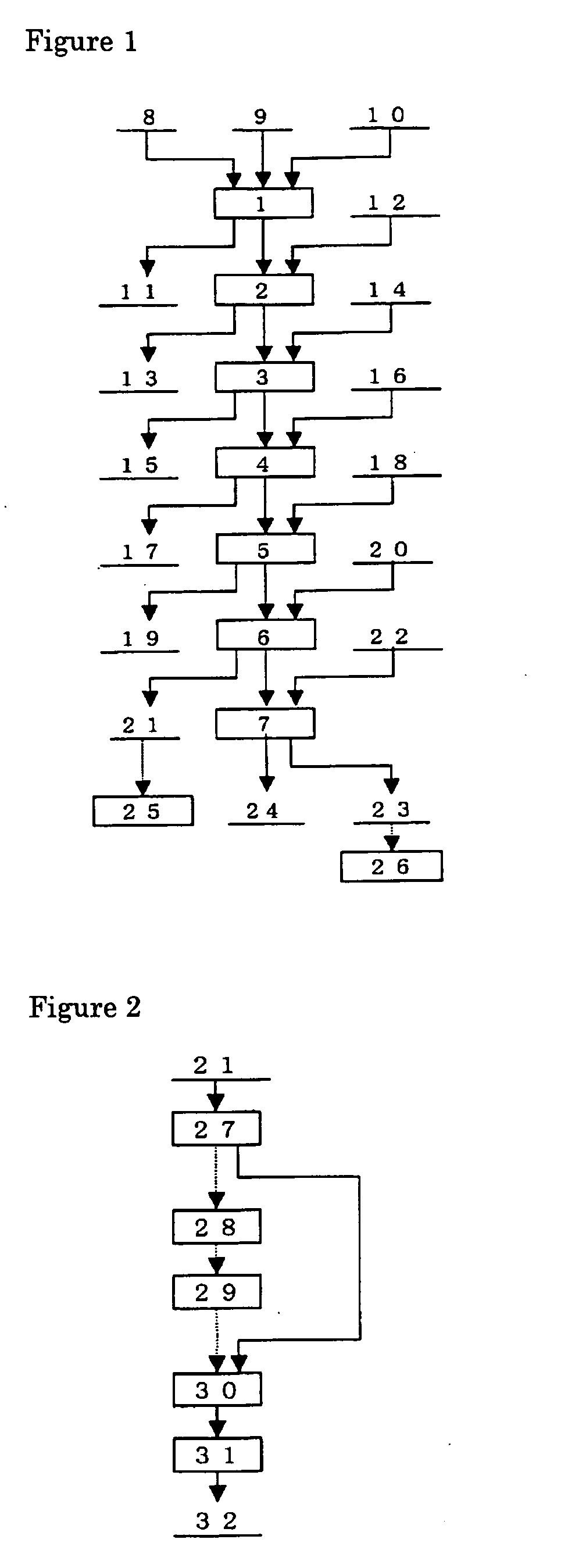
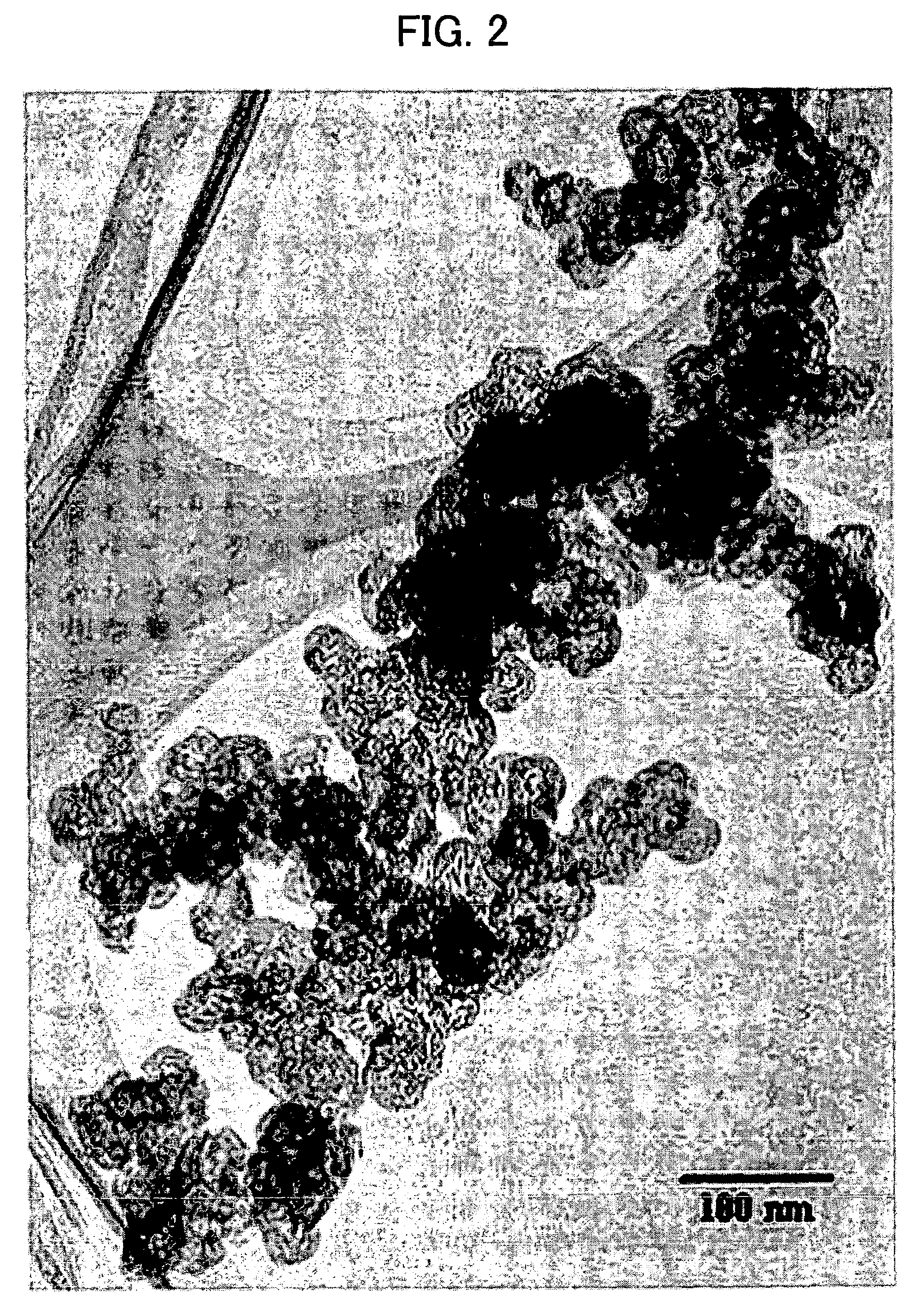
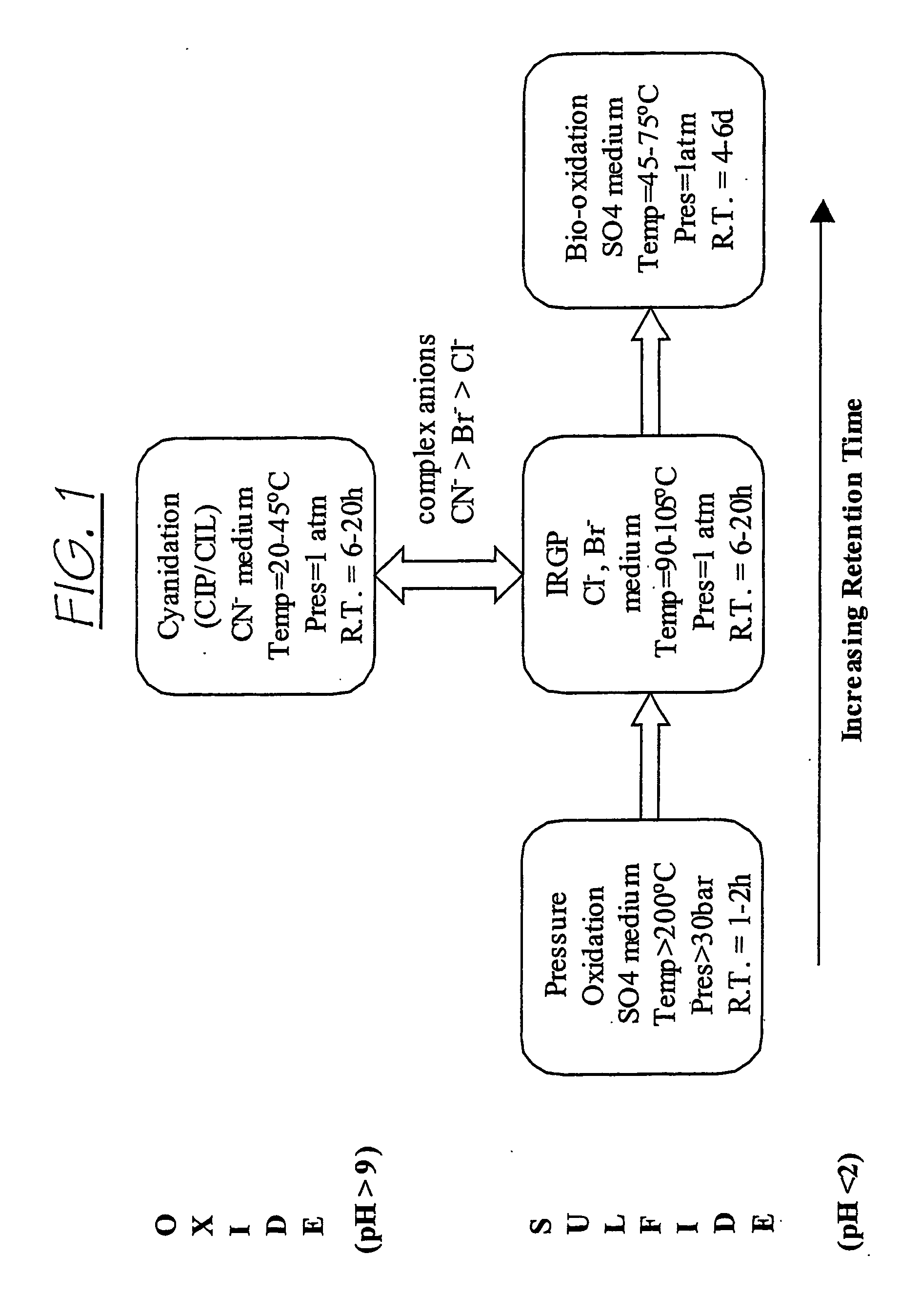
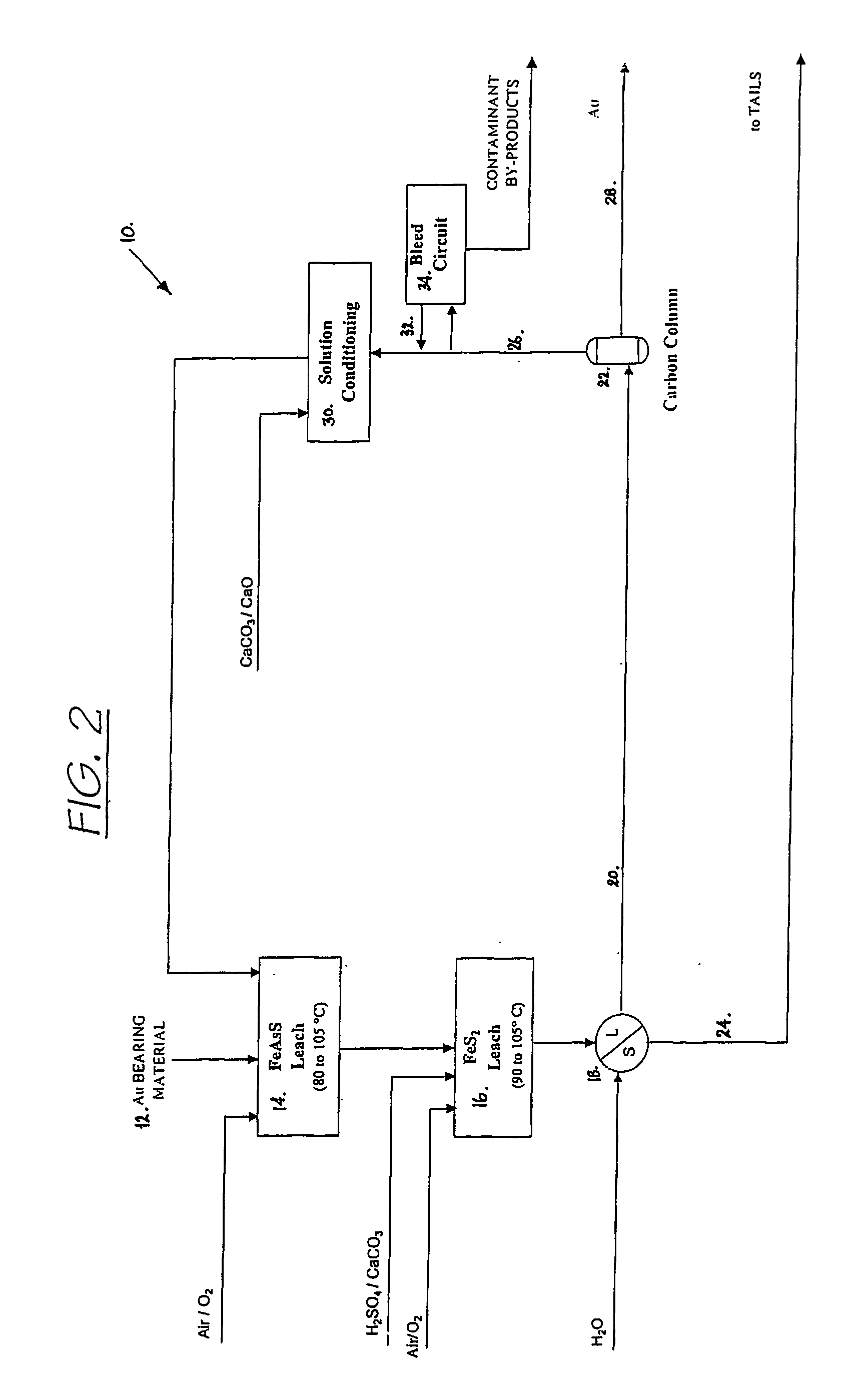
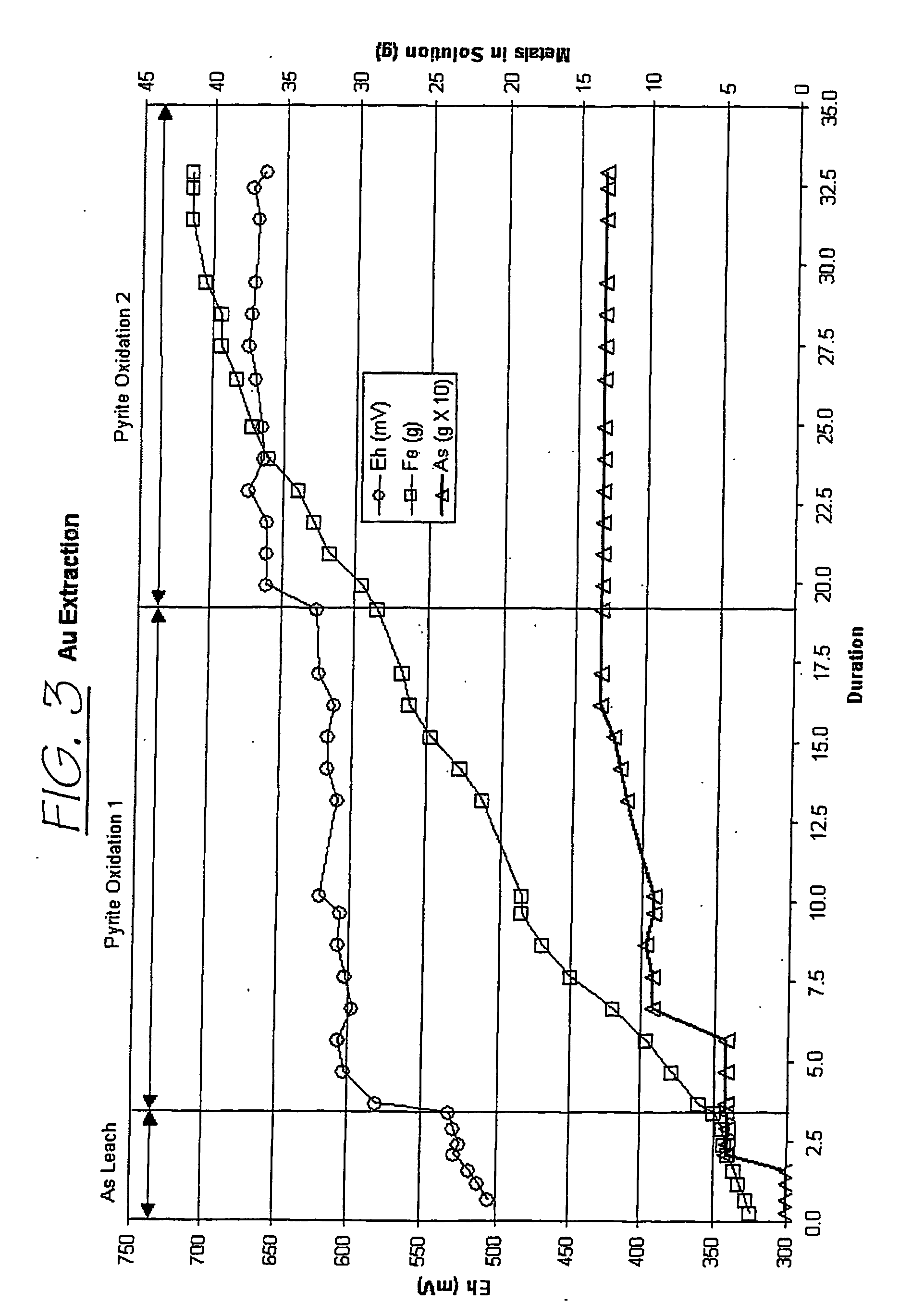
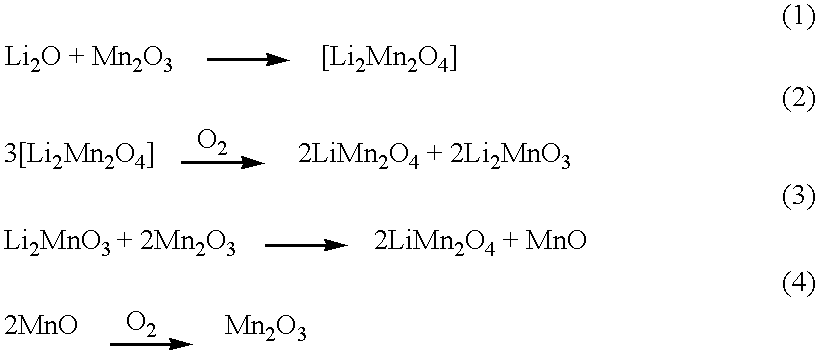Patents
Literature
699results about "Ruthenium/rhodium/palladium/osmium/iridium/platinum compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Organometallic Complex, and Light-Emitting Element, Light-Emitting Device, and Electronic Device Including the Organometallic Complex
ActiveUS20100105902A1Improve emission efficiencySolve low luminous efficiencyGroup 4/14 element organic compoundsGroup 1/11 element organic compoundsLength waveLight emitting device
An organometallic complex is provided by which favorable red-color light emission can be obtained. Further, an organometallic complex having a peak of light emission at about 620 nm is provided because the wavelength of light which is perceived as excellent red-color light is about 620 nm. Furthermore, an organometallic complex is provided by which red-color light emission with high luminous efficiency (cd / A) can be obtained. An organometallic complex represented by the following general formula (G2) and a light-emitting element, a light-emitting device, and an electronic device including the organometallic complex represented by the following general formula (G2) are provided.
Owner:SEMICON ENERGY LAB CO LTD
Method of manufacturing inorganic nanotube
InactiveUS7005391B2Material nanotechnologyPolycrystalline material growthInorganic materialsAtomic layer deposition
A method of manufacturing an inorganic nanotube using a carbon nanotube (CNT) as a template, includes preparing a template on which a CNT or a CNT array is formed, forming an inorganic thin film on the CNT by depositing an inorganic material on the template using atomic layer deposition (ALD), and removing the CNT to obtain an inorganic nanotube or an inorganic nanotube array, respectively.
