Patents
Literature
90 results about "Diphosphonates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
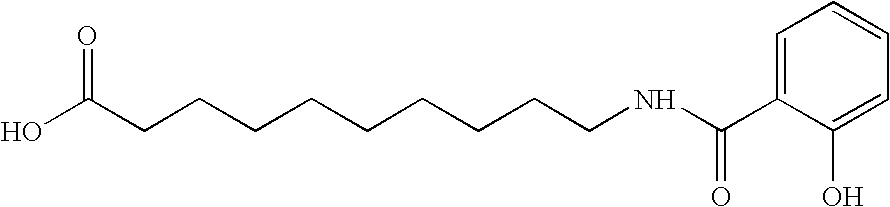
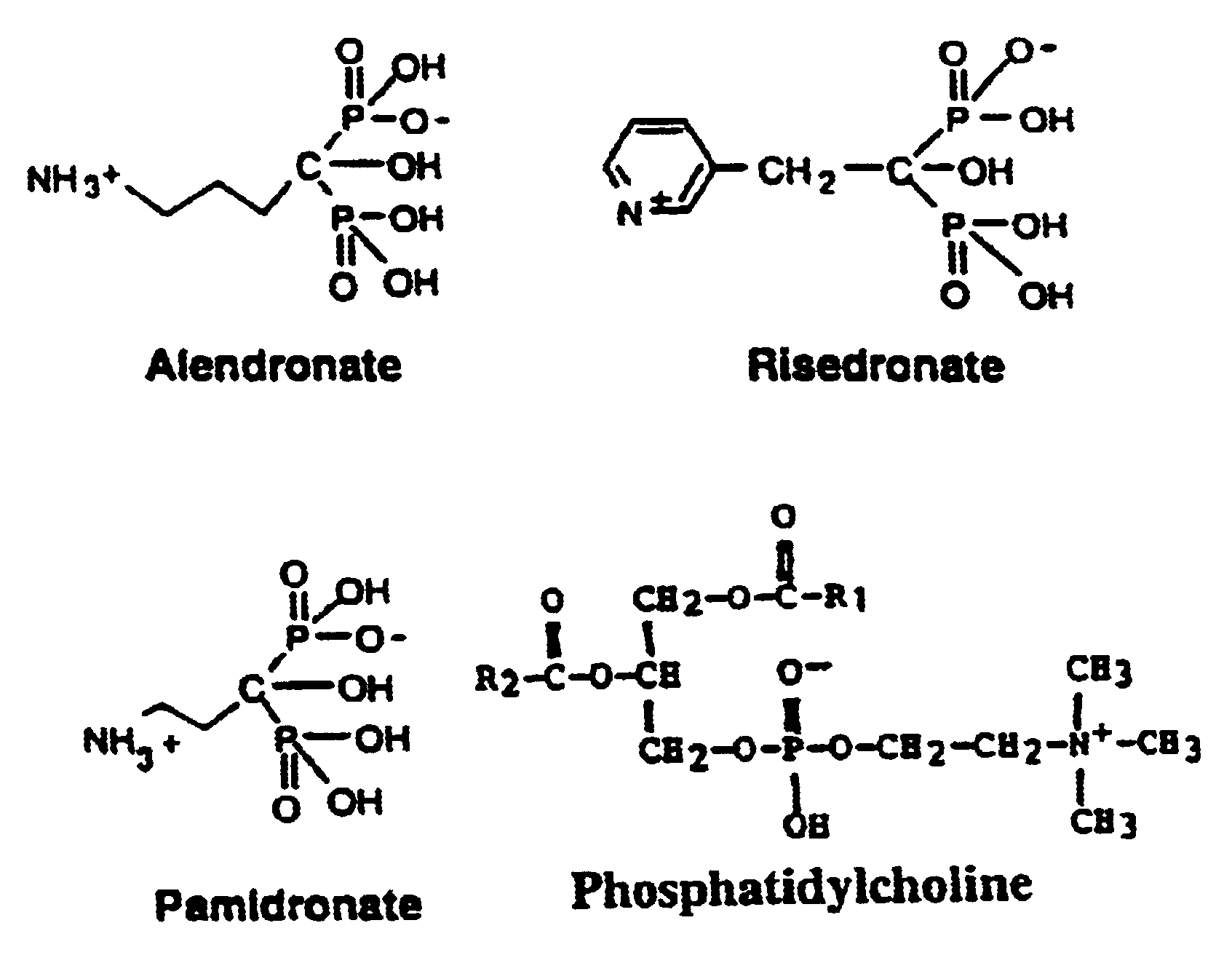
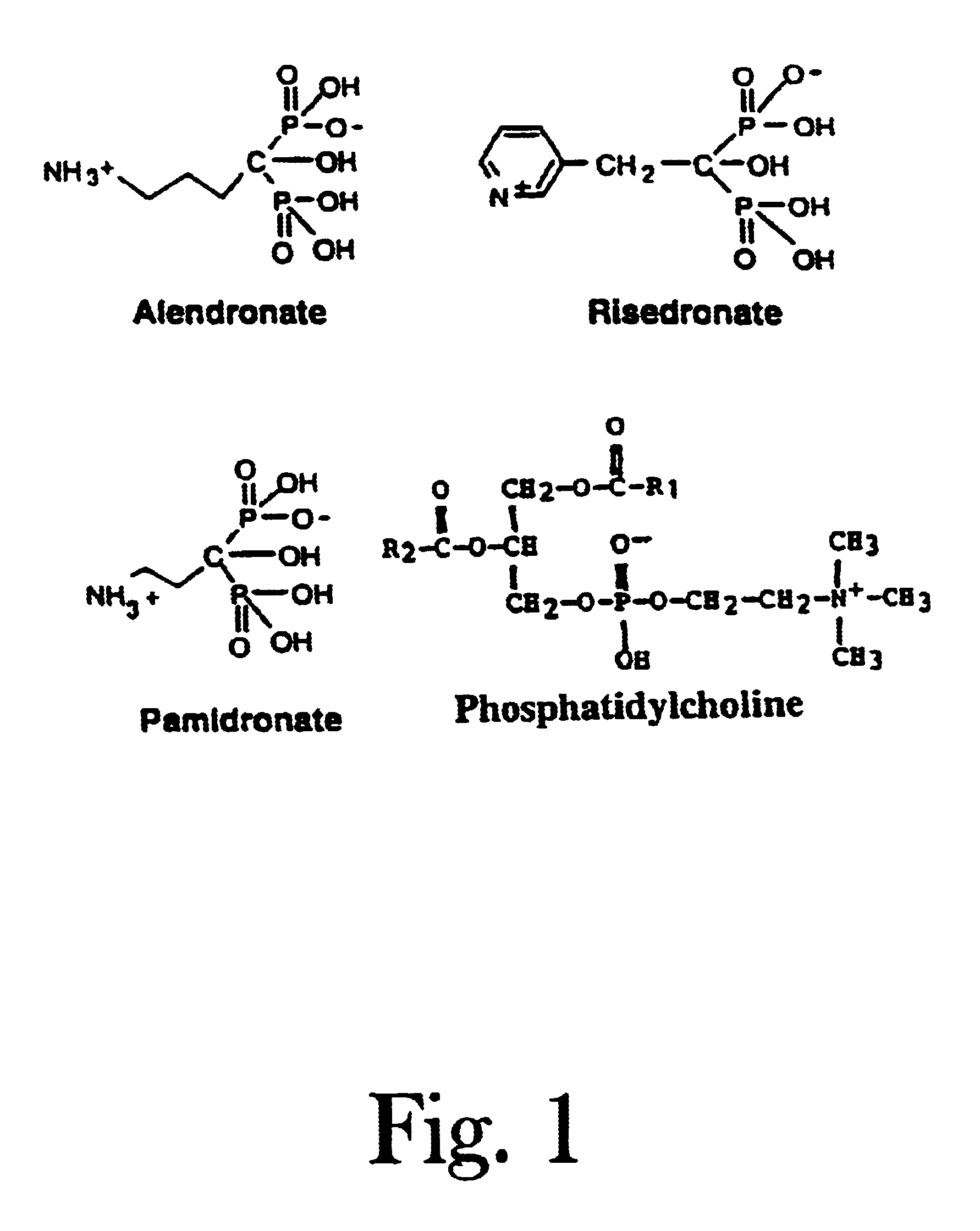
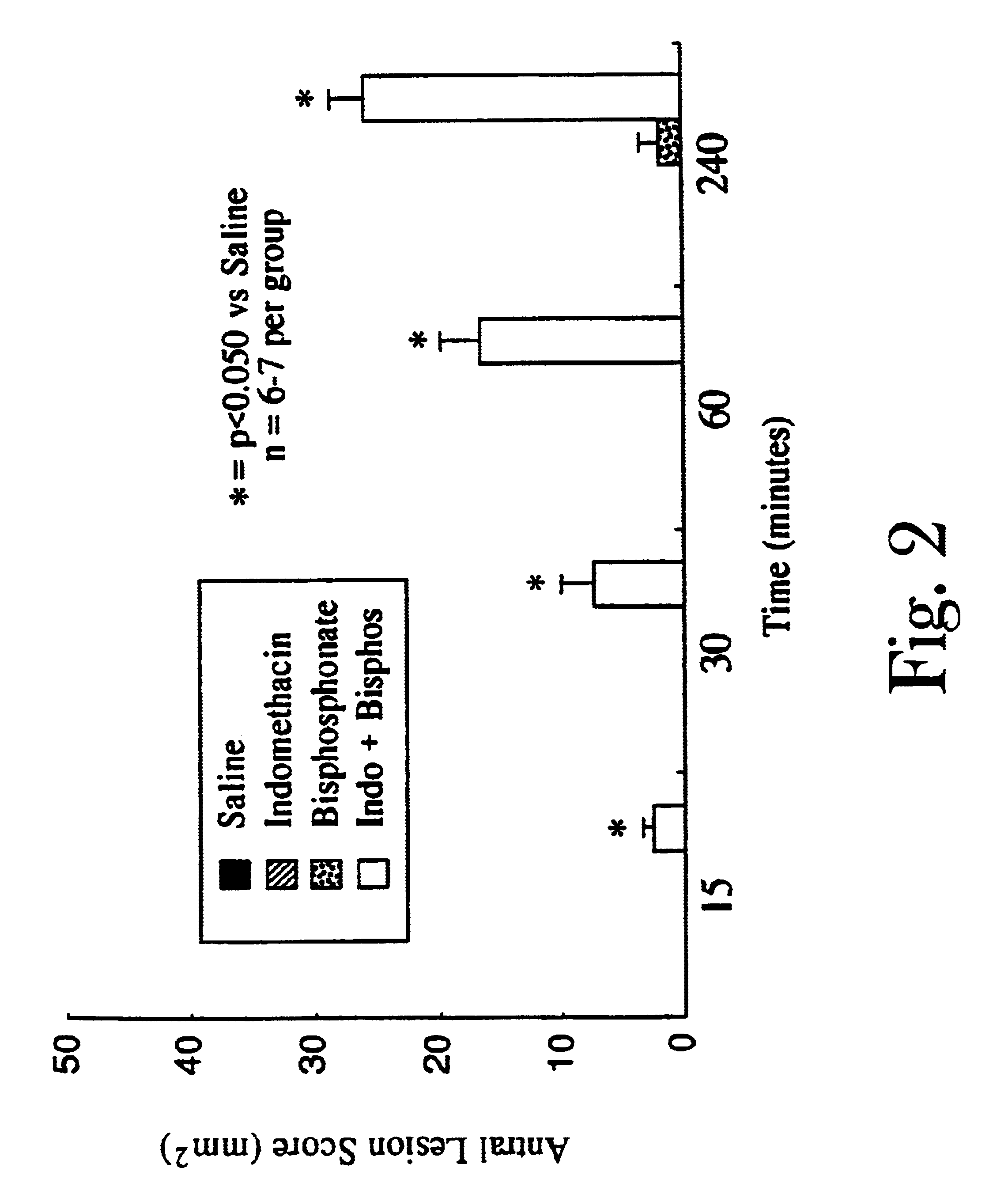
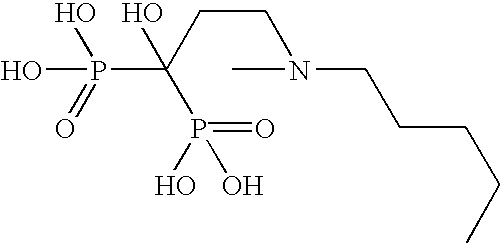
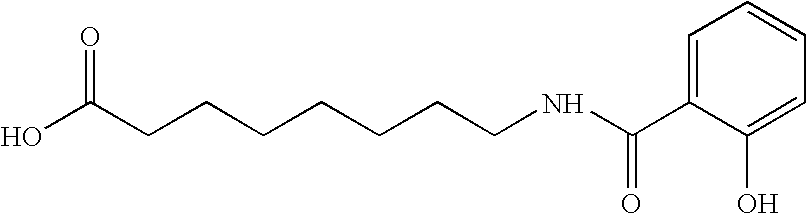
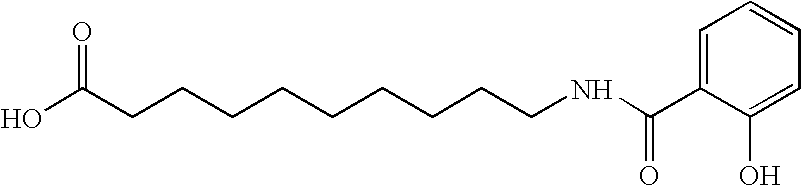
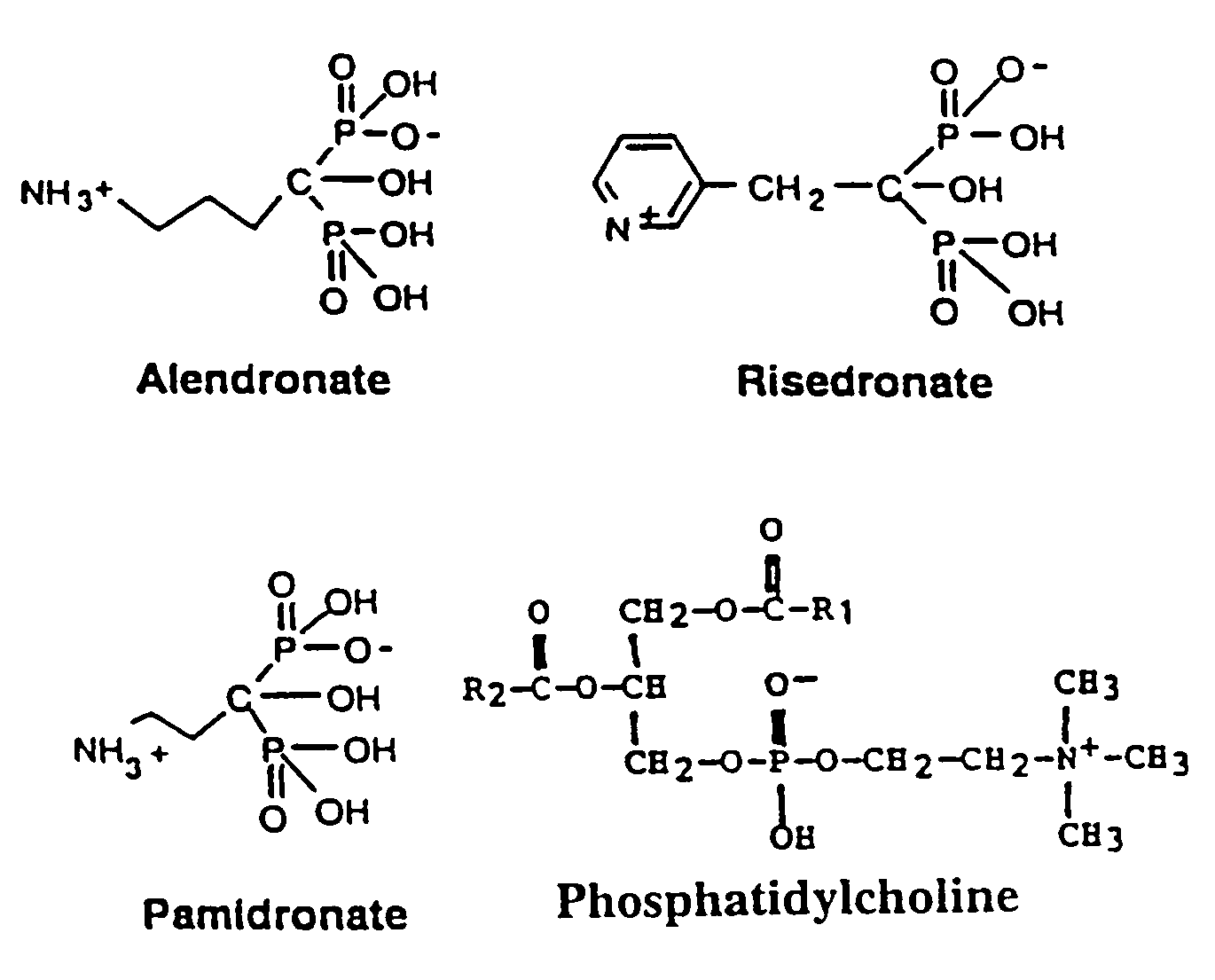
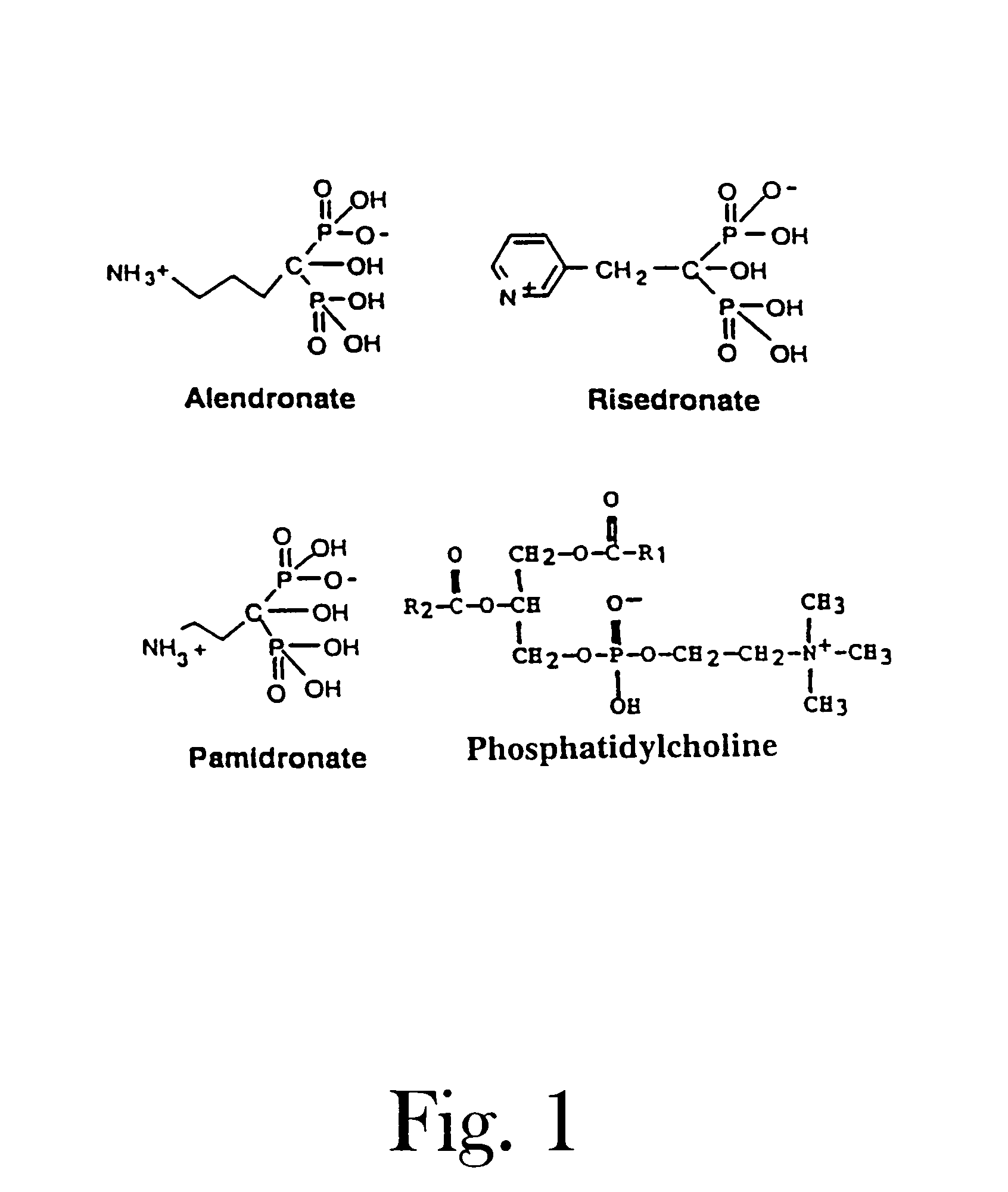
Organic compounds which contain P-C-P bonds, where P stands for phosphonates or phosphonic acids. These compounds affect calcium metabolism. They inhibit ectopic calcification and slow down bone resorption and bone turnover. Technetium complexes of diphosphonates have been used successfully as bone scanning agents.
