Patents
Literature
241 results about "Clarithromycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
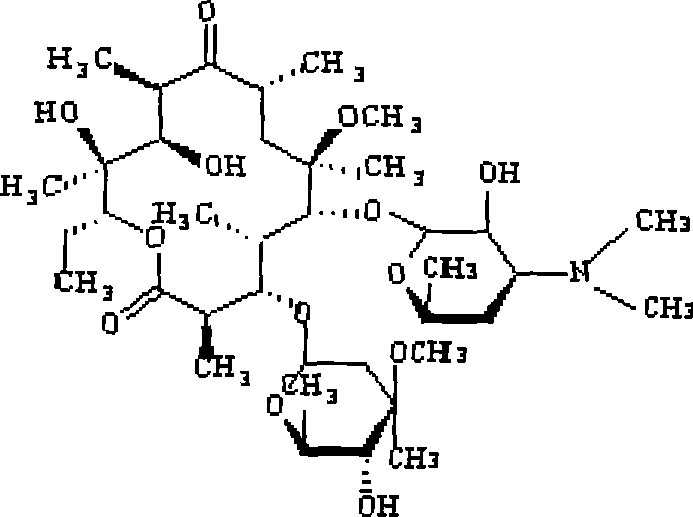
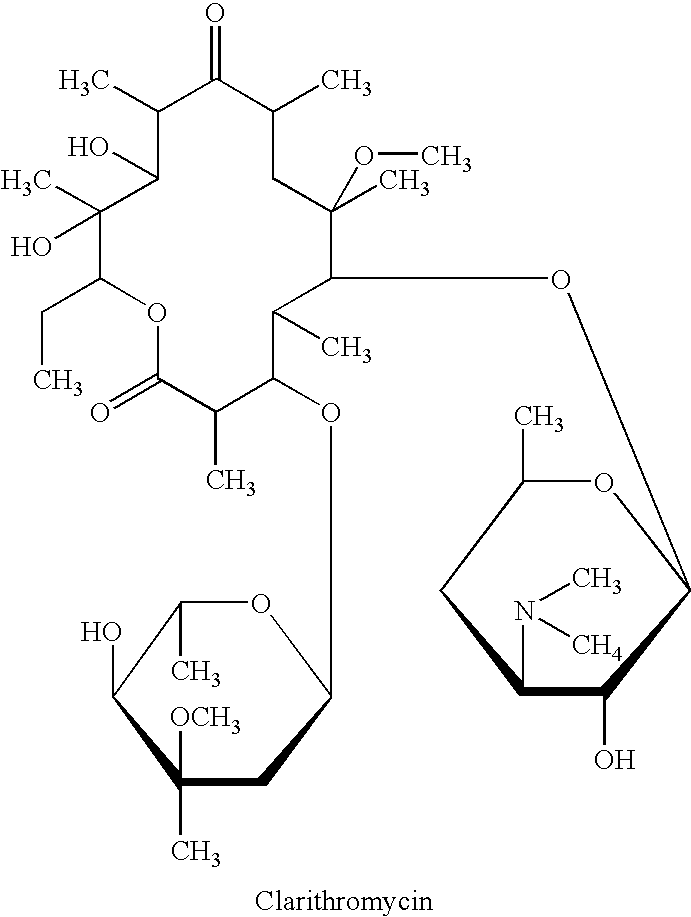
Clarithromycin is used to treat a wide variety of bacterial infections. This medication can also be used in combination with anti-ulcer medications to treat certain types of stomach ulcers. It may also be used to prevent certain bacterial infections.
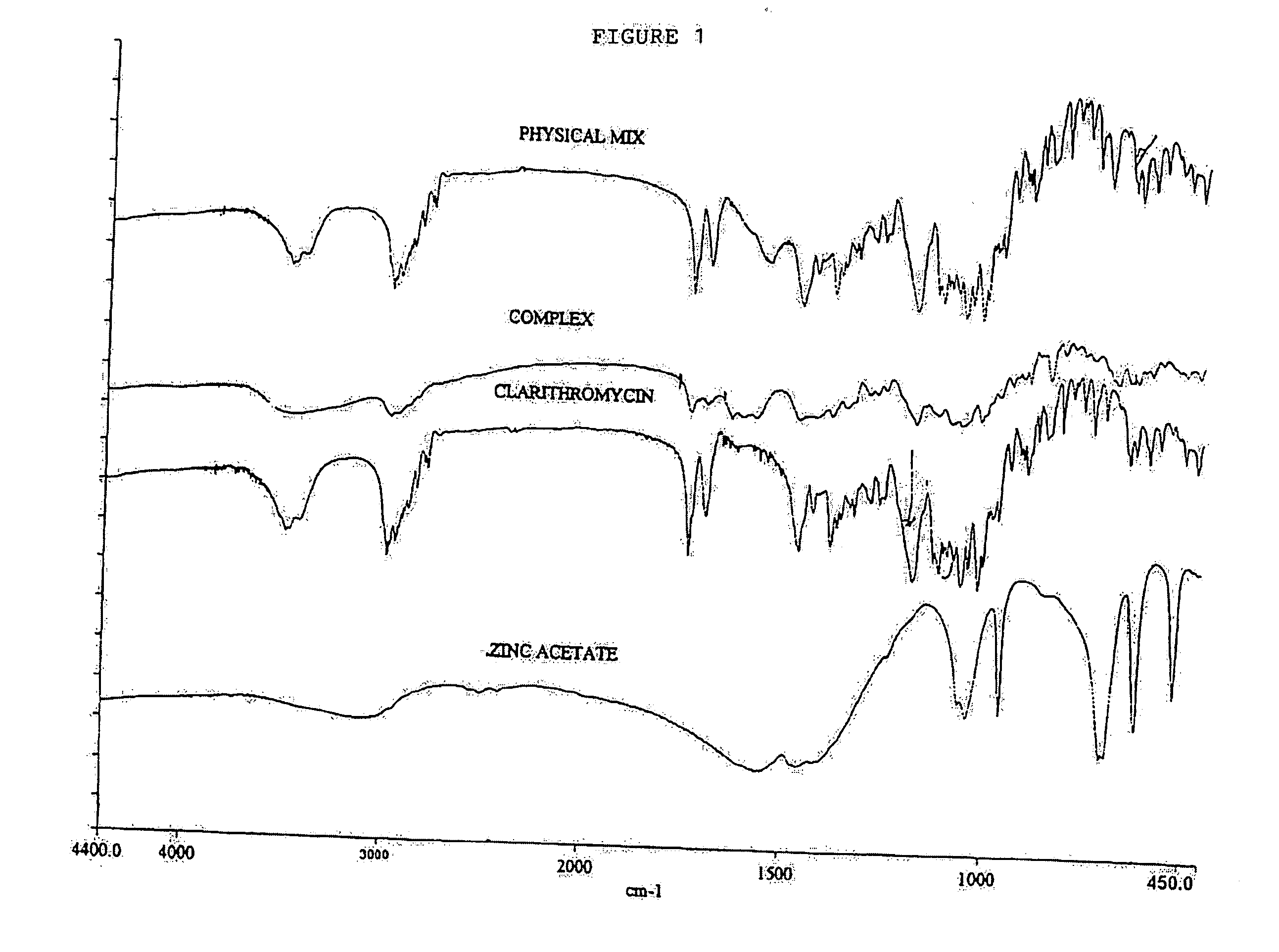
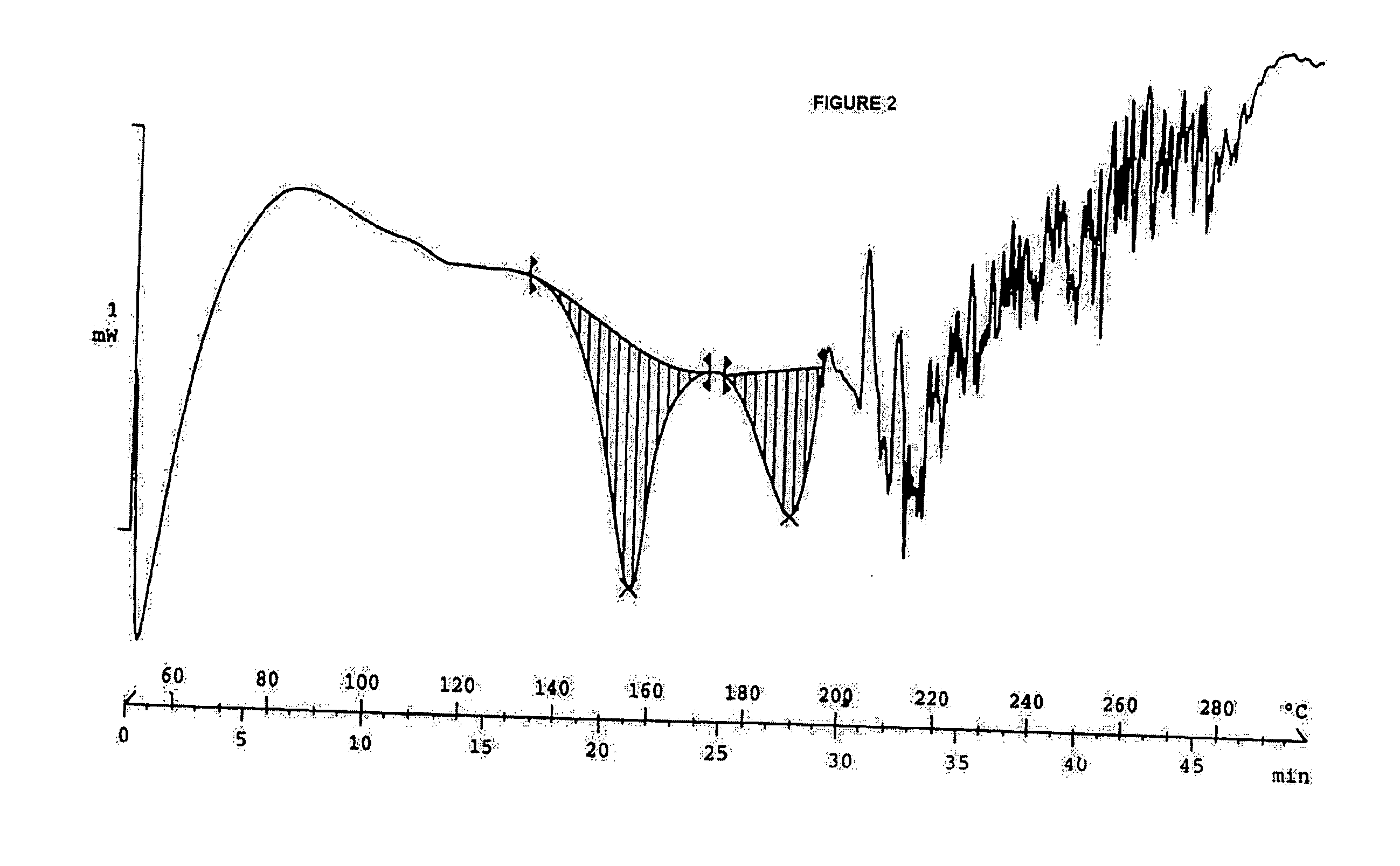
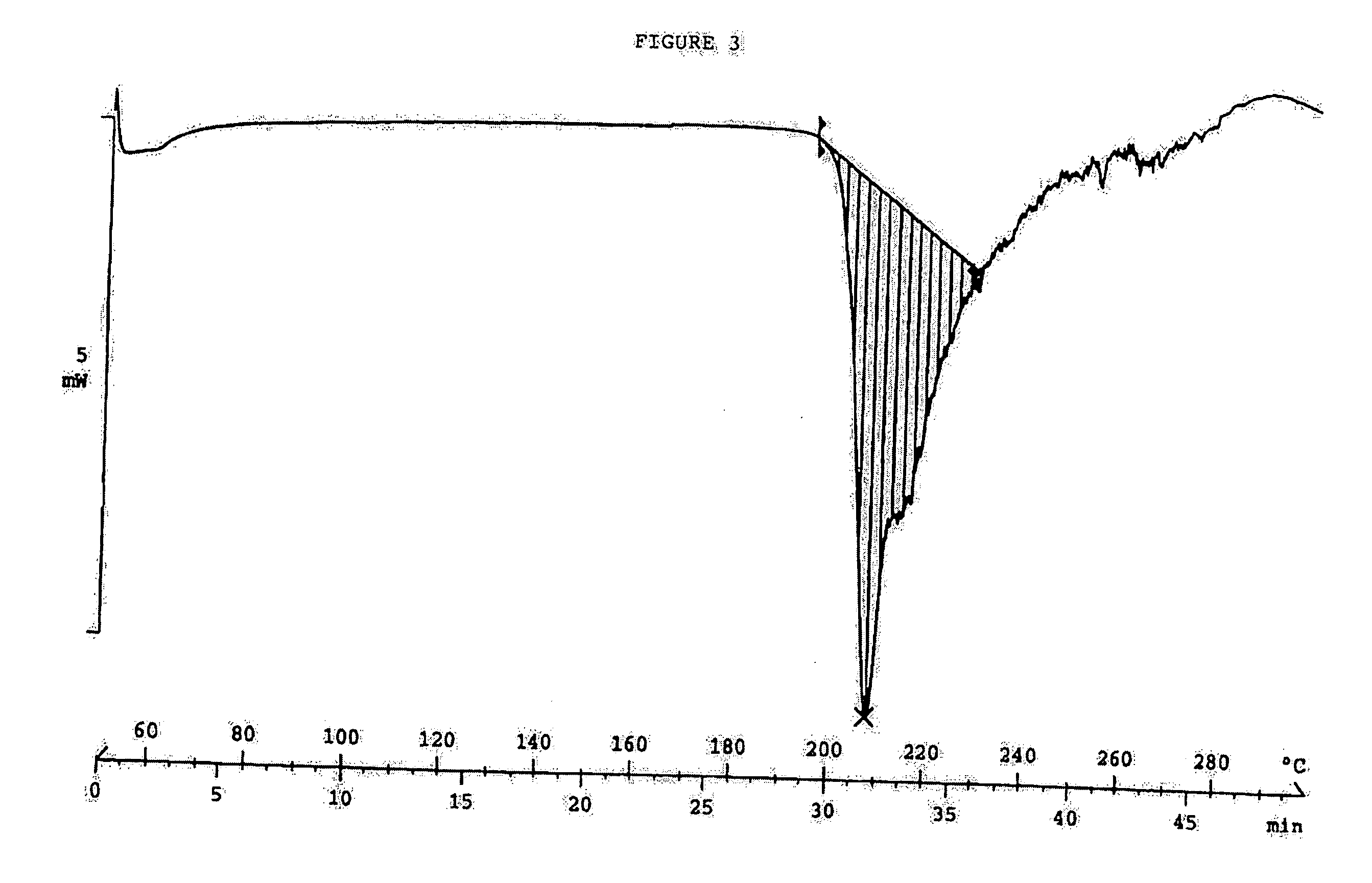
Clear, stable topical compositions of clarithromycin and processes for their preparation
The invention relates to clear, stable topical compositions of clarithromycin for the treatment of acne and processes for their preparation. The transparent topical compositions include clarithromycin, a zinc salt, a pharmaceutically acceptable vehicle and may include gelling agents.
Owner:RANBAXY LAB LTD
Nanoparticulate clarithromycin formulations
InactiveUS20070015719A1Improve bioavailabilityAntibacterial agentsPowder deliveryNanoparticleClarithromycin
The present invention is directed to compositions comprising nanoparticulate macrolides such as clarithromycin, or a salt or derivative thereof, having improved bioavailability. The nanoparticulate macrolide particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of infection and related diseases.
Owner:ELAN PHRMA INT LTD
Stable topical formulation of clarithromycin
This invention relates to a stable topical formulation of clarithromycin and its use in the treatment of acne.
Owner:RANBAXY LAB LTD
Rate-controlled delivery of macrolides
InactiveUS20030091627A1EffectiveEliminate the problemBiocideCarbohydrate active ingredientsCyclodextrinClarithromycin
There is disclosed the formulation of a poorly soluble macrolide antibiotic, such as clarithromycin together with beta-cyclodextrin, and optionally a dicarboxylic acid wherein the particles of the formulation are prepared using microfluidization techniques in a particle size in the range of from 5 to 15 microns.
