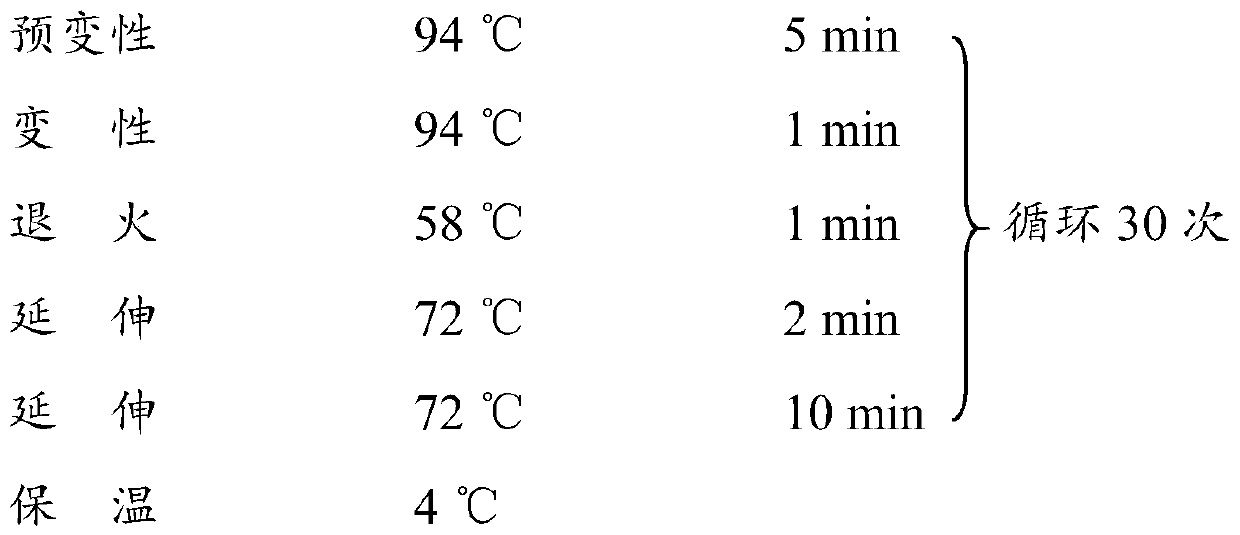
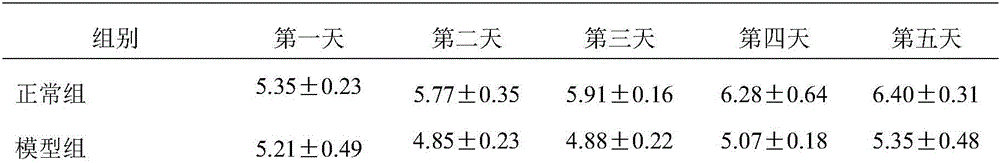
Patents
Literature
248 results about "Intragastric administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
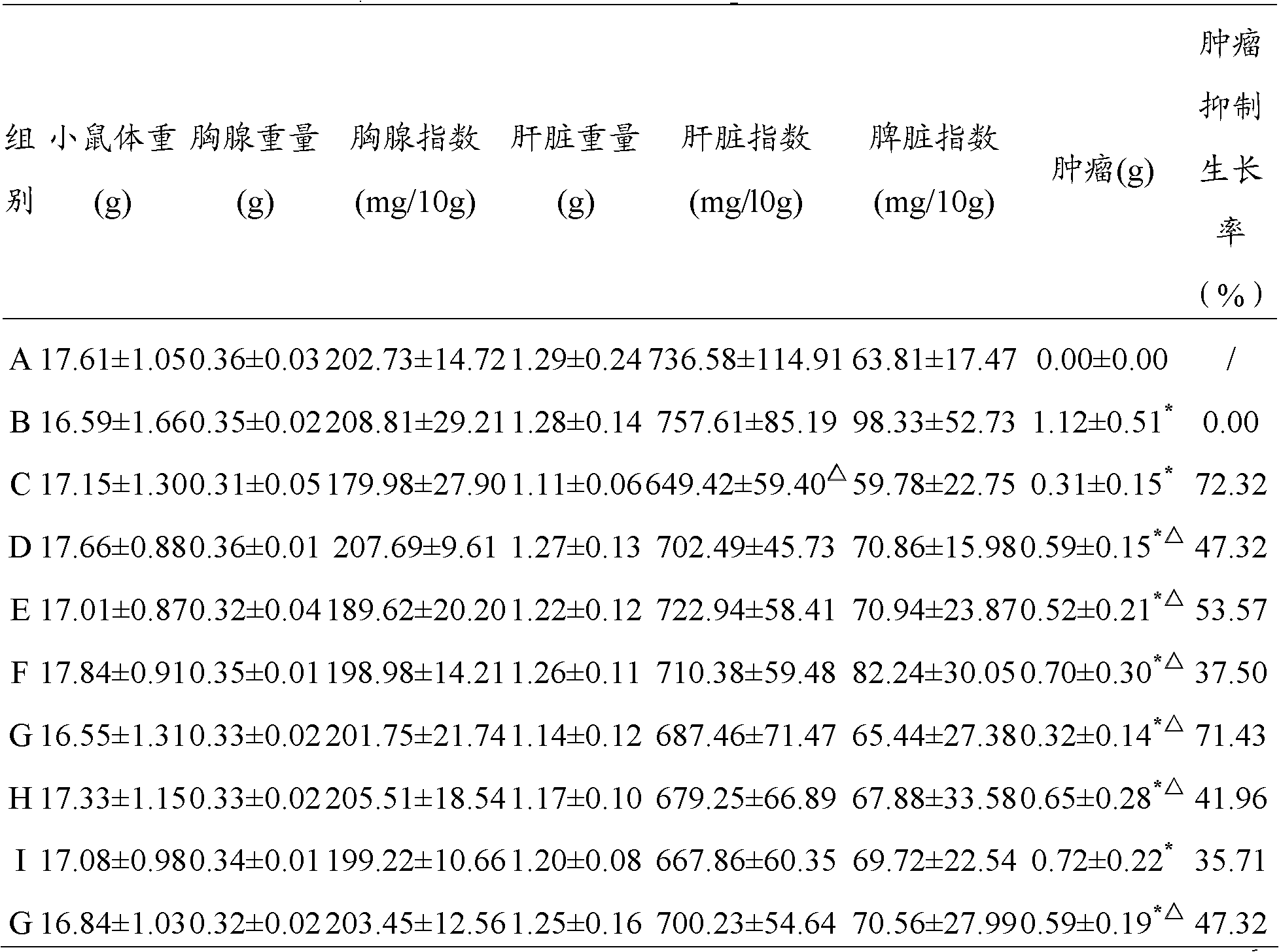
Application of Akkermansia muciniphila BAA-835 bacterial strain
InactiveCN105106245AEffective weightEffective Body Fat ControlMetabolism disorderAnimal feeding stuffMicrobiologyBacterial strain
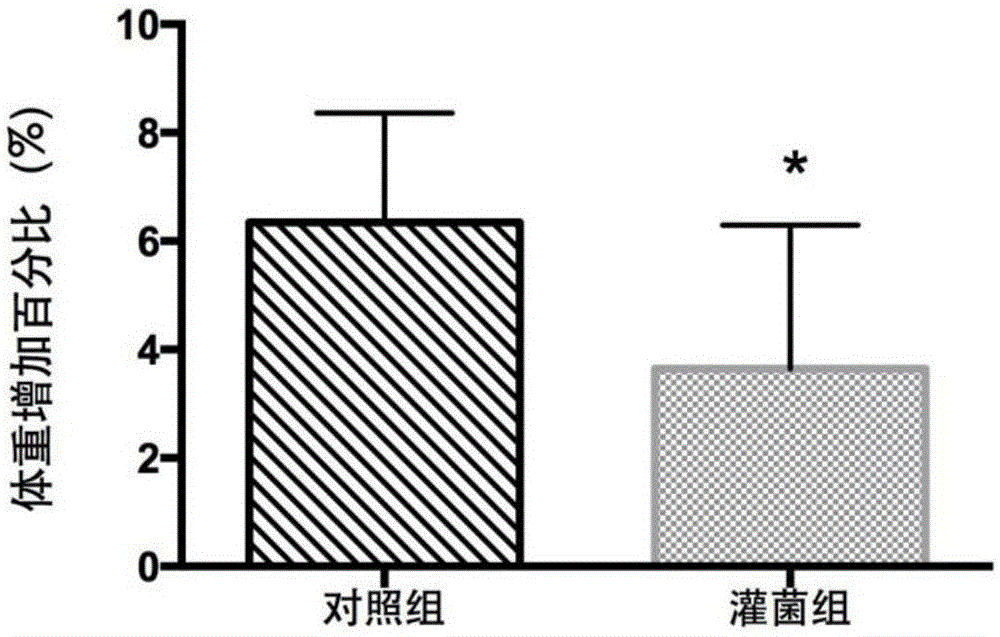
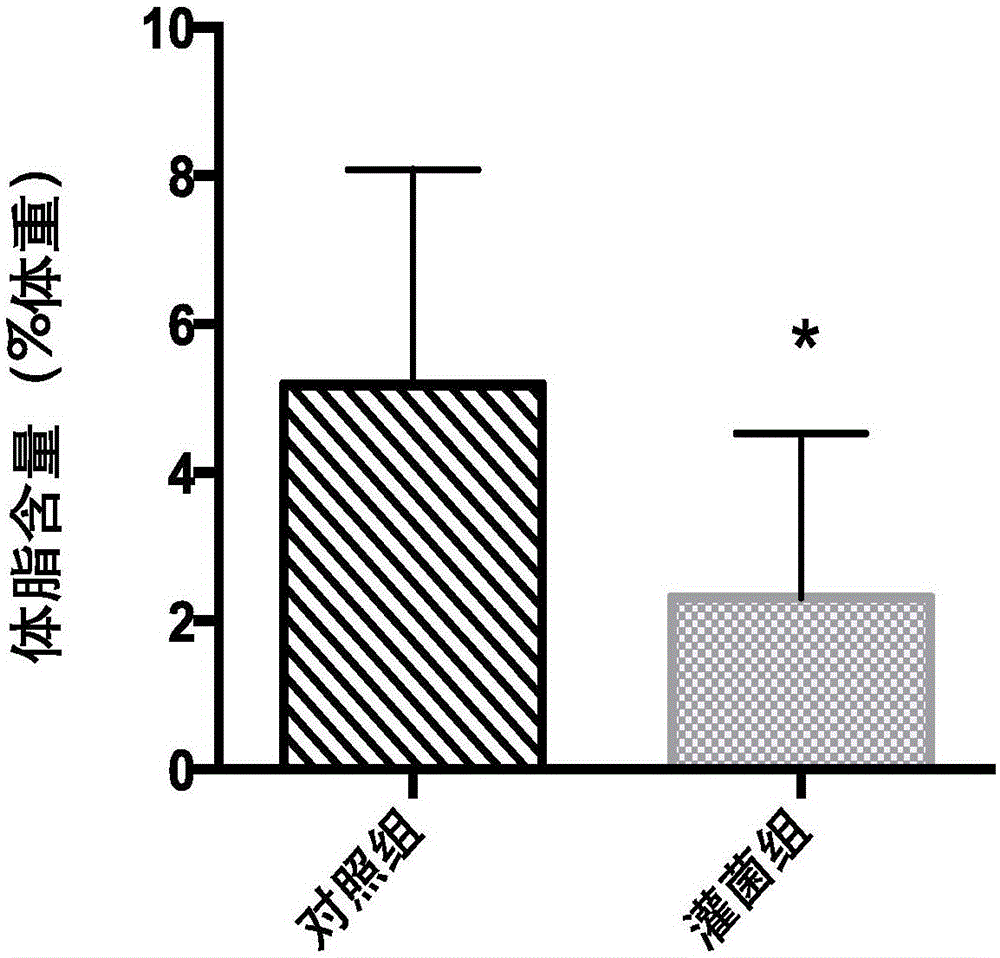
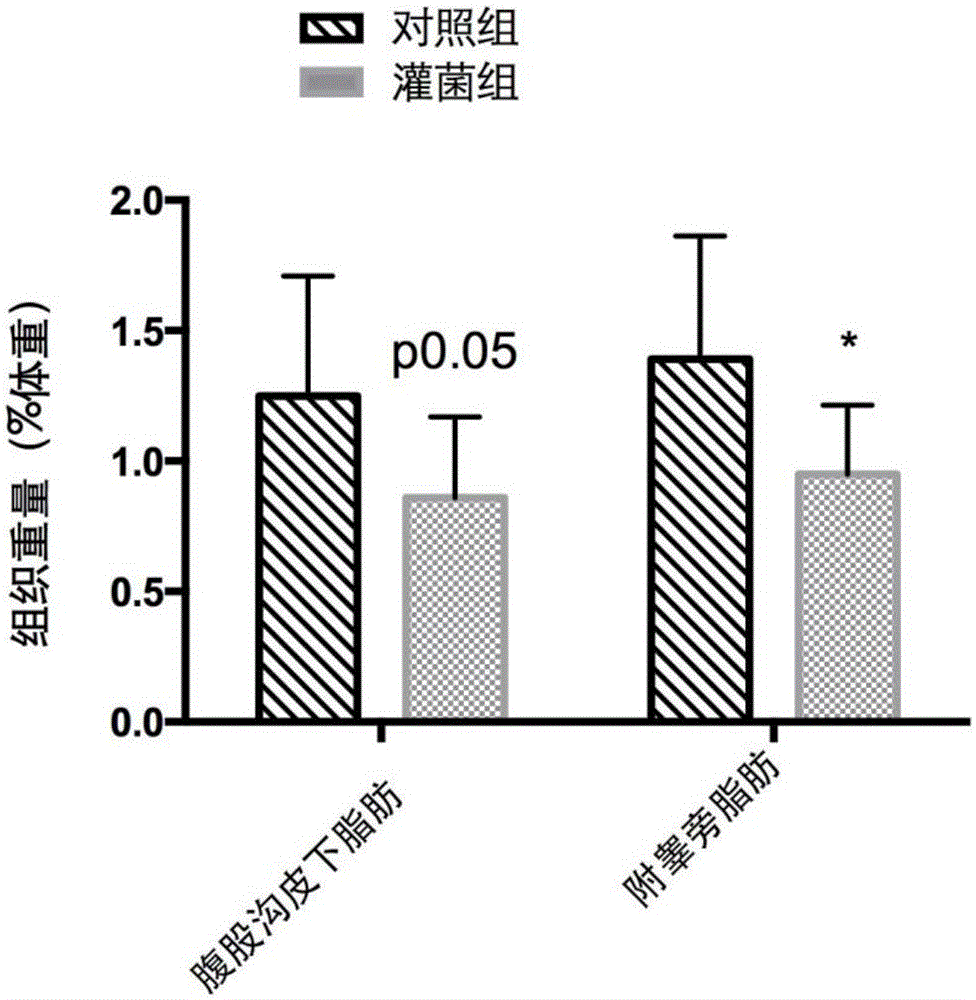
The invention belongs to the field of application of probiotics, and particularly relates to application of an Akkermansia muciniphila BAA-835 bacterial strain on preparation medicines for treating or preventing obesity. C57 / BL6 male SPF-level mice in a nest are randomly divided into a comparison group and a bacteria intragastric administration group, according to a check experiment, an Akkermansia muciniphila BAA-835 bacterial strain suspension intragastric administration experiment is carried out on the mice of the bacteria intragastric administration group once every day, and lasts for 5 weeks, through the experiment, weight and body fat of the mice in the bacteria intragastric administration group are reduced obviously, and by the Akkermansia muciniphila BAA-835 bacterial strain, content of subcutaneous fat of groins of the mice and content of fat beside epididymides of the mice are reduced quite effectively, and therefore, the bacterial strain can be used for treating or preventing the obesity.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
L.plantarum UA149 strain and application thereof
ActiveCN110055199AReduce synthesisLower uric acid levelsBacteriaMicroorganism based processesInflammatory factorsResearch Object
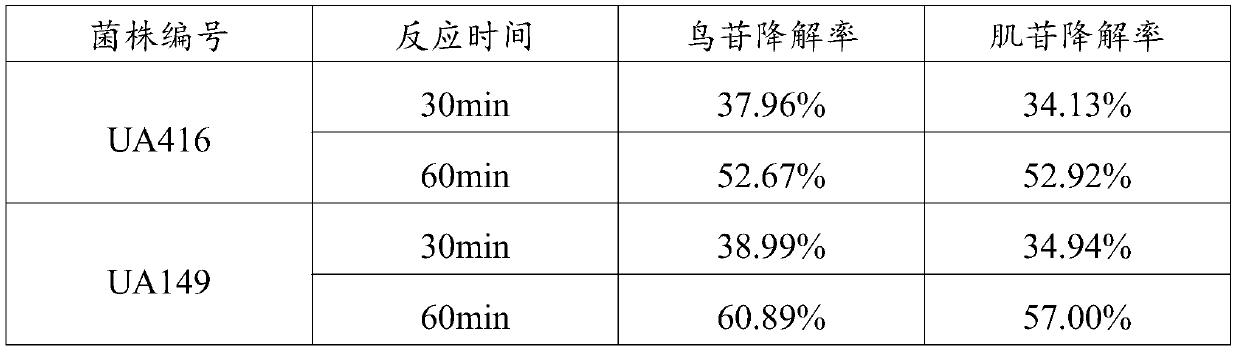
The invention discloses an L.plantarum UA149 strain and an application thereof, and belongs to the field of functional food microorganisms. The L.plantarum UA149 strain is deposited in China Typical Culture Collection Center on November 29, 2018 with the preservation number of CCTCC No: M2018842. The strain can be apply to preparing products with a uric acid reducing function or a gout resisting function. Lactic acid bacteria separated and identified from the surface of fleshy plant leaves are taken as research objects, and a new strain of lactic acid bacteria is screened through a large number of experiments. Hyperuricemia model rats are established by potassium oxazinate combined with fructose water, continuous intragastric administration of the lactobacillus plantarum UA149 strain for 14 days can significantly reduce the level of uric acid; and during gout attack, the release of inflammatory factors thromboxane and leukotriene mediated by neutrophils is reduced, the influx of neutrophils into joints is avoided, and the symptoms of redness, swelling, pain, heat and the like are reduced.
