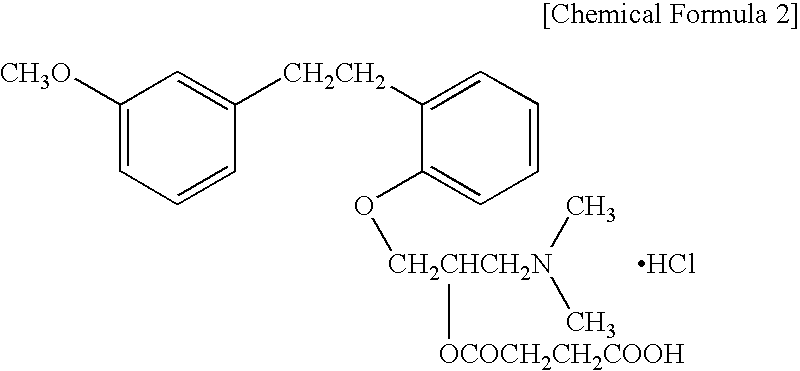
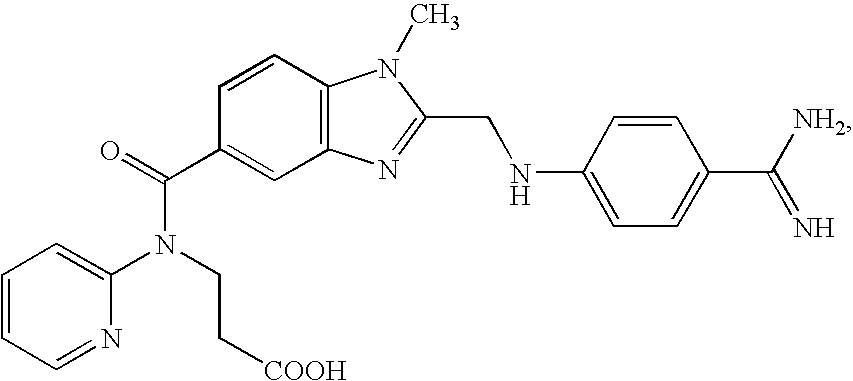
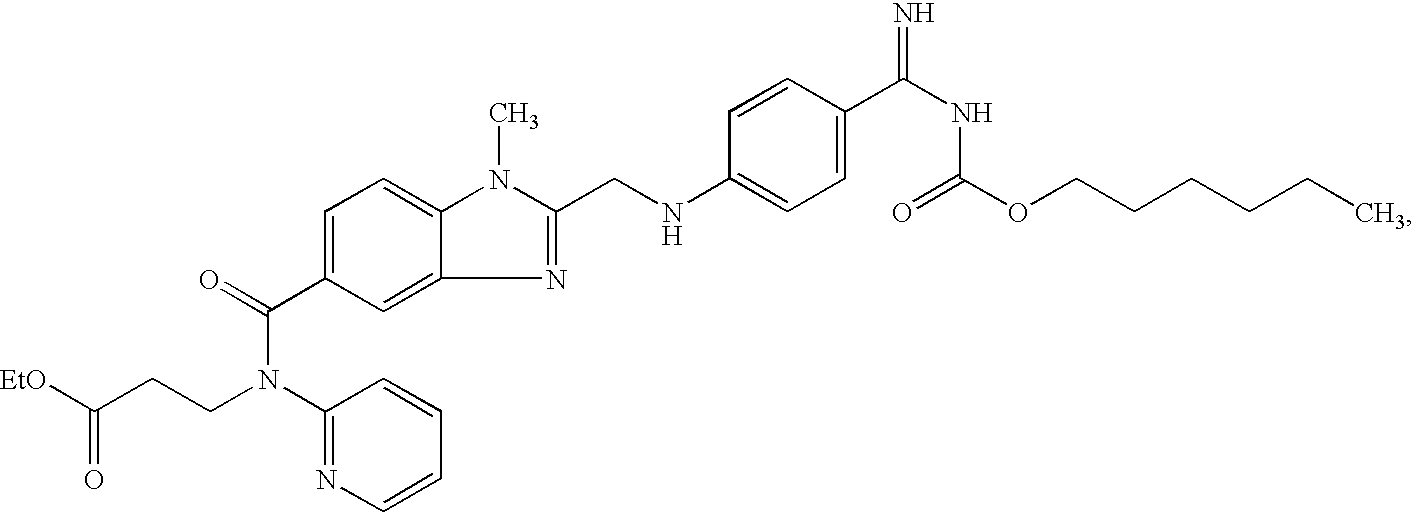
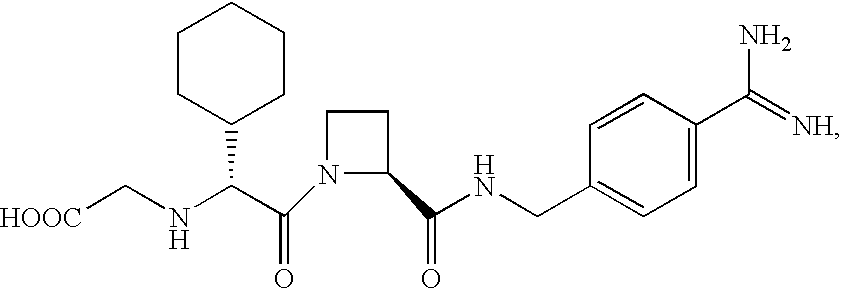
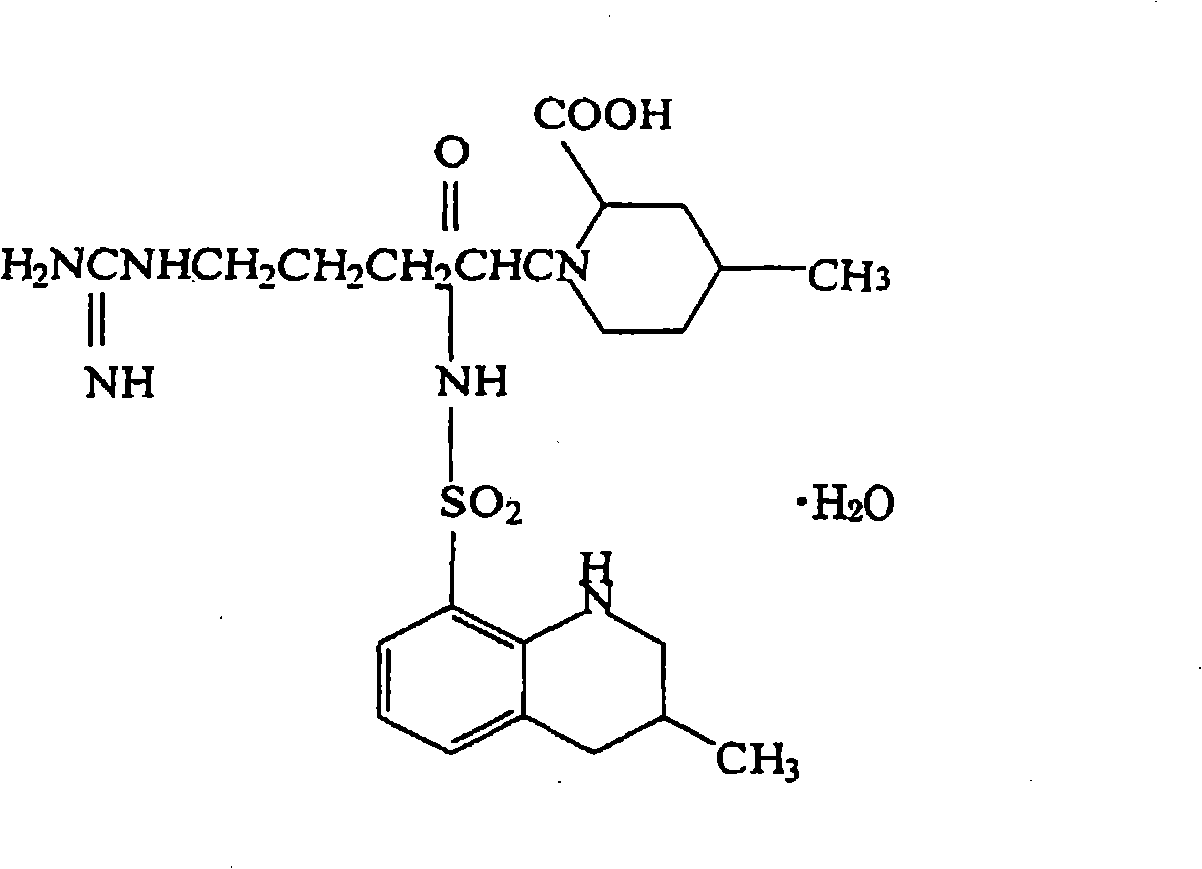
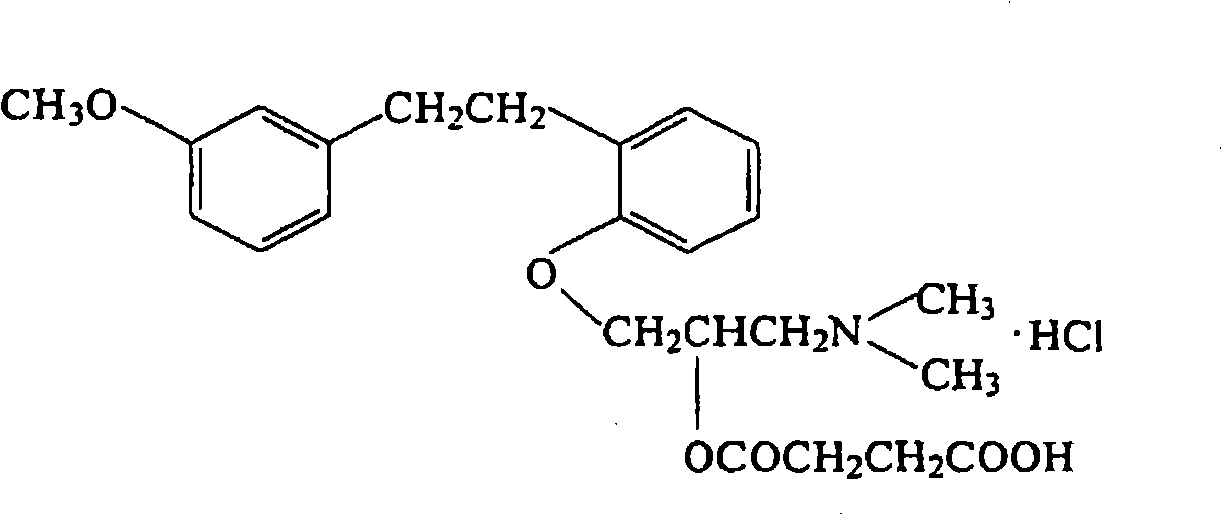
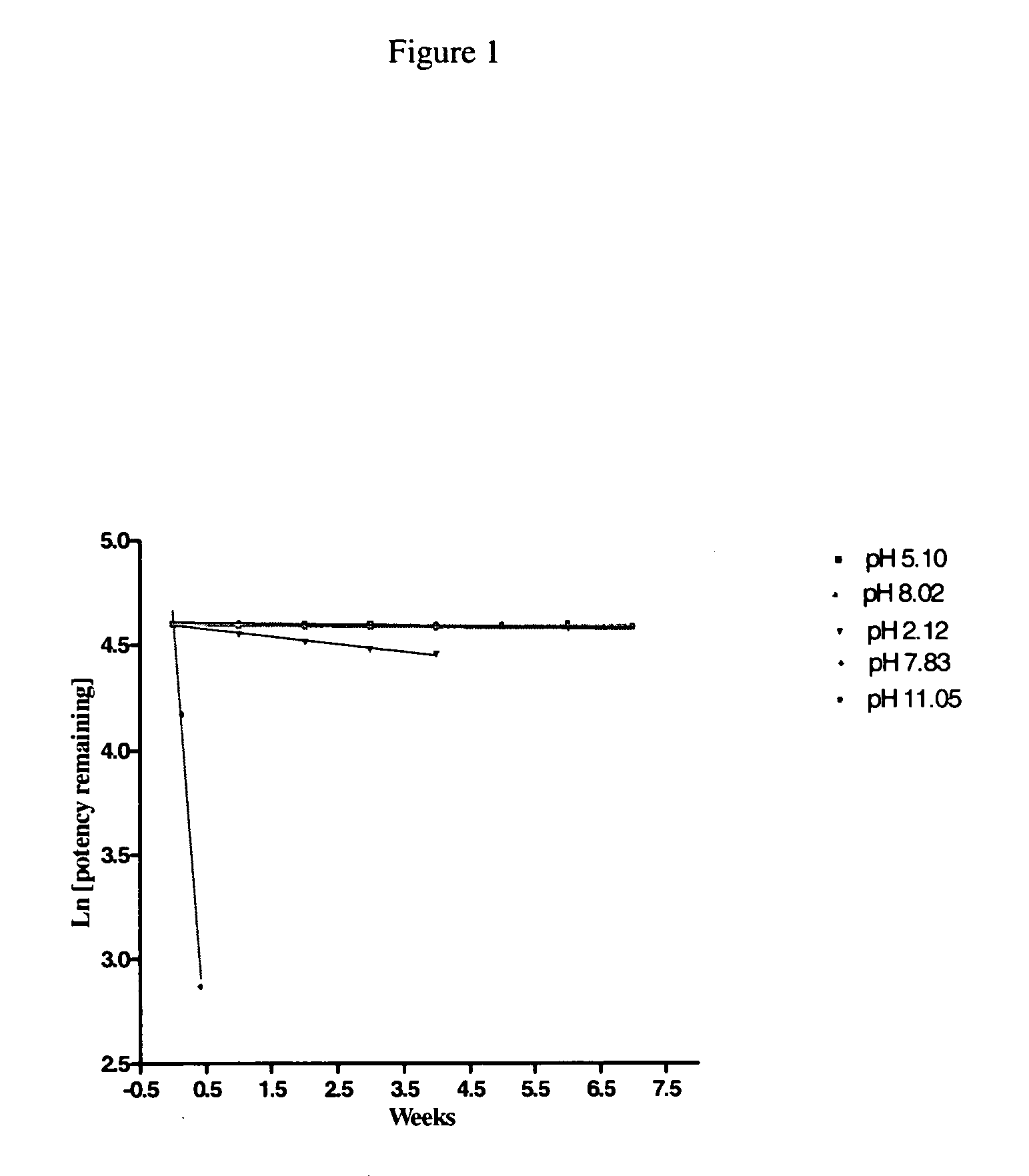
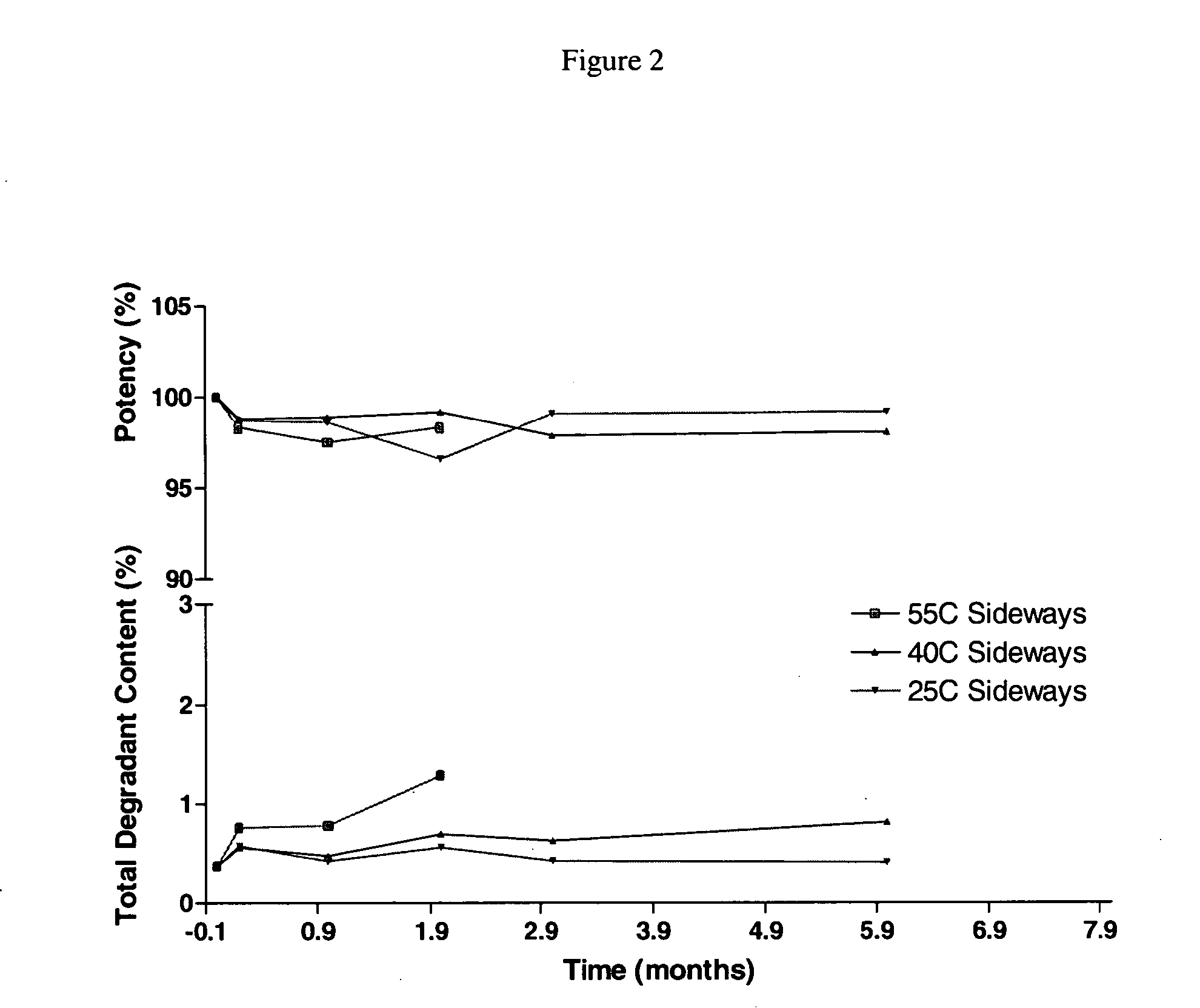
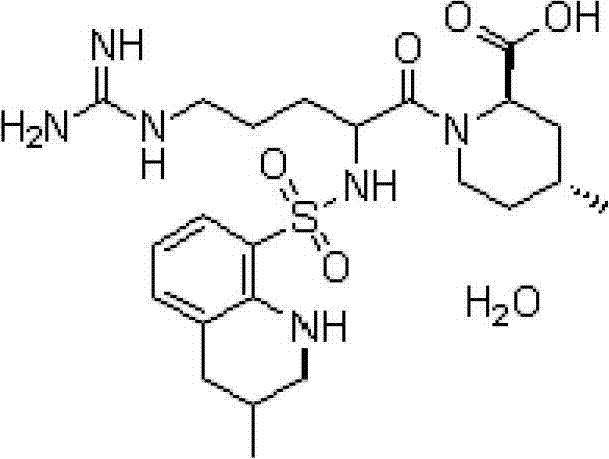
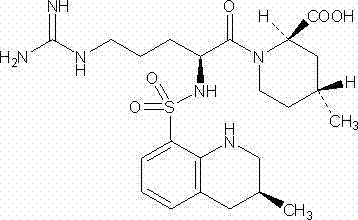
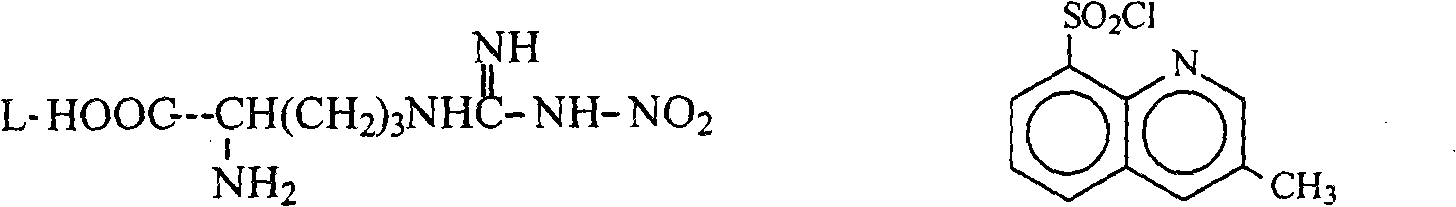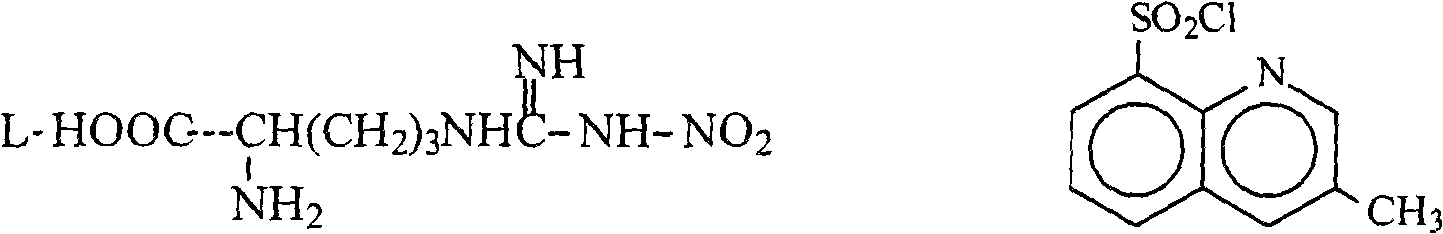Patents
Literature
94 results about "Argatroban" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
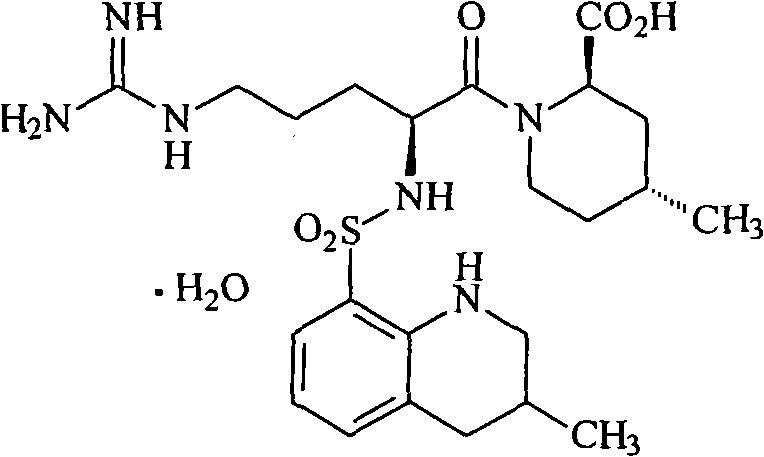
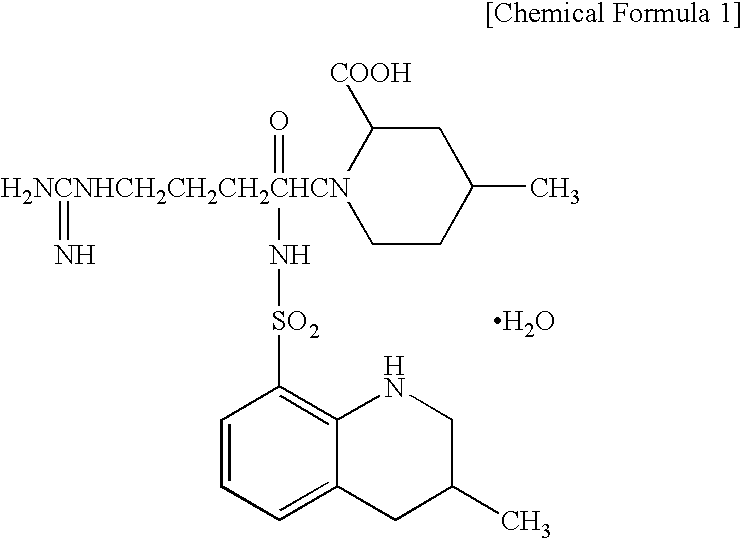
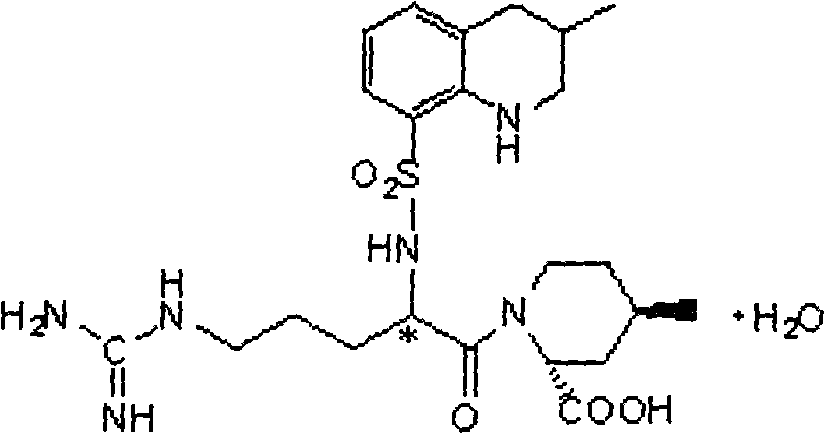
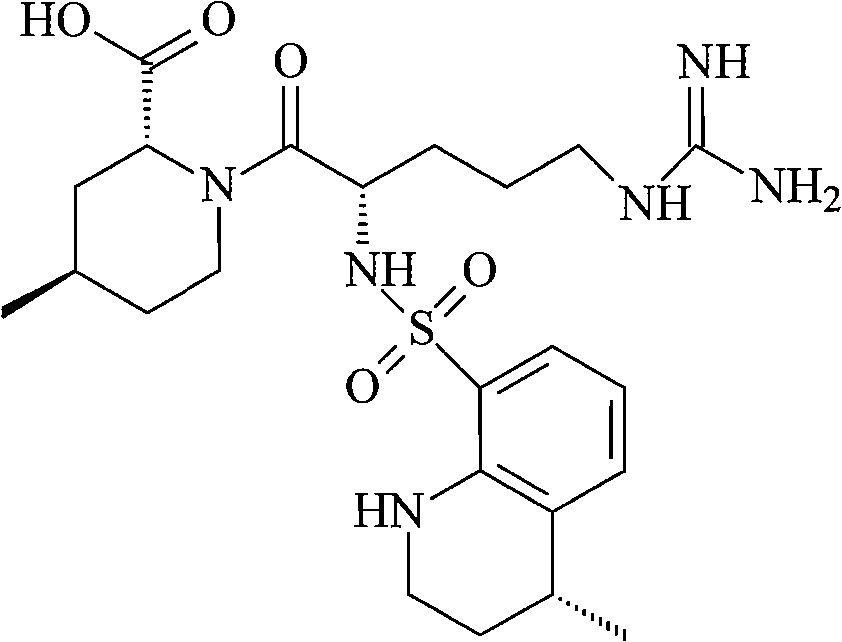
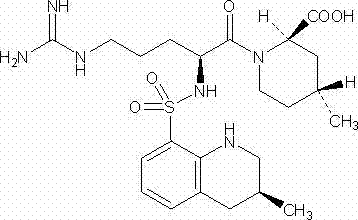
Argatroban is an anticoagulant that is a small molecule direct thrombin inhibitor. In 2000, argatroban was licensed by the Food and Drug Administration (FDA) for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT). In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.
