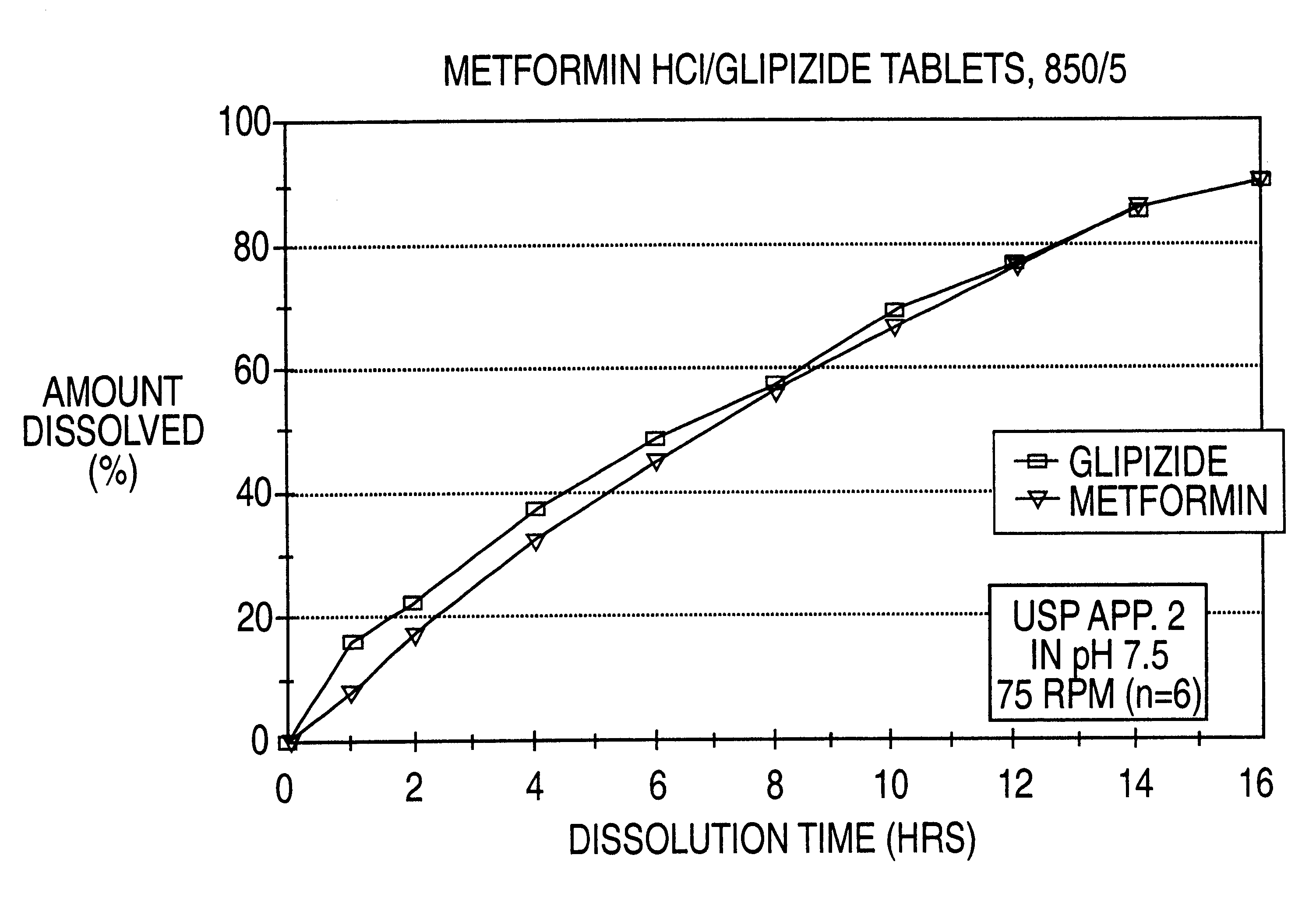
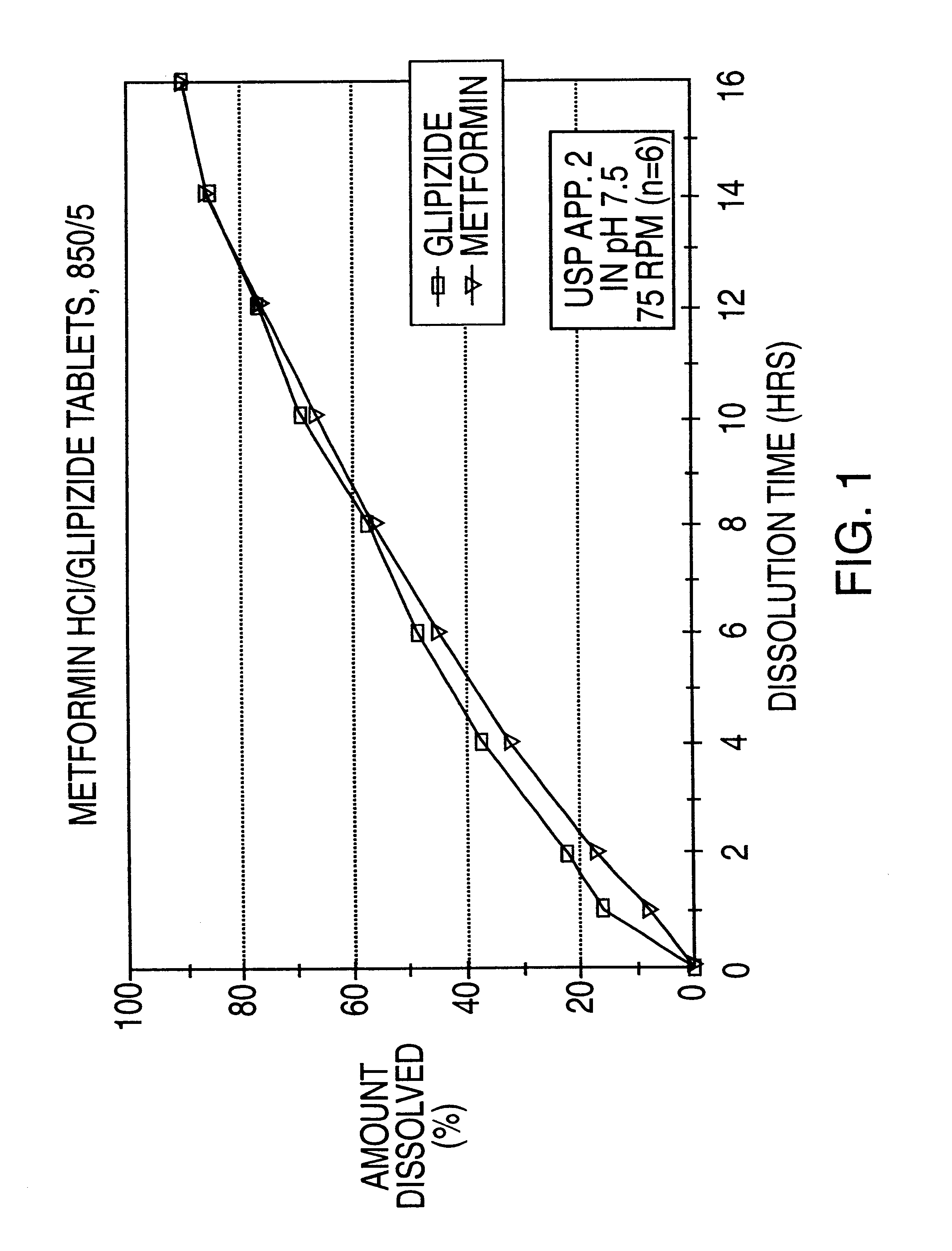
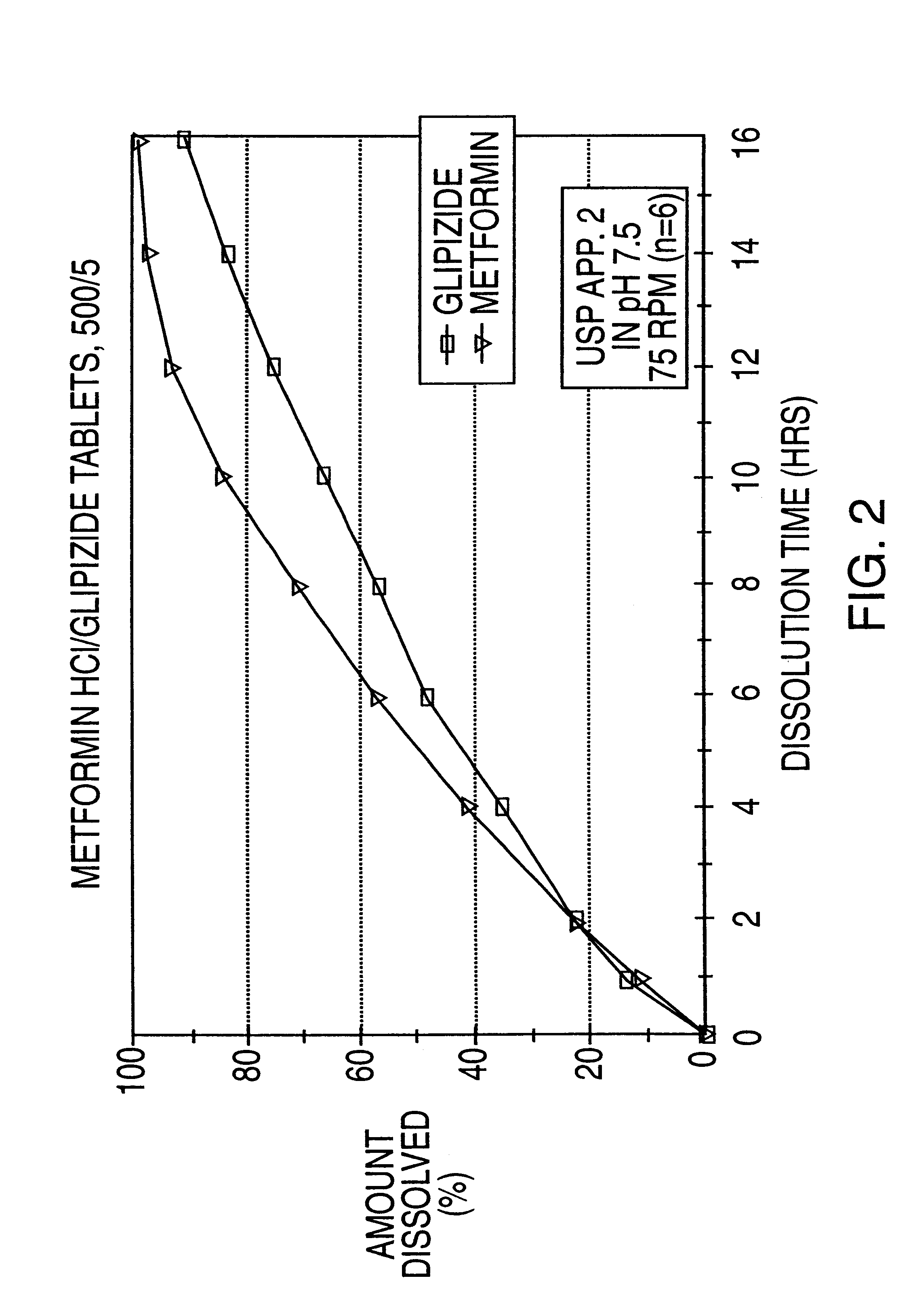
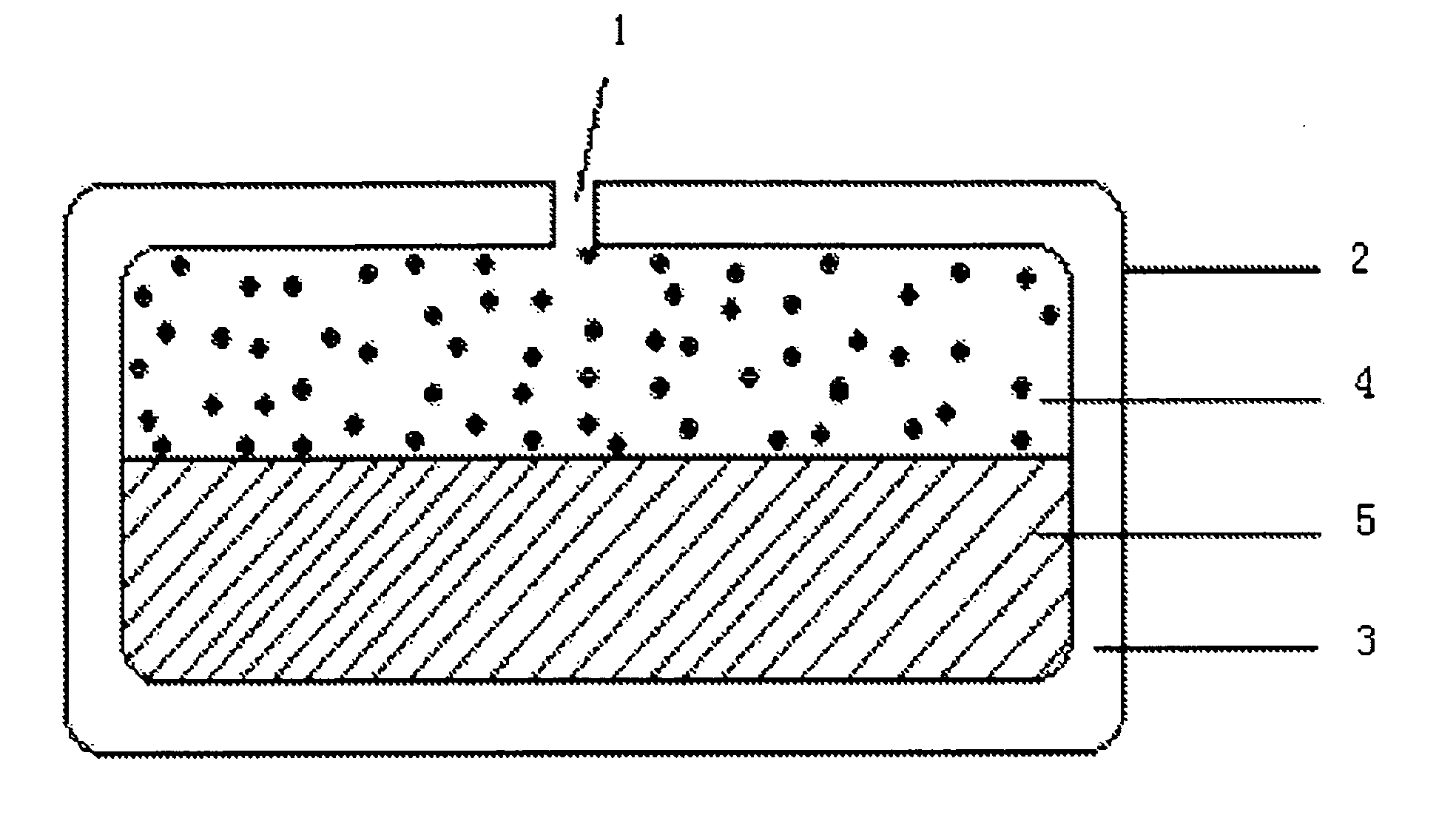
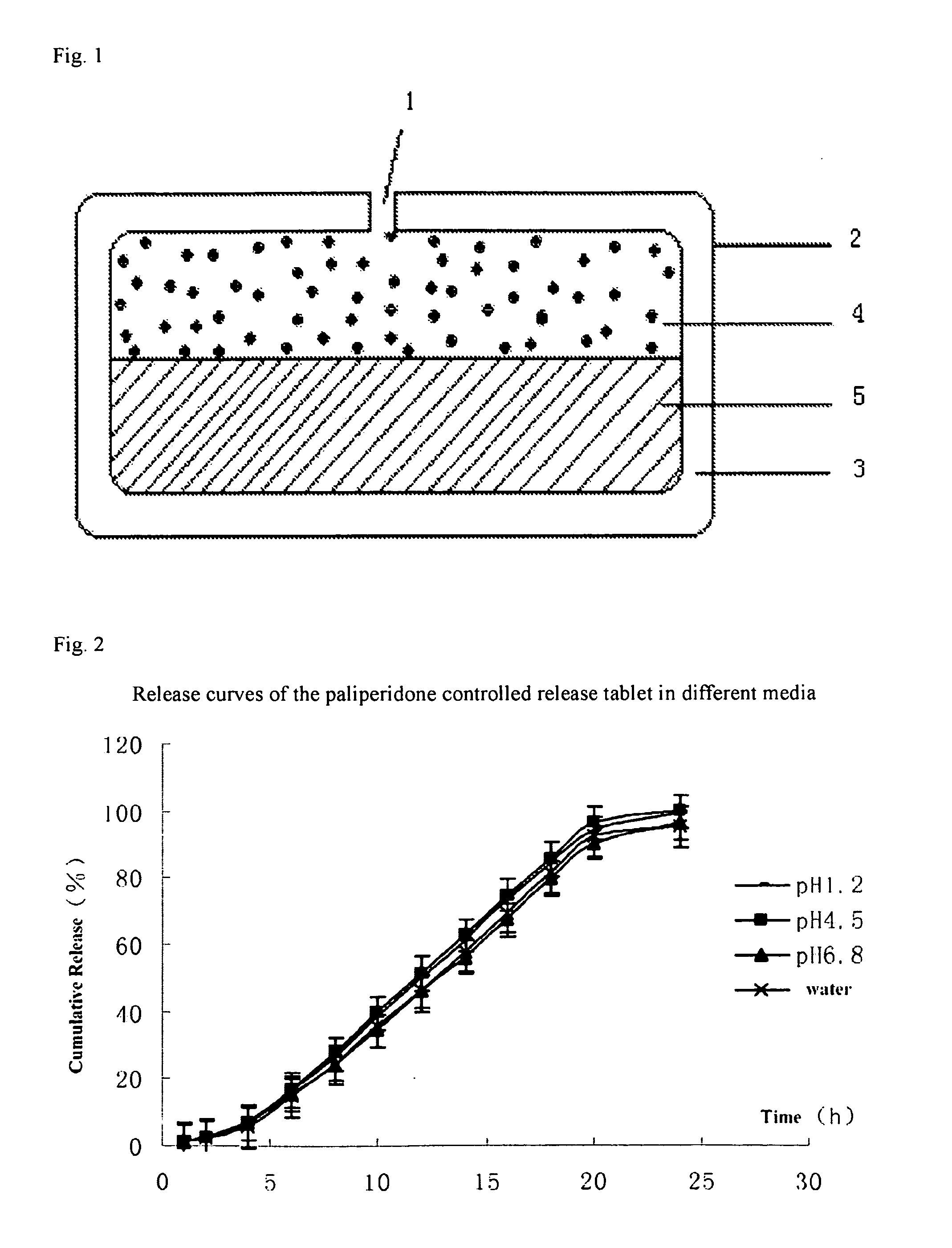
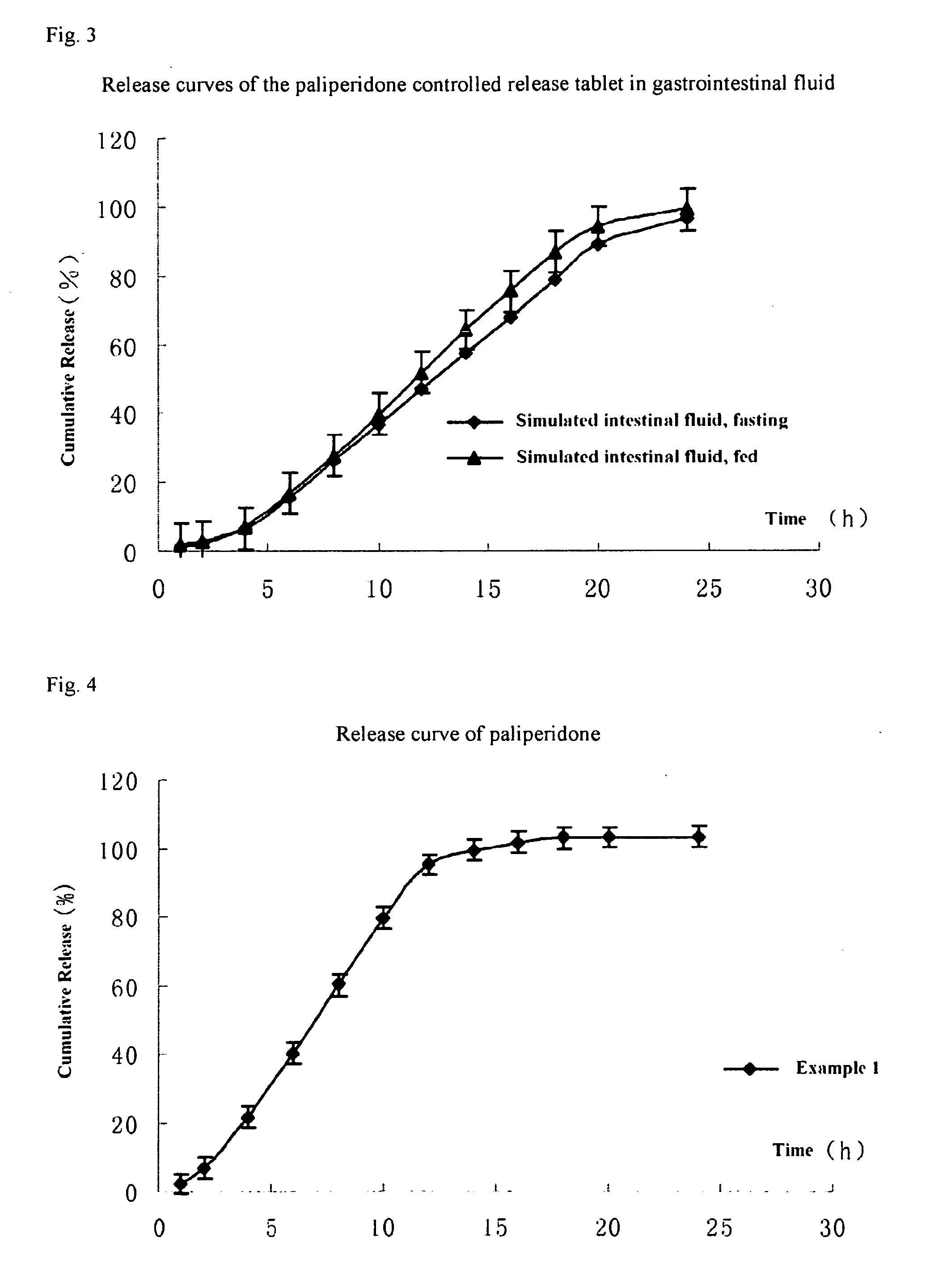
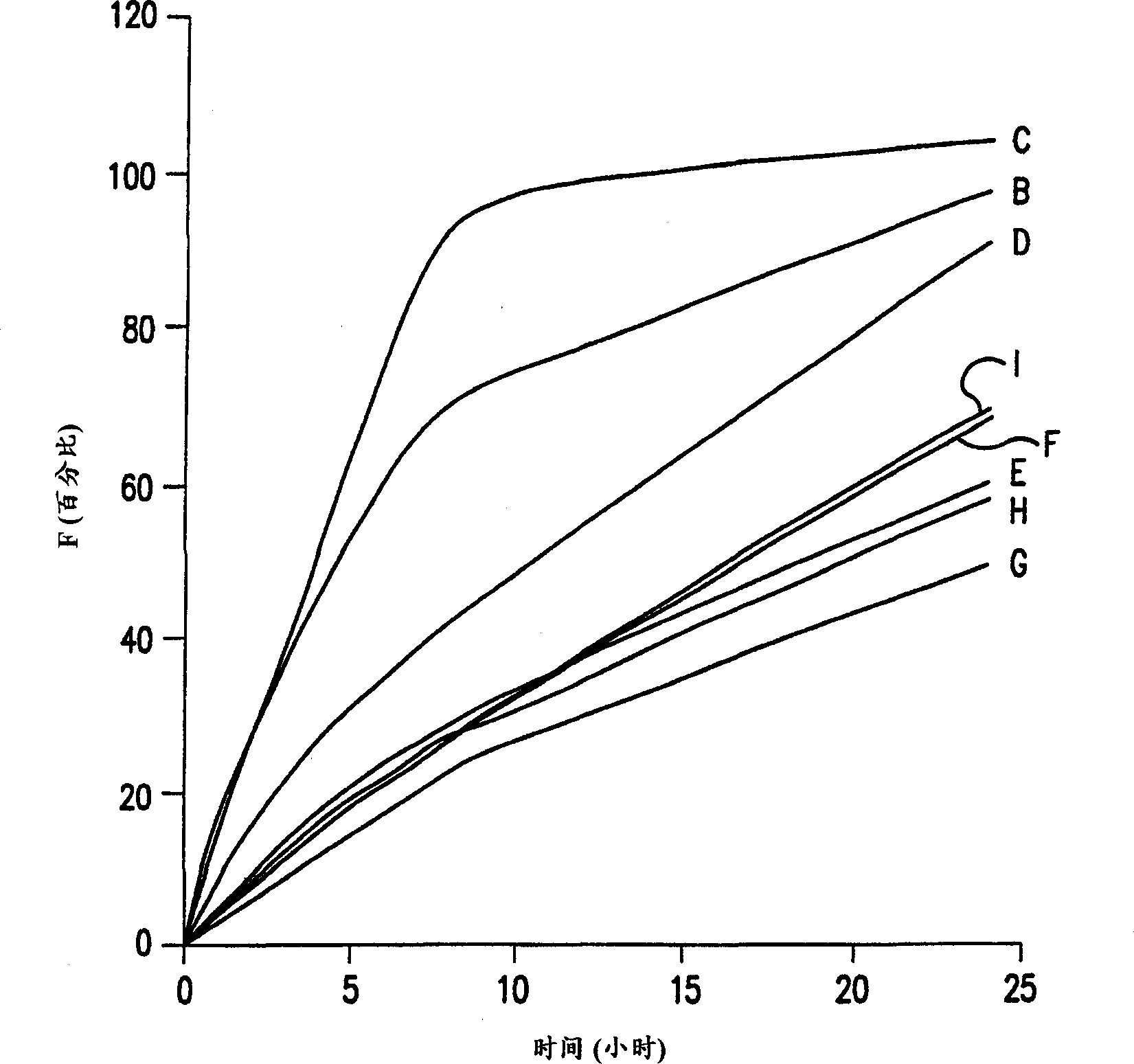
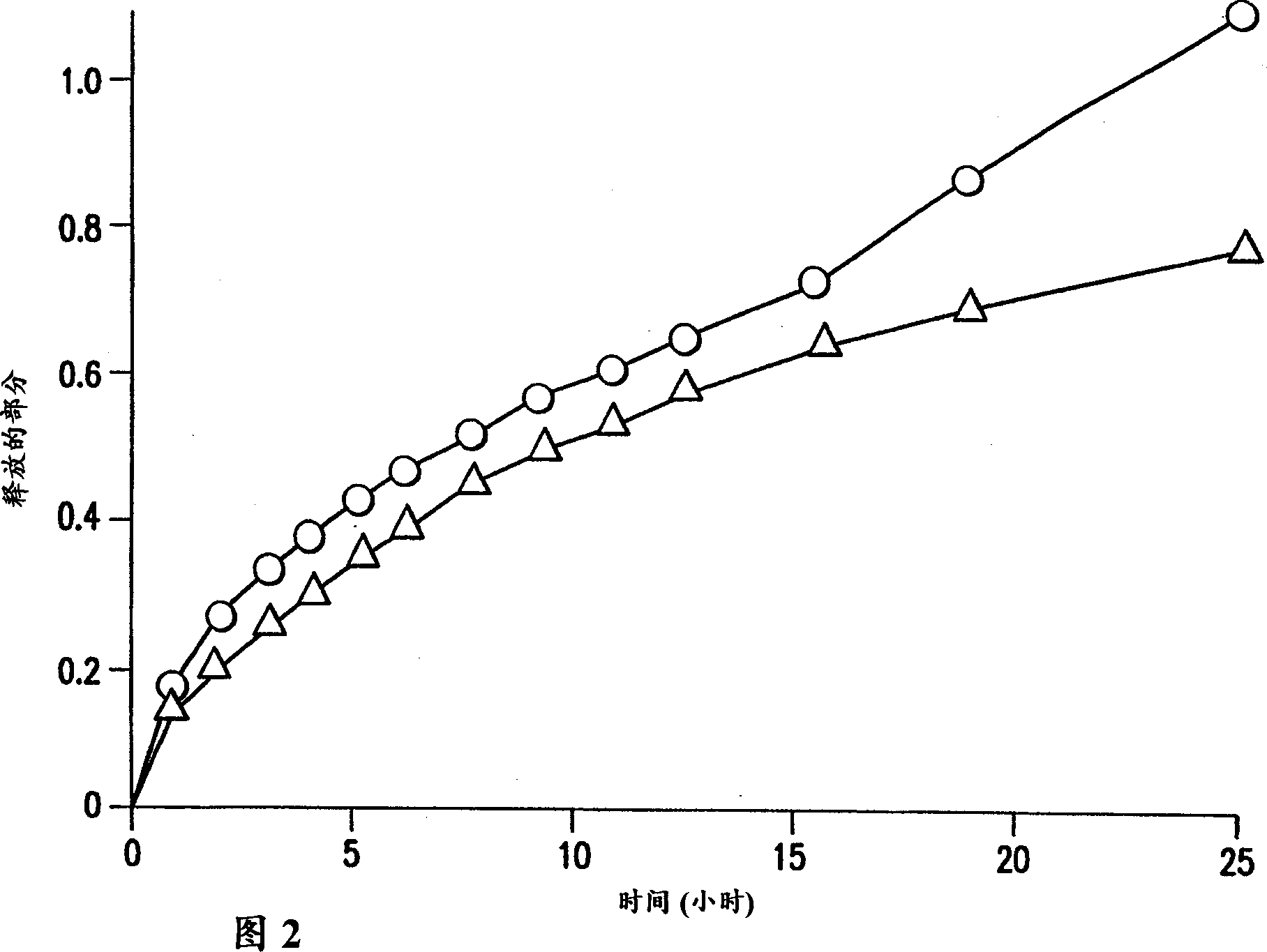
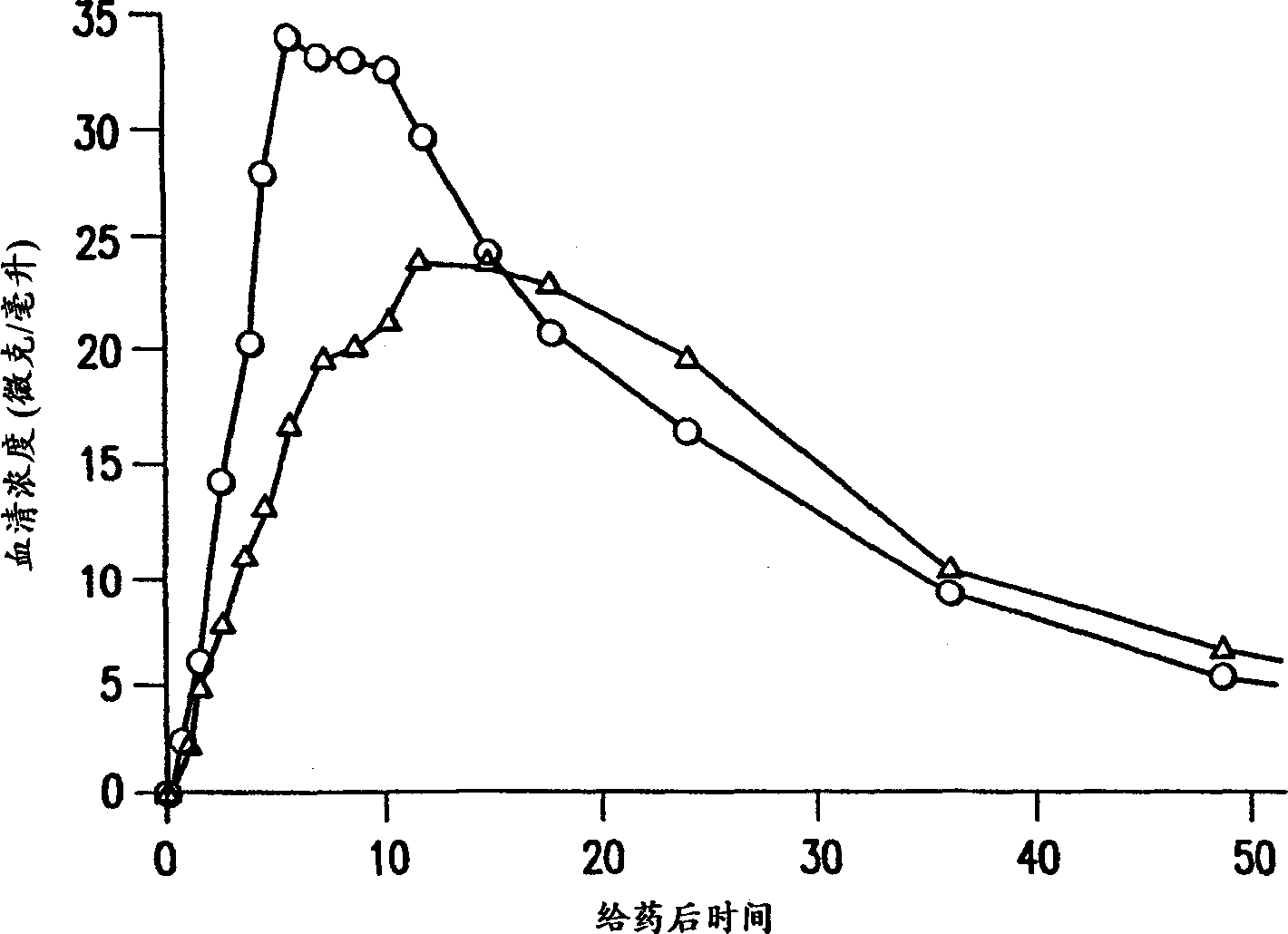
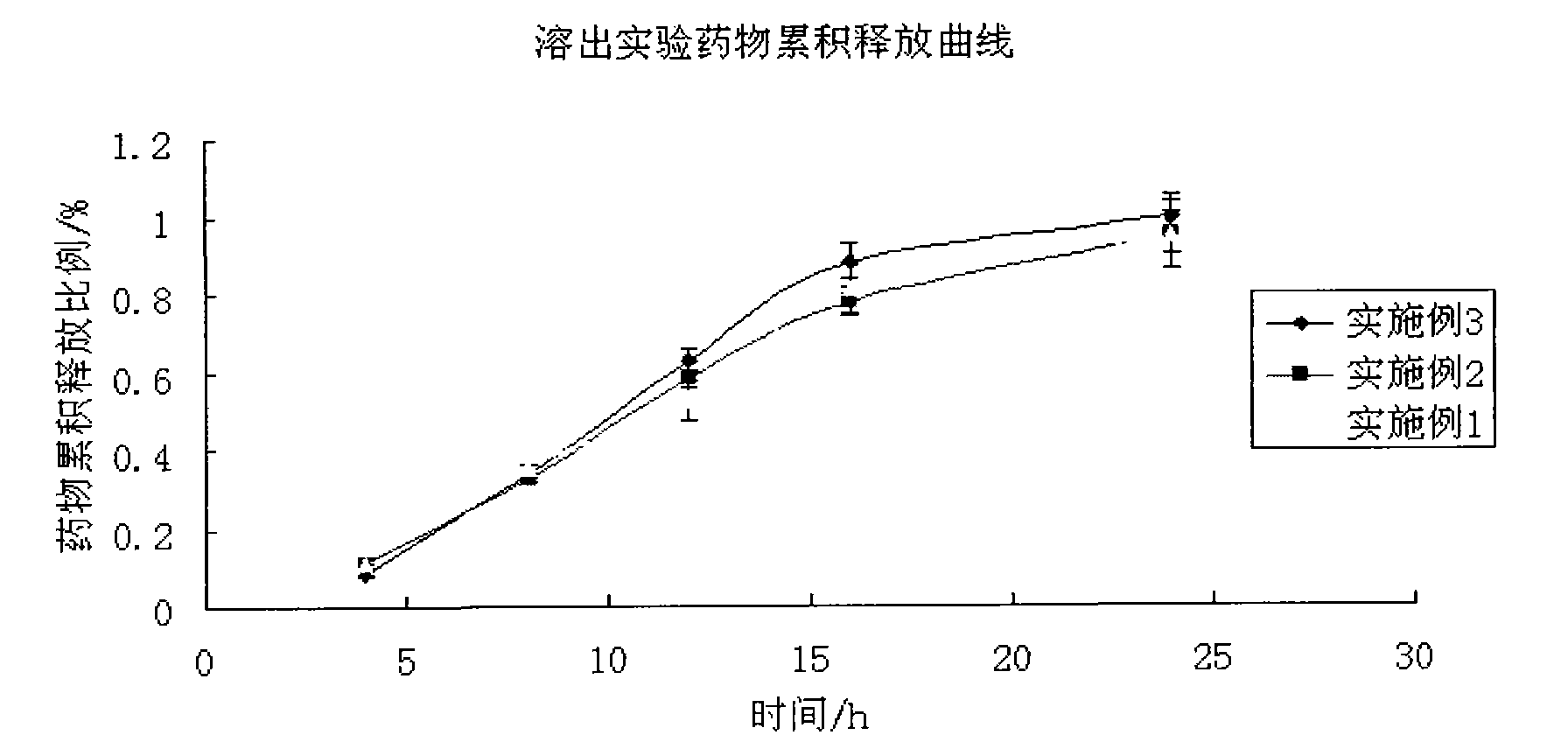
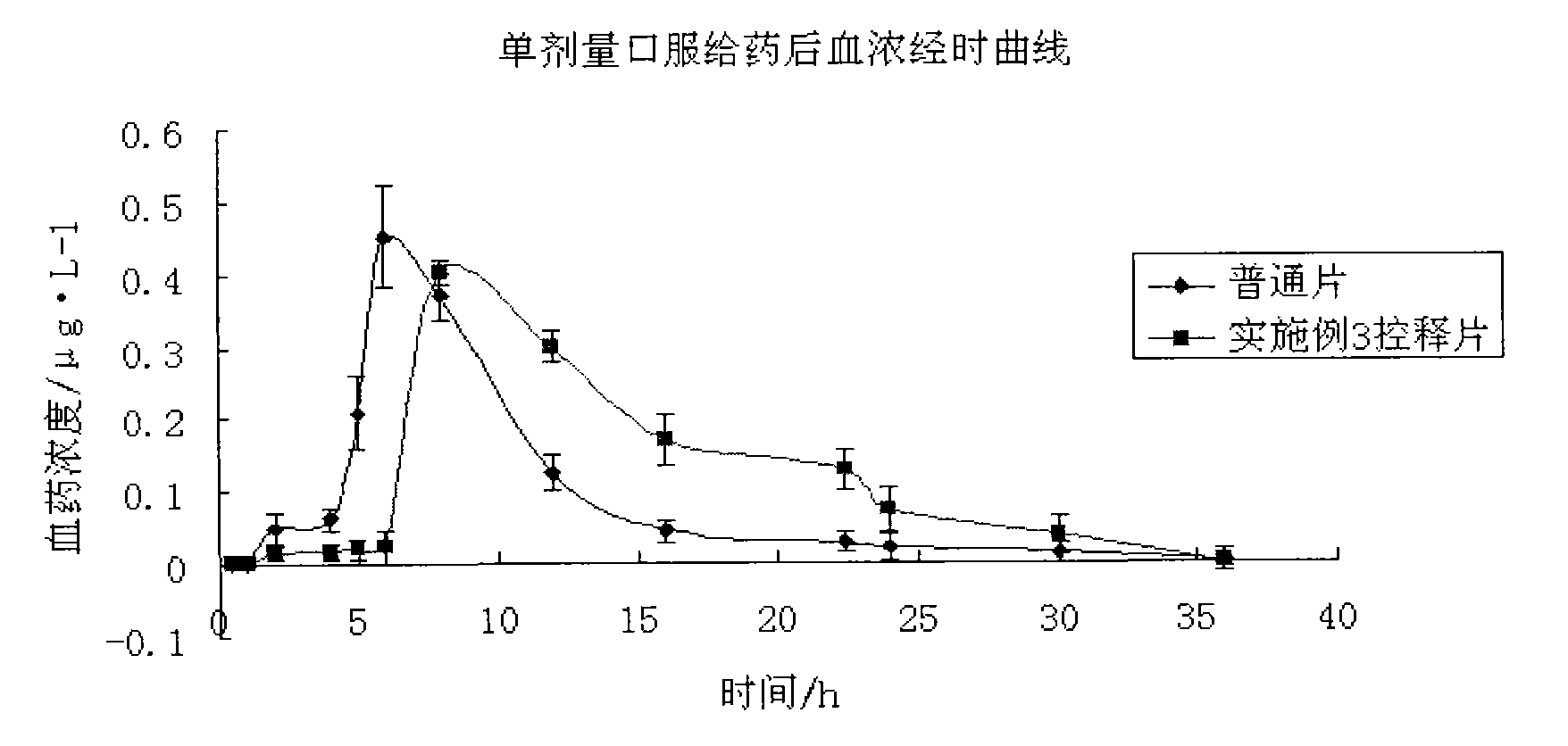
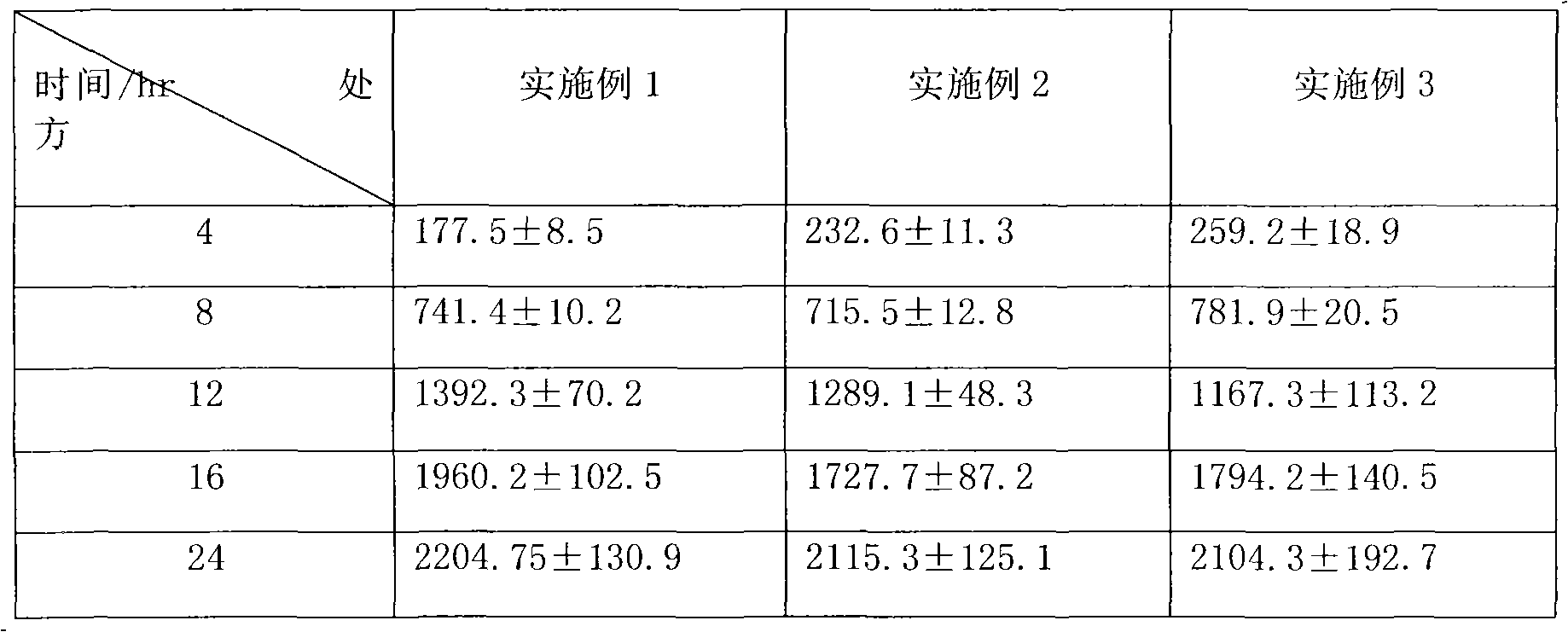
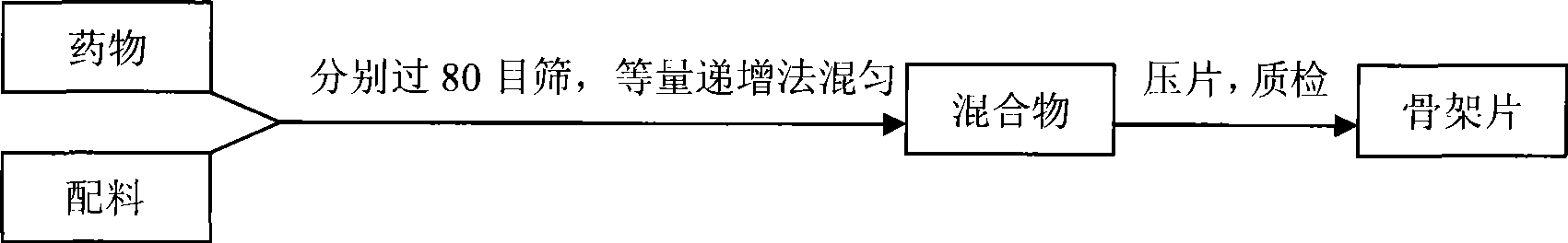
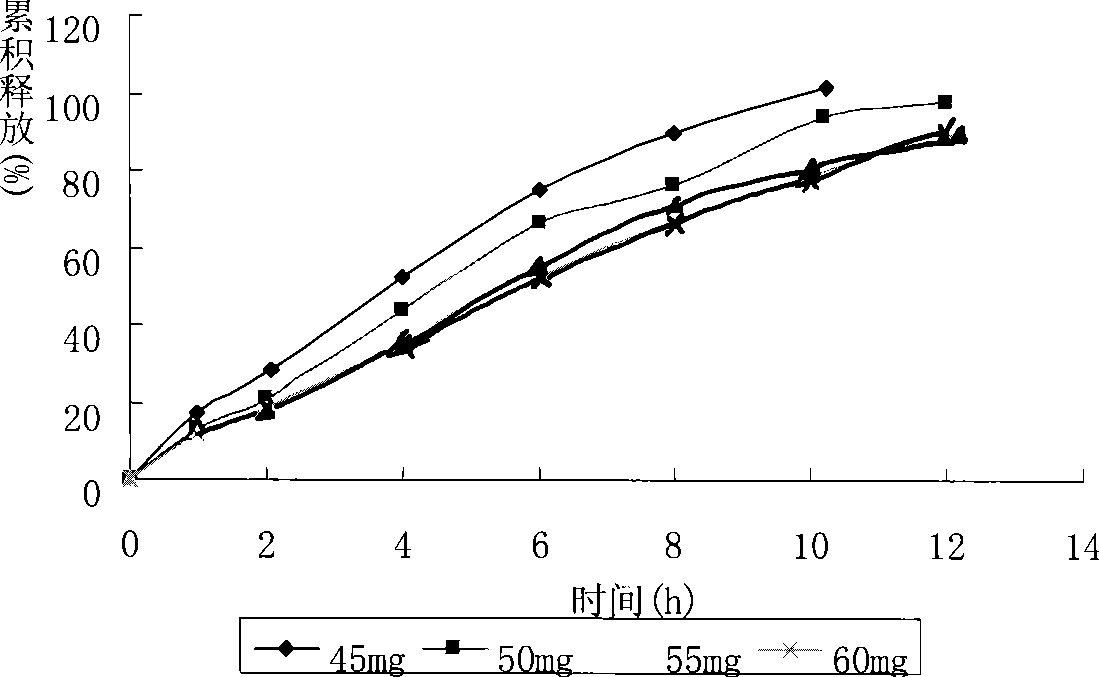
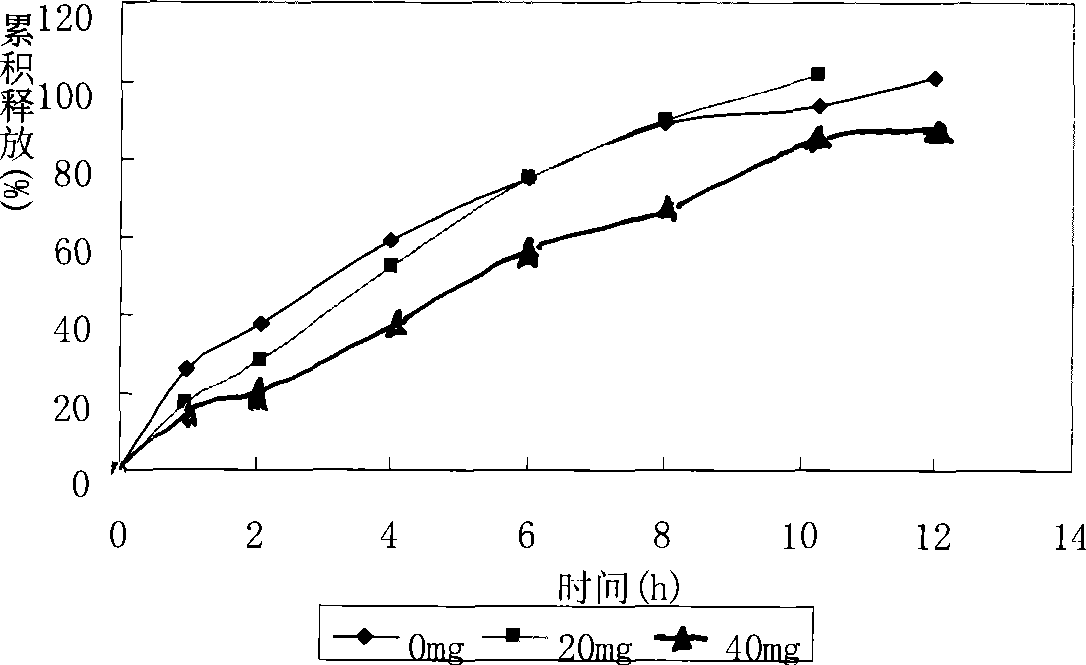
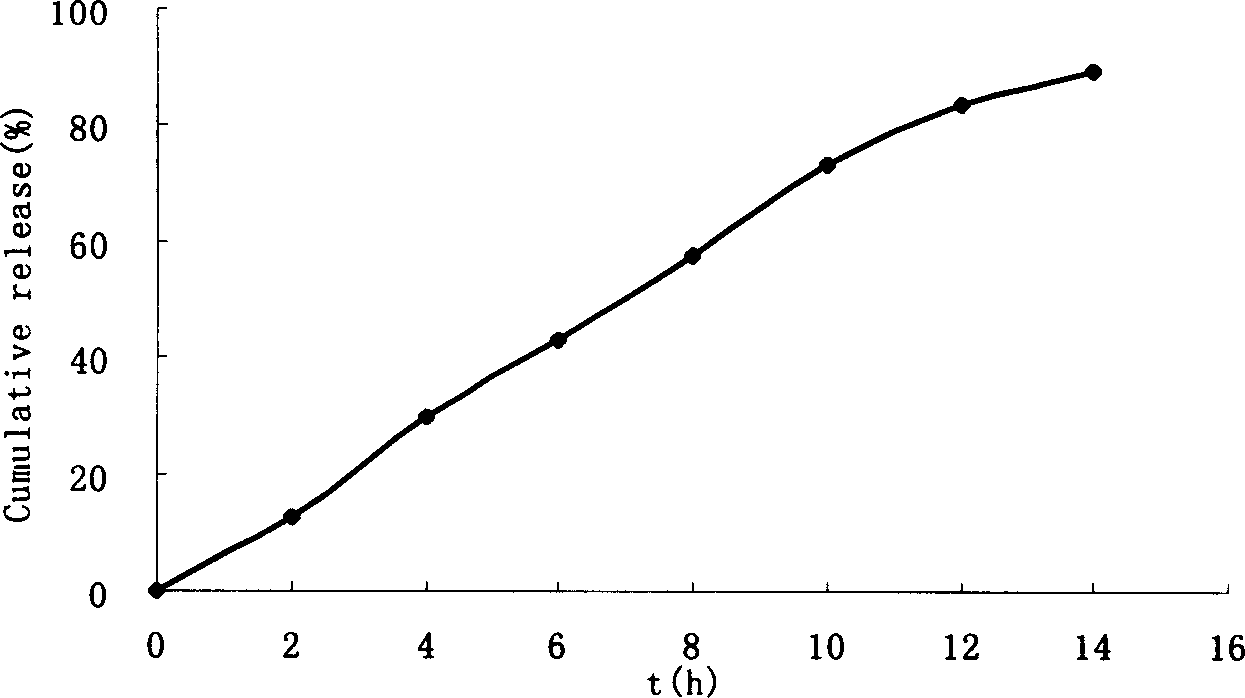
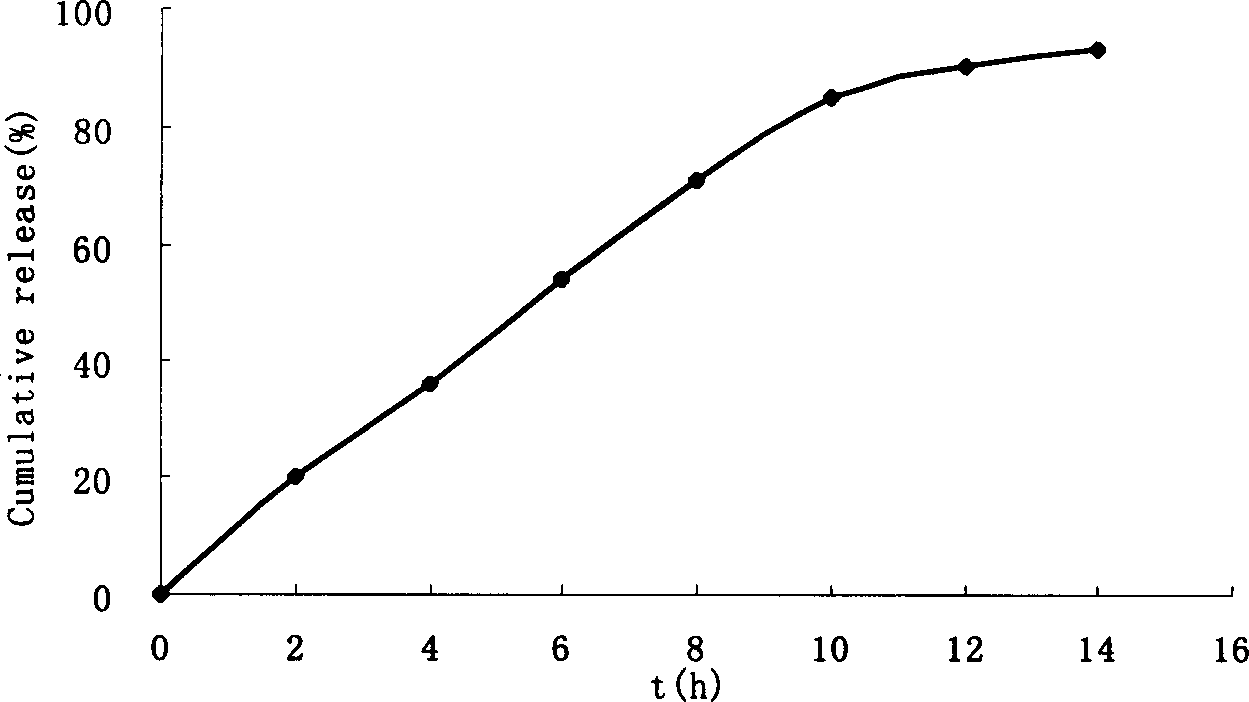
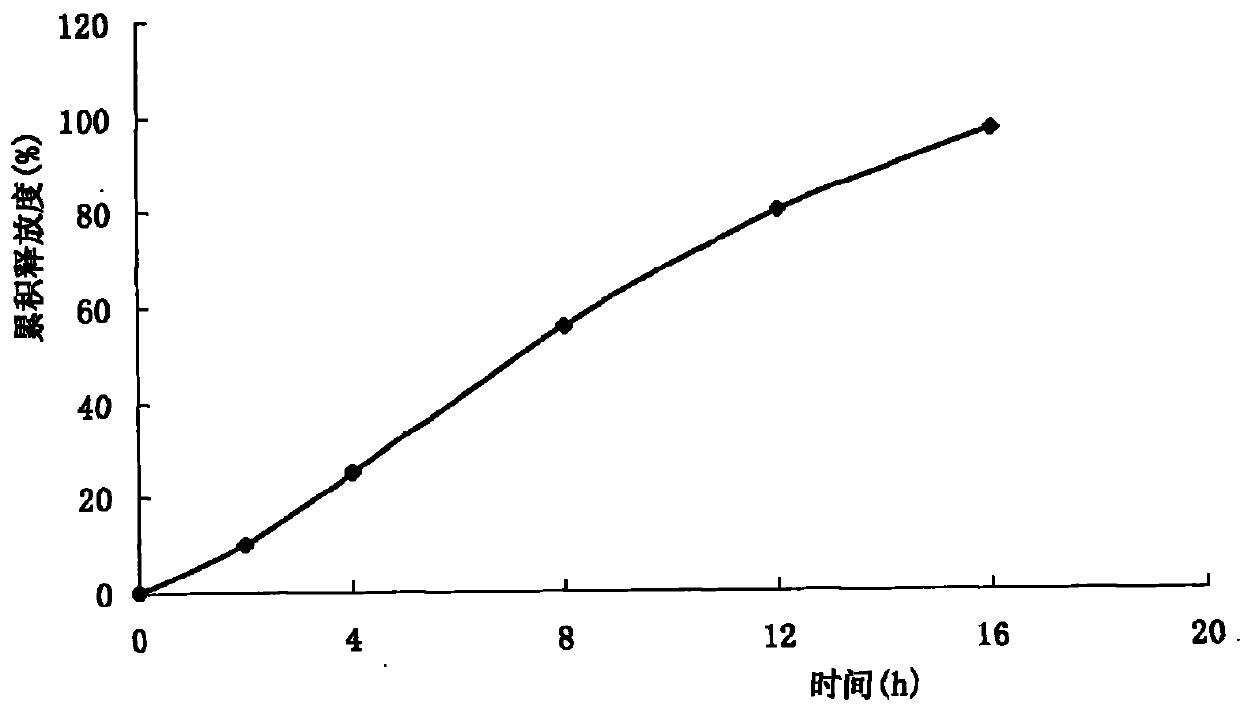
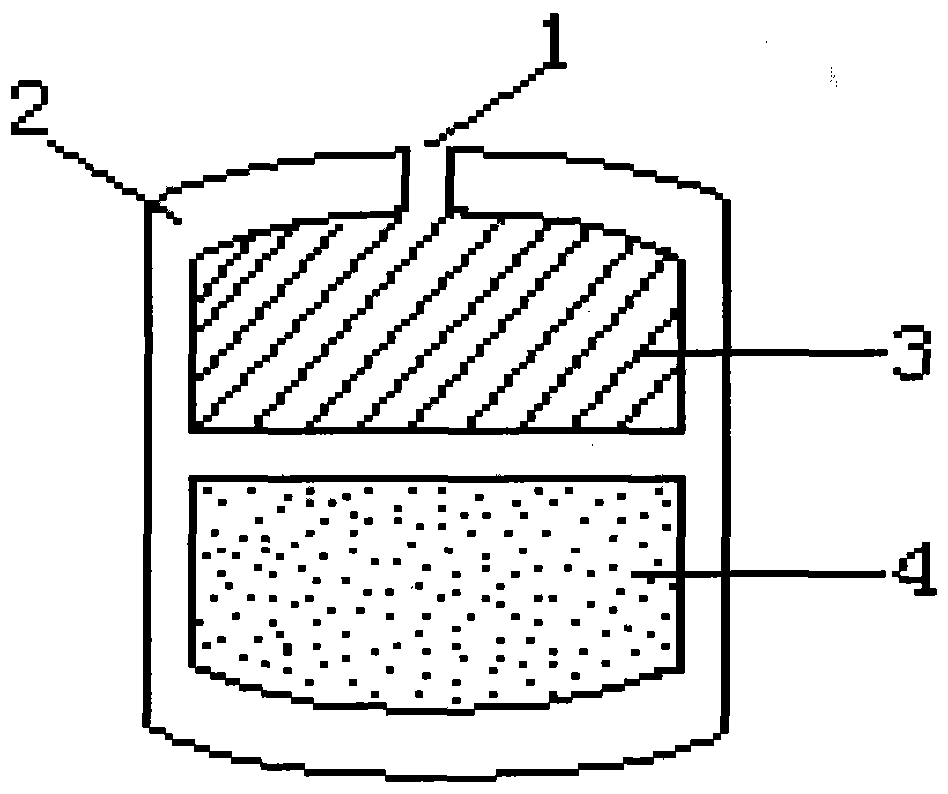
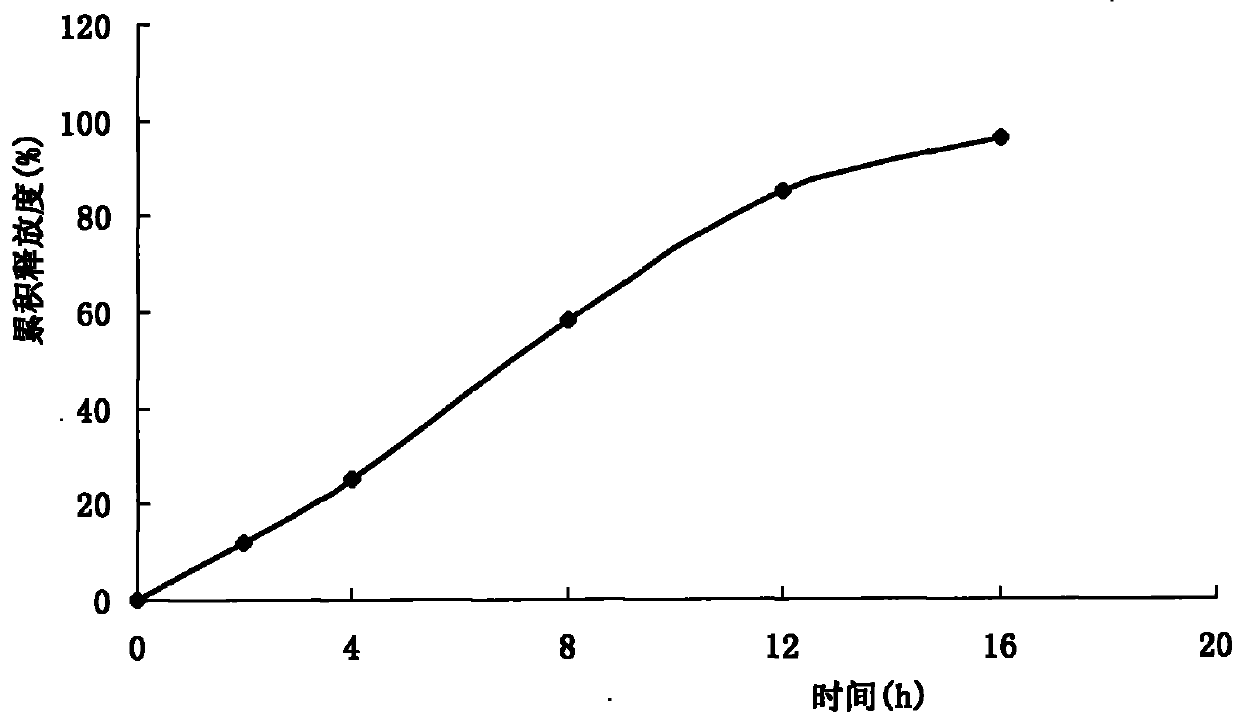
