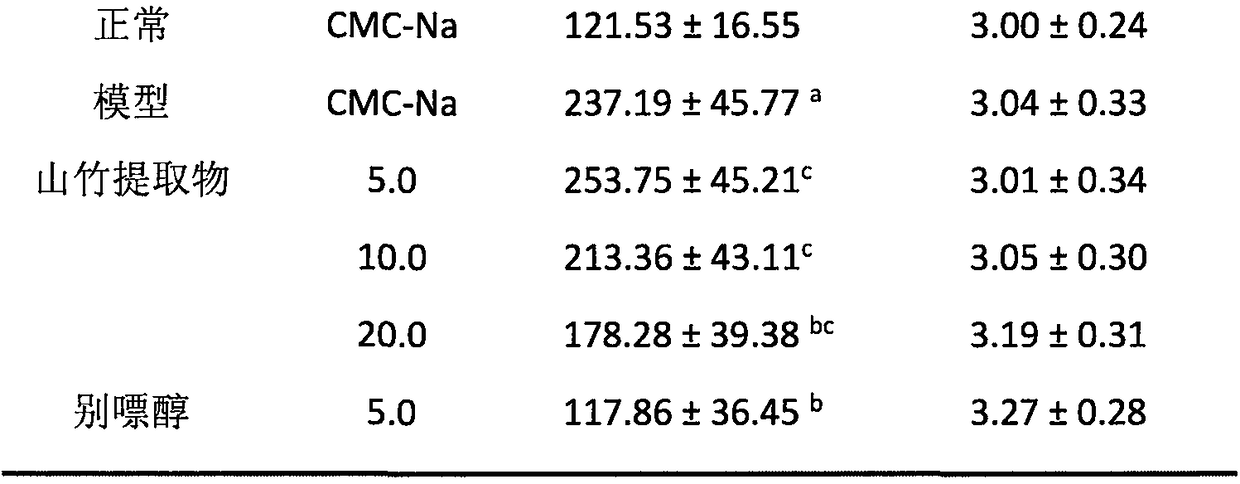
The invention relates to application of celery seed extract to preparation of medicine or health-care food for resisting to hyperuricemia and gout and further provides medicine or health-care food for treating hyperuricemia and gout. Celery seed extract serves as the active constituent, and an appropriate number of medical carriers or auxiliary constituents are added, so that a preparation is obtained. The medicine for health-care food is simple in preparation process, the celery seed extract can obviously lower serum uric acid of model mice suffering from hyperuricemia caused by oteracil potassium salt, lower the activity of xanthine oxidase to different degrees, achieve an obvious inhibition effect on arthritis of the mice, prevent and treat hyperuricemia or gout and be applied to preparation of the medicine or health-care food for resisting to hyperuricemia and gout.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
Oral preparation containing tegafur, gimeracil and oteracil potassium
ActiveCN102614183AGood effectHigh dissolution ratePill deliveryGranular deliveryOTERACIL POTASSIUMIrritation
The invention relates to an oral preparation containing tegafur, gimeracil and oteracil potassium. The oral preparation comprises the following active ingredients: tegafur with granularity of less than or equal to 180 microns, gimeracil with granularity of less than or equal to 150 microns and oteracil potassium with granularity of less than or equal to150 microns. The oral preparation reduces the irritation of the active ingredients on the gastrointestinal tract of a human body and improves pharmaceutical compliance of patients on the premise of guaranteeing that the active ingredients are dissolved out quickly.
Owner:QILU PHARMA HAINAN +1
Chemoradiotherapy with TS-1/camptothecins
InactiveUS20070036717A1Improve the level ofLess side effectsBiocideIn-vivo radioactive preparationsAbnormal tissue growthSide effect
An antitumor agent for chemoradiotherapy of rectal cancer comprising a combination of TS-1 (a combination drug of tegafur, gimeracil, and oteracil potassium at a 1:0.4:1 molar ratio) and CPT-11 (irinotecan hydrochloride). The antitumor agent of the invention can achieve marked tumor volume reduction without causing major side effects, especially by coadministering it with preoperative radiation therapy. The volume of even large tumors that are refractory to surgical resection can be reduced by coadministering the antitumor agent of the invention with preoperative radiation therapy, making the subsequent surgical resection of the tumor easier.
Owner:THE KITASATO INST
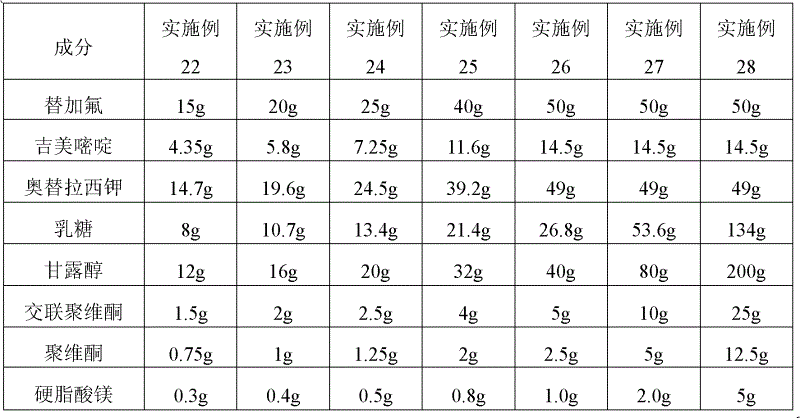
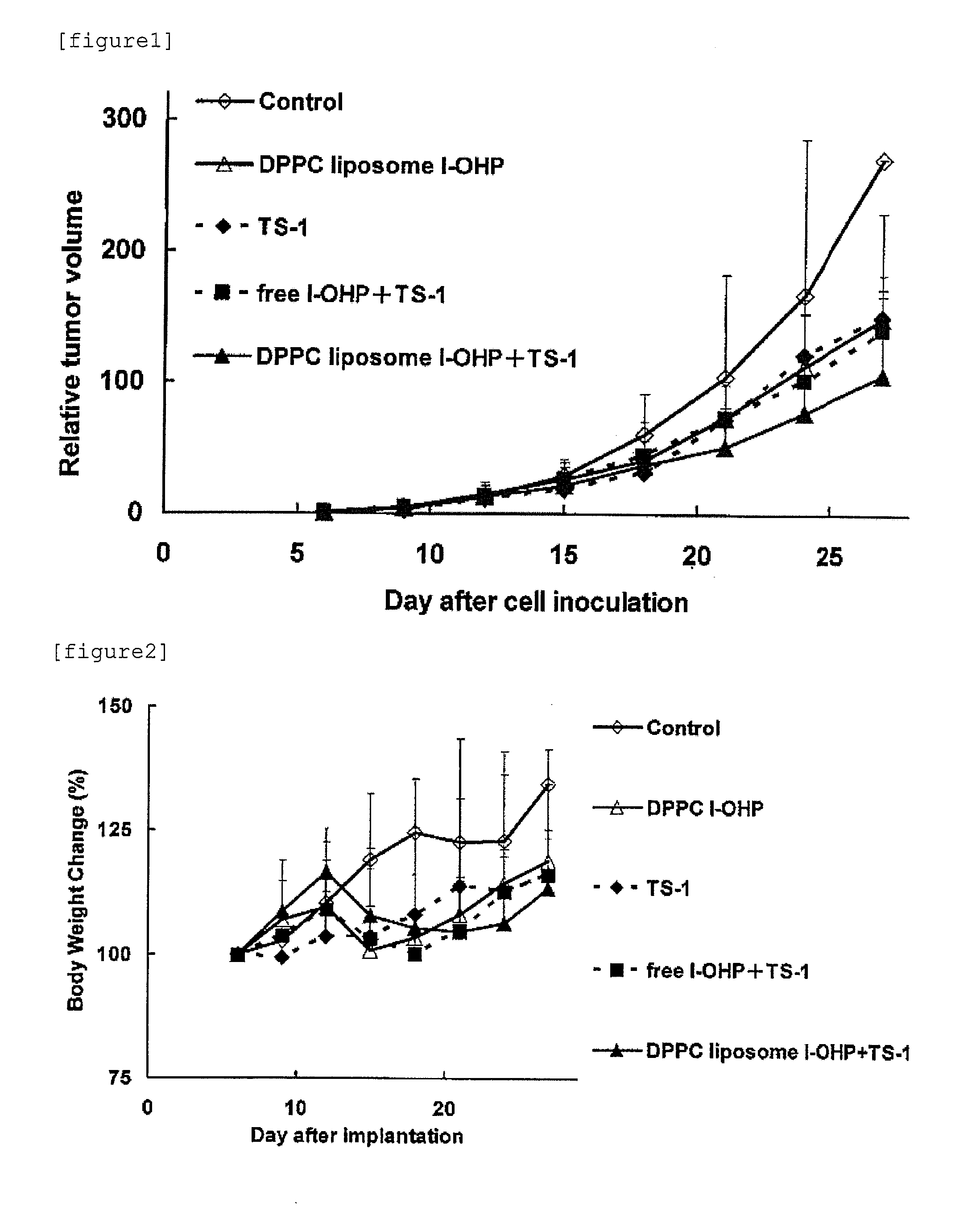
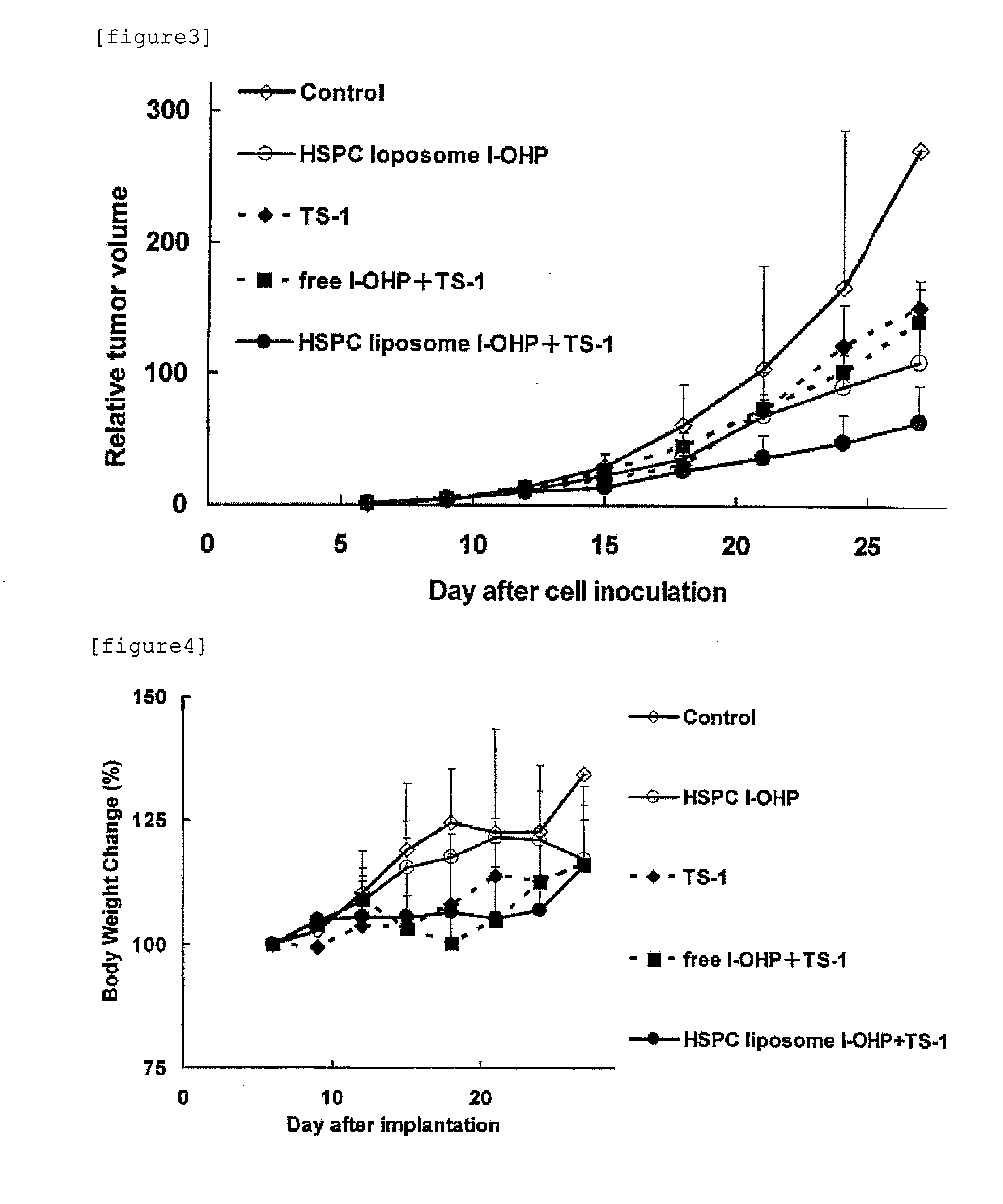
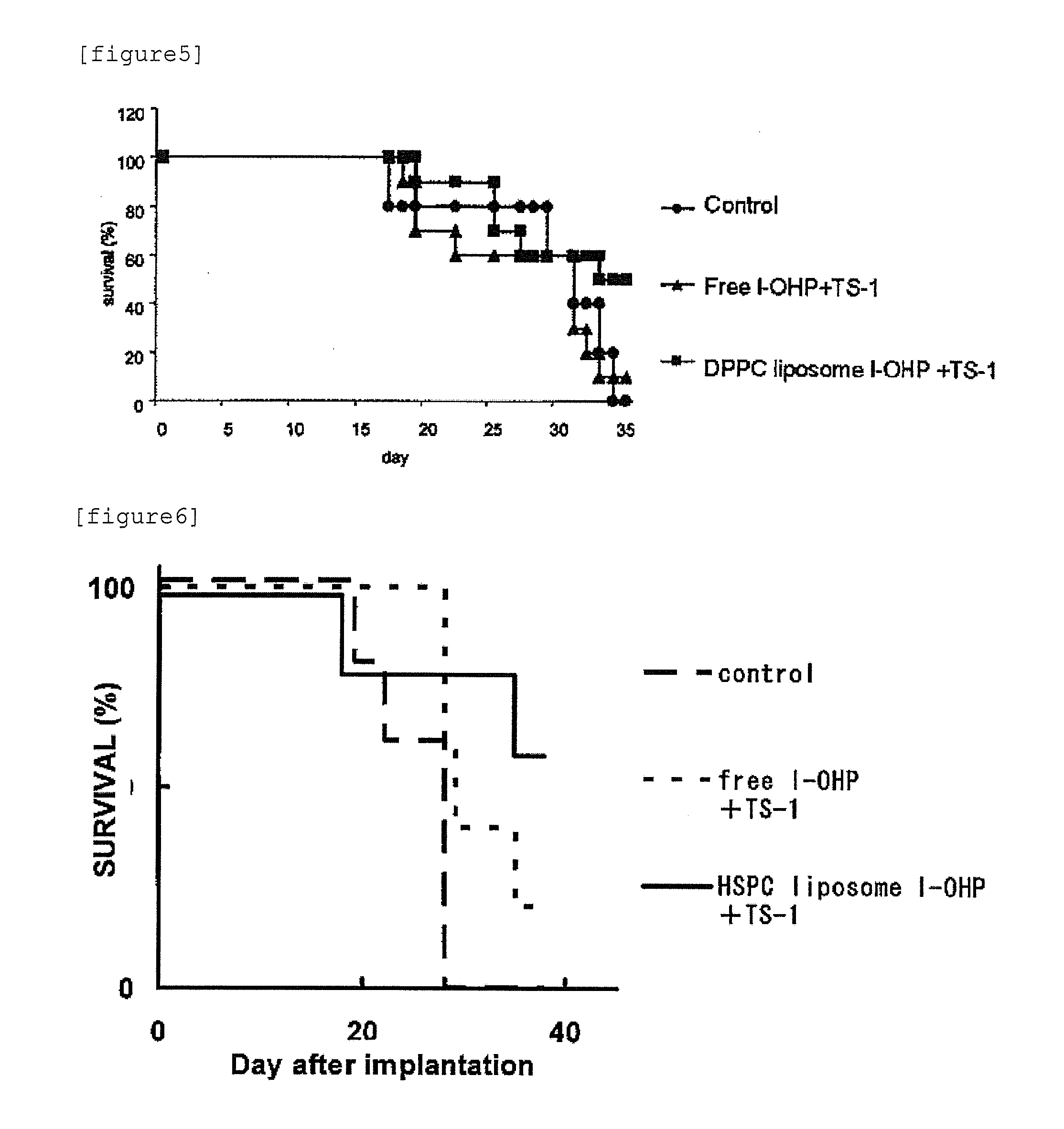
Agent for enhancing Anti-tumor effect comprising oxaliplatin liposome preparation, and Anti-tumor agent comprising the liposome preparation
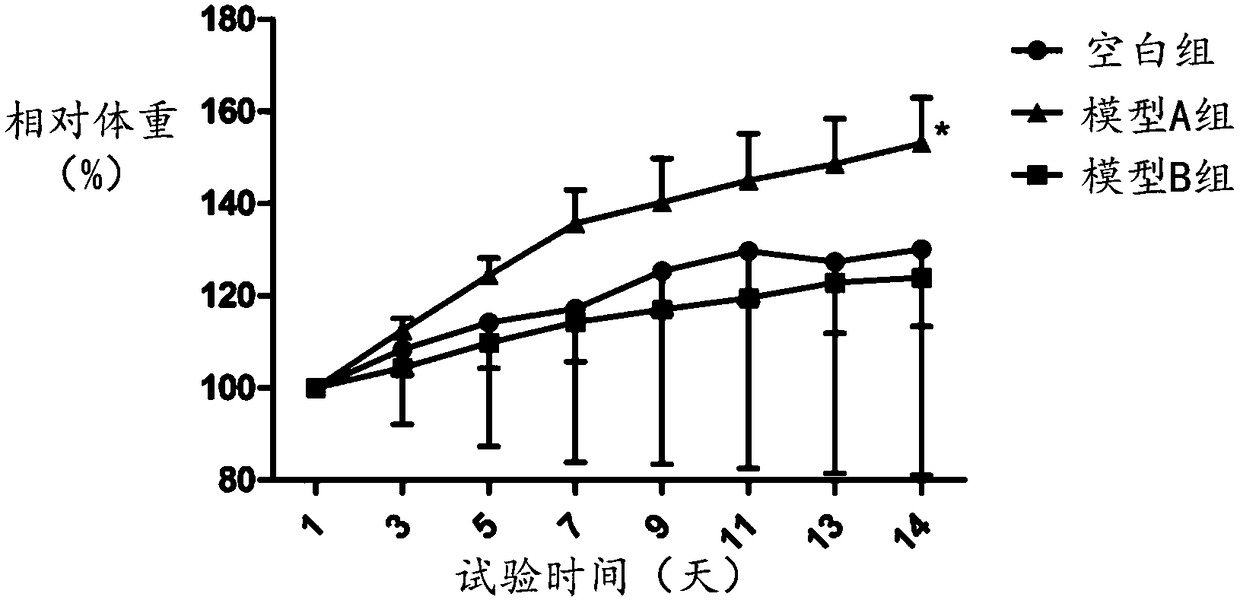
ActiveUS20100330166A1Improve anti-tumor effectIncreased toxicityBiocideAnimal repellantsSide effectOTERACIL POTASSIUM
An object of the present invention is to further enhance the antitumor effect when oxaliplatin is administered in combination with a combination drug containing tegafur, gimeracil and oteracil potassium. According to the present invention, by using oxaliplatin encapsulated in a liposome preparation, combination therapy of oxaliplatin plus a combination drug containing tegafur, gimeracil and oteracil potassium, is revealed to show remarkably enhanced antitumor effect without increasing side effects.