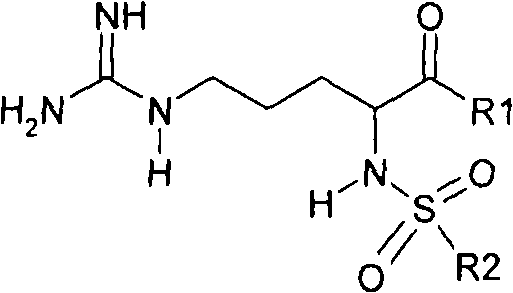
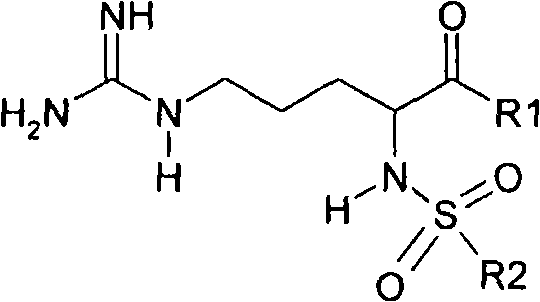
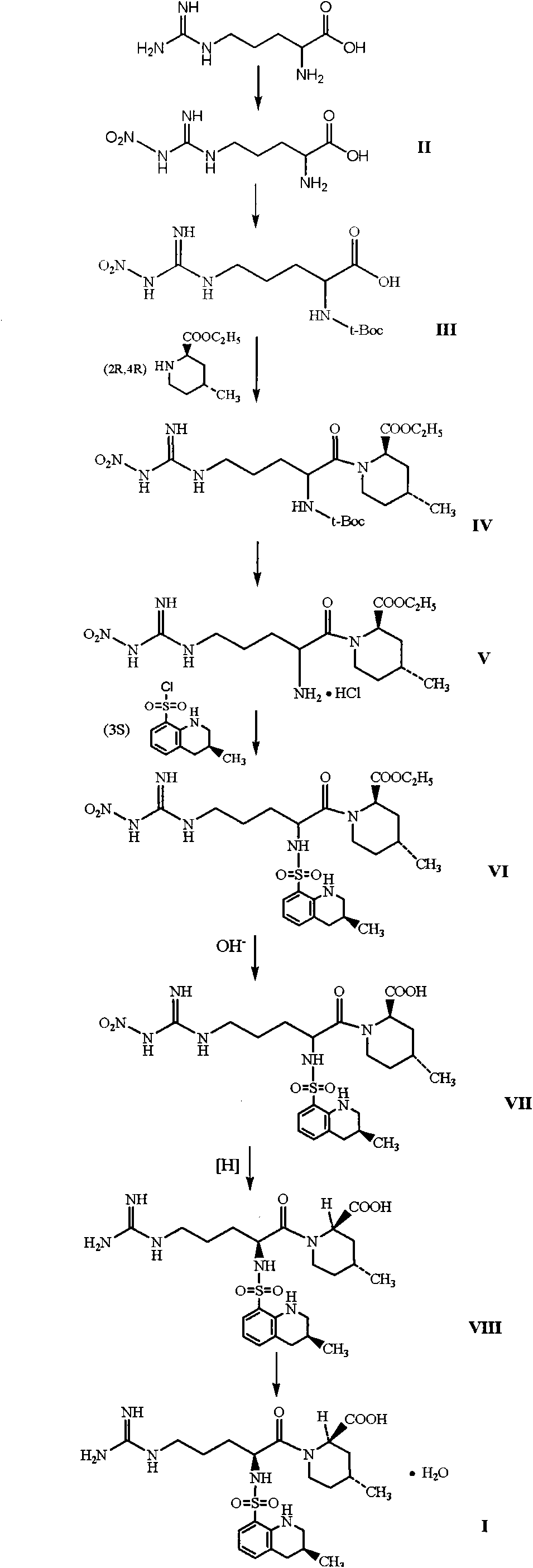
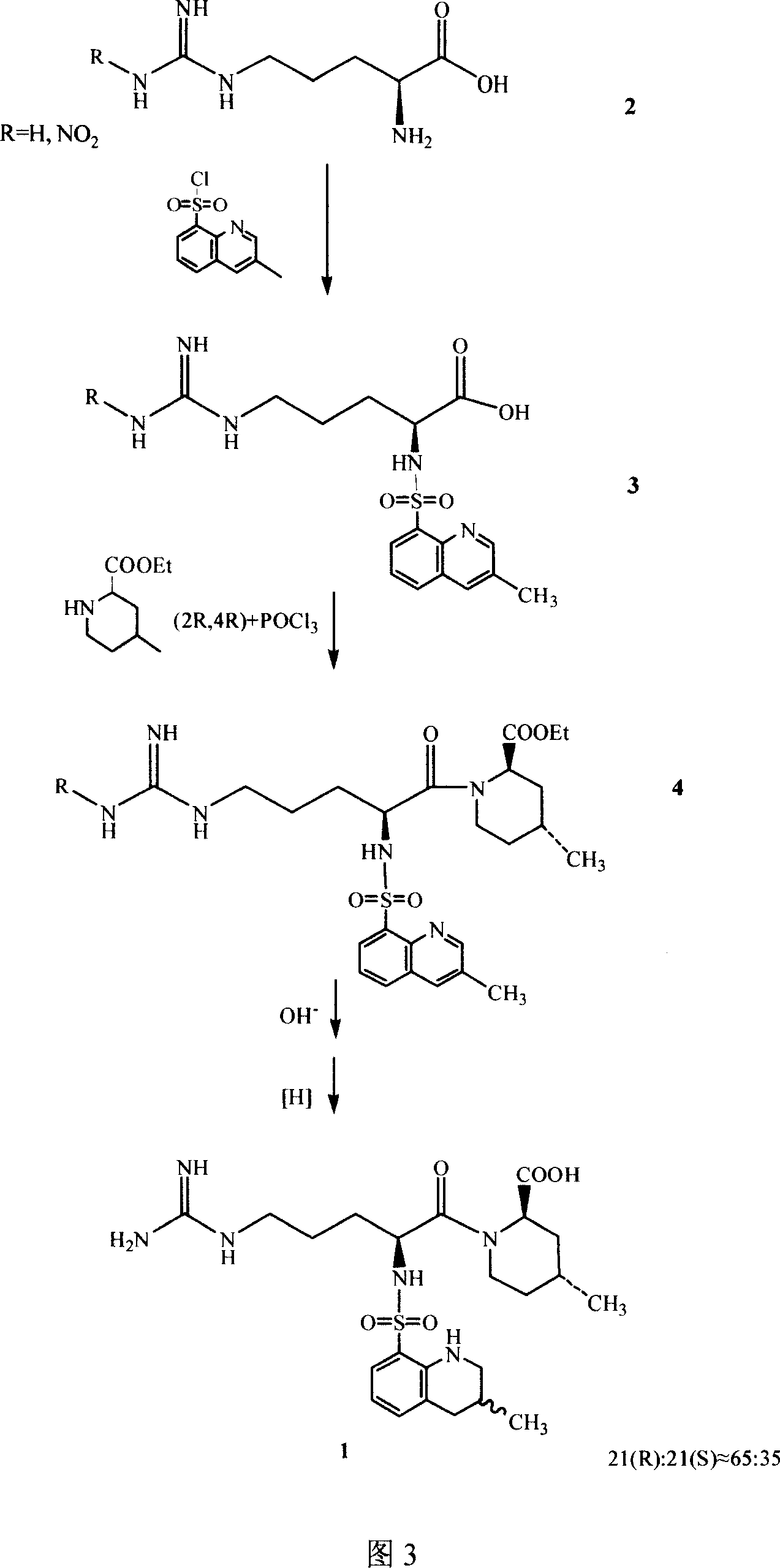
Argatroban and preparation thereof
InactiveCN101348481AThe synthesis process is simpleEasy to operateOrganic chemistryDiethyl phosphateOrganic solvent
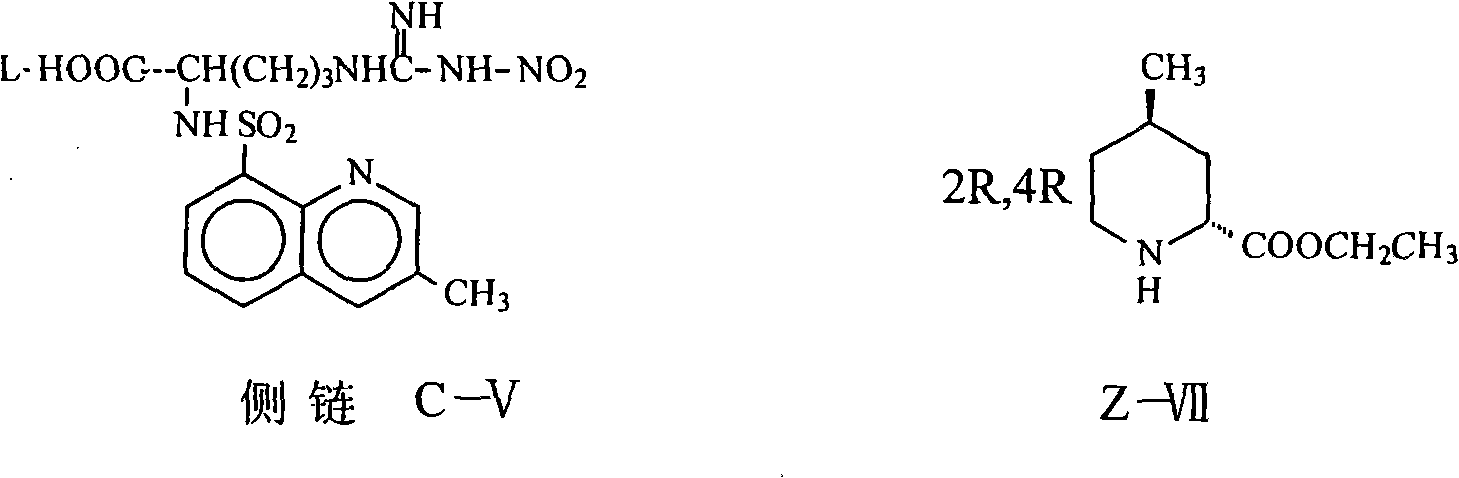
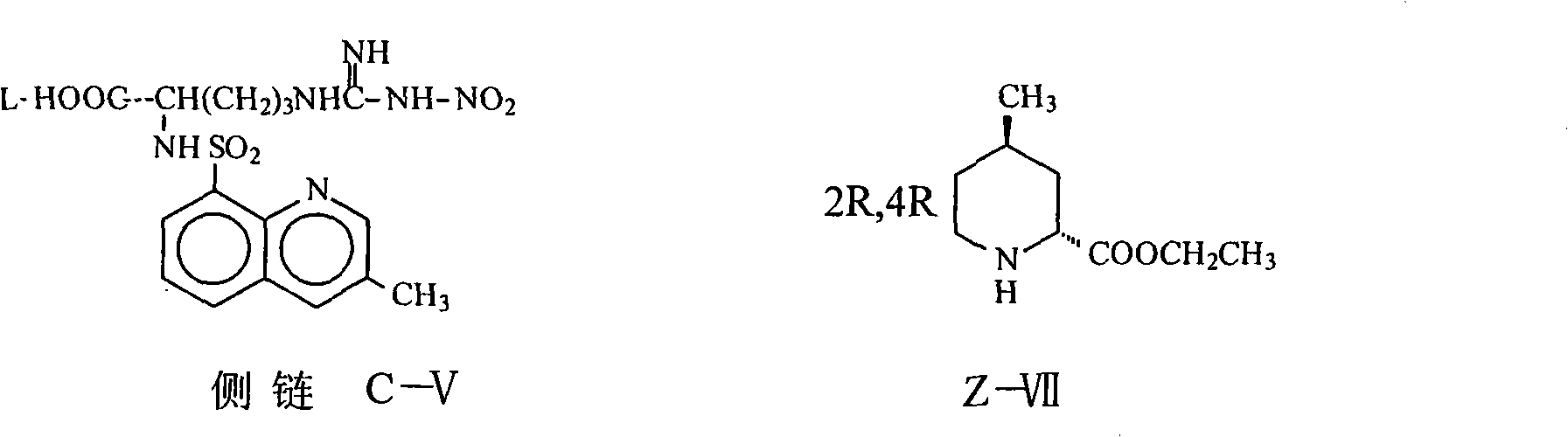
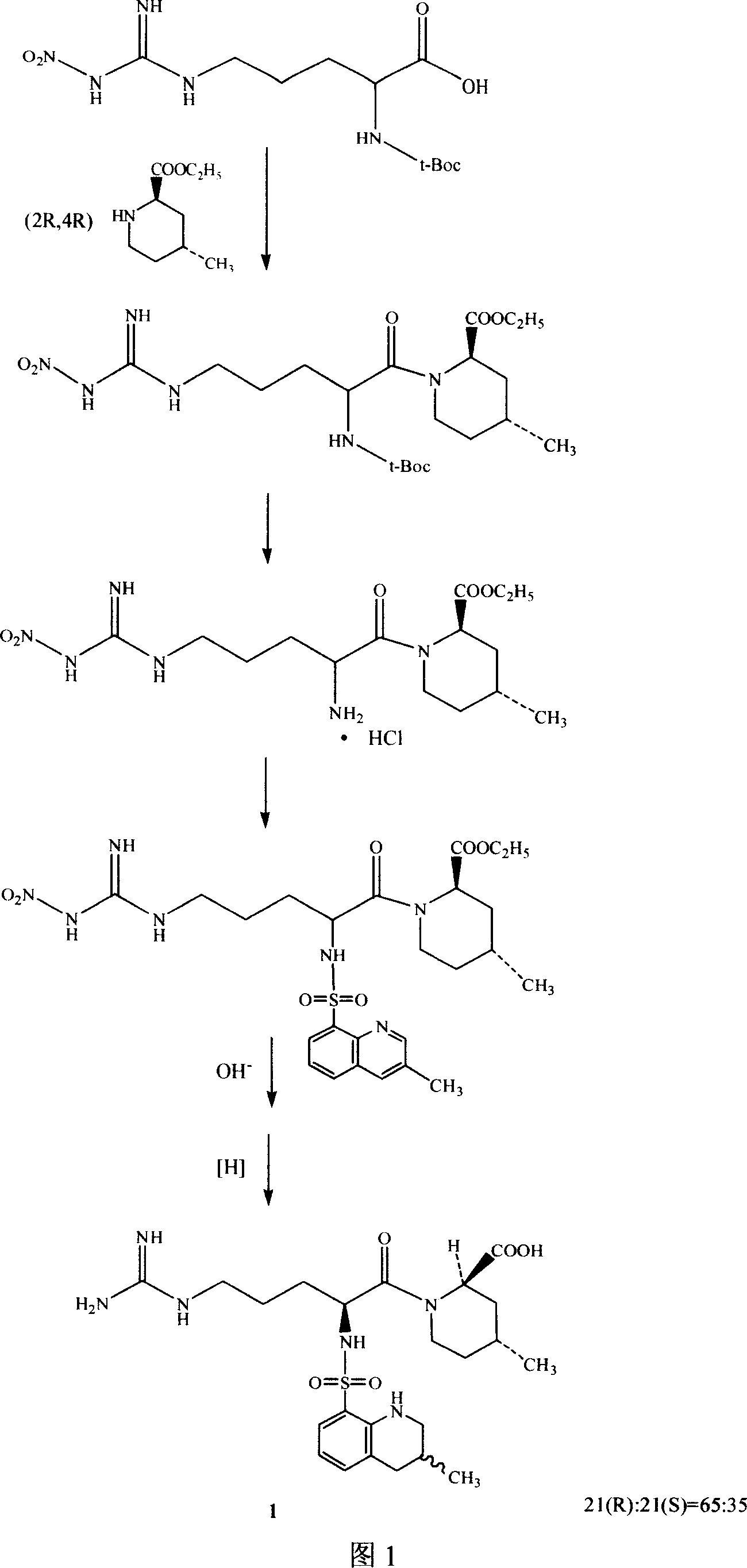
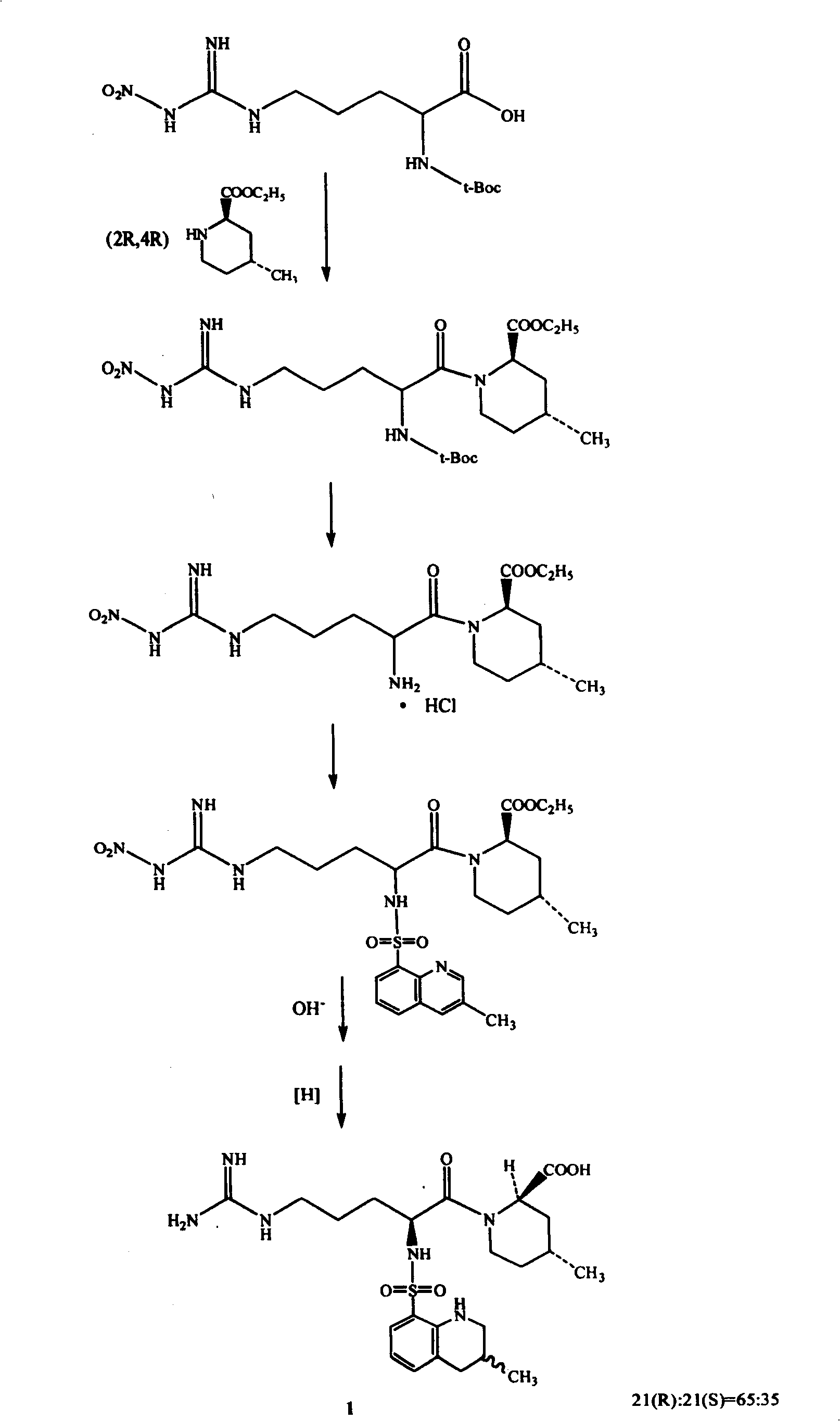
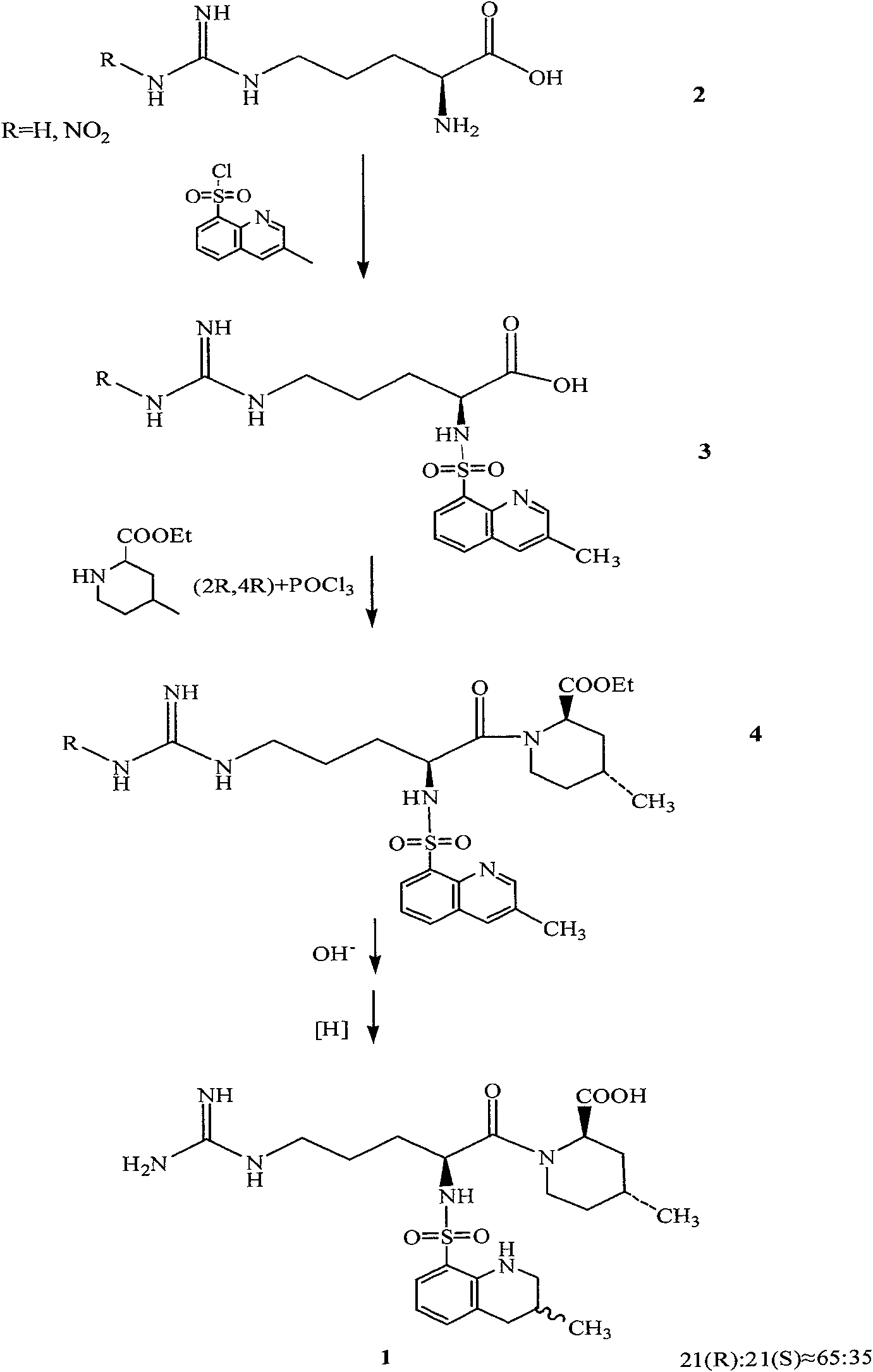
The invention relates to a method for synthesizing argatroban. The method comprises the following steps that nitryl L-arginie and quinoline sulfonchloride are condensed, and undergo amidation with piperidine ethyl formate, followed by hydrolysis and hydrogenation to obtain argatroban; the amidation is to make carboxylate (c-v) and (2R, 4R) 4MPE (Z-VII) react in an organic solvent in the presence of condensing agent, or the presence of both condensing agent and dehydration promoter, in which the molecular ratio of carboxylate (c-v): (2R, 4R) 4MPE (Z-VII): condensing agent: dehydration promoter is 1: 0.8-1.2: 0.8-1.2: 0-1.2. The condensing agent adopted by the invention is diphenylphosphoryl azide, diethylthiophosphoryl, chlorophosphoric acid diethyl or bromophosphoric acid diethyl. The invention has simplified operation, lowered cost, decreased pollution, increased yielding rate, and is suitable for large-scale industrialized production of argatroban.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV +1
Controlled Drug-Release Composition and Drug-Releasable Medical Device
InactiveUS20090048667A1Facilitated releaseSufficient amountCatheterAntithrombogenic treatmentMetal formingSarpogrelate Hydrochloride
A drug-releasable medical device contains a controlled drug-release composition comprising 100 parts by weight of an organic polymeric material which is soluble in an organic solvent and insoluble in water, 5 to 60 parts by weight of a lipid-soluble, low molecular weight release auxiliary agent and 1 to 70 parts by weight of a drug. When the composition is applied on a stent, a catheter, an organ replacement medical device, an artificial organ or the like in the form of coating or the like, the medical device is provided with a drug release function. Argatroban or sarpogrelate hydrochloride or both of them are gradually released from the surface of a stent for treating coronary artery stenosis, for example. In order to exhibit a sustained-release function for a desired period of time, the drug to be gradually released is carried in a polymeric material coated on a surface of a metal forming the stent or in a porous stent substrate.
