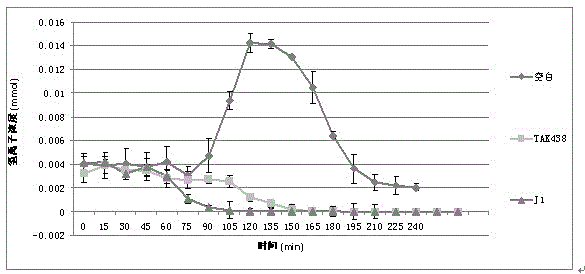
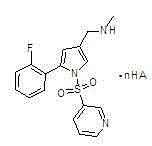
Water soluble salts of organic acid 5-(2-fluorophenyl)-N-methyl-1-(3-pyridyl sulfonyl)-1H-pyrrole-3-methylamine and injection and preparation method thereof
A technology of pyridylsulfonyl and organic acid salts, which is applied in the fields of medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc. It can solve problems related to poor water solubility, inability to meet medical needs for swallowing, restriction of acid suppression, and treatment of gastric acid Diseases and other problems, to achieve the effect of rapid onset of action, improvement of absorption kinetic parameters and bioavailability, and rapid oral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine pyroglutamate
[0021] Put 6.0 g (17.3 mmol) of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine into a 100 mL three-necked flask, and add Ethyl acetate 15 mL, isopropanol 15 mL, stirred at room temperature to dissolve. Heat the mixture to an internal temperature of 50-60 o C, add 2.24 g (17.3 mmol) of L-pyroglutamic acid. Inner temperature 50-60 o After stirring at C for 30 minutes, 15 mL of ethyl acetate was added dropwise, and the inner temperature was 50-60 o C and stirred for another 30 minutes. The mixture was cooled to room temperature and stirred in an ice bath for 1 hour. Filter, wash the filter cake with 10 mL of ethyl acetate:isopropanol (2:1), and 10 mL of ethyl acetate, respectively. Collect filter cake, reduce pressure, 45 o C was dried to give crude product 6.5 g.
[0022] Refining: Put 6.5 g of the above crude product into a 100 mL three-necked f...
Embodiment 2
[0025] 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine lactate
[0026] Put 7.6 g (22.0 mmol) of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine in a 100 mL three-necked flask, and add Ethyl acetate 30 mL, stirred at room temperature to dissolve. Heat the mixture to an internal temperature of 50-60 o C, add 1.98 g (22.0 mmol) of L-lactic acid. Inner temperature 50-60 o C was stirred for 1 hour. The mixture was cooled to room temperature and stirred for 2 hours in an ice bath. Filter and wash the filter cake with 10 mL of ethyl acetate. Collect filter cake, reduce pressure, 45 o C was dried to give crude product 6.3 g.
[0027]Refining: put 6.3 g of the above crude product into a 100 mL three-necked flask, and add 20 mL of ethanol. Stir and heat the mixture to an internal temperature of 60-70 o C was dissolved, and 190 mg of activated carbon was added. Bring the mixture to an internal temperature of 60-70 o C was...
Embodiment 3
[0030] 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylaminoethanesulfonate
[0031] Put 1.3 g (3.76 mmol) of 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine in a 25 mL three-necked flask, and add Ethyl acetate 6 mL, stirred at room temperature to dissolve. Heat the mixture to an internal temperature of 50-60 o C, add 0.41 g (3.76 mmol) of ethanesulfonic acid. Inner temperature 50-60 o C was stirred for 1 hour. The mixture was cooled to room temperature and stirred for 2 hours in an ice bath. Filter and wash the filter cake with 6 mL of ethyl acetate. Collect filter cake, reduce pressure, 45 o C was dried to obtain 0.79 g of the product, with a yield of 46.2%.
[0032] 1 H-NMR (DMSO-d 6 , 400MHz) δ (ppm): 8.91-8.90 (d, 1H), 8.71 (s, 2H), 8.58 (s, 1H), 7.90-7.89 (d, 1H), 7.82 (s, 1H), 7.66-7.63 (dd, 1H), 7.57-7.52 (q, 1H), 7.26-7.23 (t, 2H), 7.12-7.10 (t, 1H), 6.52 (s, 1H), 4.03 (s, 2H), 2.57 (s , 3H), 2.42-2.38 (q, 2H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com