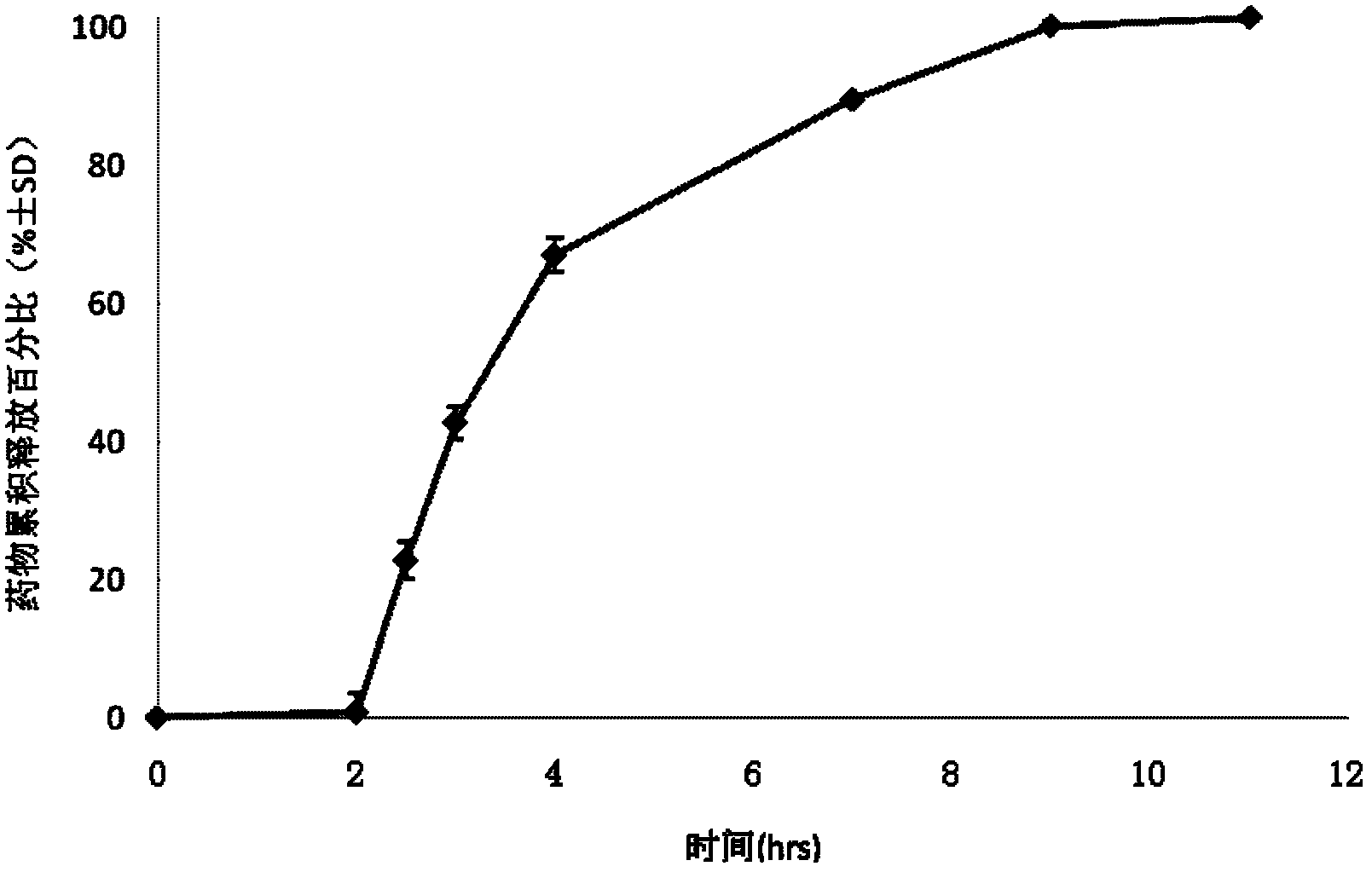
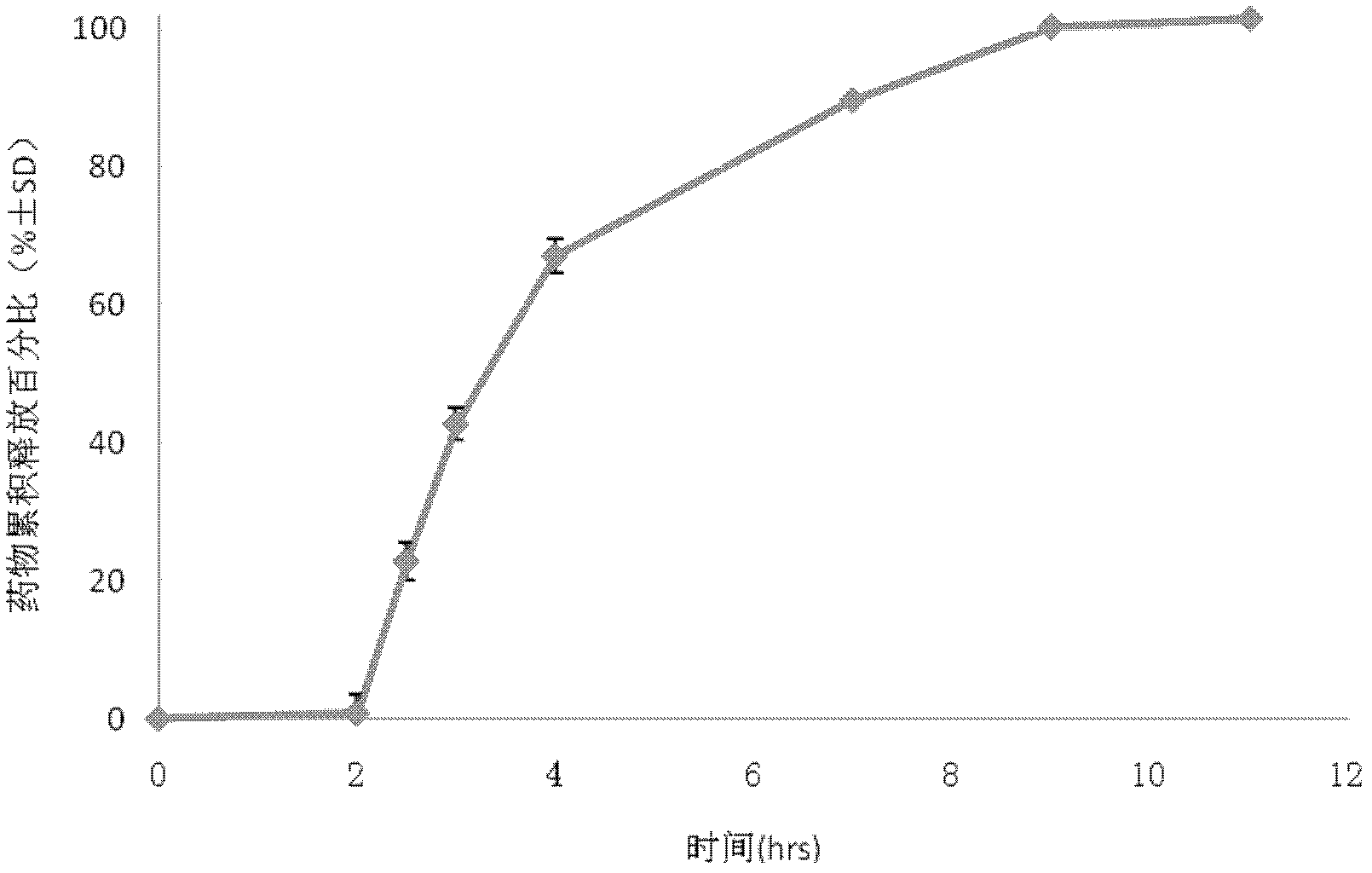
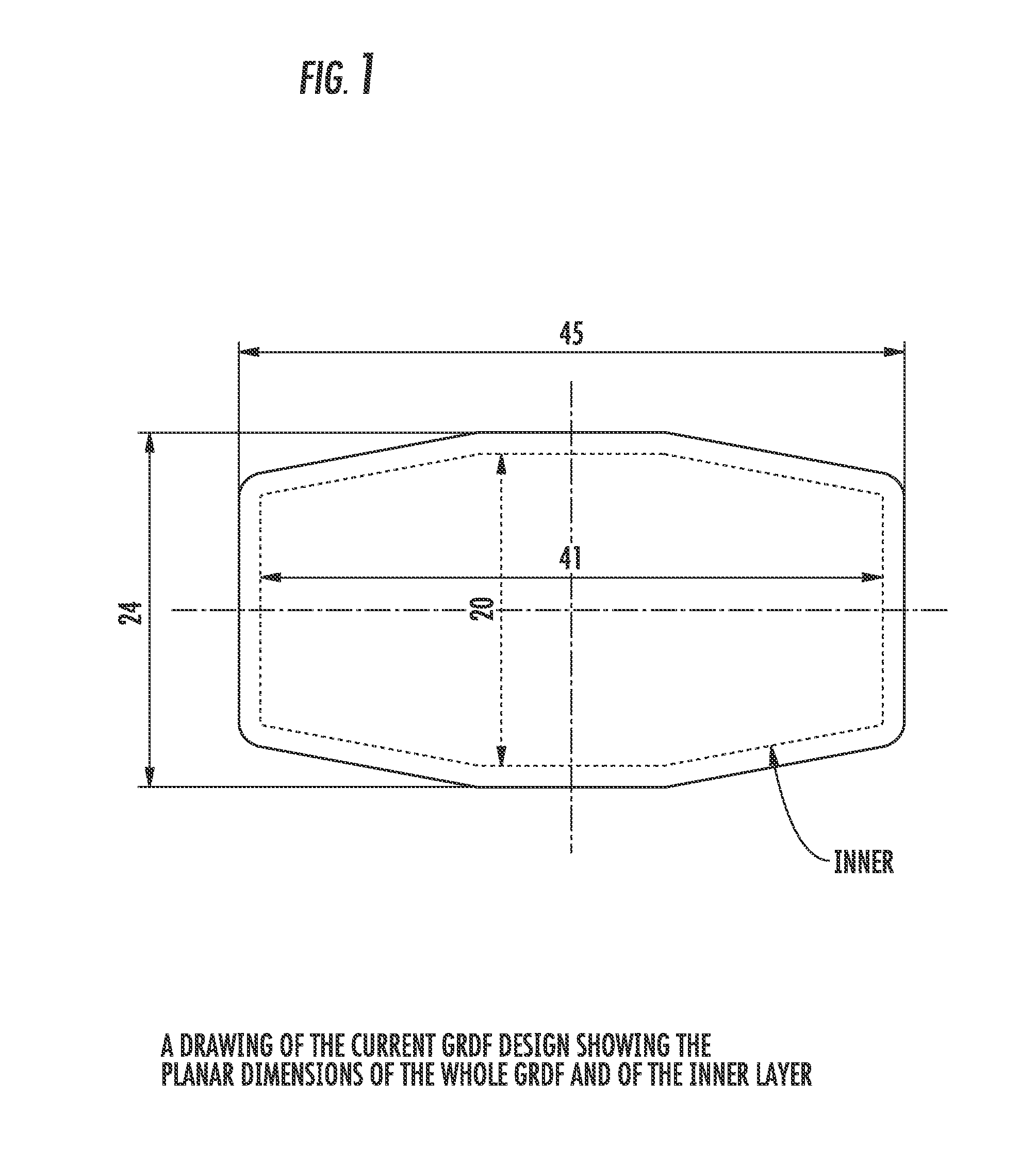
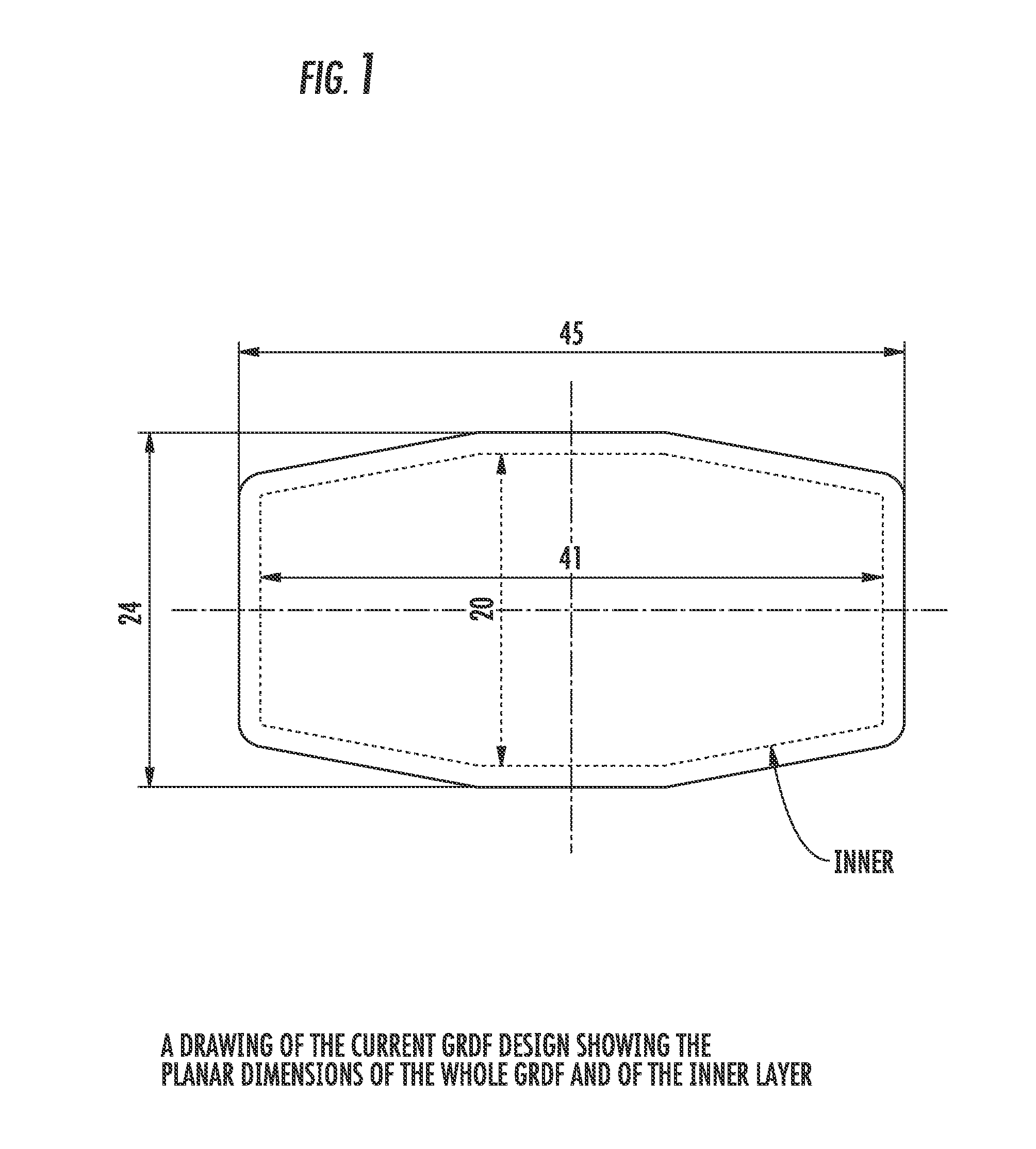
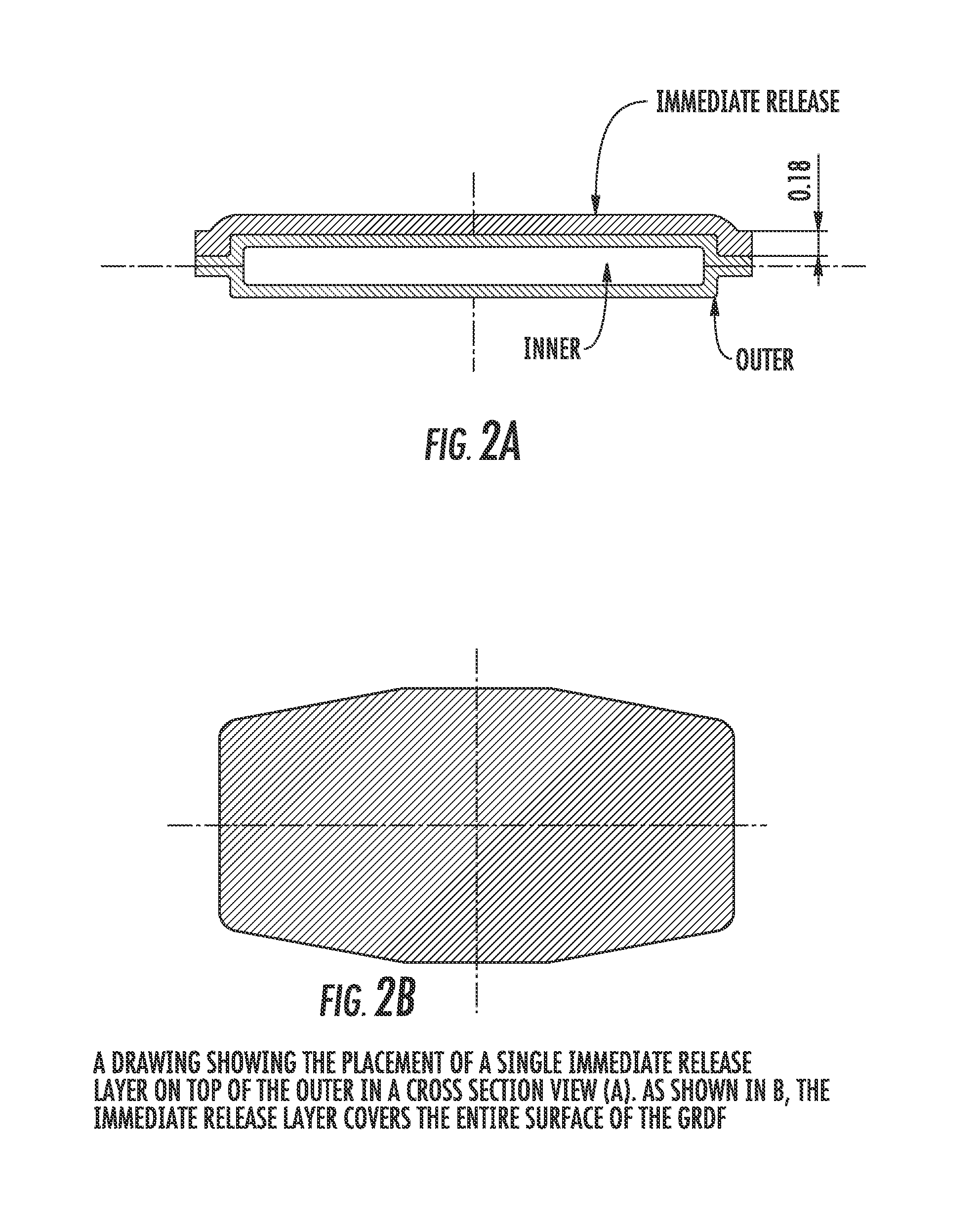
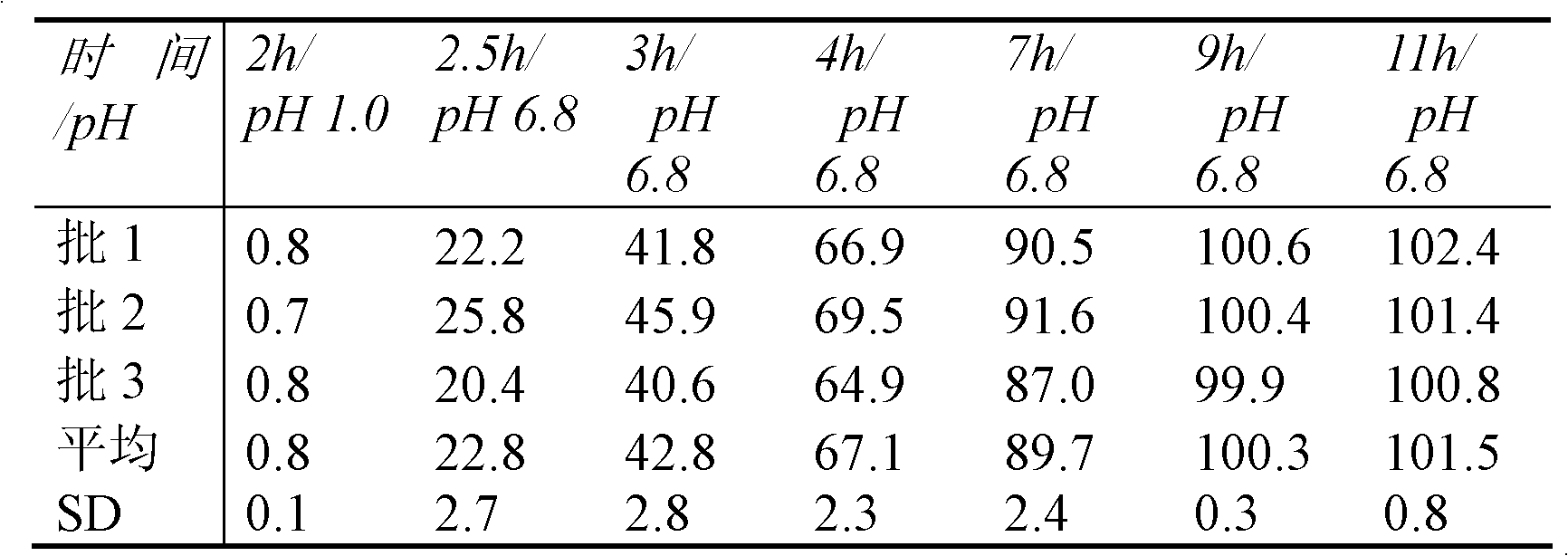Patents
Literature
905 results about "Gastric juices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
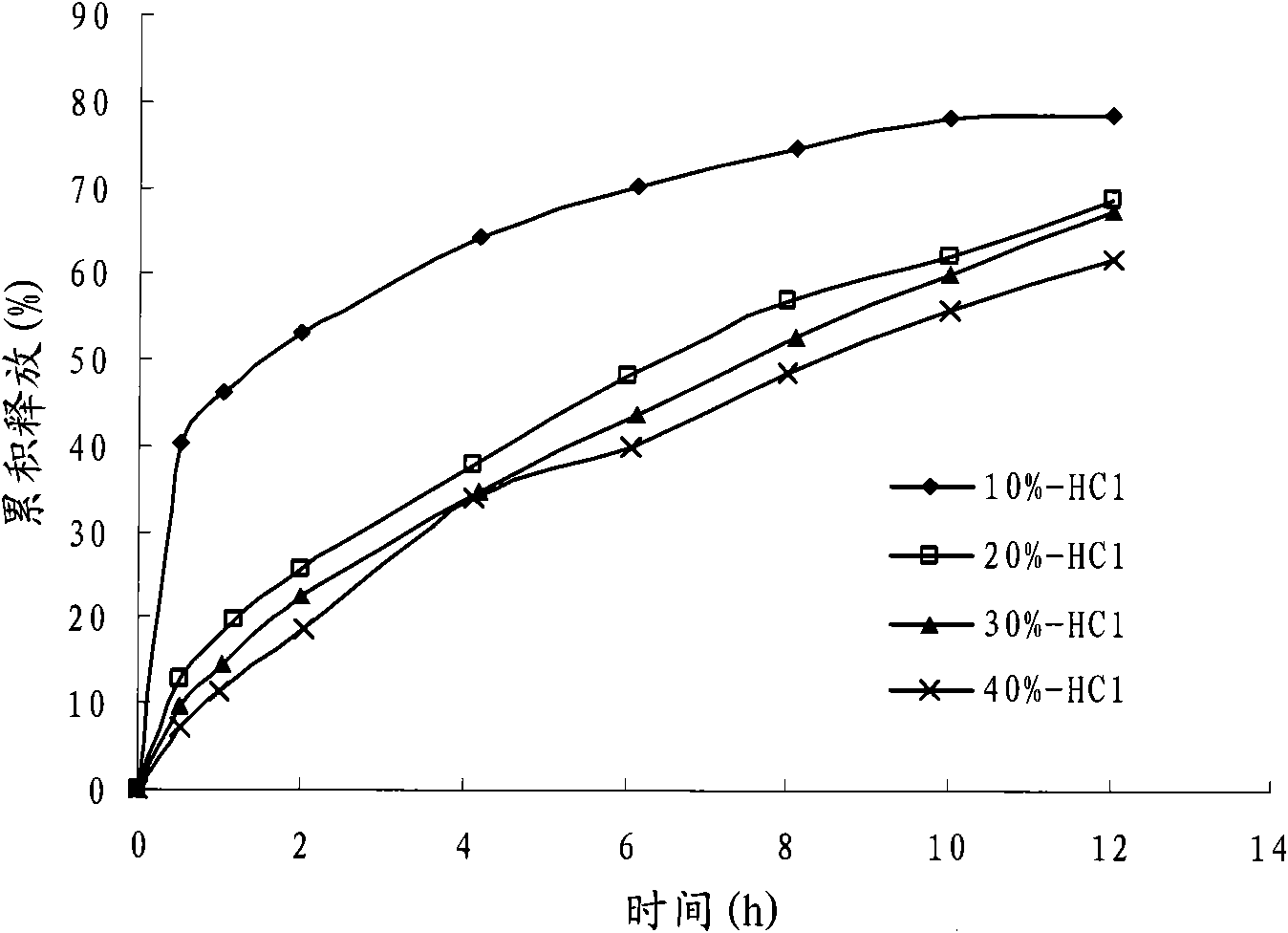
Gastric acid. Gastric acid, gastric juice or stomach acid, is a digestive fluid formed in the stomach and is composed of hydrochloric acid (HCl), potassium chloride (KCl) and sodium chloride (NaCl).
Multi-particulate, modified-release composition
