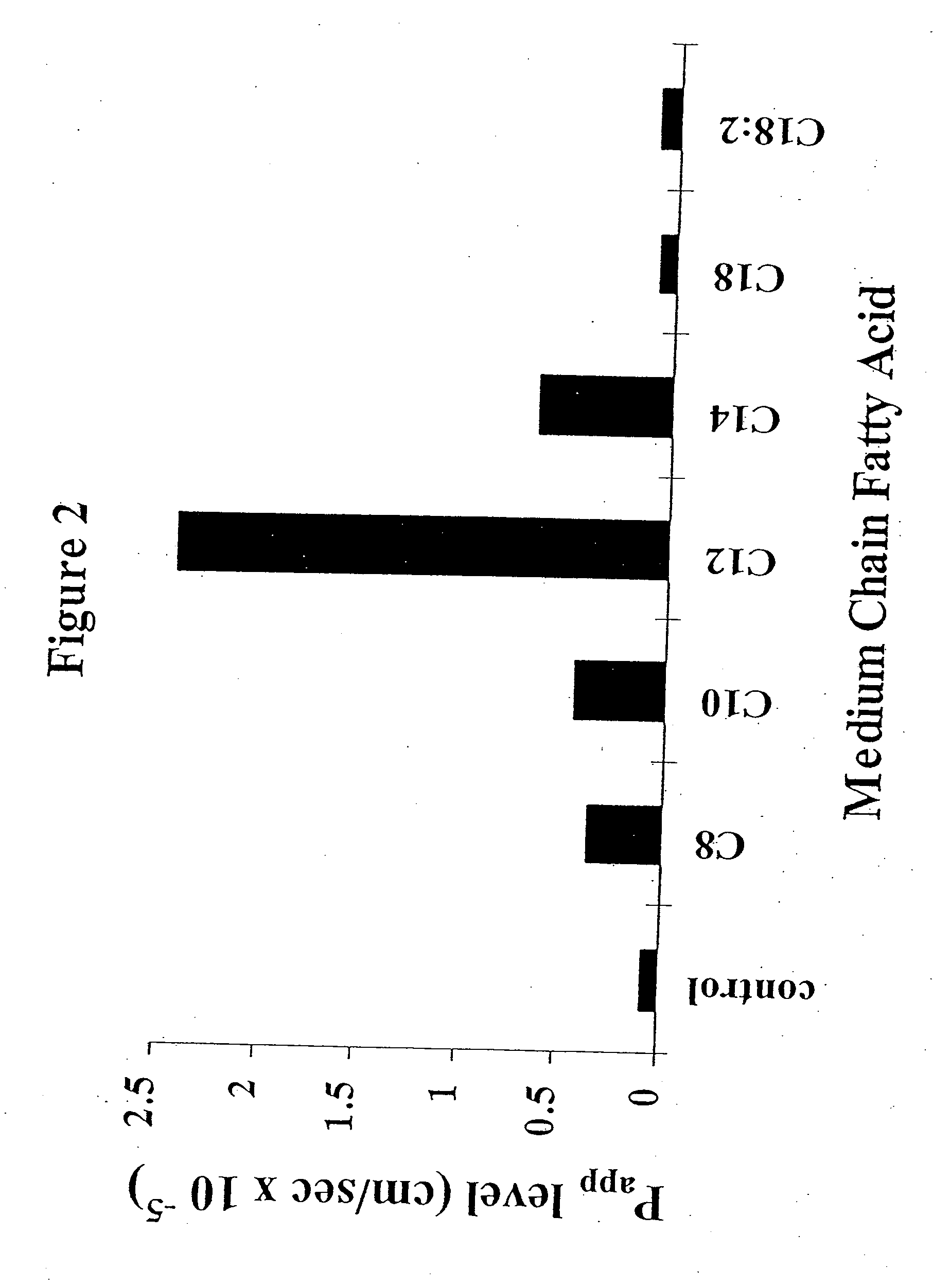
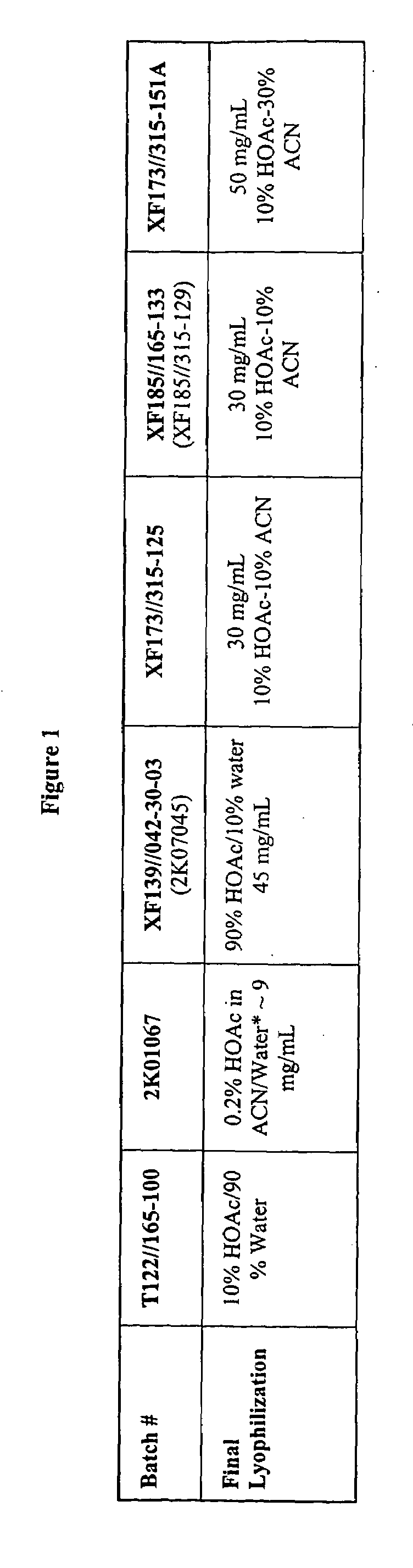
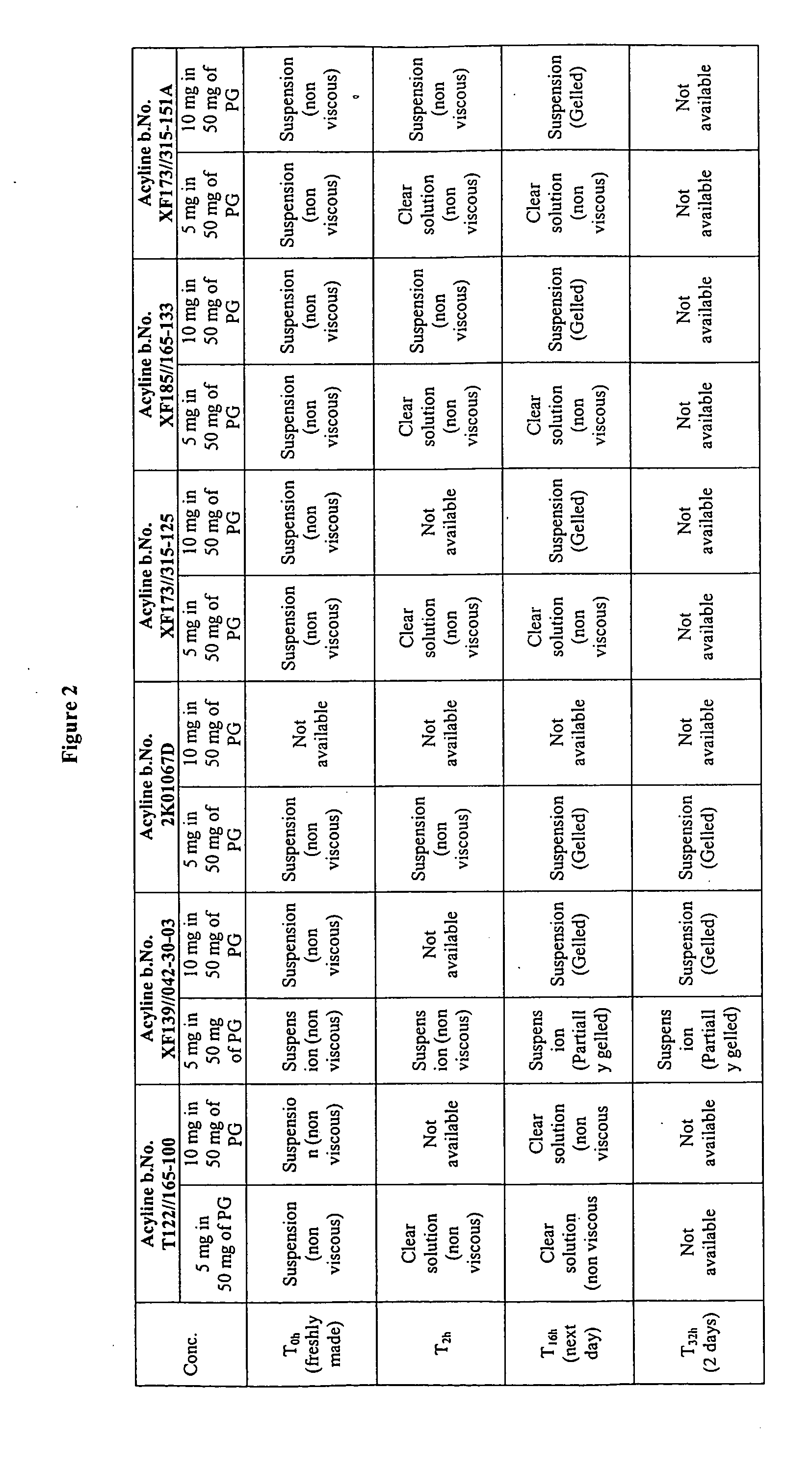
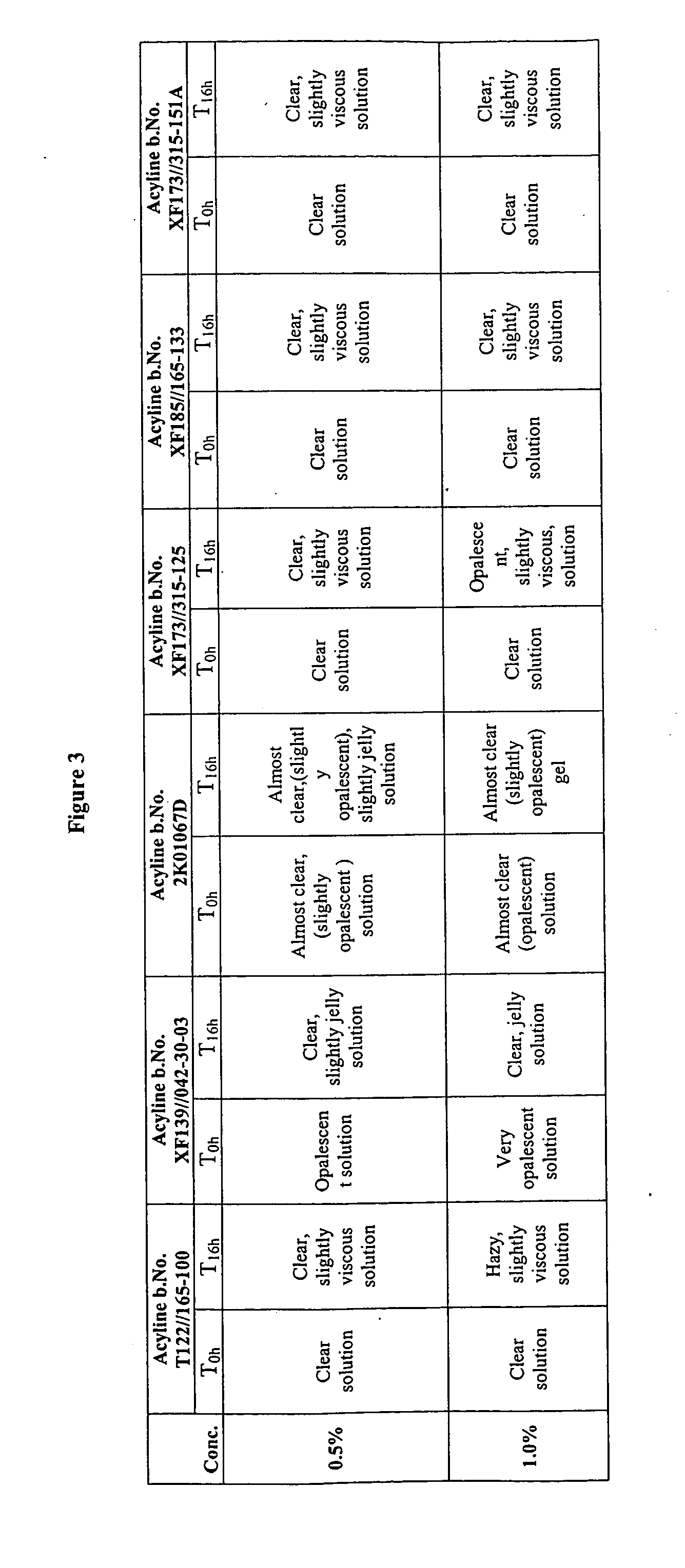
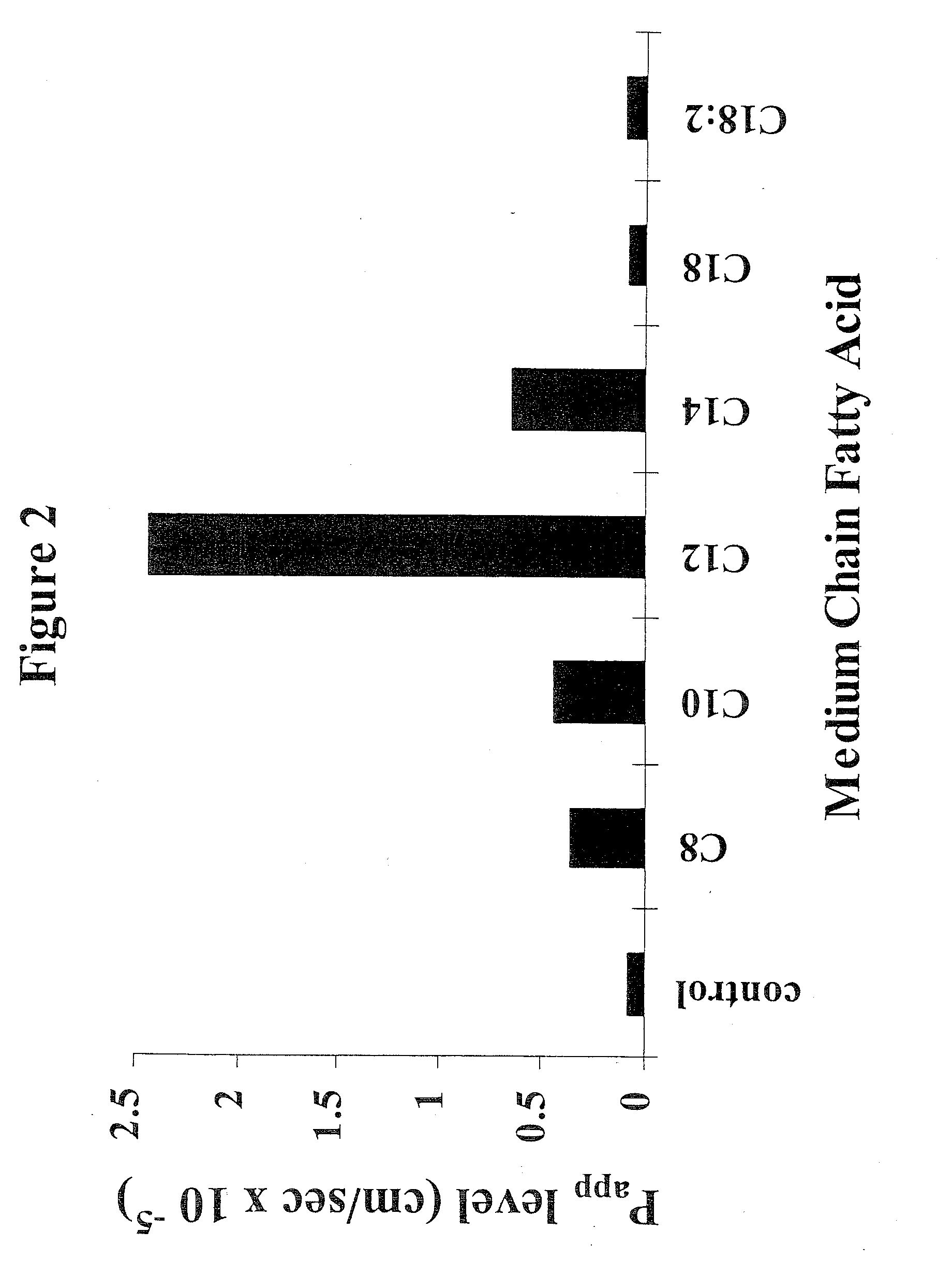
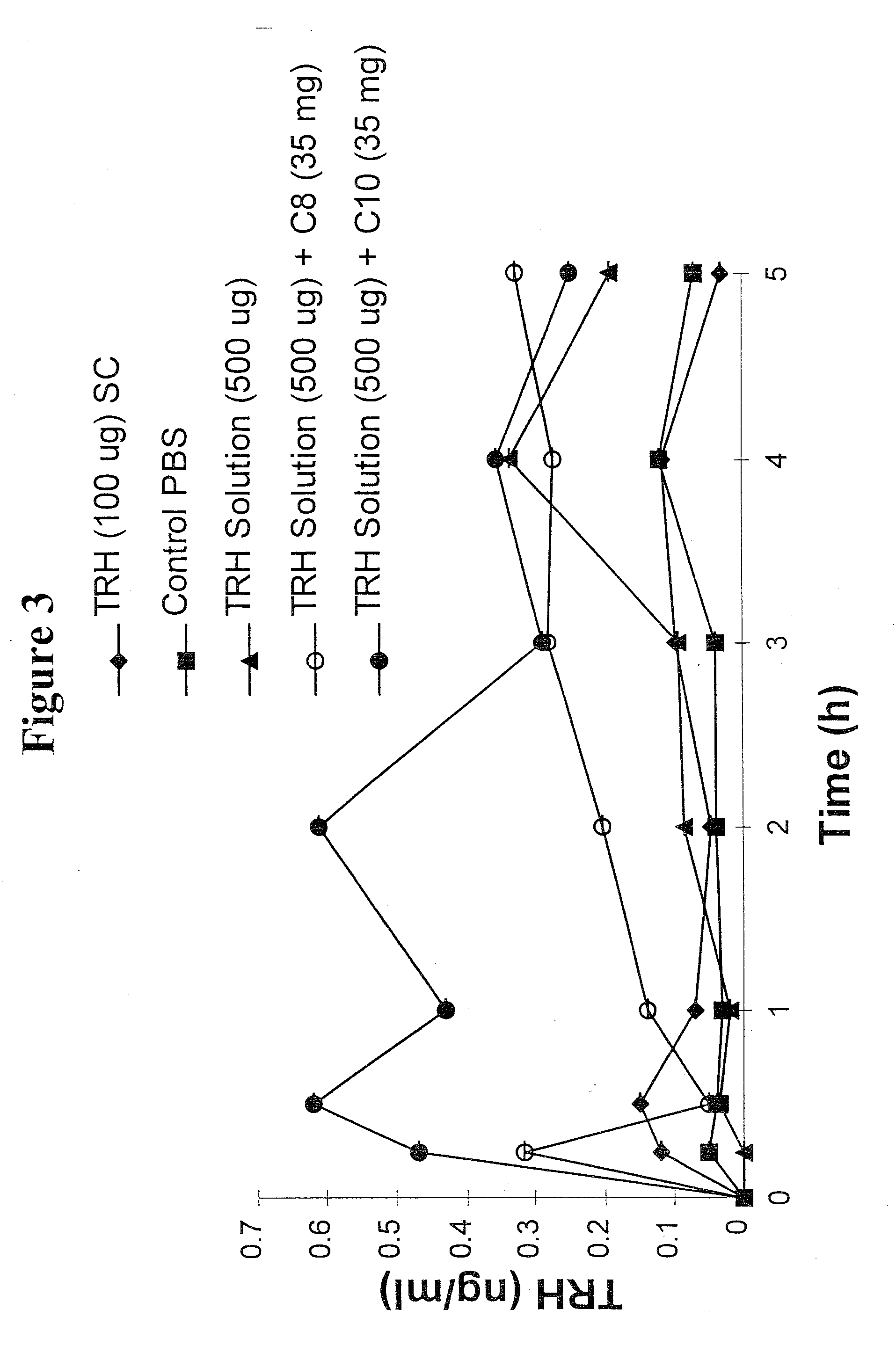
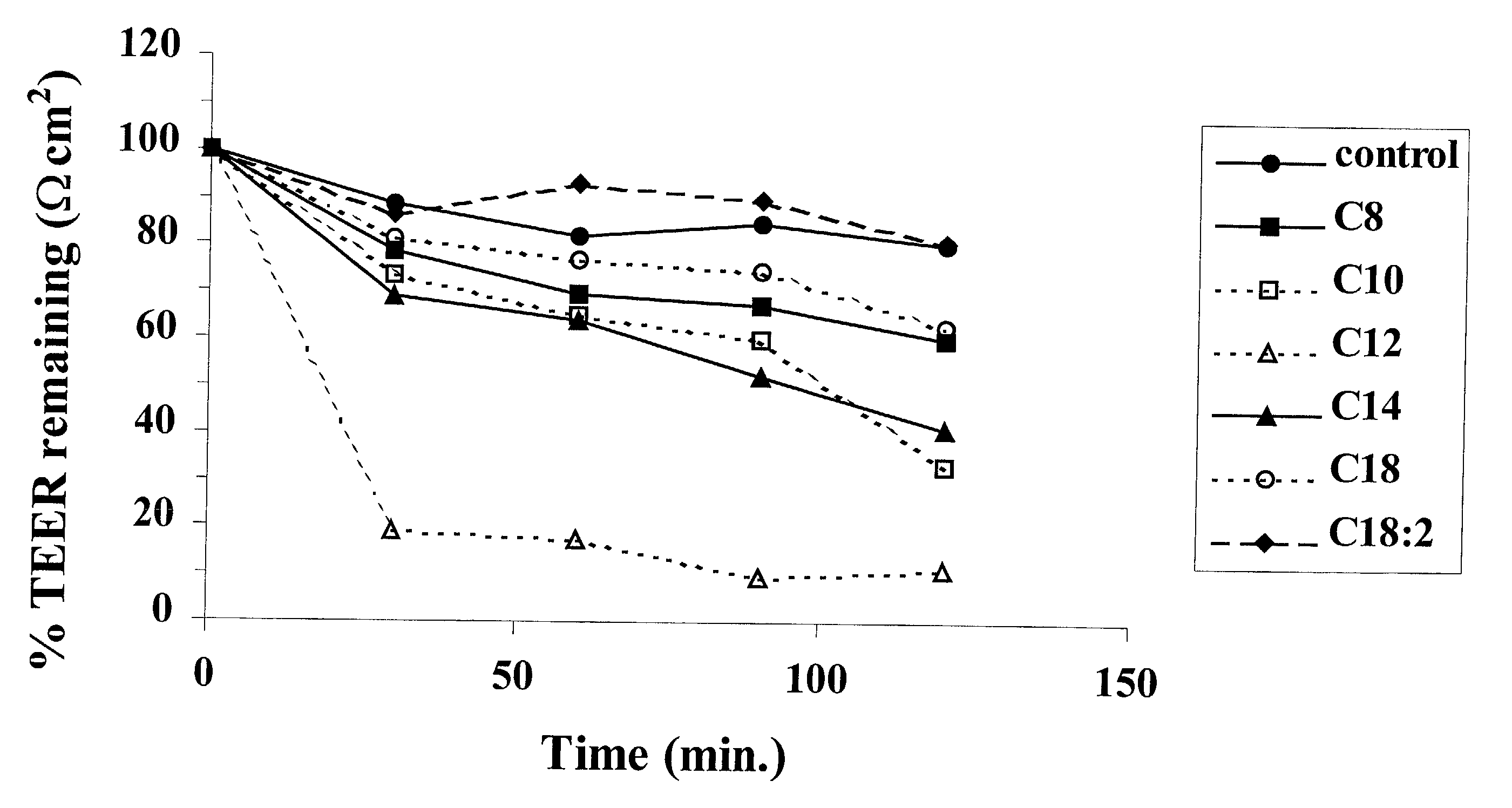
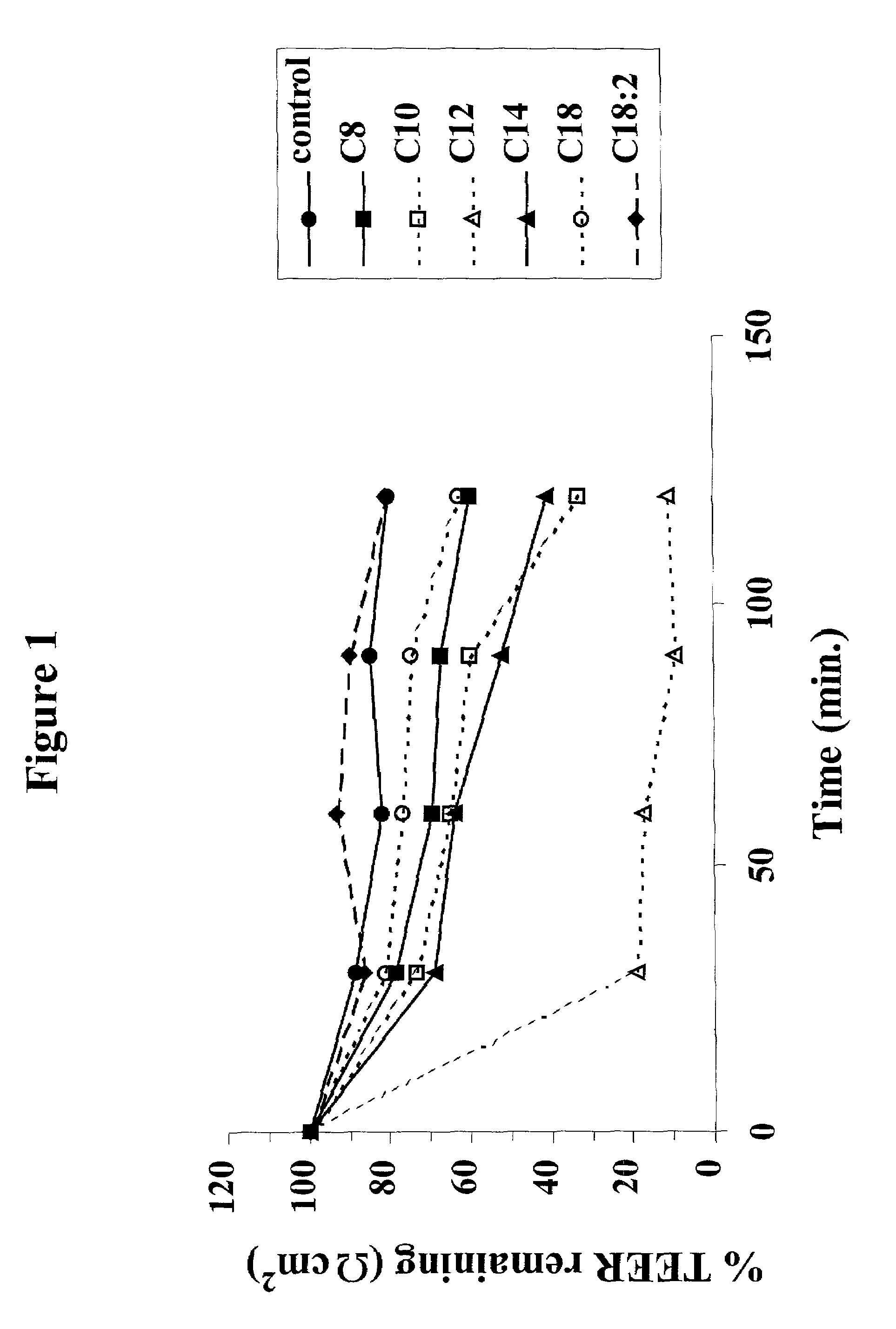
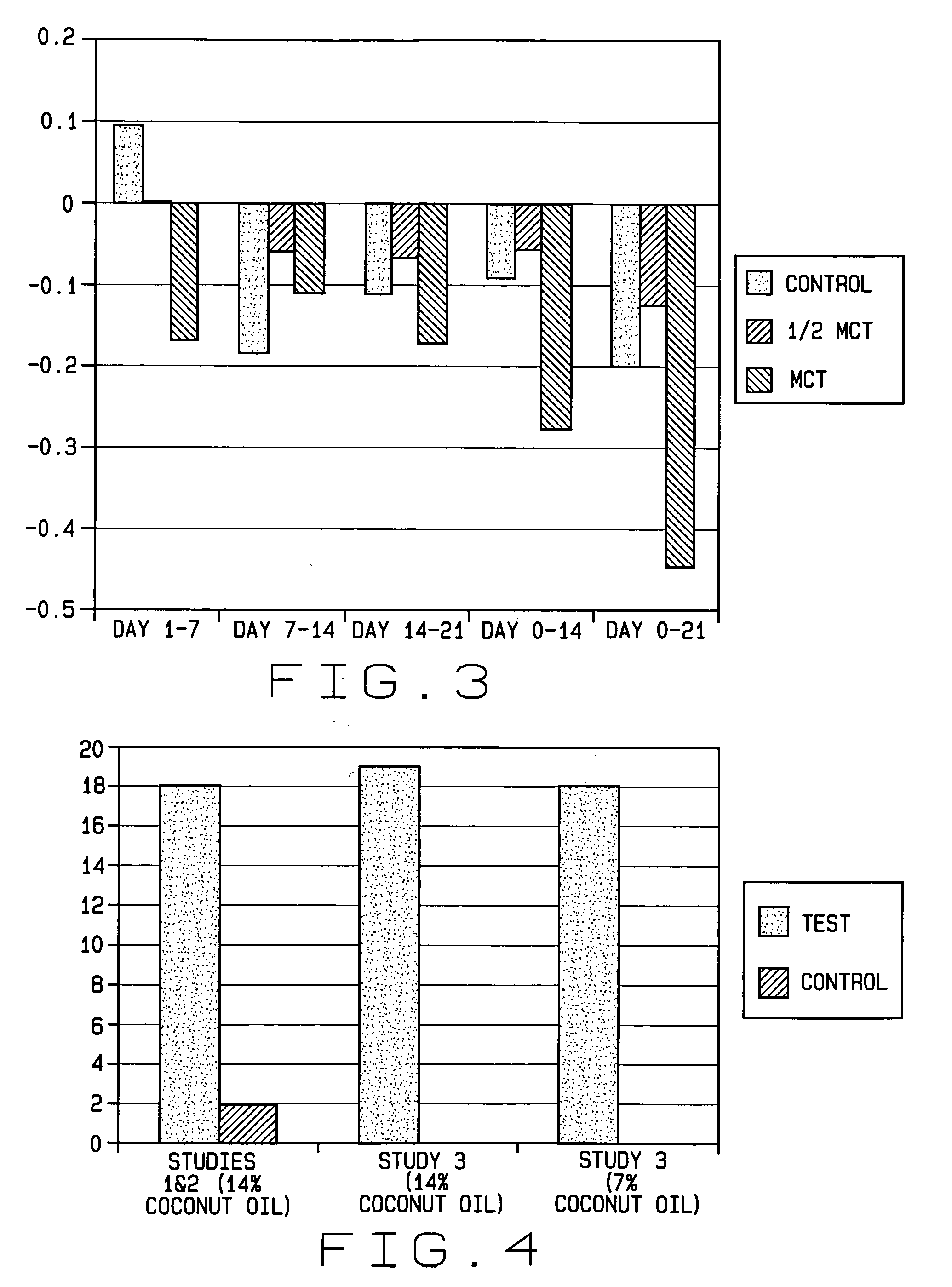
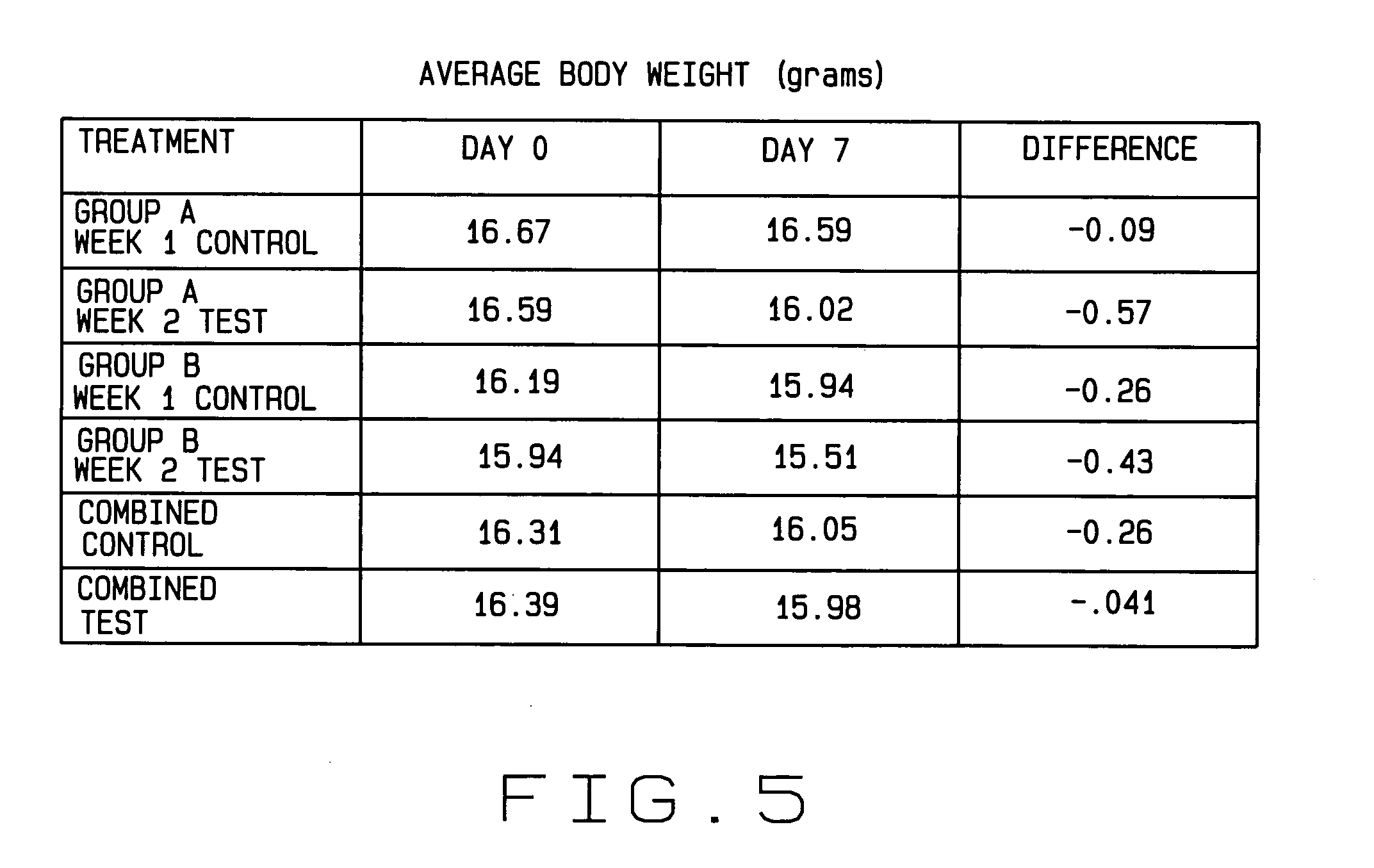
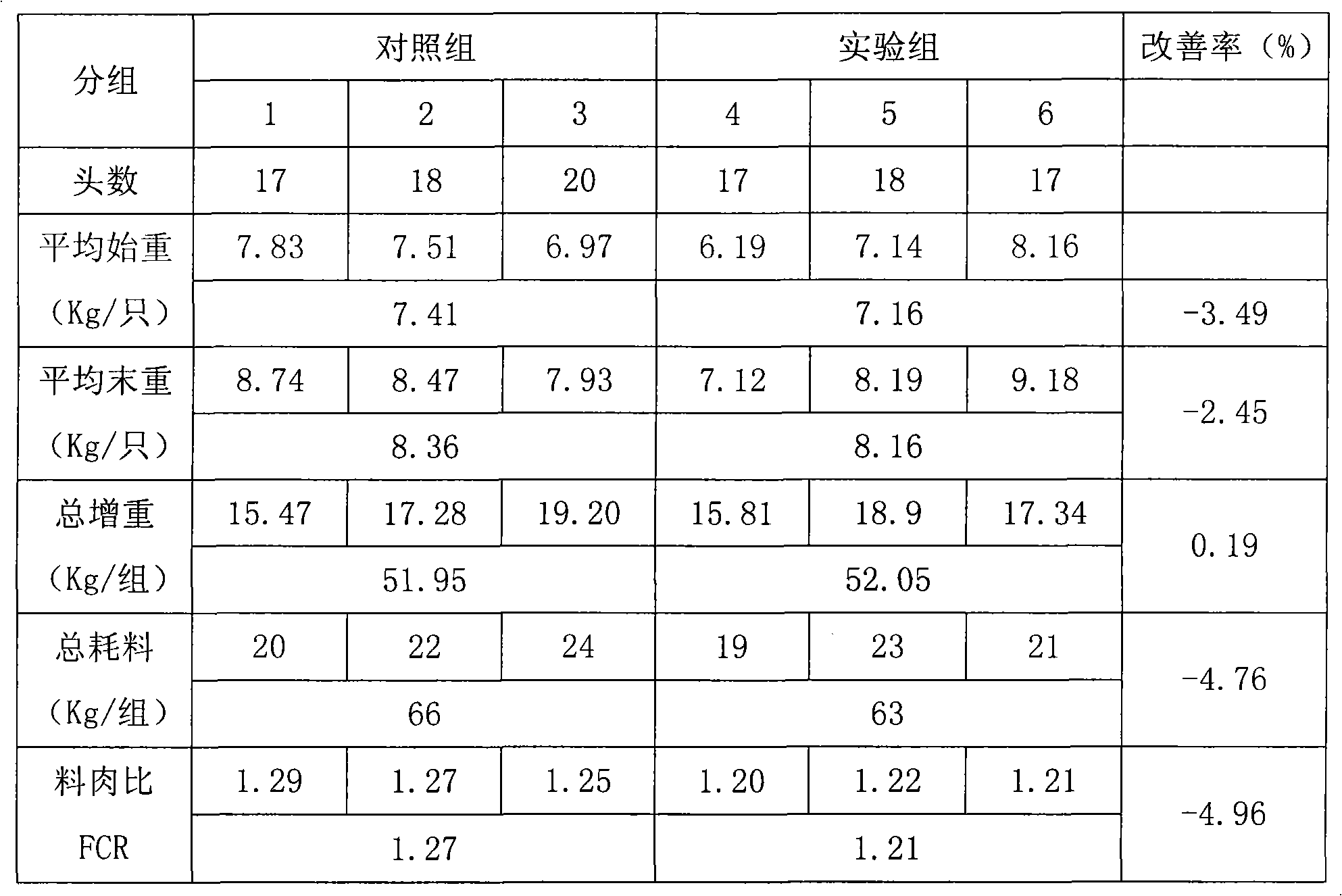