Owner:SAMSUNG ELECTRONICS CO LTD
Monodisperse noble metal nanocrystals
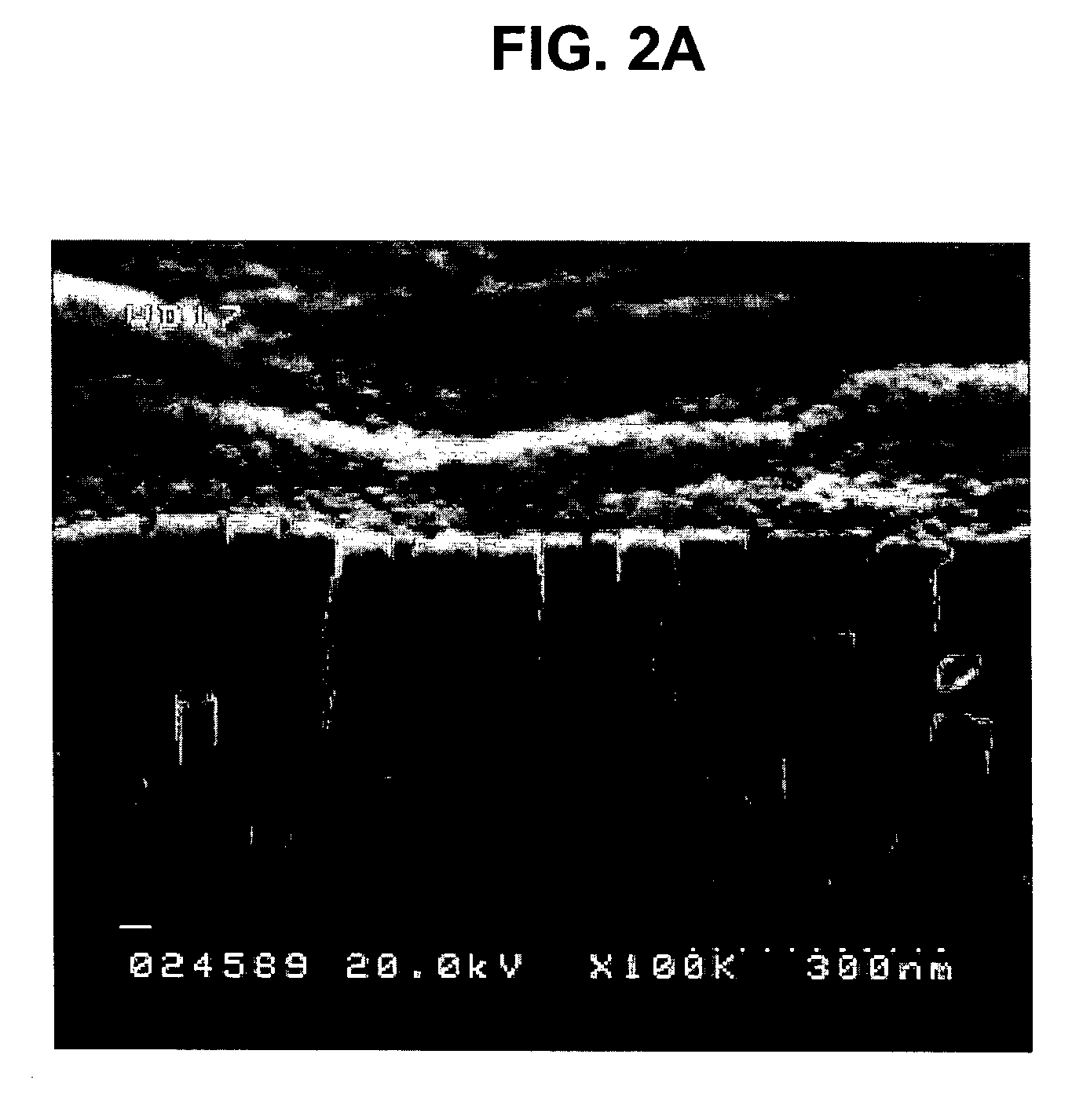
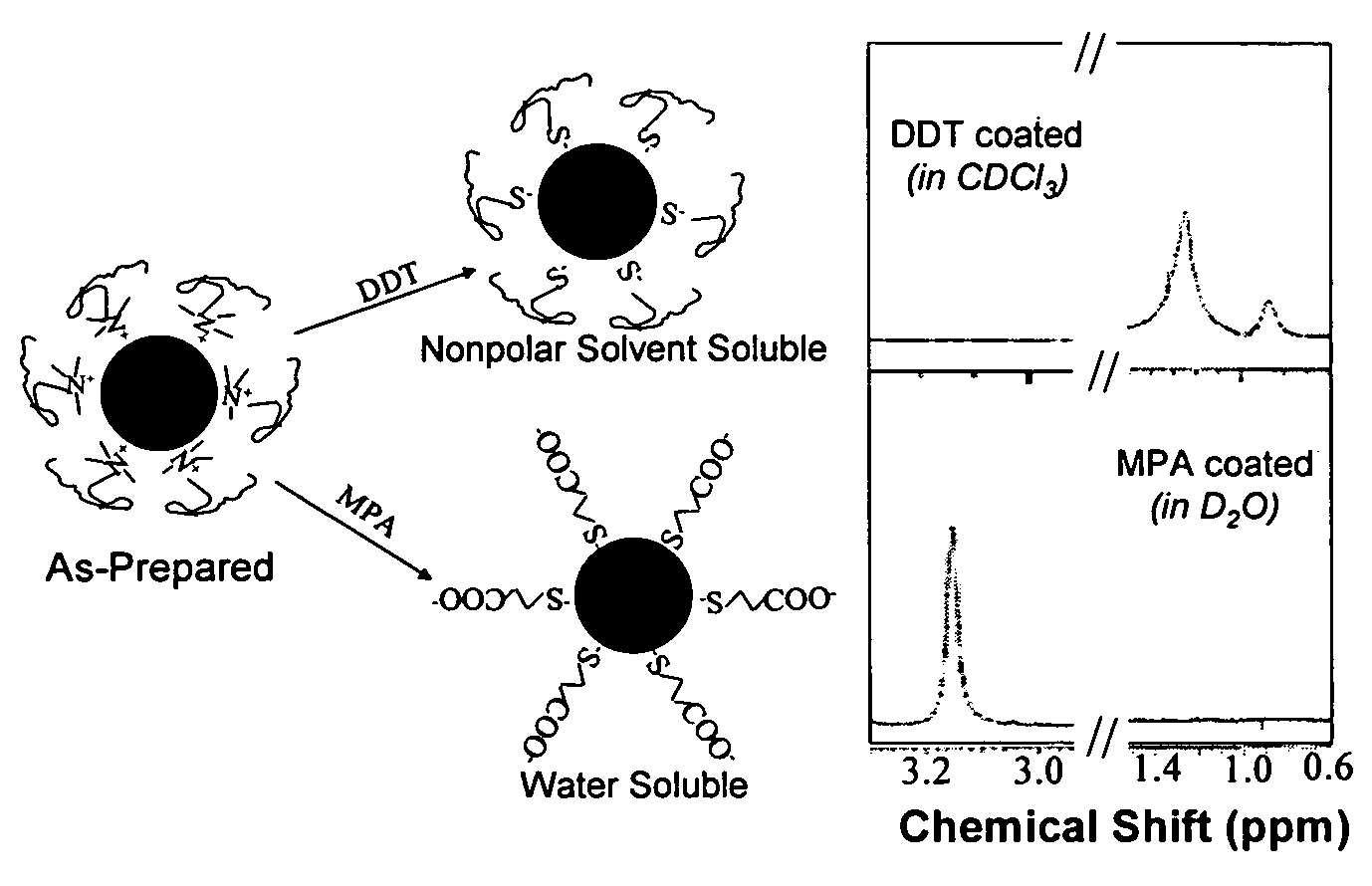
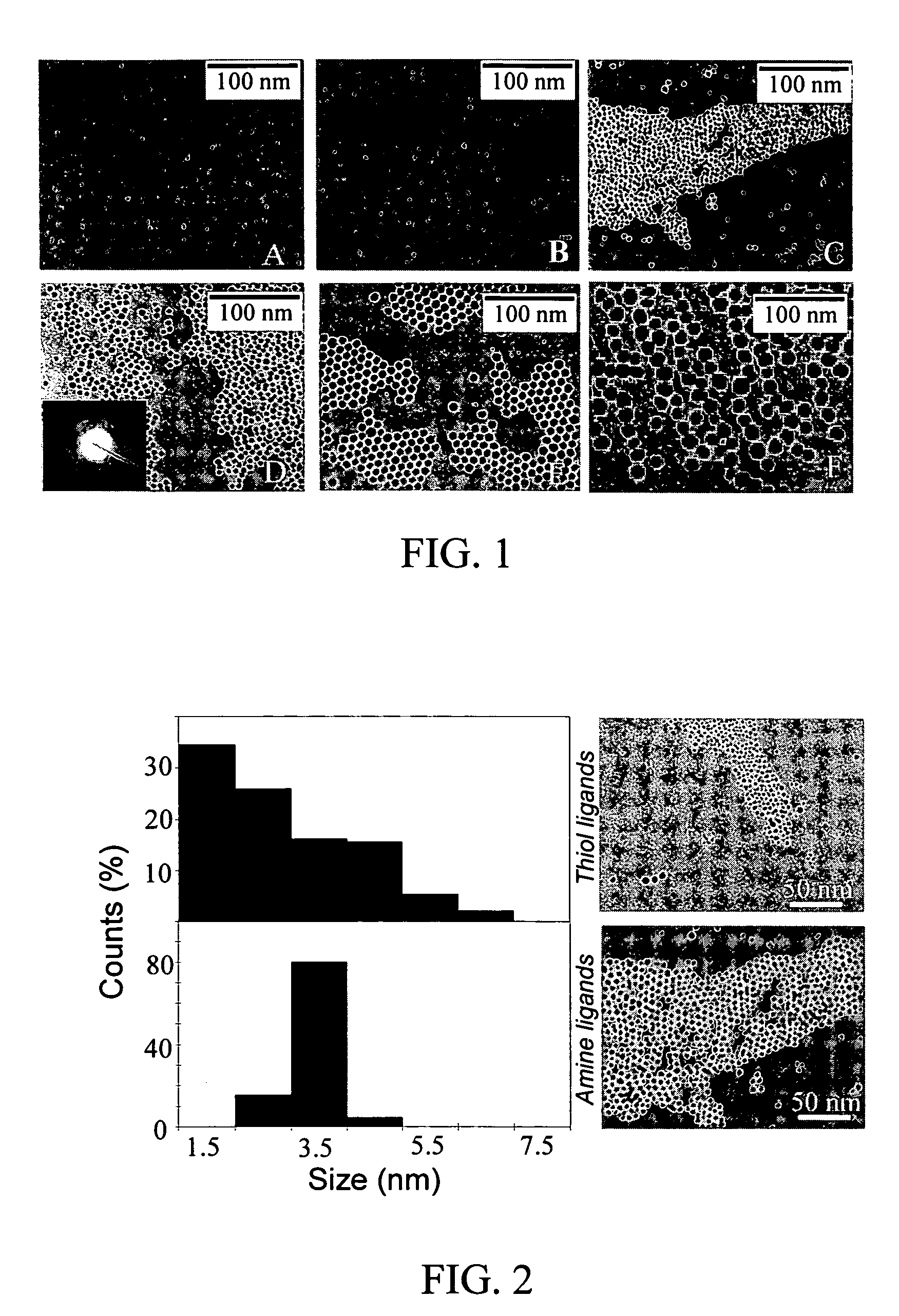
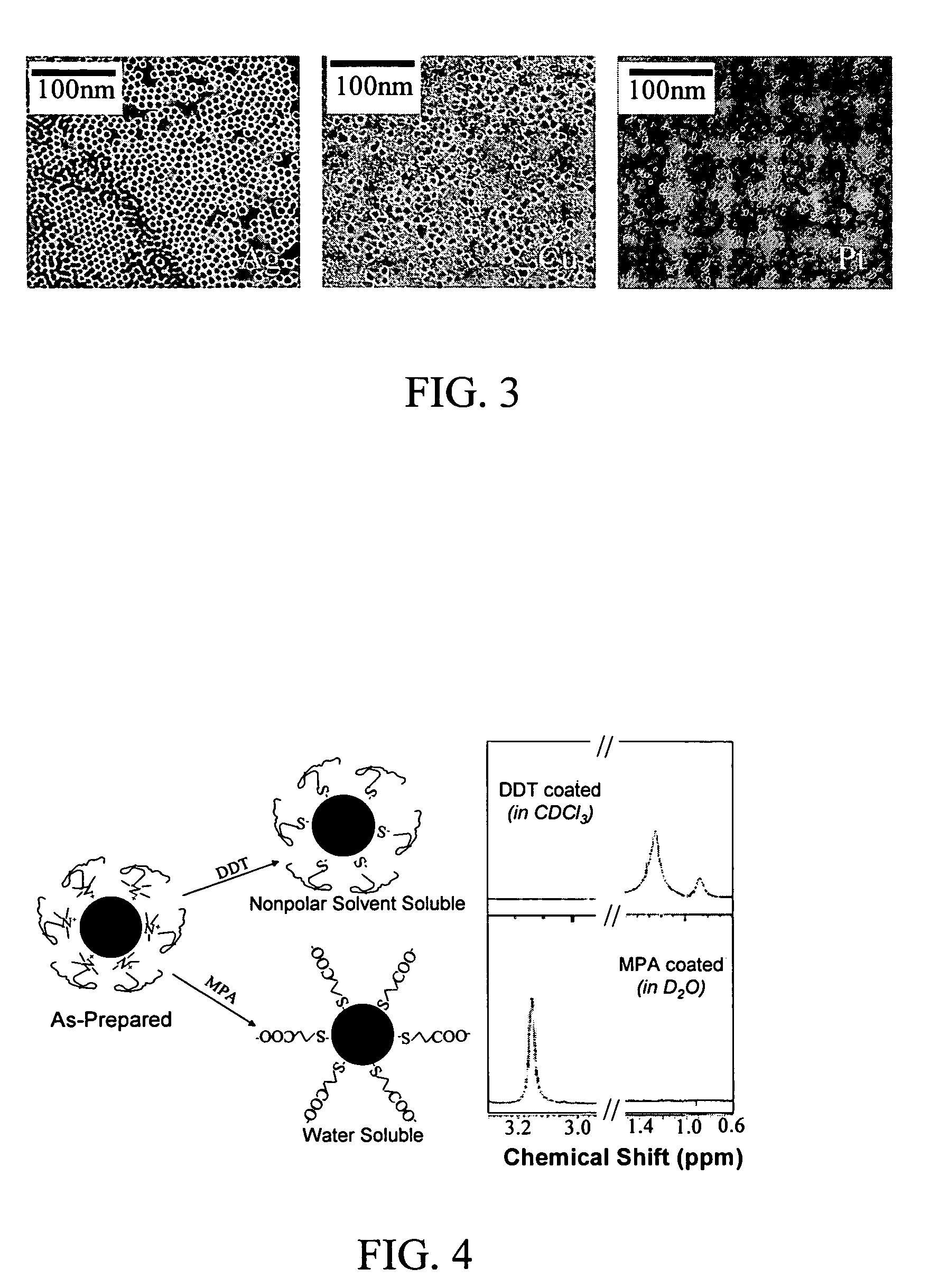
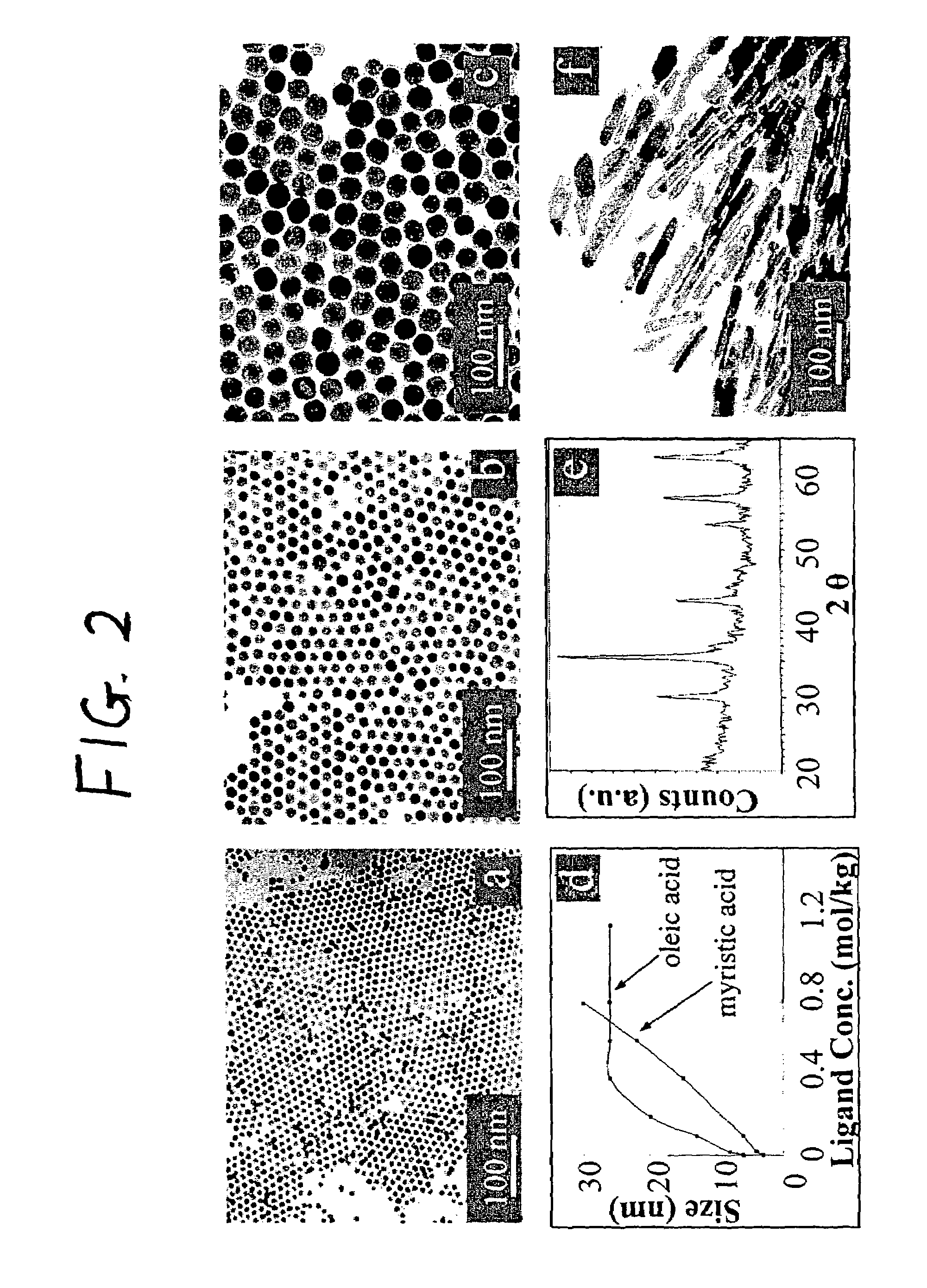
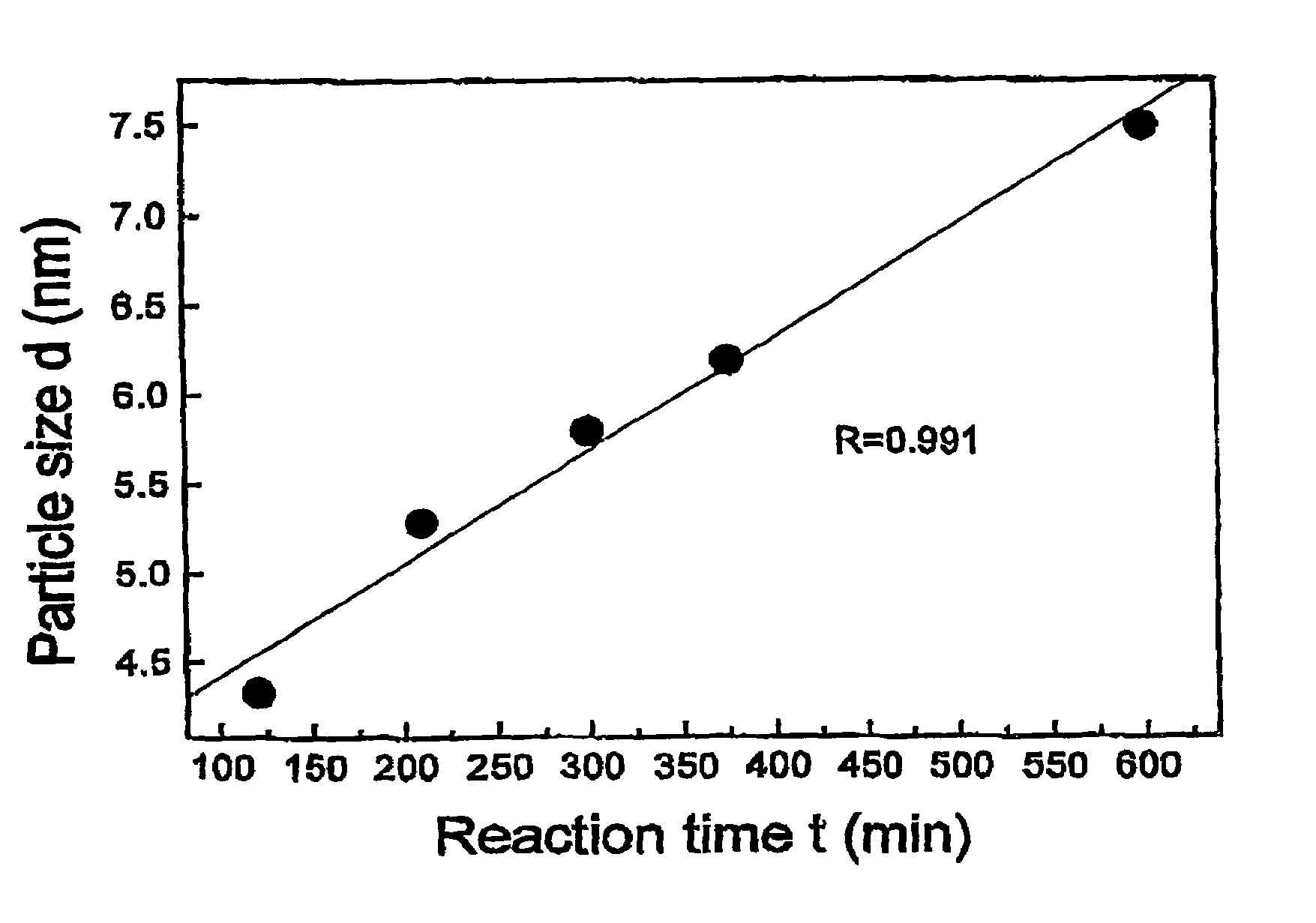
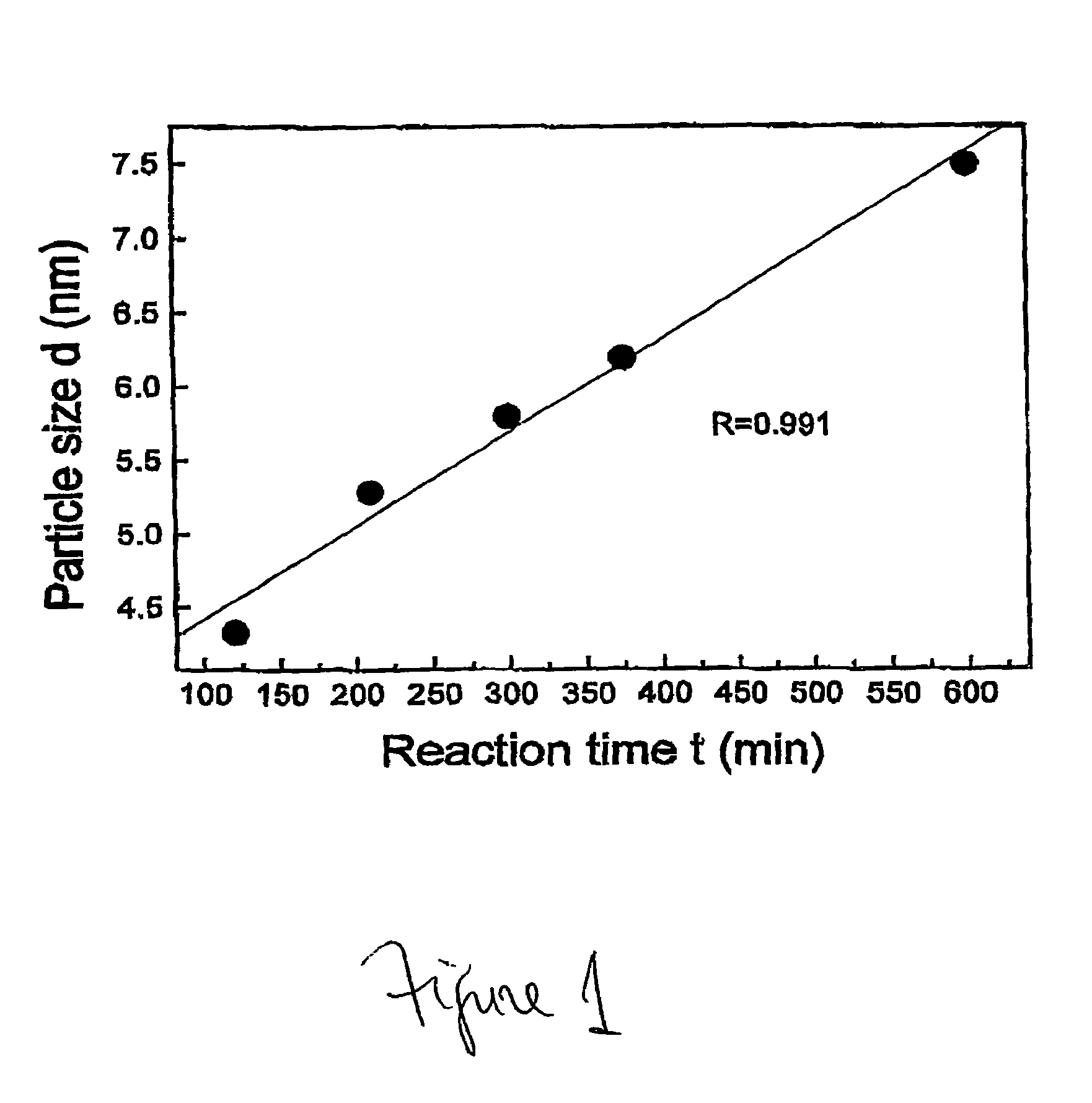
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Process for the recovery of value metals from base metal sulfide ores
InactiveUS20050118081A1Simple gas/liquidReduce the amount requiredSulfur compoundsSolid sorbent liquid separationSulfideDissolution
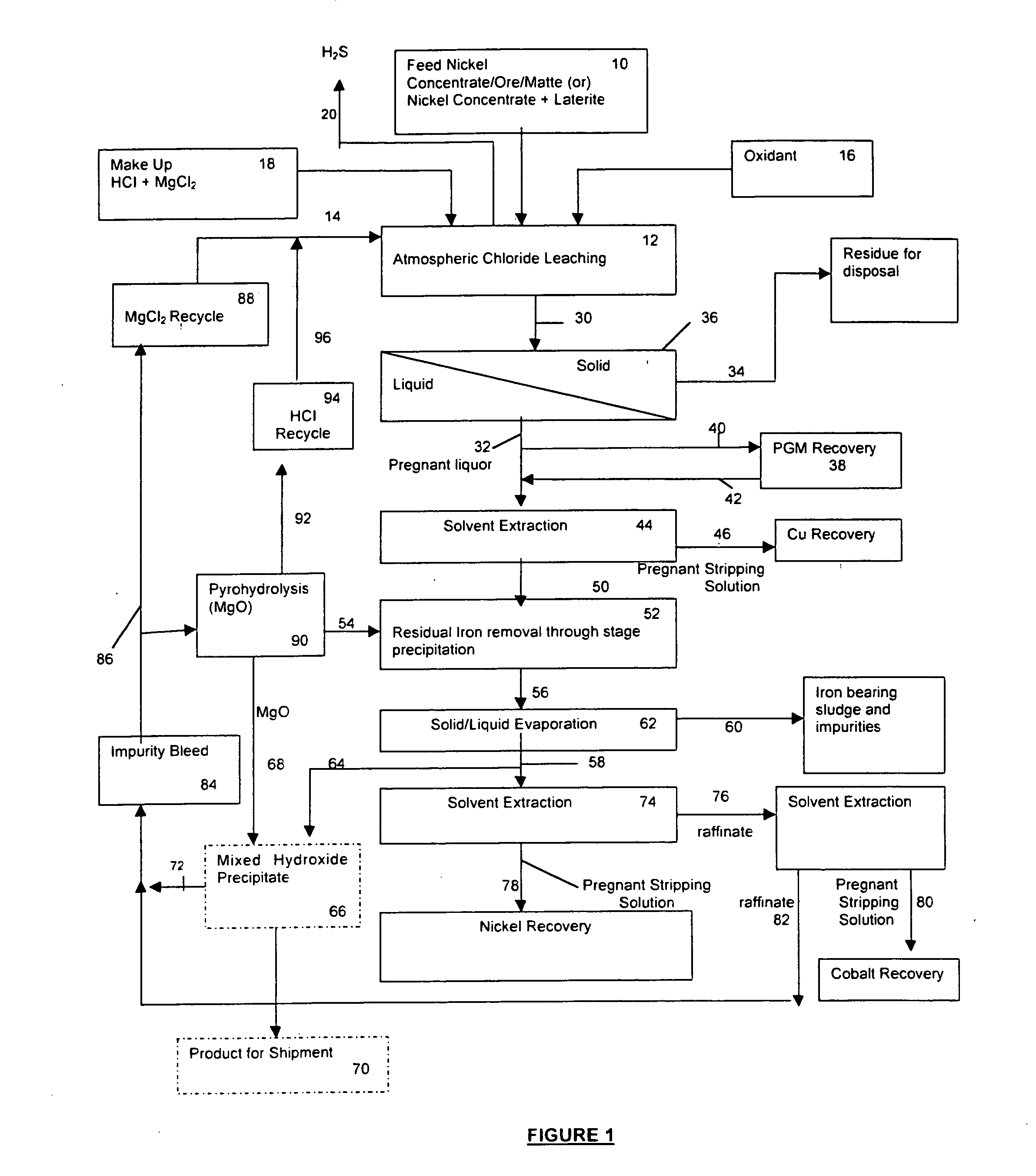
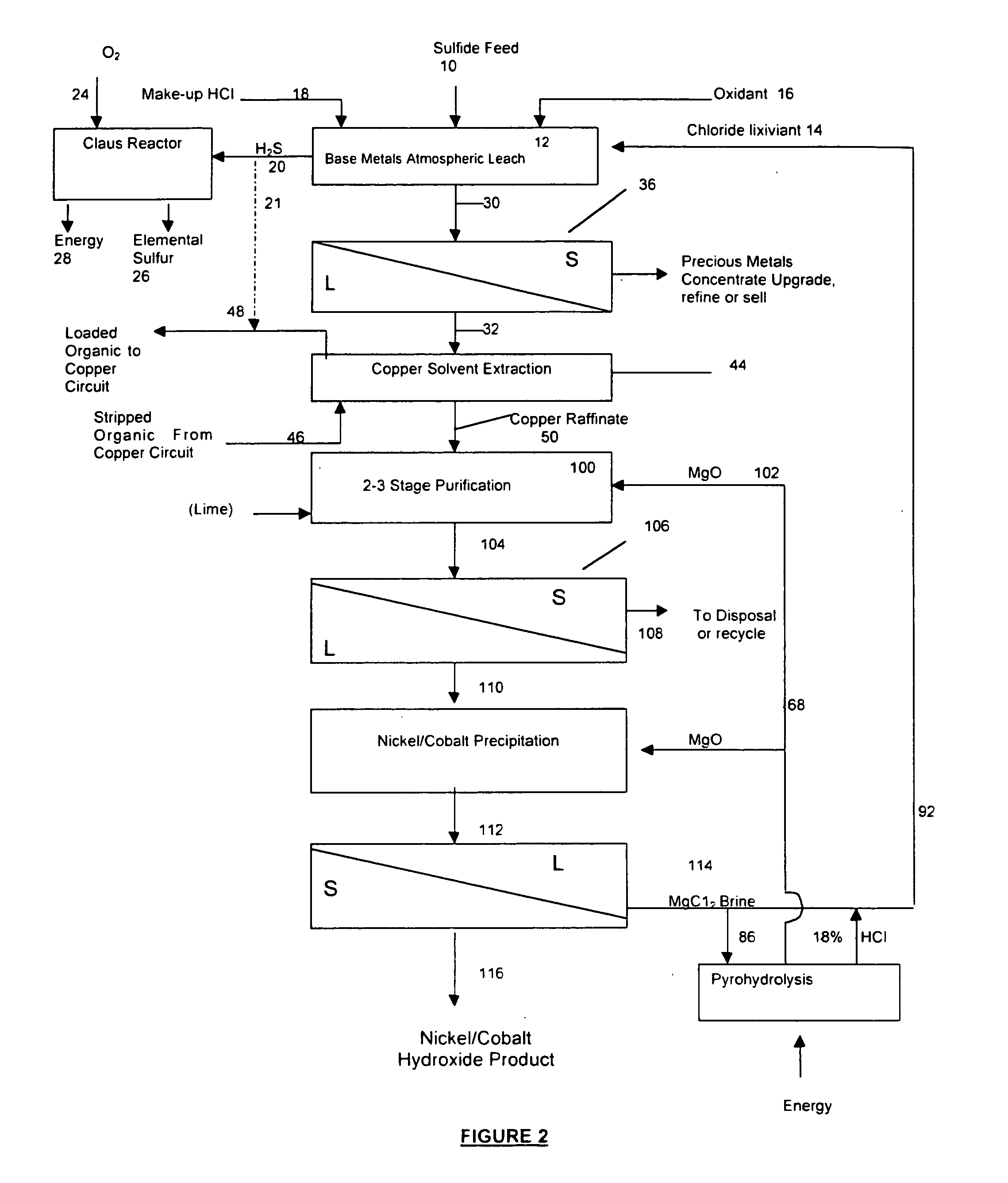