Owner:UNIVERSITY OF TOKUSHIMA +1
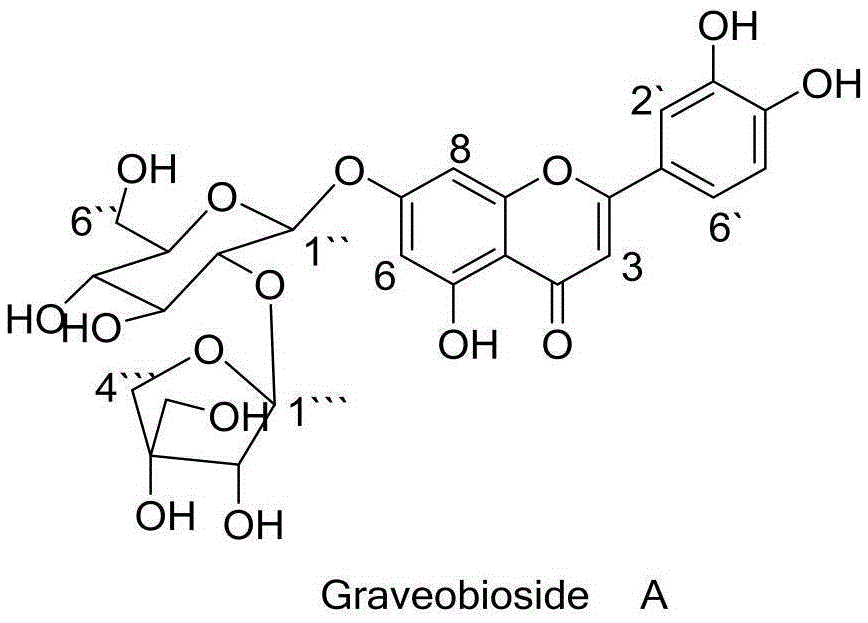
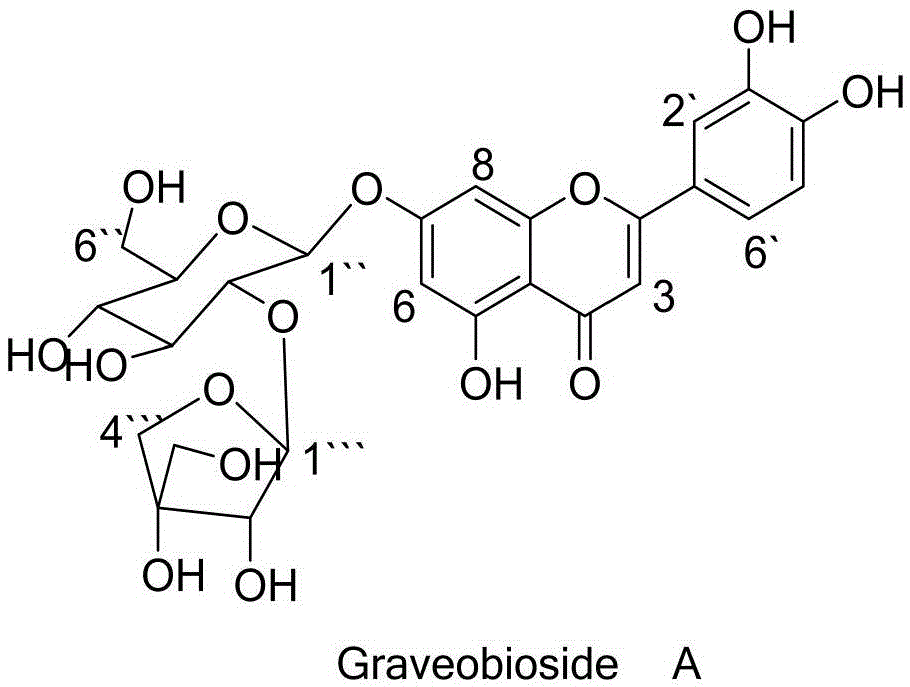
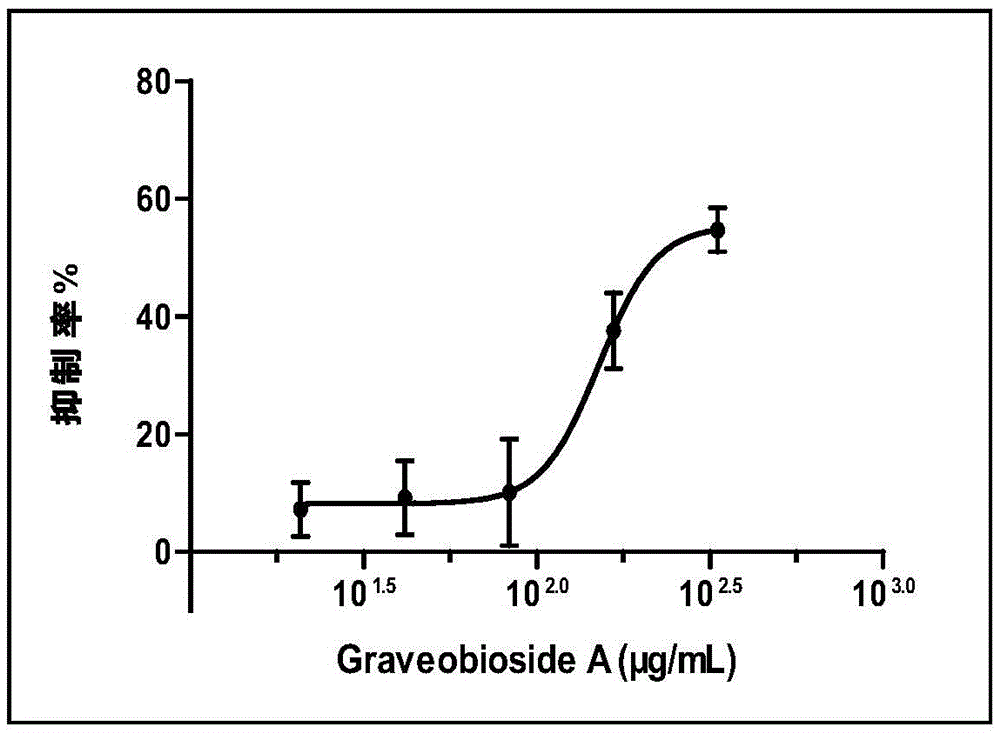
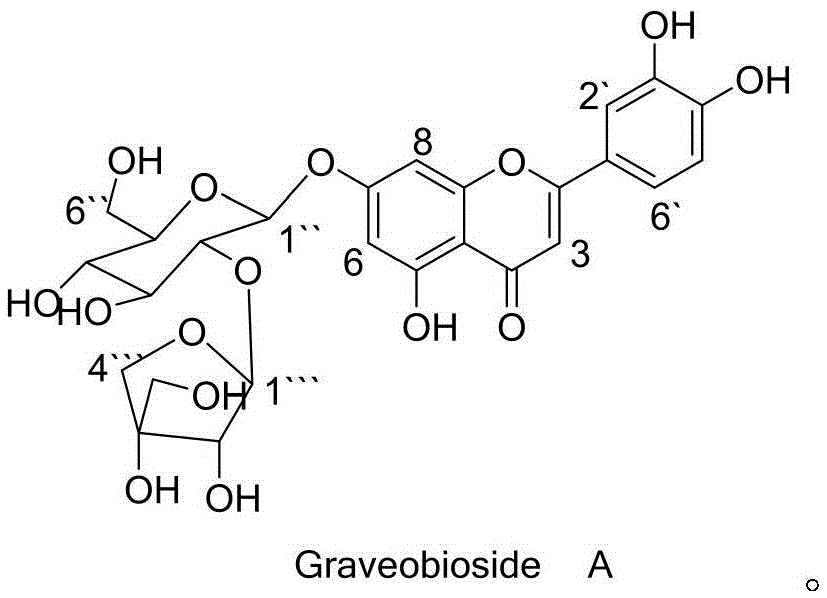
Application of Graveobioside A in preparation of drugs or healthcare food for preventing hyperuricemia and gout
The invention relates to an application of Graveobioside A in the preparation of drugs or healthcare food for preventing hyperuricemia and gout. The invention also provides a drug or healthcare food for treating hyperuricemia and gout. Graveobioside A is the active component and is mixed with a proper amount of pharmaceutical carrier or excipient to prepare preparations. Graveobioside A can prominently reduce uric acid in serum of an oteracil potassium caused hyperuricemia model mouse, reduces the activity of xanthine oxidase at different levels, and prominently inhibit the arthritis of mouse. Graveobioside A can treat and prevent hyperuricemia and gout and can be used to prepare drugs or healthcare food for preventing hyperuricemia and gout.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
Method for building animal model with hyperuricemia-combined diabetes
The invention discloses a method for building an animal model with hyperuricemia-combined diabetes. The method comprises the following steps: 1) feeding an target animal with fodder containing 10% (by mass) of yeast powder; subjecting the target animal to a lavage with a 4% (by mass) adenine water solution according to the following dosage of 100 milligram of adenine per day per kilogram of body weight until the blood uric acid level of the target animal starts falling; 2) halving the dosage of the adenine water solution in step 1), subjecting the target animal to a subcutaneous injection with an oteracil potassium emulsion twice a day according to the following dosage of 100 milligram of oteracil potassium emulsion per day per kilogram of body weight until the target animal shows clinical symptoms of diabetes, and then obtaining the animal model with the hyperuricemia-combined diabetes. The method builds the animal model with the hyperuricemia-combined diabetes for the first time, the animal model has typical disease symptoms, the quantity index has statistical significance, and studies such as glucose clamp test and immunohistochemistry also prove that the modeling is successful. Therefore, the method for building the animal model with the hyperuricemia-combined diabetes is a reliable and practical technique.