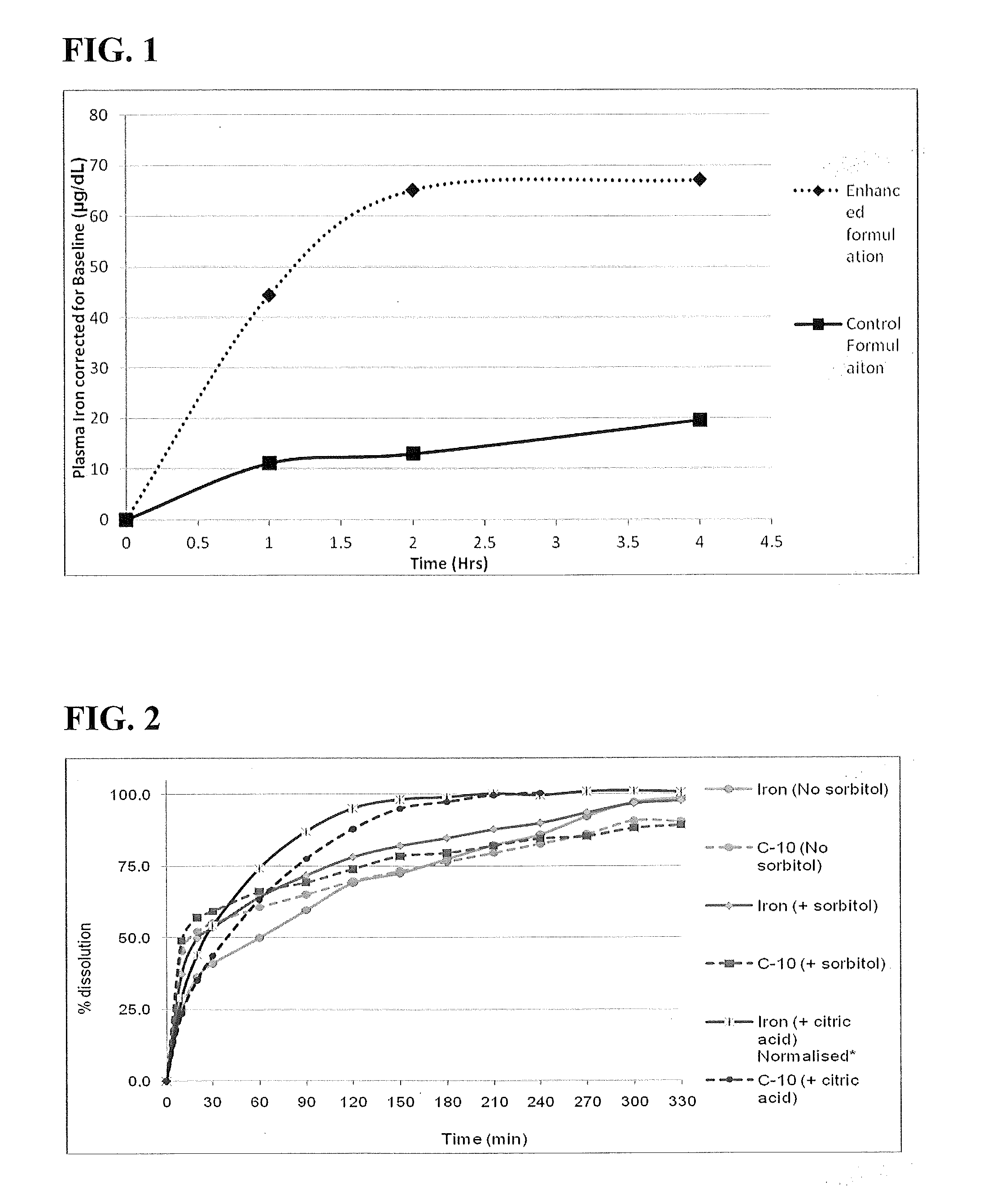
Patents
Literature
530 results about "Medium chain fatty acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
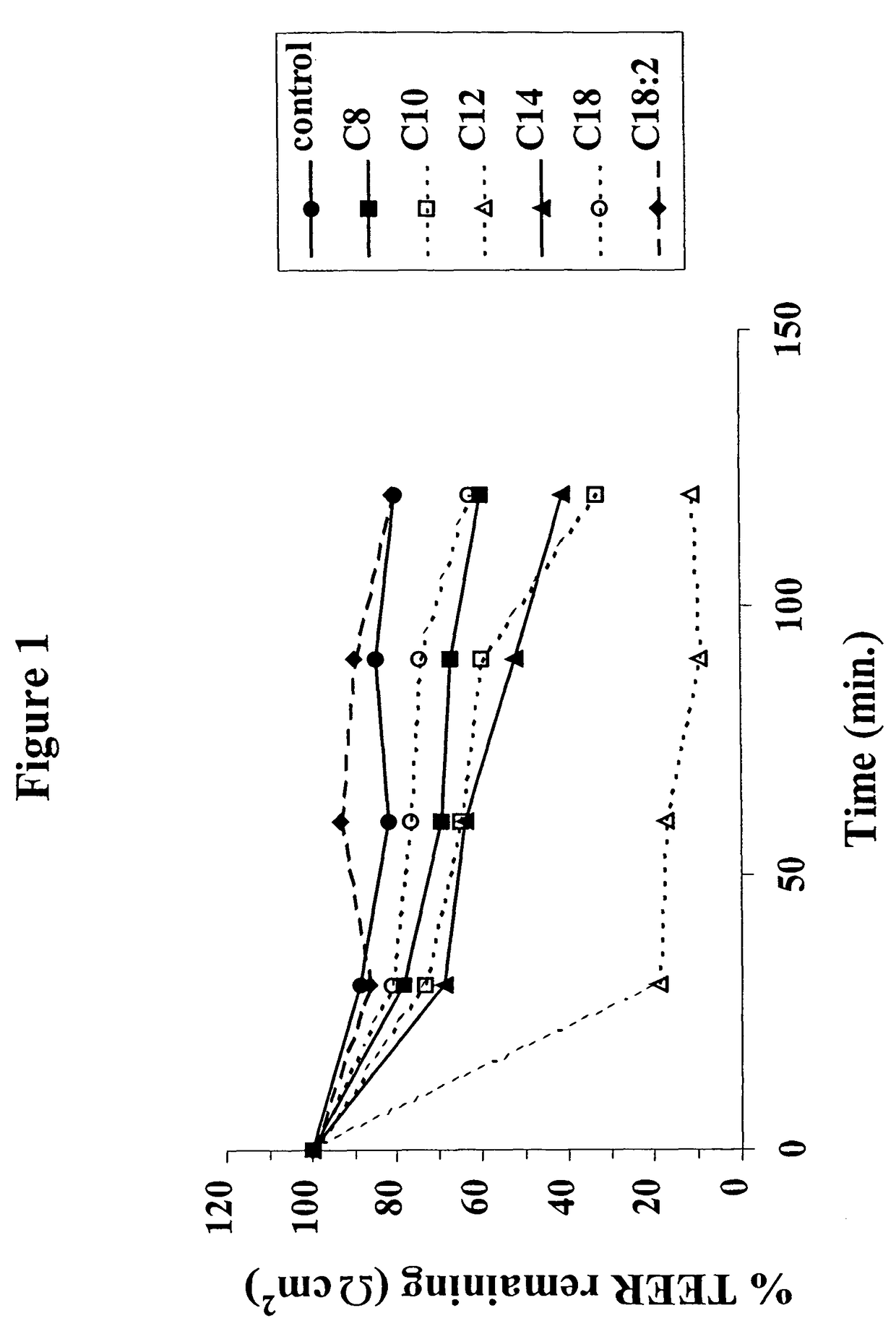
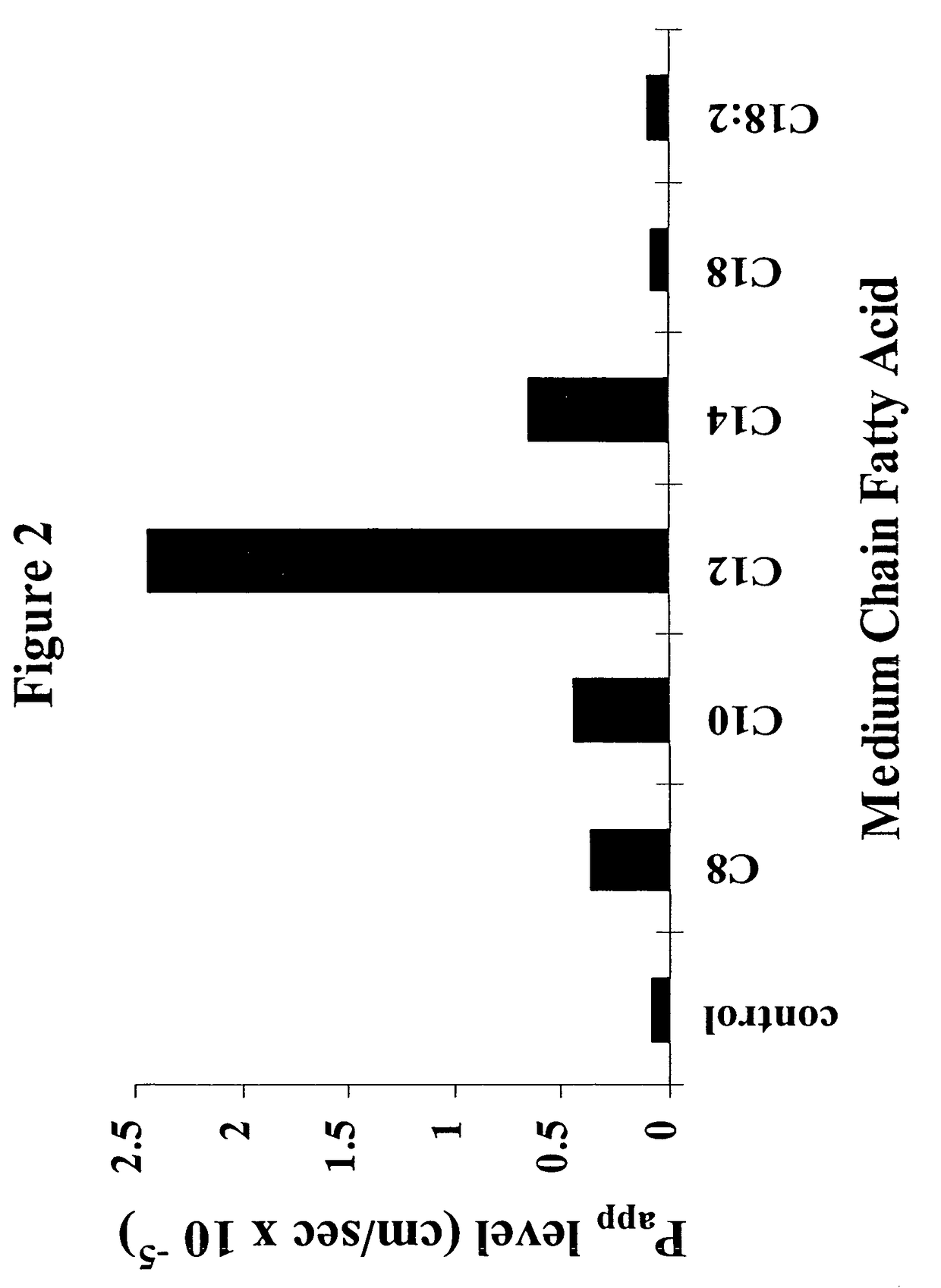
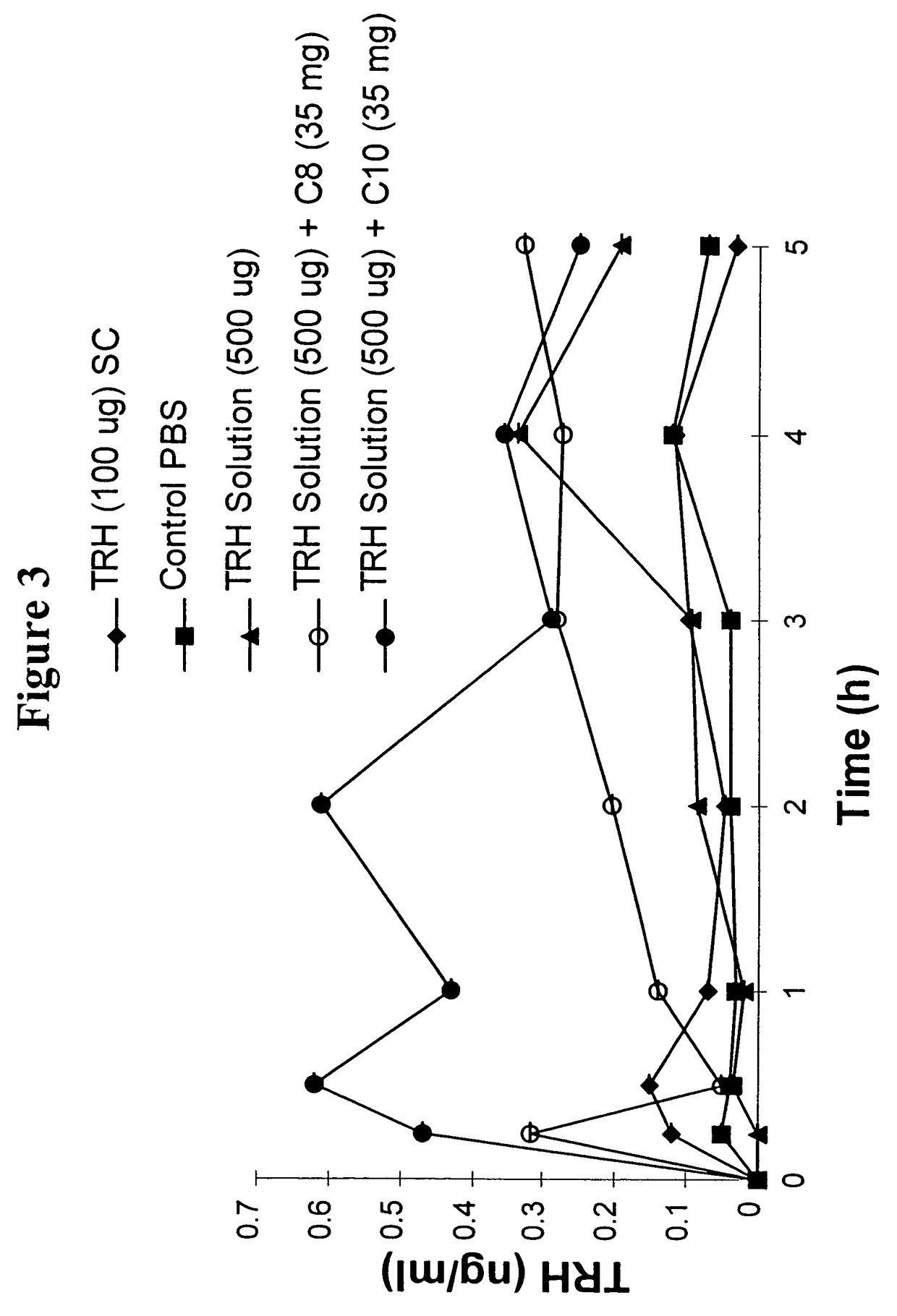
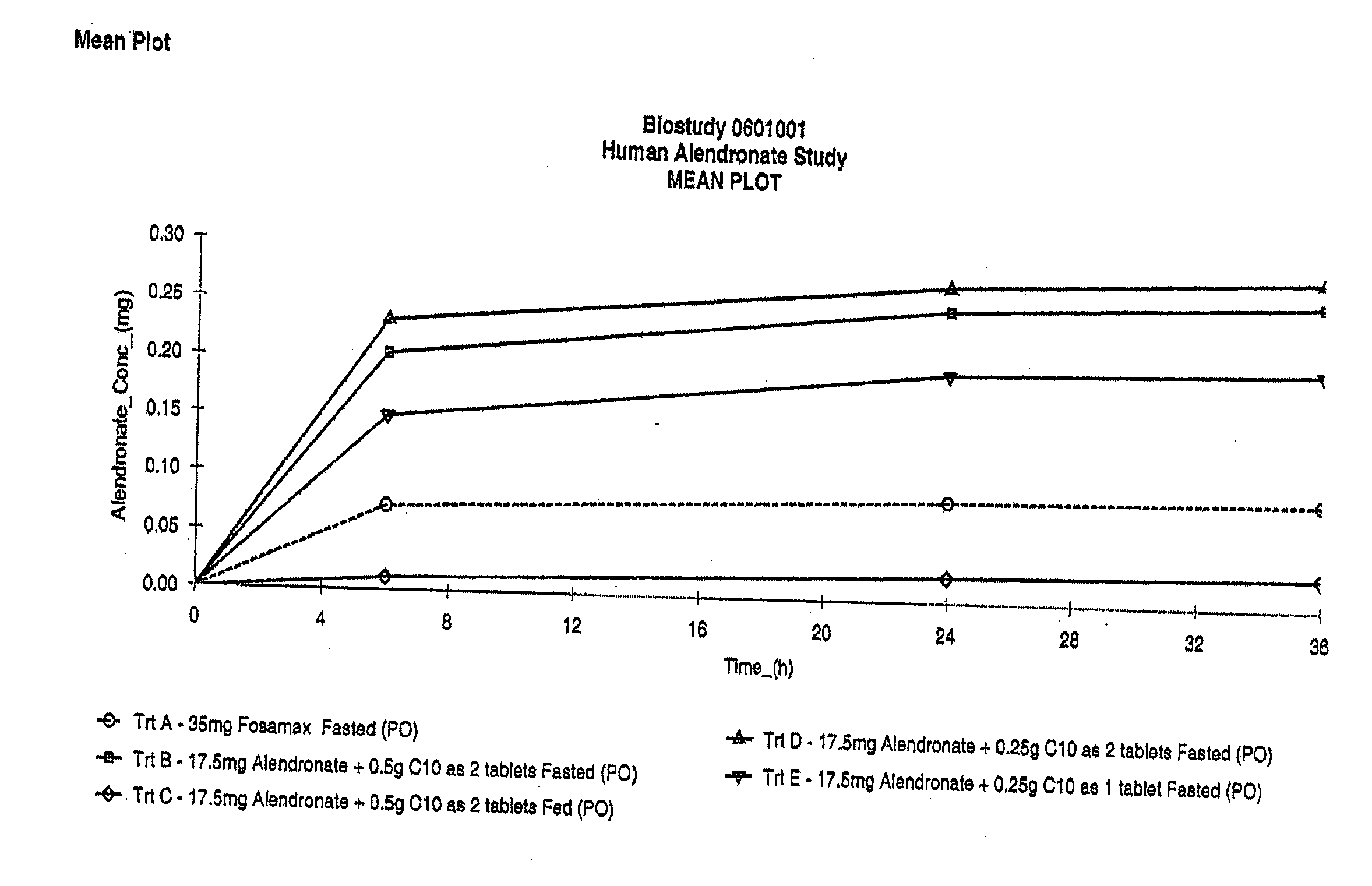
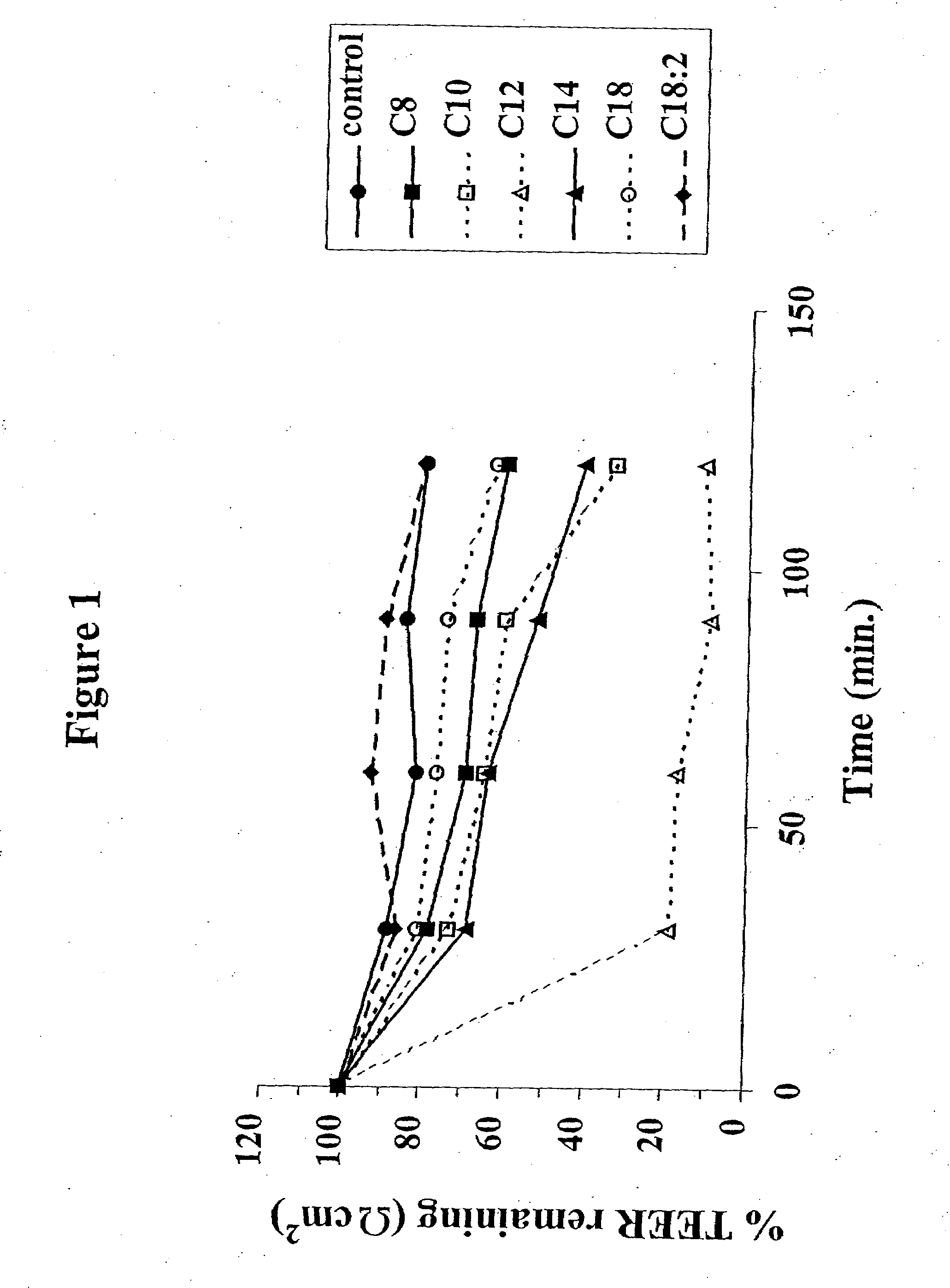
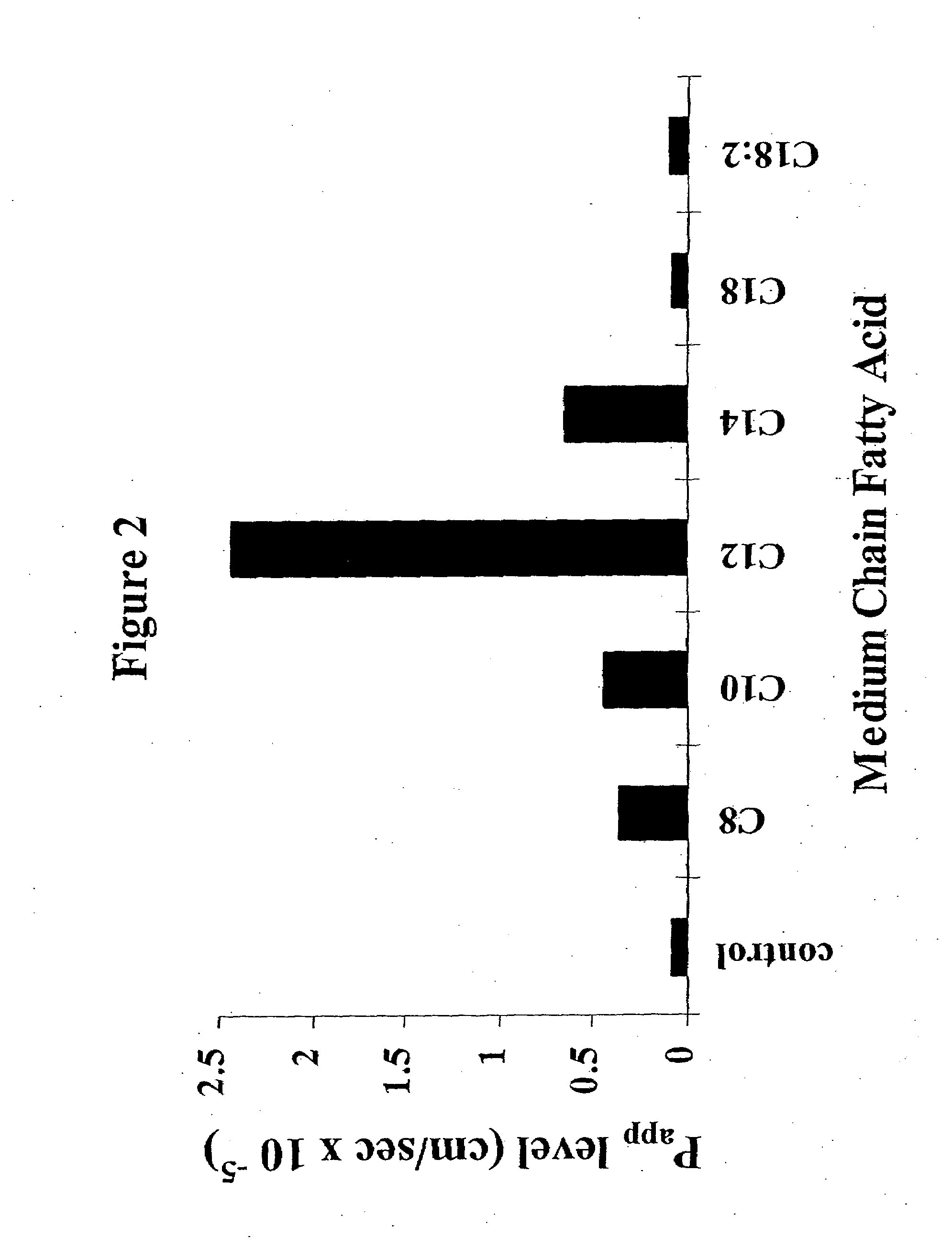
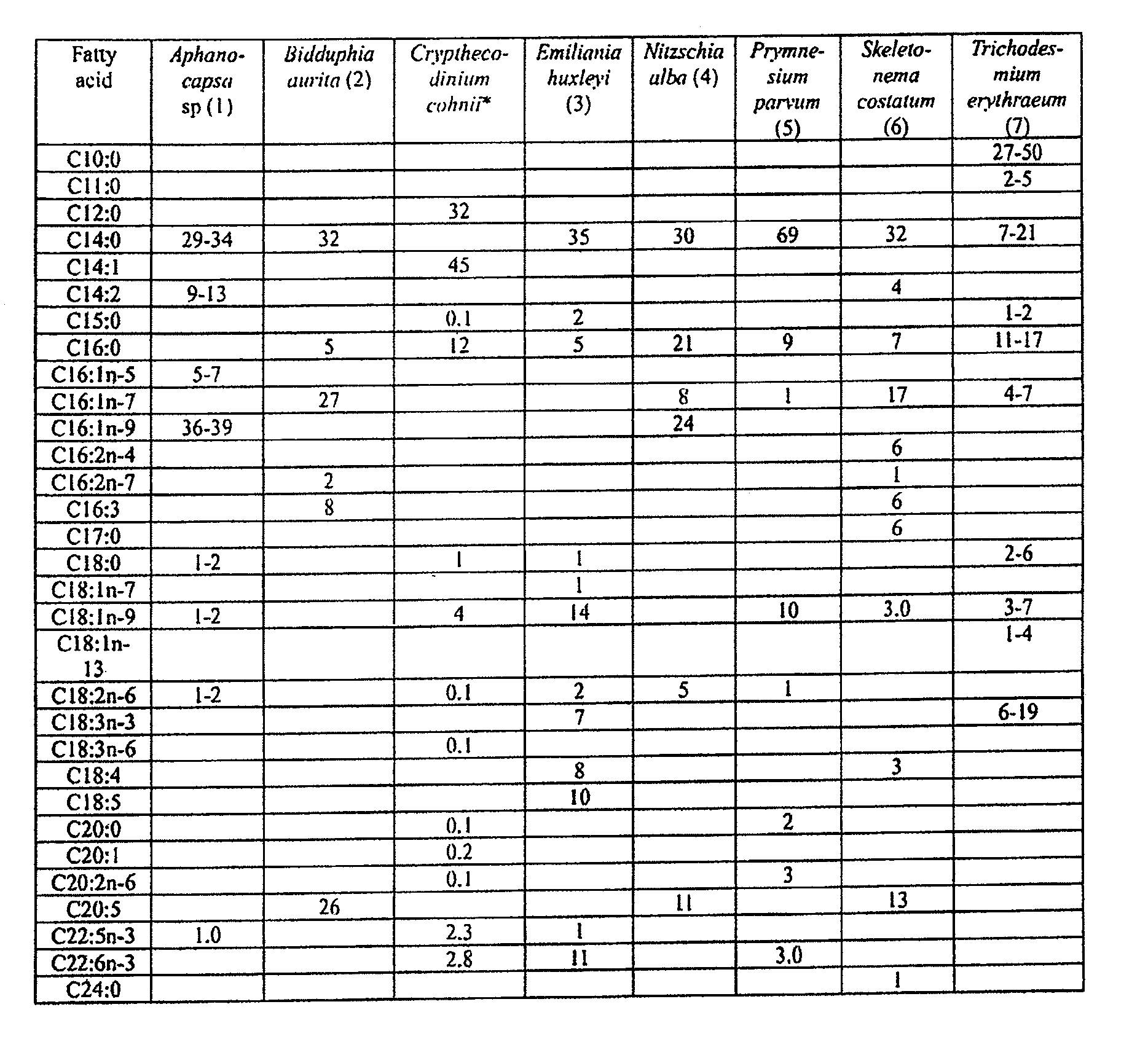
