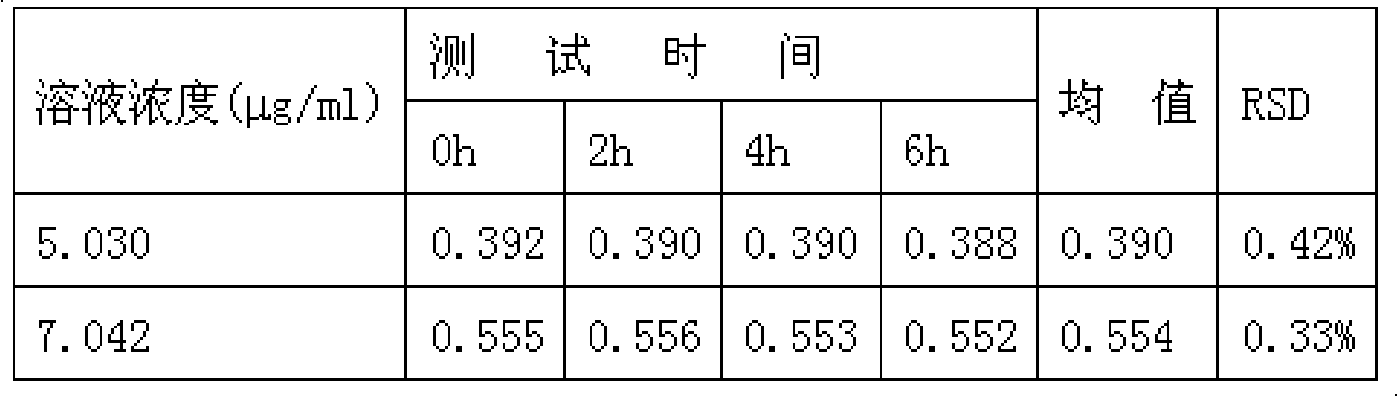
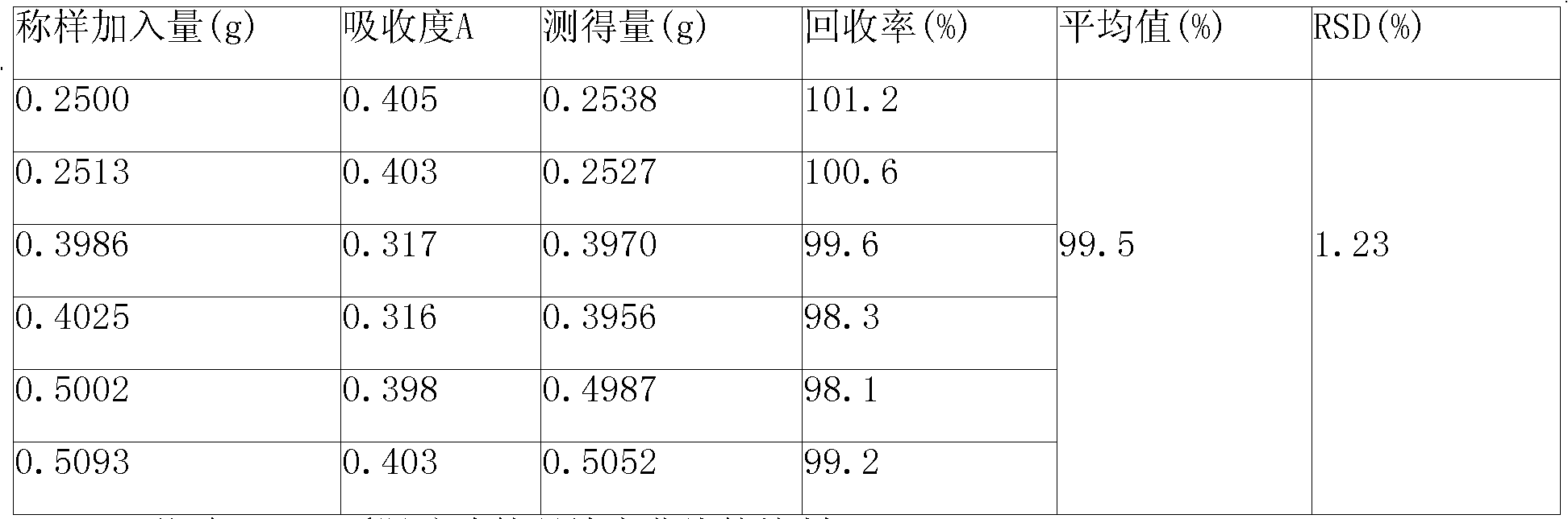
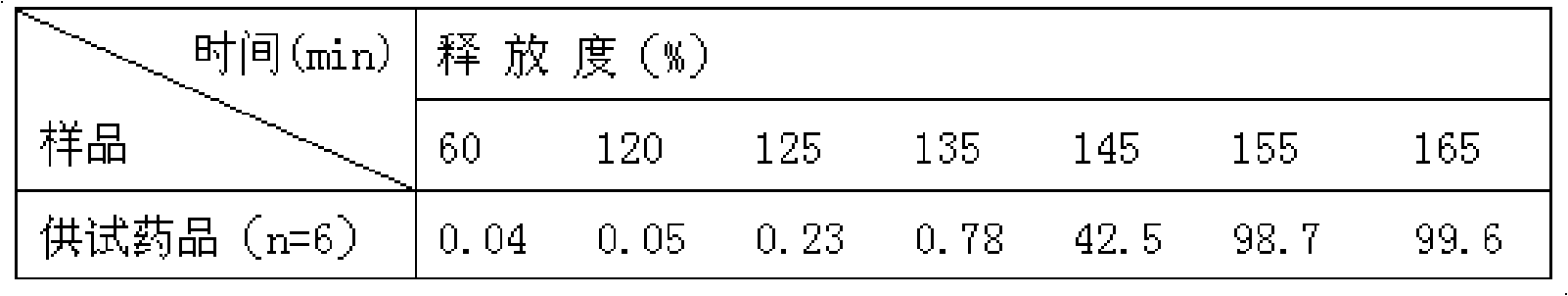
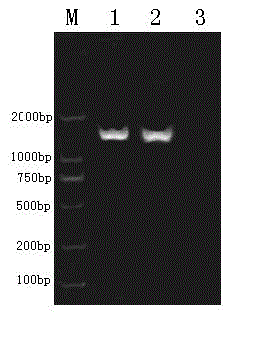
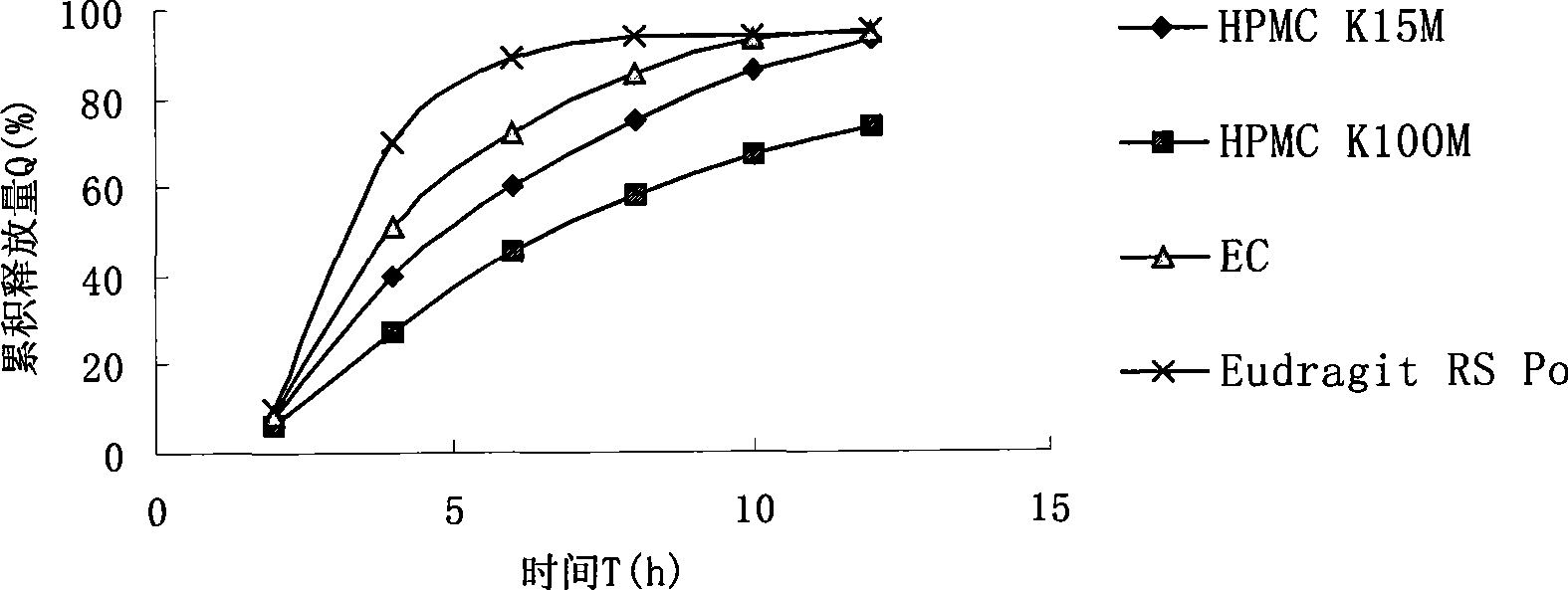
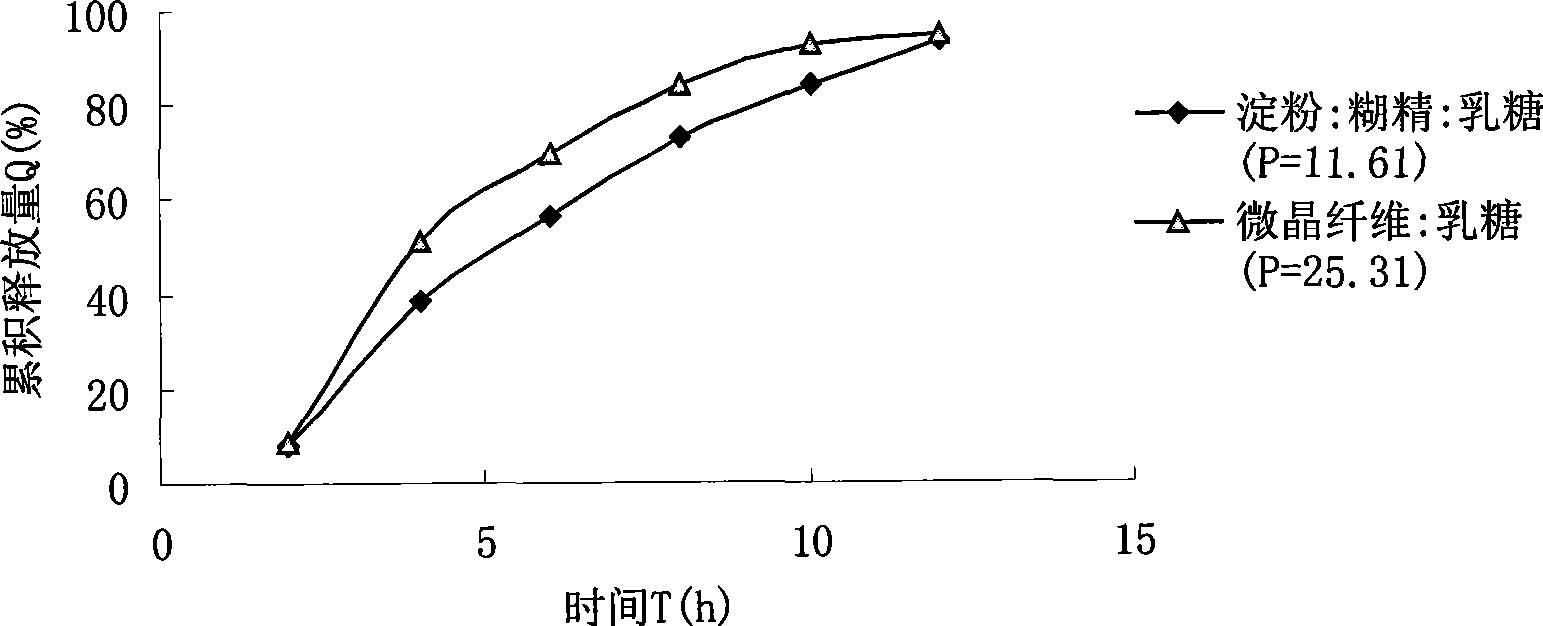
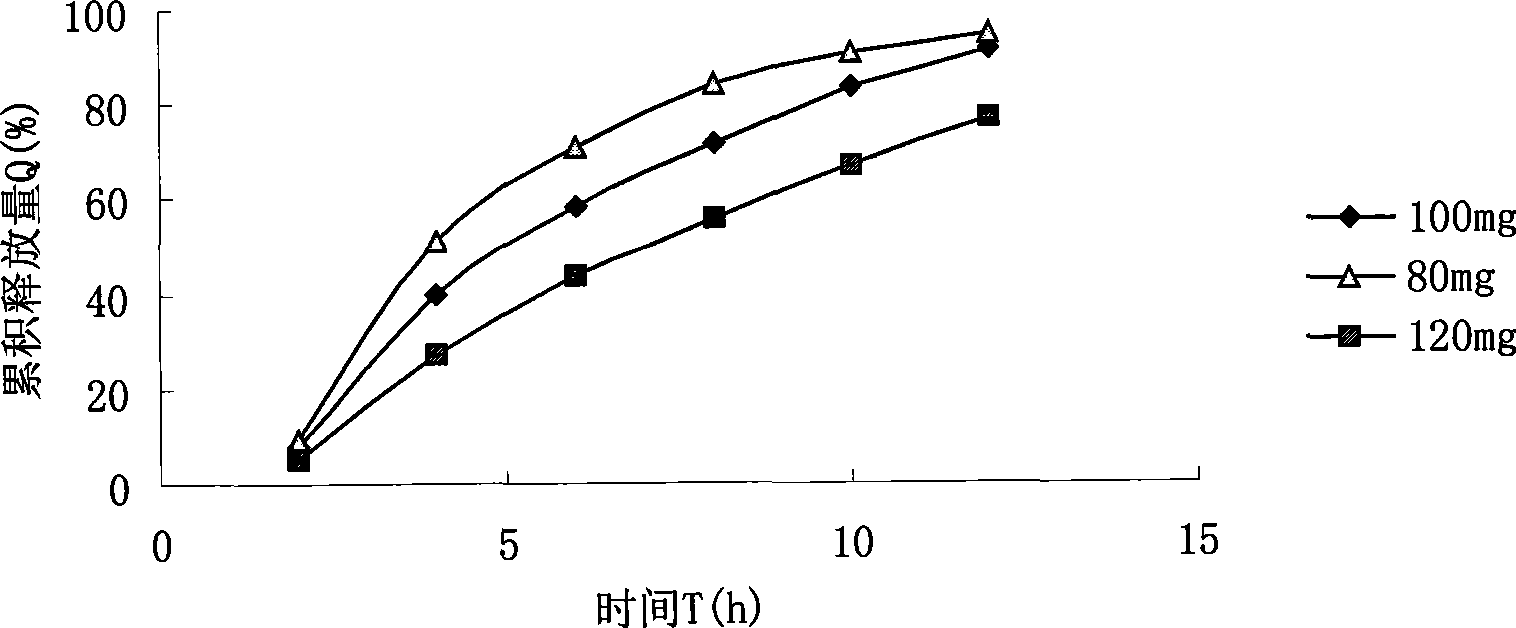
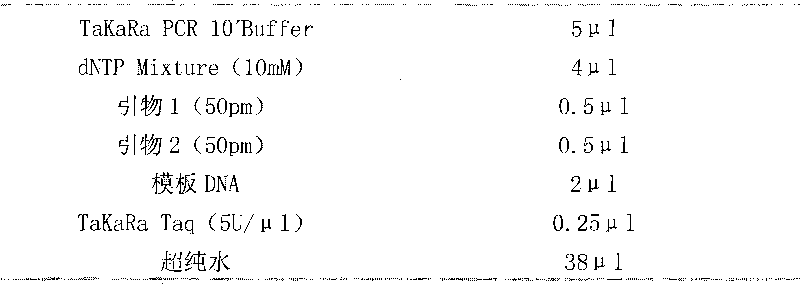
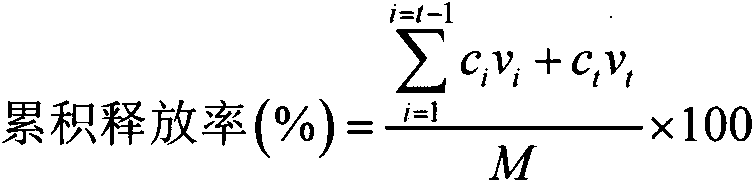Patents
Literature
340 results about "Intestinal juice" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intestinal juice the liquid secretion of glands in the intestinal lining. pancreatic juice the enzyme-containing secretion of the pancreas, conducted through its ducts to the duodenum. prostatic juice the liquid secretion of the prostate, which contributes to semen formation.