Owner:TOKAI UNIV +1
Use Of Dipyridamole For Treatment Of Resistance To Platelet Inhibitors
InactiveUS20090048173A1Reduce decreaseBiocidePeptide/protein ingredientsDipyridamolePlatelet inhibitor
Owner:EISERT WOLFGANG +1
Alcohol free formulation of argatroban
The invention provides an aqueous formulation of argatroban and of related compounds along with a reconstitutable formulation, each of which is substantially, if not totally alcohol free. The formulations are also substantially free, if not totally free, of mono-, di-, and oligo- saccharides. An especially preferred embodiment is a ready-to-use 1 mg / ml injectable dosage form having argatroban, lactobionic acid, and methionine.
Owner:SCIDOSE
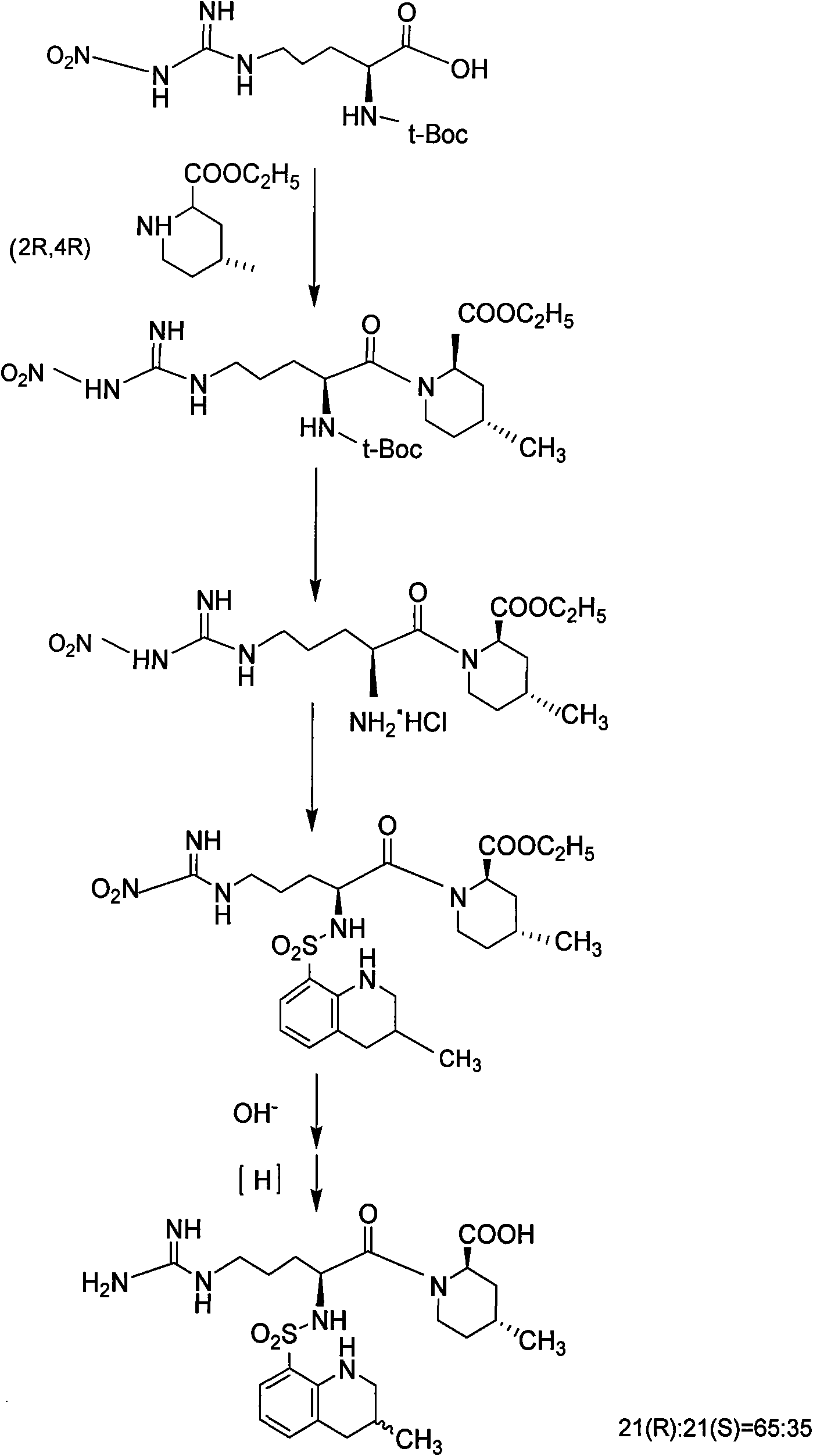
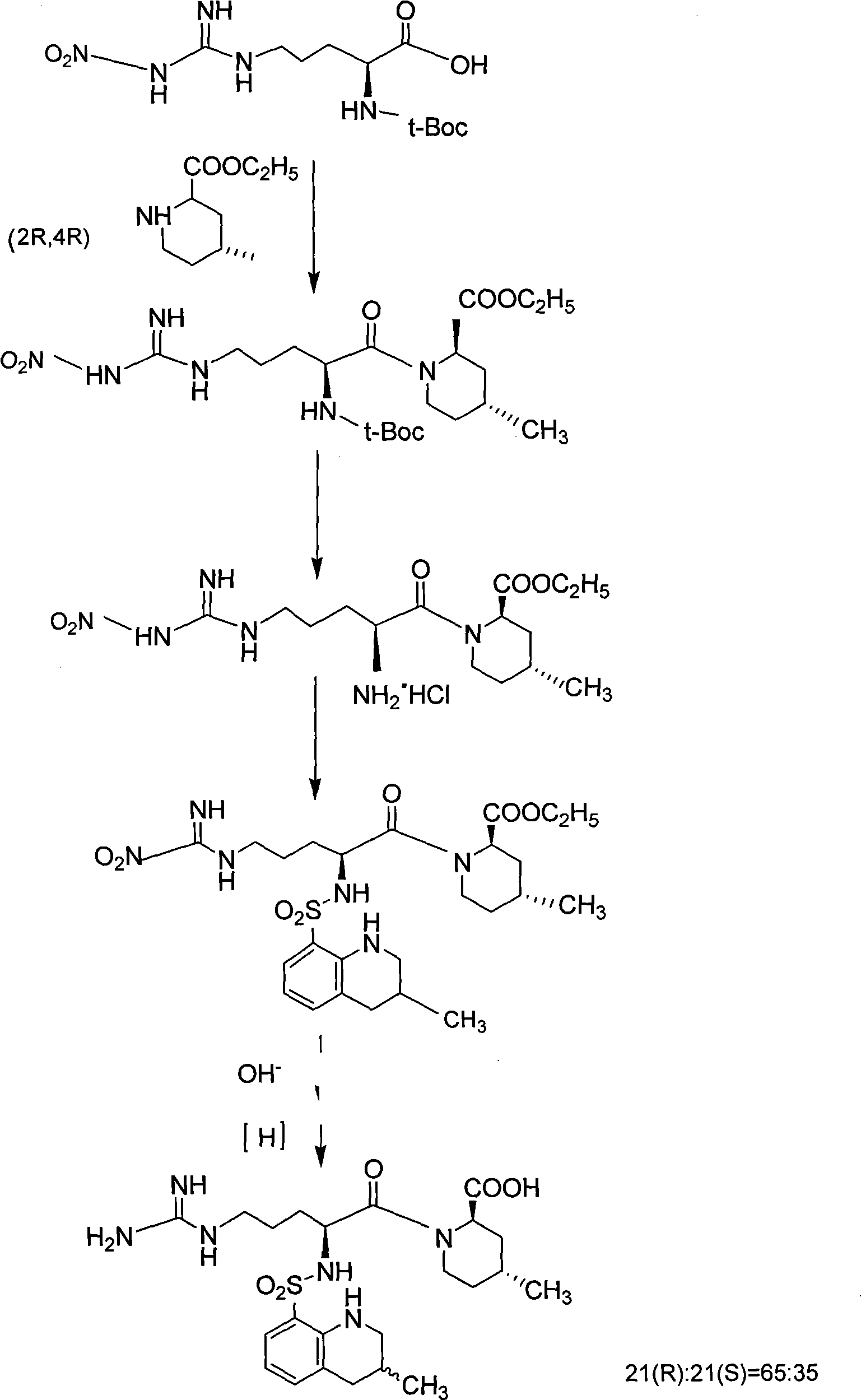
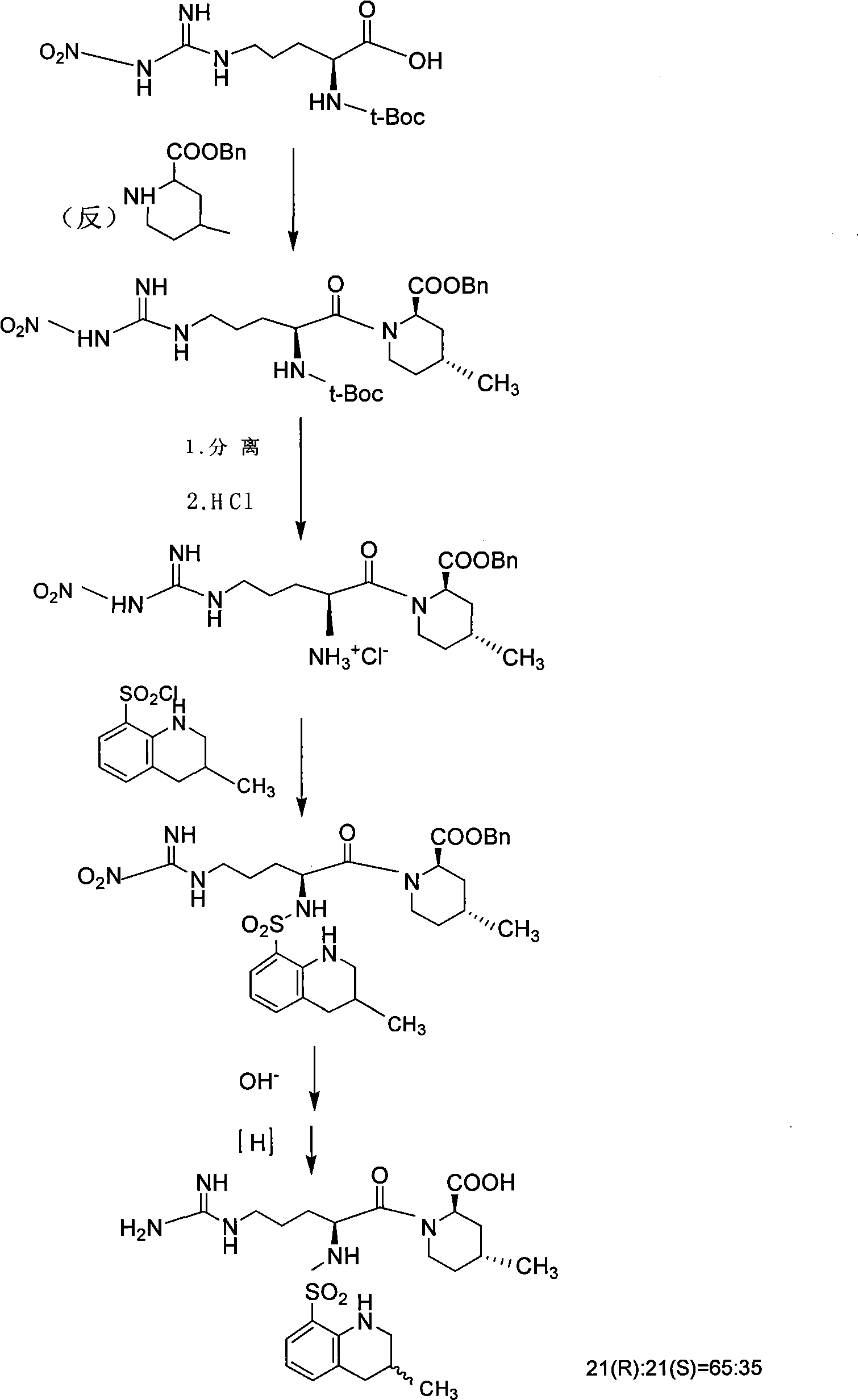
Directional synthesis method for 21(S) argatroban
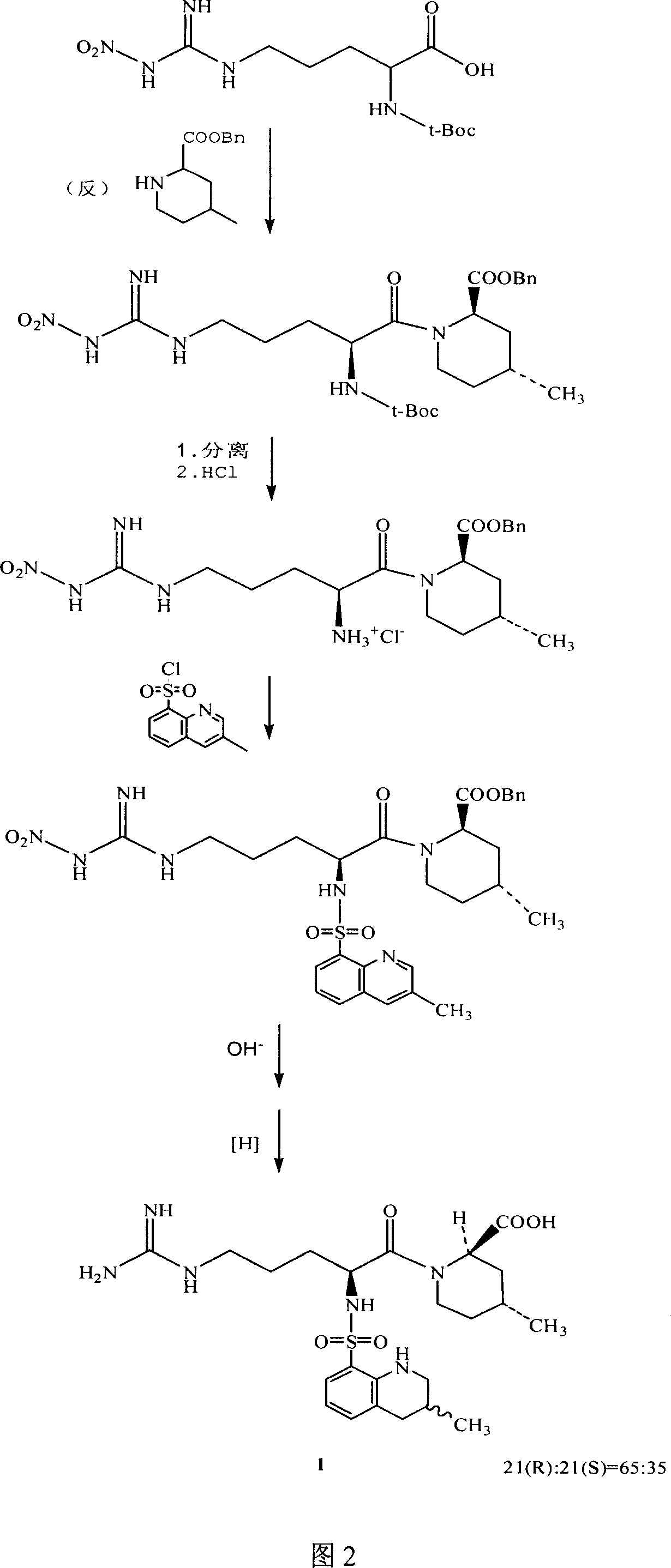
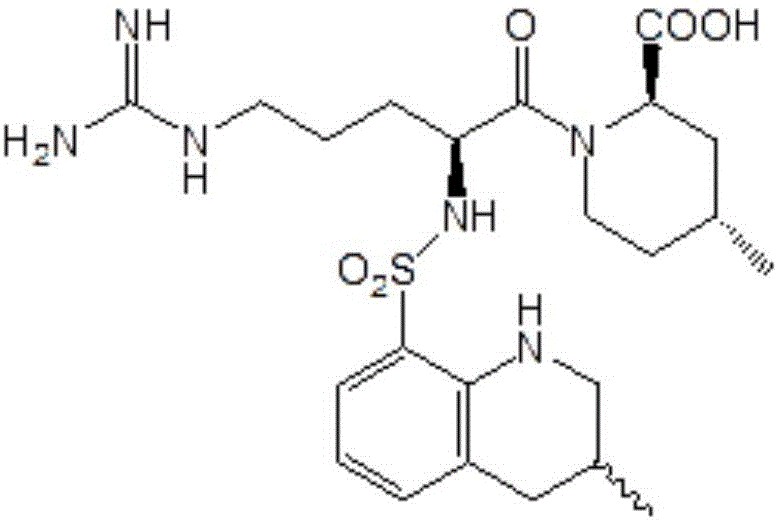
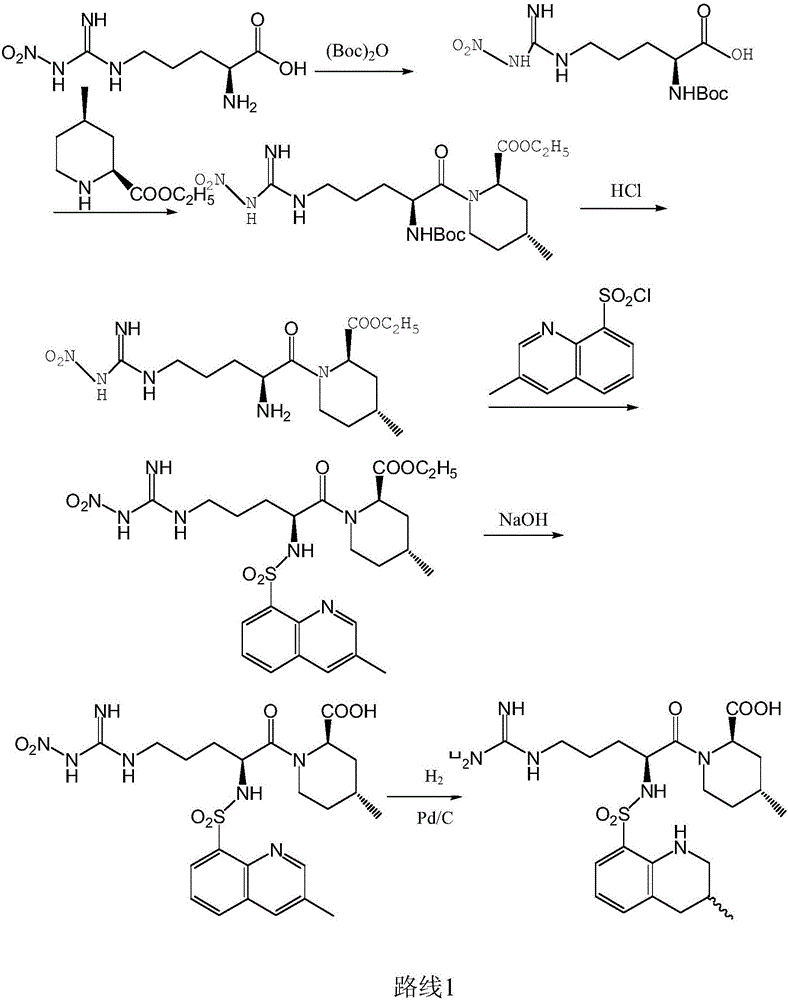
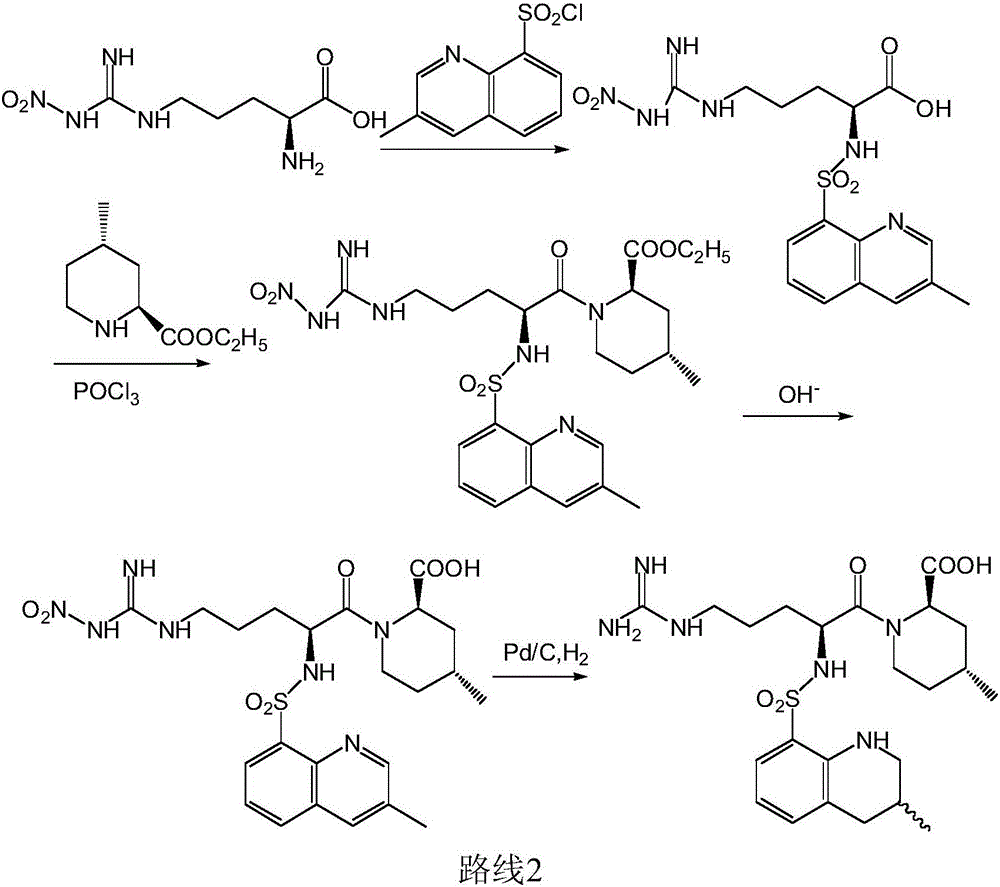
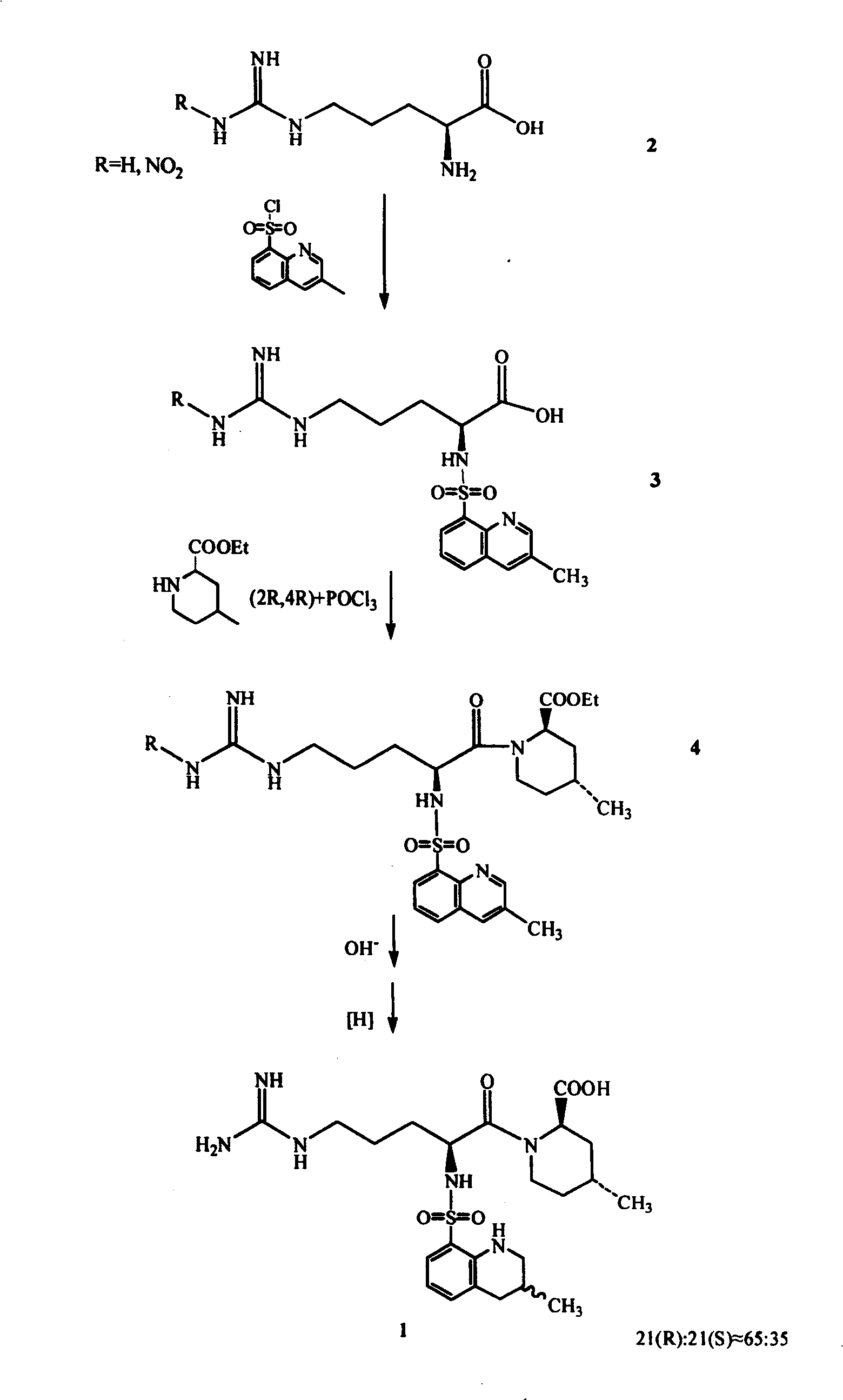
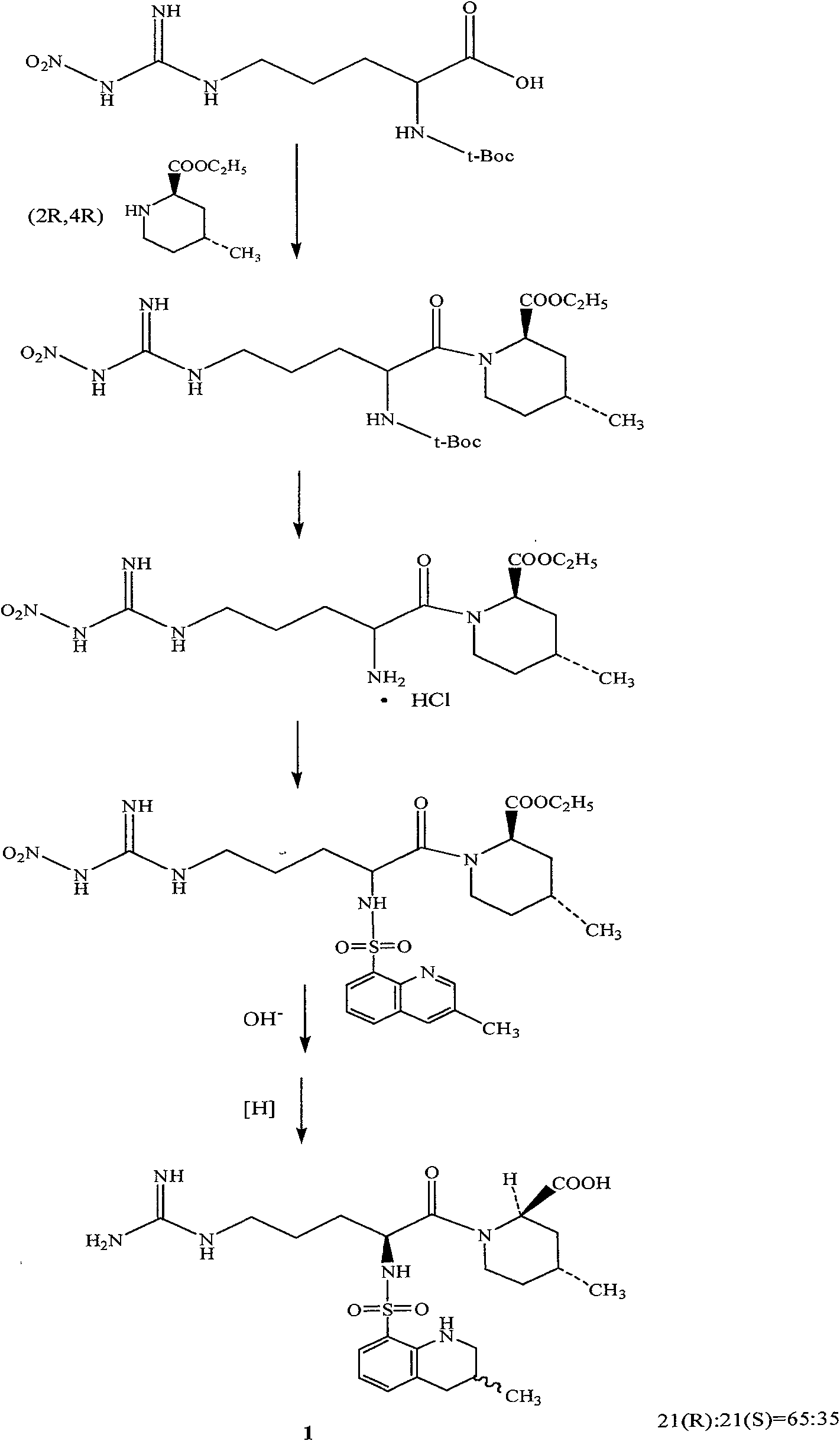
The invention discloses a directional synthesis method for 21(S) argatroban. The directional synthesis method comprises the following steps of: reacting (2R,4R)-1-[NG-nitro-L-arginyl]-4-methyl-2-piperidine ethyl formate hydrochloride serving as a raw material and (3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl chloride to obtain an intermediate (2R,4R)-1-[NG-nitro-N2-[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl]-L-arginyl]-4-methyl-2-piperidine formic ether, then hydrolyzing and acidifying the intermediate in aqueous solution of sodium hydroxide to obtain an intermediate (2R,4R)-1-[NG-nitro-N2-[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl]-L-arginyl]-4-methyl-2-piperidine formic acid, and finally hydrogenating the intermediate to obtain single diastereoisomer 21(S) argatroban by catalysis of palladium carbon.
Owner:TIANJIN WEIJIE TECH
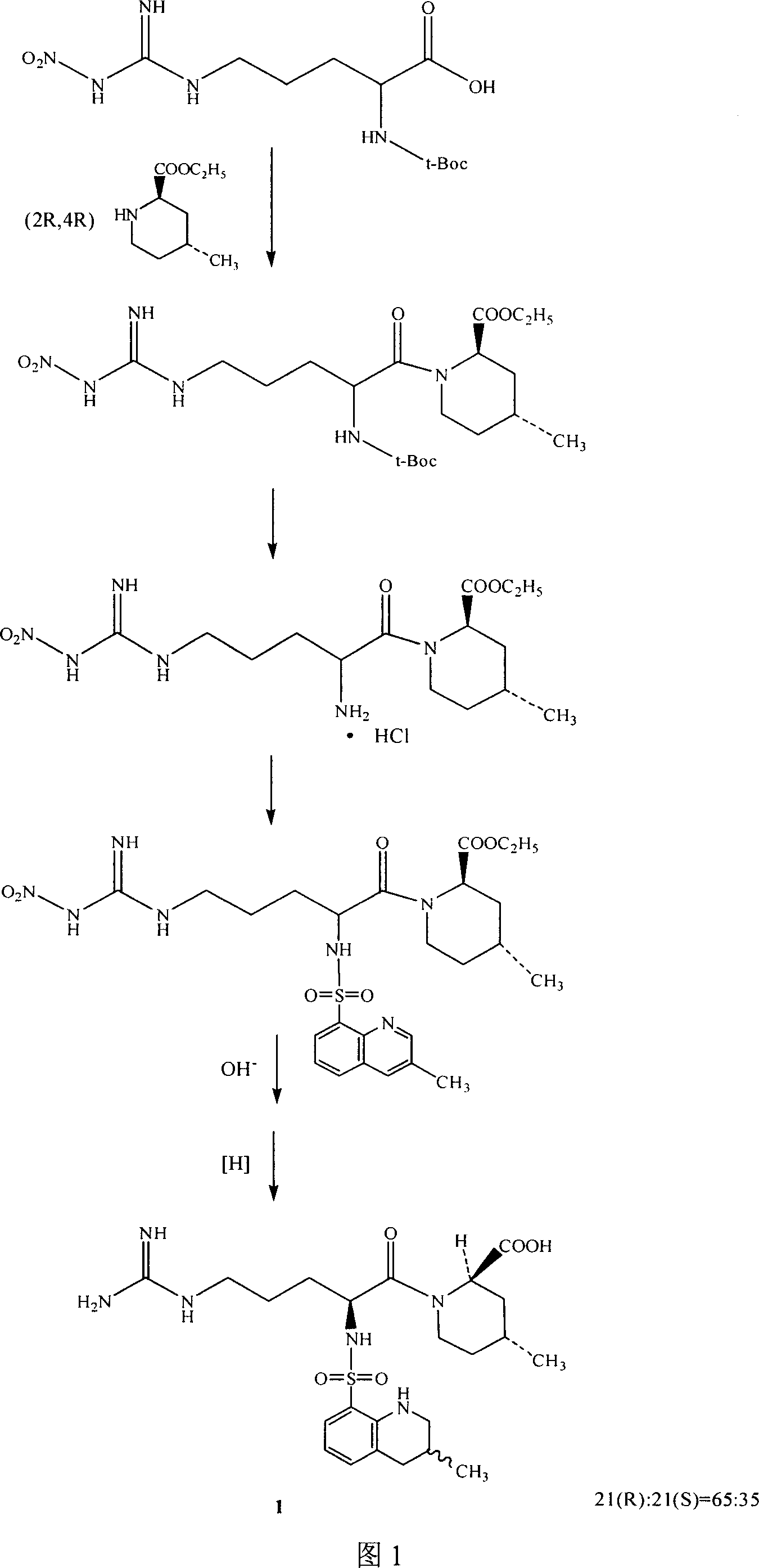
Synthetic method of argatroban and intermediate thereof