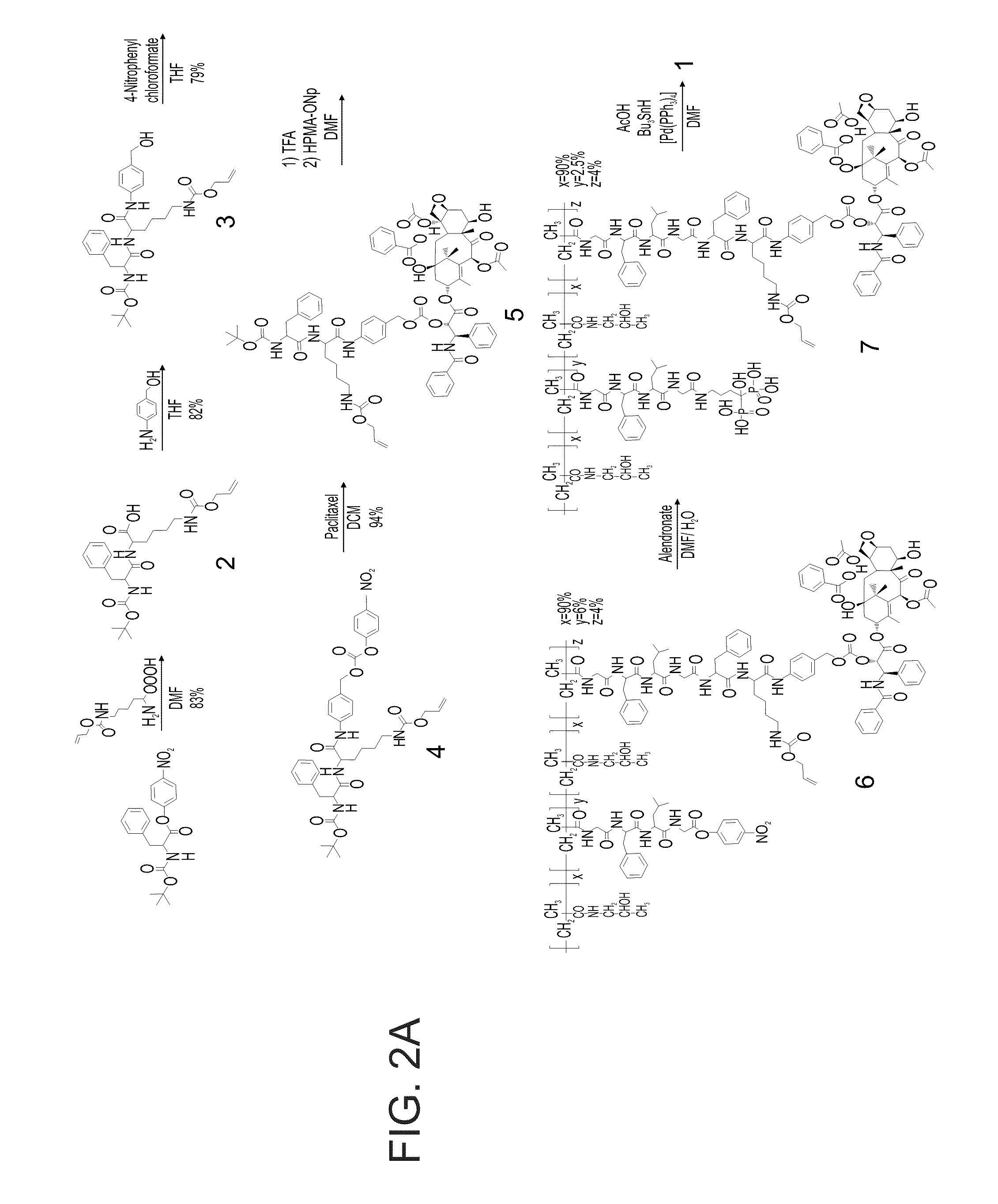
Composition and drug delivery of bisphosphonates
InactiveUS20100215743A1Reduce adverse effectsBiocideOrganic active ingredientsMedicineDiphosphonates
The present invention provides methods of treating or preventing a medical condition that is responsive to a bisphosphonate compound in a subject. The methods comprise administering to the subject a pharmaceutical composition comprising a therapeutically effective amount of the bisphosphonate no less frequently than a bi-weekly dosage schedule. In some embodiment, the bisphosphonate compound is zoledronic acid.
Owner:NOVO NORDISK AS
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
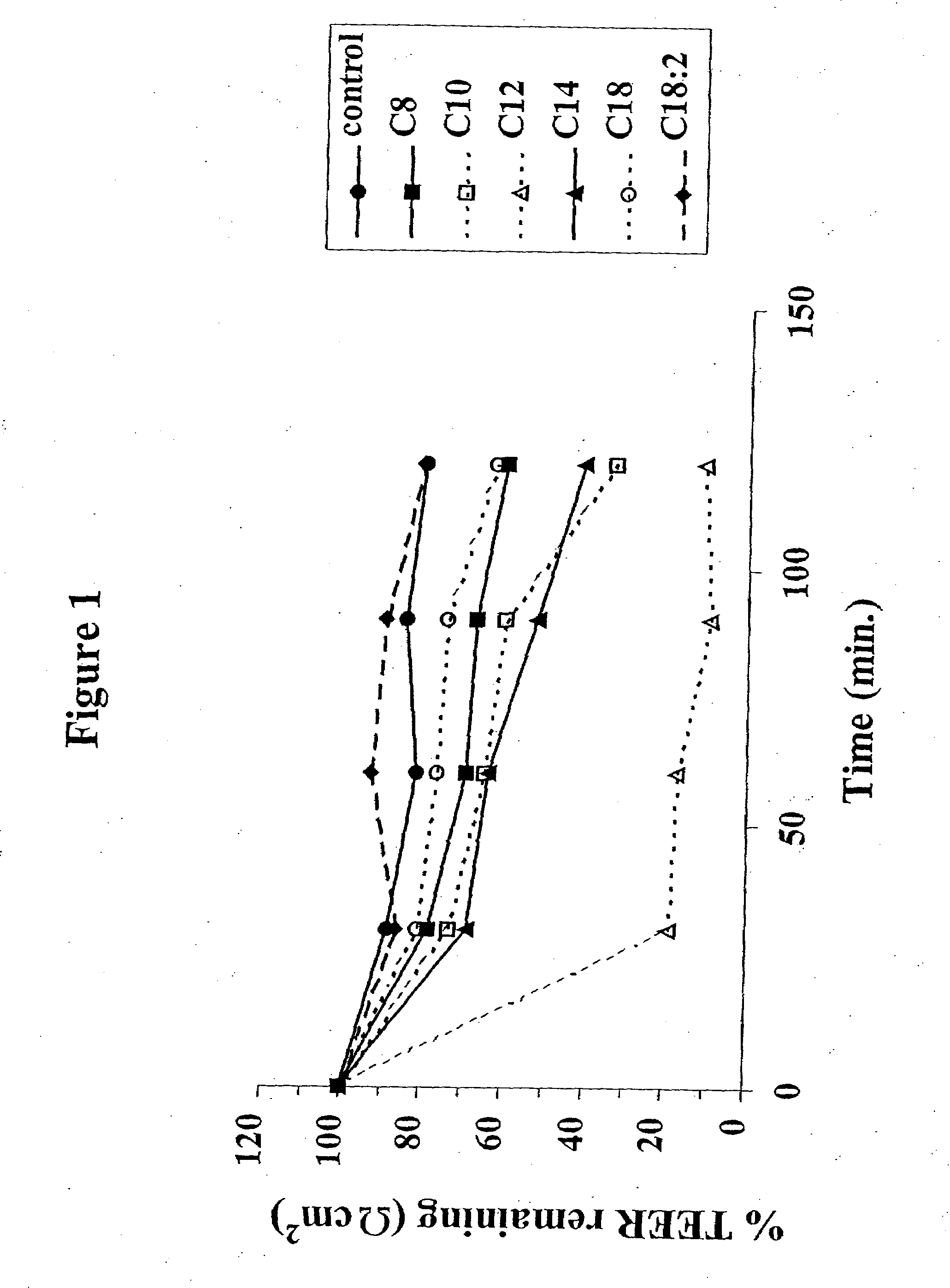
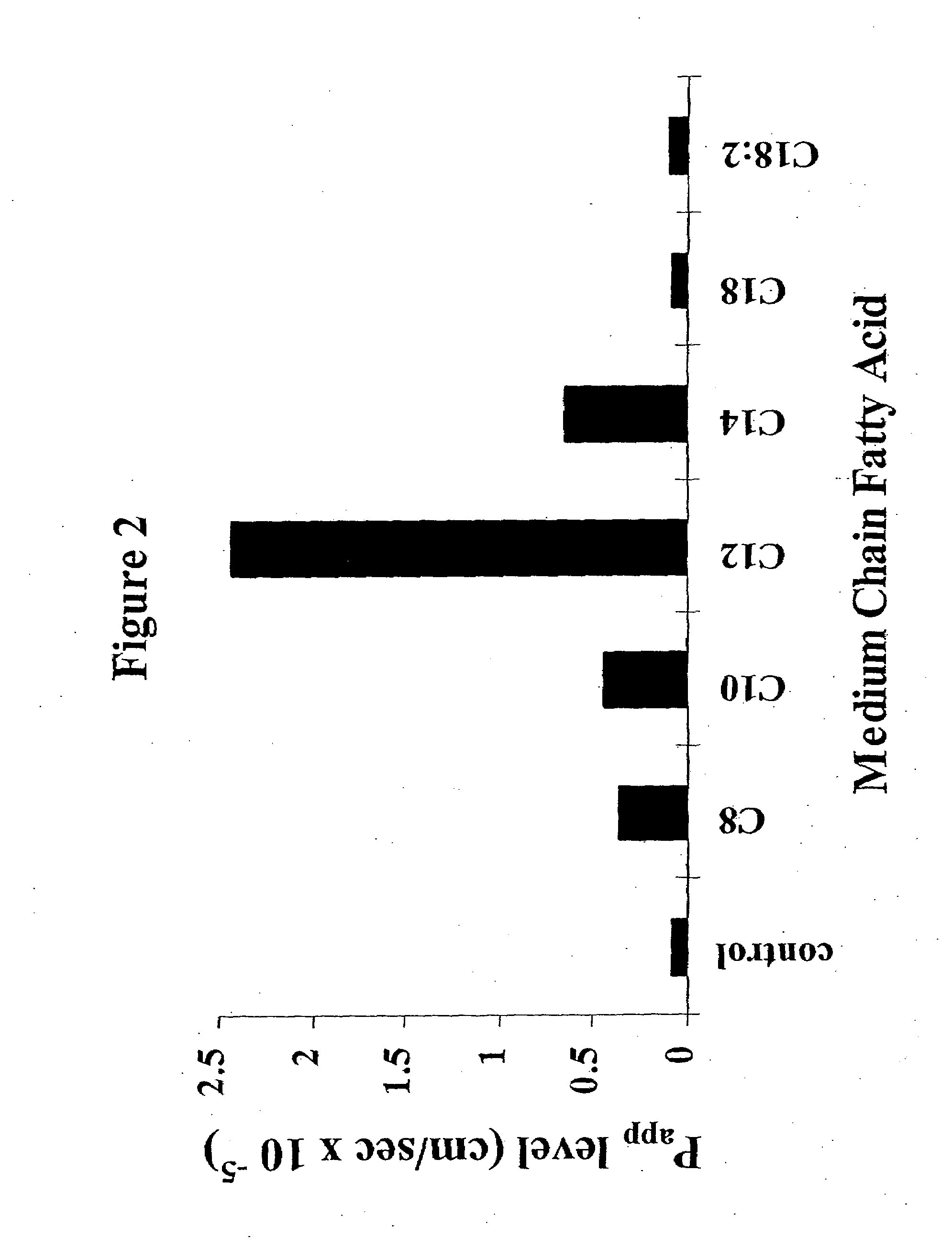
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid pharmaceutical dosage forms comprising bisphosphonates and modified amino acid carriers
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Unique compositions of zwitterionic phospholipids and bisphosphonates and use of the compositions as bisphosphate delivery systems with reduced GI toxicity
InactiveUS6943155B2Reduce GI toxicityImprove bioavailabilityBiocideInorganic active ingredientsDiphosphonatesMedicine
Compositions and methods for treating osteoporosis using the compositions are disclosed where the compositions have reduced GI toxicity and improved bio-availability and include a bisphosphonate and zwitterionic phospholipid.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Dosage forms of bisphosphonates
ActiveUS7645459B2Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyLower Gastrointestinal Tract
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Implantable medical device having biologically active polymeric casing
InactiveUS6968234B2Prevent and reduce infectionHeart defibrillatorsInternal electrodesPolyolefinActive agent
An implantable medical device has a medical unit, such as a pacemaker lead, and a casing at least partially enclosing the medical unit. The casing is formed of a base polymer such as a polyurethane, a polyurethane copolymer, a fluoropolymer and a polyolefin or a silicone rubber. The casing has biologically active agents covalently bonded to the base polymer. The biologically active agents can be attached to the base polymer as surface active end groups. As an alternative, the biologically active may be attached to a backbone the base polymer. As yet a further alternative, the biologically active agents may be attached to surface modifying end groups, which are in turn attached to the base polymer. Examples of suitable biologically active agents are microbial peptide agents, detergents, non-steroidal anti-inflammatory drugs, cations, amine-containing organosilicones, diphosphonates, fatty acids and fatty acid salts.