Multi-particulate, modified-release composition
A multi-particulate, modified-release pharmaceutical composition for the oral administration of an active ingredient to the colon, wherein said particles comprise: (a) a core comprising an active ingredient or a pharmaceutically acceptable salt or ester thereof, and optionally one or more excipients; (b) a first coating applied to the surface of the core, wherein said first coating is insoluble in gastric juice and in intestinal juice below pH 7, but soluble in colonic intestinal juice; and (c) a second coating applied to the surface of the first coating.
Owner:SANDOZ AG
Pharmaceutical formulation for the active ingredient budesonide
InactiveUS20050089571A1Reduce riskImprove solubilityOrganic active ingredientsDigestive systemDissolutionBULK ACTIVE INGREDIENT
The invention relates to a pharmaceutical formulation containing essentially a) an inner layer which can optionally be applied to a core, with the active substance budesonide, bound with a binding agent; b) a middle layer with a polymer covering agent which is soluble in intestinal juice or retardant; and c) an outer envelope or outer layer which is resistant to stomach juice, said layers being able to contain in a manner known per se other pharmaceutically usual adjutants. The inventive formulation is characterised in that the binding agent is a polymer or a copolymer with acid groups and the formulation of the inner layer without the middle and outer layer releases the bound active ingredient in a release test according to USP XXIII monography <711> dissolution with apparatus 2 (addle) at a rotational speed of 100 / min in a phosphate buffer pH 7.5 after 30 min to a value of more than 80%.
Owner:EVONIK ROEHM GMBH
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
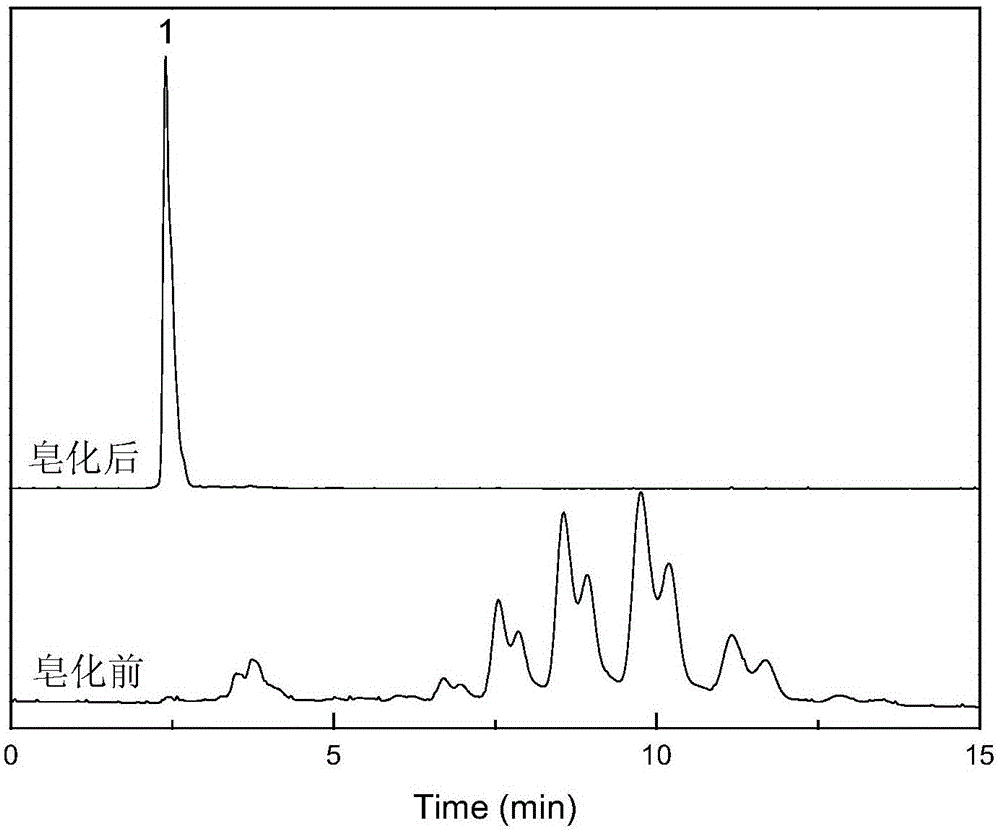
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
Enteric plant hollow capsule
ActiveCN101708171ASave the coating processSimple processPharmaceutical non-active ingredientsCapsule deliveryWater useProduction line
The invention relates to an enteric plant hollow capsule which is prepared by a method comprising: firstly adding 60-85% of pectin, 5-20% of plasticizer, 5-10% of coagulant aid and 5-10% of water used for curing agent into a reaction pot; stirring and dissolving at 60-90 DEG C, and obtaining glue stock by filtering and adjusting specific gravity; removing air bubbles and then sending into a capsule production line for the treatment such as dipping in glue, drying, cooling, demoulding, cutting, sheathing, etc. The enteric plant hollow capsule has the disintegration time limit of 3-4h in gastric juice and 30-45min in intestinal juice. Compared with the existing enteric capsule, the enteric plant hollow capsule omits the working procedure of coating, simplifies the technique, and completely uses no organic solvent, thus not only being beneficial to safe production and environmental protection, but also being beneficial to improving the efficiency and reducing the cost.
Owner:ANHUI HUANGSHAN CAPSULE CO LTD
Pickering emulsion as well as preparation method thereof
InactiveCN104403117AControllable breaking timeRelease stabilityPharmaceutical non-active ingredientsFood preparationDigestionEdible oil
The invention discloses a Pickering emulsion as well as a preparation method thereof. The Pickering emulsion comprises the following components in percentage by weight: 0.05-3% of starch nanocrystals, 0.001-2% of amino acids, 10-90% of edible oil and the balance of water. The prepared Pickering emulsion remains stable in a wide acidic range with the pH of 1-5; under an alkaline condition, the demulsification time of the emulsion can be controlled by adjusting the type of starch nanocrystals and amino acids which are compounded and used as well as the concentration and proportion of starch nanocrystals and amino acids; meanwhile, in a process of simulating human digestion, the Pickering emulsion disclosed by the invention achieves the slow-release effect of being stable in gastric juice and being released in intestinal juice. Therefore, the Pickering emulsion disclosed by the invention can be used as a safe and effective controlled release system for stomach and intestine for conveying active components in foods, health products or drugs, and has a relatively good application prospect.