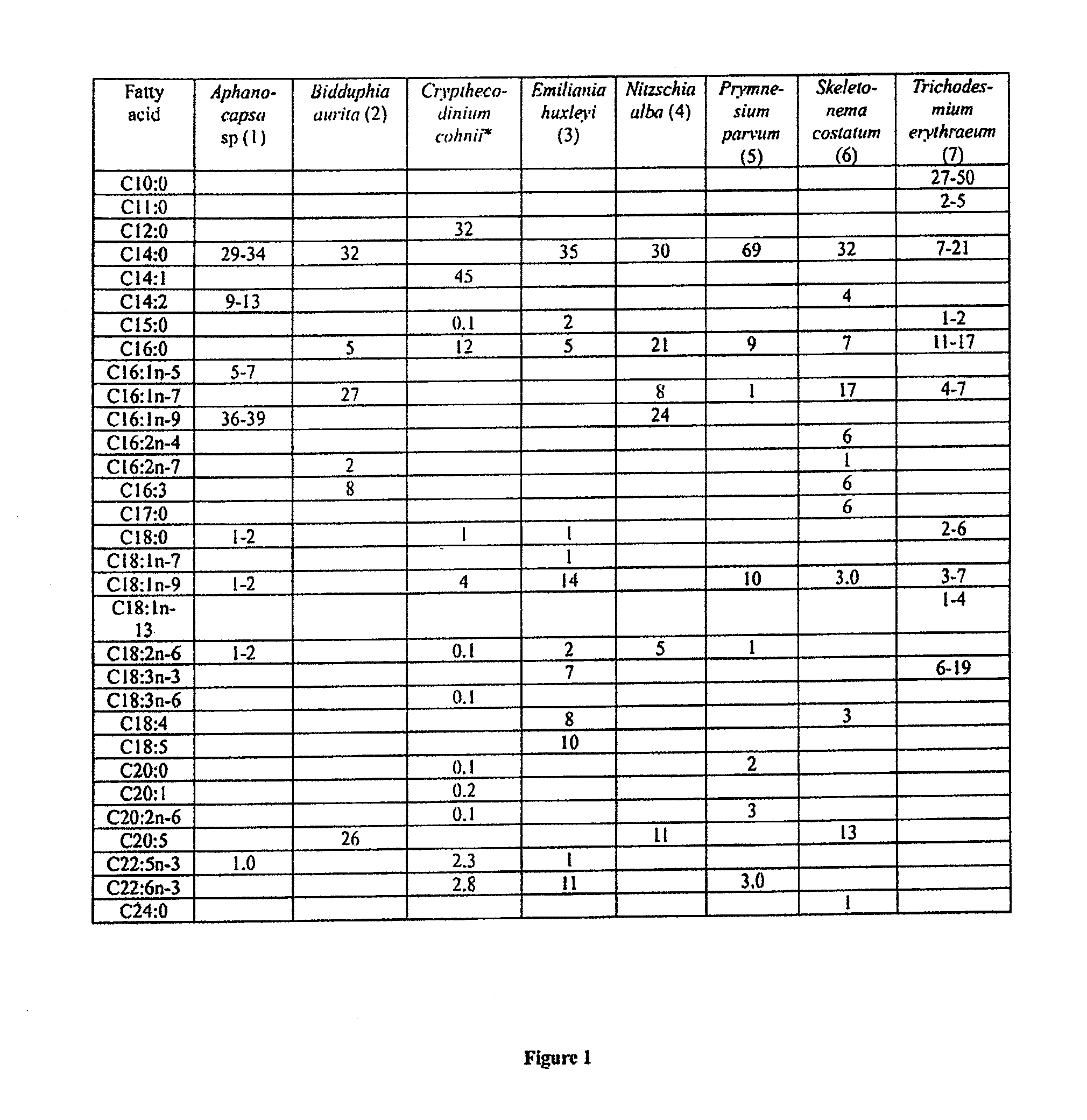
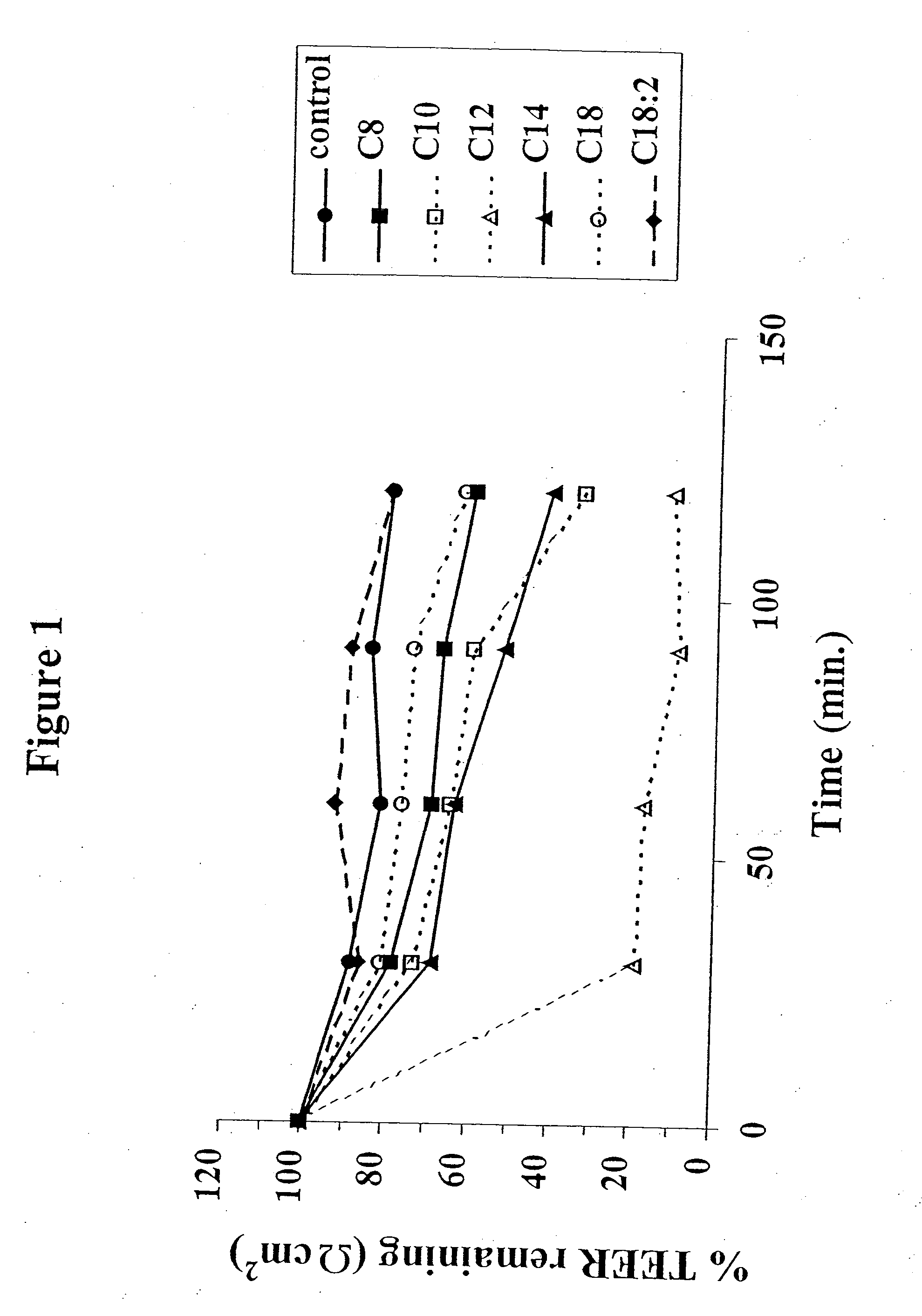
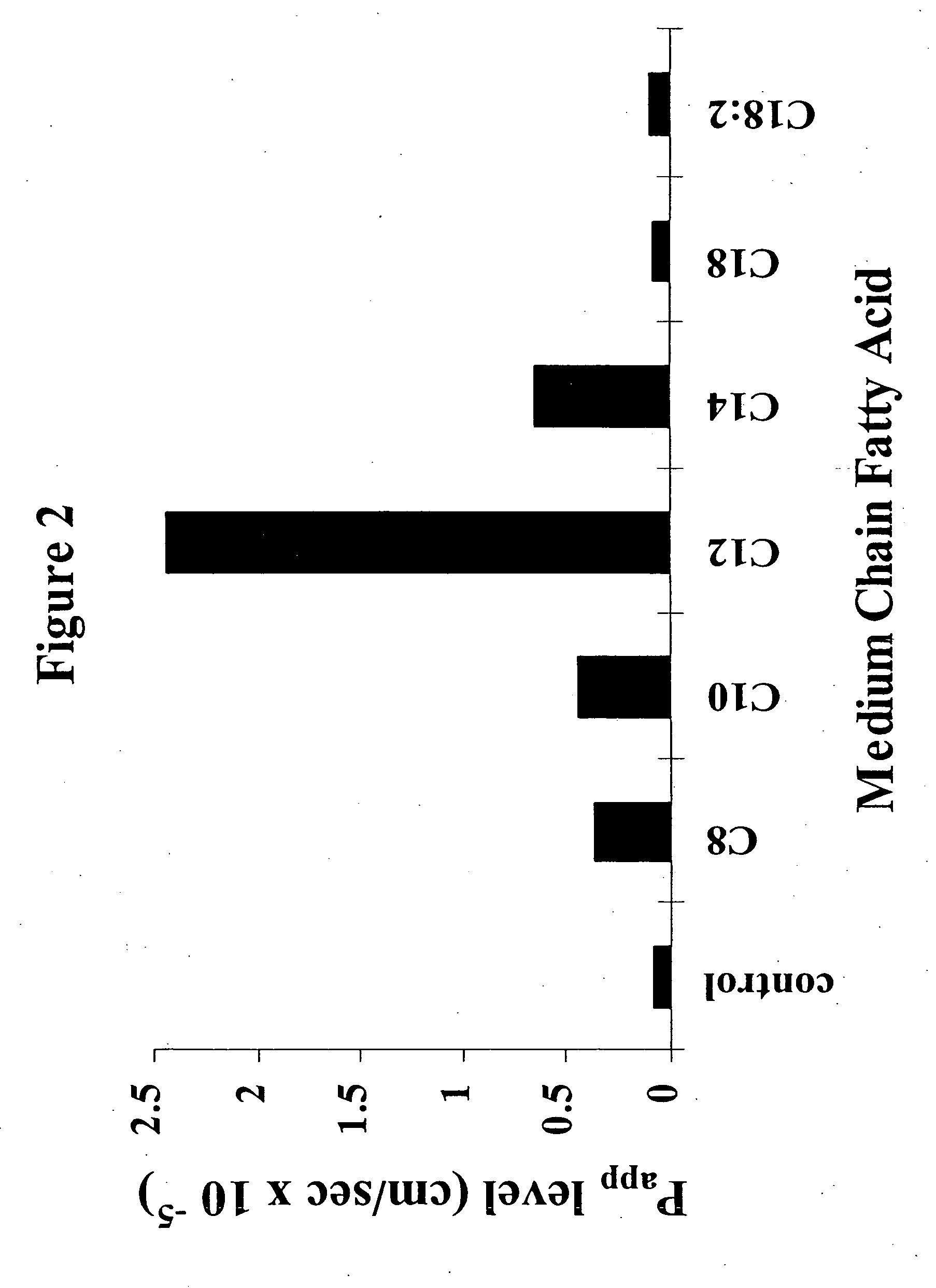
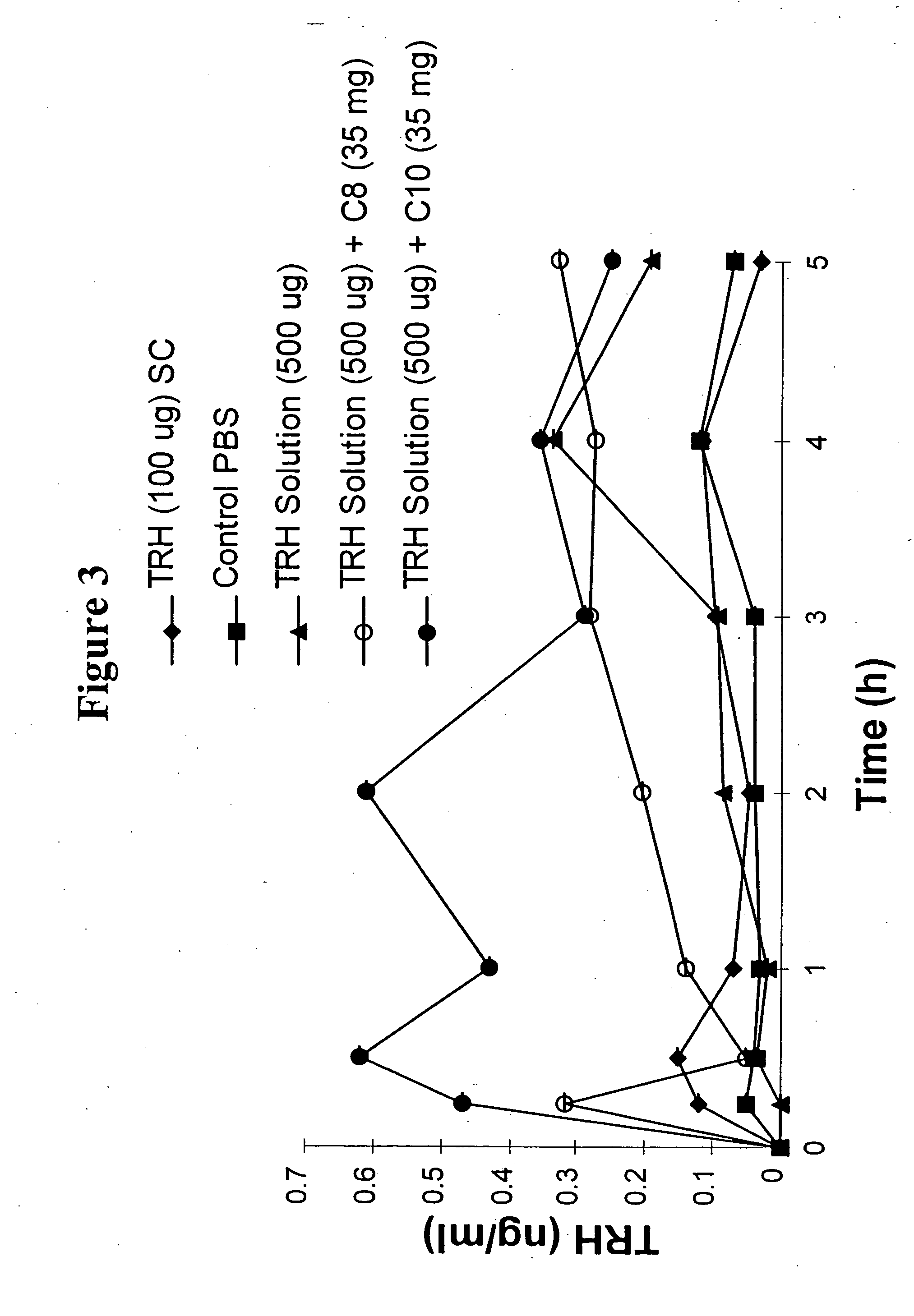
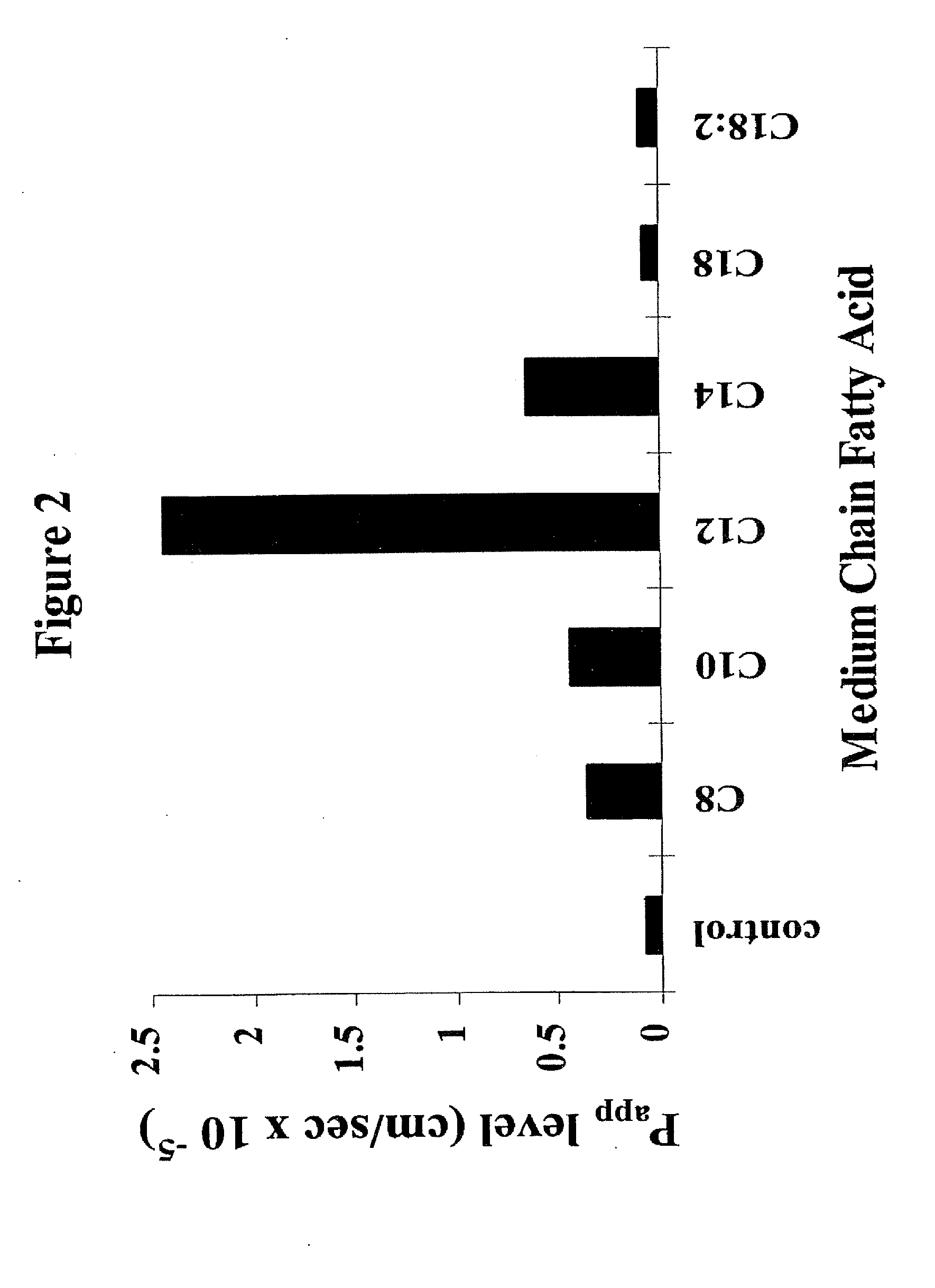
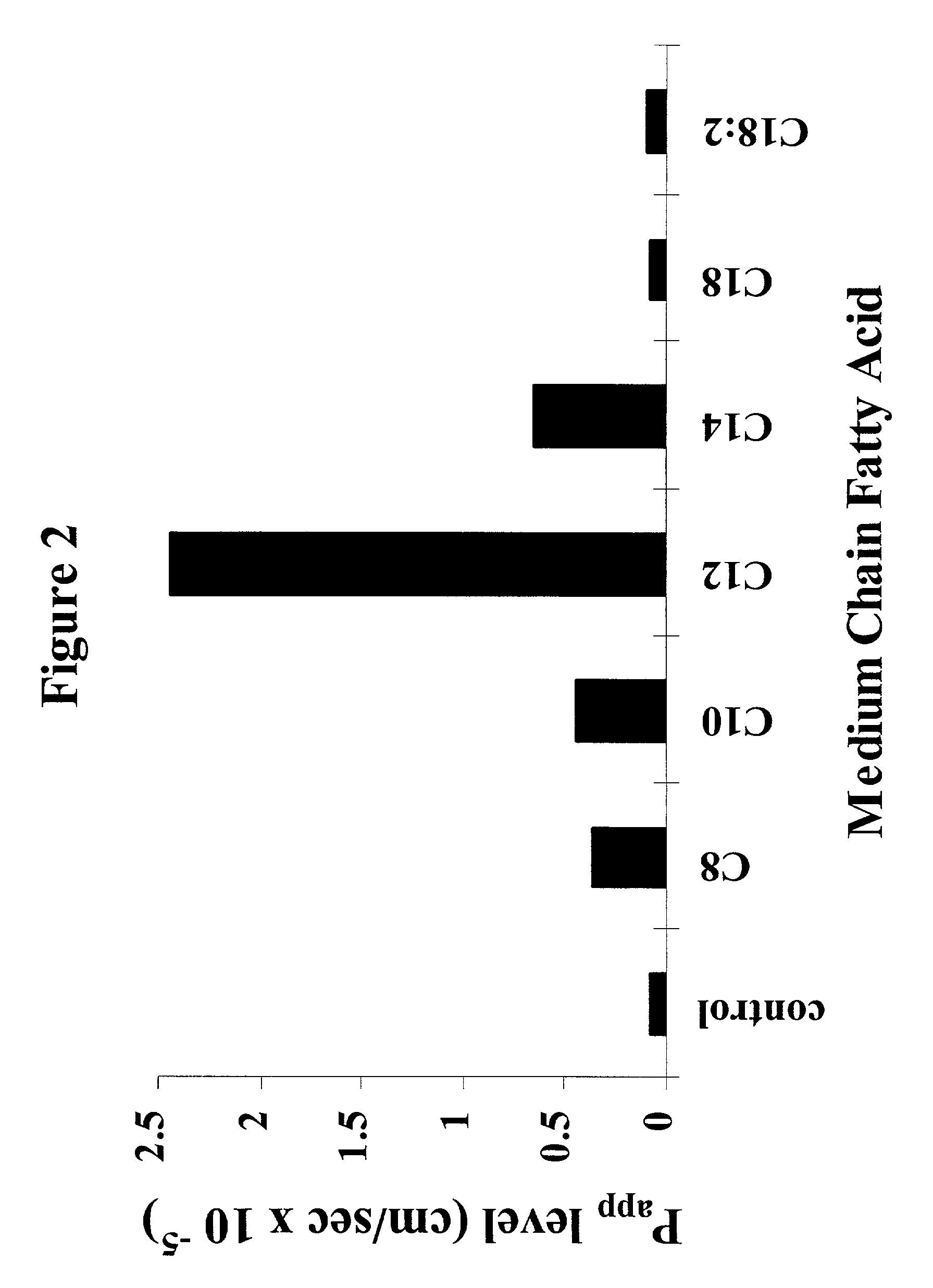
Fatty acid with 10 to 14 carbon atoms
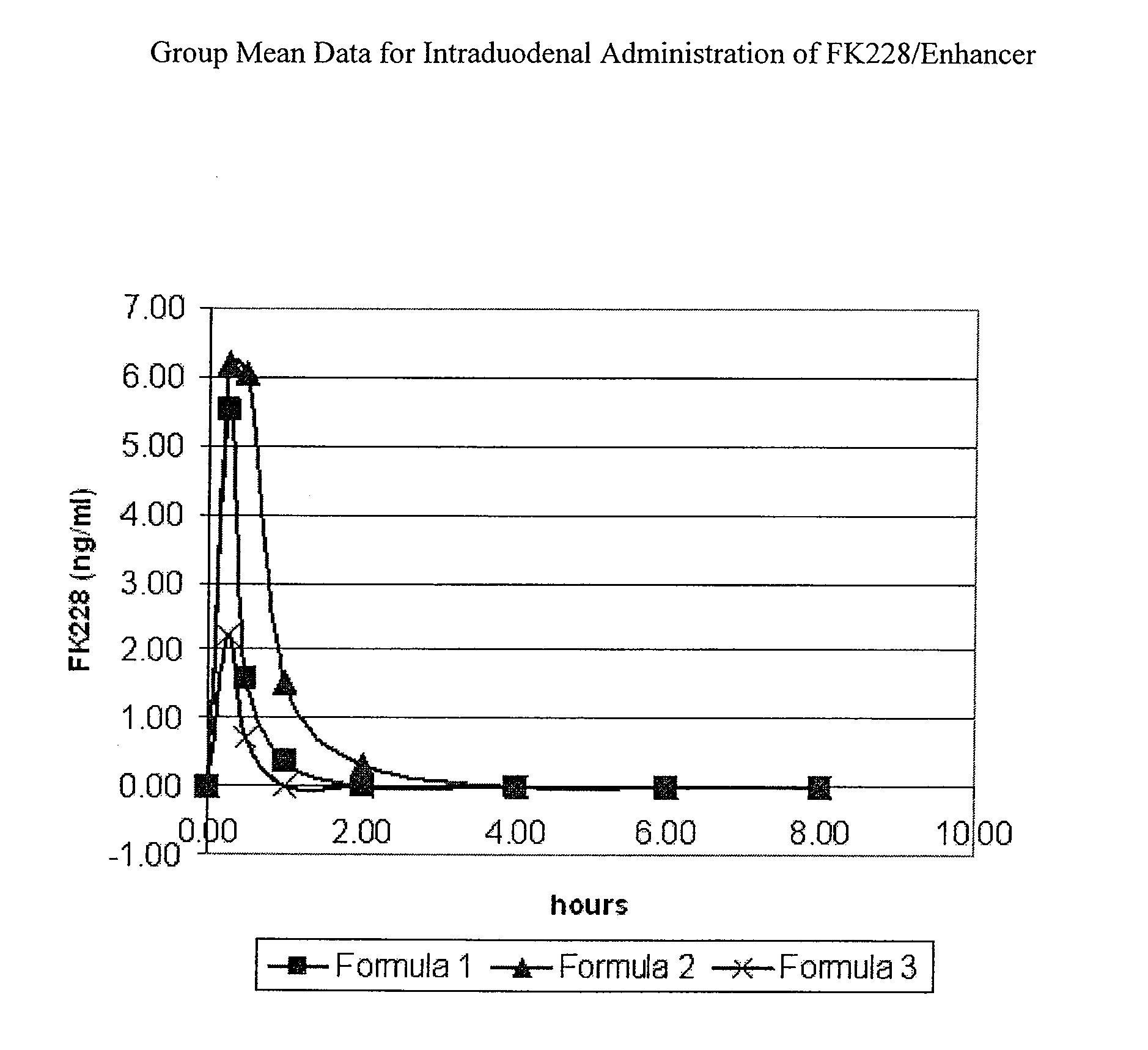
Solid oral dosage form containing an enhancer
InactiveUS8119159B2Minimizes risk of local irritationImprove oral bioavailabilityBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
