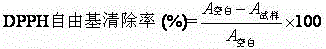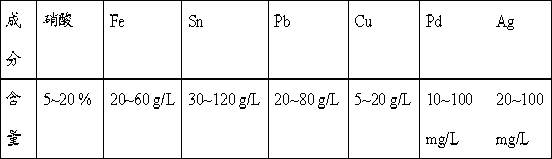Patents
Literature
250 results about "Silver sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
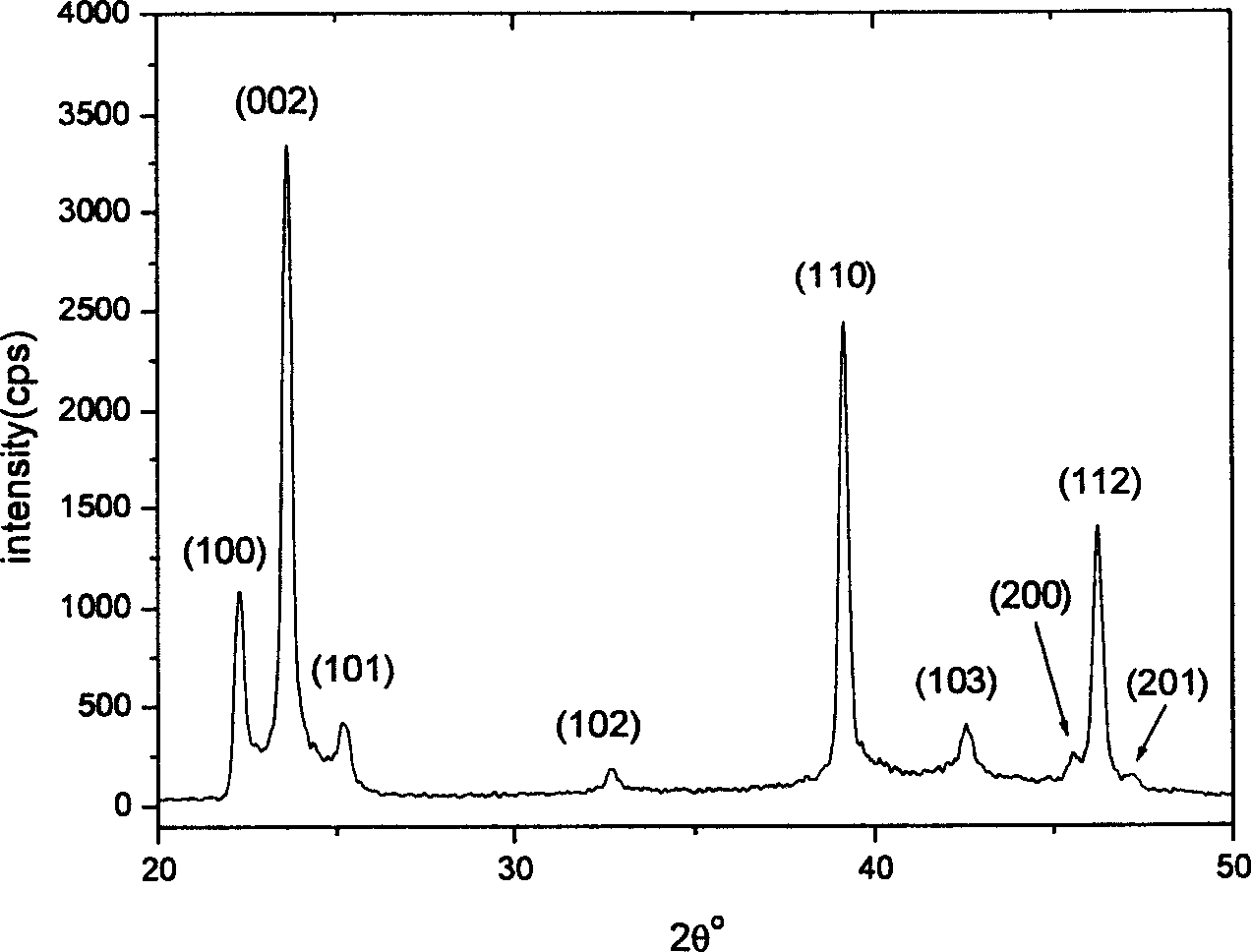
Silver sulfate (Ag₂SO₄) is an ionic compound of silver used in silver plating and as a non-staining substitute to silver nitrate. This sulfate is stable under ordinary conditions of use and storage, though it darkens upon exposure to air or light. It is minimally soluble in water.
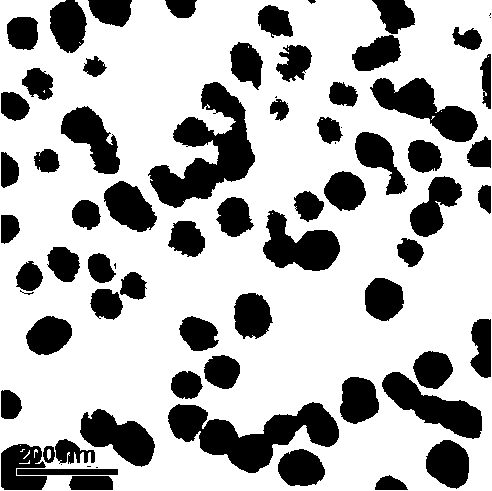
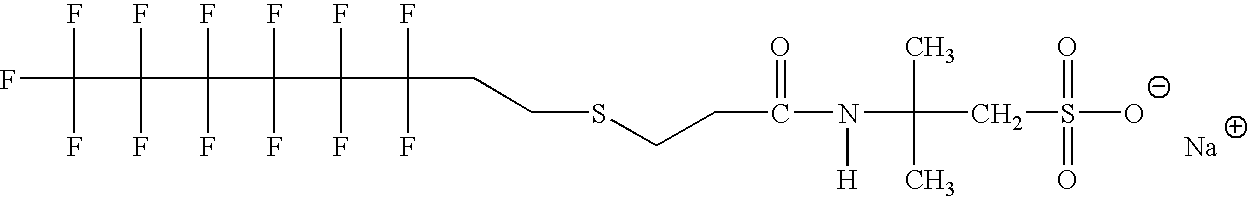
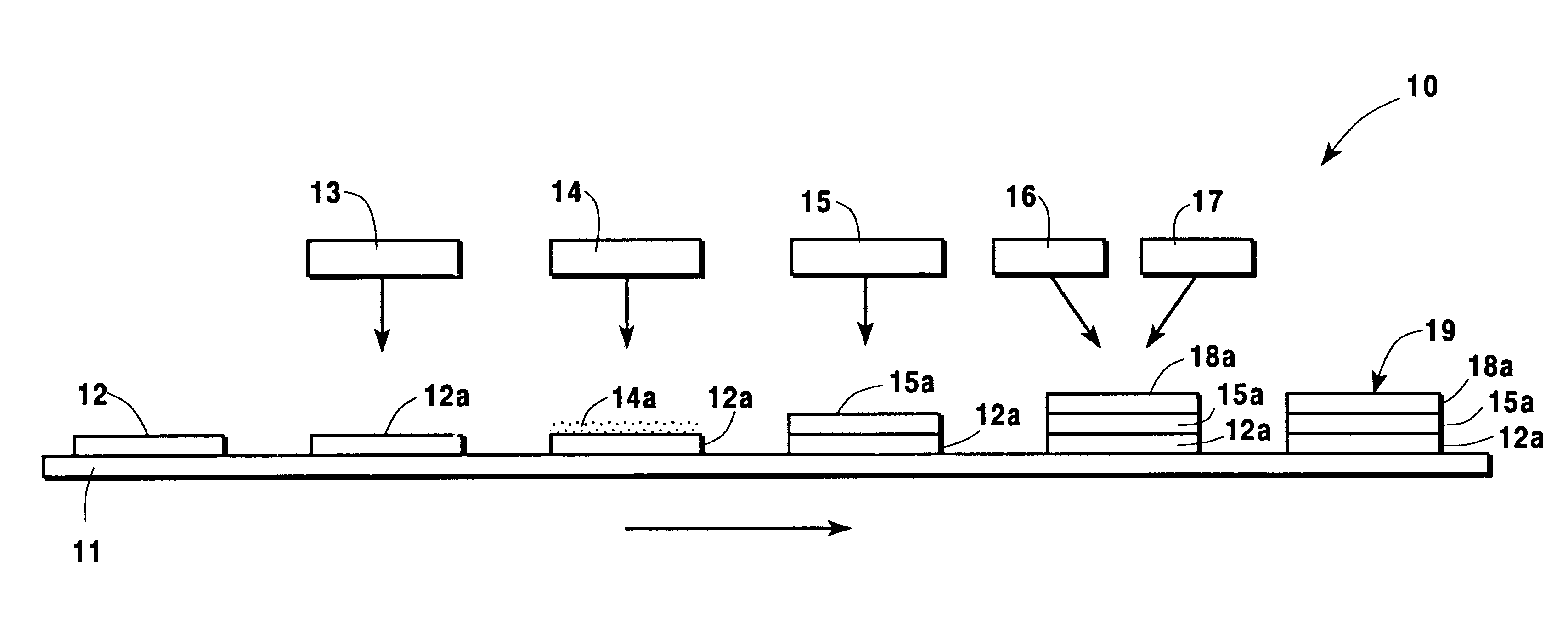
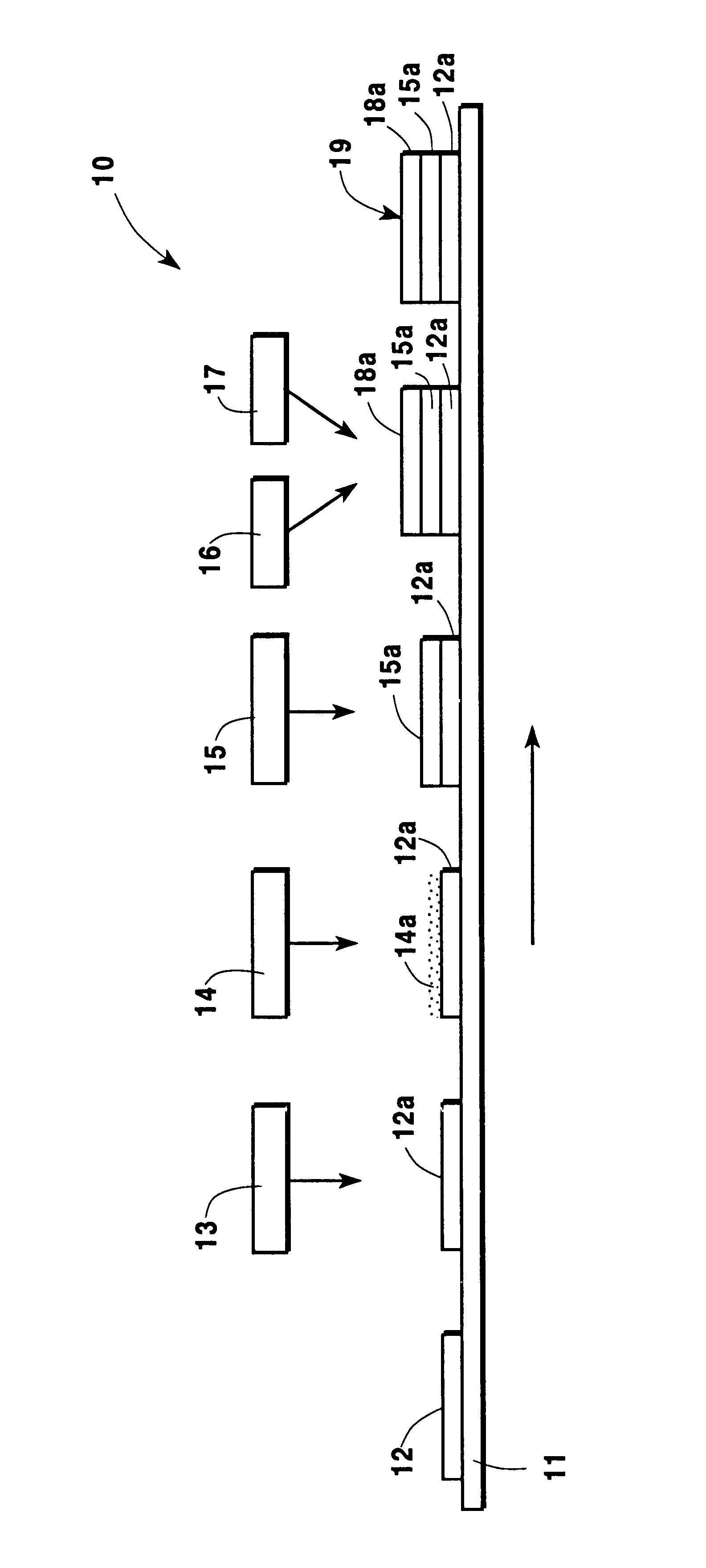
Production of silver sulfate grains using organo-sulfate or organo-sulfonate additives
ActiveUS7261867B1Uniform morphologyUniform sizePigmenting treatmentCosmetic preparationsOrganic sulfonic acidSulfonate
An aqueous precipitation process for the preparation of particles comprising primarily silver sulfate, comprising reacting an aqueous soluble silver salt and an aqueous soluble source of inorganic sulfate ion in an agitated precipitation reactor vessel and precipitating particles comprising primarily silver sulfate, wherein the reaction and precipitation are performed in the presence of an aqueous soluble organo-sulfate or organo-sulfonate additive compound, the amount of additive being a minor molar percentage, relative to the molar amount of silver sulfate precipitated, and effective to result in precipitation of particles comprising primarily silver sulfate having a mean grain size of less than 50 micrometers.
Owner:EASTMAN KODAK CO
Liquid nanometer simple substance silver antibacterial agent and preparation method thereof
InactiveCN1729787AFull complexationReduce manufacturing costBiocideAnimal repellantsLiquid stateSilver sulfate
Disclosed is a liquid state nano antimicrobial agent of Argentine simple substance, which comprises the following constituents (by weight percent): argentic salt 2-5%, nano Argentine generating agent 0.5-5%, Argentine complexing agent 0.5-8%, and water 82-97%. In the preparation, ionic type high molecular polymer is used as the Argentine complexing agent.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Treatment method for antibacterial and anti-ultraviolet cotton fabrics