Owner:JIANGNAN UNIV
One-bacterium multiple-enzyme bacterial strain as well as screening method and application thereof
The invention relates to a one-bacterium multiple-enzyme bacterial strain as well as a screening method and an application thereof. The bacterial strain is bacillus subtilis (Bacillus subtilis 1.1111) and is collected in the China center for type culture collection with the collection number of CCTCC (China center for type culture collection) No: M2011286. The bacillus subtilis (Bacillus subtilis 1.1111) can be used for preparing the bacterial strains of nine enzymes, i.e. xylanase, protease, phytase, pectinase, lipase, sweet dew glucanase, glucoamylase and the like, and the yields of the protease, the sweet dew glucanase, amylase and the glucoamylase are very high. Meanwhile, the bacterial strain is proved to have strong endurance capacity on cholate, artificial gastric juice and artificial intestinal juice by simulating the internal cholate environment, the artificial gastric juice, artificial intestinal juice and the animal test, safety and growth simulation capability are shown to a tested animal, and a foundation is laid for effectively improving the enzyme production capability of the bacterial strain, simultaneously generating the xylanase, the protease, the phytase, the pectinase, the lipase, the sweet dew glucanase and the glucoamylase and realizing one-bacterium multiple-enzyme fermentation in the fermentation process. The mutual synergistic effect among various enzymes generated by the bacterial strain is strong, and the bacterial strain can be used as a feed additive to be applied to agricultural production for livestock, fowls, aquatic livestock and the like.
Owner:HENAN UNIV OF SCI & TECH
Bacillus subtilis shou003, anti-vibrio protein and preparation method and applications of bacillus subtilis shou003 and anti-vibrio protein
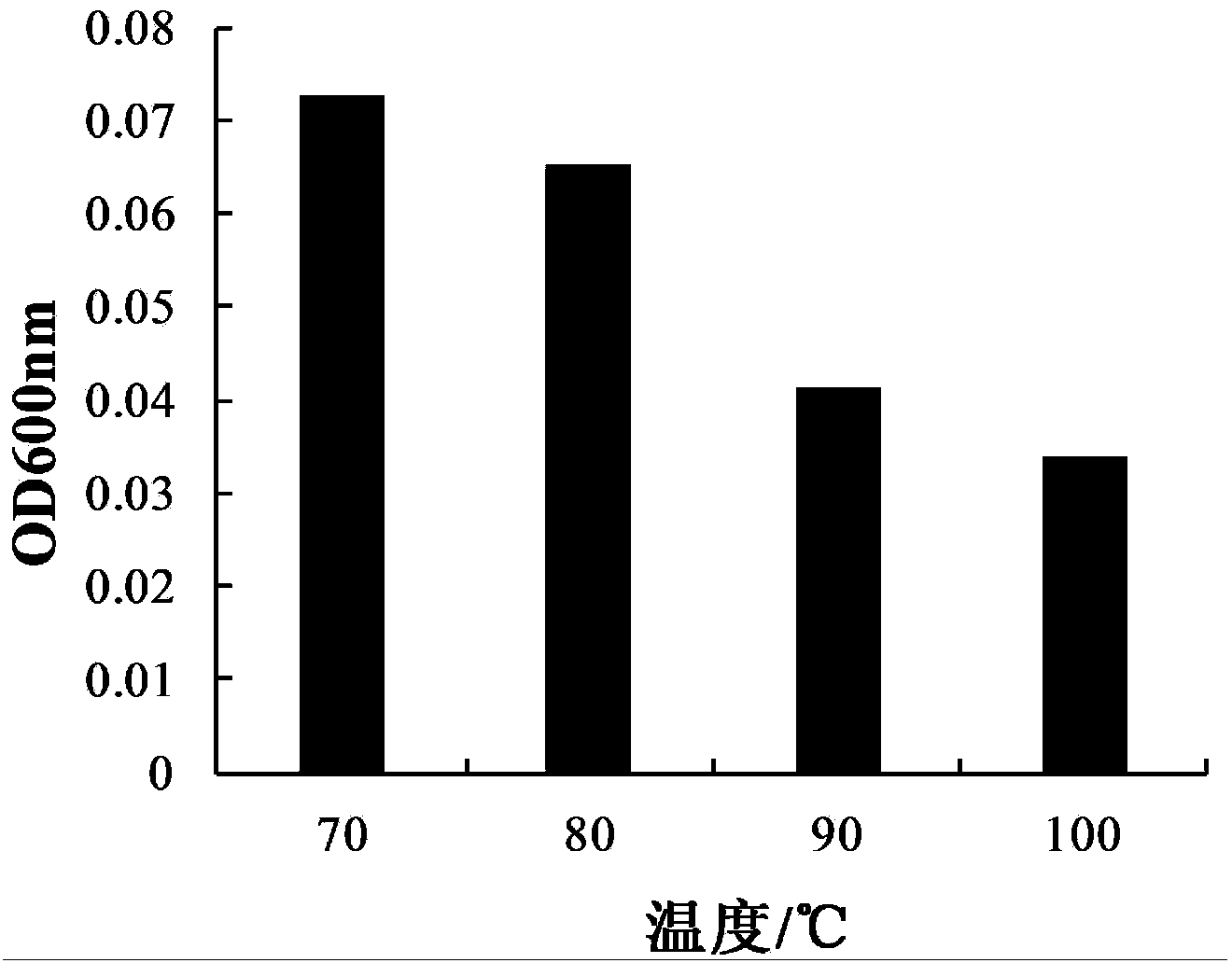
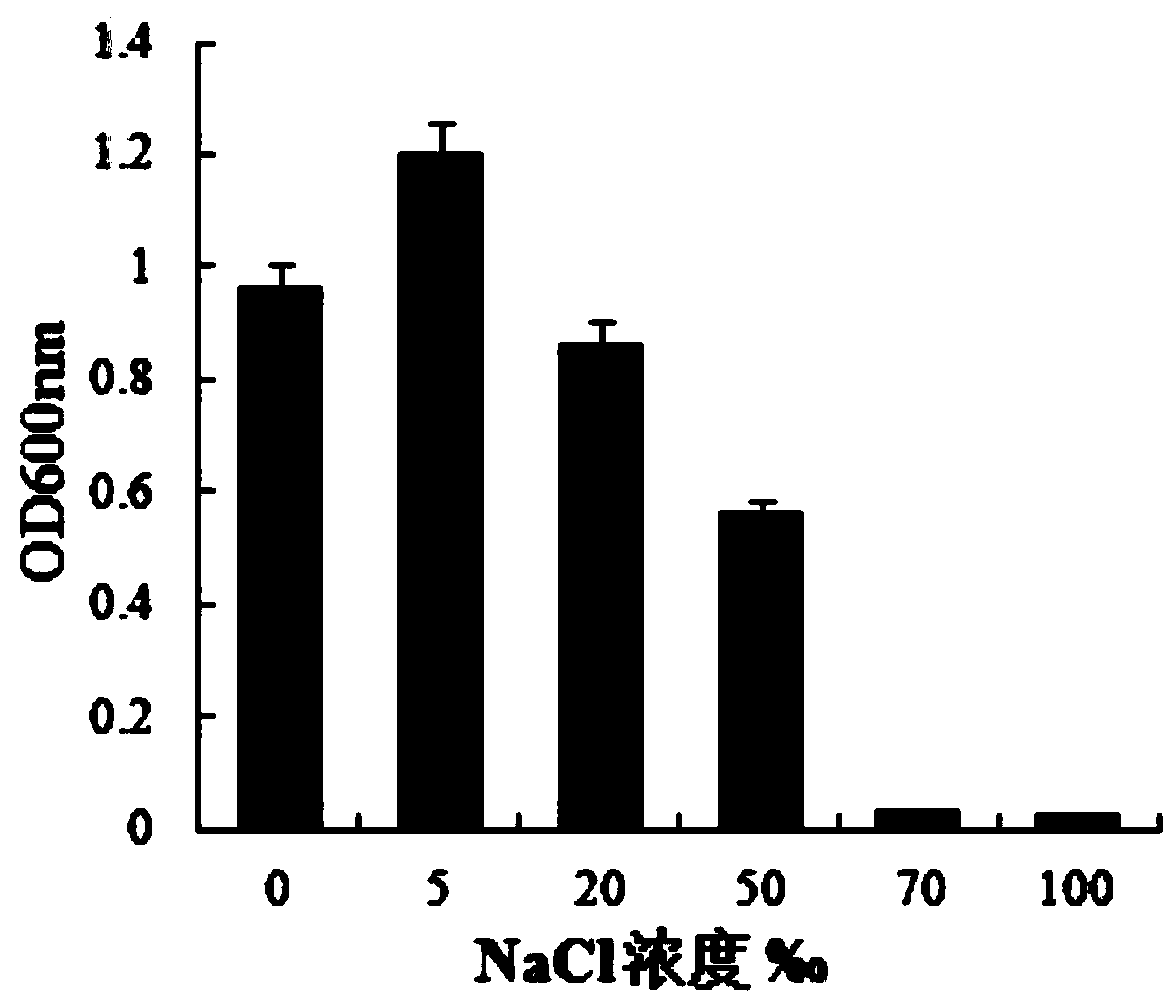
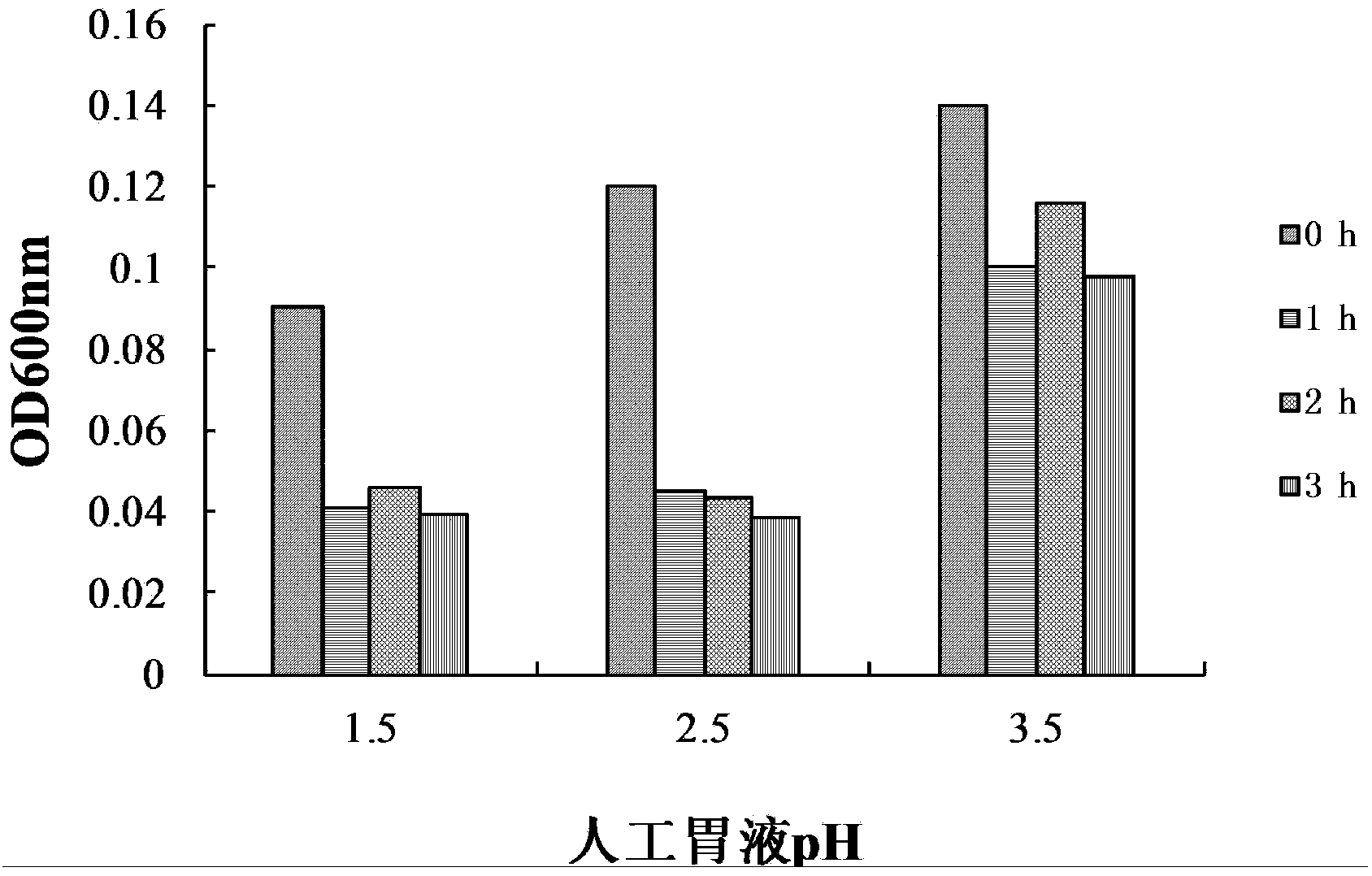
The invention provides bacillus subtilis (Bacillussubtilis) shou003, an anti-vibrio protein and a preparation method and applications of the bacillus subtilis shou003 and the anti-vibrio protein. The preparation method of the anti-vibrio protein comprises the following steps: screening out bacillus subtilis shou003 (preserved in China Center for Type Culture Collection with a number of CCTCC No.: M2013571 on November 13, 2013) from intestinal tract of a healthy large yellow croaker; and then extracting the anti-vibrio protein from the fermentation broth of bacillus subtilis shou003, wherein the amino acid sequence of the anti-vibrio protein is shown as SEQ ID No.: 1. The bacillus subtilis shou003 and the anti-vibrio protein show good effects on inhibiting aquatic pathogenic bacteria, in particular pathogenic vibrio; in addition, the bacillus subtilis shou003 shows outstanding tolerance to temperature, NaCl, gastric juice, intestinal juice and cholate and can be widely applied to the prevention of germs during aquaculture; on that basis, the optimal fermentation culture method of the bacillus subtilis shou003, and a preparation method of the anti-vibrio protein are provided.
Owner:SHANGHAI OCEAN UNIV
Treatment of allergic conditions
Orally administered sodium cromoglycate has been found to be effective in the treatment of allergic conditions such as asthma, general food allergies, ulcerative colitis, atopic eczema, chronic urticaria and irritable bowel syndrome if it is presented such that the sodium cromoglycate becomes bioavailable within 10 minutes of exposure to intestinal fluid. The sodium cromoglycate may be presented as enteric-coated tablets or individually enteric-coated pellets or microgranules packaged with disintegrant in a ratio of at least 1.2:1 disintegrant:sodium cromoglycate (w:w). Optionally, the patients are first selected to have a total serum IgE level of at least 150 iu / ml.
Owner:THORNTON & ROSS
Melatonin orally disintegrating tablet and preparation method thereof
InactiveCN101143135AMeet technical requirementsQuick effectOrganic active ingredientsNervous disorderMedicineOrally disintegrating tablet