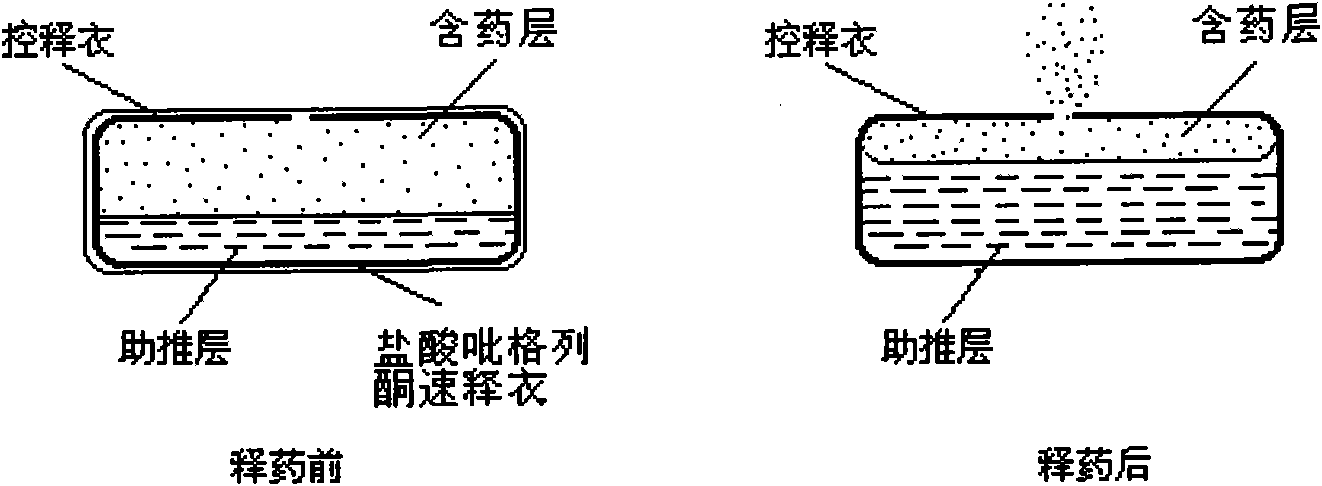
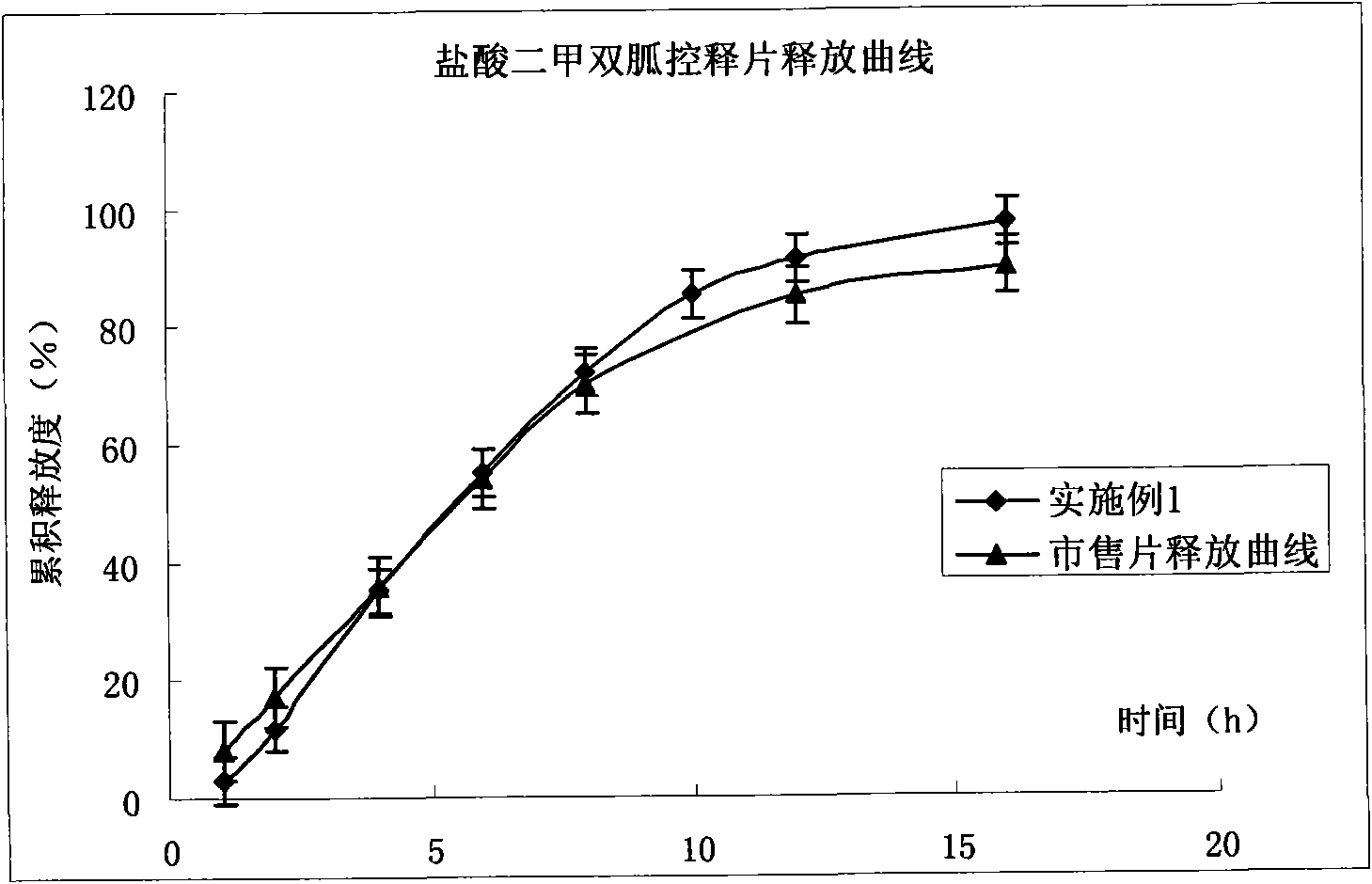
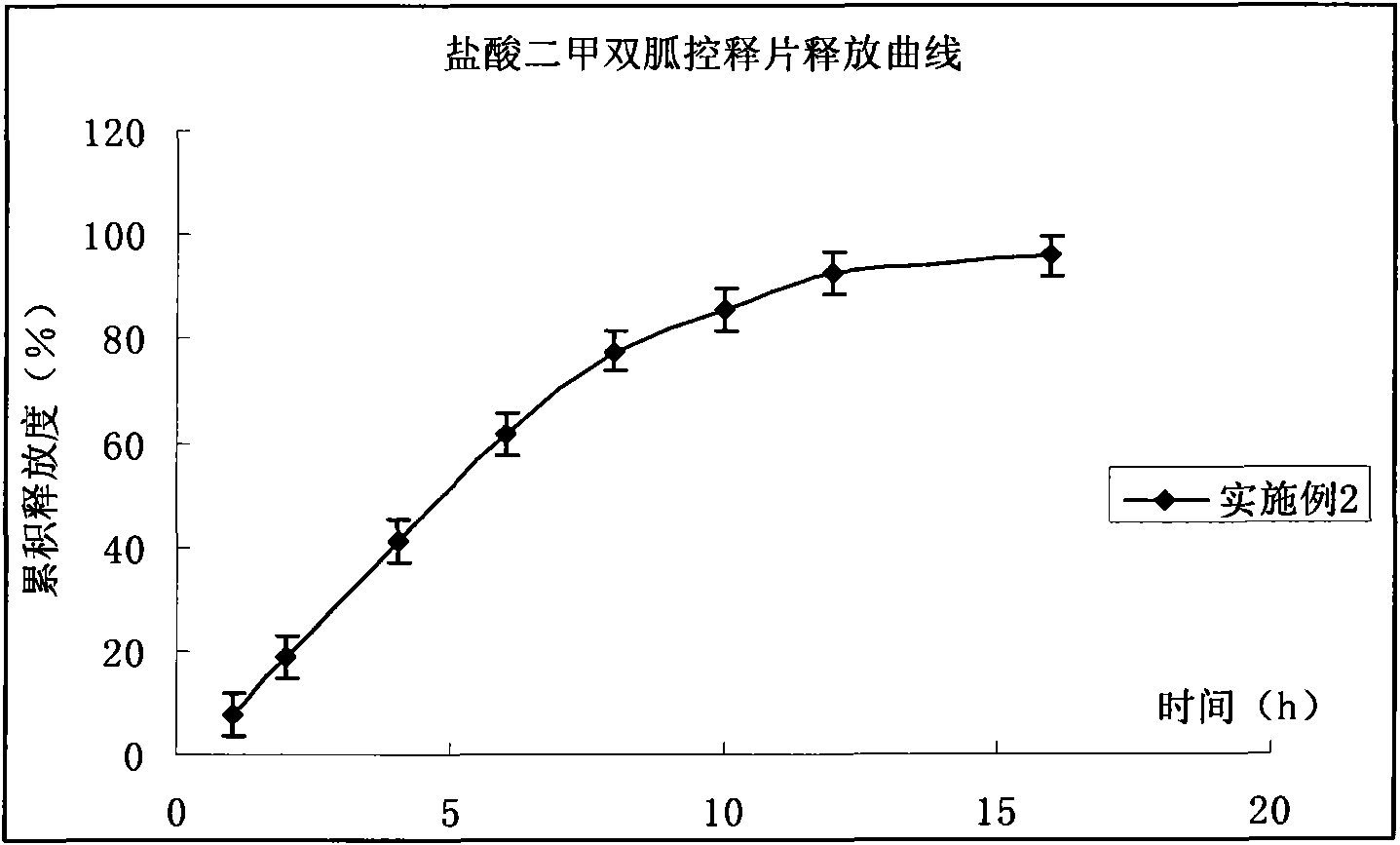
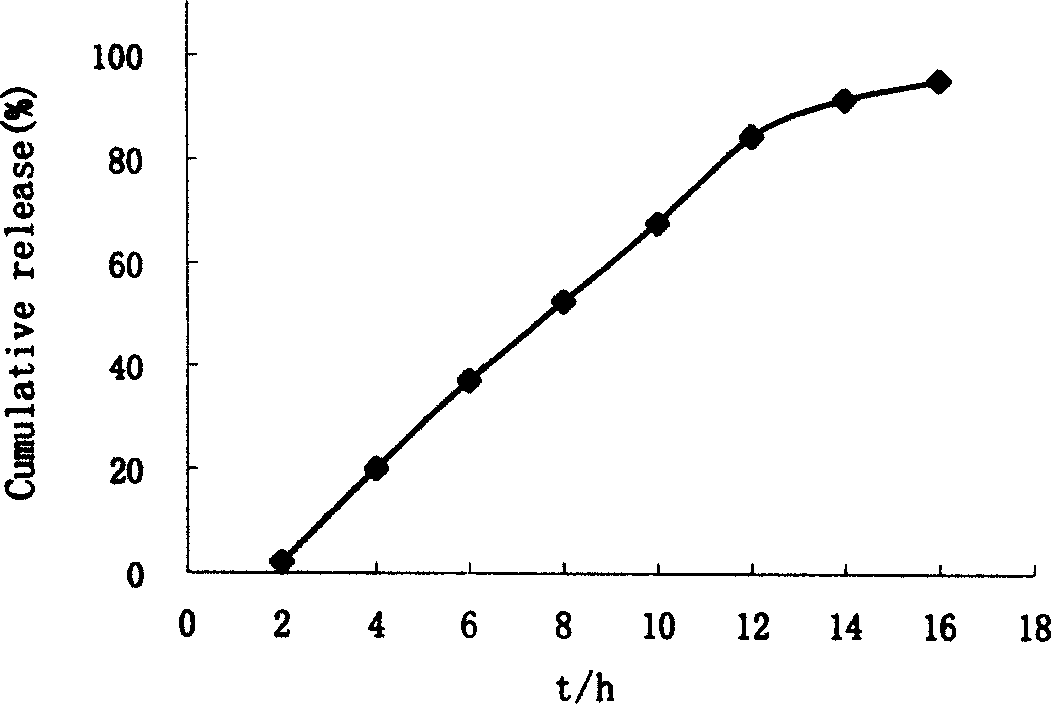
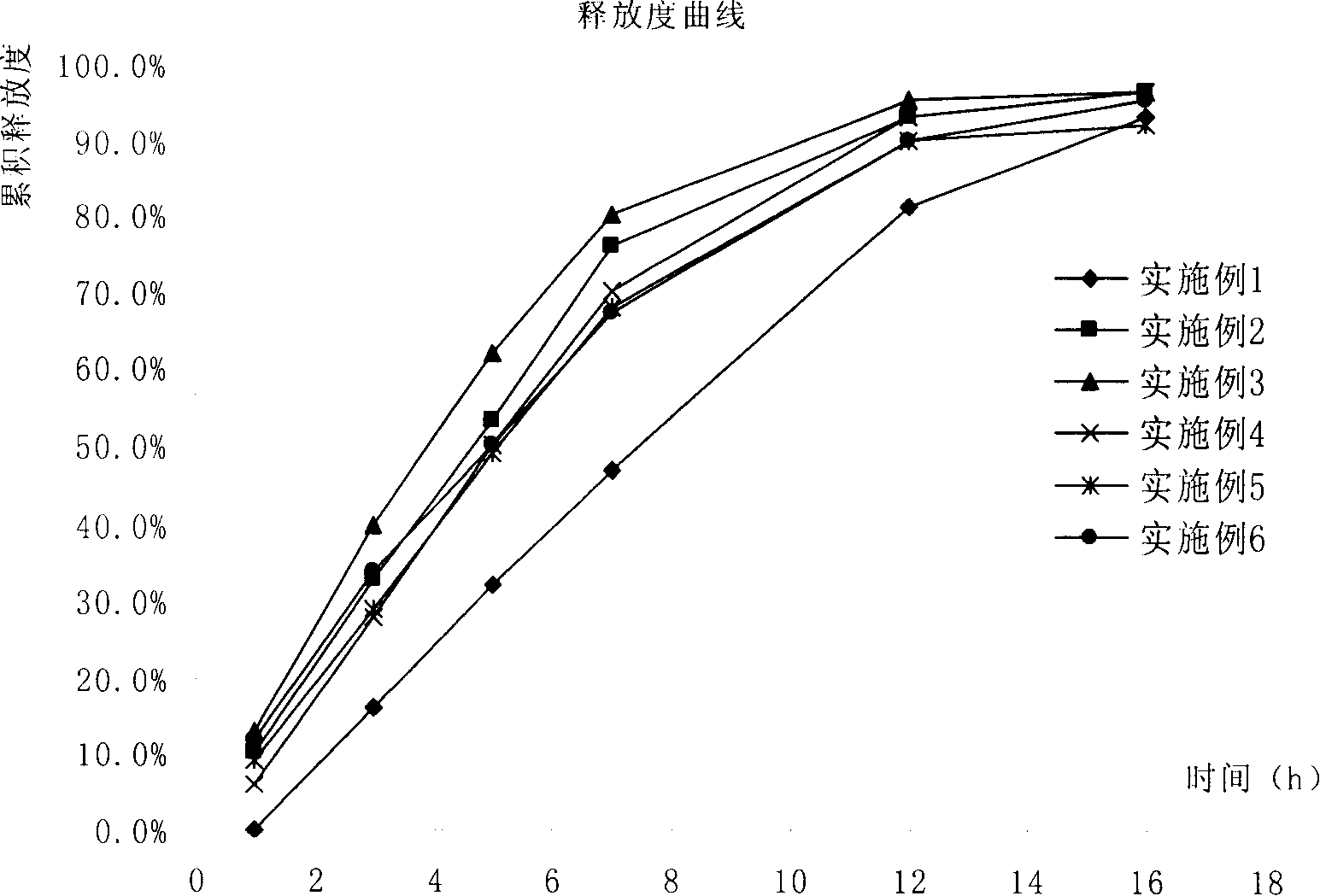
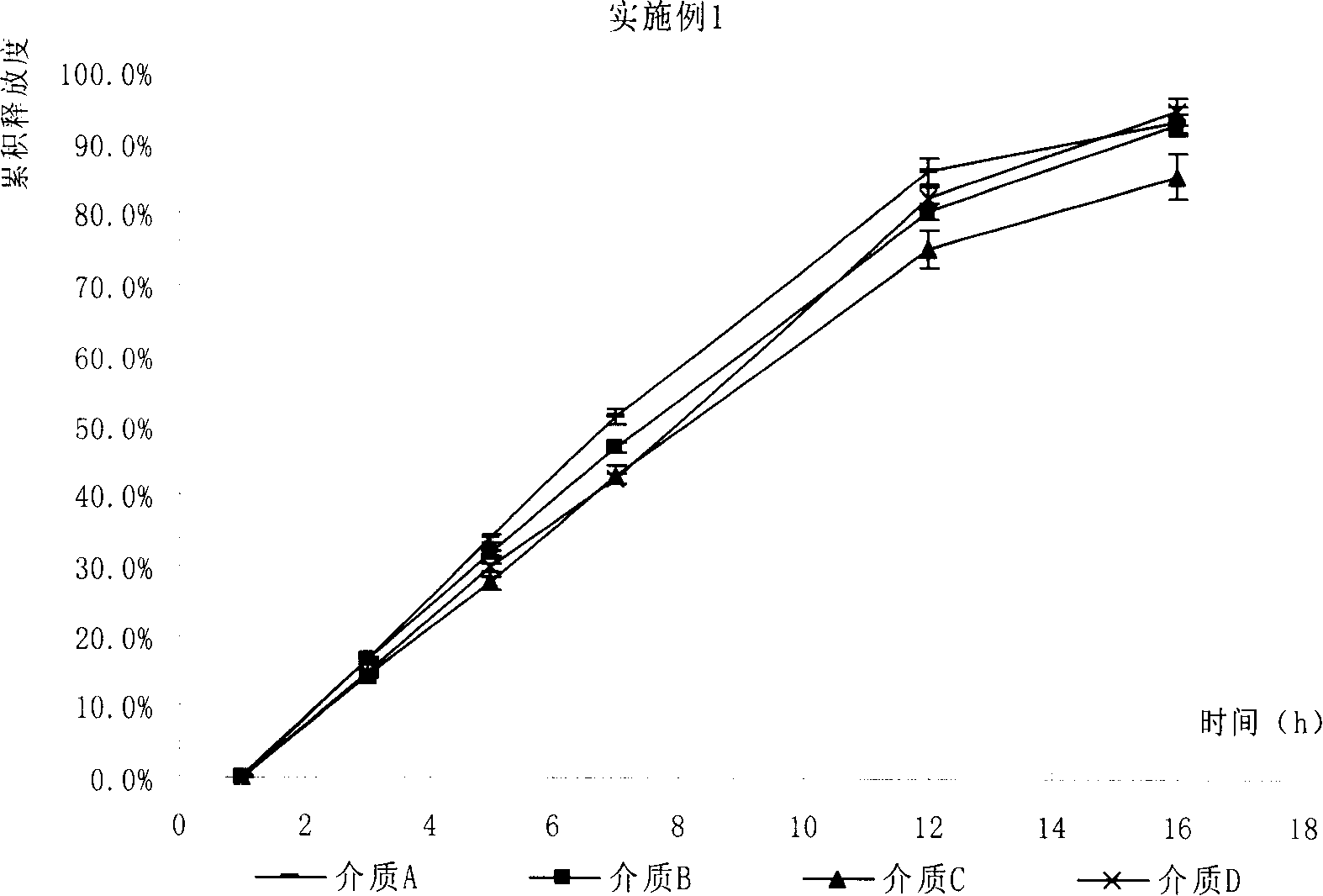
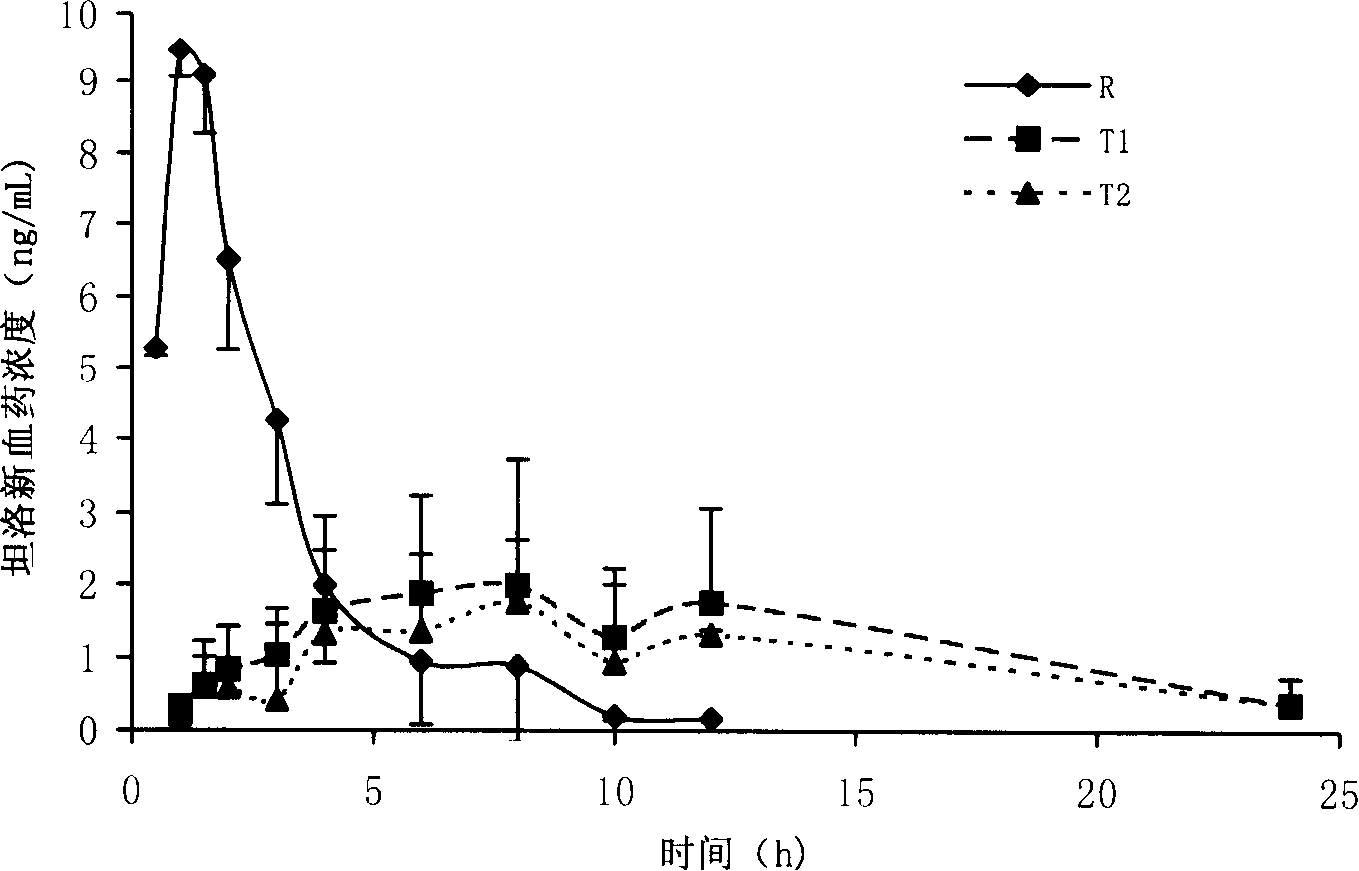
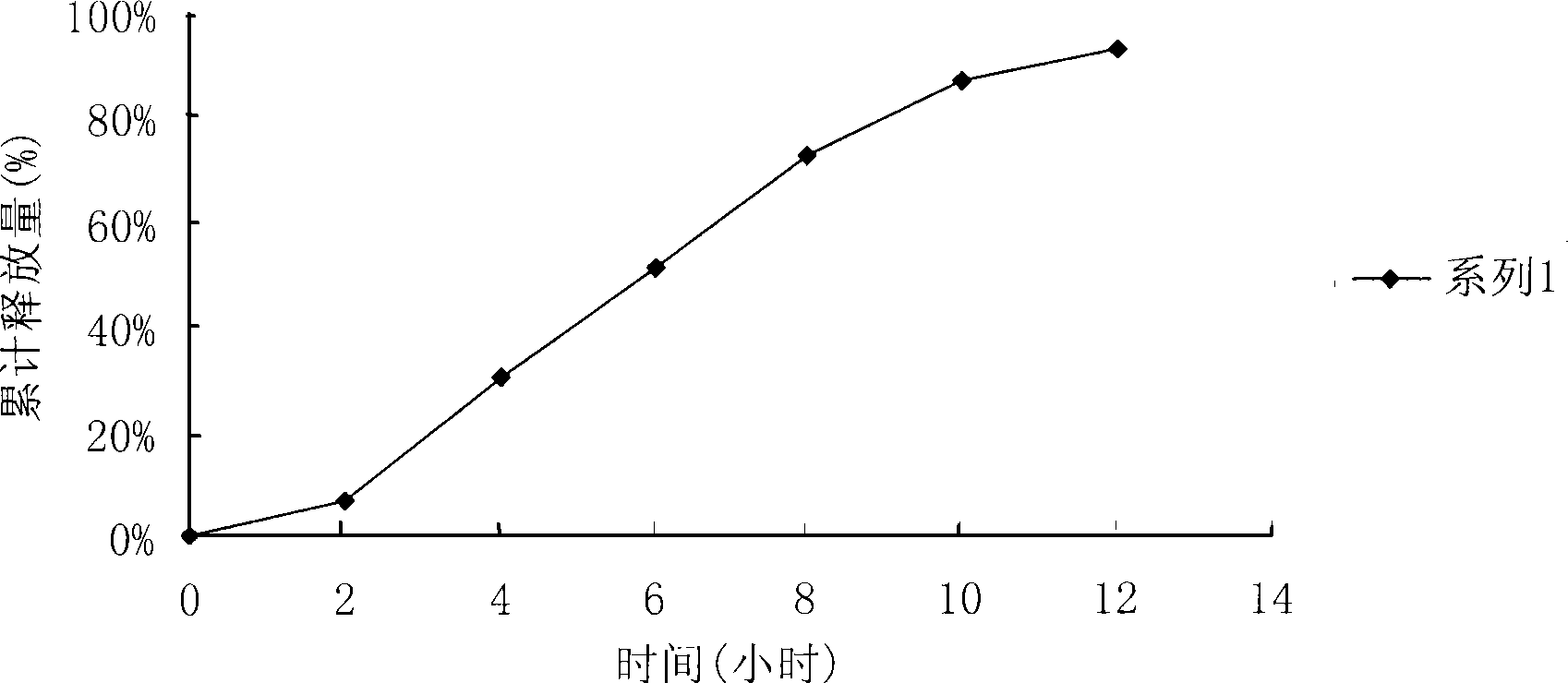
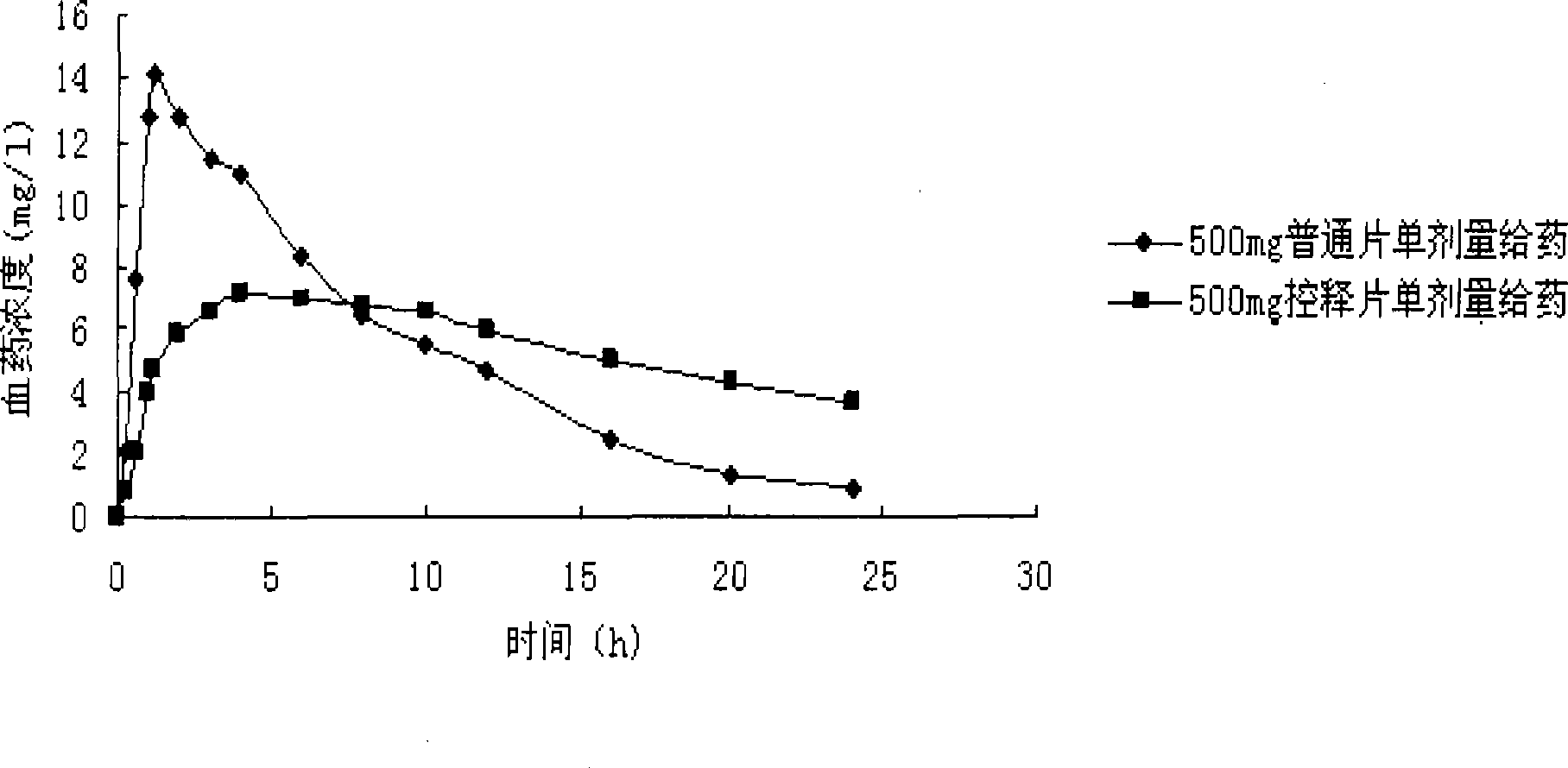
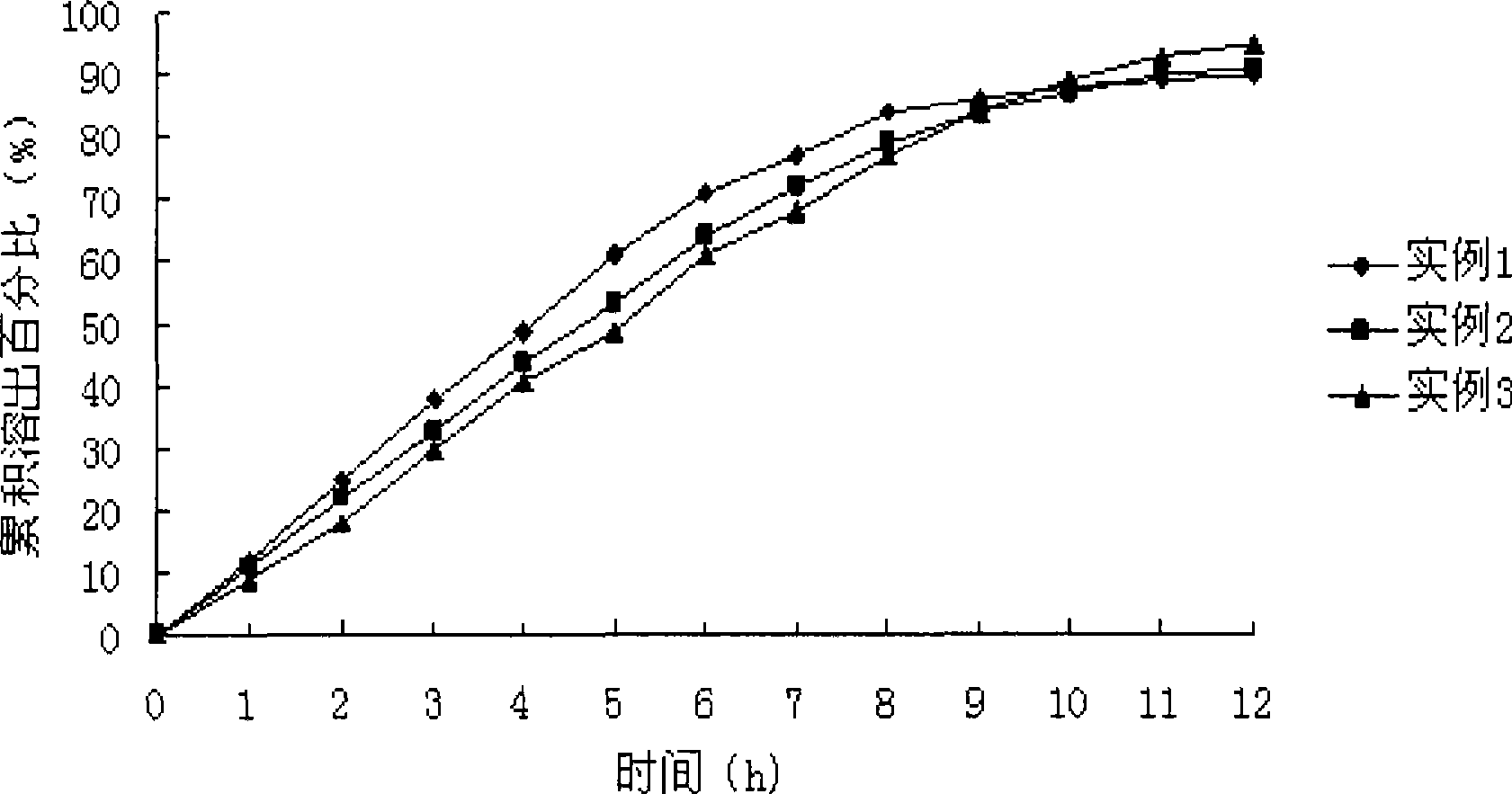
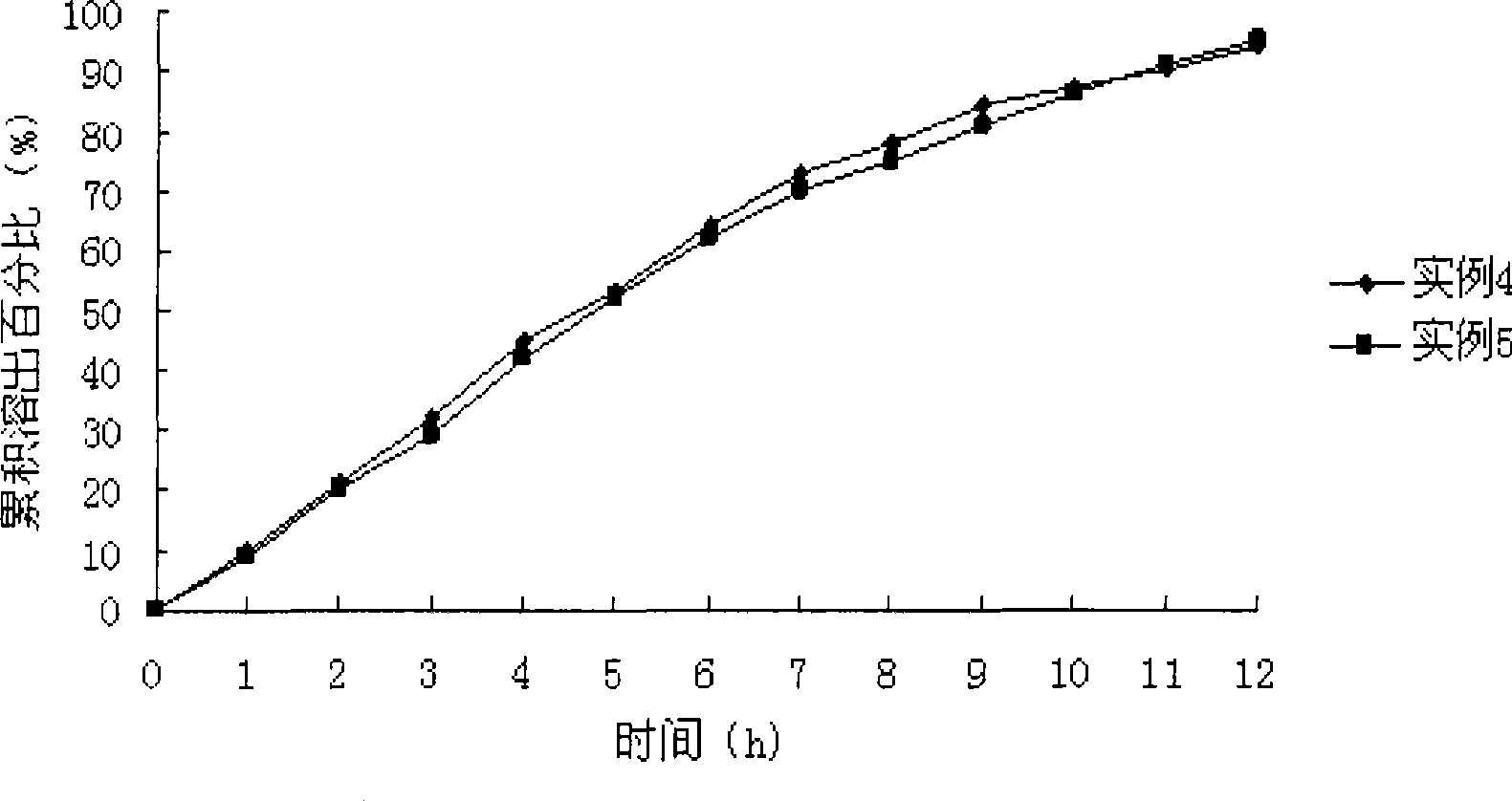
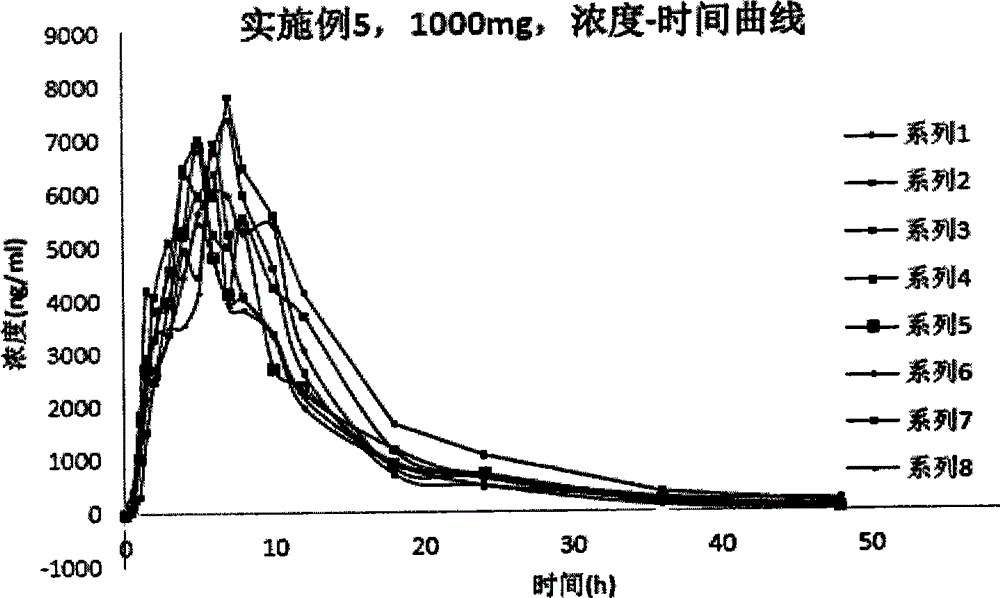
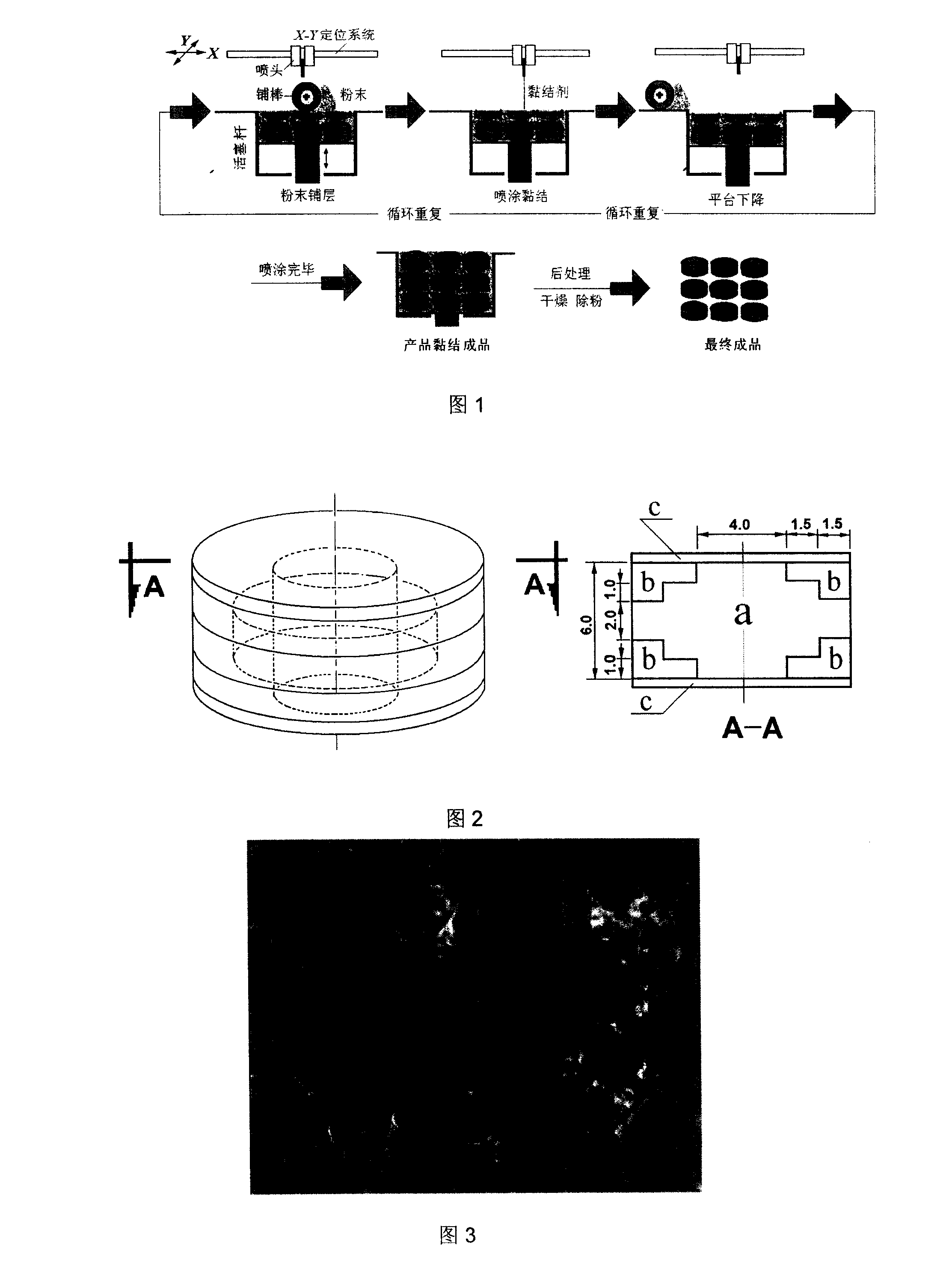
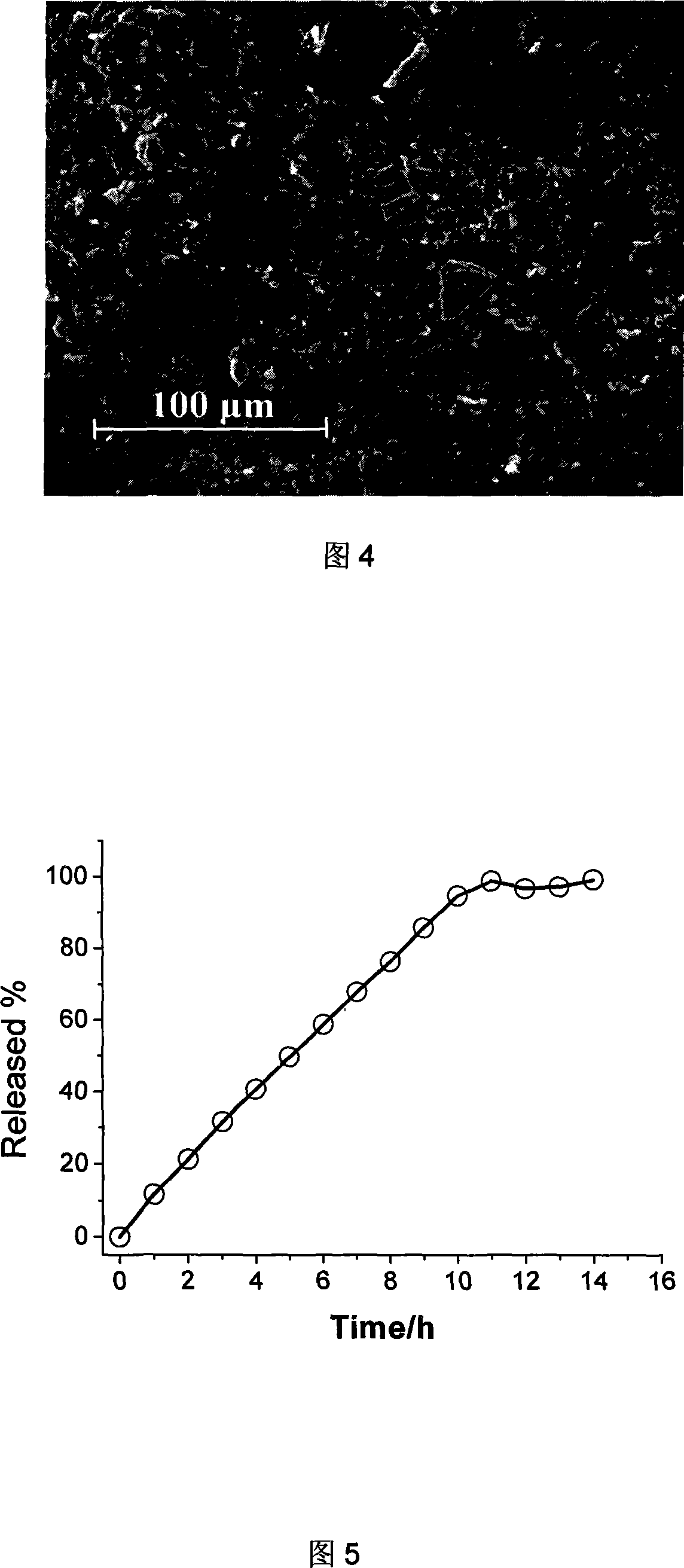
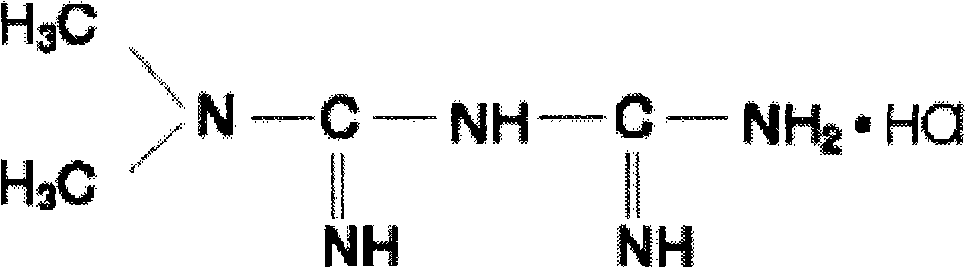Patents
Literature
424 results about "Controlled Release Tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Controlled release tablet having a unitary core
