Patents
Literature
43 results about "Sarpogrelate Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Controlled Drug-Release Composition and Drug-Releasable Medical Device
InactiveUS20090048667A1Facilitated releaseSufficient amountCatheterAntithrombogenic treatmentMetal formingSarpogrelate Hydrochloride
A drug-releasable medical device contains a controlled drug-release composition comprising 100 parts by weight of an organic polymeric material which is soluble in an organic solvent and insoluble in water, 5 to 60 parts by weight of a lipid-soluble, low molecular weight release auxiliary agent and 1 to 70 parts by weight of a drug. When the composition is applied on a stent, a catheter, an organ replacement medical device, an artificial organ or the like in the form of coating or the like, the medical device is provided with a drug release function. Argatroban or sarpogrelate hydrochloride or both of them are gradually released from the surface of a stent for treating coronary artery stenosis, for example. In order to exhibit a sustained-release function for a desired period of time, the drug to be gradually released is carried in a polymeric material coated on a surface of a metal forming the stent or in a porous stent substrate.
Owner:TOKAI UNIV +1
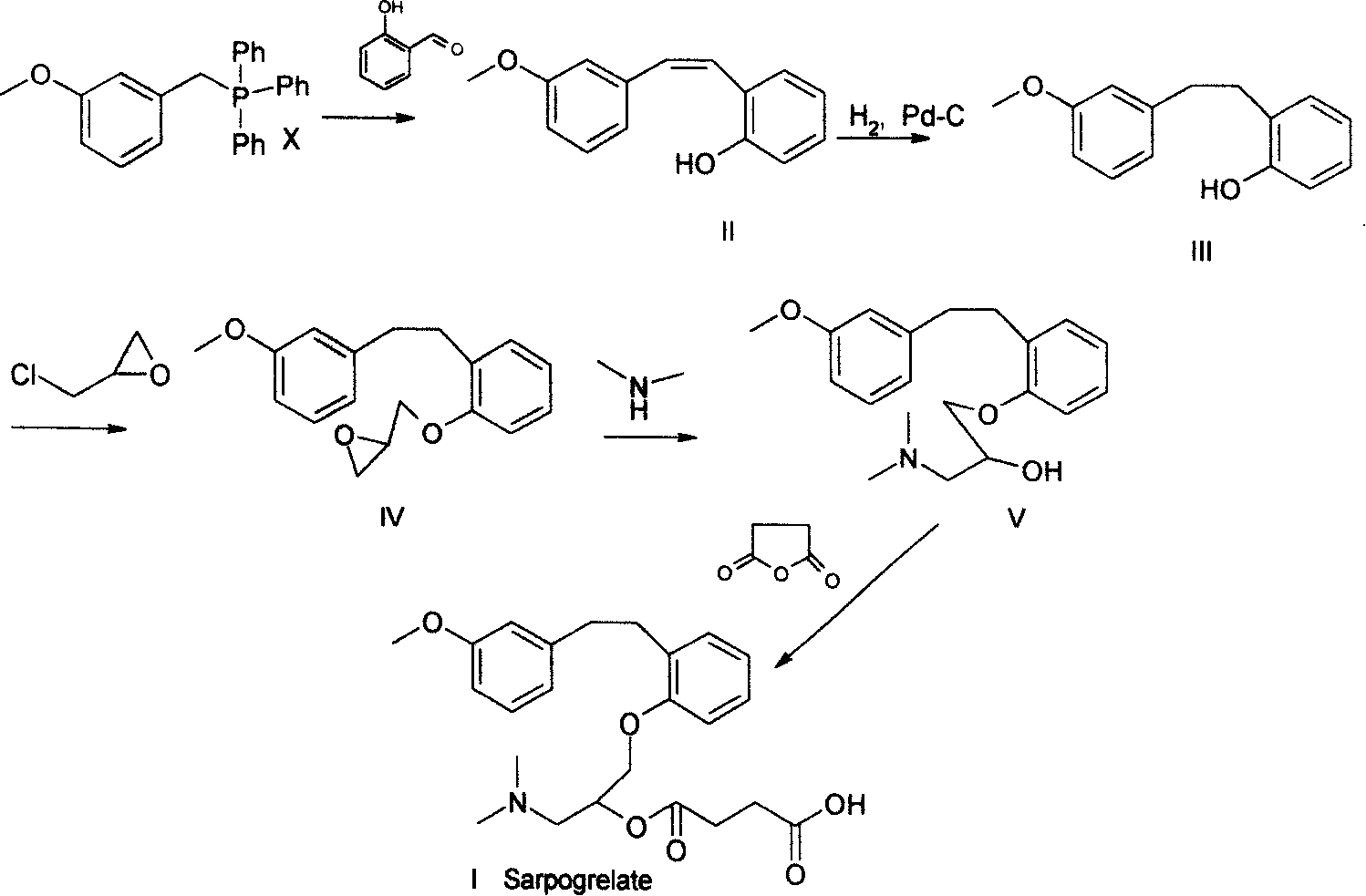
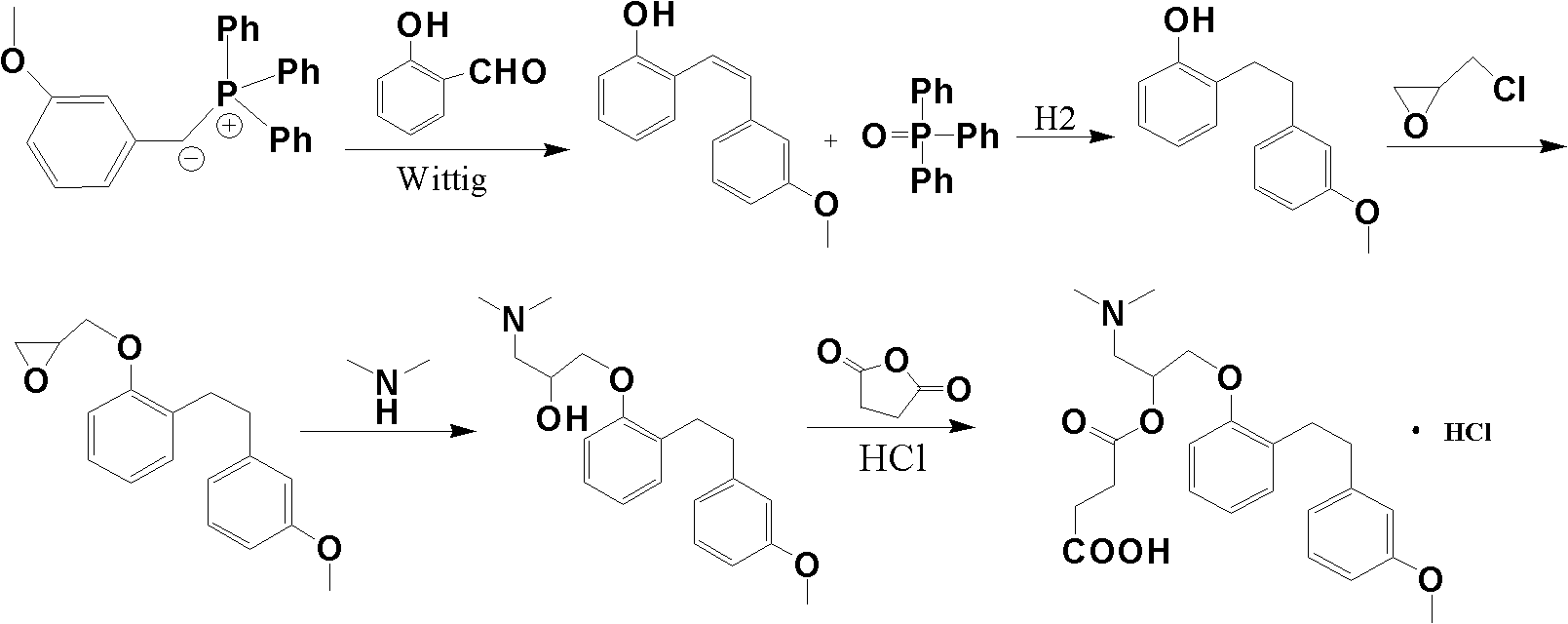
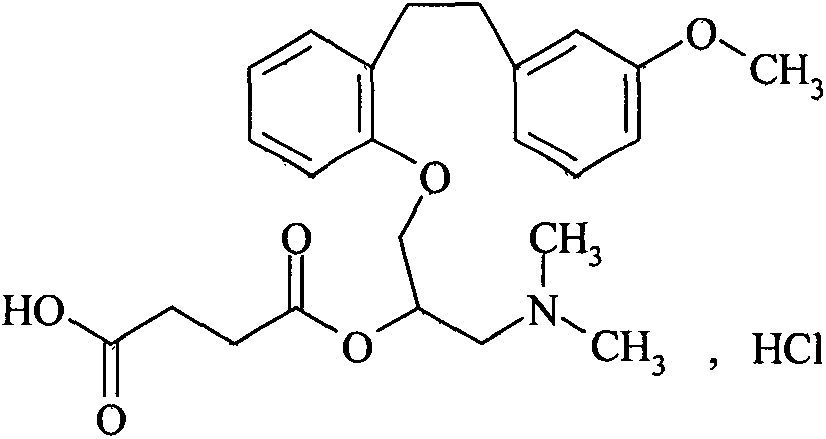
Method for preparing sarpogrelate hydrochloride
InactiveCN101239920AEase of industrial productionSimple and fast operationOrganic compound preparationAmino-hyroxy compound preparationTriphenylphosphine oxideSarpogrelate Hydrochloride
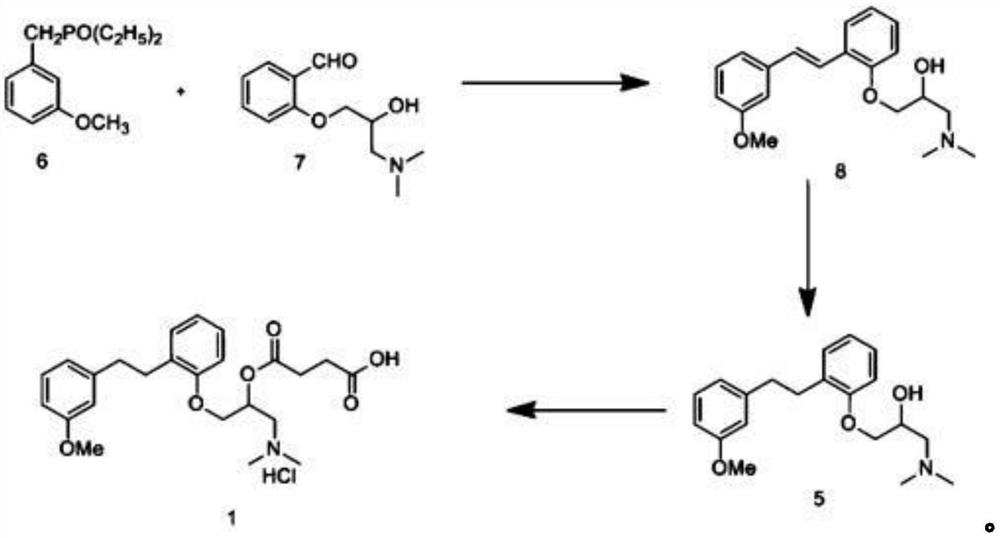
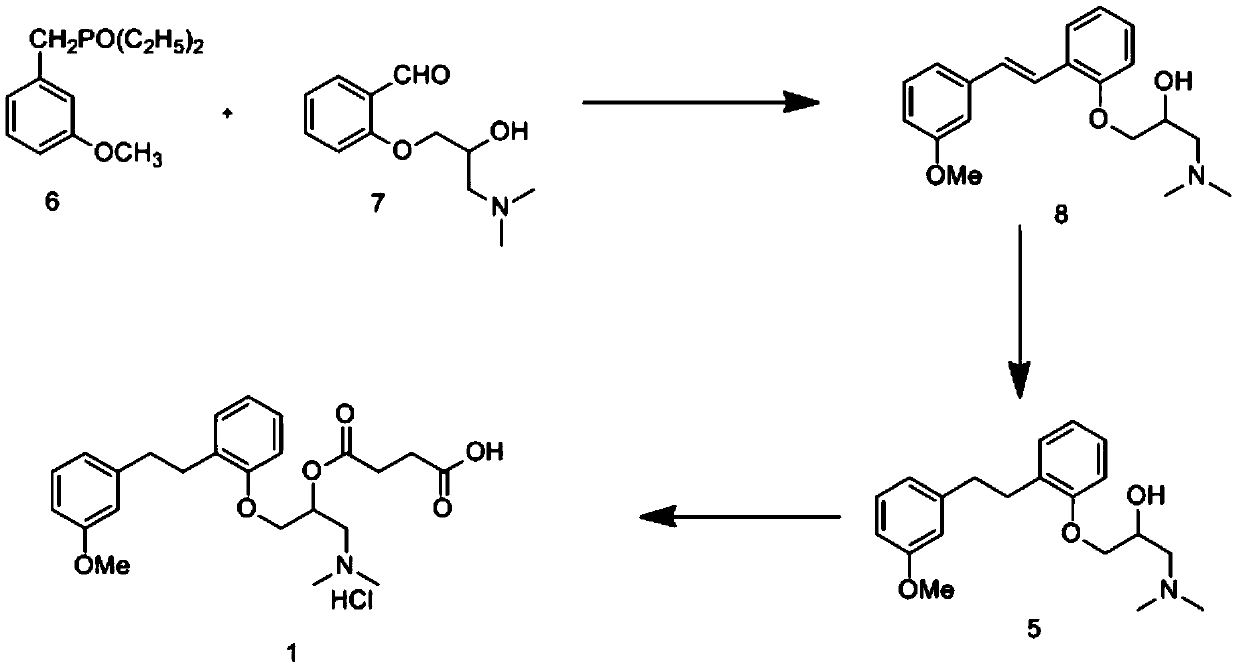
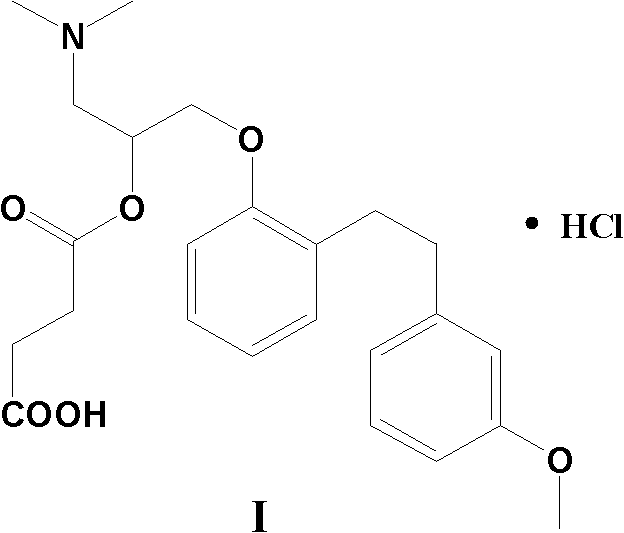
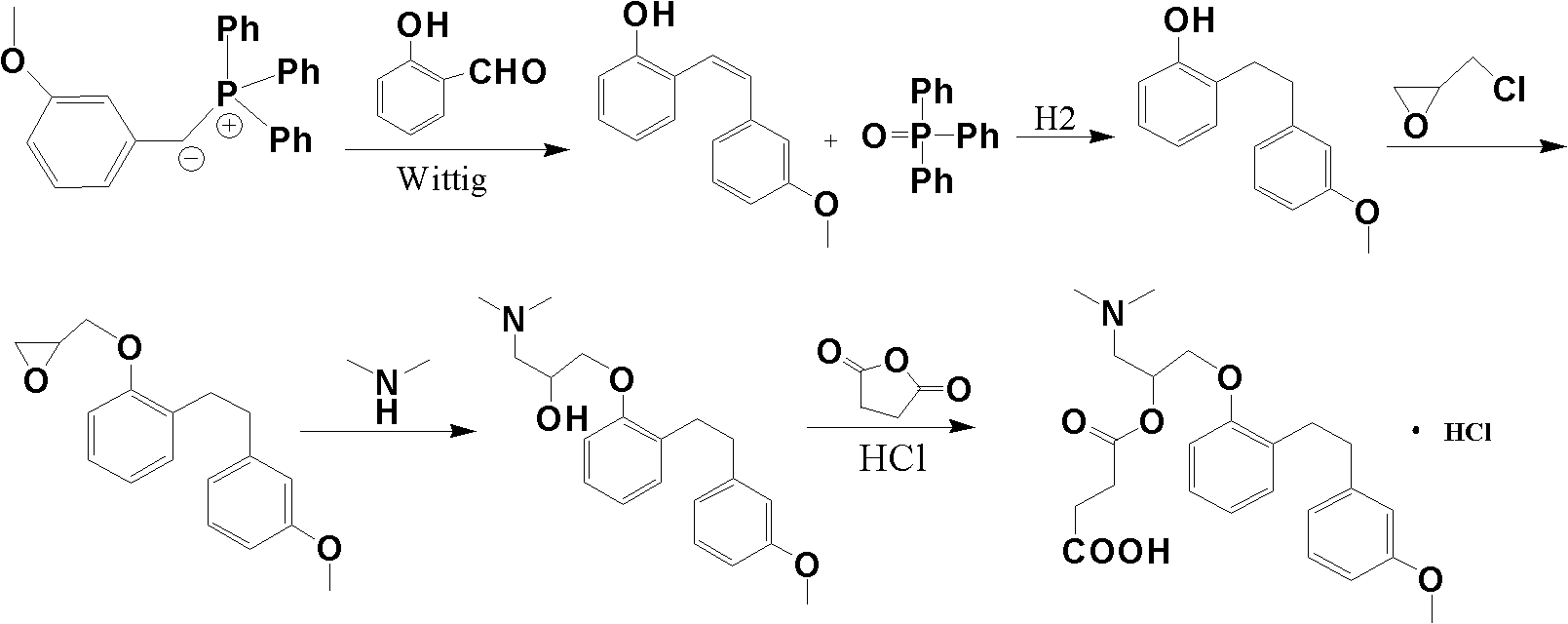
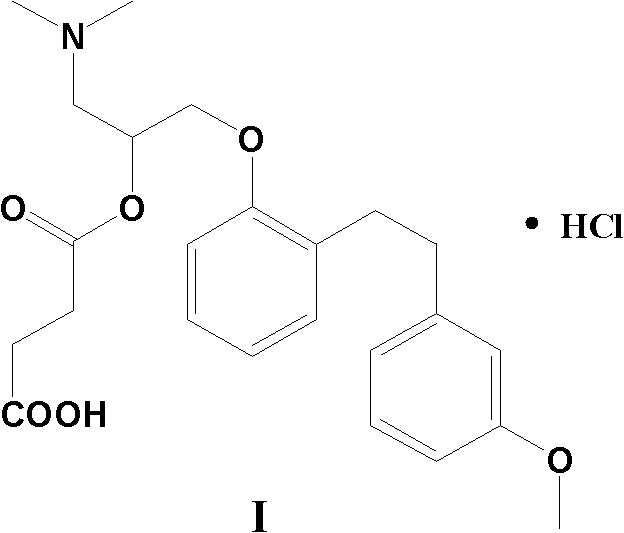
Provided is a preparing method of sarpogrelate hydrochloride, characterized in that 2-((3-methoxyl)styrylcoumarin)phenol mixture containing triphenylphosphine oxide is catalyzed and hydrogenated, and then reacted with epichlorohydrin and dimethylamine to obtain 2-(dimethylamino)-1-(o-(m-methoxyphenylethyl)phenoxymethyl)ethanol, at the same time, triphenylphosphine oxide is separated or not, reacted with succinic anhydride, salt-formed with chlorine hydride, and re-crystallization by a proper solvent, so as to obtain sarpogrelate hydrochloride.
Owner:NANJING KANGRAN PHARMA TECH
Sarpogrelate hydrochloride single layer osmotic pump regulated-release preparations and preparation thereof
InactiveCN101259112ALasting effectGood curative effectOrganic active ingredientsPill deliverySide effectAdhesive
The invention relates to the medicine technical filed, in particular to a monolayer osmotic pump controlled release preparation of sarpogrelate hydrochloride and a preparation method thereof, which is characterized by lasting drug effect, constant curative effect, slight toxic and side effect. The monolayer osmotic pump controlled release preparation of sarpogrelate hydrochloride comprises by weight percentage of 1 to 40 percent of sarpogrelate hydrochloride, 30 to 90 percent of auxiliary material that can promote osmosis, 1 to 40 percent of membrane material that performs controlled release and the rest is other auxiliary material; the diameter of a small drug release hole is 0.1 to 2.0 mm. The preparation method comprises: a certain amount of drug according to the prescription is mixed with an adhesive, a bulking agent and a co-penetrant which are respectively ground and screened, prepared into