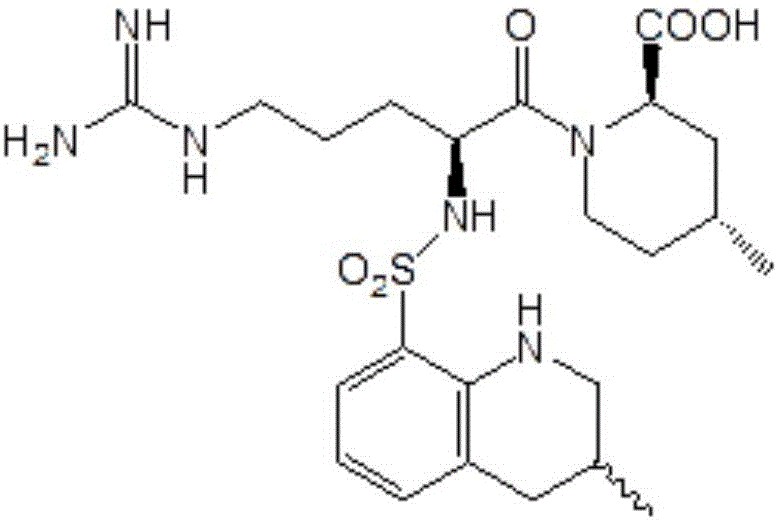
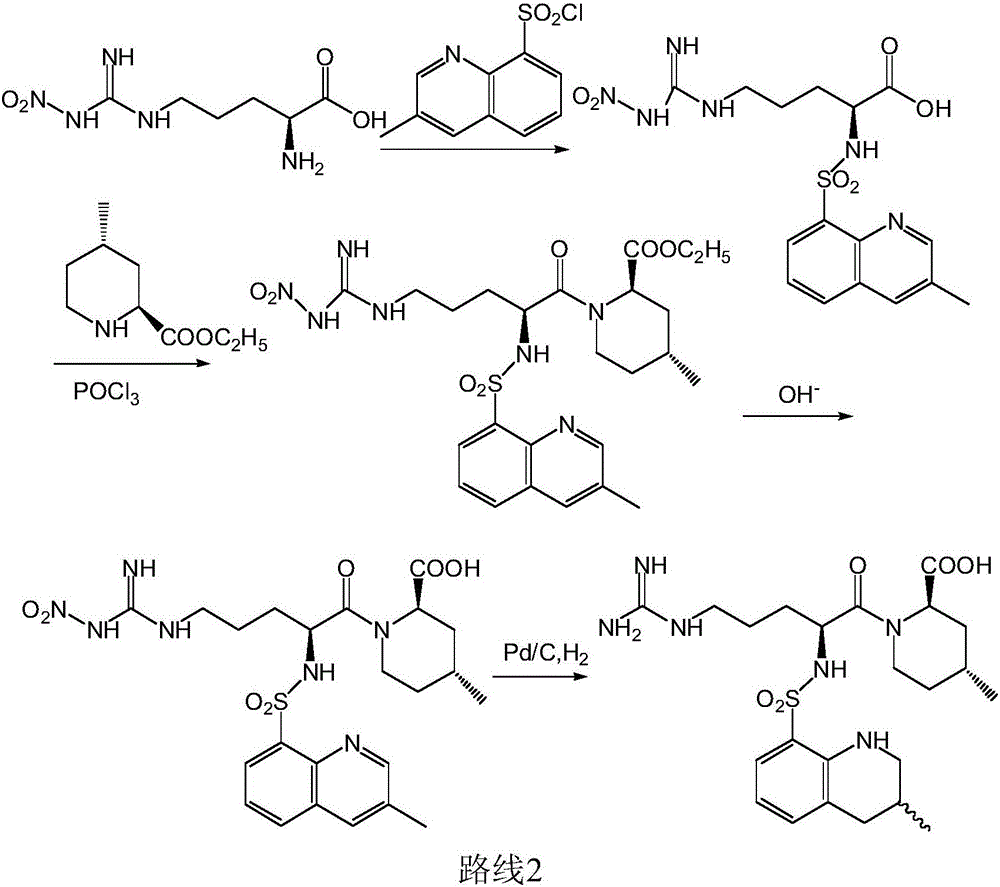
Synthesis method of argatroban
A synthetic method, the technology of argatroban, applied in the field of drug synthesis, can solve problems such as unfavorable safety production, hydrogen flammability and explosion, and unfavorable large-scale production, so as to avoid technical and safety problems, and the reaction conditions are mild and easy. Control and reduce the effect of risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
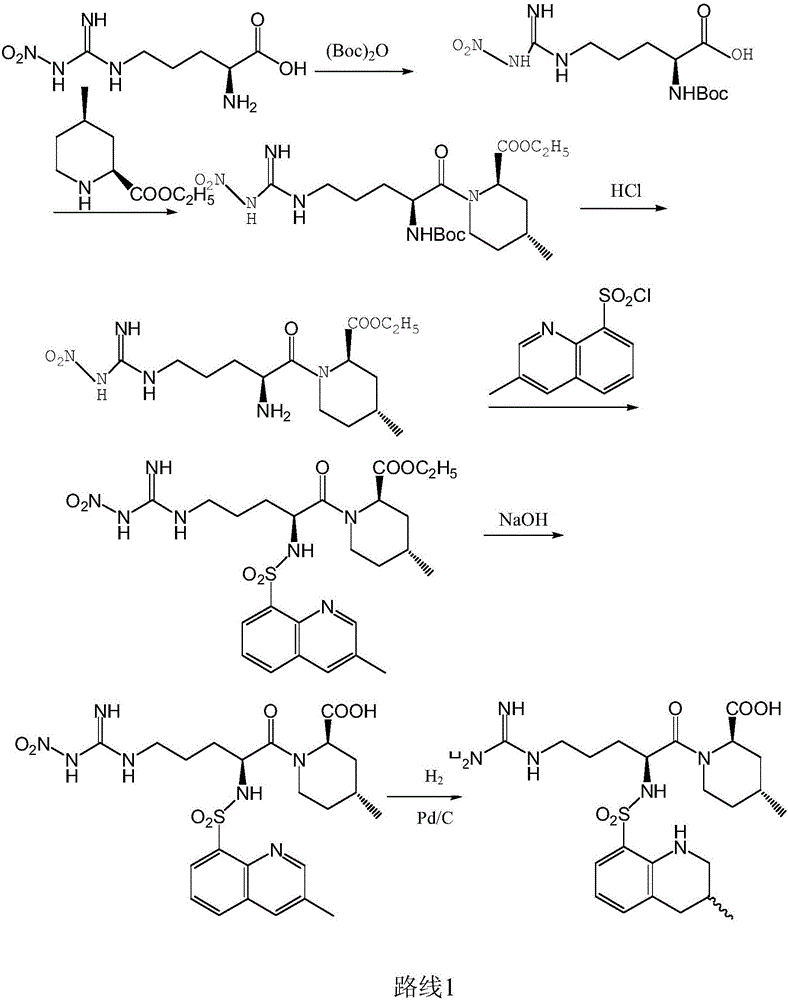
[0039] The synthetic method of compound I is as follows:
[0040] 25 g of (2R,4R)-1-[2-amino-5-[[imino(nitroamino)methyl]amino]-1-oxopentyl]-4-methyl-2 at 5°C -Ethyl piperidinecarboxylate hydrochloride (compound A) was dissolved in 200mL of chloroform, and 18.5g of triethylamine and 14.7g of 3-methyl-8-quinolinesulfonyl chloride (compound B) were added successively. The reaction was stirred at low temperature for 3 hours. After the reaction was completed, it was washed twice with 50 mL of water. The chloroform phase was dried with anhydrous sodium sulfate, and the solvent was evaporated to dryness. The residue was chromatographed on a 50 g silica gel column to obtain 32.1 g of compound I with a yield of 91 wt%.
[0041] The mass spectrum ESI-MS(m / z) of the product was detected: 578.0[M+H] + , 600.0[M+Na] + , 576.2[M-H] -
[0042]
[0043] The synthetic method of compound II is as follows:
[0044] Dissolve 30 g of the compound I prepared above in 100 mL of ethanol and ...
Embodiment 2
[0052] The synthesis method of compound I and compound II: refer to Example 1.