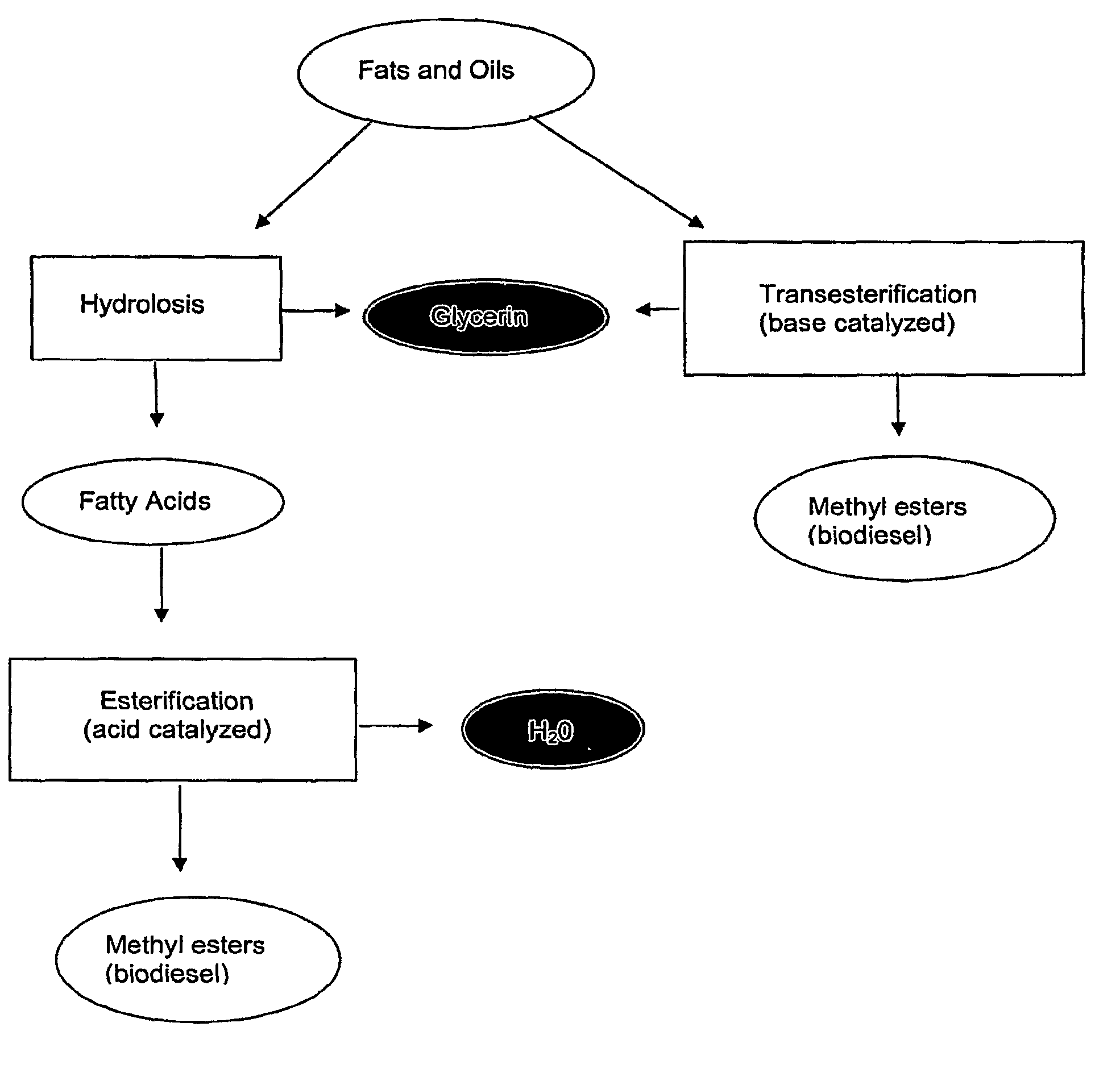
Fatty acid blends and uses therefor
ActiveUS20100058651A1Maintain good propertiesEffect cold flow propertyFatty acid esterificationFatty acids production/refiningFatty acid methyl esterMedium chain fatty acid
Provided herein are blends oils or fatty acids comprising more than 50% medium chain fatty acids, or the fatty acid alkyl esters thereof, and having low melting points. Such blends are useful as a fuel or as a starting material for the production of, for example, a biodiesel. Also provided genetically altered or modified plants, modified such that the amount of medium chain fatty acids generated by the plant are increased. Further provided is a method of predicting the melting point of a blend of fatty acid methyl esters and the use of such a method for identifying blends suitable for use as, for example, a biodiesel.