Owner:SHARMA VINAY
Method and composition for treating peridontal disease
InactiveUS20050032720A1Reduce additionalReduce inflammationBiocideElcosanoid active ingredientsClarithromycinAntibiotic Y
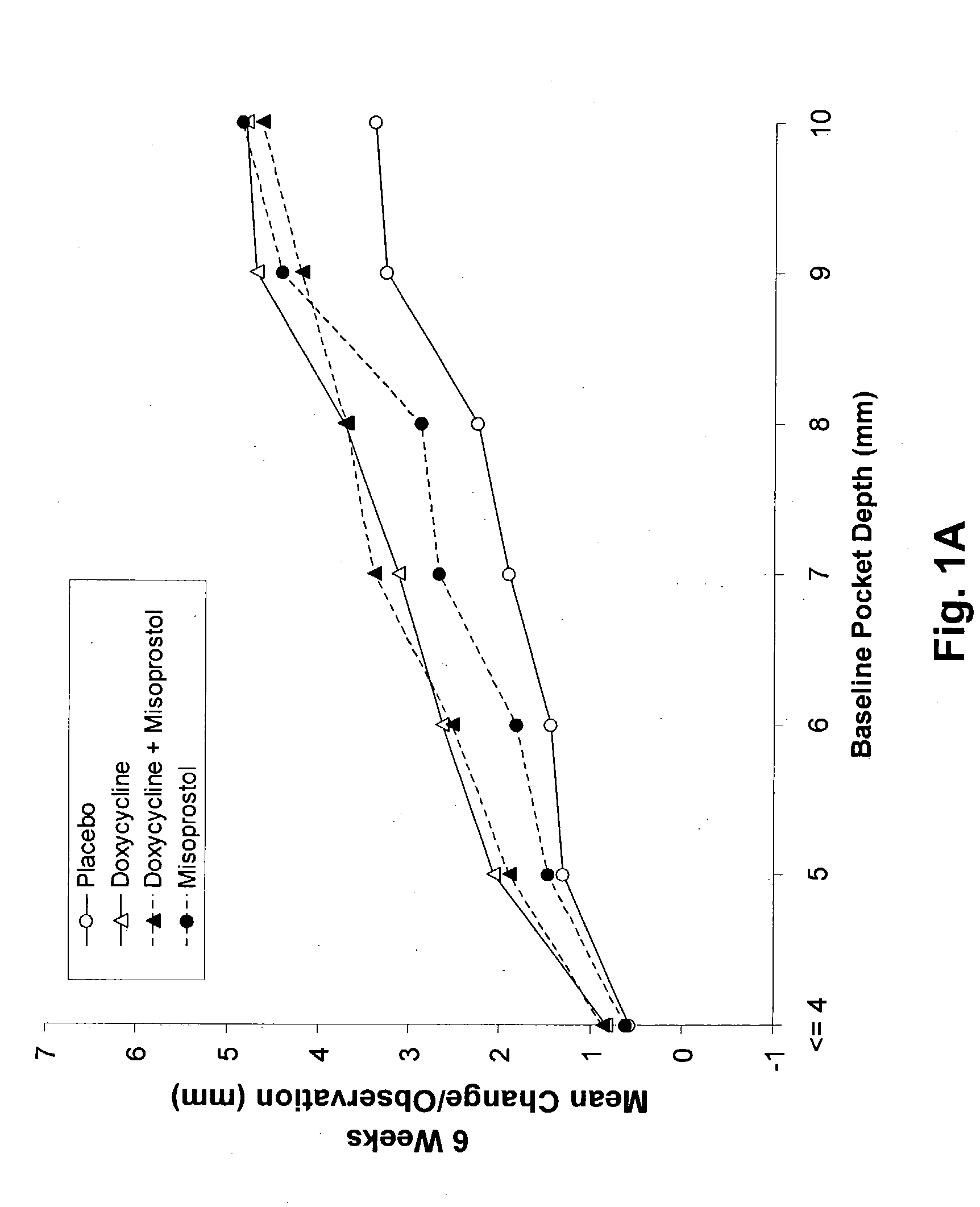
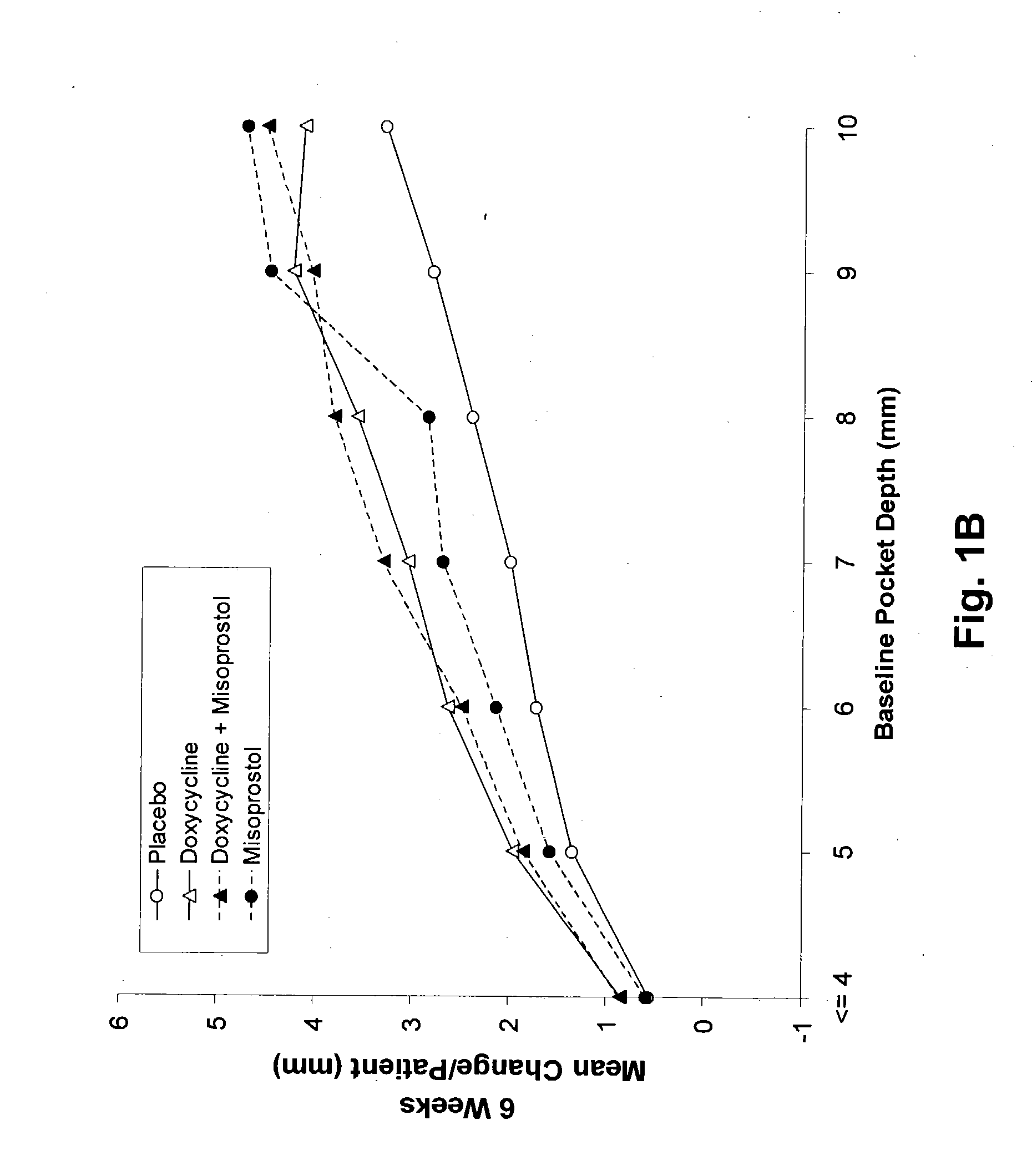
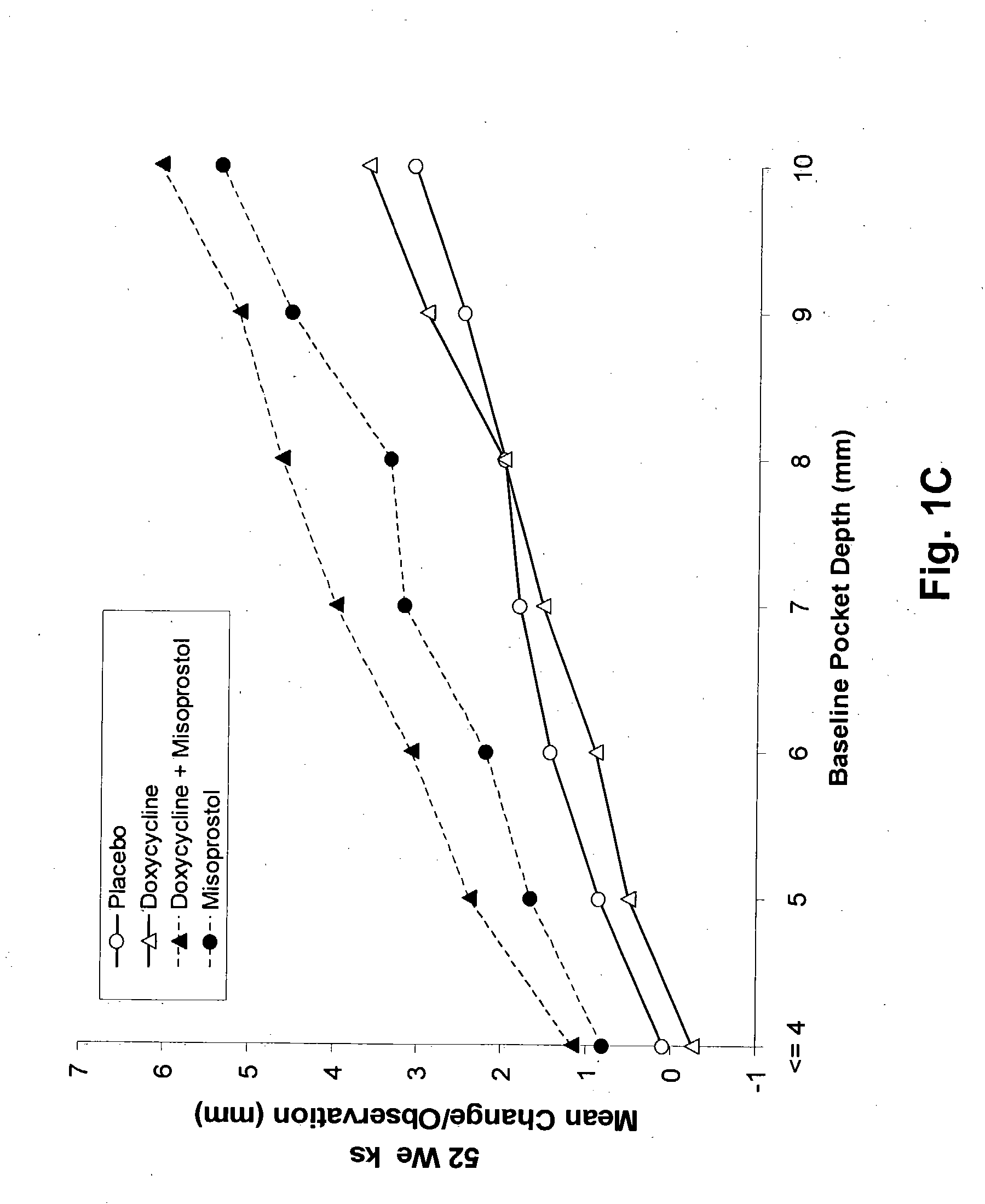
The present invention is directed to a pharmaceutical composition comprising a therapeutically effective amount of misoprostol and an effective amount of an antibiotic. A suitable antibiotic is selected from the group consisting of doxycycline, gentamicin, tobramicin, ciprofloxacin, clindamycin, clarithromycin, azithromycin and metronidazole. Preferred antibiotics are doxycycline and ciprofloxacin. More preferably, the antibiotic is doxycycline. In its second aspect, the present invention is directed to a method for treating periodontal disease in a mammalian patient comprising administering to a mammalian patient in need of such treatment a therapeutically effective amount of misoprostol and an effective amount of an antibiotic. Typically, the mammalian patient is a human.
Owner:REGENA THERAPEUTICS LC
Clarithromycin formulations having improved bioavailability
InactiveUS20050163857A1Excellent solubility propertiesPowder deliveryBiocideExtended release tabletsClarithromycin
A pharmaceutical composition includes micronized clarithromycin and exhibits improved dissolution characteristics relative to a pharmaceutical composition that includes unmicronized clarithromycin. The clarithromycin may have a particle size less than approximately 35 microns. One process for preparing an extended release tablet of the clarithromycin includes micronizing the clarithromycin; blending the micronized clarithromycin with one or more rate controlling polymers and pharmaceutically acceptable excipients; granulating the blend; and compressing to form a tablet. To treat a bacterial infection in a mammal in need of treatment, a patient may be administered a pharmaceutical composition that includes micronized clarithromycin.
Owner:RANBAXY LAB LTD
Biochip for detecting drug resistant genes of helicobacter pylori clarithromycin and preparation method and application thereof
InactiveCN101665824ADetermining drug resistanceSimple methodNucleotide librariesMicrobiological testing/measurementResistant genesClarithromycin
The invention belongs to the fields of genetic engineering and medical inspection, and relates to a biochip for detecting drug resistant genes of drug resistant genes and a preparation method and an application thereof. The biochip is prepared by the following steps: carrying out aldehyde processing for a chip base; connecting a 5' end of a probe with a molecule arm of poly (dT)10; then carrying out amino modification for the 5' end of the probe; aiming at the mutants at the following positions of helicobacter pylori 23S rRNA genes by the probe: A2115G, A2142G, A2142C, A2143G, A2143C, T2182C and G2224A; and fixing the probe on the chip base. The biochip has high sensitivity, good specificity and low detection cost, can distinguish the difference of monobasic bases, and is suitable for being popularized and used in establishment units.