The invention discloses an oral disintegrating tablet of melatonin which is made by selecting the melatonin, a disintegrator, an effervescing agent, a filling agent, an odor corrective and a lubricating agent for smashing, drying, mixing and tablet forming. The drug can be promptly disintegrated inside the mouth without water and is especially applicable to the patients with the deglutition difficulty or under the special environment, such as the elder, the children, the narcose patient etc. The drug also has good effect when used in the environment, which lacks the water, such as the outdoors, the battle field etc. The invention has simple technology and cheap cost and is provided with the advantages of short production period, simple production equipment etc. The oral disintegrating tablet which is prepared by the method of the invention has the enough rigidity to meet the requirements of the production, the packaging, the storage and the transportation, and at the same time the oral disintegrating tablet has good taste and short disintegrating time, and the inside-body disintegrating time is less than thirty seconds; the dissolved quantity inside the 37 DEG C artificial gastric juice and intestinal juice within one minute is 40 percent, and the dissolved quantity within six minutes is as high as 90 percent.
Owner:徐贵丽 +1
Water soluble salts of organic acid 5-(2-fluorophenyl)-N-methyl-1-(3-pyridyl sulfonyl)-1H-pyrrole-3-methylamine and injection and preparation method thereof
ActiveCN103951652APromote oral absorptionQuick effectOrganic active ingredientsOrganic chemistryChemical synthesisDisease
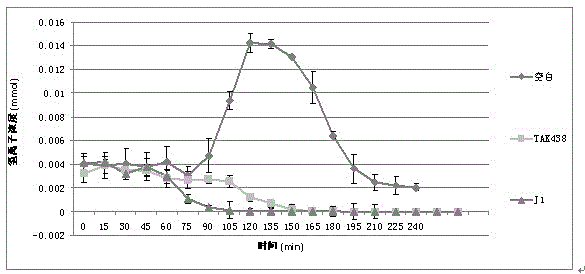
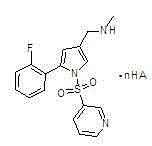
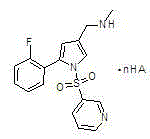
The invention belongs to the technical field of chemical synthetic medicines and particularly relates to water soluble salts of an organic acid of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridyl sulfonyl)-1H-pyrrole-3-methylamine and an injection thereof for treating gastric acid related diseases and a preparation method thereof. The invention discloses the salts of the organic acid of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridyl sulfonyl)-1H-pyrrole-3-methylamine shown as the following general formula in the specification, wherein n is 1 or 1 / 2 and HA is an organic acid. According to the salts of the organic acid, which are disclosed by the invention, the solubility of the salts in water or an artificial gastric and intestinal juice is far greater than TAK438, and the solubility of pyroglutamate and lactate in water is greater than 1g / mL.
Owner:DONGYING DAOYI BIOLOGICAL MEDICINE TECH CO LTD
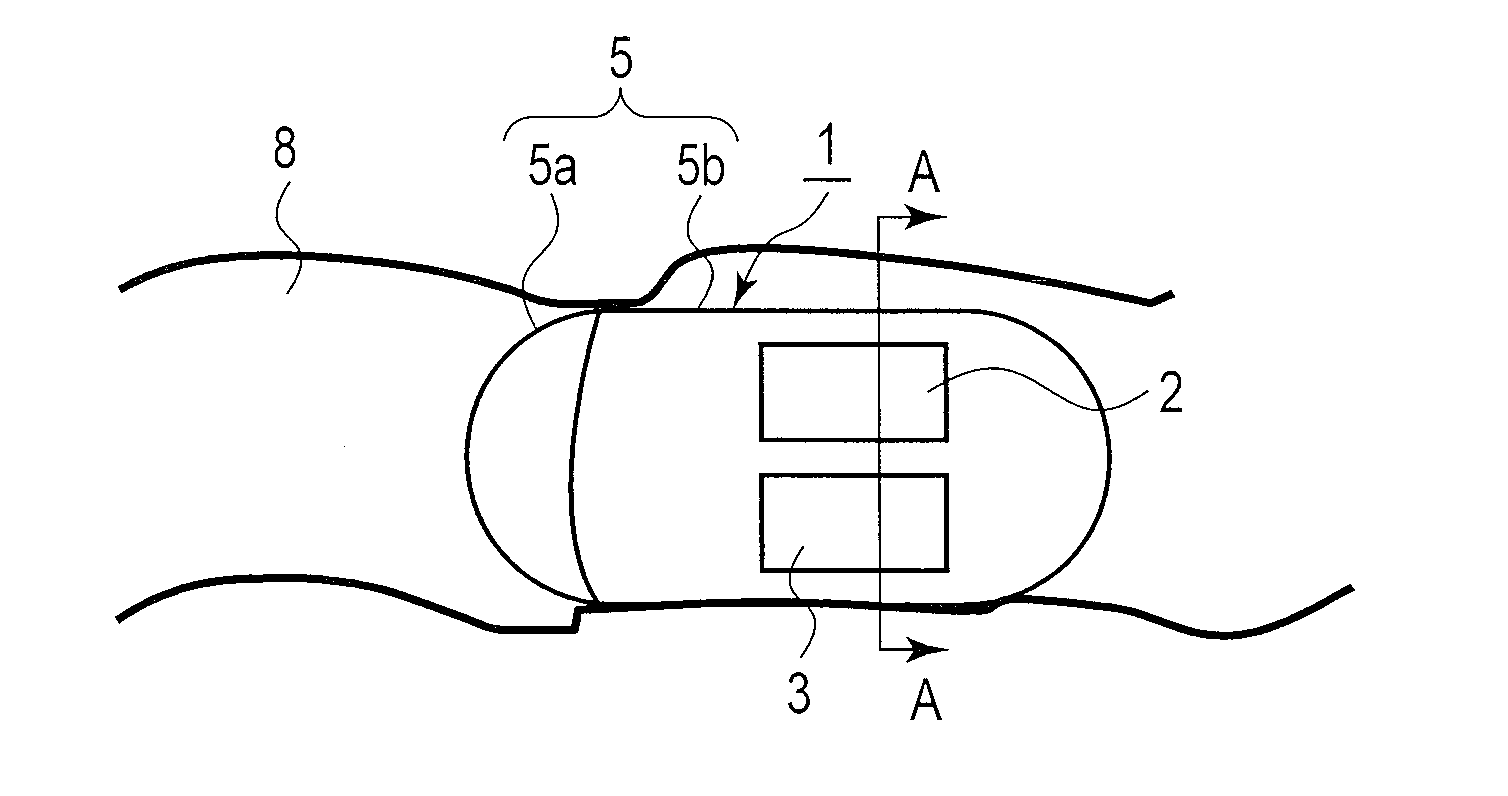
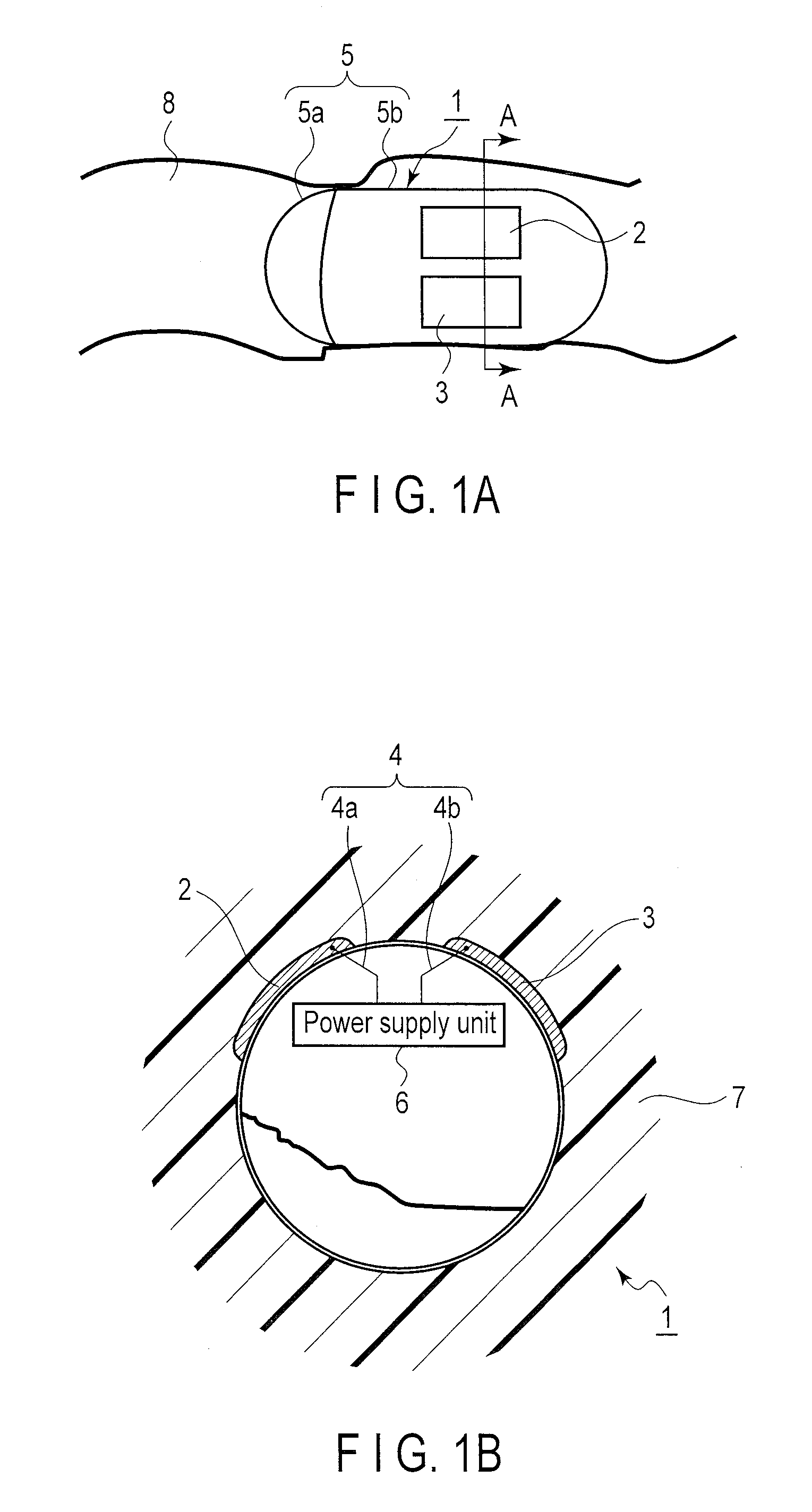
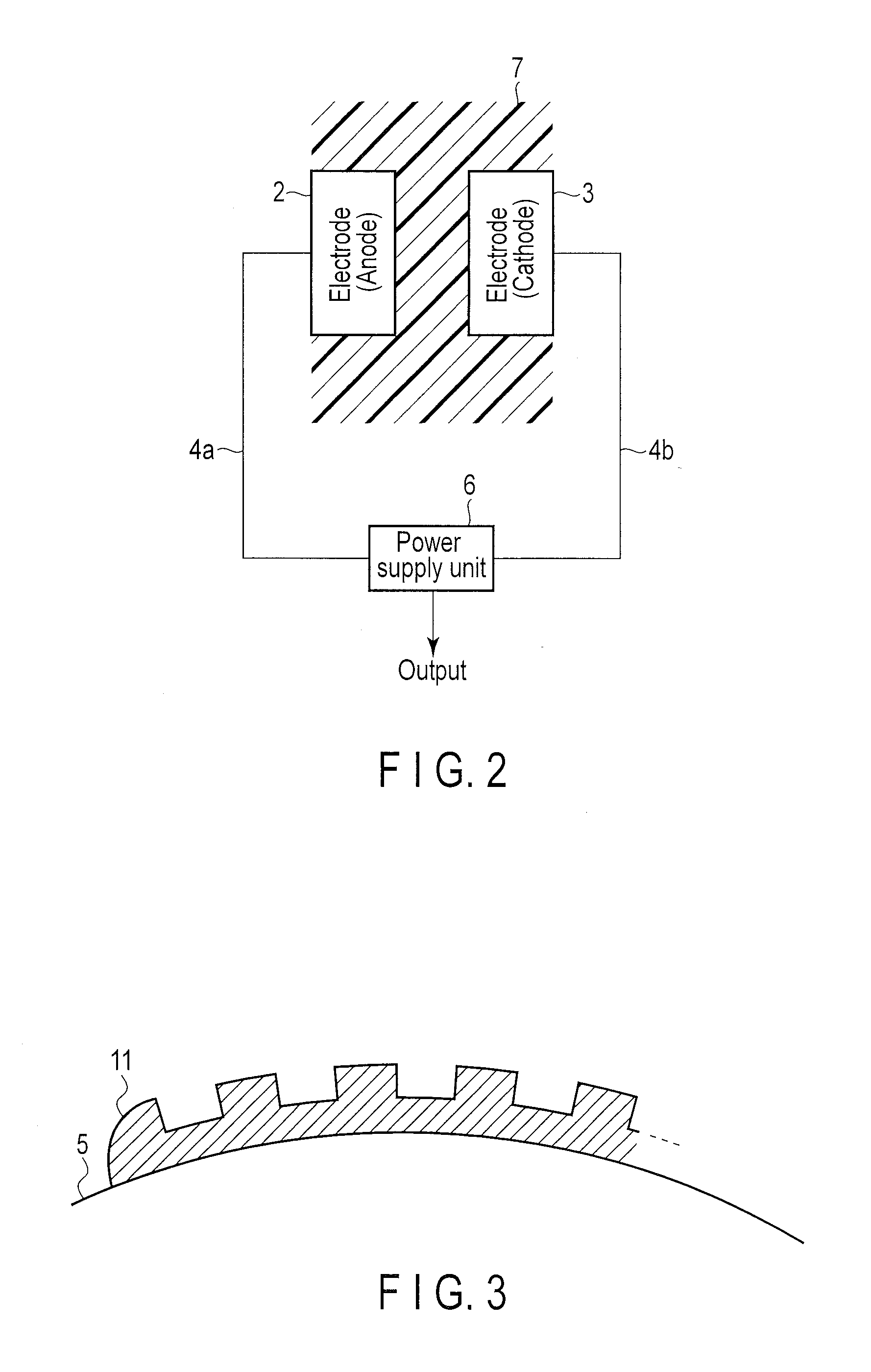
Power supply system and medical capsule device mounted with this power supply system
Provided are a power generation system which does not limit a patient's range of motion, is harmless to a living body, and can obtain a sufficient electricity generating capacity in the stomach or the intestines and a disposable medical capsule device which does not have to be collected after use. A power supply system is mounted in the capsule device, comprises an electrode pair including at least two electrodes provide on an outer wall surface of a capsule main body, for example, an aluminum electrode and a catalyst-supporting carbon electrode, generates power when immersed in an electrolytic solution consisting of gastric juice or intestinal juice, and supplies the power to constituent portions in the capsule device.
Owner:OLYMPUS CORP
Lipase and engineering strain of recombinant expression thereof
The invention provides an engineering strain of recombinant expression lipase. Lipase gene obtained by cloning in Aspergillus niger is transferred into Trichoderma reesei, so that Trichoderma reesei engineering strain can be constructed and has the preservation number of China Center for Type Culture Collection (CCTCC) No.: M2012483. The recombinant expression lipase has the optimal action pH of 5.0 and the optimal action temperature of 30 DEG C, and the tolerance of the recombinant expression lipase to gastric juice, pepsase and artificial intestinal juice can be enhanced. The lipase remarkably improves the utilization rate of oil matter in feed, thus remarkably reducing the addition of oil in the feed and reducing the cost of the feed.
