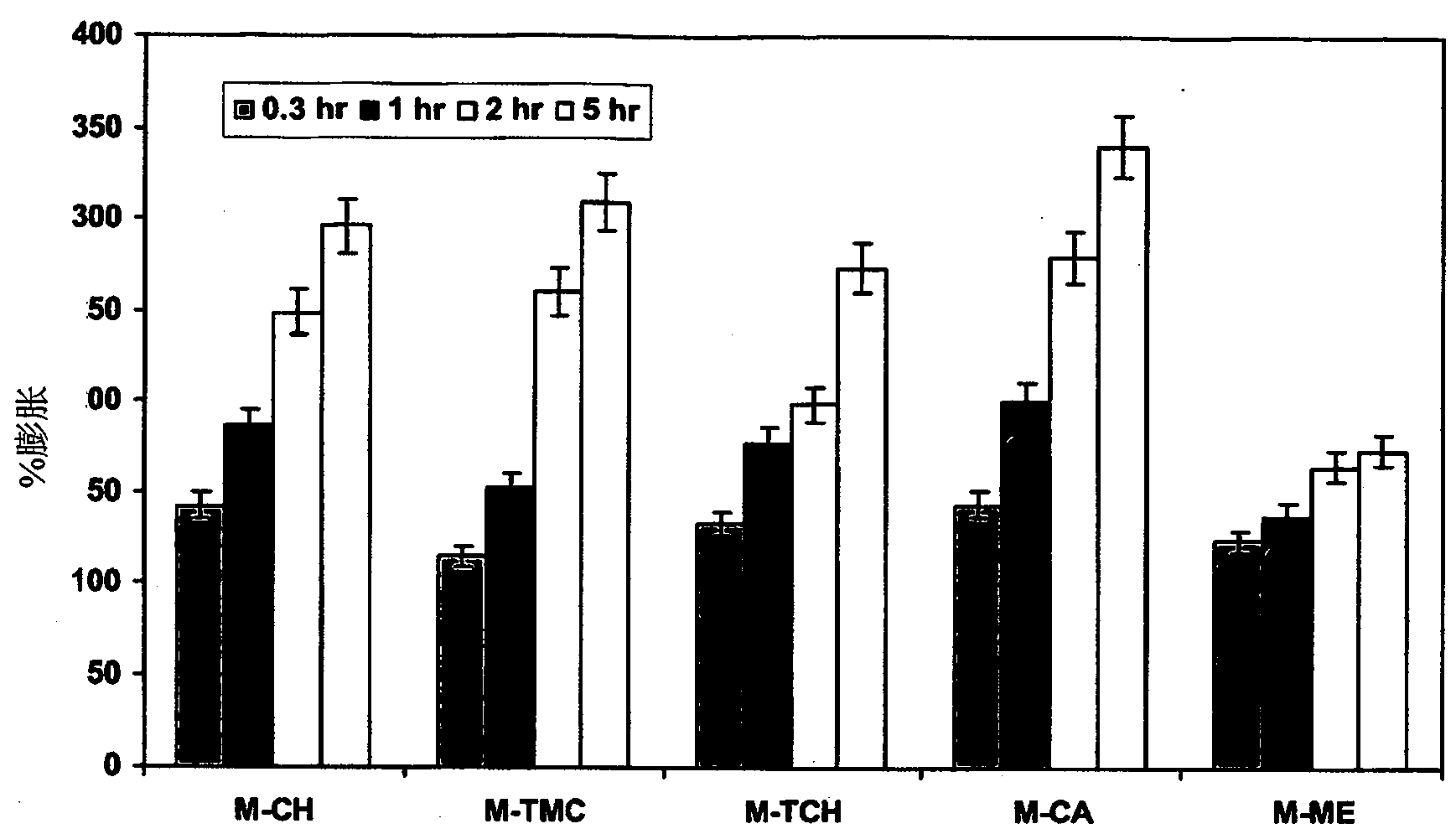Sustained release drug delivery system
A controlled release agent, USP technology, applied in the field of sustained release drug delivery systems, which can solve the problem of not specifying exactly how the drug works
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The polymers of the present invention include water-soluble or water-insoluble, and further include chitosan and chitosan derivatives, typically such as thiolated chitosan and trimethyl chitosan, carbomer, HPMC, alginic acid Salts, gums, acrylic resins, hypromellose, polyethylene oxide, and combinations thereof, etc. The pharmaceutical excipients of the present invention can be selected from the following group: binders, diluents, pH adjusters, glidants, lubricants, film-forming agents, anti-sticking agents, coating agents, colorants, and the like.
[0046] The sustained release drug delivery system may be in the form of a single unit or multiple units. It can be in the form of tablets, capsules, microspheres, pills, granules and the like. It can be contained in blisters, bottles and bags, and the like.
[0047] Drug delivery systems can be prepared using methods well known in the formulation arts, such as wet granulation, dry granulation, direct compression, mixing, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com