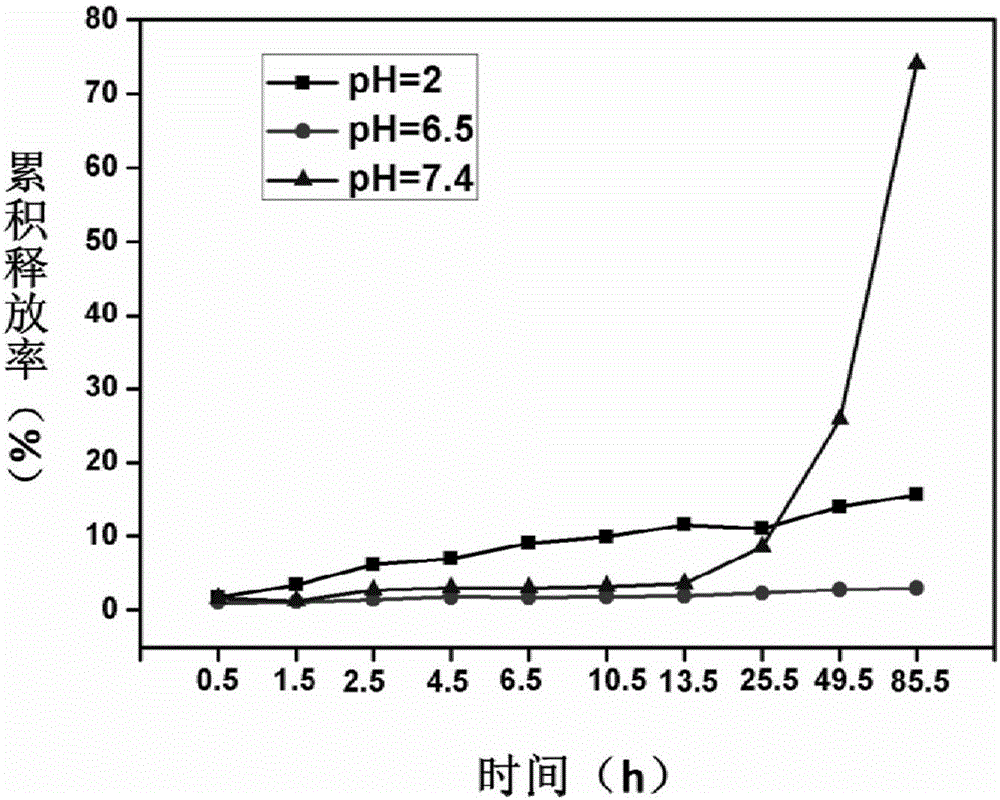
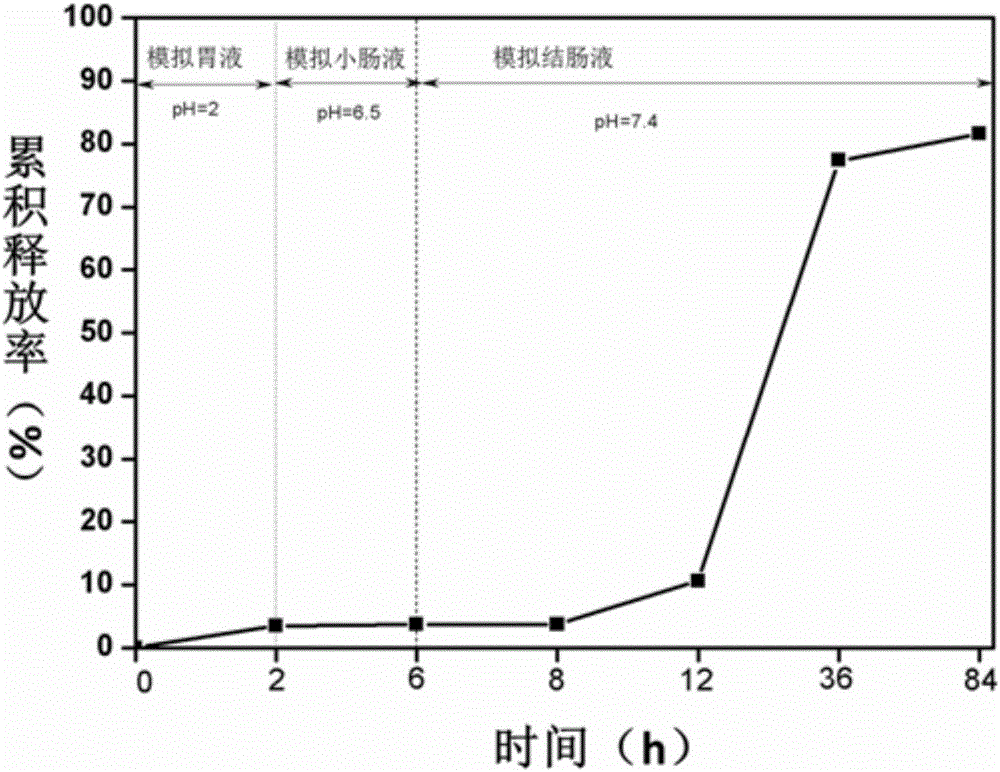
A multi-particulate, modified-release pharmaceutical composition for the oral administration of an active ingredient to the colon, wherein said particles comprise: (a) a core comprising an active ingredient or a pharmaceutically acceptable salt or ester thereof, and optionally one or more excipients; (b) a first coating applied to the surface of the core, wherein said first coating is insoluble in gastric juice and in intestinal juice below pH 7, but soluble in colonic intestinal juice; and (c) a second coating applied to the surface of the first coating.
