Controlled release dosing medicine core composition and osmotic pump preparation comprising medicine core composition
A technology of composition and osmotic pressure enhancer, which is applied in the directions of drug delivery, medical preparations of inactive ingredients, and pill delivery, etc., can solve the problems of low solubility of low water-soluble drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1( test example 1)
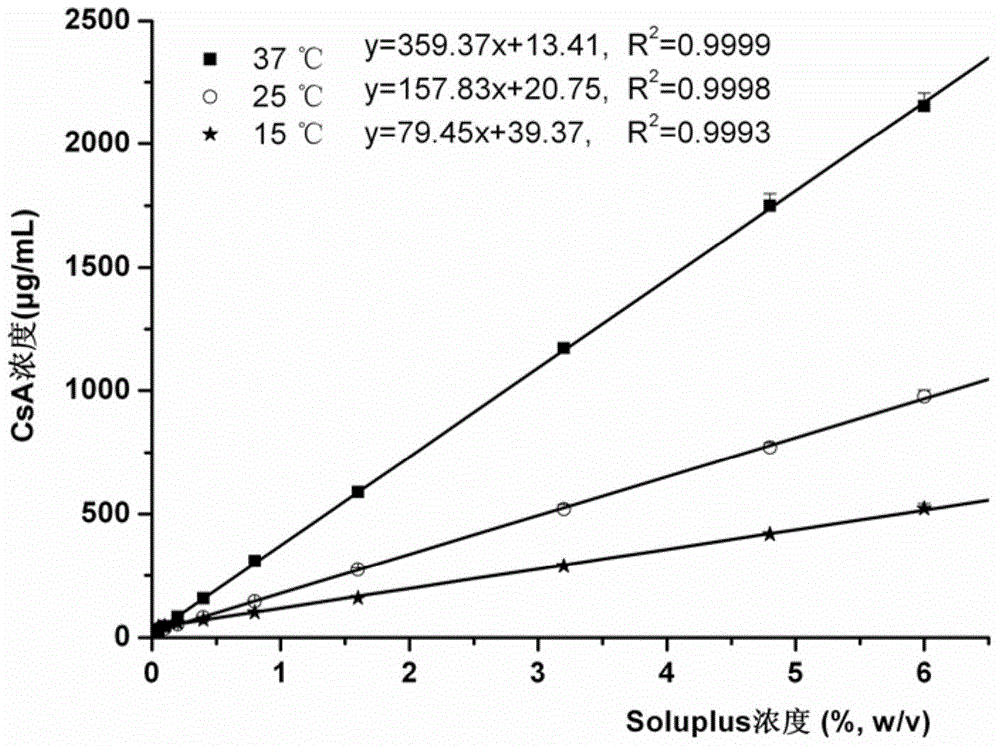
[0047] Example 1 (Test Example 1): Determination of Phase Equilibrium Solubility
[0048] The solubility of cyclosporin A (CsA) in different concentrations of Soluplus aqueous solution was determined by shaking table method. Prepare a series of Soluplus solutions with different concentrations, take 10mL of the solution, put it in a 15mL glass test tube, and then add the excess drug. The samples were equilibrated for a sufficient period of time (48 h) in a preheated shaker with temperature settings of 15, 25 and 37 °C and a rotation speed of 100 rpm. The balanced sample solution was filtered with a mixed cellulose membrane to discard the initial filtrate, and the subsequent filtrate was diluted with pure methanol to an appropriate multiple, and 20 μL was taken and injected into a high-performance liquid chromatograph, and the peak area was recorded to calculate the drug concentration.
[0049] The phase equilibrium solubility of cyclosporine A in aqueous solutions of the amphi...
Embodiment 2( test example 2)
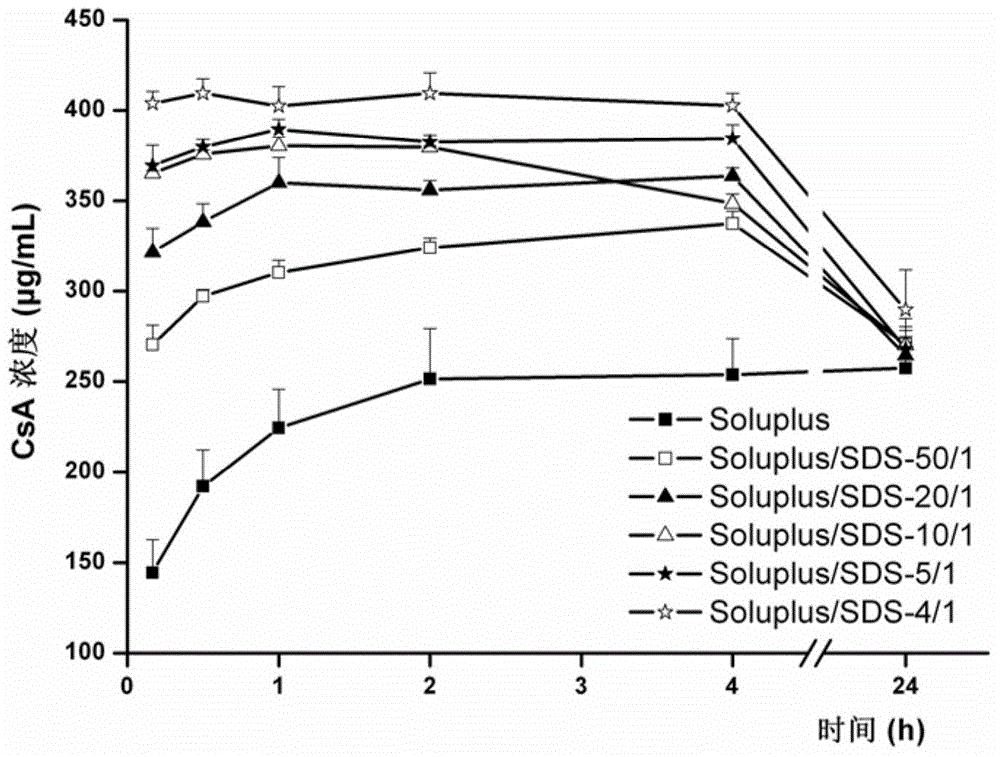
[0050] Example 2 (Test Example 2): Effect of Sodium Dodecyl Sulfate (SDS) on the Soluplus Solubilizing Drug Ability
