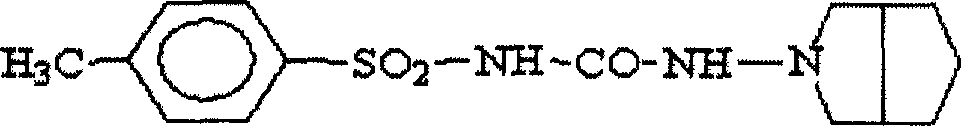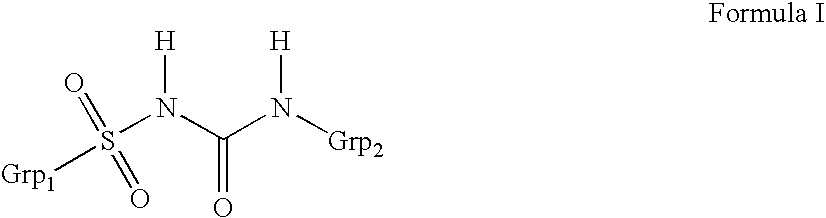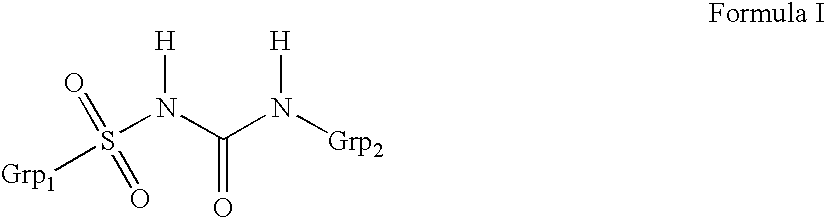Patents
Literature
122 results about "Gliclazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
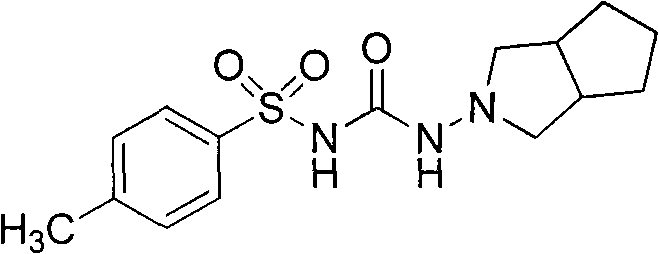
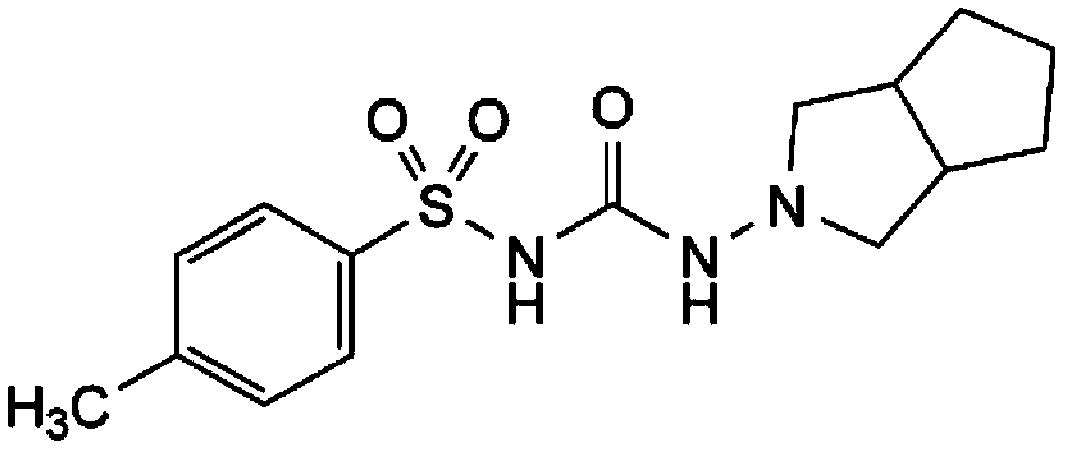
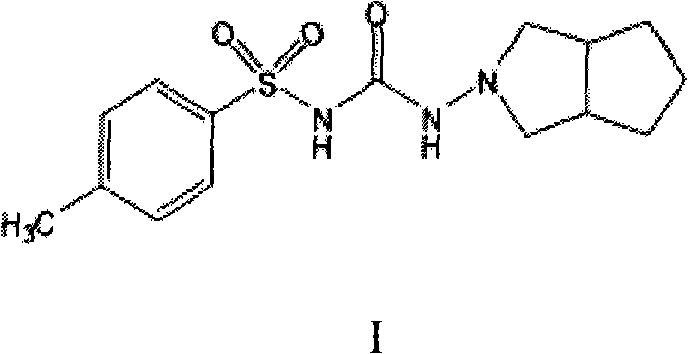
Gliclazide, sold under the brand name Diamicron among others, is an anti-diabetic medication used to treat diabetes mellitus type 2. It is used when dietary changes, exercise, and weight loss are not enough. It is taken by mouth.
Core tablet for controlled release of gliclazide after oral administration
InactiveUS6733782B1Facilitated releaseIncrease concentrationPowder deliveryMetabolism disorderControlled releaseOral medication
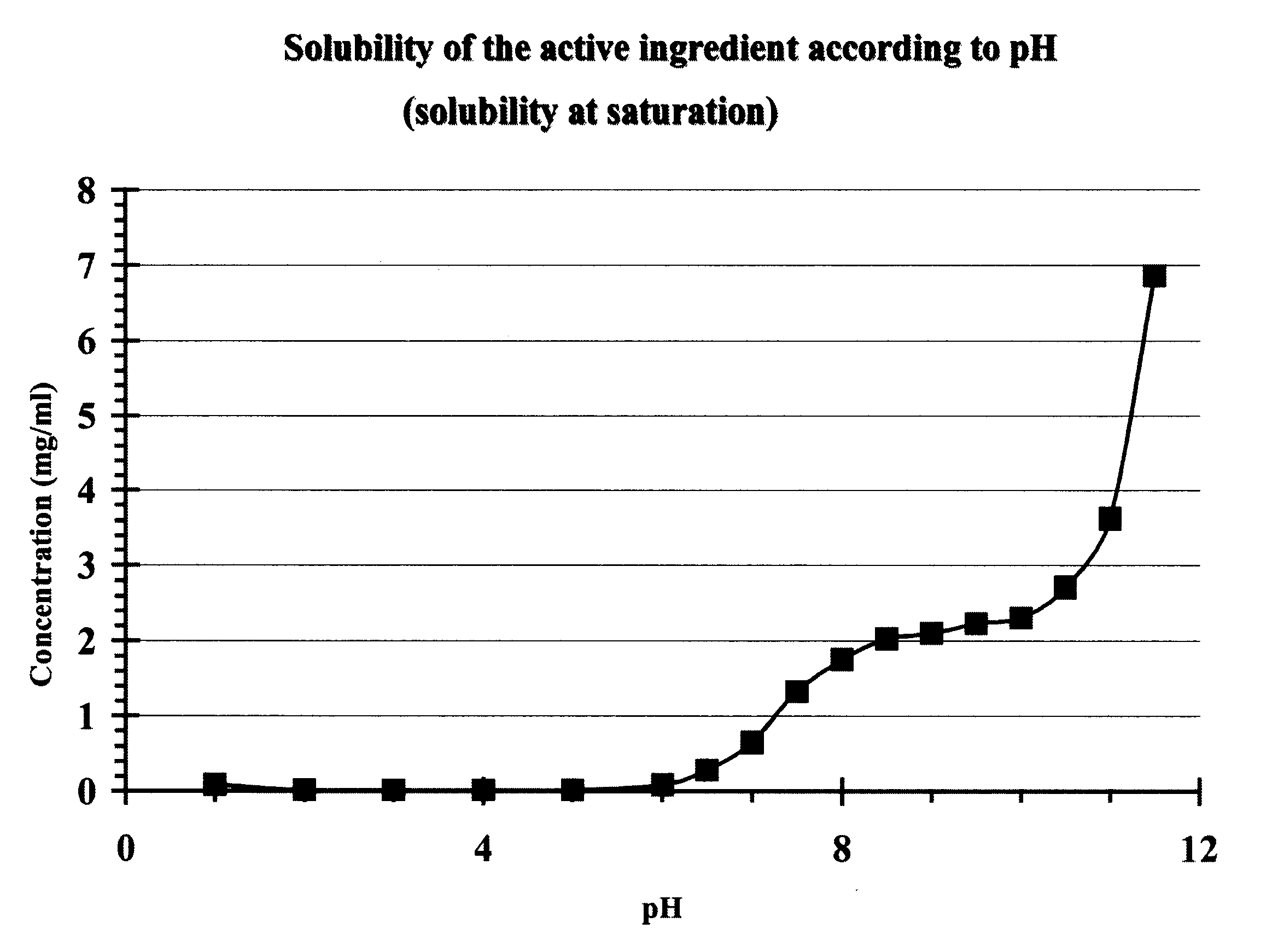
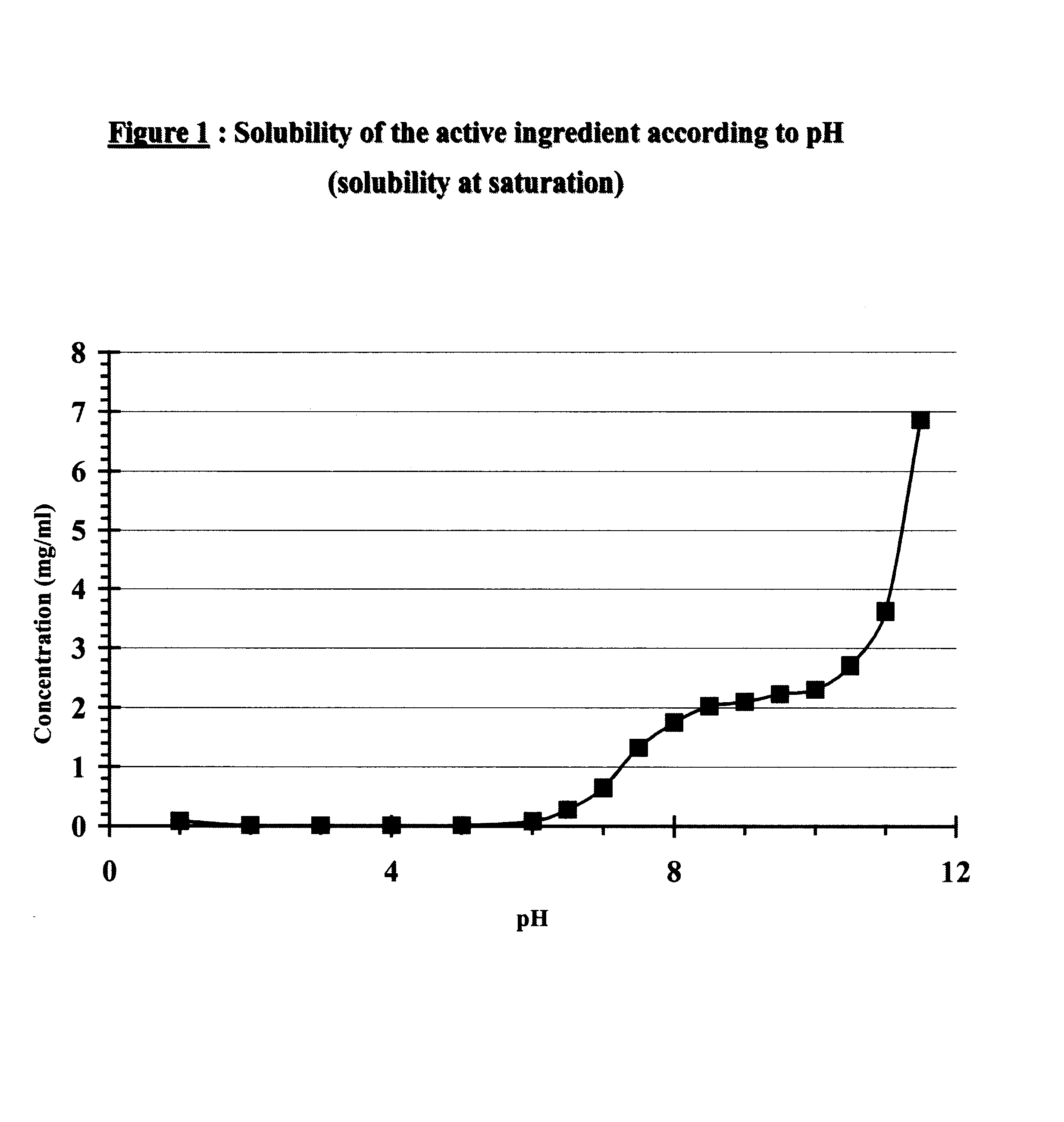
The invention relates to a matrix tablet for the prolonged release of gliclazide which ensures continuous and consistent release of the active ingredient after administration by the oral route, the release being insensitive to variations in the pH of the dissolution medium.