Owner:MEDTRONIC INC
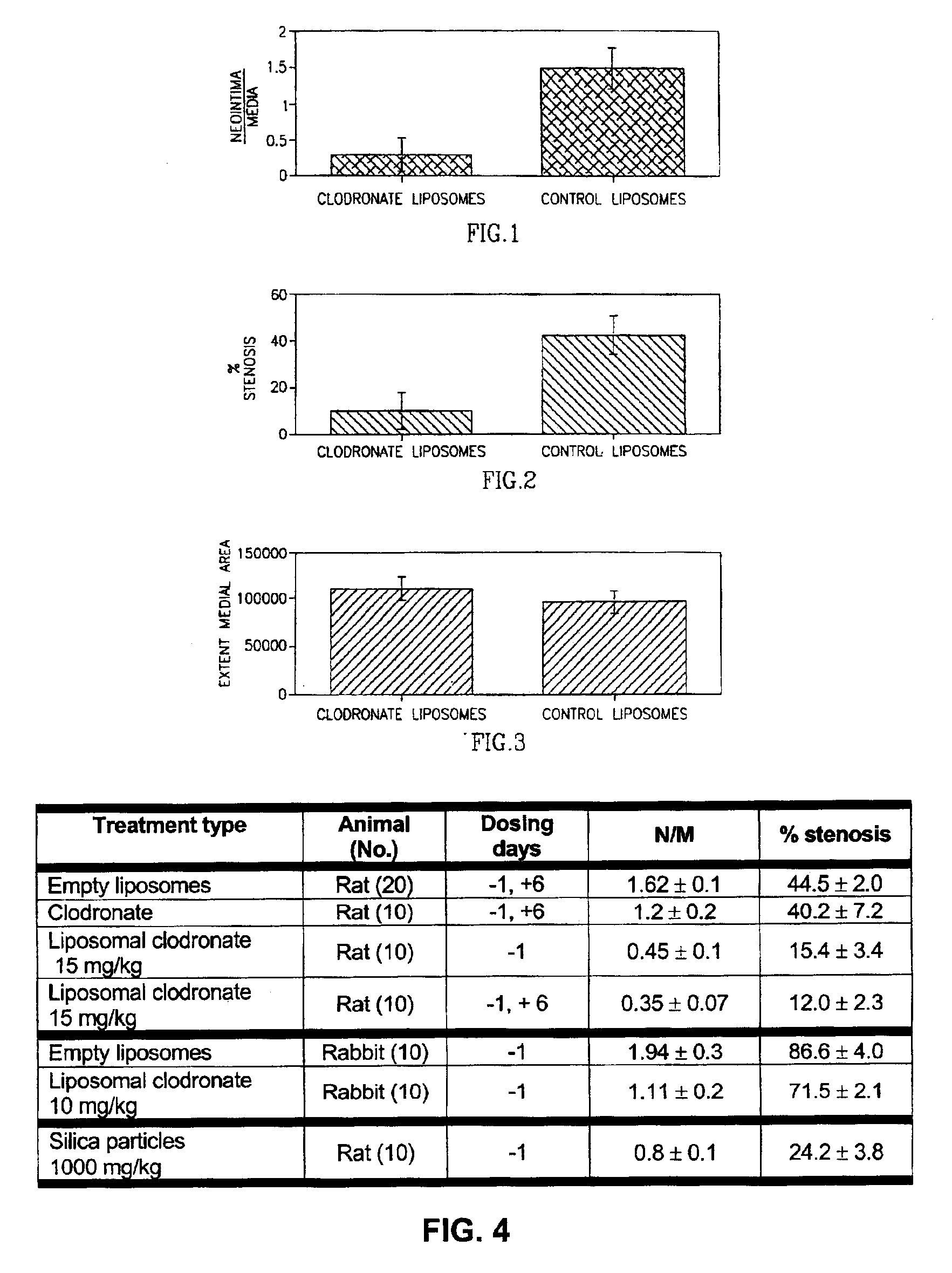
Method of inhibiting restenosis using bisphosphonates
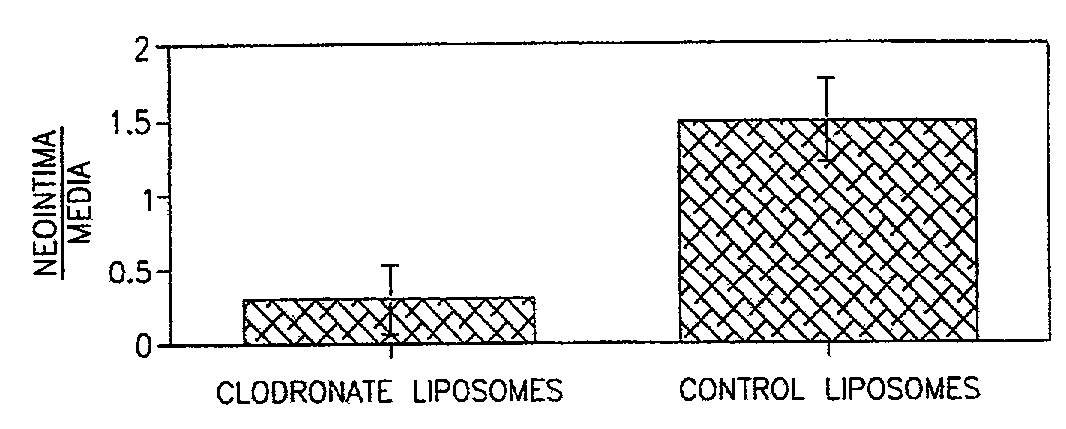
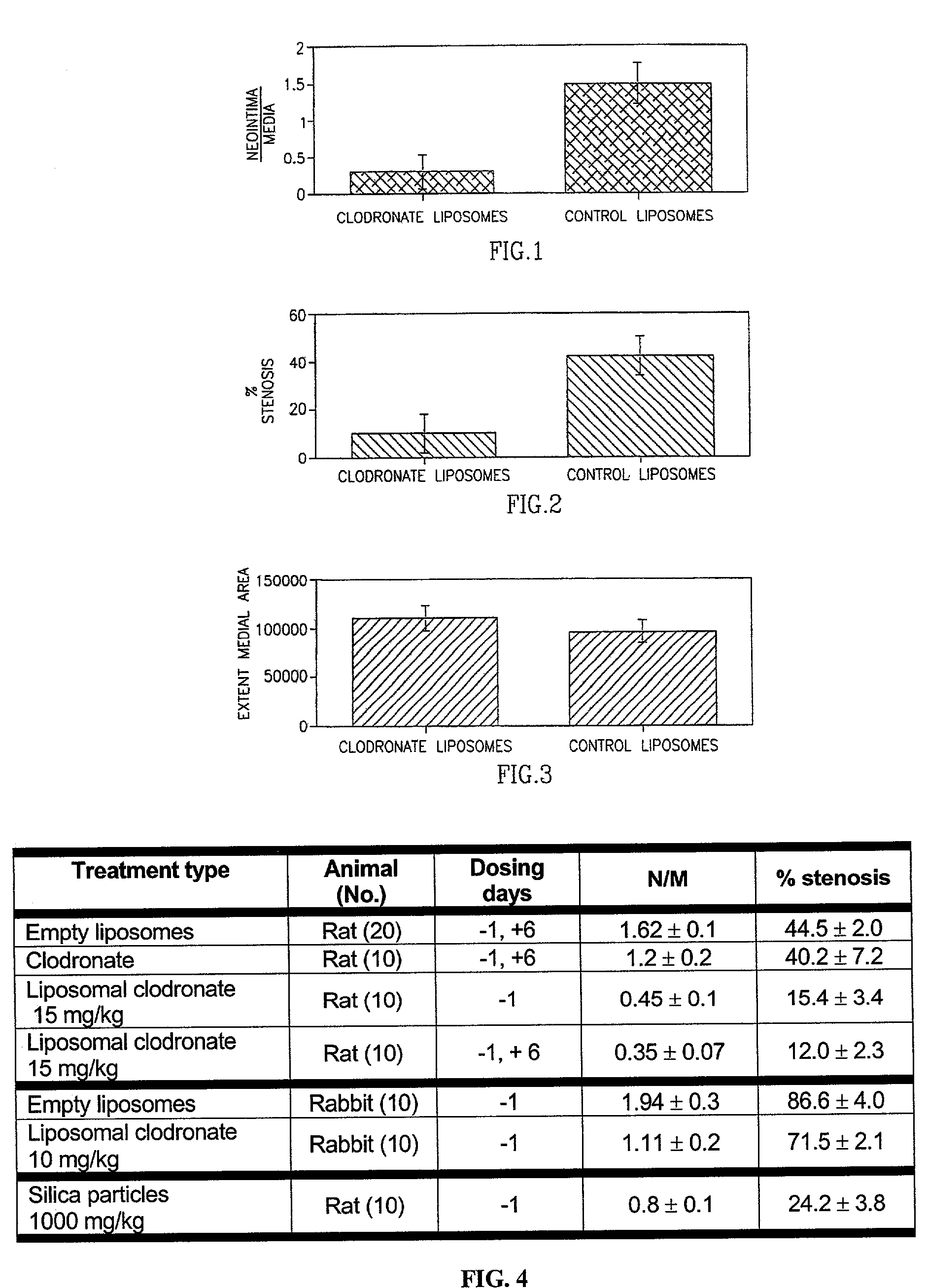
InactiveUS7008645B2Affect activityEfficient transportPowder deliveryBiocidePlatelet-Derived Growth Factor BetaParticulates
A method of inhibiting the activity or production of cytokines or growth factors associated with vascular restenosis, by administering to an individual an effective amount of an active ingredient comprising a bisphosphonate particle or a bisphosphonate particulate. The bisphosphonate may be encapsulated, embedded or adsorbed within the particle, dispersed uniformly in the polymer matrix, adsorbed on the particle surface, or in combination of any of these forms. The particles include liposomes or inert polymeric particles, such as microcapsules, nanocapsules, nanoparticles, nanospheres, or microparticles. The particulates include any suspended or dispersed form of the bisphosphonate which is not encapsulated, entrapped, or adsorbed within a polymeric particle. The particulates include suspended or dispersed colloids, aggregates, flocculates, insoluble salts and insoluble complexes of the active ingredient. The cytokines and growth factors include, but are not limited to interleukin 1-β, matrix metalloproteinase-2, and platelet-derived growth factor β (PDGFβ).
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
Microparticle compositions to modify cancer promoting cells
This invention provides pharmaceutical compositions and methods related to the prevention and treatment of primary tumors and metastatic, malignant or spreading cancers by selectively targeting cancer associated myeloid derived cells by the targeted delivery of a bisphosphonate formulated with a non-liposomal particle carrier. In some aspects, the bisphosphonate particles have one or more properties suitable for phagocytosis by cancer associated myeloid derived cells and release of the bisphosphonate within the macrophages. Advantageously, administering the particles to a subject reduces the level and / or activity of cancer associated myeloid derived cells in the subject.
Owner:JOVESIS
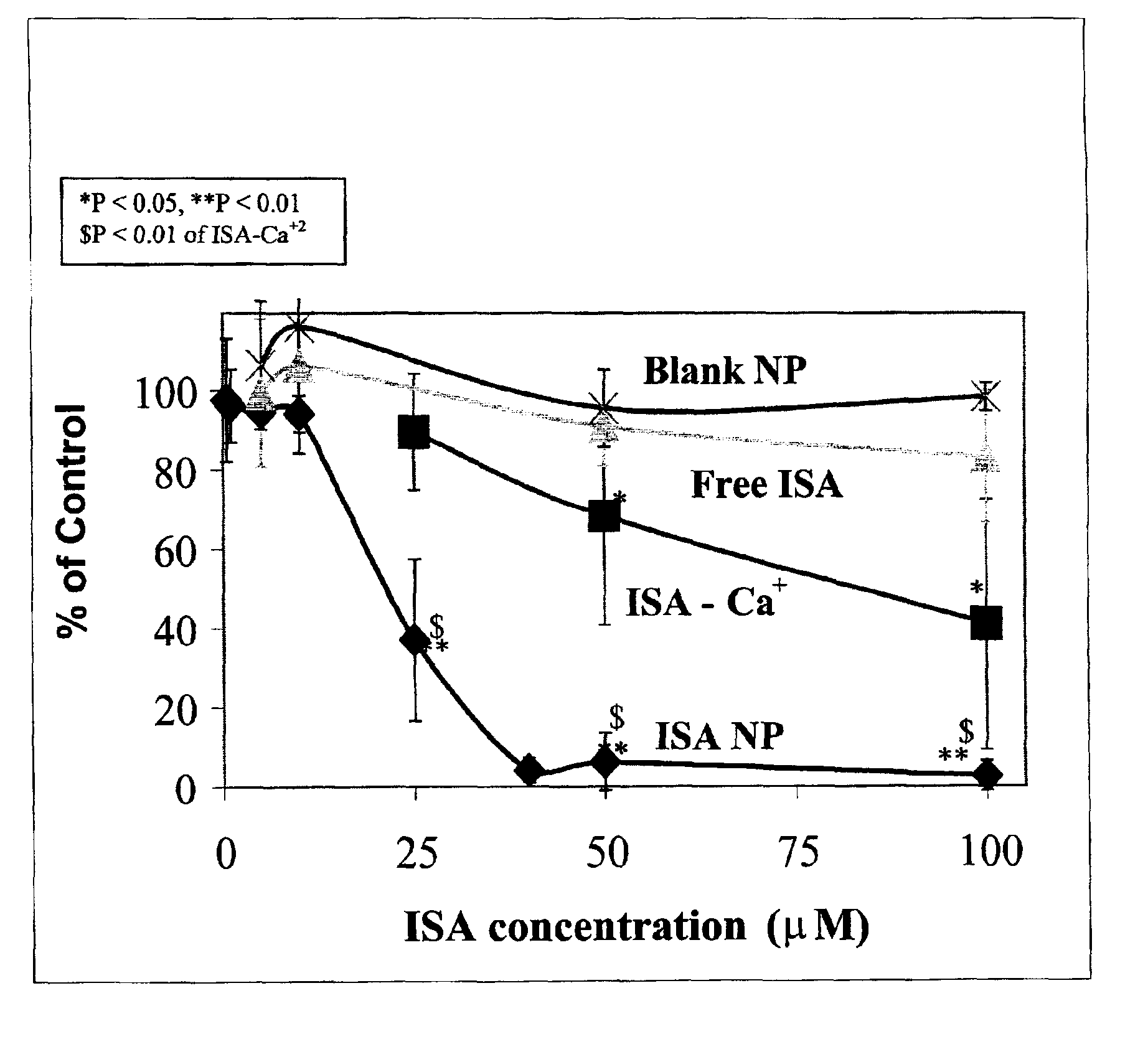
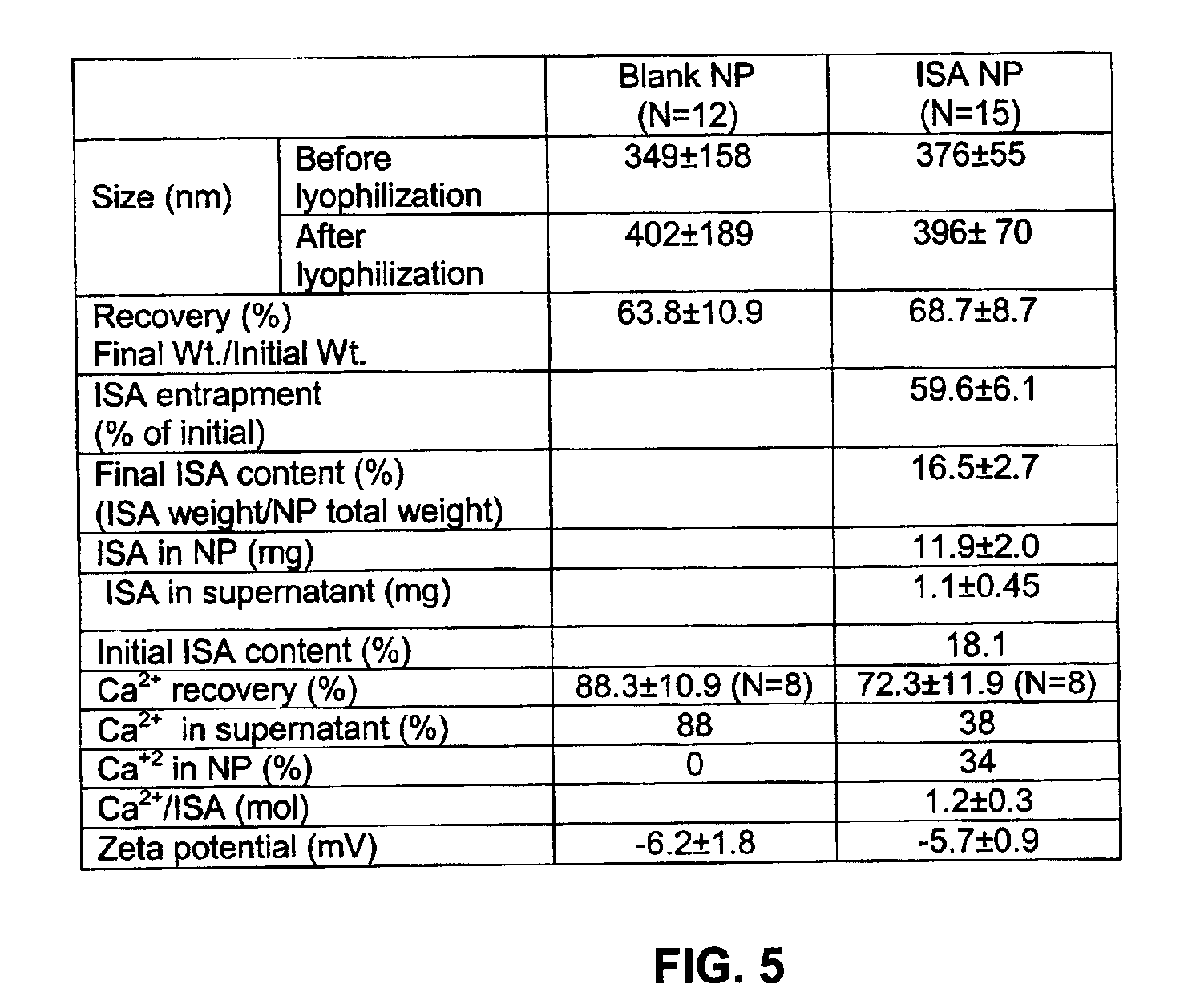
Method of treating restenosis using bisphosphonate nanoparticles
InactiveUS6984400B2Treating and preventing restenosisHeavy metal active ingredientsBiocideParticulatesDiphosphonates
A method of treating or preventing restenosis by administering to an individual an effective amount of an active ingredient comprising a bisphosphonate particle or a bisphosphonate particulate. The bisphosphonate may be encapsulated, embedded or adsorbed within the particle, dispersed uniformly in the polymer matrix, adsorbed on the particle surface, or in combination of any of these forms. The particles include liposomes or inert polymeric particles, such as microcapsules, nanocapsules, nanoparticles, nanospheres, or microparticles. The particulates include any suspended or dispersed form of the bisphosphonate which is not encapsulated, entrapped, or adsorbed within a polymeric particle. The particulates include suspended or dispersed colloids, aggregates, flocculates, insoluble salts and insoluble complexes of the active ingredient. The active ingredient effects restenosis by inhibiting the growth and proliferation of the cell types involved in the restenotic cascade, such as macrophages / monocytes, fibroblasts and smooth-muscle cells.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
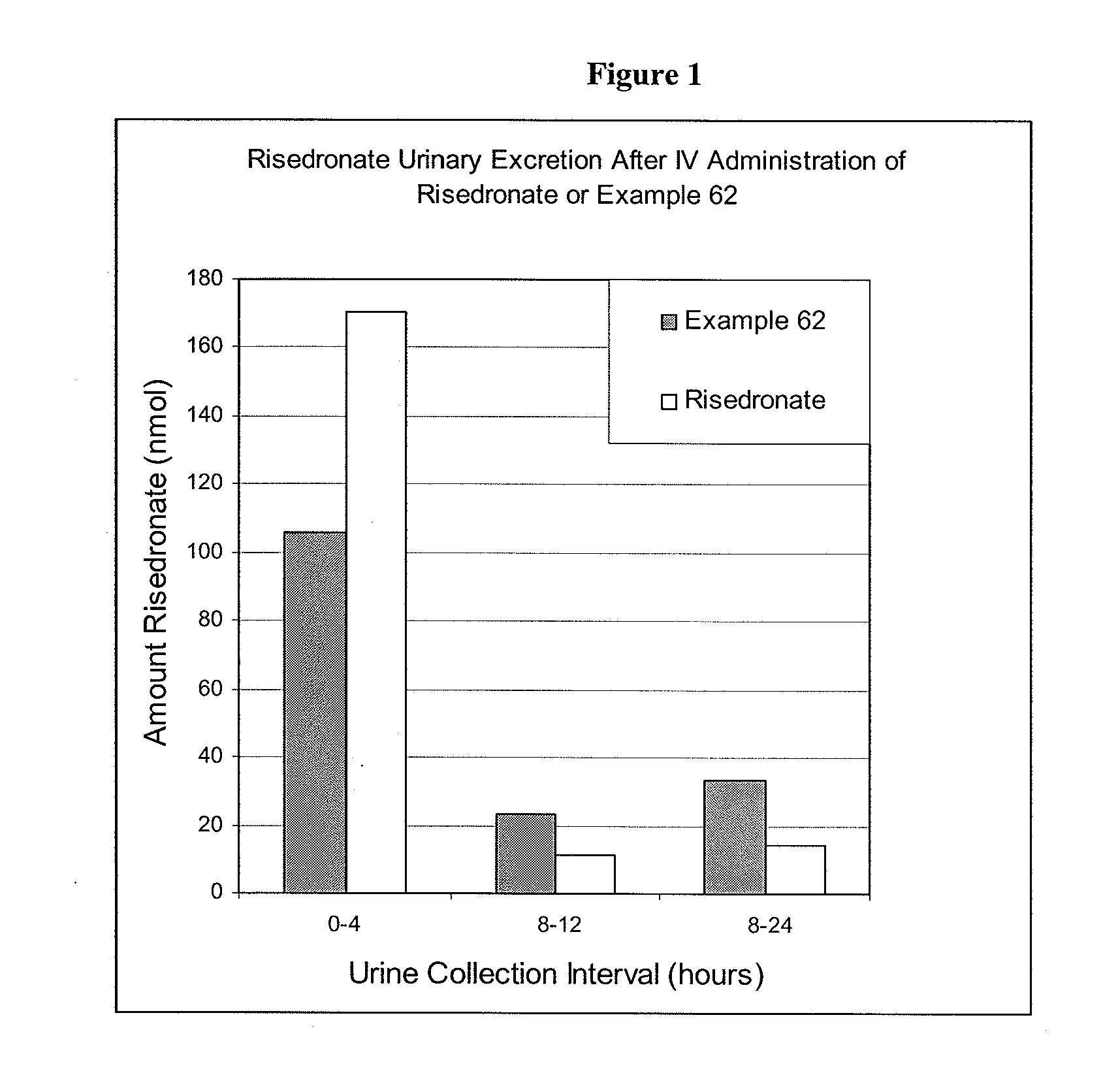
Dosage forms of risedronate
ActiveUS20060110452A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyImmediate release
Oral dosage forms of a risedronate comprised of a safe and effective amount of a pharmaceutical composition comprising risedronate, a chelating agent, and, means for effecting delayed release of the risedronate and the chelating agent in the small intestine provide immediate release of the pharmaceutical composition to the small intestine of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between risedronate and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of risedronate and the chelating agent to the small intestine, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Solid pharmaceutical dosage forms comprising bisphosphonates and modified amino acid carriers
InactiveUS20070049557A1Promote absorptionBiocidePhosphorous compound active ingredientsCalcium metabolismMetastatic bone pain
The present invention provides novel solid pharmaceutical dosage forms for oral administration comprising a bisphosphonate, or a pharmaceutically acceptable salt thereof, which bisphosphonate is present in an amount not therapeutically effective when the bisphosphonate is orally administered alone; and a modified amino acid carrier, or a pharmaceutically acceptable salt thereof, which modified amino acid carrier is present in an amount effective to facilitate absorption of the bisphosphonate in the gastrointestinal tract such that the bisphosphonate is therapeutically effective. The ratio of bisphosphonate to modified amino acid carrier is from about 1:30 to about 1:1, respectively. These novel solid pharmaceutical dosage forms are useful in the treatment or control of bone diseases and particular disorders in calcium metabolism, including, for example, osteoporosis, hypercalcaemia of cancer, and the treatment of metastatic bone pain. The present invention also provides a method for treating these diseases employing the solid pharmaceutical dosage forms and a method for preparing the pharmaceutical dosage forms.