Owner:SANDOZ AG
Enterococcus faecium for feeding and applications thereof
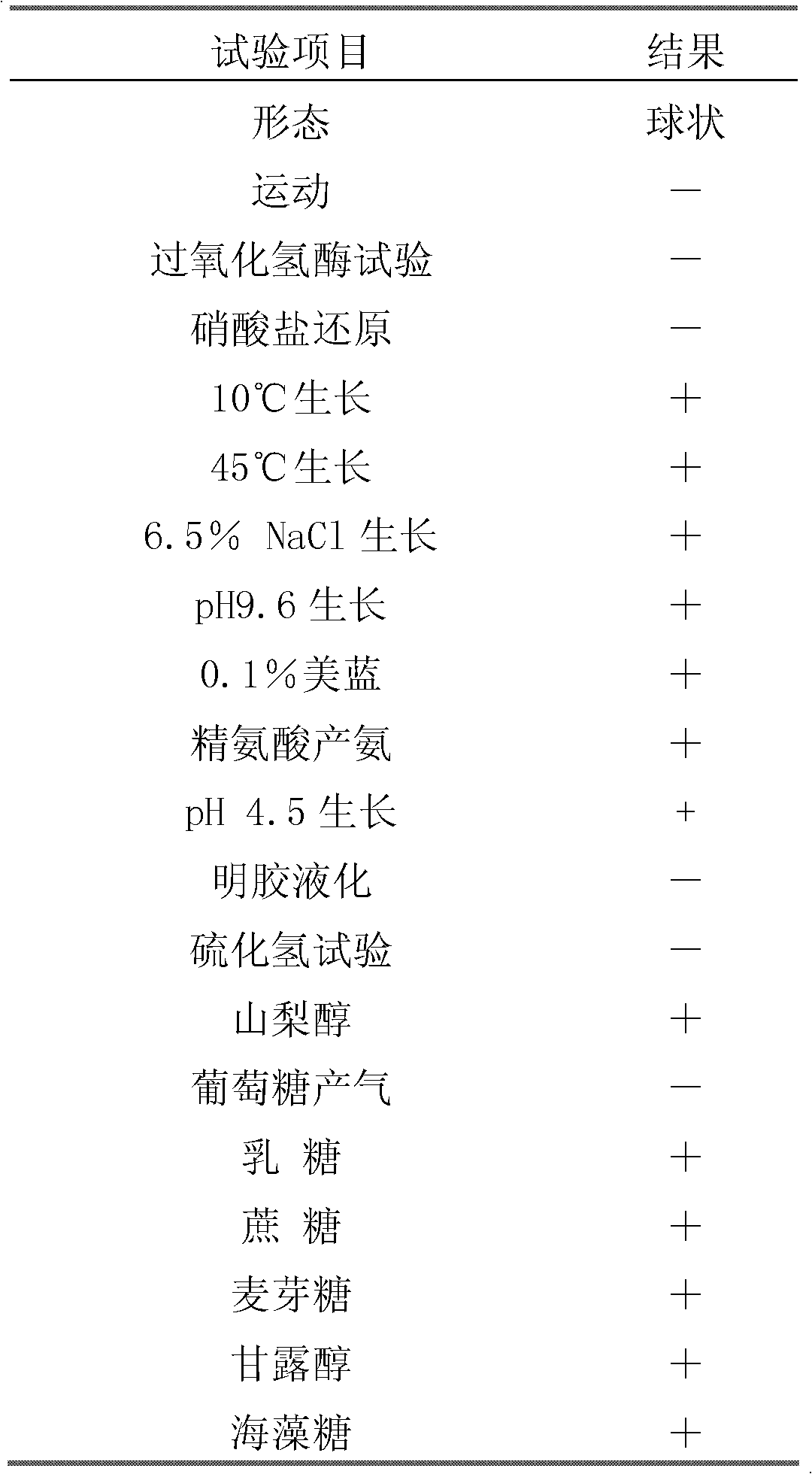
InactiveCN102304483AConducive to preservationLong storage timePowder deliveryBacteriaFreeze-dryingAntibiotic Y
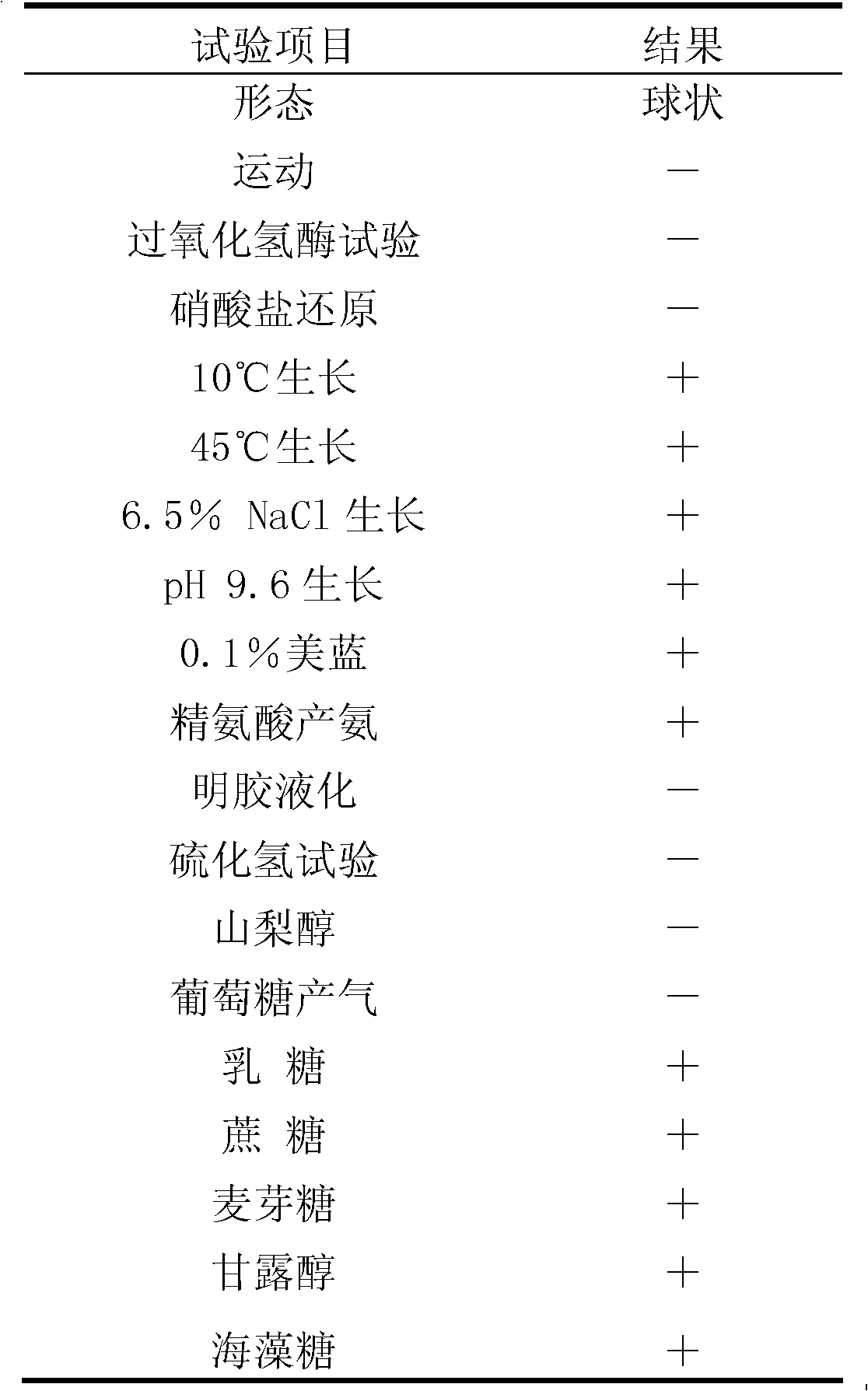
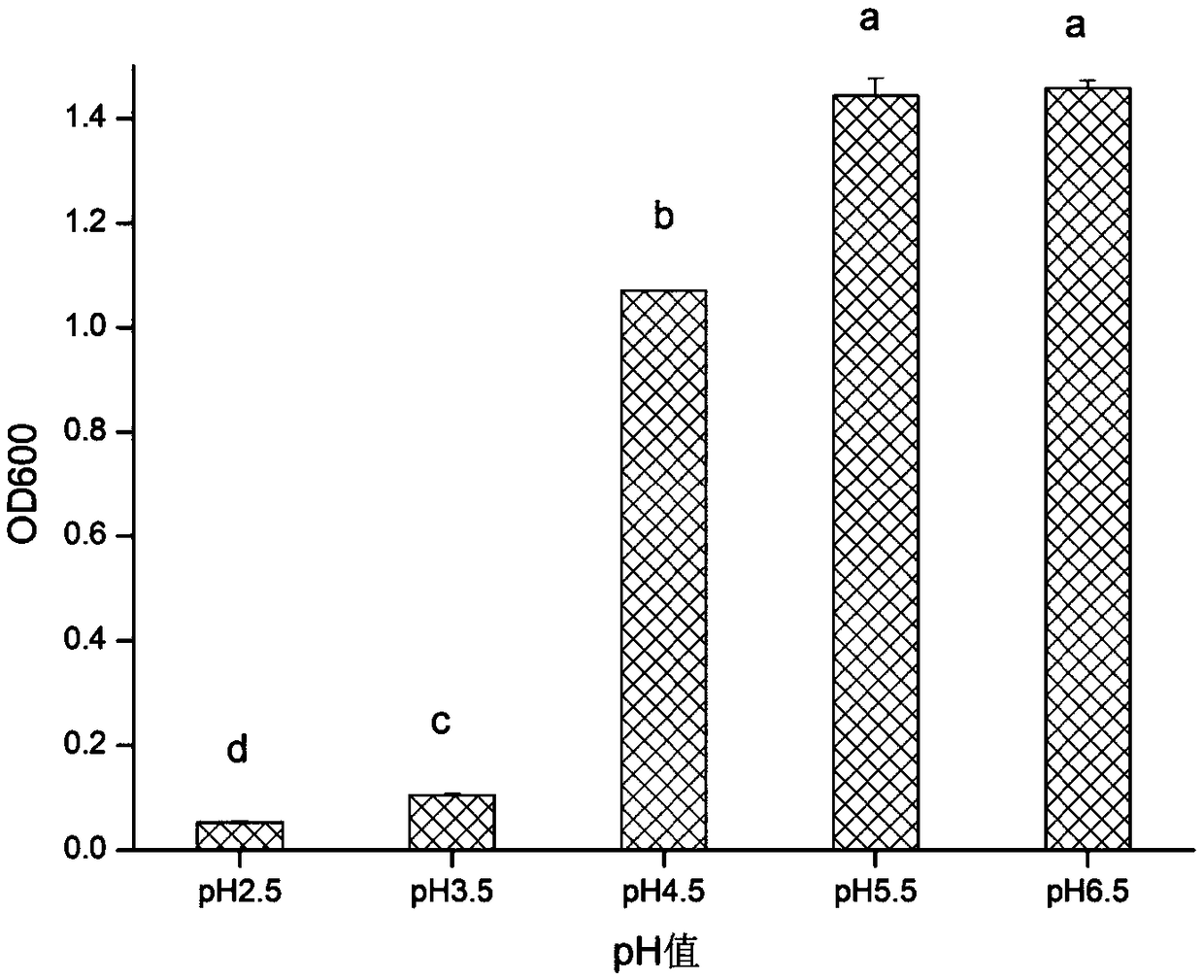
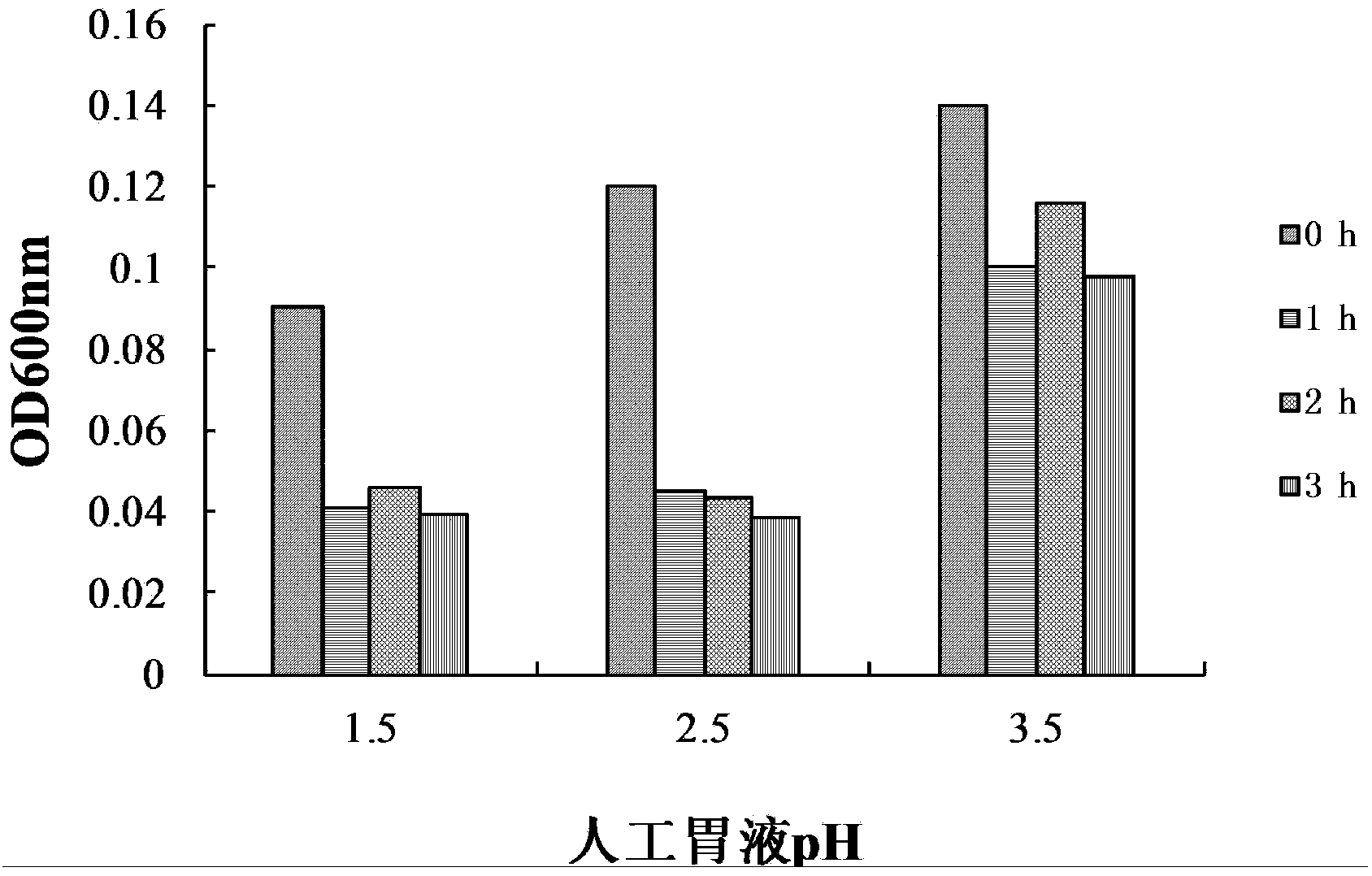
The invention relates to enterococcus faecium for feeding as well as a freeze-drying fungicide and applications thereof, and discloses enterococcus faecium LAB12 CGMCC (China General Microbiological Culture Collection Center) No.4847 which is grampositive cocci, has no spores, grows well on an MRS agar plate, forms a round bacterial colony with the diameter of 0.5-1mm within 48 hours and is used for feeding, and the bacterial colony is round, smooth and upheaved, and is a shape of grey white dewdrop; the enterococcus faecium grows in a facultative anaerobic condition; the growth temperature range is 10 DEG C-45 DEG C; the optimum growth temperature is 30 DEG C-40 DEG C; and the growth pH value is 4-10, and the optimum pH value is 6.0. The freeze-drying fungicide formed by the bacterial strain is nontoxic and harmless, is gastric juice resistant, is cholate resistant, has a high inhibitory effect for multiple harmful bacteria, has a long quality guarantee period, can be widely applied to birds and livestock breeding to strengthen the animal disease-resistant capability, and is expected to be the substitution of antibiotics for feeding.
