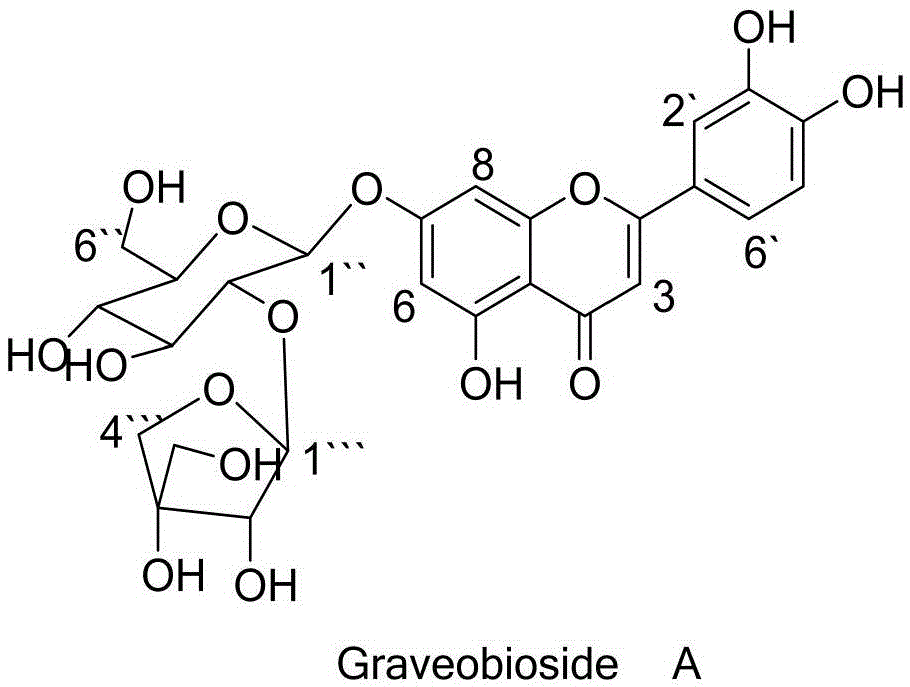
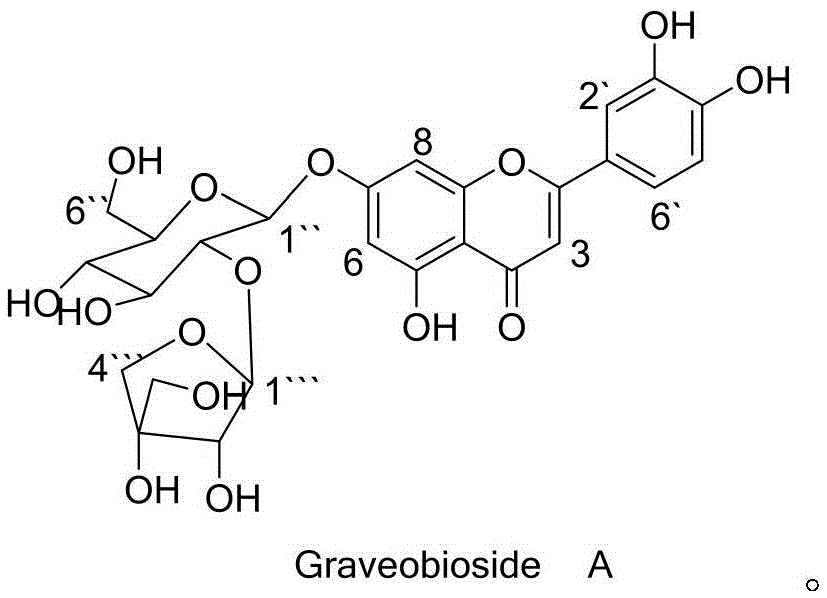
Application of Graveobioside A in preparation of drugs or healthcare food for preventing hyperuricemia and gout
A technology for hyperuricemia, health food, applied in the fields of natural medicinal chemistry and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation method of GraveobiosideA:
[0034] Celery dry seeds 10kg, with 8-15 times the amount of 30-70% (V / V) ethanol percolation extraction or heating extraction 2-4 times, each time for 0.5-2 hours, the extract is concentrated under reduced pressure to nearly no ethanol, and then concentrated Extract the liquid with 1 to 3 times of ethyl acetate for 1 to 3 times, separate the ethyl acetate and recover the ethyl acetate, pass the water layer through the D101 macroporous adsorption resin, elute with water first, then elute with 50% ethanol, Collect 0% ethanol eluate, concentrate, concentrate on silica gel column chromatography, use chloroform:methanol (90:10-0:100) gradient elution, collect eluate in sections, follow up with thin layer chromatography, and combine GraveobiosideA The fractions were concentrated, and the concentrate was purified by SephadexLH-20 gel chromatography, eluted with methanol, the eluate was collected, concentrated and dried under reduced pres...