Owner:JILIN MINGZHIYUAN BIOTECH
Euonymus alatus extract, blood-sugar-reducing activity thereof and application of euonymus alatus extract to preparation of products for reducing blood sugar
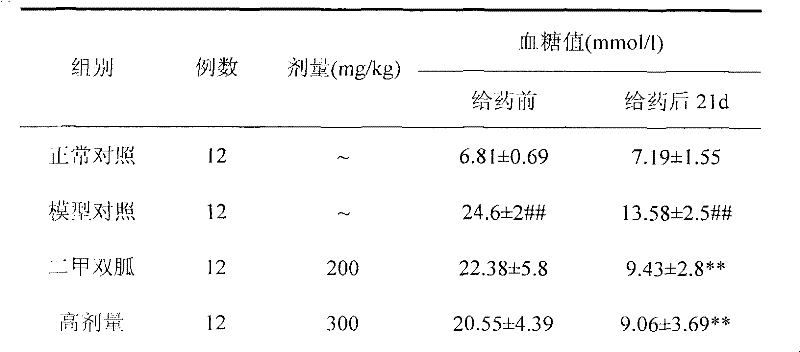
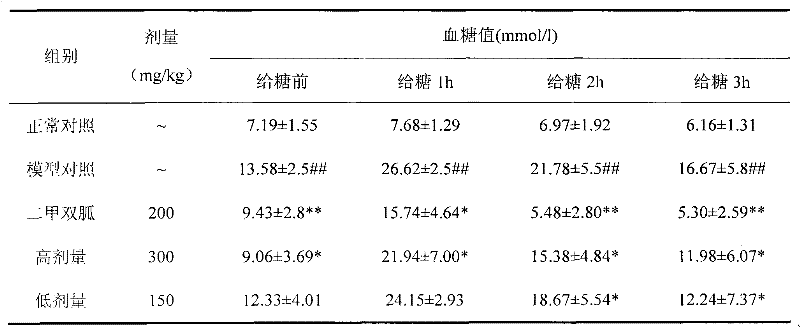
The invention relates to a euonymus alatus extract and a preparation process, a quality control method, the blood-sugar-reducing activity of the euonymus alatus extract and application of the euonymus alatus extract to the preparation of products for reducing blood sugar. The euonymus alatus extract can be prepared by any one of a solvent extracting method, a macroporous adsorbent resin method, a supercritical fluid extraction method, column chromatography, liquid-liquid counter-current partition chromatography and the like or a combination of the methods. The prepared euonymus alatus extract comprises the following active ingredients: flavonoids, phenols, alkaloids, triterpenoids, sterides, organic acids and polysaccharides, wherein the flavonoids and the phenols are main ingredients, and the content of general flavones is between 5 and 100 weight percent. Blood-sugar-reducing experiments of mice which suffer from diabetes caused by mesoxyalyurea prove that the raising of the blood sugar after the intragastric administration of glucose is inhibited effectively, and the euonymus alatus extract has a certain treatment effect on experimental diabetes caused by pancreas islet beta cell injury caused by the mesoxyalyurea, and can be applied to the preparation of the products for reducing the blood sugar.
Owner:石任兵
Application of wedelolactone in preparation of anti-pulmonary fibrosis drug
ActiveCN103816148AEnhance pharmacological effectsHas liver protectionRespiratory disorderHeterocyclic compound active ingredientsWedelolactoneHydroxyproline
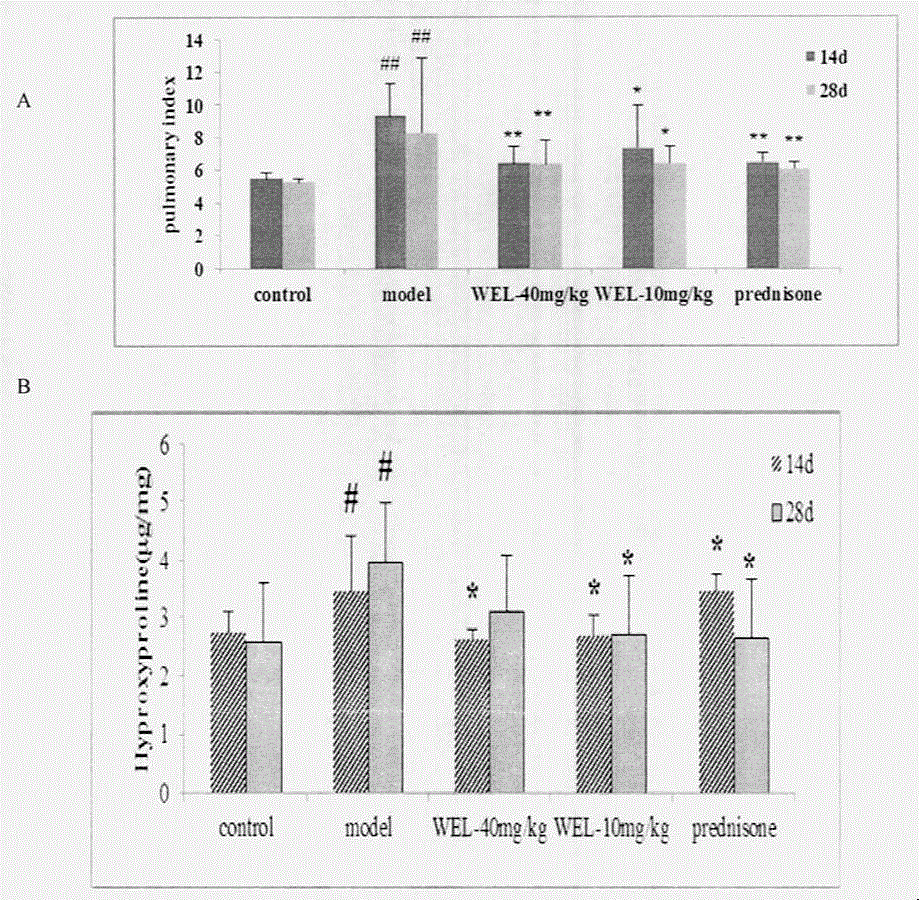
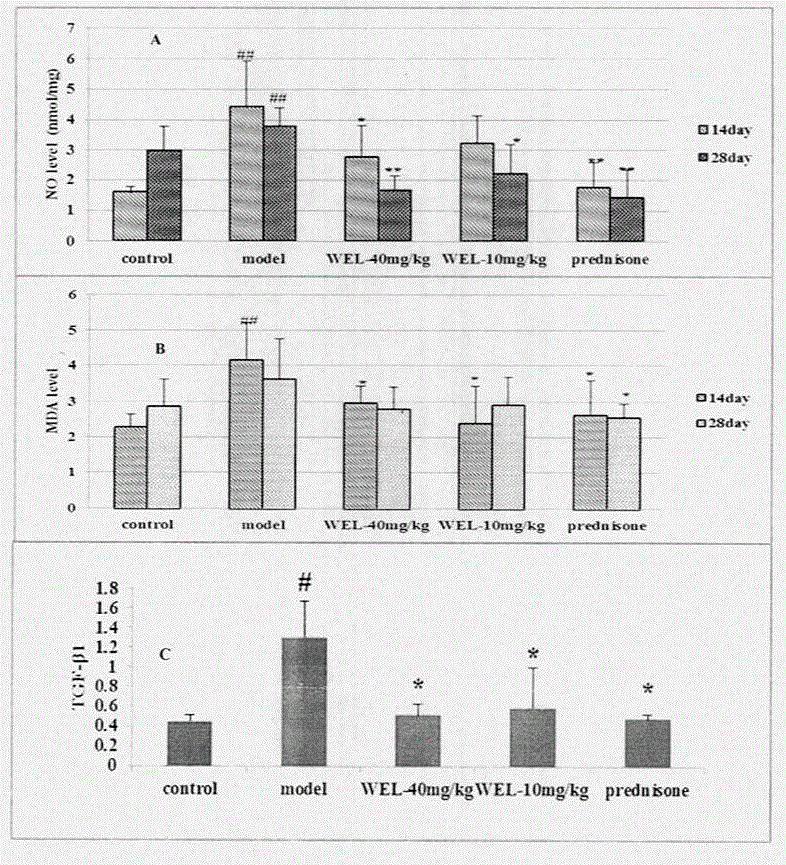
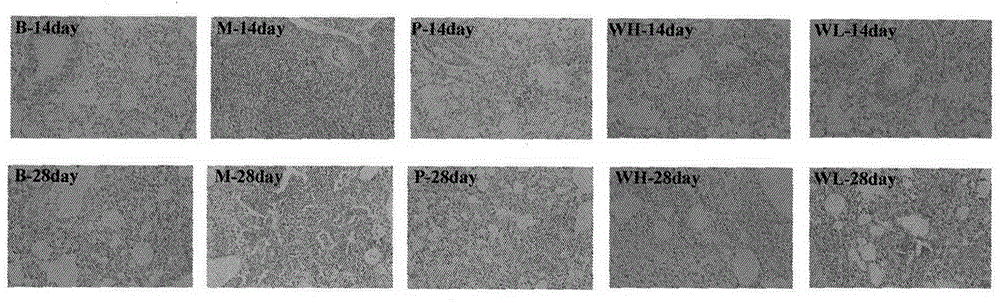
The invention relates to a drug application of a chemical substance wedelolactone for treatment of pulmonary fibrosis. The chemical substance wedelolactone is derived from a traditional Chinese medicine eclipta, and the structure is determined through spectral data; the wedelolactone can significantly improve the degree of model mouse lung tissue pulmonary fibrosis after intragastric administration, reduce NO (Nitrogen Oxide) and MDA (Methylene dioxyamphetamine) contents reflecting degree of lung injury, HYP (Hydroxyproline) content reflecting collagen deposition in the lung tissues, and cell factor TGF-beta 1 (Transforming Growth Factor) content causing pulmonary fibrosis, and inhibit human embryo lung fibroblast proliferation in vitro and HYP content of the human embryo lung fibroblast. In-vivo and in-vitro experiments show that wedelolactone can obviously improve bleomycin induced mouse pulmonary fibrosis, so that the wedelolactone can be taken as a novel drug for treating pulmonary fibrosis.