A process for leaching a value metal from a base metal sulfide ore, comprising the step of leaching the ore with a lixiviant comprising a chloride, an oxidant and hydrochloric acid is disclosed. The leaching is controlled, by use of low concentrations of hydrochloric acid and a redox potential, to effect formation of hydrogen sulfide from the base metal sulfide ore. The hydrogen sulfide is stripped from the leach solution, thereby reducing the amount of sulfate generated in the leach to very low levels. The leaching may also be conducted to limit the co-dissolution of platinum group metals and gold with the base value metals. The leach forms a value metal-rich leachate and a solids residue. The solids residue may be subsequently leached to recover the platinum group metals and gold. The value metal-rich leachate can be is oxidized and neutralized to recover the value base metals. In an embodiment, the chloride is magnesium chloride and lixiviant solution is regenerated.
Owner:JAGUAR NICKEL INC
Cathode intercalation compositions, production methods and rechargeable lithium batteries containing the same
InactiveUS6248477B1Easy to adaptOxygen/ozone/oxide/hydroxideElectrode thermal treatmentMetalSpinel group
Intercalation compositions having spinel structures with crystallites of metal oxides (M2O3) dispersed throughout the structure are provided having the general formula Li1+xMyMn2-x-yO4. Methods of producing the intercalation compositions and rechargeable lithium batteries containing the compositions are also provided.