Owner:LES LAB SERVIER
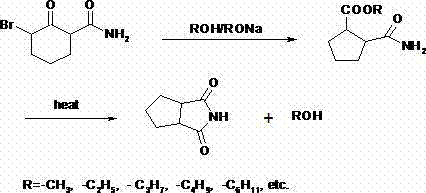
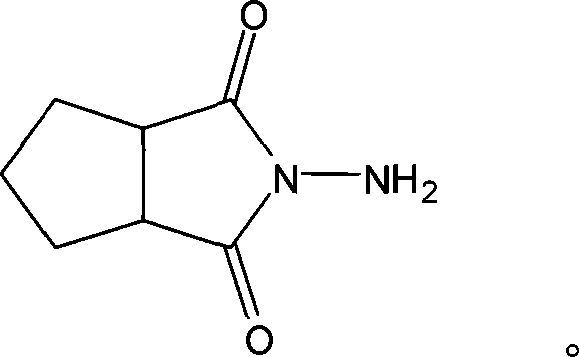
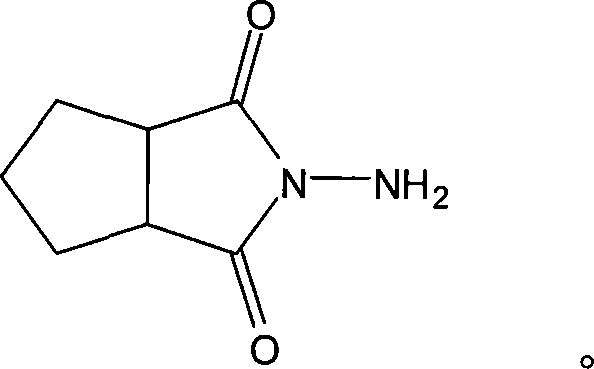
N-amino-1,2-cyclopentanediformylimine and preparation method thereof
InactiveCN101235011AFew reaction stepsReduce manufacturing costOrganic chemistryImideCombinatorial chemistry
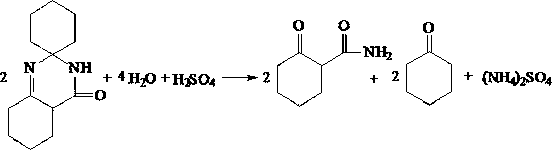
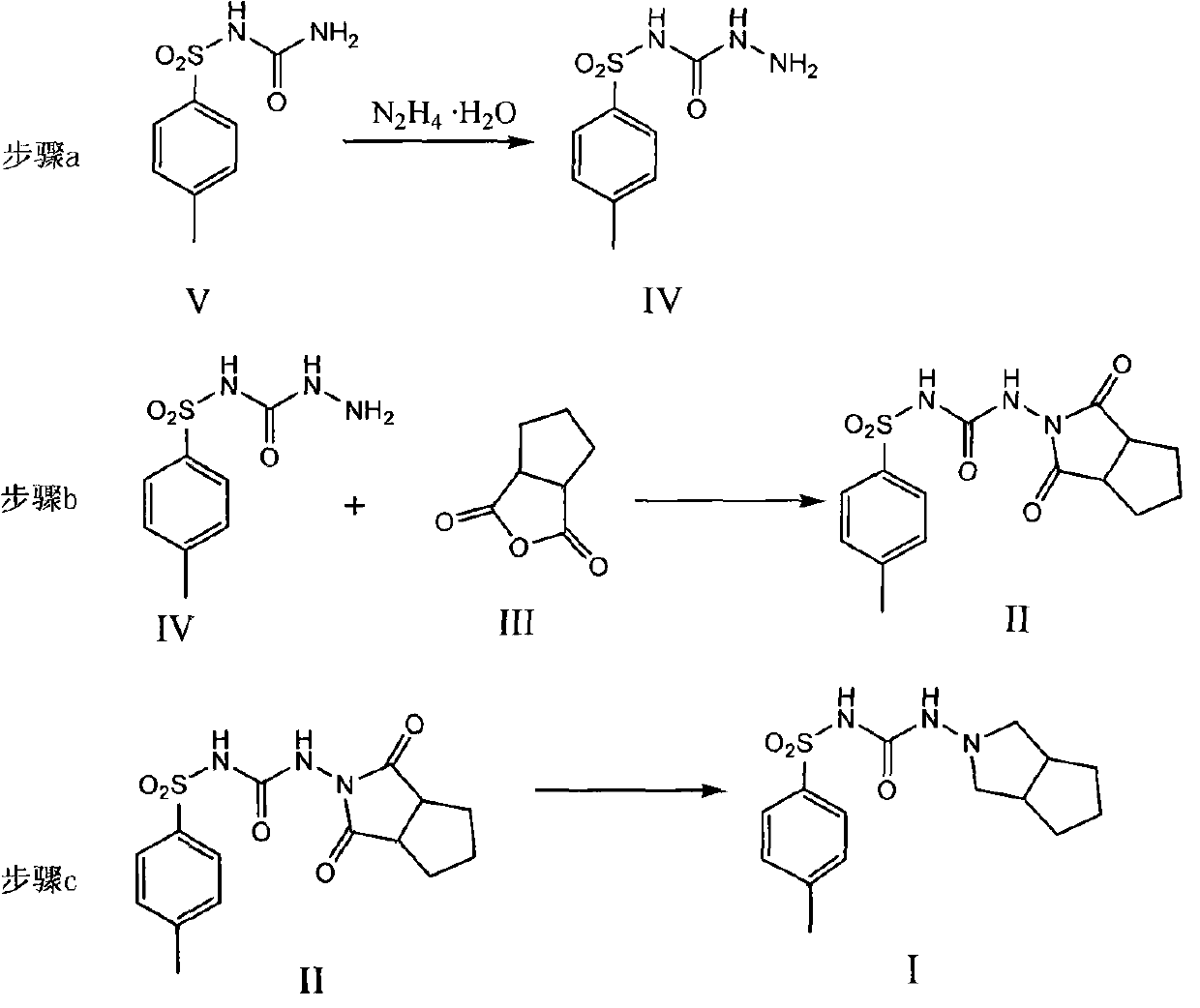
The invention discloses an N-amino-1, 2-cyclopentane dicarboximide whose formula is represented as right. The invention also discloses a corresponding preparation method which comprises using cyclopentane ortho-anhydride as raw material, carrying out hydrazine hydrate in solvent for refluxing reaction for 0.5-12h while the mol ratio of cyclopentane ortho-anhydride and hydrazine hydrate is 1:1-2.5, removing solvent after reaction, and drying to obtain N-amino-1, 2-cyclopentane dicarboximide. The N-amino-1, 2-cyclopentane dicarboximide can be used as the intermediate of gliclazide, thereby reducing the reaction route of gliclazide to reduce waste.
Owner:ZHEJIANG UNIV
Gliclazide tablet and preparation method thereof
ActiveCN103191077AReduce the impactImprove solubilityMetabolism disorderSulfonylurea active ingredientsPolyvinyl acetateSide effect
The invention discloses a gliclazide tablet. The gliclazide tablet is directly tabletted by gliclazide solid dispersoid particles and auxiliary materials accepted pharmaceutically; and the gliclazide solid dispersoid particles are prepared according to the following method: citrate and polyvinyl acetate are heated and molten in a hot melt extruder, then gliclazide is added inside to melt, and the molten liquid is extruded and granulated. The preparation method of the invention is simple in preparation production technology; a patient just takes the medicine once every day, the active components slowly release in the body of the patient, and the plasma concentration is steady; and the gliclazide has long effectiveness, so that the times of administration are reduced, the toxic and side effects are lowered, and the treatment cost is greatly reduced.
Owner:广东彼迪药业有限公司
Prescription for curing type II diabetes and complications and preparation technology thereof
InactiveCN101590101AHigh drug loadingGreat tasteOrganic active ingredientsMetabolism disorderWestern medicineTreatment effect
The invention relates to a prescription for curing type II diabetes and complications and a preparation technology thereof. The prescription for curing the type II diabetes is prepared by defining, quantifying, extracting, separating or manually synthesizing traditional Chinese medicinal materials, such as radix puerariae, radix astragali, red sage roots, and the like and adding a chemical medicine of gliclazide by a solid dispersion technology. The prescription for curing the type II diabetes is a compound preparation by compounding Chinese and Western medicines and chemical medicines, can be prepared into preparation formulations of tablets, granules, capsules, dripping pills, and the like, can effectively cure the type II diabetes, improves the body immunity and can effectively delay the occurrence and the development of the diabetes complications. The preparation technology of the compound preparation has short technological flow, low production cost, rapid effectual action, unique prescription and preparation method thereof and remarkable treatment effect.
Owner:孙民富
Serum combination marker for evaluating gliclazide applicability of type 2 diabetes mellitus and detection kit thereof
ActiveCN109682909AHigh sensitivityGood repeatabilityComponent separationMetaboliteSmall molecule metabolism
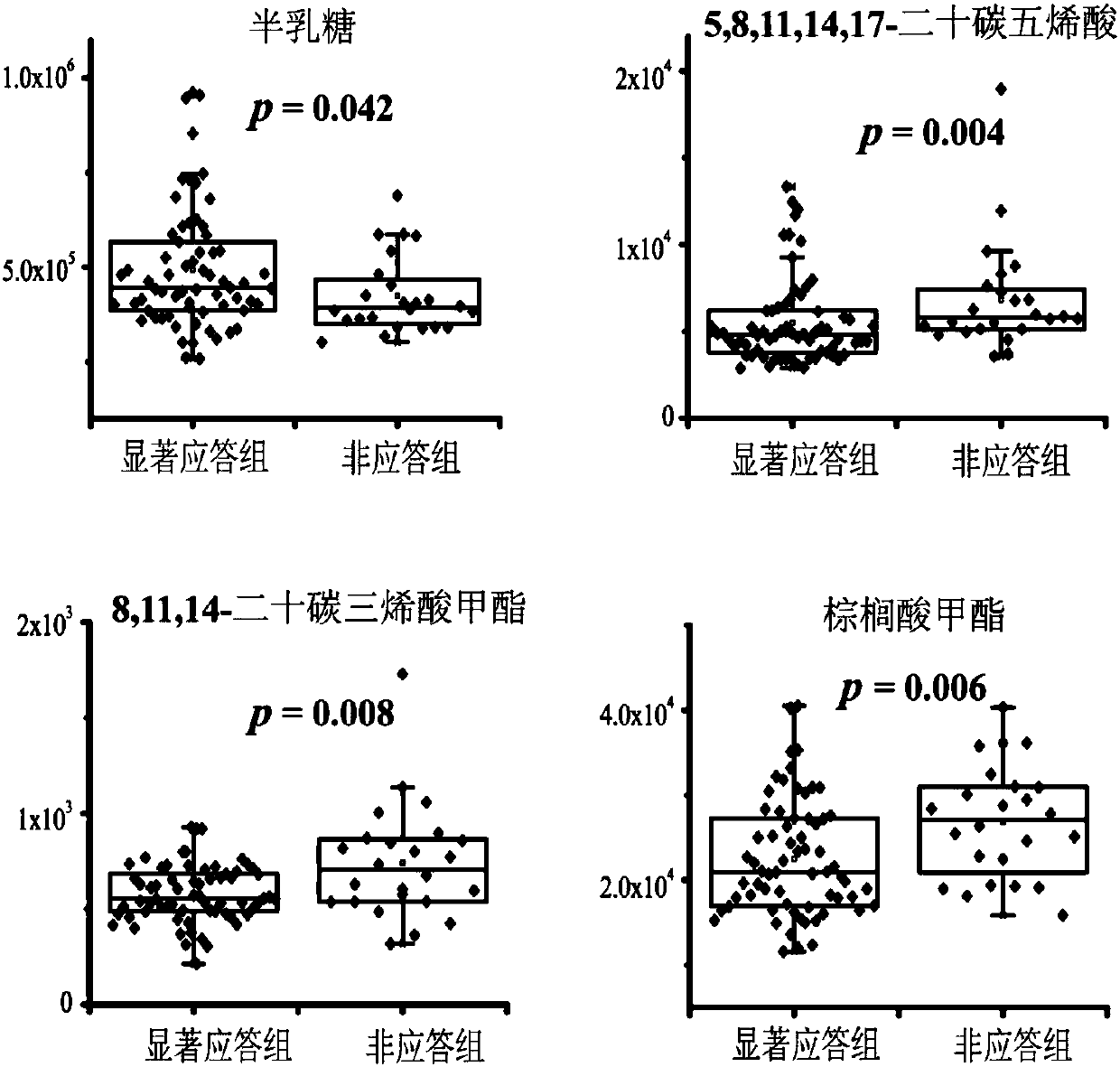
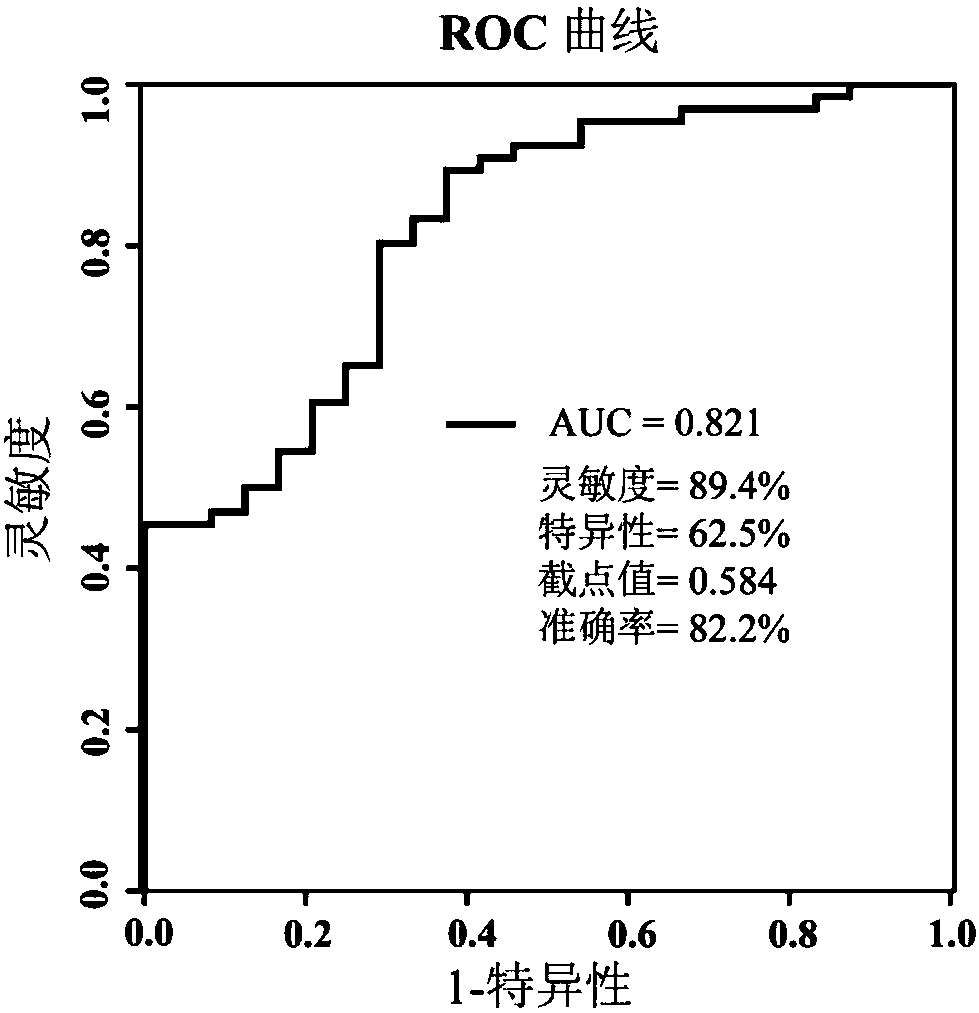
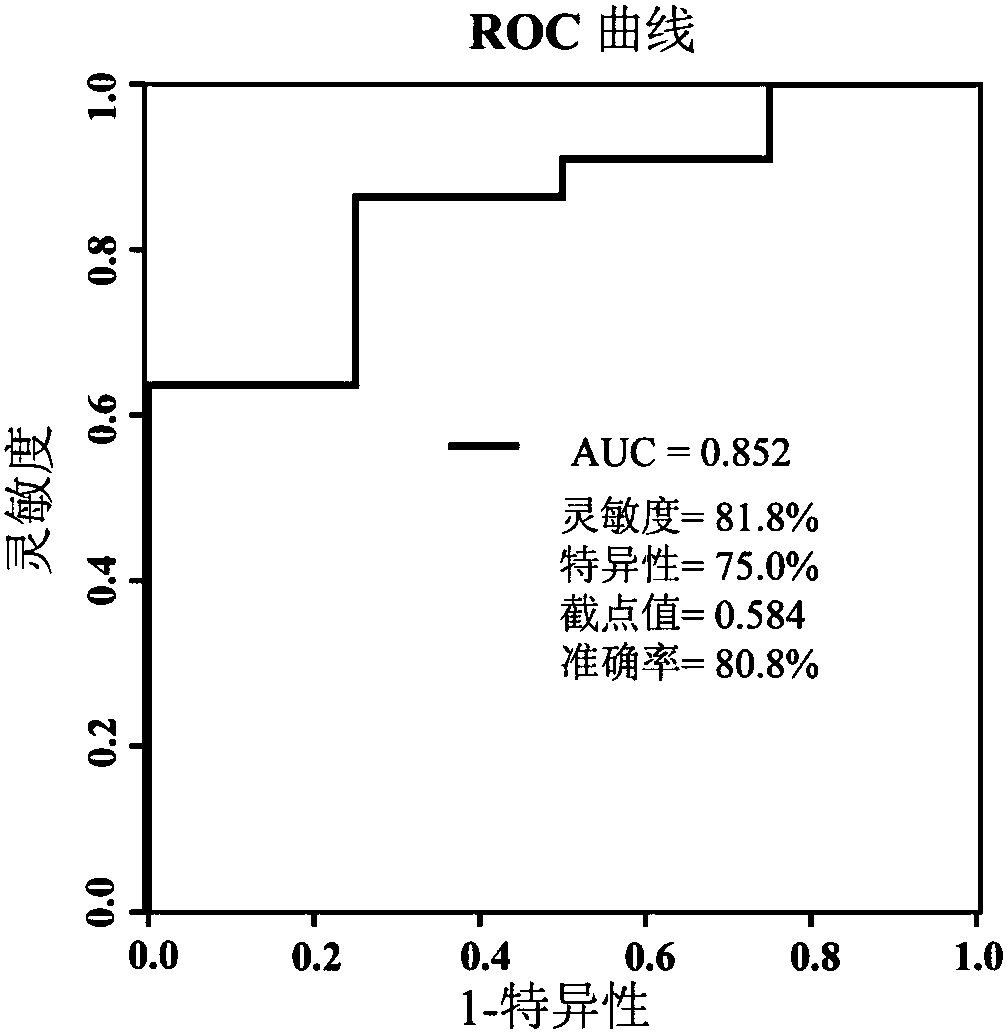
