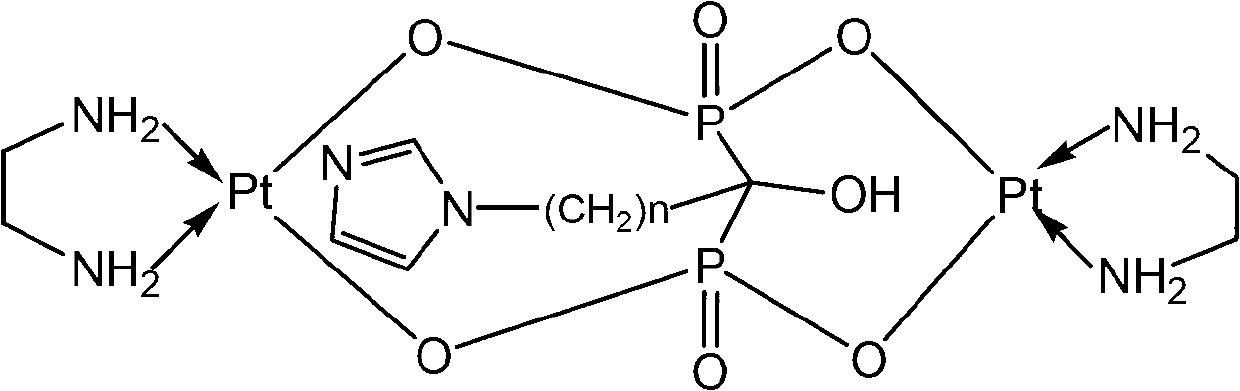
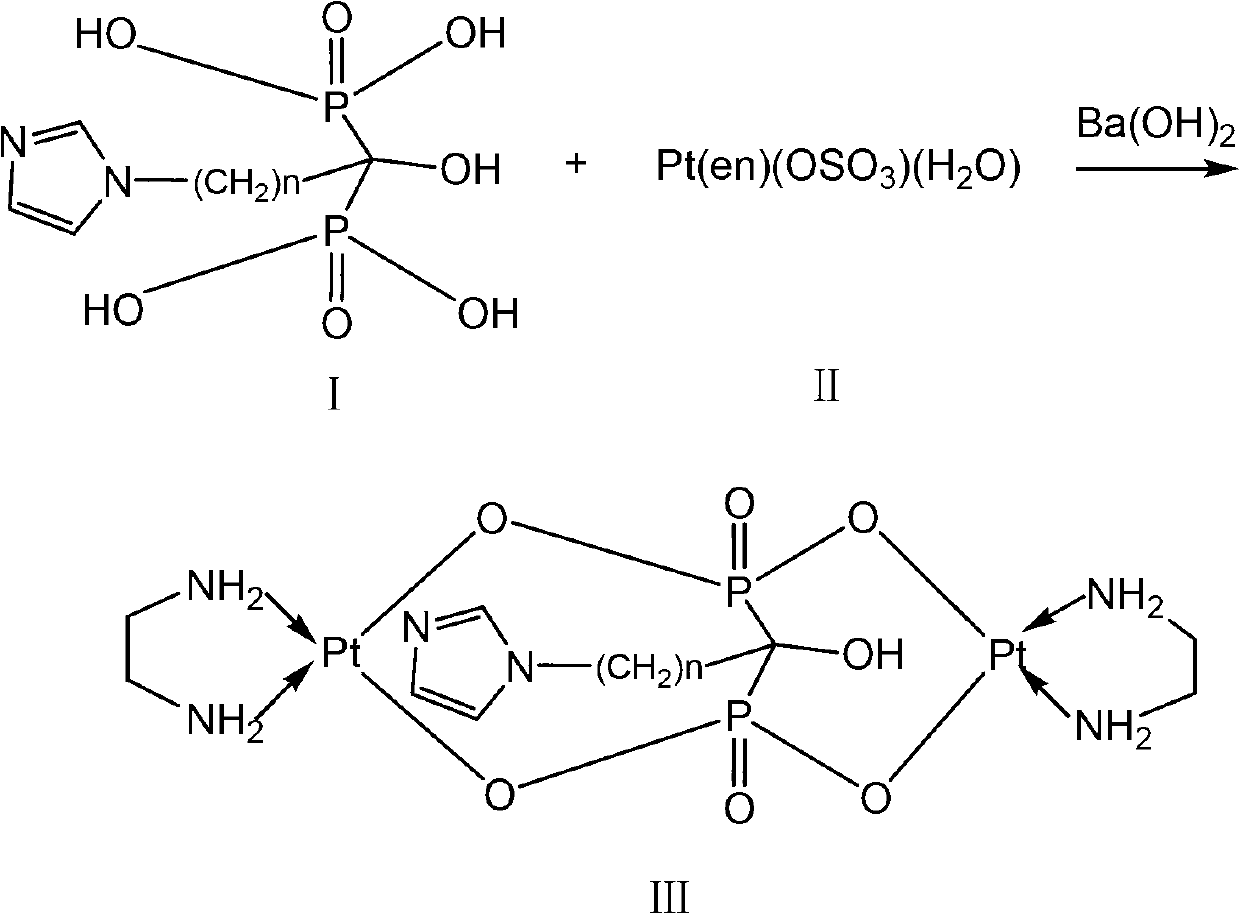
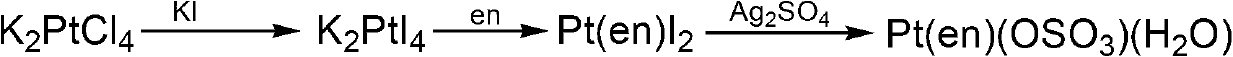Binuclear platinum (II)-diphosphonic acid coordination compound, preparation method and application thereof
A dual-nuclear platinum and complex technology, applied in the field of antitumor drugs, can solve the problems of large dosage, low activity, and many adverse reactions, and achieve the effects of small dosage, high intake, and few adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of bisphosphonic acid derivatives
[0042]
[0043] Wherein, the preparation method of the bisphosphonic acid derivative of n=2, 3, 4 or 5 is as follows:
[0044]
[0045] (1) preparation of
[0046] Dissolve 0.1mol 1H-imidazole in 75ml of CH 2 Cl 2 , then add 0.15mol of KOH, 0.0835mol of K to the above solution 2 CO 3 And the tetrabutylammonium bromide of 0.002mol, stirred reaction 30min under room temperature, then in the above-mentioned solution, add the Br of 12.8ml dropwise (CH 2 ) n COOEt, reacted for 30min, then heated in an oil bath for 7h, filtered and washed to obtain
[0047] (2) preparation of
[0048] 60mmol of the prepared in step (1) Add 100ml of water, and then add 1ml of concentrated hydrochloric acid to it, react in an oil bath for 8h under stirring conditions, concentrate and crystallize to obtain
[0049] (3) preparation of
[0050] 20mmol of the prepared in step (2) Dissolve in 4.2ml of phosphoric ...
Embodiment 2
[0083] Example 2 Pt(en)(OSO 3 )(H 2 O) Preparation
[0084]
[0085] (1) Dissolve 1mmol of potassium chloroplatinite in 5mL of water, add 8mmol of excess KI aqueous solution dropwise under stirring conditions, react at 40°C for 30min, filter, then add 1mmol of ethylenediamine to the filtrate, and react at room temperature for 5h , filtered to obtain the precipitate, which is cis-[Pt(en)I 2 ], the productive rate is 94.94%;