Owner:CHINA PHARM UNIV
Application of lucide ganoderma and ginseng medicinal mycoplasm in preparing drugs for treating lung cancer
InactiveCN102614238AGood curative effectSmall side effectsAntineoplastic agentsPlant ingredientsCisplatinAdditive ingredient
The invention provides an application of lucide ganoderma and ginseng medicinal mycoplasm in preparing drugs for treating lung cancer, wherein the lucide ganoderma and ginseng medicinal mycoplasm takes ginseng as a base material and lucide ganoderma as a strain, is obtained after bidirectional solid fermentation, contains ginsenosides Rg1, Rb1, Re, Rg3, Rh1 and various ginsenosides, as well as ginseng polysugar, ganoderan and other ingredients, and can be used for producing drugs for treating lung cancer. According to the invention, the lucide ganoderma and ginseng medicinal mycoplasm is taken as an internal treatment drug for carrying out research on a mice Lewis with lung cancer, is respectively used for the intragastric administration of the mice at the dosages of 0.5g / kg, 1.0k / kg and 2.0g / kg, meanwhile, a blank group, a model group, a cisplatin group, a Shenyi capsule group, a ginseng group, a lucide ganoderma group and a ginseng and lucide ganoderma group are taken for comparison, results show that: the lucide ganoderma and ginseng medicinal mycoplasm has the equivalent treatment effect on lung cancer as cisplatin, Shenyi capsules and other drugs, and is better than the lucide ganoderma, ginseng and the ginseng plus the lucide ganoderma.
Owner:邱智东 +2
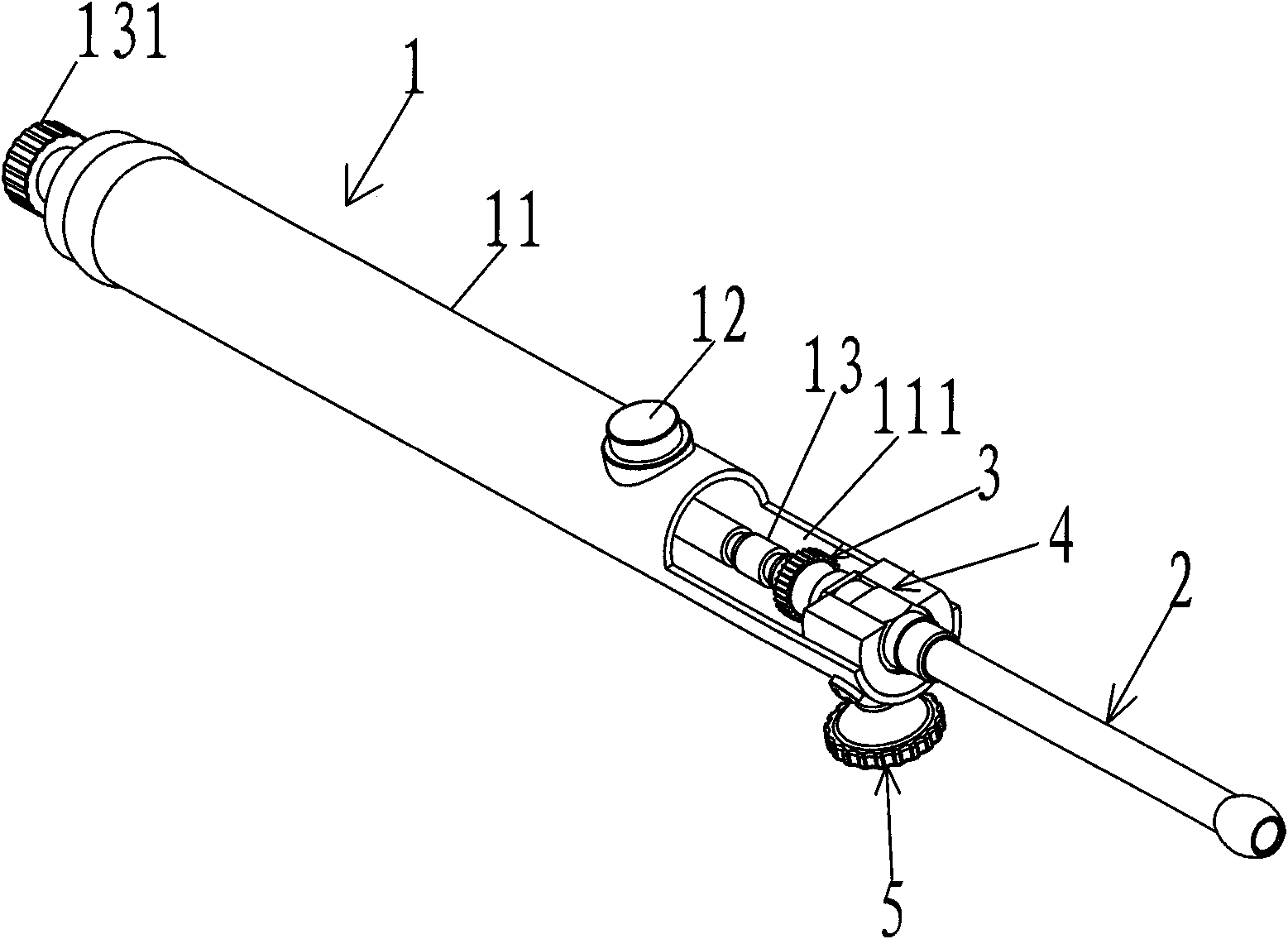
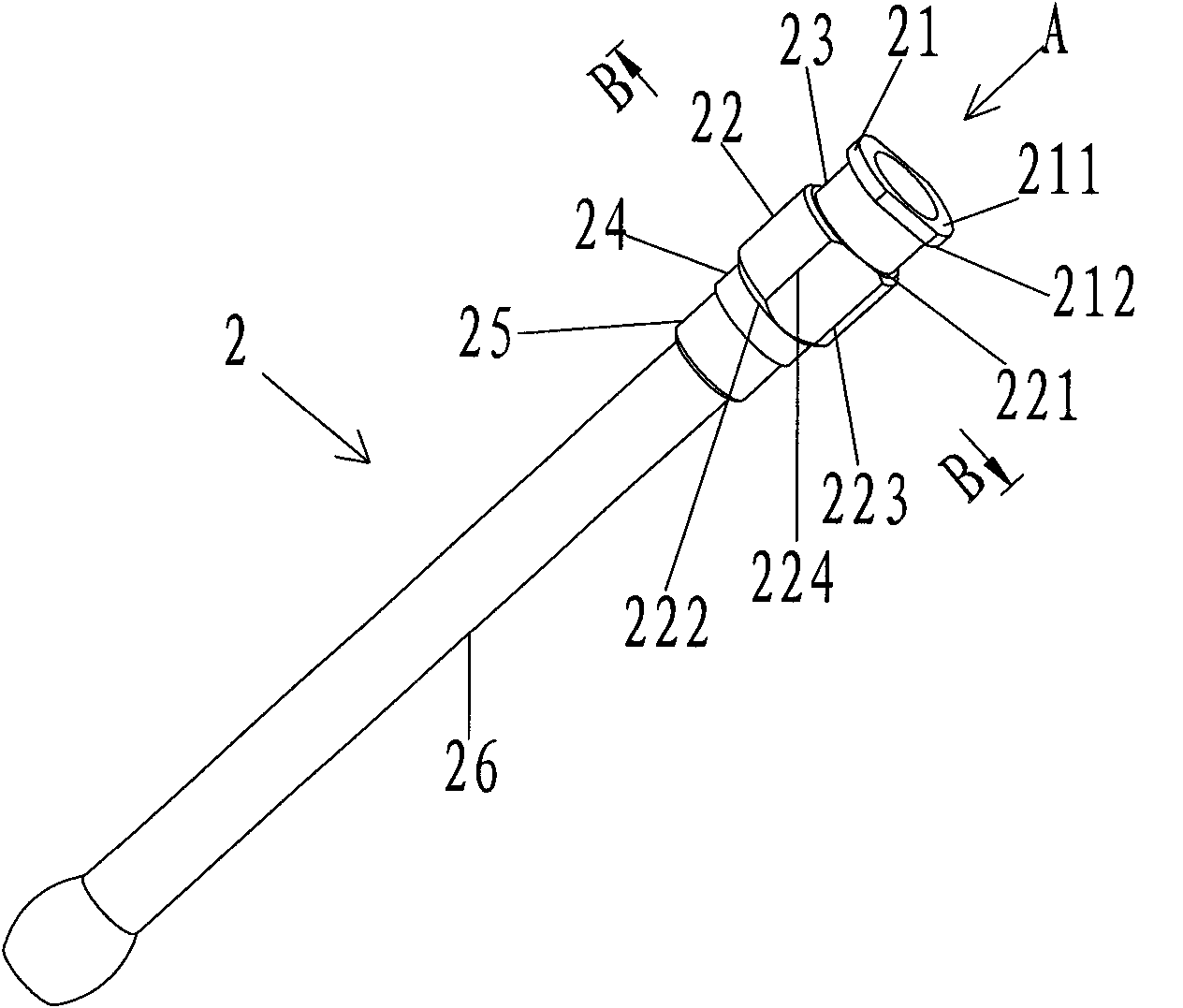
Intragastric administration device
The invention provides an intragastric administration device by which solid medicine can be fed to the stomach of an experimental animal orally. The intragastric administration device of the invention comprises a hollow needle (2) and a medicine pushing rod (3), wherein the hollow needle (2) is a hollow long rod-shaped component of which the front end can be inserted into the stomach of the experimental animal, and comprises a front end opening as a medicine outlet and a back end opening as a medicine inlet; the medicine pushing rod (3) is a solid long rod-shaped component which can be inserted into the hollow needle, and comprises a front end and a back end; and the back end of the medicine pushing rod is used for receiving external pushing force. When the medicine pushing rod is inserted from the back end opening of the hollow needle into the hollow needle containing the medicine, external pushing force can be applied to the back end of the medicine pushing rod to push the medicine by the front end of the medicine pushing rod out from the front end opening of the hollow needle into the stomach of the experimental animal.
Owner:北京晶润宏达医药科技有限公司
Method for evaluating excited degree of wine after drinking
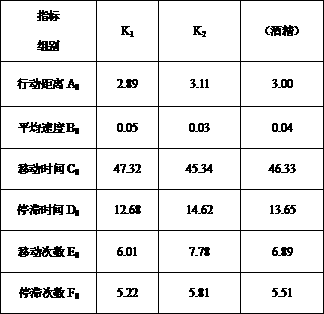
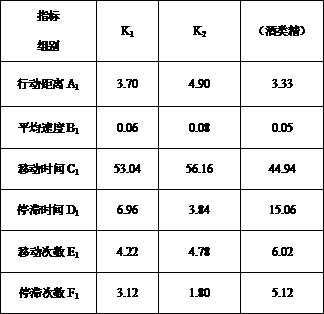
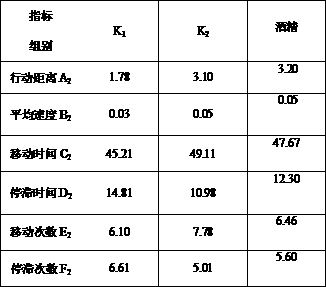
InactiveCN107908924AImprove qualityModerate drinkingChemical property predictionComponent separationProcess optimizationReal time analysis
The invention belongs to the technical field of brewing, and discloses a method for simply and effectively evaluating the excited degree of wine after drinking and application thereof. By aiming at the current conditions that the existing method relies on the manual operation on experiment data detection and drunkenness degree collection and evaluation, and the excited degree cannot be evaluated,the method is characterized in that animal fine behavioristic analysis equipment is used for performing synchronous recording and real-time analysis on one-minute movement moving tracks and behavior parameters of mice subjected to intragastric administration of a certain specific dose of distilled wine, fermentation wine or prepared wine with different or identical alcoholic strength before the wine drinking and after the wine drinking; and the behavior parameter moving distance, the average speed, the moving time, the stop time, the moving frequency and the stop frequency are subjected to synchronous real-time analysis, the mouse blood ethanol concentration is combined, and the after-drinking behavior excited degree of different kinds of wine and the behavior excitement causing degree ofthe wine sample per se can be simply, effectively and scientifically evaluated according to the wine sample and wine alcohol reference behavior parameter ratio, the behavior excitement index and the wine sample relative excitement causing degree index. The method can be applied to the evaluation of after-drinking feeling of wine samples, or the evaluation of wine sample quality, can be used for wine body design and process optimization in the production process of the distilled wine, fermentation wine or prepared wine.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
New application of scutellarin
InactiveCN104940187AReduce proteinuriaReduce direct damageOrganic active ingredientsAntipyreticHypercoagulable statesHypoproteinemia
The invention relates to the technical field of medicine, in particular to a new application of scutellarin, and provides the application of the scutellarin in preparing medicine for reducing pathological changes of kidney tissue and the application of the scutellarin in preparing medicine for treating nephritis. The new application of the scutellarin is proved by experiments to show that the intragastric administration is continuously performed on nephritis model rats with the scutellarin in the dose of 20 mg / kg and 40 mg / kg for eight weeks, the proteinuria of the model animals can be obviously reduced, hypoproteinemia, hyperlipidemia and the hypercoagulable state of blood can be corrected, the serum lipid peroxidation product content of the model rats can be lowered, the expression of TNF-alpha in the kidney tissue can be inhibited, and a protection effect on the kidney tissue and a good improvement effect on the kidney function are achieved; in addition, the maximum dose of intragastric administration on the rats is equal to and more than 18 g / kg, and obvious acute toxicity reaction and death do not occur on the animals.