Owner:北京金泰得生物科技股份有限公司
Bacillus subtilis and feed additive and fermenting agent thereof
ActiveCN102178057AStrong stress resistanceImprove immunityAnimal feeding stuffEscherichia coliBiotechnology
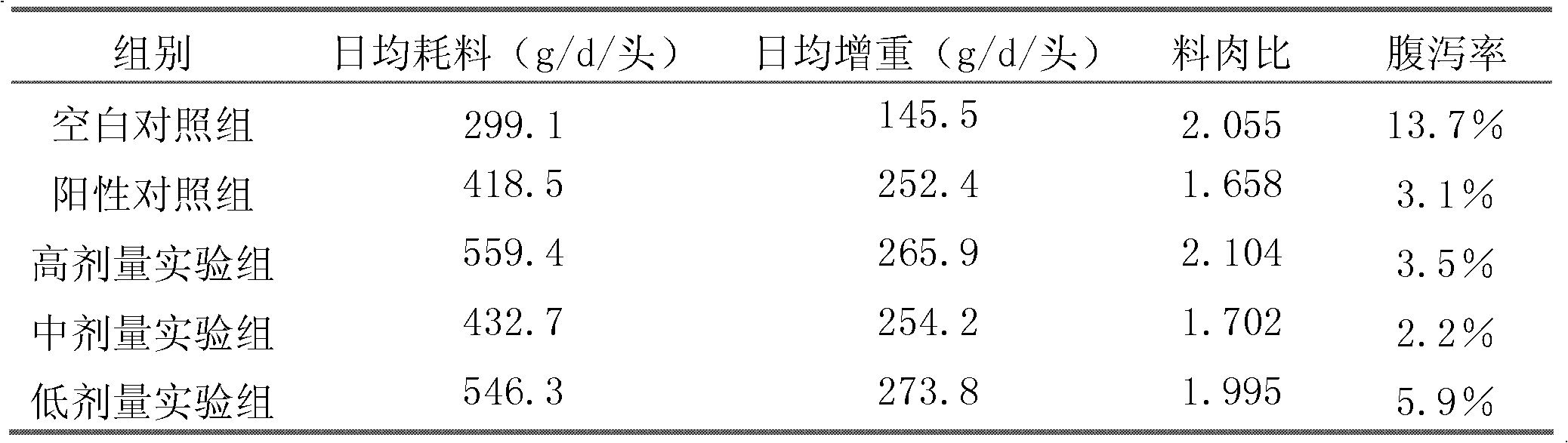
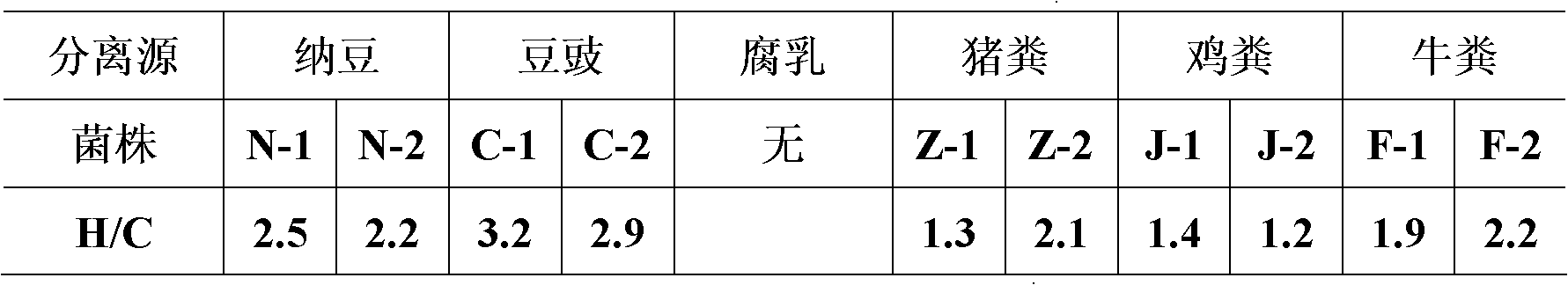
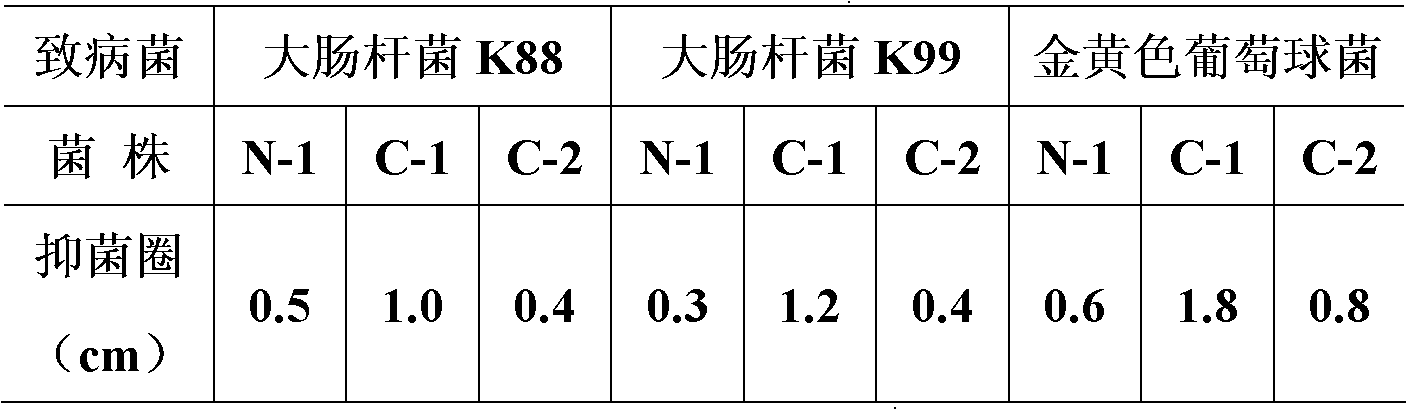
The invention belongs to the field of biological techniques and relates to a bacillus subtilis strain and a feed additive and a fermenting agent thereof. The bacillus subtilis has high stress resistance and probiotic effect, so the bacillus subtilis can tolerate artificial gastric juice with a pH value of 2.0 and a concentration of 1 percent, artificial cholate at a concentration of 0.3 percent and a granulating temperature of 80 DEG C; and the bacillus subtilis has a strong inhibiting effect on Escherichia coli K88, Escherichia coli K99 and staphylococcus aureus, high cellulase producing capacity and ability of degrading cellulose. The biological feed additive prepared by using the bacillus subtilis provided by the invention can be used in place of part of antibiotics in livestock and aquatic product culture, improve immunity in animal, improve feed conversion rate and lower culture cost. The bacillus subtilis also can be used in fermentation of bean pulp, cotton meal, vegetable mealand the like, prevent the feed from mildewing, promote the digestion of cellulose in feed and improve the utilization rate of nutrients in the feed.
Owner:BEIJING DABEINONG TECH GRP CO LTD +1
Integrated enzyme powder having effects of reducing fat and losing weight and application thereof
InactiveCN105995976AMeet nutritional needsPromote digestionYeast food ingredientsFood ingredient functionsDigestionGut flora
The invention relates to integrated enzyme powder having effects of reducing fat and losing weight. The integrated enzyme powder is characterized by being prepared from powders which are, by weight parts, 62.8-71.8 parts of integrated enzyme raw powder, 8-15 parts of konjac flour, 10 parts of pumpkin powder, 1 part of papaya powder, 3 parts of pineapple powder, 1 part of mango powder, 1 part of sweet orange powder, 1 part of snow pear powder, 3-5 parts of dietary fiber powder and 0.2 part of compound lactobacillus powder. 43 fruits, 20 vegetables, 4 cereal nuts and 11 medicinal and edible herbaceous plants and natto are selected in total to serve as fermentation raw materials; through yeast and lactobacillus fementation, generated enzymes are total nutrients needed by humans, and the enzyme powder contains activity probiotics which are resistant to acid and heat; and through testing, the probiotics in artificial gastric juice still have ultrahigh vigor; at normal temperature (37 DEG C), the probiotics still maintain activity, can regulate intestinal flora of the human body, promote digestion and avoid constipation. The mixed integrated enzyme powder not only can achieve the goal of losing weight, but also can meet the basic nutrition requirements of the human body, and it is a healthy and effective method of reducing fat and losing weight to use the enzyme powder.
Owner:厦门元之道生物科技有限公司
Pellets having a gastric juice-resistant active compound matrix
A pellet contains a pharmaceutically active substance embedded in a polymer matrix of one or more water-insoluble polymers; wherein said polymer matrix comprises 10 to 90% by weight of an anionic polymer; with the proviso that the pellet a) releases no more than 10% of said active compound in a release test according to USP in artificial gastric juice at pH 1.2 after 120 min, and b) releases at least 50% of the active compound after altogether a further 300 min at pH 6.8 and / or pH 7.5; wherein said pellet has a particle size in the range from 300 to 1100 μm.
Owner:EVONIK OPERATIONS GMBH
Lactobacillus plantarum and preparation method thereof for high-density culture and freeze-drying bacteria powder
InactiveCN102864096AStrong toleranceIncrease vitalityBacteriaMicroorganism based processesFreeze-dryingAgglutination
The invention discloses a lactobacillus plantarum and a preparation method thereof for high-density culture and freeze-drying bacteria powder, and has the technical scheme that the lactobacillus plantarum (Lactobacillus plantarum P8) is probiotics separated from the traditional fermentation milk product (yoghurt) of the Urat Middle Banner of Inner Mongolia Bayan Nur. The probiotics has the characteristics of artificial gastric juice and artificial digestive juice tolerance, cholate tolerance, intestinal agglutination action and common pathogenic entero bacteria inhibition. The bacterial strain of the bacteria is collected in the China general microbiological culture collection center with the collection number of CGMCC (China general microbiological culture collection center) No. 5468 on 18th, Nov., 2011.