ActiveCN106758216AImprove antibacterial propertiesImproves UV resistanceBiochemical fibre treatmentLight resistant fibresGrape seedUltraviolet
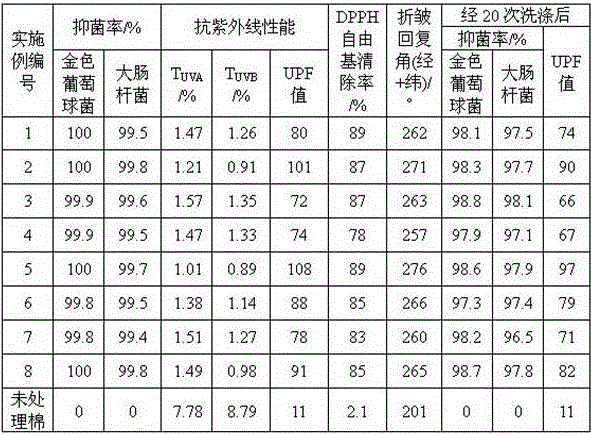
The invention discloses a treatment method for antibacterial and anti-ultraviolet cotton fabrics. The treatment method comprises the following steps: step 1, soaking the cotton fabrics with a nonionic surfactant; step 2, modifying the cotton fabrics with chitosan quaternary ammonium salt; step 3, dipping the cotton fabrics with antibacterial finishing liquid prepared by mixing 5-15 parts of a natural antibacterial agent composed of extracts of grape seeds, persimmon leaves, ginkgo leaves and licorice roots, 0.3-1 part of polyoxyethylene-40 hydrogenated castor oil and 1000 parts of water; step 4, soaking the cotton fabrics in a mixed aqueous solution containing 0.001-0.005mol / L of silver nitrate or silver sulfate or silver acetate and 1-3 g / L of soluble starch or hydroxyethyl cellulose to prepare the nano-silver attached antibacterial cotton fabrics. The prepared cotton fabrics not only have good antibacterial performance and anti-ultraviolet performance, but also have performance of resisting oxidation and scavenging free radicals, and have improved anti-wrinkle performance and good washing durability.
Owner:EASTERN LIAONING UNIV
Inorganic antiseptic of zinc oxide lattice carried silver and its prepn
ActiveCN1771807AExcellent discoloration resistanceImprove heat resistanceBiocideDisinfectantsSilver carbonateSemiconductor properties
The inorganic antiseptic consists of zinc oxide with semiconductor characteristic and Ag ion, where the Ag ion is in the mass fraction of 0.1-10 %, and the zinc oxide is one or several kinds of four-needle zinc oxide whisker, nanometer zinc oxide and common zinc oxide powder. The Ag ion is provided by one or several kinds of silver nitrate, silver carbonate, silver sulfate, silver chloride and sliver oxide, and is carried in the lattice of zinc oxide by means of high temperature solid solution technology. The inorganic antiseptic of the present invention has wide antiseptic spectrum, high efficiency, lasting antiseptic effect and high safety, and is suitable for food packing, building material, medical equipment, fabric, sanitary article and other fields.