Owner:李长贵
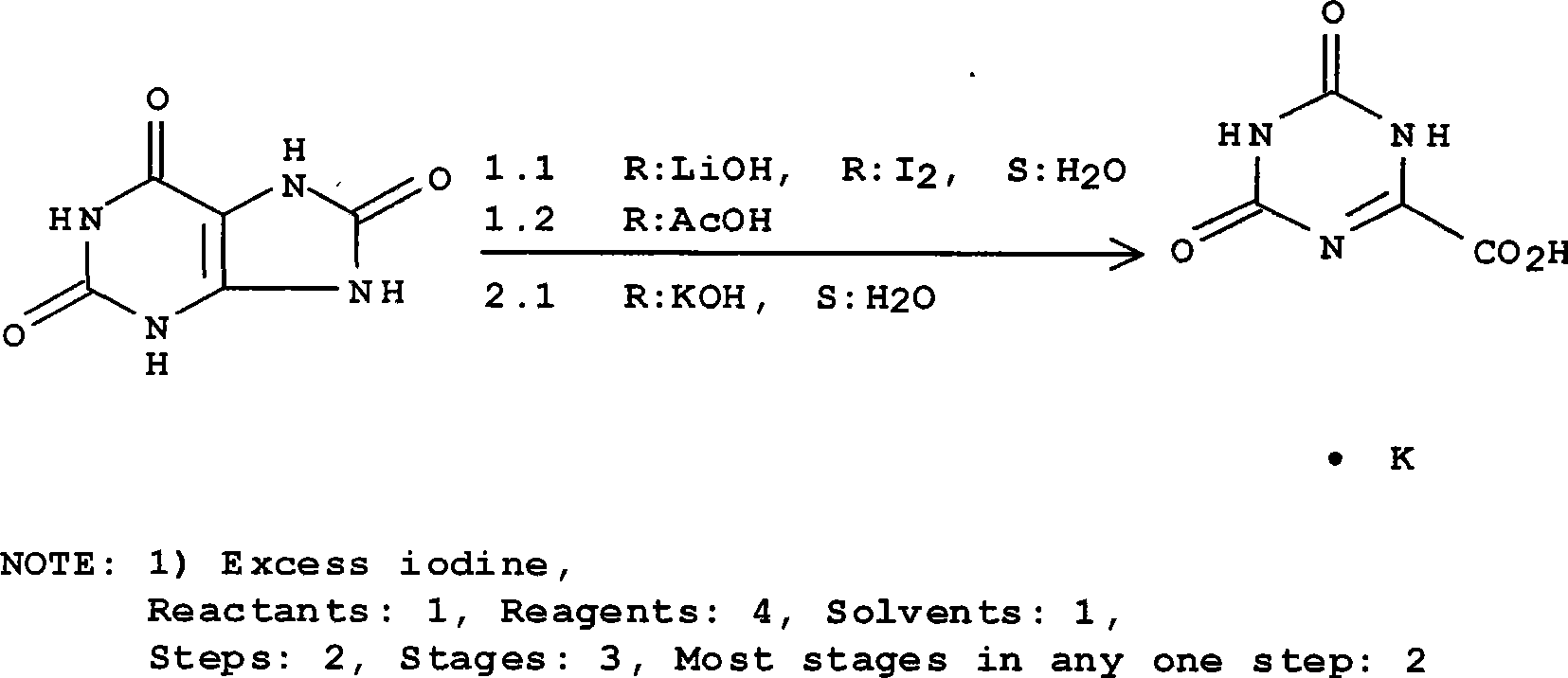
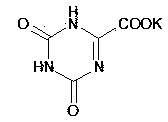
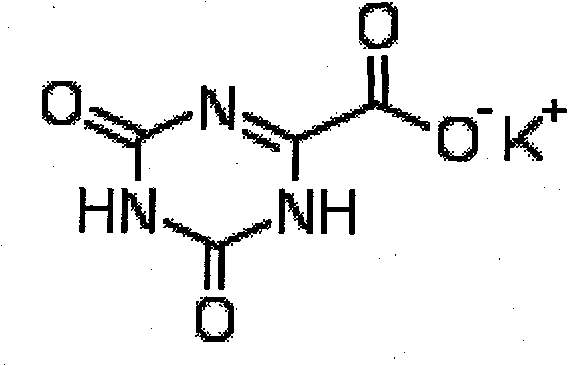
Refining method for preparing high-purity oteracil potassium
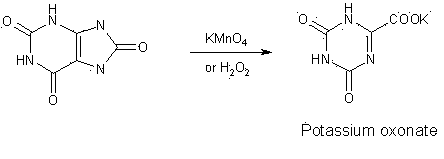
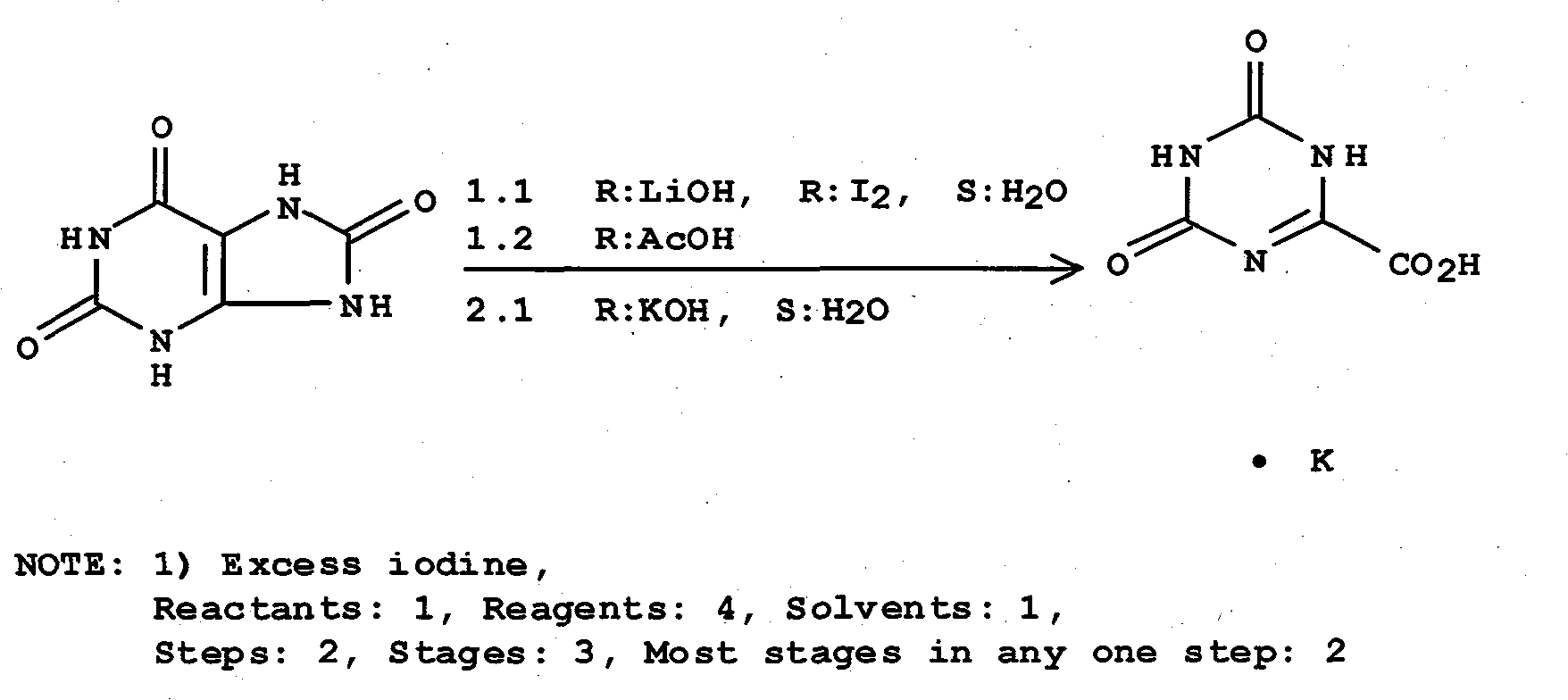
ActiveCN101475539AEasy to operateLess likely to cause side effectsOrganic chemistryPotassium oxonateOTERACIL POTASSIUM
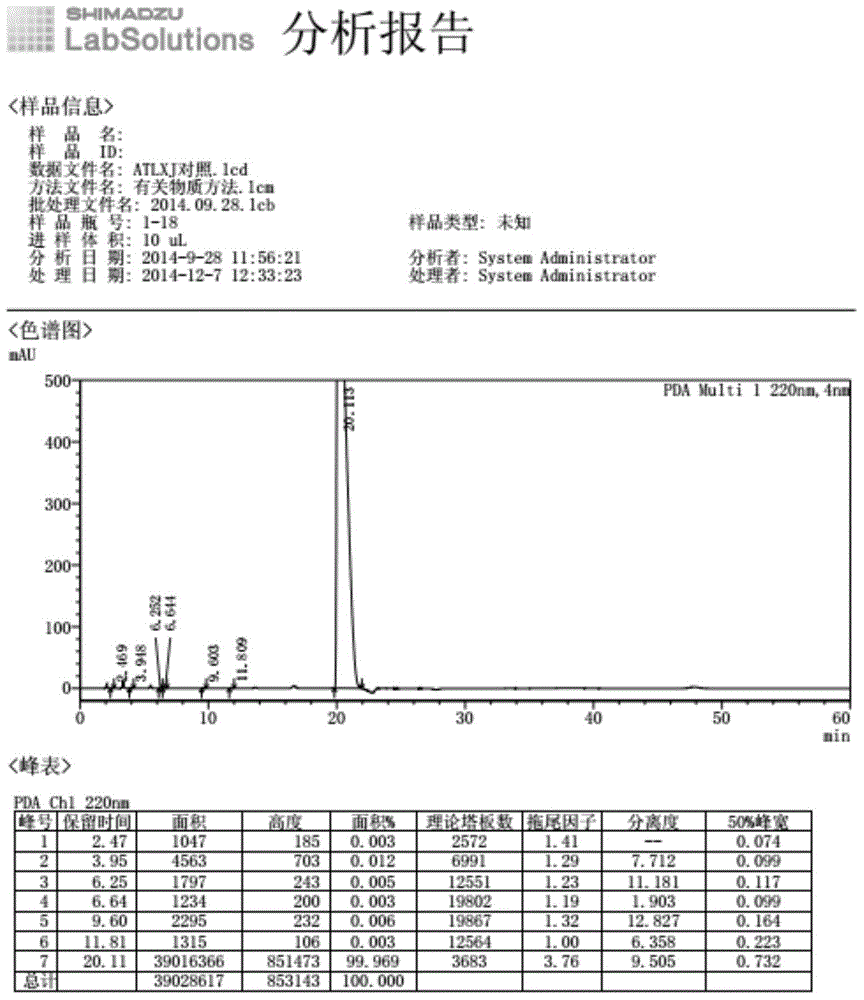
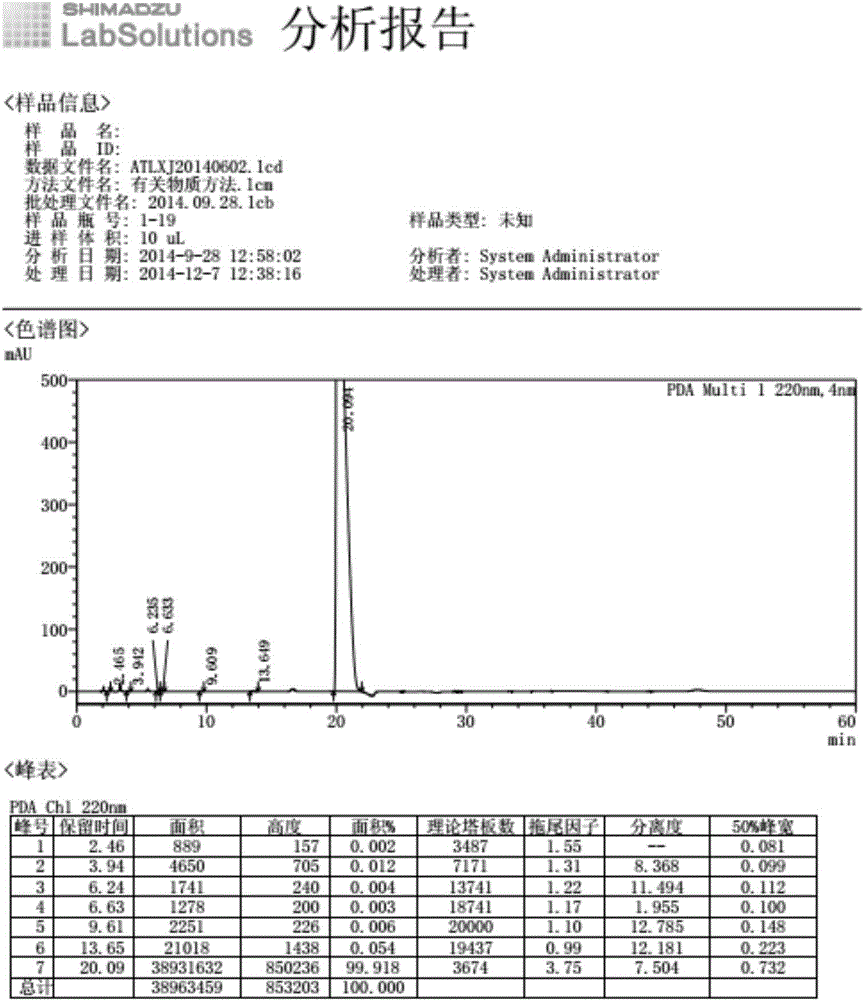
The invention provides a method for refining high-purity potassium oxonate. The method is characterized by comprising the following steps: adding an potassium oxonate crude product into water; adding alkali into the water until the crude product is completely dissolved; adding a polar solvent into the solution; adjusting the pH of the solution to neutrality by acid; filtering and separating out crystal; and drying the crystal so as to obtain the high-purity potassium oxonate. The method has the advantages of simple operation, mild reaction condition, high yield, pure product and the like, and has yield over 90 percent, purity over 99.95 percent (detected by HPLC), and a single impurity peak reduced from 0.5 percent to below 0.05 percent.
Owner:LUNAN PHARMA GROUP CORPORATION
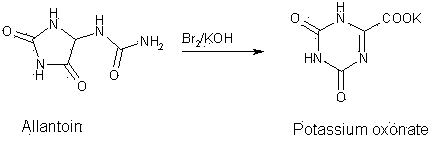
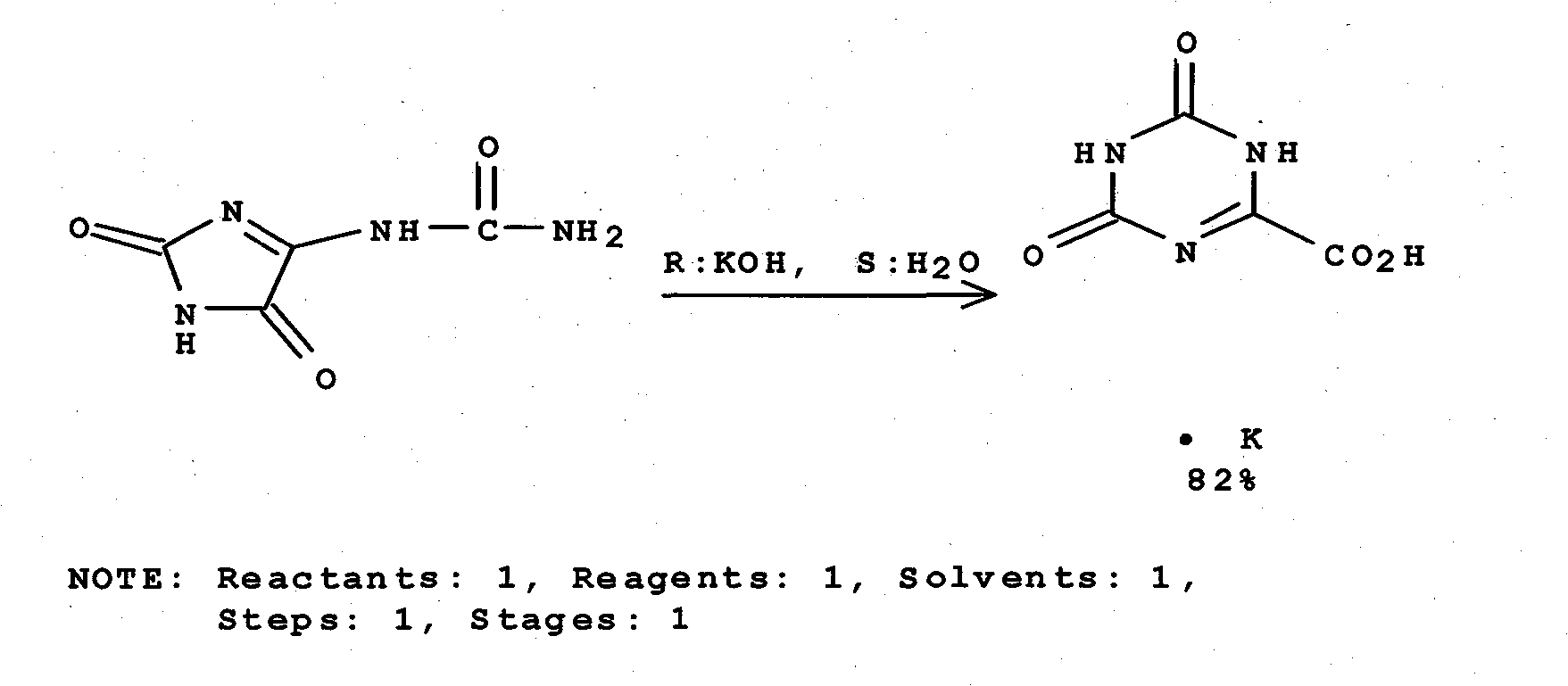
Synthesis technology for preparing oteracil potassium
The invention provides a synthesis technology for preparing oteracil potassium. With short reaction steps, the technology avoids the adoption of toxic and harmful bromine as an oxidant. The synthesis technology is characterized by comprising the following step of dissolving allantoin in a potassium hydroxide or potassium carbonate aqueous solution, wherein potassium iodide is used as a catalyst, and a pypocholoride aqueous solution is used as an oxidant. The method provided by the invention has the advantages of simplicity in operation, mild reaction conditions, high yield, high product purity and the like, and is suitable for industrial production of oteracil potassium.