Owner:KPC PHARM INC
Novel EV71 virus strain and application thereof
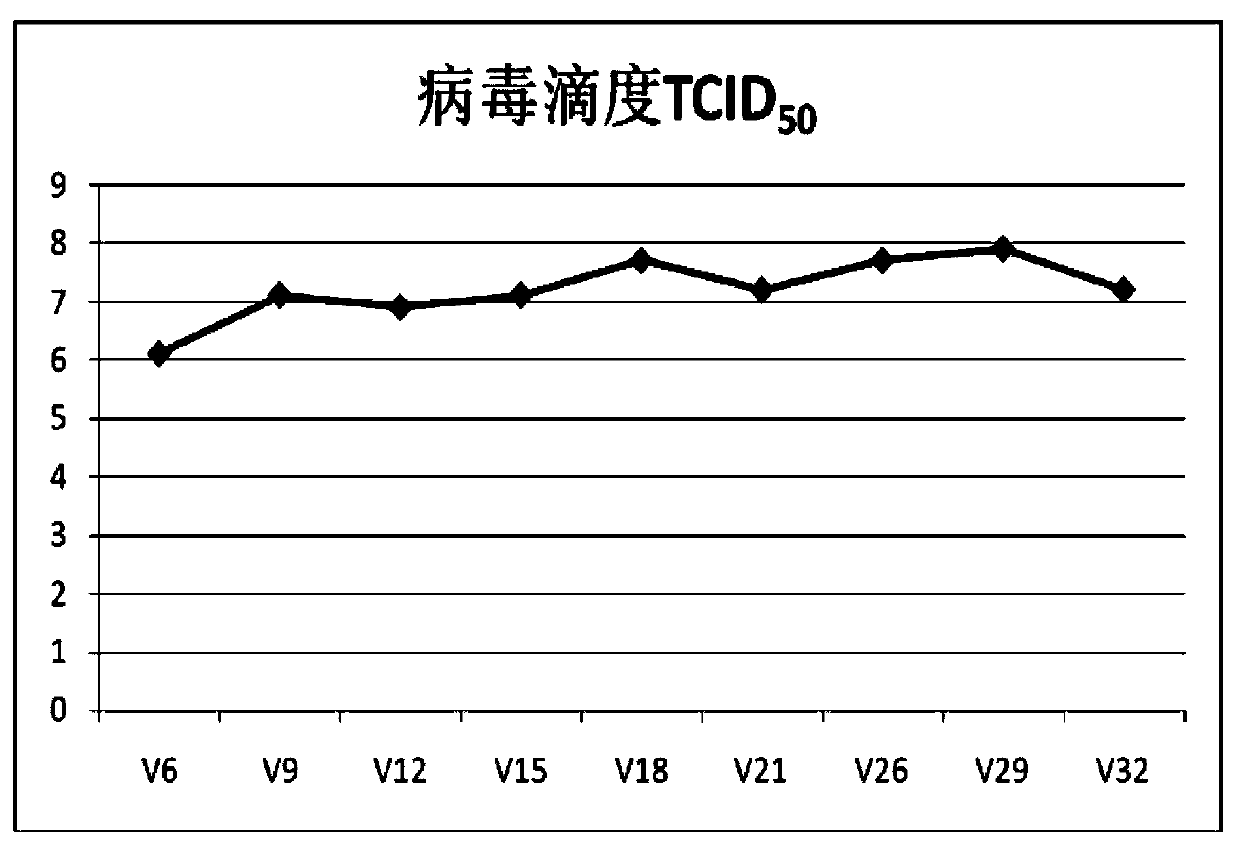
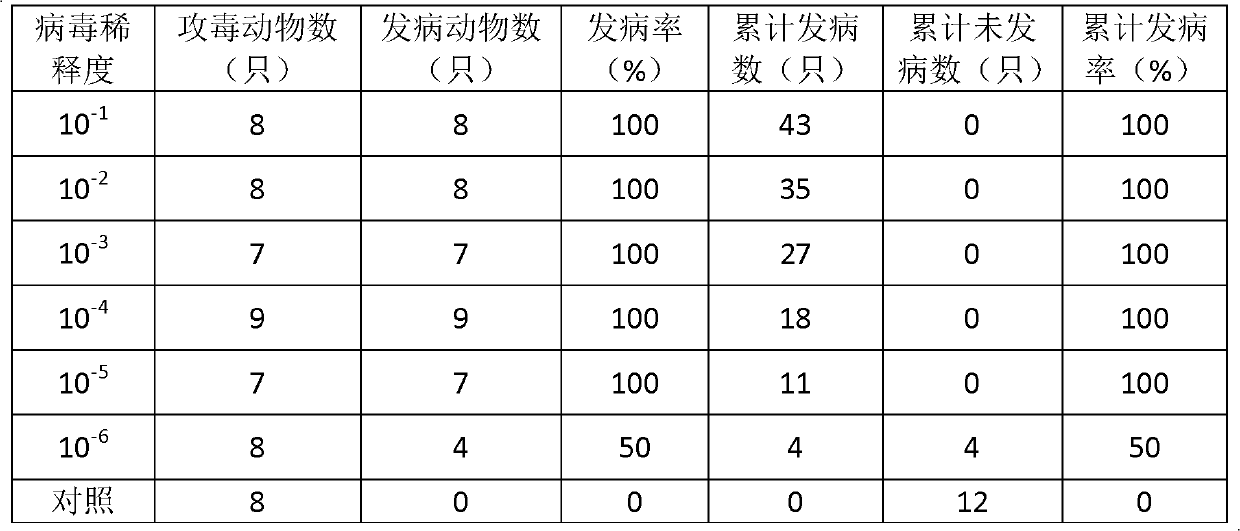
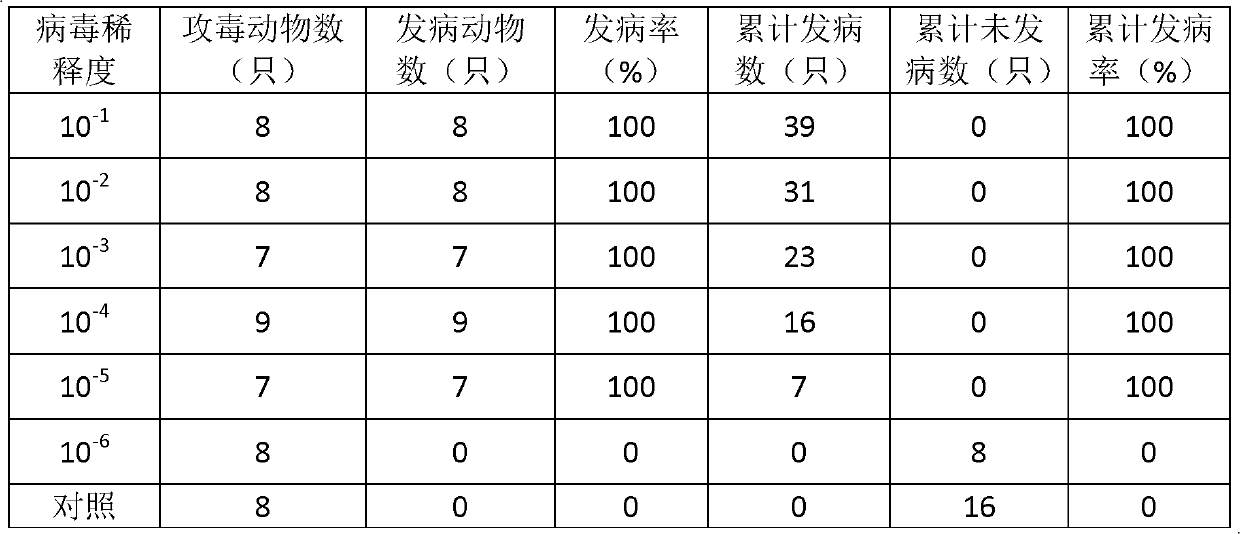
ActiveCN103374549AEasy to set upDeepen understandingViral/bacteriophage medical ingredientsMicroorganism based processesBALB/cIntraperitoneal route
The invention discloses a novel EV71 virus strain of which the whole-genome length is 7405bp and the sequence is shown in SEQID NO:1. The virus strain can infect ICR, KM, BALB / c and NIH mice; the virus dosage of larger than 1*10<2>-1*10<7> TCID50 can cause the disease attack or death of 1-17 days mice; the administration route can be intraperitoneal injection, encephalocoele injection and intragastric administration; the symptoms are typical symptoms of EV71 virus infection and are divided into the following five levels according to stages of the disease: Level 0: not attacked; L1: motion incoordination and weakness of limbs; L2: hypokinesia and paralysis of fore or posterior limbs; L3: quadriplegia and dying; and L4: death. Various animal evaluation models such as an immunogenicity animal evaluation model of hand-foot-and-mouth disease vaccine and an animal evaluation model of EV71 therapeutic drug and therapeutic vaccine, can be built conveniently.
Owner:SOUTH CHINA UNITED VACCINE INST
Application of bacillus amyloliquefaciens in prevention of diarrhea of weaned piglets
ActiveCN113288919AIncrease diversityRegulate abundanceAntibacterial agentsDigestive systemBiotechnologyDisease
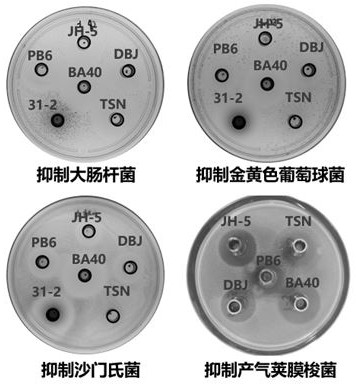
The invention relates to bacillus amyloliquefaciens and application of the bacillus amyloliquefaciens in prevention of diarrhea of weaned piglets. The invention provides a bacillus amyloliquefaciens strain BA40, which can be used for inhibiting common porcine intestinal pathogens, namely escherichia coli K88, salmonella typhimurium and staphylococcus aureus, and has the capabilities of resisting acid, bile salt and pancreatin. After the bacillus amyloliquefaciens is activated, the viable count of the bacillus amyloliquefaciens reaches 108-109 CFU / mL, 6-day-old suckling piglets are subjected to intragastric administration through the mouth, and weaning is performed after 1 mL of the suckling piglets are continuously administrated to the stomach for 24 days. The bacillus amyloliquefaciens provided by the invention can improve weaning weight gain of piglets of pigs and diversity of intestinal flora, reduce diarrhea of the weaned piglets, reduce diarrhea rate, reduce inflammatory factors, promote nutrient absorption and improve oxidation resistance and disease resistance of organisms, and provides a new way for nutrient regulation and control under antibiotic-free breeding.