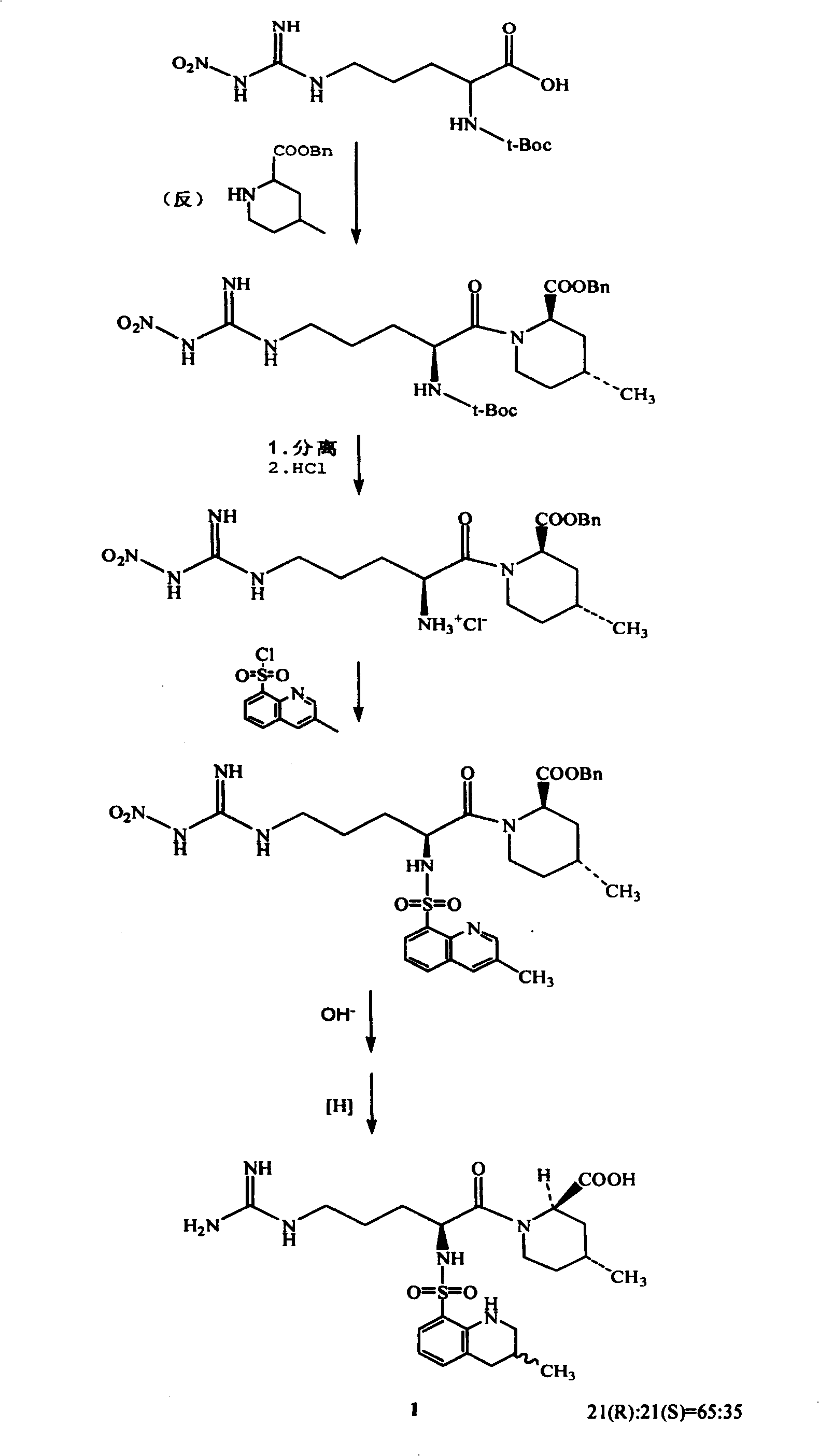
ActiveCN101348463ASolve tough problems that are hard to get rid ofMeet the needs of large-scale industrial productionOrganic chemistrySulfonyl chlorideArginine
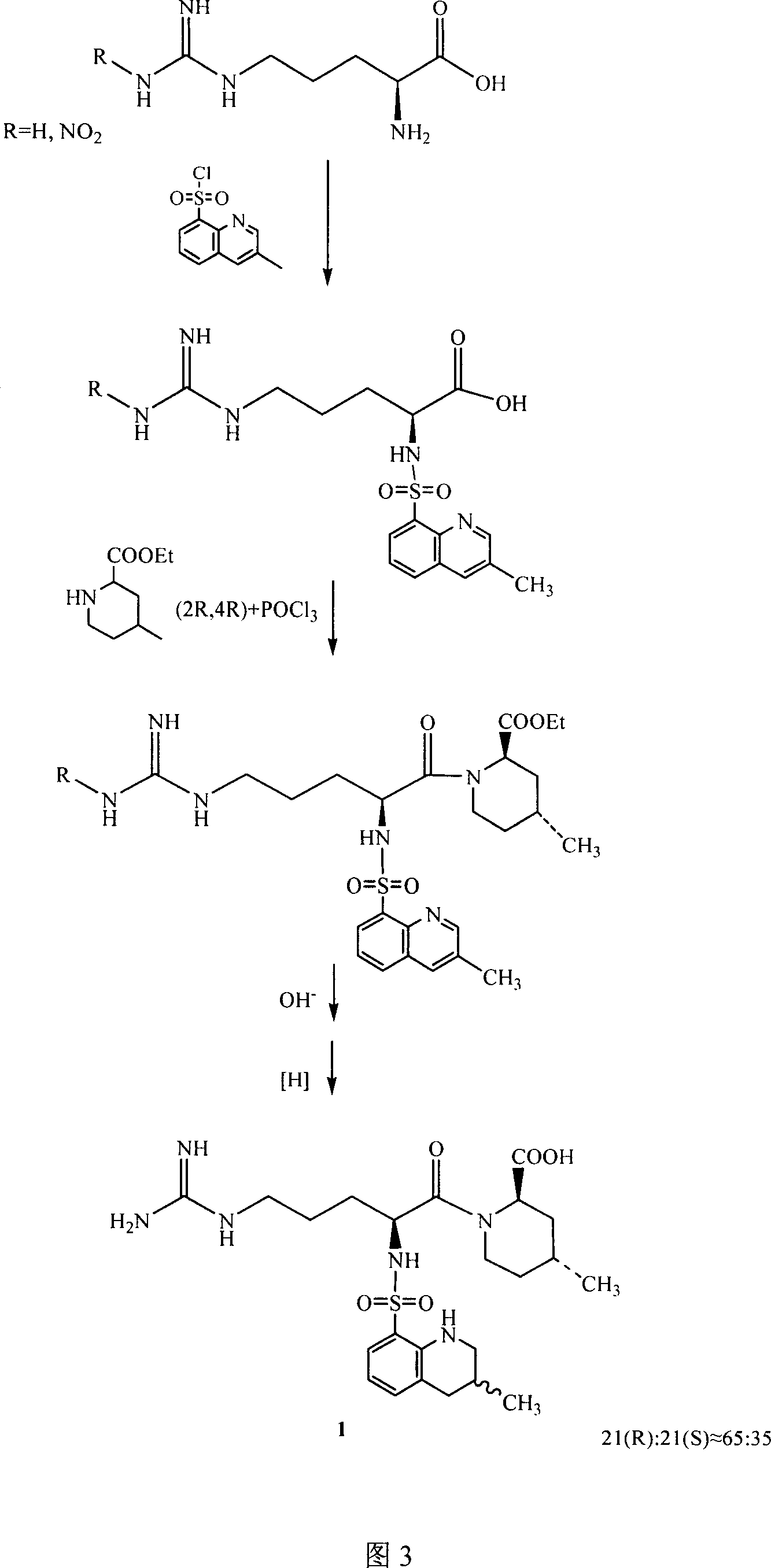
The invention relates to a method for preparing N<2>-(3-methyl-8-quinoline sulfonyl)- N-nitryl-L-arginine as the intermediate of argatroban. The method comprises the following steps that the reaction of N-nitryl-L-arginine and a 3-methyl-8-quinoline sulfonyl chloride-THF solution is carried out in an aqueous solution of sodium carbonate ; after the reaction ends, THP is collected through evaporation, and the water layer is extracted with an organic solvent, then THF is added with pH adjusted to 2-3, and then a solid saturated water layer of inorganic salt is added; THF is separated and a desiccant is added and stirred for 10-24 hours, with water content of THF measured to be less than 1 percent, then a molecular sift is added for drying, with the water content of THF controlled within 0.2 percent, and then THF reacts with (2R, 4R) 4MPE directly. The invention solves well the control on the water content of N<2>-(3-methyl-8 quinoline sulfonyl)-N N-nitryl-L-arginine, ensures the successful implementation of amide acylation, and satisfies the requirement of the large-scale industrial production.
Owner:天津药物研究院药业有限责任公司 +1
Argatroban liposome injection
InactiveCN102366410AHigh encapsulation efficiencyGood formulation stabilityDipeptide ingredientsPharmaceutical non-active ingredientsSide effectMedicine
The invention discloses an Argatroban liposome injection and a preparation method thereof. The Argatroban liposome injection with excellent quality is prepared by selecting Argatroban, sphingomyelin, octadecylamine, and tween 80 according to specific proportion. Compared with preparations in the prior art, the injection disclosed herein has improved stability and bioavailability, improved qualityof the preparation products, reduced toxic and side effect, stable drug release, and remarkable curative effect.