Owner:JIANGSU QINGJIANG PHARMA
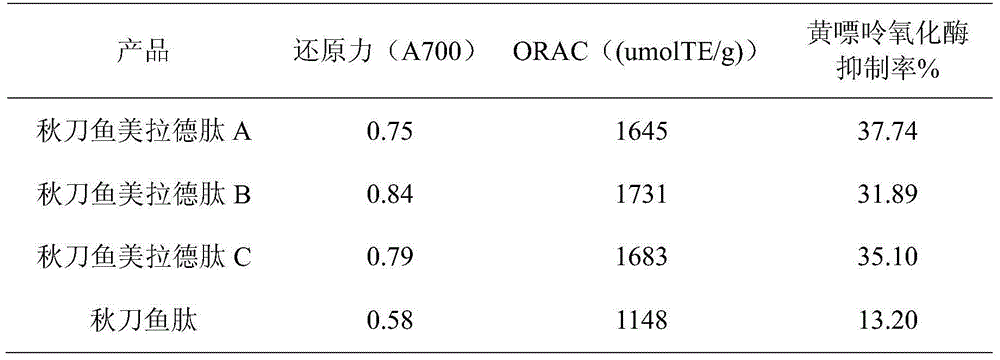
Saury maillard peptide with uric acid reducing function as well as preparation method and application of saury maillard peptide
ActiveCN104673865AIncreased uric acid-lowering activityEffective in lowering uric acidHydrolysed protein ingredientsSkeletal disorderMaillard reactionUltrafiltration
The invention discloses a saury maillard peptide with a uric acid reducing function as well as a preparation method and an application of the saury maillard peptide. The preparation method of the saury maillard peptide comprises the steps as follows: saury is minced, heated and stirred, the pH value is regulated to 4.2, centrifugal separation is performed, precipitates are taken, protease is added to the precipitates for enzymolysis, an enzyme product is centrifuged, and the supernatant is taken and filtered by an ultrafiltration membrane to obtain a saury peptide liquid; the concentrated saury peptide liquid is added with reducing sugar for reaction, and the saury maillard peptide is obtained. The method is combined with a biological enzymolysis technology, a biological membrane technology and a maillard reaction technology, biological active peptide with uric acid reducing activity is released from saury protein, target active peptide is enriched through the ultrafiltration membrane, and finally, the uric acid reducing activity of the target biological active peptide is improved with the maillard reaction technology. The saury maillard peptide prepared with the method has the remarkable uric acid reducing function, the serum uric acid level of mice with hyperuricemia induced by oteracil potassium can be remarkably reduced, and the saury maillard peptide has the remarkable kidney protection function.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing tegafur, gimeracil and oteracil potassium capsules
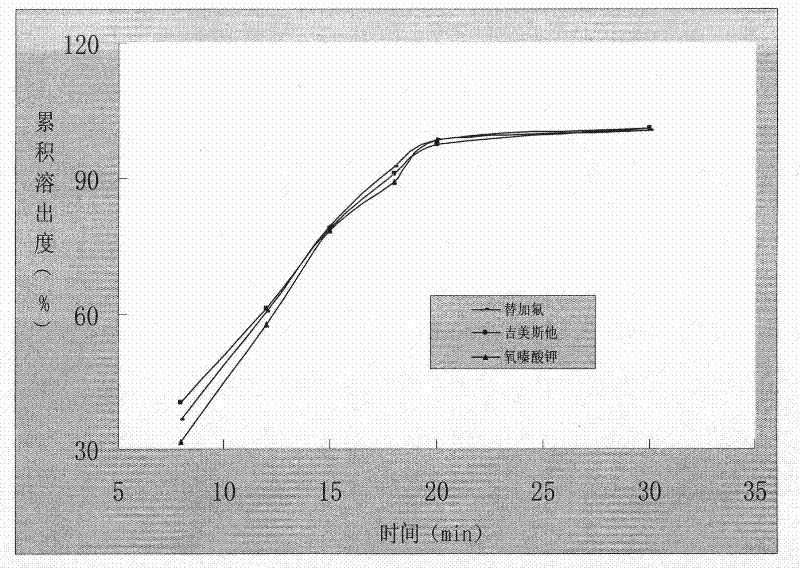
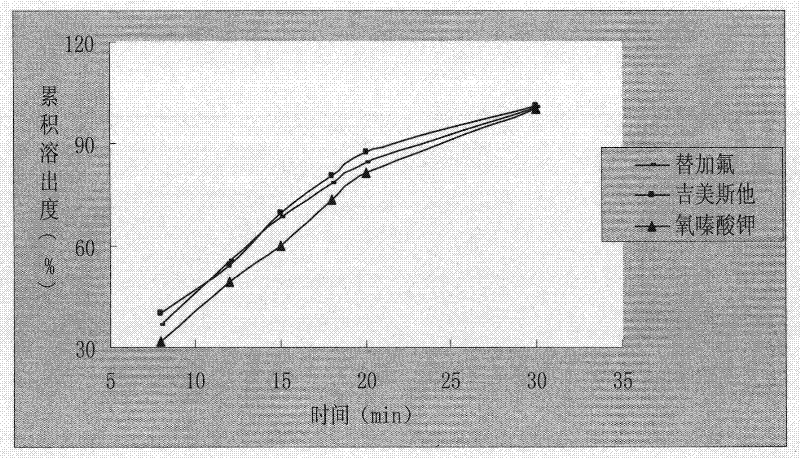
ActiveCN103211820AAvoid gatheringPromote dissolutionCapsule deliveryAntineoplastic agentsOTERACIL POTASSIUMDrug Dissolution
The invention relates to a method for preparing tegafur, gimeracil and oteracil potassium capsules. The method comprises the steps of dissolving tegafur in methanol, dissolving gimeracil in a hydrochloric acid solution, dissolving oteracil potassium in a sodium hydroxide solution, rapidly mixing the three solutions, rapidly adding microcrystalline cellulose and stirring, slowly cooling to about 4 DEG C at the same time to obtain nanoscale drug adhered to the microcrystalline cellulose, drying drug-containing particles, adding a lubricant into the dry particles, uniformly mixing and encapsulating. According to the invention, the technologies of acid-base neutralization and nanometer crystallization are creatively utilized to prepare nanoscale tegafur, gimeracil and oteracil potassium, so that the drug dissolution rate is greatly increased.
Owner:SHANDONG NEWTIME PHARMA
Building method of acute hyperuricemia renal damage mouse model
InactiveCN108785312AShort modeling timePromote reproductionHeterocyclic compound active ingredientsXanthineHypoxanthine synthesis
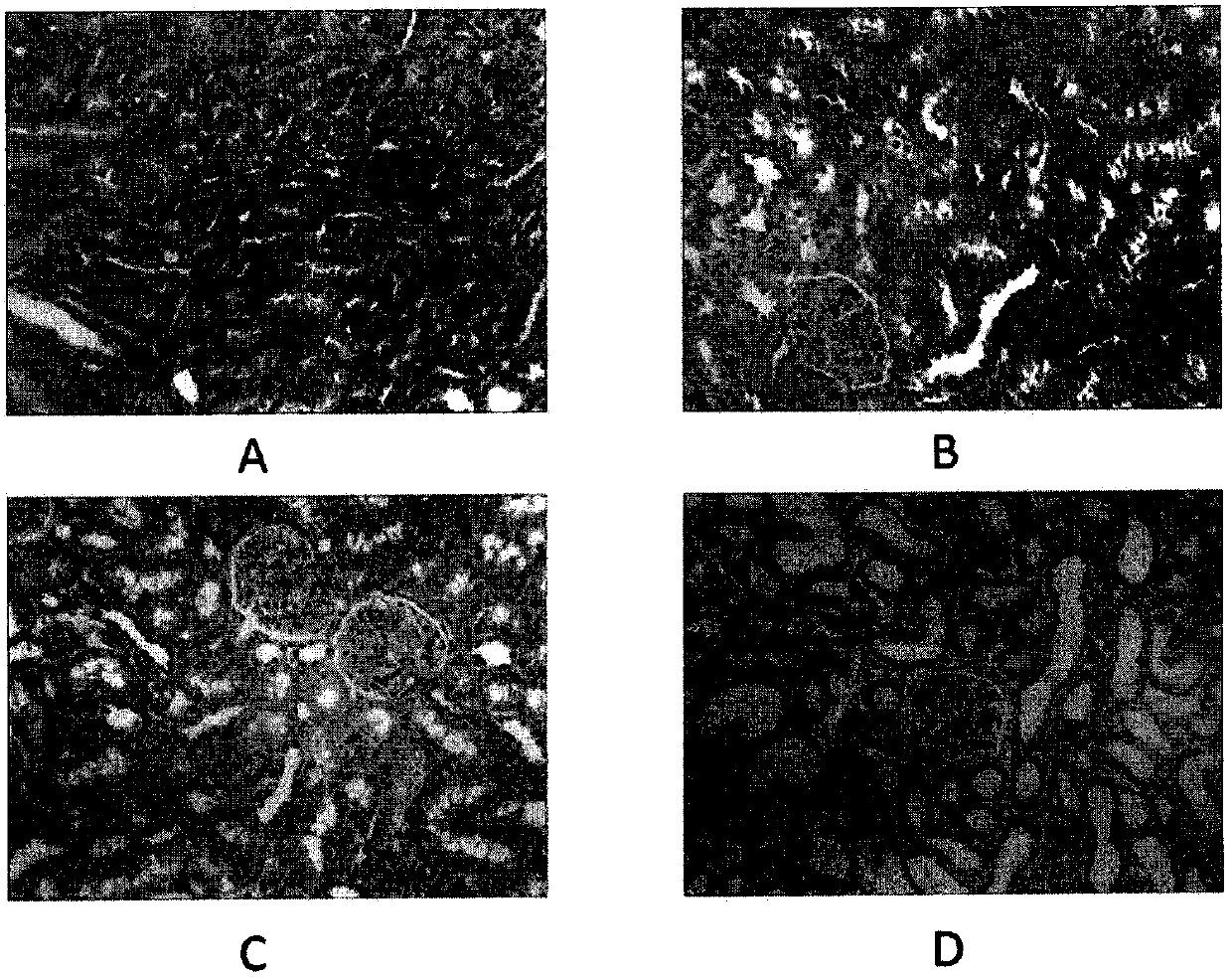