InactiveUS6284275B1Metabolism disorderSulfonylurea active ingredientsControlled Release TabletControlled release drug
A controlled release pharmaceutical tablet containing antihyperglycemic drug and a hypoglycemic drug that does not contain an expanding or gelling polymer layer and comprising a core containing the antihyperglycemic drug and the hypoglycemic drug, a semipermeable coating membrane surrounding the core and at least one passageway in the membrane to allow the drugs to be released from the core.
Owner:ANDRX LABS
Core tablet for controlled release of gliclazide after oral administration
InactiveUS6733782B1Facilitated releaseIncrease concentrationPowder deliveryMetabolism disorderControlled releaseOral medication
The invention relates to a matrix tablet for the prolonged release of gliclazide which ensures continuous and consistent release of the active ingredient after administration by the oral route, the release being insensitive to variations in the pH of the dissolution medium.
Owner:LES LAB SERVIER
Controlled-release pharmaceutical tablets
Controlled-release tablets exhibiting excellent storage stability are achieved by granulating a pharmaceutically active agent with a hydroxyalkylcelluose, blending the resulting granules with an extragranular phase composed of a particulate material that provides a sustained-release matrix, and compressing the blend into a tablet form, which may be optionally coated, such as with an enteric coating composition, to provide delayed release and / or to enhance stability of the active agent.
Owner:INTELGENX CORP
Melatonin two-layer release-controlled tablet and preparing process thereof
InactiveCN1488346AEffective plasma concentrationEffective hypnosisOrganic active ingredientsNervous disorderVitamin b6Polyethylene glycol
The invention is a melatonin double-layer controlled release tablet and making technique, using melatonin as main raw material, and composed of quick-release layer and sustained-release layer. The quick-release layerí»s components: melatonin 1-5%, Vitamin B6 3-15%, lactose 10-70%, pre-gel amylum 10-70%, hypromellose 0.03-0.3%, magnesium stearate 0.3-2% and SiO2 1.0-3.0%; the sustained layerí»s components: melatonin 2-6%, hypromellose 15-60%, stearic acid 15-65%, polyethylene glycol 10000 10-30%, and magnesium stearate 1.0-5.0%. It has efficacies of quickly hypnotizing and prolonging effective time of sleeping.
