Compositions of peptides and processes of preparation thereof
a technology of peptides and peptides, which is applied in the field of peptides, polypeptides, proteins, analogues or derivatives, can solve the problems that the stability of peptides has become a significant barrier, and achieve the effects of improving the stability of peptides, and polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
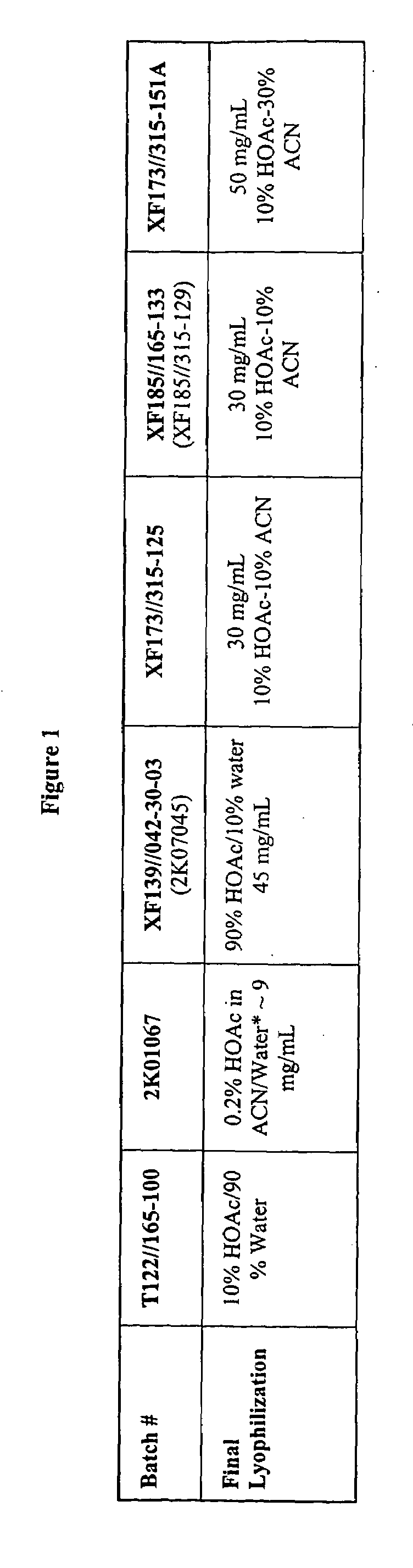
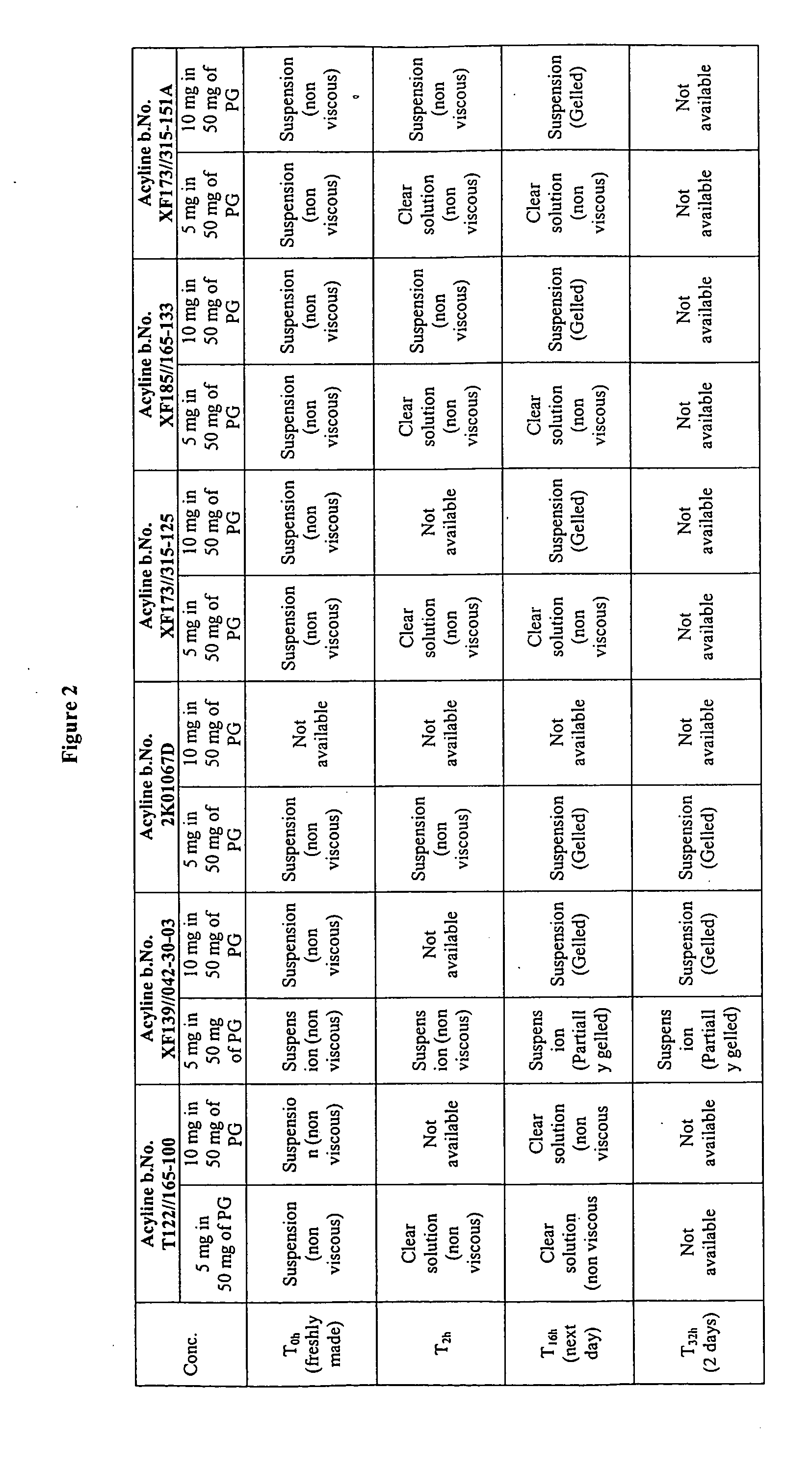
[0098]The study of gelation for different acyline batches in propylene glycol is carried out and the result is summarized in FIG. 2. The 9% and 16.7% of acyline sampls are prepared by using the acyline batches listed in FIG. 1. The 9% acyline sample of XF 173 / 315-125, XF 185 / 165-133 and XF 173 / 315-151A are prepared by using acyline prepared via use of a co-solvent during the lyophilization step. The acyline batches of XF 173 / 315-125, XF 185 / 165-133 and XF 173 / 315-151A appear clear and non-viscous after 2 hours. Other batches which are lyophilized by using water as a solvent did not appear as clear and non-viscous solutions due to the presence of gelled acyline.