Owner:HAINAN LINGKANG PHARMA CO LTD
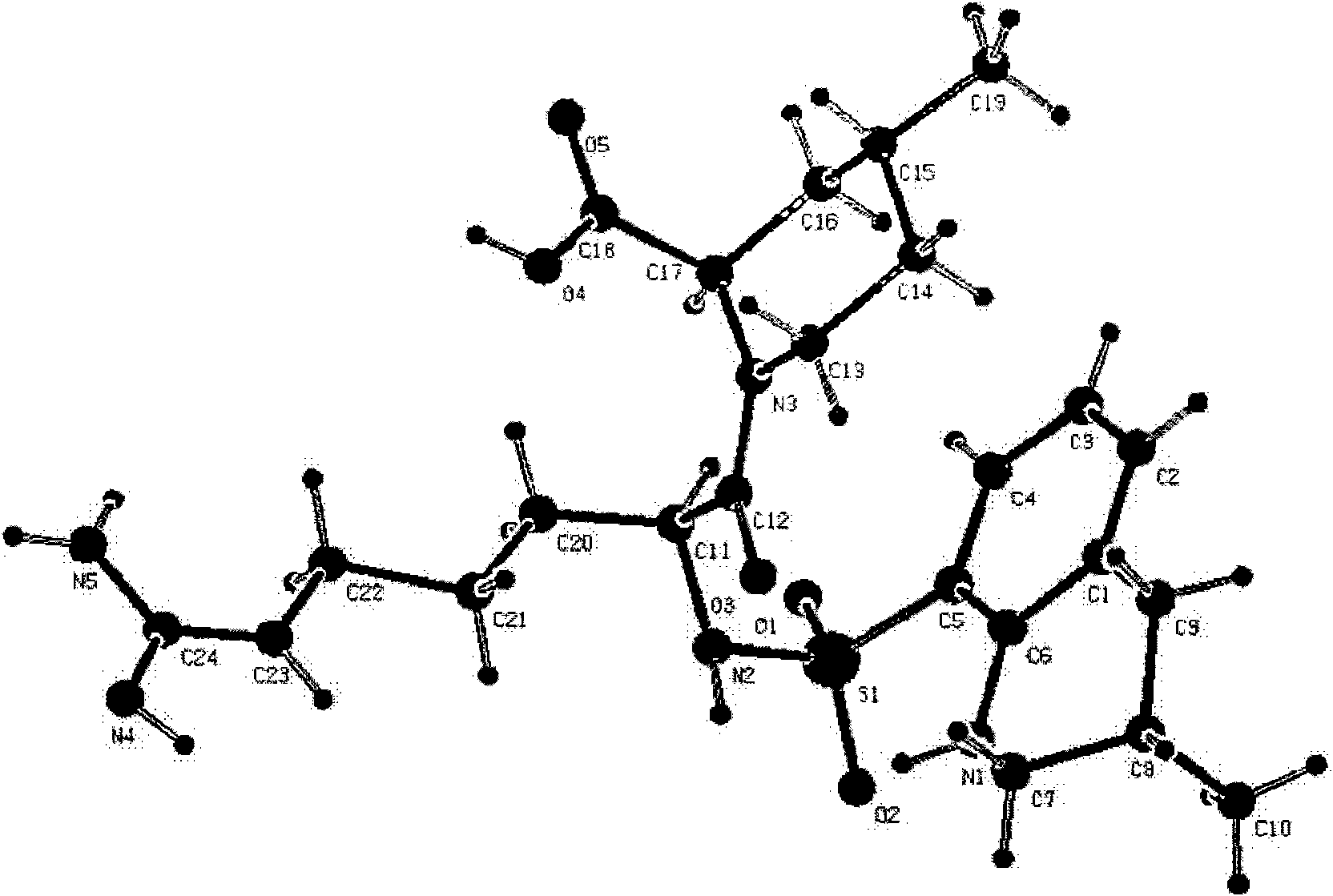
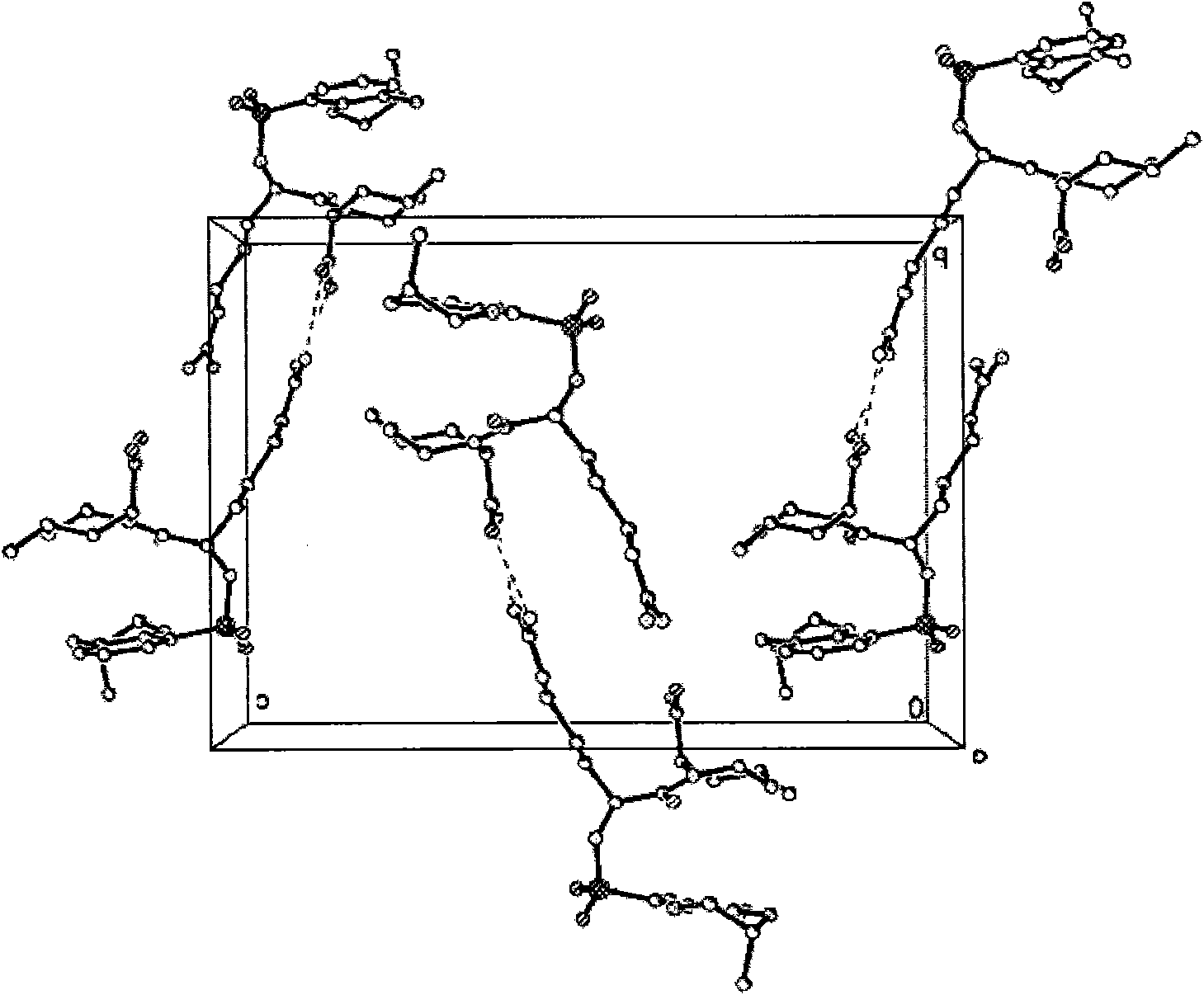
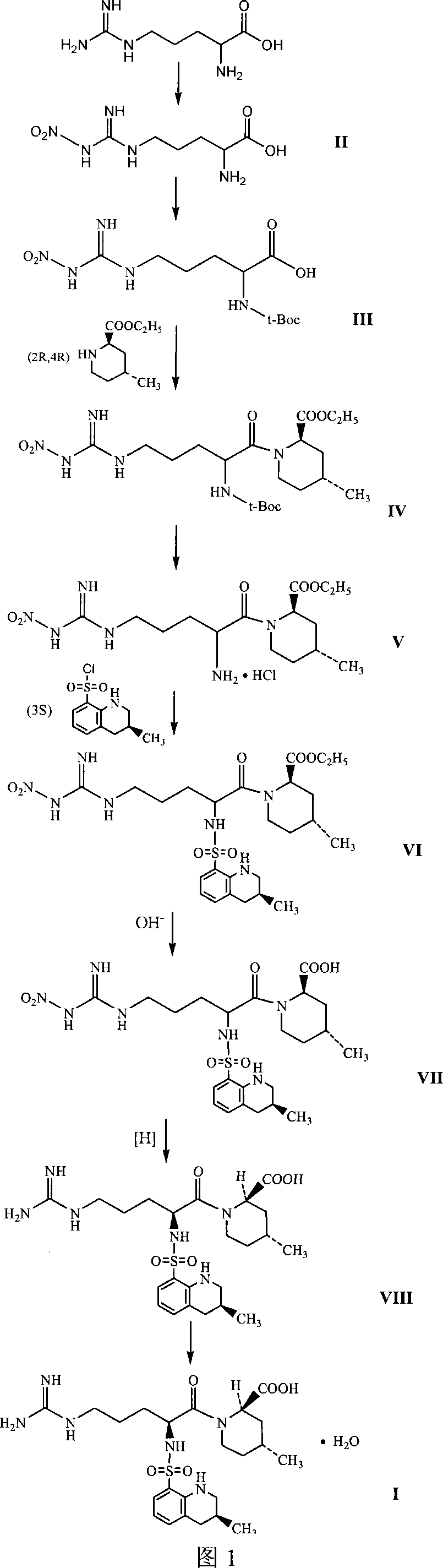
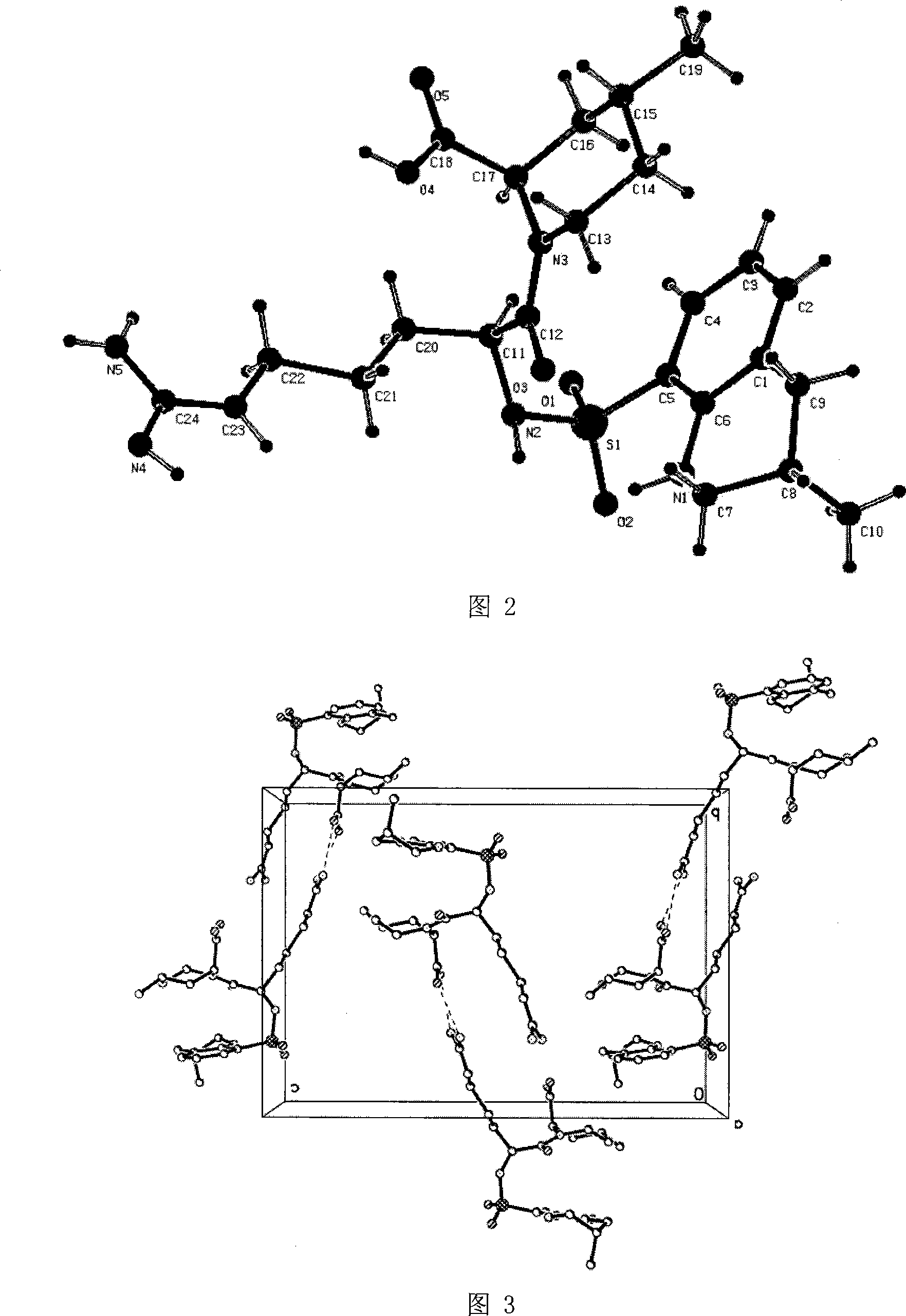
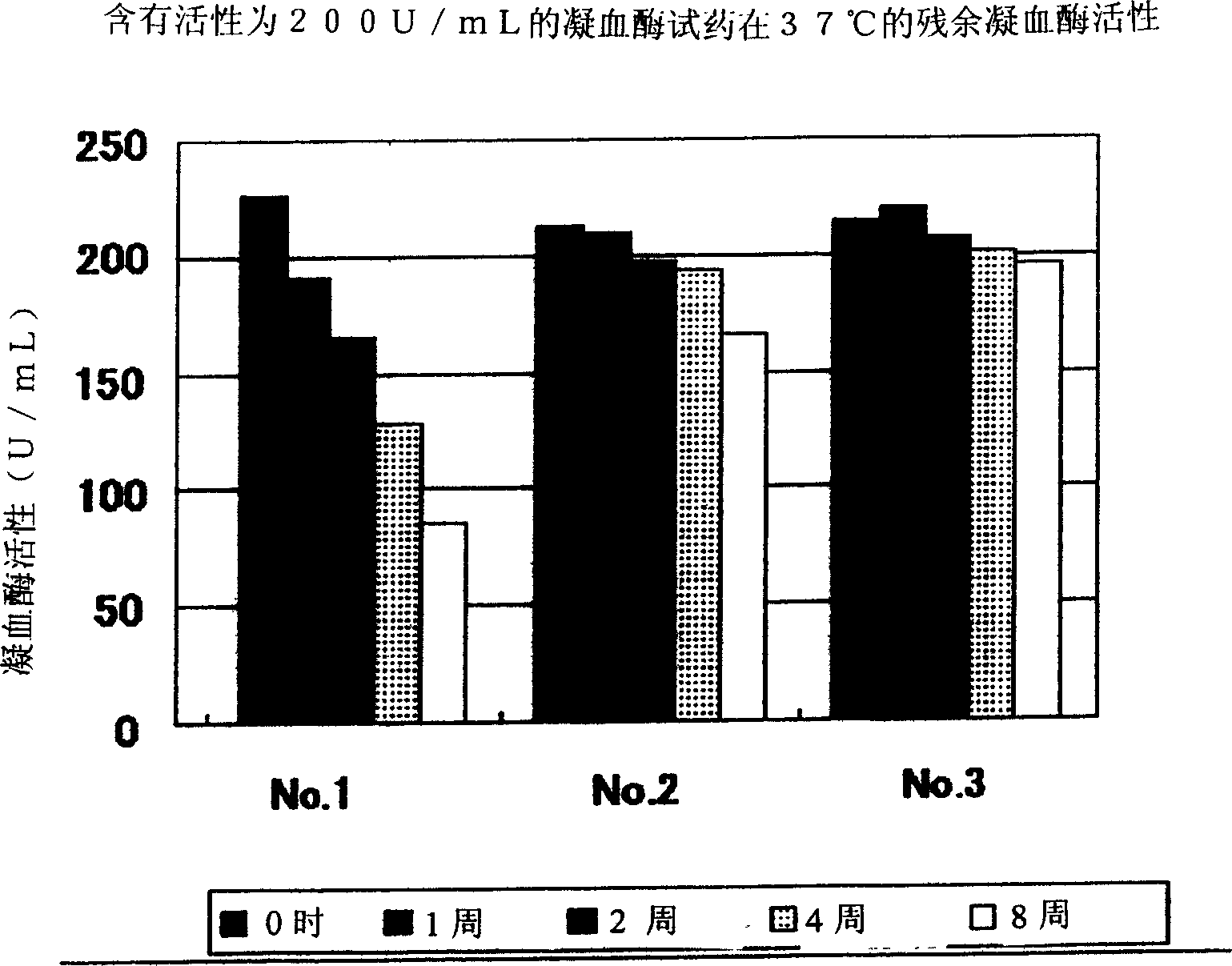
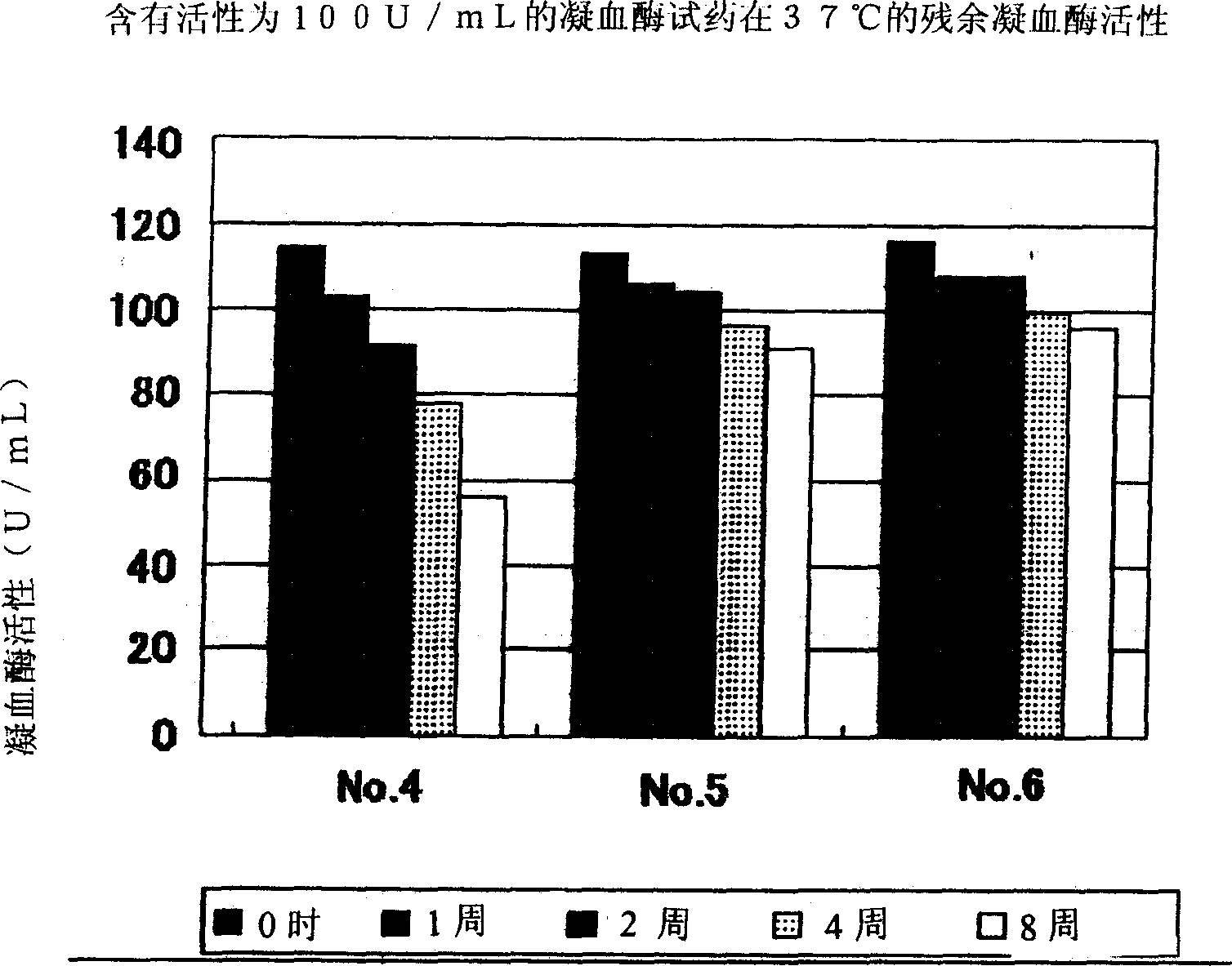
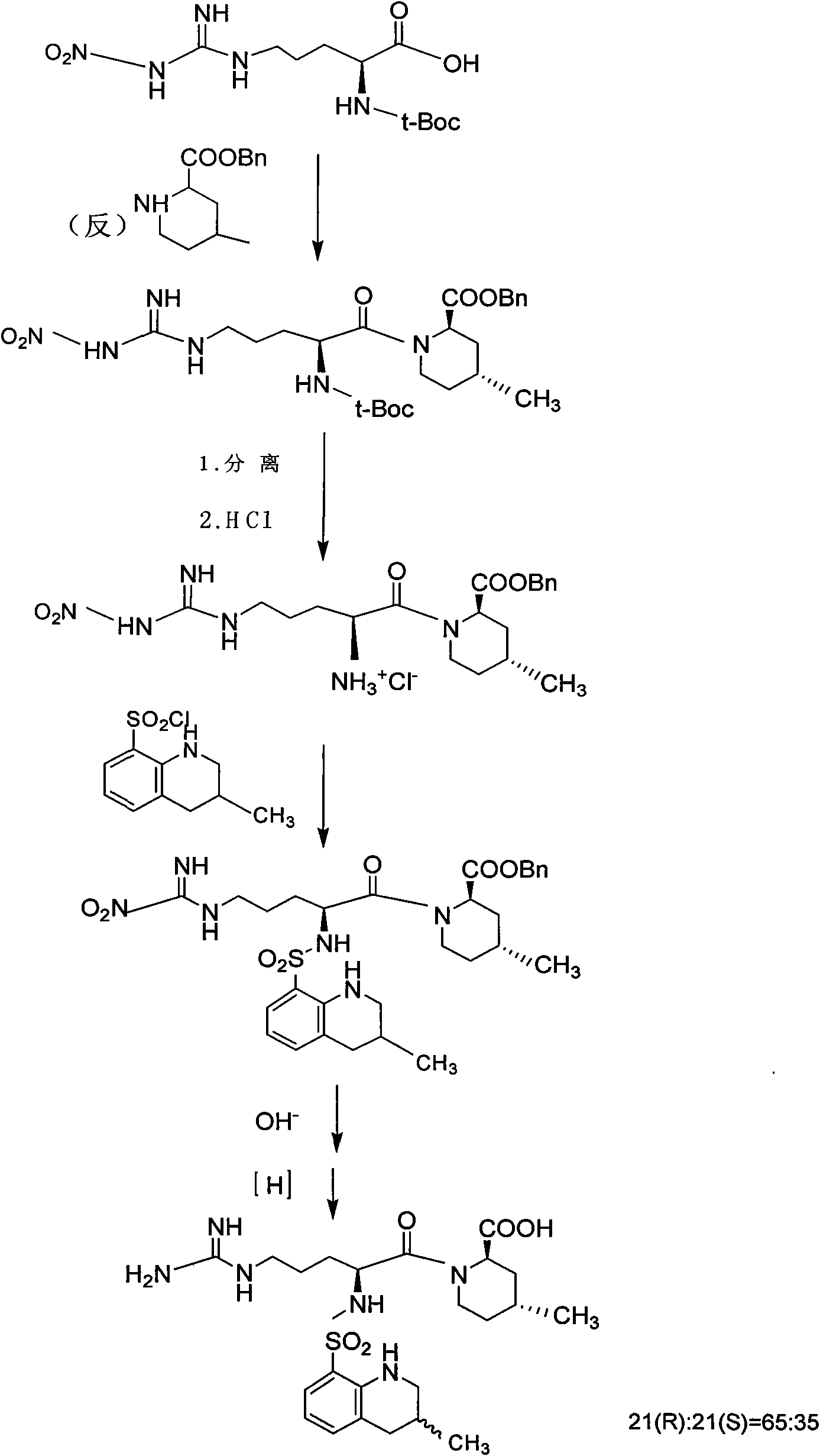
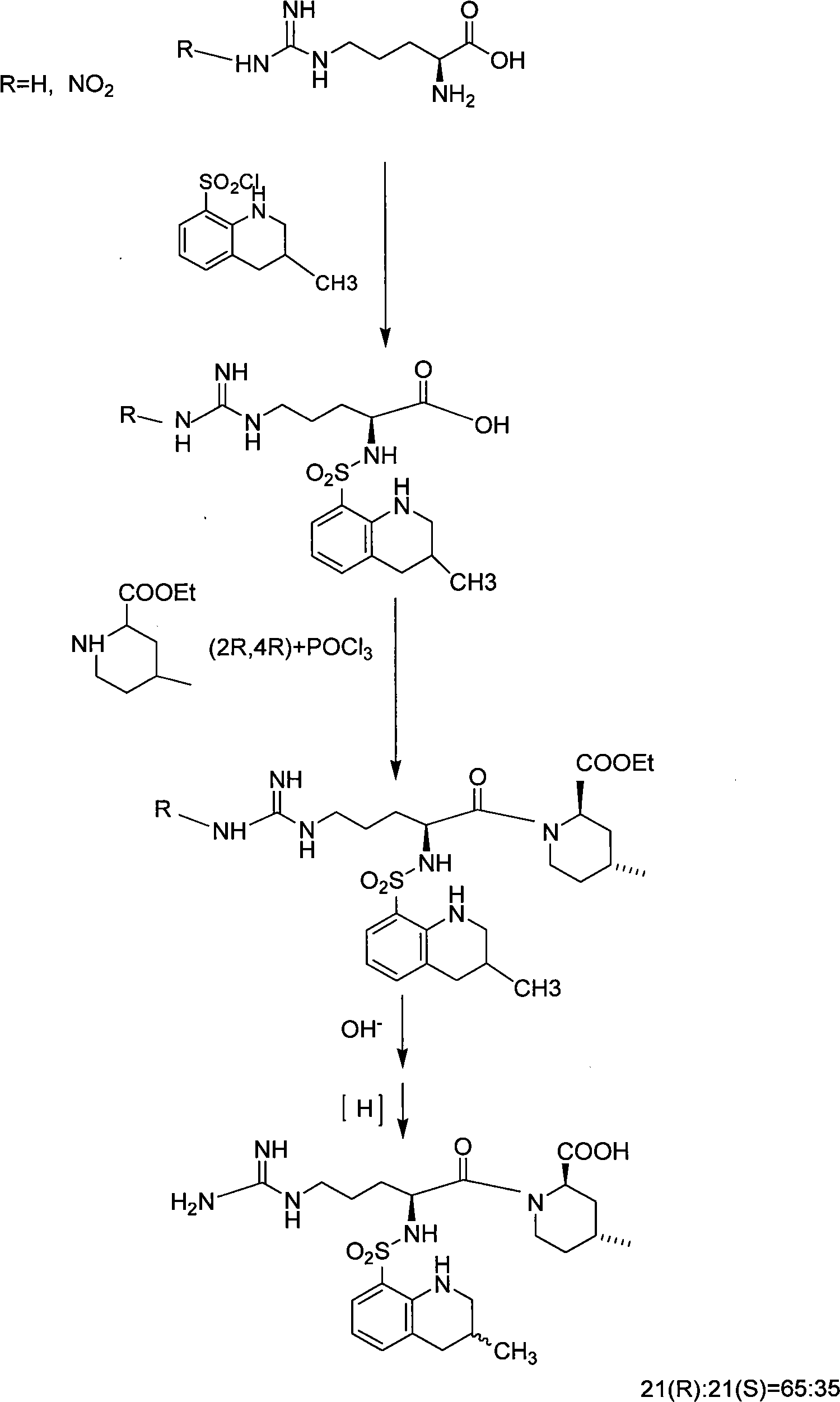
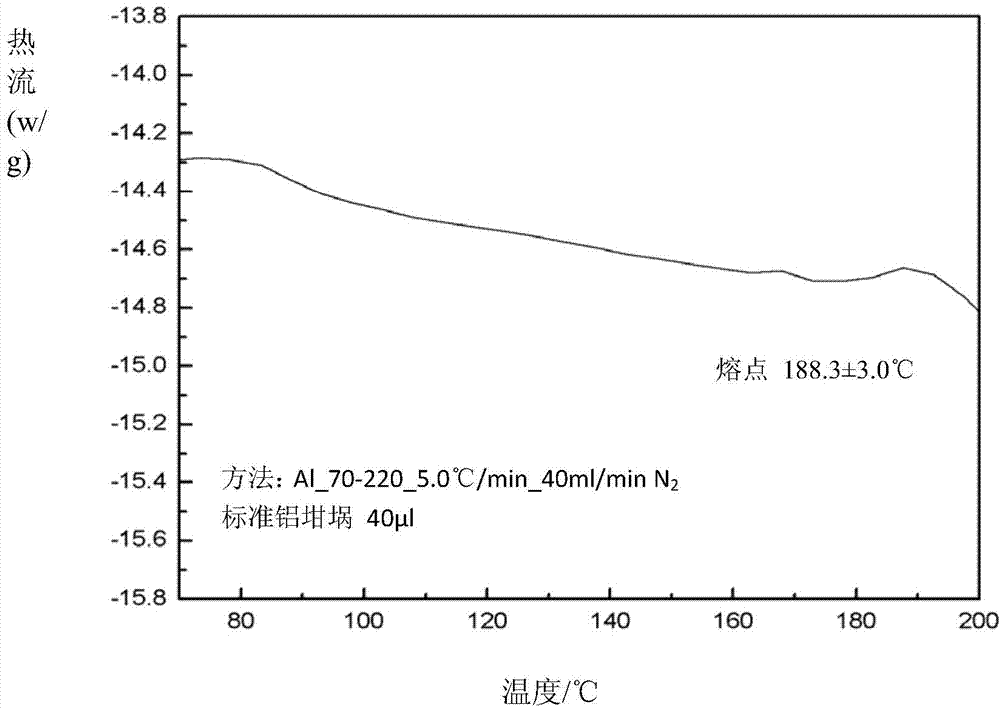
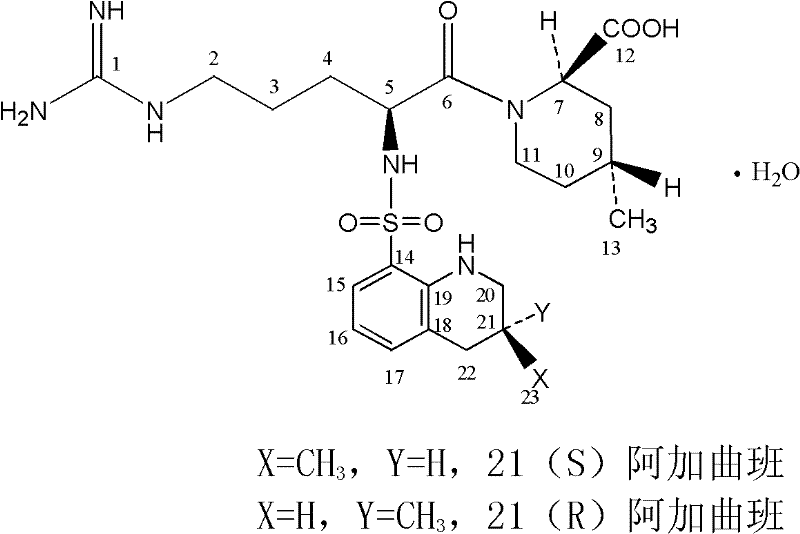
Oriented synthesis and crystal structure of 21(S) argatroban, and preparation for monohydrate thereof
InactiveCN101235031AEasy to operateLow costOrganic chemistryBlood disorderAbsolute configurationSynthesis methods
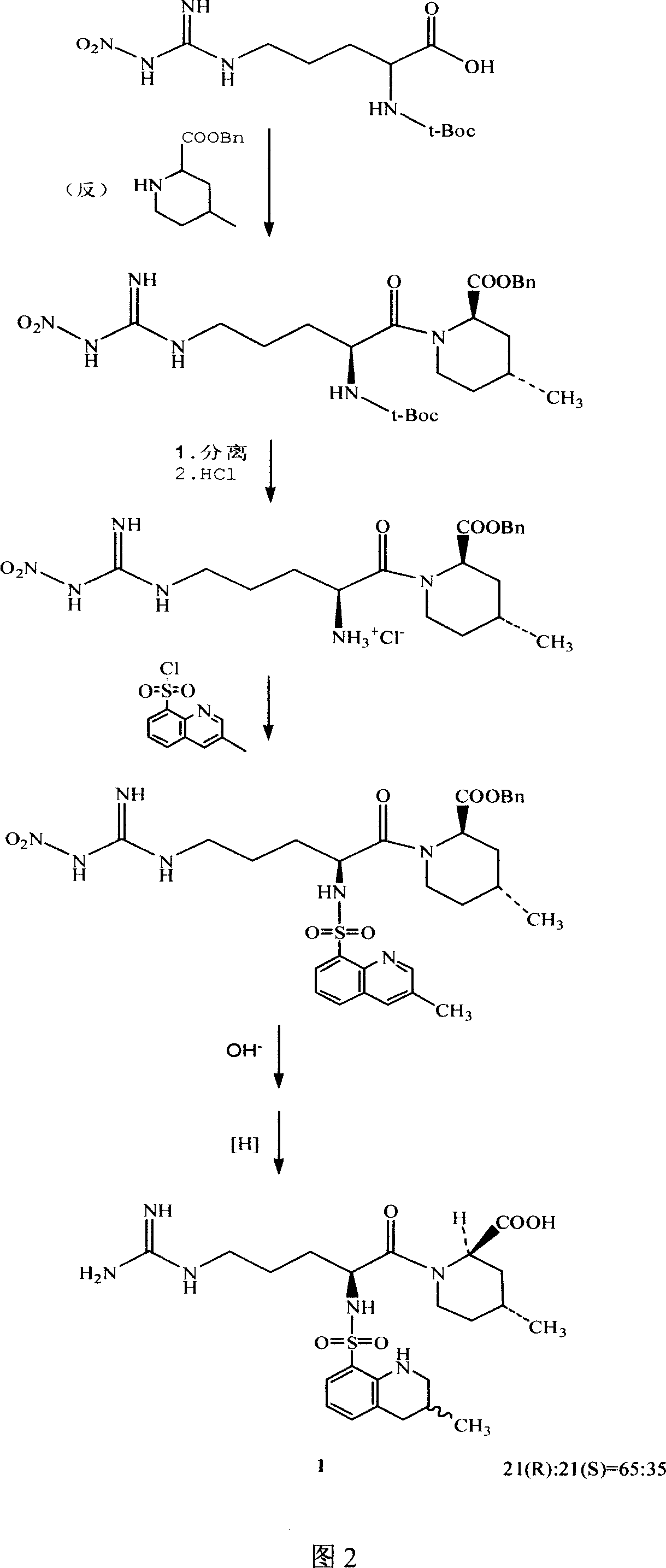
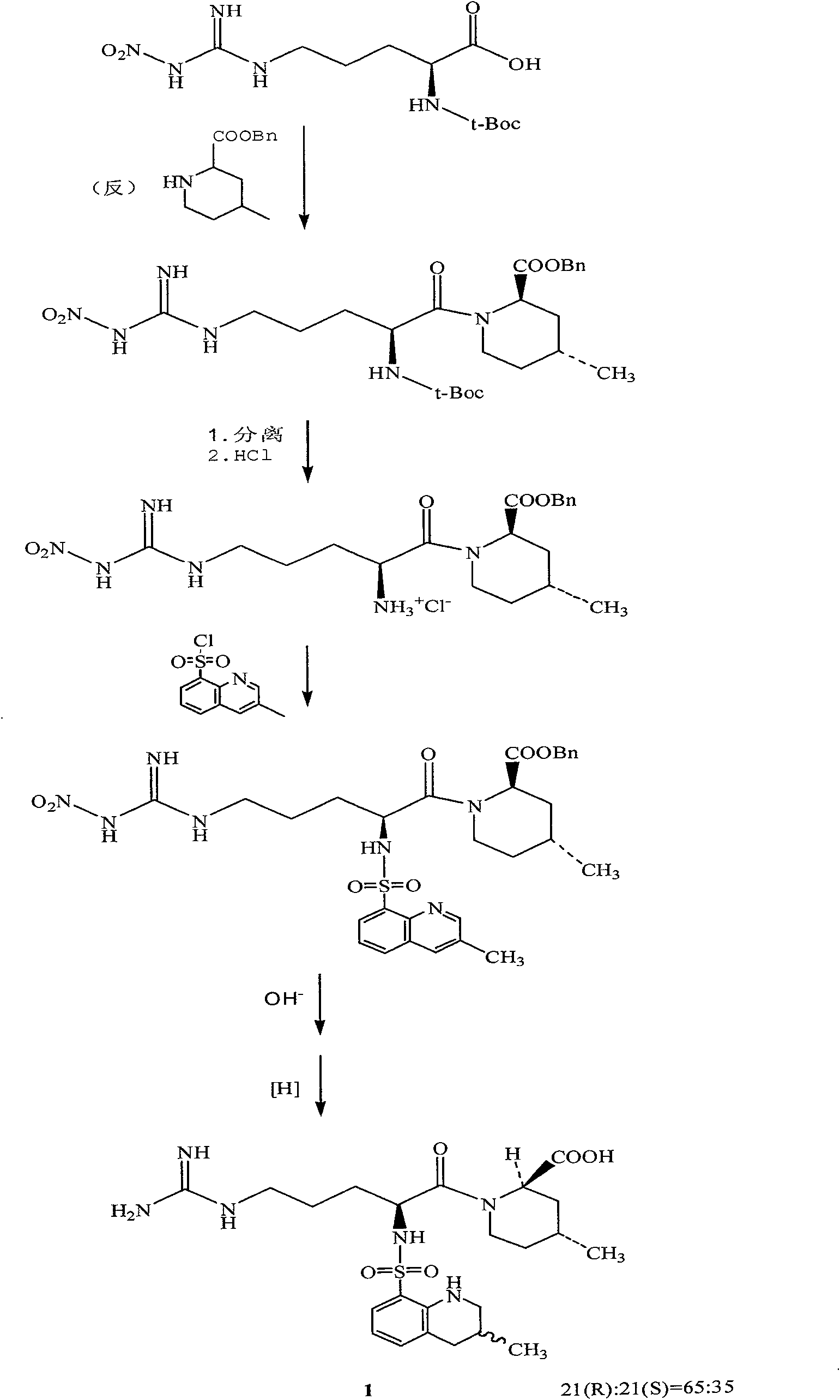
The invention relates to a 21(S) argatroban rational synthesis method, a corresponding crystal structure and a hydrate preparation method, wherein the rational synthesis uses (3S)-1, 2, 3, 4-tetrahydrochysene-3-methyl-8-quinolinesulfonyl chloride as raw material to prepare single diastereoisomer 21(S) argatroban, and uses single crystal and polycrystalline powder X diffraction method to determine the absolute configuration as 21(S) and the crystal systems I, II, and the 21(S) argatroban is soluble in hot water, via controlling the cooling speed, a 21(S) argatroban hydrate is prepared. Animal tests prove that the anticoagulant function of the 21(S) argatroban hydrate is 2-3 powers of 21(R) argatroban hydrate.
Owner:TIANJIN WEIJIE TECH
Combination comprising thrombin agent and examining agent
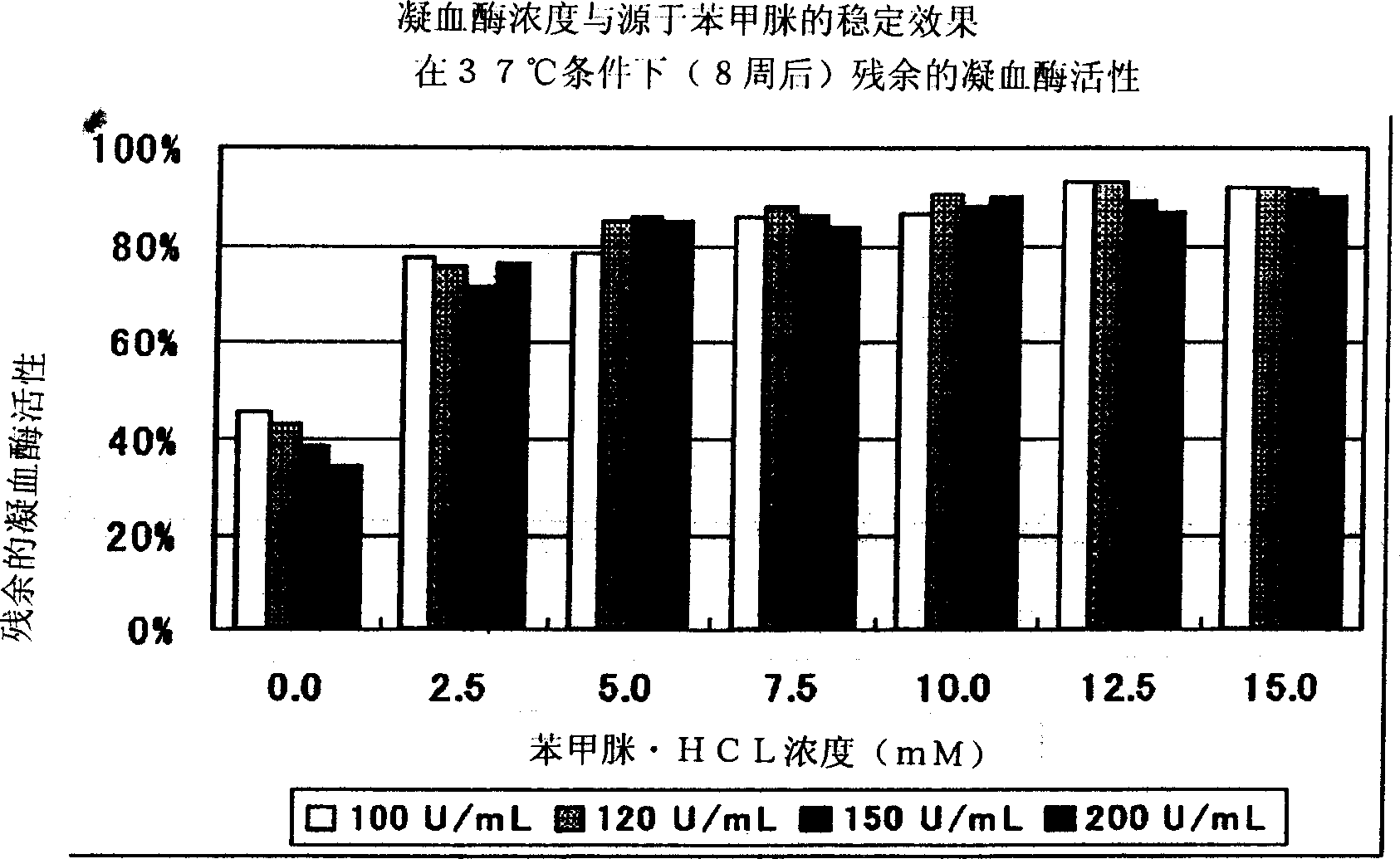
Provided is a stabilized thrombin containing a test reagent and the test reagent kit. The test reagent contains thrombin and an inhibitor having thrombin inhibiting effect, the inhibitor is a protease antagonist having thrombin inhibiting effect, and is preferably selected from the group consisting of benzamidine, p-aminobenzamidine, m-aminobenzamidine, phenylguanidine, argatroban, gabexate mesylate and nafamostat mesylate. The test reagent of the present invention can contain one or more compounds with thrombin stabilizing function, selected from the group consisting of a calcium ion, an organic acid, a surfactant and a protein. The test reagent is useful as a solidifying ability test reagent, especially, fibrinogen metering reagent.
Owner:SYSMEX CORP
Blood purification membrane with anticoagulant property and preparation method thereof
ActiveCN109224889AGood anticoagulant effectOntology performance impactSemi-permeable membranesDialysis systemsHeparin antibodyAnti coagulation
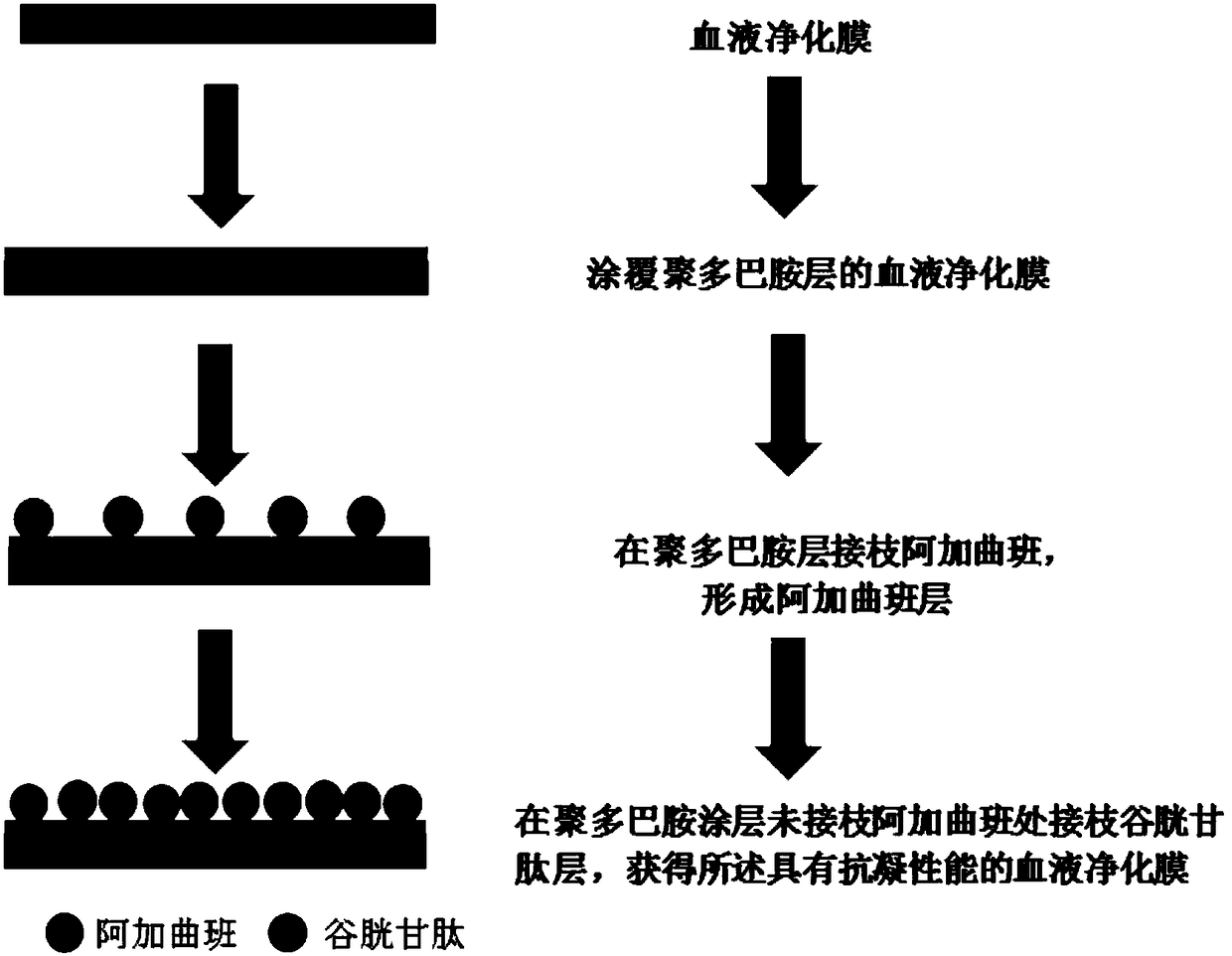
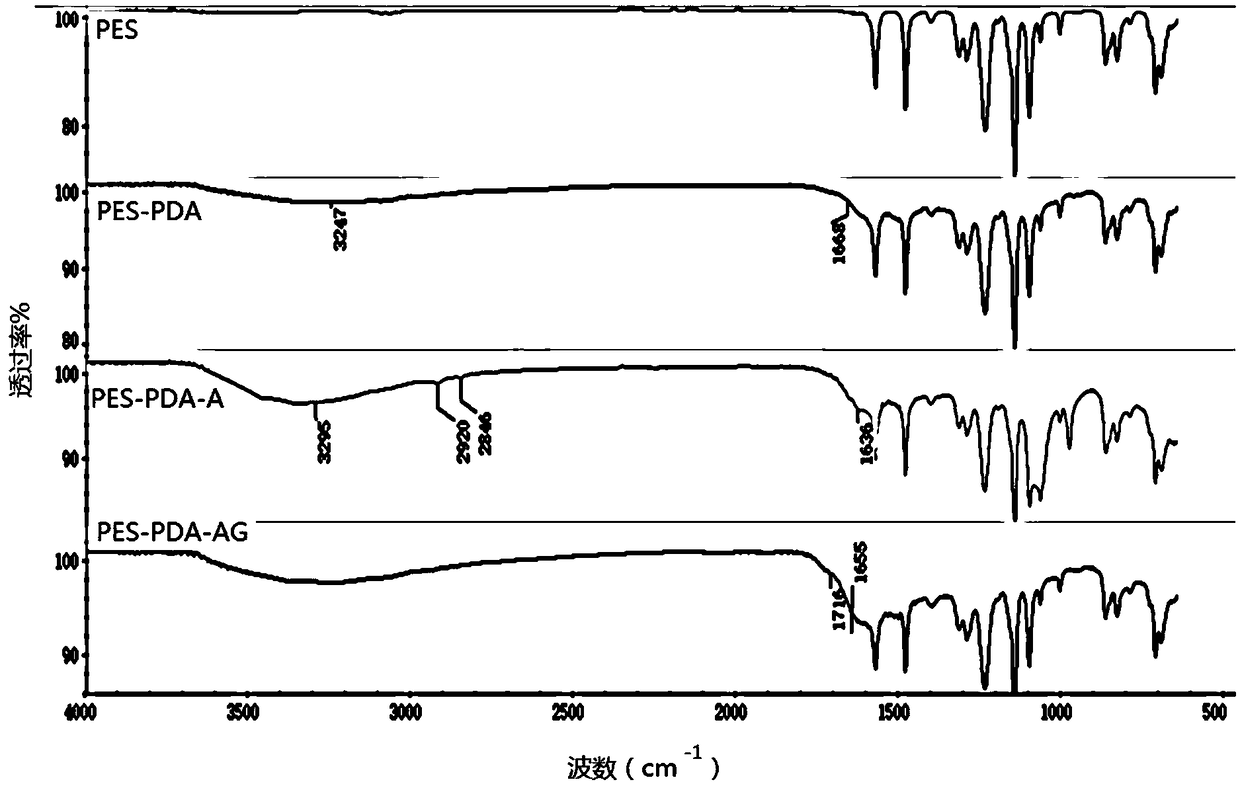
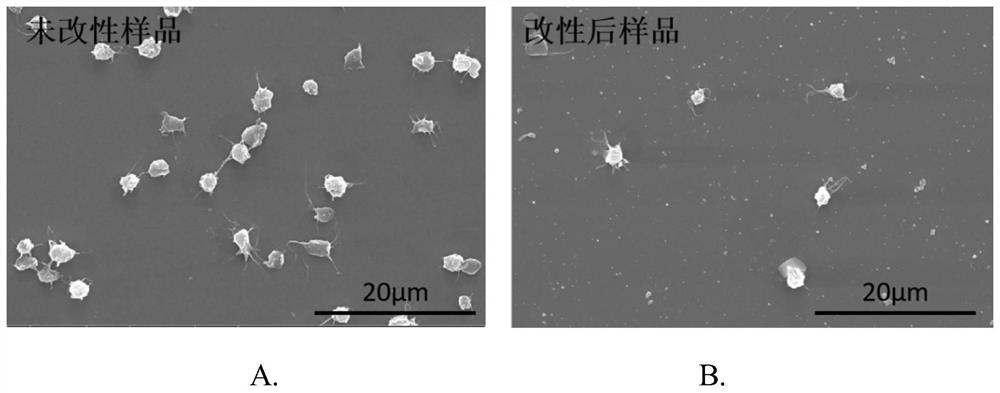
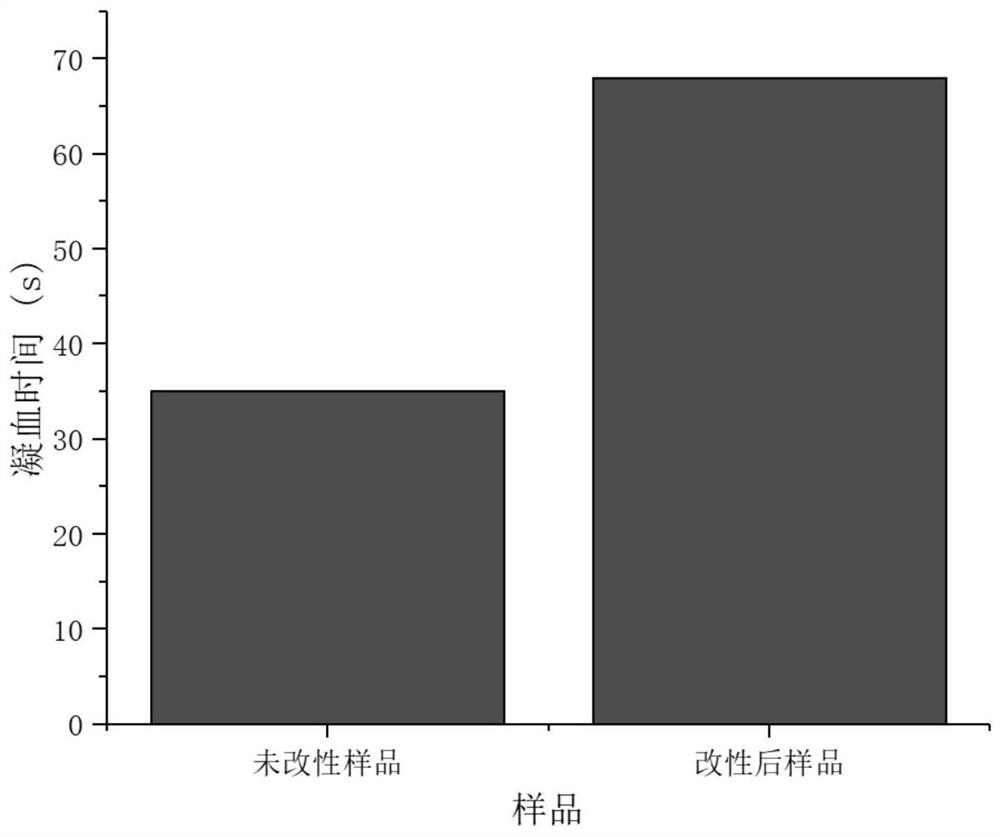
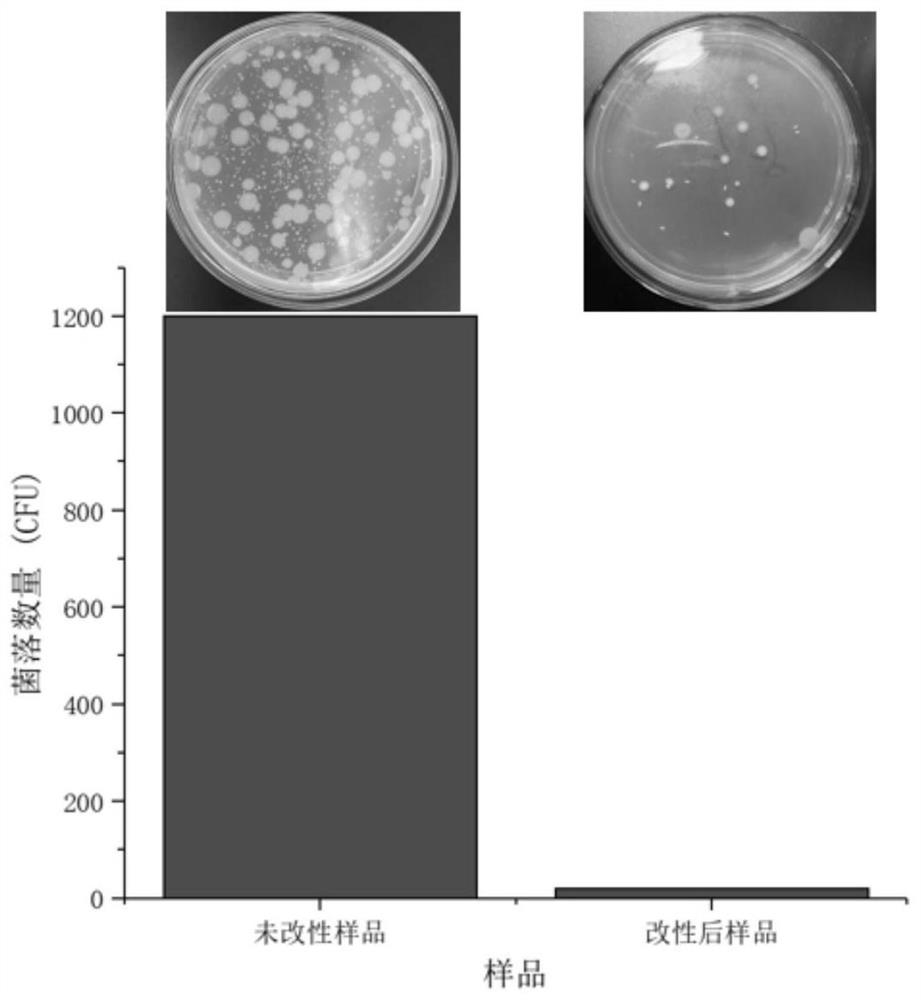
The invention discloses a blood purification membrane with an anticoagulant property and a preparation method thereof. The blood purification membrane comprises a matrix, a polydopamine layer attachedto the surface of the substrate, argatroban grafted on the polydopamine layer and glutathione. The whole surface modification process is simple in experimental conditions, has strong controllability,and is economical and environmentally-friendly; the high-efficiency anti-coagulation on the local part of a material is realized; the blood coagulation system of a body is not affected; the blood compatibility is significantly improved; and the grafted anticoagulant drug argatroban has no antigenicity and does not cause heparin-induced thrombocytopenia.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
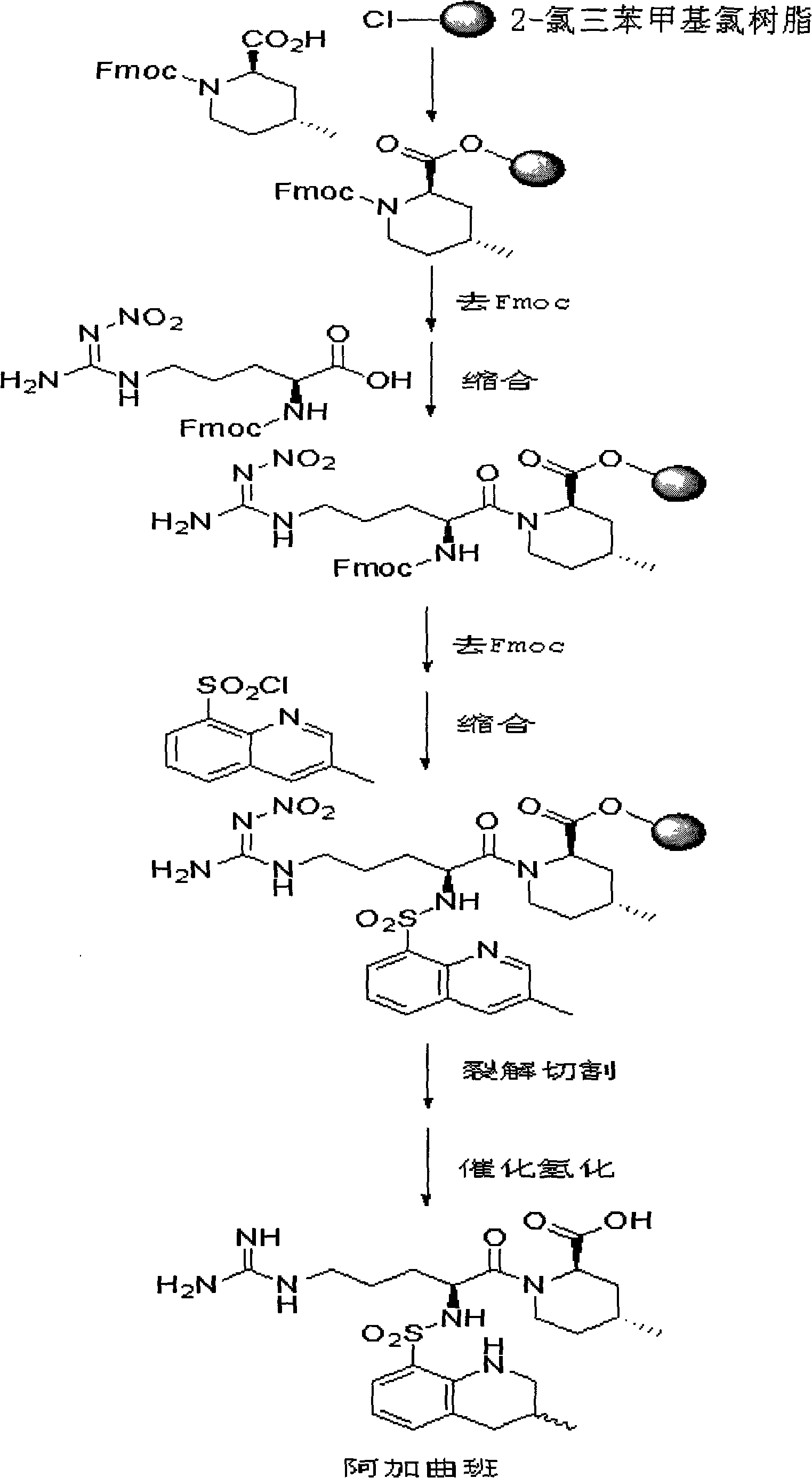
New method for synthesizing argatroban by combining solid phase method and liquid phase method
InactiveCN101560244AReduce investmentEasy to operatePeptide preparation methodsBlood disorderCombinatorial chemistryArgatroban
The invention discloses a method for synthesizing argatroban. The method comprises the following steps: the (2R, 4R)-N-Fmoc-4-methyl-2-nipecotic acid reacts with high polymer resin to obtain (2R, 4R)-N-Fmoc-4-methyl-2-nipecotic acid-resin; deprotection is carried out, Fmoc-arginyl (nitryl)-OH is coupled by a solid phase synthesis method to obtain Fmoc-arginyl (nitryl)-(2R, 4R)-4-methyl-2-nipecotic acid-resin; the deprotection is carried out again, the 3-methyl-8-quinolinesulfonyl chloride is coupled by the solid phase synthesis method to obtain 3-methyl-8-quinolinesulfonyl-arginyl (nitryl)-(2R, 4R)-4-methyl-2-nipecotic acid-resin; cracking cutting reaction is performed to the obtained resin to obtain 3-methyl-8-quinolinesulfonyl-arginyl (nitryl)-(2R, 4R)-4-methyl-2-nipecotic acid; the argatroban crude product is obtained through catalytic hydrogenation; and the argatroban is obtained through recrystallization. The method is suitable for the large-scale production and is simple and easy in operation, stable in process and low in production cost, and the total yield is up to 60 percent.
Owner:HYBIO PHARMA
Argatroban byproduct and its synthesis, separation and identification method
ActiveCN101033223AThe steps are well designedReasonable designOrganic chemistryComponent separationQuinolineArgatroban
This invention relates to an outgrowth of argatroban and a method for synthesization and separation and authentication, in which, when preparing argatroban by catalysis and hydrogenation, it makes an outgrowth with difficult separation to determine that said outgrowth is a compound by separation, authentication and synthesization, which is hydrolyzed further to get argatroban, and when testifying the hydrolyzed intermediate, it first of all recovers nitro delinking, then hydrolyzes quinoline 1, 2, 3 and 4 to get 1, and the content of compound 3 can be reduced to the allowance sphere by following the hydrogenation reactin of the intermediate.