The invention relates to a serum combination marker for evaluating the gliclazide applicability of type 2 diabetes mellitus and a detection kit thereof, in particular to novel application of small-molecule metabolites, namely galactose, 5,8,11,14,17-eicosapentaenoic acid, 8,11,14-epoxyeicosatrienoic acid methyl ester and methyl palmitate, in serum samples in preparing the kit used for evaluating the gliclazide applicability of subjects as combination markers. The invention further relates to a kit for detecting patients remarkably responding to gliclazide in the subjects. By detecting relativeconcentrations of the combination markers in the serum samples of the subjects, the variables of the combination markers are calculated on the basis of a binary logic regression equation, and then onthe basis of determined section values, whether or not the subjects are suitable for gliclazide treatment is judged. The kit can achieve high-sensitivity high-efficiency detection of the small-molecule metabolites, and has the advantages of being low in detection cost, good in repeatability and high in diagnosis sensitivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for determination of illegally added substances in traditional Chinese medicines and health-care products
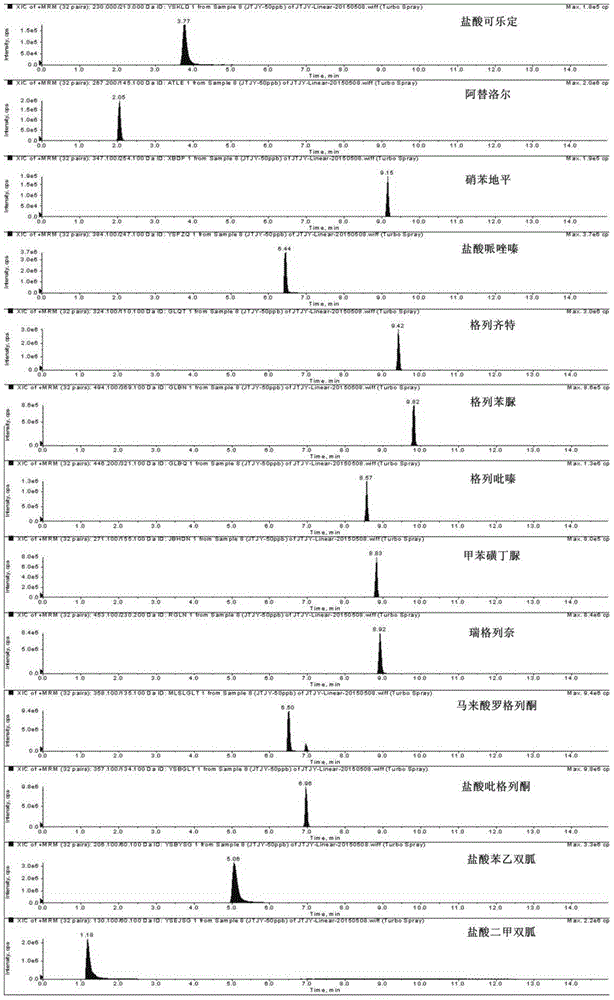
The invention discloses a method for determination of illegally added substances in traditional Chinese medicines and health-care products; with use of high performance liquid chromatography-tandem quadrupole linear ion trap mass spectrometry, the method which is capable of simultaneously qualitative and quantitative detection (multiple reaction monitoring sMRM-information dependent acquisition IDA-enhanced product ion scanning EPI detection) of 13 kinds of illegally added hypoglycemic hypotensive chemical drugs such as clonidine hydrochloride and gliclazide in the traditional Chinese medicines and the health-care products is developed. And through one-time sampling detection, not only can a quantitative result obtained, but also occurrence of a false positive result is effectively avoided through qualitative database screening. The establishment of the method provides a technical support for enacting detection standards of the illegally added hypoglycemic hypotensive chemical drugs in the traditional Chinese medicine and the health-care products.
Owner:SHANDONG ANALYSIS & TEST CENT
Gliclazide tablets (II) and preparation method thereof
PendingCN110585155AEffective controlled releaseAvoid sudden releaseMetabolism disorderSulfonylurea active ingredientsMedicineAdhesive
The invention provides gliclazide tablets (II) and a preparation method thereof, and belongs to the field of pharmaceutical preparations. According to the invention, glyceryl behenate is used as a lipid sustained-release matrix, is used as a sustained-release skeleton at the same time, and is subjected to fluidization granulation, so that solid bridges are formed among the raw materials, raw material powder is bonded, sustained-release particles are formed, the release of gliclazide can be effectively controlled, the phenomenon of sudden release of active ingredients is avoided, and the medication safety is guaranteed. Meanwhile, the glyceryl behenate is subjected to the fluidization granulation, no explosive solvent such as ethanol is required to be used for preparing an adhesive, no residual solvent is generated, and industrial mass production is facilitated.
Owner:SHANDONG LUKANG PHARMA
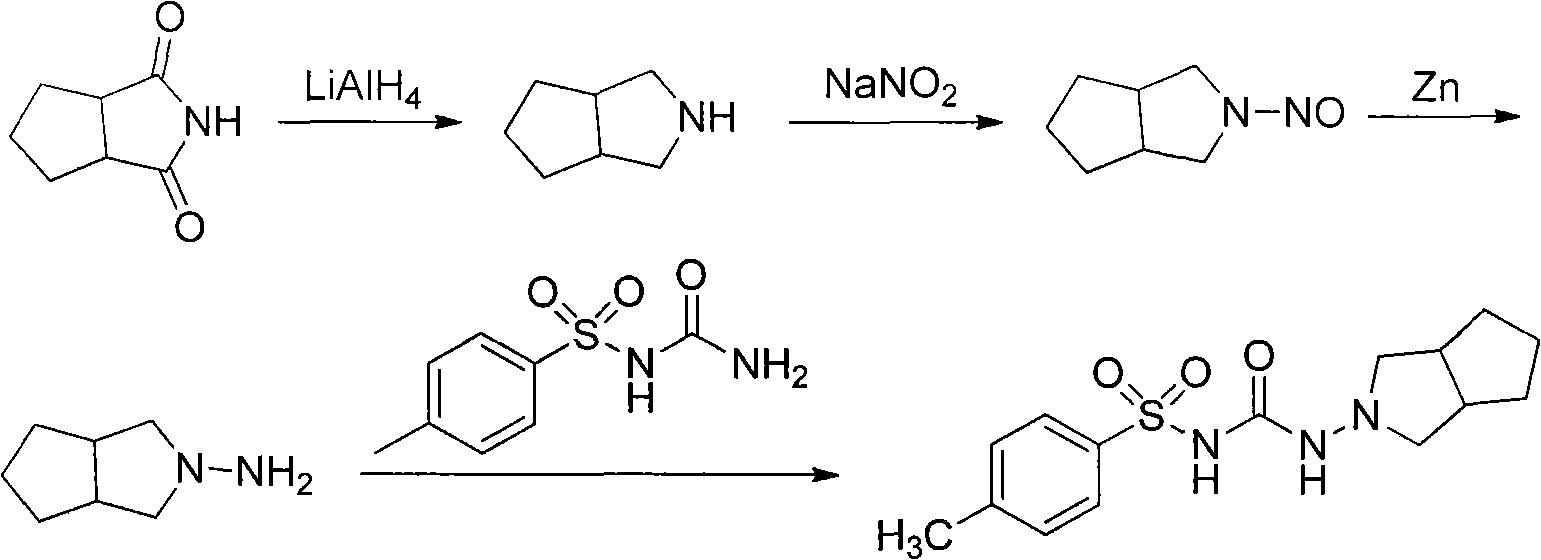
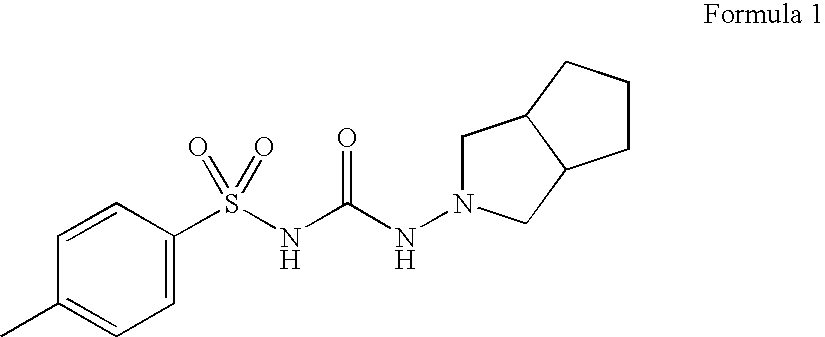
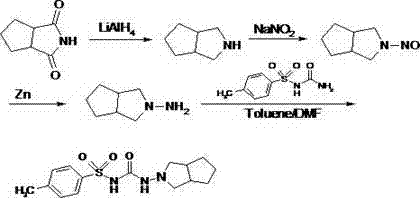
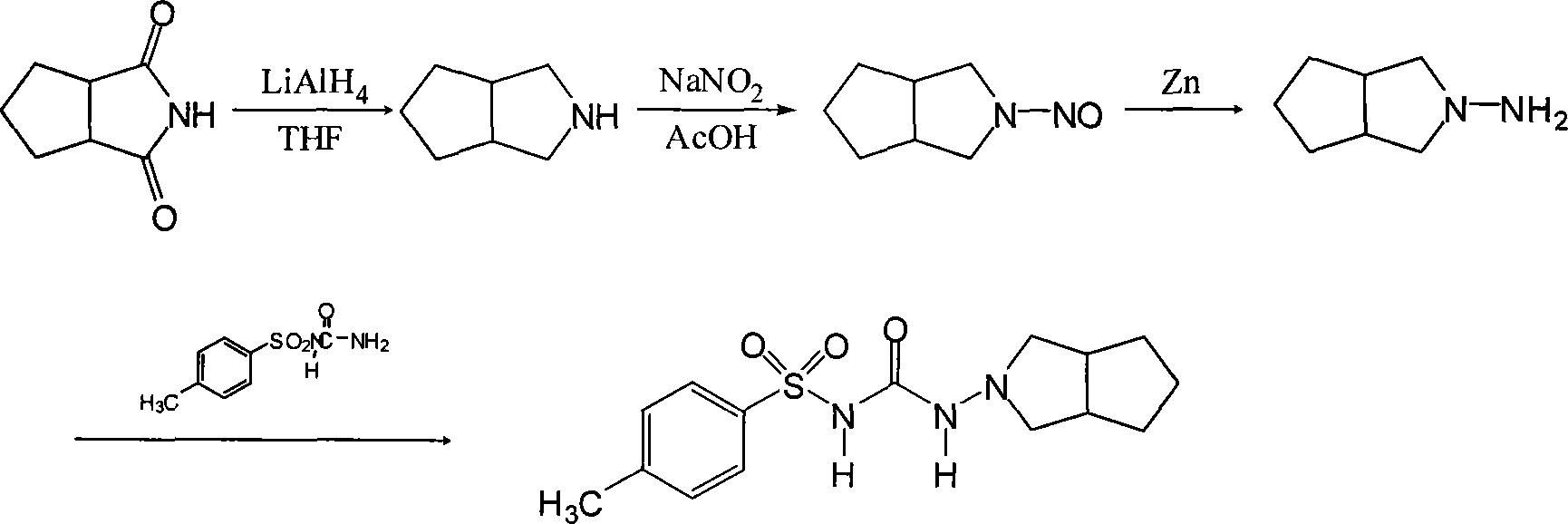
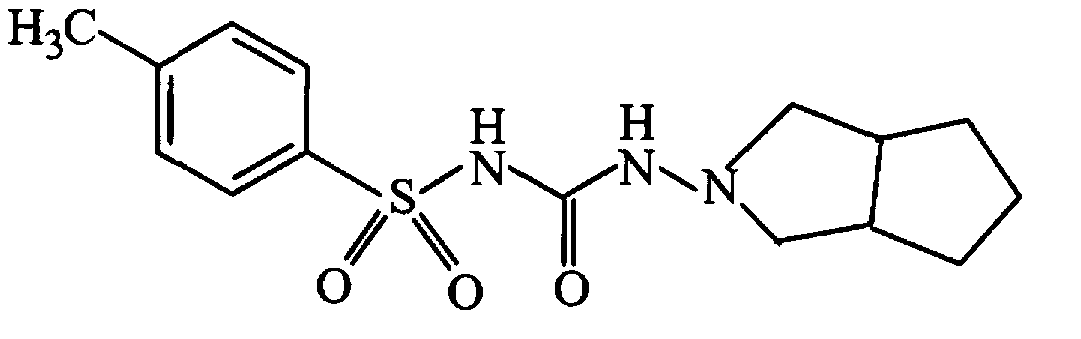
Synthetic method of N-amino-3-azabicyclo[3,3,0]octane hydrochloride
InactiveCN102382034AEmission reductionSimple processOrganic chemistryZinc oxides/hydroxidesNitrosoZinc hydroxide
The invention discloses a synthetic method of an important intermediate of gliclazide, in particular to the synthetic method of N-amino-3-azabicyclo[3,3,0]octane hydrochloride. The N-amino-3-azabicyclo[3,3,0]octane hydrochloride is prepared by the following steps of: reducing N-nitroso-3-azabicyclo[3,3,0]octane through zinc powder and hydrochloric acid, alkalizing and dissociating through potassium hydroxide or sodium hydroxide, extracting through methylbenzene or dimethylbenzene and the like, acidulating through industrial hydrochloric acid to form salts, reducing pressure, reflowing and completely distributing water, cooling and crystallizing. The synthetic method has the advantages of simple process, small alkali consumption, low discharge of wastes, high yield and low cost; and the synthetic method can be used for co-generating zinc hydroxide used as an auxiliary agent of rubber and surgical ointment and is suitable for industrial production.
