Gliclazide gastric floating tablet and preparation method thereof
The technology of gastric floating tablet and formula is applied in the field of gliclazide gastric floating tablet and its preparation, which can solve the problems of amplifying adverse reactions, fluctuations, and increasing blood concentration of gliclazide, and achieves the improvement of bioavailability and effect. Long-lasting and less toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
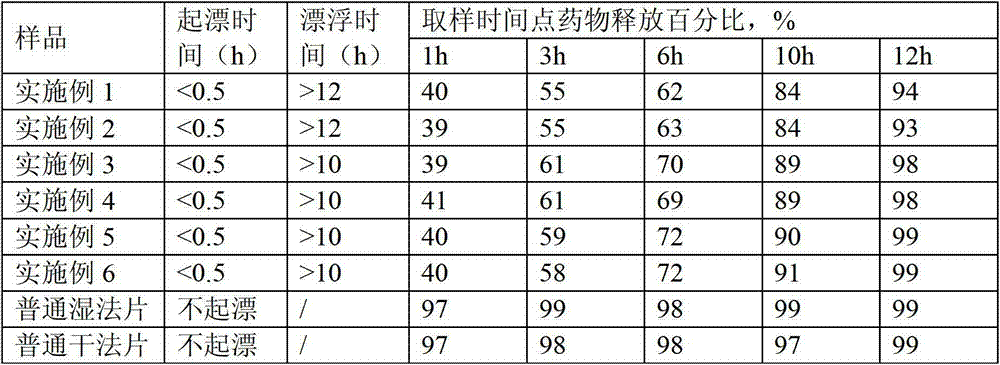
[0029] A gliclazide gastric floating tablet, the powder is directly compressed into tablets, each tablet contains 10 mg of gliclazide, the total tablet weight is 0.2 g, it includes the following components and the dosage of each component per 1000 tablets is:
[0030] Gliclazide 10g,
[0031] Gel material 60g,
[0032] Waxy material 40g,
[0033] Foaming material 5g,
[0034] Lactose 80g,
[0036] The gel material is hypromellose (HPMC).
[0037] The waxy material includes fatty acid and fatty alcohol, the fatty acid is stearic acid, the fatty alcohol is stearyl alcohol, and the amount of stearyl alcohol is 20g, and the amount of stearic acid is 20g.
[0038] The foaming material is sodium bicarbonate.
[0039] The particle size of the gliclazide is ≤100 mesh, the particle size of the gel material and the foaming material is ≤80 mesh, and the particle size of the waxy material is ≤60 mesh.
[0040] A preparation method of gliclazide gastr...
Embodiment 2
[0045] A gliclazide gastric floating tablet, shaped after wet granulation, each tablet contains 10 mg of gliclazide, the total tablet weight is 0.2 g, it includes the following components and the dosage of each component per 1000 tablets is:
[0046] Gliclazide 10g,
[0047] Gel material 60g,
[0048] Waxy material 40g,
[0049] Foaming material 5g,
[0050] Lactose 77g,
[0052] PVPK30 3g.
[0053] The gel material is hypromellose (HPMC).
[0054] The waxy material is fatty alcohol, and the fatty alcohol is stearyl alcohol.
[0055] The foaming material is sodium bicarbonate.
[0056] The particle size of the gliclazide is ≤100 mesh, the particle size of the gel material and the foaming material is ≤80 mesh, and the particle size of the waxy material is ≤60 mesh.
[0057] A preparation method of gliclazide gastric floating tablet, which comprises the following steps:
[0058] S1. Weigh each component according to the above formula, cru...
Embodiment 3
[0062] A gliclazide gastric floating tablet, the powder is directly compressed into tablets, each tablet contains 30 mg of gliclazide, the total tablet weight is 0.3 g, it includes the following components and the dosage of each component per 1000 tablets is:
[0063] Gliclazide 30g,
[0064] Gel material 120g,
[0065] Waxy material 90g,
[0066] Foaming material 20g,
[0067] Lactose 50g,
[0069] The gel material is water-soluble resin and chitosan, and the water-soluble resin is 100g, and the chitosan is 20g.
[0070] The waxy material includes beeswax and esters, the esters are triglycerides, 50g of beeswax and 40g of triglycerides.
[0071] The foaming materials are sodium carbonate and calcium carbonate, and the sodium carbonate is 10 g and the calcium carbonate is 10 g.
[0072] A preparation method of gliclazide gastric floating tablet, which comprises the following steps:
[0073] S1, weigh each component according to the above-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com