Owner:周玉贵
Taste-Masking Pharmaceutical Compositions
InactiveUS20080008765A1Reduce leakageGreat tasteAntibacterial agentsBiocideHigh osmolalityOral medication
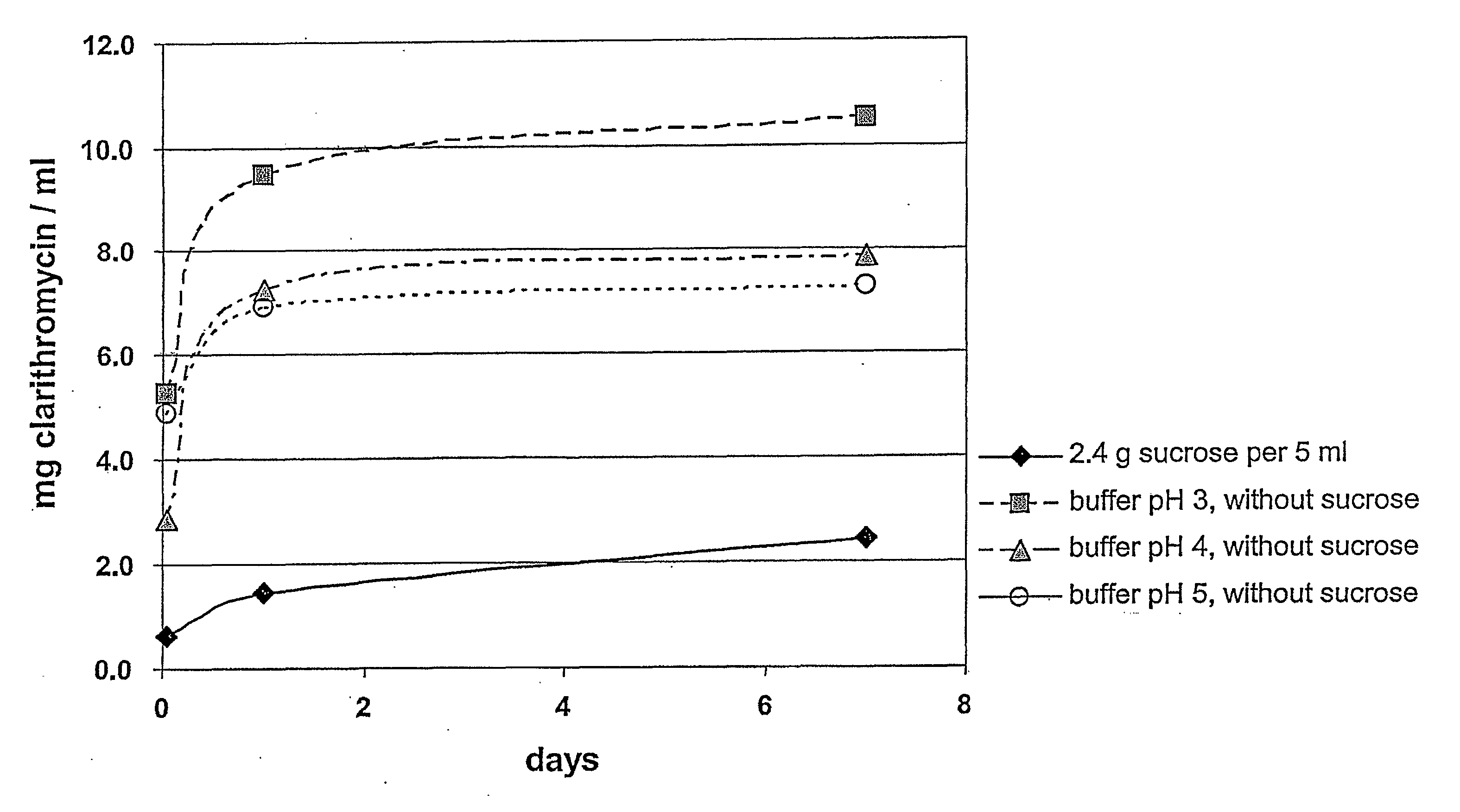
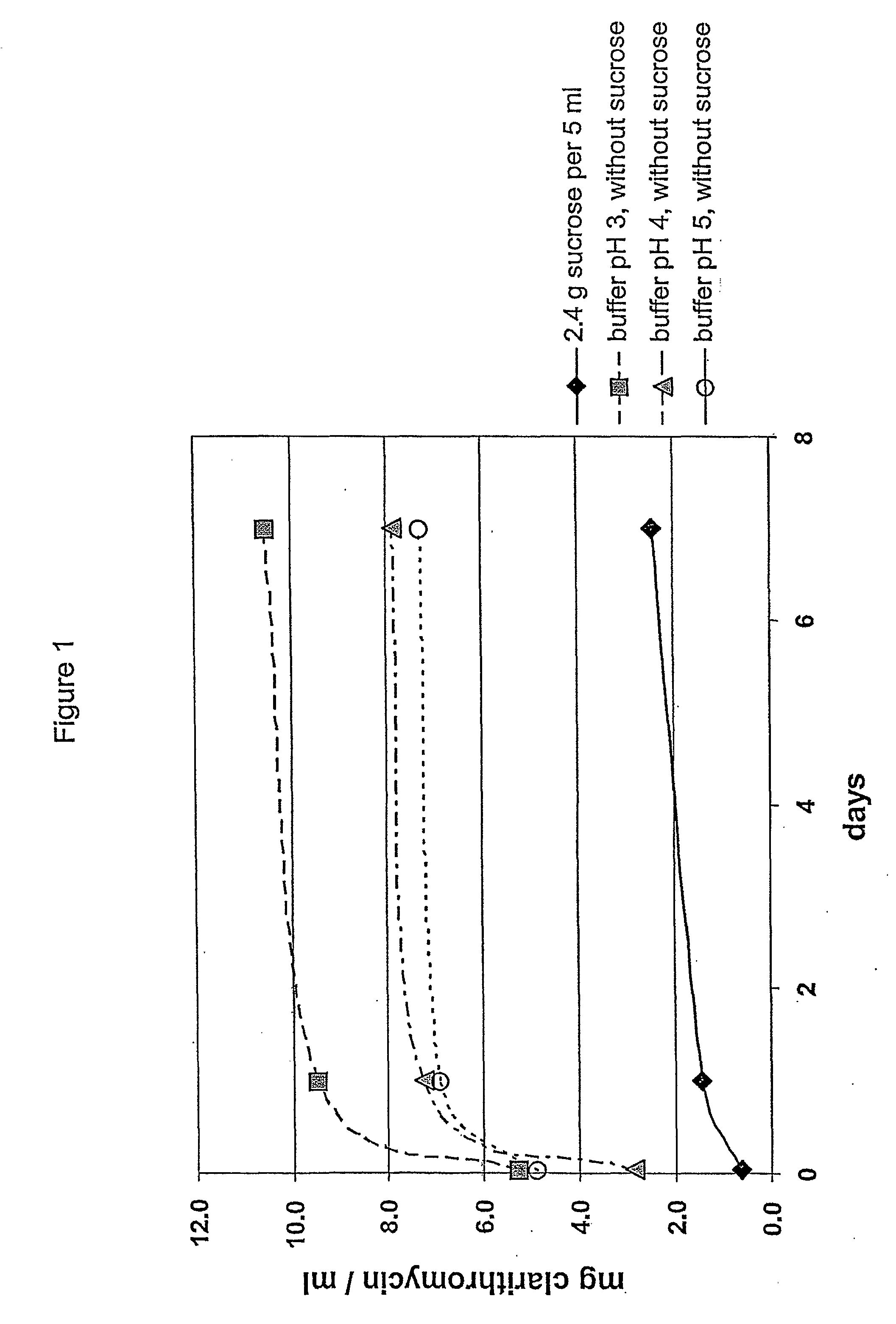
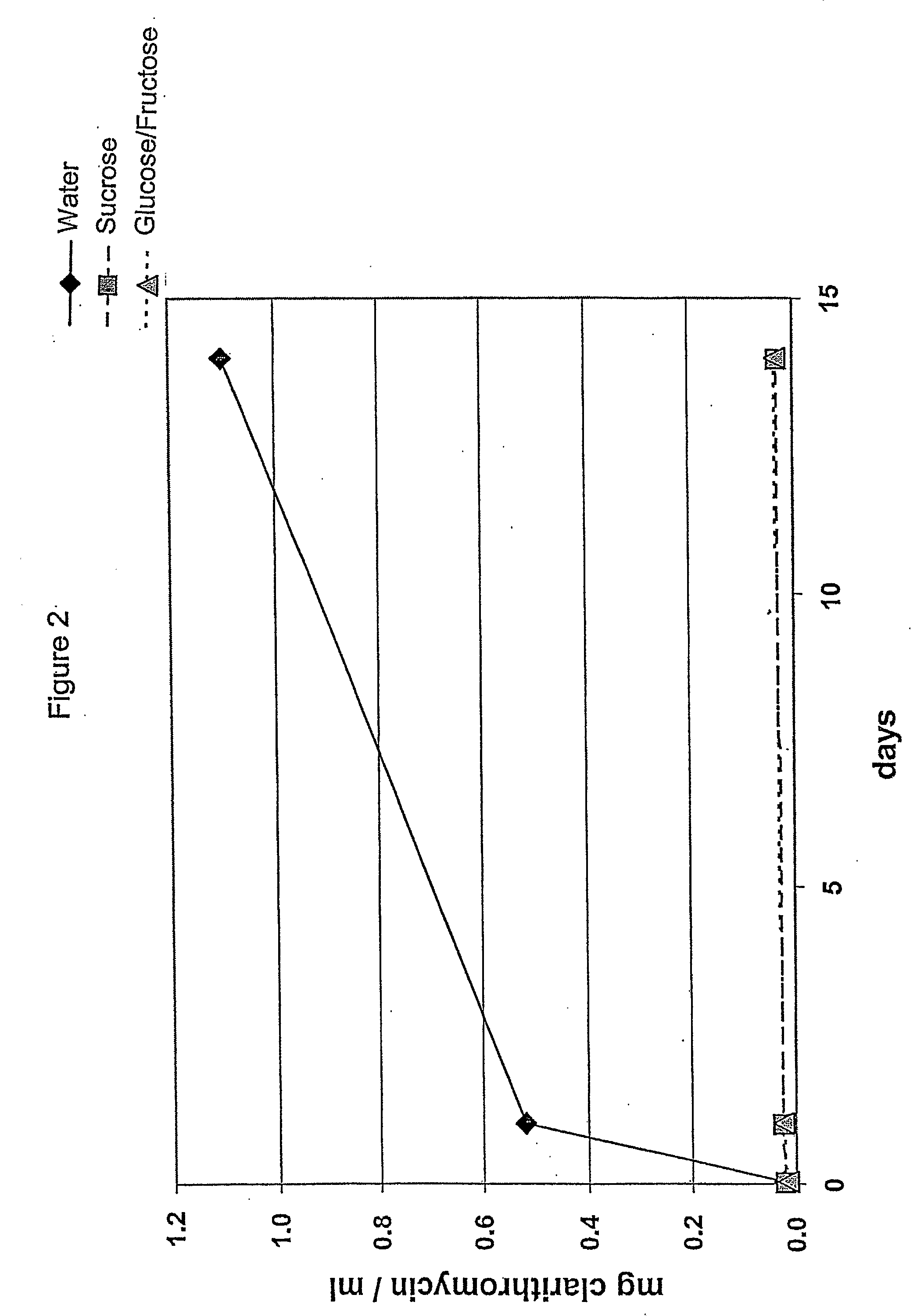
The present invention relates to a pharmaceutical composition for oral administration suitable for the preparation of a ready-to-use suspension comprising coated particles comprising an active substance having an unpleasant and / or bitter taste, such as clarithromycin, and a suspension base comprising an osmotically active substance capable of providing a high osmolality to the admixture of the suspension base with an aqueous suspending medium in the ready-to-use suspension. Said ready-to-use suspension maintains its palatability over a prolonged period of time by those defined osmotic conditions.
Owner:SANDOZ AG
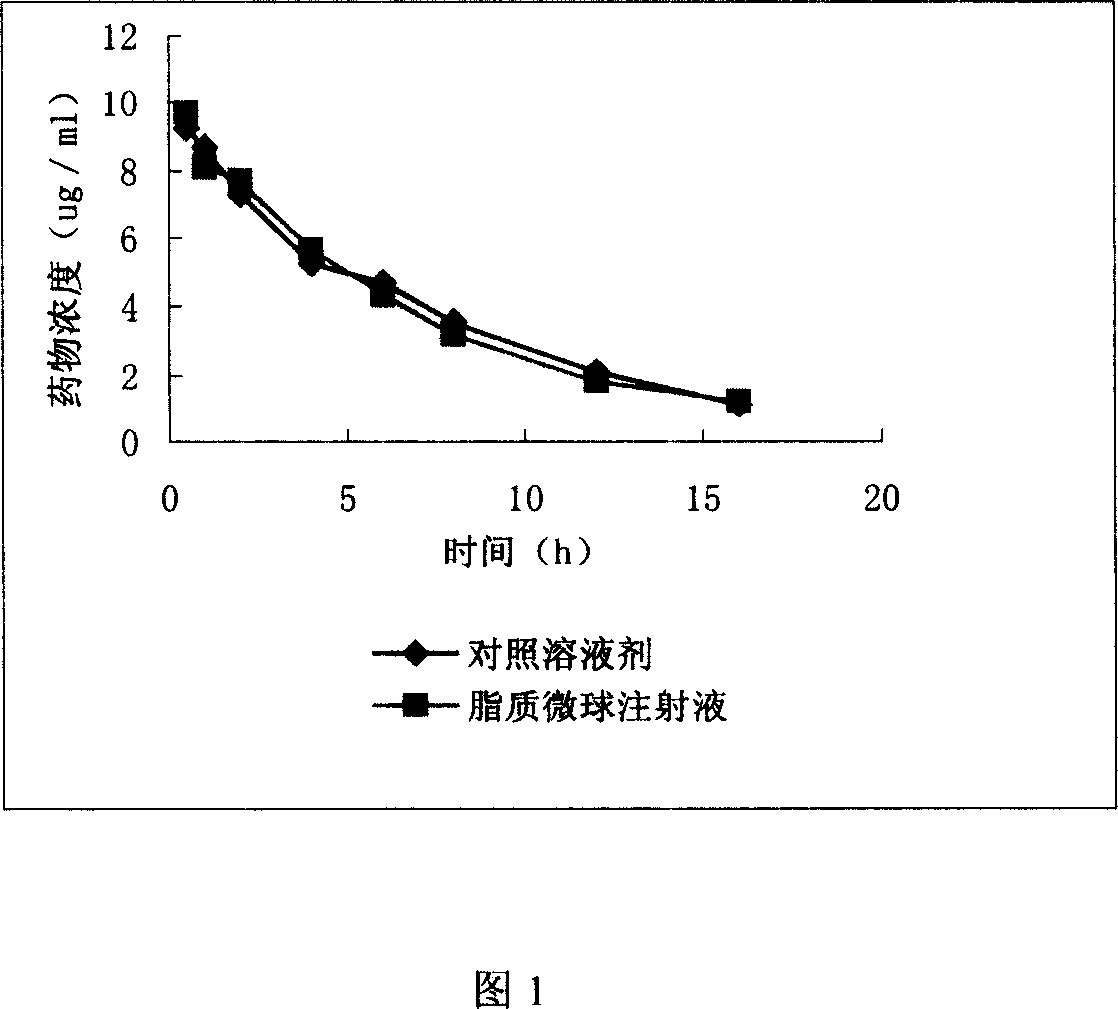
Clarithromycin liposome microsphere injection and its prepn. method
InactiveCN1947720AHigh drug loadingGuaranteed curative effectAntibacterial agentsOrganic active ingredientsMicrosphereCurative effect
A lipide microball type injection of clarithromycin with high stability and low toxin is prepared from the oil for injection, clarithromycin, surfactant, glycerin, oil-phase solubilizer, metallic chelating agent, and the water for injection. Its preparing process is also disclosed.
Owner:刘玉辉
Compound capsule and preparation method thereof
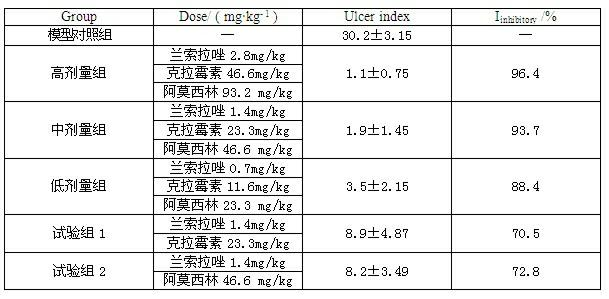
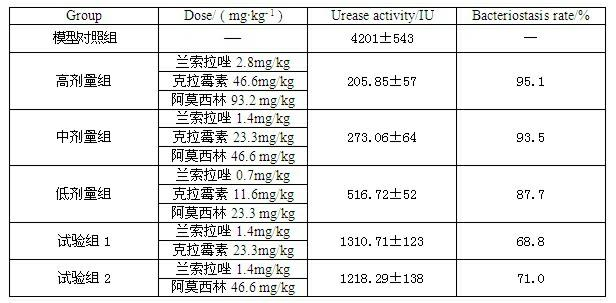
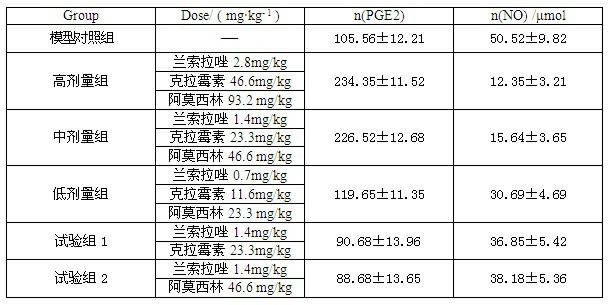
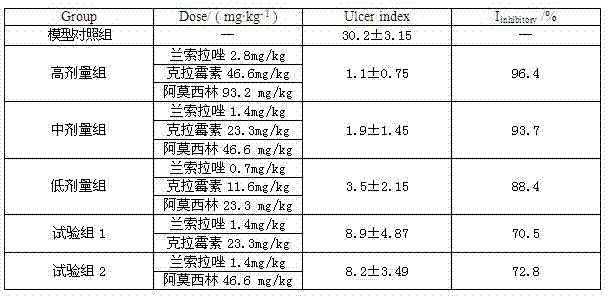
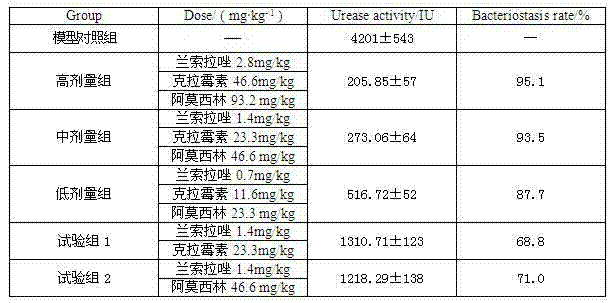
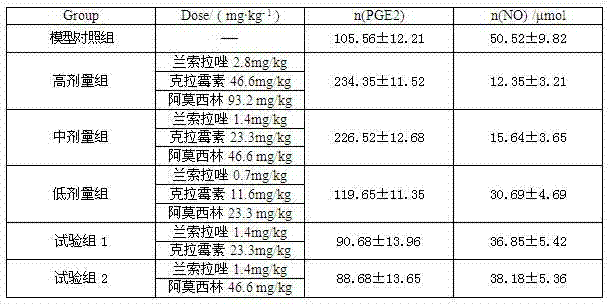
InactiveCN102091084AQuick effectReduce adverse reactionsAntibacterial agentsDigestive systemLansoprazolePeptic ulcer
The invention relates to a compound capsule and a preparation method thereof. The capsule comprises lansoprazole enteric coated pellets, clarithromycin stomach-soluble pellets and amoxicillin stomach-soluble pellets. The compound capsule is administered twice one day based on the following dose each time: lansoprazole 20-40 mg, clarithromycin 400-600 mg, and amoxicillin 900-1100 mg. The capsule provided by the invention has a distinct effect on peptic ulcer, is used for thoroughly killing helicobacter pylori, and has the advantages of quick action, strong effect, improved bioavailability, convenience in administration and low cost.