Owner:山东洪智生物科技有限公司
Preparation method of gliclazide intermediate cyclohexanone-2-methanmide
InactiveCN103159642AEasy to operateMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationCyclohexanoneFiltration
The invention discloses a preparation method of gliclazide intermediate cyclohexanone-2-methanmide. According to the preparation method, spiro-compounds are added in 9.1-9.8% of prepared dilute sulphuric acid, the temperature is raised, and then water and cyclohexanone mixed fraction are obtained. When cyclohexanone does not exist in the fraction, the water is continued to be steamed to be 65-80% of the total water amount, standing and layering are carried out, after a water layer at the bottom is layered, an oil layer at the top is placed into esters solution, the temperature of the solution is raised, backflow is conducted for half an hour, then the temperature of the solution is reduced to be below 10 DEG C and then preserved for 2 hours, and finally white or off-white gliclazide intermediate cyclohexanone-2-methanmide crystalline powder is obtained after suction filtration, washing and drying. According to the preparation method of the gliclazide intermediate cyclohexanone-2-methanmide, synthetic process is easy to operate, reaction conditions are mild, product purity is high, yield coefficients are high, and the preparation method is suitable for commercial process.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Gliclazide sustained-release tablets
ActiveCN107375224AStable release rateImprove stabilityMetabolism disorderSulfonylurea active ingredientsExtended release tabletsMedicine
The invention discloses gliclazide sustained-release tablets, which are prepared by evenly mixing drug-loading particles with pharmaceutically acceptable adjuvant materials and tabletting the obtained mixture; a preparation method of the drug-loading particles comprises the following steps: heating glyceryl behenate up to 65-70 DEG C for melting, then adding meglumine and evenly stirring in a heat preservation process, adding gliclazide and stirring until the mixture is molten, cooling for solidifying, and pelletizing, wherein the mass ratio of the gliclazide to the glyceryl behenate to the meglumine is equal to 1 to (1.5-4.5) to (0.1-0.3). The gliclazide sustained-release tablets are stable in drug release speed, better in drug stability, high in bioavailability, simple in preparation technology and suitable for industrial mass production.
Owner:浙江康德药业集团股份有限公司
Preparation method of gliclazide
The invention discloses a synthetic process for preparing gliclazide with low-cost raw materials and high yield. The synthetic process comprises the following reaction steps: 1, preparing N-amino-1,2-cyclopentane phthalic amide by a reaction of 1,2-cyclopentane phthalic anhydride and hydrazine hydrate; 2, reducing the N-amino-1,2-cyclopentane phthalic amide; and 3, preparing the gliclazide by a reaction of methylphenylsulfonylurea and the product reduced in the step 2. By adopting the synthetic process, the N-amino-1,2-cyclopentane phthalic amide is reduced by using an NaBH4 / concentrated H2SO4 system from cheap 1,2-cyclopentane phthalic anhydride; an expensive reagent LiA1H4 is avoided. Therefore, the production cost is greatly reduced.
Owner:WEIHAI WEITAI PHARMA TECH DEV
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Two-channel detection card for simultaneously detecting glibenclamide and gliclazide and detection method thereof
The invention relates to a two-channel detection card for simultaneously detecting glibenclamide and gliclazide and a detection method thereof, belonging to the technical field of detection of western medicine illegally added into Chinese medicine. The surface of a detection card casing is provided with a detection window opening and a sampling hole, a test strip is arranged in the casing, a nitrocellulose membrane is attached to the middle of a bearing back panel, a water sucking membrane is attached to one end of the bearing back panel, a sample pad is attached to the other end of the bearing back panel, two colloidal gold membranes are clamped and attached between the nitrocellulose membrane and the sample pad, wherein the colloidal gold membranes are glass fiber membranes which respectively contain an anti-glibenclamide colloidal gold labeled monoclonal antibody and anti-gliclazide colloidal gold labeled monoclonal antibody, three print display bands are intervally arranged on the nitrocellulose membrane along the traverse direction, one is a detection band containing a glibenclamide protein conjugate, another one is a detection band containing a gliclazide protein conjugate, and the third one is a quality control band containing an anti-rabbit antibody or an anti-mouse antibody, the sample pad is arranged just opposite to the sampling hole, and the nitrocellulose membrane is arranged just opposite to the detection window opening. The detection card can be used for simultaneously detecting two western medicine components illegally added into a glucose-lowering Chinese medicine sample, has the advantages of detection cost saving, rapid detection, high sensitivity, accurate result, and convenience for use.
Owner:无锡安迪生物工程有限公司
Process for preparing gliclazide slow release capsule for oral sugar reducing method
InactiveCN1615851AReduce dwell timeEasy to takeMetabolism disorderPharmaceutical delivery mechanismInsulin dependent diabetesSustained Release Capsule
The present invention discloses a preparation process of slowly releasing orally taken hypoglycemic gliclazide capsule, and belongs to the field of medicinal technology field. The capsule consists of gliclazide 10-40 wt%, calcium biphosphate 40-70 wt%, HPMC4000cp 0-20 wt%, HPMC100cp 0-20 wt%, EC100cp 0-9.3 wt%, magnesium stearate 0-2 wt% and talcum powder 0-2 wt%. The preparation process includes 60-100 mesh sieving the first mentioned five medicine materials, mixing, adding 50-75 % concentration alcohol as wetting agent and stirring to making soft material, 14-20 mesh sieve pelletizing, drying at 50-60 deg.c of 1-2 hr, 14-20 mesh sieve finishing, adding magnesium stearate and talcum powder, and capsulizing. The capsule is used in treating non-insulin dependent diabetes (type-II), is especially suitable for adult diabetes, diabetes accompanied by obesity or blood vessel pathologic change, and has the features of being safe and long in effect.
Owner:SHANGHAI JIAO TONG UNIV
Double-controlled release gliclazide sustained-release capsules and preparation method thereof
ActiveCN102058563ALong-lasting effectMaintain blood levelsMetabolism disorderSulfonylurea active ingredientsSustained release pelletsSustained Release Capsule
The invention discloses double-controlled release gliclazide sustained-release capsules and a preparation method thereof. Contents filled into the sustained-release capsules are gliclazide sustained-release pellets of which surfaces are coated with quick release film coatings. Based on 1,000 capsules, the gliclazide sustained-release pellets are prepared from the following raw materials by weight: 20 to 30 grams of gliclazide, 30 to 50 grams of microcrystalline cellulose, 40 to 60 grams of lactose, 2 to 8 grams of hydroxypropyl methylcellulose 4000cp and 30 to 60 grams of water; and the quickrelease film coatings are prepared from the following raw materials by weight: 5 to 20 grams of gliclazide, 10 to 40 grams of EudragitRL100, 5 to 30 grams of talcpowder and 300 grams of ethanol at the volume concentration of 95 percent. Compared with the prior art, the capsules provided by the invention can develop the advantages of multi-unit preparations at the same time of meeting requirementson the quick response of medicaments and keeping a certain blood concentration; and the preparation method for the capsules is simple and easy to control.
Owner:桂林华信制药有限公司
Glibenclamide, gliclazide and glipizide triple test card and test method thereof
InactiveCN102012427AEasy to manufactureLow detection costPreparing sample for investigationWestern medicineGlass fiber
The invention relates to a glibenclamide, gliclazide and glipizide triple test card and a test method thereof, belonging to the technical field of testing of western medicine components which are illegally added into traditional Chinese medicines. A test bar is arranged in a shell of the triple test card and comprises a sample pad, a plurality of sections of colloidal gold membranes, a nitrocellulose membrane and a water-absorbing membrane which are sequentially stuck on a supporting backboard, wherein the plurality of sections of the colloidal gold membranes are glass fiber membranes containing colloidal gold markers of an anti-glibenclamide antibody, an anti-gliclazide antibody and an anti-glipizide antibody sequentially, three test strips which respectively contain a glibenclamide protein conjugate, a gliclazide protein conjugate and a glipizide protein conjugate are arranged on the nitrocellulose membrane, and the triple test card additionally comprises a quality control strip containing an anti-rabbit antibody or an anti-mouse antibody. The triple test card has the advantages of being capable of simultaneously detecting glibenclamide, gliclazide and glipizide which are illegally added in a glucose-lowering traditional Chinese medicine. The test card is easy for preparation, convenient and fast for use and accurate in result, and can be used for saving the test cost.
Owner:长沙安迪生物科技有限公司
Gliclazide sustained release tablet and preparation method thereof
ActiveCN111329841AMetabolism disorderSulfonylurea active ingredientsProlonged-release tabletMagnesium stearate
The invention discloses a gliclazide sustained release tablet and a preparation method thereof. The gliclazide sustained release tablet comprises gliclazide, calcium hydrogen phosphate, maltodextrin,hydroxypropyl methylcellulose, magnesium stearate and colloidal silicon dioxide. The gliclazide sustained release tablet is prepared by the following method: mixing gliclazide, a calcium hydrogen phosphate dihydrate and maltodextrin, preparing a wet material by using pure water as a wetting agent, then granulating the wet material, and performing drying and grading to obtain medicine-containing granules; uniformly mixing the medicine-containing granules with hydroxypropyl methylcellulose, and then uniformly mixing the obtained mixture with magnesium stearate and colloidal silicon dioxide; andpressing the mixture obtained in the previous step into tablets by using a die with proper size on a rotary tablet press. The tablet provided by the present invention has excellent performance and excellent stability.
