Reagent and method for determining chemical oxygen demand of high-chloride wastewater
A technology for chemical oxygen demand and high-chlorine wastewater, which is applied in the direction of material analysis by observing the influence of chemical indicators, color/spectral characteristic measurement, and analysis by making materials undergo chemical reactions, so as to achieve short measurement time and save energy. Analyzing the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
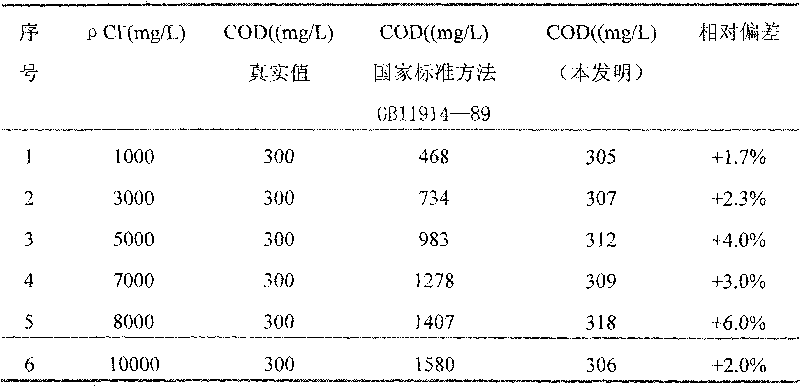
[0029] 1. Preparation of COD reagent
[0030] Weigh 10 g of reagent A and reagent B respectively, wherein the mass fraction of potassium dichromate in reagent A is 35%, the mass fraction of potassium aluminum sulfate is 50%, and the mass fraction of ammonium molybdate is 15%; The mass fraction of silver is 40%, the mass fraction of bismuth nitrate is 18%, the mass fraction of potassium chromium sulfate is 12%, and the mass fraction of mercury sulfate is 30%.
[0031] Put 10g of Reagent A in a 100mL beaker, add 75mL of distilled water, and add 5mL of 98% sulfuric acid under constant stirring until dissolved; put 10g of Reagent B in a 1000mL beaker, add 500mL of sulfuric acid, dissolve overnight or slightly heat, stir well and put 500mL brown narrow mouth bottle.
[0032] 2. COD determination steps
[0033] Step 1: Weigh 0.8502g of potassium hydrogen phthalate and dissolve it in distilled water, transfer it to a 1000ml volumetric flask, and dilute to the marked line. The COD ...
Embodiment 2
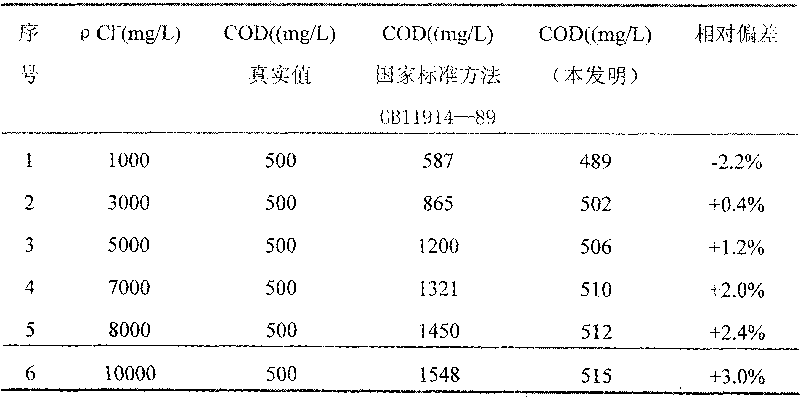
[0046] 1. Preparation of COD reagent
[0047]Weigh each 4g of A reagent and B reagent respectively, wherein the mass fraction of potassium dichromate in A reagent is 25%, the mass fraction of aluminum potassium sulfate is 40%, and the mass fraction of ammonium molybdate is 35%; The mass fraction of silver is 35%, the mass fraction of bismuth nitrate is 15%, the mass fraction of potassium chromium sulfate is 10%, and the mass fraction of mercury sulfate is 40%.
[0048] Put 4g of Reagent A in a 50mL beaker, add 30mL of distilled water, and add 2mL of 98% sulfuric acid under constant stirring until dissolved; put 4g of Reagent B in a 500mL beaker, add 200mL of sulfuric acid, dissolve overnight or slightly heat, stir well and put 500mL brown narrow mouth bottle.
[0049] 2. COD determination steps
[0050] Step 1: Weigh 0.8502g of potassium hydrogen phthalate and dissolve it in distilled water, transfer it to a 1000ml volumetric flask, and dilute to the marked line. The COD va...
Embodiment 3
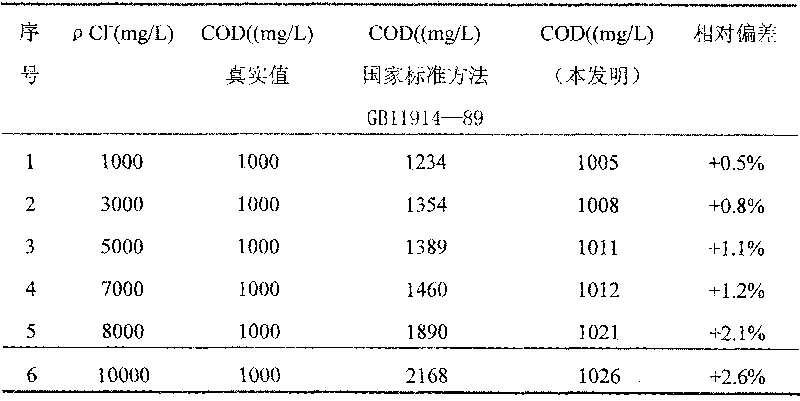
[0063] 1. Preparation of COD reagent
[0064] Weigh 6g of A reagent and B reagent respectively, wherein the mass fraction of potassium dichromate in A reagent is 40%, the mass fraction of aluminum potassium sulfate is 40%, and the mass fraction of ammonium molybdate is 20%; The mass fraction of silver is 40%, the mass fraction of bismuth nitrate is 20%, the mass fraction of potassium chromium sulfate is 20%, and the mass fraction of mercury sulfate is 20%.
[0065] Put 6g of Reagent A in a 100mL beaker, add 45mL of distilled water, and add 3mL of 98% sulfuric acid under constant stirring until dissolved; put 6g of Reagent B in a 500mL beaker, add 300mL of sulfuric acid, dissolve overnight or slightly heat, stir well and put 500mL brown narrow mouth bottle.
[0066] 2. COD determination steps
[0067] Step 1: Weigh 0.8502g of potassium hydrogen phthalate and dissolve it in distilled water, transfer it to a 1000ml volumetric flask, and dilute to the marked line. The COD value...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com