Febuxostat osmotic pump controlled release tablet for treating gout and preparation method
A technology of osmotic pump controlled release and febuxostat, which is applied in the field of pharmaceutical preparations to achieve the effect of easy and safe production and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] prescription:
[0045]
[0046]
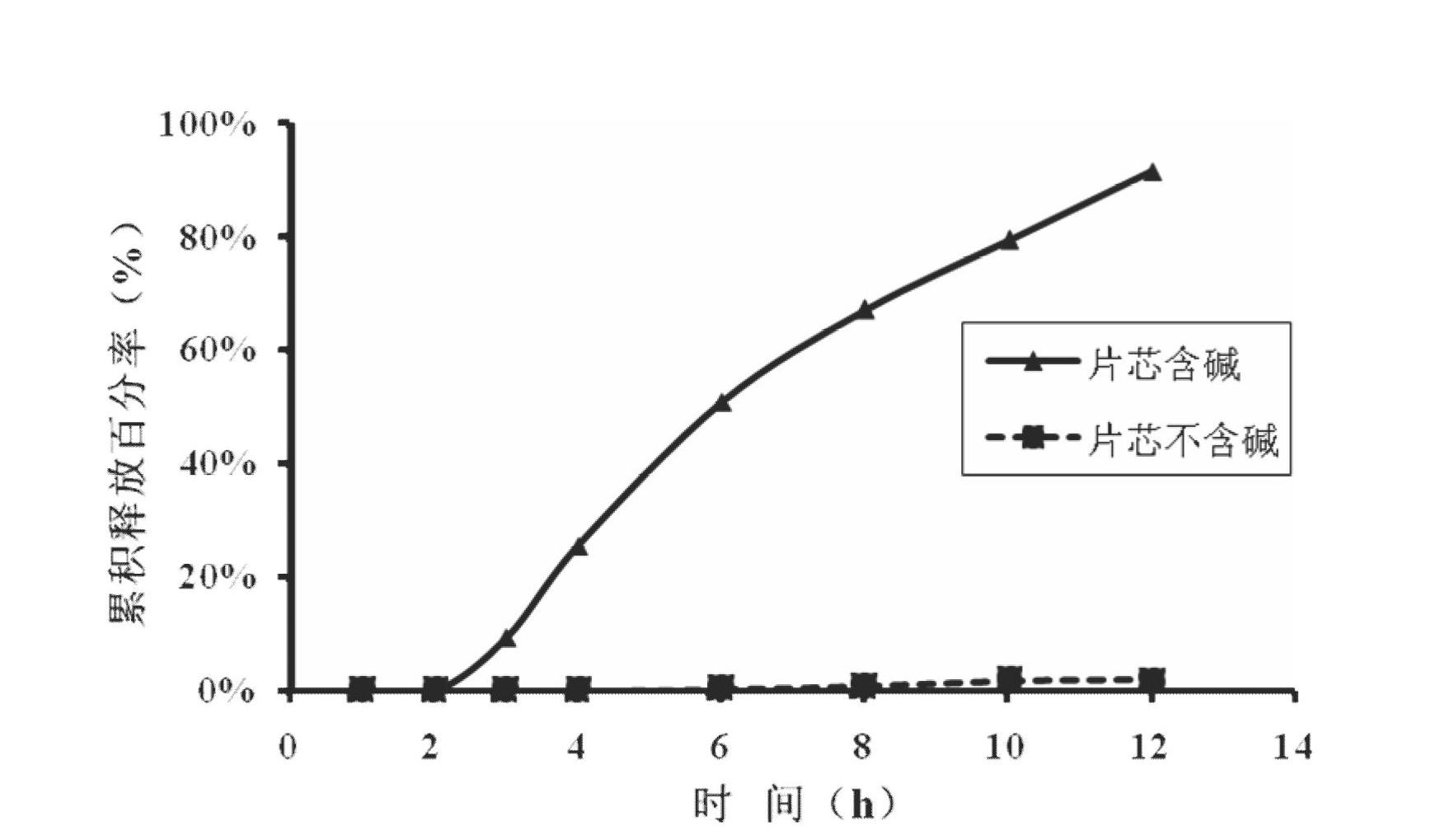
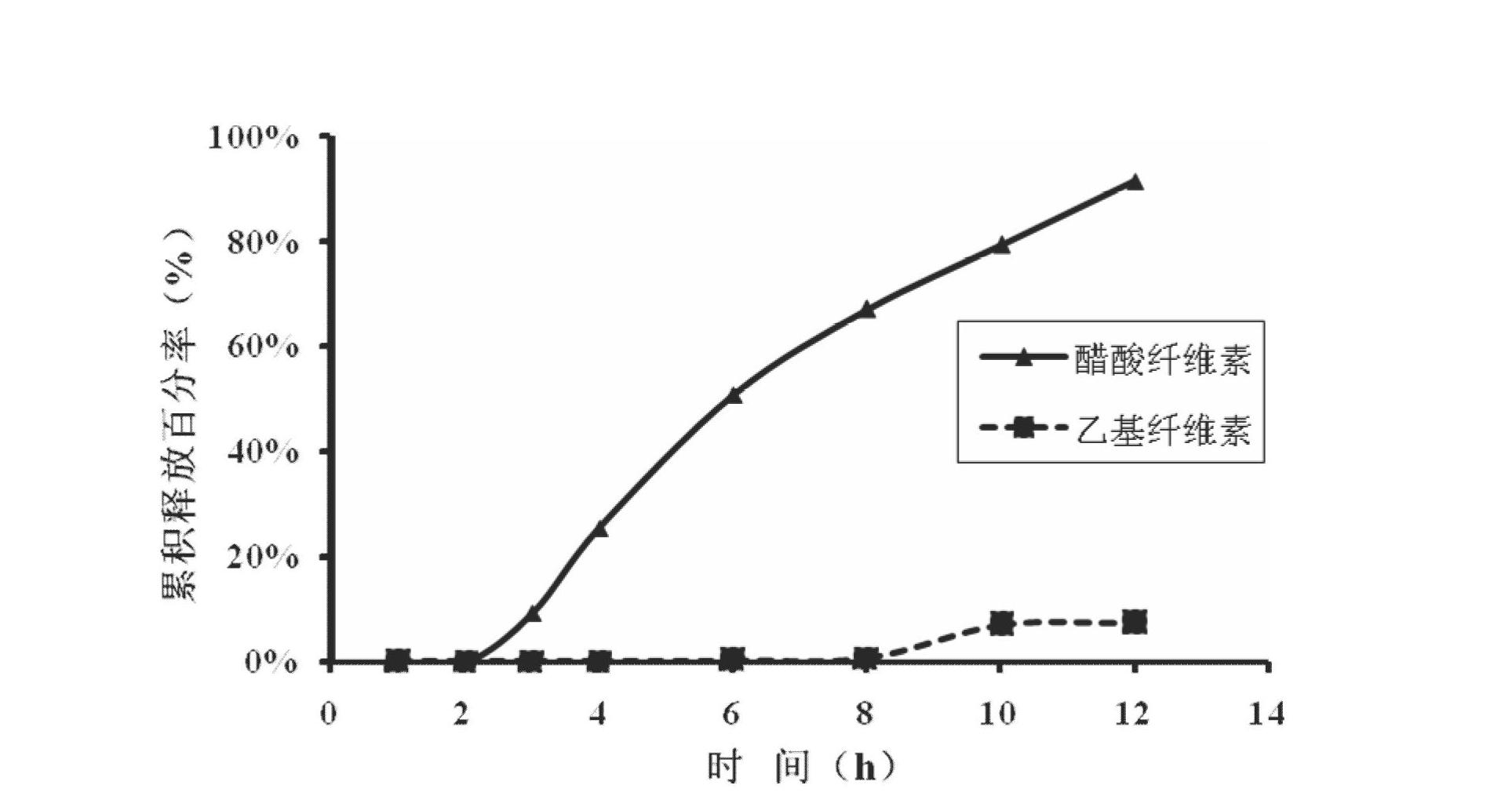
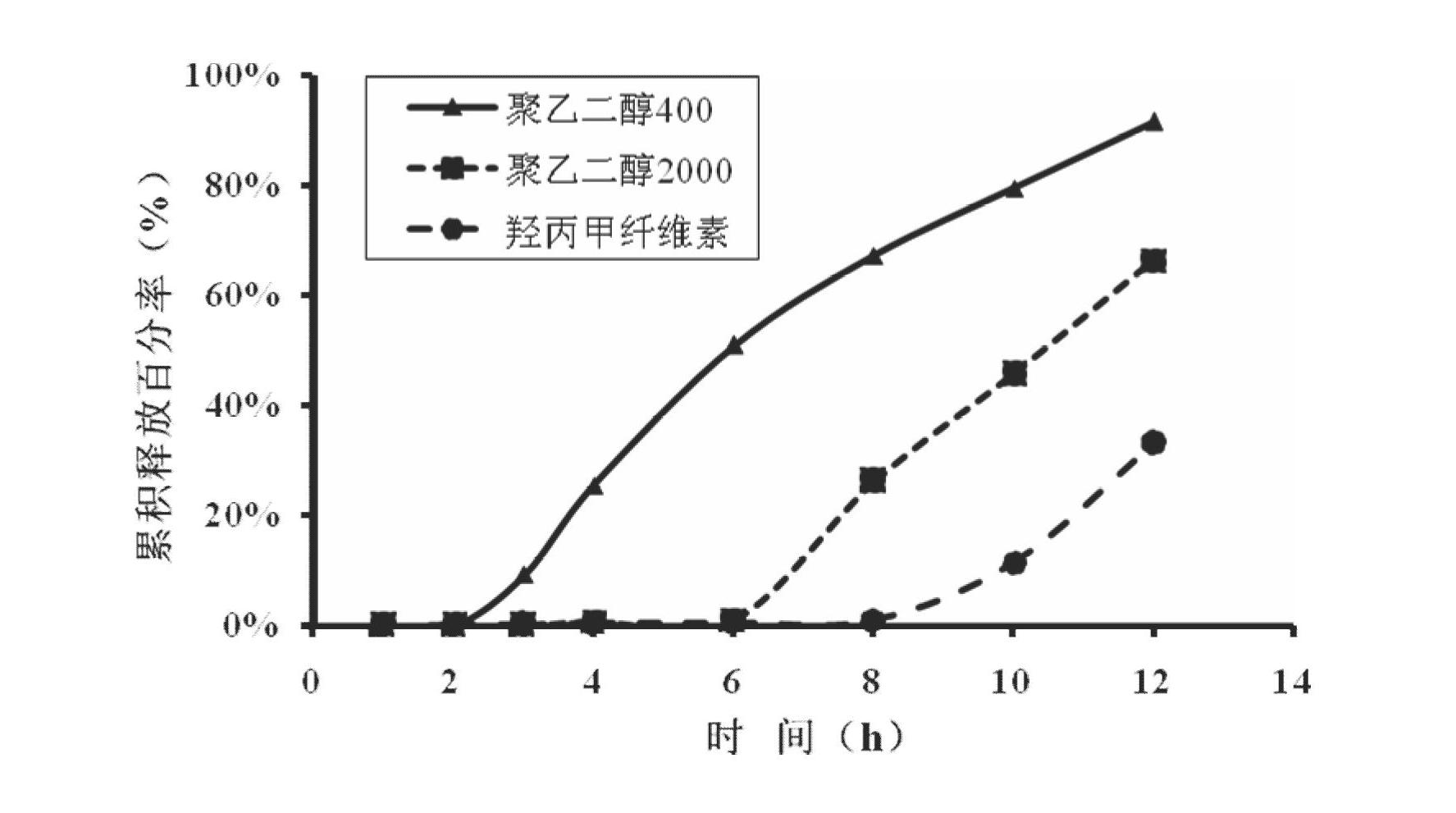
[0047] Preparation method: take 80 mesh febuxostat, lactose, sodium chloride, sodium carbonate and microcrystalline cellulose and mix evenly, add hypromellose aqueous solution with a concentration of 4%, mix evenly with a mixer to make a soft material, Granulate with a 16-mesh sieve, dry at 60°C, granulate with a 14-mesh sieve, add magnesium stearate and micronized silica gel, mix well, and press into tablets. Take cellulose acetate, polyethylene glycol 400 and diethyl phthalate, and use 500ml of acetone as a solvent to prepare a coating solution. Place the above-mentioned tablet cores in a coating pan for coating, the weight gain of the coating is 5% of the weight