Owner:SHANDONG LUKANG PHARMACEUTICAL GROUP SAITE CO LTD
Gliclazide sustained release tablets and preparation method thereof
InactiveCN105193758AComponent stabilityGood sustained release effectMetabolism disorderSulfonylurea active ingredientsMagnesium stearateGliclazide
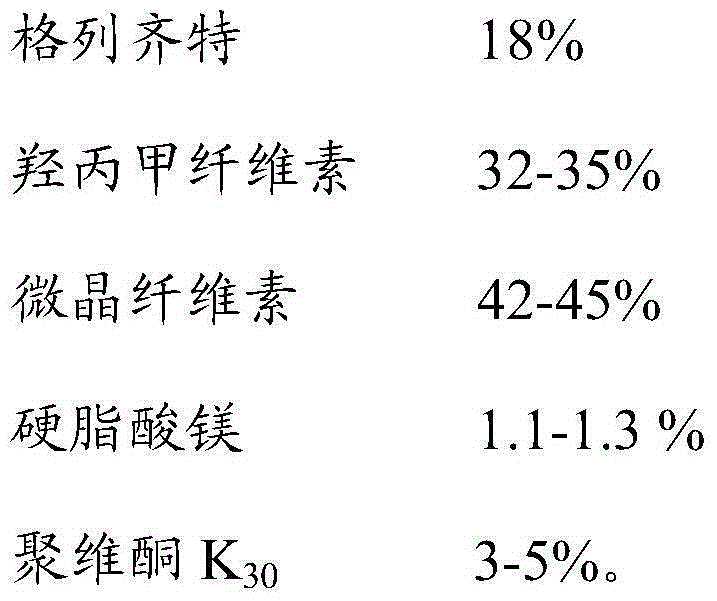
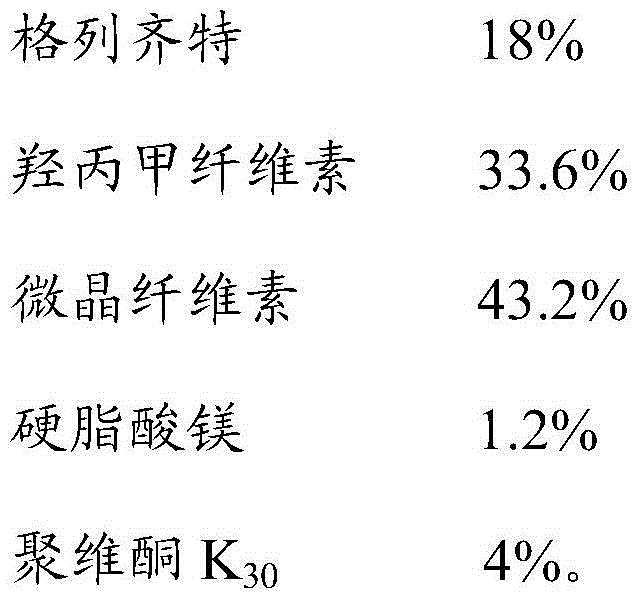
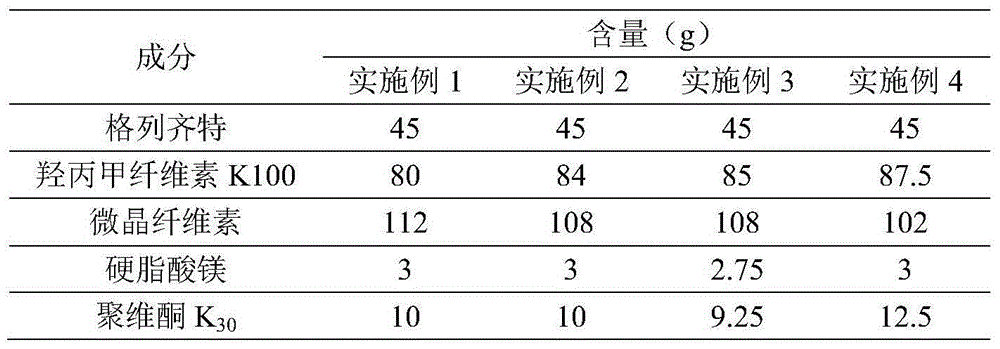
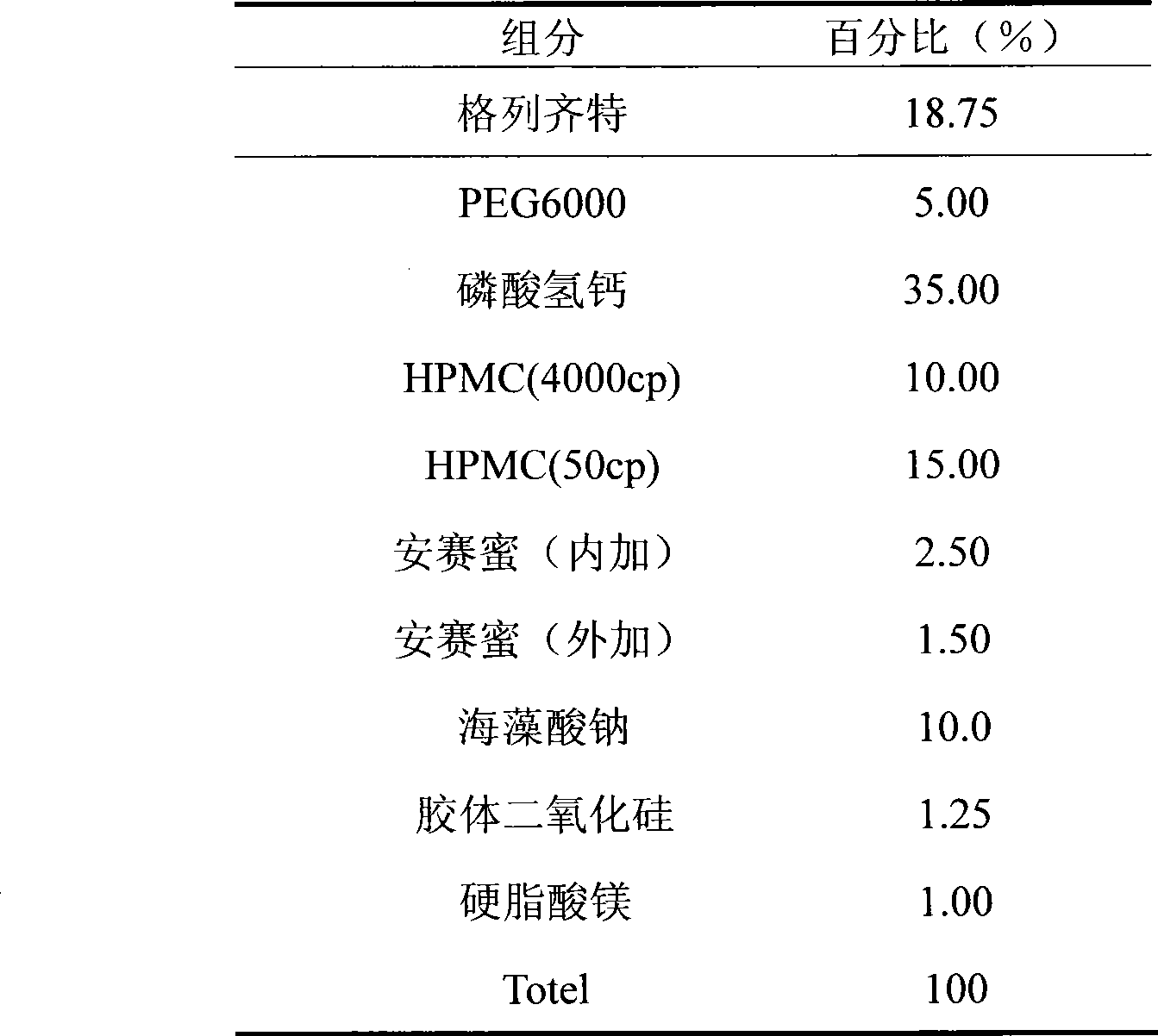
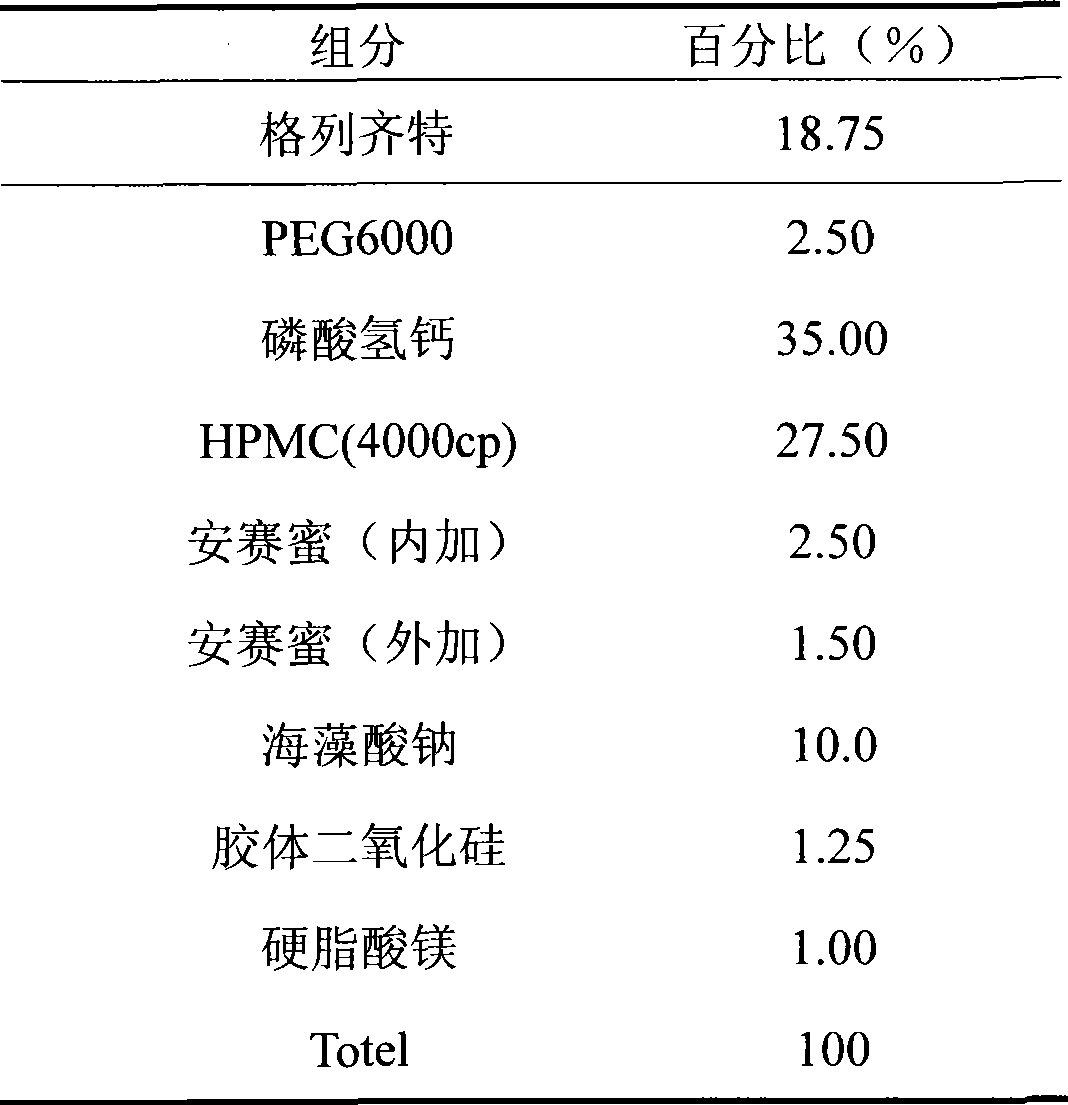
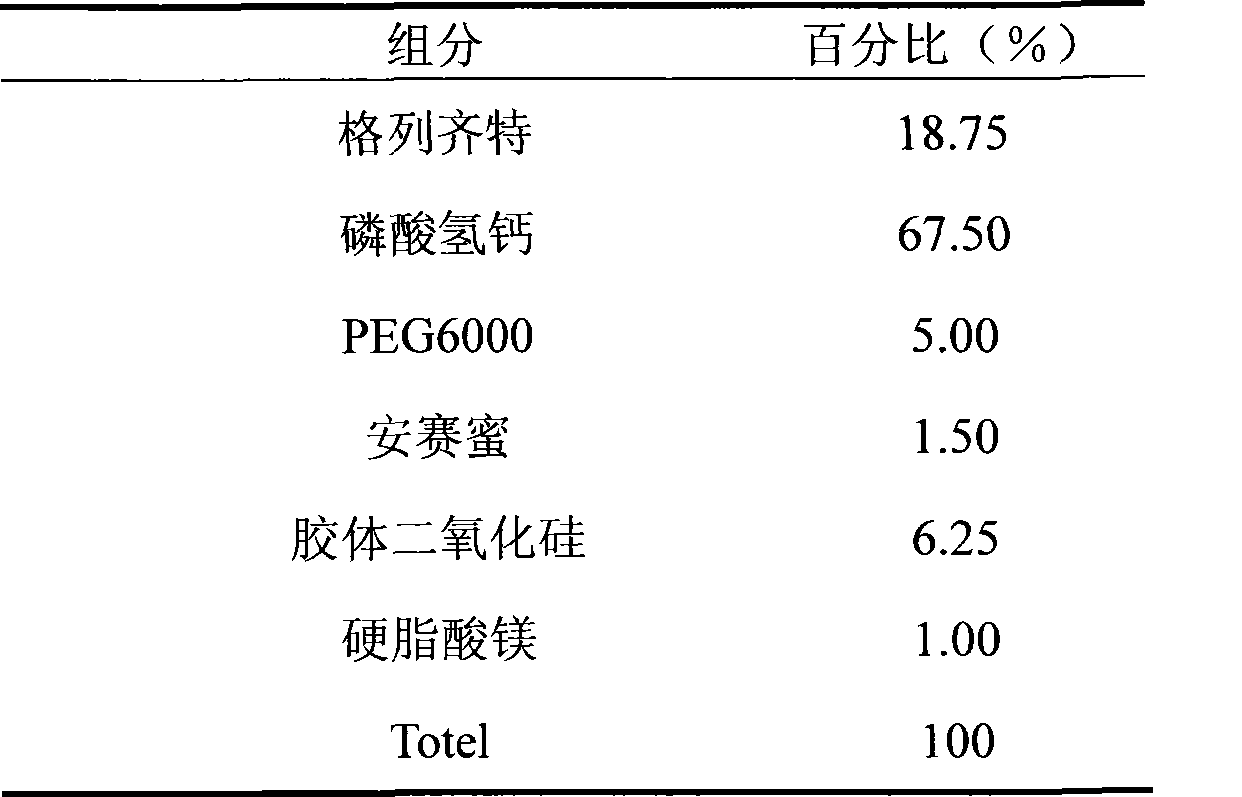
The invention discloses gliclazide sustained release tablets which are prepared from the following components in percentage by weight: 18.75% of gliclazide, 32-36% of hydroxypropyl methylcellulose, 40-50% of microcrystalline cellulose and the balance of magnesium stearate; the invention further discloses a preparation method of gliclazide sustained release tablets. The gliclazide sustained release tablets provided by the invention conform to provisions in the part II of China Pharmacopeia 2010, the components are stable, the slow-release effect is good, and the production cost is low.