Owner:王勇
Detection gene chip for helicobacter pylori infection individualized treatment and application of gene chip
ActiveCN103060455AStrong specificityConsistent specificityNucleotide librariesMicrobiological testing/measurementEtiologyIndividualized treatment
The invention relates to a biological engineering detection product and an application of the gene chip, and in particular relates to a detection gene chip for helicobacter pylori infection individualized treatment. The chip is capable of simultaneously detecting polymorphisms of helicobacter pylori clarithromycin medicine-resistant mutation sites A2142G and A2143G, carbostyril medicine-resistant mutation sites Asn87(N87K) and Asp91(D91G / Y / N), and sites 2C19*2(G6981A) and 2C19*3(G636A) related to metabolism of a proton pump inhibitor on human cytochrome enzyme CYP450. The chip detection method disclosed by the invention is rapid and accurate and is capable of identifying helicobacter pylori etiology, carrying out medicine-resistant analysis of clarithromycin and carbostyril, and analyzing metabolic individual differences of the proton pump inhibitor, so that the detection gene chip is used for guiding individualized administration of helicobacter pylori infection triplex process treatment.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
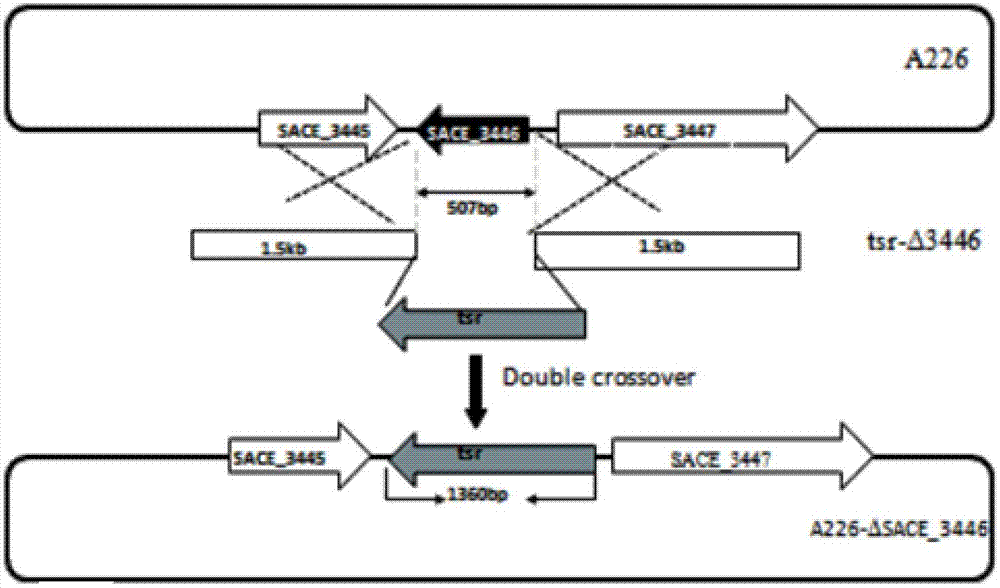
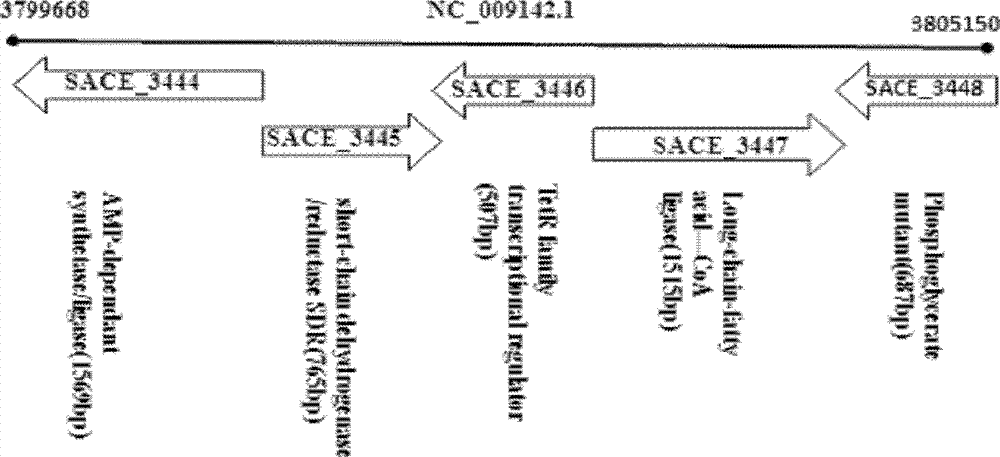
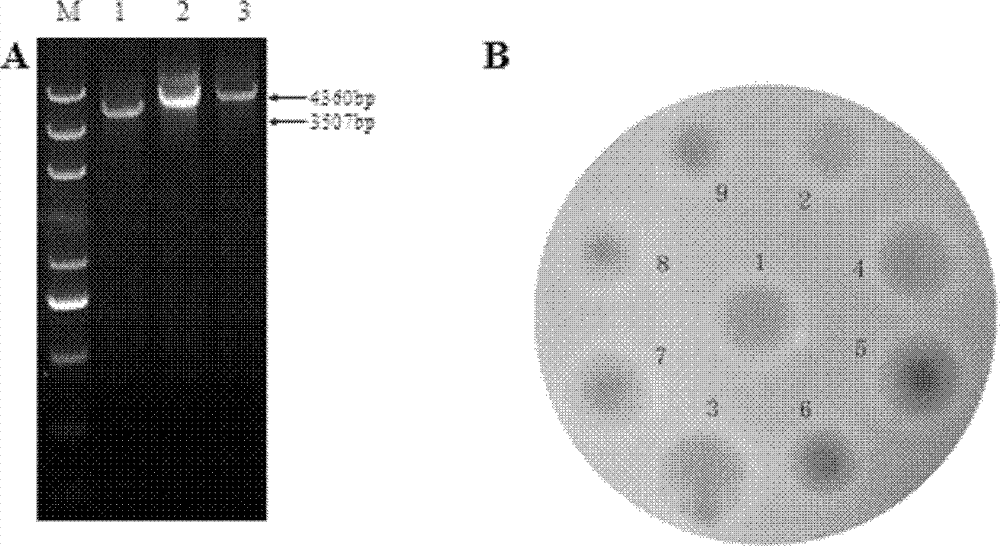
Method for improving erythrocin yield through inactivation saccharopolyspora erythraea SACE_3446 gene
The invention discloses a method for improving erythrocin yield through a negative control gene SACE_3446 on an inactivation saccharopolyspora erythraea chromosome. Saccharopolyspora erythraea is used for producing erythrocin. The erythrocin and derived drugs of the erythrocin such as clarithromycin, azithromycin and telithromycin are used widely in clinic. Erythrocin high-producing strain screening is very important in industrial production. The erythromycin biosynthesis negative control gene SACE_3446 is screened from a saccharopolyspora erythraea TetR family. Compared with erythrocin yield of an original strain, deletion mutants of the saccharopolyspora erythraea SACE_3446 is improved remarkably, the erythrocin is returned to low yield after gene complementation of the SACE_3446, and therefore the SACE_3446 gene is a erythromycin biosynthesis negative control gene. The inactivation saccharopolyspora erythraea SACE_3446 gene can improve the erythrocin yield through a genetic engineering way. Due to the fact that erythromycin biosynthesis gene control is a network system, upstream and downstream control factors acted by SACE_3446 control factors can be found by using the SACE_3446 as an object. The erythrocin yield can also be improved by changing upstream or downstream control factor genes of the saccharopolyspora erythraea SACE_3446 control factors.
Owner:ANHUI UNIVERSITY
Controlled release formulation of clarithromycin or tinidazol
The present invention relates to a controlled release pharmaceutical composition comprising amounts ranging from about 0.1 to about 4.5% w / w, of one or more of rate controlling cellulosic ether polymers.
Owner:RANBAXY LAB LTD
Clarithromycin sub-microemulsion injection and preparation method thereof
InactiveCN101411686AComply with intravenous drug requirementsNo significant change in particle sizeAntibacterial agentsOrganic active ingredientsMass ratioTG - Triglyceride
The invention belongs to the field of pharmaceutical preparation, and in particular relates to a clarithromycin submicron emulsion injection using phospholipid compound to carry medicine and a preparation method thereof. By 100 milliliters of the injection, the clarithromycin submicron emulsion injection comprises 0.05 to 0.5 gram of clarithromycin phospholipid compound in terms of clarithromycin, 10 to 20 grams of medium chain fatty acid triglyceride, 0 to 10 grams of soybean oil for injection, 0.2 to 2 grams of soya lecithin, 0.2 to 1 gram of Pluronic F-68, 0 to 0.5 gram of Tween-80, 0.01 to 0.05 gram of L-cysteine, 0.05 to 0.3 gram of sodium oleate, 2 to 5 grams of glycerin, and 70 to 90 grams of water for injection, wherein the mass ratio of clarithromycin in the clarithromycin phospholipid compound to the soya lecithin is between 1 to 1 and 1 to 12. The clarithromycin submicron emulsion injection has the advantages that the clarithromycin submicron emulsion injection has physical and chemical properties meeting requirement of medicine use for veins, not only can sterilize at high temperature, but also has favorable physical and chemical stability for long term storage, has little stimulation to the veins, improves adaptability of a patient, and is suitable for clinical application.
Owner:刘玉辉
Cryopreservation solution and cryopreservation resuscitation method for liver primary cells
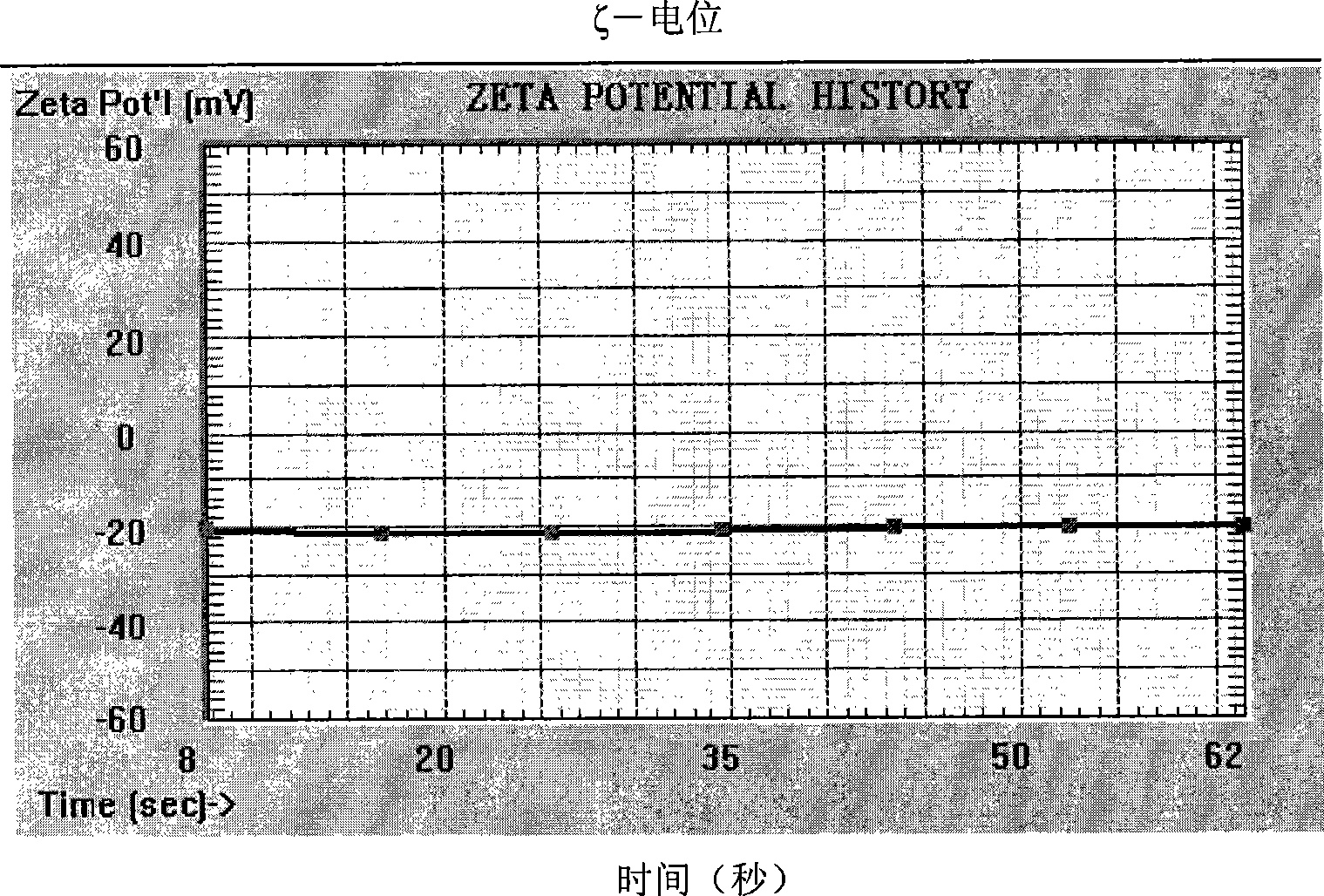
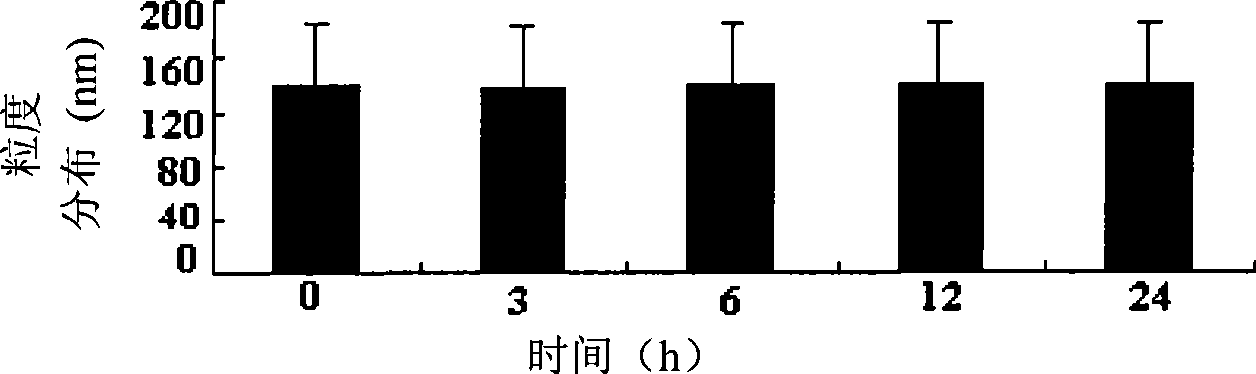
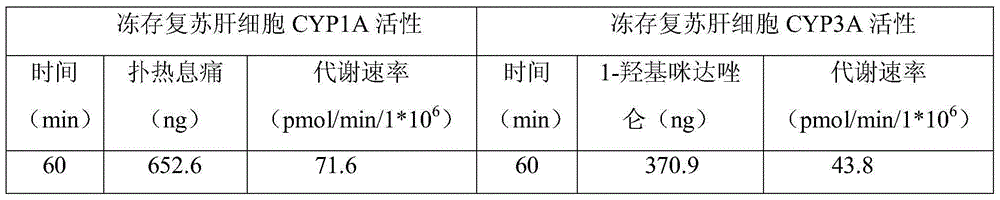
InactiveCN105010307AImprove survival rateDead animal preservationArtificial cell constructsSodium bicarbonateClarithromycin
The invention discloses a cryopreservation solution and cryopreservation resuscitation method for liver primary cells and relates to the field of biotechnologies. The cryopreservation solution is prepared according to the following formula, including 1,000g of dH2O, 15.6g of DMEM / F12 culture medium powder, 1.2g of sodium bicarbonate, 300ng of dexamethasone, 10 micrograms of liver primary cell growth factor, 25mg of insulin, 0.3g of glutamine, 25mg of gentamycin, 50mg of penicillin, 25mg of clarithromycin, 0.1g of DMSO, 10g of PEG6000, 1g of polyvinyl pyrrolidone and 1g of 1,3-propylene glycol. According to the cryopreservation solution and cryopreservation resuscitation method for the liver primary cells, the problem that the liver primary cells are relatively low in survival rate after cryopreservation resuscitation and cannot adhere to walls for drug metabolism study is solved, and the developed new cell cryopreservation solution formula and the corresponding resuscitation method can enable the cryopreservation resuscitation survival rate of the cells to be over 80%.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Silanization reaction catalyst
ActiveCN101623655AQuick responseReduce usageSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsTrimethylsilylErythromycin Oxime
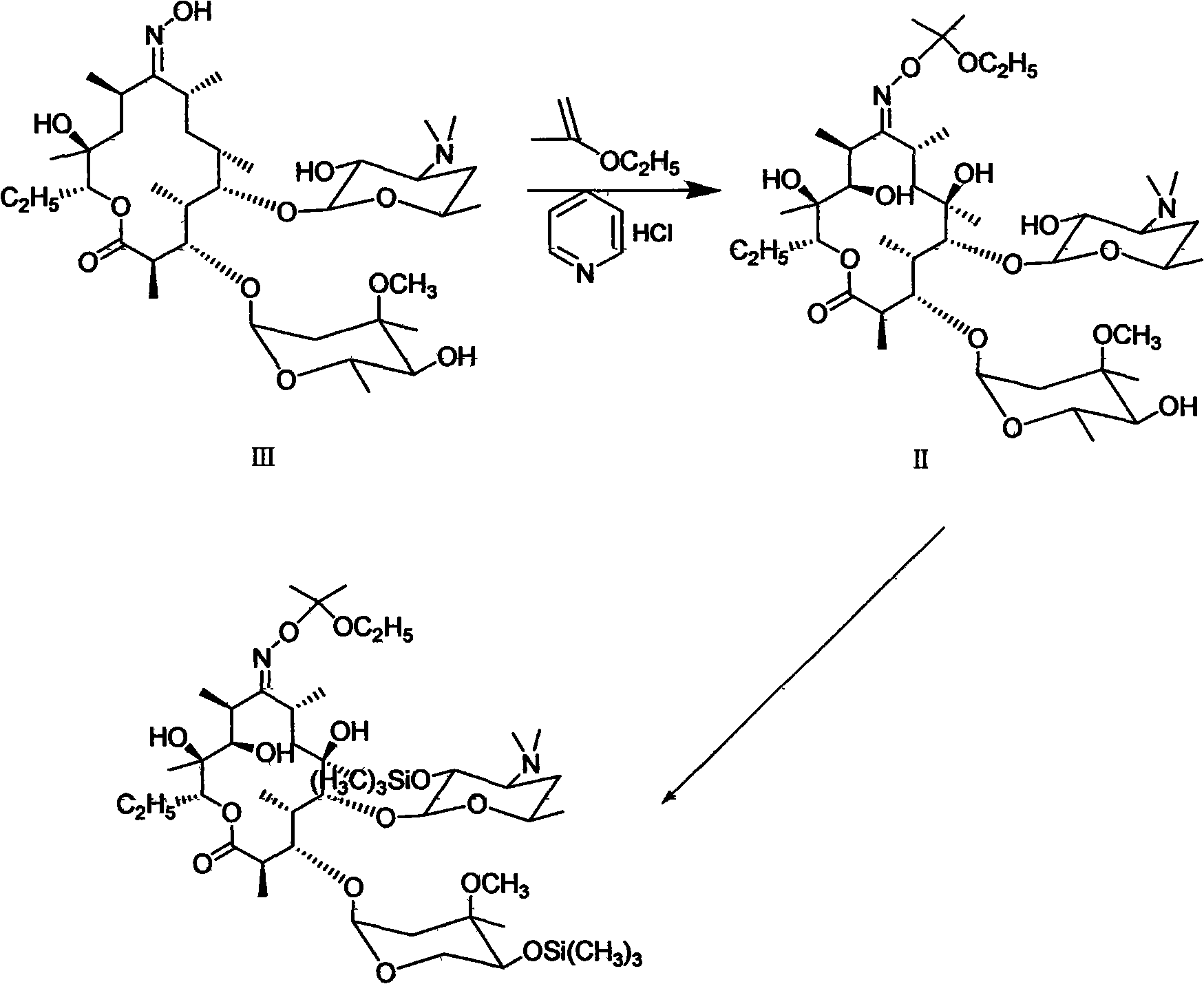
The invention mainly discloses a silanization reaction catalyst. Etherate is obtained by erythromycin oxime through etherification, (2', 4'')-O-bis(trimethylsilyl)erythromycin A-9-O-(1-ethoxy-1-methylethyl) oxime of intermediate for synthesizing clarithromycin is prepared by the silanization reaction, the catalyst is added into the silanization reaction and is hydrobromide with a general formula being RHBr, wherein R is organic alkali, or the catalyst is strong acid and weak base salt with a general formula being XBr, wherein X is weak base cations. The consumption of the catalyst is 0.01-0.4 of the erythromycin oxime according to the mol, the temperature of the silanization catalysis reaction is 0-40 DEG C and the time of the silanization catalysis reaction is 1-10h. The invention enables the process for preparing the (2', 4'')-O-bis(trimethylsilyl)erythromycin A-9-O-(1-ethoxy-1-methylethyl) oxime to be stable, the yield to be high, the cost to be low and the three wastes to be less.