[0086] (2) Add 1 mmol of cis-[Pt(en)I prepared in step (1) 2 ] was suspended in 20mL of water, and then 1mmol of silver sulfate was added thereto, reacted overnight at room temperature in the dark, filtered, and the filtrate was distilled under reduced pressure;
[0087] The vacuum distillation is carried out in two steps, the first step is vacuum distillation until the raffinate is 1 / 2 of the total volume of the original filtrate, the raffinate is filtered to obtain a new filtrate, and the new filtrate is subjected to vacuum distillation again to o...
Embodiment 3-1
[0088] Example 3-1 Preparation of binuclear platinum(II)-bisphosphonic acid complexes (bisphosphonate barium salt method)
[0089] In this embodiment, the bisphosphonic acid derivative with n=2 is selected, and the bisphosphonic acid derivative is referred to as IPrDP for short.
[0090] (1) Dissolve 0.1mmol of solid IPrDP in 5mL of water. At room temperature and under the protection of an inert gas, add 4ml of 0.025mmol / ml barium hydroxide aqueous solution dropwise into the above solution and stir for 20min to obtain white A suspension of a solid, wherein the white solid is the barium salt of IPrDP;
[0091] (2) Control the temperature of the suspension described in step (1) at 0-5°C, add 0.15 mmol of [Pt(en)(OSO 3 )(H 2 O)] dissolved in 5mL of water, dripped into the suspension described in step (1) under the protection of an inert gas (such as nitrogen), after reacting for 5h, separate the supernatant, and add Ba( Oh) 2 Adjust its pH to 4.0, continue to maintain stirr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com