soft material and palletized, dried and particle finished and then added with a lube and pressed to obtain tablet core. Coating liquid is prepared and the tablet core coated, after coating, drying is done to solidify the coating membrane, and then one side of coated tablet is provided with the small drug release hole, thus obtaining an osmotic pump controlled release tablet. The monolayer osmotic pump controlled release preparation of sarpogrelate hydrochloride of the invention has the advantages of reducing times of dosage, promoting the compliance of sufferers and satisfying the requirements of clinical medication.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL COLLEGE
Miniaturization sarpogrelate hydrochloride oral drug-giving preparation
ActiveCN1903182AHigh speedDissolution rate is fastPowder deliveryPharmaceutical non-active ingredientsSarpogrelateMiniaturization
Provided is an orally administrable preparation of hydrochloride sarpogrelate facilitating the administration by miniaturization. The miniaturized orally administrable preparation of the hydrochloride sarpogrelate has a high content of >=40% of the hydrochloride sarpogrelate per preparation, and exhibits good manufacturability by suppressing tablet trouble. The preparation also has a rapid elution rate from the preparation in the alimentary tract, and exhibits good preservation stability.
Owner:MITSUBISHI TANABE PHARMA CORP
Controlled drug release composition and drug releasing medical device
InactiveCN101309706AFacilitated releaseAdjust timingSurgeryCatheterGlycerolSarpogrelate Hydrochloride
A drug releasing medical device of the invention is provided with a controlled drug release composition containing 100 parts by weight of an organic polymeric material which is soluble in an organic solvent and insoluble in water, 5 to 60 parts by weight of a release auxiliary agent which is lipid-soluble and low in molecular weight and a 1 to 70 parts by weight of a drug. When the composition is applied to a stent, a catheter, an organ replacement medical device, an artificial organ or the like in the form of coating or the like, a drug releasing function is given to the medical device. From a surface of a stent for treating coronary stenosis, which is a preferred embodiment, argatroban, sarpogrelate hydrochloride or both of them are released gradually. In order to express a controlled release property in a desired period of time, the drug to be released gradually is carried in a polymeric material coated on a metal surface constituting the stent or in a porous stent substrate. It is preferred that the polymeric material is noncrystalline and further biodegradable and contains a tartaric acid ester, a malic acid ester or a monoester or diester of glycerine as the release auxiliary agent.
Owner:TOKAI UNIV +1
Sarpogrelate hydrochloride sustained release preparation and preparation method thereof
InactiveCN103284970ASlow release rateExtended release timeOrganic active ingredientsPharmaceutical delivery mechanismSarpogrelate HydrochlorideDrug release
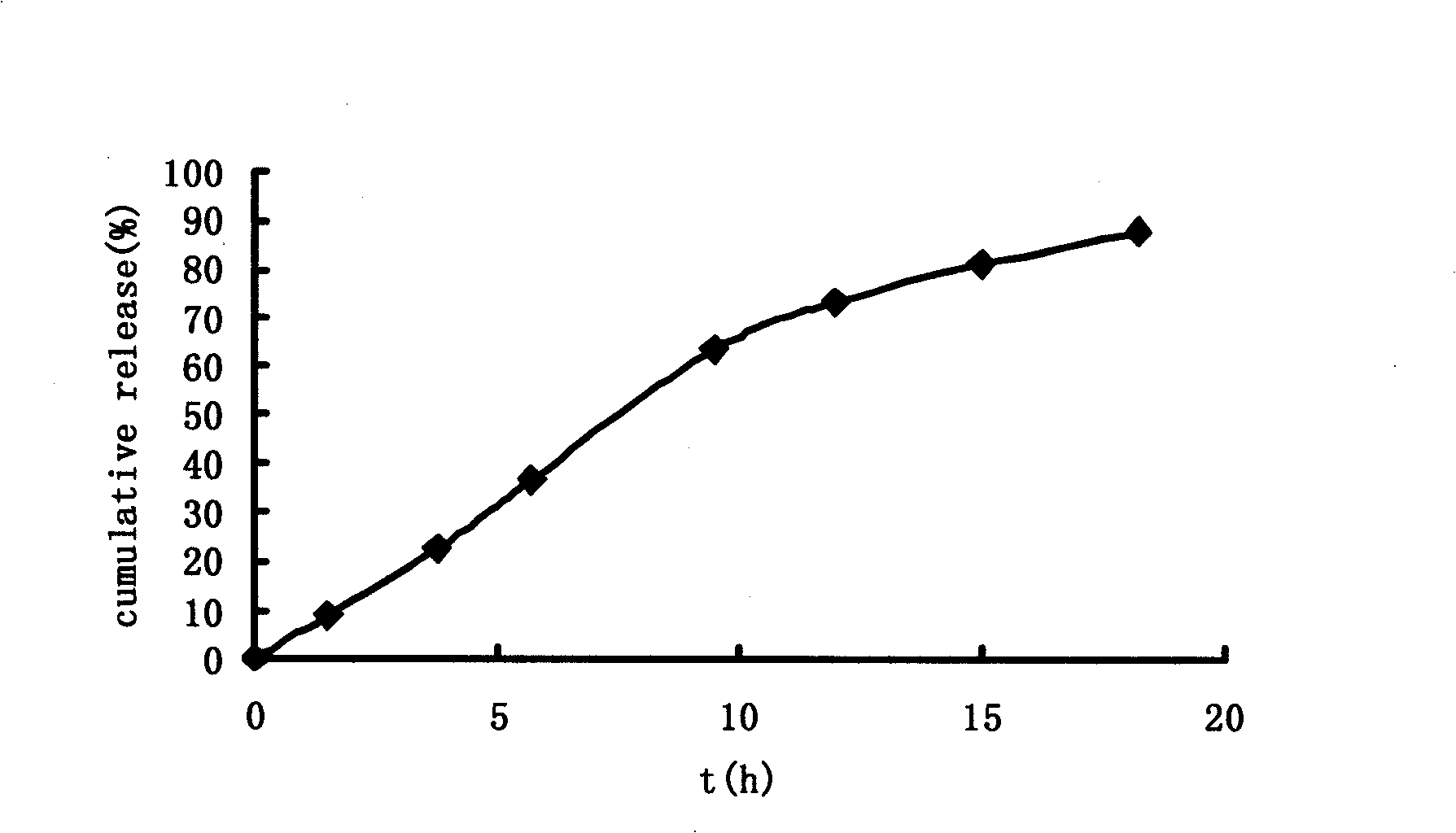
The invention relates to a sarpogrelate hydrochloride sustained release preparation and a preparation method thereof. The sarpogrelate hydrochloride sustained release preparation provided by the invention is invented for slowing down the release speed of a sarpogrelate hydrochloride medicine. The main components of the preparation provided by the invention can release medicines continuously in the gastrointestinal tract for over 24 hours.