Owner:QINGDAO VLAND BIOTECH GRP
Novel dosage form of sinomenine medicament or hydrochlorate thereof and preparation technique thereof
The invention discloses a sinomenine or an enteric-coated controlled-release tablet of hydrochloride thereof. The prepared enteric-coated controlled-release tablet hardly releases the drug in artificial simulated gastric juice, but can slowly and smoothly release the drug in artificial simulated intestinal juice; the sustained release time of the drug can achieve more than 12 hours or even 24 hours; the enteric-coated controlled-release tablet is taken once or twice daily, the plasma drug concentration in vivo is smooth, and the peak-valley phenomenon of the plasma drug concentration is reduced; as the prepared enteric-coated controlled-release tablet hardly releases the drug in stomach, the contacted concentration of the drug with the gastric mucosa is small, the stimulation of the stomach caused by the drug is alleviated. As the prepared enteric-coated controlled-release tablet sustainedly slowly releases the drug in intestinal tract, the times of the drug administration are reduced, and the patient compliance is improved, thereby being applicable to the needs of the clinical development.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
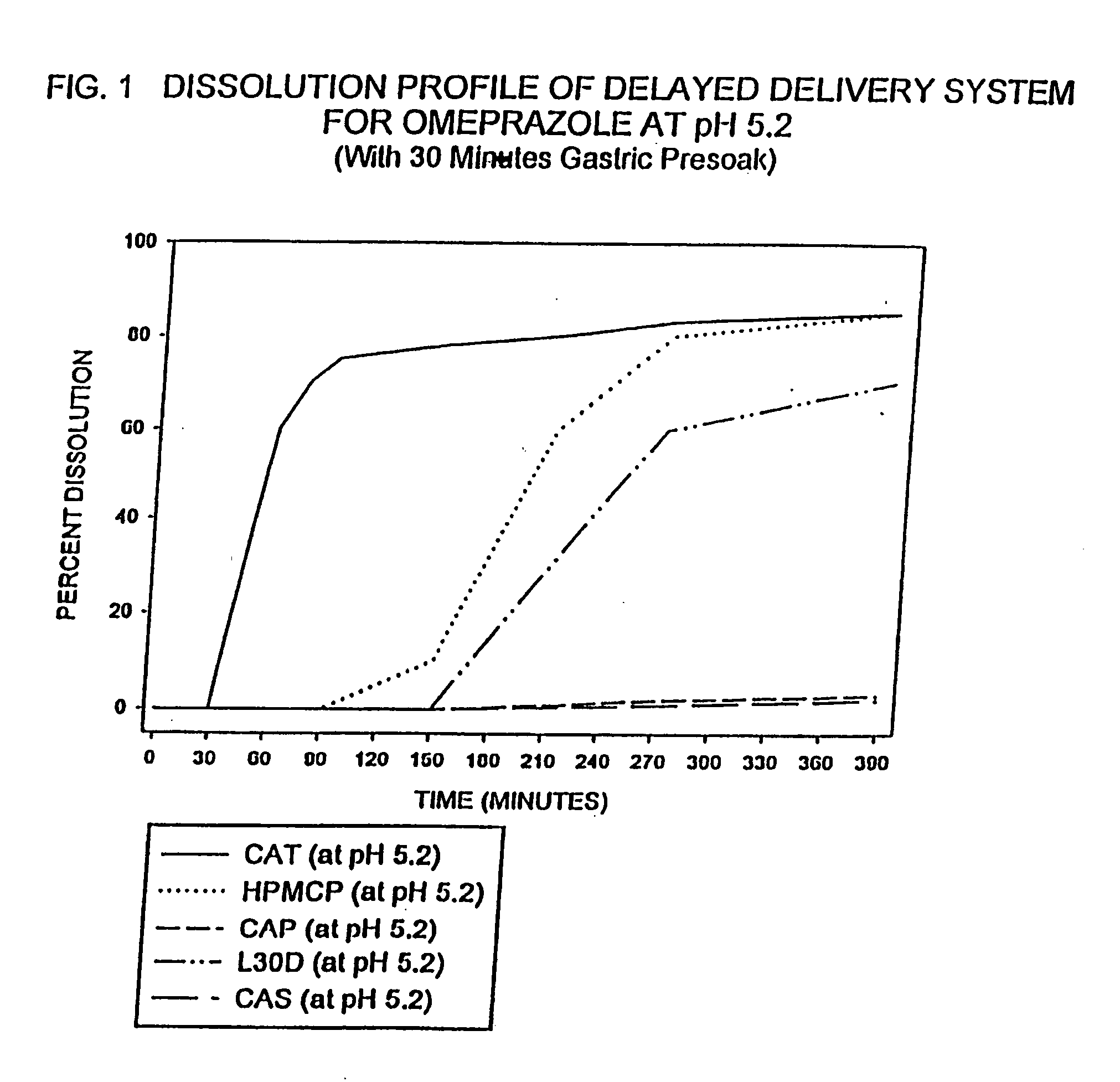
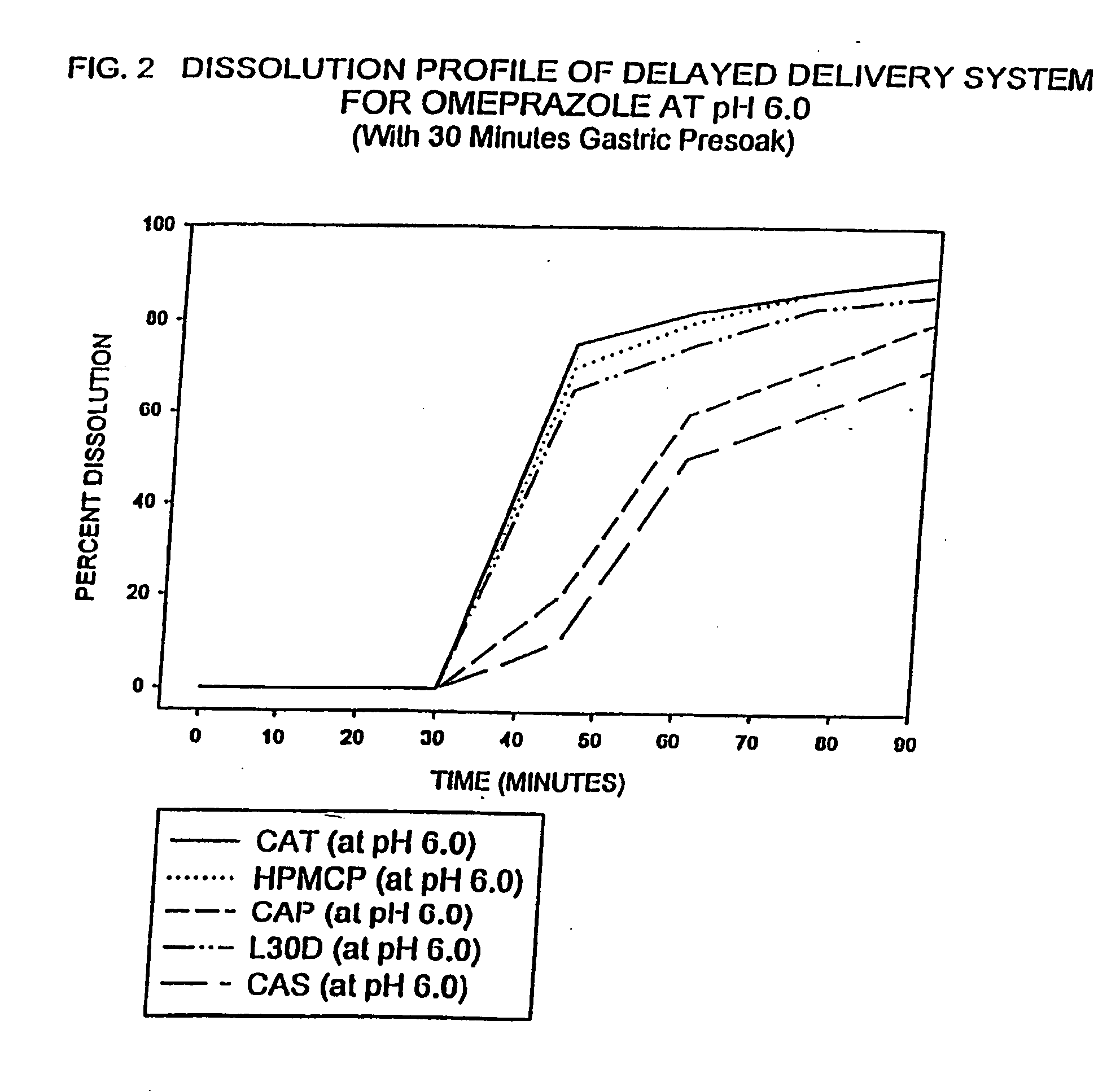
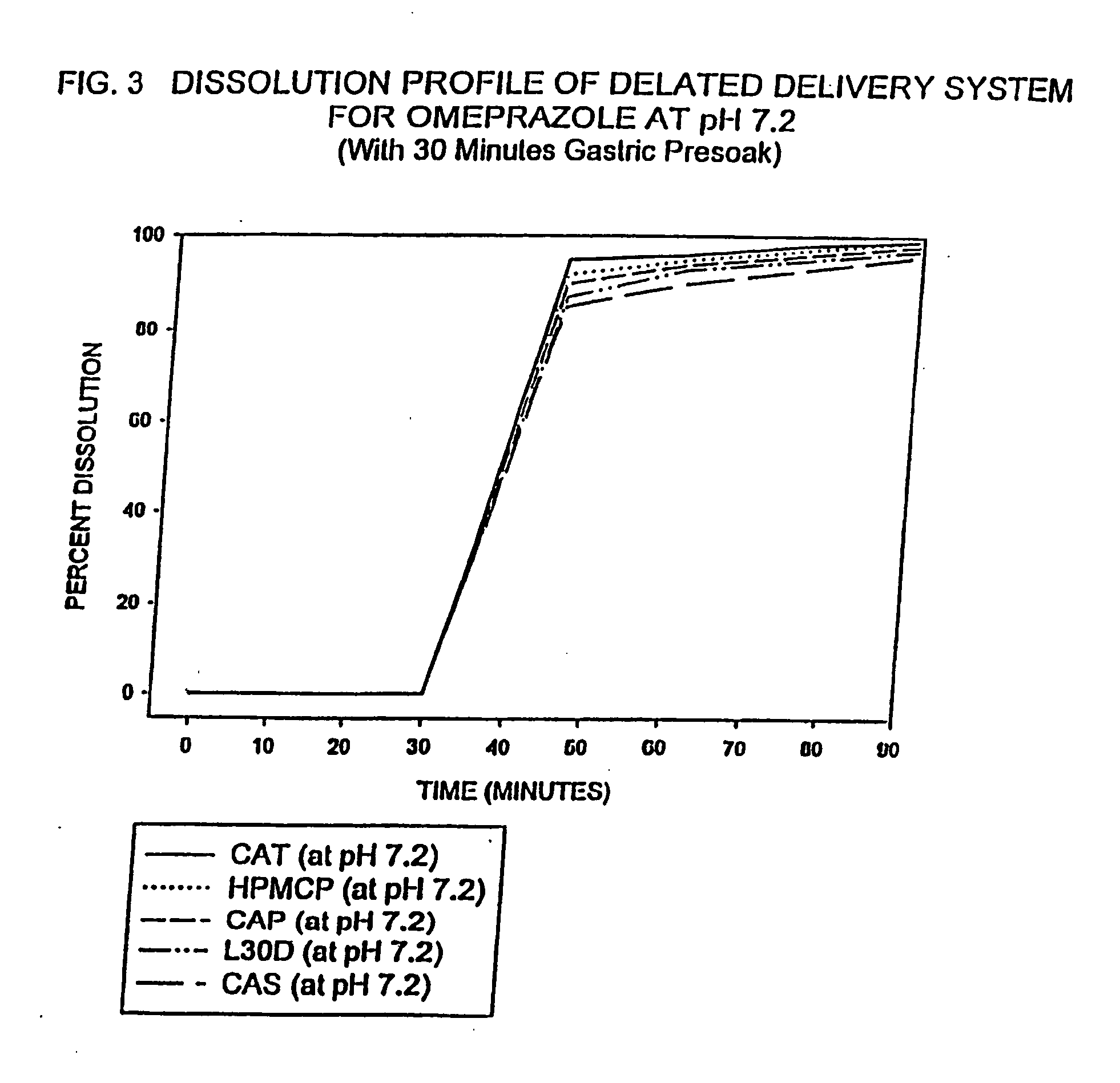
Delayed delivery system for acid-sensitive drugs
InactiveUS20050244497A1High cohesivenessImprove integrityGranular deliveryMicrocapsulesIntestinal fluidGastric fluid
The present invention relates to a delayed release drug delivery system containing omeprazole capable of site-specific delivery and pulsatile (bolus) kinetics for once-a-day dosage comprised of an alkaline core structure sequentially layered with suspensions of omeprazole; a separation barrier; and an enteric barrier. The separation barrier is coated with a pH-dependent enteric membrane, which is relatively insoluble in gastric fluid but rapidly to immediately soluble in intestinal fluid, whereby the drug is released in a pulsatile manner in the proximal segment of the gastrointestinal tract.
Owner:WOCKHARDT LTD
Lactobacillus reuteri and application thereof
ActiveCN111534446APromote growthStrong toleranceAntibacterial agentsAntimycoticsBiotechnologyProbiotic bacterium
The invention relates to the technical field of microorganisms, and particularly provides Lactobacillus reuteri and application thereof. The Lactobacillus reuteri provided by the invention is a canine-derived Lactobacillus reuteri strain which is separated from hindgut content of a healthy adult blue fox for the first time by an inventor, and the preservation number of the Lactobacillus reuteri isCCTCC NO: M 2018943. The strain has good growth performance, is high in tolerance to gastric juice and intestinal juice and good in adhesion to small intestine epithelial cells, can promote proliferation of dog intestinal tract beneficial bacteria, can inhibit growth of harmful bacteria, can enhance the immune function of pet dog organisms, has better effect than that of similar products in the market, can serve as an alternative strain of probiotic preparations, and is quite wide in application prospect.
Owner:SHENYANG BOYANG FEED
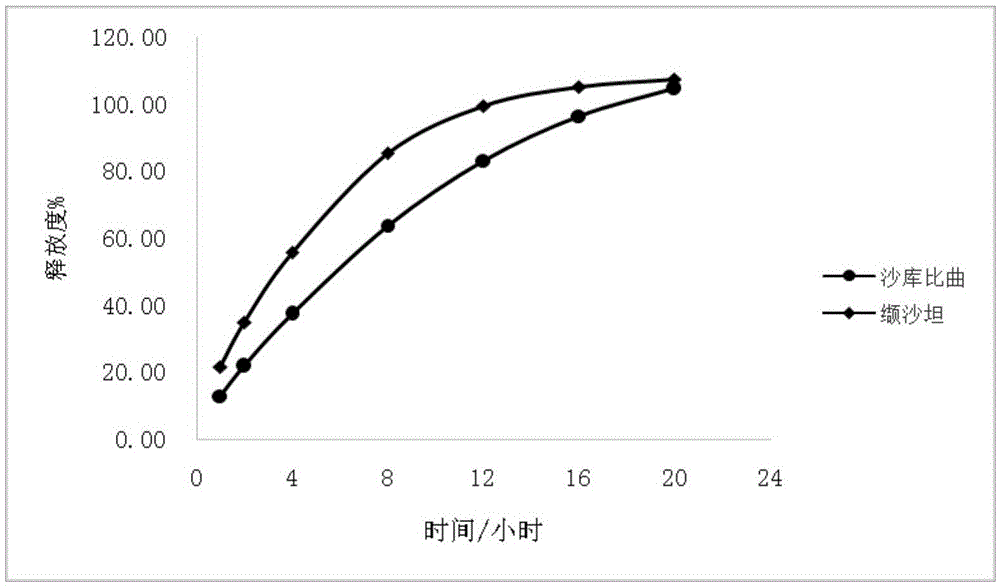
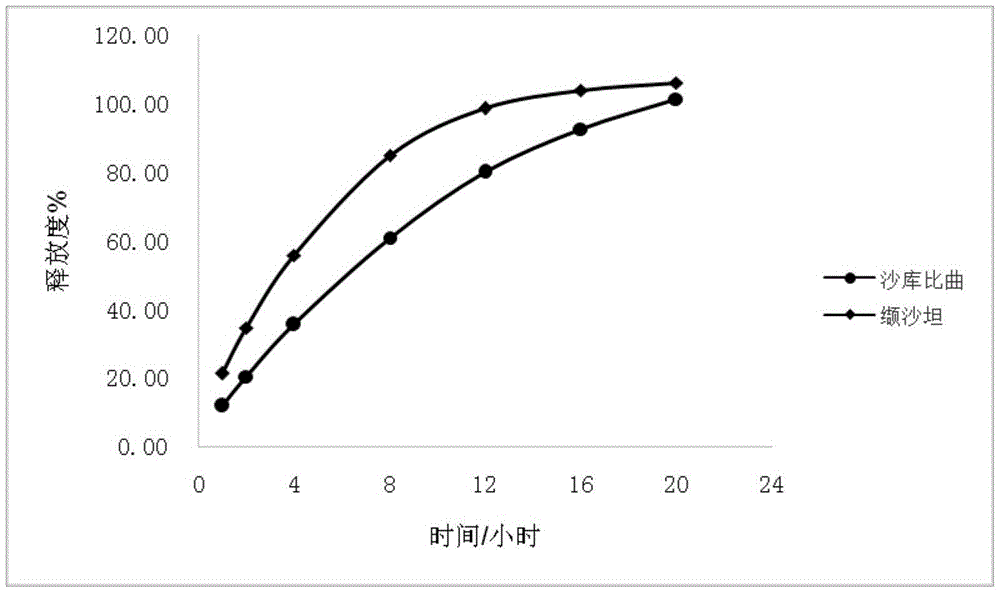
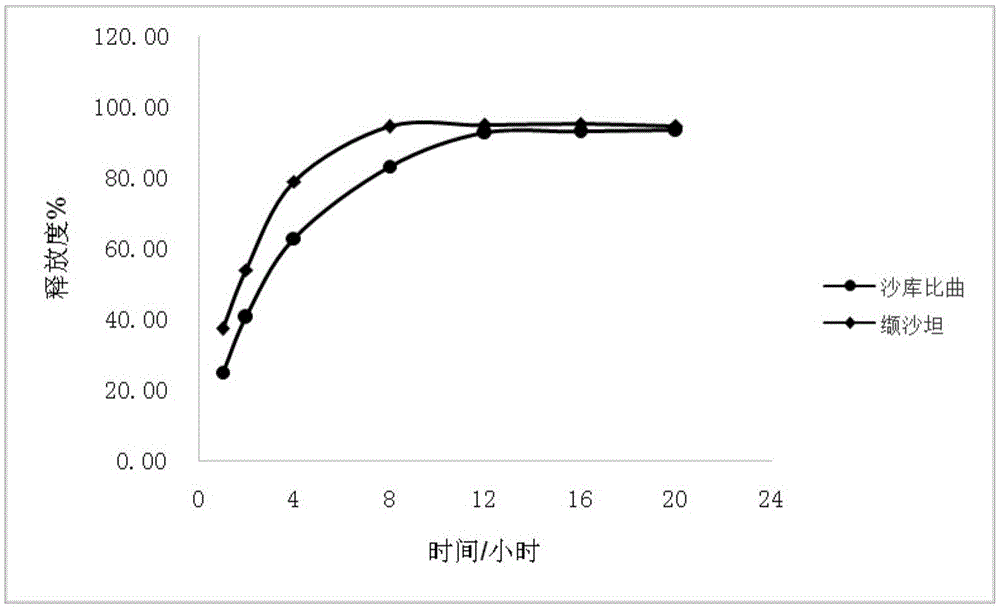
Sacubitril / valsartan sustained release agent and preparation method thereof
ActiveCN105935358ALong duration of actionExtended release characteristicsPharmaceutical non-active ingredientsCoatingsSustained Release TabletIn vitro test
The invention provides a sacubitril / valsartan sustained release agent, including 10wt%-70wt% of sacubitril / valsartan, 10wt%-50wt% of hydrophilic gel framework material, 0-80 wt% of a diluent and 0.1wt%-10wt% of a lubricant, wherein the total content of the above components is 100%. In the study of in vitro releasing rate of intestinal juice, the sacubitril / valsartan sustained release tablet reaches release of sacubitril about 80% to 12 h, release of valsartan about 80% to 8, achieves slow release of the two active ingredients, and shows slow release characteristics in vitro test.