Owner:SOUTHWEST JIAOTONG UNIV +1
Preparation method for flaky nanometer silver powder
The invention discloses a preparation method for flaky nanometer silver powder. The flaky nanometer silver powder is prepared by performing reaction on a surfactant-containing aqueous solution of silver salt and a solution of ferrous salt at temperature of 0 to 100 DEG C, wherein the silver salt is silver nitrate, silver sulfate or silver acetate; a surfactant is polyvinylpyrrolidone, hexadecyl trimethyl ammonium bromide, sodium dodecyl sulfate or polyethylene glycol; the ferrous salt is ammonium ferrous sulfate or ferrous sulfate; the weight ratio of the silver salt to the ferrous salt is 2:1 to 1:4; and the weight ratio of the silver salt to the surfactant is 50:1 to 1:2. The flaky nanometer silver powder finally obtained by the method has an undiversified shape and high purity. The method also has the advantages of short reaction time, high efficiency, mild reaction conditions, simple process and equipment, low production cost, convenience for operation, no pollution and the like, and is particularly suitable for industrial production.
Owner:JIANGSU UNIV OF TECH
Tetrammine palladium sulphate synthesis method
InactiveCN102616869AEasy to operateHigh reaction yieldRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsSynthesis methodsAmmonia
The invention discloses a novel tetrammine palladium sulphate (II) synthesis method, comprising the following steps that: palladium chloride (PdCl2) reacts with ammonia water to generate tetraammine dichloropalladium (II); and tetraammine dichloropalladium (II) reacts with silver sulfate to generate a compound of tetrammine palladium sulphate (II). The reaction operation is simple, the reaction yield is high and more than 90%, and the purity of products is high and more than 99.0%.
Owner:KUNMING INST OF PRECIOUS METALS
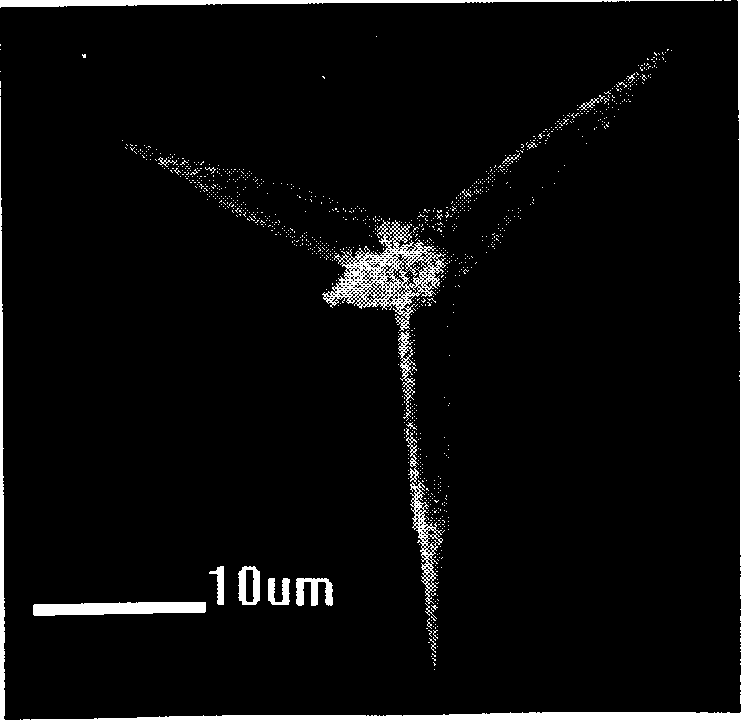
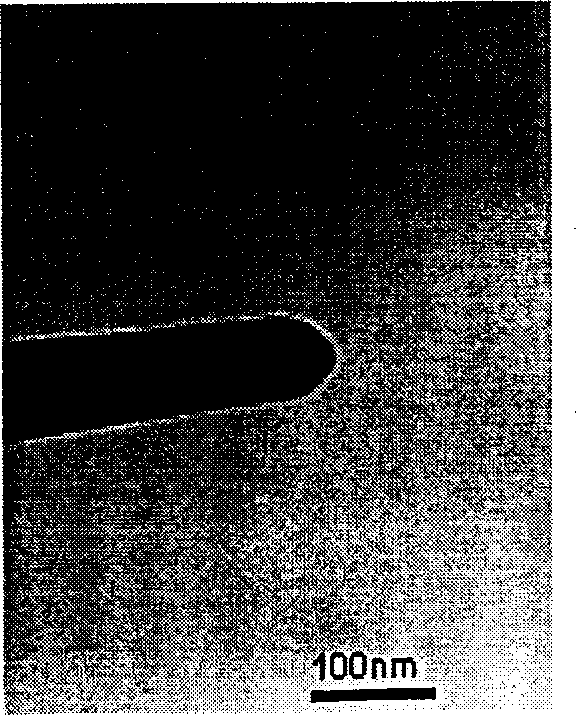
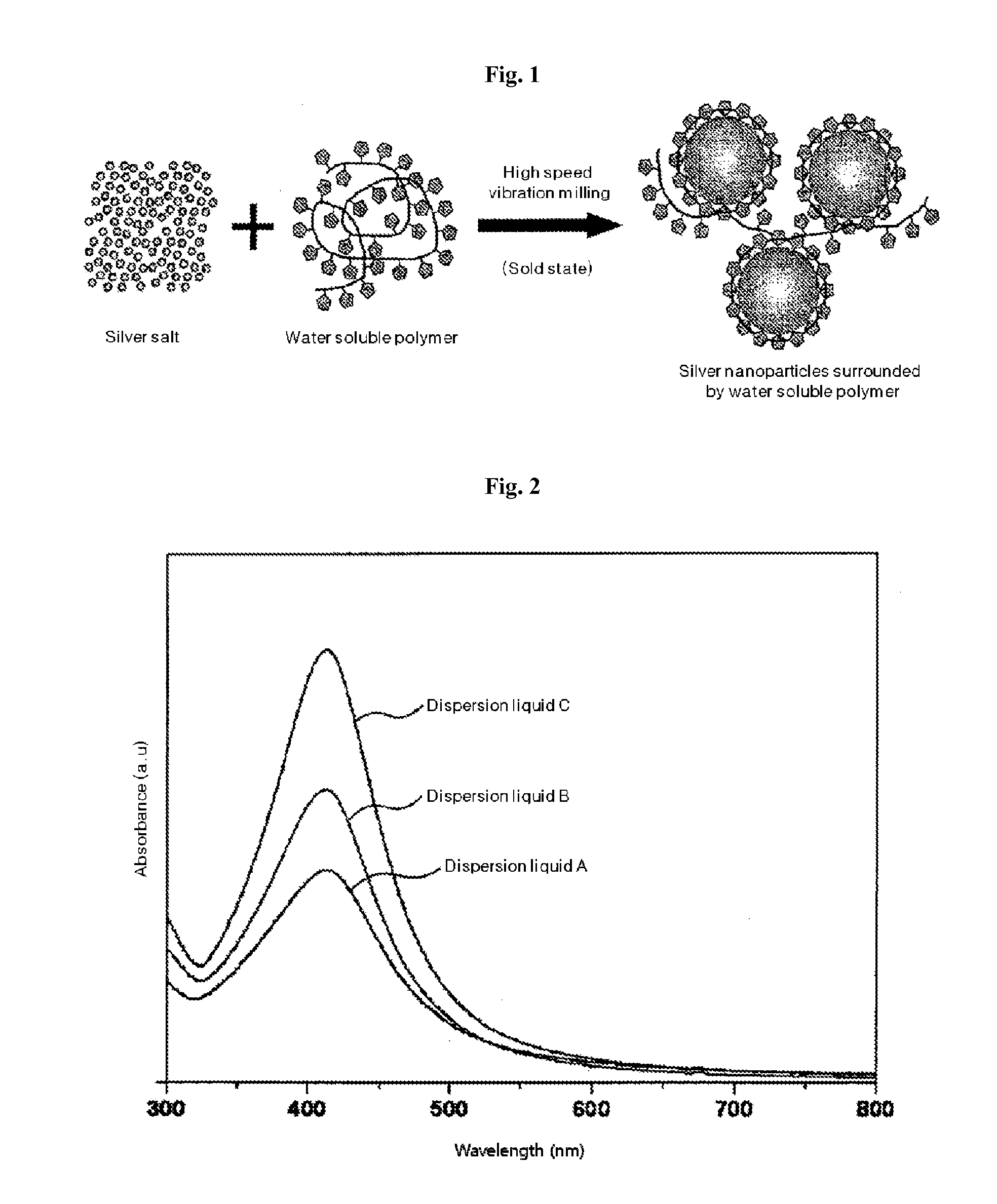
Solid state synthesis method of silver nanoparticles, and silver nanoparticles synthesized thereby
InactiveUS20120225126A1Easily and conveniently produceLow costBiocideMaterial nanotechnologySynthesis methodsWater soluble
Disclosed are a solid state synthesis method of silver nanoparticles, and silver nanoparticles synthesized thereby. The method includes mixing a silver salt and a water soluble polymer acting as both a reducing agent and a protecting agent to produce a solid mixture, and milling the solid mixture by a high-speed vibration milling process to form silver nanoparticles within the water soluble polymer. According to the present invention, silver nanoparticles can be easily and simply produced in a solid state through high speed vibration milling, thereby reducing costs for industrial production and transportation of silver nanoparticles. In addition, the synthesized silver nanoparticles can be used for a long time since the silver nanoparticles are stable in a solid state for 1 year or more.