The invention discloses a building method of an acute hyperuricemia renal damage mouse model. The method comprises the following steps of experiment mice are subjected to molding experiment for many days by using xanthine, ethambutol and oteracil potassium as molding agents; obtaining the acute hyperuricemia renal damage mouse model; observing the body weight, the serum uric acid level and the renal function change of the experiment mice and the kidney pathological change; the renal damage effect of the molding agent on the experiment mice is analyzed. The mice are used as model animals; the serum uric acid level of the mice is equivalent to the uric acid level of a hyperuricemia patient; the serum uric acid level is stable and can easily reappear; meanwhile, the combined effect of uric acid precursors of hypoxanthine is used; the uric acid excreted substances of ethambutol and uricase inhibitors of oteracil potassium are reduced; the acute hyperuricemia renal damage mouse model is built; the molding time is short; the model conforms to the clinical characteristics of human body hyperuricemia renal damage, and is applicable to screening hyperuricemia renal damage resisting medicine.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Construction method for acute hyperuricemia tree shrew model
ActiveCN103977007ATypical disease symptomsModeling dose interval safetyHeterocyclic compound active ingredientsDiseaseSerum uric acid
The invention relates to a construction method for an acute hyperuricemia tree shrew model. The method comprises the following steps: (1) selecting adult healthy tree shrew with body weight of 110 to 150 g and an age of 1 to 3 years; (2) respectively measuring fasting serum uric acid values of males and females of tree shrew selected in the step (1); (3) carrying out conventional breeding on the tree shrew selected in the step (1) and pretreating the tree shrew by grasping and releasing the tree shrew with hand; and (4) injecting the tree shrew treated in the step (3) with an oteracil potassium suspension so as to obtain the acute hyperuricemia tree shrew model, wherein the amount of the used oteracil potassium suspension is 40 mg / kg to 100 mg / kg on the basis of the body weight of the tree shrew. The model has typical disease symptoms, a quantity index has statistical significance, and serum urea nitrogen and serum creatinine values and histopathological results prove that such a model making dosage range is safe and does not result in influence on the liver and the kidney.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
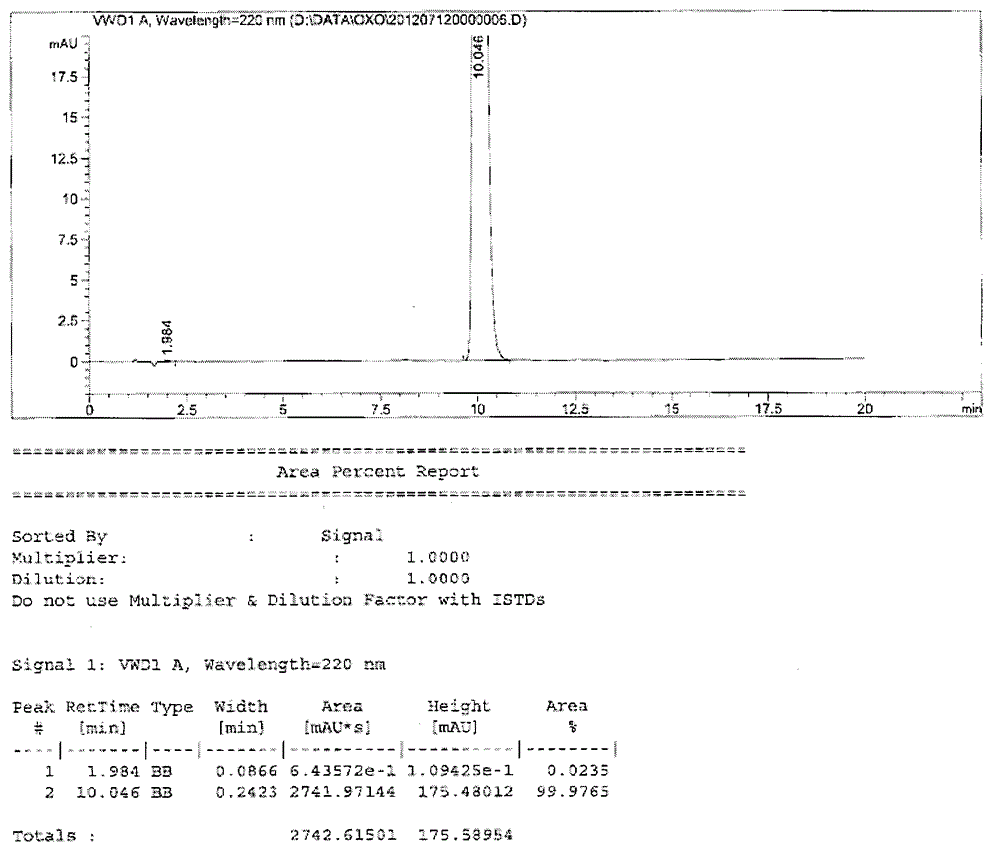
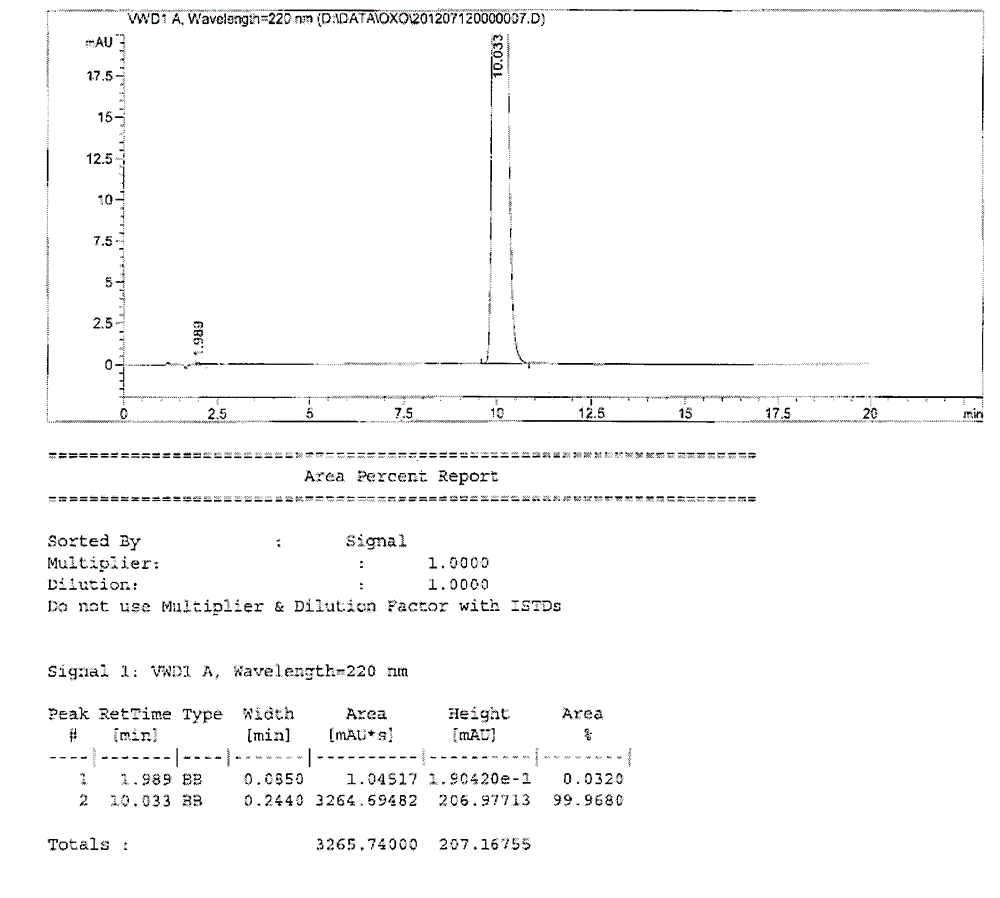
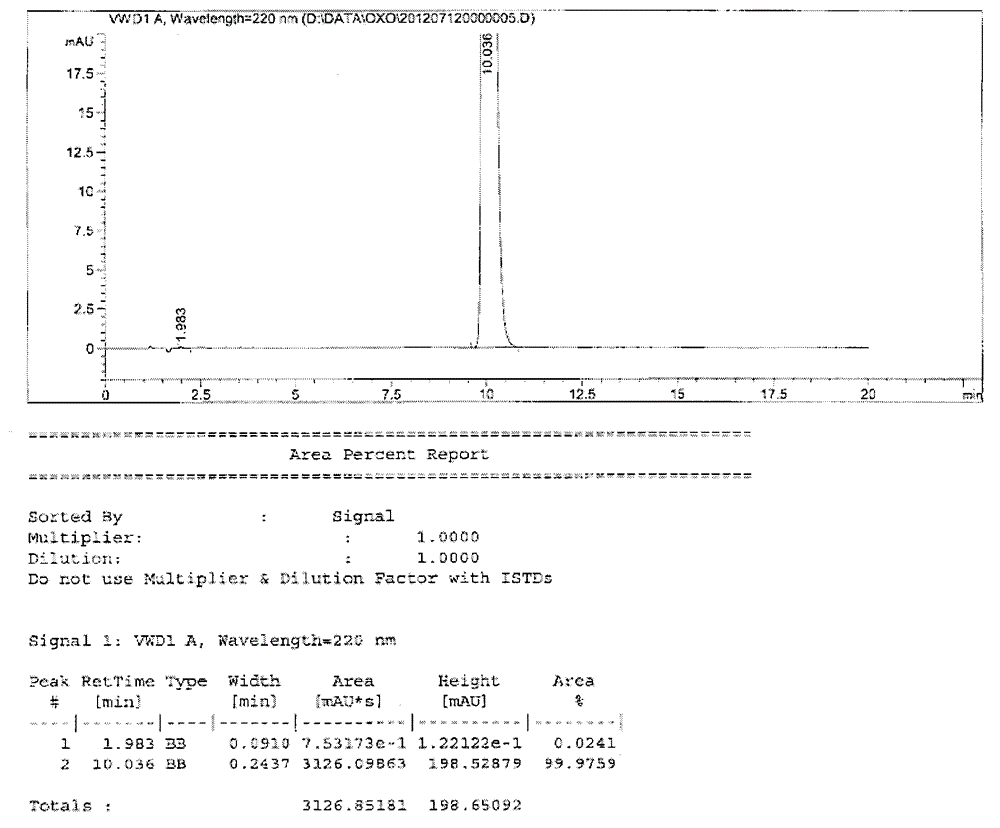
Testing method for related substances of drug combination containing tegafur, gimeracil and oteracil potassium
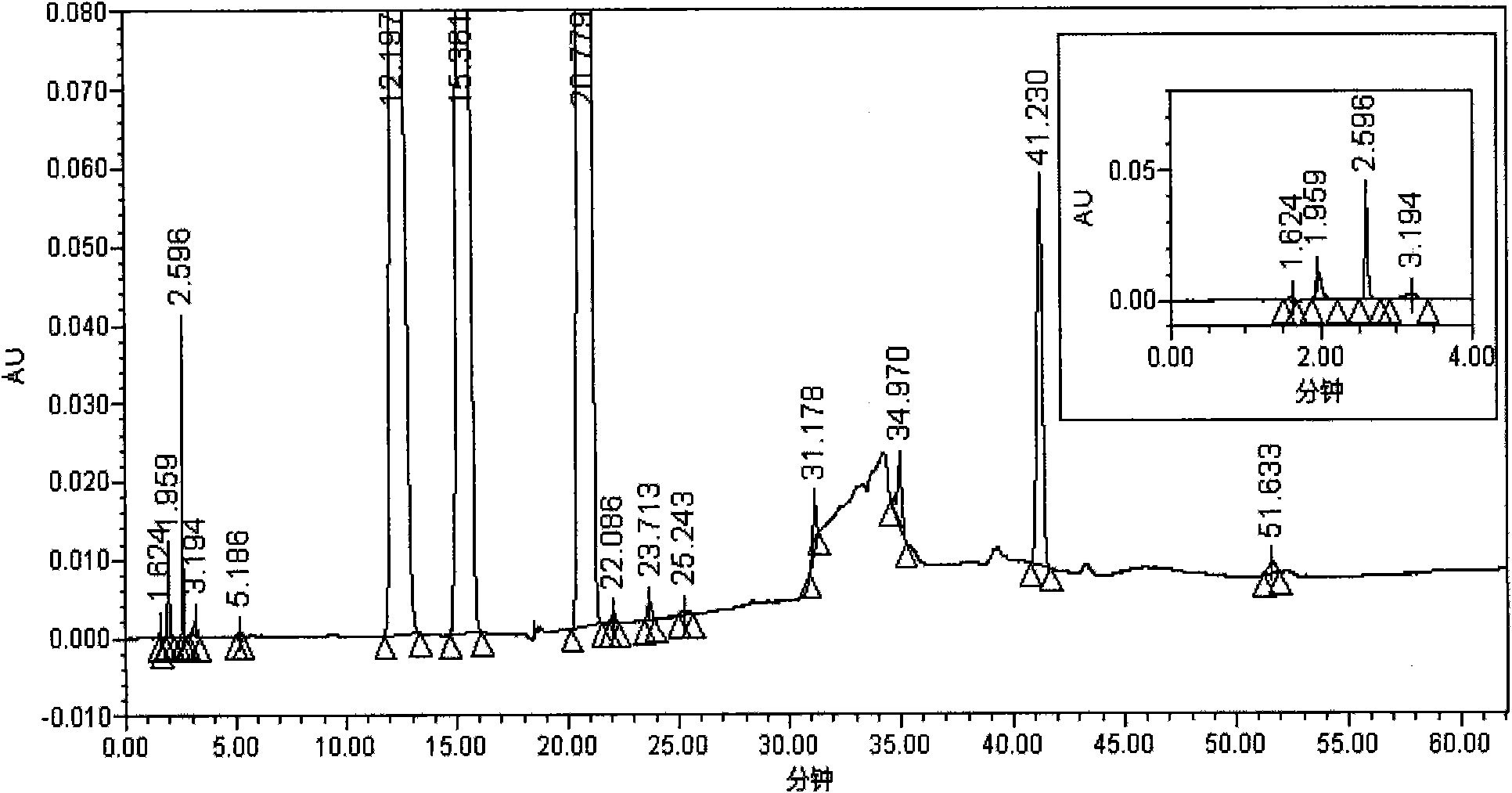
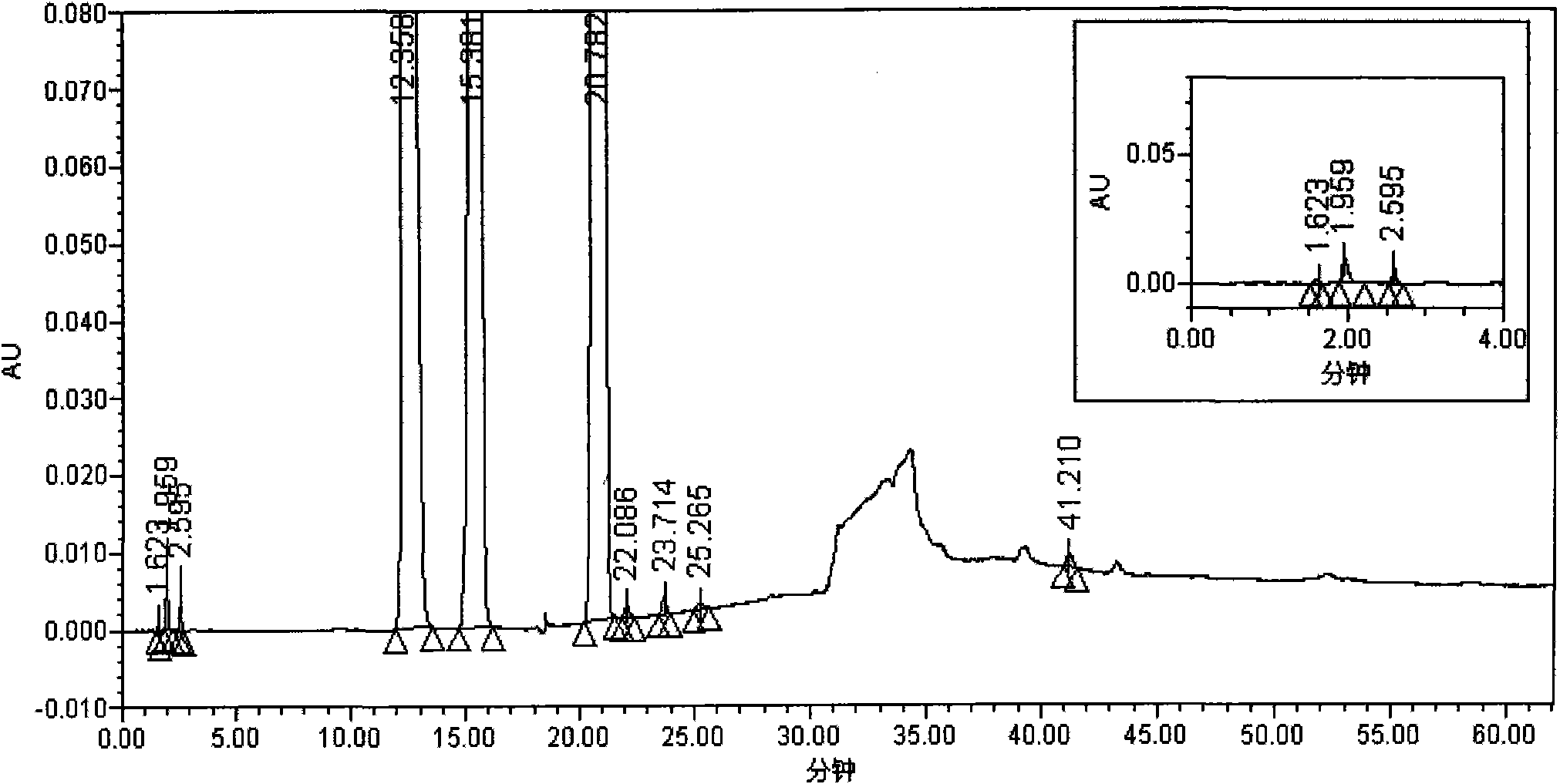
The invention relates to a testing method for the related substances of drug combination containing tegafur, gimeracil and oteracil potassium. The testing method adopts the high-efficiency liquid chromatography (gradient elution) to carry out a test, the stationary phase is a chromatographic column with octyl or octadecyl-bonded silica gel as filler, the mobile phase A is saline solution containing ion-pair reagent or the mixture of the saline solution and any one or both of methanol and acetonitrile, the mobile phase B is any one of acetonitrile and methanol or the mixture of acetonitrile and methanol, and solvent is 10 to 90 percent of aqueous acetonitrile solution, the pH of which is 10.5 to 11.5. The testing method has the advantages of good separation effect, high sensitivity, high operability and the like, and not only can increase the level of tests on the related substances of the tegafur, gimeracil and oteracil potassium drug combination but also enhances the quality control of the combination.