Owner:锦华制药株式会社
Sarpogrelate hydrochloride sustained release pellet and preparation method thereof
ActiveCN102552165ALow friabilityHigh yieldOrganic active ingredientsPharmaceutical product form changeSustained release pelletsSarpogrelate Hydrochloride
The invention belongs to the field of medicinal preparation and discloses a sarpogrelate hydrochloride sustained release pellet and a preparation method thereof. The pellet is prepared by coating a sustained release coat on a medicine contained pellet core, wherein the formula of the medicine contained pellet core comprises the following raw materials in parts by weight: 75-100 parts of sarpogrelate hydrochloride and 0-25 parts of excipient, wherein the total weight part of the sarpogrelate hydrochloride and the excipient is 100 or the weight parts of the sarpogrelate hydrochloride and the excipient are increased or reduced by the same ratio; a surfactant accounts for 0.1-8% of the total weight of the sarpogrelate hydrochloride and the excipient; and a wetting agent accounts for 0.1-30% of the total weight of the sarpogrelate hydrochloride and the excipient. By using the preparation method disclosed by the invention, the medicine contained pellet core with low friability, higher yield, smaller particle size and smooth surface can be obtained; the pellet core is convenient to be further processed; according to the invention, the medicine contained pellet core is coated with the sustained release coat so as to obtain a potassium citrate sustained release pellet; and the pellet has the advantages of controllable and stable quality in vitro and sustained release characteristics in vivo.
Owner:JINLING PHARMA
Preparation method of sarpogrelate hydrochloride photodegradation impurities
ActiveCN104356012AHigh purityEasy to operateOrganic compound preparationAmino-hyroxy compound preparationSarpogrelate HydrochlorideMass spectrometry
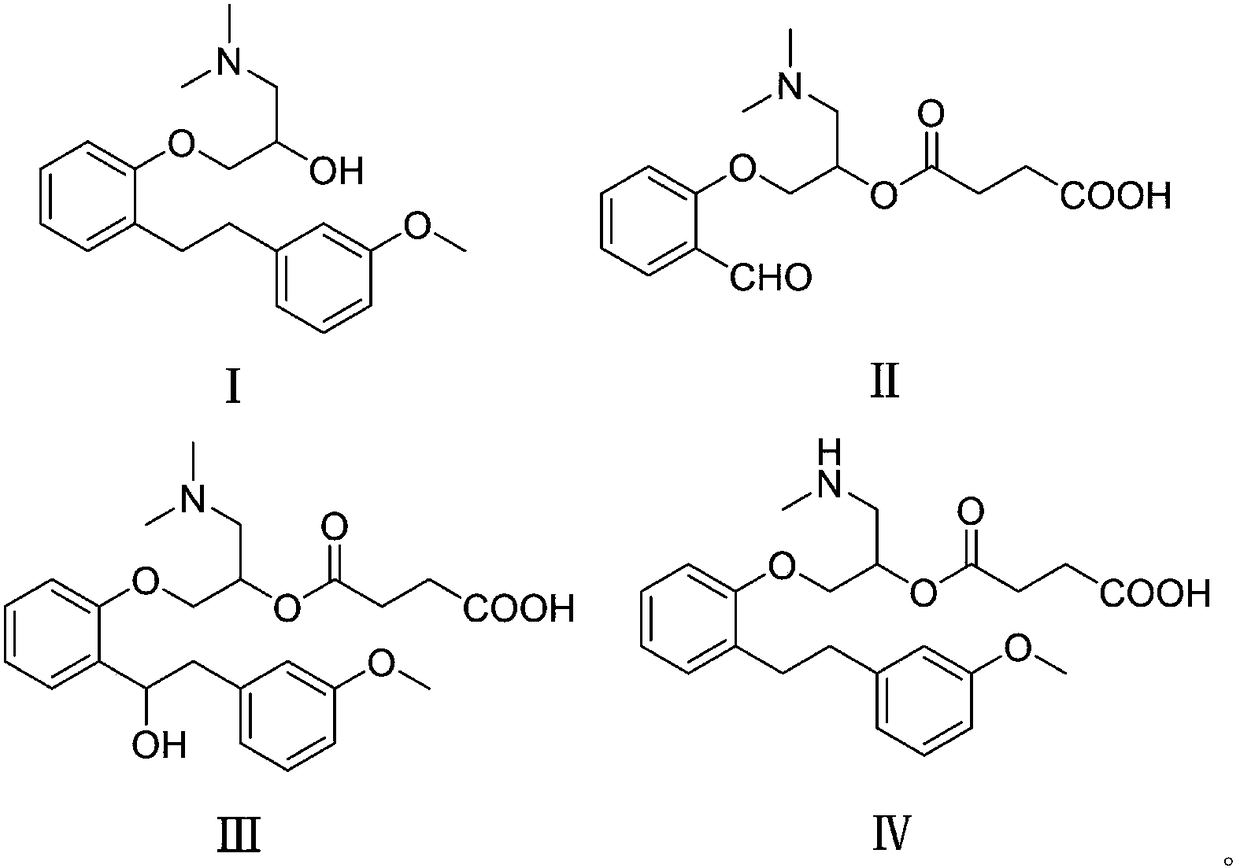
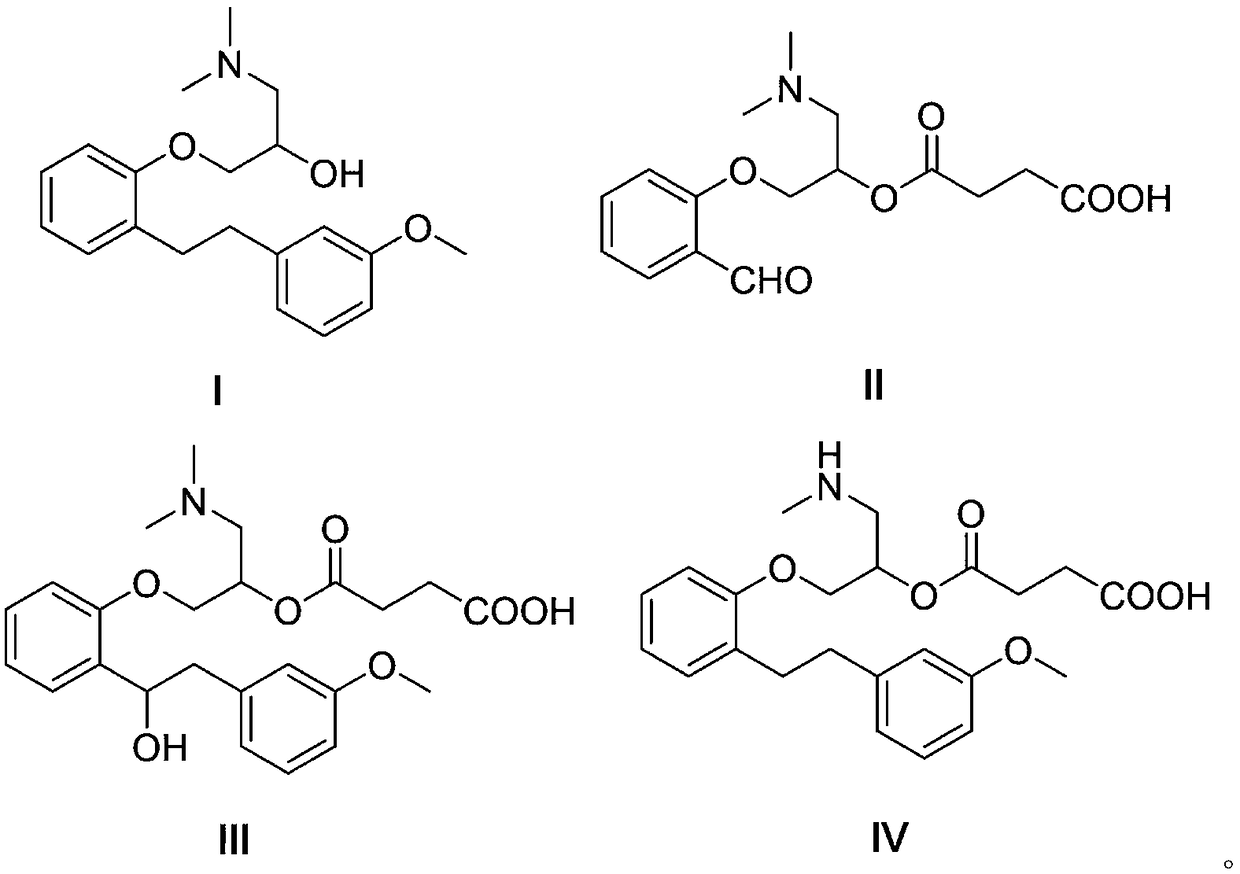
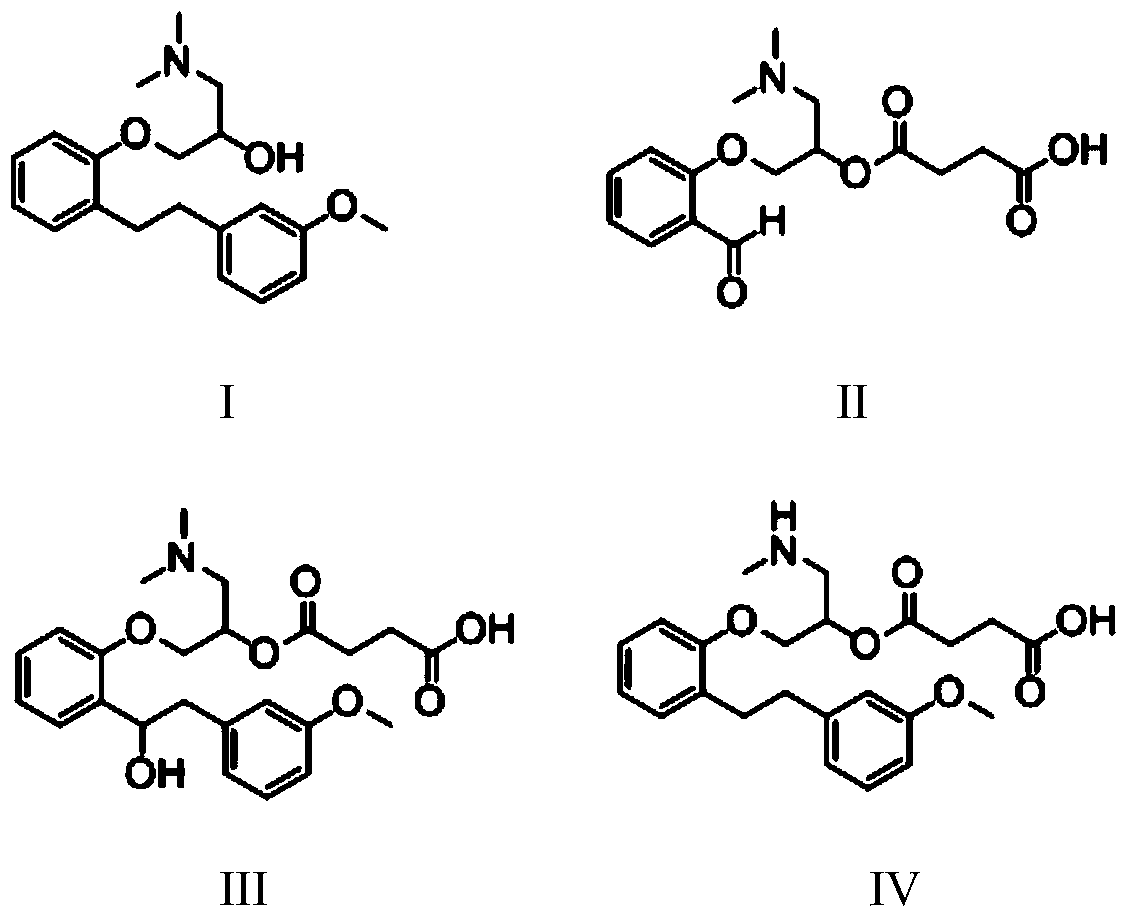
The invention belongs to the field of pharmaceutical synthesis and specifically relates to a preparation method of sarpogrelate hydrochloride photodegradation impurities. According to the preparation method, salicylaldehyde is used as a raw material to prepare a degradation impurity II by three steps of a substitution reaction, an addition reaction and an esterification reaction, etc., and a crude product undergoes column chromatography isolation such that purity reaches more than 99%; 2-[2-(3-methoxyphenyl)ethylene]phenol is used as a raw material to prepare a sarpogrelate hydrochloride impurity III by five steps of a substitution reaction, an addition reaction, an esterification reaction, an epoxidation reaction and a reduction reaction, etc., and a crude product undergoes column chromatography isolation such that purity reaches more than 99%; and [[2-[2-(3-methoxyphenyl)ethyl]phenoxyl]methyl]oxirane is used as a raw material to prepare a sarpogrelate hydrochloride gradation impurity IV by four steps of an addition reaction, amino protection, an esterification reaction and deprotection, etc., and a crude product undergoes column chromatography isolation such that purity reaches more than 99%. The preparation method is simple to operate, and reactions are mild. Product purity is high. The preparation method is suitable for impurity spectrum analysis. By the preparation method, overall yield is high.
Owner:SHANDONG QIDU PHARMA
Sarpogrelate intermediate and preparation method thereof
ActiveCN102875340AAvoid it happening againReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationSarpogrelateGrignard reagent
The invention provides a sarpogrelate intermediate and a preparation method thereof. The sarpogrelate intermediate is a compound shown as formula VI. The preparation method of the intermediate comprises a Grignard reaction of salicylic aldehyde with phenolic groups protected and a Grignard reagent. The method is mild in reaction conditions, low in energy consumption and small in pollutions, and has relatively high conversion rate and low cost of the raw materials. The obtained sarpogrelate hydrochloride has high purity with the detection purity of HPLC higher than 99%. The method is suitable for large-scale production conversion.