Owner:北京和美科盛生物技术有限公司
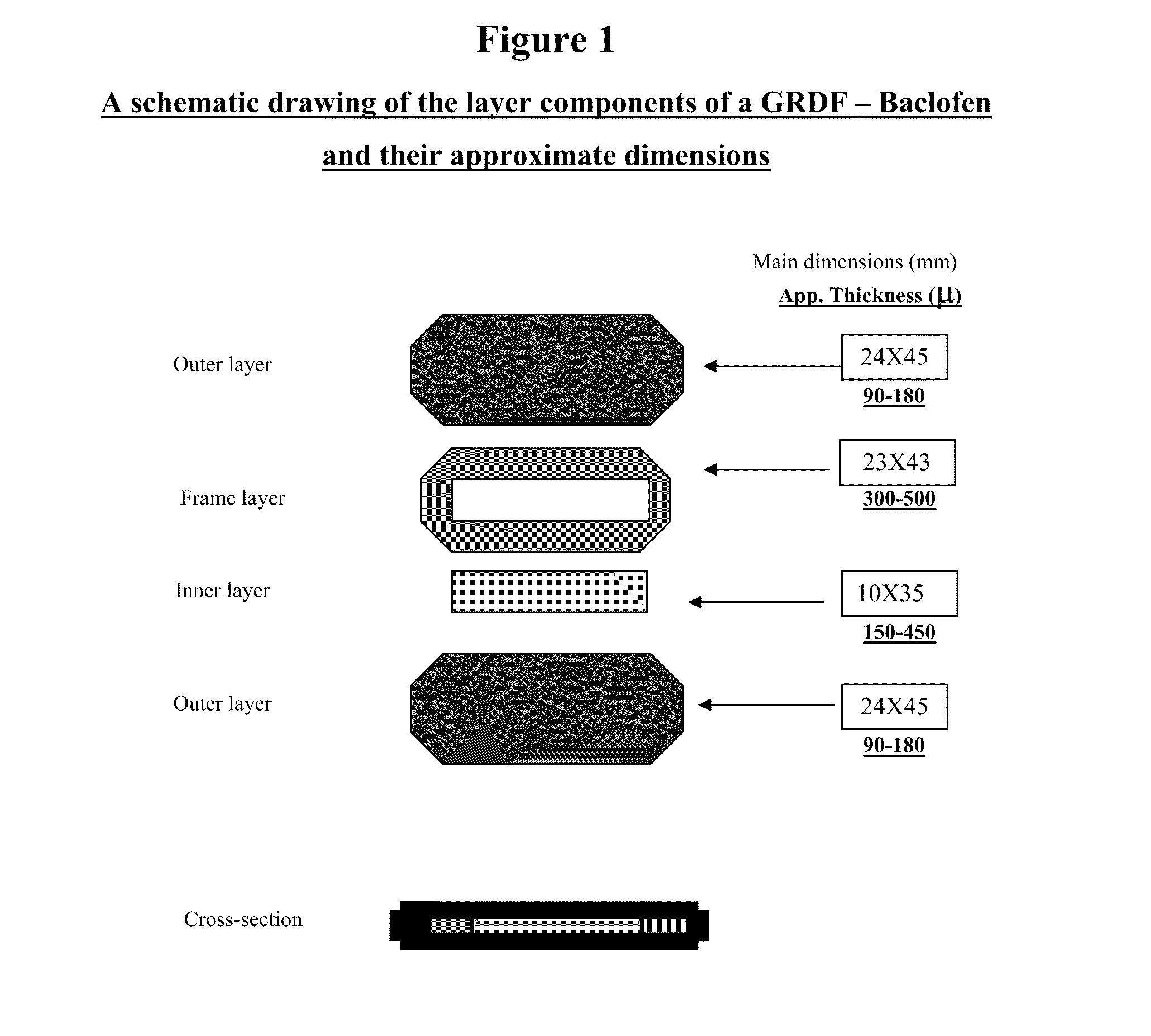
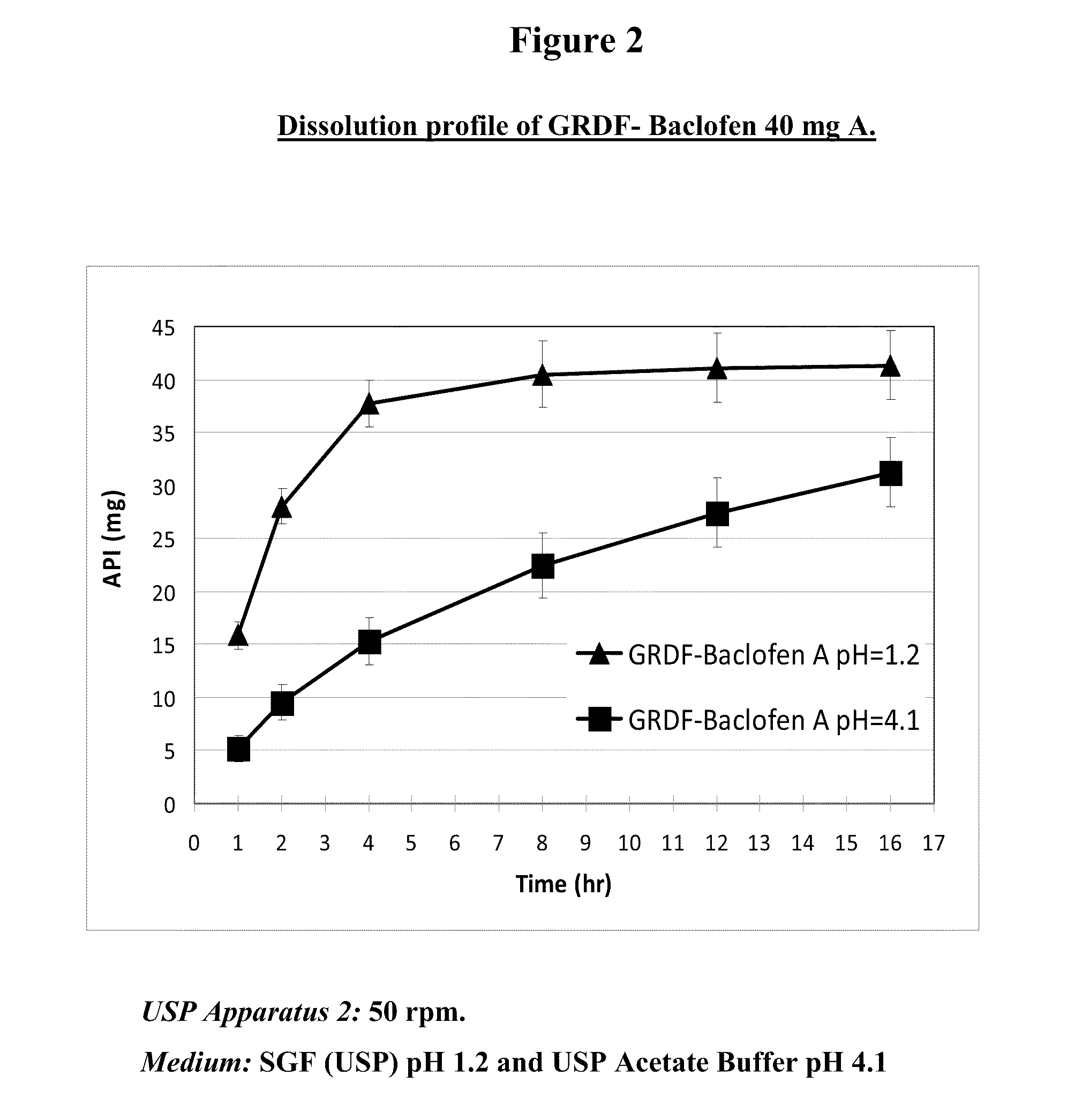
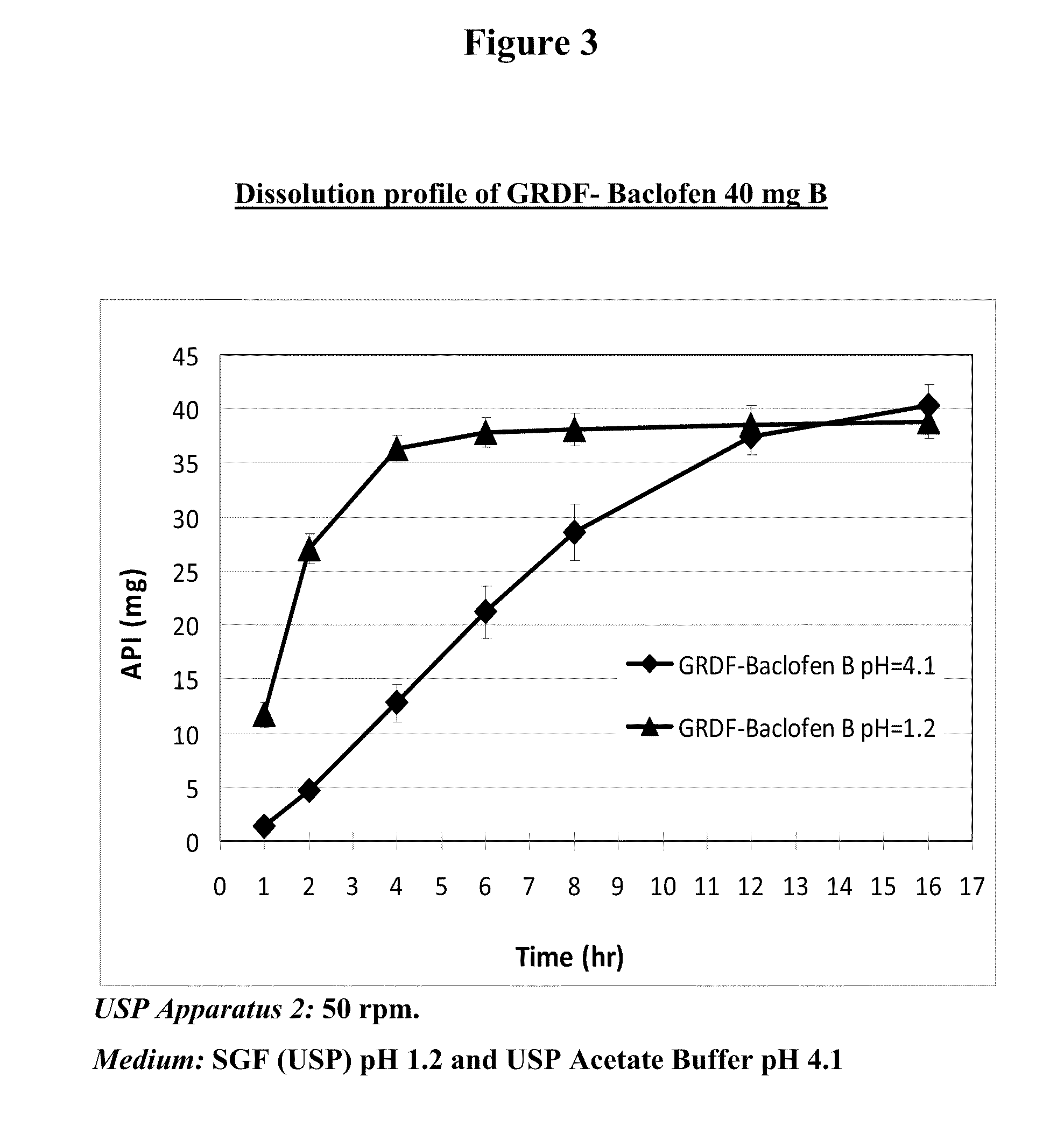
Baclofen and r-baclofen gastroretentive drug delivery systems
InactiveUS20110091542A1Reduce concentrationStrong therapeutic activityOrganic active ingredientsBiocideControlled releaseSide effect
A biodegradable, multi-layered controlled release gastroretentive baclofen or R-baclofen dosage form which is optionally divided into a first dosage of baclofen or R-baclofen for immediate release and a second dosage of baclofen or R-baclofen for controlled release in the stomach and gastrointestinal tract of a patient, folded into a capsule which disintegrates upon contact with gastric juice and the dosage form unfolds rapidly upon contact with gastric juice. The biodegradable, multi-layered gastroretentive dosage forms of the invention provide fast onset of baclofen or R-baclofen activity with prolonged absorption and minimal undesirable side effects.
Owner:INTEC PHARMA
Loaded chitosan/sodium alginate double-crosslinking hydrogel and preparation method and application thereof
ActiveCN106750398AReduce treatment riskImprove slow-release and controlled-release performancePharmaceutical non-active ingredientsMicrocapsulesSmall intestineBody fluid
The invention belongs to the field of biomedical engineering materials, and discloses a loaded chitosan (CS) / sodium alginate (SALG) double-crosslinking hydrogel with a colon target and a preparation method and an application thereof. In the invention, the hydrogel is firstly crosslinked with chitosan and then with sodium alginate, the medicine is wrapped in the chitosan, the sodium alginate is coated on the outer layer, and then double crosslinking is performed by the calcium ion and glutaraldehyde, the carboxy and amine are both fixed to form an interpenetrated network, and such a structure is not easy to be degraded and not easy to lose in the body fluid of human beings. After medicine loading, a small amount of medicine can be released after two hours in the gastric juice, and nearly no medicine is released after 4 hours in the intestine, while more medicine is released in the colon, and long-time release can be realized, which makes up the medicine burst release, medicine leakage and easy degradation and loose of materials reflected by the loaded gel prepared in the above, and colon targeting positioning and long-time release of medicine for treatment can be realized.
Owner:JINAN UNIVERSITY
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
Pellets having an active compound matrix and a polymer coating, and a process for the production of the pellets
InactiveUS20080206324A1Easy to preparePowder deliveryPharmaceutical non-active ingredientsPolymer sciencePolymer coatings
An active compound-containing pellet has a polymer coating of an anionic (meth)acrylate copolymer and a pharmaceutically active substance, embedded in a polymer matrix of one or more polymers, a particle size in the range from 300 to 1100 μm, a friability of at most 0.1%, measured using 200 g of pellets in a screening machine having a 200 μm screen, a screening diameter of 20 cm and 1.5 mm shaking amplitude at a shaking frequency of 50 l / sec for 10 min in the presence of six rubber cubes having a 1.8 cm edge length, with the proviso that the pellet releases no more than 10% of the active compound in a release test according to USP in artificial gastric juice at pH 1.2 after 120 min.
Owner:EVONIK ROEHM GMBH
Drug sustained and controlled release microparticle preparation for treating intestinal diseases, and preparation method thereof
ActiveCN102319218AEvenly distributedGuaranteed uniformityPowder deliveryOrganic active ingredientsDiseaseAnti-Adhesion Agent
The present invention discloses a drug sustained and controlled release microparticle preparation for treating intestinal diseases. The preparation comprises: a pill core containing the drug, wherein the pill core contains 5-aminosalicylic acid and an assistant material; an isolation layer for providing a smooth and flat surface for the microparticle and preventing the drug from penetrating into a sustained release coating layer, wherein the penetration of the drug into the sustained release coating layer can affect the release effect, the used material of the isolation layer comprises one or a plurality of materials selected from a water-soluble polymer and an anti-adhesion agent; the sustained release coating layer for slowly releasing the drug, wherein different drug release levels can be achieved through adjusting the thickness of the sustained release coating layer, the used material of the sustained release coating layer mainly adopts a sustained-release material; an enteric-coating layer, the enteric-coating layer is provided for avoiding the early release of the drug in gastric juice, reducing stimulation of the main drug to stomach, increasing the local concentration of the drug in the lesion location, the used material of the enteric-coating layer mainly adopts a polymer enteric material. The invention further discloses a preparation method for the microparticle preparation. According to the present invention, the drug and the sustained release coating material are uniformly dispersed on the surface of the pellet, such that the problem of mixing uniformity of the assistant material and the main drug can be effectively solved.
Owner:PIVOT PHARMA TECH SHANGHAI
Carbidopa/Levodopa gastroretentive drug delivery
A gastroretentive drug formulation for the sustained release of an active agent in the gastrointestinal tract comprises an internal layer or compartment comprising an active agent and one or more pharmaceutical excipients, of which at least one is a polymer and two membranes forming together an envelope around the inner membrane, each membrane comprising at least one polymeric combination of an enteric polymer which is not soluble in gastric juice, and an hydrophilic swelling polymer, and at least one plasticizer.