Owner:LIANGJIANG MEDICINE CO LTD
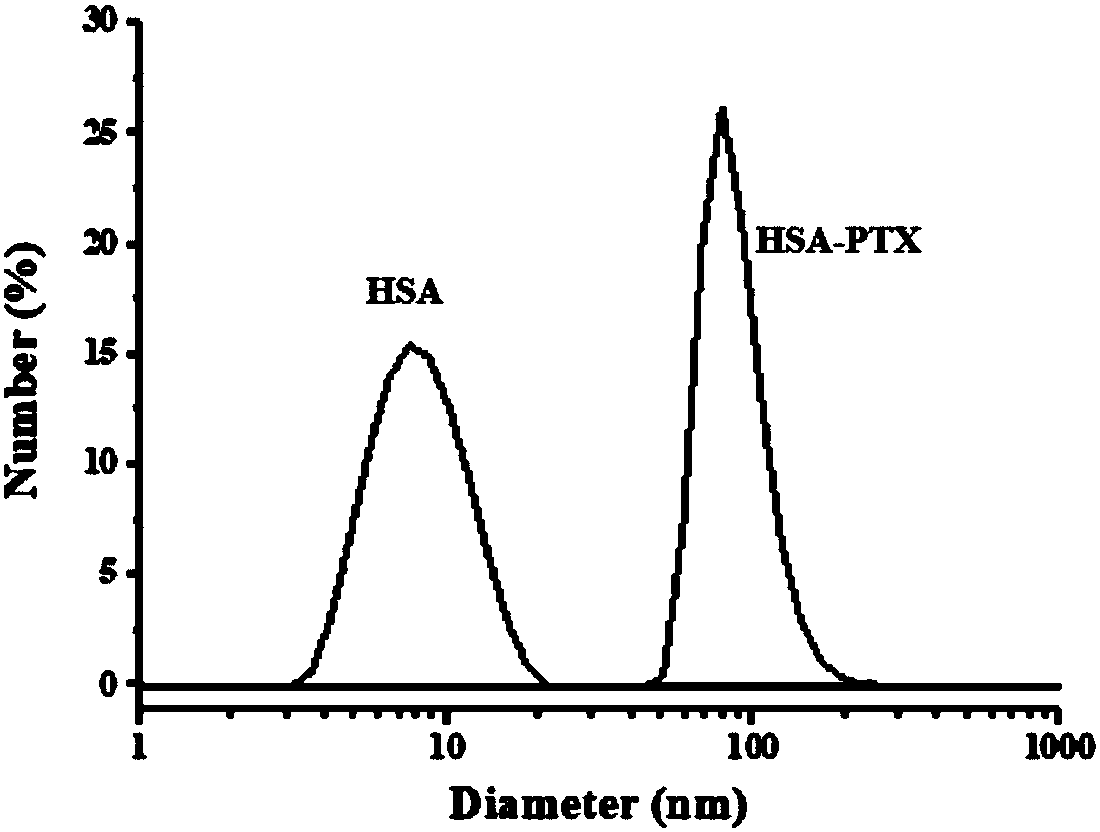
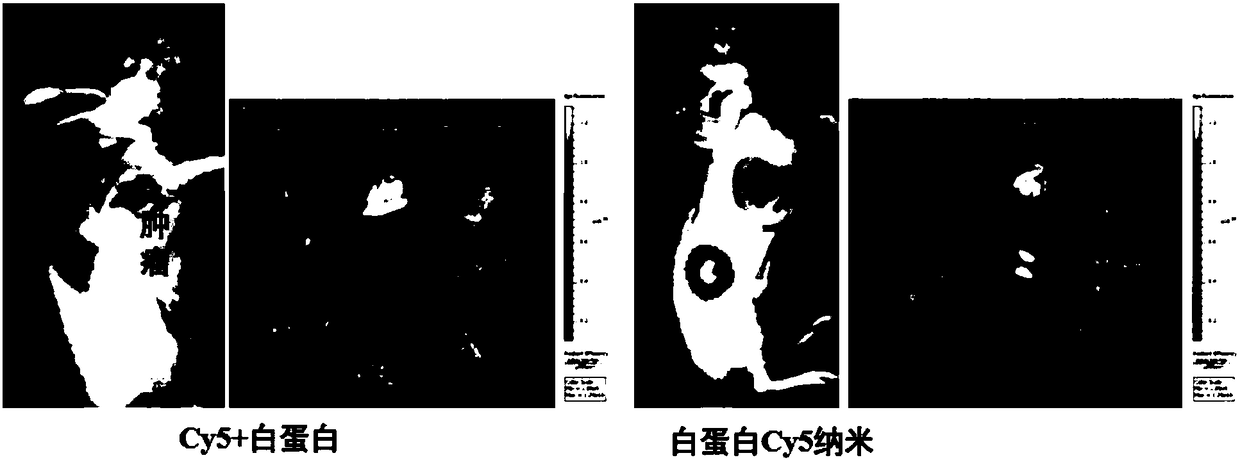
Albumin nanoparticles covering pharmacological active substances as well as preparation method and application thereof
ActiveCN108379228AHave toxic side effectsGood dispersionPowder deliveryMacromolecular non-active ingredientsLipid formationSolubility
The invention belongs to the technical field of biomedicines and discloses albumin nanoparticles covering pharmacological active substances as well as a preparation method and application thereof. Thepreparation method comprises the following steps: opening an inner space structure of albumin under the action of glutathione to form protein containing a sulfydryl group active radical; adding a selenium compound and the pharmacological active substances; obtaining the albumin nanoparticles covering the pharmacological active substances by utilizing intramolecular or intermolecular sulfydryl-selenium-sulfur bond exchange reaction and sulfydryl-disulfide bond exchange reaction and elemental selenium which is loaded at inner and outer parts of a protein cavity. The method has the advantages ofsimplicity in operation; a novel albumin binding type nano-preparation has the advantages of uniform size, good dispersity and long preservation time at room temperature, and good stability in gastric acid, intestinal juice and blood serum; the solubility, dispersity, stability and bioavailability of lipid-soluble medicines are greatly improved. Meanwhile, the albumin nano-preparation keeps the solubility of albumin and a tumor targeting enriching property very well.
Owner:HUNAN UNIV
Enteric microorganism collecting capsule
PendingCN106725634ASimple designEasy to acceptSurgical needlesVaccination/ovulation diagnosticsIntestinal microorganismsMedicine
The invention discloses an enteric microorganism collecting capsule which comprises a capsule wall and a capsule cavity formed in the capsule wall. A liquid flowing channel penetrating through the capsule wall is formed in the capsule wall, and a first enteric assembly is arranged in the liquid flowing channel to close the liquid flowing channel; a piston with a line bolt on one side is arranged in the capsule cavity in the direction perpendicular to the capsule, and the piston is arranged below the liquid flowing channel. A spring is arranged between the side, connected with the line bolt, of the piston and the inner wall of the capsule; a limiting line is connected end to end to form a closed ring, one end of the limiting line is wound around the line bolt, and the other end of the limiting line penetrates through the spring and the capsule wall and is fixed in a second enteric assembly located outside the capsule wall and closely connected with one end of the capsule wall. An enteric microorganism collecting system comprises one or more enteric microorganism collecting capsules. The enteric microorganism collecting capsule is simple in structure, convenient to use and good in intestinal juice collecting effect, fully meets clinical and scientific research needs, and has wide application prospects.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Program-controlled pig bionic digestion system and method using program-controlled pig bionic digestion system to quickly determining digestible energy value of pig feed
ActiveCN110057964AReal Digestion ApproachingConsistent activityChemical methods analysisGastric digestionAnimal science
The invention discloses a program-controlled pig bionic digestion system and a method using the program-controlled pig bionic digestion system to quickly determining a digestible energy value of pig feed. The method comprises the following steps that a feed sample is pulverized and sieved through a standard sieve; a gastric buffer solution and an intestinal buffer solution are prepared; simulatedgastric juice, simulated small intestinal juice and simulated large intestinal juice are prepared; the pulverized feed sample is filled into a simulated digester of the program-controlled pig bionic digestion system, and the simulated gastric juice is added in sequence through computer program control to simulate gastric digestion; after the intestinal buffer solution is added, the simulated smallintestinal juice is added to simulate small intestinal digestion; the simulated large intestinal juice is added to simulate large intestinal digestion; inactivation is carried out after the digestionis completed; and then cleaning is carried out, after undigested residues are obtained, a total energy value of the residues and the feed sample is tested, and the digestible energy value of the feedis calculated. The method is simple, high in precision, low in implementation cost, and the digestible energy value of the pig feed can be accurately determined within 72 hours.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Diarrhea controlling and deodorizing composition for dogs
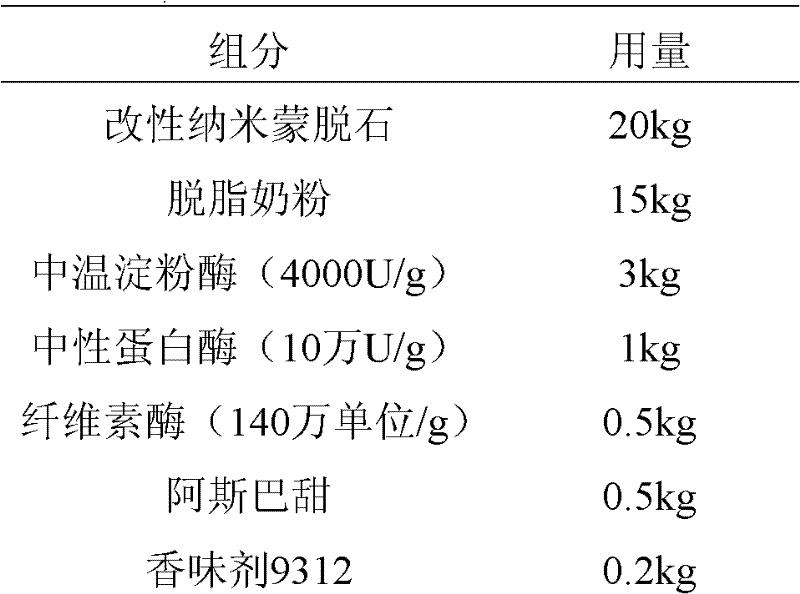
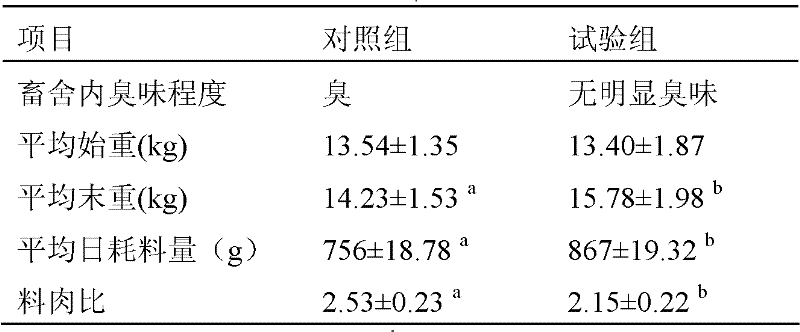
The invention discloses a diarrhea controlling and deodorizing composition for dogs. The composition is mainly composed of multiple-aperture modified nano-montmorillonite, digestive enzyme and a flavoring agent suitable for dogs. The composition can effectively inhibit dietetic diarrhea and seasonal diarrhea of dogs, has the functions of astringing intestinal juice, absorbing mycotoxin and absorbing noxious gas, can increase the nutrition density of dog food, and enhances the immunity and anti-stress ability of dogs.
Owner:TIANJIN RINGPU BIO TECH
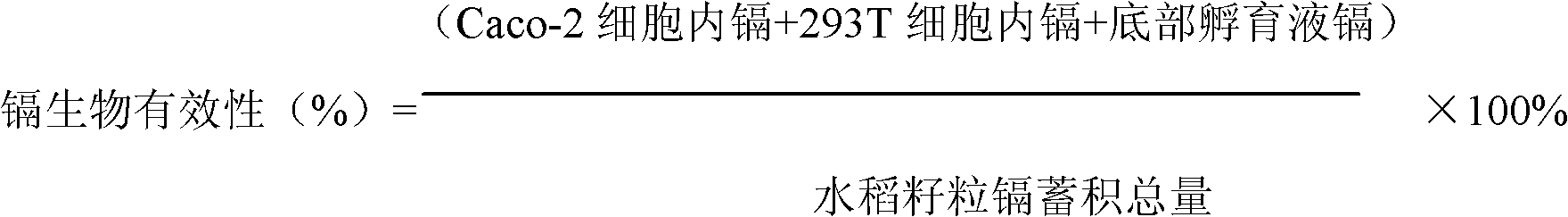
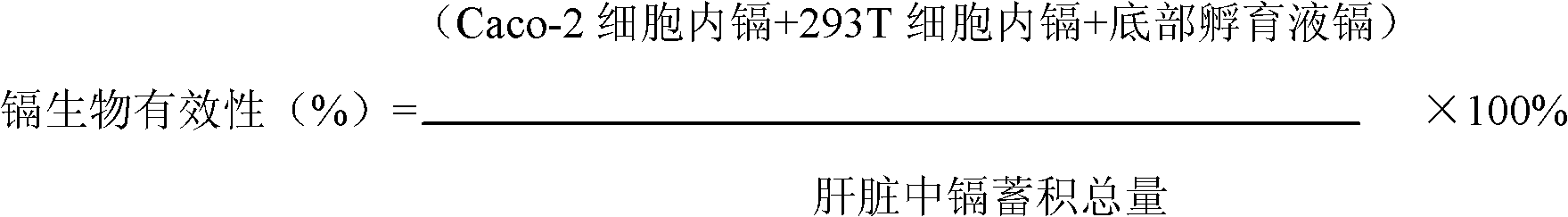
Method for building model for collectively evaluating bioavailability and toxicity of cadmium accumulation in food for human body
InactiveCN102680654AAccurate evaluationThe result is accurateMaterial analysis by electric/magnetic meansTesting foodCell layerChronic toxicity testing
The invention relates to a method for building a model for collectively evaluating bioavailability and toxicity of cadmium accumulation in food for a human body, and the method comprises the following steps of (1) food sample digestion and determination liquid acquiring: a, grinding a food sample after the food sample is collected, and sequentially digesting the food sample by sequentially adopting gastric juice and intestinal juice; and b, centrifuging the digested mixed liquid, collecting supernate to be sterilized through high pressure; (2) independently culturing Caco-2 cells and 293T cells; (3) combined culture of Caco-2 cells and 293T cells: moving an insertion groove with the Caco-2 cells into a hexagonal-porous plate with the 293T cells to be continuously cultured for 24h; (4) evaluation of bioavailability and toxicity of cadmium: placing the food sample determination liquid on a Caco-2 cell layer of a combined culture model, simultaneously adding iso-osmotic incubation liquid on a 293T cell layer, and continuing the culture for 24h; in the chronic toxicity test, maximally collectively culturing the sample for 10d; and (5) detecting indexes. The method has characteristics of simpleness in operation, easiness in controlling test conditions, small pollution, economical efficiency, accurate result and the like, and also has the advantages for collectively evaluating the bioavailability and toxicity. The method is suitable for evaluating the safety of the cadmium accumulation in grains, vegetables and animal food.