Owner:TIANJIN WEIJIE TECH
Process for preparing argatroban hydrate
The invention discloses a preparing method of Argatroban hydrate, which comprises the following steps: refluxing Argatroban and water for 0.5-1.0h; dissolving; cooling to indoor temperature; evolving white crystal; filtering; washing twice; drying to obtain medical Argatroban hydrate. The invention guarantees the constant rate of R and S without other organic impurity, which improves hydrating receiving rate between 90% and 95%.
Owner:TIANJIN WEIJIE TECH
Injection preparation containing argatroban
InactiveCN102240393ADipeptide ingredientsPharmaceutical delivery mechanismLarge volume parenteralActive component
The invention relates to an injection preparation which adopts argatroban as an active component. The injection preparation is characterized by: adopting a single optical isomer of the argatroban or a salt of the argatroban or a hydrate of the argatroban as the medicinal active component and adding a plurality of auxiliary materials with specific types and specific ratios to prepare and develop the preparation for the intravenous injection according to a technical means described in the present patent specification. The formulations of the injection preparation comprise powder injection, small volume parenteral solution or large volume parenteral solution.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Controlled drug release composition and drug releasing medical device
InactiveCN101309706AFacilitated releaseAdjust timingSurgeryCatheterGlycerolSarpogrelate Hydrochloride
A drug releasing medical device of the invention is provided with a controlled drug release composition containing 100 parts by weight of an organic polymeric material which is soluble in an organic solvent and insoluble in water, 5 to 60 parts by weight of a release auxiliary agent which is lipid-soluble and low in molecular weight and a 1 to 70 parts by weight of a drug. When the composition is applied to a stent, a catheter, an organ replacement medical device, an artificial organ or the like in the form of coating or the like, a drug releasing function is given to the medical device. From a surface of a stent for treating coronary stenosis, which is a preferred embodiment, argatroban, sarpogrelate hydrochloride or both of them are released gradually. In order to express a controlled release property in a desired period of time, the drug to be released gradually is carried in a polymeric material coated on a metal surface constituting the stent or in a porous stent substrate. It is preferred that the polymeric material is noncrystalline and further biodegradable and contains a tartaric acid ester, a malic acid ester or a monoester or diester of glycerine as the release auxiliary agent.
Owner:TOKAI UNIV +1
Solid phase method for synchronizing Argatroban
InactiveCN101519429AReduce investmentEasy to operatePeptide preparation methodsBlood disorderSide chainOrganic base
The invention discloses a solid phase method for synchronizing Argatroban, including the following steps: (1) (2R, 4R)-N-Fmoc-4-methyl-2-nipecotic acid, macromolecule resin, protective amino acid, coupling reagent and organic base are used as starting raw materials to form (2R, 4R)-N-Fmoc-4-methyl-2-nipecotic acid-resin; (2) Fmoc protection is removed, and a solid phase method is adopted to couple Fmoc-Arg(X)-OH so as to obtain Fmoc-Arg(X)-(2R, 4R)-4-methyl-2-nipecotic acid-resin; (3) the Fmoc protection is removed, and after 3-methyl-1,2,3 4-tetrahydroquinoline-sulfonic acid chloride is coupled, complete full protection Argatroban-resin is obtained; (4) complete peptide protection Argatroban-resin is reacted with a side chain separated blocking group so as to gain the crude product of the Argatroban; and (5) after the operation of recrystallization is carried out, the Argatroban with high purity is obtained. The technical process of the invention has the advantages of simple reaction operation, easy post treatment, less raw material, low cost, more than 80 percent of total yield, considerable economic and practical value as well as broad application prospect in the field of designing and synchronizing polypeptide drugs.
Owner:HYBIO PHARMA
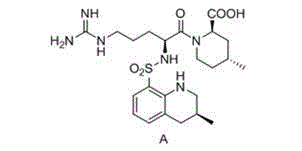
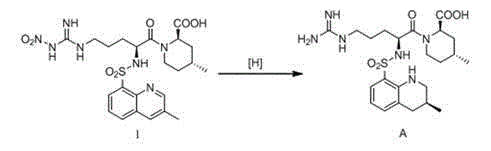
Preparation method of optically-pure argatroban
The invention belongs to the technical field of medicine, and provides a preparation method of optically-pure argatroban represented by the formula A. In the preparation method, a compound represented by a formula I is subjected to a transfer hydrogenation reaction in the presence of a chiral catalyst so as to obtain the argatroban represented by the formula A. The preparation method provided by the invention has the advantages of mild conditions, simple operation, high yield, low production cost, and suitability for massive production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Anticoagulation PVDF flat separation film and making method thereof
InactiveCN105311970AGood anticoagulant effectProlonged recalcification timeSemi-permeable membranesHemolysisALLYL SUCROSE
The invention discloses an anticoagulation PVDF flat separation film, also discloses a making method of the anticoagulation PVDF flat separation film, and belongs to the technical field of films. The making method of the anticoagulation PVDF flat separation film comprises the following steps: 1, preparing a polyacrylic acid grafted polyvinylidene fluoride copolymer PVDF-g-PAA; 2, making a PVDF-g-PAA flat separation film; and 3, making an argatroban grafted and modified PVDF-ARG film. A PVDF film is modified by micro-molecular argatroban with a high efficiency anticoagulation effect with acrylic acid as a medium in order to obtain a PVDF separation film material with a good anticoagulation effect. The anticoagulation PVDF flat separation film can obviously prolong the recalcification time of the film, substantially reduces adhesion of platelet erythrocytes on the surface of the film, reduces the hemolysis rate, improves the anticoagulation performance and greatly improves the blood compatibility. The method has the characteristics of simple process and easy industrialization enforcement.