Owner:NANJING REAL PHARMA
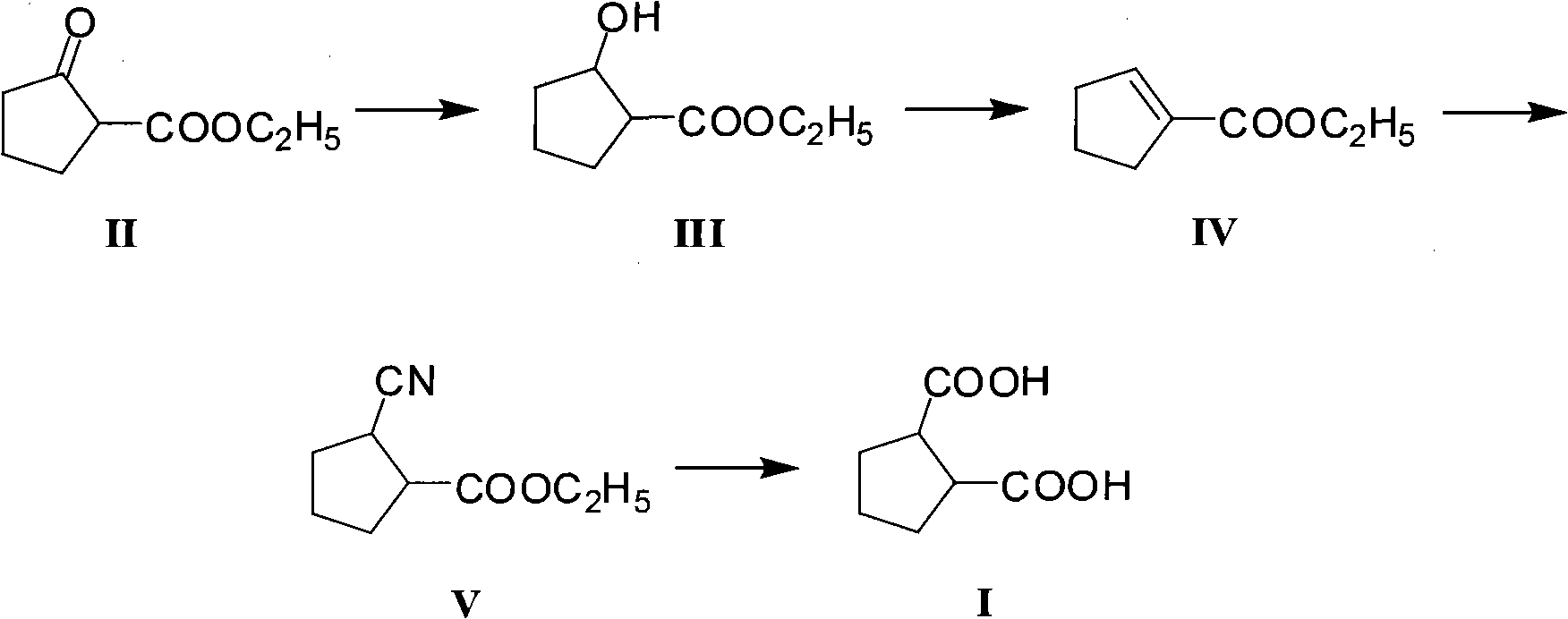
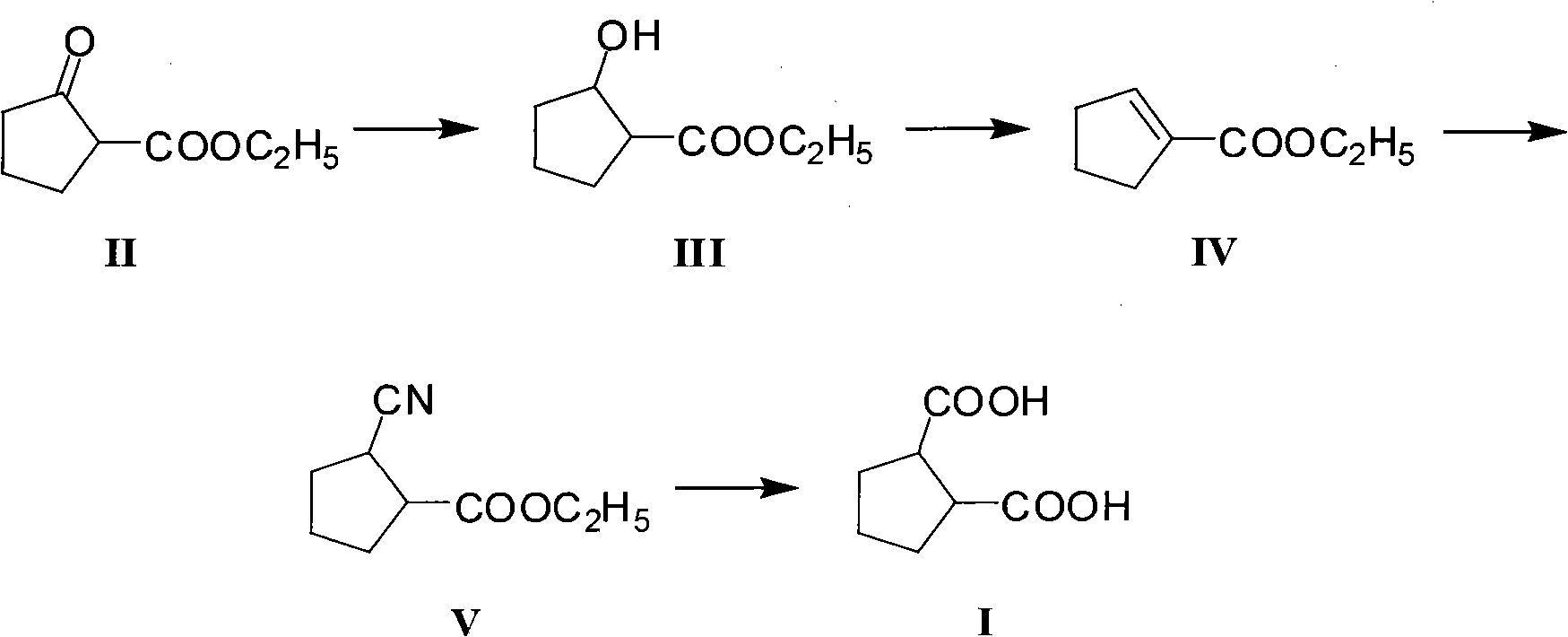
Method for preparing intermediate 1,2-dicarboxylicacid of antidiabetic medicine gliclazide
ActiveCN101962320ASufficient supply in the domestic marketLow priceOxygen-containing compound preparationOrganic compound preparationEthyl esterChemistry
The invention discloses a method for preparing intermediate 1,2-dicarboxylicacid of an antidiabetic medicine gliclazide. The method comprises the steps of reducing, eliminating, adding and hydrolyzing 2-oxocyclopentanecarboxylate serving as a raw material to prepare the product. The invention provides a brand-new synthesis route, and has the advantages wide and rich sources and low price of the raw material, mild reaction condition and simple process; and reactions in each step are conventional operation, so the production cost is reduced.
Owner:ANHUI JINDING PHARMA
Slow released preparation containing metformin hydrochloride and gliclazide and its preparing process
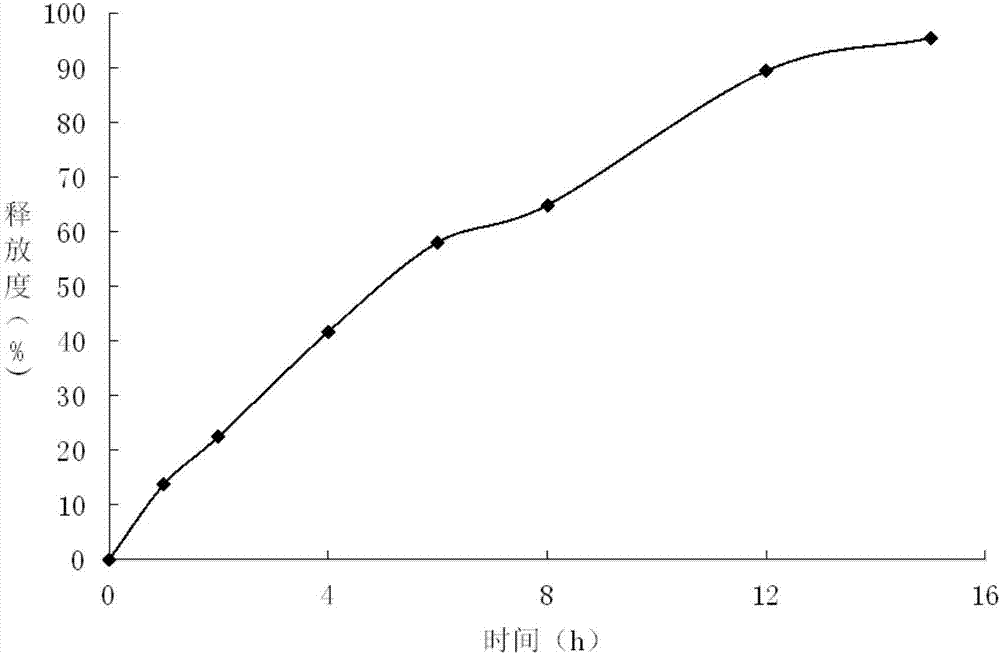
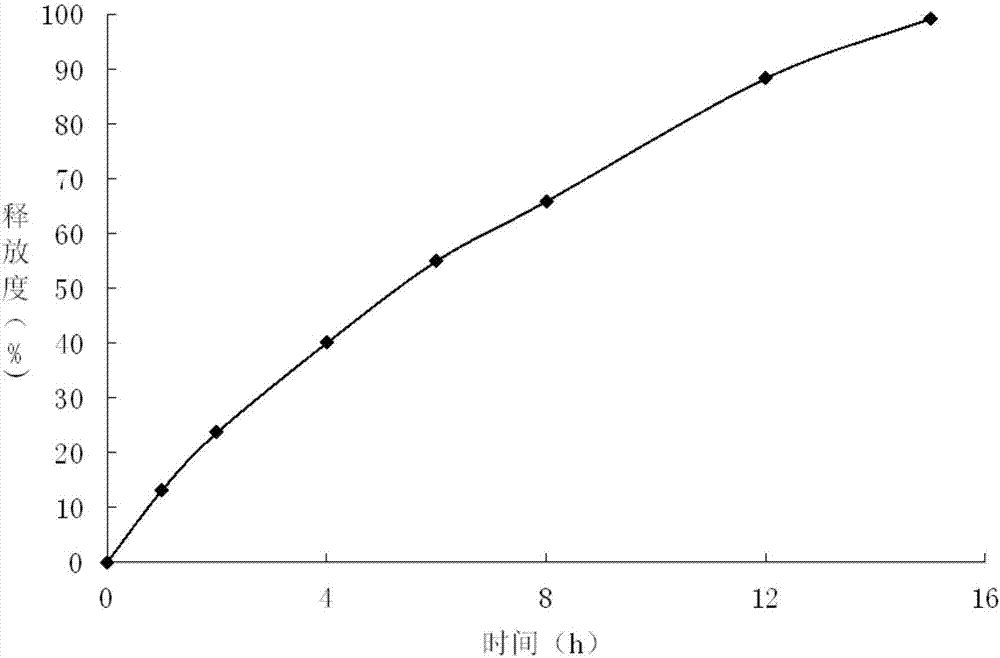
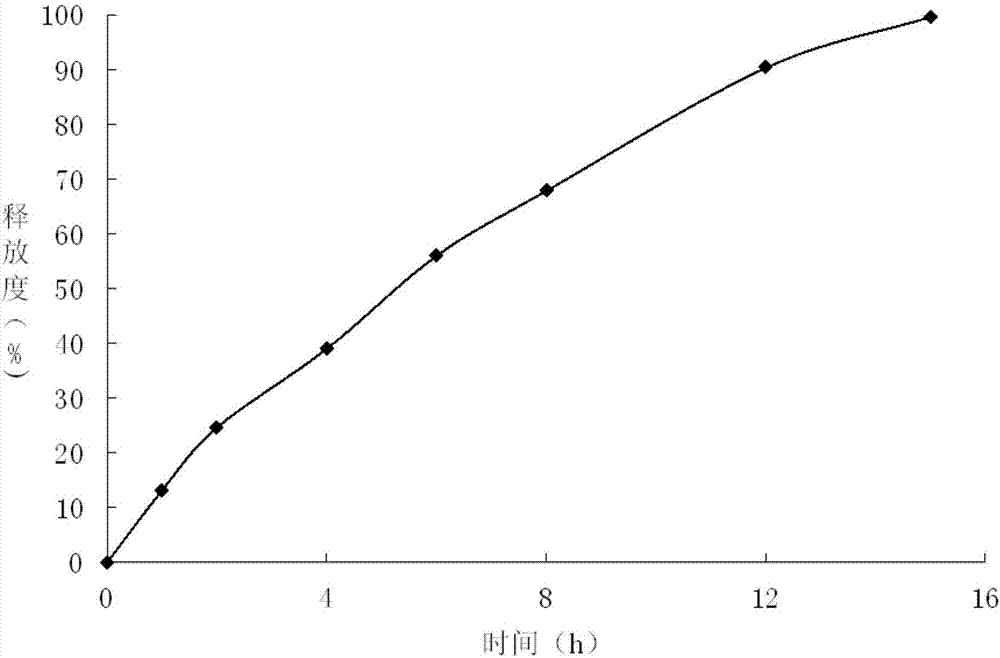
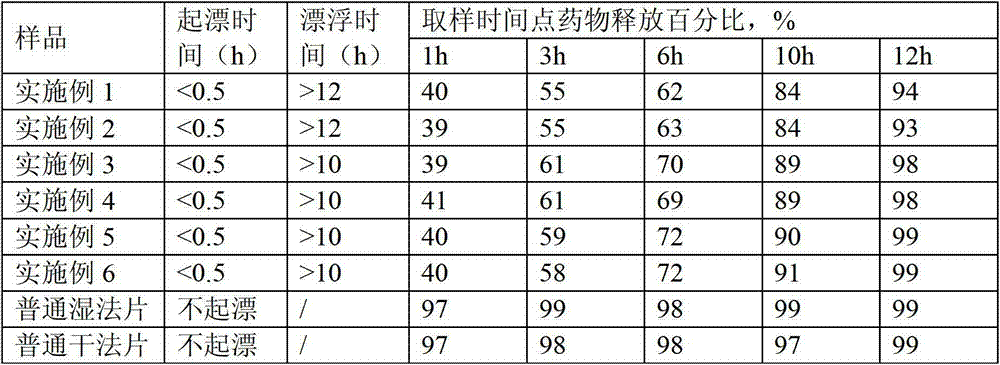
The present invention relates to a kind of slow released preparation containing metformin hydrochloride and glicazide and its preparation process. The preparation consists of slow released metformin hydrochloride and slow released glicazide. The metformin hydrochloride is released in 15-40 % in the first 1 hr, 50-70 % in the first 4 hr, and over 75 % in the first 8 hr; and the glicazide is released in 20-40 % in the first 2 hr, 40-65 % in the first 4 hr and over 75 % in the first 10 hr. The slow released preparation containing metformin hydrochloride and glicazide has mild and lasting blood sugar reduction, capacity of avoiding hypoglycemia, gastrointestinal discomfort and other untoward reaction, decreased medicine taking times and raised compliance. The present invention also discloses the extracorporeal slow releasing characteristic and preparation process of the slow released preparation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Method for using liquid chromatogram-tandem mass spectrometry to measure concentration of glipizide in plasma
InactiveCN108469479AMeet the requirements of quantitative testingEasy to operateComponent separationPlasma samplesAlcohol
The invention belongs to the field of medicine detection, and relates to a method for using liquid chromatogram-tandem mass spectrometry to measure concentration of glipizide in plasma, in particularto a method for measuring the concentration of glipizide in plasma of a beagle. The method comprises the following steps of 1, preparation of a plasma sample; 2, sedimentation of protein; 3, measuringthrough the liquid chromatogram-tandem mass spectrometry. An interior label working solution gliclazide is added in the sample, and methyl alcohol is adopted for settling, volution and centrifuging.Then the liquid chromatogram-tandem mass spectrometry is adopted for measuring the concentration of glipizide in the plasma. The method adopts methyl alcohol for settling the protein, has the advantages that liquid chromatogram-tandem mass spectrometry is conducted, the operation is simple, the detection limit is low, and the repeatability is high, and is applicable to pharmacokinetic studies, toxicokinetic studies, bioequivalence studies and the like.
