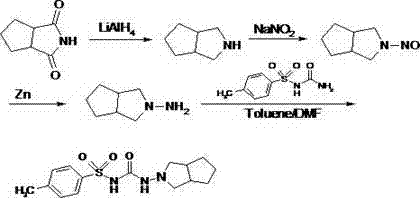
Preparation method of 1,2-cyclopentane dicarboximide
A technology of cyclopentanedicarboxamide and cyclohexanone carboxamide, which is applied in the field of chemical synthesis of organic 1,2-cyclopentanedicarboxamide, can solve the problems of high temperature energy consumption equipment, high raw material cost, The total yield is only a problem, to achieve the effect of reducing the discharge of the three wastes, reducing the production cost and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: A kind of preparation method of 1,2-cyclopentane dicarboximide, carries out following steps successively:
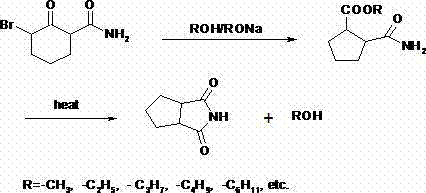
[0027] In a 250mL three-neck flask equipped with mechanical stirring, add 70ml of methanol and 30g of α-bromocyclohexanone formamide, cool down to 0-5°C, slowly add dropwise 50ml of methanol solution containing 14.8g of methanol sodium salt, after the dropwise addition , keep stirring for 30 minutes.
[0028] After the reaction is over, the device should be used for ordinary distillation to remove the alcohol solvent, then start to heat up to 180~220°C, carry out vacuum distillation to remove the generated solvent, add 150ml of ethyl acetate after cooling, add 5g of activated carbon, and heat to reflux for 1 hours, filtered while it was hot, and the filtrate was crystallized at room temperature to obtain the product 1,2-cyclopentanedicarboximide, which was vacuum-dried at 40°C for 1 hour to obtain 14.58 g of the product with a yield of 76.55%.
Embodiment 2
[0029] Embodiment 2: a kind of preparation method of 1,2-cyclopentane dicarboximide, carry out following steps successively:
[0030] In a 250mL three-neck flask equipped with mechanical stirring, add 100ml methanol and 30g α-bromocyclohexanone formamide, cool down to 0-5°C, slowly add dropwise 20ml methanol solution containing 7.4g methanol sodium salt, after the dropwise addition , keep stirring for 30 minutes.
[0031] After the reaction is over, the device should be used for ordinary distillation to remove the alcohol solvent, then start to heat up to 180~220°C, carry out vacuum distillation to remove the generated solvent, add 150ml of ethyl acetate after cooling, add 5g of activated carbon, and heat to reflux for 1 hour, filtered while hot, and the filtrate was crystallized at room temperature to obtain the product 1,2-cyclopentane dicarboximide, which was vacuum-dried at 40° C. for 1 hour to obtain 13.79 g of the product with a yield of 72.41%.
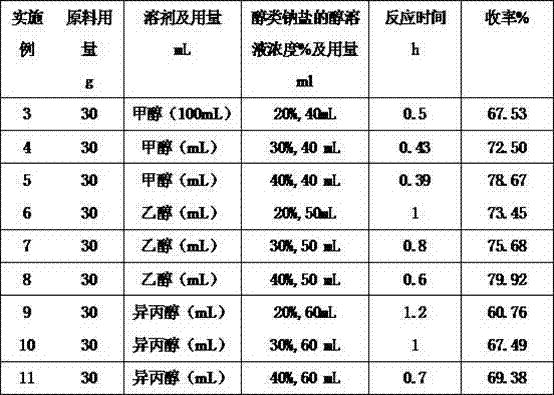
Embodiment 3~11
[0032] Embodiments 3-11: By changing the solvent, alcohol sodium salt concentration, and reaction time in the above-mentioned embodiment 1 or 2, corresponding embodiments 3-11 can be obtained. The specific content is shown in Table 1, and the product yield of each embodiment gained is shown in Table 1.
[0033] The concrete data of table 1, embodiment 3-11
[0034]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com