example 2
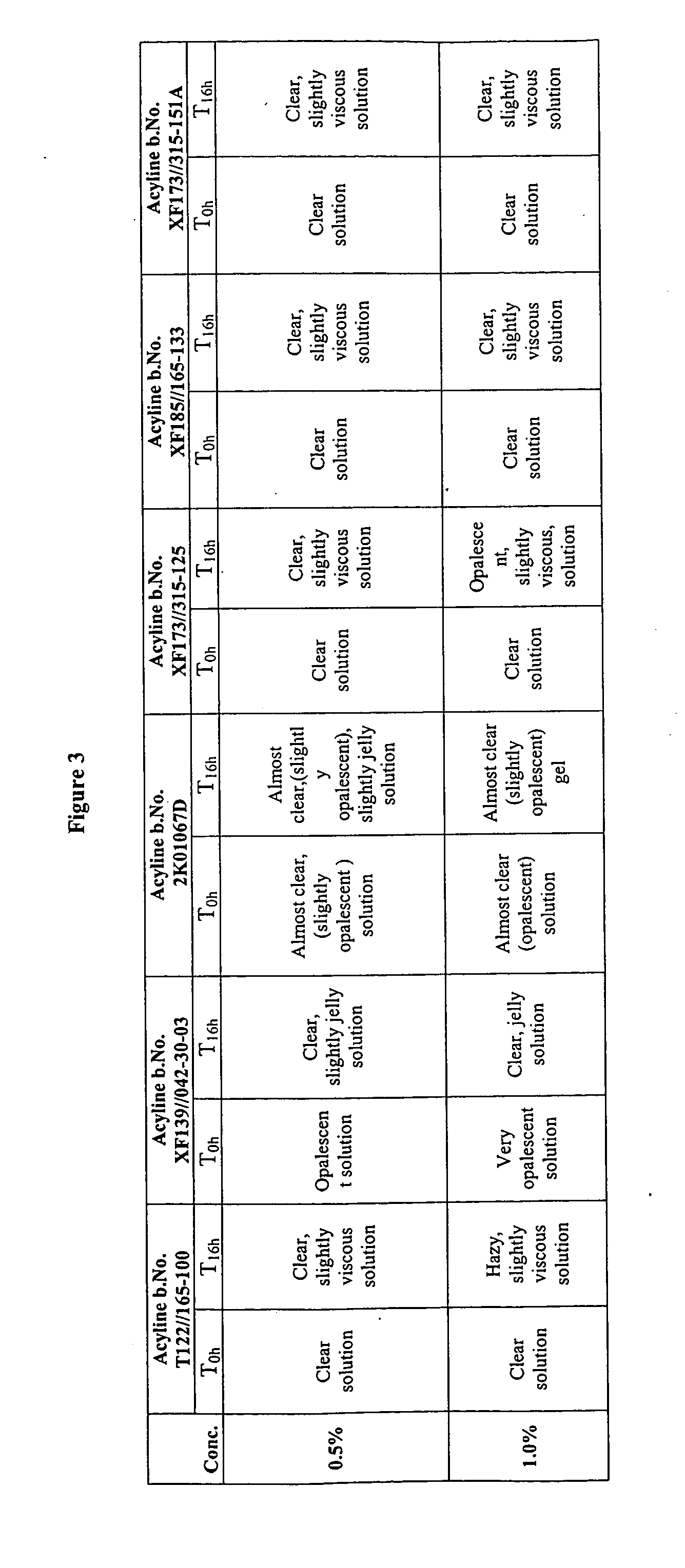
[0099]The gelation of different acyline drug batches in water is investigated and the results are summarized in FIG. 3. The 0.5% and 1% acyline samples are prepared by using the acyline batches listed in FIG. 1. The 0.5% and 1% of acyline samples of XF 173 / 315-125, XF 185 / 165-133 and XF 173 / 315-151A which are prepared by using co-solvent during the lyophilization step appear clear and non viscous upon dissolving in water.
example 3
[0100]The tendency of gelation of different acyline batches in standardized microemulsion (SM) is investigated. The result is summarized in FIG. 4(a). The formulation of the standard micro emulsion is shown in FIG. 4(b). The 5 mg and 10 mg formulations of acyline in a microemulsion are prepared by using the acyline lots synthesized in FIG. 1. 5 mg and 10 mg dose of acyline batches of XF 173 / 315-151A which are prepared by using co-solvent during the lyophilization step appeared clear and transparent after 2 hours.
2. Application of Anti-Gelling Agents in the Formulation of Acyline Compositions General Procedures of Comparison Experiments
[0101]Different concentrations of acyline composition are prepared by adding acyline to pH 6.8 buffer solution either at room temperature or 37° C. All acyline solutions contain 0.6 mg / mL sodium caprate (sodium caprate is referred as C10 in the figures). All samples are centrifuged and filtered prior to analysis. Samples are analyzed by reverse phase H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com