Owner:TIANJIN POLYTECHNIC UNIV
Argatroban formulation
ActiveUS20070049617A1Improve the effect of infusionImprove propertiesBiocideInorganic non-active ingredientsAlcoholThrombin activity
An aqueous, stable, sterile pharmaceutical composition of the thrombin inhibitor argatroban in a solution containing an acid to solubilize the argatroban, substantially free from dehydrated alcohol is described, as well as a method for its preparation.
Owner:BAXTER INT INC +1
Argatroban drug composition and preparation method and application of argatroban drug composition
InactiveCN102784382AImprove stabilityNo significant pH changePowder deliveryDipeptide ingredientsAnhydrous ethanolHydrogen
The invention provides an argatroban drug composition. Argatroban is used as a main drug, and auxiliary solution consisting of anhydrous ethanol, propylene glycol and water is used as dissolving liquid, so that the argatroban drug composition is prepared. The weight ratio of the argatroban, the anhydrous ethanol and the propylene glycol is 1:(20-50):(40-80). The pH (potential of hydrogen) values of the obtained composition before and after sterilization are stable and tend to be neutral, the content of related substances and clinical irritation are low, and the argatroban drug composition is safe in use.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of injection preparation containing argatroban
The invention relates to an injection preparation which uses argatroban as an active ingredient. It takes the single optical isomer of argatroban or its salt and hydrate as the medicinal active ingredient, adds some specific types and proportions of excipients, and prepares and develops it for intravenous injection according to the technical means described in this patent. preparations. Dosage forms include powder injection, small-volume injection or large-volume injection.
Owner:FUKANGREN BIO PHARMA
Synthesis method of argatroban
ActiveCN105837658ASuitable for industrial scale-up productionReduce riskPeptide preparation methodsHydrogenSynthesis methods
The invention discloses a synthesis method of argatroban .The method includes the step of making a compound II of a structure shown in the formula II make contact with and react with a hydrogen donor under the catalytic transfer hydrogenation conditions through the catalyst to obtain an argatroban rough product, wherein the hydrogen donor is formic acid or formate, and the formula II can be seen in the description .By means of the method, formic acid or formate serves as the hydrogen donor, the compound II is subjected to hydrogenation and reduction to obtain argatroban .By means of the method, in the hydrogenation and reduction process of the compound II, catalytic transfer hydrogenation reaction replaces catalytic hydrogenation reaction, operation steps are simplified, the problems on the aspects of technology and safety caused by hydrogen application are solved, reaction dangerousness is reduced, reaction conditions are mild and easy to control, and the method is more suitable for industrial application and production of argatroban.
Owner:河北广祥制药有限公司
Process for preparing argatroban hydrate
The invention discloses a preparing method of Argatroban hydrate, which comprises the following steps: refluxing Argatroban and water for 0.5-1.0h; dissolving; cooling to indoor temperature; evolving white crystal; filtering; washing twice; drying to obtain medical Argatroban hydrate. The invention guarantees the constant rate of R and S without other organic impurity, which improves hydrating receiving rate between 90% and 95%.
Owner:TIANJIN WEIJIE TECH
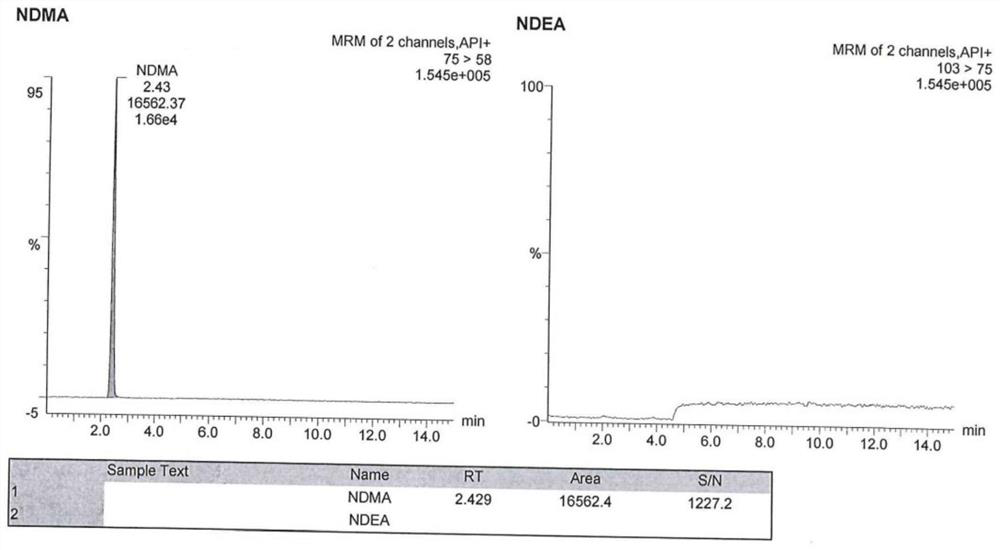
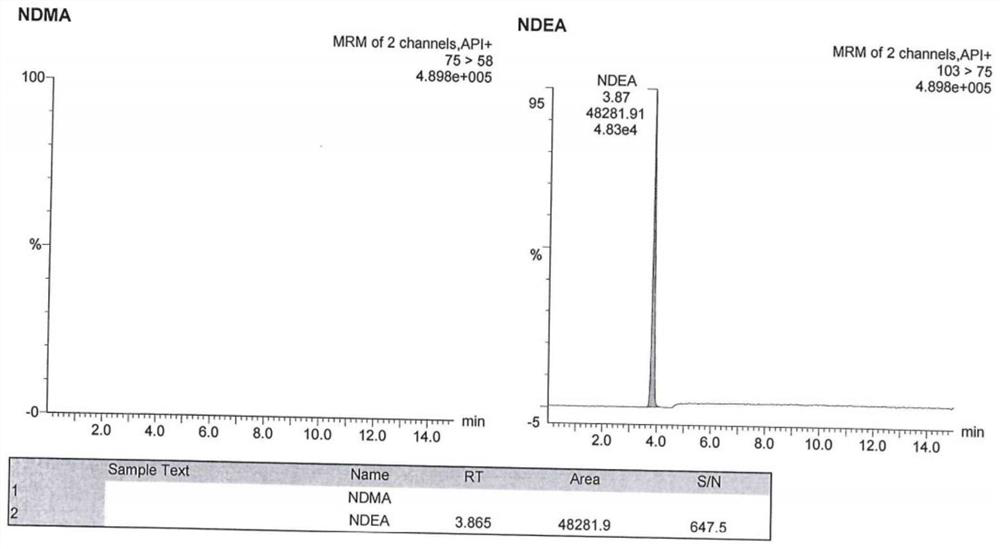
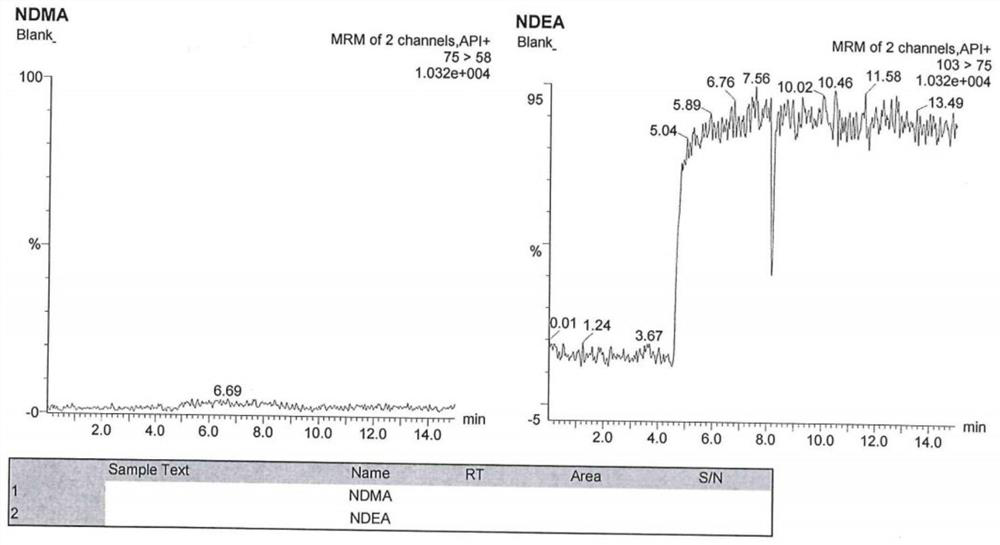
Method for analyzing N-nitrosodimethylamine and N-nitrosodiethylamine in argatroban bulk drug or preparation
ActiveCN112946107AHigh cancer riskHigh sensitivityComponent separationAgainst vector-borne diseasesNitrosoBiology
The invention provides a method for analyzing N-nitrosodimethylamine and N-nitrosodiethylamine in an argatroban bulk drug or preparation, which adopts an ultra-high performance liquid chromatography (UPLC) and mass spectrometry combined method, and comprises the following steps: (1) preparing a test solution and a reference solution: dissolving a sample to be detected by using methanol as a solvent to prepare the test solution; dissolving standard substances of N-nitrosodimethylamine and N-nitrosodiethylamine by taking methanol as a solvent to prepare a reference substance solution; (2) taking the test solution and the reference substance solution, respectively injecting samples, detecting by using an ultra-high performance liquid chromatography-mass spectrometer, and recording a chromatogram map; and calculating the contents of N-nitrosodimethylamine and N-nitrosodiethylamine according to an external standard method. According to the liquid chromatography-mass spectrometry method provided by the invention, the sample treatment process is simple to operate, the sensitivity is high, the detection result is accurate, the stability is good, and the method can be used for quality control of argatroban bulk drugs or preparations.