Owner:INTEC PHARMA
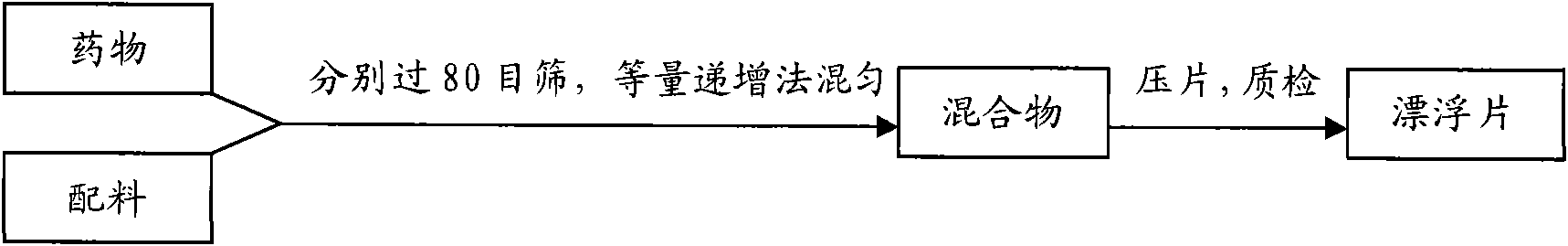
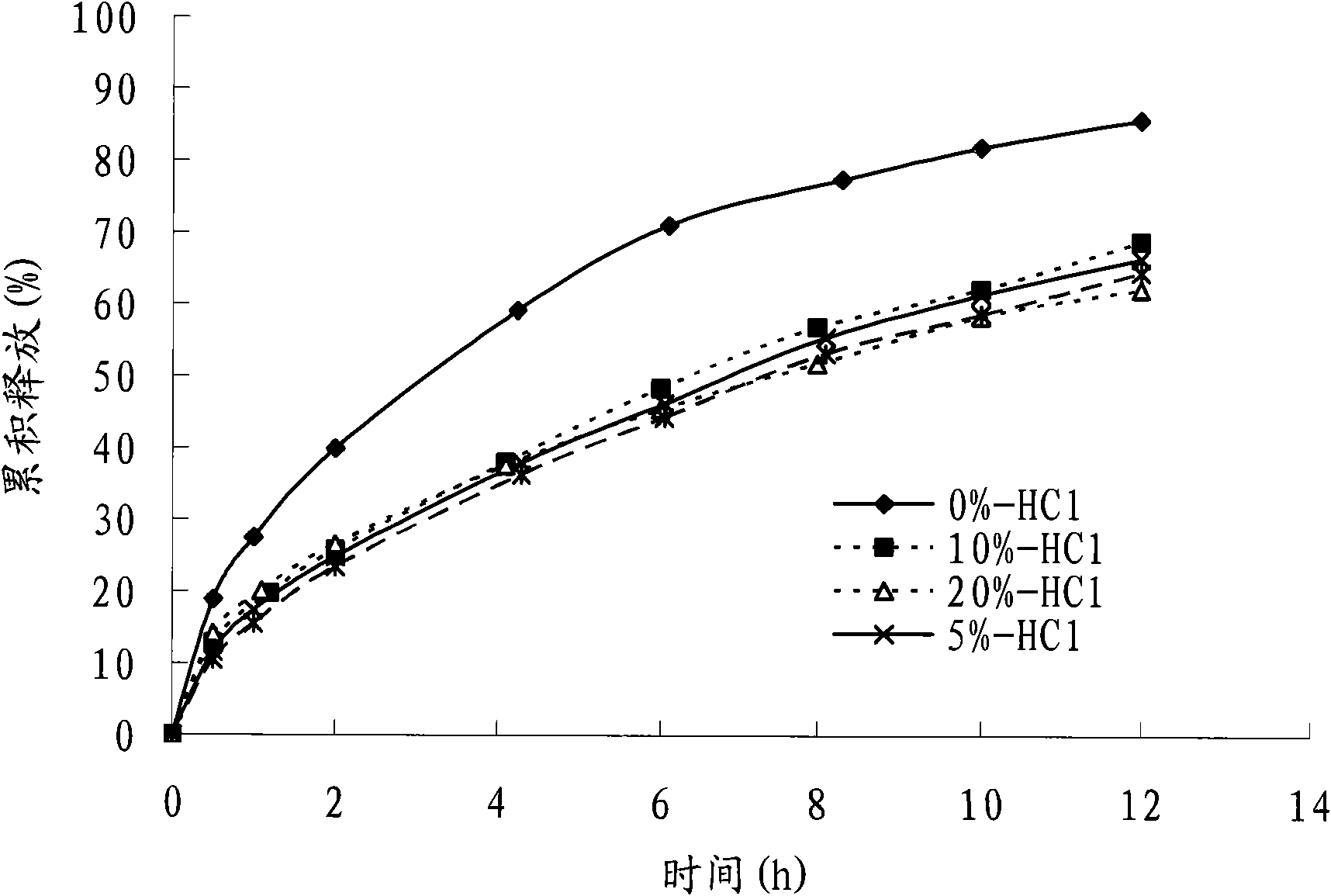
Palbociclib gastric-floating tablet and preparation method thereof
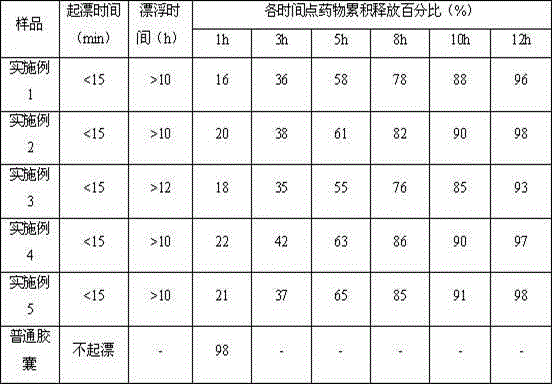
ActiveCN104887641ALow drift timeReduce dosageOrganic active ingredientsPill deliveryUse medicationPharmaceutical drug
The invention belongs to the technical field of medicine, and relates to a palbociclib gastric-floating tablet and a preparation method thereof. The palbociclib gastric-floating tablet comprises, by mass, 10%-30% of palbociclib, 20%-50% of hydroxypropyl methylcellulose, 20%-40% of bleaching auxiliaries, 2%-10% of foaming agents, 0%-25% of microcrystalline cellulose and 0.5%-3% of magnesium stearate. A dry granulating technology or a wet granulating technology can be used as the preparation technology. The palbociclib gastric-floating tablet is high in bioavailability, has a slow release tendency, and effectively lowers the total dosage. The palbociclib gastric-floating tablet and the preparation method thereof have the unique advantages that two different mechanisms are used for preparing the gastric-floating tablet, and accordingly the prepared tablet can keep floating in gastric juice by more than 10 hours and continuously release drugs in the hydrochloric acid solution with the pH being 1.2; the problem that the bioavailability is low due to the fact that drugs are extremely difficult to dissolve after the pH is higher than four is effectively solved; the medicine taking frequency is reduced; toxic and side effects are lightened; and the complaisance of a patient is effectively improved.
Owner:上海润泰医药科技有限公司
Method for preparing probiotic brown lactobacillus beverage product with large amount of bacteria
ActiveCN101731330AStrong toleranceImprove the detection rateMilk preparationBiotechnologyMaillard reaction
The invention discloses a method for preparing a probiotic brown lactobacillus beverage product with large amount of bacteria, which comprises the steps of Maillard reaction, probiotic fermentation and post treatment, wherein the probiotic fermentation step comprises: hydrating and sterilizing defatted milk powder or defatted fresh milk, reacting the defatted milk powder or the defatted fresh milk for 2.5 hours at the temperature of between 95 and 98 DEG C till the milky yellow becomes brown at the end point of the reaction, cooling the reaction product to between 37 and 38 DEG C, inoculating the reaction product to a Lactobacillus casei strain, statically fermenting the strain for 48 to 72 hours at the temperature of between 37 and 38 DEG C till the pH reaches 3.8 to 4.0, and cooling the fermentation product to between 2 and 6 DEG C; and the post treatment steps comprises: preparing base solution by using sugar, high fructose corn syrup, pectin and purified water, wherein the weight ratio of the base solution to the probiotic fermentation solution is 1 to 3; and the pH is adjusted to between 3.5 and 3.6; homogenizing the base solution, cooling the base solution to between 2 and 6 DEG C, canning products after passing detection, maturing the products for 4 to 8 hours at the temperature of between 2 and 6 DEG C, and delivery the products from a storage. The number of the living bacteria in the shelf life of the product can reach 1*108 to 1*1,010cfu / ml; and the product has zero fat and super clear mouthfeel, has no viscosity, has high probiotic detection rate, and can effectively keep or recover the intestinal microbial balance.
Owner:SHANDONG DEYI DAIRY IND
Enteric plant hollow capsule
ActiveCN101708171ASave the coating processSimple processPharmaceutical non-active ingredientsCapsule deliveryWater useProduction line
The invention relates to an enteric plant hollow capsule which is prepared by a method comprising: firstly adding 60-85% of pectin, 5-20% of plasticizer, 5-10% of coagulant aid and 5-10% of water used for curing agent into a reaction pot; stirring and dissolving at 60-90 DEG C, and obtaining glue stock by filtering and adjusting specific gravity; removing air bubbles and then sending into a capsule production line for the treatment such as dipping in glue, drying, cooling, demoulding, cutting, sheathing, etc. The enteric plant hollow capsule has the disintegration time limit of 3-4h in gastric juice and 30-45min in intestinal juice. Compared with the existing enteric capsule, the enteric plant hollow capsule omits the working procedure of coating, simplifies the technique, and completely uses no organic solvent, thus not only being beneficial to safe production and environmental protection, but also being beneficial to improving the efficiency and reducing the cost.
Owner:ANHUI HUANGSHAN CAPSULE CO LTD
Stomach dissolved film coating pre-mix dose and preparing method thereof
InactiveCN101199854AGood ratioGood performancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsLow speedActive agent
The invention relates to a gastric-juice-soluble film-coating premix and the preparation method. The components include film-forming material (principal film-forming material and non-principal film-forming material), plasticizer, antisticking agent, surfactant and unorganic colorant. The preparation method of the gastric-juice-soluble film-coat premix is that: principle film-forming material is put into a super-mixer whose temperature is between 20 to 80 DEG C to mix at a low speed for 5minutes,and then after the plasticizer and the surfactant being added, to mix at a high speed for 20 minutes to get dispersive medium; then the dispersive medium, the lubricant and the unorganic colorant are added into the super-mixer to be dispersed at a high speed for 30 minutes; then the non-principal film-forming material and the plasticizer are added to mix at a low speed for 5 minutes; the finished product can be got after being screened. The gastric-juice-soluble film-coat premix which adopts complete hydrosolvent system is of moderate viscosity, so that good suspending state can be maintained; relatively low permeability can ensure the stability of the preparation; with improved production efficiency, improved moistureproof effect of the traditional Chinese medicine, more delicate coat and brighter color, the invention can better meet the requirements of the solid medicine for the film-coating.
Owner:AILEYI MEDICINE MATERIAL SCI & TECH TIANJIN CITY
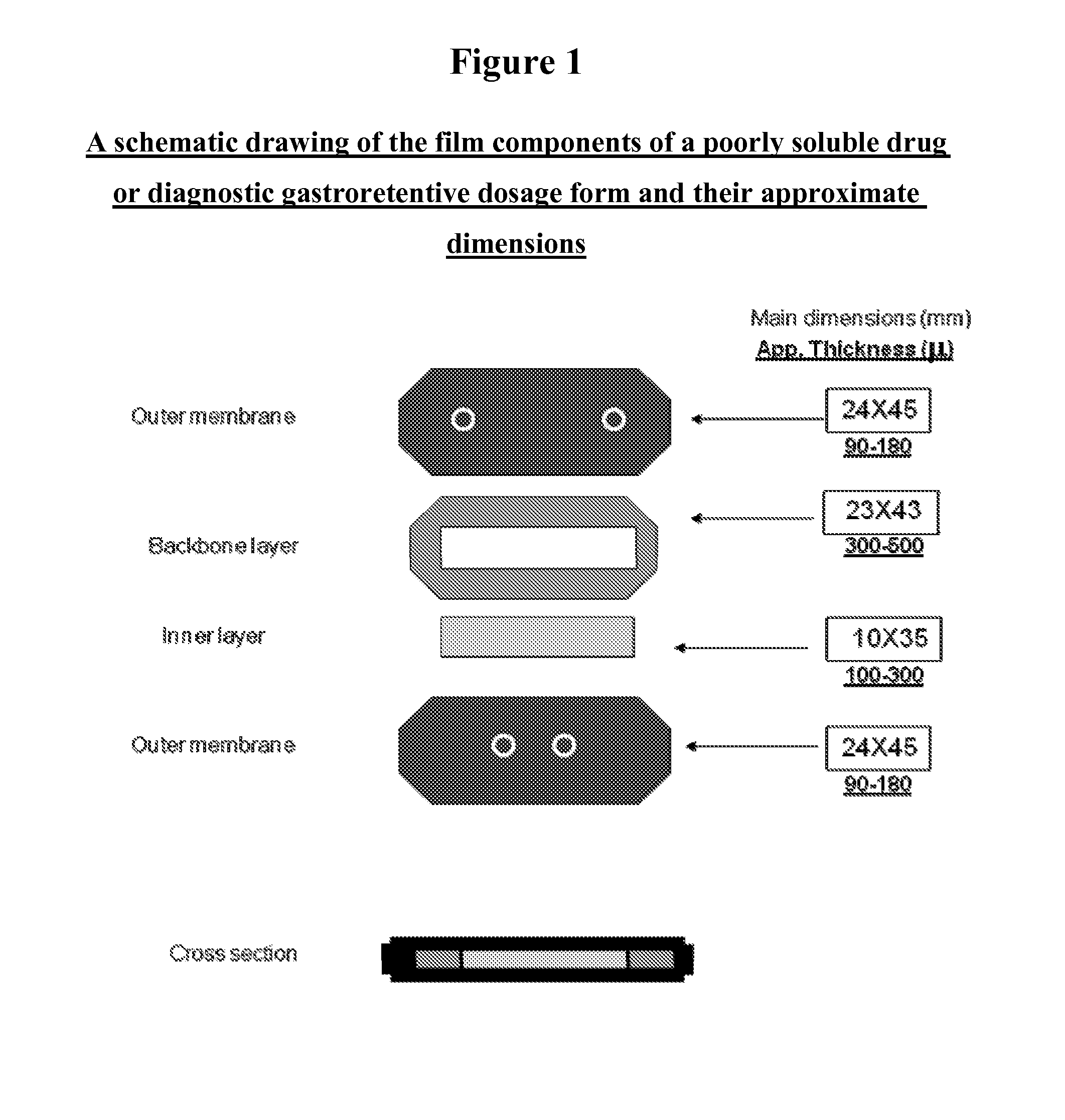
Novel gastroretentive dosage forms of poorly soluble drugs
Disclosed is a multi-layered gastroretentive dosage form for the controlled release of a poorly soluble drug or diagnostic in the stomach and gastrointestinal tract of a patient, folded into a capsule which disintegrates rapidly and the said multi-layered dosage form unfolds rapidly upon contact with the gastric juice. The mechanisms of the gastric retention are not dependent on and do not influence the materials and methods used in controlling the release of the said poorly soluble drug.
Owner:INTEC PHARMA
Carbidopa/lipodopa gastroretentive drug delivery
A gastroretentive drug formulation for the sustained release of an active agent in the gastrointestinal tract comprises an internal layer or compartment comprising an active agent and one or more pharmaceutical excipients, of which at least one is a polymer and two membranes forming together an envelope around the inner membrane, each membrane comprising at least one polymeric combination of an enteric polymer which is not soluble in gastric juice, and an hydrophilic swelling polymer, and at least one plasticizer.