Owner:CIBUS
Fatty acid blends and uses therefor
ActiveUS8029579B2Maintain good propertiesEffect cold flow propertyFatty oils/acids recovery from wasteFatty acid esterificationPolymer scienceFatty acid methyl ester
Owner:CIBUS
Solid oral dosage form containing an enhancer
InactiveUS20070148228A1BiocideCyclic peptide ingredientsDelayed Release Dosage FormPharmaceutical drug
The invention relates to a pharmaceutical composition and oral dosage forms comprising an HDAC inhibitor in combination with an enhancer to promote absorption of the HDAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
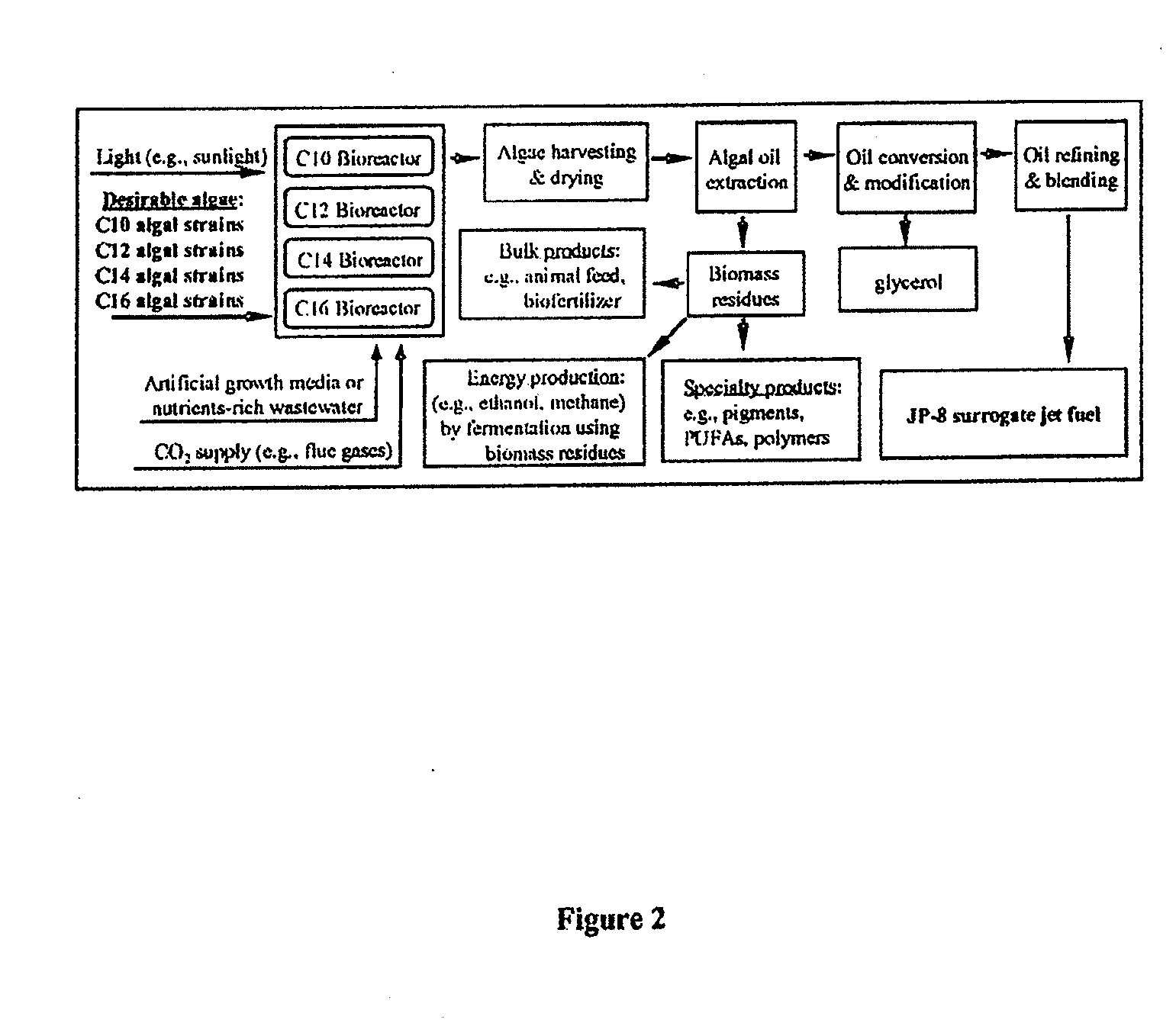
Co-culturing algal strains to produce fatty acids or hydrocarbons
Owner:ARIZONA STATE UNIVERSITY
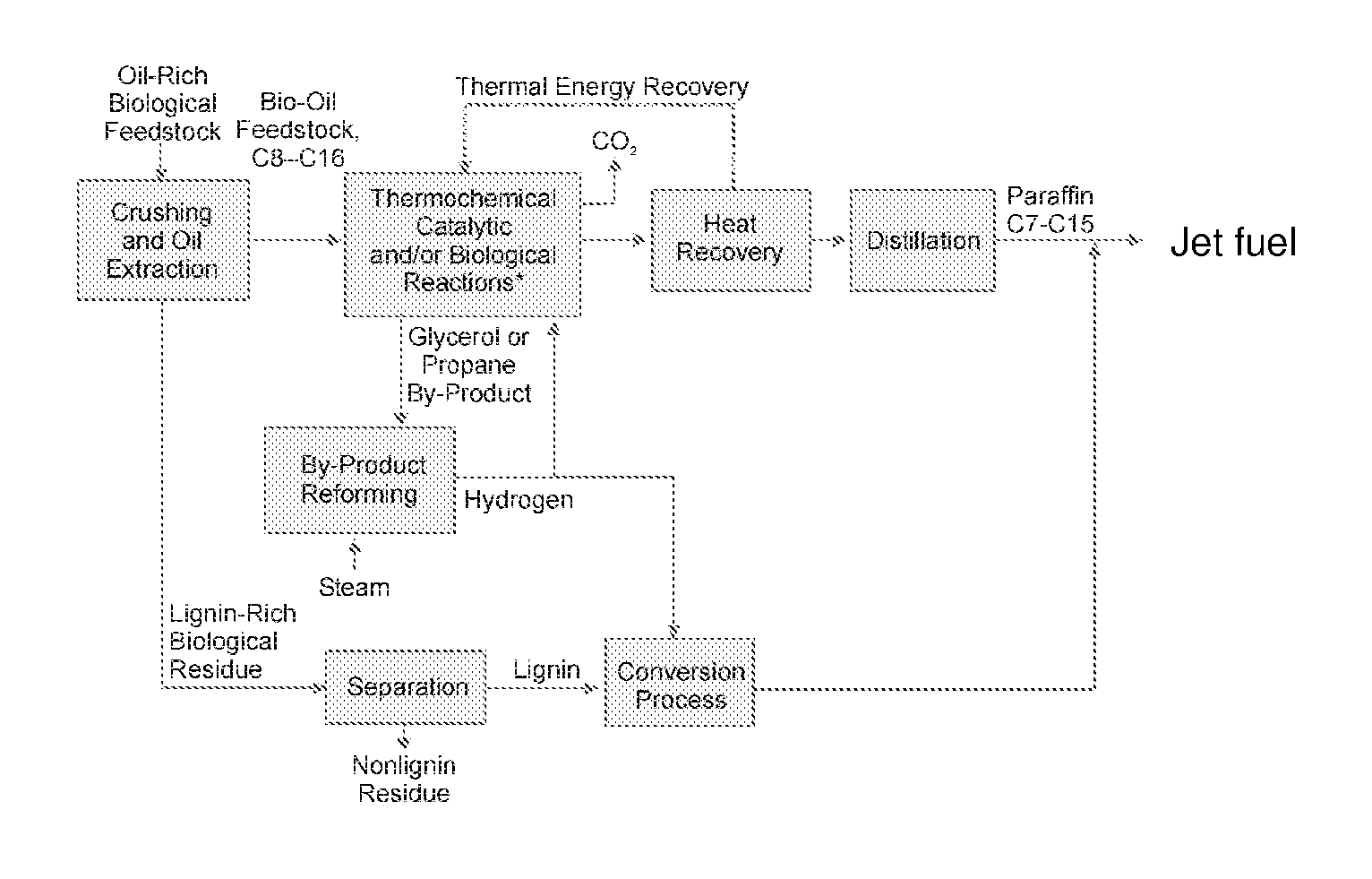
Optimal energy pathway to renewable domestic and other fuels
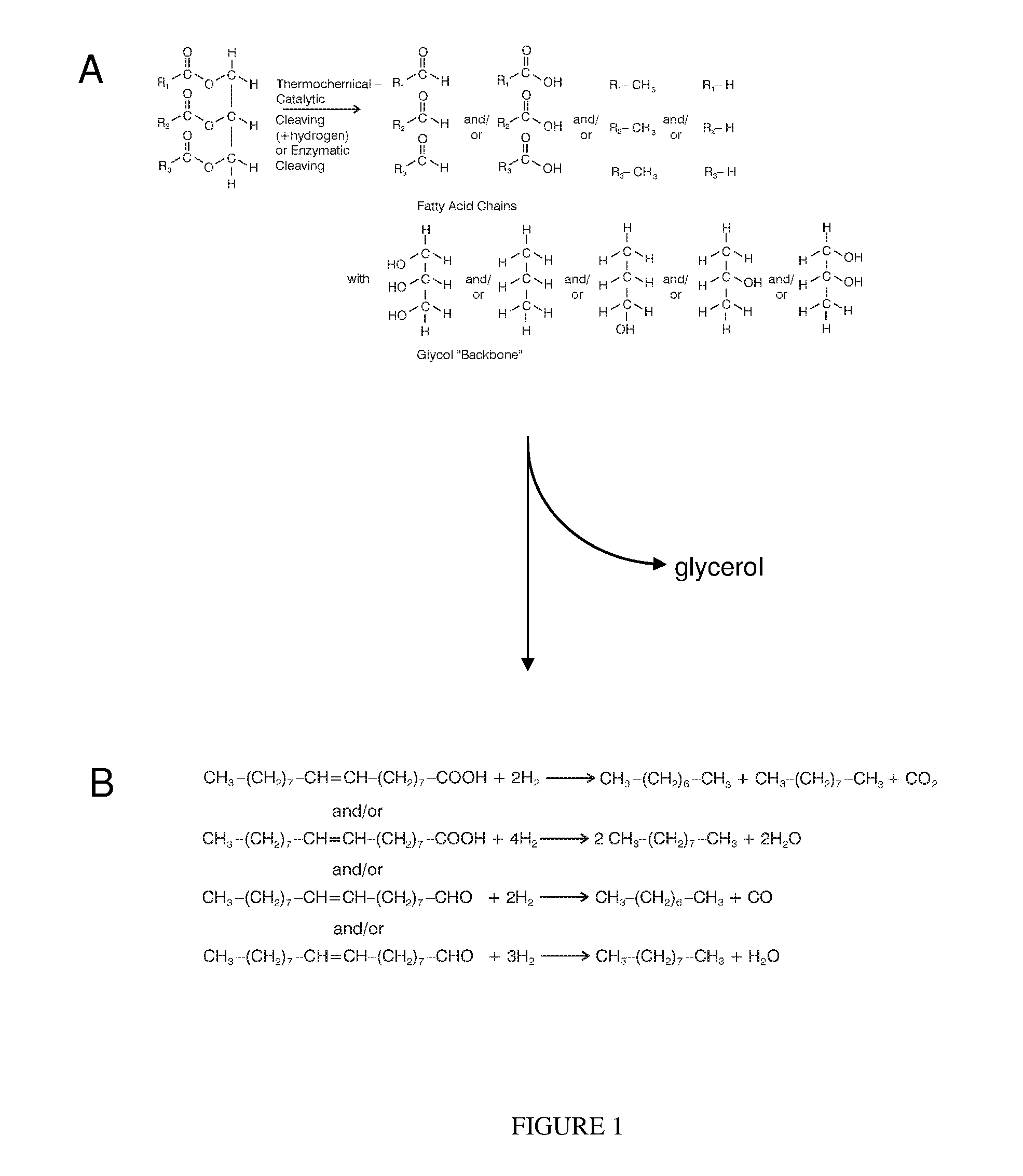
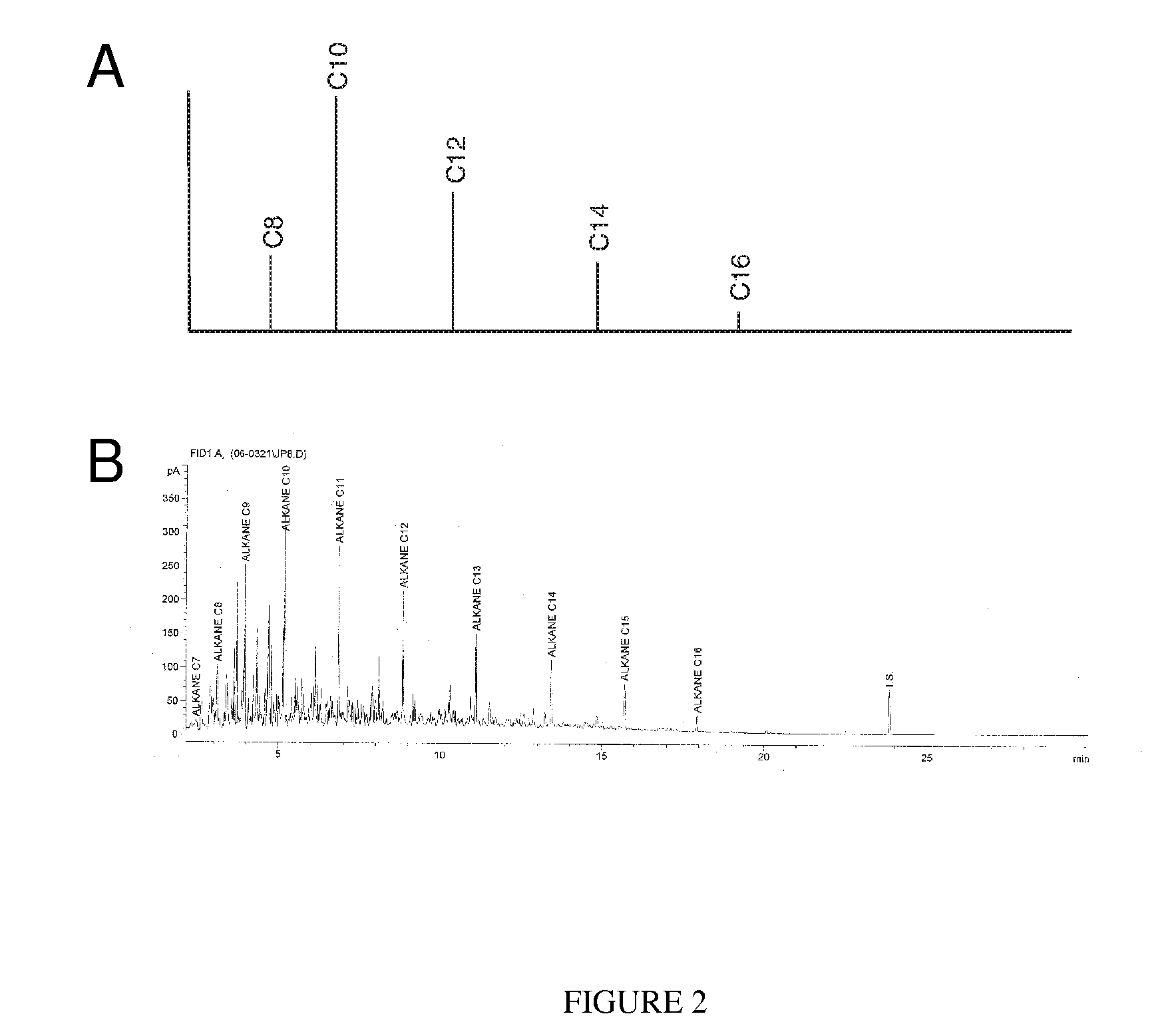
A novel, energy efficient process of producing jet fuel is disclosed herein. The process is based on utilizing a medium chain fatty acid source such as cuphea oil, which precludes the need for high-energy fatty acid chain cracking to achieve the shorter molecules needed for jet fuels and other fuels with low-temperature flow requirements. In an embodiment, a process for producing a jet fuel comprises providing a medium chain fatty acid source. The method also comprises cleaving the one or more medium chain fatty acid groups from the glycerides to form glycerol and one or more free fatty acids. The method further comprises decarboxylating the one or more medium chain fatty acids to form one or more hydrocarbons for the production of the jet fuel.
Owner:ENERGY & ENVIRONMENTAL RES CENT FOUNDATIO
Solid Oral Dosage Form Containing an Enhancer
The invention relates to a pharmaceutical composition, particularly oral dosage forms, comprising a DAC inhibitor in combination with an enhancer to promote absorption of the DAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or derivative thereof having a carbon chain length of from 6 to 20 carbon atoms. In certain embodiments, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
Compositions of peptides and processes of preparation thereof
InactiveUS20090280169A1Improve stabilityNervous disorderPeptide/protein ingredientsEtherCarbon chain
The present invention provides composition of comprising a therapeutically effective amount of at least one peptide, polypeptide, analog or derivative thereof and a sufficient amount of at least one stabilizing agent to improve the stability of the peptide, polypeptide, an analog or derivative thereof, wherein at least one stabilizing agent is a medium chain fatty acid salt, an ester, an ether, or a derivative of a medium chain fatty acid and has a carbon chain length of from about 4 to about 20 carbon atoms or is a surface active agent. The method for preparation of a composition of a peptide, polypeptide, protein, an analog and / or derivative thereof is also provided. The process comprises mixing the peptide, polypeptide, protein, an analog or derivative thereof with a sufficient amount of at least one stabilizing agents to improve the stability of the peptide, polypeptide, protein, an analog or derivative thereof, and the agent is a medium chain fatty acid salt, an ester, an ether, or a derivative of a medium chain fatty acid and has a carbon chain length of from about 4 to about 20 carbon atoms or is a surface active agent.
