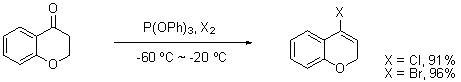Process method for synthesizing 4-site heteroatom-substituted cyclohexenyl halide
A technology of cyclohexenyl and process method, which is applied in the field of synthesis of organic compounds, can solve the problems of harsh reaction conditions and difficult control, and achieve the effects of mild process conditions, easy operation, avoiding ultra-low temperature reaction and column chromatography purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
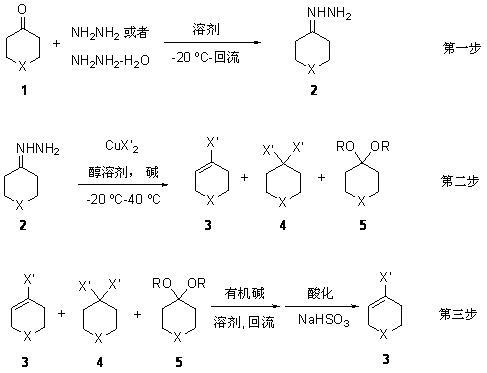
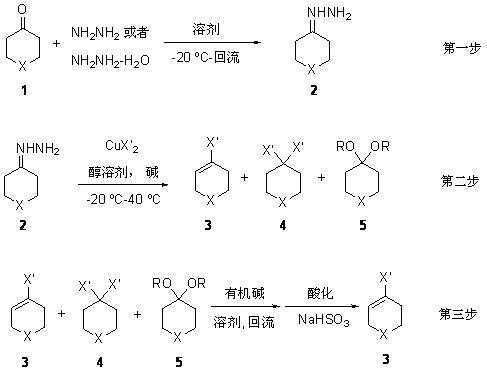
[0034] The first step: Tetrahydropyran-4-hydrazine ( 2a , X = O) to synthesize
[0035] Under argon protection, add 70-80% hydrazine hydrate (10 moles) and 400 ml of ethanol to a 1-liter reaction flask equipped with a constant-pressure addition funnel, and stir at room temperature for 20 minutes. Cool down to -10 o C. Slowly add tetrahydropyran-4-one (0.5 mole) in ethanol (100 ml) solution dropwise, the dropwise addition is completed in 8-10 hours, keep warm and continue to react for 3-5 hours. After the complete reaction of the raw materials was detected by TLC (using the exclusive color reagent 2,4-dinitrophenylhydrazine for color development). After rotary evaporation of the solvent and vacuum distillation, 52 grams of the product tetrahydropyran-4-hydrazylhydrazone were obtained with a yield of 91%.
[0036] The second step: tetrahydropyran-4-enyl chloride ( 3a ) and tetrahydropyran-4-dichloro ( 4a ) (X = O, X' = Cl) synthesis:
[0037] Under the protection of argon,...
Embodiment 2
[0041] The first step: tetrahydrosulfone pyran-4-hydrazine ( 2b , X = SO 2 )synthesis
[0042] Under argon protection, add tetrahydrosulfone pyran-4-one (0.5 mol) and 450 ml of ethylene glycol dimethyl ether to a 1-liter reaction flask equipped with a condenser tube and a constant pressure addition funnel, and stir at room temperature After 20 minutes, start to slowly add 70-80% hydrazine hydrate (20 moles) dropwise. After the dropwise addition is complete, heat up to reflux for 3-5 hours. After the complete reaction of the raw materials was detected by TLC (using the exclusive color reagent 2,4-dinitrophenylhydrazine for color development). After rotary evaporation of the solvent and distillation under reduced pressure, 75 grams of the product tetrahydrosulfonepyran-4-hydrahydrazone were obtained, with a yield of 93%.
[0043] The second step: tetrahydrothiopyran-4-enyl bromide ( 3b ) and tetrahydrothiopyran-4-dibromo ( 4b ) (X = S, X’ = Br) synthesis:
[0044] Under ar...
Embodiment 3
[0048] The first step: nitrogen benzyl cyclohexyl-4-hydrazine ( 2c , X = NBn) to synthesize
[0049] Under argon protection, add nitrogen benzylcyclohexyl-4-one (0.5 mol) and 400 ml of toluene to a 1-liter reaction flask equipped with a constant pressure addition funnel, and stir at room temperature for 20 minutes. Cool down to -20 o C, Start to slowly add anhydrous hydrazine (2.0 mol) dropwise, the dropwise addition is completed in 8-10 hours, keep warm and continue the reaction for 3-5 hours, then rise to room temperature and stir overnight. After the complete reaction of raw materials was detected by TLC (UV lamp 254 nm). After rotary evaporation of the solvent and distillation of excess hydrazine hydrate under reduced pressure, n-hexane was added to make a slurry to obtain 100 g of the product nitrogen benzylcyclohexyl-4-one, with a yield of 98%.
[0050] The second step: azbutoxycarbonyl cyclohex-4-enyl chloride ( 3c ) and Azebutoxycarbonylcyclohexyl-4-dichloro ( 4c ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com