Owner:EMISPHERE TECH INC
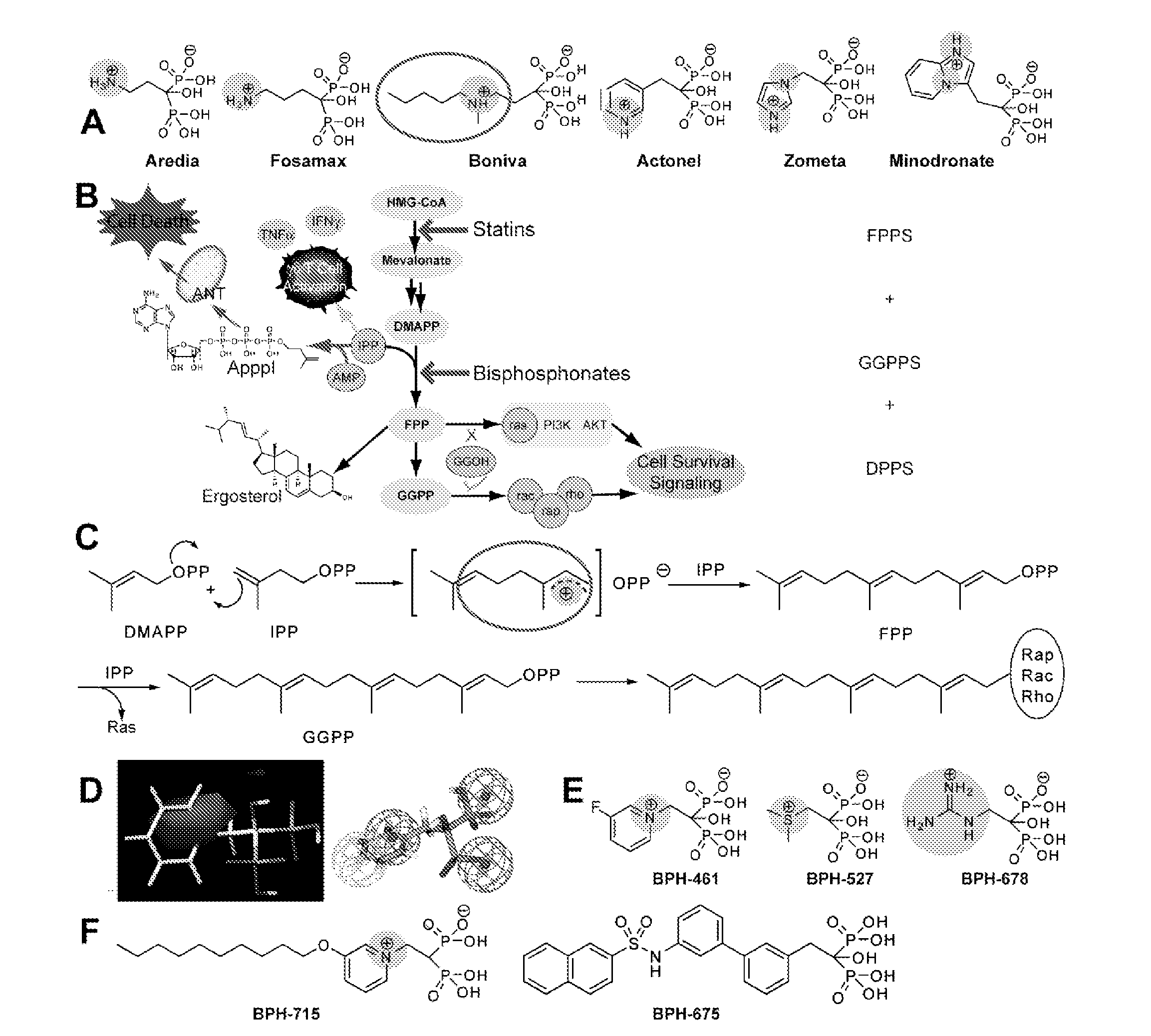
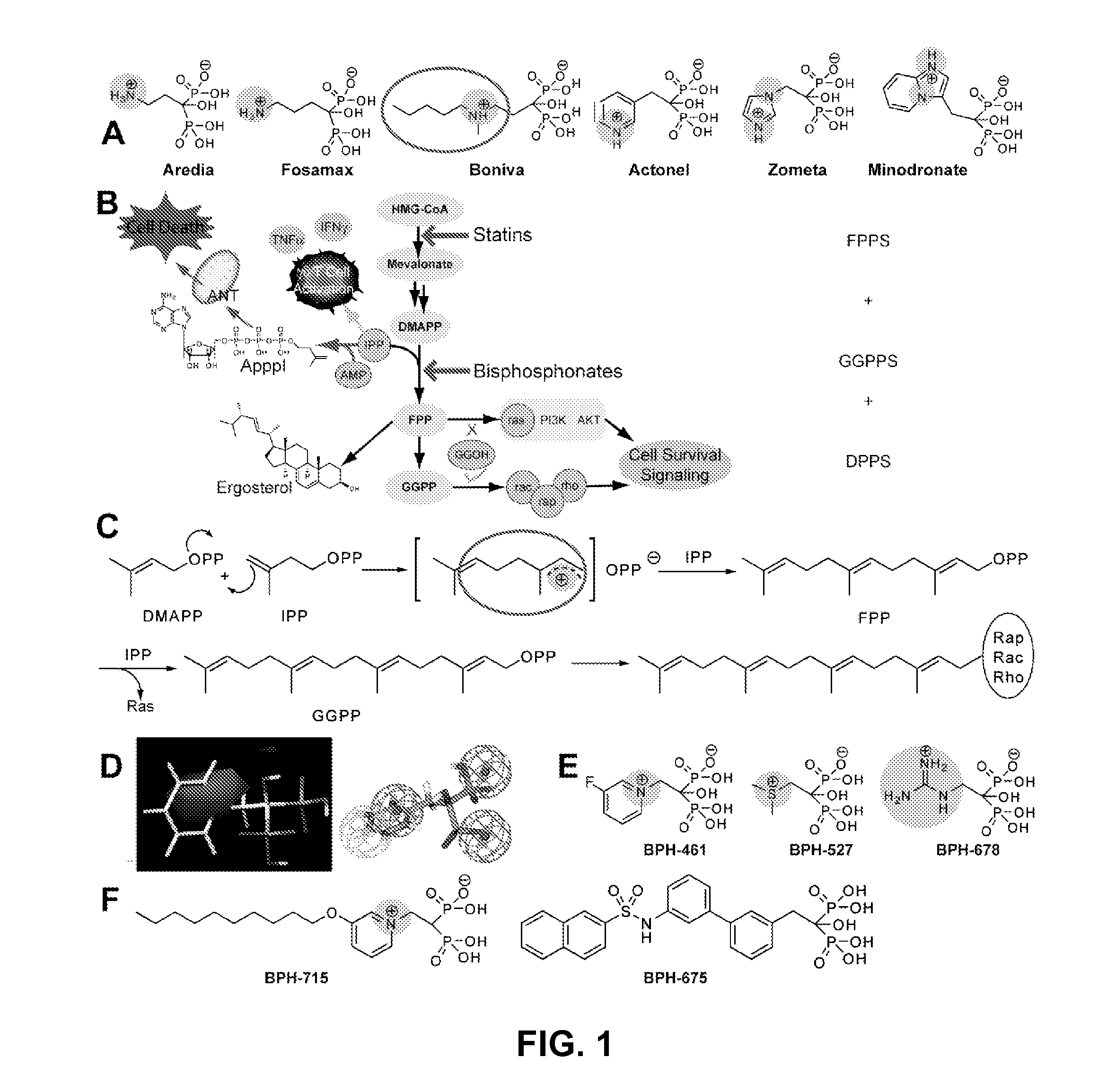
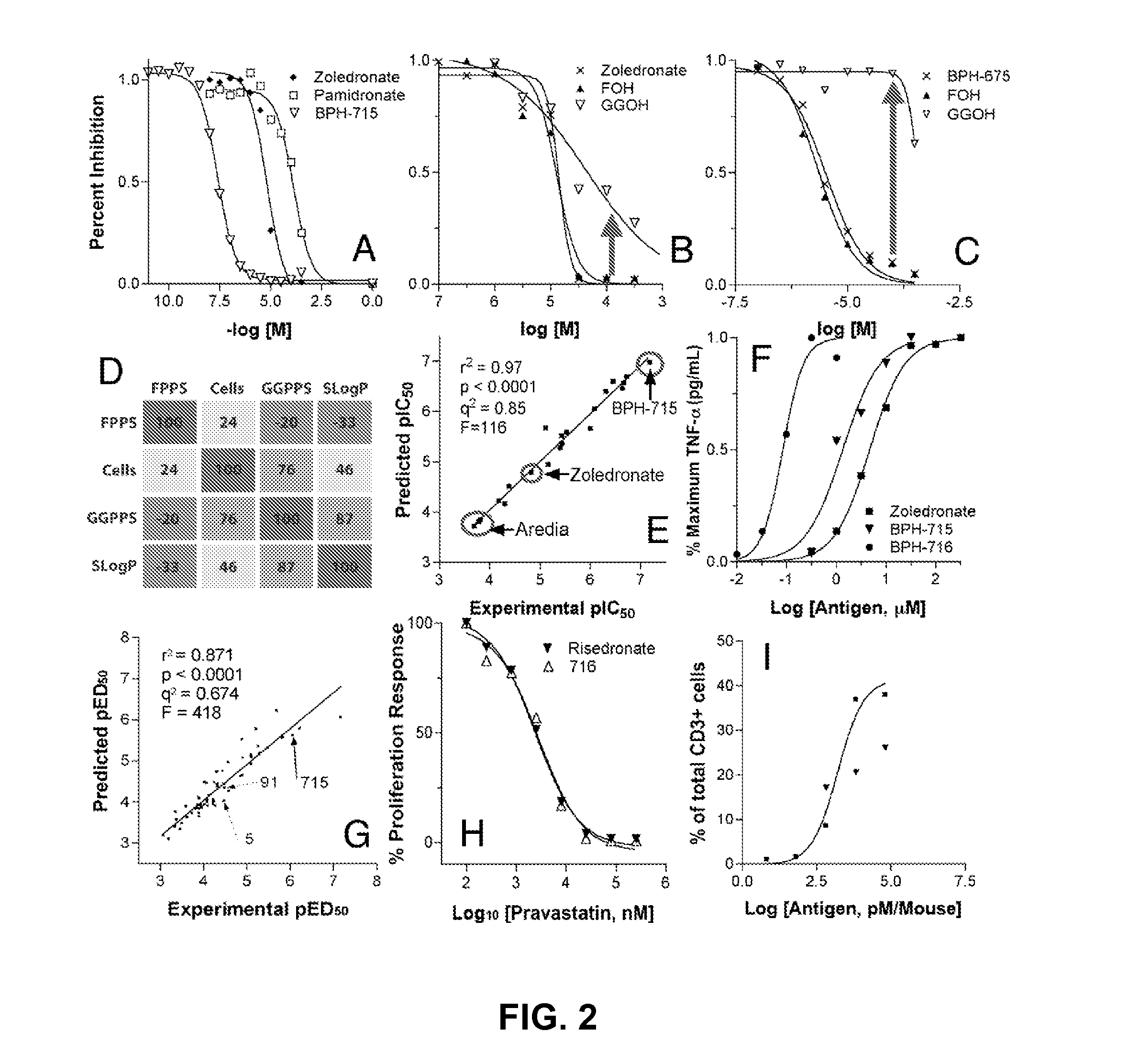
Bisphosphonate Compounds and Methods with Enhanced Potency for Multiple Targets including FPPS, GGPPS, AND DPPS
InactiveUS20080255070A1High activityGood effectBiocideMicrobiological testing/measurementCancer cellDiphosphonates
The disclosure provides, inter alia, novel bisphosphonate compounds and methods of making and using such compounds. In certain embodiments, compounds of the invention include bisphosphonates that are capable of selectively inhibiting one or more of farnesyl diphosphate synthase (FPPS), geranylgeranyl diphosphate synthase (GGPPS), and decaprenyl pyrophosphate synthase (DPPS). In preferred embodiments, compounds of the invention are capable of selectively inhibiting two or more of FPPS, GGPPS, and DPPS. In embodiments, compounds and methods of the invention demonstrate superior activity levels, such as in the anti-cancer context, immunostimulation context, and other contexts, which in several cases exceed the activity levels of previous generation bisphosphonate drugs by orders of magnitude. In embodiments, the invention provides compounds and methods in connection with research and therapeutic applications, e.g., for tumor or cancer cell growth inhibition, activation of gammadelta T cells, inhibition of certain enzymes related to the mevalonate metabolic pathway, bone resorption diseases, cancer, immune disorders, immunotherapy, and infectious diseases.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
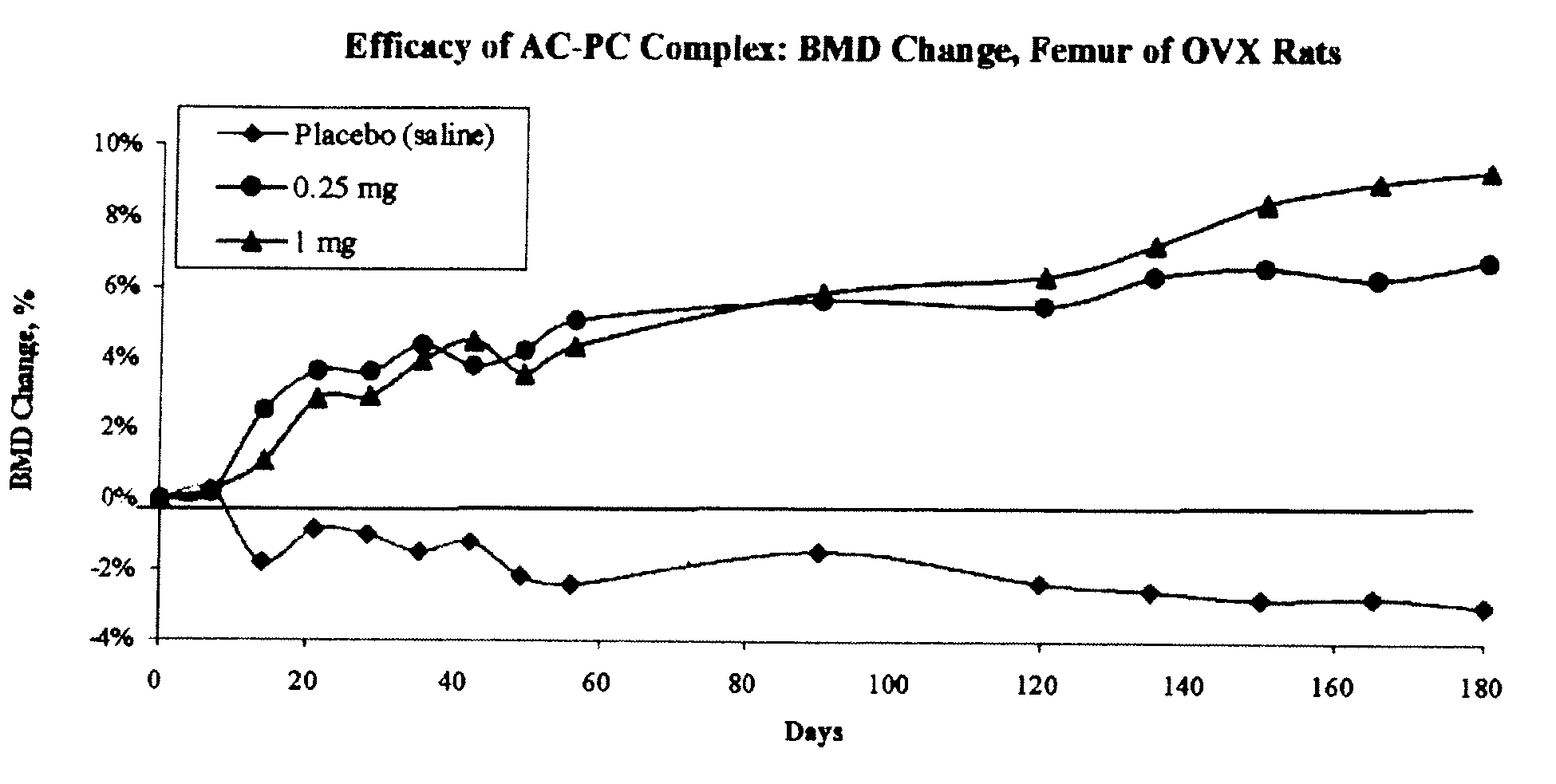
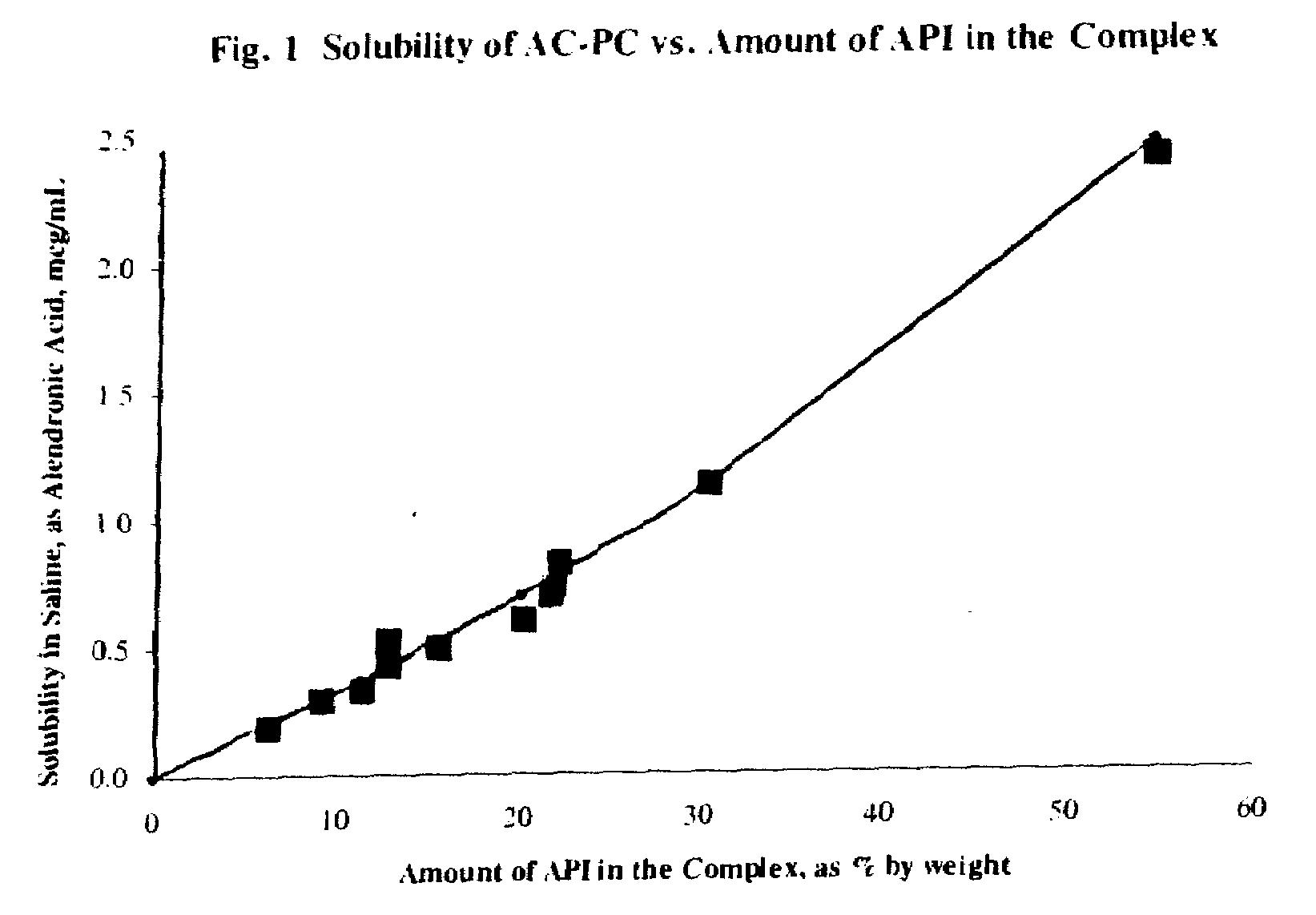
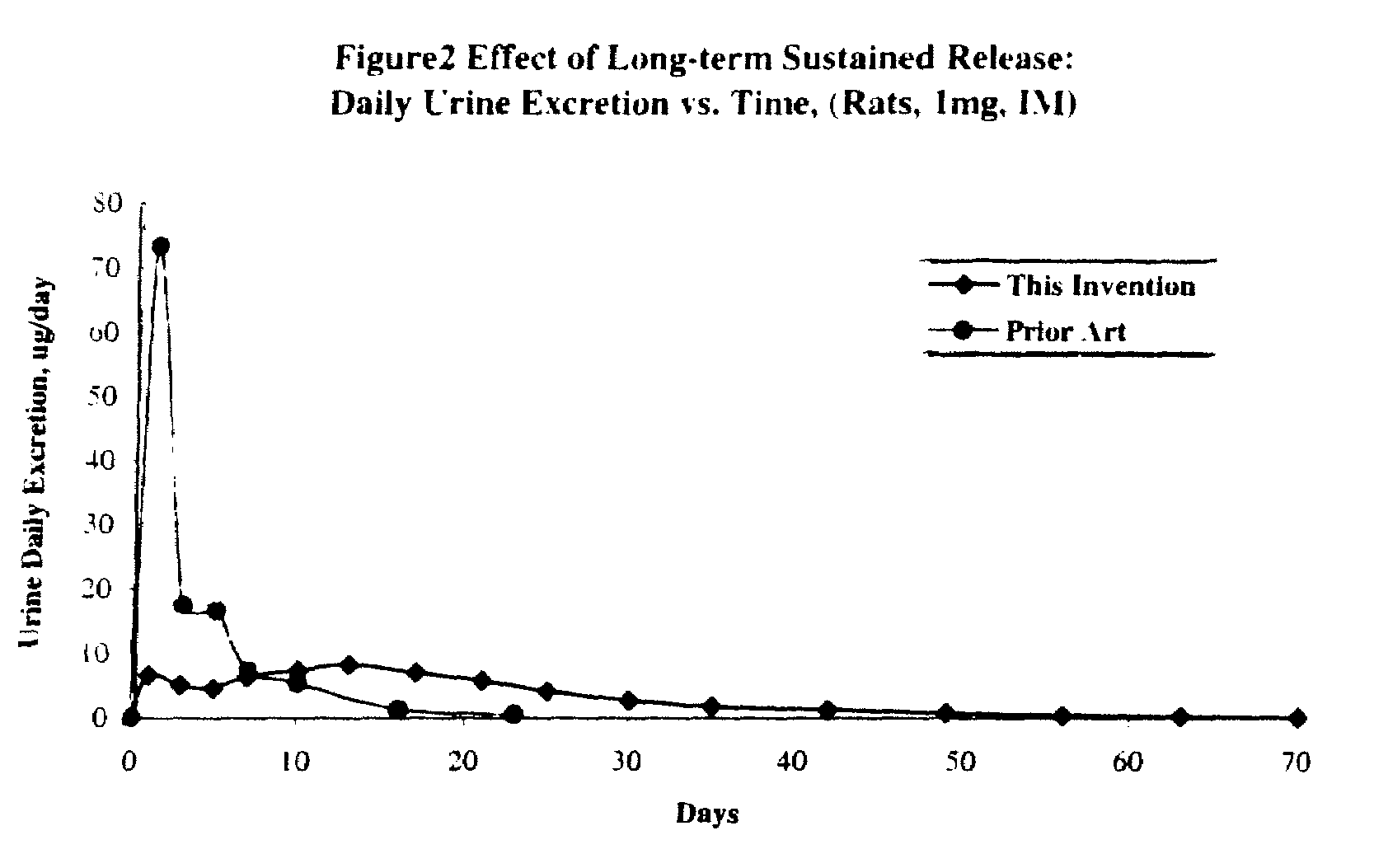
Long term sustained release pharmaceutical composition containing aqueous suspension of bisphosphonate
ActiveUS20080153784A1Side effect of substantially reduced and eliminatedHigh densityBiocideInorganic phosphorous active ingredientsDiphosphonatesOxoacid
Pharmaceutical compositions for long-term sustained release of bisphosphonate drugs are provided. In one embodiment, the composition includes an aqueous suspension of a solid which includes a salt of a bisphosphonate drug and a salt of pentavalent phosphorus oxoacid. The compositions can be used to treat a variety of bone diseases, including osteoporosis.
Owner:AMPHASTAR PHARMA INC
Low Dosage Forms Of Risedronate Or Its Salts
InactiveUS20080287400A1Effective absorptionReduce absorptionBiocideOrganic active ingredientsBisphosphonate therapyPatient compliance
Oral dosage forms comprising risedronate or a salt thereof, a chelating agent, and means for effecting delayed release of the risedronate (or salt) immediate release of the oral dosage form to the small intestine of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between the risedronate (or salt) and food or beverages, which interaction results in the active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, disclosed is delivery of risedronate and the chelating agent to the small intestine, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:WARNER CHILCOTT CO LLC