Owner:NOVO NORDISK AS
Compositions and methods for producing elevated and sustained ketosis
ActiveUS9138420B2Rapid and sustained elevationImprove the level ofHydroxy compound active ingredientsMetabolism disorderSignificant elevationKetogenic diet
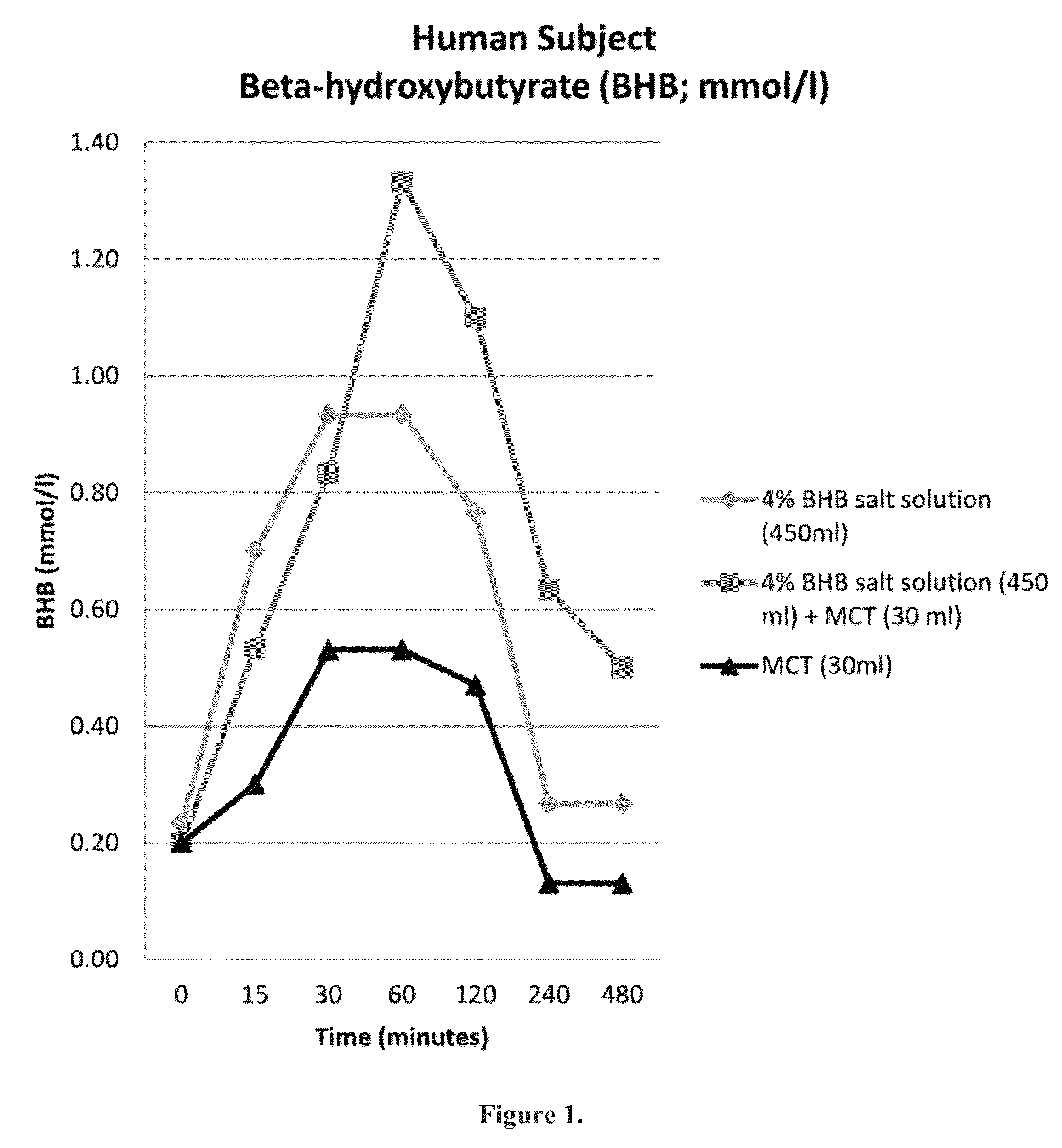
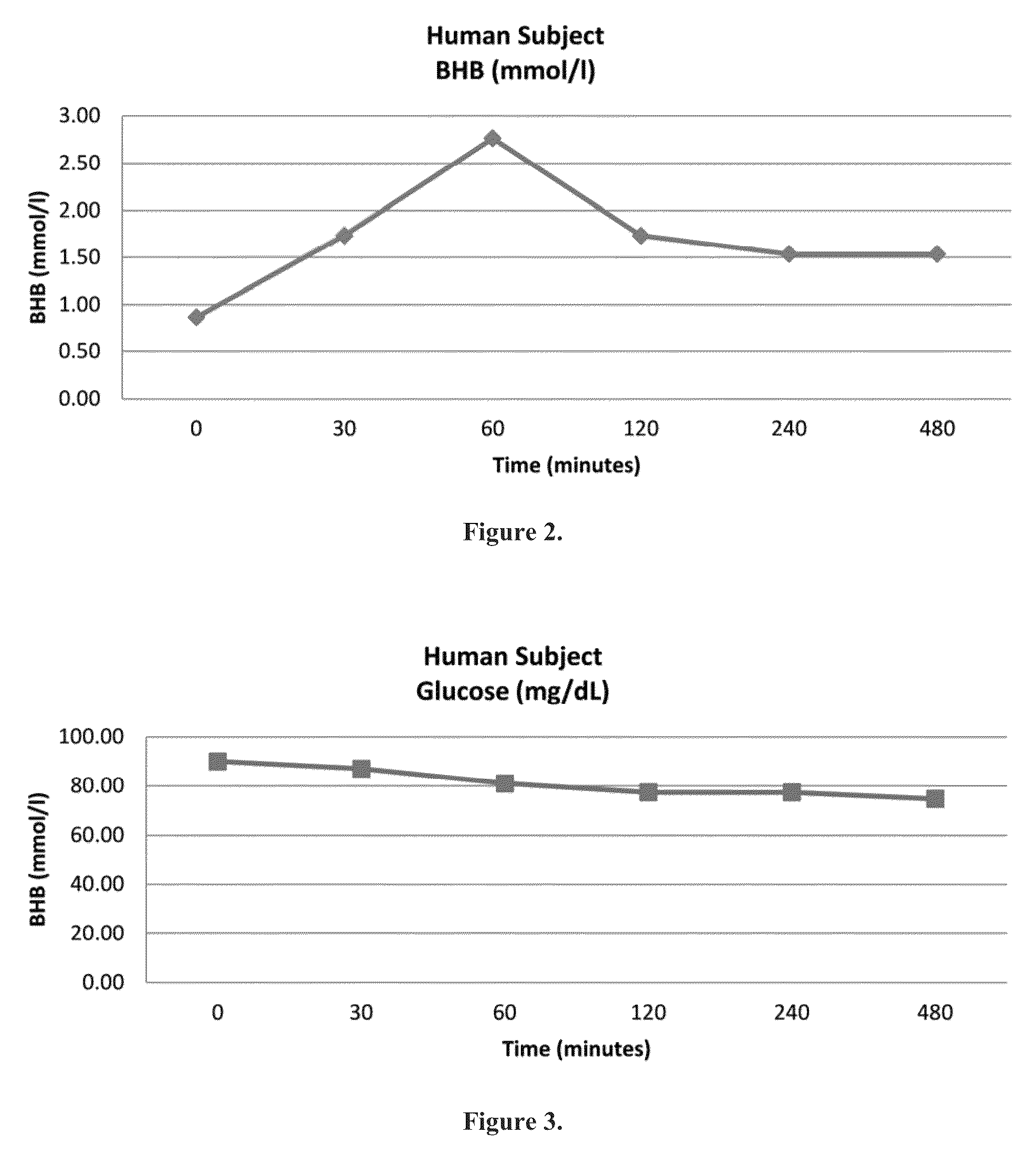
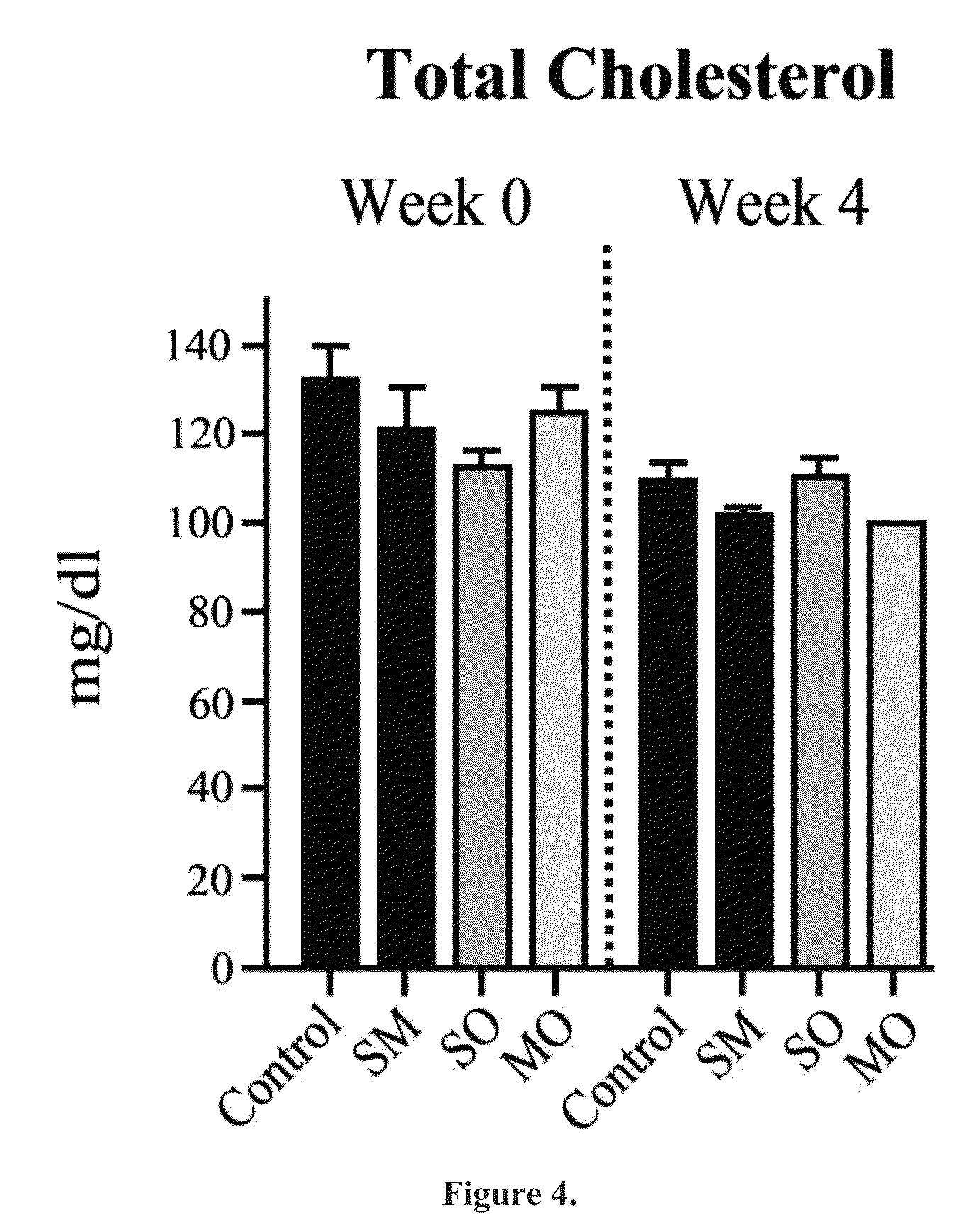
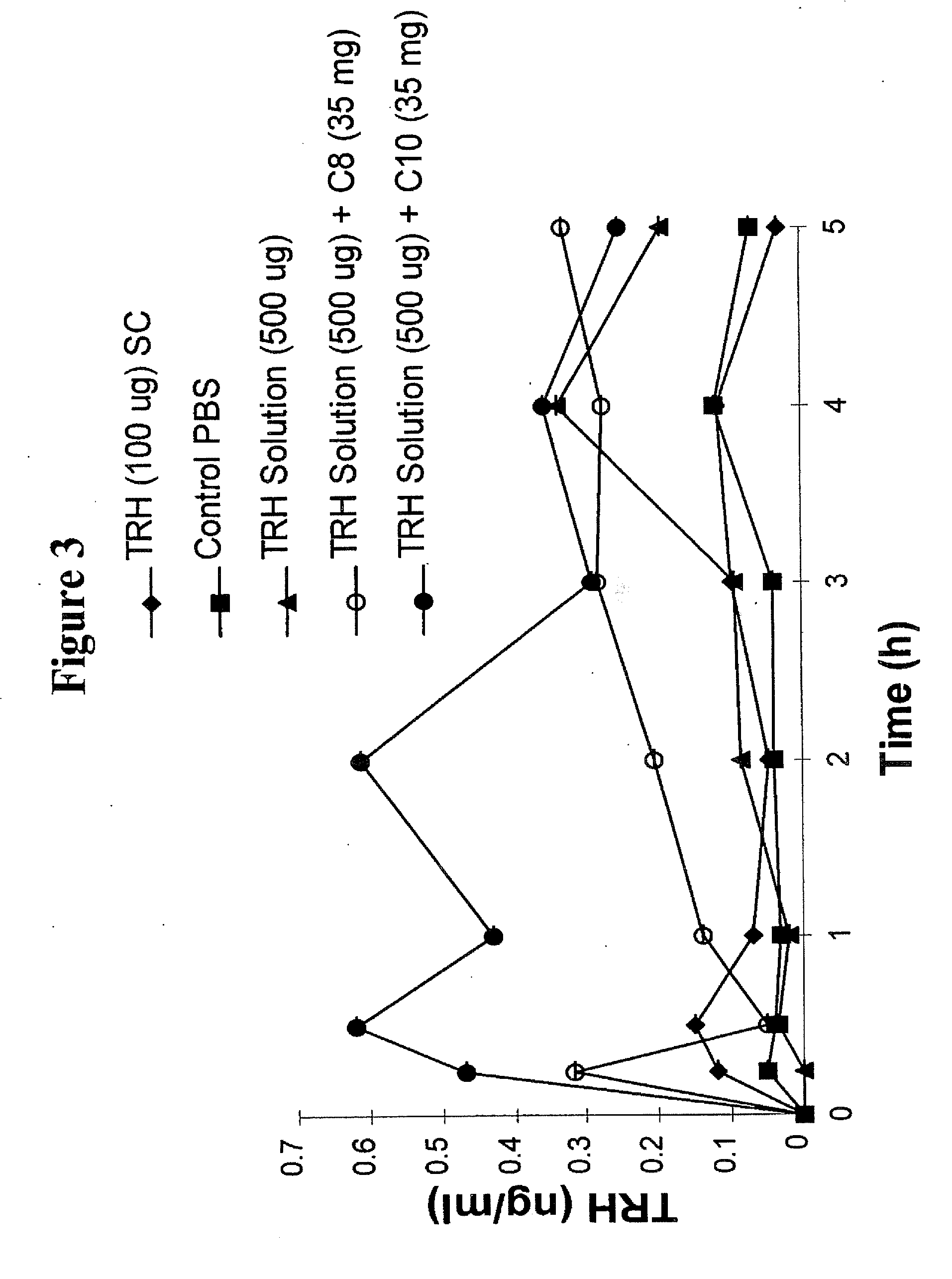
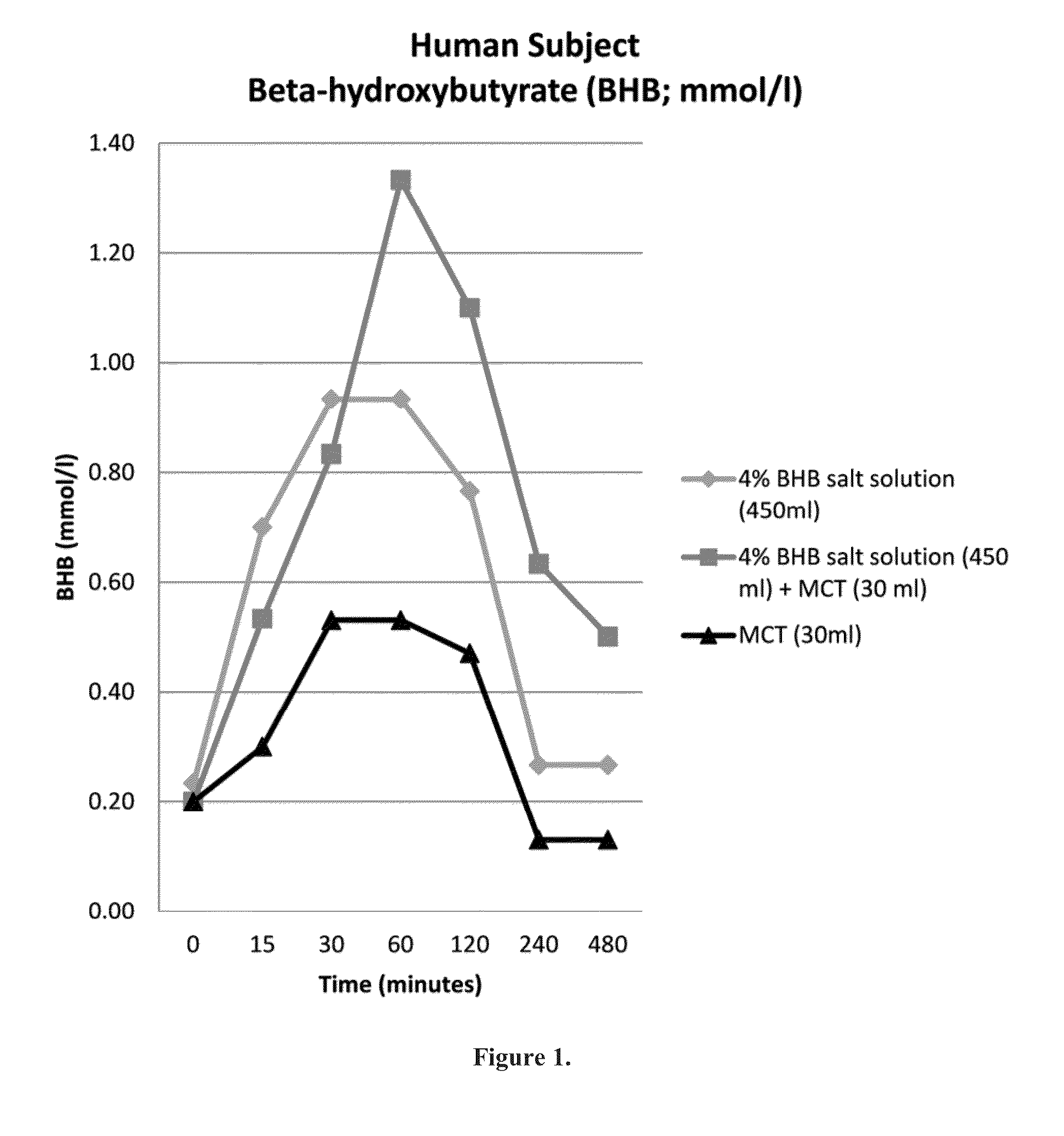
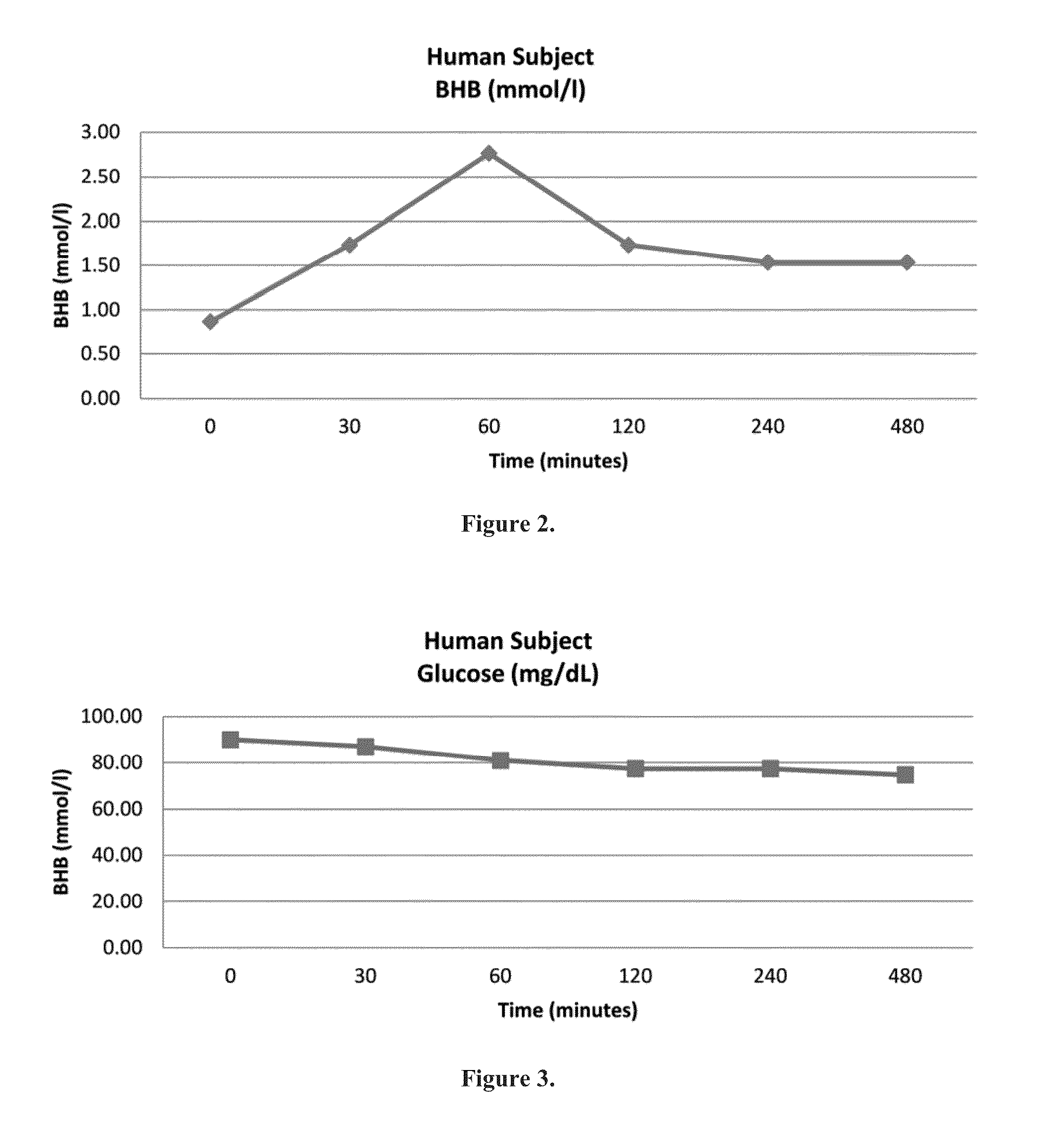
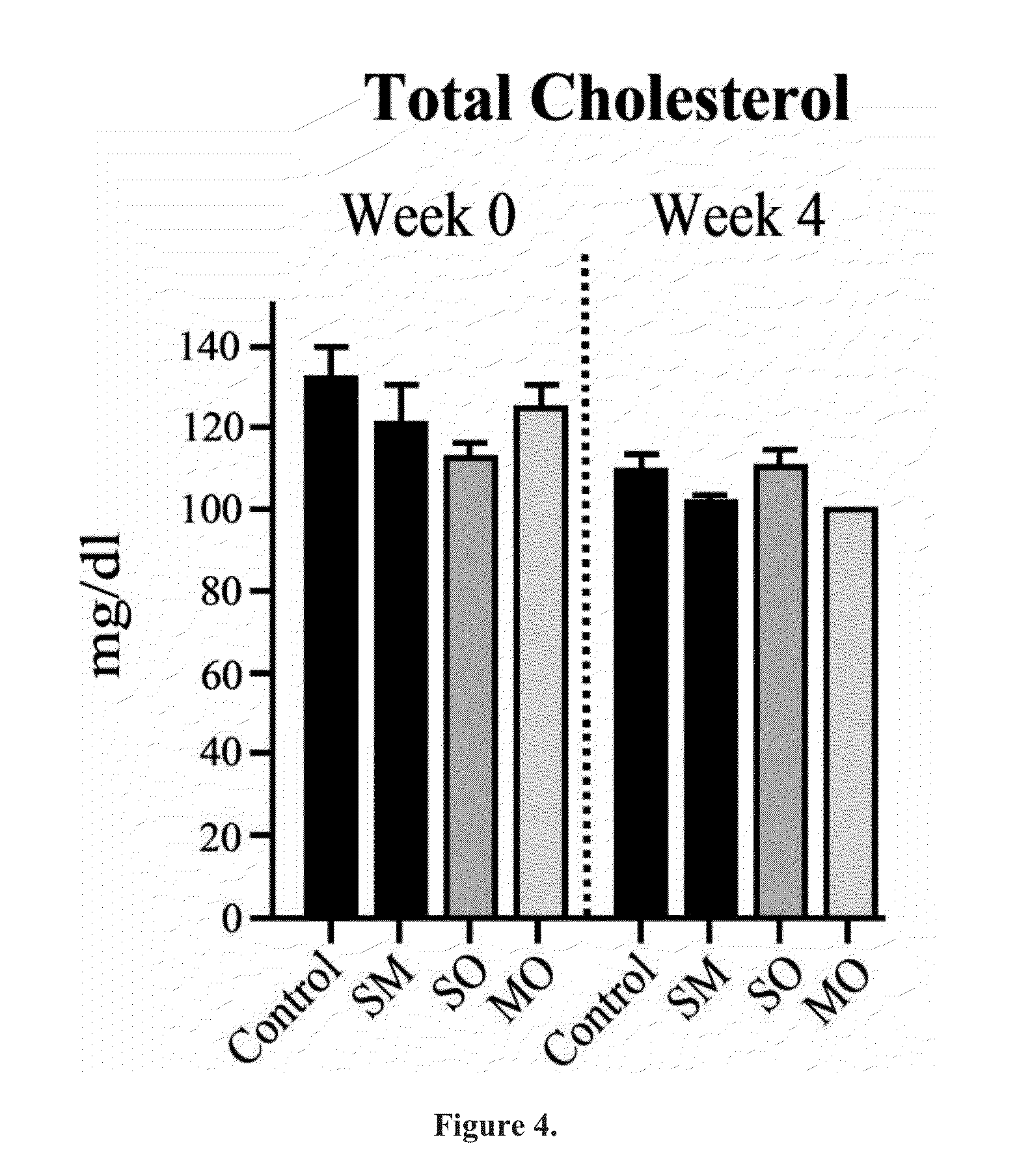
Beta-hydroxybutyrate mineral salts in combination with medium chain fatty acids or an ester thereof such as medium chain triglycerides were used to induce ketosis, achieving blood ketone levels of (2-7 mmol / L), with or without dietary restriction. The combination results in substantial improvements in metabolic biomarkers related to insulin resistance, diabetes, weight loss, and physical performance in a short period of time. Further, use of these supplements to achieve ketosis yields a significant elevation of blood ketones and reduction of blood glucose levels. Use of these substances does not adversely affect lipid profiles. By initiating rapid ketosis and accelerating the rate of ketoadaptation, this invention is useful for the avoidance of glucose withdrawal symptoms commonly experienced by individuals initiating a ketogenic diet, and minimizes the loss of lean body mass during dietary restriction.
Owner:UNIV OF SOUTH FLORIDA
Solid oral dosage form containing an enhancer
InactiveUS20070196464A1Improve oral bioavailabilityMinimizes risk of local irritationBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid oral dosage form containing an enhancer
InactiveUS20080275001A1BiocideOrganic active ingredientsDelayed Release Dosage FormAdditive ingredient
The invention relates to a solid oral dosage form comprising a pharmaceutically active ingredient in combination with an enhancer which enhances the bioavailability and / or the absorption of the active ingredient. Accordingly, a solid oral dosage form comprises a drug and an enhancer wherein the enhancer is a medium chain fatty acid ester, ether or salt or a derivative of a medium chain fatty acid, which is, preferably, solid at room temperature and which has a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Compositions and methods for producing elevated and sustained ketosis
ActiveUS20140350105A1Rapid and sustained elevationImprove metabolic healthBiocideHydroxy compound active ingredientsKetoneSignificant elevation
Beta-hydroxybutyrate mineral salts in combination with medium chain fatty acids or an ester thereof such as medium chain triglycerides were used to induce ketosis, achieving blood ketone levels of (2-7 mmol / L), with or without dietary restriction. The combination results in substantial improvements in metabolic biomarkers related to insulin resistance, diabetes, weight loss, and physical performance in a short period of time. Further, use of these supplements to achieve ketosis yields a significant elevation of blood ketones and reduction of blood glucose levels. Use of these substances does not adversely affect lipid profiles. By initiating rapid ketosis and accelerating the rate of ketoadaptation, this invention is useful for the avoidance of glucose withdrawal symptoms commonly experienced by individuals initiating a ketogenic diet, and minimizes the loss of lean body mass during dietary restriction.