Owner:CHONGQING TAIJI MEDICAL RES INST CO LTD
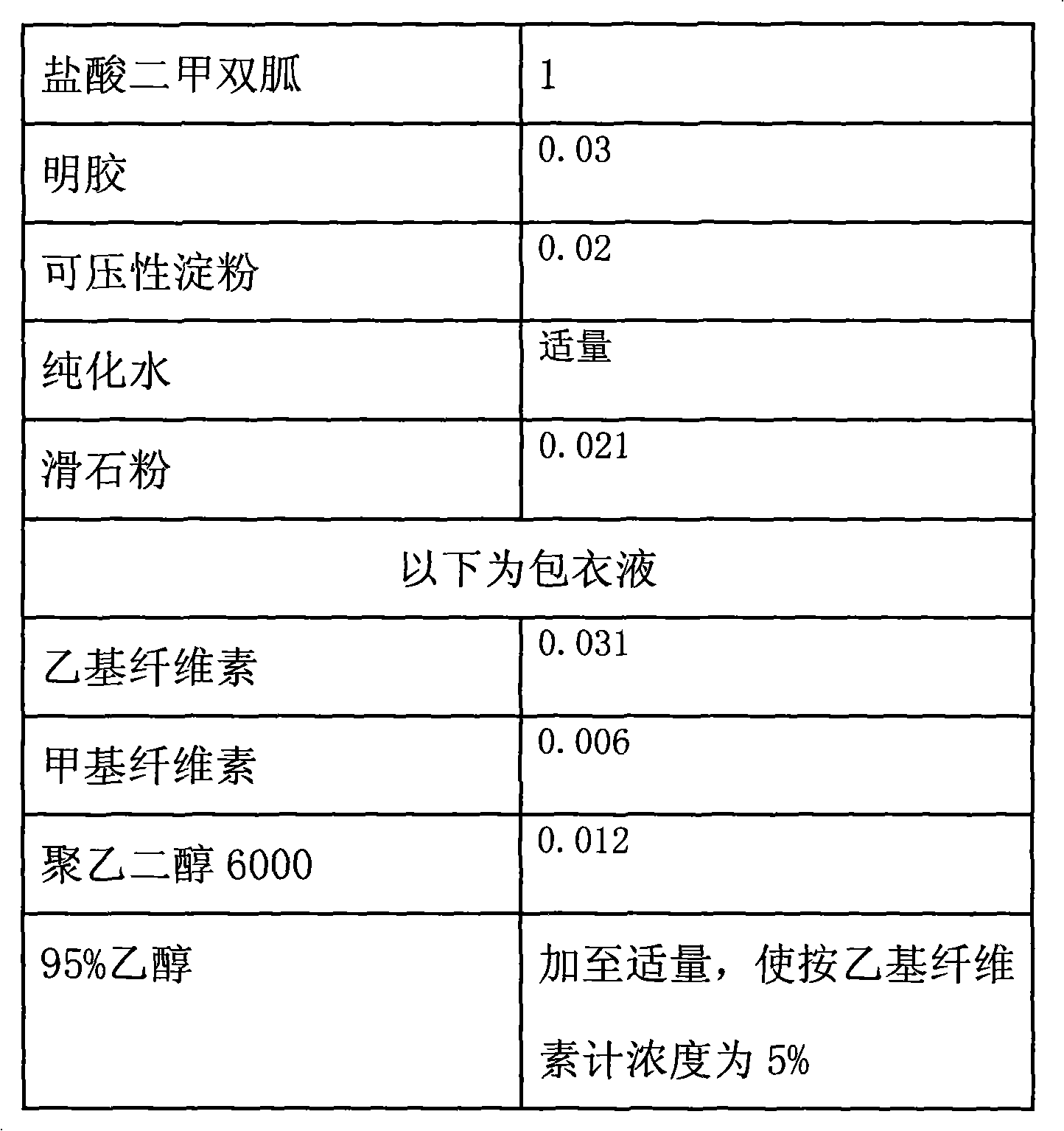
Metformin hydrochloride controlled-release tablet and preparation method thereof
ActiveCN101579325ALess weight gainIncrease production capacityOrganic active ingredientsMetabolism disorderPharmaceutical industryMetformin Hydrochloride
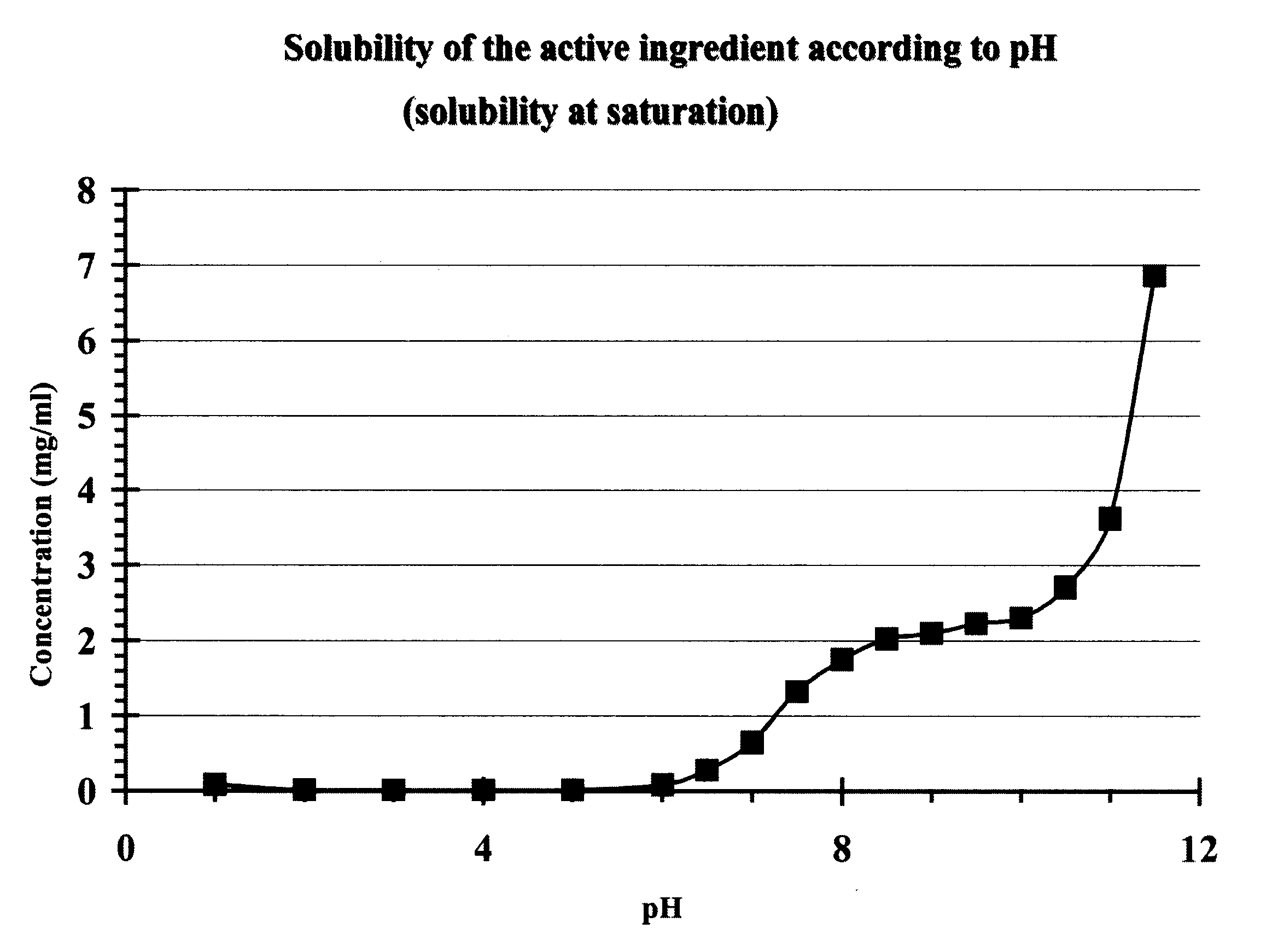
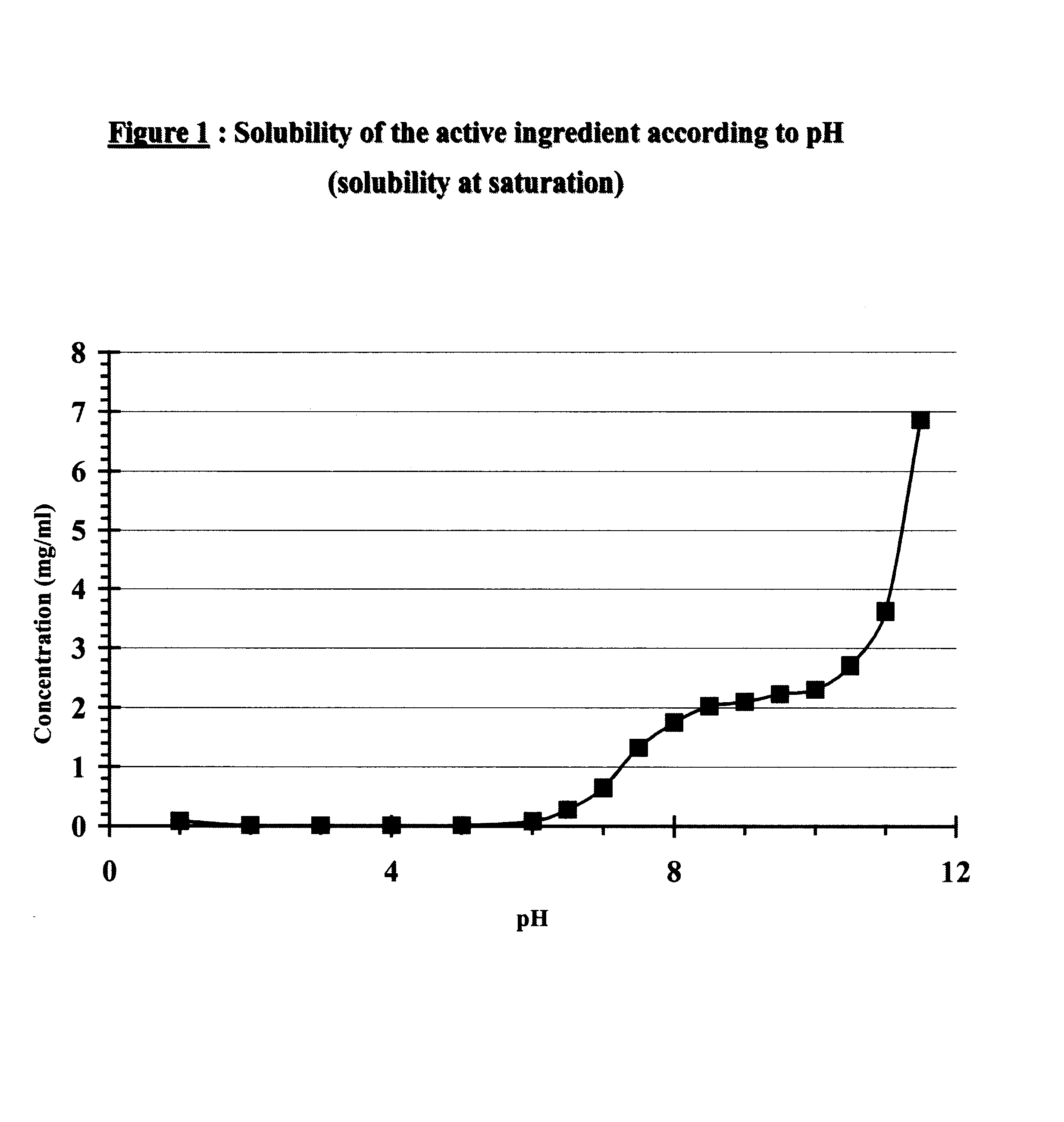
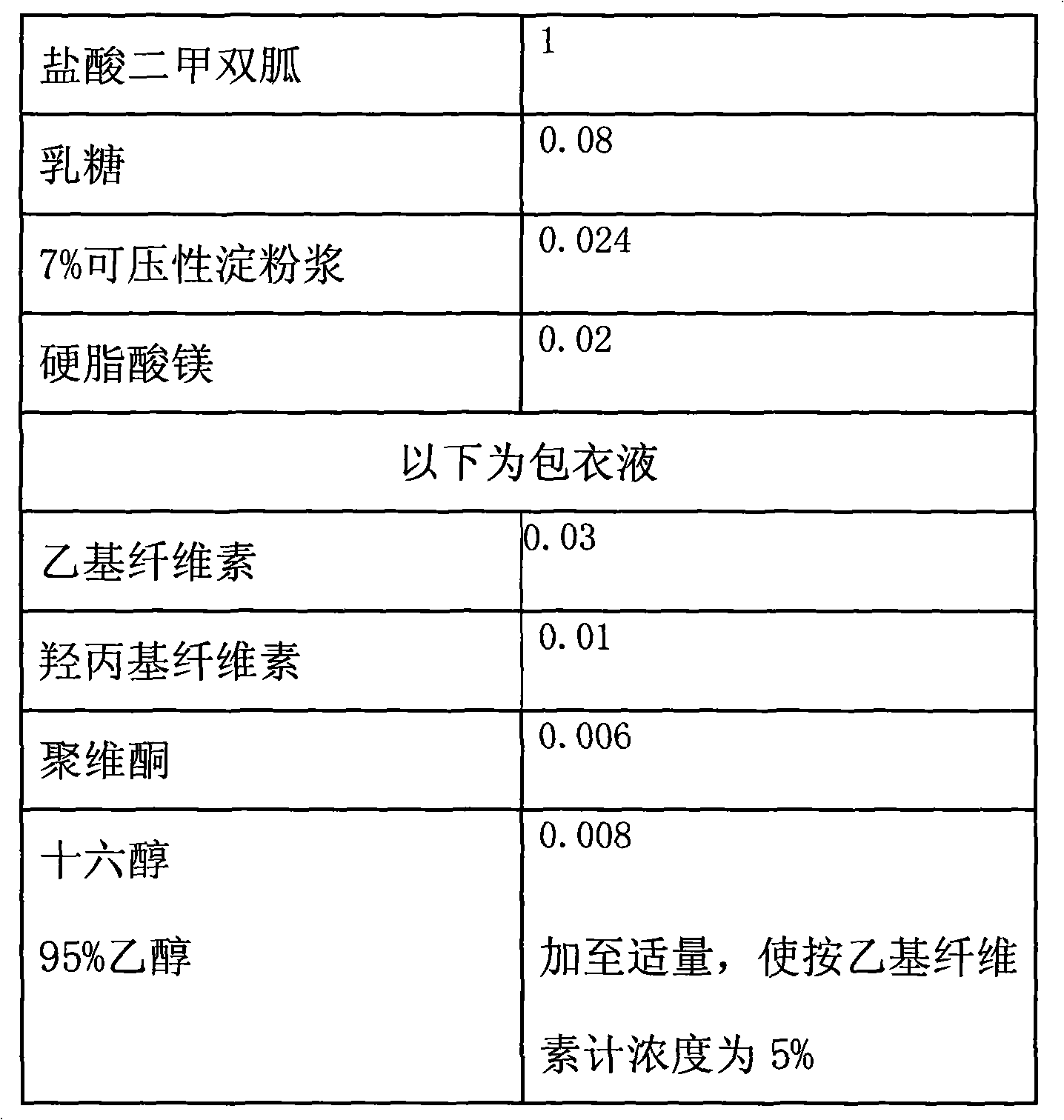
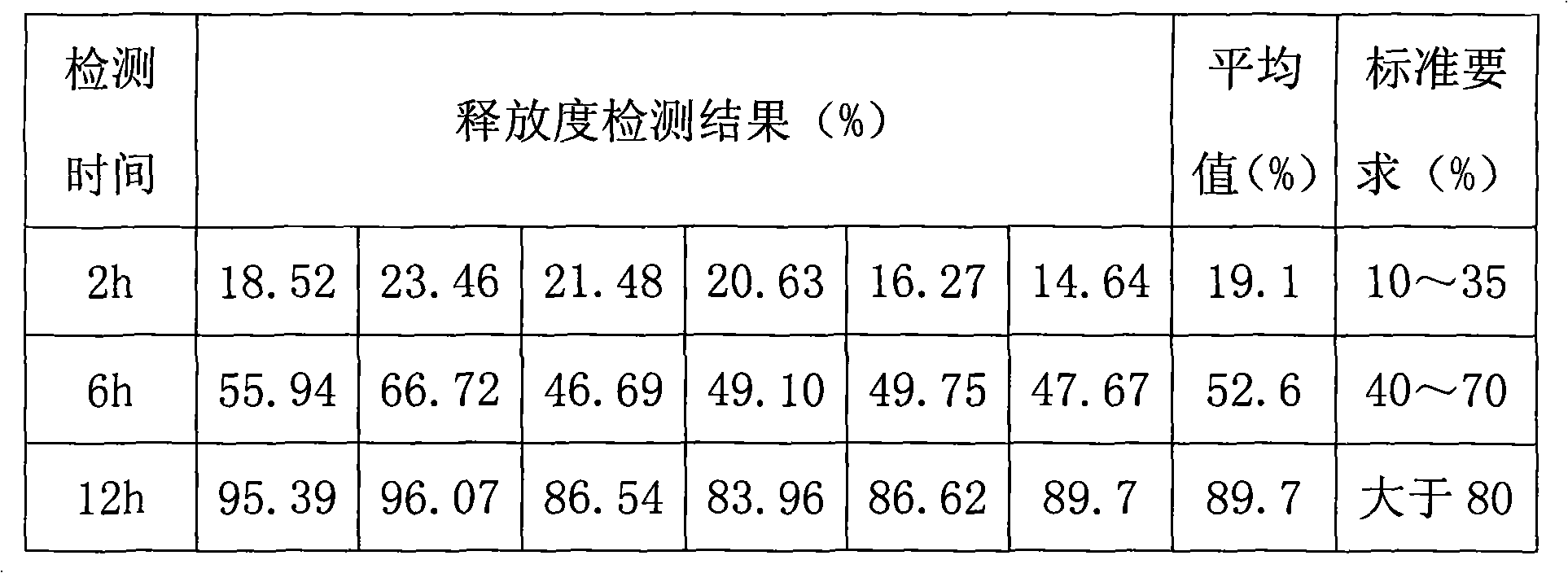
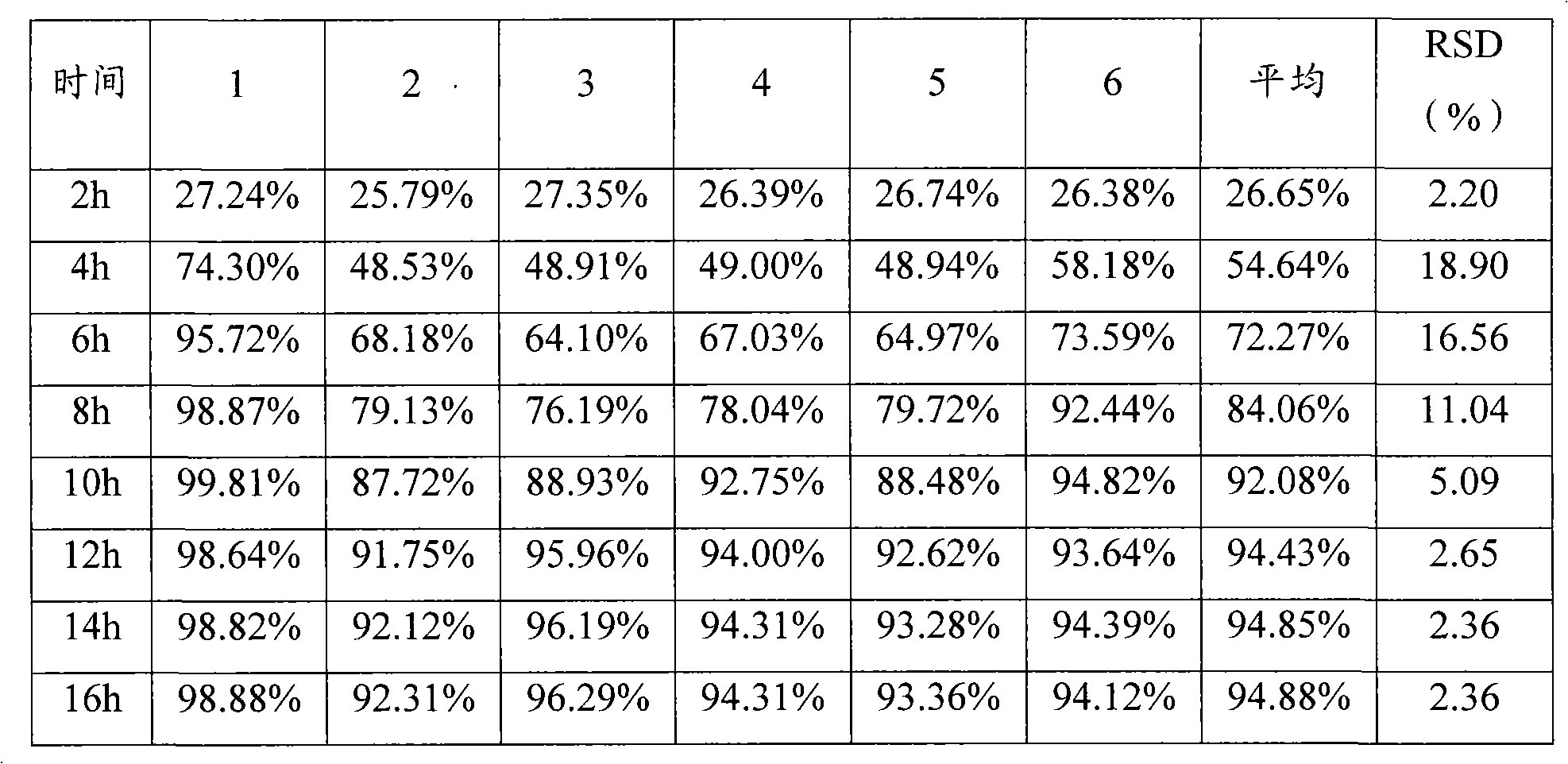
The invention provides a metformin hydrochloride controlled-release tablet, which comprises a metformin hydrochloride controlled-release tablet with effective dose and pharmaceutical accessories and is characterized in that the metformin hydrochloride controlled-release tablet uses the common tabletting, and the controlled-release effect is controlled by totally depending on the film coating technique. The coating adopted by the invention is a releasing system comprising the prescription which can cause the medicine to reach release degree standard in vitro. The preparation method in the invention is simple and convenient; the process conditions are easy to control and suitable for batch production, can use conventional production equipment in pharmaceutical industries for economically and conveniently producing the metformin hydrochloride controlled-release tablet on a large scale, can effectively and stably cause the release degree of the metformin hydrochloride controlled-release tablet in the second hour to be 10% to 35%, the release degree to be 40% to 70% in the sixth hour and the release degree to be more than 80% in the twelfth hour.
Owner:CHONGQING CONQUER PHARML
Oral controlled release tablet
A method of reducing the risk of alcohol-induced dose-dumping of a therapeutically active ingredient comprising administering to human subjects who have ingested alcohol an oral controlled release tablet; said tablet comprising:a core comprisingan upper compressed layer comprising a swelling agent, anda lower compressed layer comprising at least one therapeutically active ingredient, and pharmaceutically acceptable excipient wherein at least one excipient is a release rate controlling excipient and wherein the percent by weight of excipients that are soluble in alcohol does not exceed 35% by weight of the layer and;a coating surrounding the said core, the coating comprising a polymer insoluble in an aqueous medium comprising from 0% v / v to 40% v / v of alcohol, whereby upon contact with aqueous gastrointestinal fluids, the upper compressed layer swells to cause removal of the coating from the upper surface of the upper compressed layer and then said upper layer disintegrates allowing the release of the active ingredient from the defined surface area of the upper surface of said lower compressed layer with the coating covering its bottom and side surfaces.
Owner:SUN PHARMA INDS
Method for alleviating signs and symptoms of spasticity
ActiveUS8426470B2Lower Level RequirementsAlleviation of signBiocideOrganic active ingredientsSedative EffectsImmediate release
A method of alleviating signs and symptoms of spasticity in human patient comprising orally administering to said human patients once in a day a controlled drug delivery system comprising an effective daily dose of baclofen or its pharmaceutically acceptable salt. The controlled drug delivery system is operable to produce a level of sedation lower than a sedation produced by three times a day immediate release tablets. A total daily dosage of the controlled release tablets and a total daily dosage of the three times a day immediate release tablets remain same.
Owner:SUN PHARMA INDS
Paliperidone double-layered osmotic pump controlled release tablet and preparation method thereof
ActiveUS20120301547A1Increase release rateRapid onsetOrganic active ingredientsBiocideDrug release rateHydrophilic polymers
A paliperidone double-layered osmotic pump controlled release tablet and the preparation method thereof are disclosed. The double-layered osmotic pump controlled release tablet comprises a rigid membrane, a push layer, a drug layer, an isolation layer and an aesthetic coating, wherein the rigid membrane contains a semi-permeable polymer, a porogen and / or a plasticizer and has one or more drug release orifices on one end, the push layer comprises an expanding material, an osmotic agent, a binder, a colorant and a lubricant, the drug layer contains a pharmaceutically active ingredient, a hydrophilic polymer, an osmotic agent, a colorant, a lubricant and an antistatic agent, the isolation layer is located between the inner surface of the rigid membrane and the push layer, and contains a hydrophilic polymer. The paliperidone double-layered osmotic pump controlled release tablet shows an increasing drug release rate at early stage and keeps a constant drug release rate at later stage.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Controlled release formulation of divalproex sodium
A controlled release tablet formulation which permits once daily dosing in the treatment of epilepsy comprises from about 50 weight percent to about 55 weight percent of an active ingredient selected from the group consisting of valproic acid, a pharmaceutically acceptable salt or ester of valproic acid, divalproex sodium, and valpromide; from about 20 weight percent to about 40 weight percent hydroxypropyl methylcellulose; from about 5 weight percent to about 15 weight percent lactose, from about 4 weight percent to about 6 weight percent microcrystalline cellulose, and from about 1 weight percent to about 5 weight percent silicon dioxide having an average particle size ranging between about 1 micron and about 10 microns; all weight percentages based upon the total weight of the tablet dosage form. Also disclosed are pre-tableting granular formulations, methods of making the granular formulations and tablets, and a method of treating epilepsy employing the controlled release tablet formulations of the invention.
Owner:ABBOTT LAB INC
Nifedipine osmotic pump controlled release tablet and preparation method thereof
InactiveCN102138912AMaintain blood levelsGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismNifedipineControlled Release Tablet
The invention provides a nifedipine osmotic pump controlled release tablet comprising a drug-containing layer tablet core, a booster layer tablet core, a coating membrane and a single drug-release pore on the surface of the controlled release tablet at one side of the drug-containing layer tablet core. The nifedipine osmotic pump controlled release tablet provided by the invention has stable medicament release velocity, basically realizes zero drug release within 0-20h and basically fully releases drug; therefore, the dosing number of a patient is reduced, and more stable blood drug concentration can be realized after a patient takes the drug. The nifedipine osmotic pump controlled release tablet provided by the invention is a safe, effective, stable, controllable and conveniently-applied medicament new preparation for clinically treating hypertension.
Owner:CHINA PHARM UNIV
Colchicine framework controlled release tablets or capsules
InactiveCN101485637AStable release rateHigh Cumulative Release RateOrganic active ingredientsSkeletal disorderHydrophilic polymersSide effect
The invention discloses a colchicine framework controlled-release tablet or capsule, which comprises colchicines and framework materials having controlled-release function, wherein the framework materials contain hydrophilic polymers of which the weight against the weight of the tablet is at least 20 percent and other pharmaceutic adjuvants; and the tablet or capsule comprises the following components in percentage by weight: 0.01 to 1.0 percent of colchicine, 20 to 85 percent of hydrophilic polymer, 1 to 30 percent of retarder, 20 to 60 percent of bulking agent, and 0.1 to 2 percent of lubricant. Release degree test results show that more than 90 percent of drugs are continuously released in vitro in 12 hours; and compared with the common tablet, the tablet or capsule has the advantages of obviously reducing daily taking frequency, lowering toxic and side effects of the drugs and improving the compliance and curative effect of patients.
Owner:普尔药物科技开发(深圳)有限公司
Novel dosage form of sinomenine medicament or hydrochlorate thereof and preparation technique thereof
The invention discloses a sinomenine or an enteric-coated controlled-release tablet of hydrochloride thereof. The prepared enteric-coated controlled-release tablet hardly releases the drug in artificial simulated gastric juice, but can slowly and smoothly release the drug in artificial simulated intestinal juice; the sustained release time of the drug can achieve more than 12 hours or even 24 hours; the enteric-coated controlled-release tablet is taken once or twice daily, the plasma drug concentration in vivo is smooth, and the peak-valley phenomenon of the plasma drug concentration is reduced; as the prepared enteric-coated controlled-release tablet hardly releases the drug in stomach, the contacted concentration of the drug with the gastric mucosa is small, the stimulation of the stomach caused by the drug is alleviated. As the prepared enteric-coated controlled-release tablet sustainedly slowly releases the drug in intestinal tract, the times of the drug administration are reduced, and the patient compliance is improved, thereby being applicable to the needs of the clinical development.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Nifedipine controlled-releasing tablet and preparation method thereof
ActiveCN101167700AShort time lagQuick effectOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineMedicine
The invention discloses nifedipine controlled release medicament, which contains a pastille layer and a boosting layer with the proportion of 1: 0.5-3 by weight, wherein the pastille layer contains nifedipine and carrying agent which is the homopolymer of vinylpyrrohdone and / or copolymer of vinylpyrrohdone and is 40-99% of the pastille layer, the boosting layer includes infiltration promoting polymer which is 10-80% of the boosting layer by weight, insoluble polymer which is 10-80% of the boosting layer by weight, and the other component is osmotic pressure accelerant The rate of medicament releasing controlled by nifedipine can make the medicament release the nifedipine in 24 hours by administering drug once a day.
