Doxazosin-mesylate controlled-releasing tablet and preparation method thereof
A technology of doxazosin mesylate and controlled-release tablets is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc. application, increasing the difficulty of quality control, restricting large-scale production and other issues, to meet the needs of clinical medication, overcome technical difficulties, and reduce side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Tablet core prescription
[0040] Composition g / 1000 tablets
[0041] Doxazosin mesylate 4.86
[0042] Tartaric acid 30
[0043] Sodium dodecyl sulfonate 5
[0044]Lactose 70
[0045] Mannitol 70
[0046] Polyethylene oxide (200,000 molecular weight) 3
[0047] Gum Arabic 17
[0048] Polyethylene oxide (5 million molecular weight) 5
[0049] Dextrin 44
[0051] (2) Preparation method
[0052] Raw and auxiliary materials are respectively passed through 80 mesh sieves. Weigh according to the prescription ratio, mix evenly, use ethanol as wetting agent to make soft material, granulate with a 20-mesh sieve, dry the wet granules at 50°C, granulate with an 18-mesh sieve, and mix the dry granules with a lubricant Compress the tablet to get the tablet core.
[0053] Dissolve cellulose acetate and polyethylene glycol 1500 (weight ratio 5:1) in a mixed solvent of acetone and isopropanol to prepare a semipermeable membrane coating solu...
Embodiment 2
[0055] (1) Tablet core prescription
[0056] Composition g / 1000 tablets
[0057] Doxazosin mesylate 4.86
[0058] Citric acid 35
[0059] Sodium dodecyl sulfonate 5
[0060] Sucrose 40
[0061] Lactose 80
[0062] Polyethylene oxide (100,000 molecular weight) 5
[0063] Gum Arabic 20
[0064] Sodium Alginate 20
[0065] Starch 40
[0067] (2) Preparation method