Owner:GWANGJU INST OF SCI & TECH
Silver thiosulfate complex or silver-ammonia complex-containing hygroscopic silver-containing product and preparation method thereof
ActiveCN104083800ABroad spectrum antibacterialHigh antibacterial activityFibre treatmentTwo or more solvent application treatmentAdditive ingredientMoisture absorption
The invention relates to a silver thiosulfate complex or silver-ammonia complex-containing hygroscopic silver-containing product and a preparation method thereof. The invention discloses a hygroscopic silver-containing antibacterial product which comprises a silver thiosulfate complex or a silver-ammonia complex as an antibacterial ingredient, the silver thiosulfate complex or the silver-ammonia complex is evenly distributed and combined on the inner side and / or surface, the hygroscopic silver-containing antibacterial product comprises 0.01-10 wt% of silver, and the moisture absorption ability of the hygroscopic silver-containing antibacterial product is 6g / g and above. The invention also provides a preparation method of the hygroscopic silver-containing antibacterial product as well as a silver-containing antibacterial product dressing prepared from the hygroscopic silver-containing antibacterial product. The hygroscopic silver-containing antibacterial product has a very wide antibacterial spectrum, shows strong antibacterial activity to gram-negative bacteria and gram-positive bacteria, is rapid in effect, has light stability, and can be widely applied to chronic infections exudative wounds.
Owner:FOSHAN UNITED MEDICAL TECH
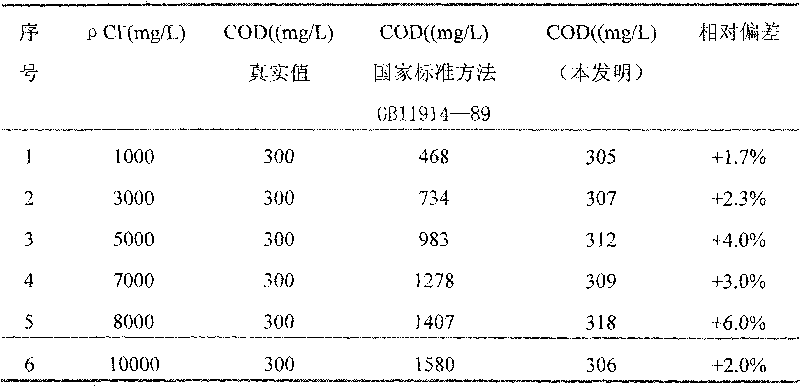
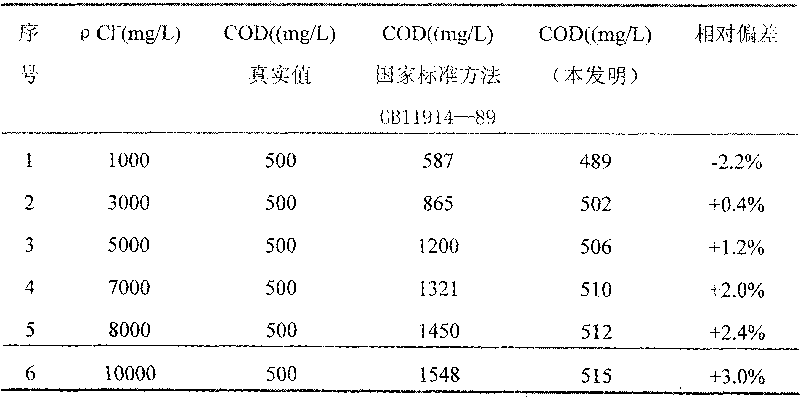
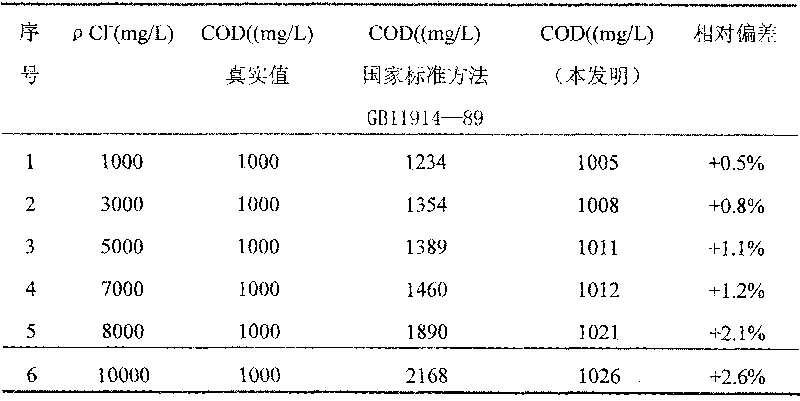
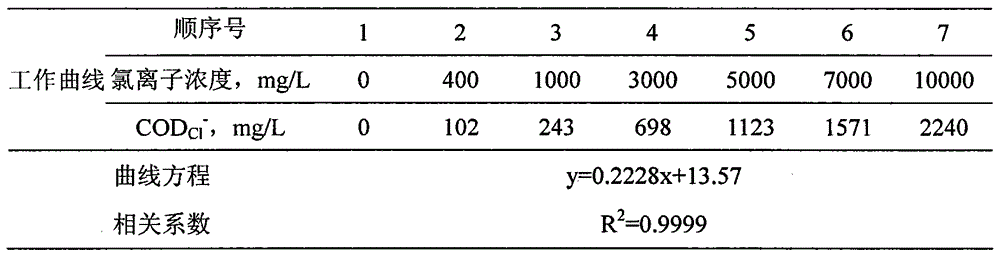
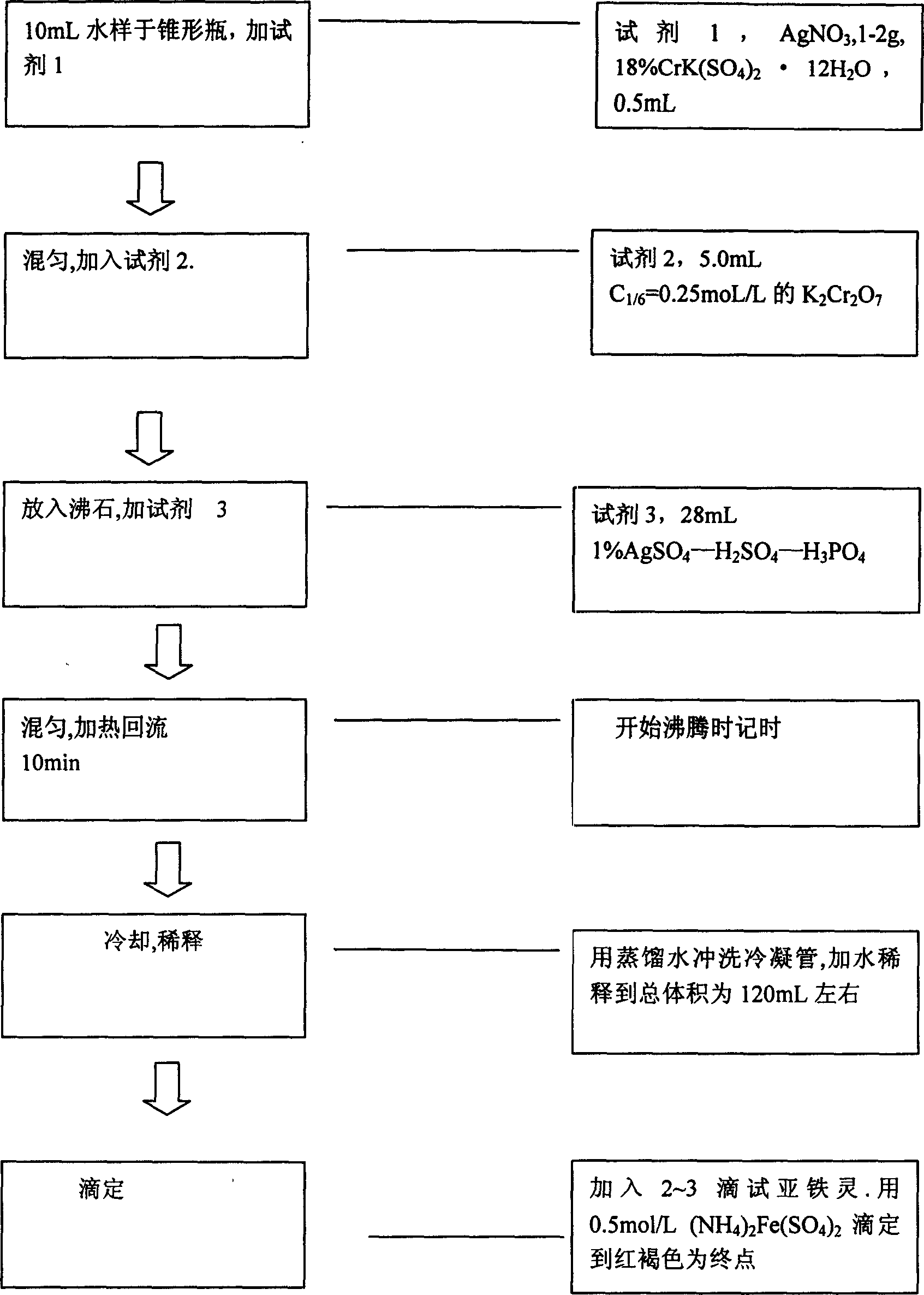
Reagent and method for determining chemical oxygen demand of high-chloride wastewater
InactiveCN101713739AShort measurement timeShort analysis timeMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsDigestionChromium
The invention discloses a reagent and a method for determining the chemical oxygen demand of high-chloride wastewater. The reagent consists of a reagent A and a reagent B, wherein the reagent A comprises 20 to 40 mass percent of potassium dichromate, 20 to 50 mass percent of aluminum potassium sulfate, and 10 to 60 mass percent of ammonium molybdate; and the reagent B comprises 20 to 40 mass percent of silver sulfate, 10 to 20 mass percent of bismuth nitrate, 10 to 20 mass percent of chromium potassium sulfate, and 20 to 60 mass percent of mercury sulfate. The method comprises the steps of: during the determination, sequentially adding the reagent A and the reagent B into a water sample, placing the obtained solution into a digestion device for digestion, cooling the obtained product, and then performing colorimetric determination. The reagent and the method are suitable for wastewater with the chloride ion concentration of between 1,000 and 10,000mg / L; and the reagent has the characteristics of accurate formula proportion, accurate and reliable determined data, short determination time, simple determination steps, convenient operation and the like; and the method can perform batch determination.