Owner:UNIV OF SOUTH FLORIDA
Algal medium chain length fatty acids and hydrocarbons
InactiveUS20120135478A1Easy to convertUnicellular algaeLiquid carbonaceous fuelsChain lengthNannochloropsis
The present invention provides methods and compositions for production of algal-based medium chain fatty acids and hydrocarbons. More specifically, the invention relates to a Nannochloropsis algal strain and mutants that produces high amounts of C16 fatty acids and hydrocarbons. The present invention provides methods and compositions for production of algal-based medium chain fatty acids and hydrocarbons. More specifically, the invention relates to a Nannochloropsis algal strain and mutants that produces high amounts of C16 fatty acids and hydrocarbons.
Owner:ARIZONA STATE UNIVERSITY
Mixed salt compositions for producing elevated and sustained ketosis
ActiveUS20170258745A1Improved metabolic healthLimiting, preventing, or improving an electrolyte imbalancePowder deliveryDispersion deliveryPotassiumExcipient
Ketogenic compositions including a beta-hydroxybutyrate (BHB) mixed salt are formulated to induce or sustain ketosis in a subject to which the ketogenic compositions are administered. The BHB mixed salt is formulated to provide a biologically balanced set of cationic electrolytes, and is formulated to avoid detrimental health effects associated with imbalanced electrolyte ratios. A ketogenic composition includes BHB salts of at least sodium, potassium, calcium, and magnesium. The BHB salts may also include at least other component such as a BHB compound containing other cations, such as transition metal cations (e.g., zinc or iron), a BHB-amino acid salts, medium chain fatty acid source, vitamin D3, flavorant, or other excipient.
Owner:AXCESS GLOBAL SCI LLC
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20100028421A1BiocidePeptide/protein ingredientsDelayed Release Dosage FormAdditive ingredient
The invention relates to a solid oral dosage form comprising a pharmaceutically active ingredient in combination with an enhancer which enhances the bioavailability and / or the absorption of the active ingredient. Accordingly, a solid oral dosage form comprises a drug and an enhancer wherein the enhancer is a medium chain fatty acid ester, ether or salt or a derivative of a medium chain fatty acid, which is, preferably, solid at room temperature and which has a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Appetite suppressing diet bar
InactiveUS6884454B2Improve the level ofAppetite suppressantVitamin food ingredientsFood preparationAnimal scienceMedium-chain triglyceride
A method to decrease feed intake in humans by ingesting a diet bar comprising of whole soybean and medium chain triglycerides and / or medium chain fatty acids, said bar containing an adequate level of nutrients to serve as a meal.
Owner:PIMENTEL JULIO LIONEL
Solid oral dosage form containing an enhancer
The invention relates to a solid oral dosage form comprising a pharmaceutically active ingredient in combination with an enhancer which enhances the bioavailability and / or the absorption of the active ingredient. Accordingly, a solid oral dosage form comprises a drug and an enhancer wherein the enhancer is a medium chain fatty acid ester, ether or salt or a derivative of a medium chain fatty acid, which is, preferably, solid at room temperature and which has a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Comprehensive nutritional and semi-digested formulated food for special medical use, and preparation method thereof
ActiveCN104082739AImprove immunityBlood sugar controlSugar food ingredientsFood ingredient functionsDiseaseVegetable oil
The invention provides a comprehensive nutritional and semi-digested formulated food for special medical use, and a preparation method thereof. Every 100 g of the comprehensive nutritional and semi-digested formulated food for special medical use comprises 5-15 g of hydrolyzed whey protein, 3-8 g of egg white protein, 1-4 g of soy isolate protein, 1-4 g of concentrated whey protein, 0.5-2 g of sodium caseinate, 37-60 g of carbohydrate with a low GI value, 1-4 g of fructose oligosaccharide, 0.005-0.05 g of carboxymethyl pachymaran, 15-22 g of vegetable oil powder, 4-10 g of a medium-chain fatty acid, 3-8 g of a mineral premix and 0.2-1.4 g of a vitamin premix. A finished product is prepared by mixing the above components uniformly. With a synergistic effect of the above components, partial digestion functions of a patient after operation can be taken fully used to keep, maintain and promote comprehensive restoration of the digestion functions of the patient; an immune function can be regulated; and body disease-resistant ability can be enhanced, thereby influencing development and conversion of the disease.
Owner:广州纽健生物科技有限公司
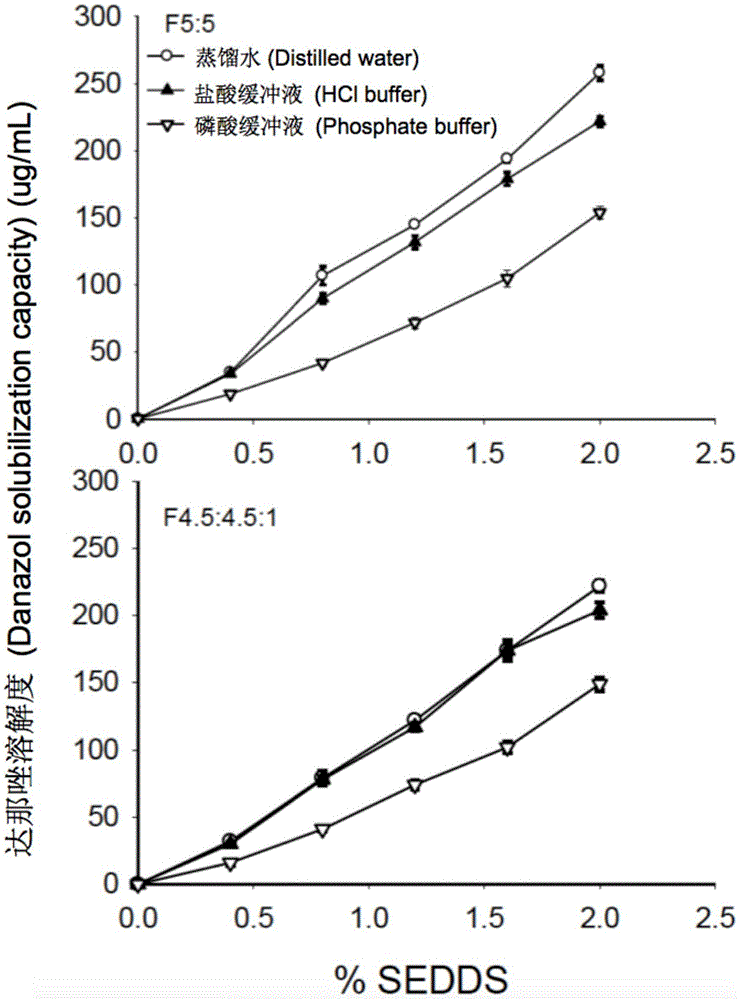
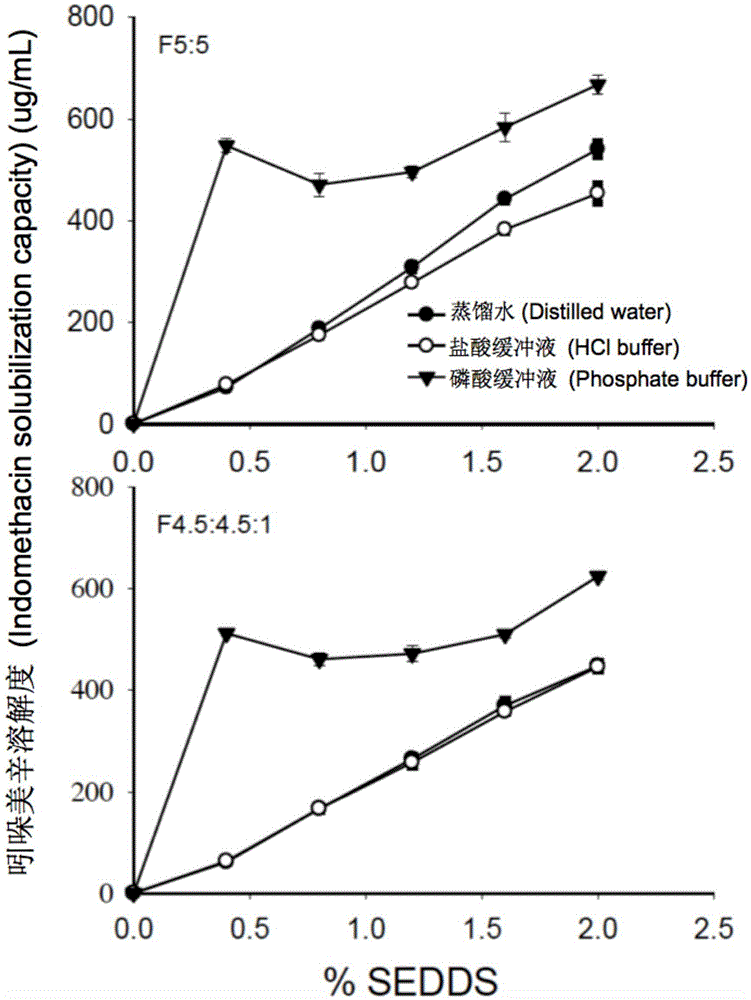
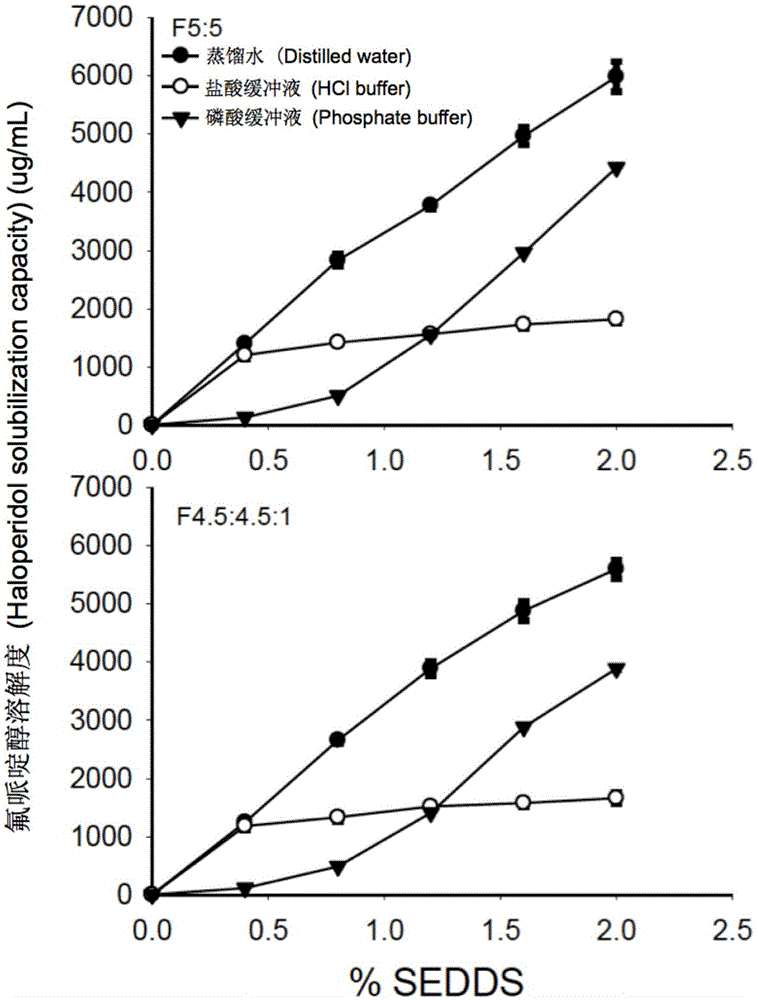
Self-emulsifying drug delivery system for improving bioavailability of insoluble medicine, and application thereof
ActiveCN105535979AGood water solubilityIncrease dissolution rateOrganic active ingredientsCapsule deliverySolubilityOil phase
The invention provides a novel self-emulsifying drug delivery system (SEDDS). An SEDDS carrier material comprises a surfactant and an oil phase containing Capmul MCMs and medium chain fatty acids, is suitable for loading pH-dependent (weakly acidic and weakly alkaline) and a pH-independent (neutral) insoluble medicines, greatly improves the solubility of the medicines to realize optimum bioavailability, and has important application values in the development of preparations of the insoluble medicines.
Owner:李素华
Medium carbon chain fatty acid powder grease and preparation method thereof
ActiveCN101919453AImprove stabilityAvoid the risk of ingestionEdible oils/fatsDipotassium hydrogen phosphateGlucose-Fructose Syrup
The invention discloses medium carbon chain fatty acid powder grease and a preparation method thereof. The medium carbon chain fatty acid powder grease comprises the following components in percentage by weight: 40%-60% of MCT (Medium Chain Fatty Acid) grease, 4%-6% of sodium caseinate, 0.5%-2.0% of monoglyceride, 0.3%-0.7% of sodium stearoyl lactylate, 1%-2% of dipotassium hydrogen phosphate, 0.2%-0.4% of silicon dioxide and the balance of glucose syrup. The invention greatly enhances the stability of the MCT grease, extends the application of the MCT grease in the field of foods, prevents the risk of excessive grease intake and also reduces the potential hazards of excessive antioxidant intake.
Owner:JIAHE FOODS IND CO LTD
Compositions and Methods for Improving Cardiovascular Health
InactiveUS20090197820A1Improve muscle massHigh strengthBiocideMuscular disorderEssential amino acidCardiovascular health
Compositions and methods for improving cardiovascular health in the elderly by delivering a selection of essential amino acids, phytosterols, stanols, and isoflavones, which may be supplemented with a low glycemic carbohydrate and / or a medium chain fatty acid.
Owner:ENERGY LIGHT
Production method for medium-chain and long-chain oil fatty acid
InactiveCN103897811AResolution timeSolve the problem of energy consumptionFatty acid esterificationFatty acids production/refiningAlkaline waterLong chain fatty acid
The invention discloses a production method for medium-chain and long-chain oil fatty acid. The production method comprises the following steps: carrying out transesterification reaction, saturated alkaline water neutralization, adsorbent desoaping, vacuum flashing and the like to medium-chain fatty acid triglycerides of C6-C12 and long-chain fatty acid triglycerides of C16-C24 in a reactor filled with lipase, to obtain the long-chain oil fatty acid in the product. The method solves the difficulty that the medium-chain and long-chain oil fatty acid in the prior art is complicated, and has the characteristics of being short in reaction time of the reactor, and low in energy consumption.
Owner:河北康睿达脂质有限公司
Composition for animal consumption
InactiveUS20050100584A1Reduce weight gainOrganic active ingredientsBiocideBiotechnologyWeight gaining
This invention is directed generally to compositions (including foods, supplements, treats, toys, etc.) for animal consumption, particularly compositions that tend to aid in weight loss or reduction in weight gain, and particularly compositions that comprise one or more medium chain fatty acid triglycerides (“MCT”). This invention also is directed generally to methods for using such compositions. This invention is further directed generally to processes for making such compositions.
Owner:HILLS PET NUTRITION INC
Polyphyly fat mixture for feeding animals and microcapsule applying mixture
InactiveCN101647512ACompatible with growth performanceProductive full expressionAnimal feeding stuffAccessory food factorsLong chain fatty acidSodium Caseinate
The invention discloses a polyphyly fat mixture for feeding animals, which is formed by mixing more than two greases. The mixture comprises long chain fatty acid and medium chain fatty acid, and the weight ratio of the long chain fatty acid to the medium chain fatty acid is 30-90:60-10. A microcapsule applying the polyphyly fat mixture for feeding animals comprises the following raw materials in percentage by weight: 25-80 percent of olyphyly fat mixture, 0.8-4 percent of complex emulsifying agent F, 0.8-4 percent of sodium stearoyl-2-lactylate, 0.3-5 percent of sodium caseinate and 10-70 percent of encrusting substance. The polyphyly fat mixture accords with the growth performance of animals and ensures that the production performance of the animals can be fully expressed. Experiments prove that after feeding the polyphyly fat mixture, the fresh of piggies is improved by 3.96 compared with piggies fed with single derived fat feed. By adopting the fatty acid mode and fat products, after feeding nursing sows, the weight of weaning litter of the piggies is improved by 3.96 percent. After feeding the polyphyly fat mixture, the fresh of broiler chickens is improved by 3.96 percent compared with broiler chickens fed by single derived fat.
Owner:SINGAO
Pharmaceutical Compositions of Iron for Oral Administration
InactiveUS20120189692A1Increases bioavailability of ironBiocideHeavy metal active ingredientsOral medicationPhysiology
The present invention generally relates to orally administered pharmaceutical compositions of iron compounds with medium chain fatty acid salts. The invention further relates to methods of using the pharmaceutical compositions to treat iron deficiency and related disorders.
Owner:NOVO NORDISK AS
Structural type triglyceride rich in medium-chain fatty acid and preparation method thereof
ActiveCN104988190AWide variety of sourcesReduce energy consumptionFermentationCarbon numberPalmitates
The invention provides a structural type triglyceride rich in medium-chain fatty acid and a preparation method thereof. The provided triglyceride is prepared from medium-chain fatty acid with a carbon number of 8-12, palmitic acid, glycerin, and long-chain unsaturated fatty acid / fatty acid ester through catalytic reactions. In the provided triglyceride, medium-chain fatty acid accounts for 11 to 40 wt% of total fatty acid; Sn-2 position saturated fatty acid accounts for 52 to 80 wt% of total saturated fatty acid; Sn-1,3 position unsaturated fatty acid accounts for 80 to 95 wt% of total unsaturated fatty acid, the content of Sn-USU type triglyceride is 40 to 80%, and the content of triglyceride palmitate is 3 to 8%. The provided triglyceride has the characteristics of medium-chain triglyceride and long-chain triglyceride; compared with the common medium / long-chain fatty acid edible oil, the provided triglyceride has richer structures, according to the product characteristics, and the provided triglyceride can be used as a food nutrient supplement and added in different food formulas. The preparation method has the characteristics of high production efficiency, low energy consumption, mild reaction conditions, little using amount of enzyme, more precise procedure, and effectively-reduced cost.
Owner:XIAMEN SHUANGBEI BIOTECH CO LTD
Resourceful treatment method for organic waste
ActiveCN107363076AReduces chemical oxygen demand (COD)Promote emission standardsSolid waste disposalCarbon chainWaste treatment
The invention relates to the technical field of treatment and resourceful utilization of organic waste and aims at providing a resourceful treatment method for organic waste. The resourceful treatment method can improve the economic benefits of fermentation products. According to the adopted technical scheme, the method includes the steps of lactic acid fermentation, carbon chain lengthening and medium-chain fatty acid extracting, wherein according to carbon chain lengthening, microorganisms for synthesizing medium-chain fatty acid are added for anaerobic fermentation; and the organic waste is biodegradable organic waste. The brand new organic waste treatment method is provided, in other words, the technology for synthesizing medium-chain fatty acid as the main product through coupling of organic matter hydrolysis lactic acid generating and carbon chain lengthening is provided. The principle of the technology is obviously different from a previous anaerobic biochemical treatment technology, harmlessness and stability of organic waste are achieved, meanwhile, medium-chain fatty acid which is easy to extract and has high economic benefits can be generated, organic matter such as ethyl alcohol does not need to be additionally added, the technological cost is greatly reduced, and the economic benefits of the technology are obviously reduced.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Child care pig compound feed without tensile body and preparation method thereof
InactiveCN102919573AIncrease feed intakeImprove immunityFood processingAnimal feeding stuffFeed conversion ratioPhytase
The invention relates to child care pig compound feed without a tensile body, and the child care pig compound feed is prepared from the following components: corn, peeled soybean meal, puffed soybean meal, fermented soybean meal, steam white fish meal, flour, soybean oil, mountain flour, calcium hydrogen phosphate, edible salt, child car pig compound premix, coated slow-release acidifier, L-lysine hydrochloride, DL methionine, L-thronine, tryptophan, octanoic / decanoic acid glyceride, yeast nucleic acid, xylo-oligosaccharides, sodium glutamate, sodium butyrate, the root of Chinese pulsatilla, coptis chinensis, golden cypress, ash bark, choline chloride and phytase. The child care pig compound feed without the tensile body provided by the invention starts from the physiological characteristics of a child care pig, adopts multiple combinations of raw materials, is complementary in nutrition, designs amino-acid-balanced, calcium-phosphate-balanced and electrolyte-balanced daily ration, adds no antibiotic, adopts medium-chain fatty acid and natural Chinese herbal medicine additive for health protection, and can be used for improving food consumption of the child care pig, reducing the feed conversion ratio, improving intestinal health of the child care pig, improving immunity and drawing a skeleton of the child care pig, so that a good foundation is laid for later-stage growth.
Owner:HUAIAN ZHENGCHANG FEED
Medium-chain length fatty acids, glycerides and analogues as stimulators of erythropoiesis
ActiveUS20060128800A1Satisfies needMeet actual needsBiocidePharmaceutical non-active ingredientsRed blood cellErythropoiesis
Use of a composition comprising a compound of any of formulae I, II, Ila, III and Illa; or a combination thereof wherein each R1 is independently C7-11 alkyl; A and B are independently H or CO—R1; R2 is H or C1-4 alkyl; M is a metal monocation (k=1) or dication (k=2); Y is 0 or NH; and Z is 0, NH, CH2O or a bond; for the manufacture of a medicament for stimulating erythropoiesis. Preferably, the composition further comprises human erythroporietin.
Owner:PROMETIC PHARMA SMT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com