Owner:ZHEJIANG GUOBANG PHARMA
Enteric coated preparation of Clarithromycin
An entenic clamycin and its preparing process are disclosed. Said enteric clamycin contains one or more units and each unit is composed of a core prepared from clamycin and one or more medicinal excipients and an enteric polymer film coated on the surface of said core.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Kit used for detecting helicobacter pylori drug resistance gene mutation via multiple fluorescent PCR
InactiveCN107099611AReal-time detectionSubsequent sequencingMicrobiological testing/measurementMicroorganism based processesClarithromycinGene mutation
The invention provides a kit used for detecting helicobacter pylori drug resistance gene mutation via multiple fluorescent PCR. The kit can be used for detecting helicobacter pylori drug resistance gene mutation of gastric mucosa tissue samples, and determining whether drug resistance is generated. The kit comprises following drugs and drug resistance gene mutation sites: clarithromycin (2142A>G, 2143A>G, 2182C>T), tetracycline (926-928AGA>TTC), quinolones (Asn87-Lys, Ala88-Val, Asp91-Gly), and amoxicillin (S414R, Y484C, and P600T). The kit can be used for obtaining accurate results in a short time, and is suitable for guidance of personalized medicine of helicobacter pylori in clinical treatment.
Owner:嘉兴雅康博生物技术有限公司
Compound capsule and preparation method thereof
InactiveCN102091084BQuick effectReduce adverse reactionsAntibacterial agentsDigestive systemLansoprazolePeptic ulcer
The invention relates to a compound capsule and a preparation method thereof. The capsule comprises lansoprazole enteric coated pellets, clarithromycin stomach-soluble pellets and amoxicillin stomach-soluble pellets. The compound capsule is administered twice one day based on the following dose each time: lansoprazole 20-40 mg, clarithromycin 400-600 mg, and amoxicillin 900-1100 mg. The capsule provided by the invention has a distinct effect on peptic ulcer, is used for thoroughly killing helicobacter pylori, and has the advantages of quick action, strong effect, improved bioavailability, convenience in administration and low cost.
Owner:王勇
Methylation reaction in clarithromycin synthesis process and recycling method of methylation reagent
The invention mainly discloses methylation reaction in a clarithromycin synthesis process and a recycling method of a methylation reagent. In the methylation reaction, methyl benzenesulfonate is used as the methylation reagent, potassium hydroxide is used as a catalyst, and methyl tertbutyl ether and dimethyl sulfoxide are used as solvents. After the methylation reaction, water is added for stratification, the dimethylsulfoxide (DMSO) layer is recycled and dried to obtain P-methyl benzene potassium sulfonate, dimethylsulfoxide is added for reaction to obtain P-methyl benzene sulfonyl chloride, and P-methyl benzene methyl sulfonate is regenerated via reaction with methanol. The generation of bismethyl impurities can be avoided effectively. Meanwhile, P-methyl benzene methyl sulfonate is regenerated via reaction of the dimethylsulfoxide and the methanol, and thus the recycling of the methylation reagent is realized.
Owner:ZHEJIANG GUOBANG PHARMA