Owner:HENAN UNIV OF SCI & TECH
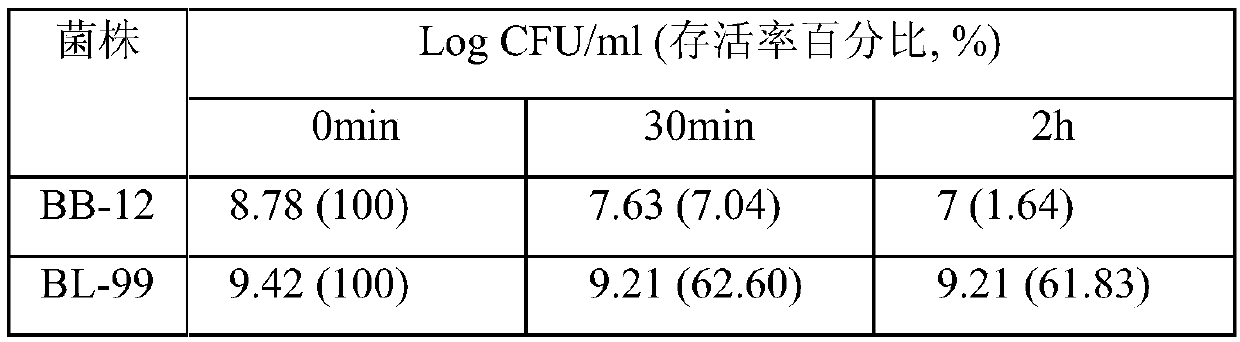
Bifidobacterium lactis bl-99 with function of enhancing immunity and application thereof
ActiveCN110964657AImprove immunityImprove immunity and other problemsBacteriaAnimal feeding stuffBiotechnologyNatural Killer Cell Inhibitory Receptors
The invention provides bifidobacterium lactis BL-99 with a function of enhancing the immunity and application thereof and belongs to the technical field of microbes. The bifidobacterium lactis BL-99 is preserved in the China General Microbiological Culture Collection Center CGMCC on April 26, 2018, has a preservation number of CGMCC No.15650, has high gastric acid tolerance and intestinal juice tolerance, can remarkably increase antibody-producing cells and half value of hemolysin HC50 and activate the activity of NK cells, can be used for preparing foods and the like with a function of enhancing the immunity, and has an extensive application prospect.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
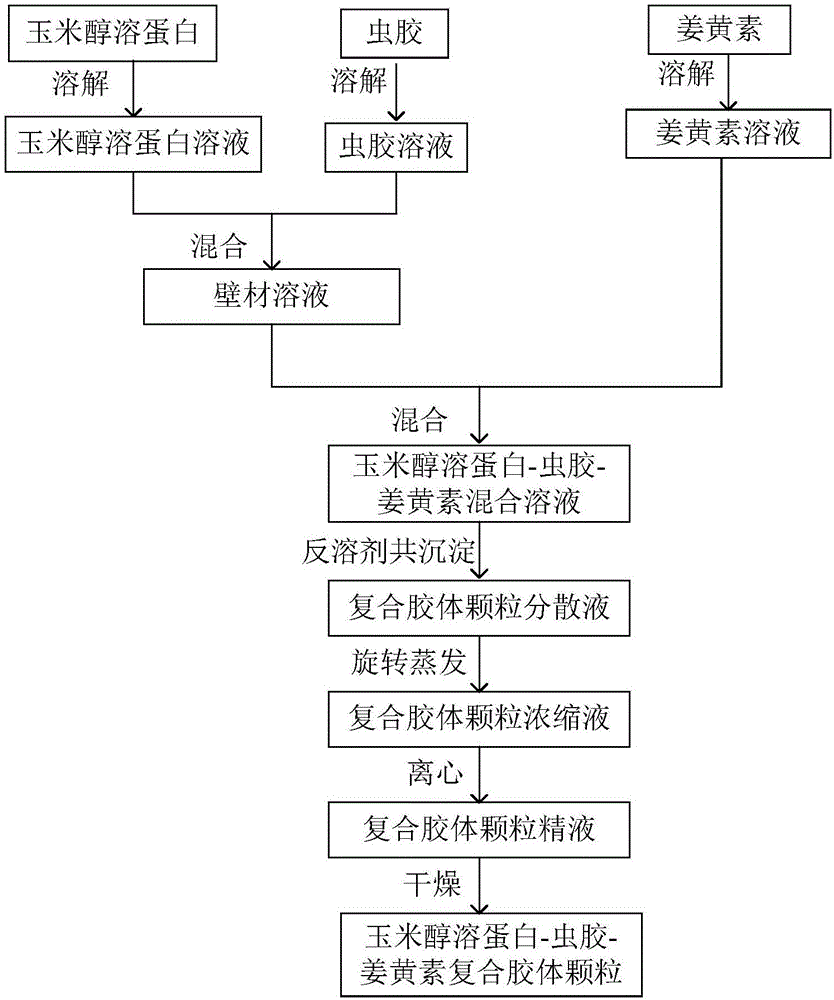
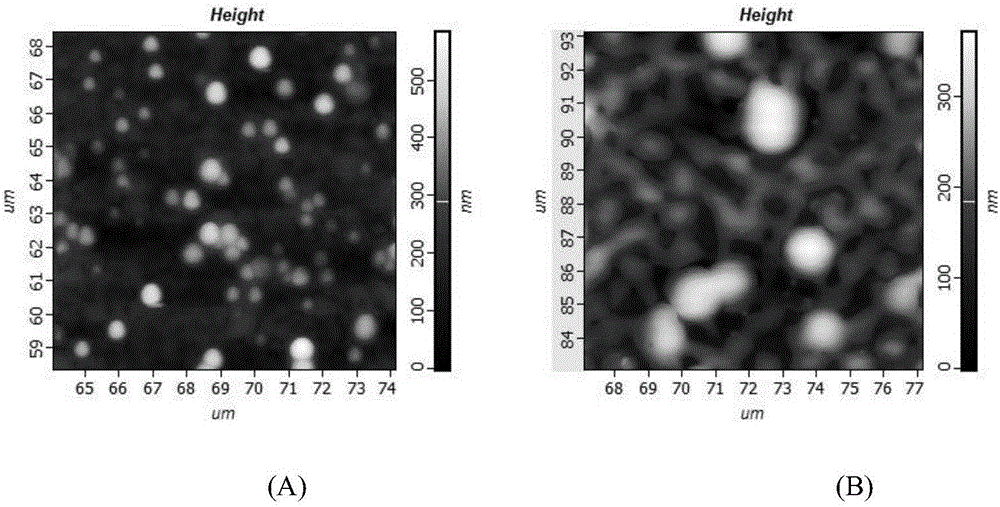
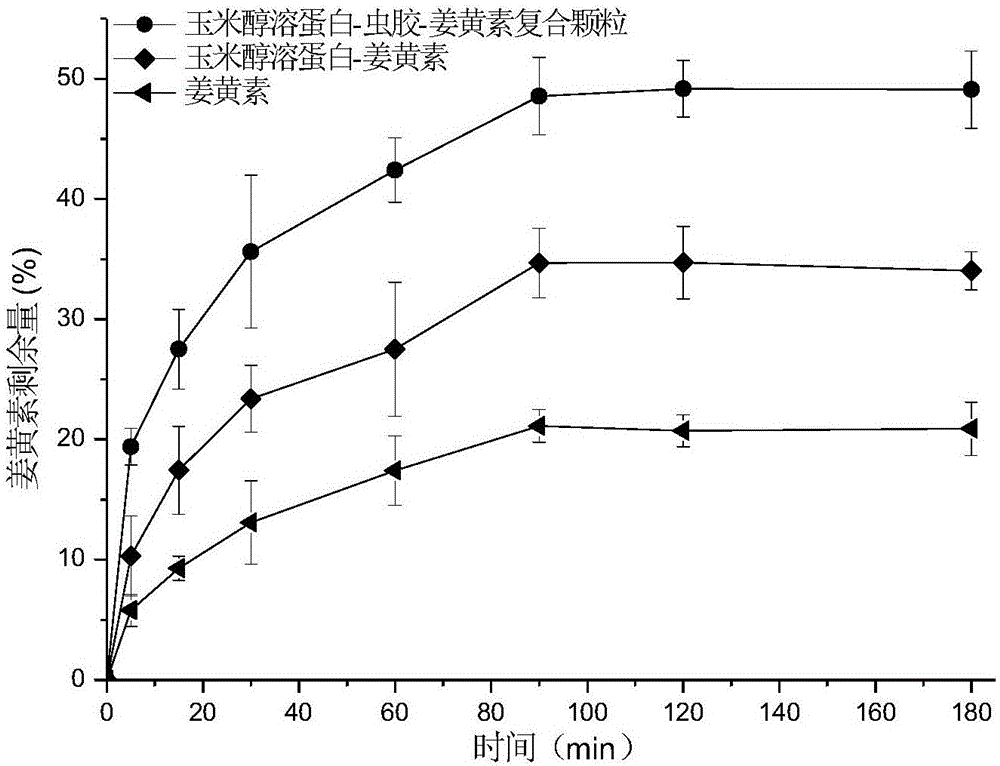
Corn prolamine-shellac-curcumin compound colloidal particles and preparation method thereof
InactiveCN106822035AAntioxidantImprove bioavailabilityAntipyreticAnalgesicsIntestinal fluidAqueous solubility
The invention discloses corn prolamine-shellac-curcumin compound colloidal particles and a preparation method thereof and belongs to the technical field of preparation of the compound colloidal particles. The compound colloidal particles take corn prolamine and shellac as wall materials and take curcumin as a core material, and are prepared by adopting an anti-solvent co-precipitation method; the mass ratio of the corn prolamine to the shellac ranges from (5 to 1) to (1 to 2) and the mass ratio of the wall materials to the curcumin ranges from (10 to 1) to (1 to 2); the corn prolamine, the shellac and the curcumin are combined through hydrogen bonds and a hydrophobic interaction effect. According to the compound colloidal particles provided by the invention, the embedding rate on the curcumin is more than 90 percent; the water solubility of the curcumin is extremely increased and the light degradation and thermal degradation speeds of the curcumin are effectively reduced; the bioavailability of the curcumin is remarkably improved, controlled release of the curcumin is realized to a certain extent and a releasing speed of the curcumin in intestinal fluid is reduced; According to the corn prolamine-shellac-curcumin compound colloidal particles provided by the embodiment of the invention, a new concept and a new way can be provided for a steady state of functional factors.
Owner:CHINA AGRI UNIV
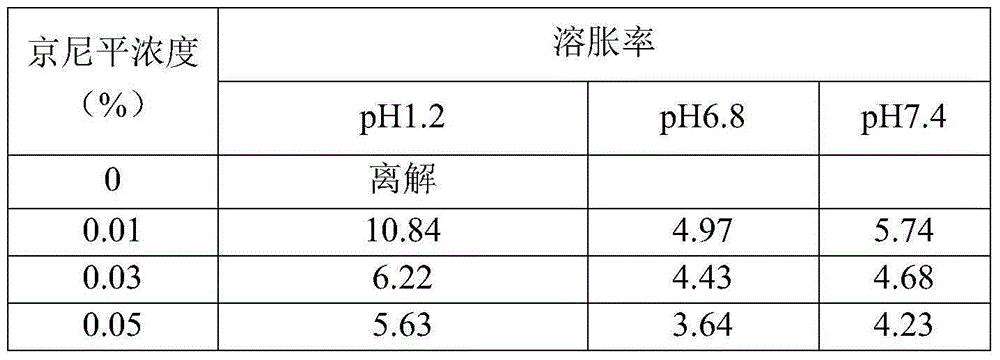
Intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system and preparation method and application thereof
ActiveCN105011343AFree from destructionAchieve targeted releaseFood shapingFood ingredient as encapsulating agentIntestinal fluidCentrifugation
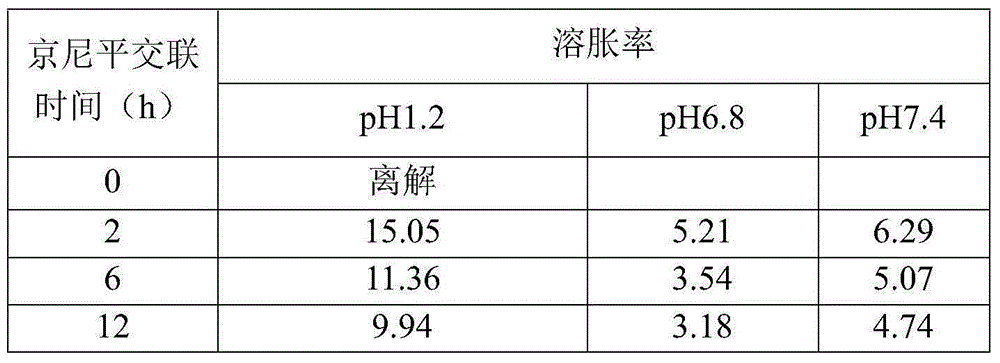
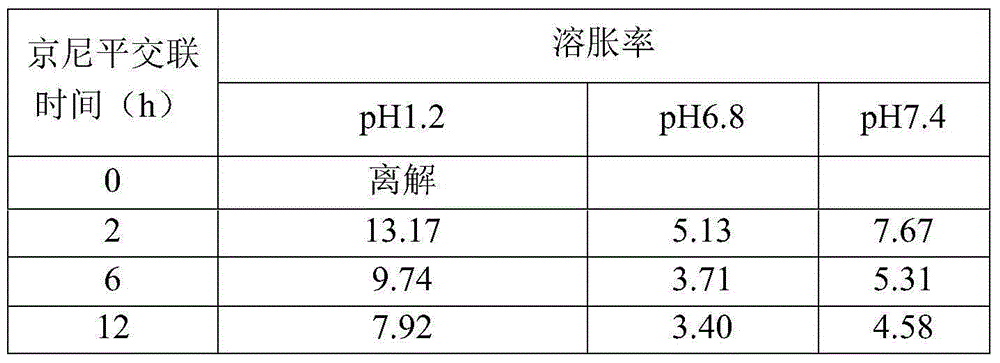
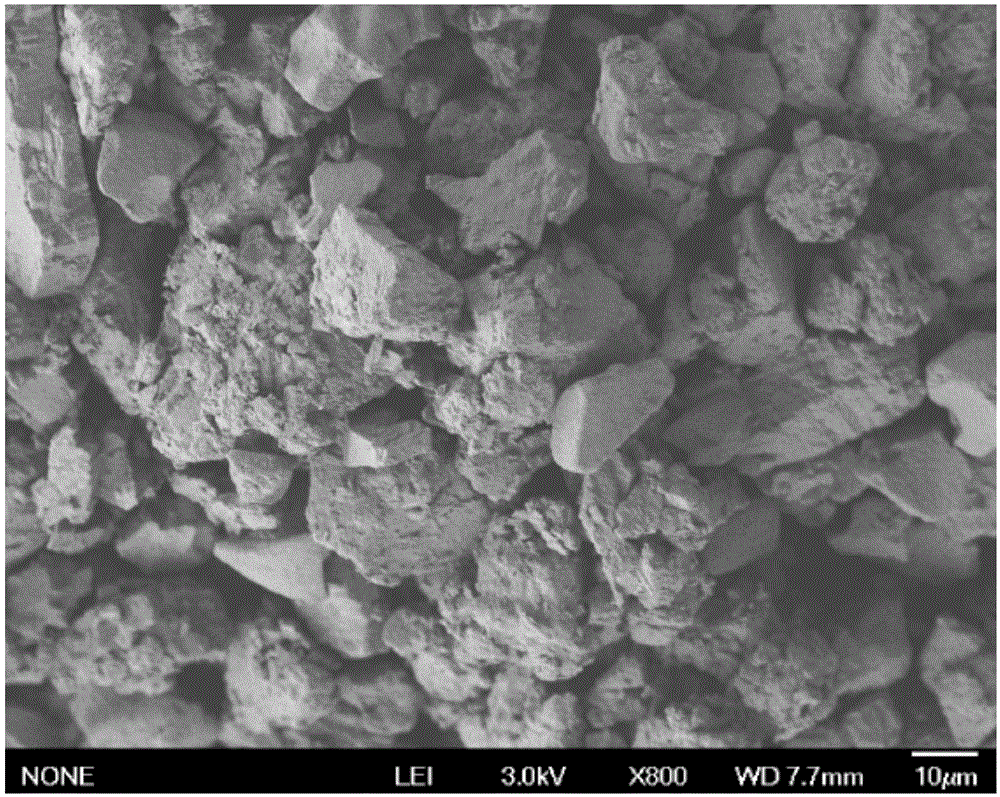
The present invention provides an intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system and a preparation method and an application thereof. Carboxymethyl chitosan and arabic gum are polycations and polyanions to constitute a complex coacervationsystem. By adjusting pH value, the carboxymethyl chitosan and the arabic gum are subjected to complex coacervation reaction, and the complex coacervation phase is collected by centrifugation, crosslinked by genipin, and freeze-dried to obtain the intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system. The intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system can be used either for embedding water-soluble functional components, but also for embedding fat-soluble functional components, can protect core materials against damage caused by strongly acidic gastric environment, safely delivery the core materials to the intestinal tracts to conduct targeted releases, and expand the practical application field of the complex coacervation microencapsulation technology. The intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system does not dissociate in a simulated gastric fluid and swells in a simulated intestinal fluid, and has a strong stability, and the process is simple, safe, efficient, and easy for mass production.