Owner:JIANGSU POLYTECHNIC UNIVERSITY +1
Cellulose environment-friendly synthesis method of nano silver particles
The invention discloses a cellulose environment-friendly synthesis method of nano silver particles. The cellulose environment-friendly synthesis method comprises the following steps of: placing cellulose and silver sulfate in a polytetrafluoroethylene lining, adding deionized water, fully stirring to ensure that the cellulose, the silver sulfate and the deionized water are uniformly mixed; and loading the lining in a high-pressure autoclave and placing in an oven, heating for 10-24h at a temperature of 180-210 DEG C, taking out and then naturally cooling; centrifuging or suction-filtering and separating a cooled product to obtain the nano silver particles. According to the cellulose environmental-friendly synthesis method, other chemical agents are not needed to be introduced, renewable biomass resources can be utilized, and the convenience is brought for the operation; the synthesized nano silver particles are controllable in morphology and size, uniform in dispersion, and high in yield; and the synthesized nano silver particles are not agglomerated.
Owner:PUTIAN UNIV
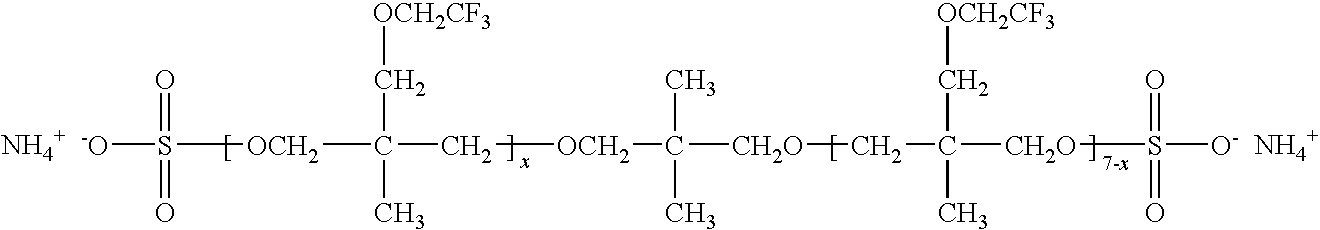
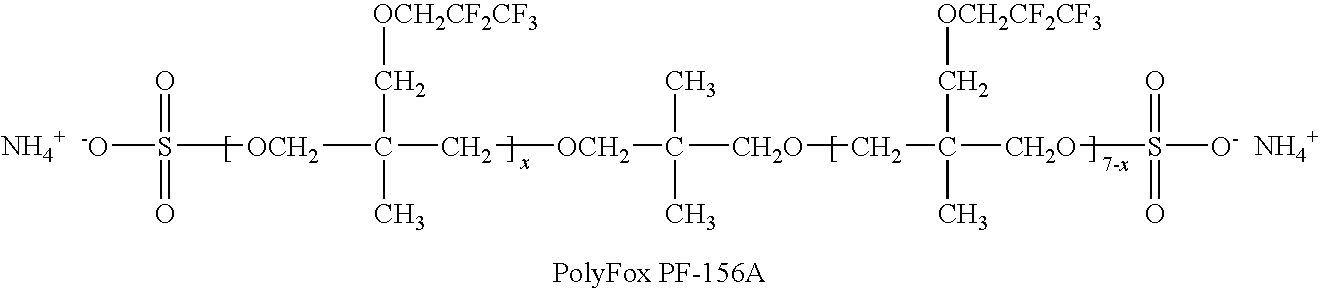
Production of silver sulfate grains using a fluorinated additive
InactiveUS20090258984A1Uniform morphologyUniform sizePigmenting treatmentCosmetic preparationsMicrometerWater soluble
An aqueous precipitation process for the preparation of particles comprising primarily silver sulfate, comprising reacting an aqueous soluble silver salt and an aqueous soluble source of inorganic sulfate ion in an agitated precipitation reactor vessel and precipitating particles comprising primarily silver sulfate, wherein the reaction and precipitation are performed in the presence of an aqueous soluble fluorinated additive, the amount of additive being a minor molar percentage, relative to the molar amount of silver sulfate precipitated, and effective to result in precipitation of particles comprising primarily silver sulfate having a mean grain-size of less than 50 micrometers.
Owner:EASTMAN KODAK CO
Antibacterial and antifungal protection for ink jet image
InactiveUS20130189499A1Effective in providing antibacterialEffective in antifungal protectionBiocideDecorative surface effectsColor imageSolvent
A method of forming a clear ink jet coating or a colored ink jet image on a substrate is disclosed. The coating or colored image provides antibacterial and antifungal protection. The method includes providing a source of ink jet ink having a mixture of solvent and a silver salt biocide including a silver sulfate biocide having a concentration range of 0.0005 to 0.5 weight %, applying the clear ink or colored ink in an image wise fashion to a substrate, and fixing the clear or colored ink to the substrate whereby an effective coating or image article is formed that provides antibacterial and antifungal protection.
Owner:EASTMAN KODAK CO
Prepn process of nano silver iodide powder
InactiveCN1673093AHigh purityLow costNanostructure manufactureIodide preparationSilver iodideGranularity
The present invention relates to the preparation process of nanometer silver iodide powder. Clear silver sulfate water solution containing silver nitrate in 0.1-0.4 M / L and complexing agent in 0.034-0.2 M / L is dropped slowly into clear potassium iodide water solution containing potassium iodide in 0.1-0.4 M / L, complexing agent in 0.034-0.2 M / L and dispersant in 0.01-0.04 M / L at normal temperature and normal pressure via stirring, and the product is let stand to deposit to obtain nanometer silver iodide deposit. The nanometer silver iodide deposit is filtered, washed and dried at 80-100 deg.c for 1-2.5 hr to obtain nanometer silver iodide powder with granularity below 100 nm. The process is easy to control, pollution-free and suitable for industrial production, and the product has high quality and low cost.
Owner:JILIN UNIV
One-pot cellulose hydrothermal synthesis method for preparing high-draw-ratio silver nanowire
InactiveCN103357890AEasy to operateUniform sizePolycrystalline material growthFrom normal temperature solutionsCelluloseFiltration
The invention discloses a one-pot cellulose hydrothermal synthesis method for preparing a high-draw-ratio silver nanowire. The method comprises the following steps: adding cellulose, silver sulfate, cetyl trimethyl ammonium bromide and deionized water into a polytetrafluoroethylene liner at one time, and performing full stirring and uniform mixing; filling the liner into a high-pressure kettle, and putting the liner into an oven or a muffle furnace; heating for 10 to 24 h at the temperature of 180 to 200 DEG C, and taking the liner out for natural cooling; and performing centrifugal separation or suction filtration separation on a cooled product to obtain the high-draw-ratio silver nanowire. By using renewable biomass resources, the method is easy to operate; the silver nanowire is generated by one-pot; and the average diameter of the obtained silver nanowire is 90 to 350 nm, the draw ratio is more than 200, and the silver nanowire is uniform in size and high in yield.