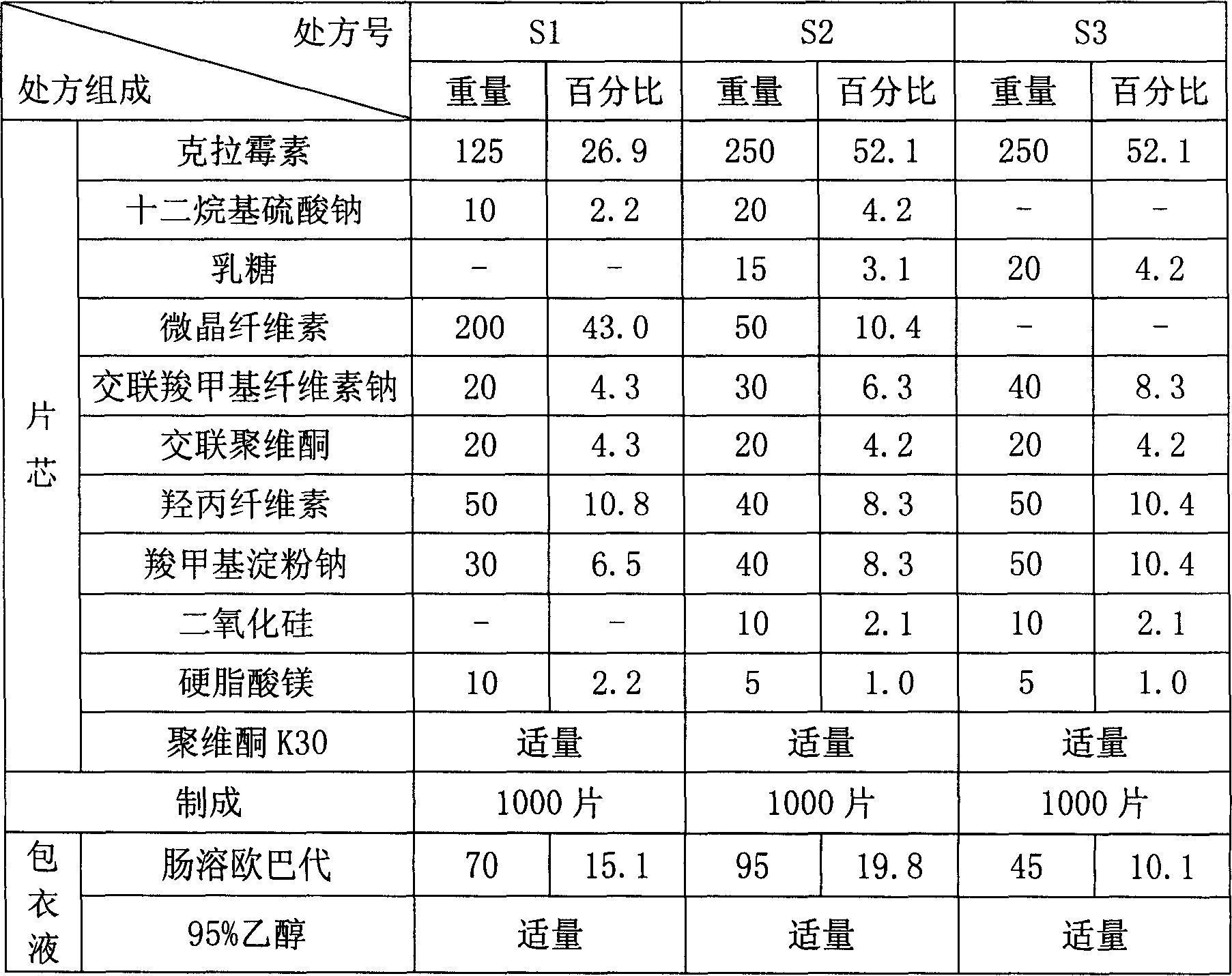
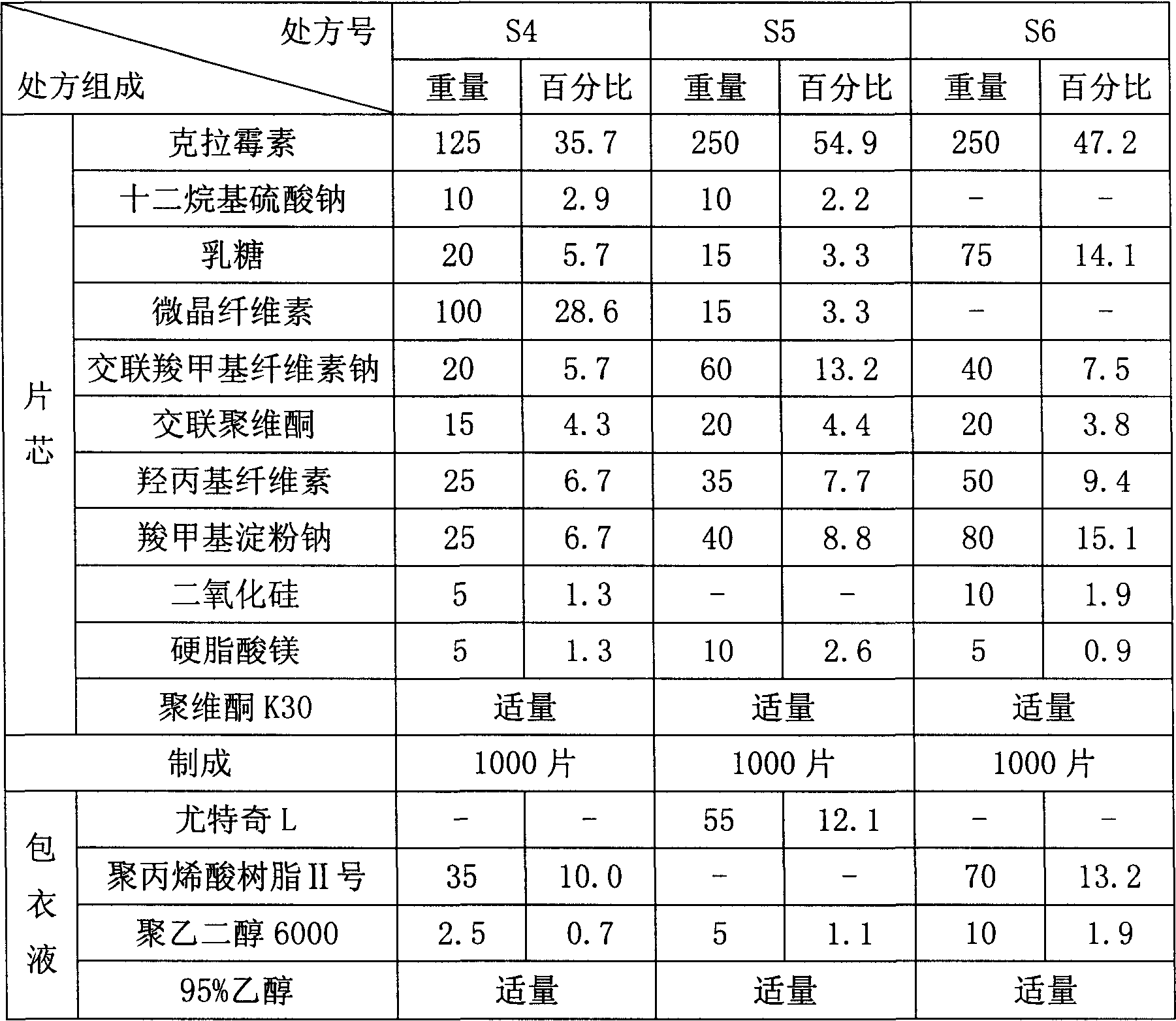
Clarithromycin dispersible tablet preparation method
InactiveCN104337778AQuality improvementStable useAntibacterial agentsOrganic active ingredientsSolubilityPrill
The present invention provides a clarithromycin dispersible tablet preparation method, which comprises: carrying out ultrafine crushing on clarithromycin and part of lactose into micro-powder with the diameter of less than 10 [mu]m to obtain mixed powder A; taking the remaining filler and part of a disintegrant, and mixing to form mixed powder B; mixing an adhesive and purified water to prepare an aqueous solution with the mass fraction of 1-10%; mixing the mixed powder B with the mixed powder A in an equal increase manner, placing into a three-dimensional mixer to mix for 30-40 min, adding to the adhesive aqueous solution to prepare a soft material, and screening with a 16-24 mesh sieve; drying at a temperature of 50-70 DEG C, and screening the whole particle with a 16-24 mesh sieve; externally adding the remaining disintegrant, a lubricant and a sweetener to the particles to obtain a material C; and tableting the material C to obtain the clarithromycin dispersible tablets. According to the invention, the clarithromycin and part of the lactose are subjected to ultrafine crushing, such that the particle size is small, the specific surface area is increased, the adsorption property and the solubility are correspondingly increased, the bitterness of the product can be reduced, and the patient compliance can be improved; and with the process, the clarithromycin dispersible tablet with characteristics of stable quality, rapid drug dissolution and no bad odor can be prepared.
Owner:哈药集团人民同泰医药股份有限公司
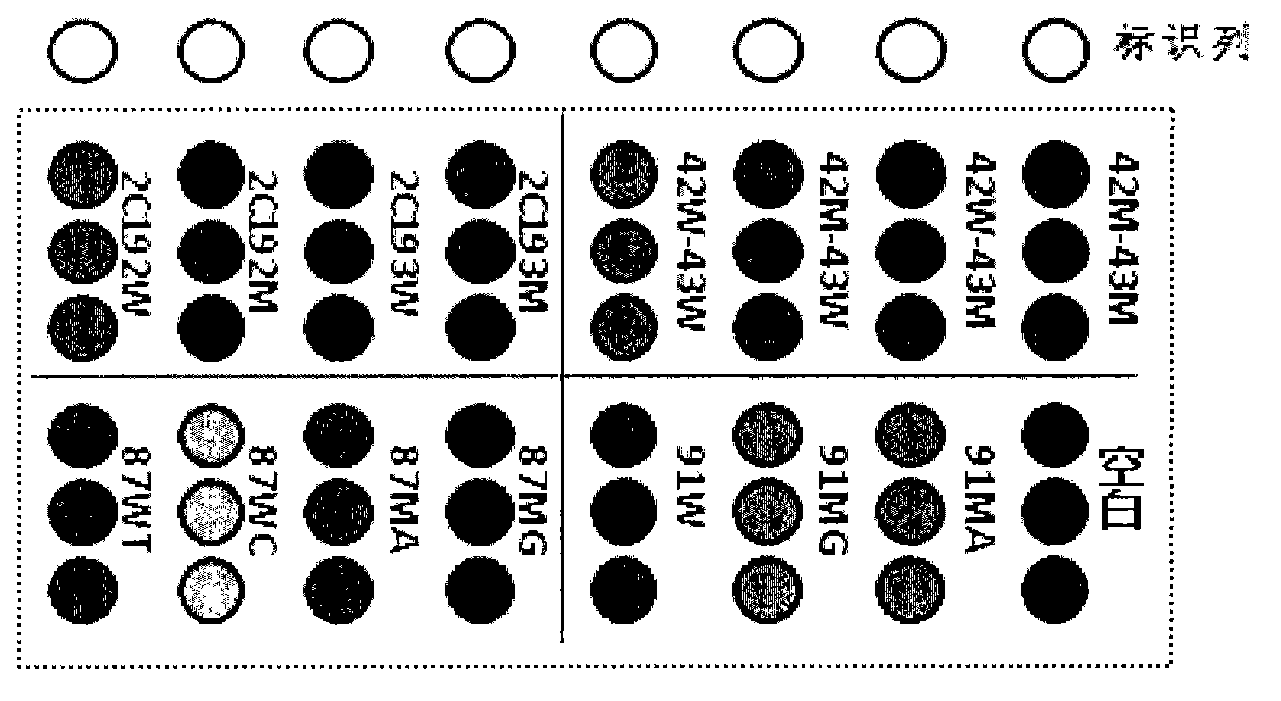
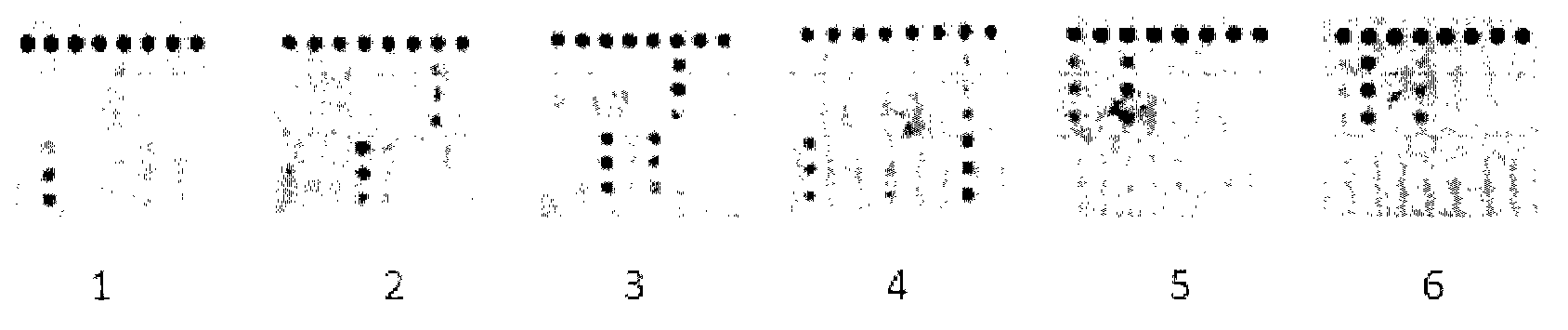
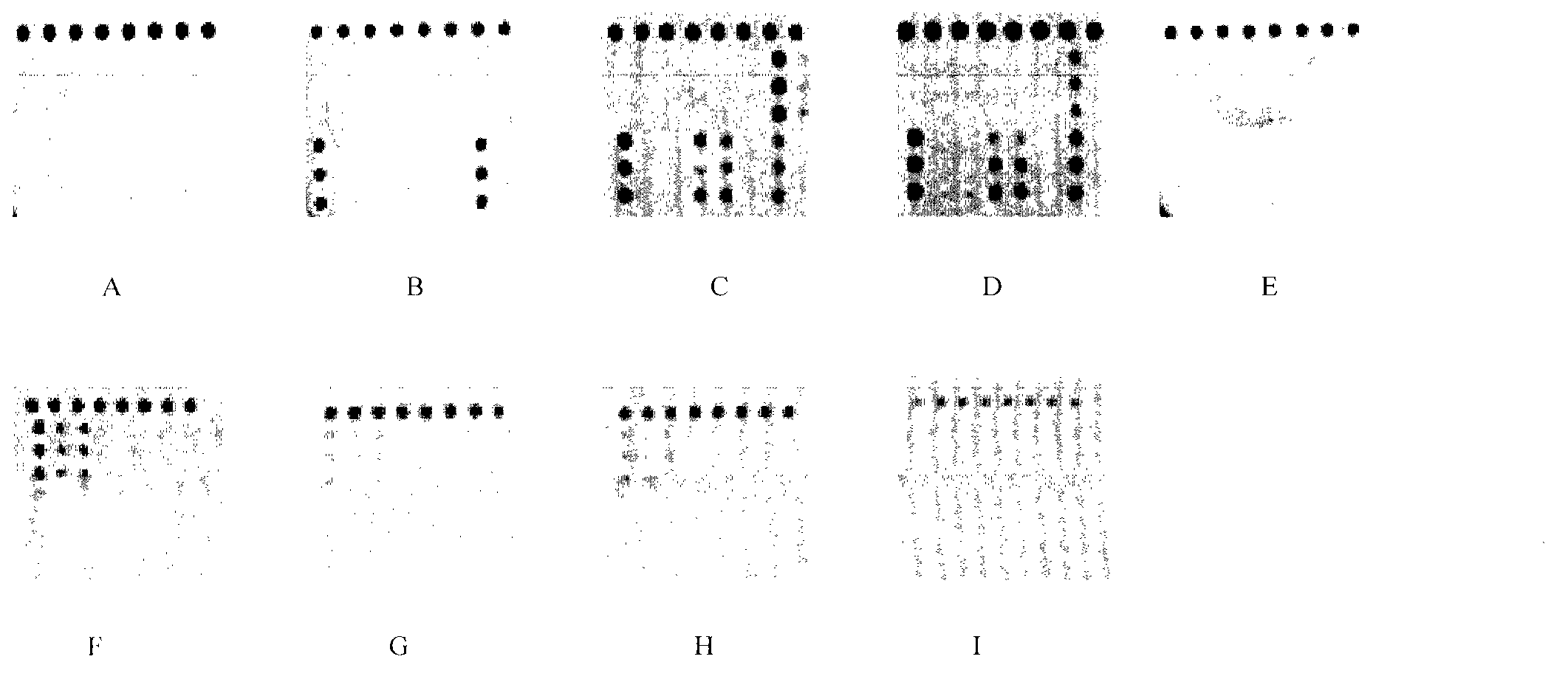
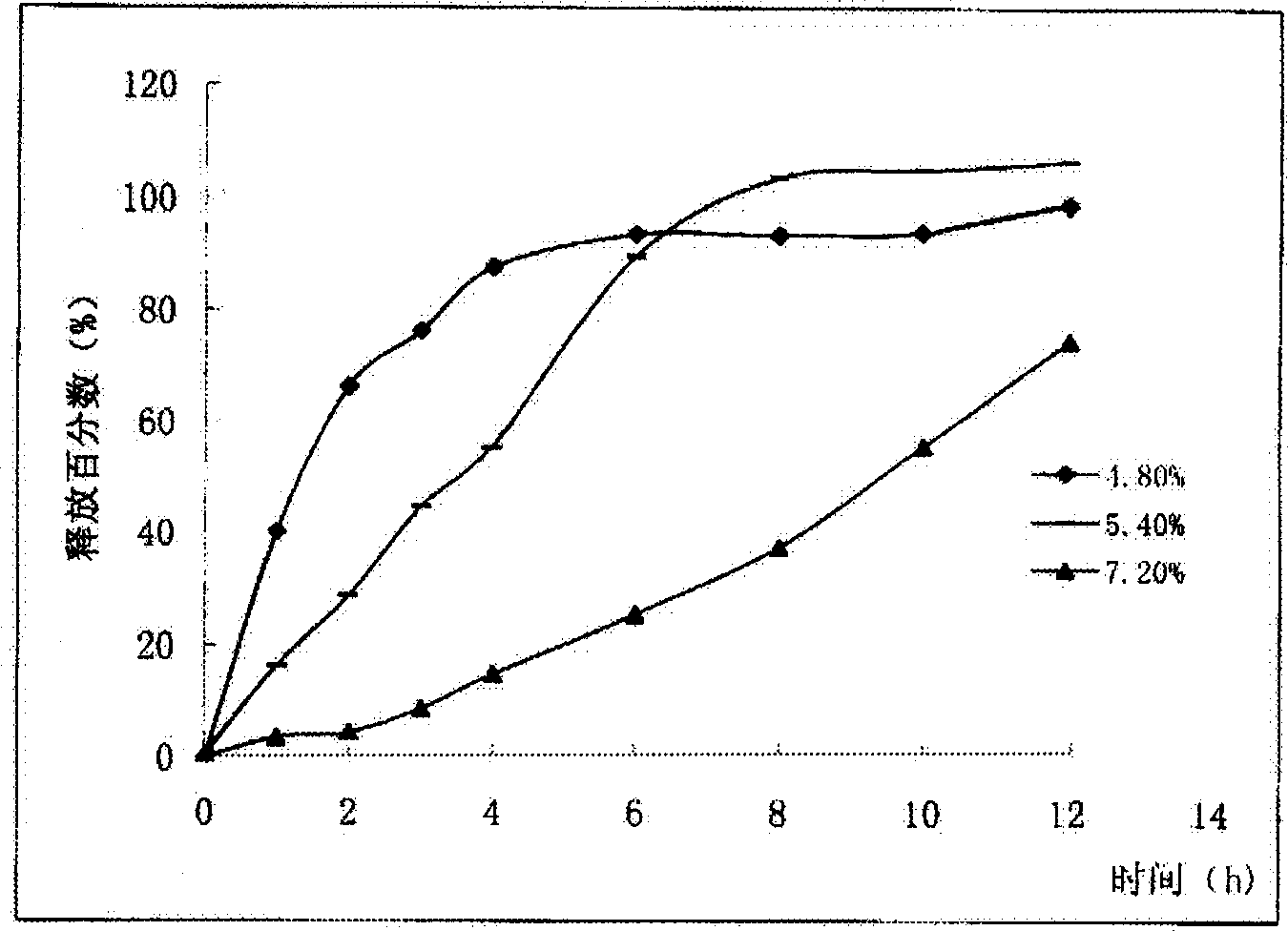
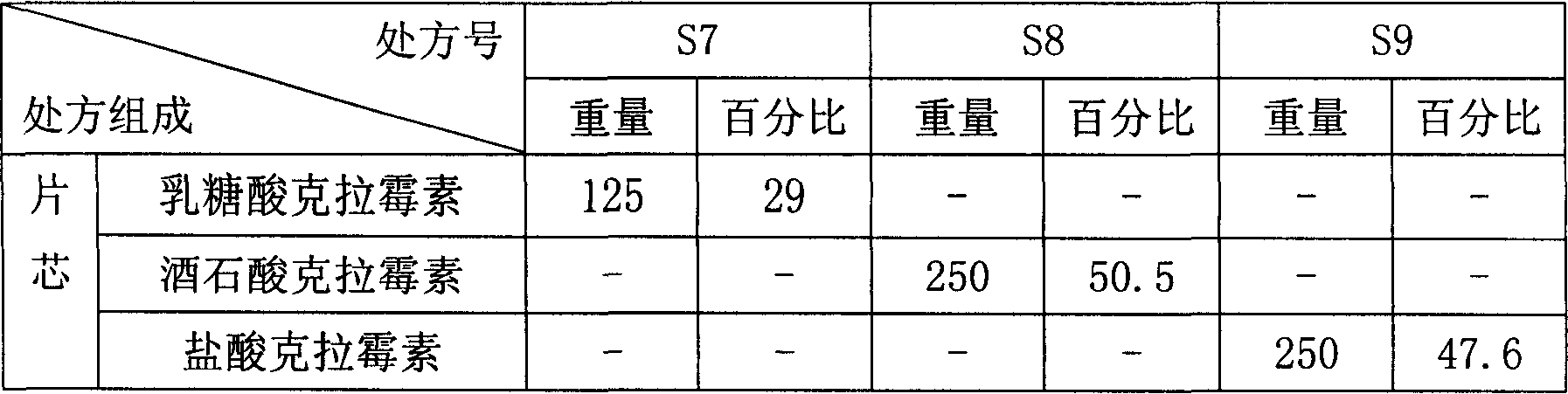
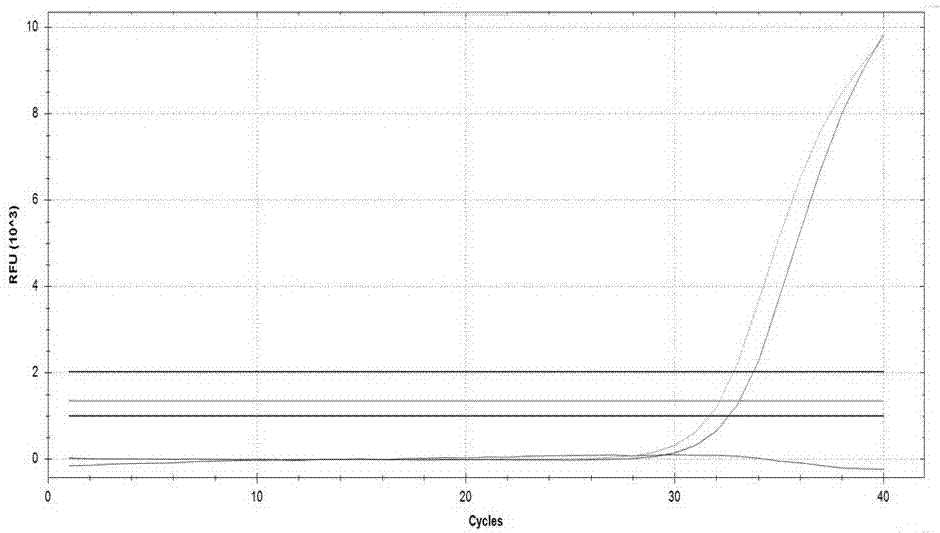
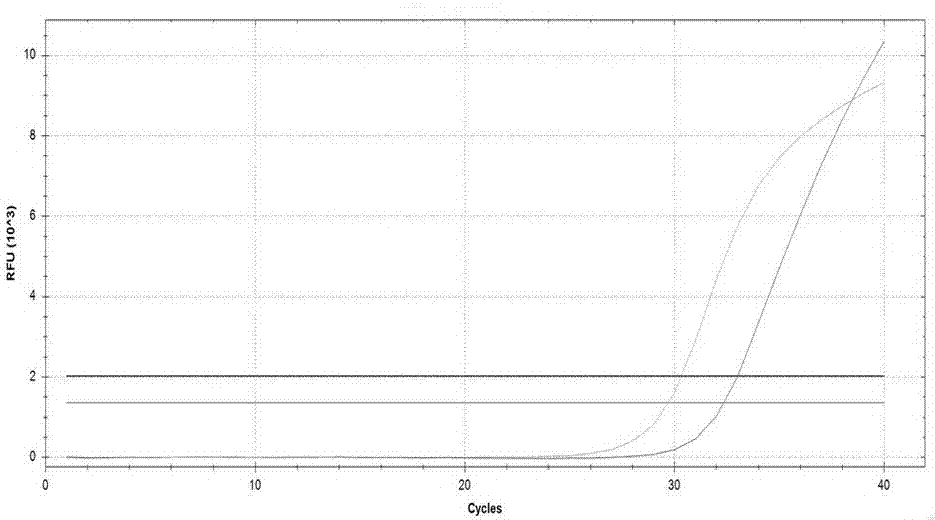
Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection
ActiveCN107243008AReduce healingLong treatment cycleAntibacterial agentsOrganic active ingredientsMycobacterium InfectionsMycobacterium abscessus Infections
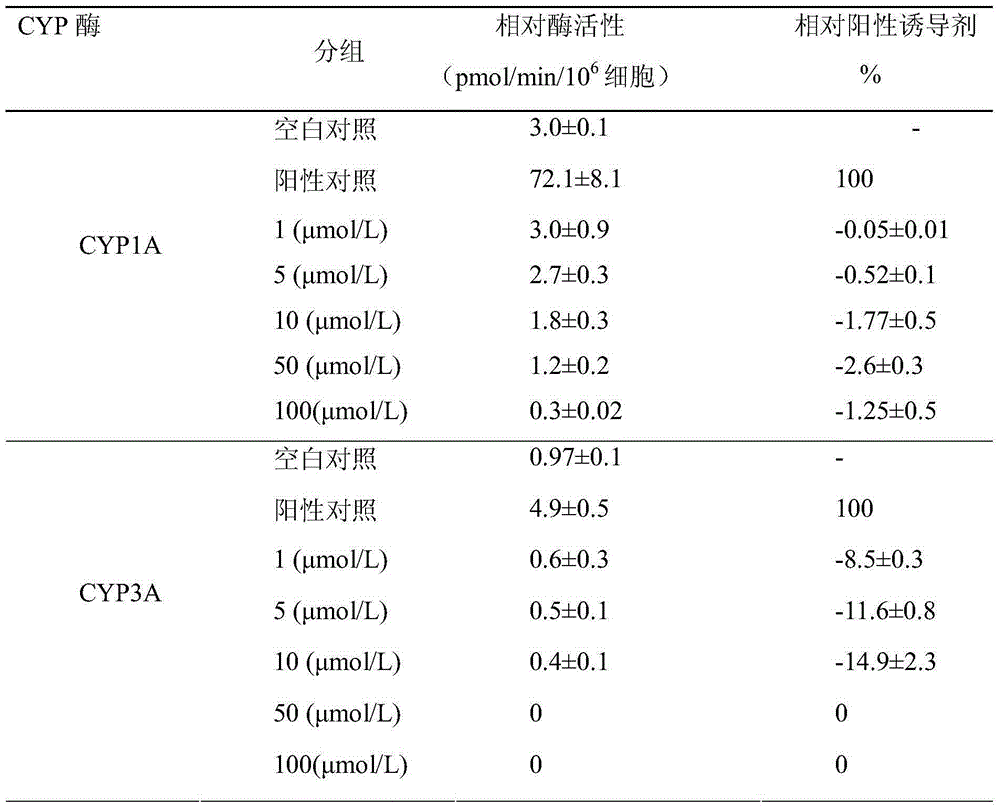
The invention discloses novel application of a pyrazolo[1, 5-a]pyridine compound and a composition for treatment of Mycobacterium abscessus infection. The pyrazolo[1, 5-a]pyridine compound can be used for preparation of an anti-Mycobacterium abscessus synergist of clofazimine, combined use of the two can reach a synergistic effect, and a low concentration pyrazolo[1, 5-a]pyridine compound can reinforce the anti-Mycobacterium abscessus effect of clofazimine. The active components of the composition for treatment of Mycobacterium abscessus infection provided by the invention contain clofazimine and / or macrolide antibiotic, and the pyrazolo[1, 5-a]pyridine compound serving as a synergist. The composition has the characteristics of rapid effect, no relapse after treatment, and efficacy significantly superior to the combined use of clarithromycin and amikacin, and overcomes the shortcomings of easy generation of induced drug-resistance and poor long-term treatment effect in existing macrolide antibiotic clinical treatment.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI +1