[0051] Fix the concentration of Soluplus (0.04%) unchanged, add different amounts of SDS, and investigate the effects of Soluplus / SDS (w / w) ratios of 50 / 1, 20 / 1, 10 / 1, 5 / 1 and 4 / 1 on the drug The effect of solubilization ability. Among them, SDS aqueous solution alone did not increase the equilibrium solubility of the drug at the maximum concentration used in this study (0.01%). Fill 100mL of different media into the glass cups respectively, at 37°C, with a mechanical paddle speed of 100rpm, and add DMSO solution equivalent to 1mL of CsA (the concentration of CsA is 100mg / mL) into each cup. Take 2 mL of the sample solution at 0.25, 1, 2, 4 and 24 hours respectively, quickly filter it with a disposable organic phase needle filter, discard 1 mL of the initial filtrate, take the subsequent filtrate and add pure acetonitrile to dilute an appropriate multiple, and ...
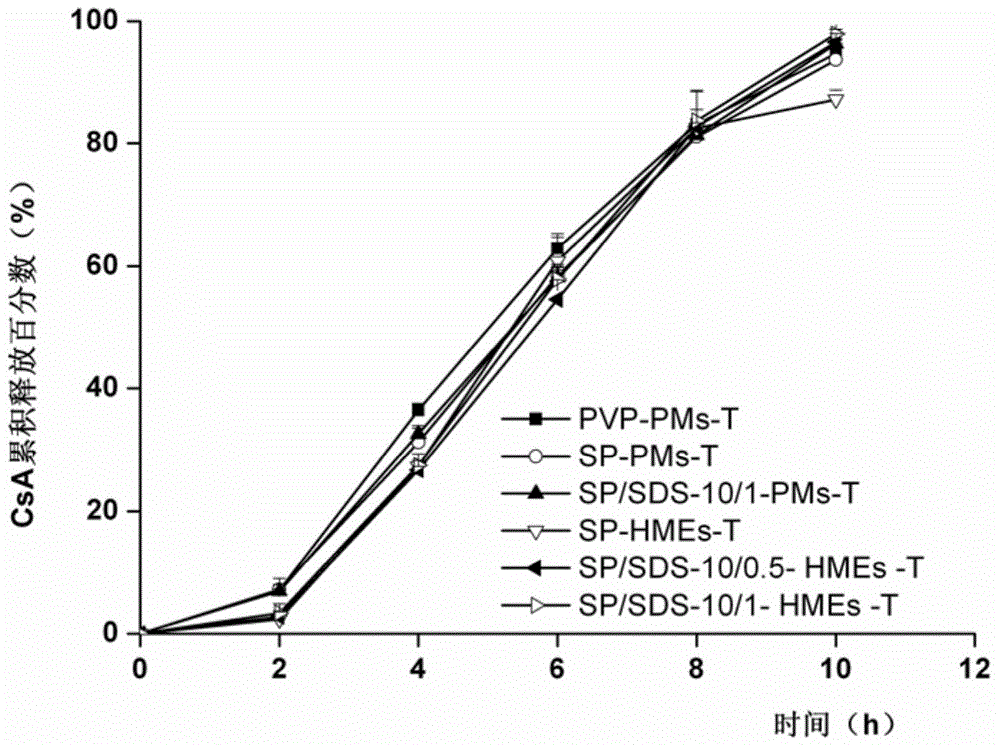
Embodiment 3
[0053] Example 3 (comparative example): prescription and preparation process of ordinary push-pull cyclosporin A-loaded osmotic pump tablets (PVP-PMs-T)
[0054] (1) chip
[0055]
[0056] The drug-containing layer is directly mixed with powder, and the CsA, PVP30, micropowder silica gel and PolyoxWSR N80 are mixed evenly according to the prescription amount by an equal-volume incremental method to obtain the drug-containing layer.
[0057]
[0058] The booster layer is granulated. Process: After mixing PEO, HPMC, NaCL, PVP K30 and iron red through a 60-mesh sieve evenly according to the prescription amount, add 95% ethanol liquid to make a soft material with a 24-mesh sieve. Granules, dried at 45°C for 2 hours, sieved with a 22-mesh sieve, then added magnesium stearate, mixed evenly, and set aside.
[0059] A 10.5# die is used for tableting, and the drug-containing layer and the booster layer are pressed into a double-layer tablet core, and the tableting pressure is be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com