[0053] The synthetic method of argatroban: 25g (0.0455mol) compound II of aforementioned preparation is dissolved in 500mL ethanol, add 7.5g palladium loading capacity and be the palladium charcoal catalyst of 10wt% (add in the form of wet palladium charcoal with moisture content 70wt%) ) and 21g (0.456mol) formic acid, stirred and reacted at 40°C for 12h, the reaction was completed, filtered, evaporated to dryness of ethanol, the residue was dissolved in 200mL ethyl acetate, washed twice with 100mL water, separated, and 25g anhydrous sodium sulfate was added to the organic phase Dry for 6 hours, filter, and evaporate the solvent to dryness to obtain 21.2 g of white solid with a yield of 91.8 wt%. Then recrystallized with 425mL of 95wt% ethanol to obtain 18.4g of white powder, and the recrystallization yield was 86.7wt%.
[0054] After testing the proton nuclear magnetic resonance spectrum (1H.NM...
Embodiment 3
[0056] The synthesis method of compound I and compound II: refer to Example 1.
[0057] The synthetic method of argatroban: 30g (0.0546mol) of compound II prepared above was dissolved in 450mL of methanol, and 12g of palladium loading was added as a palladium carbon catalyst (in the form of wet palladium carbon with a moisture content of 70wt%) that was 10wt%. and 44.5g (0.654mol) sodium formate, stirred and reacted at 30°C for 14h, the reaction was completed, filtered, evaporated to dry methanol, the residue was dissolved in 240mL ethyl acetate, washed twice with 120mL water, separated, and 30g anhydrous sodium sulfate was added to the organic phase After drying for 6 hours, filtering, and evaporating the solvent to dryness, 24.4 g of a white solid was obtained, and the yield was 88 wt%. Then recrystallized with 490mL of 95wt% ethanol to obtain 20.3g of white powder, and the recrystallization yield was 83.2wt%.
[0058] After testing the proton nuclear magnetic resonance spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Recrystallization yield | aaaaa | aaaaa |
| Recrystallization yield | aaaaa | aaaaa |
| Recrystallization yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com