Owner:PUTIAN UNIV
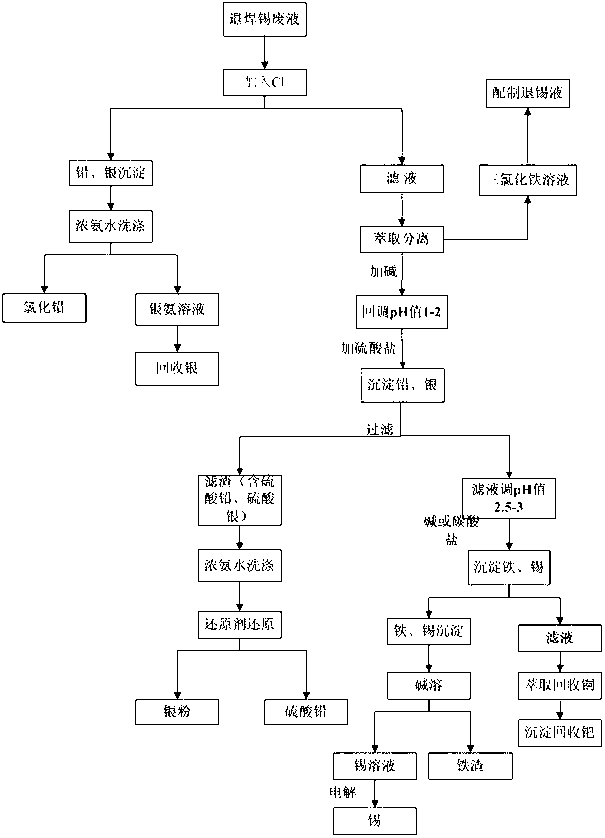
Processing method for waste liquid from stripping tin scolding
ActiveCN103031437AReduce wasteReduce pollutionLead halidesMultistage water/sewage treatmentWater chlorinationLead(II) sulfate
The invention provides a processing method for waste liquid from stripping tin scolding, which comprises the following steps: in the waste liquid from stripping tin scolding, adding a chlorate for reacting, then filtering the solution, washing the filter residue by a concentrated ammonia liquor for dissolving the silver chloride sediment, recovering silver by a washing lotion to acquire pure lead chloride, extracting a filtrate by an extractant to obtain ferric iron, wherein a strip liquor is a ferric trichloride solution which can be prepared to a tin stripping liquid, adding sulfate for depositing lead and silver, wherein the reacted filter residue is a mixed sediment of lead sulphate and silver sulfate, washing by the concentrated ammonia liquor, reducing to obtain a silver powder and lead sulphate; filtering and depositing lead and silver, depositing tin and iron, filtering to obtain the sediment and dissolving by alkali, and then electrolyzing to obtain tin, electrolyzing the residual filtrate to recovery copper, and depositing palladium in the solution to obtain the sediment for recovering palladium. The processing method has reasonable and useful process flow, and performs comprehensive recovery and utilization on valuable metal in the waste liquid from stripping tin scolding, the resource waste is reduced, and the environmental pollution is reduced.
Owner:JIANGXI GREEN ECO MFG RESOURCE CYCLE
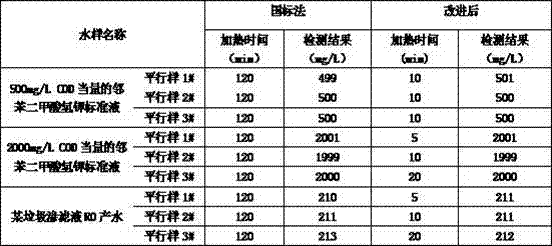
Analysis method for detecting chemical oxygen demand in water
InactiveCN106932532AIncrease dosageFast oxidationChemical analysis using catalysisChemical analysis using titrationRefluxSulfate
The invention discloses an analysis method for detecting chemical oxygen demand (COD) in water. The method comprises the steps of: putting a water sample into a reaction container, adding mercury sulfate and stirring till the mercury sulfate is completely dissolved; adding a potassium dichromate standard solution; connecting a condenser to the reaction container, and adding a sulfuric acid-silver sulfate solution from the upper opening of the condenser while shaking the container; placing the reaction container over a heating device, performing reflux heating, and taking the reaction container down till the test solution is cooled; flushing the inner wall of a condensation tube with distilled water, and taking the condensation tube down; adding distilled water into the cooled test sample, and shaking uniformly; adding a ferroin indicator solution into the diluted test sample, titrating with a ferrous sulfate standard solution till the test solution suddenly becomes red brown, and recording the milliliter value of the used ferrous sulfate standard solution. By partially optimizing and improving the original national method, the method can greatly shorten the time of COD detection.
Owner:CHENGDU MEIFUTE MEMBRANE TECHNOLOGY CO LTD
Silver film incorporating protective insoluble metallic salt precipitate
InactiveUS6432200B2Improve corrosion resistanceImprove metal resistanceInorganic chemistryMirrorsProduction lineWater insoluble
An apparatus is provided for making mirrors having enhanced reflective layer resistance to corrosion. The reflective layer of the mirror, typically silver, is contacted, preferably simultaneously, with a first solution containing a specific cation and a second solution containing a specific anion, or alkaline material which forms hydroxyl ions, the specific cation and specific anion or hydroxyl ion reacting to form a water insoluble precipitate on the silver surface. The mirror may then be painted to provide additional corrosion resistance to the mirror. The apparatus device for forming the precipitate eliminates the need for a device to form a copper layer on the silver surface and the device may be incorporated into existing mirror production lines as a replacement for the copper layering device. Also provided are mirrors made using the apparatus of the invention. A preferred cation containing solution contains tin (e.g., SnCl2) and a preferred anion containing solution contains hydroxyl ions (e.g, NaOH).
Owner:SWIMC LLC
Method for treating wastewater of rubber antioxidant (RD) production device
ActiveCN102442735ALow costSolve the emission problemLiquid carbonaceous fuelsMultistage water/sewage treatmentAntioxidantPolystyrene
The invention discloses a method for treating wastewater of a rubber antioxidant (RD) production device, which is used for preparing active ingredients (water and an additive) of coal water slurry by synthesizing a polymer surfactant and adding the polymer surfactant into wastewater to prevent environmental pollution caused by the discharge of wastewater of the RD production device and realize reutilization of the of wastewater of the RD production device. The method comprises the following steps of: weighing 2-10 parts by mass of polystyrene, adding into 20-60 parts by mass of concentrated sulfuric acid serving as a sulfonating agent, and adding 0.01-0.1 part of silver sulfate; raising the temperature to 80-98 DEG C, and reacting under the stirring condition for 3-5 hours to obtain polystyrolsulfon acid serving as a reaction product; and adding the reaction product into 400-1,000 parts of wastewater of the RD production device, and adjusting the pH to 7 with sodium hydroxide to obtain a sodium polystyrene sulfonate solution serving as a coal water slurry additive.
Owner:CHINA PETROLEUM & CHEM CORP +1
Production of silver sulfate grains using carboxylic acid additives
InactiveUS20090258218A1Reduce cakingLimited and avoidedInorganic active ingredientsCell electrodesWater solubleCarboxylic acid
Owner:EASTMAN KODAK CO
Preparation method of silver-copper coating powder
The invention discloses a preparation method of silver-copper coating powder. The preparation method comprises the following steps of: weighing 5-200 grams of copper powder, adding 10-500 milliliters of dilute acid solutions with the concentration of 1%-10%, placing into an ultrasonic stirrer, and stirring for 1-30 minutes; filtering, adding the dilute acid solutions for washing, and finally repeatedly washing the copper powder by using deionized water till washing liquid is neutral; adding the copper powder subjected to oxidation treatment to a solution with a dissolved complexing agent, placing into the ultrasonic stirrer, and stirring for 1-30 minutes to obtain a reducing solution; then adding silver sulfate to the reducing solution at the amount of 0.05-0.5 gram / minutes, and then continuously reacting for 30-60 minutes after the silver sulfate is completely added, wherein the molar ratio of the complexing agent solution to the silver sulfate solution is 2:1; adding 1-50 milliliters of ammonia water to remove the silver sulfate and the complexing agent which are remained in a reaction solution after the reaction is finished; and finally filtering the reaction solution, washing, and drying to obtain the silver-copper coating powder. The preparation method of the silver-copper coating powder, which is disclosed by the invention, simplifies the craft process, is conductive to controlling the integral reaction and ensures that a formed silver coating layer is compact.