Owner:沈阳信达泰康医药科技有限公司
Pharmaceutical composition for treating diabetes
PendingCN106974951AImprove fatigue resistancePromote recoveryMetabolism disorderSulfonylurea active ingredientsMedicineRemove blood
The invention discloses a pharmaceutical composition for treating diabetes. The pharmaceutical composition is prepared from 8 to 16 parts by weight of gliclazide, 1 to 3 parts by weight of vitB6, 0. 025 to 0.1 parts by weight of viB12, 300 to 500 parts by weight of American ginseng and 300 to 500 parts by weight of pseudo-ginseng. The American ginseng in the pharmaceutical composition can balance blood sugar (bilaterally adjust blood sugar), has effects of nourishing yin and tonifying yang, reinforcing the vital essence and relieving collapse, resisting oxidation and removing free radicals, and can obviously improve human immunity through long-term use. Pseudo-ginseng in the pharmaceutical composition can improve microcirculation, can accelerate complication recovery, can reduce blood fat and blood sugar, has effects of promoting blood circulation to remove blood stasis, relaxing tendons and activating collaterals, resisting oxidation and aging and removing free radicals, and can produce good hypoglycemic synergism with gliclazide and good free radical removal synergism with American ginseng. The vitB6 and viB12 have a function of regulating nerves.
Owner:李忠
Extended release gliclazide formulations
The present invention relates to an extended release (XL) pharmaceutical tablet composition for oral administration and methods of its manufacture. More particularly, the present invention relates to an extended release gliclazide formulation which does not increase the blood glucose level in human patients.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Liquid formulations of compounds active at sulfonylurea receptors
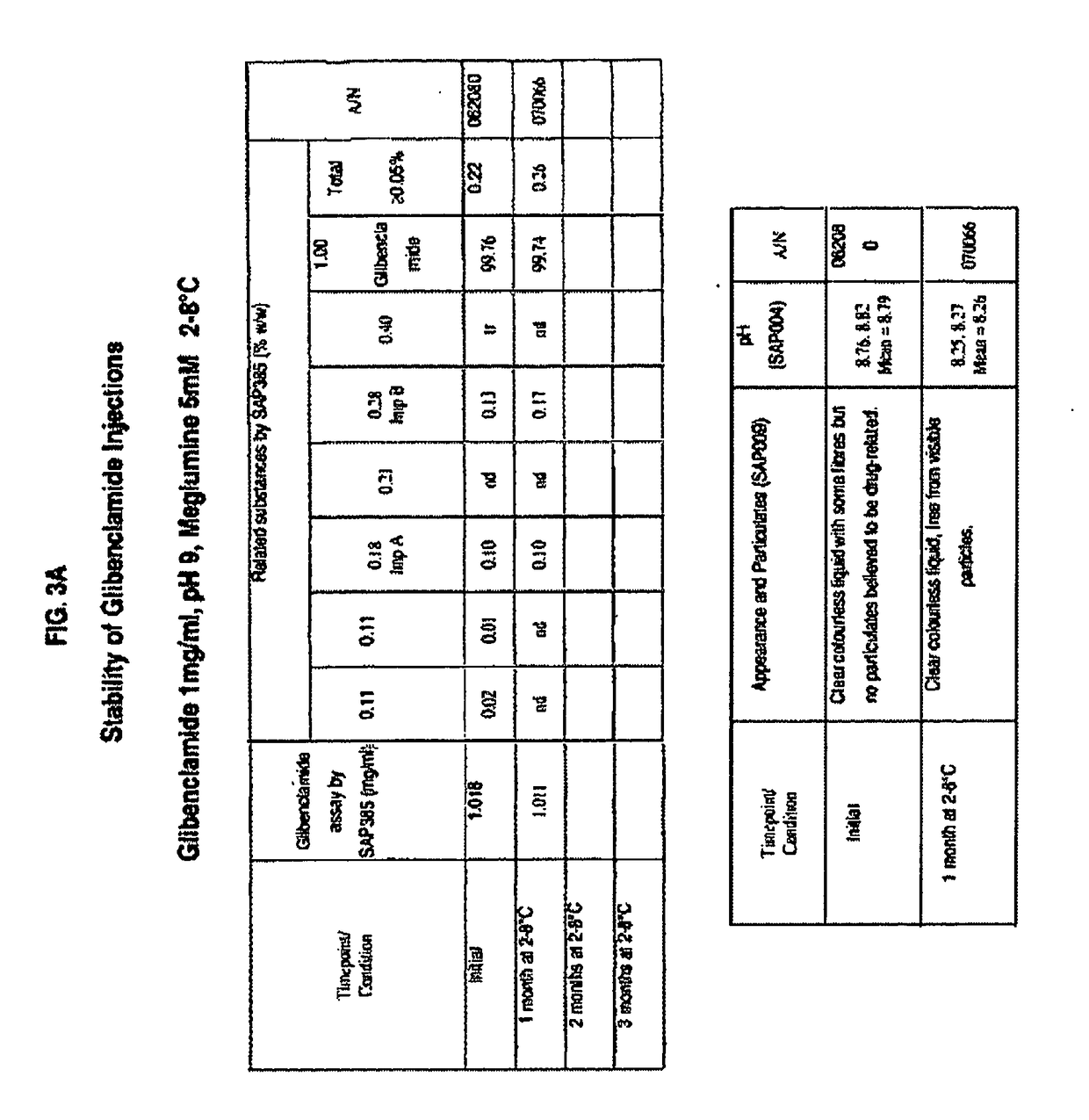
ActiveUS20170216321A1Rapid and readily controlled increase in circulating drug concentrationsQuick cureBiocideMetabolism disorderMeglitinideWater based
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intravenous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
Gliclazide gastric floating tablet and preparation method thereof
ActiveCN103110601AImprove bioavailabilityPromote absorptionMetabolism disorderSulfonylurea active ingredientsWaxSide effect
The invention discloses a gliclazide gastric floating tablet. The gliclazide gastric floating tablet comprises the following ingredients in proportion by weight: 5-15 parts of gliclazide, 20-60 parts of gel material, 20-50 parts of wax material, 1-10 parts of foaming material, 15-40 parts of lactose, 1-5 parts of magnesium stearate, and 0-5 parts of PVPK30 (Polyvinylpyrrolidone-K30). The invention also discloses a preparation method of the gliclazide gastric floating tablet. The gliclazide gastric floating tablet has the following beneficial effects: the gliclazide gastric floating tablet can keep a floating state in gastric juice, gastric transit time is 8-12 hours, the tablet can keep sustained medicine release in an acidic gastric juice environment, therefore, medicine release is constant and complete, medicine bioavailability is improved, administration dosage and times are reduced, medicine absorption at a certain part is promoted, and the toxic and side effect is relieved.
Owner:CHENGDU HENGRUI PHARMA
Method for synthesizing gliclazide and intermediate thereof
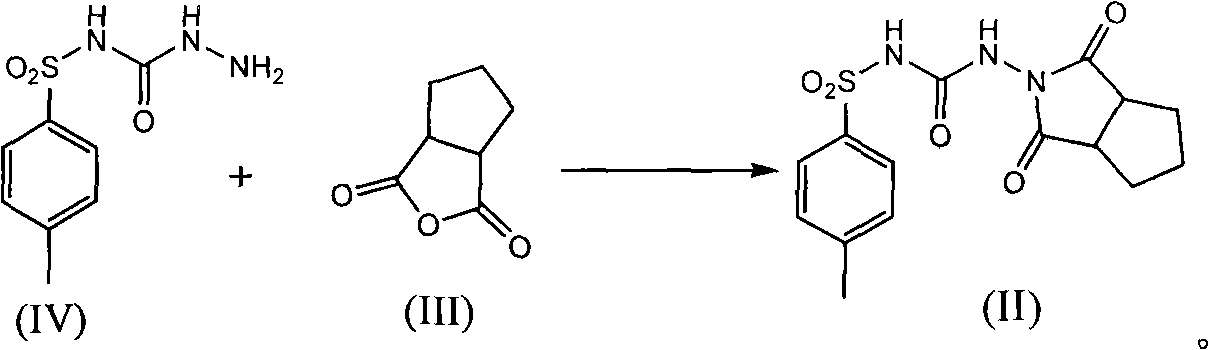
ActiveCN102050778AHigh yieldReduce usageSulfonic acid amide preparationP-tolylsulfonylureaCondensation reaction
The invention relates to a method for synthesizing gliclazide and an intermediate thereof, which comprises the following steps of: performing a condensation reaction of p-toluenesulfonylurea serving as a raw material and hydrazine hydrate to obtain a compound IV; reacting the compound IV with 1,2-cyclopentanedicarboxylic anhydride to obtain a compound II; and finally performing a reduction reaction of the compound II to obtain the gliclazide, wherein the compound II is a new intermediate compound. The gliclazide is synthesized by the method, amino heterocycles are not used, and the problem of oxidizing the raw material easily is solved fundamentally.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
HPLC-MS/MS (High Performance Liquid Chromatography-Mass Spectrum/Mass Spectrum) technique-based method for detecting blood concentration of NMDA (N Methyl D Aspartate) receptor antagonist JCC-02
ActiveCN108918722AFew samplesEasy pretreatmentComponent separationChromatographic separationNR1 NMDA receptor
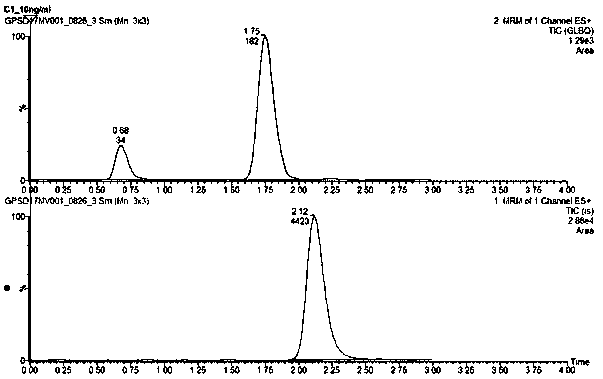
The invention provides an HPLC-MS / MS (High Performance Liquid Chromatography-Mass Spectrum / Mass Spectrum) technique-based method for detecting blood concentration of a NMDA (N Methyl D Aspartate) receptor antagonist JCC-02 and relates to the field of drug analysis. The method comprises the following steps of: sequentially adding methanol, acetonitrile and an internal standard working solution intoplasma of an SD (Sprague Dawley) rat after intragastric administration of the JCC-02, eddying and dissolving supernatant by using a mobile phase to obtain a preprocessed sample, wherein the internalstandard working solution was a gliclazide methanol solution; and carrying out gradient elution by taking acetonitrile-formic acid water mixed solution as a mobile phase and by adopting HPLC-MS / MS technique, carrying out chromatographic separation by using a Venusil ASB C8 chromatographic column, detecting through a second-stage mass spectrometry and carrying out quantitative analysis. The methodhas the advantages of being strong in specificity, high in sensitivity, small in sample sampling amount, simple and rapid in preprocessing and short in analysis period; and proved by methodology, themethod is accurate and reliable and is suitable for drug concentration determination of the JCC-02 in the plasma of the SD rat and pharmacokinetic study.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Pharmaceutical composition containing gliclazide
The invention relates to pharmaceutical composition with gliclazide and preparation method thereof. By producing solid dispersion, the invention improves the releasing rate of the medicament, so as to achieve better sustained-release purpose. The pharmaceutical composition applied to the treatment of diabetes, and reduces the frequency of injecting medicine and helps stabilize the blood concentration of the patients, meanwhile, the adaptability is increased too.