Owner:NEW FOUNDER HLDG DEV LLC +2
Sarpogrelate hydrochloride sustained-release preparation and preparation method
InactiveCN101933918AOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientSarpogrelate Hydrochloride
The invention provides a low-release sarpogrelate hydrochloride medicament. The main ingredients of the medicament can be continuously released in the gastrointestinal tract for over 10h.
Owner:苏州世林医药技术发展有限公司
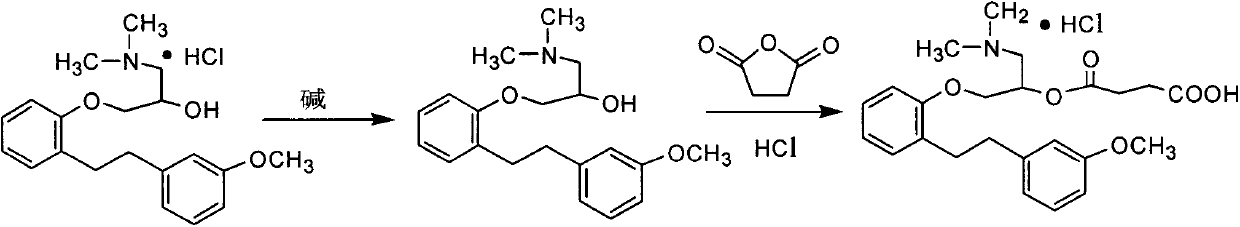
Preparation method for sarpogrelate hydrochloride
InactiveCN102372643AQuality improvementEasy to operateOrganic compound preparationAmino-hyroxy compound preparationAlcoholEthylene oxide
The invention discloses a preparation method for sarpogrelate hydrochloride. The method comprises the steps of preparation of the intermediate [[2-(3-methoxyphenyl)ethyl]phenoxyl]methyl]oxirane, 1-dimethylamino-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxyl]2-propyl alcohol and a finished product in order. According to the invention, ethanol with a concentration of 95% is used as a solvent, and substituted sodium hydride is changed into sodium hydroxide or potassium hydroxide or the like; therefore, cost is reduced, operation is simple, and the finished product has stable quality.
Owner:TIANJIN KELIN CHEM
Preparation method of sarpogrelate hydrochloride
ActiveCN102875396AAvoid it happening againReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationSarpogrelateSarpogrelate Hydrochloride
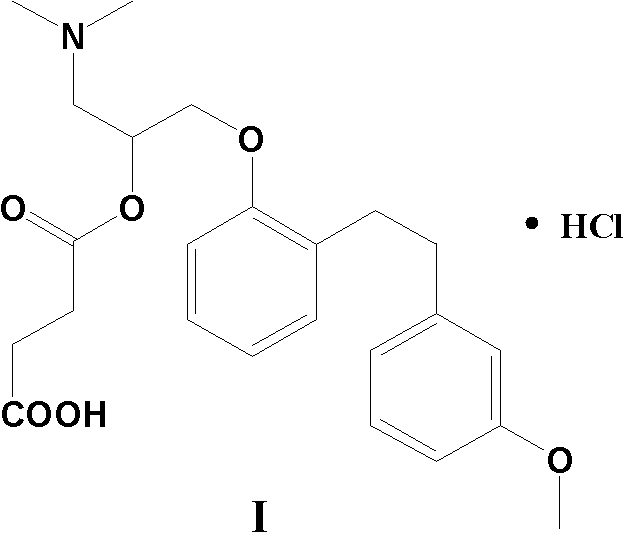
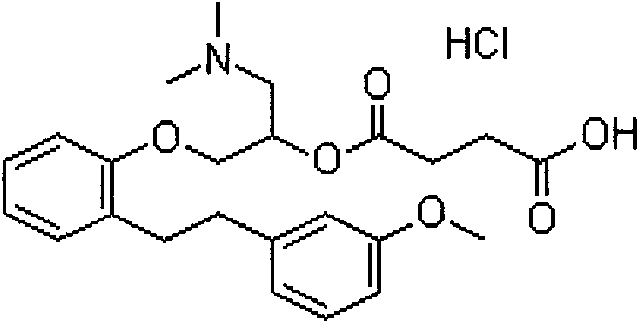
The invention provides a preparation method of sarpogrelate hydrochloride. The sarpogrelate hydrochloride is shown as Formula I. The method comprises the following steps of 1) performing the following dehydration reaction on sarpogrelate intermediate shown as formula VI in the presence of an acid dehydrating agent to obtain a compound shown as formula VII; and 2) further preparing the sarpogrelate hydrochloride from the compound shown as the formula VII. The method is mild in reaction conditions, low in energy consumption and small in pollution, and has relatively high conversion rate and low raw material cost. The obtained sarpogrelate hydrochloride has high purity with detection purity of HPLC over 99%, and is suitable for large-scale production conversion.
Owner:NEW FOUNDER HLDG DEV LLC +2
Method for detecting dimethylamine contained in sarpogrelate hydrochloride intermediate
InactiveCN108226338AThe detection method is simpleSimple methodComponent separationSarpogrelate HydrochlorideHydrochloride
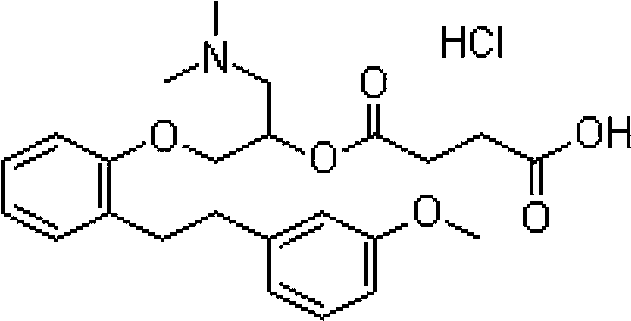
The invention discloses a method for detecting dimethylamine and dimethylamine hydrochloride in a sarpogrelate hydrochloride intermediate C. The method is simple, and favorable in precision, accuracy,reproducibility and durability. The method can be used for detecting the dimethylamine and the dimethylamine hydrochloride in the sarpogrelate hydrochloride intermediate C, i.e. 1-(dimethylamino)-3-[2-[2-(3-methoxyphenyl) ethyl] phenoxy]-2-propanol hydrochloride.
Owner:TIANJIN CHASE SUN PHARM CO LTD
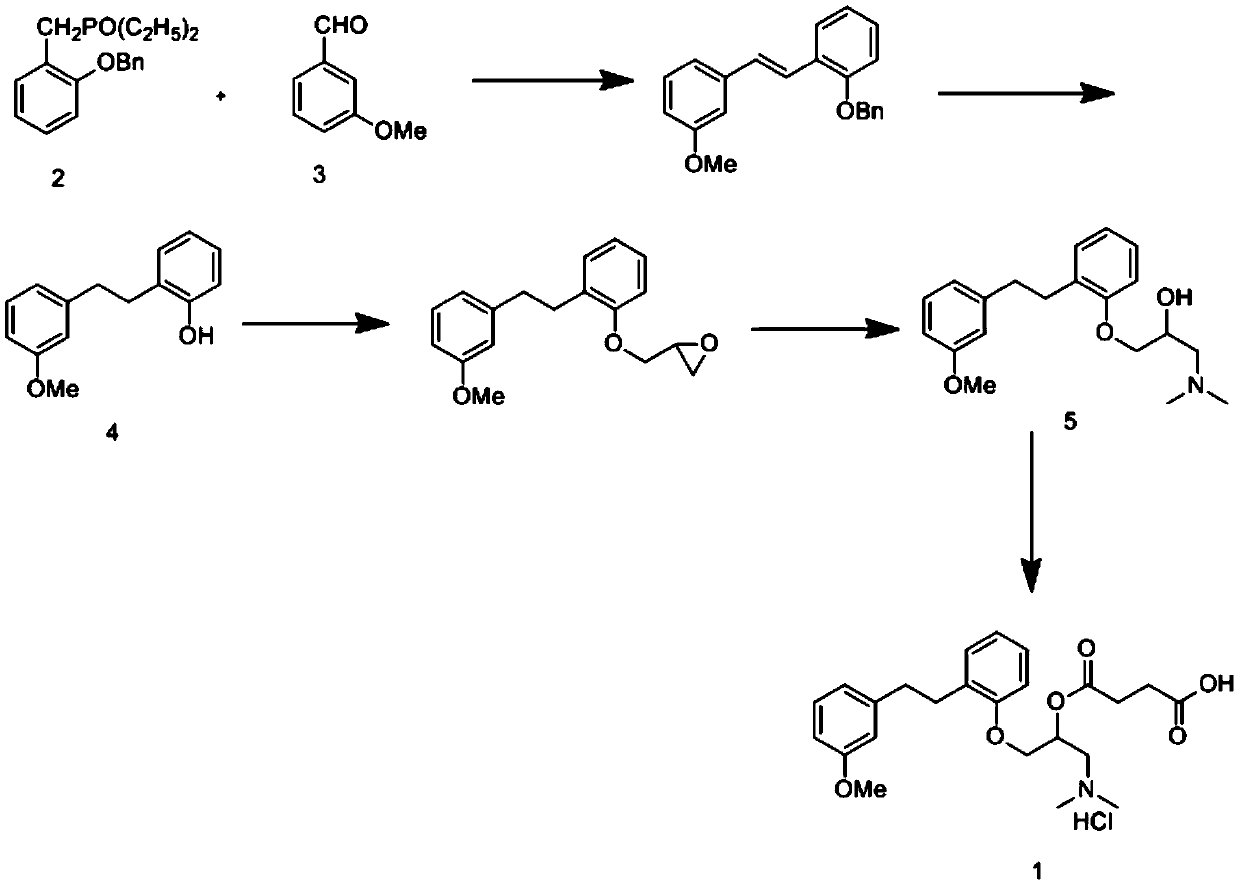
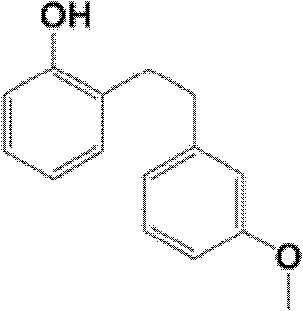