[0068] Raw and auxiliary materials are respectively passed through 80 mesh sieves. Weigh according to the prescription ratio, mix evenly, use ethanol as wetting agent to make soft material, granulate with a 20-mesh sieve, dry the wet granules at 50°C, granulate with an 18-mesh sieve, and mix the dry granules with a lubricant Compress the tablet to get the tablet core.
[0069] Dissolve cellulose acetate and polyethylene glycol 1500 (weight ratio 5:1) in a mixed solvent of acetone and isopropanol to prepare a semipermeable membrane coating solution. Coat the tablet cores in a ...
experiment example 1
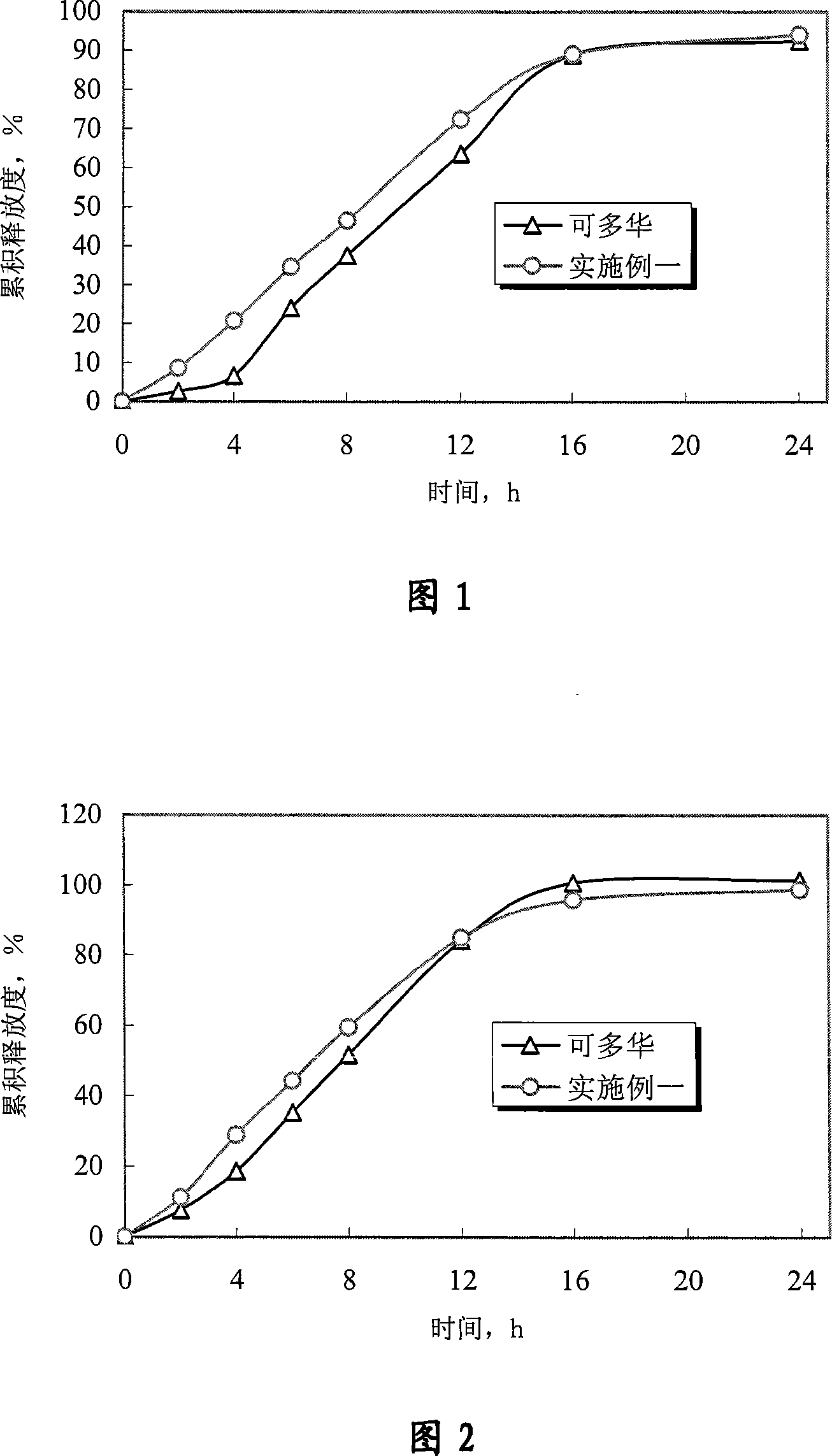
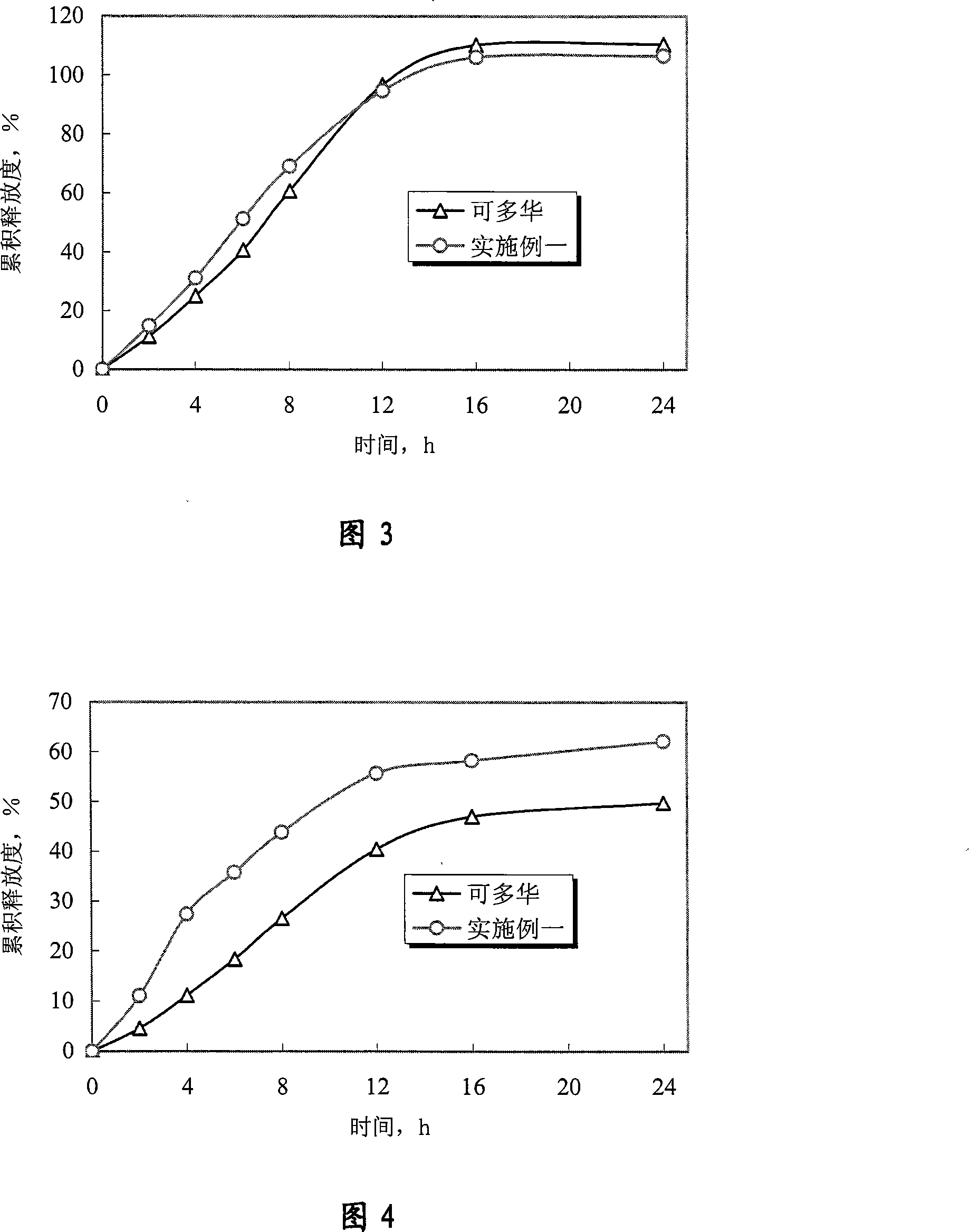
[0070] Experimental example 1 Determination experiment of release degree
[0071] 1. Sample
[0072] The doxazosin mesylate controlled-release tablet of the present invention is prepared according to Example 1 of the present invention; "Keduohua" is purchased from the market.
[0073] 2. Experimental method
[0074] With Chinese Pharmacopoeia version in 2005 two appendices X D first method (adopting the device of dissolution rate second method, rotating speed is 75 revolutions per minute), measure the cumulative release of doxazosin mesylate, and preparation of the present invention and Marketed Kaduohua (produced by Pfizer of the United States, repackaged by Pfizer of Dalian) was compared. In order to further illustrate that the controlled release of the drug by the osmotic pump preparation is not affected by the environment, according to the relevant guiding principles and methods of the Chinese Pharmacopoeia 2005 edition, the standard medium (hydrochloric acid solution (7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com