Owner:JIANGSU BOQIAN NEW MATERIALS
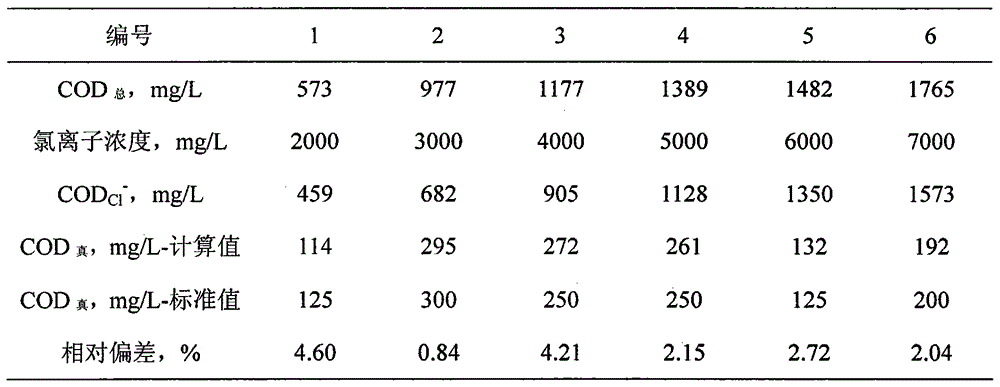
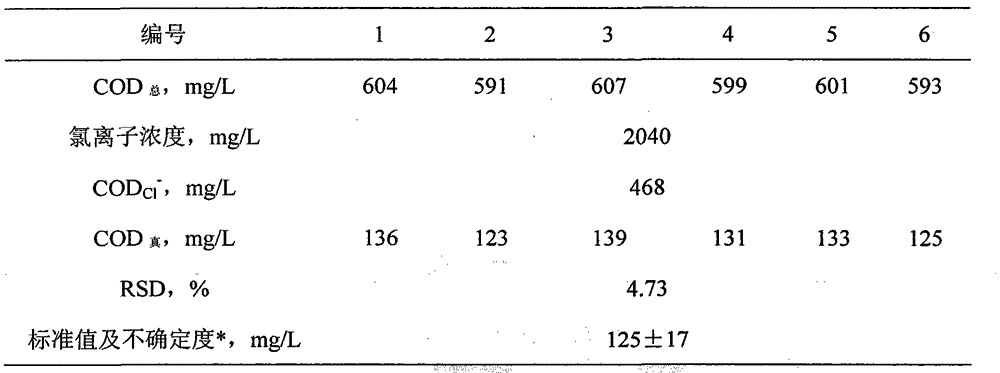
Method for measuring chemical oxygen demand of high-chlorine wastewater
InactiveCN104949970AMaterial analysis by observing effect on chemical indicatorSulfateCalibration curve
The invention discloses a method for measuring the chemical oxygen demand of high-chlorine wastewater. The method is applicable to the scope that the chemical oxygen demand (COD) is higher than or equal to 100 mg / L and the chloridion concentration is lower than or equal to 1%. When the COD and the chloridion concentration exceed the scope, the method can be available through proper dilution of a sample. The method comprises the following steps: (1) preparation of a concentrated sulfuric acid saturated copper sulfate catalyst; (2) drawing of a CODCl<-> chloridion calibration curve; (3) measurement of the chloridion content of a sample solution according to a silver nitrate titration method; (4) measurement of the total COD of the sample solution : selecting a defined amount of the sample solution for measurement of the total COD of the sample solution according to the method in step (2); (5) calculation of the difference between the total COD and CODCl<->, namely the actual COD of the sample solution. The method has the advantages that adding of mercury sulfate and silver sulfate is not needed, so that the cost is low, and secondary environmental pollution is avoided; the method is simple and easy to operate, and accurate and reliable in measurement result.
Owner:ZHENGZHOU PONY TESTING TECH
Application of organic framework porous solid acid
ActiveCN102861611AHigh sulfonate contentSkeleton structure is stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitrogenRoom temperature
The invention relates to application of organic framework porous solid acid. A catalyst for catalytic esterification and acylation reaction is prepared by the steps of adding a divinyl benzene monomer into a solvent prepared by mixing azobisisobutyronitrile, tetrahydrofuran and water, performing hydro-thermal treatment at the normal temperature under the constant pressure, and volatilizing the solvent at the room temperature to obtain polydivinylbenzene; grinding the polydivinylbenzene into 200-mesh powder and performing degassing treatment under the nitrogen condition; adding polydivinylbenzene blocks into 1,1',2-methyl chloroform to explode and crack into small blocks, wherein the small blocks swell; evenly mixing 1,1',2-methyl chloroform and concentrated sulfuric acid, adding silver sulfate serving as a catalyst, adding processed polydivinylbenzene, performing sulfonation, filtering and dioxane washing till a solution is neutral, performing drying, using dilute sulphuric acid to activate, performing washing till the solution is neutral and performing drying. The catalyst is used for the catalytic esterification and the acylation reaction and has high catalytic activity and conversion rate.
Owner:PETROCHINA CO LTD
Reagent for measuring chemical oxygen demand (COD) and method for preparing digestion solutions of reagent
InactiveCN104330405AReduce usageReduce the amount of analysisMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationAmmonium ferrous sulfateSulfate
The invention discloses a reagent for measuring chemical oxygen demand (COD) and a method for preparing digestion solutions of the reagent. The reagent comprises an H reagent silver sulfate and a J reagent, wherein the mass fraction of potassium dichromate in the J reagent is 28.0%-36.0% and the mass fraction of mercury sulfate in the J reagent is 64.0%-72.0%. The preparation method of the digestion solutions of the reagent comprises the following steps: adding 1g of silver sulfate into 100ml of concentrated sulfuric acid to prepare the digestion solution of the H reagent; the preparation method of the digestion solution of the J reagent comprises the steps of dissolving the J reagent into 80mL-100mL of distilled water, adding 6.00mL-10.00mL of concentrated sulfuric acid, cooling until the temperature reaches a room temperature after dissolving, and then diluting by use of water until 120mL of solution is achieved. The dosage of the digestion solutions prepared by use of the J reagent and the H reagent of the reagent for measuring COD is greatly reduced so that the analysis cost can be greatly reduced. Compared with the national recommended standards, an ammonium ferrous sulfate standard titration solution does not need to be prepared for titration, and the analysis steps are simplified, and the reagent for measuring COD is simple, convenient and quick, and meets the requirements of an enterprise on fast detection.
Owner:XINYU IRON & STEEL CO LTD
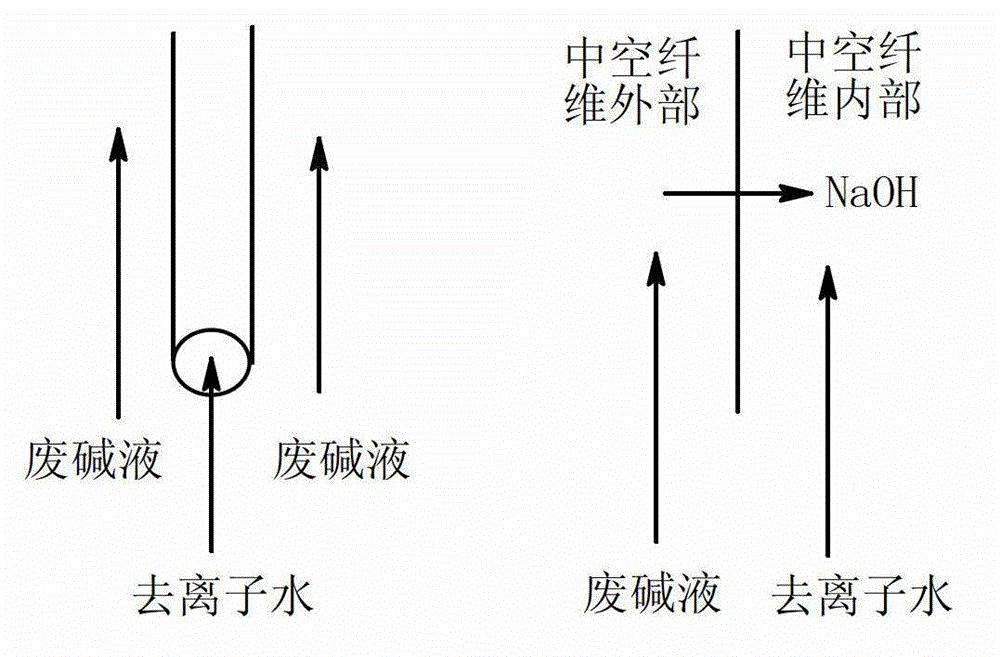
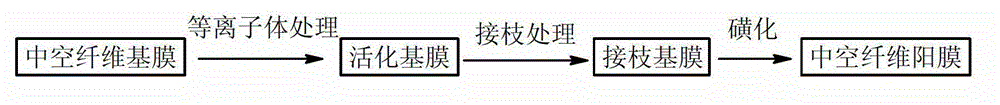
Preparation method of hollow fiber dispersion and dialysis alkali recycling membrane
ActiveCN102908916ALarge amount of processingSmall footprintSemi-permeable membranesHollow fibreFiltration membrane
The invention discloses a preparation method of a polymeric membrane and particularly relates to a preparation method of a hollow fiber dispersion and dialysis alkali recycling membrane. The preparation method comprises the steps of selecting a hollow fiber ultra-filtration membrane of polypropylene, polyacrylonitrile or nylon as a hollow fiber base membrane; putting the base membrane into a plasma chamber to carry out plasma activation; immersing the activated hollow fiber base membrane into a solution of styrene, divinylbenzene and benzoyl peroxide to carry out functional group grafting; and sulfonating the hollow fiber base membrane with the grafted functional group by taking silver sulfate as a catalyst and taking concentrated sulfuric acid as a sulfonating agent. The preparation method has the advantages that a membrane material in unit area has the characteristics of large processing amount, small occupied area of an assembly made of the membrane and the like. The preparation method has a wide application prospect.