Owner:OCEAN STAR INT
Monolayer osmotic pump controlled releasing tablets of nimodipine
InactiveCN1552323ALasting effectGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismCellulose acetatePolyethylene glycol
A release controlled nisoldipine tablet with single-layer osmotic pump for treating hypertension, angina pectoris, heart failure, etc contains nisoldipine, the release-controlling auxidiary chosen from sodium chloride, potassium chloride, glyceride behenate, etc, and other auxiliaries. Its advantages are sure and durable curative effect and low toxic by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Tablet of isosorbide mononitrate
ActiveCN101732276AEasy to pumpStable release ratePill deliveryHeterocyclic compound active ingredientsMedicineSemipermeable membrane
The invention relates to a tablet of isosorbide mononitrate, in particular to a double-layer osmotic pump controlled release tablet of isosorbide mononitrate, which belongs to the field of medicine preparation. A single-chamber double-layer osmotic pump tablet of the isosorbide mononitrate is characterized in that a semi-transparent coating film cover a double-layer core body consisting of a medicine-containing layer and a boosting layer; and the coating film is provided with a medicine releasing pore on the surface of the medicine-containing layer. The gastrointestinal tract water enters a double-layer tablet chip through the semi-transparent film; the medicines forms a mixed suspension liquid or solution when contacting water in the medicine-containing layer; a penetration enhancer enables the solution of the medicine-containing layer to be hypertonic so that an osmotic pressure difference exists between the inner side and the outer side of the film, which is beneficial to pumping the medicines out; and the pressure is generated in the boosting layer through water absorption, dissolution and expansion of a penetrating agent so as to further boost a medicine liquid to eject the pore.
Owner:LUNAN BETTER PHARMA
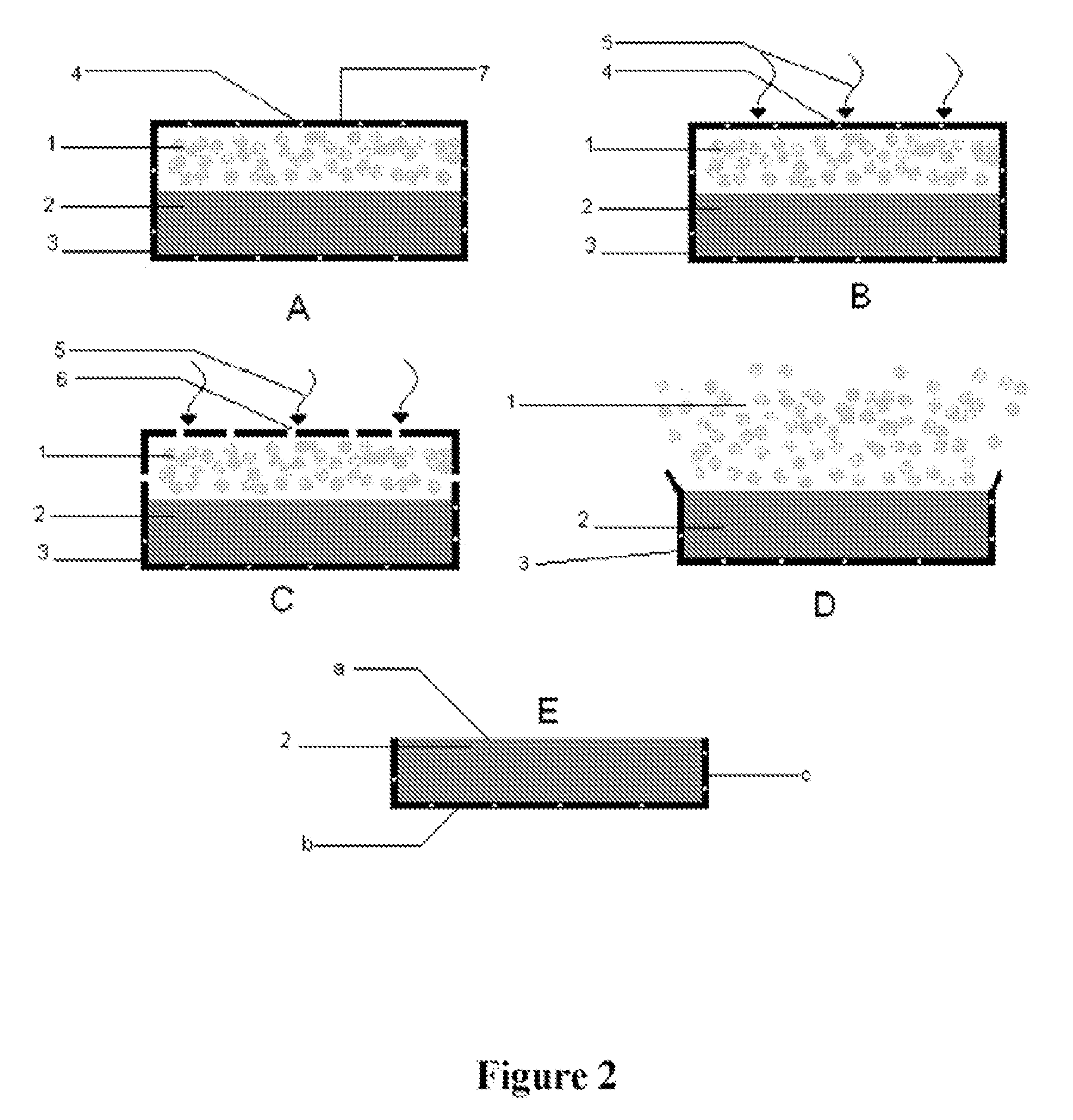
Compound pioglitazone hydrochloride/metformin hydrochloride bilayer osmotic pump controlled release preparation and preparation method thereof
InactiveCN102008472AImprove controllabilityStable storageOrganic active ingredientsMetabolism disorderCoated tabletsControl release
The invention provides a compound preparation of pioglitazone hydrochloride and metformin hydrochloride bilayer osmotic pump controlled release tablets. The compound preparation structurally comprises the following parts from inside to outside in sequence: a tablet core comprising a drug layer and a digestive layer, an insulation coating layer, a controlled release coating film with drug release holes, a rapid pioglitazone hydrochloride release layer and an unnecessary attractive coating. The invention also provides a preparation method of the compound preparation of pioglitazone hydrochloride and metformin hydrochloride bilayer osmotic pump controlled release tablets. The preparation method comprises the following steps of: (1) preparing the drug layer; (2) preparing the digestive layer; (3) tabletting the tablet core; (4) coating insulation coating layer; (5) coating the controlled release coating; (6) punching the coated tablet: (7) coating the rapid pioglitazone hydrochloride release layer; and (8) coating the attractive coating.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Metformin controlled release tablet
The invention relates to a metformin controlled release tablet compound which contains an effective dose of metformin and polyoxyethylene and selectively contains or excludes one or more pharmaceutically acceptable auxiliary materials. The invention also relates to a metformin controlled release tablet which comprises a single-layer tablet core and a coating membrane covering the tablet core, wherein medicine release hole(s) is / are arranged on one side or two sides of each tablet, the tablet core contain an effective dose of the metformin and the polyoxyethylene and selectively contains one or more pharmaceutically acceptable auxiliary materials, and the coating membrane is a semi-permeable membrane.
Owner:冯岩
Pain relieving bilayer controlled-release tablet and preparation method thereof
ActiveCN103655505AImprove physical stabilityTightly boundAntipyreticAnalgesicsSide effectBlood concentration
The invention provides a pain relieving bilayer controlled-release tablet which comprises a quick release layer and a slow release layer, wherein holes are formed in the slow release layer; the holes are filled with quick release particles; the quick release layer and the quick release particles consist of pain relieving drugs and pharmaceutical adjuvant; the slow release layer consists of pain relieving drugs, slow release materials and pharmaceutical adjuvant. The pain relieving bilayer controlled-release tablet has the following technical effects: 1) the bilayer tablet has better physical stability than a common bilayer tablet, so that the storage and the transportation are convenient; 2) the disintegration time limit of the quick release layer of the bilayer controlled-release tablet is 10-30 seconds through the detection of the dissolving experiment; the slow release layer presents the zero-level release mode; the medicine taking effectiveness and safety of patients are largely improved. In the preparation process of the bilayer tablet, the quick release particles are filled in the holes. The quick disintegration of the quick release layer after the medicine taking is guaranteed through the drug release mode of combining the quick release with the slow release, so that the blood concentration can quickly achieve the range of a therapeutic window; the slow release layer is slowly released in a longer time period to continuously maintain the treatment effect; the toxic or side effects are effectively controlled.
Owner:越洋医药(广州)开发有限公司
Sarpogrelate hydrochloride single layer osmotic pump regulated-release preparations and preparation thereof
InactiveCN101259112ALasting effectGood curative effectOrganic active ingredientsPill deliverySide effectAdhesive
The invention relates to the medicine technical filed, in particular to a monolayer osmotic pump controlled release preparation of sarpogrelate hydrochloride and a preparation method thereof, which is characterized by lasting drug effect, constant curative effect, slight toxic and side effect. The monolayer osmotic pump controlled release preparation of sarpogrelate hydrochloride comprises by weight percentage of 1 to 40 percent of sarpogrelate hydrochloride, 30 to 90 percent of auxiliary material that can promote osmosis, 1 to 40 percent of membrane material that performs controlled release and the rest is other auxiliary material; the diameter of a small drug release hole is 0.1 to 2.0 mm. The preparation method comprises: a certain amount of drug according to the prescription is mixed with an adhesive, a bulking agent and a co-penetrant which are respectively ground and screened, prepared into soft material and palletized, dried and particle finished and then added with a lube and pressed to obtain tablet core. Coating liquid is prepared and the tablet core coated, after coating, drying is done to solidify the coating membrane, and then one side of coated tablet is provided with the small drug release hole, thus obtaining an osmotic pump controlled release tablet. The monolayer osmotic pump controlled release preparation of sarpogrelate hydrochloride of the invention has the advantages of reducing times of dosage, promoting the compliance of sufferers and satisfying the requirements of clinical medication.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL COLLEGE
Preparation method of glipizide osmotic pump controlled release tablet
ActiveCN102133205AFast dissolutionEasy to acceptMetabolism disorderSulfonylurea active ingredientsSolubilityTreatment effect
The invention relates to a preparation method of a glipizide osmotic pump controlled release tablet. A glipizide tablet core is prepared by the following steps of semipermeable membrane wrapping, laser boring and damp-proof layer wrapping. The preparation method is characterized in that the preparation of the glipizide tablet core comprises preparation of solid dispersoid and preparation of the tablet core. In the preparation method, a solid dispersion technology is utilized, so that the dissolving speed of medicines is improved; the solubility of the glipizide is improved through the fluxing auxiliary materials; and a proper amount of penetration promoter is added to adjust the osmotic pressure so as to achieve constant-speed or approximately-constant-speed release. The invention has the advantages that the process is simple, the cost is low, the release tablet is easy to accept by patients and the treating effect is good.
Owner:SHANDONG XINHUA PHARMA CO LTD
Doxazosin-mesylate controlled-releasing tablet and preparation method thereof
ActiveCN101167728AImprove complianceStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectGeneration rate
The invention discloses methanesulfonic acid doxazosin controlling releasing tablets and a process for preparation, which contains a single-layer core, insoluble semi-permeable membrane, single drug-releasing eyelet on each side of the tablet, and depends on the osmotic pressure difference between inside and outside of the semi-permeable membrane medium. The invention realizes the control of linear releasing of methanesulfonic acid doxazosin by single-layer core structure, and can remain effective and steady blood and drug concentration. The curative effect is improved, the generation rate of side effect is reduced, and the productive technology is greatly simplified.
Owner:HEFEI LIFEON PHARMA
Lisinopril controlled-release tablet and preparation method thereof
InactiveCN103006612AThe effect of delayed releaseAchieve the effect of releaseDipeptide ingredientsPharmaceutical delivery mechanismFoaming agentWater insoluble
The invention relates to a lisinopril controlled-release tablet and a preparation method thereof, belonging to the field of pharmaceutic preparation technology and solving the problem that existing lisinpopril controlled-release tablet cannot delay release and release mildly. The tablet consists of core of the tablet containing lisinopril and a coating wrapped outside the core of the tablet, the core of the tablet containing the lisinopril comprises the following components by weight percent: 5.0%-20% of lisinopril, 25%-55% of framework material, 20%-50% of filler, and1.0%-3.0% of lubricant, the coating comprises the following components in weight percent: 60%-90% of water insoluble coating material and 10%-40% of pore-foaming agent. The method comprises the preparation of core of the tablet containing lisinpopril, the preparation of coating liquor, and the preparation of the tablet by wrapping the coating liquor outside the core of table so as to form the coating. The lisinopril controlled-release tablet can delay release and release mildly, and the method is simple and beneficial to industrialization.
Owner:石雷 +1
Nisoldipine double layer penetrated pump control releasing tablets
InactiveCN1439372AReduce the number of dosesEasy to takeOrganic active ingredientsPharmaceutical delivery mechanismCellulose acetateLeft ventricular size
A release controllable nisoldipine with dual-layer osmotic pump for treating cardiovascular diseases including hypertension, angine pectoris, left ventricular disfunction and congestive heart failure contains nisoldipine (5-20 wt.%), polyoxyethene and / or sodium chloride and / or hydroxypropyl methylcellulose as the release controlling agent of medicine layer, polyoxyethene and / or hydroxypropyl methylcellulose and / or ethylcellulose as the release controlling agent of boosting layer, and acetate cellulose and / or ethylcellulose and / or hydroxypropyl methylcellulose and / or polyethanediol as release-controlling membrane.
Owner:SHENYANG PHARMA UNIVERSITY
Tamsulosin hydrochloride double-layer osmotic pump controlled-releasing tablet and preparation method thereof
InactiveCN101167701AStable concentrationRelease constant speed evenlyInorganic non-active ingredientsPharmaceutical delivery mechanismExcipientMoisture
The invention provides double-layer osmotic pump tablets of tamsulosin ehydrochloride and a process for preparation. The medicament contains tamsulosin ehydrochloride and acceptable medical polymeric excipient, and is characterized in that the invention has excellent zero-level controlled releasing, pH level of environment, movements of the stomach and intestine, and food, has little effect on releasing action and food, and has no effect on the internal pharmacokinetics parameter. According to the percentage by weight, the preparation contains tamsulosin ehydrochloride 0-2%, excipient in pastille layer with the function of controlled-releasing 30-70% excipient in boosting layer with the function of controlled releasing 30-70%, and the rest percentage of other excipient. The of process for preparation the double layer permeable pump controlled-release tablets of tamsulosin ehydrochloride comprises (1) the preparation of pastille layer, (2) the preparation of boosting layer, (3) the compressing of the two layers, (4) the coating of the double layer tablets, (5) the perforating of the coated tablets, (6) the packing of moisture proof cost. The invention is clinically used for the treatment of paruria symptom like frequent micturition, diuresis at night, dysuria caused by prostatic hyperplasia.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Fenofibrate osmotic pump controlled release preparation and preparation method thereof
InactiveCN101422443AAdjust the rate of constant releaseImprove complianceOrganic active ingredientsMetabolism disorderSide effectFoaming agent
The invention belongs to the field of medicament preparation and discloses a fenofibrate osmotic pump type controlled release preparation and a preparation method thereof. The preparation is formed by an osmotic pump tablet core and a controlled release coat coated outside the tablet core. The weight of the osmotic pump controlled release tablet core is as follows: 250mg / tablet of fenofibrate, 25mg to 100mg / tablet of osmotic active matter, 200mg of 300mg / tablet of accessory which can lead the medicament in a medicine containing layer to be easily released, 75 to 150mg / tablet of accessory which leads the medicament to be easily released in a promoting layer and proper amount of other accessories. The weight of the osmotic pump coat is as follows: 15g to 30g / 100 tablets of semi-transparent membrane coat macromolecule material and 2 to 10g / 100 tablets of pore-foaming agent. The invention can effectively adjust the release speed of the medicament by adjusting the tablet core and the prescription of the coat and obtain more stable and durable effective blood medicine concentration, thereby reducing the side effect and the taking times of the medicament, ensuring that the suction of themedicament is not affected by whether the patient eats or not and the environment in a body, and improving the compliance of a sufferer. The invention can be broadly applied to curing hyperlipemia.
Owner:SHENYANG PHARMA UNIVERSITY
Levetiracetam osmotic pump controlled release tablet and preparation method thereof
InactiveCN101422442ASmooth and sustained releaseReduce toxic and side effectsNervous disorderPharmaceutical delivery mechanismSide effectActive matter
The invention belongs to the technical field of medicament and provides a Levetiracetam osmotic pump controlled-release tablet and a preparation method thereof. The invention consists of the accessory of the Levetiracetam playing the effect of release control and a semi-transparent membrane; in the invention, proper accessory and medicament are mixed to press a tablet core at first; then a layer of semi-transparent membrane is coated outside the tablet core; then at least one small hole is punched on the semi-transparent membrane so as to lead active matters to be released from the semi-transparent membrane, thereby controlling the release of the medicament. Compared with a common preparation, the controlled-release preparation prepared by the invention has the advantages of small wave range of the blood medicine concentration, reducing toxic and side effect, being taken once in one day and improving the compliance of sufferers. The controlled-release preparation is applied on the adjunctive therapy for the partial seizure of epileptics in clinic.
Owner:SHENYANG PHARMA UNIVERSITY
Febuxostat osmotic pump controlled release tablet for treating gout and preparation method
InactiveCN102641255AOrganic active ingredientsInorganic non-active ingredientsCellulose acetateAdhesive
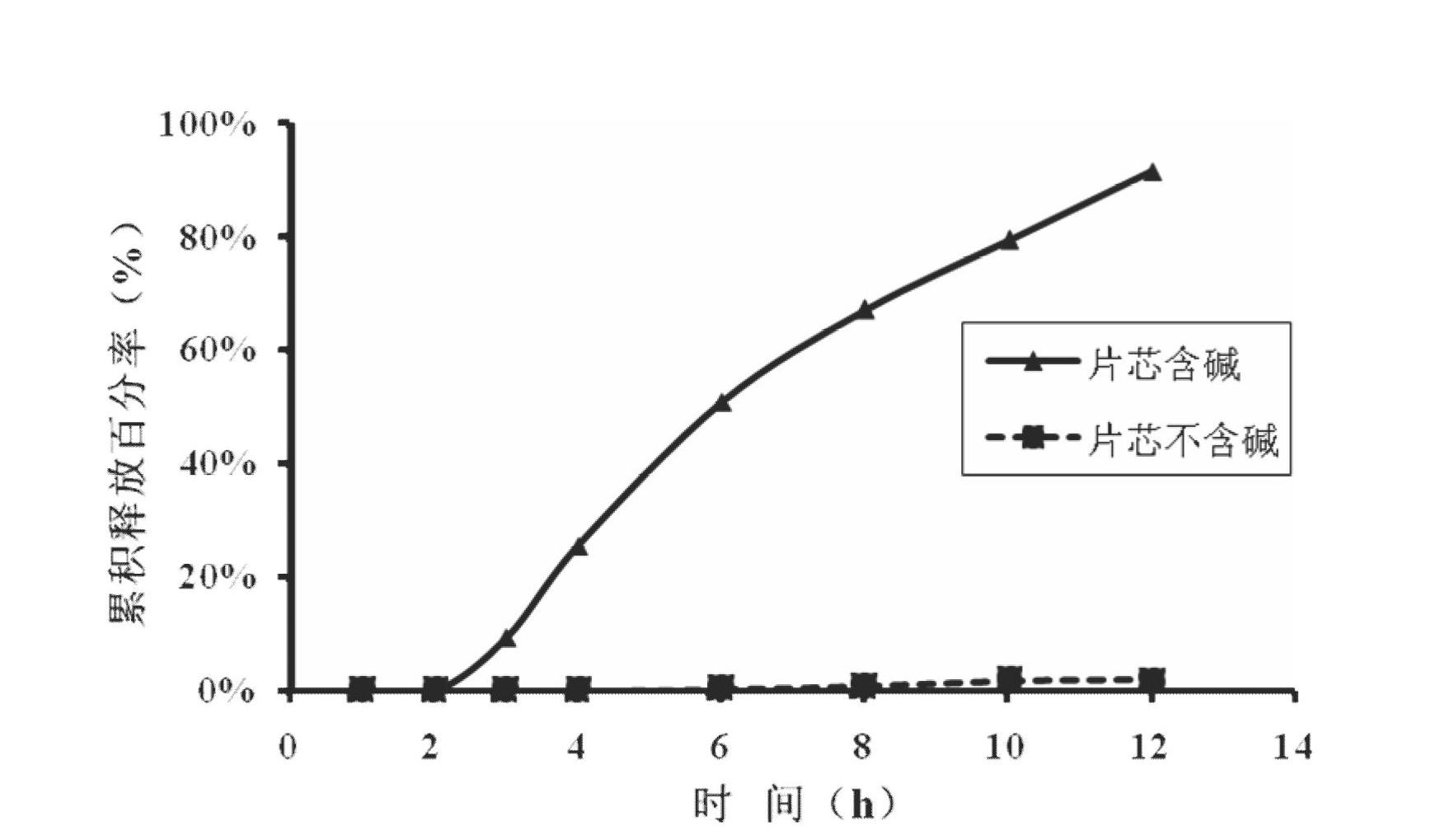
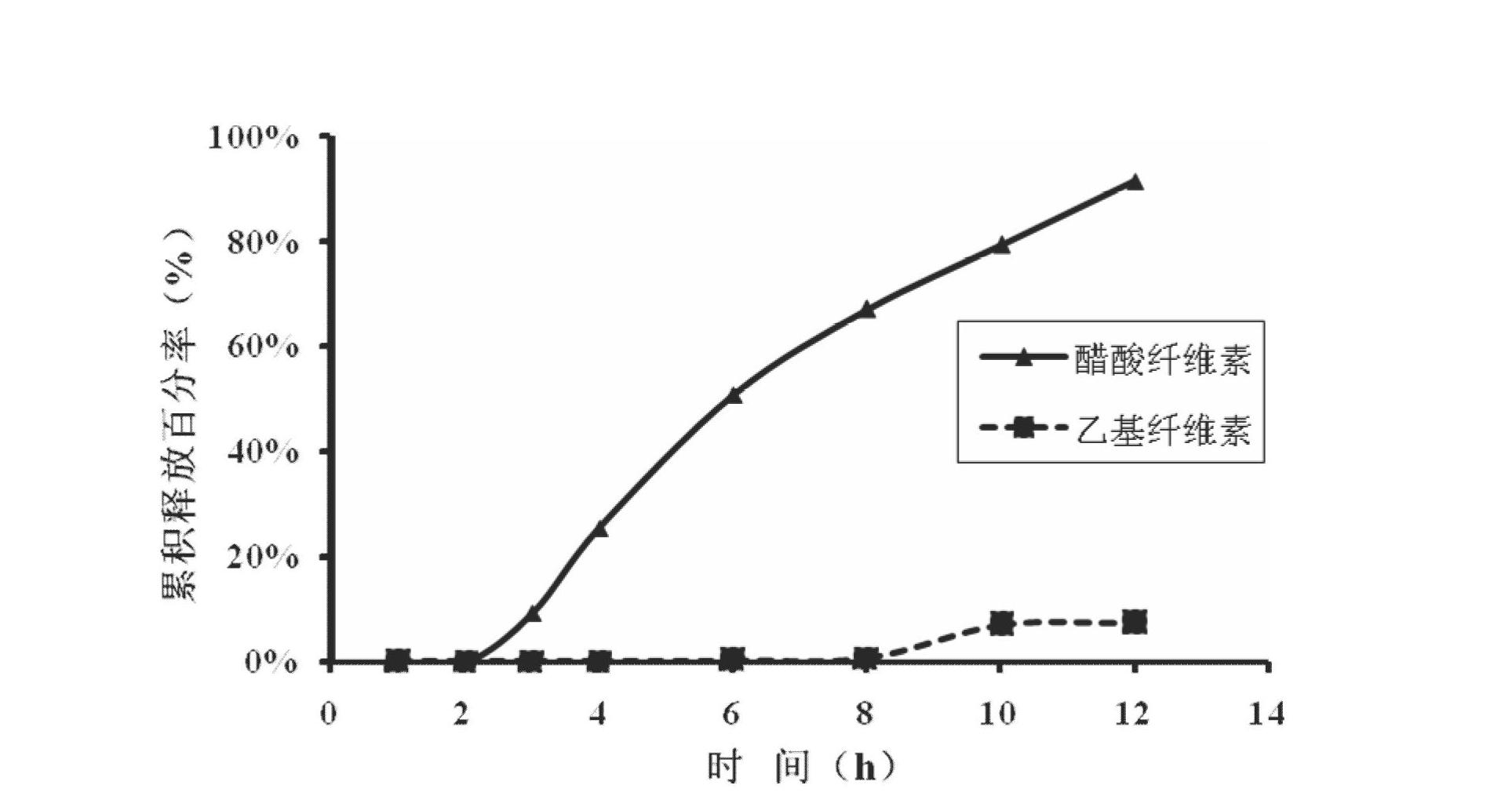
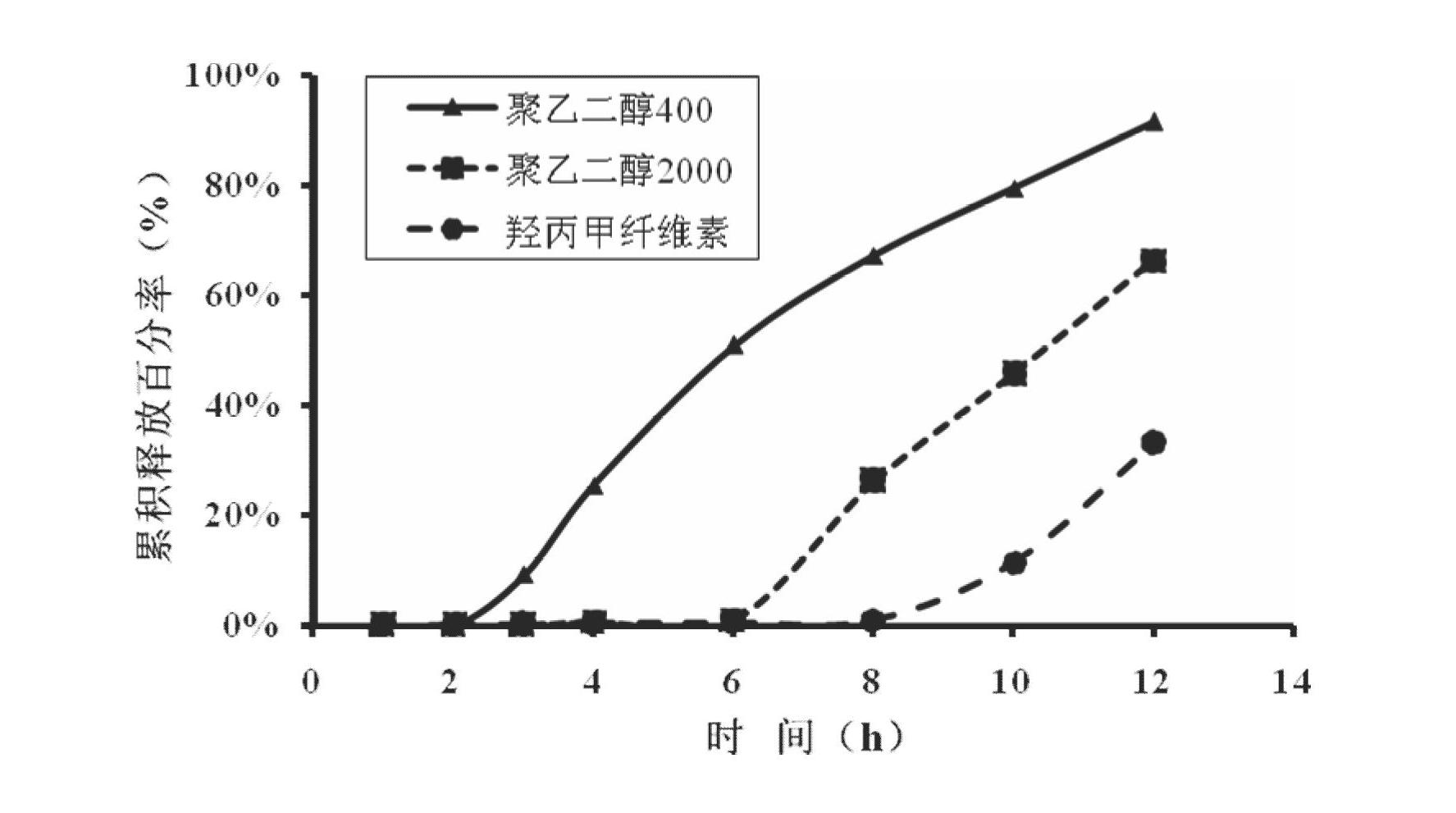
The invention relates to a medical preparation containing a Febuxostat compound and with special physical shape as a characteristic, in particular to a Febuxostat osmotic pump controlled release tablet for treating gout. The osmotic pump controlled release agent comprises a core tablet and a coat and is characterized in that the core tablet is composed of the following components by weight: 10% to 40% of Febuxostat, 30% to 60% of osmotic pressure active substance, 4% to 20% of alkaline substance, 0% to 30% of filler, 1% to 5% of adhesive and 0.2% to 2% of lubricant. The coat is composed of the following components by weight: 40% to 70% of cellulose acetate, 20% to 40% of polyethylene glycol 400 and 10% to 20% of phthalic acid diethyl ester. The tablet resolves the problem that the Febuxostat is slightly soluble medicine and is hard to release by adding the alkaline substance into the core tablet, is capable of releasing over 90% of medicine within 12 hours by screening optimization of film formation materials, porogen and plasticizers in the coat, and satisfies zero level dynamic medicine releasing characteristics. The Febuxostat osmotic pump controlled release tablet for treating gout can be used for treating gout and has the advantages of being durable in effect, safe and easy to produce and prepare.