Owner:鲁南新时代生物技术有限公司
Postoperative adjuvant chemotherapy for gastric cancer
InactiveUS20090318453A1Improve survival rateLow recurrence rateBiocideDigestive systemSurgical operationPotassium
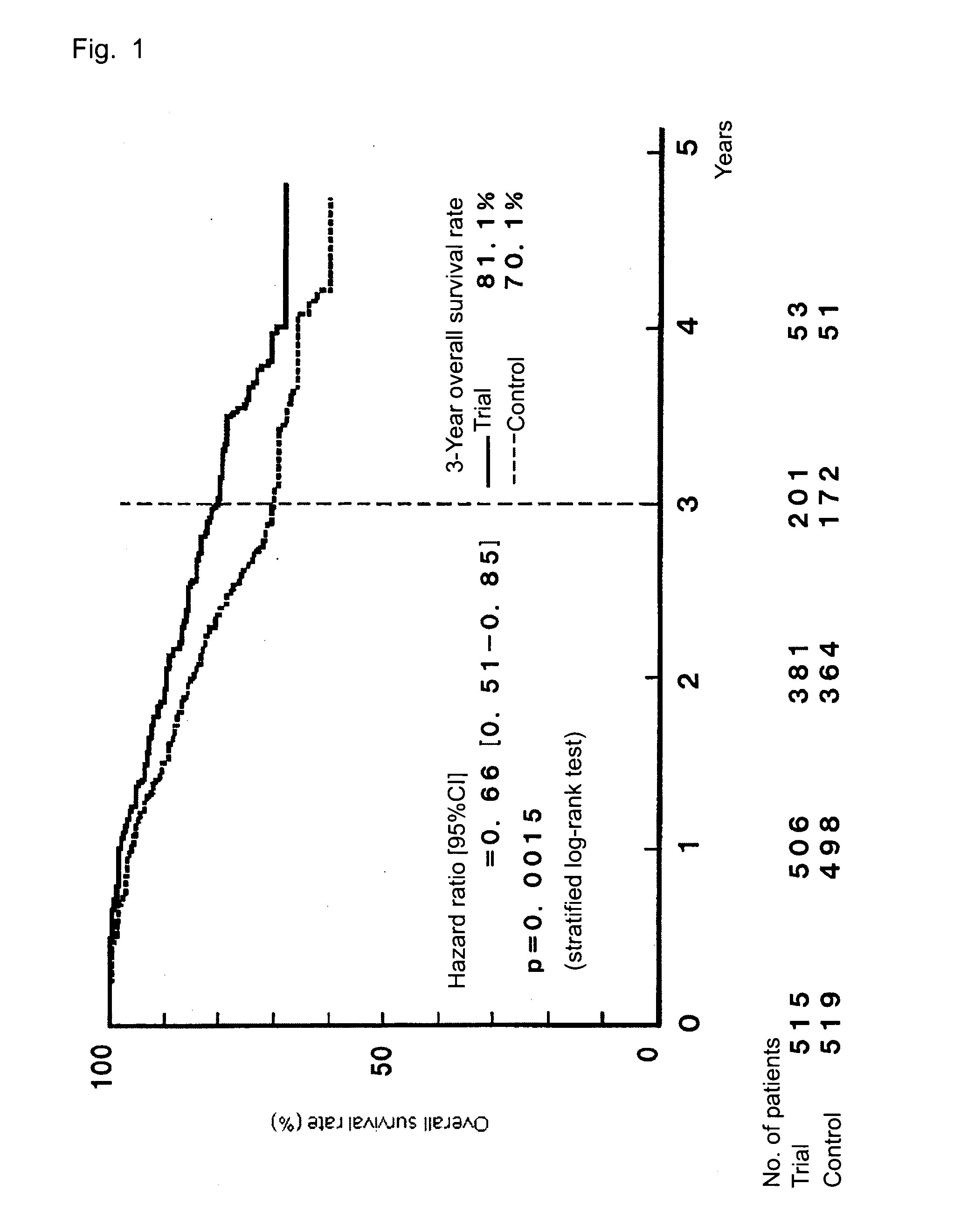
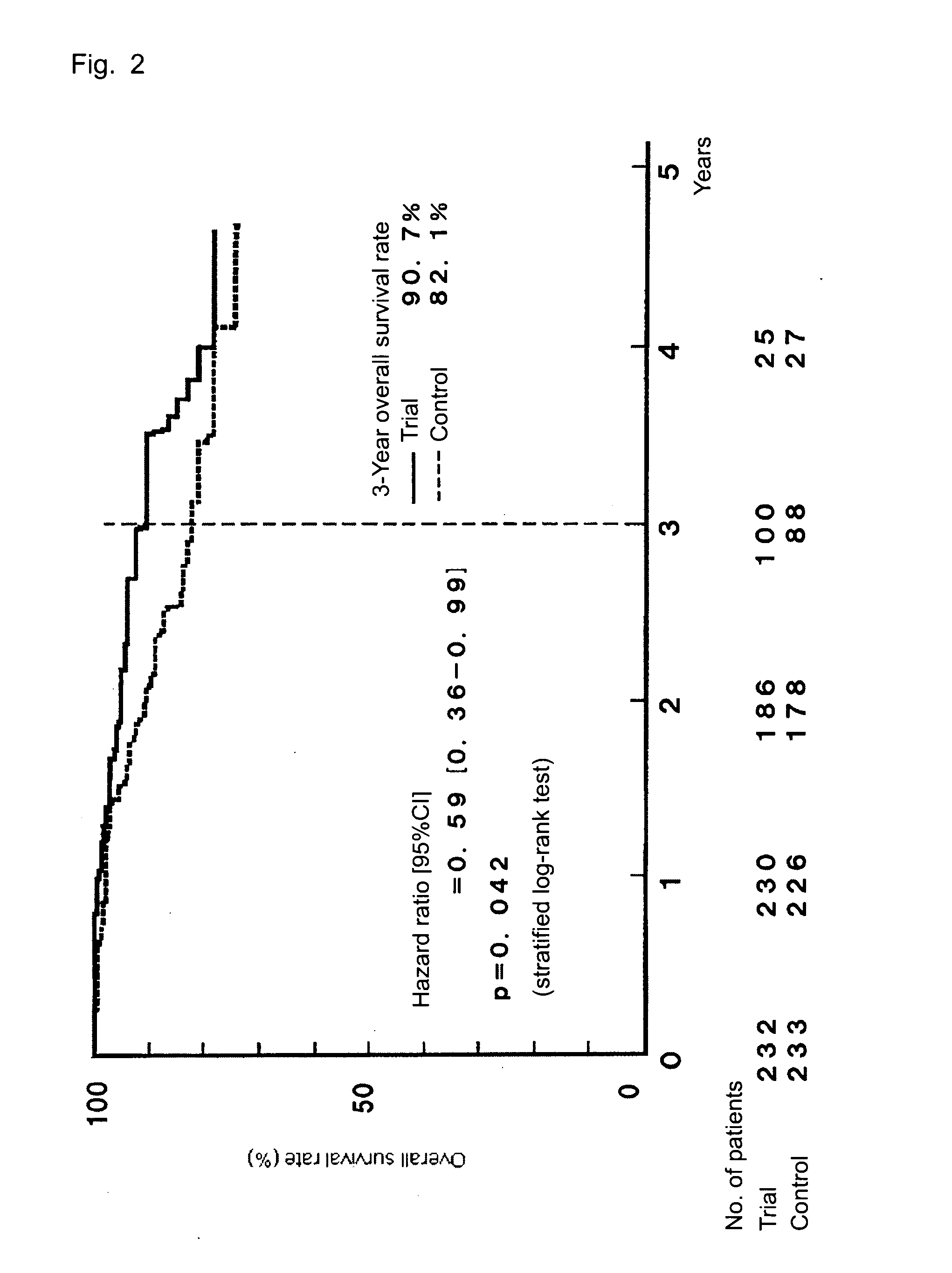
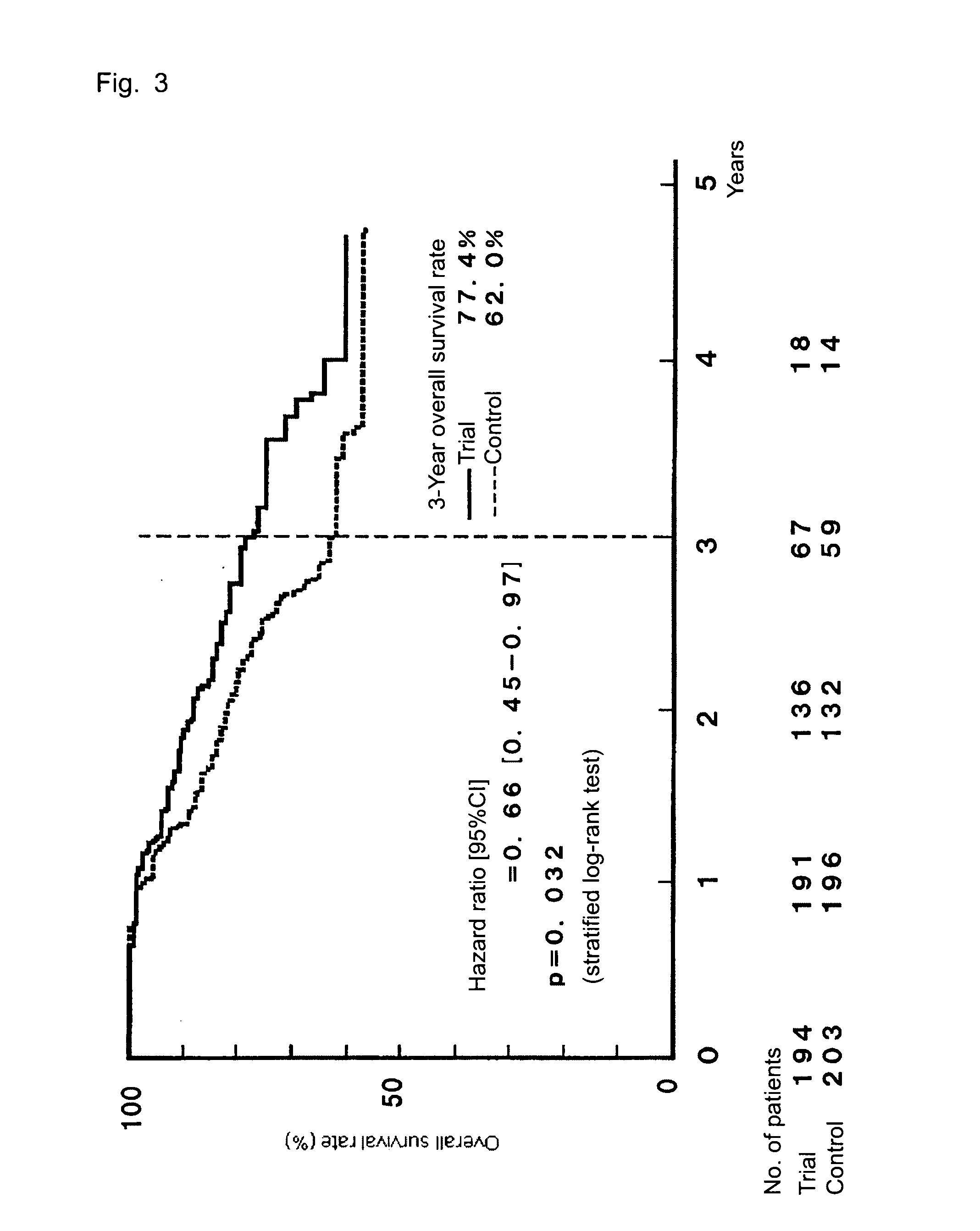
The invention provides a method for treatment of gastric cancer, which method is a postoperative adjuvant chemotherapy for gastric cancer, comprising orally administering, to a patient with gastric cancer in stage II, IIIA, or IIIB in a classification of the stage of gastric cancer, a pharmaceutical composition containing Tegafur, Gimeracil, and Oteracil potassium at a molar ratio of 1:0.4:1, at a dose of 50 to 150 mg / day as Tegafur dose and according to an administration schedule including, from within 45 days after a surgical operation of gastric cancer, drug administration for 28 consecutive days, followed by a rest period of 7 to 14 consecutive days.The chemotherapy reduces the relapse rate after a surgical operation, thereby improving the survival rate.
Owner:TAIHO PHARMA CO LTD
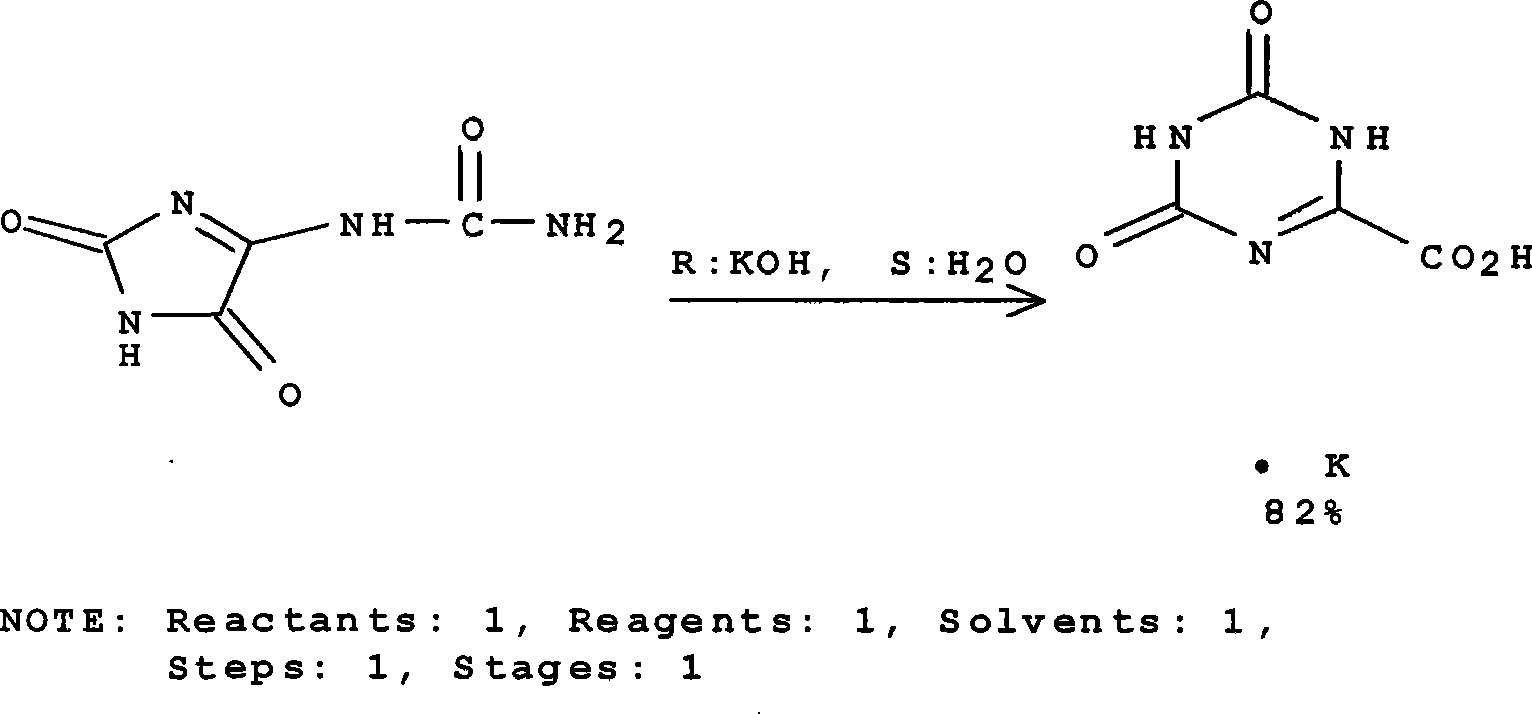
Oteracil potassium preparation method
InactiveCN104610180ANo pollutionRaw materials are easy to obtainOrganic chemistryPotassium hydroxidePotassium iodine
The invention discloses an oteracil potassium preparation method whcih comprises the following steps: allantoin is dissolved in the aqueous solution of potassium hydroxide or potassium carbonate, potassium iodide is used as a catalyst, potassium persulfate is used as an oxidant to synthesize oteracil potassium. Reaction steps of the method are short, the environmentally-friendly and cheap oxidant is used, use of toxic and harmful oxidant can be avoided, and the method has the advantages of simple operation, mild reaction condition, high yield, pure product, no any environmental pollution and the like, and is suitable for industrial production.
Owner:BEIJING MEDISAN TECH +1
Constructing method for subacute hyperuricemia renal damage mouse model
InactiveCN108524553AGood effectReasonably constructedCompounds screening/testingHeterocyclic compound active ingredientsYeastOTERACIL POTASSIUM
The invention discloses a constructing method for a subacute hyperuricemia renal damage mouse model. The method comprises the following steps that yeast cream and oteracil potassium are taken as a molding agent, a molding experiment is constantly carried out on an experiment mouse for multiple days, a subacute hyperuricemia renal damage mouse model is acquired, wherein during the molding experiment process, the yeast cream is given to the experiment mouse every day, and after the yeast cream is given to the experiment mouse in the last day of the molding experiment, the oteracil potassium is given to the experiment mouse; the weight, serum uric acid level, renal function change and renal pathology change of the experiment mouse are observed, and damage of the molding agent to the experiment mouse is analyzed. According to the constructing method, the mouse is taken as a model animal, the yeast cream in which a uric acid precusor substance is added for a long term is adopted, the oteracil potassium as a uricase inhibitor is taken as the molding agent and is used, and the subacute hyperuricemia renal damage mouse model with stable effect is in accordance with clinical features and issuccessfully constructed.