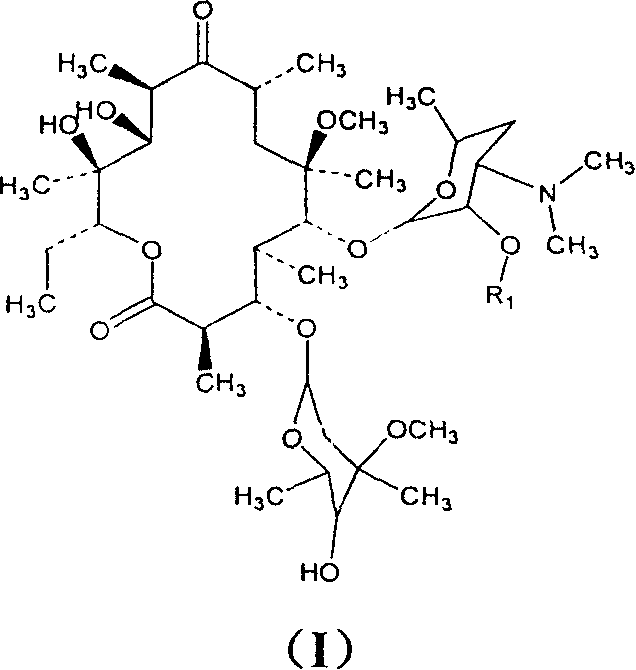
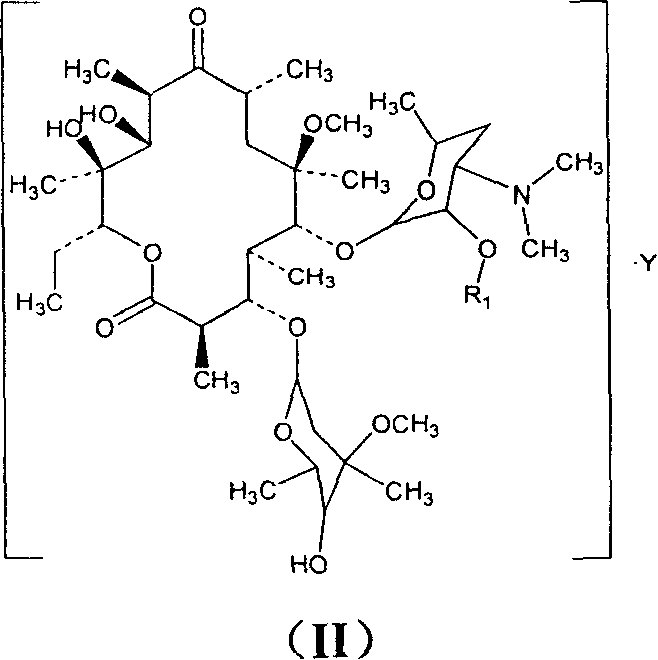
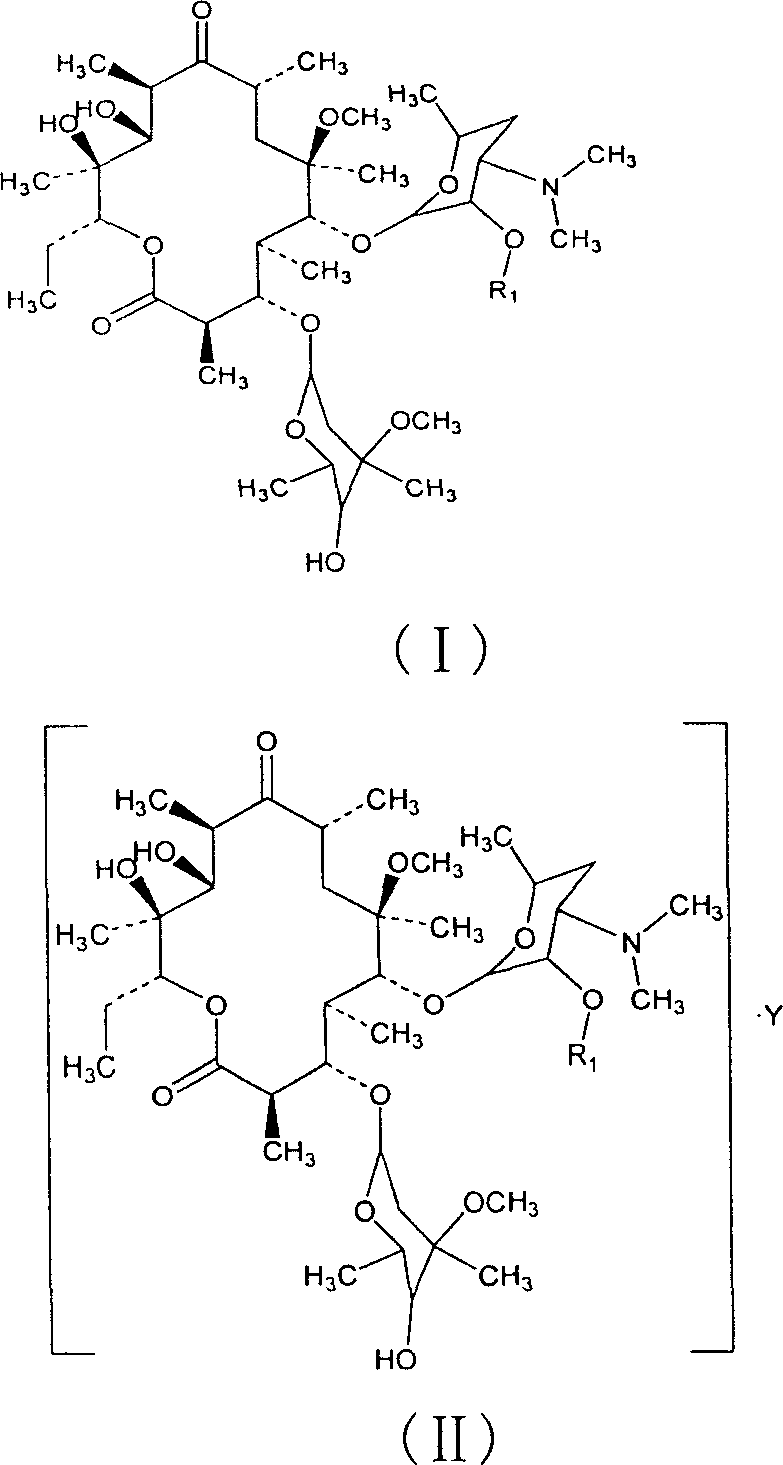
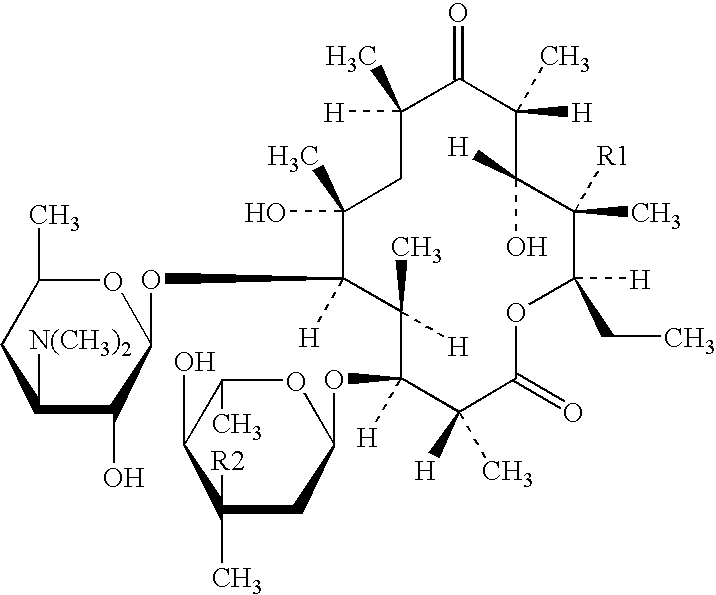
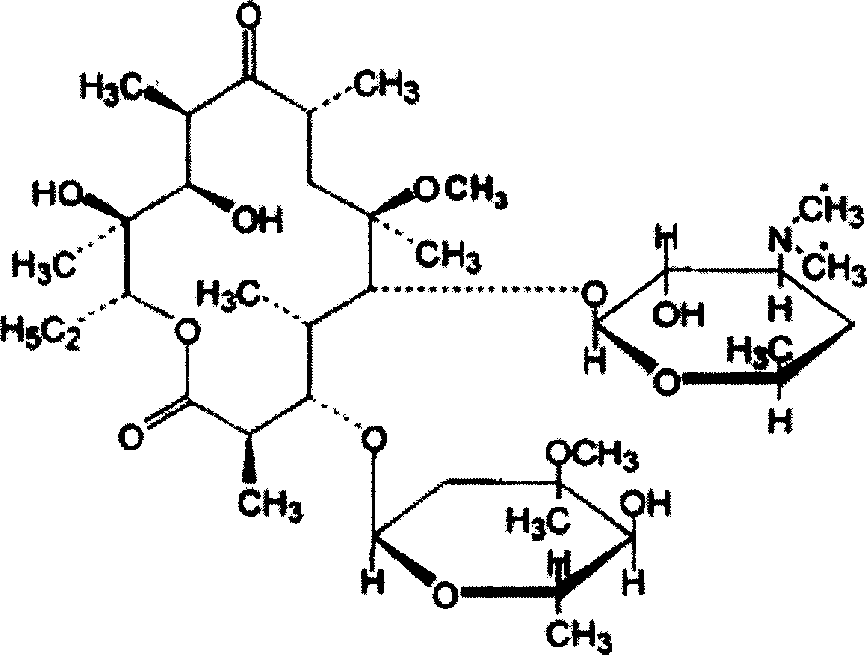
Clarithromycin derivatives and its preparation process and pharmaceutical application
The invention relates to Clarithromycin derivatives, the preparing process and medicinal use, the Clarithromycin derivatives have general formulas of (I), (II), these compounds and the medicinal compositions of them can be used for treating infectious diseases caused by sensitive organisms and other pathogenic agents.
Owner:济南思创生物技术有限公司
Clarithromycin injection
InactiveCN1452977AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsActive componentWater soluble
The present invention relates to one kind of Clarithromycin injection, and is especially one bacteria-free powder and solution of water soluble Clarithromycin salt for injection. The injection of the present invention has water soluble Clarithromycin salt, preferably hydrochloride and tartrate of Clarithromycin, as its active component. The present invention also provides the preparation processof bacteria-free powder and solution of water soluble Clarithromycin salt for injection.
Owner:GUANGZHOU PUIS PHARMA FACTORY
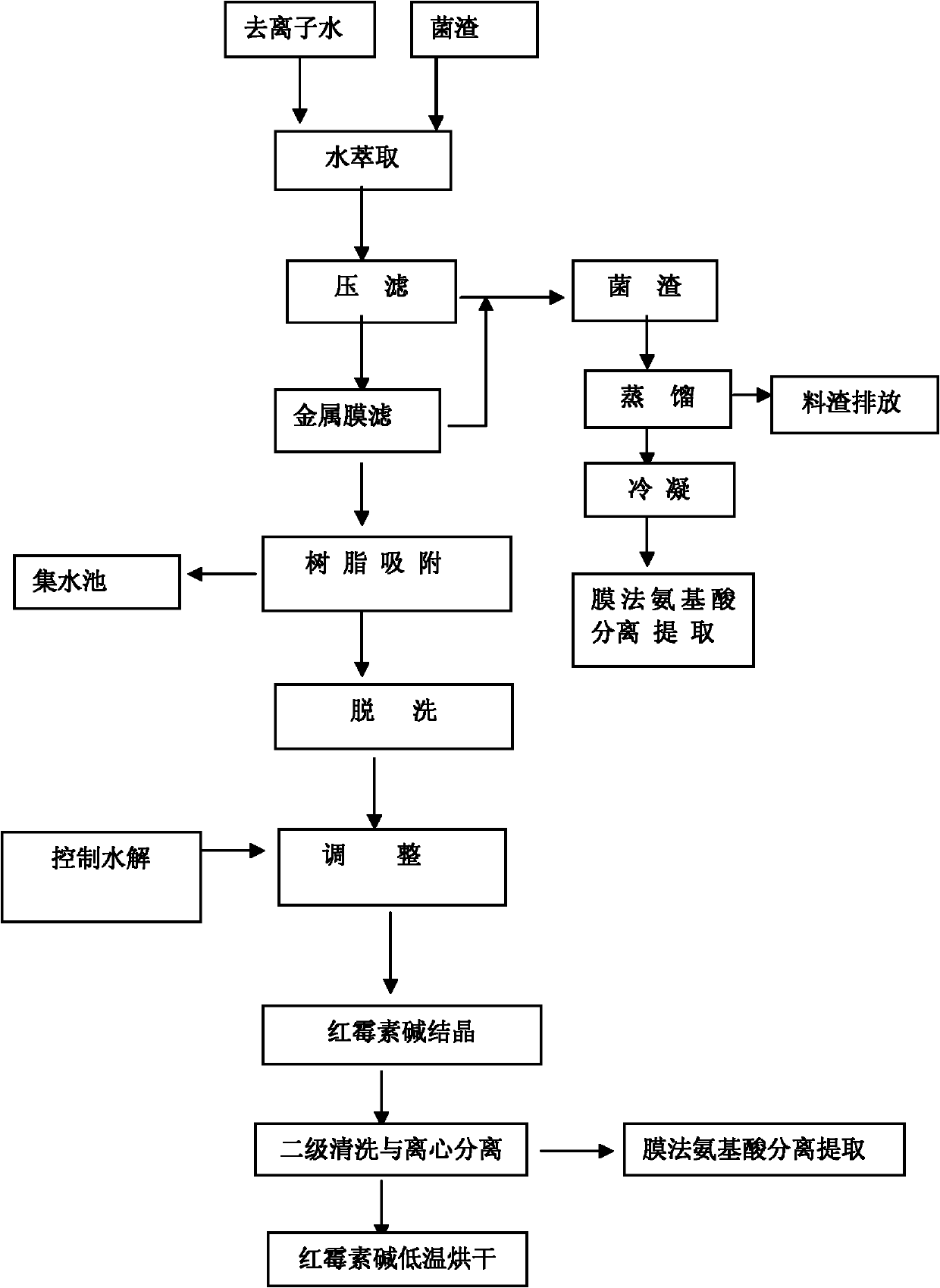
Technology for extracting amino acids from residual medicine dregs generated in production of erythromycin
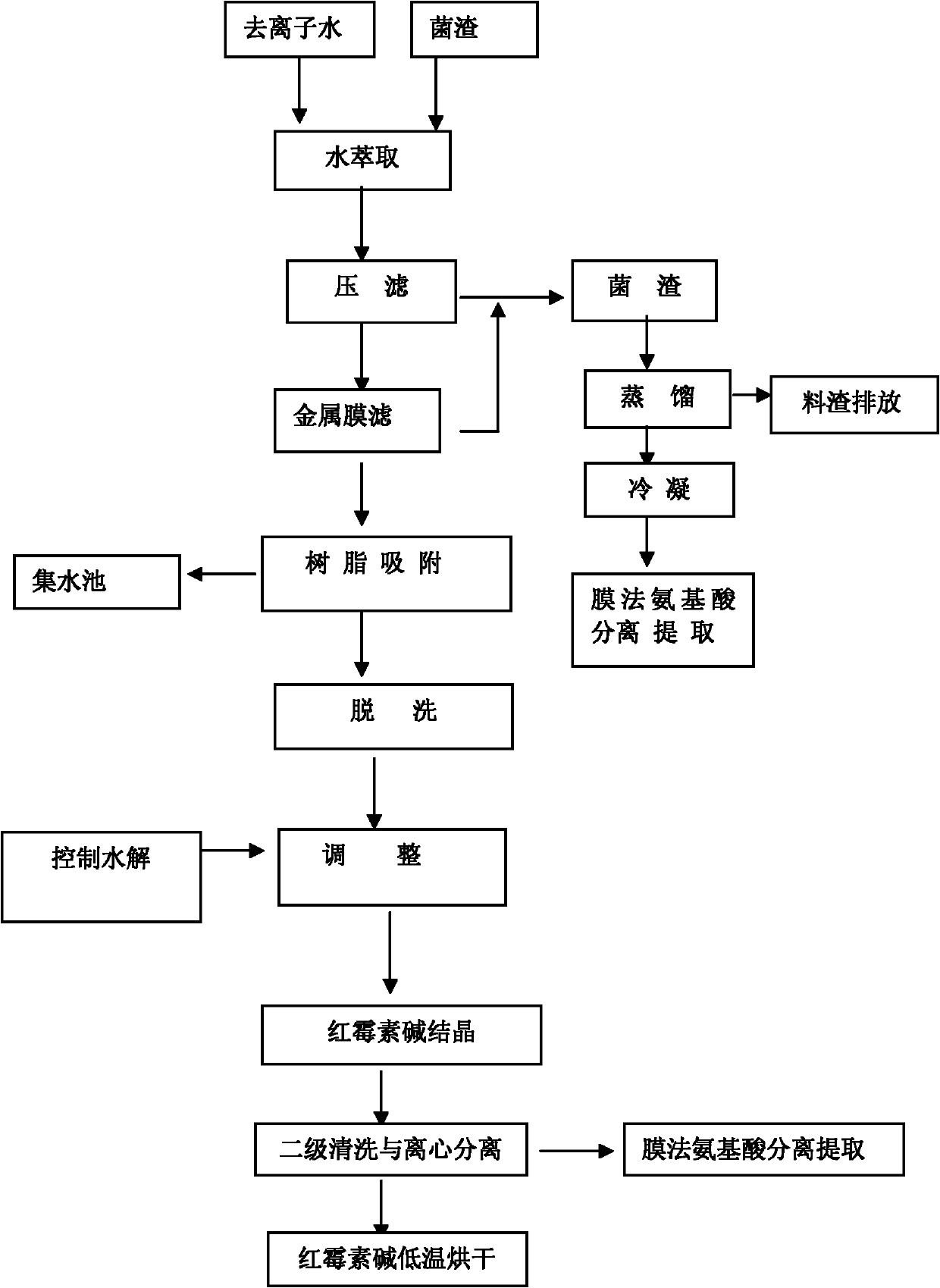
ActiveCN102167669ARealize harmless useAvoid pollutionSugar derivativesOrganic compound preparationDistillationClarithromycin
The invention provides a technology for extracting amino acids from residual medicine dregs generated in the production of erythromycin. The technology comprises the following steps of: extracting the residual medicine dregs of erythromycin with deionized water and then carrying out filter pressing; distilling the solid obtained through filter pressing a lower temperature between150 and 200 DEG C, extracting amino acids from gas generated through distillation through a membrane method; firstly filtering filtrate obtained through filter pressing by using a metal membrane, mixing the filtered solid with the solid obtained through filter pressing and performing the above treatments, absorbing the filtrate obtained through metal membrane filtering by using ion exchange resin, eluting with alcoholic solvent and finally precipitating out and crystallizing clarithromycin by a control hydrolysis method, cleaning and centrifugalizing, performing vacuum drying on the solid obtained through centrifugalization to prepare clarithromycin; and separating and extracting amino acids from the liquid phase through the membrane method. By adopting the technology, the harmless application of fungus dregs can be realized and at least 10 tons of pure amino acid products can be extracted from 1000 tons of erythromycin fungus dregs; and the erythromycin content of the discharged waste treated by the technology provided by the invention is less than 1ppm, which completely meets the national discharge standard.