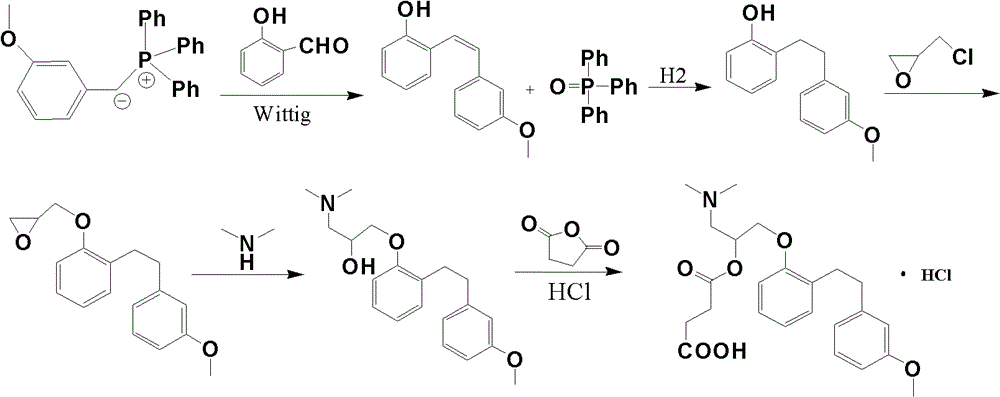
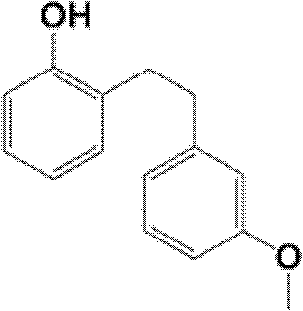
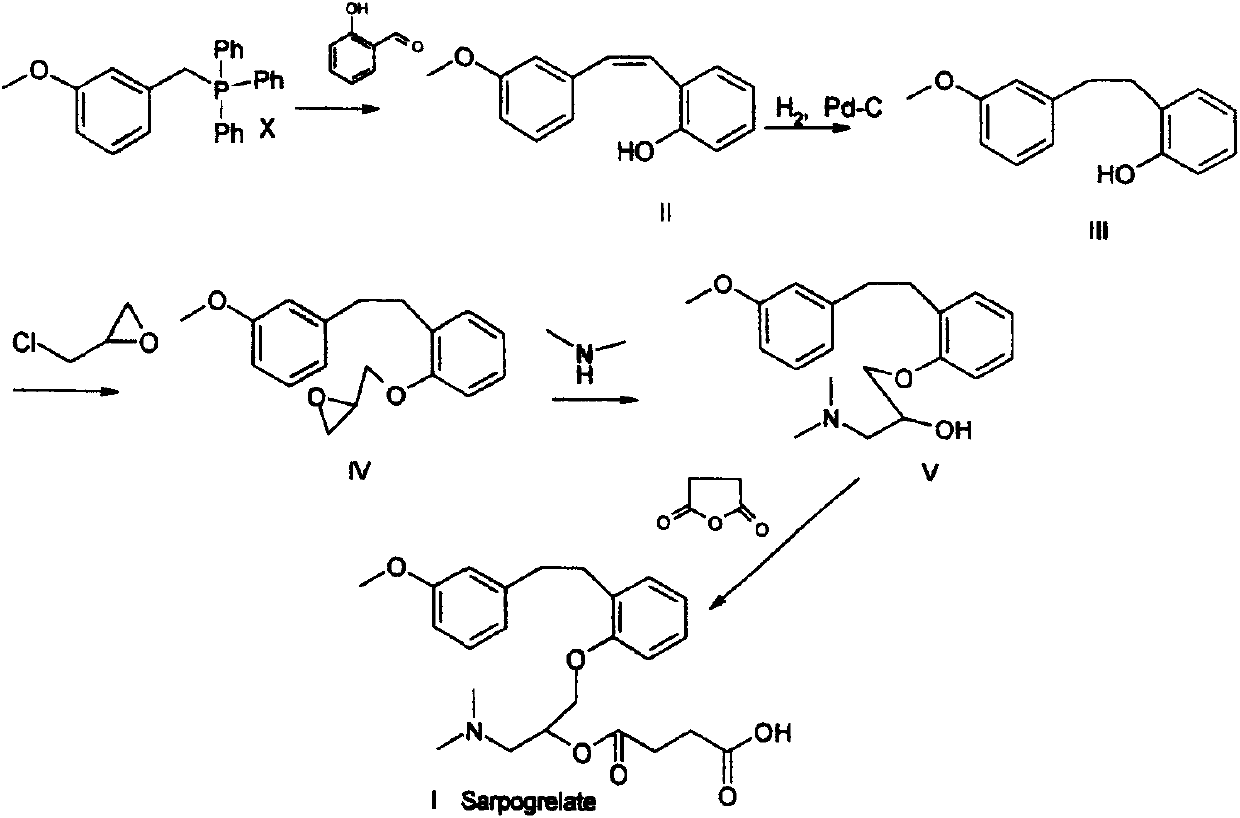
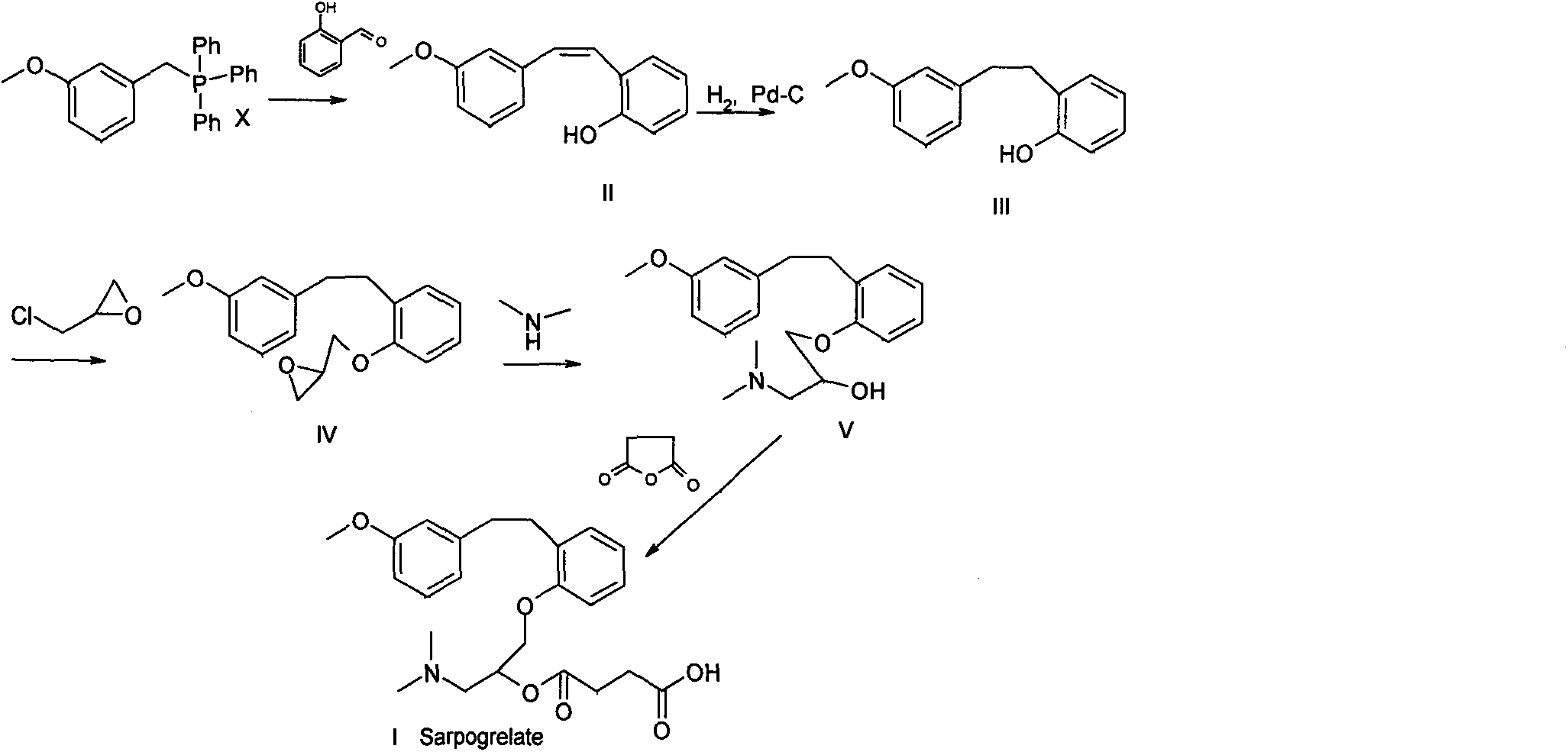
Synthetic method of sarpogrelate intermediate 2-[2-(3-methoxyphenyl)ethyl]phenol
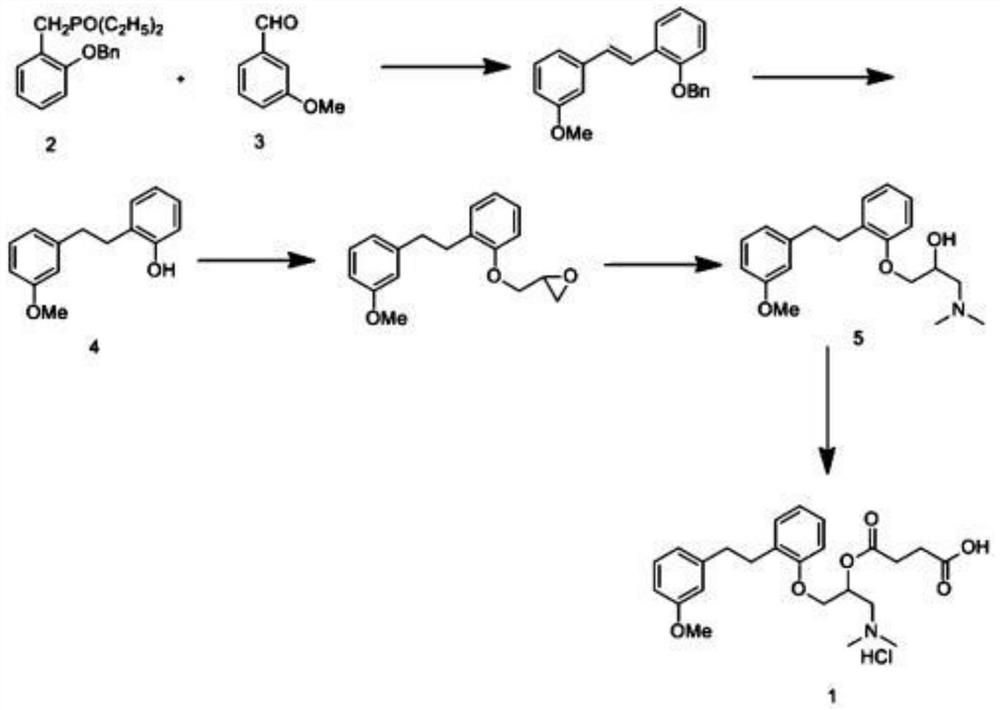
ActiveCN105906486ASimplified processing stepsReduce loss rateOrganic compound preparationGroup 5/15 element organic compoundsChlorobenzeneTriethylphosphite
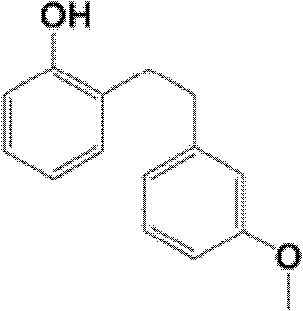
The invention discloses a synthetic method of a sarpogrelate intermediate 2-[2-(3-methoxyphenyl)ethyl]phenol and relates to the technical field of organic synthesis. In the method, with m-methoxyl chlorobenzene, which is easy to obtain and is low in cost, as a raw material, the method includes the steps of: 1) performing condensation to the raw material with triethyl phosphite; and 2) performing condensation with o-phenylmethoxyl benzaldehyde (which is prepared from salicylic acid and chlorobenzene), performing hydrogenation to alkylate a double bond, and crystallizing a product with petroleum ether to prepare a white crystal target product. The method simplifies process steps, reduces loss ratio and is 99.5% in product purity. The 2-[2-(3-methoxyphenyl)ethyl]phenol is used for preparing sarpogrelate hydrochloride, wherein single impurity is less than 0.1% in content and the product purity reaches more than 99.8%.
Owner:安徽修一制药有限公司
Solid drug composition containing sarpogrelate hydrochloride
ActiveCN106580909AOrganic active ingredientsPharmaceutical non-active ingredientsHardnessSarpogrelate Hydrochloride
The invention relates to a solid drug composition containing sarpogrelate hydrochloride, a preparation process and an application. Through selecting components and dosage of every auxiliary material in the preparation, a sarpogrelate hydrochloride coating tablet is obtained; the tablet is proper in disintegration; the hardness, and friability, and other indexes can reach the requirement of the coating process; the solid drug composition is featured by simple production process, convenience in production and stable quality.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Sarpogrelate hydrochloride tablet and preparation method thereof
ActiveCN105769800AImprove stabilitySatisfy Dissolution BehaviorOrganic active ingredientsPharmaceutical non-active ingredientsSpecific modelSarpogrelate Hydrochloride
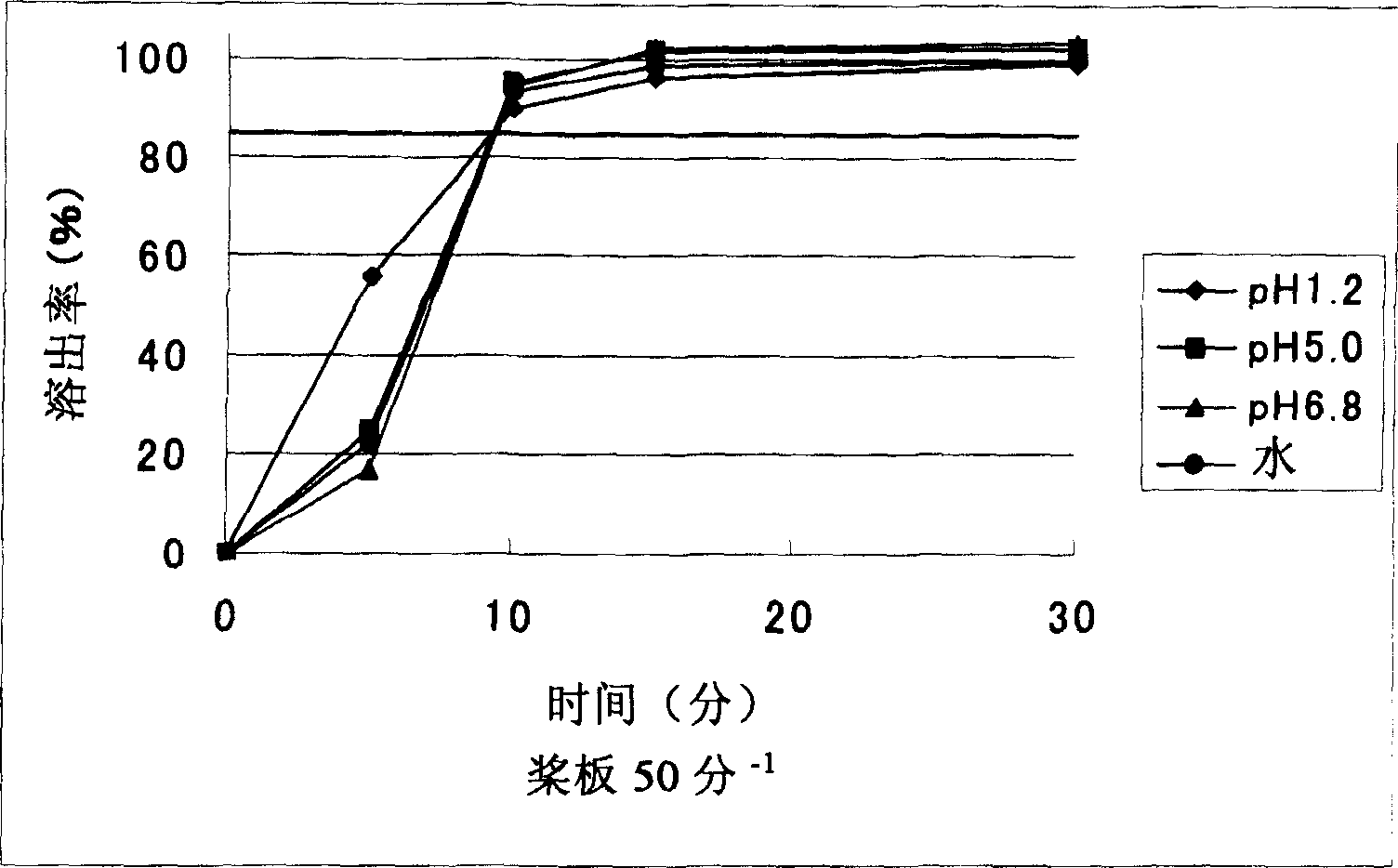
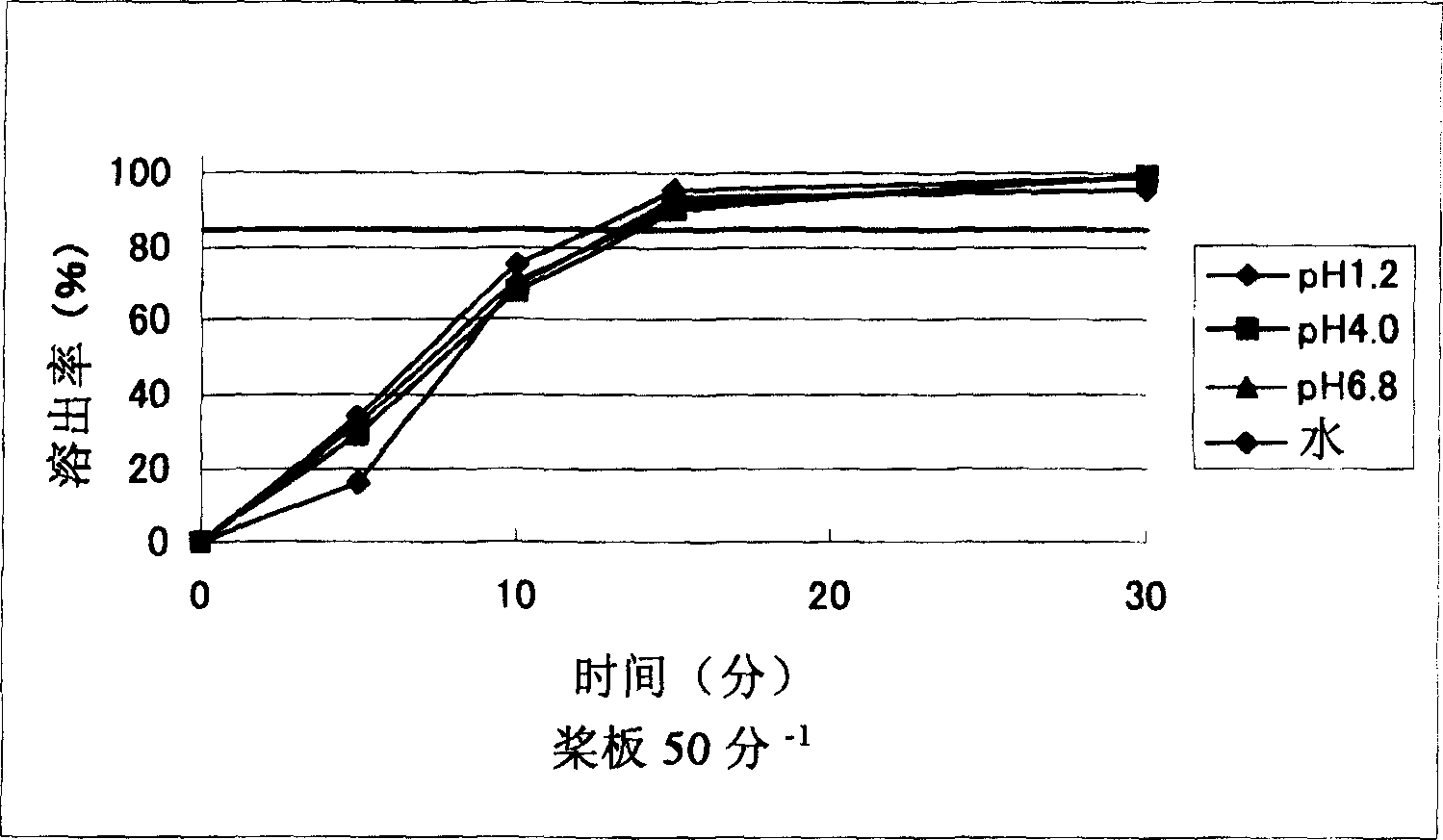
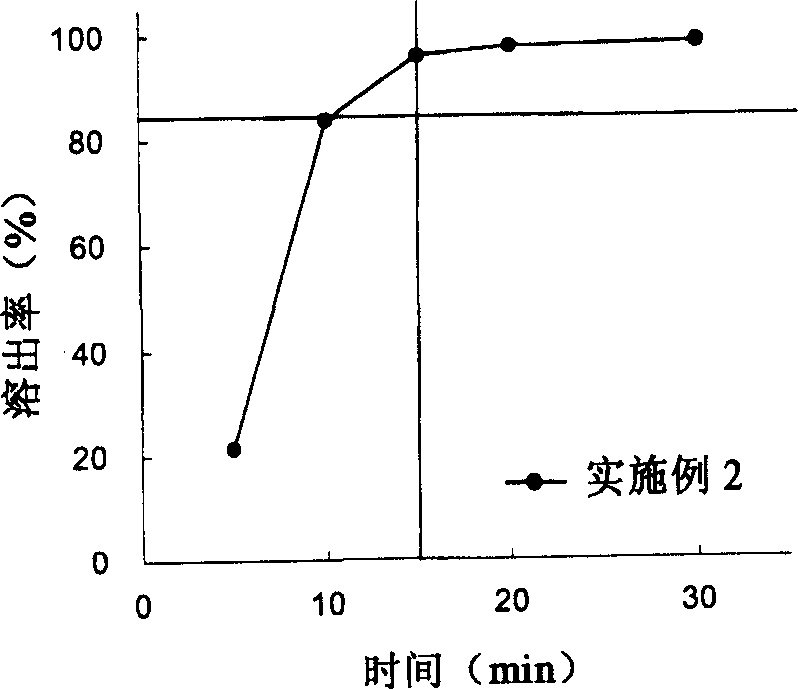
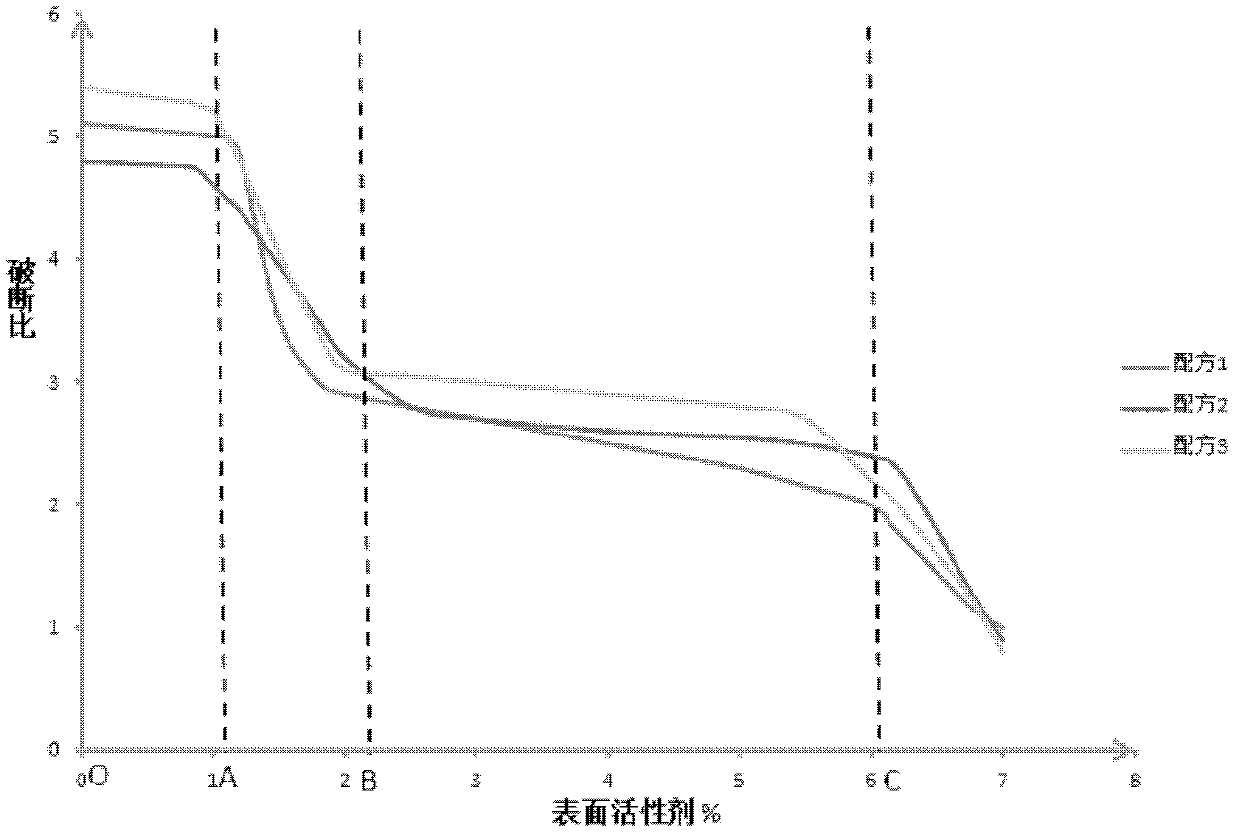
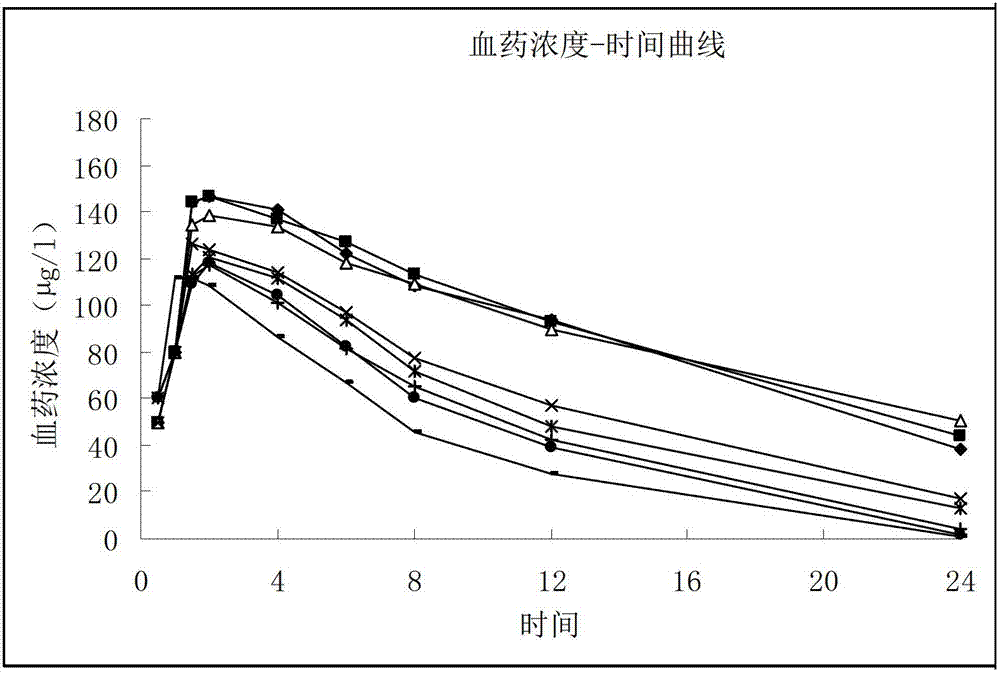
The invention provides a sarpogrelate hydrochloride tablet. The sarpogrelate hydrochloride tablet is composed of the following raw and accessory materials by weight: 100 parts of sarpogrelate hydrochloride, 60 to 100 parts of microcrystalline cellulose PH302, 4 to 8 parts of citric acid, 2 to 6 parts of magnesium stearate, 8 to 12 parts of aerosil and 5 to 6 parts of Opadry (registeration trademark) 295F6800. The tablet has low preparation cost and better stability compared with commercially available preparations. The invention also provides a preparation