Owner:WUHAN POLYTECHNIC UNIVERSITY
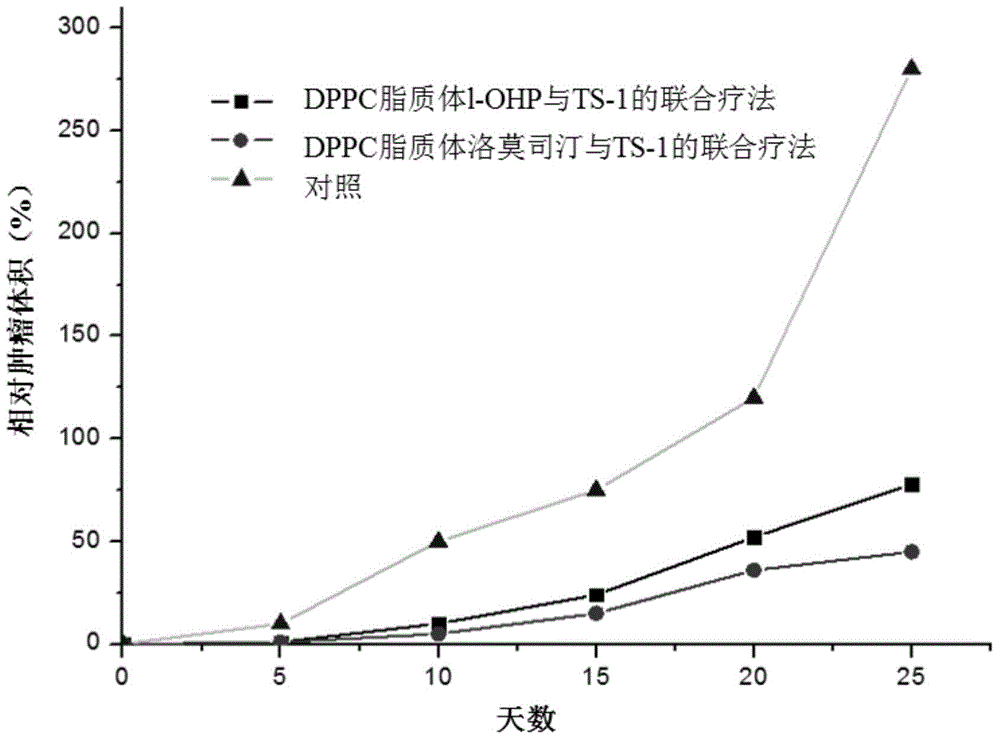
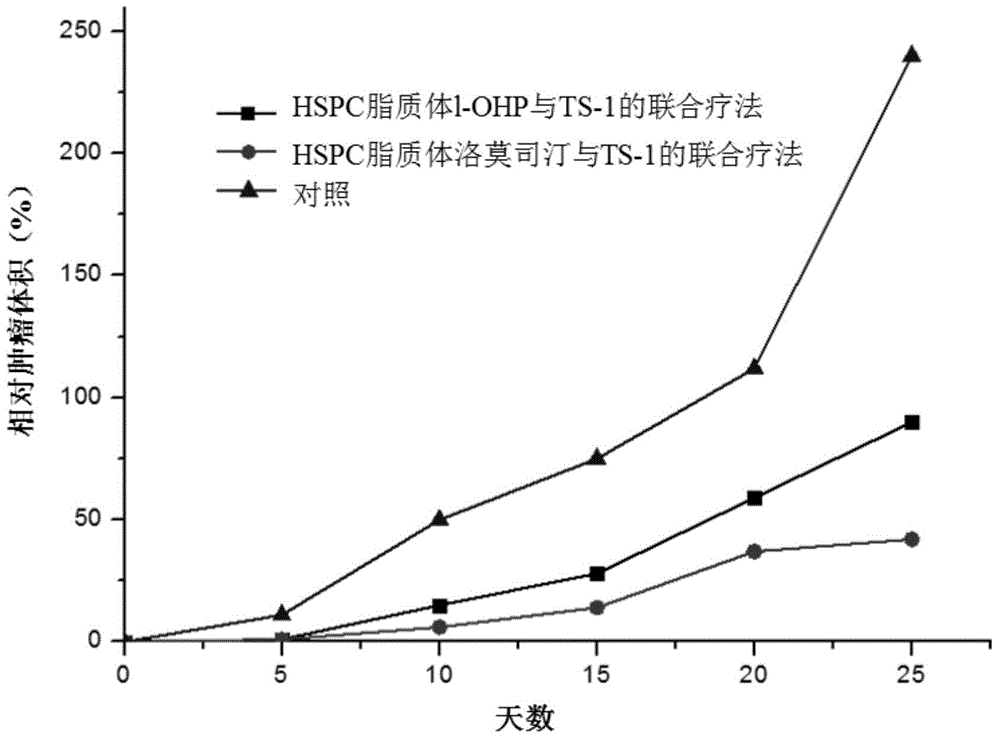
Anti-tumor effect enhancer and anti-tumor preparation
InactiveCN104434925AImprove anti-tumor effectImprove anti-tumor activityAntineoplastic agentsLiposomal deliveryOTERACIL POTASSIUMWilms' tumor
The invention relates to an anti-tumor effect enhancer and an anti-tumor preparation. The anti-tumor effect enhancer is a lipidosome preparation containing an effective dose of lumostine, and can be used for improving the anti-tumor activity of a combined medicine containing effective doses of tegafur, gimeracil and oteracil potassium. Compared with the prior art, the anti-tumor effect enhancer has the advantage of stronger anti-tumor enhancing effect for the combined medicine containing tegafur, gimeracil and oteracil potassium.
Owner:朱忠良
Refining method of oteracil potassium
The invention relates to the technical field of medicines and chemical industry, and specifically relates to a refining method of oteracil potassium. The refining method comprises the steps of heating and dissolving oteracil potassium crude product in aqueous solution containing alkaline matter, filtering, adding one or more of polar organic solvent to filtrate, cooling and crystallizing, and filtering to obtain high-purity oteracil. By adopting the refining method, oteracil potassium with the purity more than 99.96% can be obtained and the yield achieves more than 90%. The refining method has the advantages of being easy and simple to operate, low in production cost and high product purity, and is suitable for industrial production and use.
Owner:NANJING CHIA TAI TIANQING PHARMA
Antitumor effect potentiator and antitumor agent
InactiveCN1761665AImprove anti-tumor effectNo significant increase in toxicityOrganic chemistryPharmaceutical non-active ingredientsSide effectOTERACIL POTASSIUM
It is intended to provide: an antitumor effect potentiator containing a therapeutically efficacious amount of Tegafur, Gimeracil in an amount efficacious for potentiating the antitumor effect and Oteracil potassium in an amount efficacious in inhibiting side effects, characterized by containing at least one component selected from the group consisting of folinic acid and its pharmaceutically acceptable salts in an amount efficacious for potentiating the antitumor effect as the active ingredient; an antitumor agent characterized by containing a therapeutically efficacious amount of Tegafur, Gimeracil in an amount efficacious for potentiating the antitumor effect and Oteracil potassium in an amount efficacious in inhibiting side effects and , together with at least one component selected from the group consisting of folinic acid and its pharmaceutically acceptable salts in an amount efficacious for potentiating the antitumor effect as the active ingredient; a method of potentiating the antitumor effect of an antitumor agent characterized by comprising administering the above-described antitumor effect potentiator to a patient; and a method of treating cancer characterized by comprising administering the above antitumor agent to a patient.
Owner:TAIHO PHARMA CO LTD
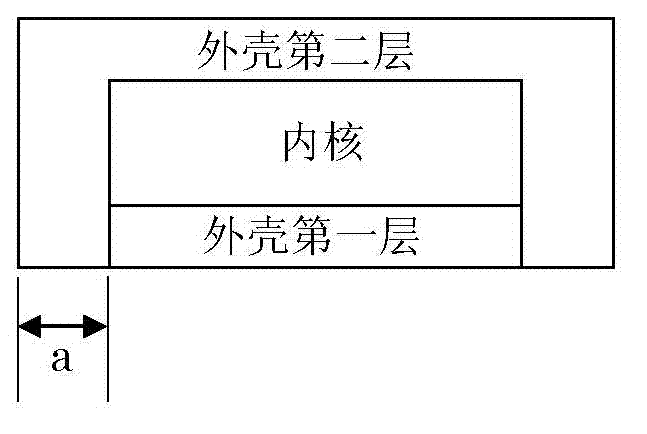
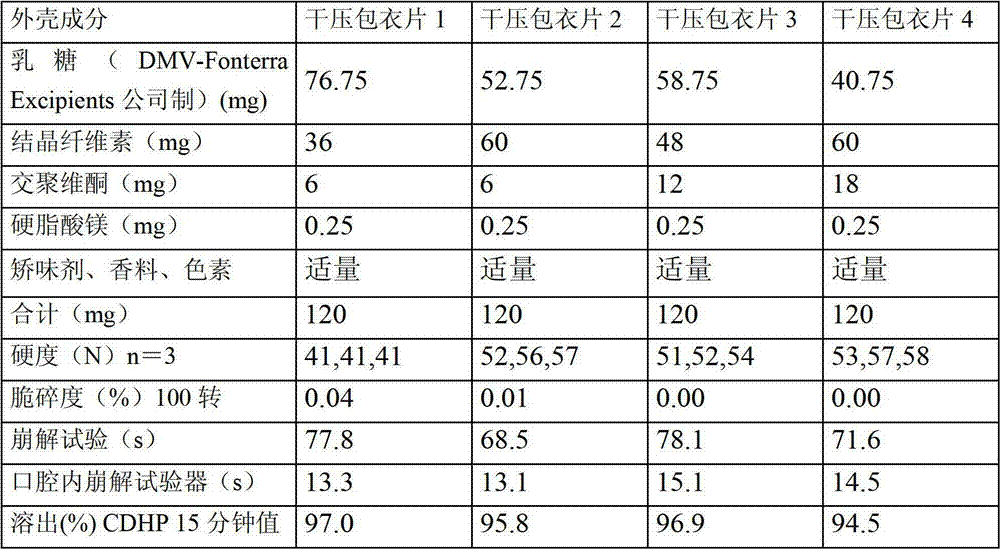
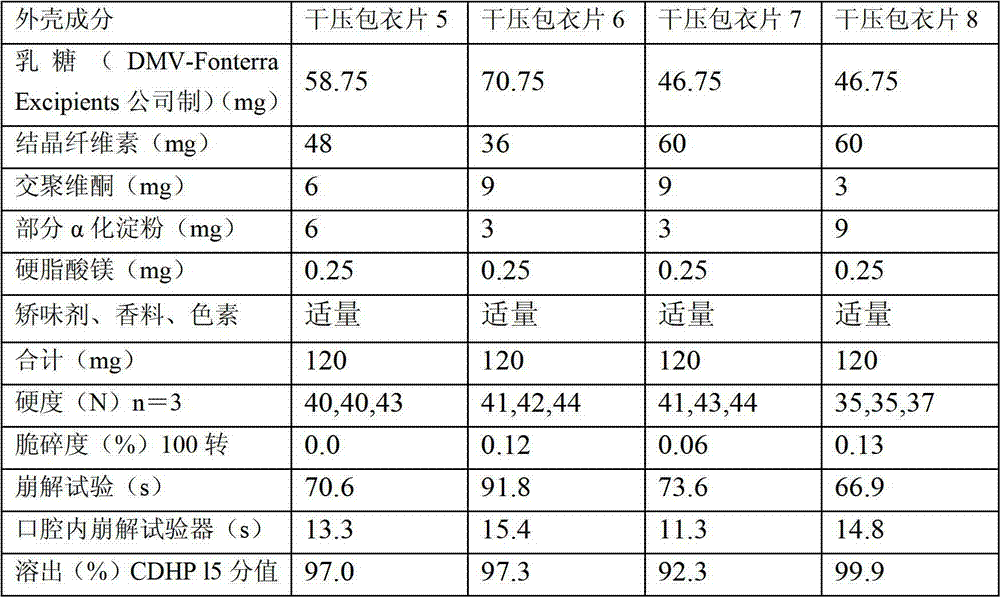
Dry pressed coating tablet containing tegafur, gimeracil and oteracil potassium
InactiveCN102895212ADisintegrates quicklyReduce riskPharmaceutical delivery mechanismPharmaceutical non-active ingredientsOTERACIL POTASSIUMChemistry
The invention provides a dry pressed coating tablet which is composed of an inner core and an outer core, and the inner core contains (a)tegafur, (b) gimeracil and (c)oteracil potassium which can be taken as effective components.
Owner:TAIHO PHARMA CO LTD
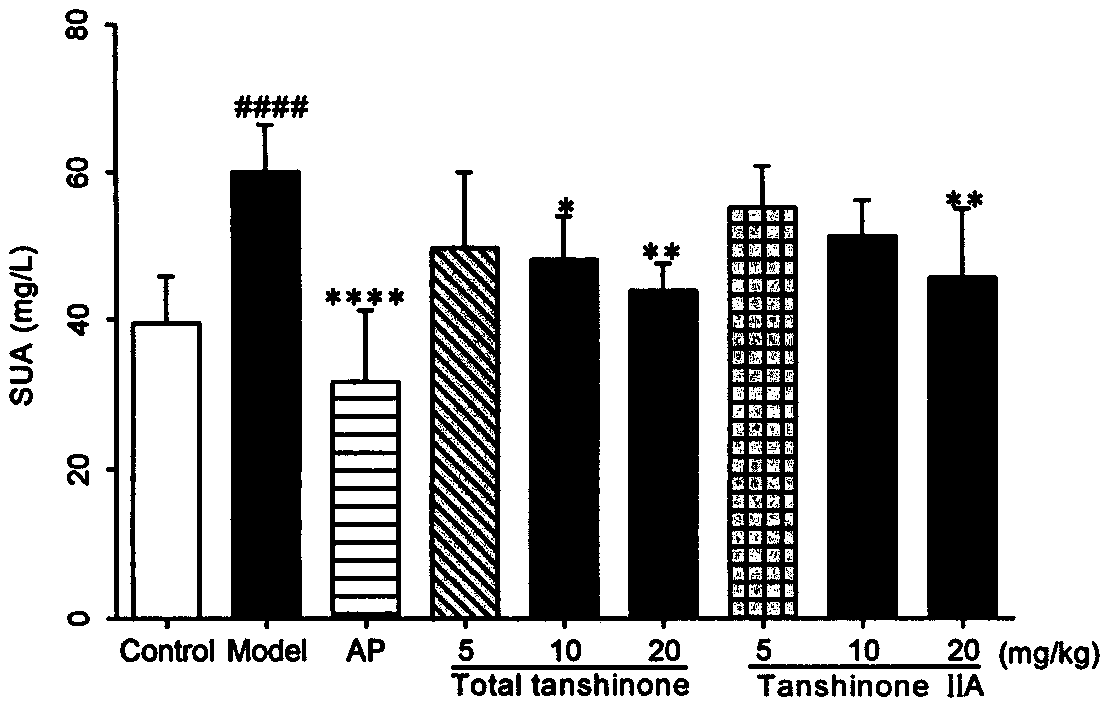
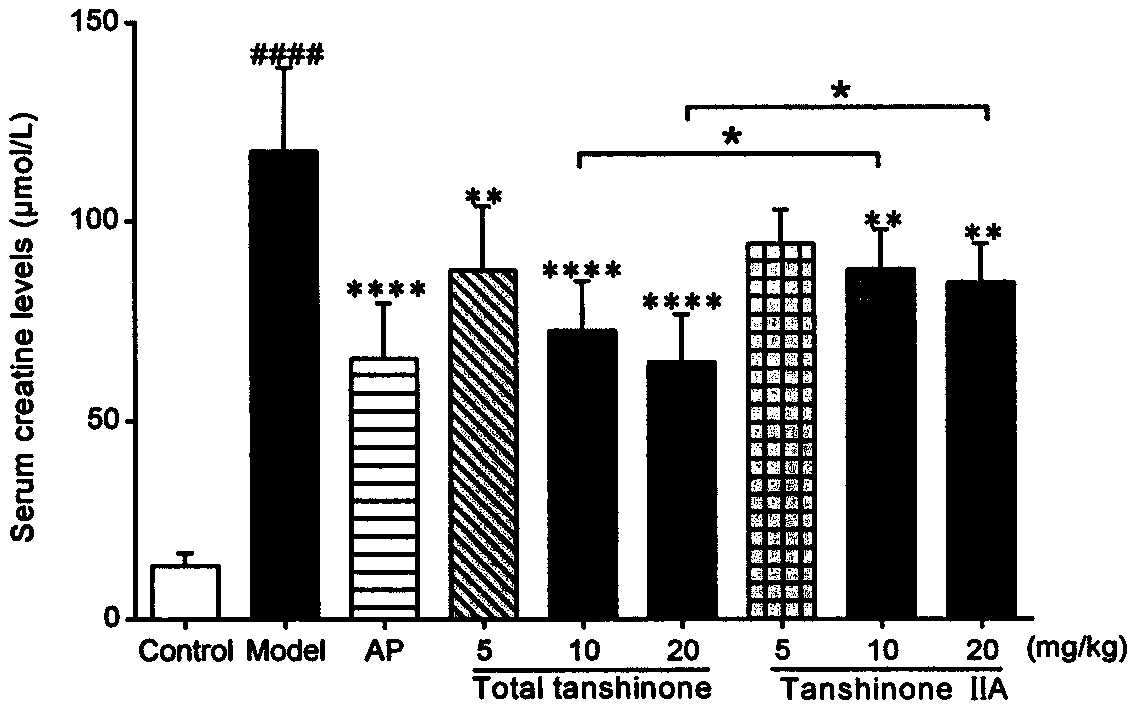
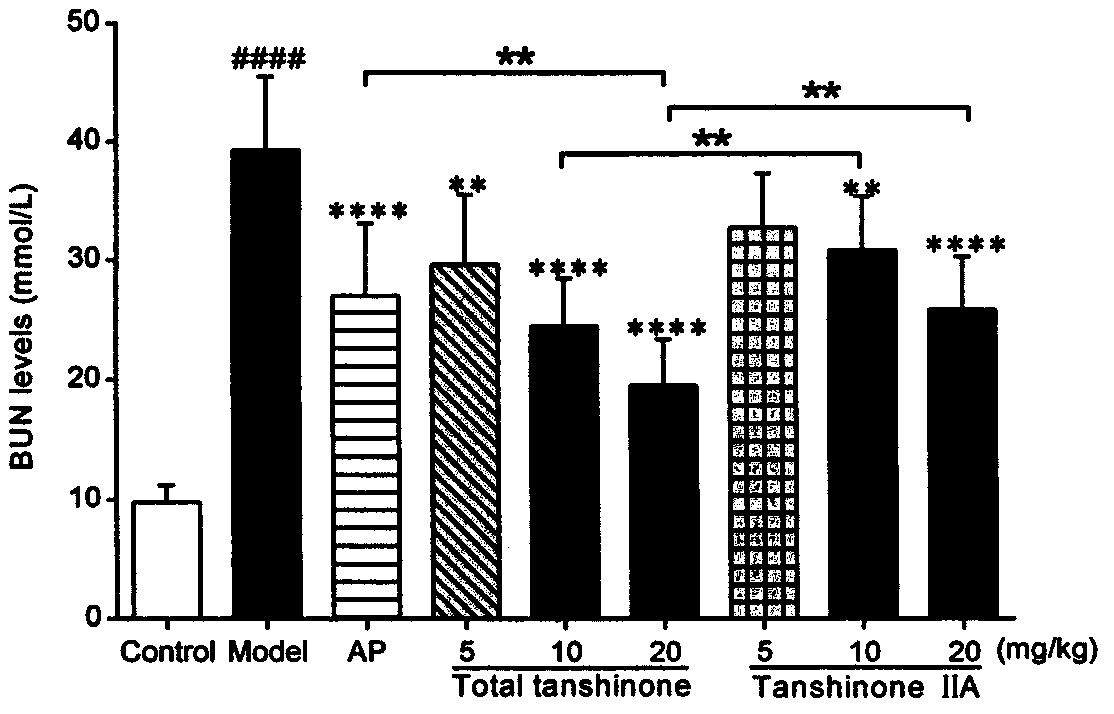
Use of tanshinone in resisting uric acid nephropathy
InactiveCN107913277AGood treatment effectLower creatinineOrganic active ingredientsSkeletal disorderAntioxidantAdditive ingredient
The invention relates to the field of natural medicine, in particular to a use of ethyl acetate extract of Salvia miltiorrhiza, namely tanshinone, in the preparation of drugs to resist uric acid nephropathy, and a use thereof in the preparation of antioxidant drugs. The drugs containing tanshinone are applied to treat uric acid nephropathy, particularly to prevent or treat hyperuricemia, gout andhyperuricemia-induced chronic nephropathy. Tanshinone mainly includes diterpene phenanthraquinone ingredients such as tanshinone IIA, 15,16-dihydrotanshinone I, cryptotanshinone and miltirone; owing to their synergy, the ingredients have better therapeutic effect in terms of prevention and treatment of uric acid nephropathy than single tanshinone IIA and the clinically common drug allopurinol under single dosage. When the four ingredients, tanshinone IIA, 15,16-dihydrotanshinone I, cryptotanshinone and miltirone, are combined in the optimal ratio of 6:3:6:4 into a tanshinone mixture, best prevention and treatment effect is provided for mouse uric acid nephropathy; through the use of the tanshinone combinations, Kunming mice co-modeled with adenine and oteracil potassium are significantly lower than model groups in terms of blood uric acid, creatinine and urea nitrogen, and kidney protection is achieved.