of the tablet cores, and after coating, put them in an oven at 40°C for 12 hours to age, and each tablet contains 50 mg of febuxostat. Take 1-2 tablets orally daily. The release rate of the febuxostat osmotic pump controlled-release tablet for the treatment of gout is...
Embodiment 2
[0049] prescription:
[0050] Tablet prescription:
[0051]
[0052] Coating prescription (the weight gain of the coating is 7% of the core weight):
[0053]
[0054]
[0055] Preparation method: take 80 mesh febuxostat, mannitol, sodium chloride, sodium hydroxide and mix with precrossed starch, add starch slurry with a concentration of 5%, mix evenly with a mixer to make soft material, 18 mesh Sieve and granulate, dry at 60°C, sieve with a 16-mesh sieve, add magnesium stearate, mix well, and press into tablets. Take cellulose acetate, polyethylene glycol 400 and diethyl phthalate, and use 500ml of acetone as a solvent to prepare a coating solution. Place the above-mentioned tablet cores in a coating pan for coating, the weight gain of the coating is 7% of the weight of the tablet cores, and after coating, put them in an oven at 40°C for aging for 12 hours, and each tablet contains 25 mg of febuxostat. Take 2-4 tablets orally every day. Adopt above-mentioned metho...
Embodiment 3
[0057] prescription:
[0058] Tablet prescription:
[0059]
[0060] Coating prescription (the weight gain of the coating is 5% of the weight of the tablet core):
[0061]
[0062] Preparation method: take 80 mesh febuxostat, glucose, sodium chloride, sodium carbonate and dextrin, mix evenly, add 5% povidone aqueous solution, mix by hand to make soft material, and granulate with 16 mesh sieve , dried at 60°C, granulated with a 14-mesh sieve, added with magnesium stearate and talcum powder, mixed evenly, and compressed into tablets. Take cellulose acetate, polyethylene glycol 400 and diethyl phthalate, and use 500ml of acetone as a solvent to prepare a coating solution. Place the above-mentioned tablet cores in a coating pan for coating, the weight gain of the coating is 5% of the weight of the tablet cores, and after coating, put them in an oven at 40°C for 12 hours to age, and each tablet contains 50 mg of febuxostat. Take 1-2 tablets orally daily. Adopt above-menti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com