method for the sarpogrelate hydrochloride tablet to overcome contradiction between minimal accessory materials and particle mobility. Research results in the invention prove that when sarpogrelate hydrochloride and citric acid are mixed and crushed to specific particle sizes in advance and then mixed with microcrystalline cellulose of a specific model, the requirement on mobility of mixed particles during direct pressing of powder is met and the dissolving-out behavior of the tablet is realized; and results of stability test show that the tablet has better stability compared with commercially available preparations.
Owner:SHANDONG JINHE DRUG RES DEV
Sarpogrelate hydrochloride sustained release pellet and preparation method thereof
ActiveCN102552165BLow friabilityHigh yieldOrganic active ingredientsPharmaceutical product form changeSustained release pelletsSarpogrelate Hydrochloride
The invention belongs to the field of medicinal preparation and discloses a sarpogrelate hydrochloride sustained release pellet and a preparation method thereof. The pellet is prepared by coating a sustained release coat on a medicine contained pellet core, wherein the formula of the medicine contained pellet core comprises the following raw materials in parts by weight: 75-100 parts of sarpogrelate hydrochloride and 0-25 parts of excipient, wherein the total weight part of the sarpogrelate hydrochloride and the excipient is 100 or the weight parts of the sarpogrelate hydrochloride and the excipient are increased or reduced by the same ratio; a surfactant accounts for 0.1-8% of the total weight of the sarpogrelate hydrochloride and the excipient; and a wetting agent accounts for 0.1-30% of the total weight of the sarpogrelate hydrochloride and the excipient. By using the preparation method disclosed by the invention, the medicine contained pellet core with low friability, higher yield, smaller particle size and smooth surface can be obtained; the pellet core is convenient to be further processed; according to the invention, the medicine contained pellet core is coated with the sustained release coat so as to obtain a potassium citrate sustained release pellet; and the pellet has the advantages of controllable and stable quality in vitro and sustained release characteristics in vivo.
Owner:JINLING PHARMA
Sarpogrelate hydrochloride lipidosome solid preparation
InactiveCN103040749ASmall particle sizeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolSarpogrelate Hydrochloride
The invention relates to a sarpogrelate hydrochloride lipidosome solid preparation and a preparation method thereof. The preparation method comprises the following steps: selecting sarpogrelate hydrochloride, dilauroyl phosphatidylcholine, phosphatidyl ethanolamine, phosphatidyl glycerol and cholesterol with specific weight ratio to prepare sarpogrelate hydrochloride lipidosome with excellent quality; and preparing the solid preparation by the common preparation method. Compared with the conventional preparation, the preparation provided by the invention improves the stability, the bioavailability and the product quality of the preparation and reduces the toxic or side effect.