Owner:HANGZHOU WATER TREATMENT TECH DEV CENT
Quick detecting method for needed oxygen COD cr of mercuryless salt high-chlorine waste water chemical
InactiveCN1598548AImprove reliabilityGood reproducibilityMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationOxygenContamination
The invention discloses a quick measuring method for mercury salt less high chlorine waste water chemical oxygen demond quantity CODcr. Its character lies in: the method uses standard potassium dichromate method, uses MnSO4 and NiSO4 to replace Ag2SO4, and checks the CODcr in water sample. The method is reliable, the energy consumption and reagent cost are saved; and because of using AgNO3 and CrK (SO4)2 to replace severe toxici HgSO4 as combined masking agent, solves the secondary contamination to environment.
Owner:DALIAN JIAOTONG UNIVERSITY
Method for analyzing content of COD (chemical oxygen demand) in sewage with high chloride ion concentration
InactiveCN103645186AEliminate distractionsImprove accuracyMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationChemical oxygen demandDigestion
The invention discloses a method for analyzing the content of COD (chemical oxygen demand) in sewage with high chloride ion concentration. The method comprises the following steps: adding 5ml of 1mol / L silver nitrate solution into a sewage sample, mixing, uniformly shaking, precipitating, adding water for dilution till 25mL, performing centrifugal separation, taking 3mL of supernatant liquid, placing into a digestion pipe, adding 1mL of 10% mercury sulfate solution, 1mL of 0.025mol / L potassium dichromate oxidant solution and 5mL of 1% sulfuric acid-silver sulfate catalyst solution, plugging, uniformly shaking, digesting for 15min, taking out, cooling, and determining the COD content in the sample by applying a colorimetric method. According to the method, a silver salt precipitation method and a dilution method are combined and applied, AgNO3 is added into the chlorine-containing sewage sample to precipitate the chloride ions and further eliminate the interference of the chloride ions on the accuracy of COD determination result, the dilution method is further adopted to reduce the influence of nitrate radicals introduced by silver nitrate on an analytical system, the method is suitable for analyzing the content of COD in the sewage sample with the chlorine content of 1200-10000mg / L, and the accuracy in detection result is greatly improved.
Owner:南京中锗科技有限责任公司
Digestion solution formula for fast determining chemical oxygen demand
InactiveCN101839901AReduce dosageLow priceChemical analysis using catalysisSolubilityPotassium dichromate
The invention provides a digestion solution formula for fast determining chemical oxygen demand, relating to the field of digestion solution formulas of chemical oxygen demand after dissolved substances and suspended substances in a water sample are treated by potassium dichromate oxidation treatment. The digestion solution formula for fast determining chemical oxygen demand comprises the following three prepared solutions: a solution as a catalyst prepared by dissolving 2.00-2.15g of silver sulfate in 500ml of sulfuric acid, a solution as an oxidant prepared by dissolving 2.45g of potassium dichromate dried to constant weight in 100ml of water and 400ml of the sulfuric acid and a solution as a cocatalyst prepared by dissolving 0.80-15.15g of aluminum potassium sulfate, 0.48g of ammonium tetramolybdate and 2.25-5.15g of citric acid in 500ml of concentrated sulfuric acid. The digestion solution formula has the advantages of short reaction time, stable reaction and less silver sulfate dosage.
Owner:程俊彪
Silver-containing foam structure
ActiveUS20100196501A1Reduce the amount requiredFacilitated releaseAntibacterial agentsPowder deliveryWound dressingSilver carbonate
A method of producing a hydrophilic polyurethane foam structure containing a silver salt, chosen from the group of silver sulphate, silver citrate, silver acetate, silver carbonate, silver lactate and silver phosphate, or a mixture of these salts includes the steps of (a) providing a water phase containing a surfactant, and at least one silver salt, wherein the at least one silver salt is dispersed in the water phase; (b) providing a isocyanate-terminated polyether having functionality of more than (2); (c) mixing the water phase and the isocyanate-terminated polyether, immediately transferring the resulting mixture to a mould whereby a foam structure is obtained; and (d) drying the foam structure until it has a moisture content of at most 10% (wt). The hydrophilic polyurethane foam structure produced by the method and a wound dressing containing the foam structure are also described.
Owner:MOLNLYCKE HEALTH CARE AB
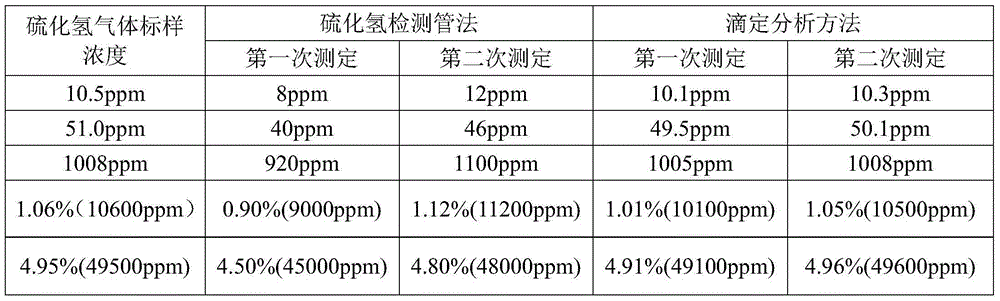
Method for measuring content of hydrogen sulfide in gas by titrimetric analysis
InactiveCN104865251AEasy to handleAvoid pollutionMaterial analysis by observing effect on chemical indicatorRepeatabilitySilver sulfate
The invention relates to a detection method and particularly relates to a method for measuring the content of hydrogen sulfide in gas by titrimetric analysis. The method is characterized by comprising the following steps: quantifying the volume V1 of the gas containing the hydrogen sulfide, and absorbing the gas with a saturated silver sulfate solution; (2) additionally arranging a methyl red-methylene blue indicator into the saturated silver sulfate solution, performing titration with a standard sodium hydroxide solution, stopping titration when the color of the mixed solution is changed from purple red into greyish green, and accurately calculating the content of the hydrogen sulfide in the gas according to the volume V and the concentration C of the consumed standard sodium hydroxide, wherein a calculation formula is that H2S(ppm)=C*V*22.4*10<6> / (2V1). The method can be used for measuring 5-200,000ppm of the hydrogen sulfide in the gas, a repeatability error is not greater than 2% of two measured results, and a reproducibility error is not greater than 5% of the two measured results.
Owner:SHANDONG JINCHENG HEAVY OIL RES INST OFCHEM TECH
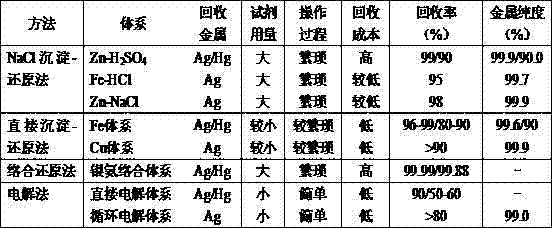
Resource treatment method of CODCr (chemical oxygen demand chromium) measurement waste liquid
InactiveCN102807293AHigh recovery rateSimple processMultistage water/sewage treatmentLiquid wasteChemical oxygen demand
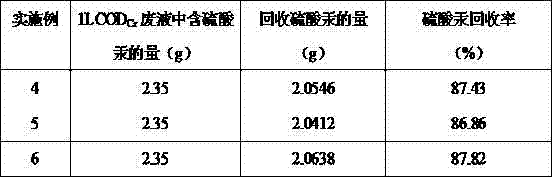
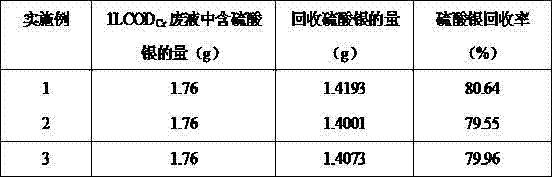
The invention discloses a method for recovering four heavy metals, namely silver, mercury, iron and chromium, and a sulfuric acid from CODCr (chemical oxygen demand chromium) measurement waste liquid, and belongs to the technical field of the resource treatment of waste liquid. The method mainly comprises a technology of recovering Ag2SO4 by using a NaCl-H2SO4 method, a technology of recovering the H2SO4 by using a strongly basic anion exchange resin acid retardation principle, a technology of recovering Hg2SO4 by using a Na2S-HCl-NaOH-H2SO4 method and a technology of recovering a Fe2O3, Cr2O3 and Na2SO4 solution by using a sequential precipitation method. The method provided by the invention is simple in process and is easy to operate; the recovery rates of the silver sulfate and the mercury sulfate are high and respectively reach 80.6% and 87.8%; the recovery rate of the sulfuric acid reaches 82%; the secondary pollution cannot be caused; the product quality is good; the silver sulfate and the mercury sulfate which are recovered are both up to the standard of a commercially available reagent and can be recycled in CODCr measurement; and the recovery cost is low.
Owner:NANJING COLLEGE OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com