Owner:广州百仕肽生物科技有限公司
Micro-nano material for preventing and/or treating myelosuppression and application thereof
InactiveCN106109494ARapid prevention and repair of inhibitionPrevention and repair of inhibitionDigestive systemCarbon active ingredientsMicro nanoHematopoietic cell
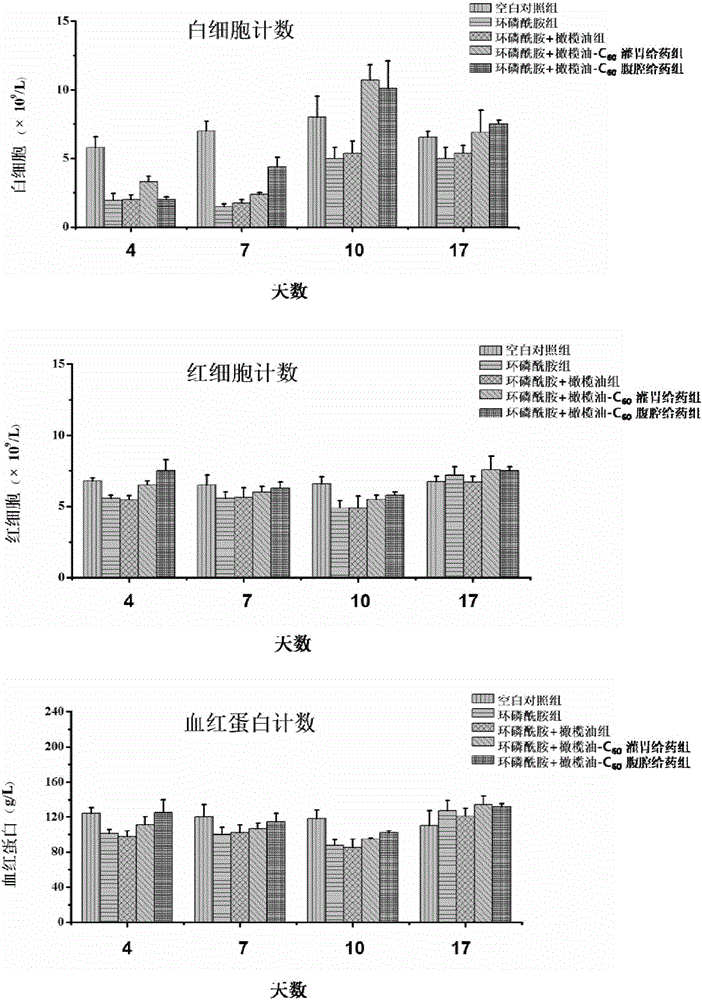
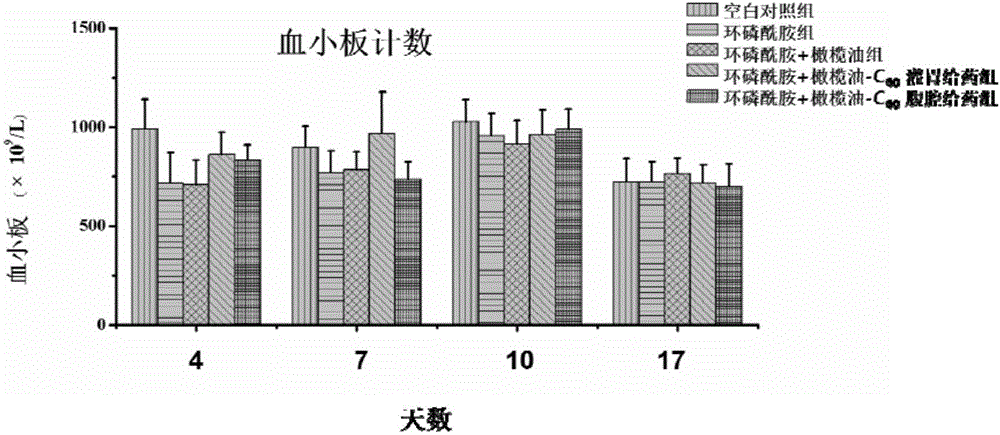
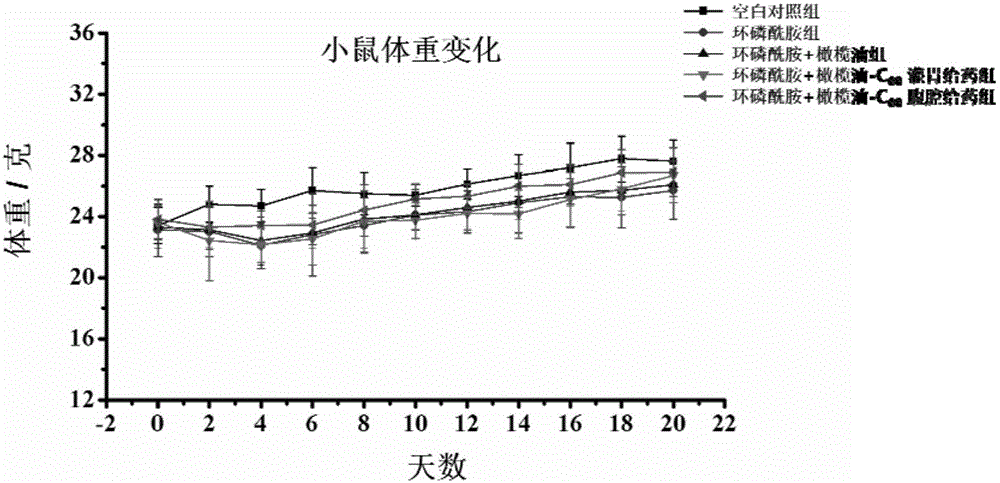
The invention discloses a micro-nano material for preventing and / or treating myelosuppression and application thereof. A fullerene and / or metal fullerene micro-nano material can be applied to preparing medicine with at least one of the following properties of preventing and / or treating myelosuppression, removing free radicals, preventing and / or treating at least one of leukocyte reduction, platelet reduction, hemoglobin reduction and monocyte reduction caused by myelosuppression, preventing bone marrow cells and / or hematopoietic cells, improving the function of antioxidant systems of organisms and protecting liver tissue, spleen tissue and kidney tissue. The fullerene and / or metal fullerene micro-nano material can be metabolized out of organisms, is enriched in visceral organs and marrow through blood circulation after intragastric administration or intraperitoneal injection, has an excellent function of preventing myelosuppression induced by chemotherapy in bodies, can effectively reduce toxic and side effects of chemotherapy on marrow and other organs, and is still obvious in protection effect after multiple treatment courses.
Owner:BEIJING FUNAKANG BIOTECH CO LTD
Building method of rotavirus infection suckling mouse experiment model
InactiveCN106420842AQuick buildShort modeling timeViral/bacteriophage medical ingredientsRotavirus RNAAcute gastroenteritis
The invention relates to a building method of a rotavirus infection suckling mouse experiment model. The method concretely comprises the following steps that 7-day-old ICR suckling mice are used as experimental animals; a 1ml improved injector is used; 0.2ml of rotavirus liquid is given in an intragastric administration mode. The model provided by the invention can simulate features of infant rotavirus infection acute gastroenteritis; the rotavirus infection suckling mouse experiment model is fast built; the sufficient number of mice infecting the rotavirus are obtained; the stability is good; the repeatability is realized; the model building success rate is high and is nearly 100 percent. In addition, the technical scheme provided by the invention has the advantages that the weight of the selected low-age animals is light; the obtaining is easy; the used virus dose is small; the experiment cost is reduced; the method is applicable to batch modeling.
Owner:湖北华冠中科生物药业有限公司 +3
Application of Plukenetia volubilis husk extract in preparation of blood pressure lowering medicines
ActiveCN106038672AThe effect is stable and peacefulGood blood pressure effectCardiovascular disorderPlant ingredientsCaptoprilSide effect
The invention discloses an application of a Plukenetia volubilis husk extract in preparation of blood pressure lowering medicines. An extraction method for the Plukenetia volubilis husk extract comprises the following steps of crushing Plukenetia volubilis husks, adding a solvent into the crushed Plukenetia volubilis husks, carrying out decocting extraction, filtering out the husks, filtrating an extracting solution, and carrying out concentration, thereby preparing the Plukenetia volubilis husk extract. According to the application, the natural plant extract is adopted, so that toxic or side effects on human bodies caused by Western medicines such as captopril are avoided. The extraction method is easy and feasible and is flexible in manner. Through carrying out an intragastric administration test on rats by using the Plukenetia volubilis husk extract, a research result shows that the Plukenetia volubilis husk extract has a remarkable blood pressure lowering effect, so that the condition that the Plukenetia volubilis husks contain blood pressure lowering functional components is deduced. The Plukenetia volubilis husk extract belongs to plant extracts and is stable and mild in effect, no obvious toxic or side effects is discovered at present, and the abuse of the traditional Western medicines that sequelae of diabetes are aggravated is avoided while the Plukenetia volubilis husk extract plays a role in lowering blood pressure.
Owner:杜冰 +1
Method for tracing absorbable proteins in traditional Chinese medicine
ActiveCN103308494AEliminate distractionsRealize active tracerFluorescence/phosphorescenceMass spectrometryFluorophore
The invention discloses a method for tracing absorbable proteins in a traditional Chinese medicine. The method comprises the following steps: 1, marking a traditional Chinese medicine protein extract product by using a fluorophore; 2, carrying out intragastric administration of the marked traditional Chinese medicine protein extract to an experiment mode animal; 3, acquiring the serum of the experiment mode animal, concentrating, carrying out native protein electrophoresis , cutting the obtained gel in direction vertical to the electric field direction to form a plurality of gel blocks, and detecting the fluorescence intensities and diffusion coefficients of all the gel blocks; 4, respectively carrying out in-gel enzymatic hydrolysis of screened gel blocks by using trypsin, carrying out real-time fluorescence monitored molecular sieve chromatography of the obtained enzymatic hydrolysis products, recovering enzymatic hydrolysis products having fluorescence signal peaks, and carrying out mass spectrum determination; and 5, determining the absorbable proteins according to mass spectrum determination results. The method will make great contributions to the disclosure of the action mechanisms of traditional Chinese medicinal macromolecules, and lays a theoretic foundation for promoting the development of the traditional Chinese medicine and pharmacy.
Owner:EXPERIMENTAL RES CENT CHINA ACAD OF CHINESE MEDICAL SCI
Application of Platycodon grandiflorum total saponins in the treatment and prevention of mycoplasma pneumoniae infectious diseases
ActiveCN102274264AInfectious Disease Prevention and ControlAvoid Overshooting ProblemsAntibacterial agentsRespiratory disorderBALB/cTherapeutic effect
The invention relates to application of a platycodon root total saponin to medicaments for treating and preventing mycoplasma pneumoniae infectious diseases, and relates to application of platycodon root total saponin to medicaments. The platycodon root total saponin is used as the active ingredient of the medicaments for treating and preventing the mycoplasma pneumoniae infectious diseases, and is a composition of one or more of saponins of platycogenic acid, polygalic acid, platycogenic acid and platycogenic acid A lactone. The platycodon root total saponin has the effect of resisting mycoplasma pneumoniae; in-vitro experiments indicate that a minimal inhibitory concentration (MIC) value of resisting mycoplasma pneumoniae (MP) is between 16 and 64 mu g / ml, and the minimal bactericidal concentration (MBC) is between 64 to 128 mu g / ml; and in-vivo experiments indicate that after 8 mg / Kg and 16 mg / Kg of platycodon root total saponins are subjected to intragastric administration on BALB / C mice subjected to MP injection by nasal dropping for 10 days, the pathological injury of lungs of the mice can be relieved obviously, and the immunologic function of organisms is regulated, so the platycodon root total saponin has a treatment effect on the MP infection.
Owner:黑龙江省中医研究院
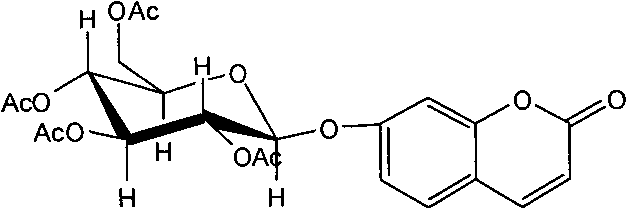
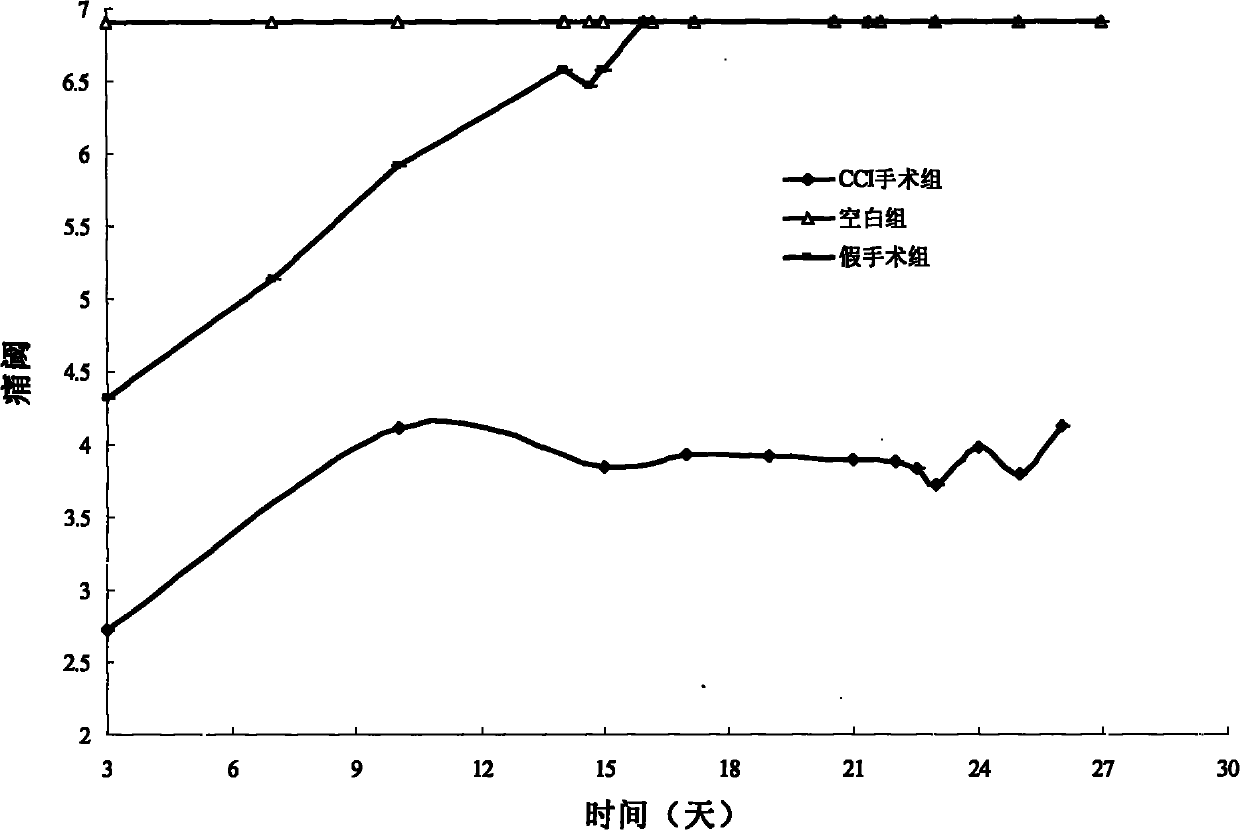
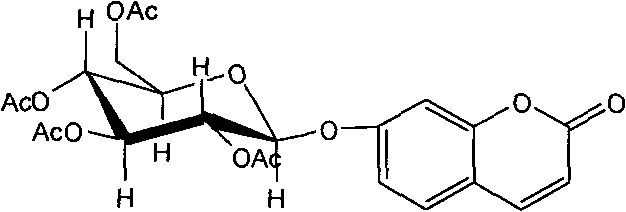
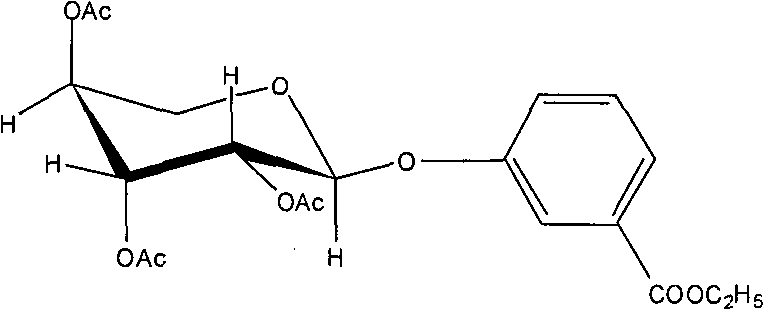
Application of 7-O-beta-D-acetylation sugar-coumarin compounds in treating chronic neuropathic pains
The invention relates to application of 7-O-beta-D-acetylation sugar-coumarin compounds in treating chronic neuropathic pains. The 7-O-beta-D-acetylation sugar-coumarin compounds comprise a pharmaceutically acceptable salt, ester or a solvate thereof, and the application relates to the application of the salt, the ester or the solvate in preparing medicaments for treating the chronic neuropathic pains, wherein a glycosyl part is arbitrary pyranose or furanose, the hydroxy of which is totally acetylated; a representative compound is 7-O-beta-D-acetylation glucose-coumarin; and the structural formula is disclosed in the specification. On a classical sciatic nerve chronic compression injury (CCI) model, through single intragastric administration and continuous intragastric administration for 7 days, the compound shows definite treatment effect of resisting nervous pains. At a dose point appointed by an experiment, the compound can obviously improve the mechanical stimulus pain threshold, the nervous pain resistant efficacy is equivalent to that of Gabapentin which is a contrast medicament, and the duration is superior to that of the Gabapentin. Proved by research results, the compound can be used for treating the chronic neuropathic pains.