Owner:CHINA PHARM UNIV
Antitumor effect fortifier, antitumor agent and method of therapy for cancer
The present invention provides an antitumor effect potentiator, a method for treating cancer using a plurality of pharmaceutical preparations having excellent antitumor activity, and an antitumor preparation. In particular, the present invention provides an antitumor effect potentiator for potentiating the antitumor activity of an antitumor preparation comprising tegafur in a therapeutically effective amount, gimeracil in an amount effective for potentiating an antitumor effect, and oteracil potassium in an amount effective for inhibiting a side effect, the antitumor effect potentiator comprising cis-oxalate( 1R , 2R -diaminocyclohexane)platinum(II) in an amount effective for potentiating the antitumor effect; a method for treating cancer comprising the step of concomitantly administering tegafur in a therapeutically effective amount, gimeracil in an amount effective for potentiating an antitumor effect, oteracil potassium in an amount effective for inhibiting a side effect, and cis-oxalate( 1R,2R- diaminocyclohexane)platinum(II) in an amount effective for potentiating the antitumor effect; an antitumor preparation in a pharmaceutical form comprising a plurality of pharmaceutical agents each of which contains one of the active ingredients consisting of tegafur, gimeracil, oteracil potassium, and cis-oxalate( 1R , 2R -diaminocyclohexane)platinum(II), or each of which contains such active ingredients in any combination, or in a pharmaceutical form comprising a single pharmaceutical agent containing all the active ingredients; and a kit.
Owner:TAIHO PHARMA CO LTD
Duplication method of rat continuous hyperuricemia model
InactiveCN108030784AEasy to masterGood application prospectSkeletal disorderHeterocyclic compound active ingredientsIntraperitoneal routeFructose
The invention belongs to the technical field of experimental model building, and relates to a duplication method of a rat continuous hyperuricemia model. The method comprises the following four steps:animal selection, adaptive feeding, model building and model application; the method comprises the following specific steps: performing adaptive feeding on selected healthy rats in a clean animal feeding room for 1 week, and ensuring that rats with normal morphological characteristics are used for building a model; and injecting 500 mg.kg<-1> uricase inhibitor oteracil potassium to the rats withnormal morphological characteristics at room temperature every day in an intraperitoneal injection administration manner, and meanwhile giving 10 percent fructose drinking water and sufficient diet for 24 hours without interruption. Administration to rats and molding are continuously performed every day, and administration is continued for 3 weeks, a sample rat hyperuricemia model is obtained; andthe built rat hyperuricemia model is applied, so that a hypeluricemia model with typical disease characteristics is obtained. The method is simple to operate, and short in duplication cycle, can ensure that the hyperuricemia level of the rats can be obviously improved, can further properly simulate the clinical features of hyperuricemia, and has a good application prospect.
Owner:CHINA PHARM UNIV
A kind of capsule preparation containing tegafur, gemesta and oxonate potassium and preparation method thereof
ActiveCN101574326BWidely evenly distributedLess irritatingCapsule deliveryMacromolecular non-active ingredientsAdditive ingredientPotassium
The invention relates to a capsule preparation containing tegafur, gemestat and oxonate potassium and a preparation method thereof, which is characterized in that the capsule preparation contains tegafur pellets, gemesta pellets and oxonate potassium pellets , wherein the pellets are composed of active pharmaceutical ingredients, diluents, binders and surfactants; the pellets are prepared by extrusion-spheronization method, centrifugal-fluidization method and coating pot pan-pellet method, and then other excipients are added, Finally the capsules are filled. The capsule prepared by the invention can release the drug stably and quickly in the body, improves the stability of the drug, has better dissolution rate and bioavailability, and reduces the adverse reaction of the drug.
Owner:LUNAN PHARMA GROUP CORPORATION
Tegafur and gimeracil compound injecta
ActiveCN102309491AReduce gastrointestinal side effectsQuick effectOrganic active ingredientsPharmaceutical delivery mechanismChemistryOTERACIL POTASSIUM
The invention discloses tegafur and gimeracil compound injecta, comprising tegafur, gimeracil, one or several kinds of auxiliaries suitable for injecta, and the balance of water for injection, wherein the tegafur and the gimeracil serve as active constituents, and the tegafur and gimeracil compound injecta is characterized in that the molar ratio of tegafur to gimeracil to oteracil potassium is 1:(0.2-0.4):1.
Owner:TIANJIN JINYAO GRP
Novel application of garcinia mangostana L pericarp extract in prevention and treatment of hyperuricemia and gout
InactiveCN108785338AStrong uric acid-lowering effectLower serum uric acid levelsSkeletal disorderPlant ingredientsDiseaseSerum ige
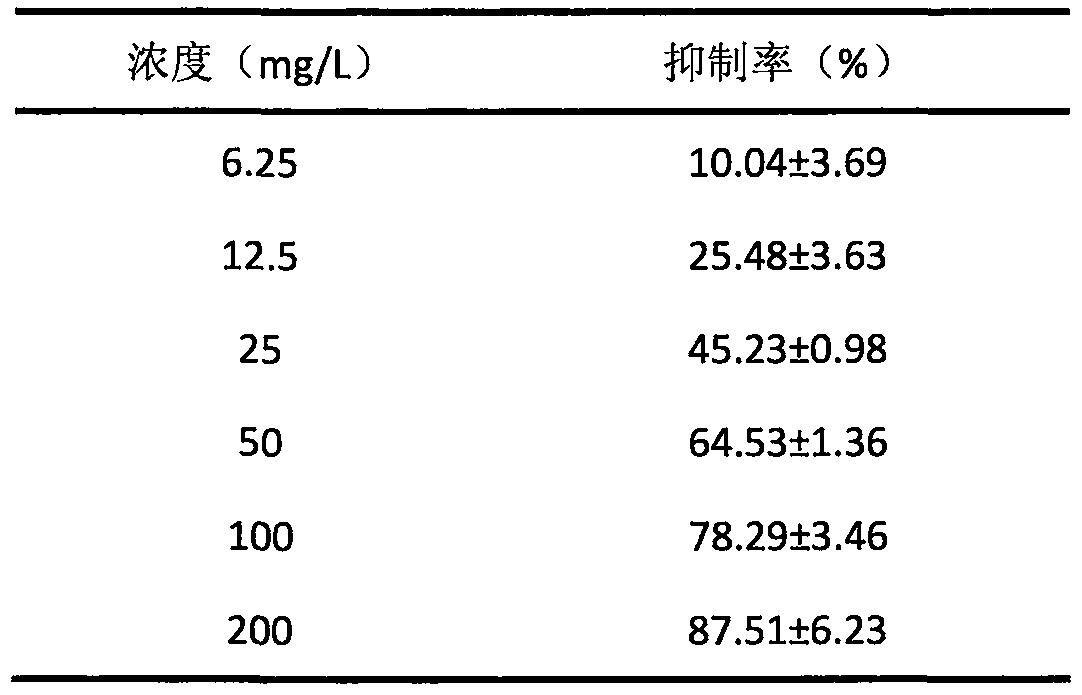
The invention relates to novel application of garcinia mangostana L pericarp extract in prevention and treatment of hyperuricemia and gout. Researches find that the garcinia mangostana L pericarp extract is capable of in-vitro inhibiting activities of xanthine oxidase, and IC50 of the garcinia mangostana L pericarp extract is 31.39ml / L. in vivo studies show that the garcinia mangostana L pericarpextract has a strong uric acid lowering effect, by feeding 20mg / kg of the garcinia mangostana L pericarp extract to a mouse with oteracil potassium induced hyperuricemia intragastrically, the serum uric acid level of the mouse can be obviously lowered, which means the uric acid lowering effect of the garcinia mangostana L pericarp extract is related to inhibition of the xanthine oxidase activities, and generation of the uric acid is inhibited. By feeding 2.0g / kg of the garcinia mangostana L pericarp extract to the mouse intragastrically, no animal dies, and no obvious toxic and side effect isfound, so that the garcinia mangostana L pericarp extract can be used for preparing oral medicaments for treating the gout and the hyperuricemia and medicament and health care products for diseases related to the hyperuricemia.
Owner:KUNMING MEDICAL UNIVERSITY +1
Refining method for preparing high-purity oteracil potassium
ActiveCN101475539BEasy to operateLess likely to cause side effectsOrganic chemistryPotassium oxonateOTERACIL POTASSIUM
The invention provides a method for refining high-purity potassium oxonate. The method is characterized by comprising the following steps: adding an potassium oxonate crude product into water; adding alkali into the water until the crude product is completely dissolved; adding a polar solvent into the solution; adjusting the pH of the solution to neutrality by acid; filtering and separating out crystal; and drying the crystal so as to obtain the high-purity potassium oxonate. The method has the advantages of simple operation, mild reaction condition, high yield, pure product and the like, andhas yield over 90 percent, purity over 99.95 percent (detected by HPLC), and a single impurity peak reduced from 0.5 percent to below 0.05 percent.
Owner:LUNAN PHARMA GROUP CORPORATION
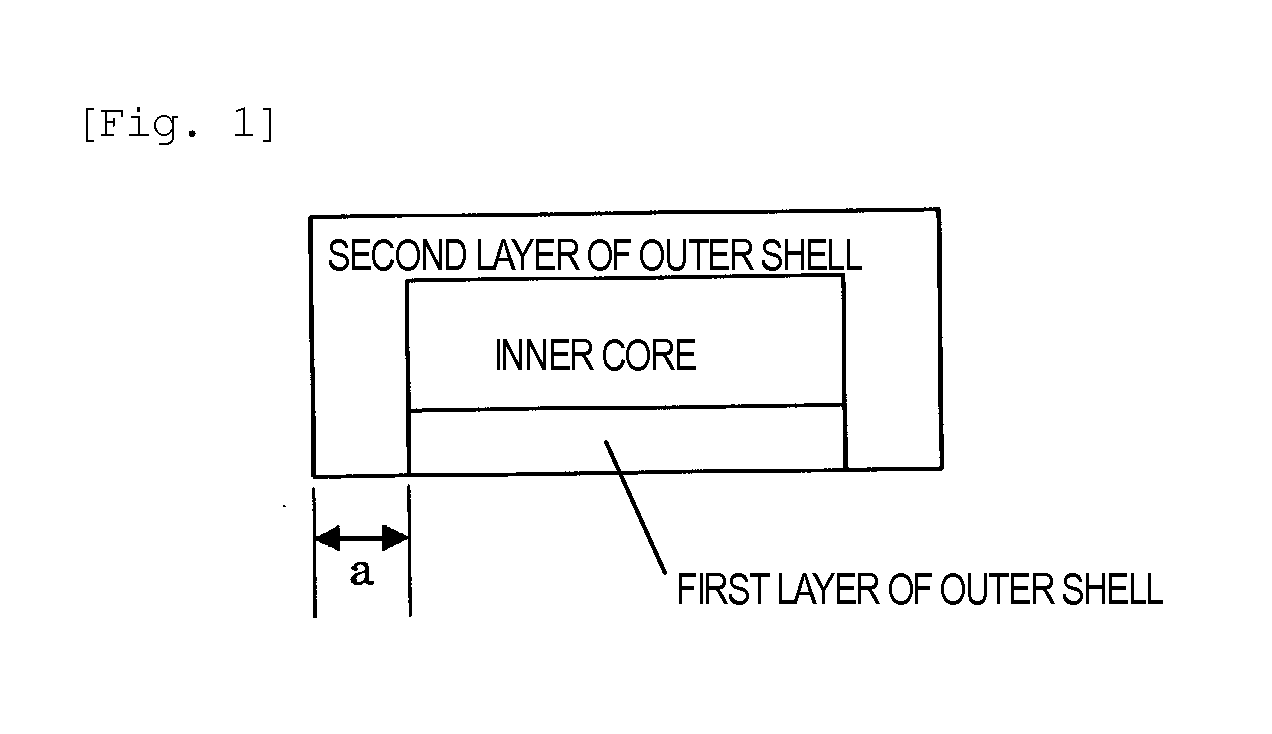
Dry-coated tablet containing tegafur, gimeracil and oteracil potassium
InactiveUS20140335174A1Reduce riskAdequate disintegrationBiocidePharmaceutical non-active ingredientsCoated tabletsOTERACIL POTASSIUM
The present invention provides a dry-coated tablet comprising: an inner core containing, as active ingredients, (a) tegafur, (b) gimeracil, and (c) oteracil potassium; and an outer shell.
Owner:TAIHO PHARMA CO LTD
Tegafur, gimeracil and oteracil potassium compound injecta
InactiveCN102309492AReduce gastrointestinal side effectsQuick effectPharmaceutical delivery mechanismAntineoplastic agentsOTERACIL POTASSIUMChemistry
The invention discloses tegafur, gimeracil and oteracil potassium compound injecta, comprising tegafur, gimeracil, oteracil potassium, one or several kinds of auxiliaries suitable for injecta, and the balance of water for injection, wherein the tegafur, the gimeracil and the oteracil potassium serve as active constituents, and the tegafur, gimeracil and oteracil potassium compound injecta is characterized in that the molar ratio of tegafur to gimeracil to oteracil potassium is 1:(0.2-0.4):1.
Owner:TIANJIN JINYAO GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com