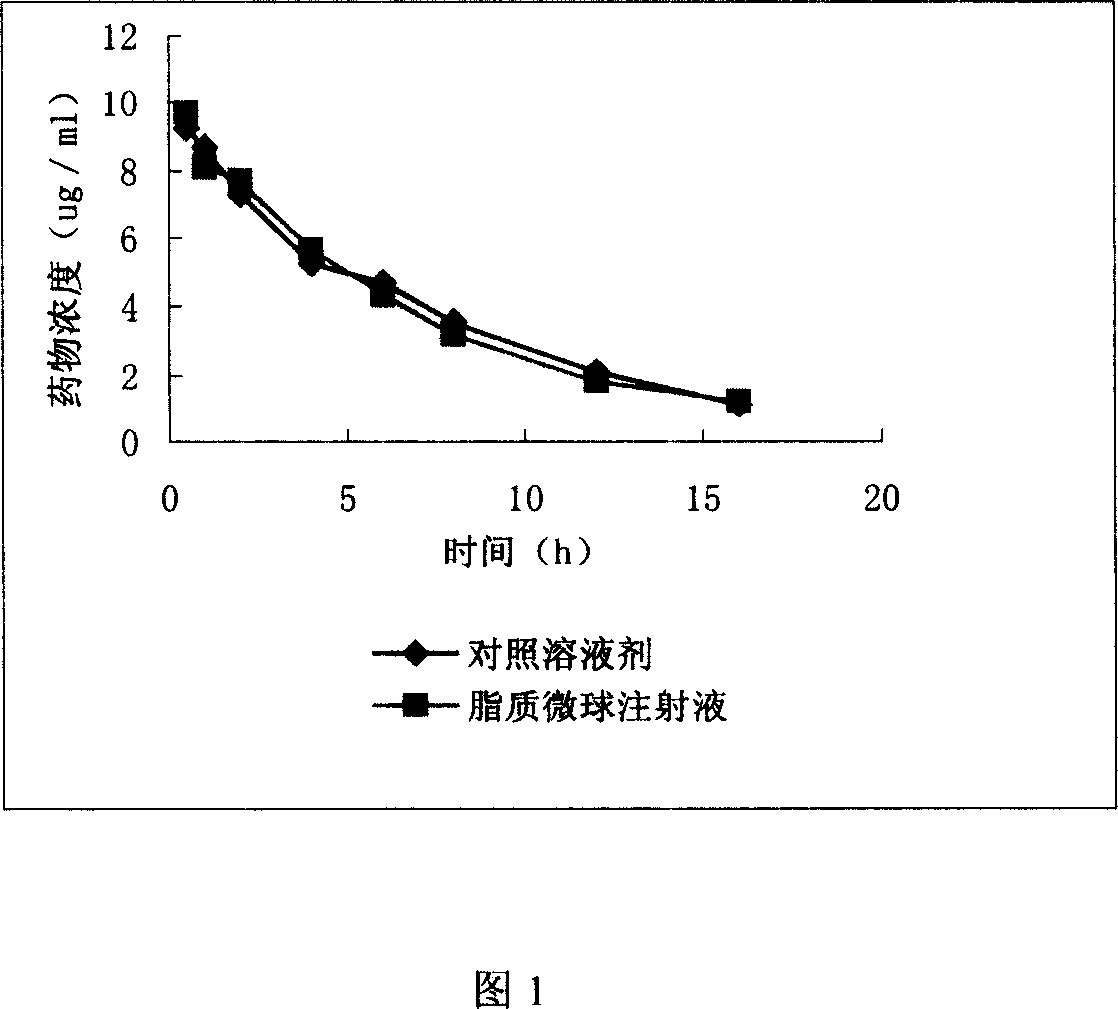
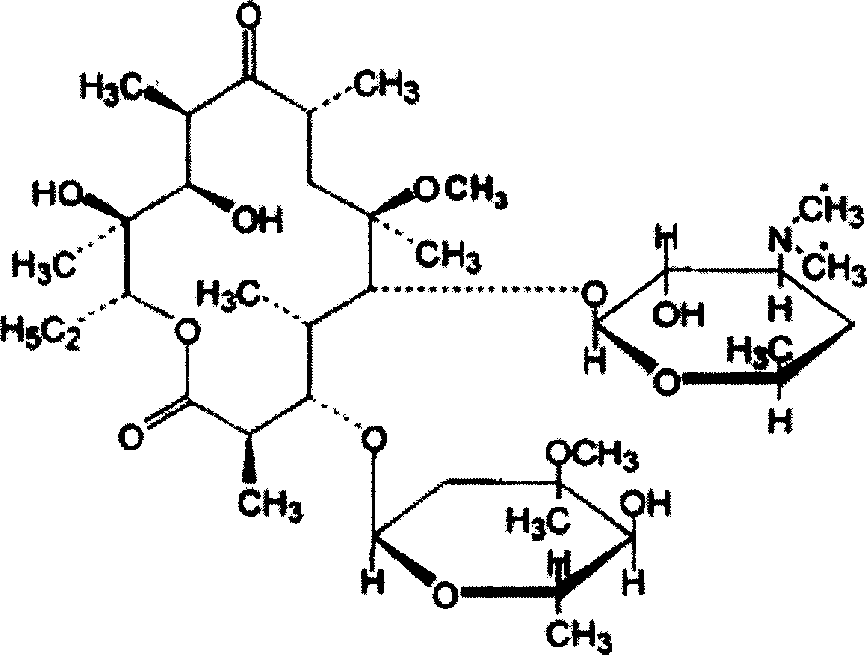
Clarithromycin liposome microsphere injection and its prepn. method
A technology of clarithromycin and lipid microspheres, applied in the field of medicine, can solve problems such as impossible preparation, and achieve the effects of reducing toxicity and irritation, providing drug efficacy and increasing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: a clarithromycin lipid microsphere injection, which comprises the following components:
[0041] Castor Oil for Injection 10g
[0042] Clarithromycin 0.5g
[0043] Lecithin 3g
[0044] Glycerin 2.5g
[0045] Caproic acid 0.6g
[0046] Water for injection 100ml
[0047] Its preparation method is as follows:
[0048] 1) Dissolve 0.5g of clarithromycin in 0.6g of caproic acid, mix with 10g of castor oil for injection to form an oil phase, stir magnetically and heat to 60°C;
[0049] 2) Disperse 3g of lecithin and 2.5g of glycerin in 80ml of water for injection, magnetically stir to disperse evenly, and heat to 60°C as the water phase;
[0050] 3) Add the oil phase to the water phase under magnetic stirring, and continue stirring for 10 minutes after the addition to obtain colostrum;
[0051] 4) Homogenizing the prepared colostrum at 30,000 rpm for 10 minutes, repeating it 3 times to obtain a milky white emulsion;
[0052] 5) add remaining 20ml water f...
Embodiment 2
[0053] Embodiment 2: a clarithromycin lipid microsphere injection, which comprises the following components:
[0054] Corn oil for injection 33g
[0055] Clarithromycin 0.7g
[0056] Soy Lecithin 0.3g
[0057] Glycerin 8.8g
[0058] Oleic acid 3g
[0059] Water for injection 64ml
[0060] Its preparation method is as follows:
[0061] 1) Dissolve 0.7g of clarithromycin in 3g of oleic acid, then mix with 33g of corn oil for injection to form an oil phase, magnetically stir and heat to 50°C;
[0062] 2) Disperse 0.3g soybean lecithin and 8.8g glycerin in 50ml water for injection, stir magnetically to disperse evenly, and heat to 50°C as the water phase;
[0063] 3) Add the oil phase to the water phase under magnetic stirring, and continue stirring for 15 minutes after the addition to obtain colostrum;
[0064] 4) Homogenizing the prepared colostrum at 20,000 rpm for 15 minutes, repeating it 3 times to obtain a milky white emulsion;
[0065] 5) add remaining 14ml water fo...
Embodiment 3
[0066] Embodiment 3: a clarithromycin lipid microsphere injection, which comprises the following components:
[0067] C7-C9 Fatty Acid Triglycerides 10g
[0068] Clarithromycin 1g
[0069] Tween 80 3g
[0070] Glycerin 5g
[0071] Pelargonic acid 2g
[0072] Water for injection 79ml
[0073] Its preparation method is as follows:
[0074] 1) Dissolve 1 g of clarithromycin in 2 g of nonanoic acid, then mix it with 10 g of C7-C9 fatty acid triglyceride to form an oil phase, magnetically stir and heat to 80°C;
[0075] 2) Disperse 3g of Tween 80 and 5g of glycerin in 60ml of water for injection, stir magnetically to disperse evenly, and heat to 80°C as the water phase;
[0076] 3) Add the oil phase to the water phase under magnetic stirring, and continue stirring for 12 minutes after the addition to obtain colostrum;
[0077] 4) Homogenizing the prepared colostrum at 20,000 rpm for 15 minutes, repeating it 3 times to obtain a milky white emulsion;
[0078] 5) add remaining...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com