Owner:YUNNAN UNIV
Establishment method of endoplasmic reticulum stress induced mouse acute liver injury model
The invention provides an establishment method of an endoplasmic reticulum stress induced mouse acute liver injury model. The establishment method orderly comprises the following steps: carrying out intragastric administration treatment on mice by use of an endoplasmic reticulum stress inducer with a dosage of 0.2ml / 10g for each mouse, and completing the establishment of the mouse acute liver injury model in 8-48 hours after administration, wherein the endoplasmic reticulum stress inducer is prepared by a method comprising the steps of taking (0.5-4)mg of tunicamycin for dissolving 100 microliters of dimethyl sulfoxide to obtain a preservation solution, taking 100 microliters of preservation solution and adding 19.9ml of double distilled water to 20ml, and thus completing the preparation of the endoplasmic reticulum stress inducer. The mouse model of the induced acute liver injury is established by inducing the endoplasmic reticulum stress effect; through the study on the mechanism of action of the endoplasmic reticulum stress, the self regulation ability of cells in the stress state can be further known, and the occurrence mechanism of the liver diseases can be further known; therefore, new intervention and treatment measures can be adopted for the liver diseases to achieve the purposes of preventing and treating diseases.
Owner:BENGBU MEDICAL COLLEGE
Eu-commin intragastric administration agent for cows and preparation method thereof
InactiveCN103816209ASecure Anniversary UseHigh retention ratePharmaceutical delivery mechanismSexual disorderBiotechnologyButterfat
The invention provides an eu-commin intragastric administration agent for cows and a production method thereof. The production method comprises the following steps: dry eucommia leaves are extracted twice continuously, and each time extraction time is 1.5-2 h; an extract liquid is subjected to stilling and precipitating by a natural clarifying agent, supernate is pumped out, lower suspension is centrifugalized, and filtrate is mixed with the supernate; an ultrafiltration membrane is separated, then a dialyzate over-wind type nanofiltration membrane is separated and condensed, dialyzate is pumped in an extracting tank for recycling, and trapped fluid is condensed till the concentration is 20-30%; nanofiltration trapped fluid is pumped in a high-temperature instant sterilization machine for sterilization, and enters into a filling machine for filling under the sterile condition. The eu-commin intragastric administration agent can enhance the immunity of the organism of the cows, effectively controls the recessive mastitis and the clinical mastitis, can reduce the somatic cell counts of the cows remarkably, improves the tendency of milk production, and has no remarkable influence on the fermentation function of cow rumen, the butter-fat content and the milk protein rate. Meanwhile, the eu-commin intragastric administration agent is an aqueous solution, is convenient to use, and has no side effects.
Owner:ZHANGJIAJIE EVERGREEN BIOLOGICAL SCI
Application of beta-nicotinamide mononucleotide (NMN) in products for preventing alopecia or promoting hair growth
InactiveCN111588729APromote growthNo side effectsOrganic active ingredientsDermatological disorderPhysiologyNicotinamide mononucleotide
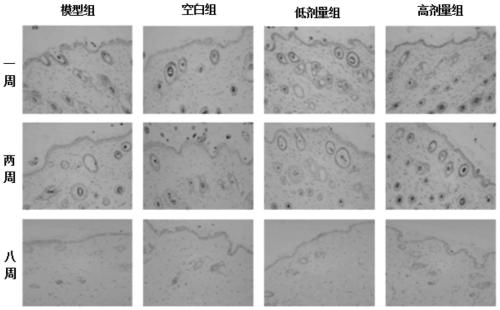
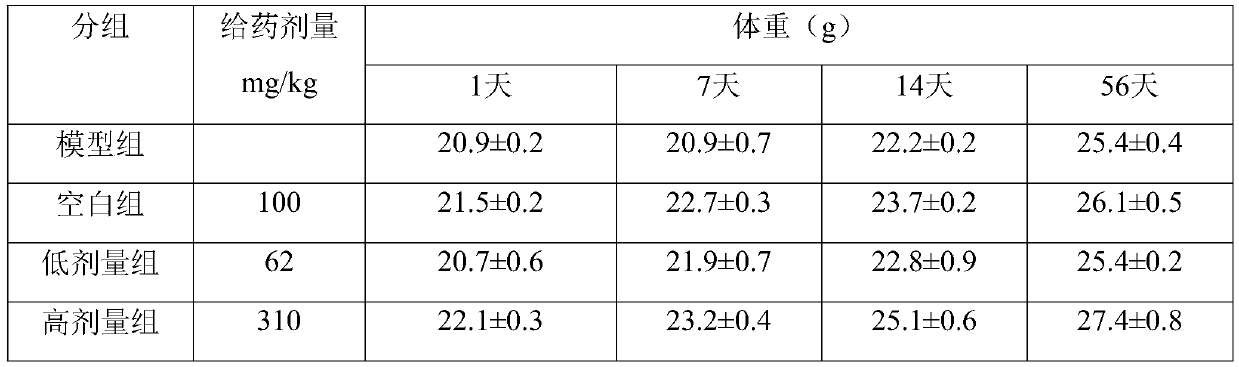
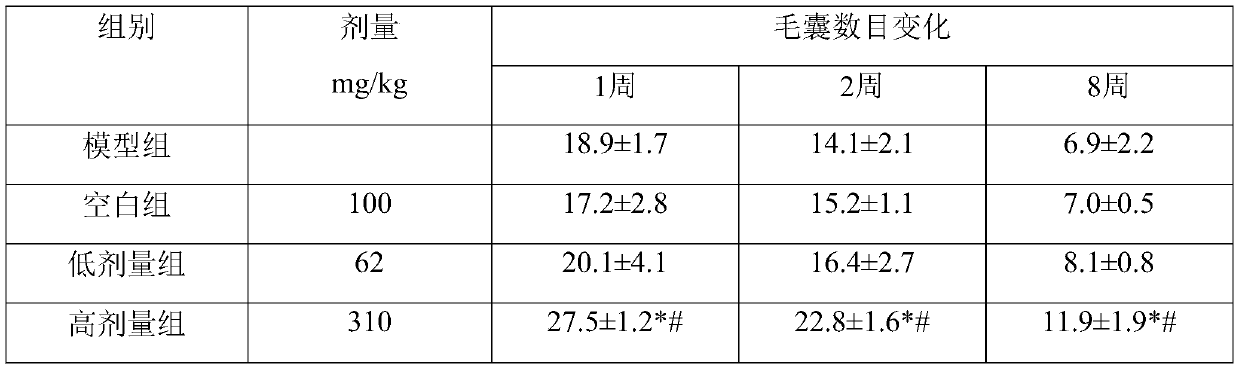
The invention discloses an application of beta-nicotinamide mononucleotide (NMN) in products for preventing alopecia or promoting hair growth, and relates to the technical field of products for preventing alopecia or promoting hair growth. Research results show that mice in a control group and mice in a test group have no significant difference in weight in a whole test period, and the growth condition of the mice is good, which illustrates that the NMN has no toxic or side effect; compared with a blank group and a model group, after 8 weeks of high-dose intragastric administration, the hair of the mice grows more densely, the numbers of corium layer hair follicles in hair removal areas of the high-dose group are slightly reduced, the structures of residual hair follicles are basically complete, sebaceous glands are slightly reduced, but the improvement of the low-dose group is not obvious, and thus it is suggested that the NMN can promote hair growth of the depilated mice.
Owner:WUXI MAGTEIN BIOMEDICAL TECH CO LTD
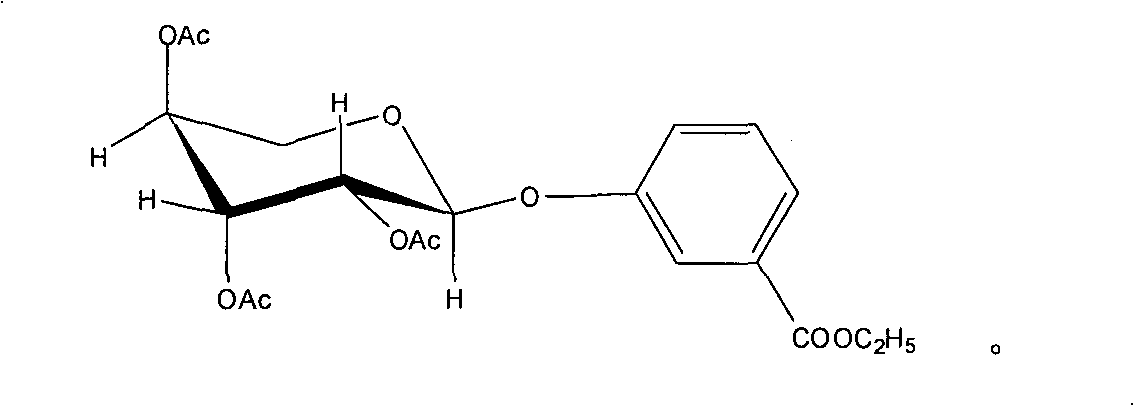
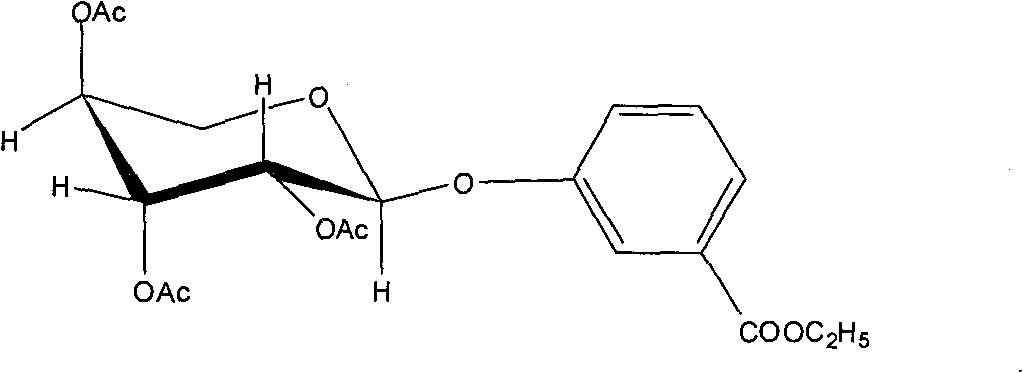
Application of 3-carbethoxyphenyl-beta-D-acetylated glucoside in treating depression
The invention relates to application of 3-carbethoxyphenyl-beta-D-acetylated glucoside in treating depression. The 3-carbethoxyphenyl-beta-D-acetylated glucoside comprises a pharmaceutically acceptable salt, ester or a solvate thereof, and the application relates to the application of the salt, the ester and the solvate in preparing medicaments for treating the depression, wherein a glycosyl part is arbitrary pyranose or furanose, the hydroxy of which is totally acetylated; a representative compound is 3-carbethoxyphenyl-beta-D-acetylated glucoside; and the structural formula is disclosed in the specification. The compound is used for treating the depression, on two depression behavior models, through 45mg / Kg of single intragastric administration to a mouse of a KM species, the representative compound 3-carbethoxyphenyl-beta-D-acetylated glucoside shows definite depression resistant effect, and the depression resistant effect of the compound is higher than that of Hilieidum with equivalent quantity. A test on the spontaneous activity of the mouse also indicates that an effective dose point does not show the effect of interfering in the spontaneous activity of the tested animal.
Owner:YUNNAN UNIV
Quantitative analysis method of plasma-drug concentration of novel compound WSJ-557 in SD rat plasma
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Application of wedelolactone in preparing drug for resisting ulcerative colitis
InactiveCN105030763AImprove colitis symptomsGood treatment effectDigestive systemHeterocyclic compound active ingredientsInflammatory factorsWedelolactone
The invention relates to an application of Chinese traditional herb monomer wedelolactone as a drug for treating ulcerative colitis. According to the application, wedelolactone is obtained from Chinese traditional herb material eclipta, and the structure of wedelolactone is determined according to spectrum data. Through intragastric administration of wedelolactone, the body weight change of a model mouse is obviously changed, the length of the colon is increased, the inflammation level of the colon is obviously hanged, the NO content reflecting the inflammation degree of the colon is reduced, the activity of myeloperoxidase (MPO) in the colon tissue is reduced, release of inflammatory factors IL-8 of Caco-2 cells excited byIL-1beta is inhibited in vitro. The results of in-vivo and in-vitro experiments show that wedelolactone under certain dosage can obviously inhibit release of the inflammatory factors of the colon tissue, so that the acute ulcerative colitis of the mouse caused by dextran sulphate sodium salt (DSS) can be obviously improved, and therefore, wedelolactone has a novel application as a drug for treating or improving ulcerative colitis.