Owner:四川金本科技有限公司
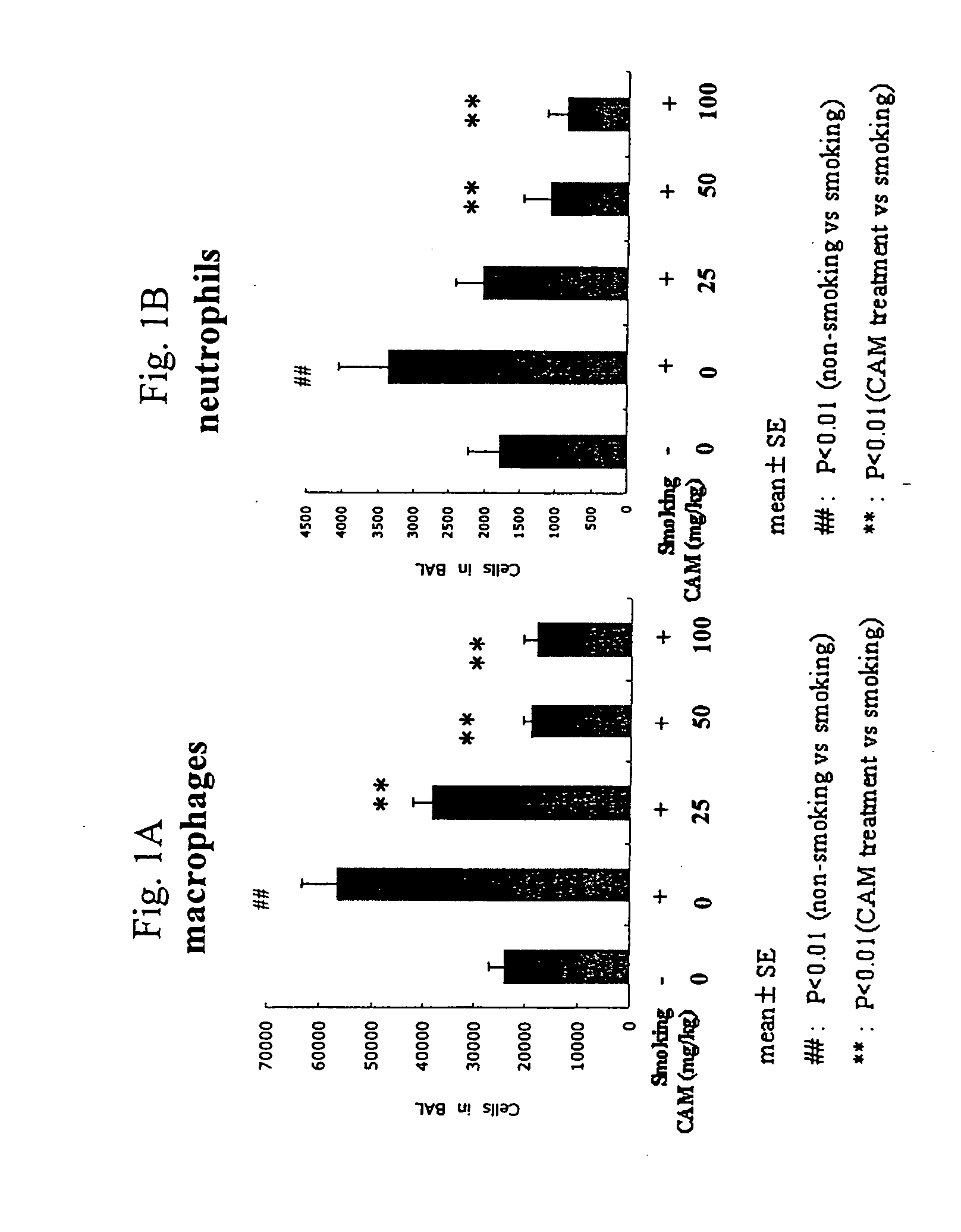
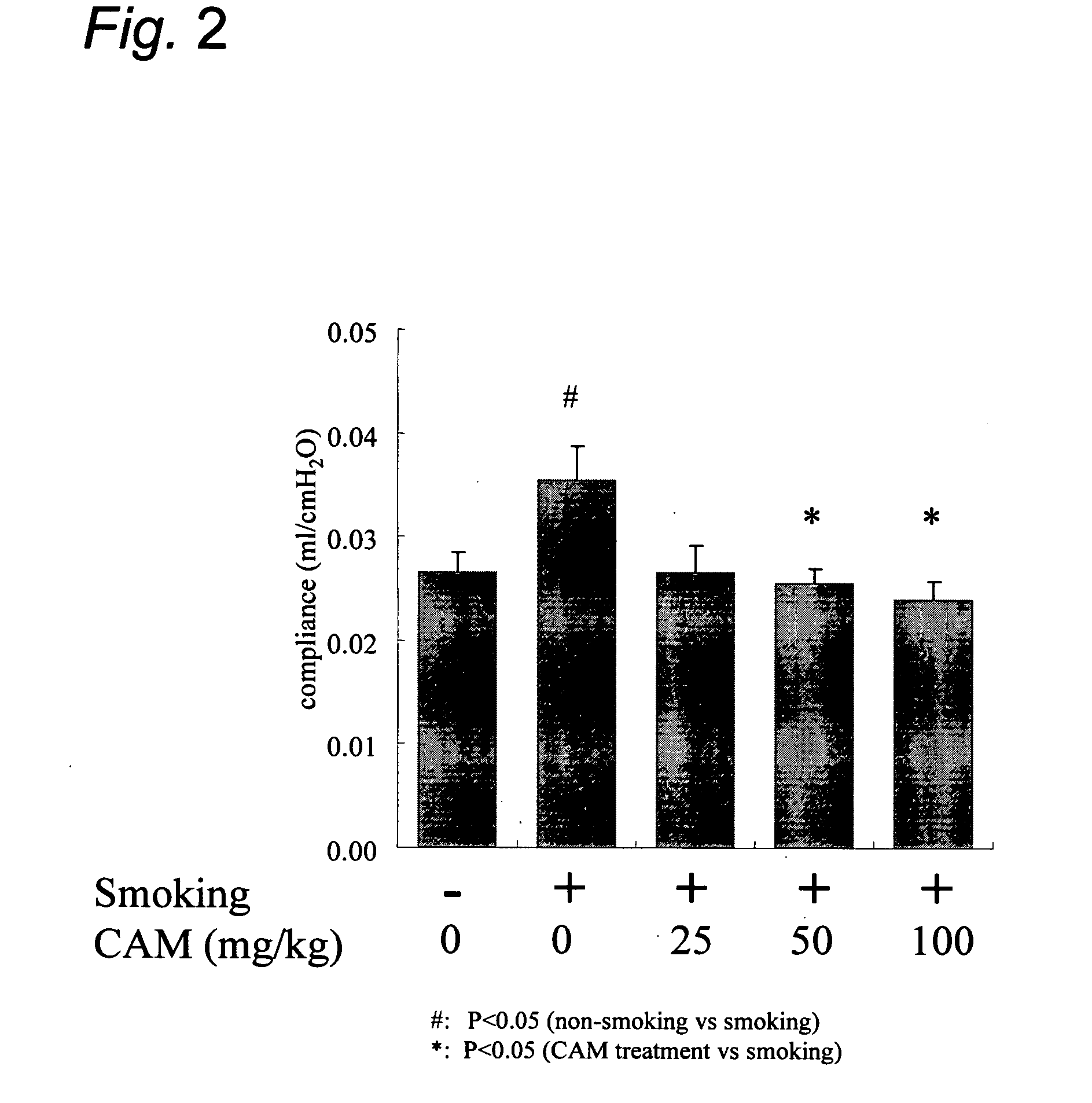
Clarithromycin or a salt thereof for the treatment or prevention of pulmonary disorders caused by the destruction of pulmonary alveoli
InactiveUS20060293261A1Effective therapyBiocideCarbohydrate active ingredientsClarithromycinAir pollution
For the purpose of treatment and / or prevention of pulmonary disorders caused by the destruction of pulmonary alveoli resulting from smoking, air pollution, noxious gas, etc., there are provided, among others, a method of administering clarithromycin or a salt thereof to a mammal and a pharmaceutical composition comprising clarithromycin or a salt thereof.
Owner:TAISHO PHARMACEUTICAL CO LTD
Clamycin medicinal preparation
InactiveCN1475220AImprove stabilityLow costOrganic active ingredientsSenses disorderPharmaceutical drugClarithromycin
Owner:GUANGZHOU PUIS PHARMA FACTORY
Teste masking pharmaceutical composition
InactiveUS20040175418A1Great tasteAvoid premature releaseAntibacterial agentsBiocideOral medicationClarithromycin
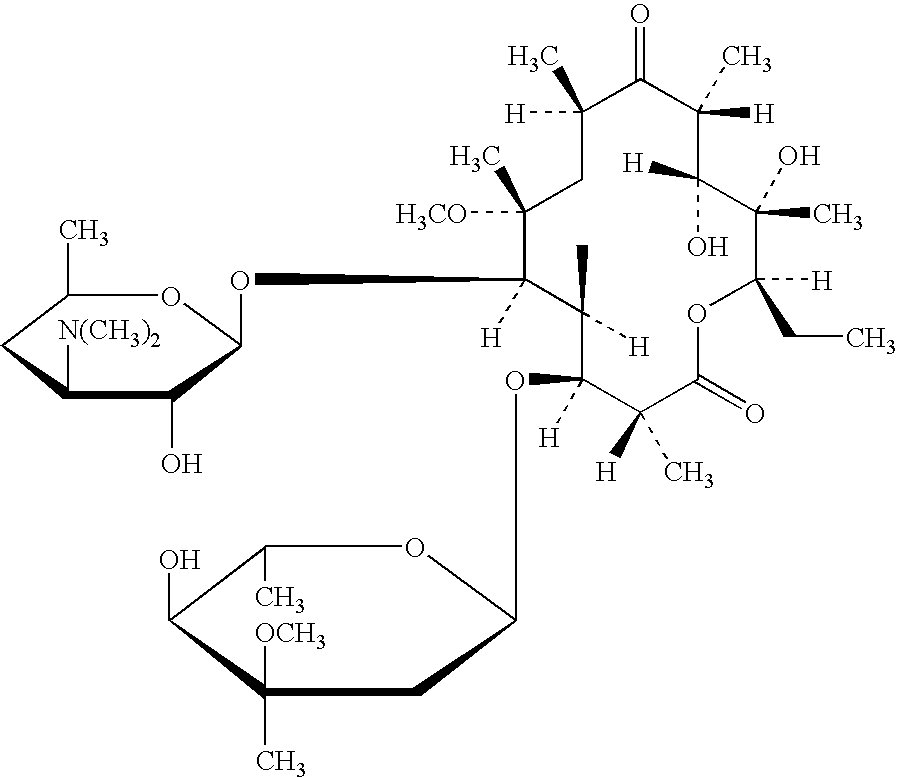
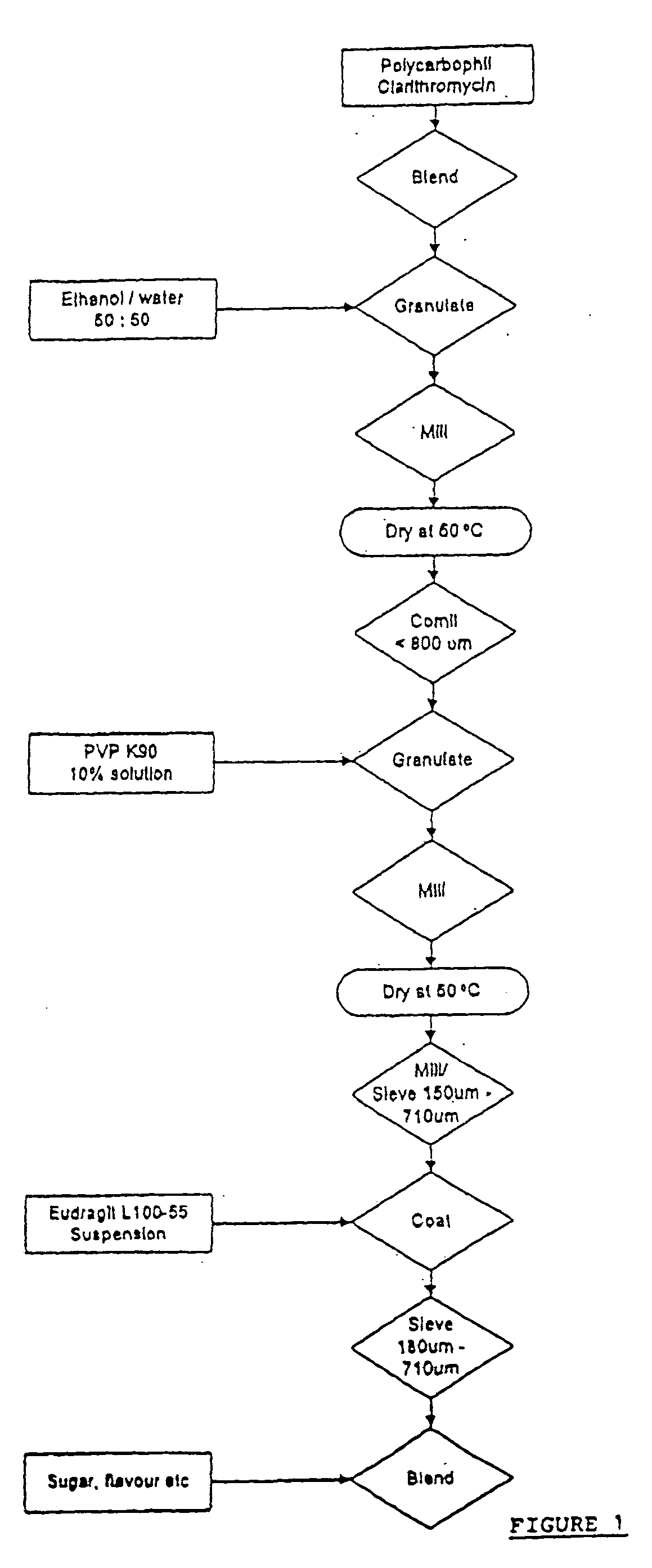
The invention describes a composition suitable for oral administration comprising and antibiotic macrolide and a polycarbophil. The antibiotic macrolide is preferably clarithromycin. The polycarbophil is reported to have surprising taste-masking properties in combination with the antibiotic and acts by inhibiting the undesirable release of the antibiotic component in the mouth or stomach. Several methods of preparing granules of the antibiotic macrolide said polycarbophil are also described.
Owner:PACIFIC PHARMA LTD
Method for preparing clarithromycin granule without bitter taste
InactiveCN101502492AImprove flexibilityGood flexibility and good film coating, which makes it have good crack resistanceAntibacterial agentsOrganic active ingredientsPrillOral medication
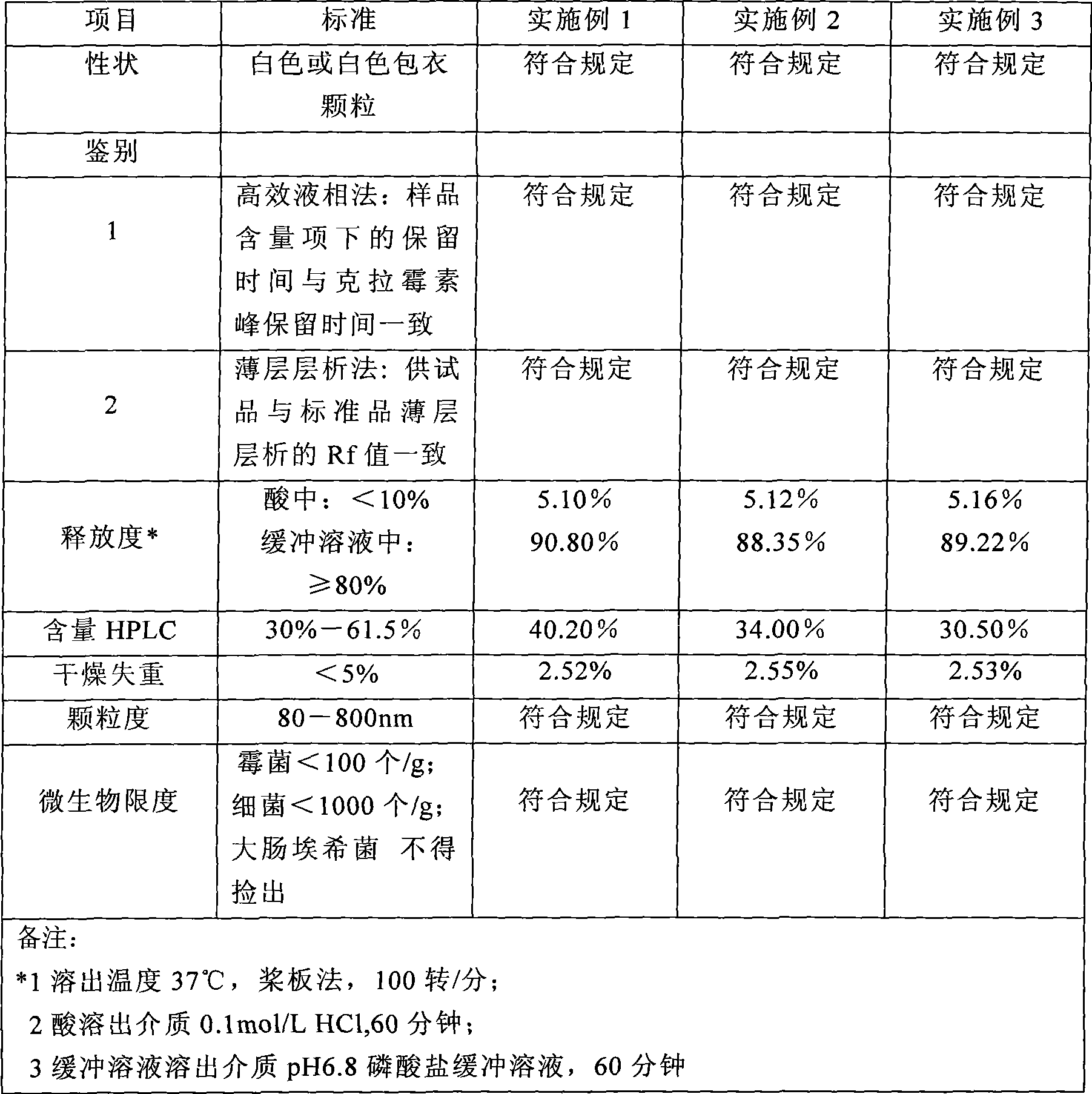
The invention relates to a method for preparing clarithromycin debitterized granules, which comprises the following steps: (1) preparing solution of coating: preparing solutions A, B and C respectively, then, mixing the solutions evenly, and adding pure water for later use; and (2) coating: coating 2,000g of 60 to 120-mesh powdery clarithromycin with the coating solution with the specific coating parameters being fluidized coating / drying; preheating the coating system to the temperature of 38 DEG C, and spraying the coating system with the inlet temperature being 36 to 60 DEG C, the atomization pressure being 0.08 to 0.45 MPa and the flow rate of the spray solution being 2 to 120 ml / min; and proceeding with drying for 20min and collecting pellets with the pellet diameter being 80 to 800 Mum. The invention has the advantages that the materials are easily obtainable, the cost is low and the preparation method is simple and environment-friendly, therefore, the method is favorable for industrialized production; moreover, the prepared granules solve the problem that the bitter taste is insufferable during the oral administration and make the forms of the oral preparation richer and the range of those who take the granules wider, and the granules are particularly suitable for children to take.
Owner:上海微丸医药开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection](https://images-eureka.patsnap.com/patent_img/6dedd4ce-d271-4b08-8f49-f5b5c4194c5a/HDA0001269169880000011.png)
![Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection](https://images-eureka.patsnap.com/patent_img/6dedd4ce-d271-4b08-8f49-f5b5c4194c5a/HDA0001269169880000012.png)
![Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection Novel application of pyrazolo[1, 5-a]pyridine compound and composition for treatment of Mycobacterium abscessus infection](https://images-eureka.patsnap.com/patent_img/6dedd4ce-d271-4b08-8f49-f5b5c4194c5a/HDA0001269169880000021.png)