Compositions comprising bisphosphonate and an antifolate
Compositions and methods for the treatment of arthritis, particularly rheumatoid arthritis and osteoarthritis. These compositions include at least one antifolate and at least one bisphosphonate, or pharmaceutically acceptable salts thereof
Owner:CYPRESS BIOSCI
Unique compositions of zwitterionic phospholipids and bisphosphonates and use of the compositions as bisphosphate delivery systems with reduced GI toxicity
InactiveUS7354912B2Low toxicityImprove bisphosphonate bio-availabilityBiocideInorganic active ingredientsDiphosphonatesMedicine
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of treating or preventing osteoporosis comprising administering to a patient in need thereof an effective amount of pharmacuetical composition comprising benzamidine derivative or it's salt, and bisphosphonate
The present invention provides a method of treating or preventing osteoporosis comprising administering to a patient in need thereof an effective amount of pharmaceutical composition comprising benzamidine derivative or its salt, and bisphosphonate for the purpose of using simultaneously, separately, or sequentially as active ingredients. As a prophylactic or therapeutic composition for osteoporosis, the combination treatment of the benzamidine derivative and the bisphosphonate compound exhibits excellent inhibitory effect on osteoclast differentiation than the total effect of each individual treatment, thereby being used for the prevention or treatment of osteoporosis.
Owner:DONG WHA PHARM CO LTD
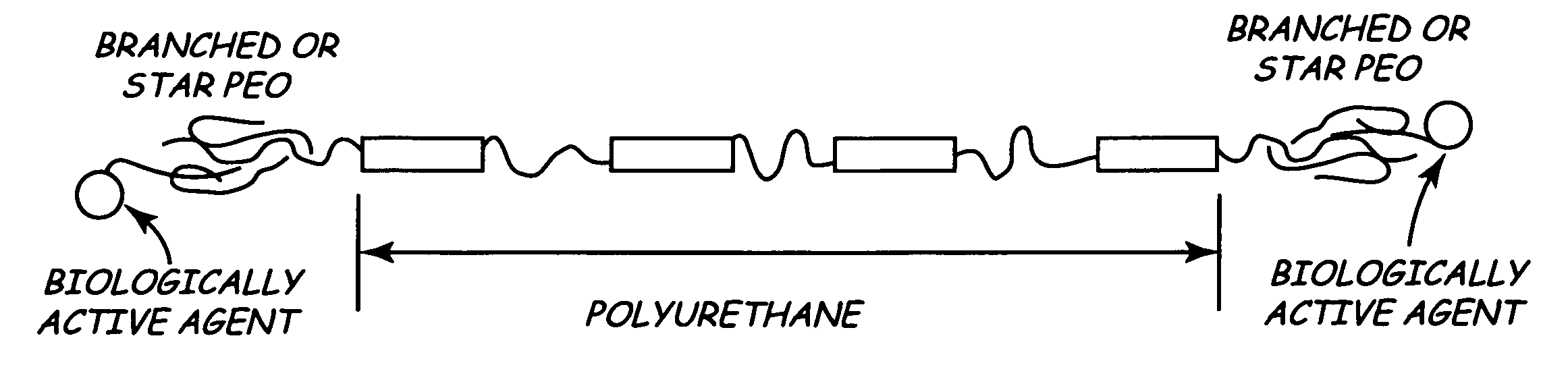
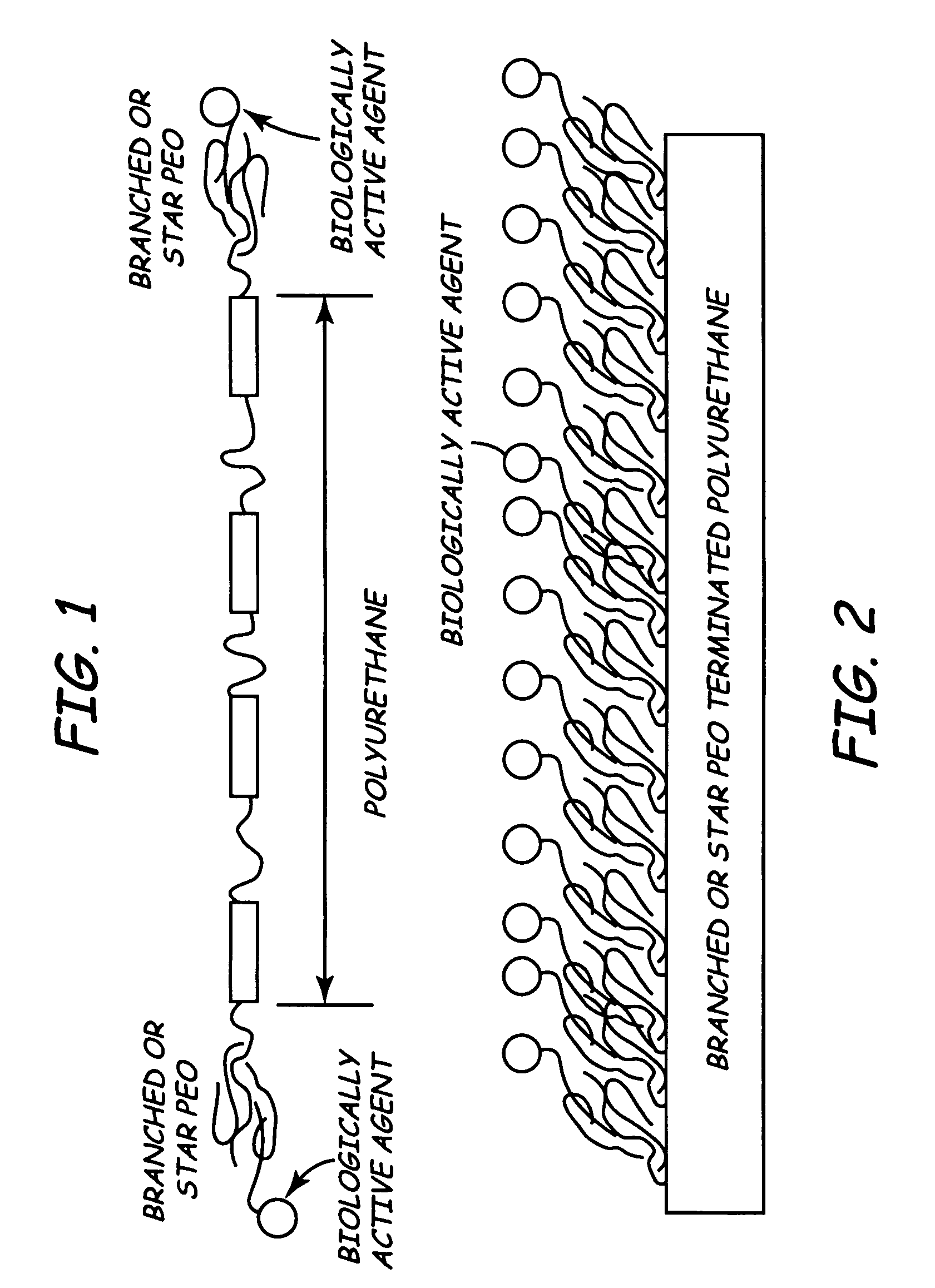
Branched polyethylene oxide terminated biomedical polymers and their use in biomedical devices
A biomedical polymer has a substantially linear base polymer; and branched polyethylene oxide covalently bonded to the base polymer as surface active end groups. The branched polyethylene oxide has at least two, more particularly at least four, and still more particularly at least six branches. Suitable base polymers include epoxies, polyurethanes, polyurethane copolymers, fluoropolymers, polyolefins and silicone rubbers. Biologically active agents may be attached to the branched polyethylene oxide. Suitable biologically active agents include microbial peptide agents, detergents, non-steroidal anti-inflammatory drugs, cations, amine-containing organosilicones, diphosphonates, fatty acids, fatty acid salts, heparin and glucocorticosteroids. The biological polymer may be used as a casing for a medical unit of an implantable medical device, such as a pacemaker. In this case, the casing at least partially encloses the medical unit.
