Preparation and application of novel oral insulin nanoparticles
A kind of use, amino acid technology, applied in the field of new-type specific derivative oral nanoparticles and its preparation, can solve the problems of insulin dependence, pain and inconvenience, easy to cause infection and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
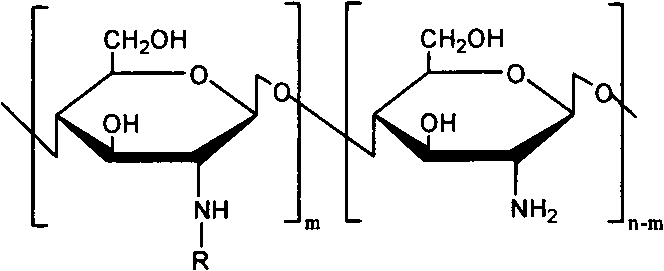
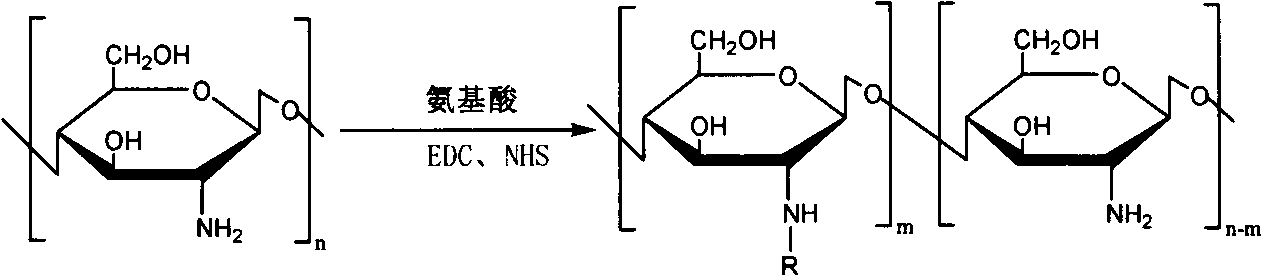
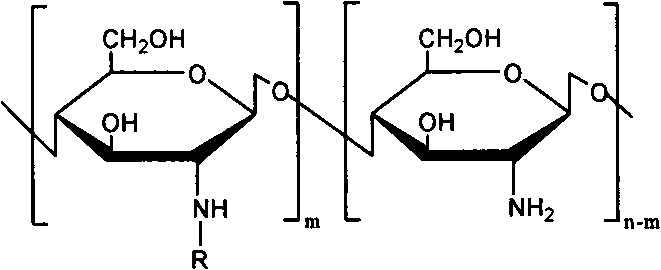
[0027] The preparation of embodiment 1N-arginine chitosan
[0028] Get chitosan (Mw 5000, 10000, 100000, 200000, 400000) 1g and add in 100ml 1% acetic acid aqueous solution, stir at room temperature, add a certain amount of arginine (2.92g), NHS (1.94g) and EDC (7.8 g), adjust the pH to 5.5 with acetic acid, react for 48 hours, dialyze with a dialysis bag (MWCO 3500), filter, freeze-dry, and obtain 0.86 g of milky white floc after drying, that is, N-arginine chitosan.
[0029] FTIR: 1620, 1129cm -1 (arginine)
[0030] The degree of deacetylation measured by elemental analysis was 88%, and the degree of substitution of arginine was 11%.
Embodiment 2
[0031] Embodiment 2 Preparation and particle size determination of N-arginine chitosan nanoparticles comprising insulin
[0032] Take 10 mg of N-arginine chitosan, dissolve it in 5 mL of water, and adjust its pH to 5.5 with 0.1 M HCl. Weigh 5mg of insulin, dissolve it in 5mL of 0.01M HCl, and adjust its pH to 8.0 with 0.1M NaOH. Get each 2mL of N-arginine chitosan aqueous solution and insulin solution, mix, stir at room temperature 20min, product solution has blue opalescence, measures nanoparticle particle size with Zetasizer 3000HS particle size analyzer (Malvern Instruments, Malvern, UK) is 120-200nm.
Embodiment 3
[0033] Example 3 N-arginine chitosan promotes the experiment of gastrointestinal absorption of insulin
[0034] After weighing 15 Wistar rats, 70mg·kg -1 The dose weighed STZ (streptozotocin), with 0.7ml citric acid buffer (0.1mol L -1 , pH4.4) dissolved, and immediately intraperitoneally injected into rats. After feeding for 3 days, blood sugar was measured, and the blood sugar value was higher than 16.67mmol·L -1 Rats can continue the experiment. Experimental animals were randomly divided into 3 groups, and fasted for 12 hours before administration, without water. The first group is the insulin control group, which is given intragastric insulin solution (30IU / kg); the second group is the N-arginine chitosan group (the degree of substitution of arginine is 11%), which is given intragastric insulin N-arginine chitosan nanoparticle solution (30IU / kg); the third group is subcutaneous injection group, given subcutaneous injection of insulin solution (2IU / kg). Blood was colle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com