Embodiment 2
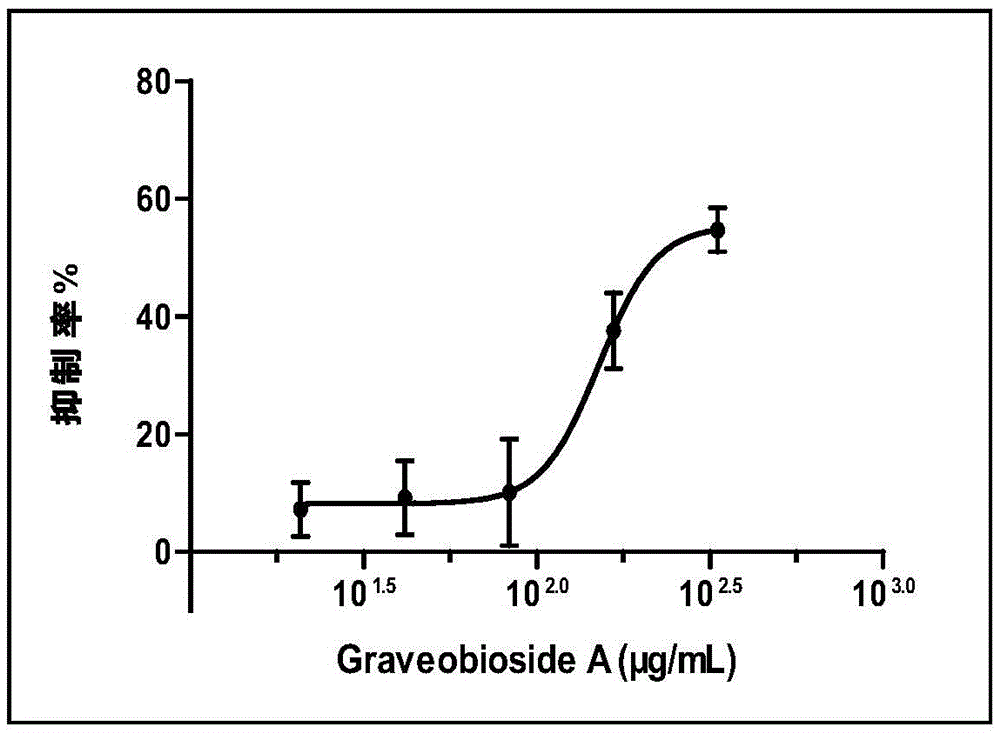
[0046] Effect of GraveobiosideA on hyperuricemia induced by oxonic acid potassium salt in mice:
[0047] The three dosage groups of Graveobioside A in Example 1 are 20, 10, and 5 mg / kg respectively, and are prepared into solutions with concentrations of 1, 0.5, and 0.25 mg / mL with pure water; allopurinol tablets are prepared with 2 mg / mL concentration with pure water suspension.
[0048] Seventy-two male ICR mice of 21-24 g were randomly divided into 6 groups according to body weight: normal control group; model control group; positive control allopurinol tablet 40 mg / kg group; Graveobioside A20, 10, 5 mg / kg group. Except for the positive control allopurinol tablet, which was intragastrically administered once on the day of the experiment, the animals in the other groups were intragastrically administered once a day according to the dose, for 3 consecutive days. On the 2nd day, the mice were fasted overnight (12h), and in the morning of the next day, except the normal control...
Embodiment 3
[0057] Effect of GraveobiosideA on carrageenan-induced paw swelling in mice:
[0058] The three dosage groups of Graveobioside A in Example 1 were 200, 100, and 50 mg / kg respectively, and were prepared into solutions with concentrations of 1, 0.5, and 0.25 mg / mL with pure water; mg / mL suspension.
[0059] Male ICR mice of 19-21 g were selected and randomly divided into 5 groups according to body weight: control group; positive control groups of indomethacin tablet 10 mg / kg, Graveobioside A20, 10, 5 mg / kg, 12 in each group. Except for the indomethacin tablet which was administered by intragastric administration only once on the day of the experiment, the animals in the other groups were intragastrically administered once a day according to the dose for 3 consecutive days, and the control group was given 20mL / kg.bw of pure water. 30 minutes after the last administration, 0.05 mL / foot of 1% carrageenan was subcutaneously injected into the right hind foot of the mice in each grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com