Owner:MEDTRONIC INC
Method to ameliorate osteolysis and metastasis
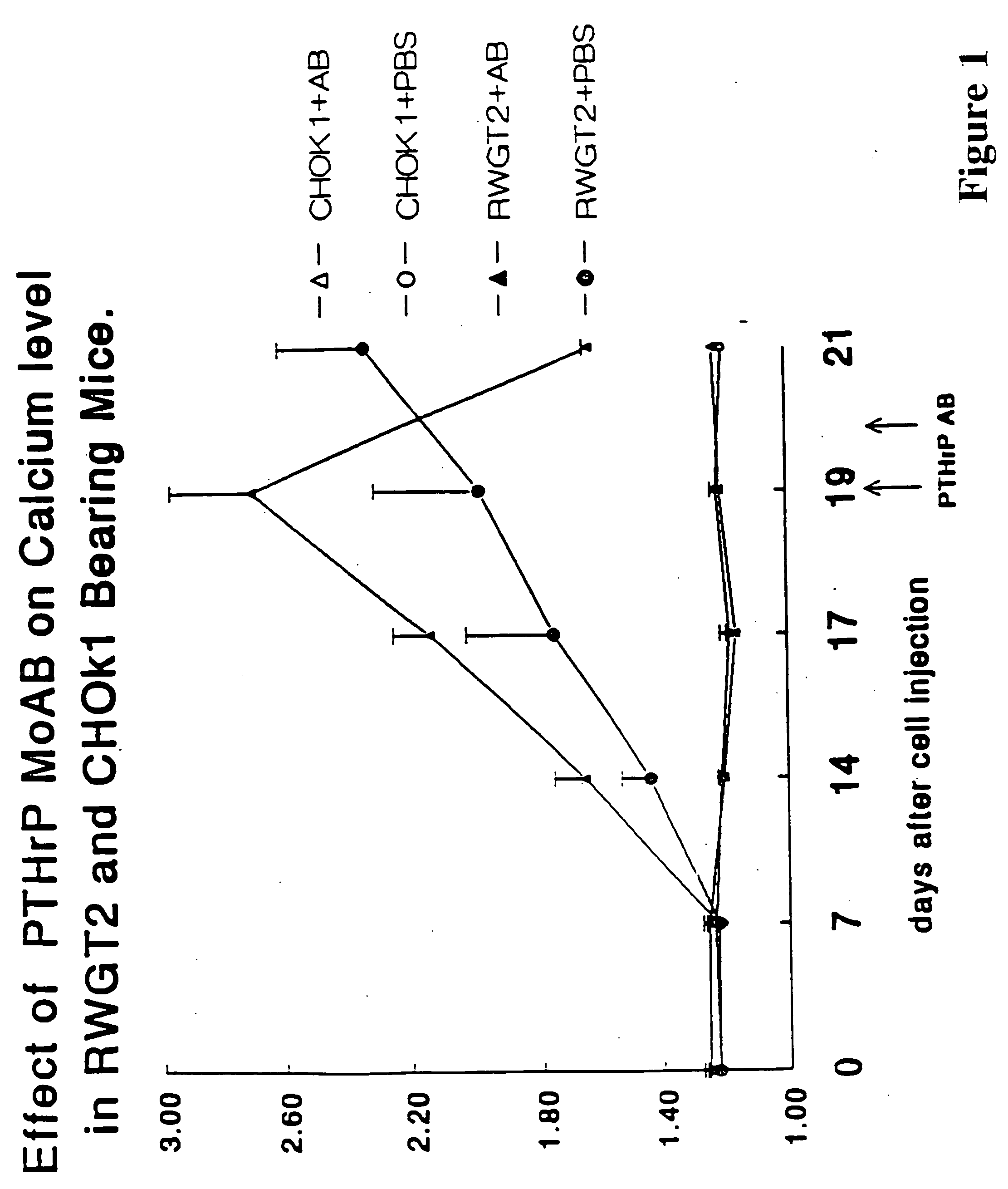
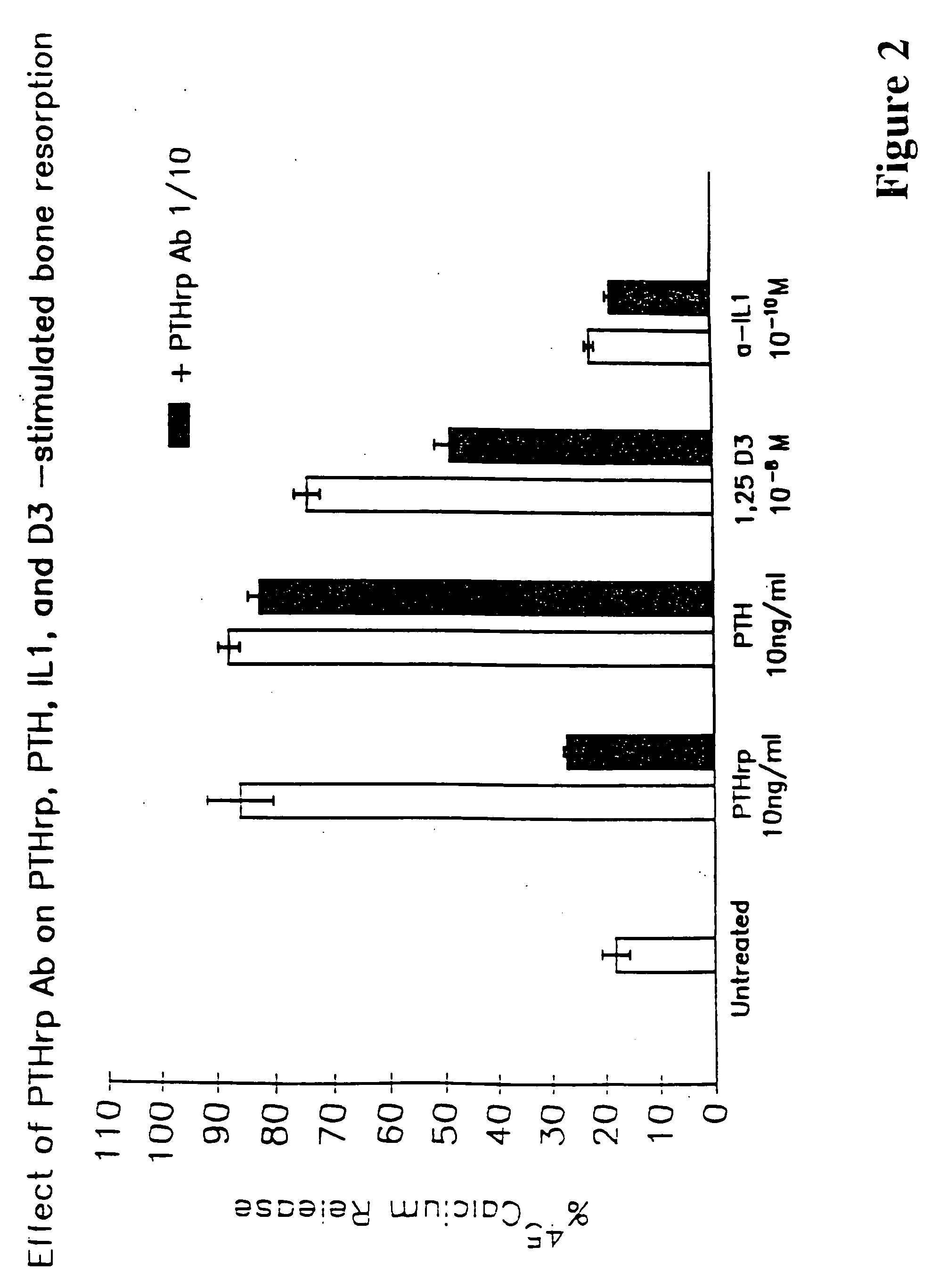
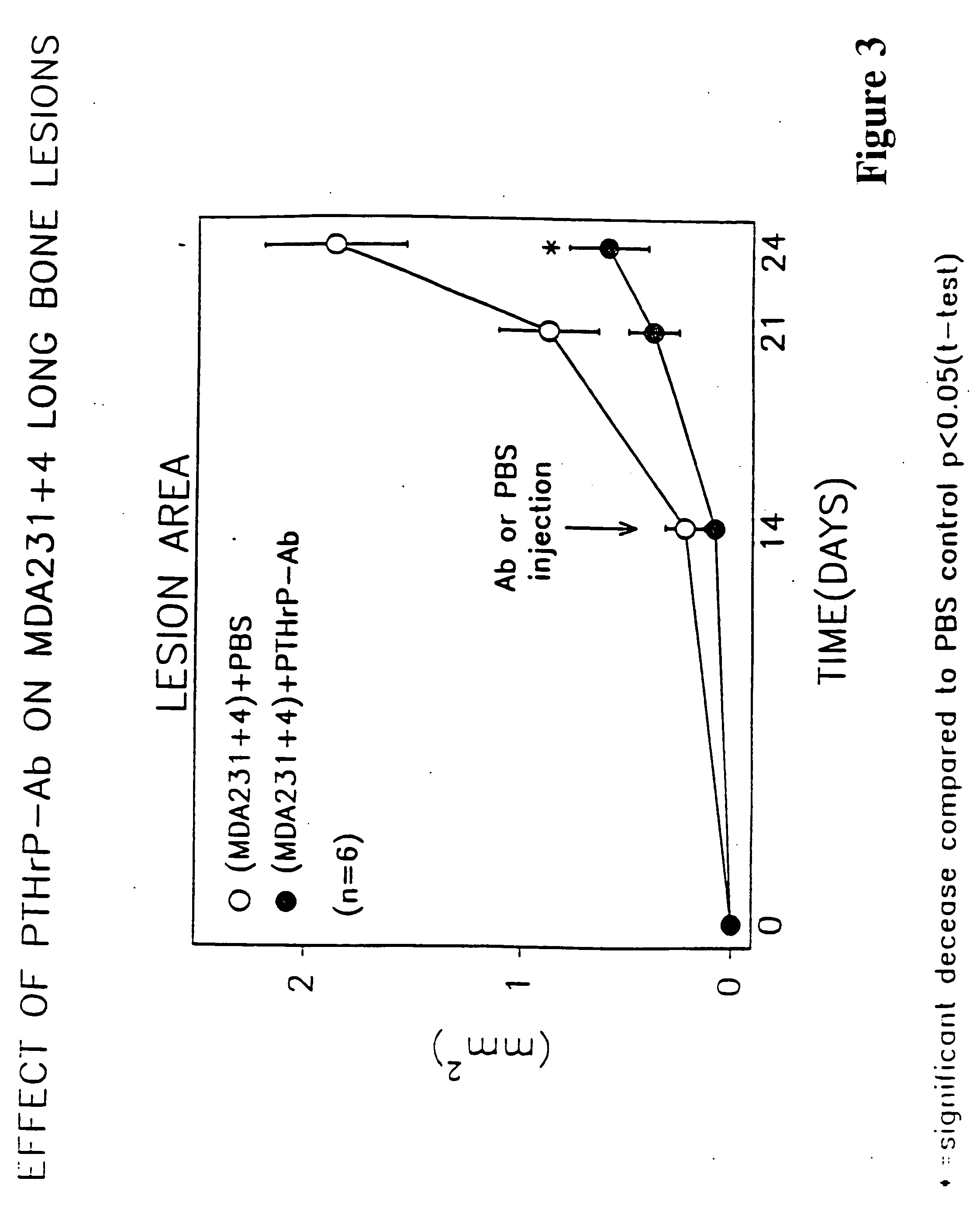
A therapeutically effective amount of an antibody for a compound selected from the group consisting of PTHrp, TGFα, IL-1α, IL-1β, IL-6, Lymphotoxin, TNF, PGE; 1,25 dihydroxy vitamin D3 and an antigenic fragment thereof used in the treatment of cancer metastasis to bone and cancer cell growth in bone as well as osteolysis and symptomatic sequelae thereof. An antibody immunoreactive with parathyroid hormone-related protein (PTHrp) is particularly preferred. Antibodies with human characteristics are included in the invention for application of the invention method to human subjects. Also, the antibody can be administered in an injectable is formulation in combination with a therapeutically effective amount of a bisphosphonate or pyrophosphate having the general structure formula wherein X is a linking moiety allowing for the interconnection of the phosphonate groups, and pharmaceutically acceptable salts, hydrates and partial hydrates thereof. The antibody and bisphosphonate act synergistically in the treatment of cancer metastases to bone and symptomatic sequelae thereof and particularly as regards bone resorption.