Owner:EMD ACQUISITION LLC
Lithium composite oxide particle for positive electrode material of lithium secondary battery, and lithium secondary battery positive electrode and lithium secondary battery using the same
InactiveUS20060134521A1Improving low-temperature load characteristicGood paintabilityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsAlkali metal oxidesElectrical batteryComposite oxide
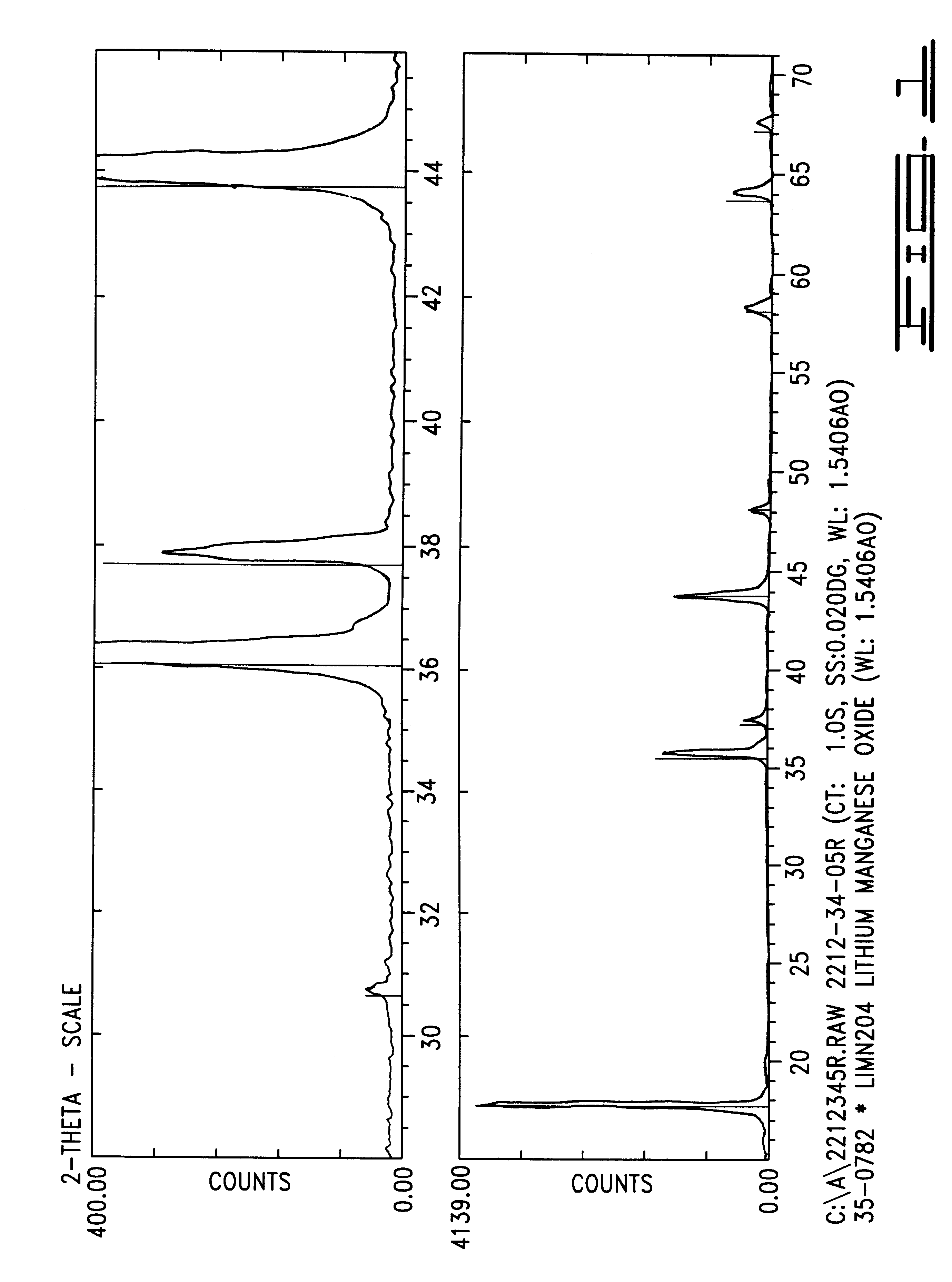
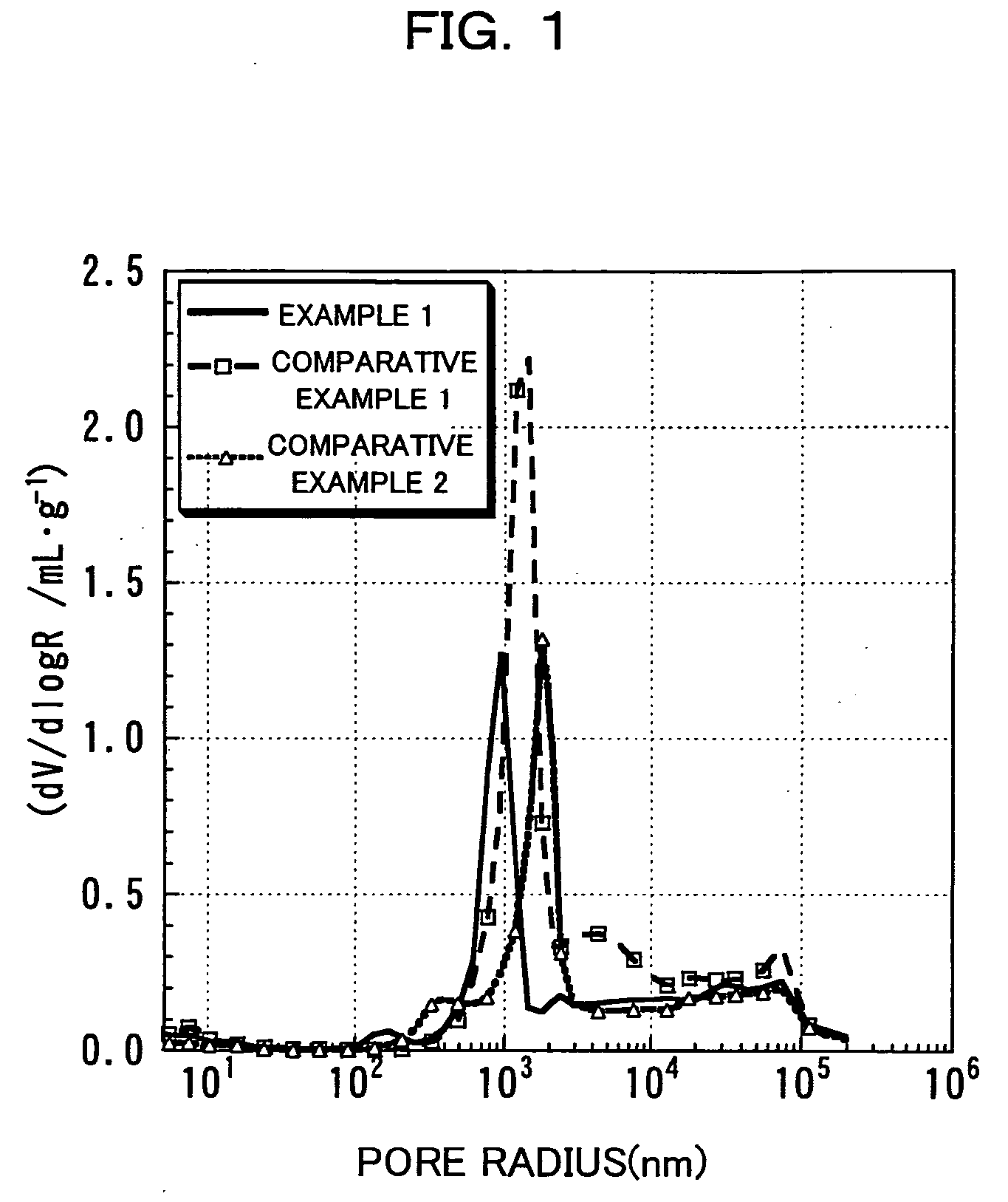
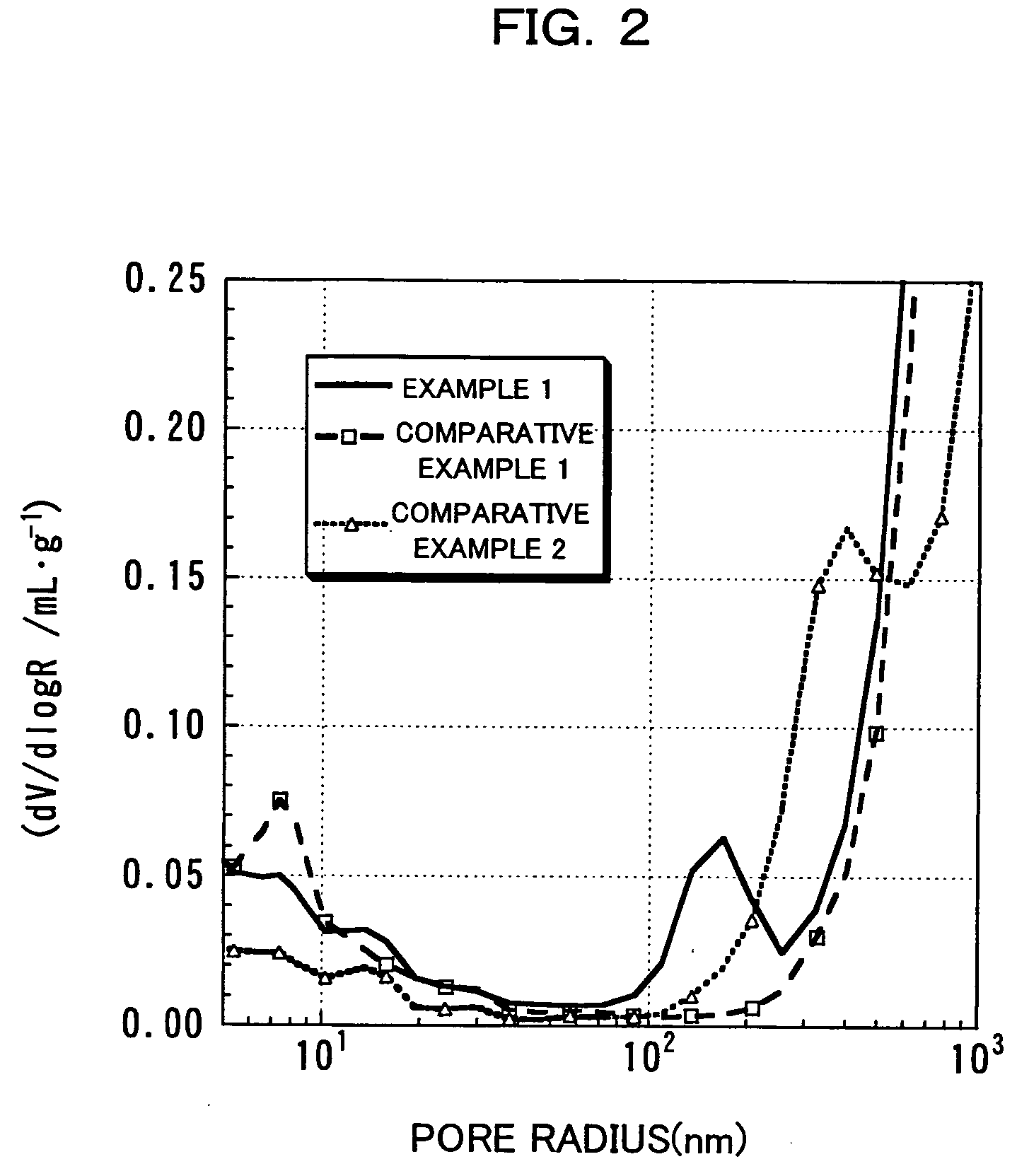
An excellent positive electrode material for a lithium secondary battery is provided that can increase low-temperature load characteristics of the battery as well as improving coatability. When measured by mercury intrusion porosimetry, the material meets Condition (A) and at least either Condition (B) or Condition (C). Condition (A) : on a mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.02 cm3 / g or smaller. Condition (B): on the mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.01 cm3 / g or larger. Condition (C): the average pore radius is within 10-100 nm, and the pore-size distribution curve has a main peak (with peak top at a pore radius of within 0.5-50 μm) and a sub peak (with peak top at a pore radius of within 80-300 nm).