Owner:INTEC PHARMA
Pickering emulsion as well as preparation method thereof
InactiveCN104403117AControllable breaking timeRelease stabilityPharmaceutical non-active ingredientsFood preparationDigestionEdible oil
The invention discloses a Pickering emulsion as well as a preparation method thereof. The Pickering emulsion comprises the following components in percentage by weight: 0.05-3% of starch nanocrystals, 0.001-2% of amino acids, 10-90% of edible oil and the balance of water. The prepared Pickering emulsion remains stable in a wide acidic range with the pH of 1-5; under an alkaline condition, the demulsification time of the emulsion can be controlled by adjusting the type of starch nanocrystals and amino acids which are compounded and used as well as the concentration and proportion of starch nanocrystals and amino acids; meanwhile, in a process of simulating human digestion, the Pickering emulsion disclosed by the invention achieves the slow-release effect of being stable in gastric juice and being released in intestinal juice. Therefore, the Pickering emulsion disclosed by the invention can be used as a safe and effective controlled release system for stomach and intestine for conveying active components in foods, health products or drugs, and has a relatively good application prospect.
Owner:JIANGNAN UNIV
One-bacterium multiple-enzyme bacterial strain as well as screening method and application thereof
The invention relates to a one-bacterium multiple-enzyme bacterial strain as well as a screening method and an application thereof. The bacterial strain is bacillus subtilis (Bacillus subtilis 1.1111) and is collected in the China center for type culture collection with the collection number of CCTCC (China center for type culture collection) No: M2011286. The bacillus subtilis (Bacillus subtilis 1.1111) can be used for preparing the bacterial strains of nine enzymes, i.e. xylanase, protease, phytase, pectinase, lipase, sweet dew glucanase, glucoamylase and the like, and the yields of the protease, the sweet dew glucanase, amylase and the glucoamylase are very high. Meanwhile, the bacterial strain is proved to have strong endurance capacity on cholate, artificial gastric juice and artificial intestinal juice by simulating the internal cholate environment, the artificial gastric juice, artificial intestinal juice and the animal test, safety and growth simulation capability are shown to a tested animal, and a foundation is laid for effectively improving the enzyme production capability of the bacterial strain, simultaneously generating the xylanase, the protease, the phytase, the pectinase, the lipase, the sweet dew glucanase and the glucoamylase and realizing one-bacterium multiple-enzyme fermentation in the fermentation process. The mutual synergistic effect among various enzymes generated by the bacterial strain is strong, and the bacterial strain can be used as a feed additive to be applied to agricultural production for livestock, fowls, aquatic livestock and the like.
Owner:HENAN UNIV OF SCI & TECH
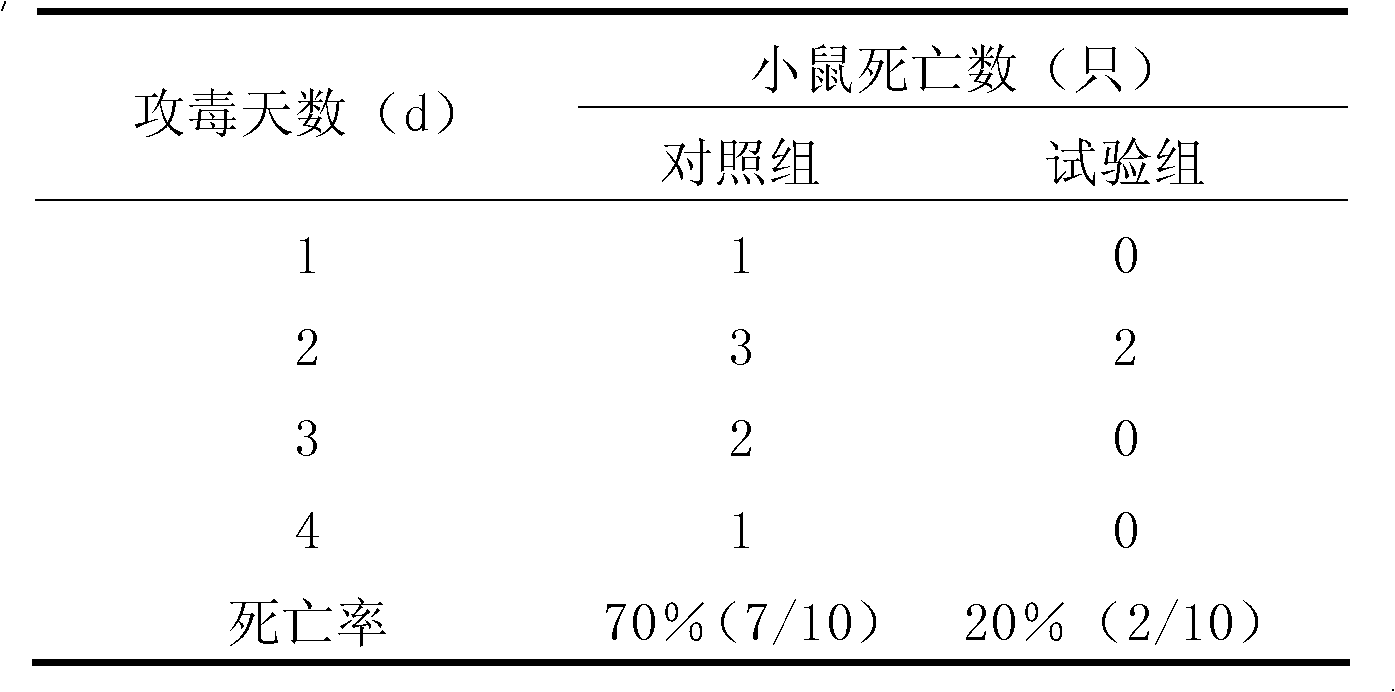
A Strain of Enterococcus Faecalis for Feed and Its Application
InactiveCN102277325AEnhanced inhibitory effectImprove the living environmentAntibacterial agentsBacteriaDiseaseOptimal growth
The invention discloses an Enterococcus faecalis strain SBD, of which the collection number is CGMCC No.4848. The strain is a Gram positive strain and is spherical according to observation with microscope, and the strain does not form spores; the strain can well grow on a Mann, Rogosa and Sharpe (MRS) agar plate and can grow into a round, smooth and raised bacterial colony like a grey white dew and with a diameter of 0.5 to 1 millimeter within 48 hours; and the strain can grow in a facultatively anaerobic environment, the growth temperature range is from 10 to 50 DEG C, the optimal growth temperature is 30 to 40 DEG C, and the growth pH value range is from 4 to 10 and the optimal pH value is 6.5. The bacterial preparation made by the strain is nontoxic and safe, can resist gastric juice and cholate, has a strong inhibition effect on various harmful bacteria and can be widely used in livestock breeding industry (by directly adding into daily ration or drinking water of animals) to increase the disease resistance in animals; and the preparation is expected to replace antibiotic for feed purpose and can obviously improve average weight of weaned piglets, reduce a feed-to-meat ratio and improve living environment of livestock and therefore has a bright prospect.
Owner:北京金泰得生物科技股份有限公司
Lactobacillus plantarum CQ02-108 and application thereof to preparation of fermented sausages
ActiveCN108949645AInhibition of reproductionGrowth inhibitionSugar food ingredientsBacteriaEscherichia coliStaphylococcus cohnii
The invention discloses lactobacillus plantarum CQ02-108 and application thereof to preparation of fermented sausages. The lactobacillus plantarum CQ02-108 is preserved in China General Microbiological Culture Collection Center (CGMCC) on July 17, 2018; and the preservation number is CGMCC No. 16121. The lactobacillus plantarum CQ02-108 can be used for producing proteinase and rapidly producing acid, does not cause viscosity, produce gas, produce biological amine, produce H2O2, produce H2S and produce pigments, and also has the advantages of high-salt concentration tolerance, high acidity tolerance, inhibition of growth of escherichia coli and staphylococcus aureus, bile salt resistance and gastric juice tolerance. When the lactobacillus plantarum CQ02-108 is used for fermenting the sausages, the quantity of viable bacteria of lactic acid bacteria in the fermented sausages can be remarkably improved and the pH (Potential of Hydrogen) value of the sausages is reduced; and the growth ofharmful bacteria is inhibited and the quality of the fermented sausages can be remarkably improved.
Owner:NANJING AGRICULTURAL UNIVERSITY
Bacillus subtilis shou003, anti-vibrio protein and preparation method and applications of bacillus subtilis shou003 and anti-vibrio protein
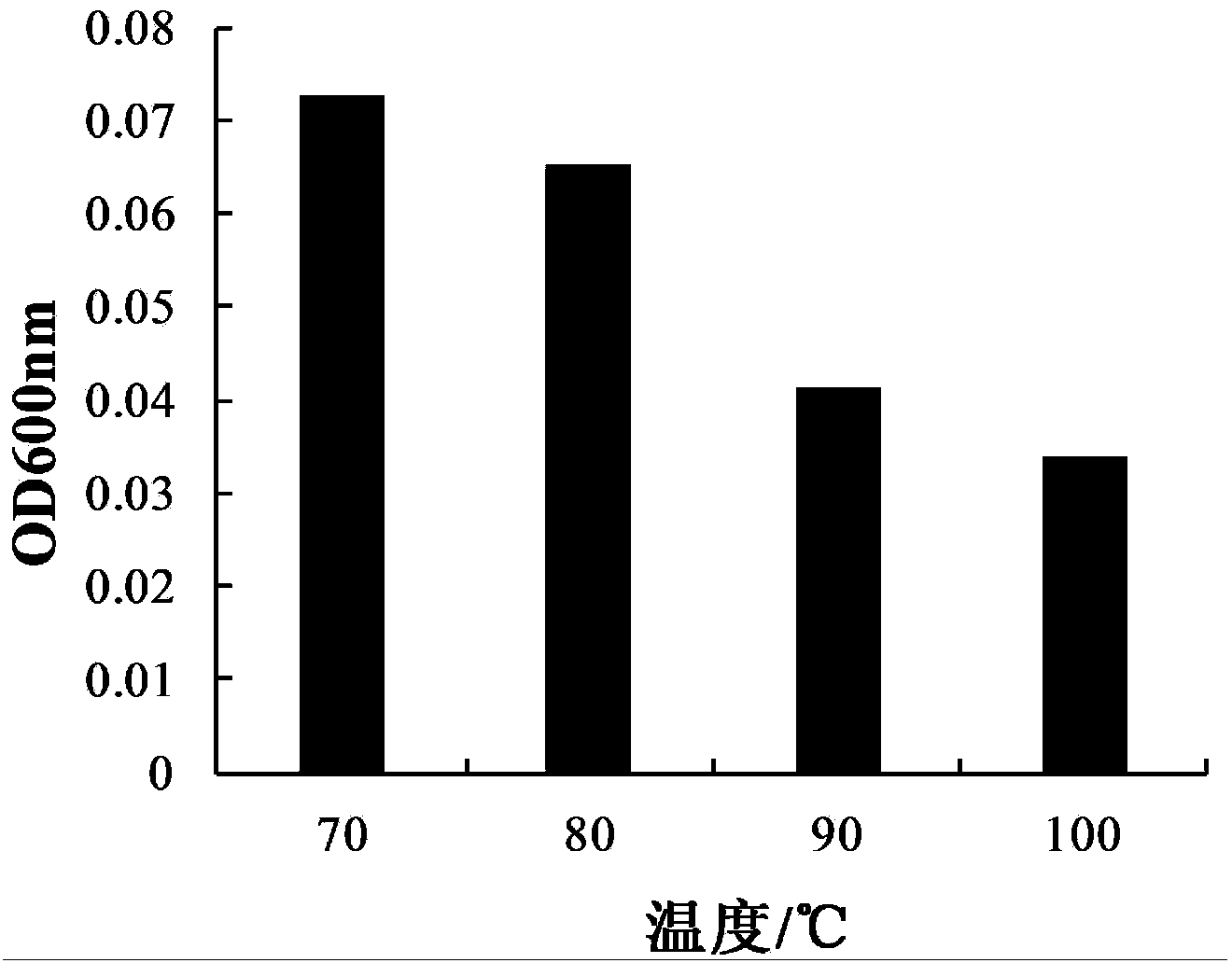
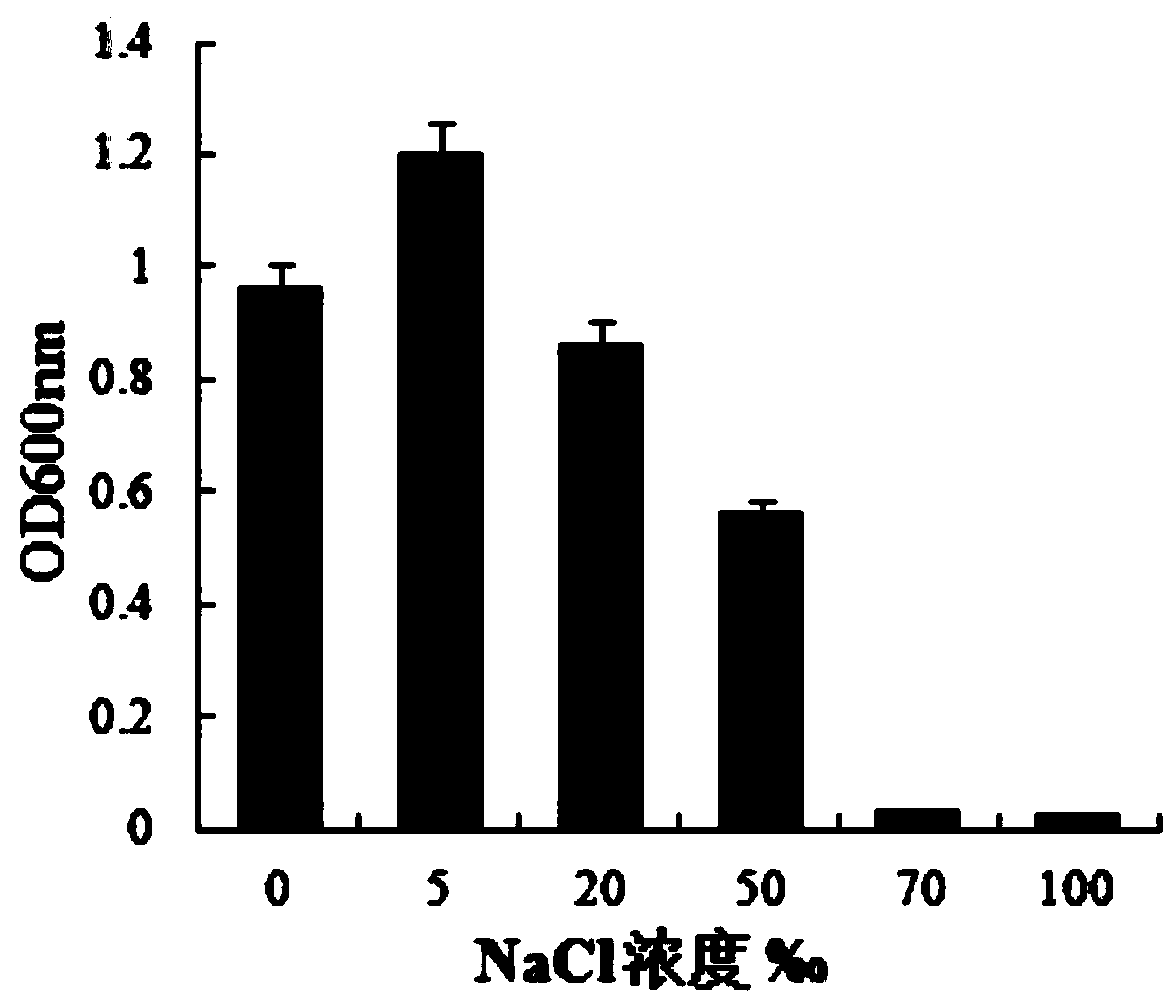
The invention provides bacillus subtilis (Bacillussubtilis) shou003, an anti-vibrio protein and a preparation method and applications of the bacillus subtilis shou003 and the anti-vibrio protein. The preparation method of the anti-vibrio protein comprises the following steps: screening out bacillus subtilis shou003 (preserved in China Center for Type Culture Collection with a number of CCTCC No.: M2013571 on November 13, 2013) from intestinal tract of a healthy large yellow croaker; and then extracting the anti-vibrio protein from the fermentation broth of bacillus subtilis shou003, wherein the amino acid sequence of the anti-vibrio protein is shown as SEQ ID No.: 1. The bacillus subtilis shou003 and the anti-vibrio protein show good effects on inhibiting aquatic pathogenic bacteria, in particular pathogenic vibrio; in addition, the bacillus subtilis shou003 shows outstanding tolerance to temperature, NaCl, gastric juice, intestinal juice and cholate and can be widely applied to the prevention of germs during aquaculture; on that basis, the optimal fermentation culture method of the bacillus subtilis shou003, and a preparation method of the anti-vibrio protein are provided.