Owner:XENOTECH CALIFORNIA
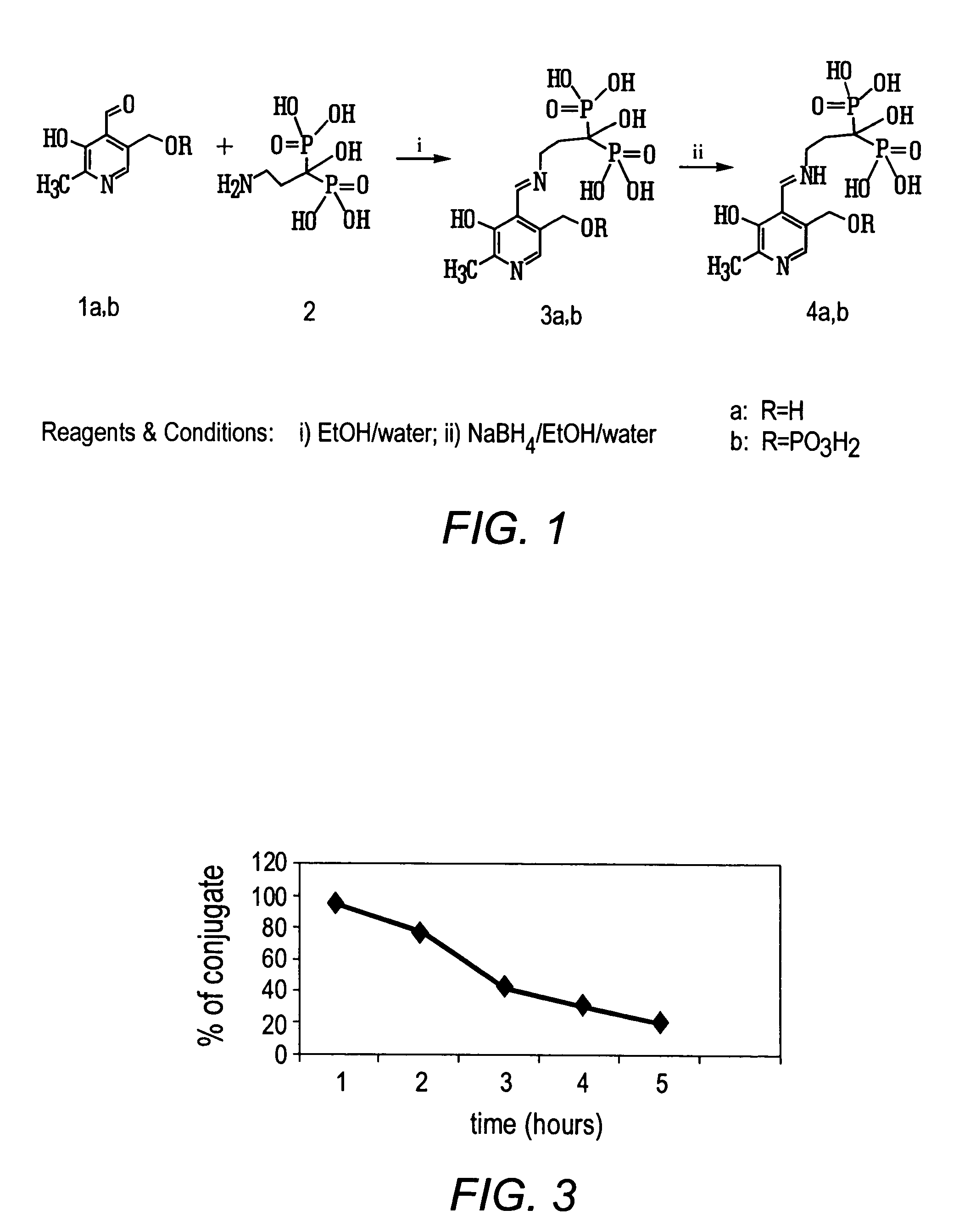
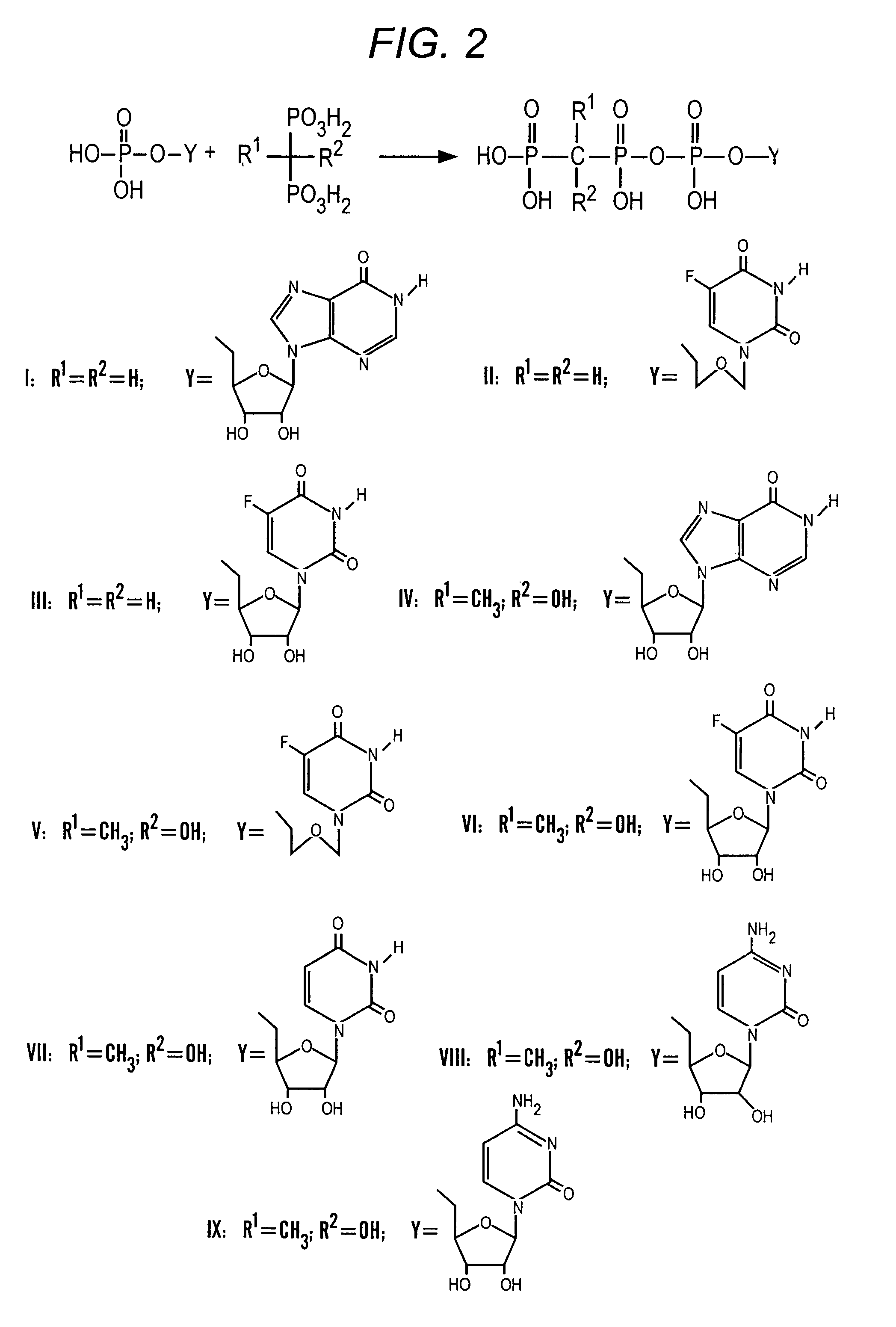
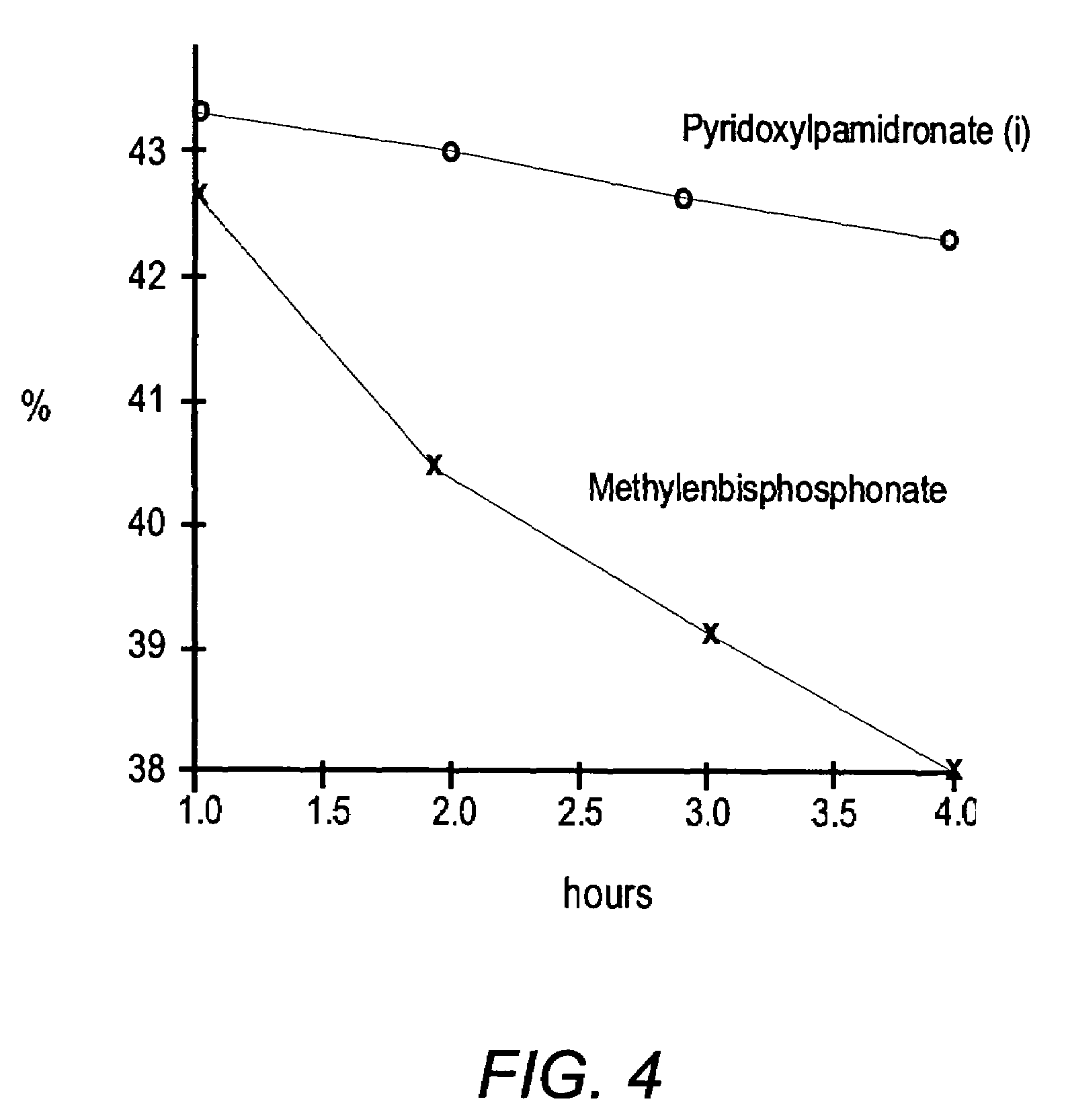
Bisphosphonate conjugates and methods of making and using the same
The present invention provides novel bisphosphonate conjugates, pharmaceutical compositions comprising bisphosphonate conjugates and methods of using such analogs in the treatment of bone cancer, bone-related diseases and diseases of the soft tissues surrounding bones.
Owner:MBC PHARMA
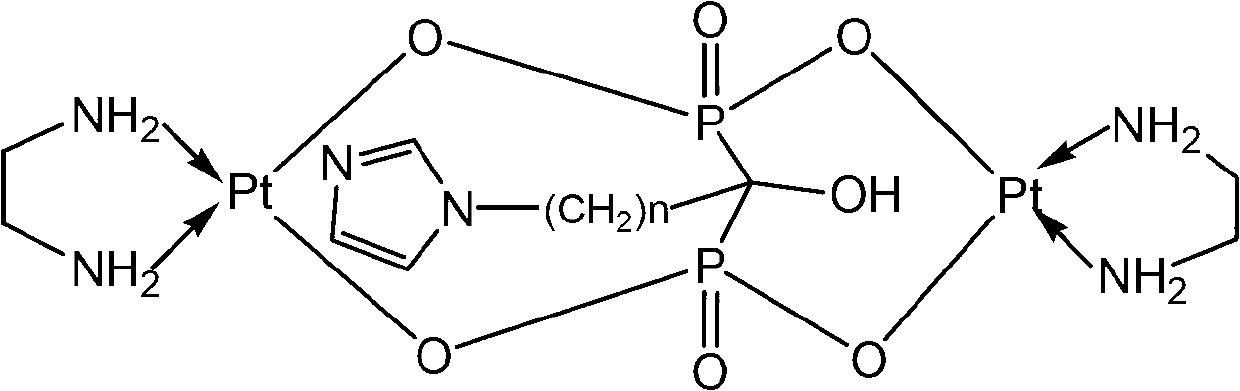
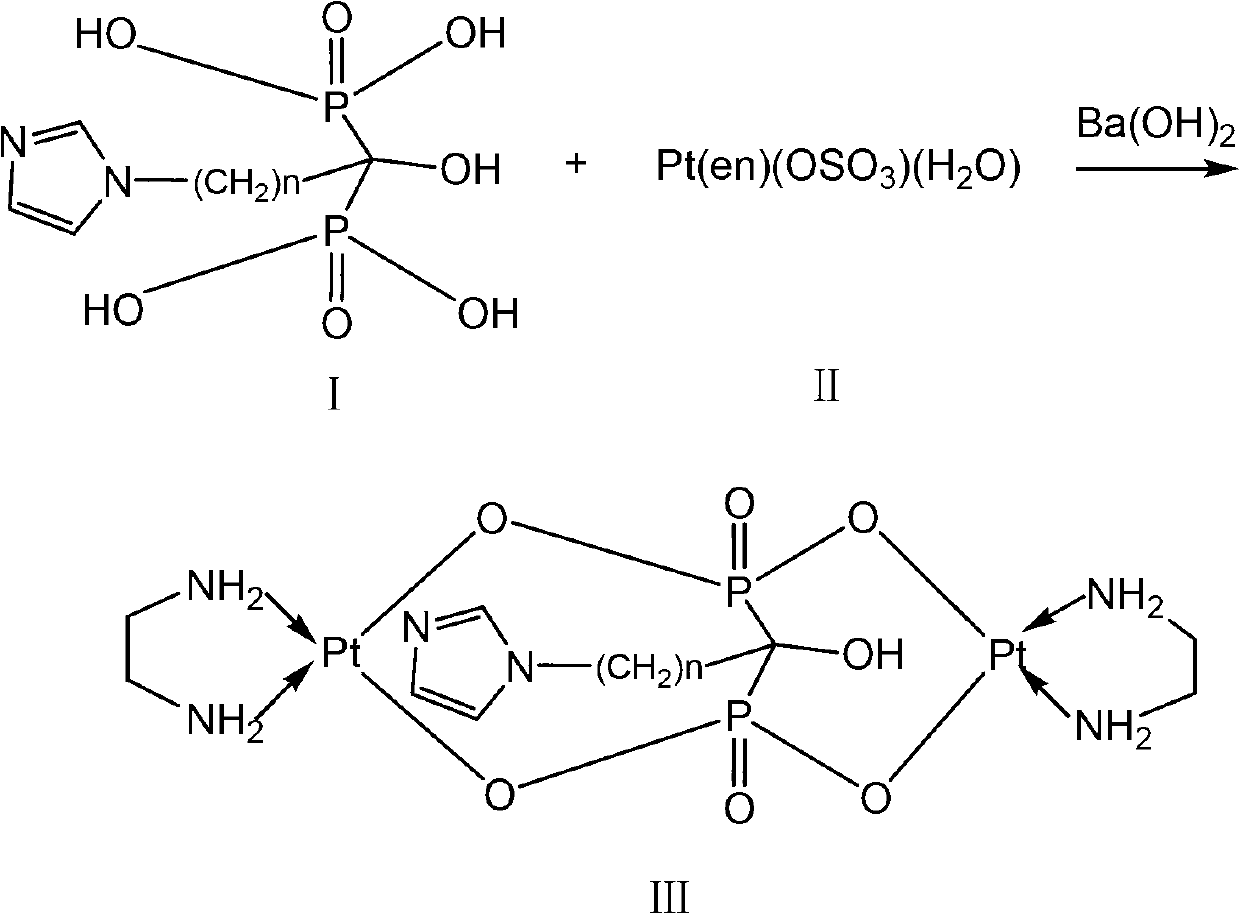
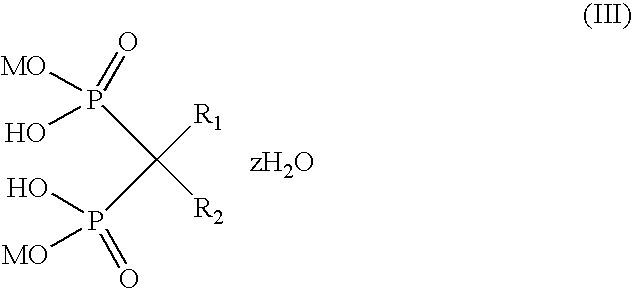
Binuclear platinum (II)-diphosphonic acid coordination compound, preparation method and application thereof
ActiveCN102731580AIncrease intakeExtended stayOrganic active ingredientsOrganic chemistryPlatinumDiphosphonates
The invention provides a binuclear platinum (II)-diphosphonic acid coordination compound, a preparation method and an application thereof, and belongs to the field of anti-cancer drugs. The binuclear platinum (II)-diphosphonic acid coordination compound has the structural formula shown in the description, wherein n is 2, 3, 4 or 5. The preparation method of the binuclear platinum (II)-diphosphonic acid coordination compound comprises the following steps: diphosphonic acid derivative and Pt(en)(OSO3)(H2O) are reacted under the function of Ba(OH)2 to obtain the binuclear platinum (II)-diphosphonic acid coordination compound. According to the compound, a link arm of C between diphosphonates is expanded; quinary aromatic amine (namely imidazole) is introduced in, so that at the initial usage stage of the coordination compound, the coordination compound has high intake in tumour cells and long residence time; and the aggregation concentration of the coordination compound in other non-targeted tissues is greatly reduced.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Treatment and prevention of dental pathology in humans and non-human animals
ActiveUS20120231057A1Reduce riskTreating and preventing and reducing riskPowder deliveryBiocideParticulatesDisease
The present invention relates to methods and compositions for inhibiting, treating, and preventing dental diseases in human and non-human animals, particularly domesticated companion animals. More particularly, the present invention relates to the unexpected discovery that the combination of micron-sized particulate bioactive glass and a topical bisphosphonate yields a composition that is capable of treating and / or preventing dental problems such as periodontal disease, tooth decay and tooth resorption in animals, particularly small mammals such as cats.