Owner:北京德众万全医药科技有限公司
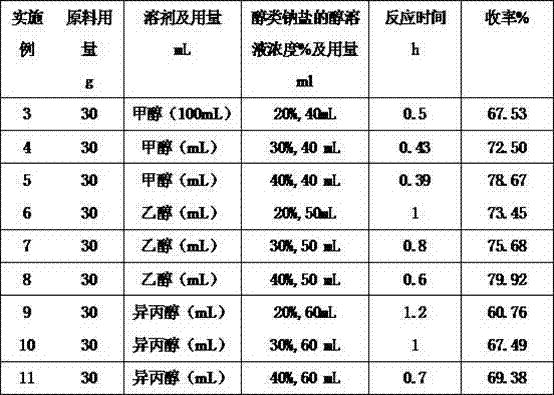
Preparation method of 1,2-cyclopentane dicarboximide
The invention discloses a preparation method of 1,2-cyclopentane dicarboximide disclosed as a chemical structural formula on the right. The preparation method comprises the following steps: reacting the raw material alpha-bromocyclohexanone formamide with alcohol and alcohol sodium salt at low temperature for 0.5-1.5 hours, wherein the mol ratio of alpha-bromocyclohexanone formamide to alcohol sodium salt is 1:(1-2); and after the reaction finishes, removing the solvent, continuing heating to 180-220 DEG C to carry out condensation, removing the alcohol solvent, and recrystallizing with a proper solvent to obtain the 1,2-cyclopentane dicarboximide. The 1,2-cyclopentane dicarboximide prepared by the method disclosed by the invention can be used as an intermediate of gliclazide; and compared with the 1,2-cyclopentane dicarboximide prepared by the traditional ammonia water technique, the 1,2-cyclopentane dicarboximide prepared by the method disclosed by the invention has the advantages of high yield, low energy consumption, less wastewater and the like, and provides a feasible technical route for industrial production.
Owner:山东洪智生物科技有限公司
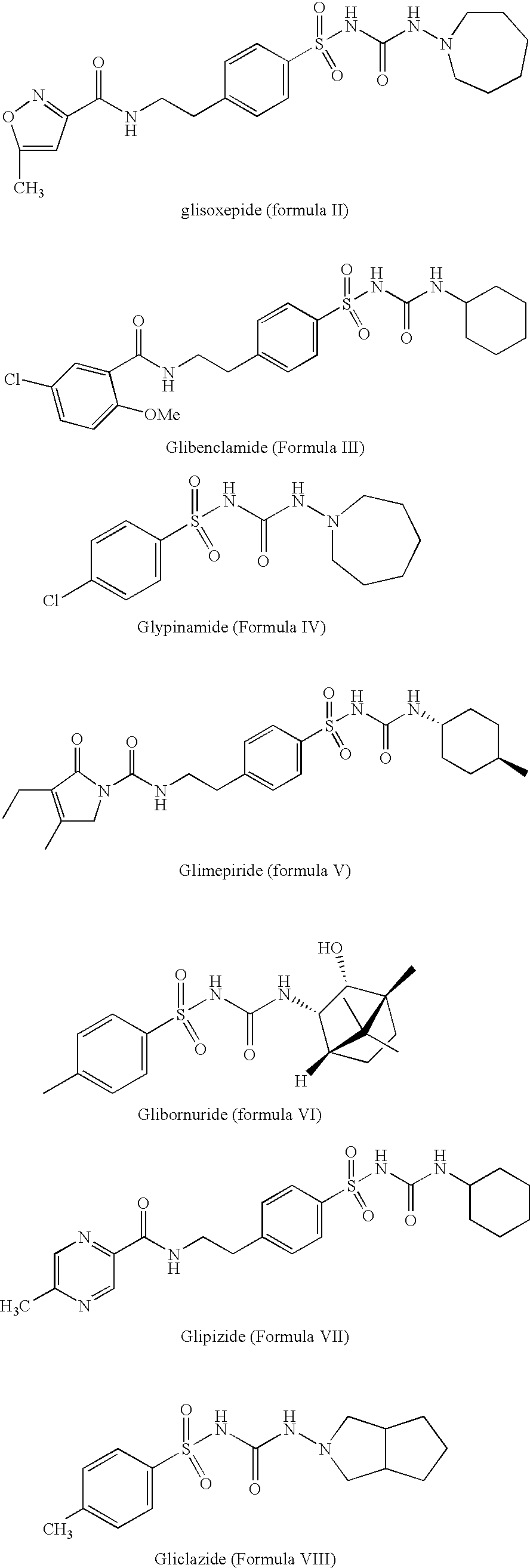
Method for manufacture of compounds related to the class of substituted sulfonyl urea anti-diabetics
InactiveUS20070255056A1Quick responseEfficient purificationOrganic compound preparationSulfonic acid amide preparationGlibornurideDiabrezide
The present invention relates to a process for preparation of sulfonyl urea compounds in high conversion rates and purity. More specifically, this invention relates to a process for manufacture of sulfonyl urea class of anti-diabetic pharmaceutical drugs in higher purity and yield. The process may effectively and economically be used to produce anti-diabetic drugs, such as glimepiride, glipizide, gliclazide, glibenclamide, glibornuride, and glisoxepide.
Owner:IPCA LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


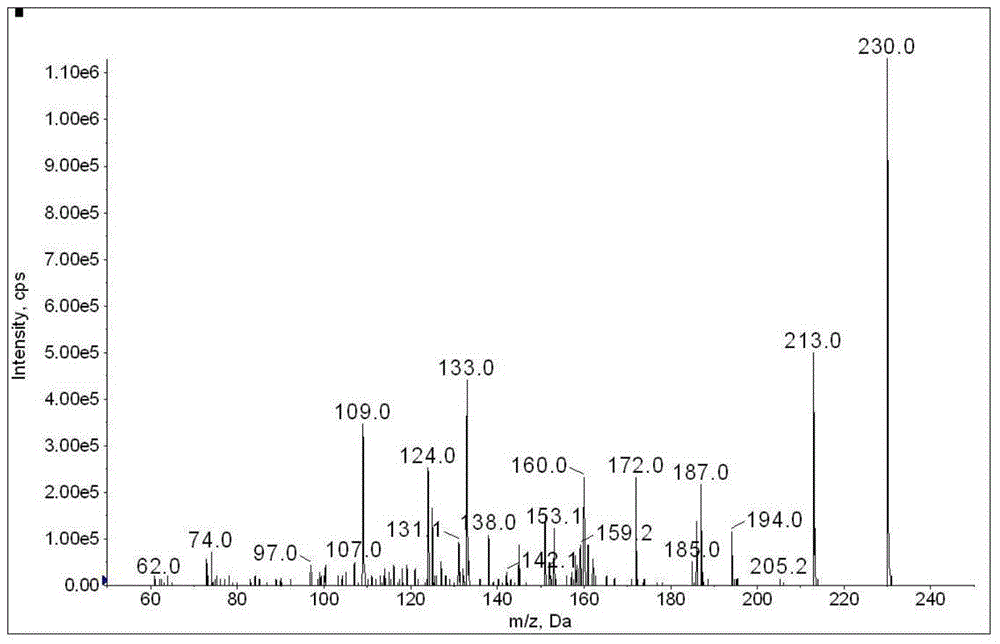
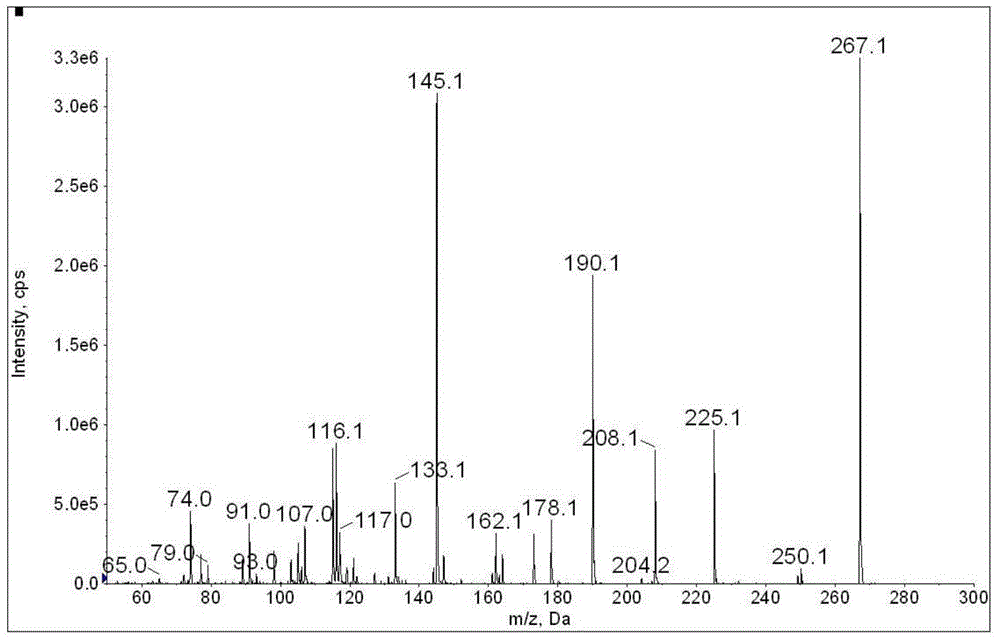
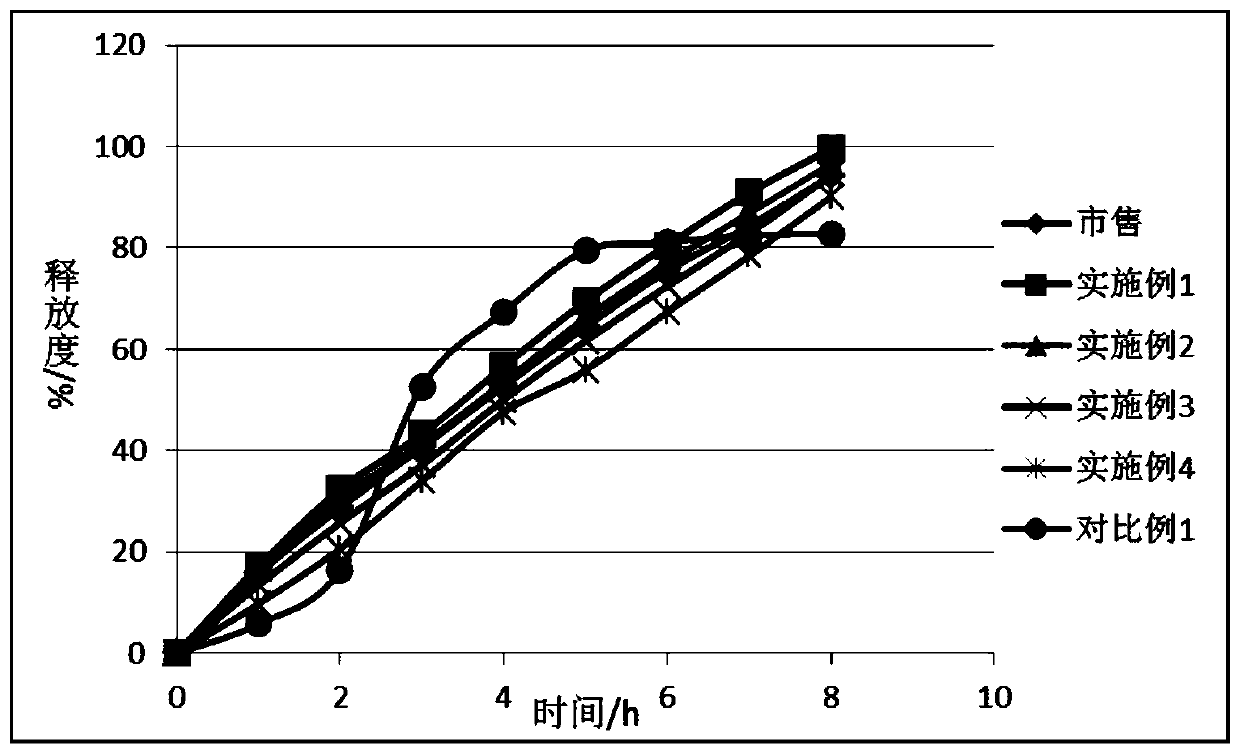
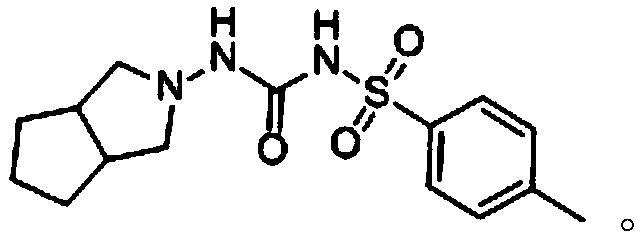

![Synthetic method of N-amino-3-azabicyclo[3,3,0]octane hydrochloride Synthetic method of N-amino-3-azabicyclo[3,3,0]octane hydrochloride](https://images-eureka.patsnap.com/patent_img/760201bd-256c-4f0e-9446-fba4eb04329b/FSA00000265866100011.PNG)