Owner:海南路易丹尼生物科技有限公司
Preparation method of high-purity sarpogrelate hydrochloride
ActiveCN103242179BOrganic compound preparationAmino-hyroxy compound preparationSarpogrelate HydrochlorideSolvent
The invention provides a preparation method of high-purity sarpogrelate hydrochloride. The method comprises the following steps of: carrying out alkali dissociation on 1-dimethylamino-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy-2-propanol hydrochloride which is taken as a raw material, reacting the dissociated 1-dimethylamino-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy-2-propanol hydrochloride with succinic anhydride to obtain an ester, acidifying the ester to obtain a sarpogrelate hydrochloride crude product, and purifying the sarpogrelate hydrochloride crude product by using butanone as a recrystallization solvent to obtain the sarpogrelate hydrochloride, wherein the purity of the obtained sarpogrelate hydrochloride is higher than 9.9%, the any individual impurity of the obtained sarpogrelate hydrochloride is less than 0.1%, and the yield of the obtained sarpogrelate hydrochloride is higher than 90%. The preparation method adopts the single solvent for recrystallization, which facilitates solvent recovery, so that the preparation method is suitable for industrial production.
Owner:深圳万乐药业有限公司
Method for preparing sarpogrelate hydrochloride
InactiveCN101239920BEase of industrial productionSimple and fast operationOrganic compound preparationAmino-hyroxy compound preparationTriphenylphosphine oxideSarpogrelate Hydrochloride
Provided is a preparing method of sarpogrelate hydrochloride, characterized in that 2-((3-methoxyl)styrylcoumarin)phenol mixture containing triphenylphosphine oxide is catalyzed and hydrogenated, and then reacted with epichlorohydrin and dimethylamine to obtain 2-(dimethylamino)-1-(o-(m-methoxyphenylethyl)phenoxymethyl)ethanol, at the same time, triphenylphosphine oxide is separated or not, reacted with succinic anhydride, salt-formed with chlorine hydride, and re-crystallization by a proper solvent, so as to obtain sarpogrelate hydrochloride.
Owner:NANJING KANGRAN PHARMA TECH
Method for detecting related substances of sarpogrelate hydrochloride intermediate
ActiveCN108205034ASimple methodGood reproducibilityComponent separationAlcoholSarpogrelate Hydrochloride
The invention discloses a method for detecting related substances of sarpogrelate hydrochloride intermediate C. The method is simple, and the precision, accuracy, reproducibility and durability are good. The method can be used for quality control of the related substances of the sarpogrelate hydrochloride raw material intermediate C, namely 1-(dimethylamino)-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]-2-propyl-alcohol hydrochloride.
Owner:TIANJIN CHASE SUN PHARM CO LTD
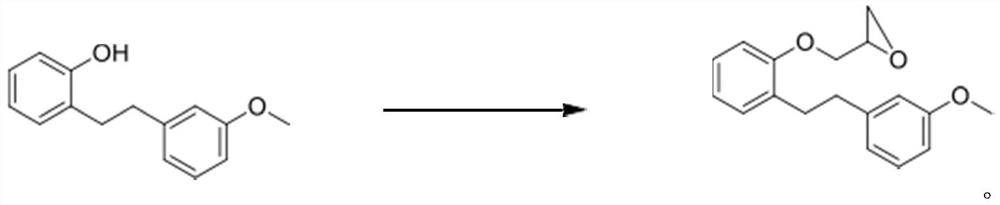
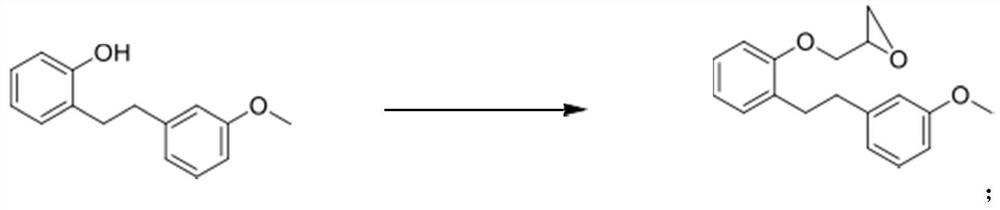
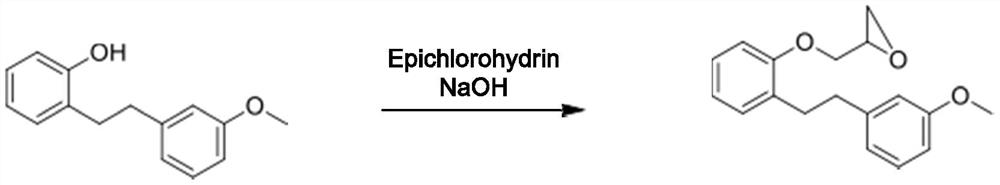
Novel method for preparing sarpogrelate hydrochloride
PendingCN111807976AEasy to operateSuitable for industrial productionOrganic compound preparationAmino-hyroxy compound preparationEthyl groupSolvent free
The invention aims to disclose sarpogrelate hydrochloride prepared by a convenient and environment-friendly method, relates to a solvent, in particular to a solvent which is prepared by free condensation of 2-[2-(3-methoxyphenyl) ethyl] phenol and epoxy chloropropane (2-[2-(3-metoxypherol) ethyl] pherol withepoxychloropropane). Under atmospheric pressure, dimethylamine is used for ring opening ofethylene oxide of the obtained intermediate, and then the intermediate reacts with succinic anhydride to obtain sarpogrelate. The obtained free alkali is treated by using isopropanol IPA.HCl to obtainthe sarpogrelate hydrochloride. Crystals of hydrochloride are formed in acetone, and a pure product is obtained. According to the preparation method, solvent-free reaction is adopted in the first step, atmospheric pressure reaction is adopted in the second step, and thus the preparation method is suitable for industrial production.
Owner:无锡道科森医药有限公司
The synthetic method of sagrellate intermediate 2-[2-(3-methoxyphenyl) ethyl] phenol
ActiveCN105906486BSimplified processing stepsReduce loss rateOrganic compound preparationGroup 5/15 element organic compoundsChlorobenzeneTriethylphosphite
The invention discloses a synthetic method of a sarpogrelate intermediate 2-[2-(3-methoxyphenyl)ethyl]phenol and relates to the technical field of organic synthesis. In the method, with m-methoxyl chlorobenzene, which is easy to obtain and is low in cost, as a raw material, the method includes the steps of: 1) performing condensation to the raw material with triethyl phosphite; and 2) performing condensation with o-phenylmethoxyl benzaldehyde (which is prepared from salicylic acid and chlorobenzene), performing hydrogenation to alkylate a double bond, and crystallizing a product with petroleum ether to prepare a white crystal target product. The method simplifies process steps, reduces loss ratio and is 99.5% in product purity. The 2-[2-(3-methoxyphenyl)ethyl]phenol is used for preparing sarpogrelate hydrochloride, wherein single impurity is less than 0.1% in content and the product purity reaches more than 99.8%.
Owner:安徽修一制药有限公司
One-pot preparation method for photodegradation of impurities Ⅰ, Ⅱ, ⅳ, ⅳ of sarcogrelate hydrochloride
ActiveCN107337608BIncrease profitImprove efficiencyOrganic compound preparationAmino-hyroxy compound preparationChromatographic separationSarpogrelate Hydrochloride
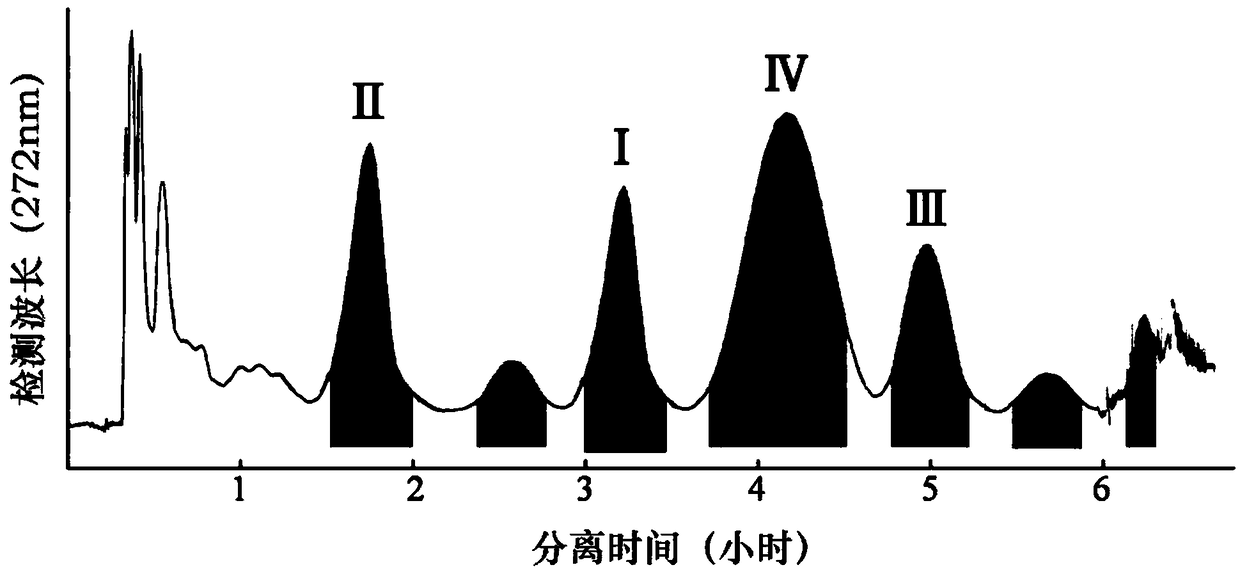
The invention discloses a one-pot method for preparing sarpogrelate hydrochloride photodegradation impurities I, II, III and IV. The method comprises the steps as follows: S1, photodegradation: 2-4 mg / mL of a solution is prepared by dissolving sarpogrelate hydrochloride in water, is treated at the high temperature of 80-90 DEG C for 1-2 h and then sequentially irradiated by a 1200,000-lux cold white fluorescent lamp for 4-6 h and a 200 Wh / m<2> ultraviolet fluorescent lamp for 3-5 h, finally, the degraded solution is concentrated and frozen to dryness, and a degraded mixture is obtained; S2, high-speed countercurrent chromatography: the degraded mixture is dissolved with a fixed phase and a mobile phase with the same volume to serve as a sample solution, ethyl acetate-ethanol-water-formic acid (4:1:5:0.025, v / v / v / v) serves as a two-phase solvent system, the upper phase is the fixed phase, the lower phase is the mobile phase, and the impurities I, II, III and IV are prepared through one-time separation under the conditions that the main engine rotational speed is 800 r / min, the flow velocity is 2.0 mL / min and the detection wavelength is 272 nm. The method adopts few steps and has high efficiency.
Owner:绍兴市逸晨医疗科技有限公司
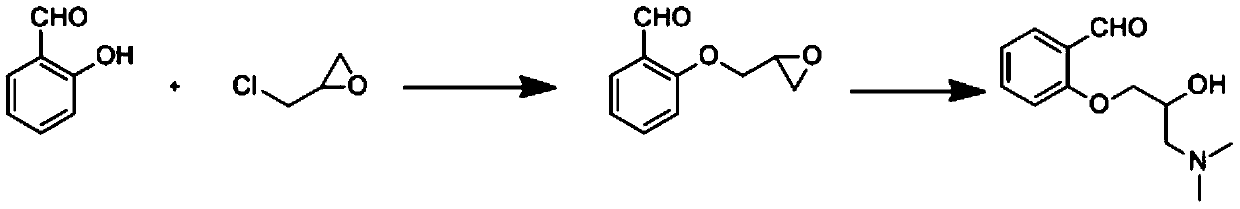
Synthetic method of sarpogrelate hydrochloride intermediate 2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde
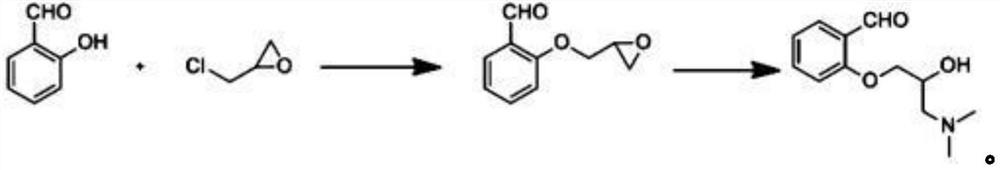
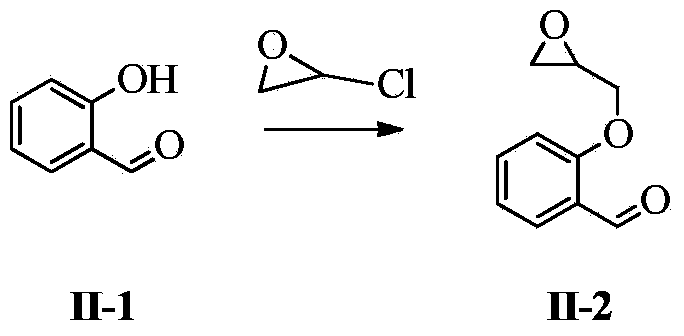
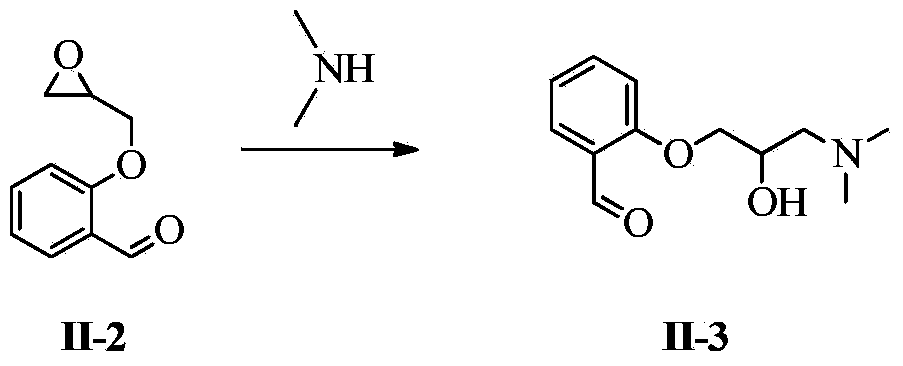
PendingCN114394905ALow input costEasy to synthesizeOrganic compound preparationAmino-hyroxy compound preparationSalicylaldehydeOrganic synthesis
The invention belongs to the technical field of organic synthesis of medicines, and particularly relates to a synthetic method of sarpogrelate hydrochloride intermediate 2-(3-dimethylamino-2-hydroxyl) propoxy benzaldehyde, which comprises the following steps: by taking salicylaldehyde as a raw material, carrying out etherification reaction on the salicylaldehyde and epoxy chloropropane in the presence of an acid-binding agent to generate intermediate 2-(oxirane-2-methoxy) benzaldehyde; carrying out amination reaction on the 2-(3-dimethylamino-2-hydroxy) propoxy benzaldehyde and dimethylamine to generate 2-(3-dimethylamino-2-hydroxy) propoxy benzaldehyde; the epichlorohydrin is used as a reaction solvent at the same time; no additional reaction solvent is added in the etherification reaction and the amination reaction. The 2-(3-dimethylamino-2-hydroxyl) propoxybenzaldehyde is prepared from salicylaldehyde through two-step reaction, no additional reaction solvent is added, the cost of the reaction solvent is reduced, and meanwhile, environmental pollution caused by direct discharge of the reaction solvent or energy consumption input increased by recovery of the reaction solvent is avoided.