Owner:MITSUBISHI CHEM CORP
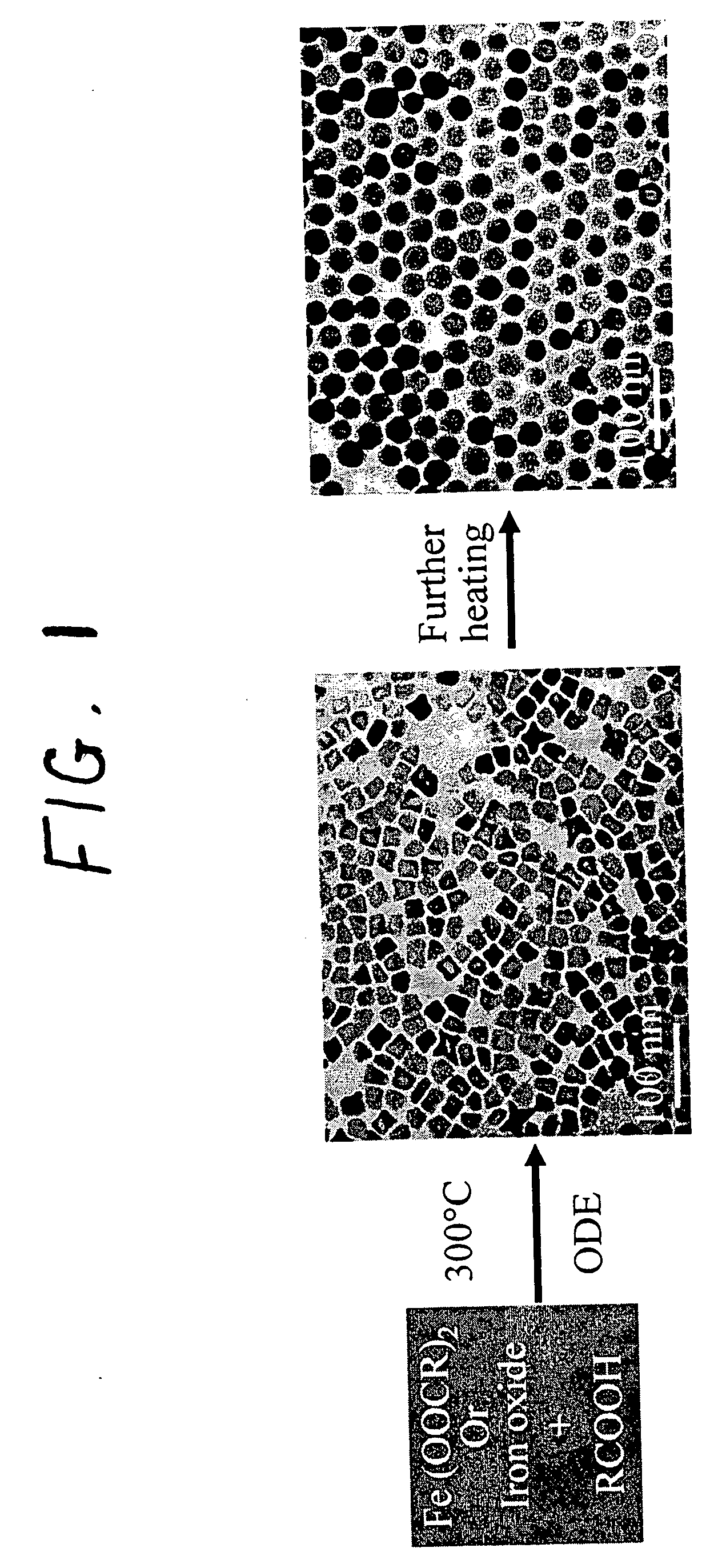
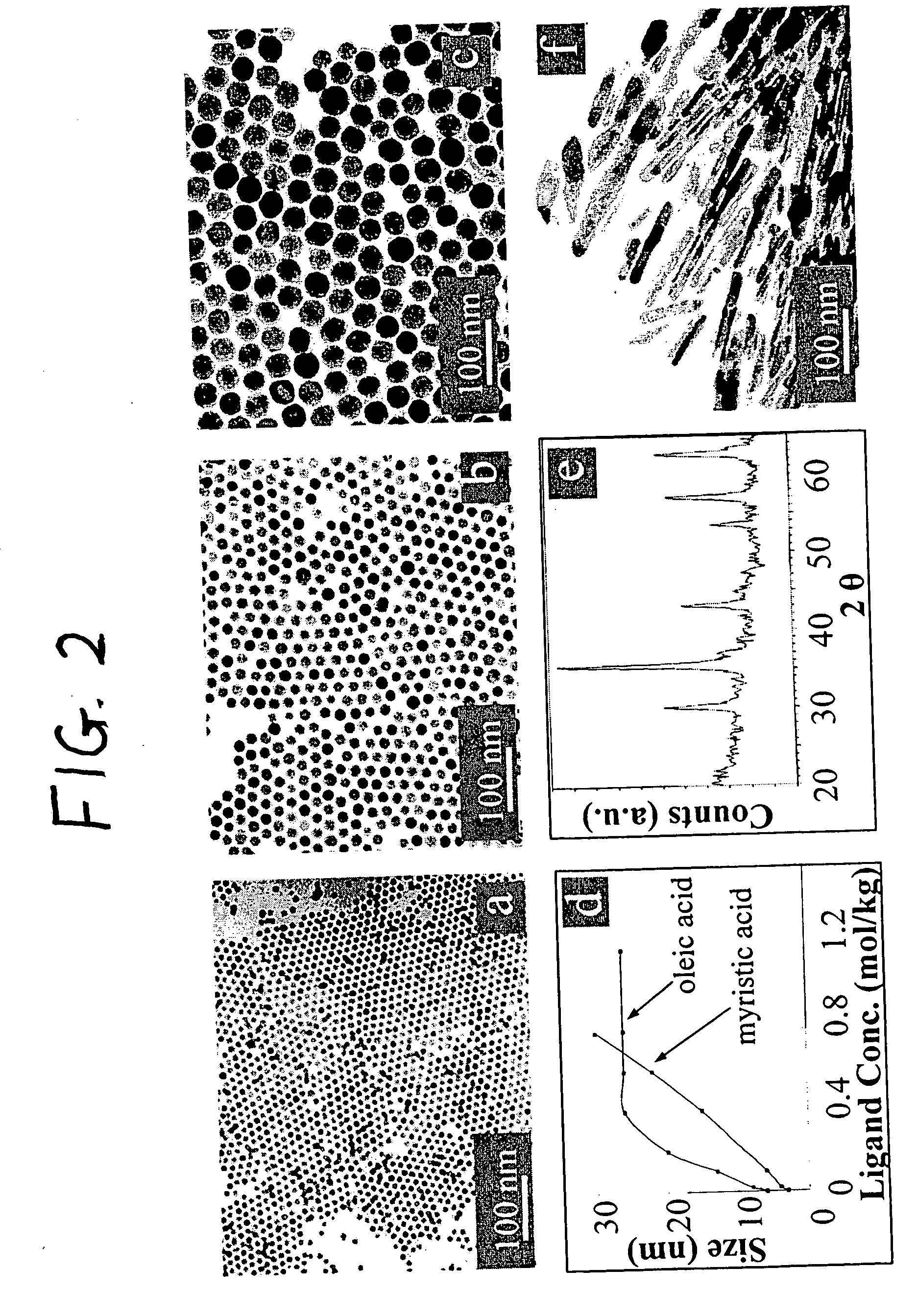
Synthetic control of metal oxide nanocrystal sizes and shapes
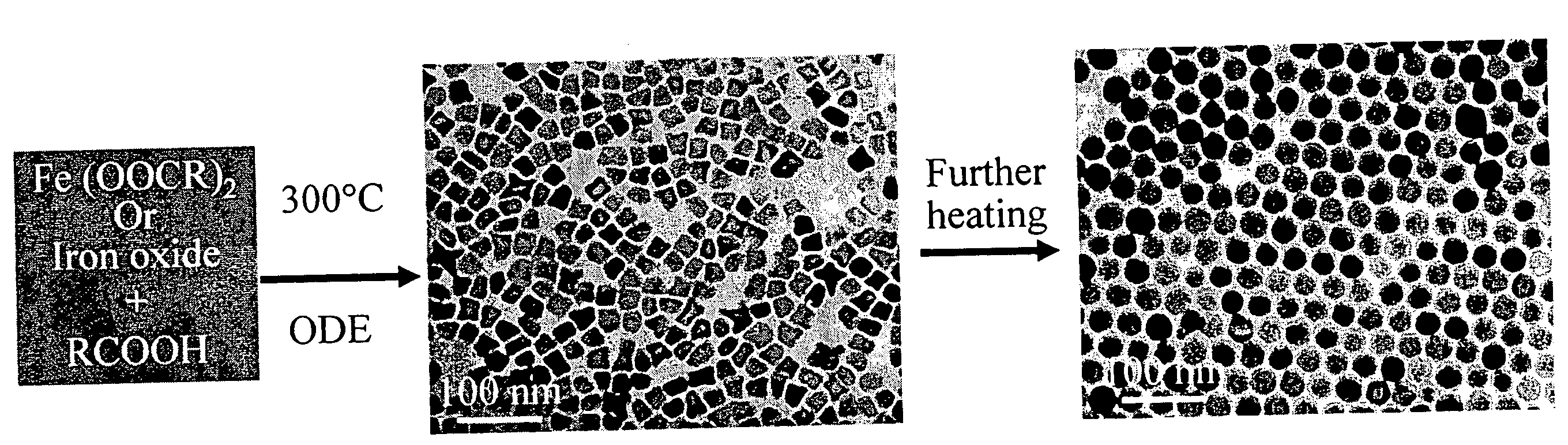
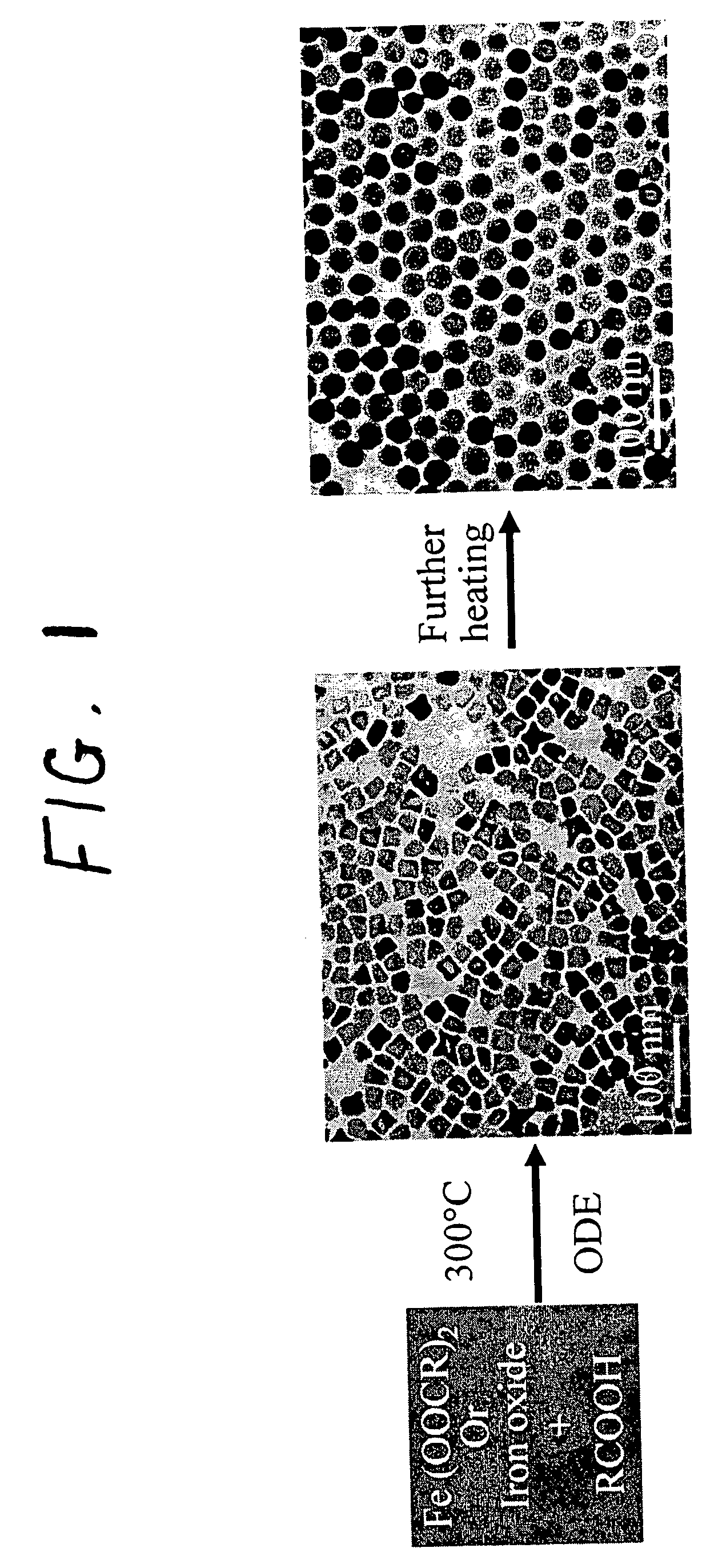
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Production of mechanically exfoliated graphene and nanoparticle composites comprising same
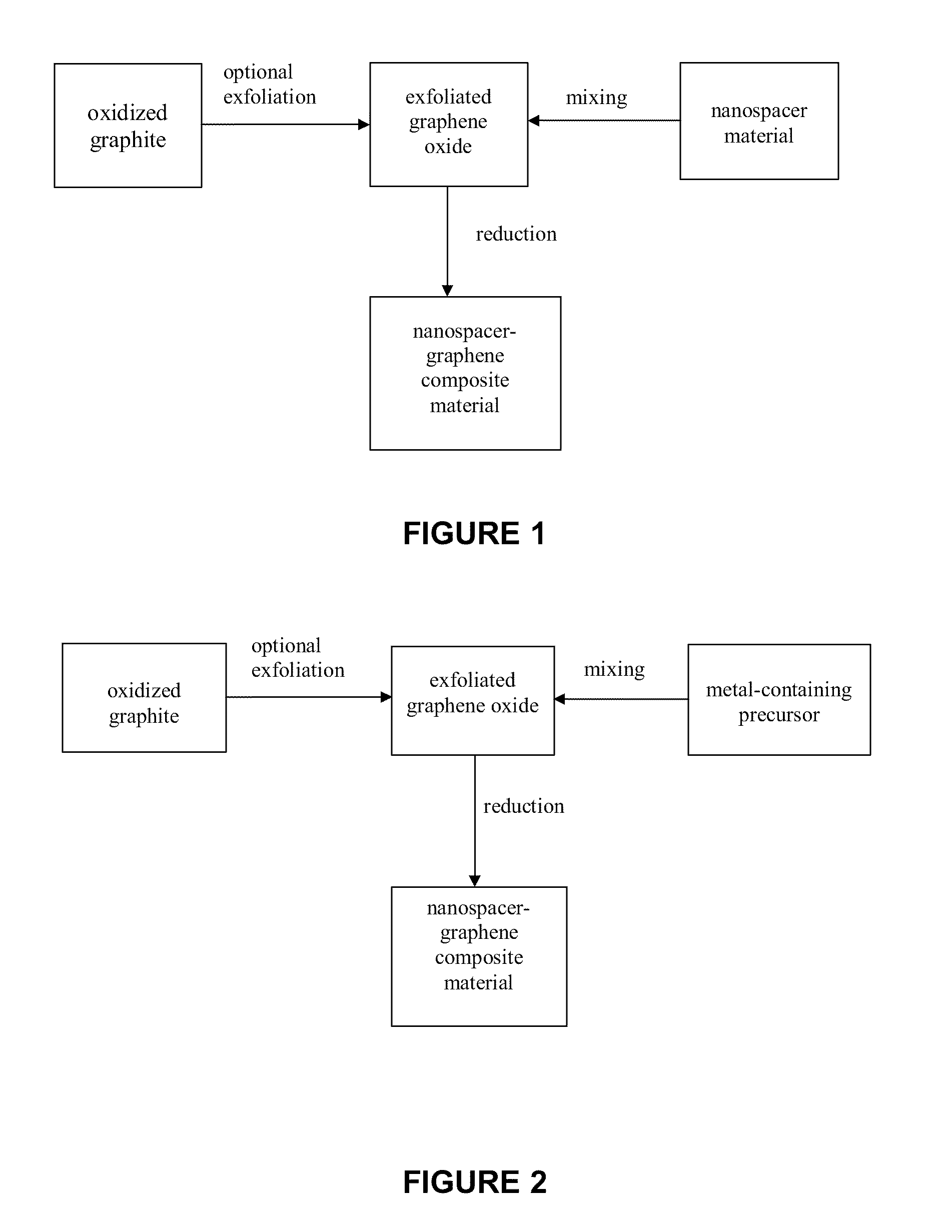
InactiveUS20110284805A1Minimizing aggregationMaterial nanotechnologyNon-metal conductorsNanoparticleGraphene
A method for producing nanospacer-graphene composite materials (i.e., mechanically-exfolitated graphene), wherein the graphene sheets are interspersed with nanospacers, thereby maintaining the 2D characteristics of the graphene sheets. The nanospacer-graphene composite material is highly porous, has a high surface area and is highly electrically conductive and may be optically transparent.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Apparatus and method for preparing cerium oxide nanoparticles
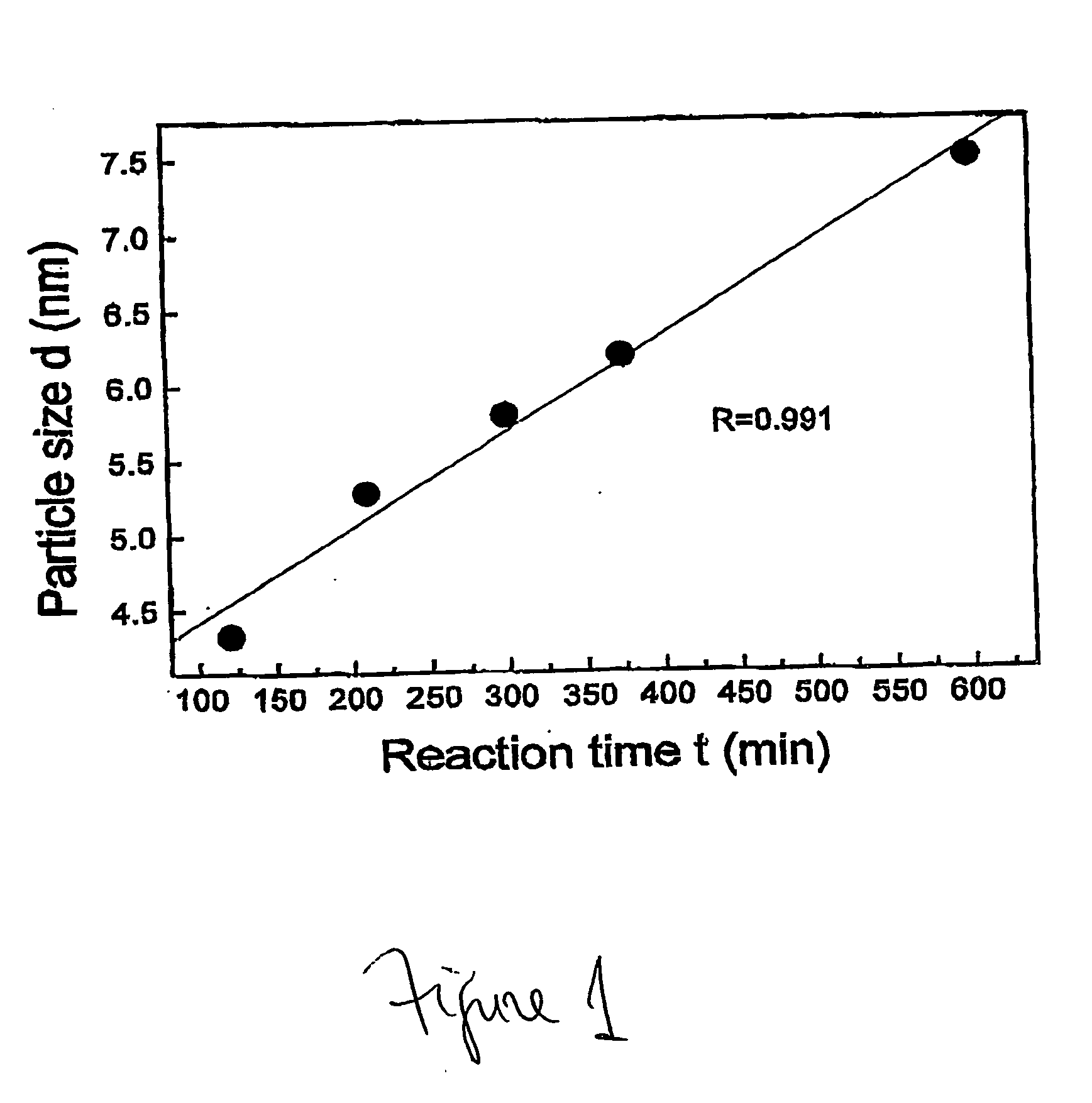
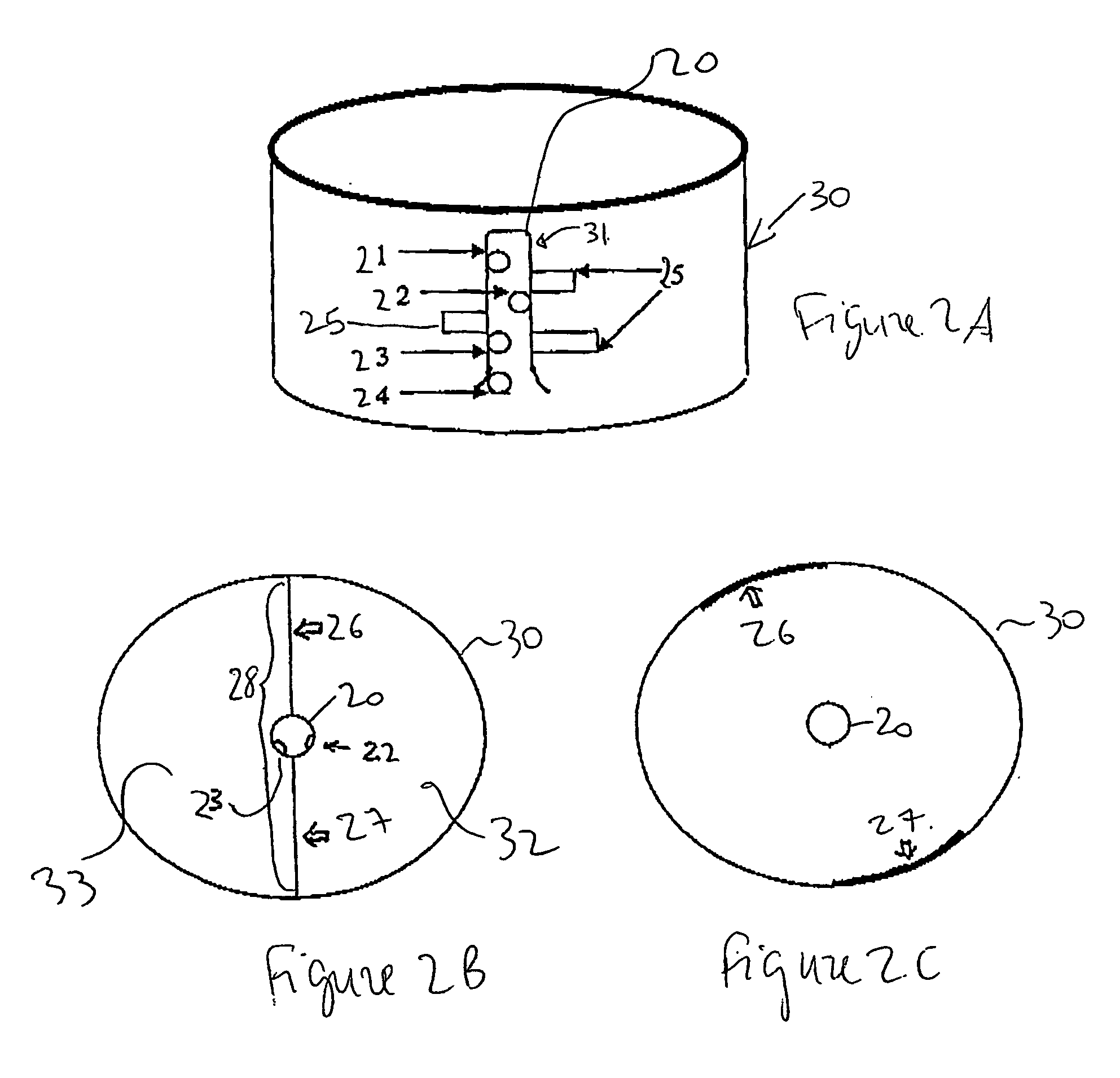
InactiveUS20050031517A1Quick responseReaction time is thus limitedMaterial nanotechnologyMixing methodsCerium nitrateNanoparticle
This invention provides a method for preparing cerium oxide nanoparticles with a narrow size distribution. The cerium oxide nanoparticles obtained by the method of the invention are nearly all crystalline. The method comprises providing a first aqueous solution comprising cerium nitrate and providing a second aqueous solution comprising hexamethylenetetramine. The first and second aqueous