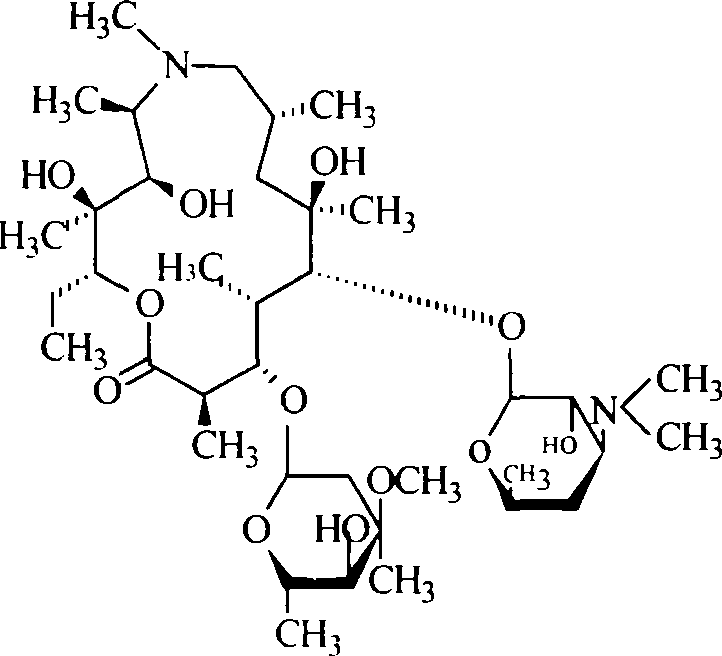
Slow released dry azithromycin suspension and its prepn
A technology of azithromycin and dry suspension, which is applied in the field of medicine to achieve the effects of reducing the generation of drug-resistant strains, low production costs, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation process has the following steps:
[0024] ①Crush the azithromycin in the prescription and pass it through a 120-mesh sieve for use. ②Use 0.5% ammonia solution as solvent to prepare 0.6% acrylic resin No. II solution; ③Add the prescription amount of azithromycin obtained in step ① to the solution obtained in step ② and stir to prepare a suspension; ④ Add a prescription amount of talc to the suspension obtained in step ③ and stir evenly; ⑤ spray-dry the material obtained in step ④ with an inlet temperature of 160-180°C and an outlet temperature of about 90°C to prepare azithromycin sustained-release microspheres; ⑥Determine the content of the microspheres, and after calculation, add the corresponding excipients such as diluting deodorant and suspension according to the proportion, and then granulate.
Embodiment 2
[0027] The preparation process has the following steps:
[0028] ①Crush the azithromycin in the prescription and pass it through a 120-mesh sieve for use. ②Prepare an aqueous solution containing 3% gelatin and 0.5% sodium carboxymethylcellulose; ③Add the prescription amount of azithromycin obtained in step ① to the solution obtained in step ② and stir to make a suspension; ④in step ③ Add the prescription amount of talc to the obtained suspension and stir evenly; ⑤Spray-dry the material obtained in step ④, make the azithromycin sustained-release microspheres at an inlet temperature of 160-180℃ and an outlet temperature of about 90℃; ⑥this The content of the microspheres is determined, and after calculation, the corresponding auxiliary materials such as diluting deodorant and suspension are added in proportion to granulate, and then it is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com