Owner:SOUTHERN MEDICAL UNIVERSITY
Tofacitinib controlled release tablet, preparation method and application of Tofacitinib controlled release tablet
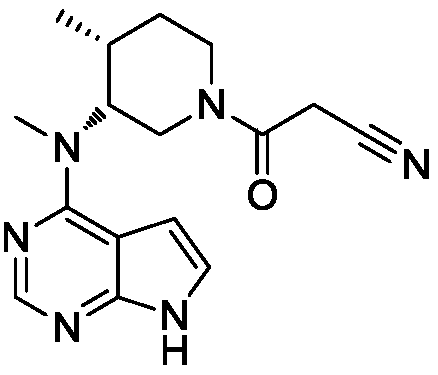
ActiveCN111150711AReduce adverse effectsReduce manufacturing costOrganic active ingredientsNervous disorderOrganic solventTofacitinib
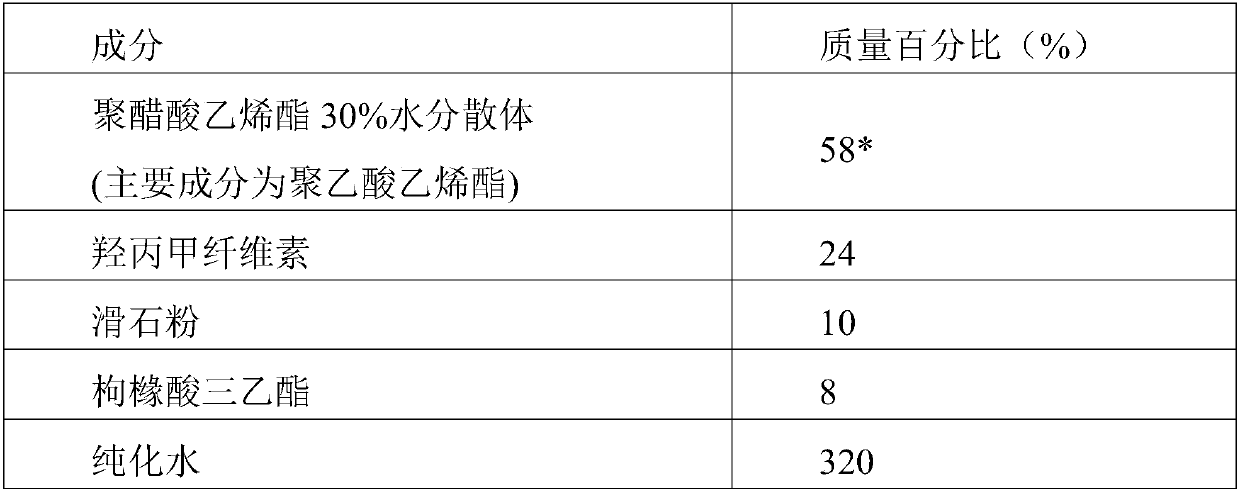
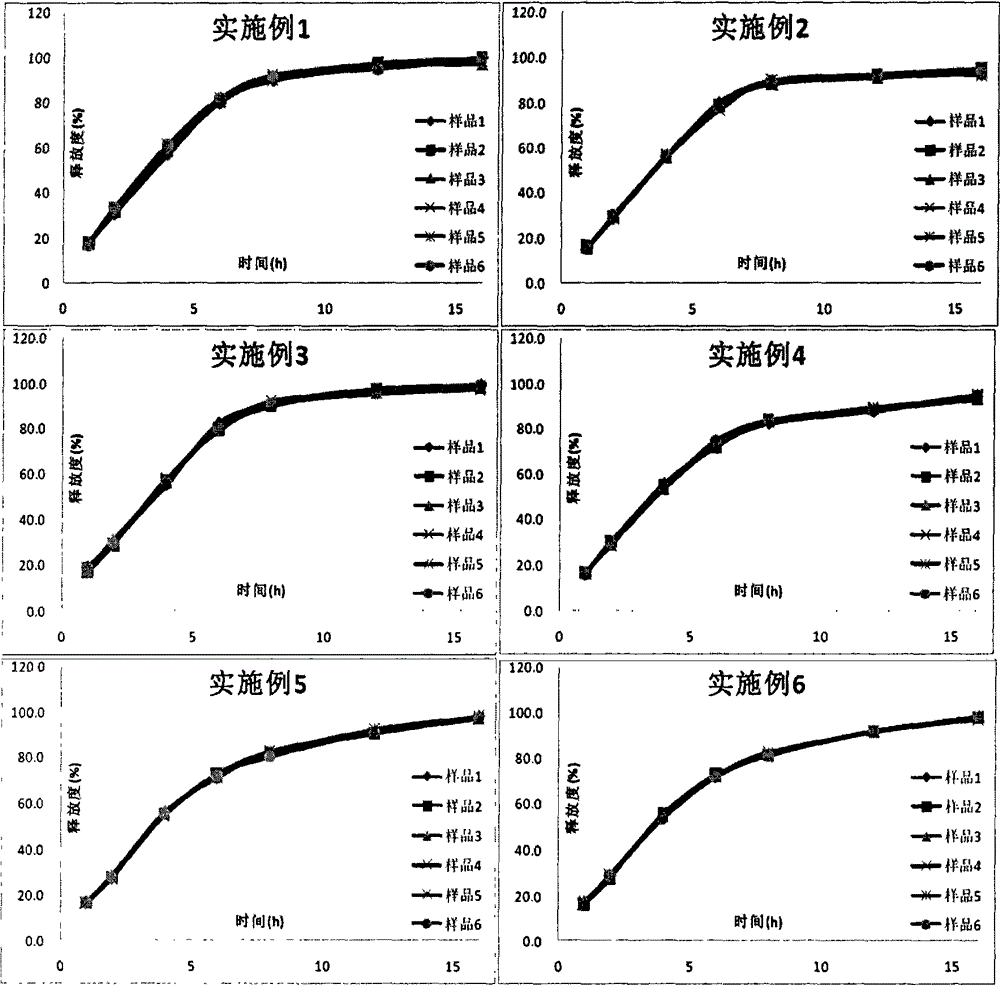
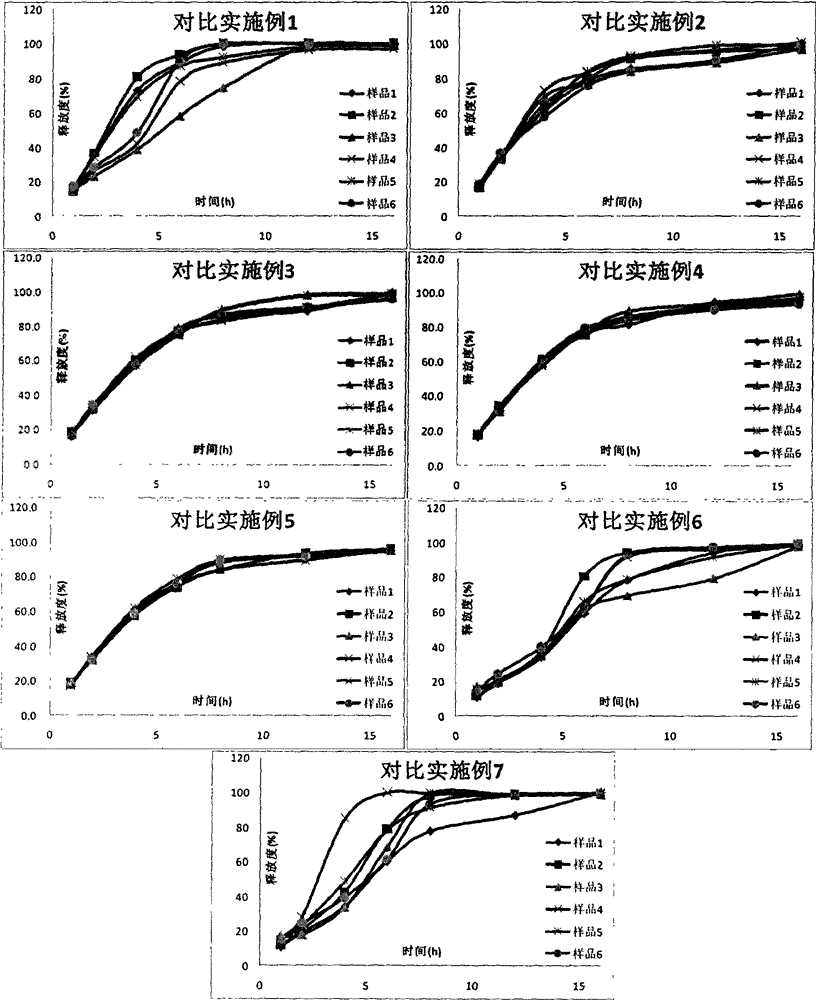
The invention discloses a tofacitinib controlled release tablet, a preparation method and application of the tofacitinib controlled release tablet. The tofacitinib controlled release tablet consists of a medicine-containing tablet core and a controlled release coating film, wherein the medicine-containing tablet core contains a tofacitinib raw material and an auxiliary material; and the controlledrelease coating film contains a film-forming material, a pore-forming agent and / or an excipient. The preparation process of the tofacitinib controlled release tablet requires no laser drilling or special precision equipment, and is simple, easy to implement, low in production cost and short in period, and no organic solvent is used in a coating liquid preparation process, so that adverse effectson the human body and the environment are avoided, and the preparation process is suitable for industrial production.
Owner:SHANGHAI BOCIMED PHARM RES CO LTD
Metformin hydrochloride osmotic pump controlled release tablet and preparation method thereof
ActiveCN105878204AImproved controlled releaseGood compressibilityOrganic active ingredientsMetabolism disorderRelease modulatorAdhesive
The invention relates to the field of pharmaceutical preparations and particularly provides a controlled release tablet of metformin hydrochloride and a preparation method of the controlled release tablet. The controlled release tablet provided by the invention contains a tablet core, an insoluble semi-permeable membrane and a drug release micro-pore, wherein the tablet core contains metformin hydrochloride, an adhesive, a release regulator, an absorption accelerant and a lubricating agent. The metformin hydrochloride in the controlled release tablet provided by the invention can be stably released at a constant speed, so that absorption of drugs is more facilitated, and a better in vivo pharmacokinetic curve is realized. Moreover, the preparation method of the controlled release tablet is simple and easy in process, and the technical defect of poor compressibility of the tablet is solved.
Owner:HEFEI LIFEON PHARMA
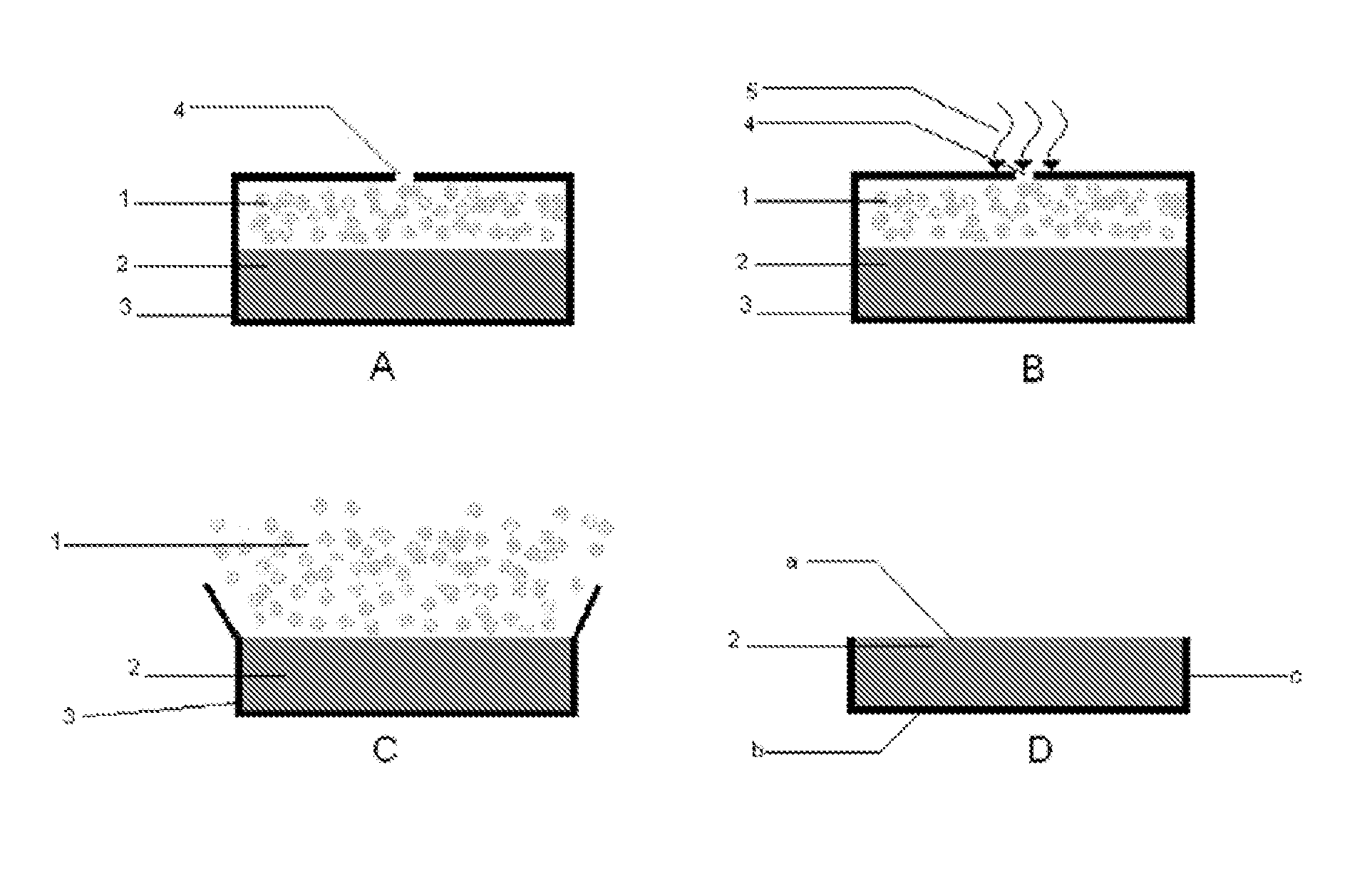
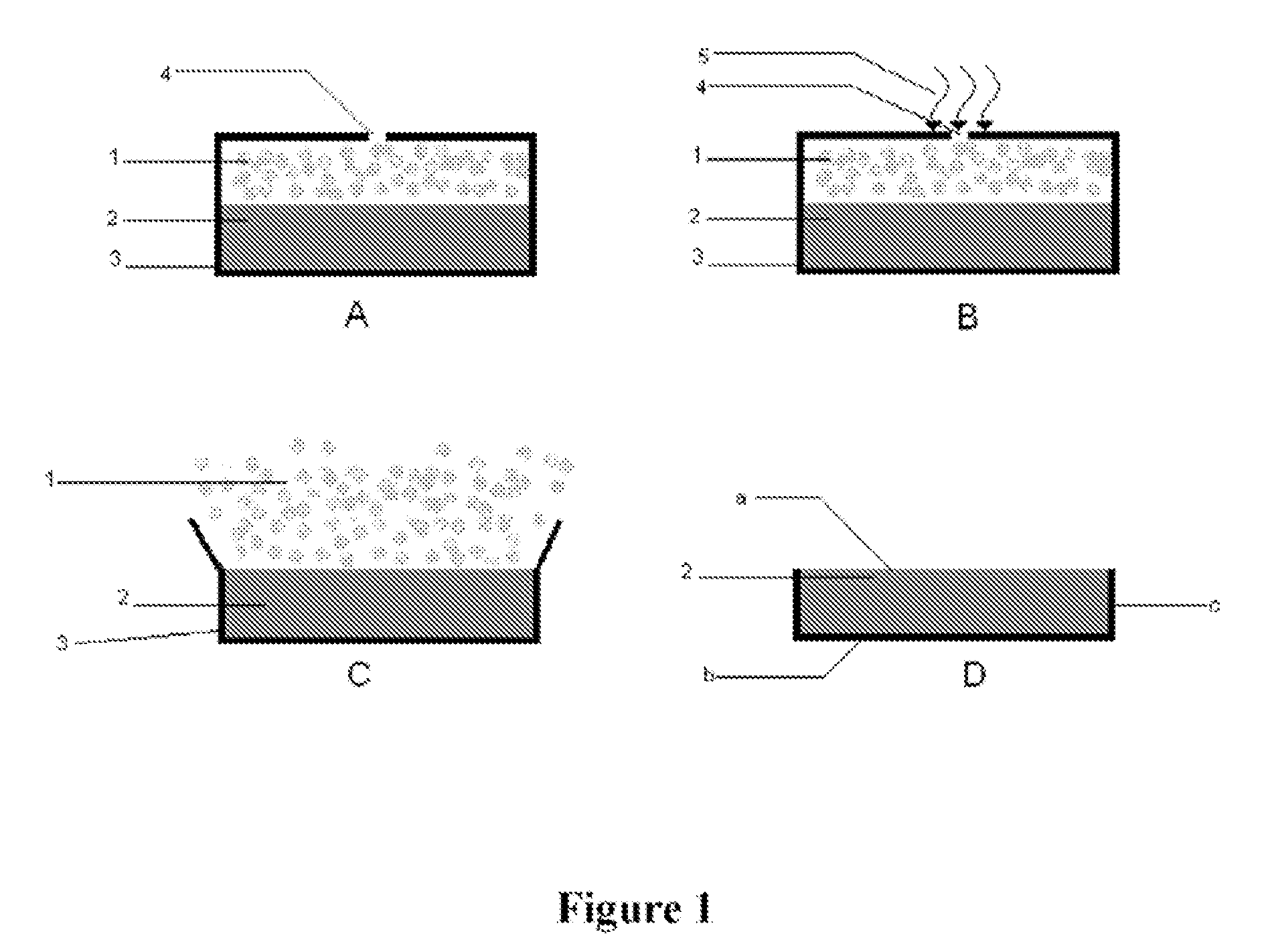
Zero level drug administration oral administration controlled-release tablet and preparation thereof
InactiveCN101244045AGood release reproducibilityEasy to makePharmaceutical delivery mechanismPharmaceutical product form changeOral medicationMedicine
The invention relates to a zero order administration oral controlled release tablet and a preparation method of the tablet. The top surface and the bottom surface of the column tablet is formed by bonding the polymer accessories, namely the retarded release material, the medicine loading area in the middle part is formed by bonding and spraying the printing liquid loading the medicine. The two faces at the axial direction are sealed by ethyl cellulose, which makes the medicine discharged at two dimensions at the radial direction. The selective areas are sprayed with the printing liquid with medicine to obtain the character of the same amount of medicine distribution at the ring surfaces with different diameters and the other areas are sprayed with the printing liquid without medicine and then are formed by bonding. The zero order administration oral controlled release tablet has the advantages of simple technology, high degree of automation and favorable reproducibility of medicine discharge.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com