Owner:SHANGHAI OCEAN UNIV
Lactobacillus fermentum HY01 with effects of intestinal tract function regulation and colitis prevention, and uses thereof
InactiveCN107164263AStrong toleranceRelieve infiltrationMilk preparationBacteriaLactobacillus fermentumIn vivo
The present invention discloses Lactobacillus fermentum HY01 with effects of intestinal tract function regulation and colitis prevention, and uses thereof, wherein the preservation number of the Lactobacillus fermentum HY01 is CCTCCM2015792. According to the present invention, the in vitro tolerance of the Lactobacillus fermentum HY01 is strong, wherein the survival rate of the Lactobacillus fermentum HY01 is 103.73+ / -8.60% after the Lactobacillus fermentum HY01 is treated for 3 h in the artificial gastric juice with the pH value of 3.0, the Lactobacillus fermentum HY01 can grow at the bile salt concentration of 0.3%, the growth efficiency is 21.62+ / -0.86% of the growth efficiency of the bile salt-free culture, and the Lactobacillus fermentum HY01 has good in vitro tolerance; and the in vivo mouse test results show that the Lactobacillus fermentum HY01 can alleviate the colonic shortening and the colonic edema of the colitis mice, and the inflammatory cell infiltration and the mucosal injury in colon, such that the Lactobacillus fermentum HY01 has the colitis prevention effect.
Owner:SOUTHWEST UNIV
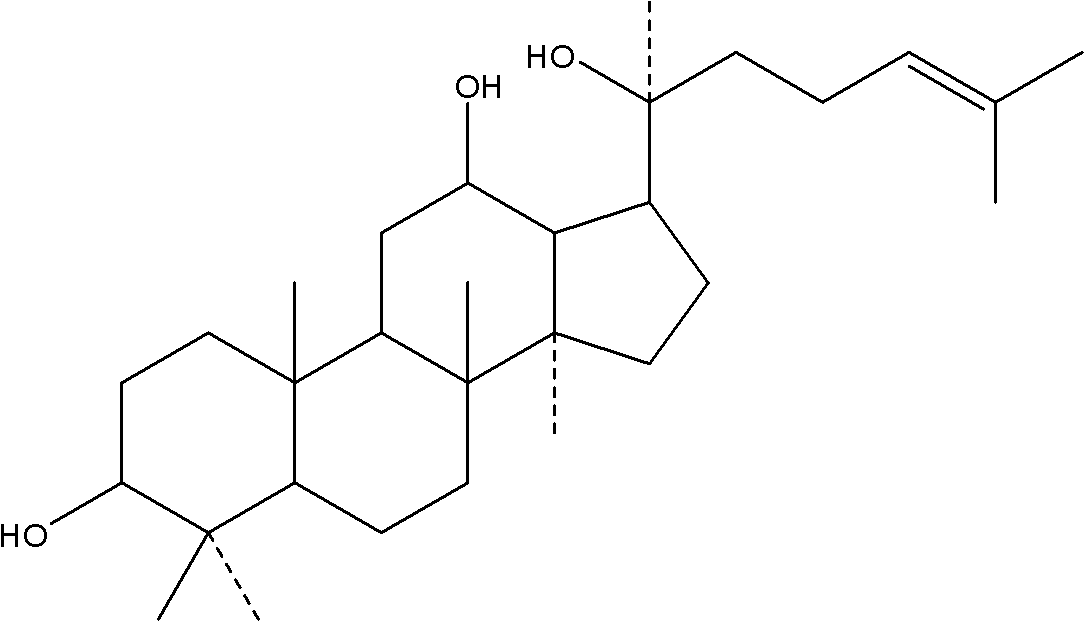
Preparation, medical application and composition of 20S-protopanaxadiol beta-cyclodextrin inclusion compound
ActiveCN102178956AEfficient manufacturingAntibacterial agentsOrganic active ingredientsMedicineProtopanaxadiol
The invention relates to a 20S-protopanaxadiol beta-cyclodextrin inclusion compound which can be used for preparing medicines and foods for resisting tumors, protecting the liver, reducing blood fat, reducing blood sugar, promoting gastric secretion, promoting appetite, improving digestion functions, enhancing immunity, resisting oxidization and resisting senescence. The invention also relates toa preparation process as well as composition of the inclusion compound.
Owner:兰州和盛堂制药股份有限公司
Colchicines gastric floating sustained-release tablet and method for preparing same
InactiveCN101536990ASchematic diagram of the preparation processOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a colchicines gastric floating sustained-release tablet, which comprises active components of colchicines and pharmaceutic adjuvant according to the weight ratio of 1:24-1,999, wherein the pharmaceutic adjuvant comprises a hydrophilic gel framework material, a effervescing agent, a floating assistant material, a filler, a pH value regulator and a lubricant. The colchicines gastric floating sustained-release tablet can swell quickly in gastric juice or a similar gastric juice medium and can float on the gastric juice for at least 4 hours. The invention also relates to a method for preparing the colchicines gastric floating sustained-release tablet. The colchicines gastric floating sustained-release tablet can reach the effective blood-drug concentration quickly after being taken and then release drugs slowly, and can maintain the balanced blood-drug concentration so as to reduce the dose times, relieve the toxic side effect and improve the bioavailability.
Owner:普尔药物科技开发(深圳)有限公司
Tabletting candy containing probiotics microcapsules and preparation method of tabletting candy
The invention relates to a tabletting candy containing probiotics microcapsules. The tabletting candy contains the probiotics microcapsules of which the mass percentage is not more than 25%, and the balance of auxiliary materials for candy, wherein the probiotics microcapsules contain the following components: at least one kind of lactic acid bacteria, colloid and functional substances, and the functional substances are functional lactic acid bacteria and exopolysaccharide and / or lactic acid bacteria lysate produced by the lactic acid bacteria; the probiotics microcapsules are prepared through the steps of mixing the lactic acid bacteria, the functional substances and a colloid water solution, solidifying the mixture, pelleting the solidified mixture and sieving pellets; the tabletting candy is prepared through the steps of mixing the probiotics microcapsules and the auxiliary materials for the candy, pelleting the mixture by a dry method, and tabletting the pellets. According to the technical scheme adopted by the tabletting candy provided by the invention, probiotics are prepared into the microcapsules, and then the microcapsule and base materials for the candy are mixed and tabletted, so that the stability of the tabletting candy is improved, the tolerance of the tabletting candy to gastric juice of a human body and high cholate is excellent; therefore, after entering the human body, the tabletting candy can furthest exert the effects.
Owner:JIANGSU WECARE BIOTECHNOLOGY CO LTD
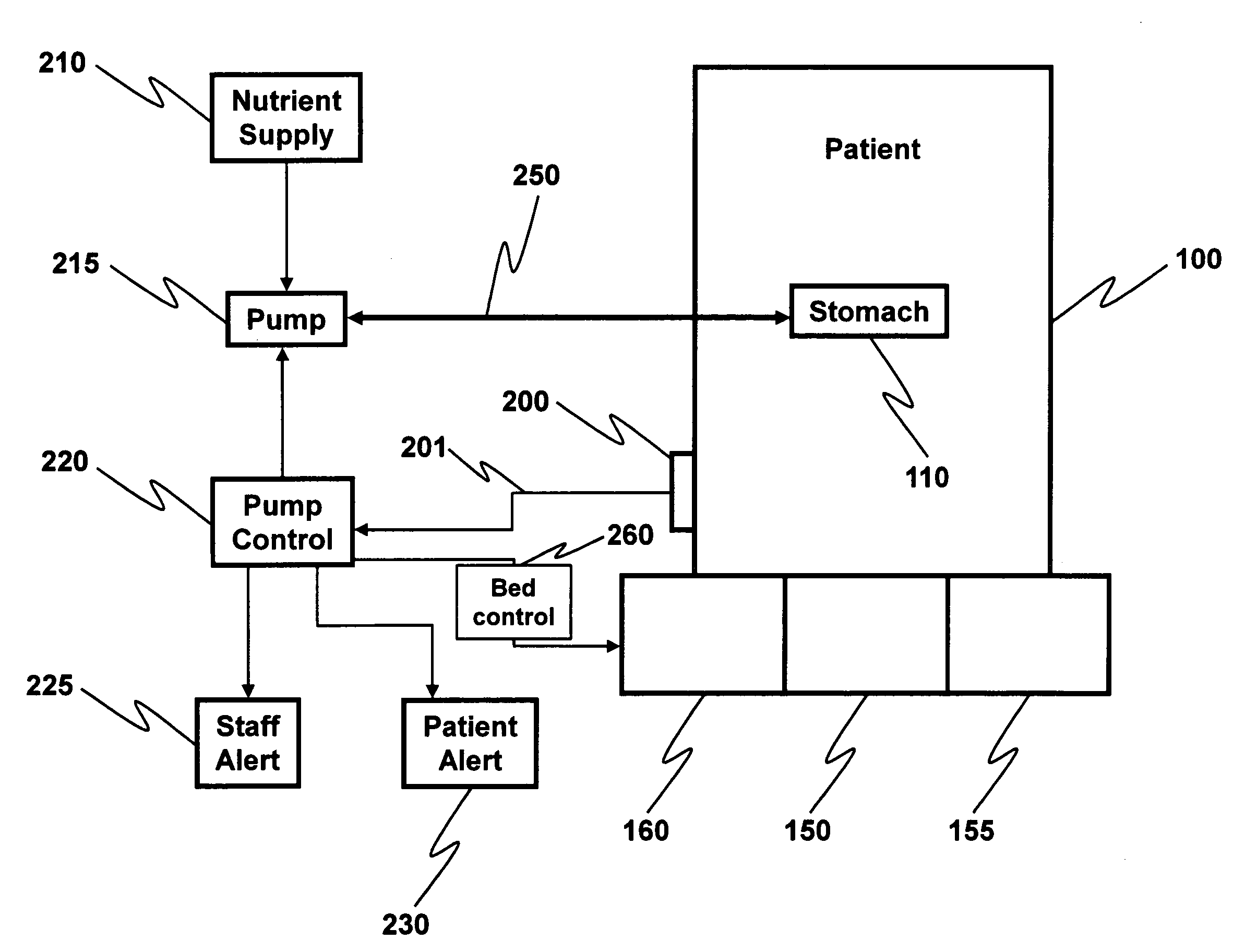
Anti-aspiration device with content monitoring functionality
InactiveUS20080086076A1Reduce eliminateEasy to controlElectrotherapyMedical syringesHospitalized patientsCombined use
A patient stomach fullness sensor is employed in conjunction with an optional patient angle sensor to shut off or to reverse the flow of fluid in a gastric tube when the combination of stomach fullness and patient angle relative to the horizontal becomes sufficient to indicate that gastric juices may enter the esophagus or go even higher. In this way incidents of aspirational pneumonia in hospitalized patients is significantly reduced or eliminated.
Owner:GERBER ALLEN
Melatonin orally disintegrating tablet and preparation method thereof
InactiveCN101143135AMeet technical requirementsQuick effectOrganic active ingredientsNervous disorderMedicineOrally disintegrating tablet
The invention discloses an oral disintegrating tablet of melatonin which is made by selecting the melatonin, a disintegrator, an effervescing agent, a filling agent, an odor corrective and a lubricating agent for smashing, drying, mixing and tablet forming. The drug can be promptly disintegrated inside the mouth without water and is especially applicable to the patients with the deglutition difficulty or under the special environment, such as the elder, the children, the narcose patient etc. The drug also has good effect when used in the environment, which lacks the water, such as the outdoors, the battle field etc. The invention has simple technology and cheap cost and is provided with the advantages of short production period, simple production equipment etc. The oral disintegrating tablet which is prepared by the method of the invention has the enough rigidity to meet the requirements of the production, the packaging, the storage and the transportation, and at the same time the oral disintegrating tablet has good taste and short disintegrating time, and the inside-body disintegrating time is less than thirty seconds; the dissolved quantity inside the 37 DEG C artificial gastric juice and intestinal juice within one minute is 40 percent, and the dissolved quantity within six minutes is as high as 90 percent.
Owner:徐贵丽 +1
Lipase and engineering strain of recombinant expression thereof
The invention provides an engineering strain of recombinant expression lipase. Lipase gene obtained by cloning in Aspergillus niger is transferred into Trichoderma reesei, so that Trichoderma reesei engineering strain can be constructed and has the preservation number of China Center for Type Culture Collection (CCTCC) No.: M2012483. The recombinant expression lipase has the optimal action pH of 5.0 and the optimal action temperature of 30 DEG C, and the tolerance of the recombinant expression lipase to gastric juice, pepsase and artificial intestinal juice can be enhanced. The lipase remarkably improves the utilization rate of oil matter in feed, thus remarkably reducing the addition of oil in the feed and reducing the cost of the feed.
Owner:QINGDAO VLAND BIOTECH GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com