Owner:ANHUI HERYI CHEM
Sarpogrelate hydrochloride lipidosome solid preparation
InactiveCN103040749BSmall particle sizeUniform particle size distributionLiposomal deliverySide effectCholesterol
The invention relates to a sarpogrelate hydrochloride lipidosome solid preparation and a preparation method thereof. The preparation method comprises the following steps: selecting sarpogrelate hydrochloride, dilauroyl phosphatidylcholine, phosphatidyl ethanolamine, phosphatidyl glycerol and cholesterol with specific weight ratio to prepare sarpogrelate hydrochloride lipidosome with excellent quality; and preparing the solid preparation by the common preparation method. Compared with the conventional preparation, the preparation provided by the invention improves the stability, the bioavailability and the product quality of the preparation and reduces the toxic or side effect.
Owner:海南路易丹尼生物科技有限公司
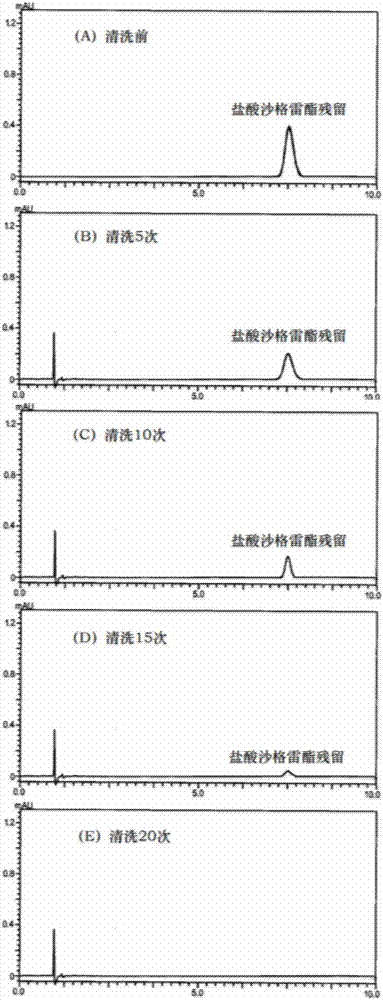
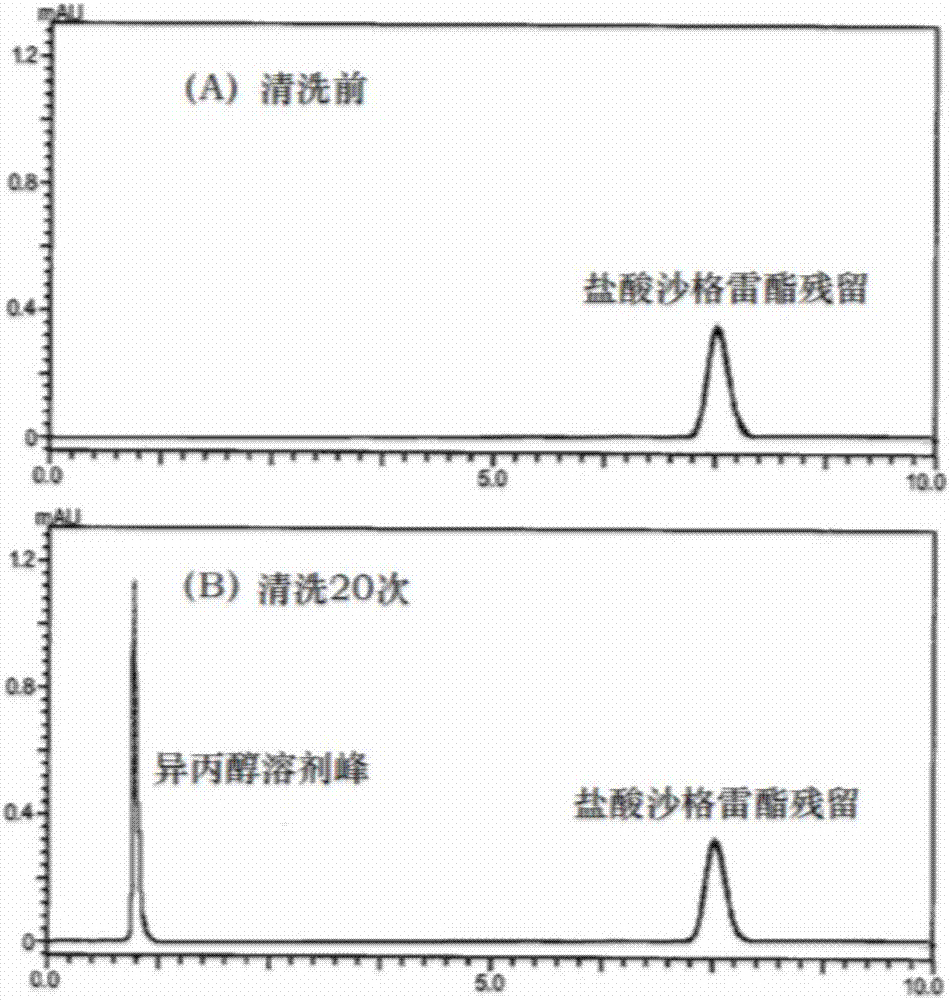
Method for removing residuals of sarpogrelate hydrochloride in high-performance liquid chromatography sample injection valve
InactiveCN107262442AImprove cleaning efficiencyImprove work efficiencyCleaning using liquidsSolubilityArteriolar Vasoconstriction
The invention discloses a method for removing residuals of sarpogrelate hydrochloride in a high-performance liquid chromatography sample injection valve. The sample injection valve is cleaned through multiple times of sample injection of a blank solvent, wherein the blank solvent is a N-methyl pyrrolidone aqueous solution with the volume percent fraction of 50%-70%. Sarpogrelate hydrochloride is a 5-HT2 receptor blocker, can inhibit platelet aggregation and vasoconstriction and has an anti-thrombotic effect and a microcirculation improving effect. The compound is very liable to remain in the sample injection valve although the water solubility is very good, then a small peak can occur at the sarpogrelate peak appearance position even though the blank solvent is injected, a quantitation limit and detection limit determination experiment of sarpogrelate hydrochloride can not be carried out at all due to the phenomenon, and the sarpogrelate hydrochloride content determination accuracy is influenced. According to the method, the efficiency of removing the sarpogrelate hydrochloride residues in the sample injection valve is high, and the working efficiency can be improved.
Owner:南京普氟生物检测技术有限公司
Method for synthesizing 2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde as intermediate of sarpogrelate hydrochloride
InactiveCN109824526ALow input costEasy to synthesizeOrganic compound preparationAmino-hyroxy compound preparationOrganic synthesisBenzaldehyde
The invention discloses a method for synthesizing 2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde as an intermediate of sarpogrelate hydrochloride, and relates to the technical field of pharmaceutical organic synthesis.Salicylaldehyde is used as a raw material, the salicylaldehyde and epichlorohydrin are subjected to etherification reaction in the presence of a deacid reagent to generate theintermediate 2-(ethylene oxide-2-methoxy) benzaldehyde,and then the intermediate 2-(ethylene oxide-2-methoxy) benzaldehydeis aminated with dimethylamine to generate 2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde.According to the method for synthesizing the 2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde as the intermediate of the sarpogrelate hydrochloride,2-(3-dimethylamino-2-hydroxy) propoxybenzaldehyde is prepared by two steps with the salicylaldehyde, no additional reaction solvent is added, the input cost of the reaction solvent is reduced, and the pollution to the environment caused bythe direct discharge of the reaction solvent or the increased energy consumption input of the recovery of the reaction solvent is avoided.
Owner:ANHUI HERYI CHEM
Sarpogrelate intermediate and preparation method thereof
ActiveCN102875340BAvoid it happening againReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationGrignard reagentSarpogrelate Hydrochloride
The invention provides a sarpogrelate intermediate and a preparation method thereof. The sarpogrelate intermediate is a compound shown as formula VI. The preparation method of the intermediate comprises a Grignard reaction of salicylic aldehyde with phenolic groups protected and a Grignard reagent. The method is mild in reaction conditions, low in energy consumption and small in pollutions, and has relatively high conversion rate and low cost of the raw materials. The obtained sarpogrelate hydrochloride has high purity with the detection purity of HPLC higher than 99%. The method is suitable for large-scale production conversion.
Owner:NEW FOUNDER HLDG DEV LLC +2
Use of sarpogrelate hydrochloride impurities I for inducing differentiation of bone marrow mesenchymal stem cells
InactiveCN107714681AGood water solubilityImprove bioavailabilityOrganic active ingredientsDrug compositionsSolubilityOsteoblast
The invention discloses the use of sargrelate hydrochloride impurity I for inducing differentiation of bone marrow mesenchymal stem cells. The present invention provides the application of sarcogrelate hydrochloride impurity I in the preparation of drugs for inducing osteogenic differentiation of bone marrow mesenchymal stem cells. The cognition level of prior art to sarcogrelate hydrochloride impurity I; In order to overcome the problem of poor water solubility and low bioavailability of sarcogrelate hydrochloride impurity I-povidone K ‑30 solid dispersion, the preparation can significantly improve the water solubility of impurity Ⅰ of sarcogrelate hydrochloride, thereby improving its bioavailability.
Owner:南京普氟生物检测技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthetic method of sarpogrelate intermediate 2-[2-(3-methoxyphenyl)ethyl]phenol Synthetic method of sarpogrelate intermediate 2-[2-(3-methoxyphenyl)ethyl]phenol](https://images-eureka.patsnap.com/patent_img/255b1aa1-e06c-420b-989d-f6c949b9c616/BDA0000969687850000021.PNG)

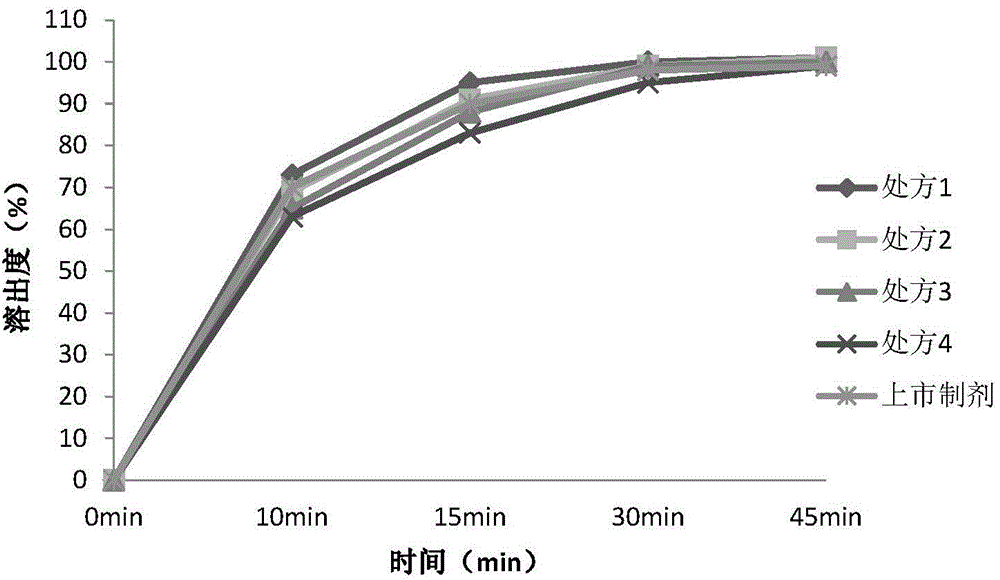

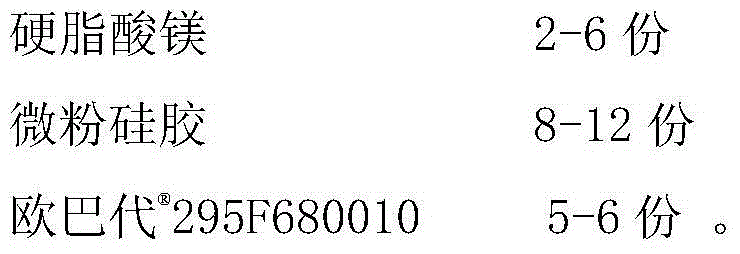



![The synthetic method of sagrellate intermediate 2-[2-(3-methoxyphenyl) ethyl] phenol The synthetic method of sagrellate intermediate 2-[2-(3-methoxyphenyl) ethyl] phenol](https://images-eureka.patsnap.com/patent_img/1b7c2ff2-d23c-4d90-8352-0a8ffdb70ddd/BDA0000969687850000021.png)