Owner:CHINA PHARM UNIV
Fusion gene GAD-GLP-1 and culture method of diabetes diet therapy type cucumber
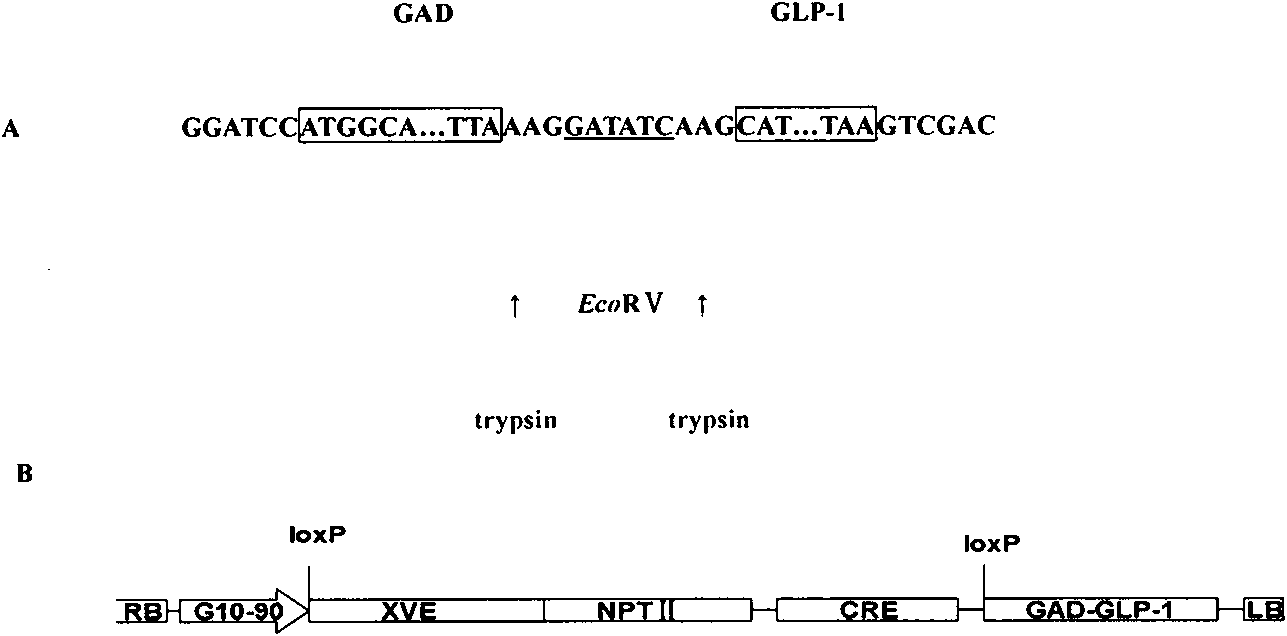
InactiveCN101824423ALower blood sugar levelsImprove diabetes symptomsBacteriaMetabolism disorderEnzyme digestionDiabetes model
The invention relates to a fusion gene GAD-GLP-1 and a culture method of diabetes diet therapy type cucumber. The fusion gene GAD-GLP-1 provided by the invention is shown as a sequence list, and comprises a section of glutamate decarboxylase gene GAD and a section of glucagon-like peptide-1 gene GLP-1, the glutamate decarboxylase gene GAD and the glucagon-like peptide-1 gene GLP-1 are connected through an EcoRV site, two lysines are respectively added at both sides of the EcoRV site, and the four amino acids simultaneously realize the effect of peptide connection. The invention is characterized in that the fusion gene in a cloning vector pMD-GAD-GLP-1 is connected into a Marker-free plant expression vector pX6 after the BamHI and SalI enzyme digestion recovery, a selective-marker-free plant expression vector pX6-GAD-GLP-1 is obtained through construction, and the selective-marker-free plant expression vector pX6-GAD-GLP-1 is converted into agrobacterium rhizogenes LBA4404 to be construction into engineered strains. The invention successfully converts the GAD-GLP-1 fusion gene into a cucumber self-bred line 2M1 by an agrobacterium rhizogenes mediate method, and obtains transgene plants of stably expressed GAD-GLP-1 fusion protein. Through the intragastric administration on diabetes model bandicoot of the transgene cucumber provided by the invention, the blood sugar level can be reduced, and the diabetes symptom can be relieved.
Owner:NANKAI UNIV
Application of philippine violet herb total phenol extract to treatment of hyperuricemia
The invention relates to the field of natural medicaments, in particular to application of a philippine violet herb total phenol extract to treatment of hyperuricacidemia. Pharmacological tests prove that joint swelling of gouty arthritis of rats due to sodium urate crystals can be relieved obviously and the tread treatment of the rats can be improved by intragastric administration of the philippine violet herb total phenol extract. The philippine violet herb total phenol extract has the obvious inhibitory effect on hyperuricemia and urate nephropathy of mice due to a uricase inhibitor, adenine and ethambutol, but does not exert obvious influence on urinary volume, so the philippine violet herb total phenol extract has the effect of promoting the excretion of uric acid and has no effect of diuresis; and due to the single function of the philippine violet herb total phenol extract, doctors do not need to consider the influence of diuretic factors on patients during clinical application.
Owner:CHINA PHARM UNIV
Lactococcus lactis subsp.lactis HFY14 and application thereof
PendingCN114686402AImprove immune organ indexImprove kidney functionBacteriaMicroorganism based processesBiotechnologyStaphylococcus lactis
The invention discloses a lactococcus lactis subsp. Lactis HFY14 and application thereof, and belongs to the technical field of biology, the lactococcus lactis subsp. Lactis is named as HFY14, the preservation number is CGMCC No.16647, the strain is separated from naturally fermented yoghurt, and the strain has the advantages that the strain can be used for preparing the lactococcus lactis subsp. Lactis HFY14 and the application of the lactococcus lactis subsp. Lactis HFY14; animal experiments, histopathologic observation and other experiments prove that the renal dysfunction of lupus nephritis mice induced by hypophytane through intragastric administration of viable bacterial liquid of the strain can be obviously improved, and the effect is close to that of a medicine prednisone. The invention provides a theoretical basis for the subsequent development and utilization of the lactococcus lactis subsp. Lactis HFY14 and the application of the lactococcus lactis subsp. Lactis HFY14 as a probiotic to improve the renal function of nephropathy, and the strain has the application potential of performing long-term intervention on lupus nephritis to improve the renal function.
Owner:善恩康生物科技(苏州)有限公司
HPLC-MS/MS (High Performance Liquid Chromatography-Mass Spectrum/Mass Spectrum) technique-based method for detecting blood concentration of NMDA (N Methyl D Aspartate) receptor antagonist JCC-02
ActiveCN108918722AFew samplesEasy pretreatmentComponent separationChromatographic separationNR1 NMDA receptor
The invention provides an HPLC-MS / MS (High Performance Liquid Chromatography-Mass Spectrum / Mass Spectrum) technique-based method for detecting blood concentration of a NMDA (N Methyl D Aspartate) receptor antagonist JCC-02 and relates to the field of drug analysis. The method comprises the following steps of: sequentially adding methanol, acetonitrile and an internal standard working solution intoplasma of an SD (Sprague Dawley) rat after intragastric administration of the JCC-02, eddying and dissolving supernatant by using a mobile phase to obtain a preprocessed sample, wherein the internalstandard working solution was a gliclazide methanol solution; and carrying out gradient elution by taking acetonitrile-formic acid water mixed solution as a mobile phase and by adopting HPLC-MS / MS technique, carrying out chromatographic separation by using a Venusil ASB C8 chromatographic column, detecting through a second-stage mass spectrometry and carrying out quantitative analysis. The methodhas the advantages of being strong in specificity, high in sensitivity, small in sample sampling amount, simple and rapid in preprocessing and short in analysis period; and proved by methodology, themethod is accurate and reliable and is suitable for drug concentration determination of the JCC-02 in the plasma of the SD rat and pharmacokinetic study.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Sustained release drug delivery system
The invention discloses a controlled release dosage form comprising a therapeutically effective amount of a pharmaceutically active agent, illustrated by Acyclovir, that would release in about 12 hours not more than about 90% of the said active agent in a simulated gastric juice in a first order rate of release in a USP type 1 dissolution test, and not containing a solubilizer or a swelling enhancer or both, comprising (a) a tablet made from polymer matrix of at least two biocompatible polymers, illustrated by Carbopol 974P and polyethylene oxide, the said pharmaceutically active agent and pharmaceutically permitted excipients; the said tablet capable of rapid swelling without disintegration in the said simulated gastric juice to a size that shall result in its gastric retention in the stomach and start controlled release of the said active agent by starting controlled erosion as well as diffusion immediately after coming into contact with the said gastric juice, or (b) microspheres of ungrafted chitosan or a chitosan derivative illustrated by thiolated chitosan and trimethyl chitosan, or Carbopol incorporating the said active agent, wherein the said pharmaceutically active agent is not a polymeric molecule and after administration in stomach, the said microspheres adhare to the gastric mucosa for a long time releasing the active agent in a controlled way.
Owner:BIOPLUS LIFE SCI PVT
Porcine rotavirus (PRV) VP fusion protein reconstruction body as well as preparation method and application thereof
ActiveCN108359015AGut-piercing activityAvoid enteringPolypeptide with localisation/targeting motifBacteriaMucosal Immune ResponsesIntraperitoneal route
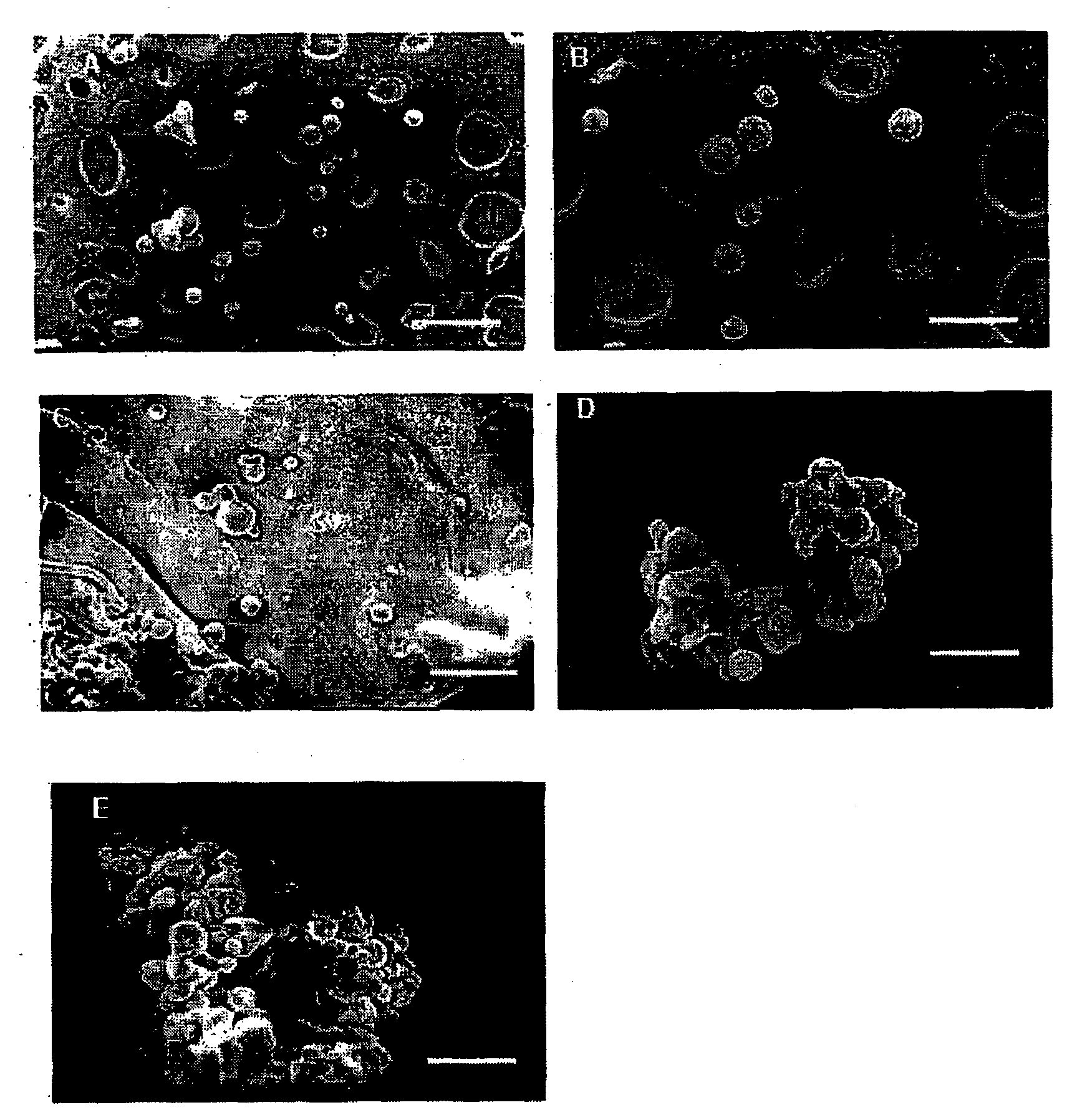
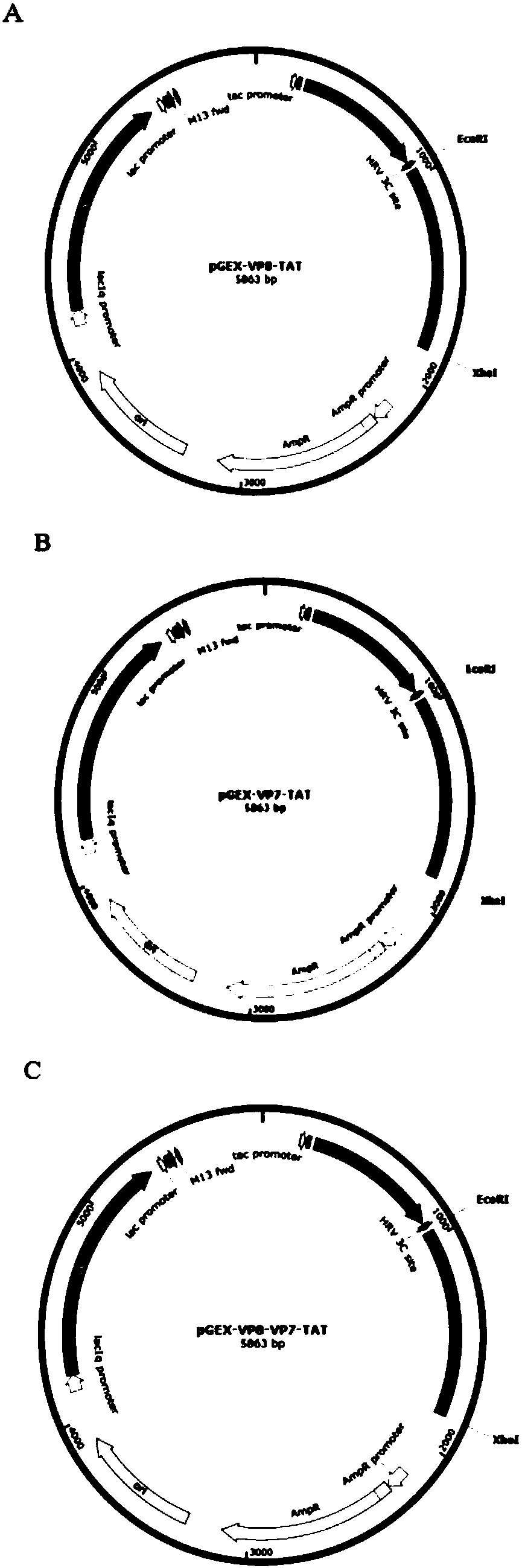
The invention discloses a porcine rotavirus (PRV) VP fusion protein reconstruction body as well as a preparation method and application thereof. The fusion protein VP8-VP7-TAT provided by the invention comprises truncation fragments VP8 and VP7 of a PRV coat shell structure protein VP4 and eleven core amino acids bonded to a TAT protein transduction peptide basic amino acid enrichment region at anend C. A mouse is immunized with the fusion protein VP8-VP7-TAT by means of intraperitoneal injection or oral intragastric administration, so that the organism can be effectively inducted to generatea humoral immune response and a mucosal immune response, and high immunogenicity is achieved. Through fusion expression of PRV proteins VP8, VP7 and TAT, a novel method is provided for the preventionof PRV infection, and a basis is laid for the development of a novel PRV oral vaccine.
