Silanization reaction catalyst
A silylation and catalyst technology, which is applied in the field of preparation of macrolide drug intermediates and other compounds, can solve the problems of high dosage of hexamethyldisilazane, no introduction of test data, and rising cost of auxiliary materials, etc. The effect of shortened reaction time, low production cost and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
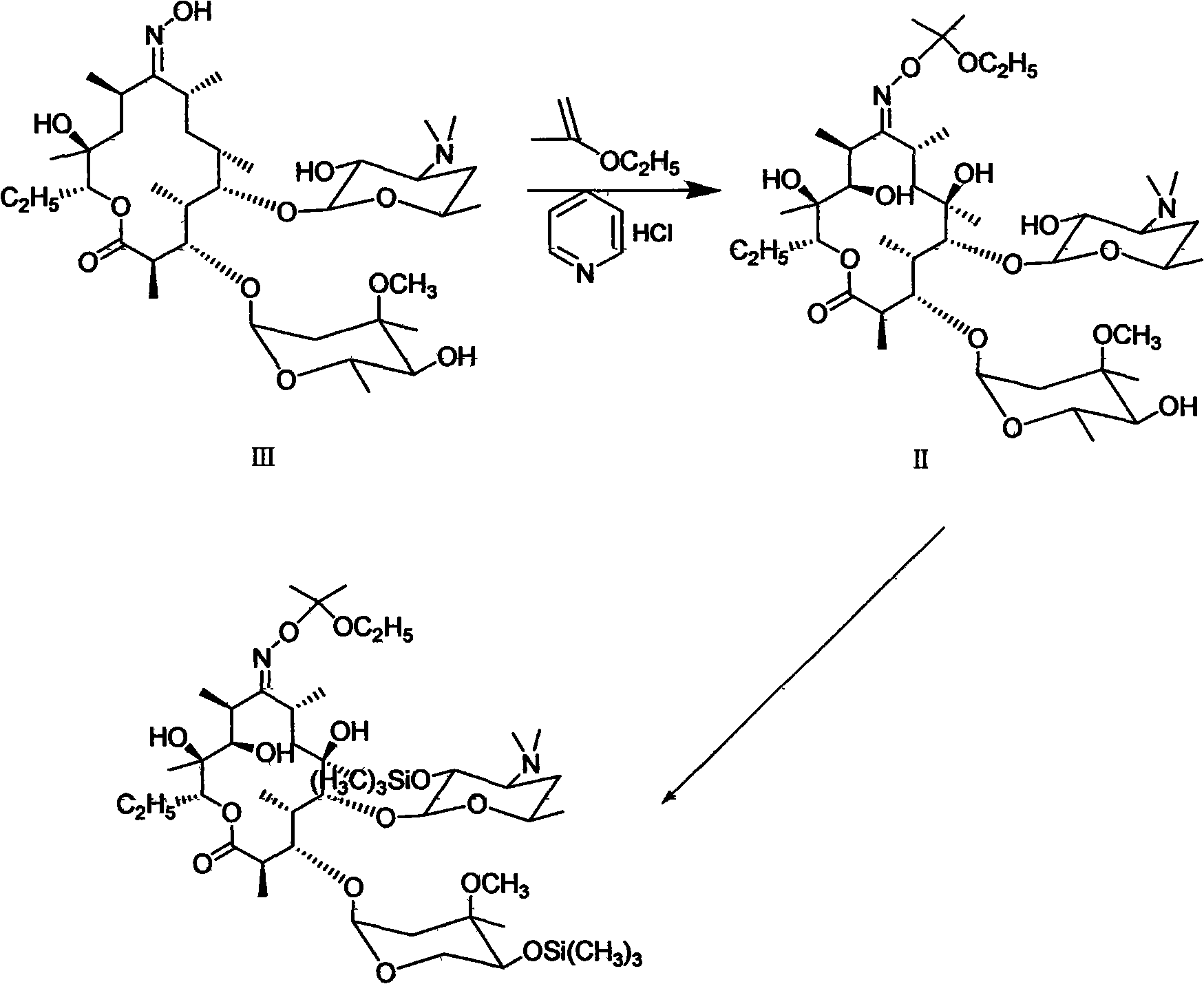
[0024] Step 1: add 20.0 grams (0.0267mol) erythromycin A oxime, 200ml dichloromethane in the 500 milliliter four-necked reaction flask that stirrer, thermometer are equipped with, add 5.6 grams (0.0487mol) pyridine hydrochloride and 5.3 grams under stirring (0.0616mol) 2-ethoxypropene, reacted at 20-25°C for 15 minutes.
[0025] Step 2: Following step 1, add 5.7 g (0.0354 mol) of hexamethyldisilazane and react at 20-30° C. for 3 hours. After the reaction is over, add 60 ml of water to terminate, then adjust the pH to 9-10 with 20% sodium hydroxide solution, then wash with 60 ml of saturated brine and 60 ml of water successively, and concentrate and crystallize under reduced pressure to obtain 26.6 g, with a content of 75.8%. , of which the unsilanized content is 11.6%. Acetone was refined to obtain 21.2 grams of refined product, with a content of 92.6%, and a molar yield of 81.1%.
Embodiment 2
[0027] Step 1: Same as Step 1 in Embodiment 1.
[0028] Step 2: Following step 1, add 5.7 g (0.0354 mol) of hexamethyldisilazane, react for 5 minutes, add 0.8 g (0.005 mol) of pyridinium hydrobromide, and react at 20-30° C. for 2 hours. After the reaction is over, add 60 ml of water to terminate, then adjust the pH to 9-10 with sodium hydroxide solution, then wash with 60 ml of saturated brine and 60 ml of water successively, and concentrate and crystallize under reduced pressure to obtain 28.9 g, with a main content of 86.3%. , the non-silanized impurity was 0.7%. Acetone is refined to obtain 24.9 grams of fine product, the content is 96.0%, and the molar yield reaches 95.5%.
Embodiment 3
[0030] Step 1: Same as Step 1 in Embodiment 1.
[0031] Step 2: Following step 1, add 5.7 g (0.0354 mol) of hexamethyldisilazane, react for 5 minutes, add 0.04 g (0.00027 mol) of pyridinium hydrobromide, and react at 20-30° C. for 10 hours. After the reaction was finished, add 60 ml of water to terminate, then adjust the pH to 9-10 with sodium hydroxide solution, then wash with 60 ml of saturated brine and 60 ml of water successively, and distill and concentrate under reduced pressure to obtain 27.7 g of crystals with a main content of 84.3%. , the unsilanized impurity was 2.9%. Acetone is refined to obtain 23.6 grams of fine product, the content is 95.4%, and the molar yield reaches 90.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com