Owner:QINGDAO AGRI UNIV
Method for preparing lutein feed additive
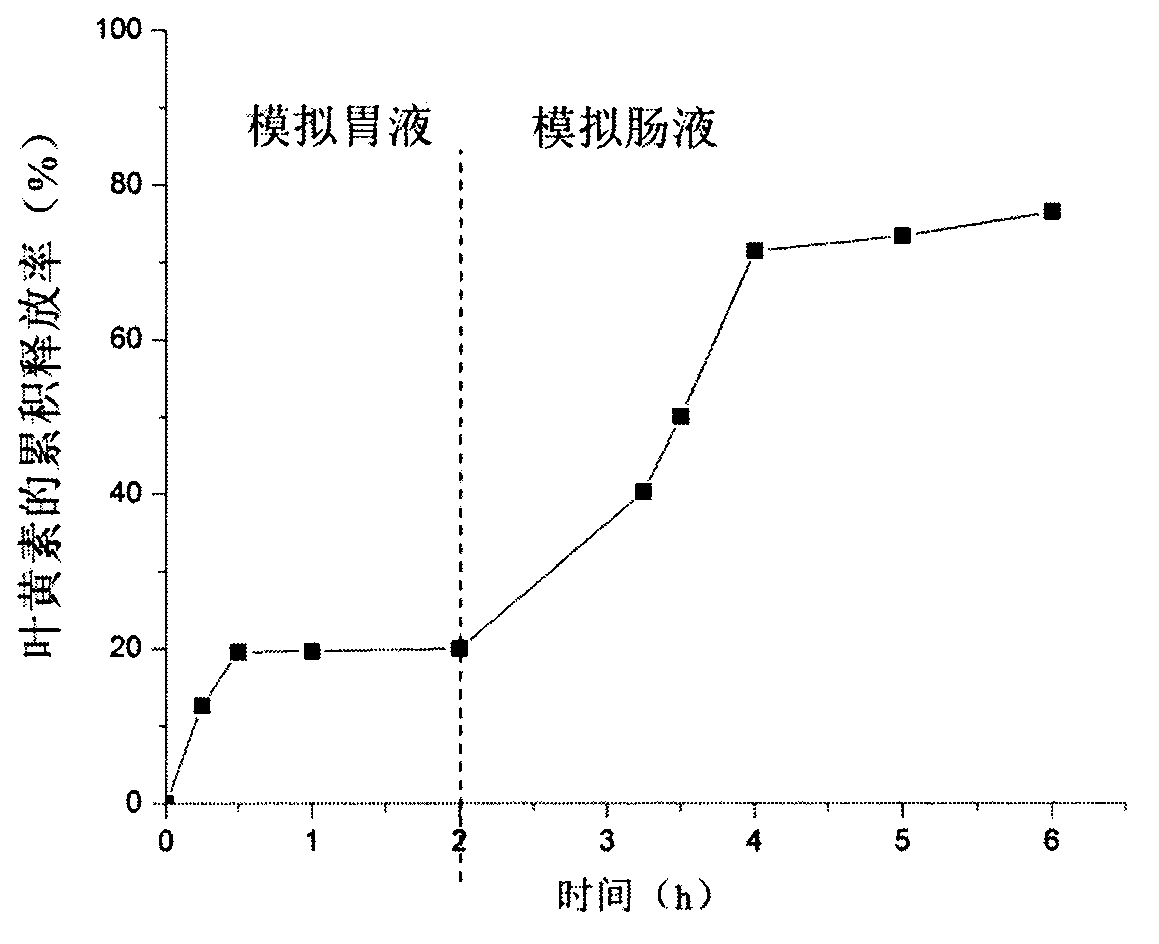
ActiveCN105053556APoor chemical stabilityGood chemical stabilityOrganic chemistryAnimal feeding stuffControlled releaseGlycerol
Owner:ZHEJIANG NEW WEIPU ADDITIVE +3
Method for microencapsulating cholesterol-degrading lactic acid bacteria
InactiveCN101724589ALow priceImprove acid resistanceBacteriaMetabolism disorderAcid-fastNon toxicity
The invention discloses a method for microencapsulating cholesterol-degrading lactic acid bacteria, which comprises the steps of the activation of the cholesterol-degrading lactic acid bacteria, the preparation of immobilized cholesterol-degrading lactic acid bacteria, the preparation of microcapsules by a W / O / W multiphase emulsion method, and freeze-drying. The cholesterol-degrading lactic acid bacteria microcapsules prepared by adopting the multiphase emulsion method (W / O / W) have the characteristics of stability, non-toxicity and the like due to the fact that wall materials thereof all belong to natural polymer materials; the release rate of the microcapsules in intestinal juice within 2h is over 87.4 percent, and the viable count can reach 7.03*109cfu / mL; the viable count can still reach 6.81*106cfu / mL after the microcapsules are placed for 3h at the temperature of 50 DEG C, and can still reach 6*108cfu / mL after the microcapsules are stored for 30d; in vitro cholesterol degradation tests, the degradation rate of cholesterol is over 36.07 percent; and the microcapsules have better acid resistance, enteric property, high temperature resistance and cholesterol degradation, and the yield is about 86.62 percent. Capsule materials and carrier materials have low cost, so the cost is reduced.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Xanthophylls nano dispersion liquid with control release property as well as preparation method
InactiveCN103892165AAchieve protectionSimple ingredientsFood preparationUltra high pressureFreeze-drying
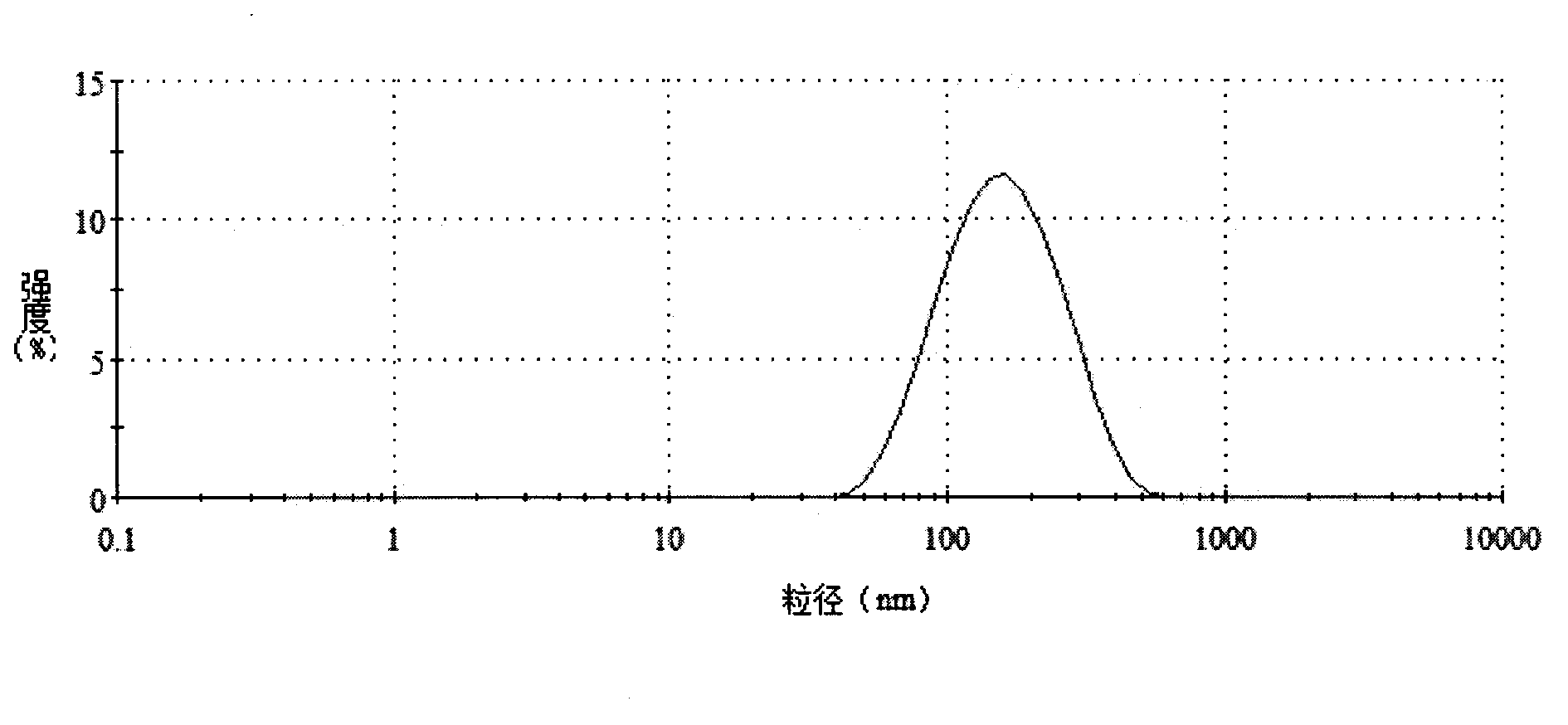
The invention belongs to the technical field of functional healthcare food, and discloses a xanthophylls nano dispersion liquid with a control release property. The preparation method of the xanthophylls nano dispersion liquid with the control release property comprises the following steps: dissolving casein-glucan copolymer into water, then rapidly injecting a water phase in an ethyl alcohol phase (in which xanthophylls is dissolved) in stirring by adopting an injection method, removing ethyl alcohol in a system by adopting a rotary evaporation method, carrying out ultra-high-pressure homogenization treatment so as to obtain the xanthophylls nano dispersion liquid, and carrying out freeze-drying to obtain powder with good dispersion. The nano dispersion liquid prepared by the invention has high xanthophylls encapsulation efficiency higher than 90%, has the average particle size of 110-150nm, and the xanthophylls is at the amorphous state. According to the invention, the bioavailability of the xanthophylls is improved, and the xanthophylls nano dispersion liquid as a xanthophylls supplement can be widely applied to industries such as of food industry and health care product industry. The xanthophylls nano dispersion liquid with the control release property and the preparation method thereof have the characteristics that directional grafted copolymer of casein and glucan acts as a wall material, so that the aims of controlling the release of xanthophylls in gastrointestinal tracts, uniformly dispersing in intestinal juice and improving the bioavailability are achieved.
Owner:JIANGNAN UNIV
Levofloxacin hydrochloride tablet and preparation method thereof
ActiveCN103520124AReduce alkalinityGuaranteed not to precipitateAntibacterial agentsOrganic active ingredientsAlkalinityAlcohol
The invention discloses a levofloxacin hydrochloride tablet and a preparation method of the levofloxacin hydrochloride tablet. The preparation is prepared by blending and tabletting medicine-containing particles, citric acidenteric particles and a lubricant, wherein the citric acidenteric particles is prepared by dissolving the citric acid and the polyvinyl acetatephthalic acid ester in ethyl alcohol. According to the invention, the citric acid is wrapped in an enteric material and is not dissolved in the stomach, the citric acid is dissolved and released in the intestines, so that the alkalinity of the intestinal juice is reduced, the levofloxacin hydrochloride is enabled not to be separated out, and therefore, the bioavailability of the medicine is improved.
Owner:NANJING REDWOOD FINE CHEM CO LTD
Famciclovir slow-releasing capsule for anti-virus treatment and its producing method
ActiveCN1714793ASlow blood concentrationBlood levels rise slowlyDigestive systemPharmaceutical delivery mechanismAnti virusDrug content
The present invention relates to a kind of slow-releasing famciclovir capsule for antiviral treatment, and features that the slow-releasing famciclovir capsule is prepared through coating famciclovir medicine with supplementary material to form the pellet core, coating the pellet core with inner release controlling layer of water insoluble polymer, re-coating with outer release controlling layer of enteric polymer to form release controlling famciclovir pellet, and encapsulating famciclovir pellet to form the slow-releasing famciclovir capsule. In artificial gastric juice and artificial intestinal juice, the slow-releasing famciclovir capsule has its medicine releasing degree of 10-30 %, 30-70 % and over 70 % separately in 1.5 hr, 3 hr and 8 hr. The pellet is measured to have medicine content of 56.8-65.2 %.
Owner:LIVZON PHARM GRP INC
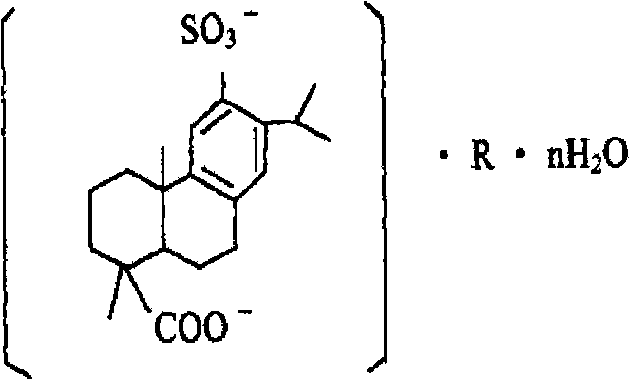
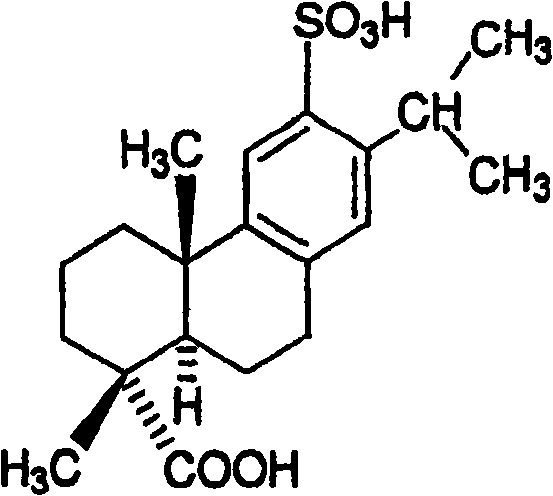
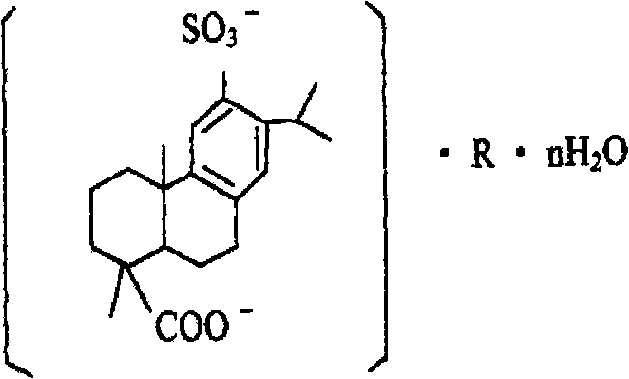
Disodium sulfodehydroabietate (DSDA) and composition and application thereof
ActiveCN101590031AGood curative effectQuick resultsOrganic active ingredientsDigestive systemDiseaseIntestinal structure
The invention discloses disodium sulfodehydroabietate (DSDA) and a composition, a preparation method and application thereof in medicaments for preventing hyperacid peptic ulcer and gastrointestinal inflammation. A patent application disclosed by CN00818150.0 is of the opinion that monosodium sulfodehydroabietate is superior to disodium salt, but does not further disclose test conditions and data conclusion; moreover, the pharmacological action and clinical application of the disodium salt deserve further research. The invention compares the in-vitro acid making effects of monosodium salt and the disodium salt, the dissolution of the monosodium salt and the disodium salt in artificial gastric juice and intestinal juice as well as the clinical efficacies in treating gastric ulcer, acute gastritis and acute attack of chronic gastritis. Results show that DSDA has the advantages that DSDA has stronger acid-making capability during treating the gastric ulcer, acute gastritis and acute attack of chronic gastritis and faster dissolution speed in stomach and intestine; and medicaments prepared from the DSDA have better curative effect and quick response.
Owner:ZHEJIANG ASEN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com