Owner:HACK GARY D
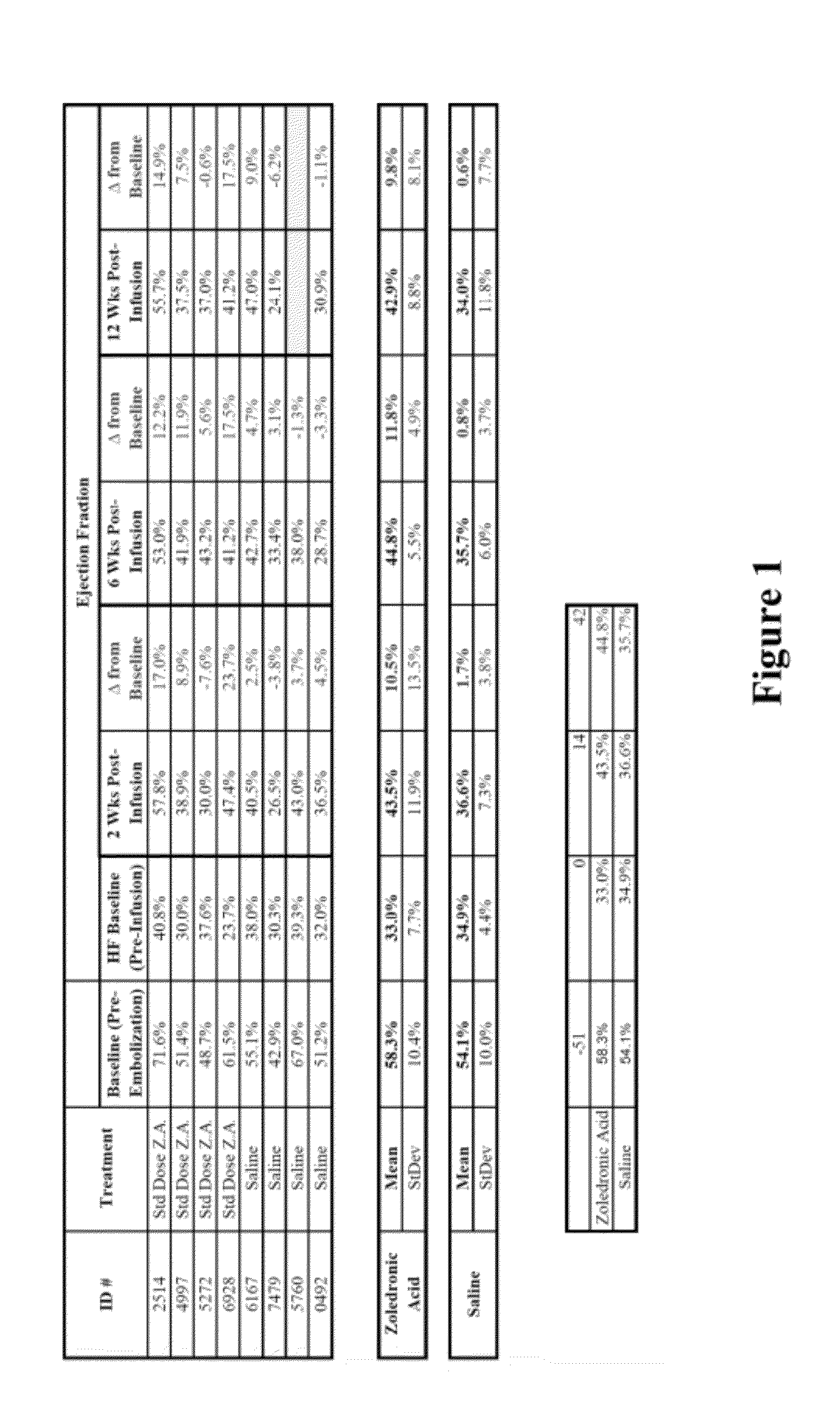
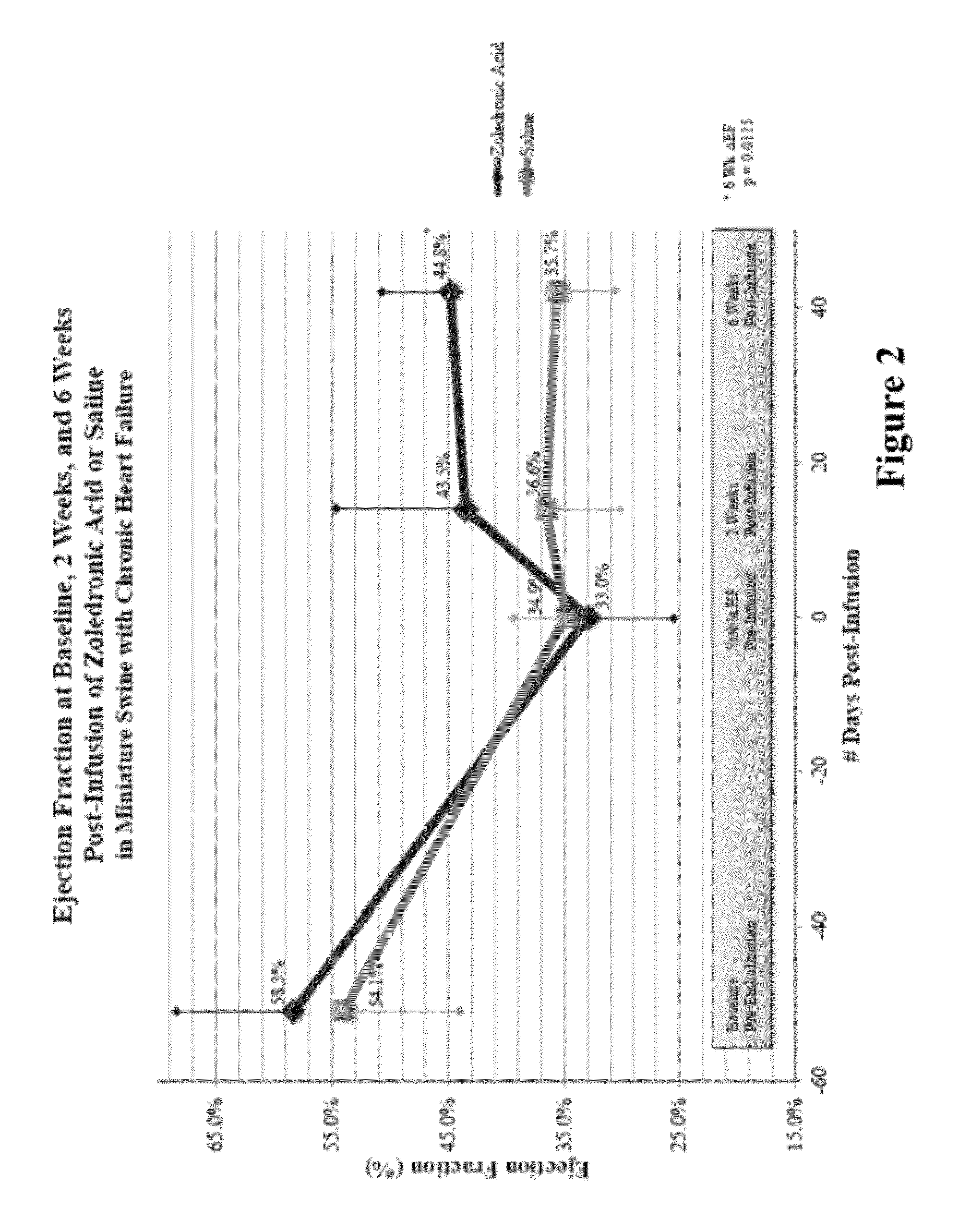
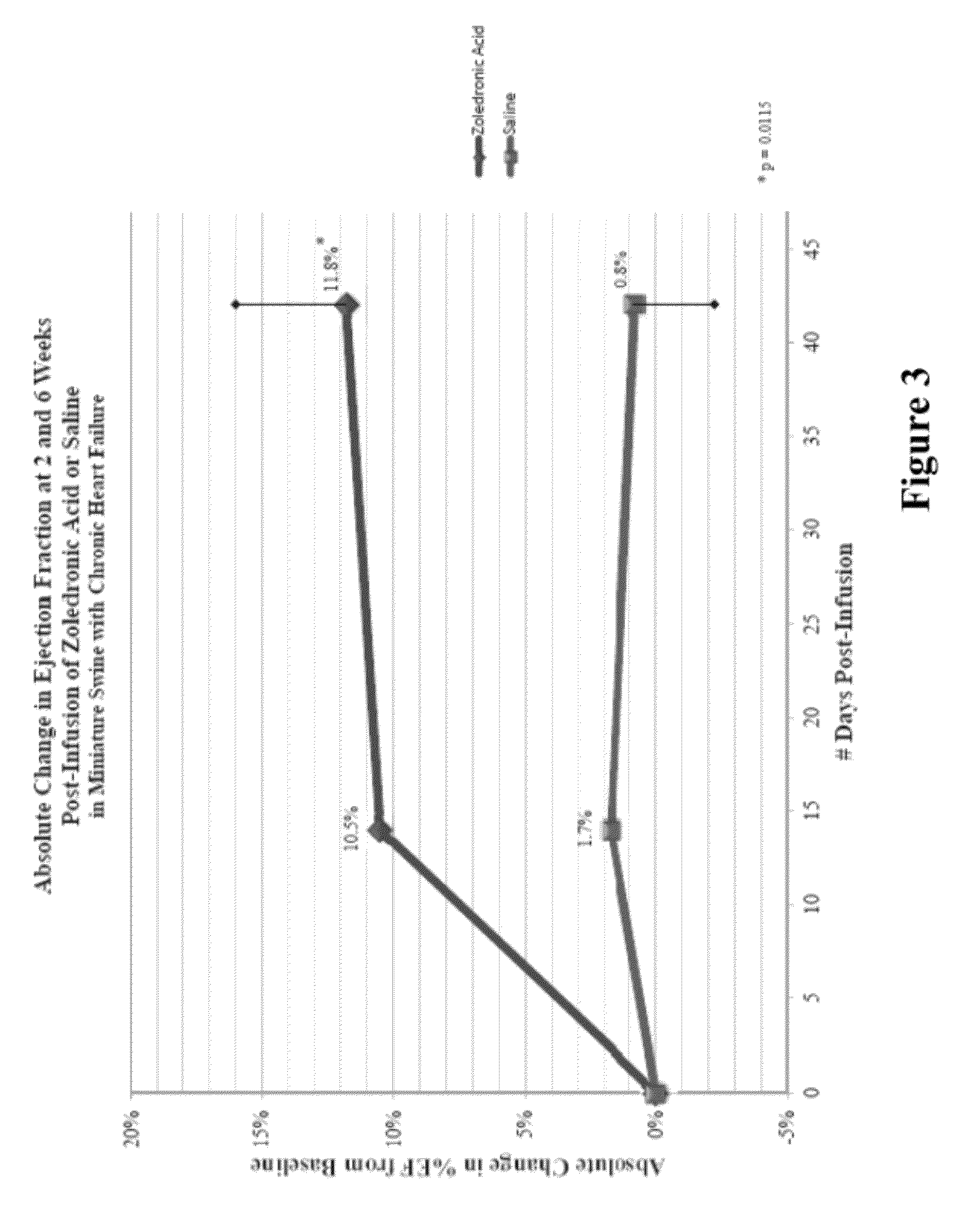
Bisphosphonate compositions and methods for treating heart failure
ActiveUS20120208786A1Treat and prevent heart failureRemarkable effectBiocidePhosphorous compound active ingredientsCardiac dysfunctionDiphosphonates
The present invention provides for methods and compositions for treating and / or preventing cardiac dysfunction by administering to subject a therapeutically effective amount of a bisphosphonate, functional analogue or a pharmaceutically effective salt thereof.
Owner:DUKE UNIV
Low dosage forms of risedronate or its salts
ActiveUS20100113394A1Effective absorptionReduce absorptionOrganic active ingredientsBiocideBisphosphonate therapyImmediate release
Oral dosage forms comprising risedronate or a salt thereof, a chelating agent, and means for effecting delayed release of the risedronate (or salt) immediate release of the oral dosage form to the small intestine of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between the risedronate (or salt) and food or beverages, which interaction results in the active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, disclosed is delivery of risedronate and the chelating agent to the small intestine, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
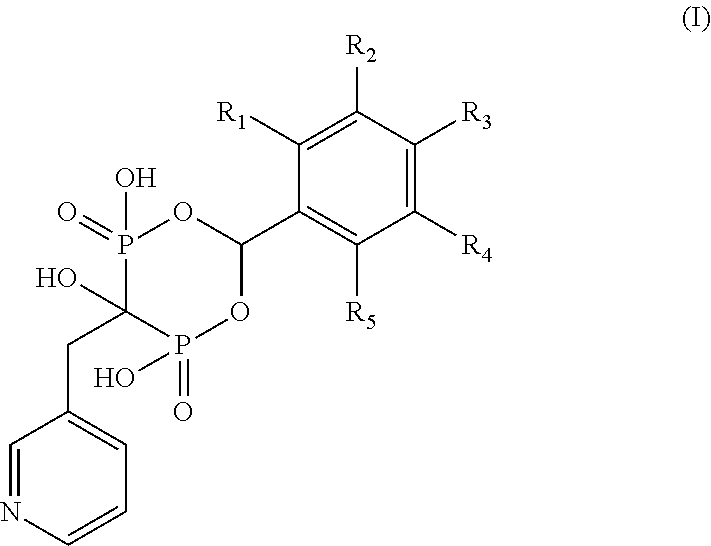
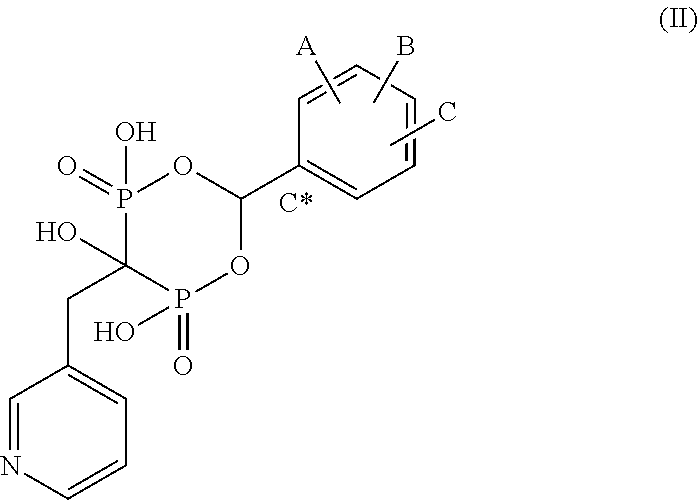
Bisphosphonate compounds
Novel bisphosphonate cyclic acetal compounds are disclosed, as well as methods of preparing the compounds, pharmaceutical compositions including the compounds, and administration of the compounds in methods of treating bone metabolism disorders, such as abnormal calcium and phosphate metabolism.
Owner:APTALIS PHARMA
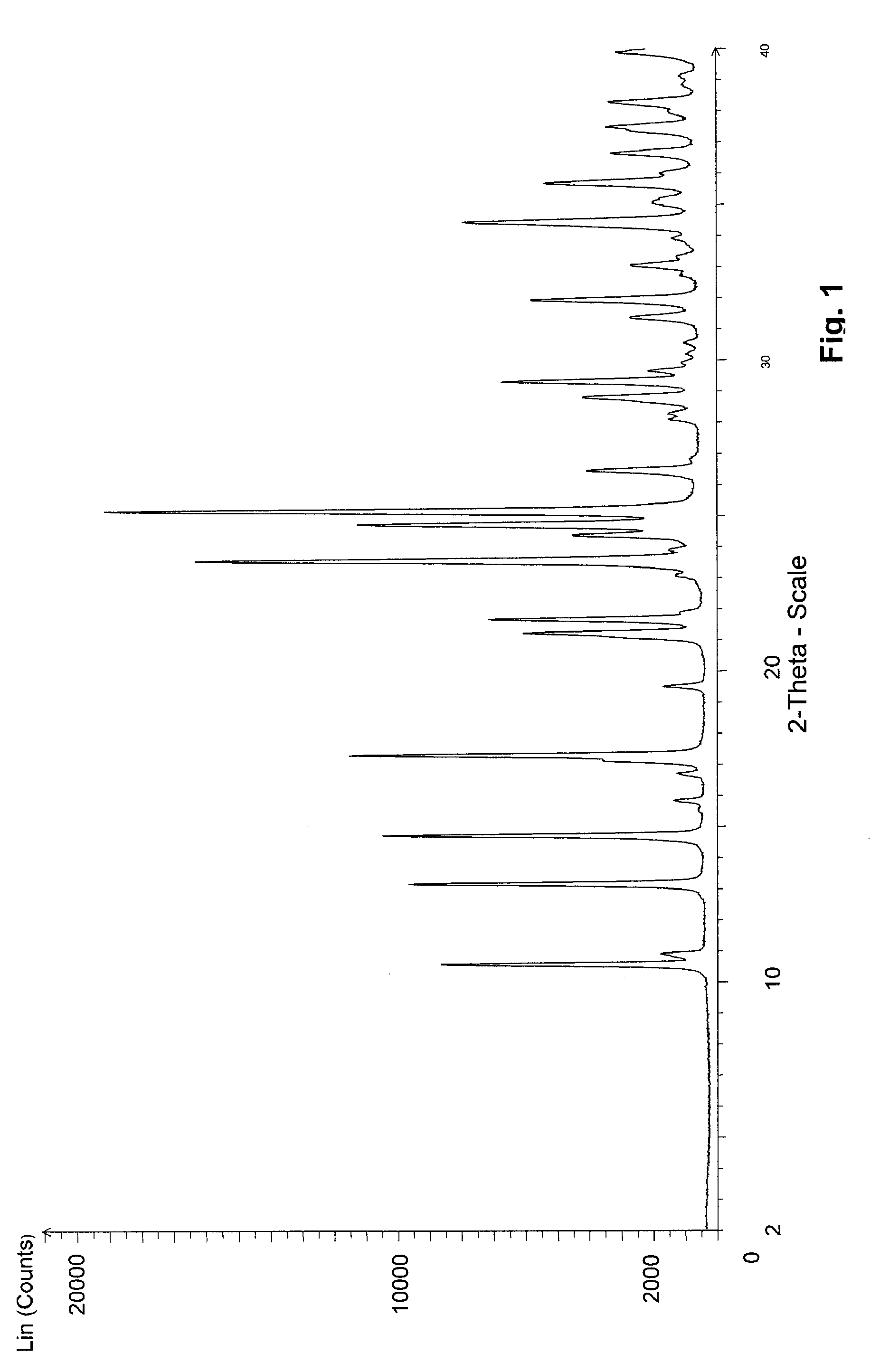
Pharmaceutical composition with bisphosphonate
The present invention relates to depot formulations comprising a poorly water soluble salt of a bisphosphonate forming together with one or more biocompatible polymers, to poorly water-soluble salts of such bisphosphonates, to crystalline forms of the free compounds and the salts and to other related aspects, where the compounds are of the Formula (I), where R1 and R2 are as described in the specification. Compounds of the Formula (I) and their forms mentioned in the disclosure are useful for the treatment of bone-related disorders and cancer.
Owner:NOVARTIS AG
Conjugates of a polymer, a bisphosphonate and an Anti-angiogenesis agent and uses thereof in the treatment and monitoring of bone related diseases
Conjugates of polymers or copolymers having attached thereto an anti-angiogenesis agent and a bisphosphonate bone targeting agent, and processes of preparing same, are disclosed.Pharmaceutical compositions containing these conjugates and uses thereof in the treatment of bone related disorders are also disclosed.
Owner:RAMOT AT TEL AVIV UNIV LTD
Low dosage forms of risedronate or its salts
ActiveUS20100113395A1Effective absorptionReduce absorptionBiocideOrganic active ingredientsBisphosphonate therapyPatient compliance
Oral dosage forms comprising risedronate or a salt thereof, a chelating agent, and means for effecting delayed release of the risedronate (or salt) immediate release of the oral dosage form to the small intestine of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between the risedronate (or salt) and food or beverages, which interaction results in the active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, disclosed is delivery of risedronate and the chelating agent to the small intestine, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Implantable formulations of bisphosphonic acids
InactiveUS20100247607A1Sustained releaseBiocideGroup 8/9/10/18 element organic compoundsMedicineDiphosphonates
The invention relates to devices, methods and formulation for subcutaneous administration of a bisphosphonate. In such a device, a drug core, comprising a bisphosphonate, is disposed in a tube. The devices may be administered to a patient in need of subcutaneously wherein the release of the bisphosphonate is desired to provide sustained release of a therapeutically effective dose of the bisphosphonate.
Owner:PSIVIDA INC
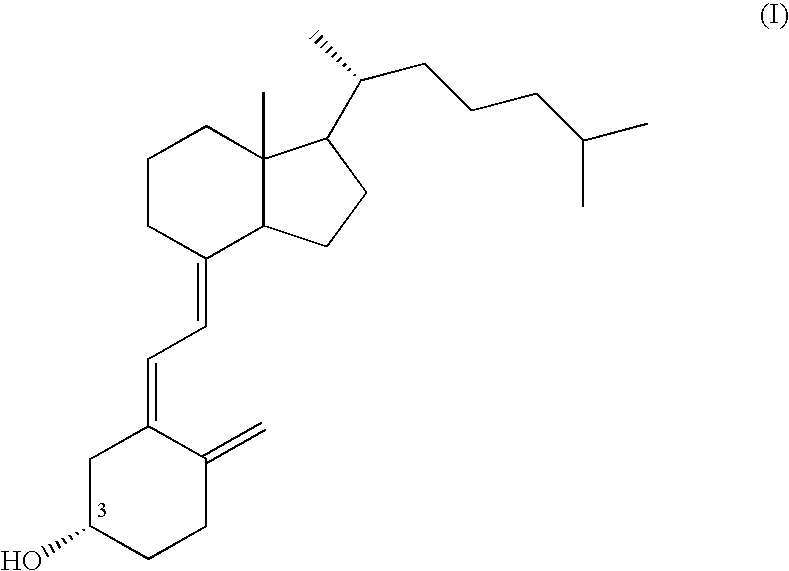
Complex formulation for preventing or treating osteoporosis which comprises solid dispersion of vitamin d or its derivative and bisphosphonate
InactiveUS20100048511A1Improved vitamin D stabilityImprove stabilityBiocideNanomedicinePatient complianceDiphosphonates
Provided is a solid dispersion comprising vitamin D or a derivative thereof and a cyclodextrin; a complex formulation for the prevention or treatment of osteophorosis, which includes the solid dispersion and a bisphosphonate; and a method for preparing said complex formulation. The complex formulation can maintain a constant therapeutic level of vitamin D or a derivative thereof through its improved drug stability, while enhancing the patient compliance by minimizing inconvenience and adverse effects when administered to patients.
Owner:HANMI SCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com