Owner:CSPC OUYI PHARM CO LTD
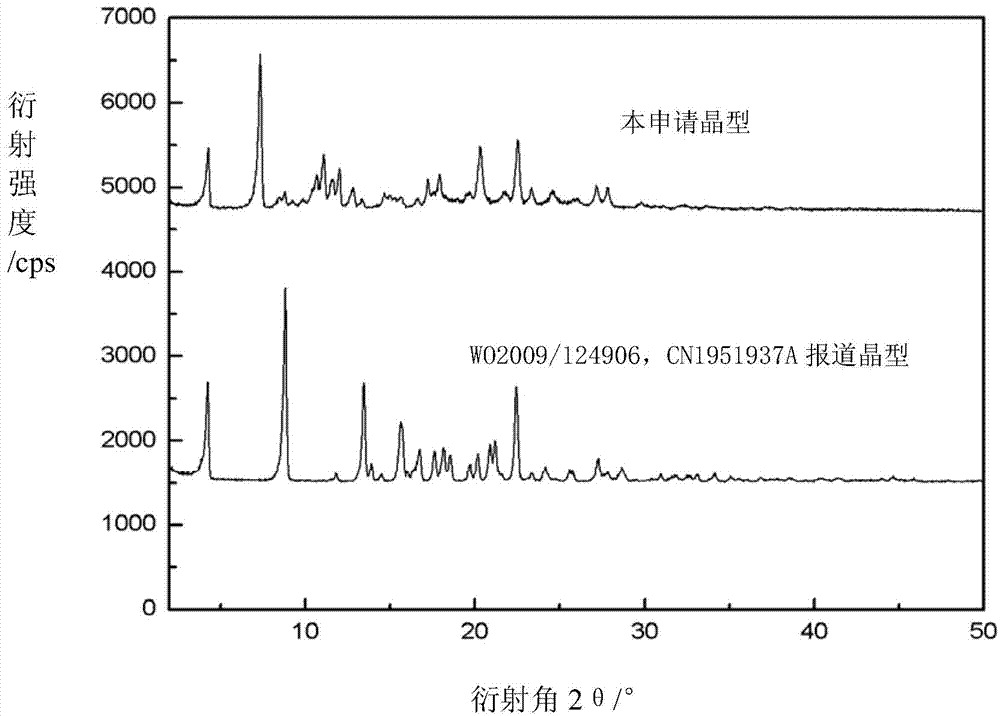
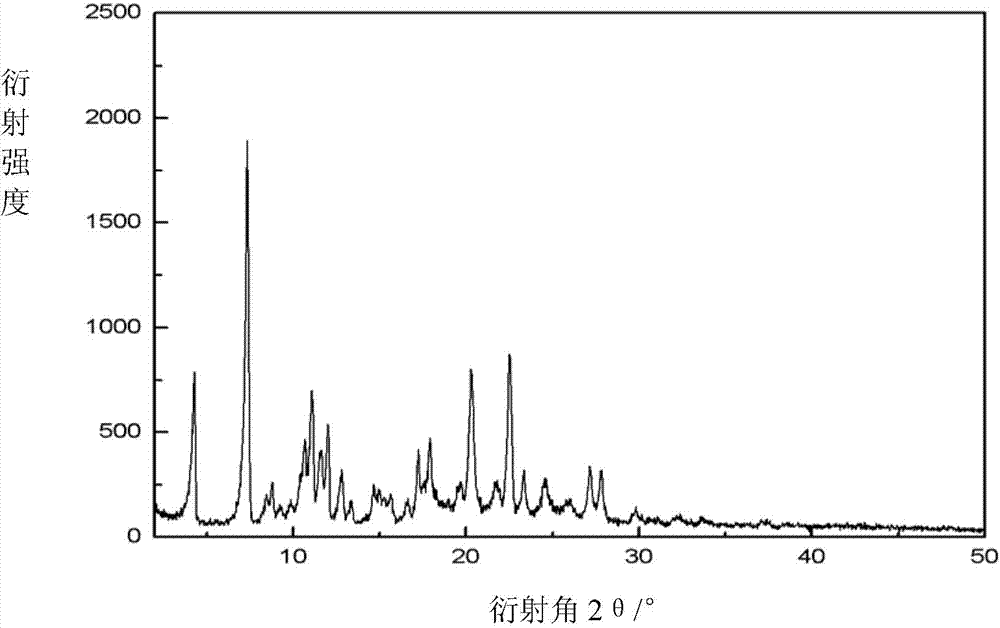
Novel crystal form of argatroban and preparation method thereof
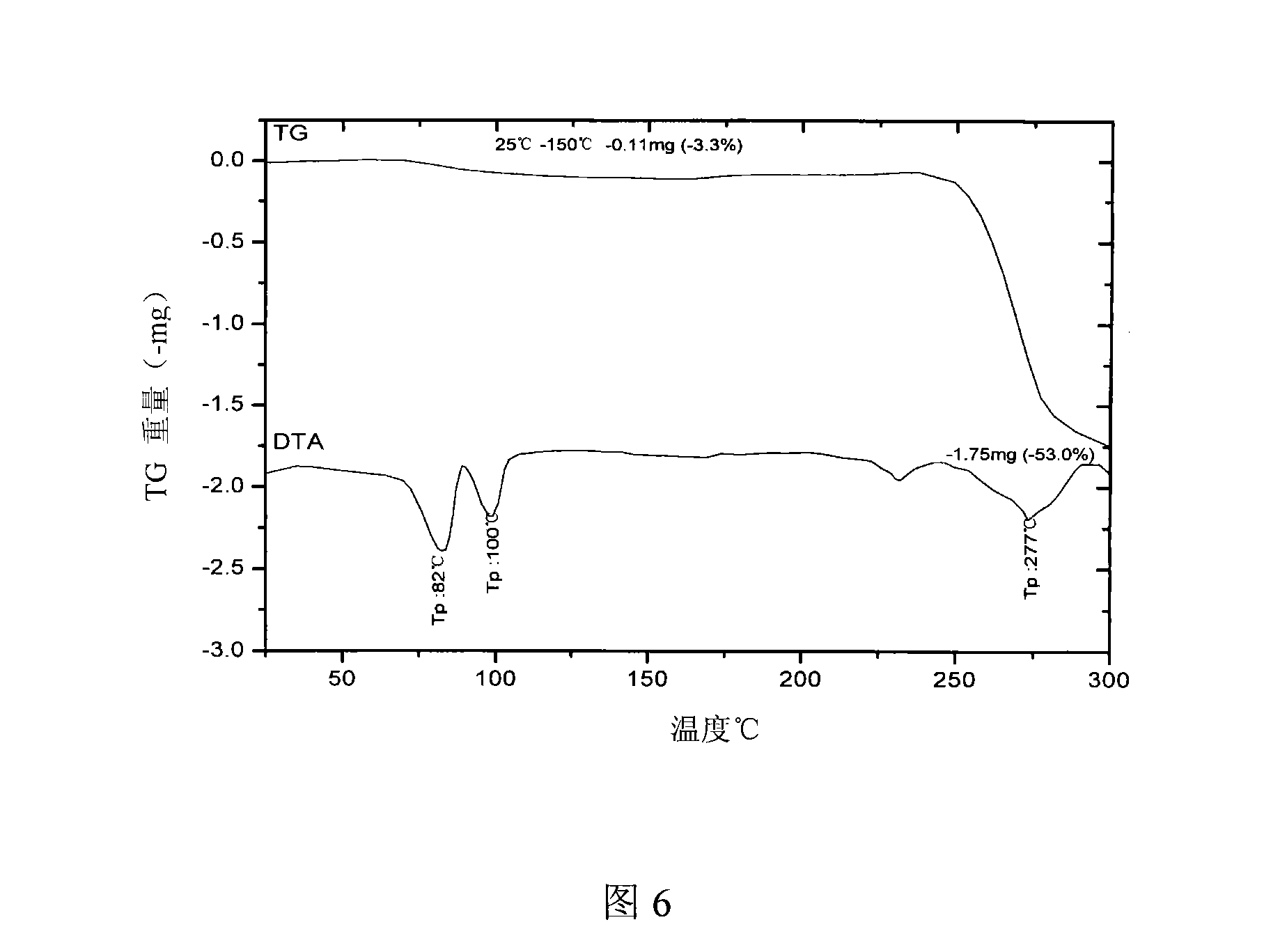
The invention relates to a novel crystal form of argatroban and a preparation method thereof. The novel crystal form has characteristic peaks at 4.3+ / -0.1, 7.4+ / -0.1, 8.5+ / -0.1, 8.8+ / -0.1, 10.4+ / -0.1, 10.7+ / -0.1, 17.2+ / -0.1, 17.9+ / -0.1, 20.3+ / -0.1, 22.5+ / -0.1, 27.8+ / -0.1 and the like by diffraction angle 2theta. The preparation method comprises the following steps: dissolving an argatroban coarse product in a mixed solvent of ethanol and water to prepare argatroban liquor; heating to 40-75 DEG C, and stirring, dissolving and clarifying; then, cooling and recrystallizing, filtering, vacuum drying to obtain the novel crystal form of argatroban. The product is uniformly paved in an opened culture dish, wherein the thickness of a sample is less than 5mm and the sample is hermetically placed in a drier at 50-80 DEG C, X-Ray Diffraction detections are carried out on samples respectively in 30 days and 60 days, the results are compared with the result at the 0th day, and the result shows that the novel crystal form of argatroban does not changed and is good in stability.
Owner:TIANJIN UNIV +1
21 (R) argatroban intravenous injection containing acid as solubiliser
InactiveCN102228677AImprove solubilityEnhanced infusion propertiesDipeptide ingredientsPharmaceutical non-active ingredientsSolubilityChemical reaction
The invention discloses a 21 (R) argatroban intravenous injection containing at least one acid as a solubiliser. The intravenous injection is characterized in that at least one acid is utilized for improving the dissolvability of 21 (R) argatroban; at least one buffering agent is utilized for maintaining pH; and at least one penetration regulator is utilized for enhancing transfusion characteristics. A test result shows that the intravenous injection can avoid effectively hydrolysis and other chemical reactions, can be stored stably under light and heat conditions, and can be preserved at room temperature for at least 24 month.
Owner:TIANJIN WEIJIE TECH
Alcohol free formulation of argatroban
ActiveUS7589106B2Improve solubilityReduce usageBiocidePharmaceutical delivery mechanismAlcohol freeArgatroban
An aqueous formulation of argatroban and of related compounds is disclosed along with a reconstitutable formulation, each of which is substantially, if not totally alcohol free. The formulations are also substantially free, if not totally free, of mono-, di-, and oligo-saccharides. An especially preferred embodiment is a ready-to-administer 1 mg / ml injectable dosage form having argatroban, lactobionic acid, and methionine.
Owner:EAGLE PHARMACEUTICALS INC
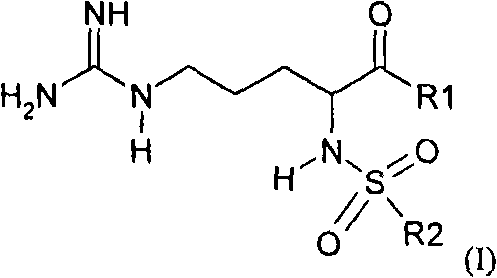
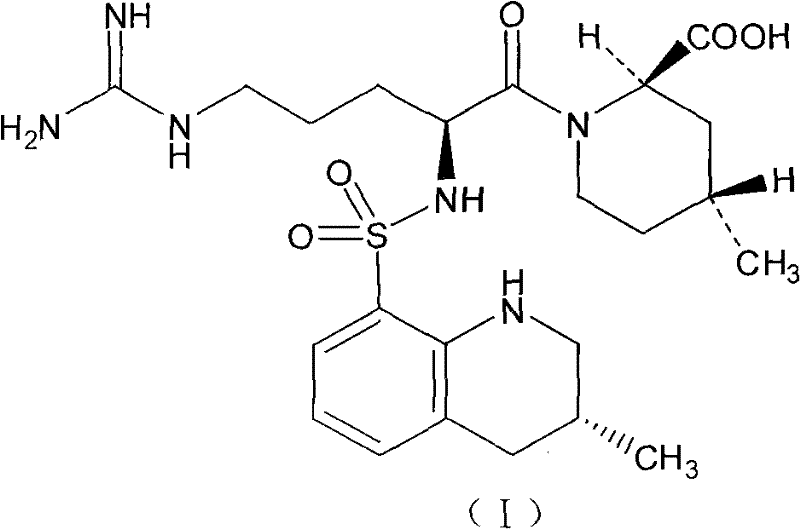
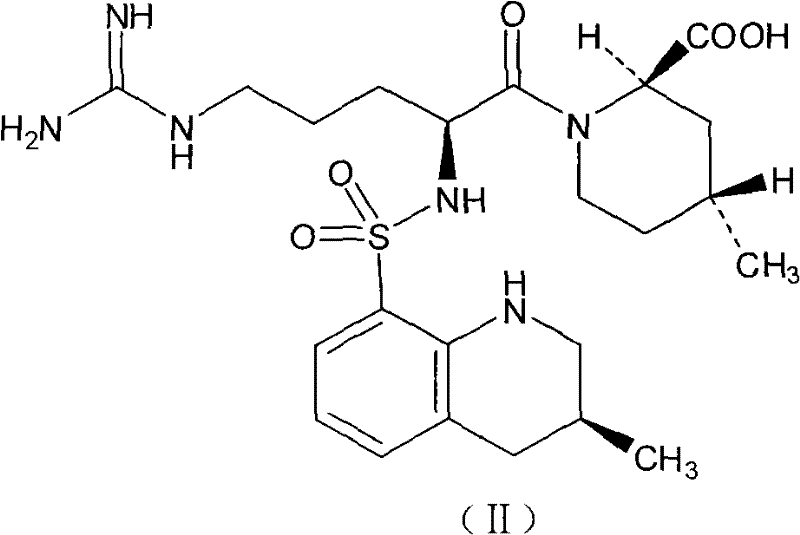
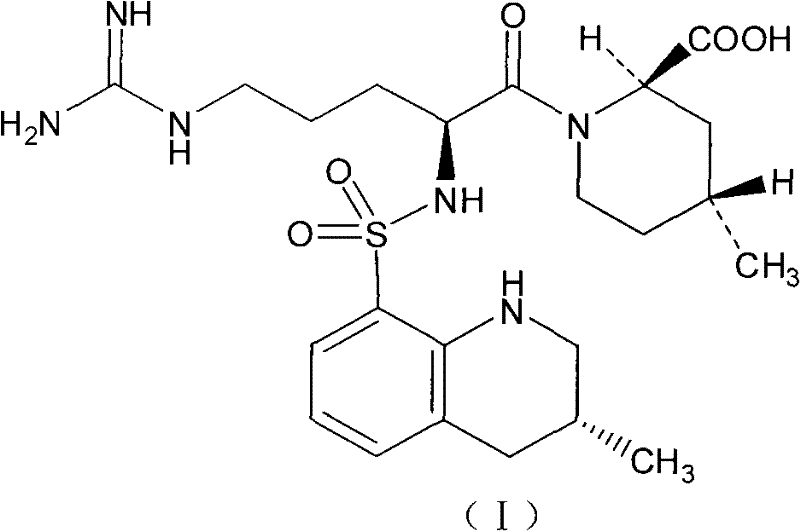
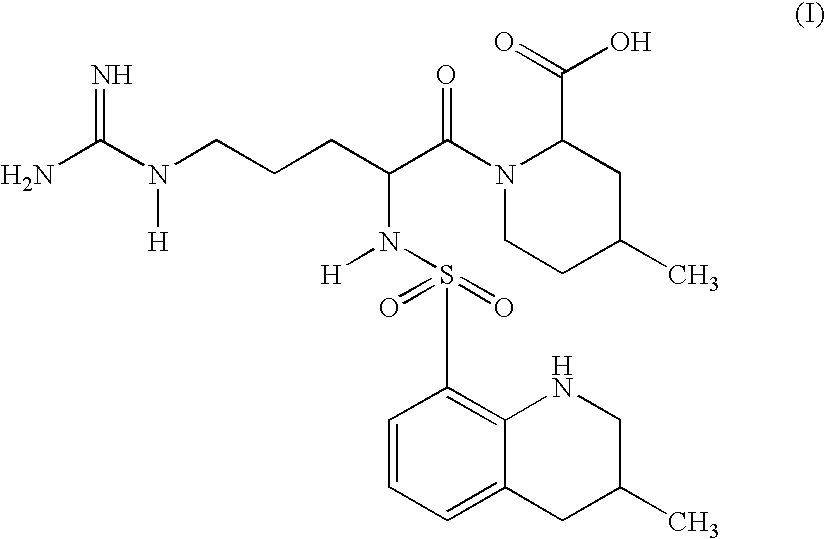
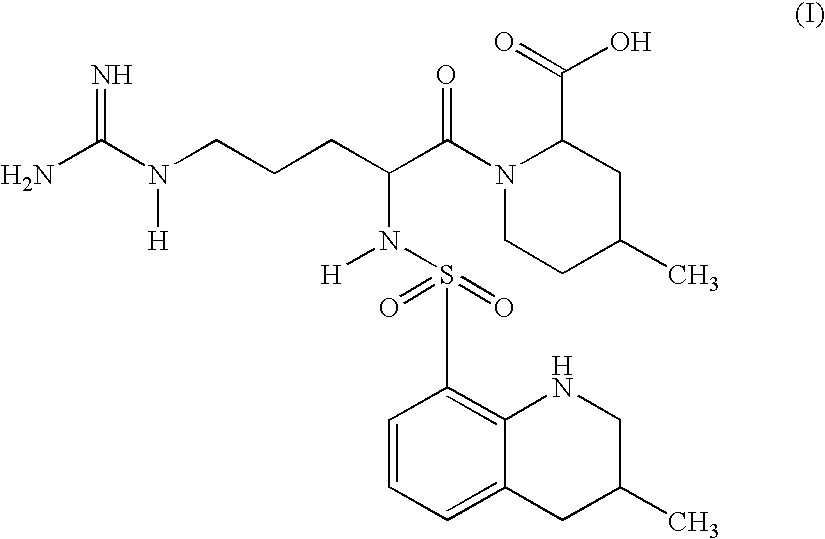
Argatroban analogue and preparation method and medical application thereof
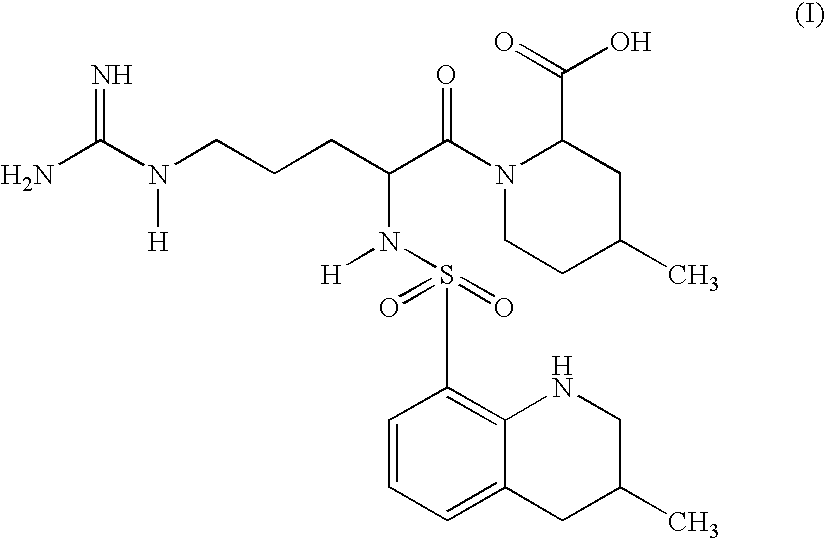
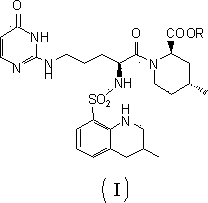
ActiveCN104098647AHigh dissolution rateImprove bioavailabilityDipeptide ingredientsPeptidesOral medicationPharmaceutical drug
The invention relates to an argatroban analogue expressed in the general formula (I), a preparation method, a pharmaceutical composition containing the argatroban analogue and medical application that the argatroban analogue is adopted as a therapeutic agent, especially as a thrombin inhibitor, wherein the definitions of all substituent groups in the general formula (I) (refer to the Specification) are the same as the definitions in the specification and the argatroban analogue provided by the invention is for oral administration.
Owner:安庆生命科技园发展有限公司
Anticoagulant and metal-organic framework compound dual-modified in-vitro blood circulation pipeline coating and preparation method thereof
InactiveCN113082301AReduce the risk of bacterial infectionAvoid inactivationPharmaceutical containersMedical packagingHemodialysisAnticoagulant Agent
The invention discloses an anticoagulant and metal-organic framework compound dual-modified in-vitro blood circulation pipeline coating and a preparation method thereof. The anticoagulant of the coating is selected from heparin, hirudin, citric acid or argatroban, and the metal-organic framework compound in the coating is MOF-Ag or MOF-Cu; the coating is obtained by fixing the metal-organic framework compound on the inner wall of an in-vitro blood circulation pipeline through dopamine and then fixing the anticoagulant on a dopamine layer with the metal-organic framework compound in a chemical grafting mode. The coating is high in stability, can improve blood compatibility and has excellent anticoagulation and antibacterial effects; and the in-vitro blood circulation pipeline with the coating can be applied to conventional hemodialysis, blood adsorption and continuous blood purification treatment.
Owner:重庆天外天生物技术有限公司
Argatroban separation method
The invention discloses a separating method of optically-active pure isomer 21 (S)-Argatroban, which comprises the following steps: adopting 21(S) and 21(R) Argatroban as raw material; heating to reflux based on mixture of alcohol and water as solvent for 5-10h; cooling; stewing; filtering; obtaining white crystal product; drying; repeating 2-6 times; combining mother liquid; stripping solvent; detecting the content of 21 (S) through high-effect liquid-phase chromatography not less than 98%.
Owner:TIANJIN WEIJIE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com