Owner:WUHAN UNIV
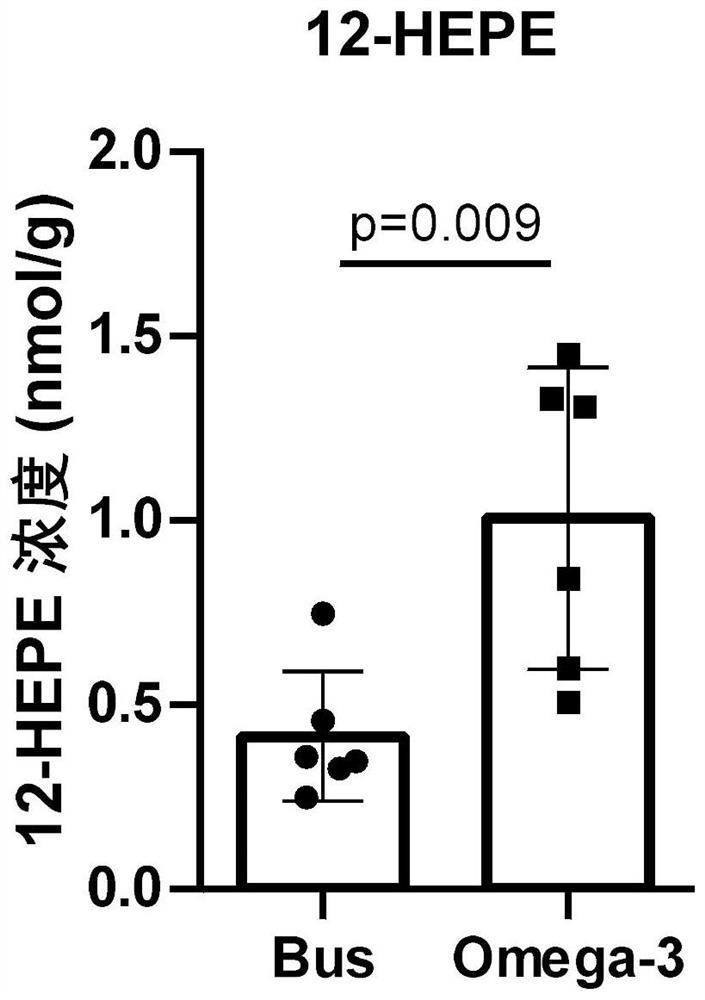
Application of 12-HEPE or pharmaceutically acceptable fatty acid thereof in alleviation of spermatogenesis disorder
ActiveCN113855659APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
The invention discloses novel application of 12-HEPE or pharmaceutically acceptable fatty acid thereof, and comprises the application of the 12-HEPE or the pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. The 12-HEPE or the pharmaceutically acceptable fatty acid thereof can play a therapeutic role by promoting proliferation and differentiation of the endogenous spermatogonium of a mouse damaged by busulfan, and experiments prove that recovery of the thickness and the integrity of the spermatogenic epithelium of the testis and the recovery of the sperm concentration of the tail of epididymiscan be observed through intragastric administration of the 12-HEPE into the NOA mouse induced by the busulfan, meanwhile, proliferation and differentiation of spermatogonium are promoted, expression of the paracrine factors of cells is supported, so that the 12-HEPE can be used as a new target for alleviating the spermatogenesis dysfunction of the testis of the NOA mouse, and a new method is provided for treating the non-obstructive azoospermia.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +3
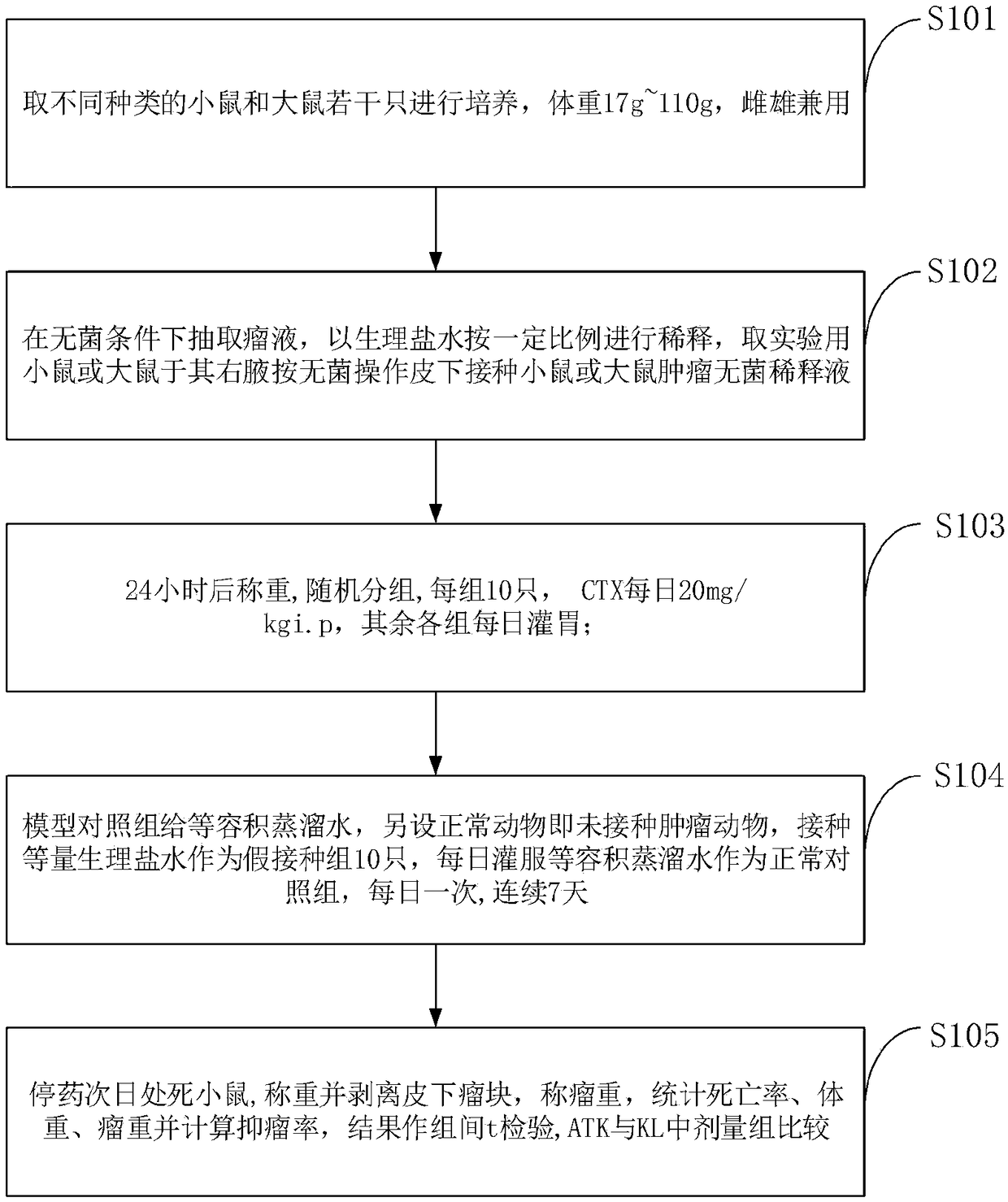
Anti-tumor experimental animal model and construction method thereof
InactiveCN109329207AVerification process is shortHave pursuasive powerMammal material medical ingredientsAnimal husbandryVaccinationZoology
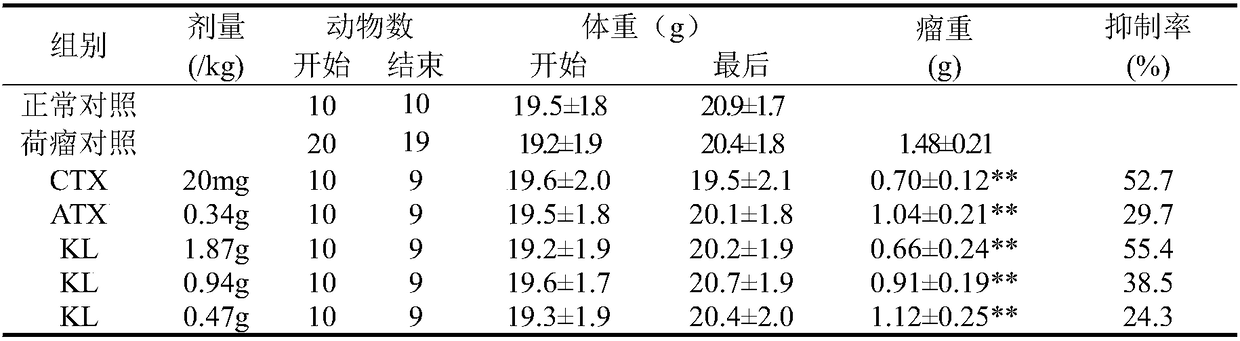
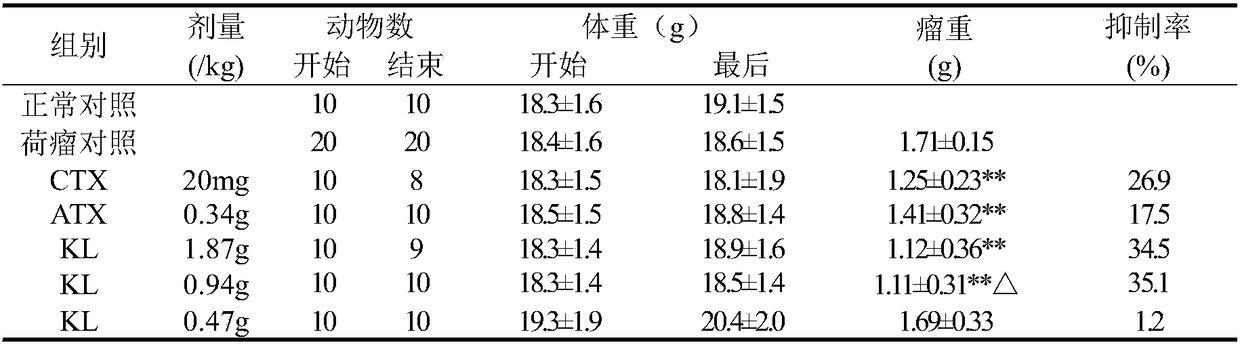
The invention belongs to the technical field of vertebrate breeding, and discloses an anti-tumor experimental animal model and a construction method thereof. The right armpits of mice or rats for experiments are taken for subcutaneous vaccination of a mouse or rat tumor sterile diluent according to the sterile operation, and weighing and grouping are performed after 24 hours; weighing is performedafter 24 hours, random grouping is performed, 10 mice or rats fall into one group, 20 mg / kgi.p of CTX is applied every day, and intragastric administration is performed on the other groups; a constant amount of distilled water is given to a model control group, the equal amount of normal saline is inoculated on normal animals which are animals with no inoculated tumor as ten animals as a fake inoculation group, the constant volume of distilled water is fed every day to the normal animals as a normal control group, and the water is fed every day for 7 days. According to the animal experimentalmodel, a tumor sterile diluent is directly injected into experimental animals under the sterile condition, tumors grow inside the animals, the verification process is short, and by weighing the tumorweight and calculating the inhibition rate, the verification effect is convictive.
Owner:胡学东
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com