solutions are mixed to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles. The nanoparticles that are formed are then separated from the mixture. A further aspect of the present invention is an apparatus for preparing cerium oxide nanoparticles. The apparatus comprises a mixing vessel having a first compartment for holding a first aqueous solution comprising cerium nitrate and a second compartment for holding a second aqueous solution comprising hexamethylenetetramine. The mixing vessel has a retractable partition separating the first and second compartments. When the retractable partition is retracted, rapid mixing of the first aqueous solution with the second aqueous solution takes place to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles therein.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Thermoelectric material and thermoelectric converting element using the same
Compounds are expressed by general formula of AxBC2-y where 0<=x<=2 and 0<=y<1, and have CdI2 analogous layer structures; A-site is occupied by at least one element selected from the group consisting of Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Zr, Nb, Mo, Ru, Rh, Pd, Ag, Cd, Hf, Ta, W, Re, Ir, Pt, Au, Sc, rare earth elements containing Y, B, Al, Ga, In, Tl, Sn, Pb and Bi; B-site is occupied by at least one element selected from the group consisting of Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, W, Ir, and Sn; C-site is occupied by at least one element selected from the group consisting of S, Se and Te; the compounds exhibit large figure of merit so as to be preferable for thermoelectric generator / refrigerator.
Owner:NEC CORP
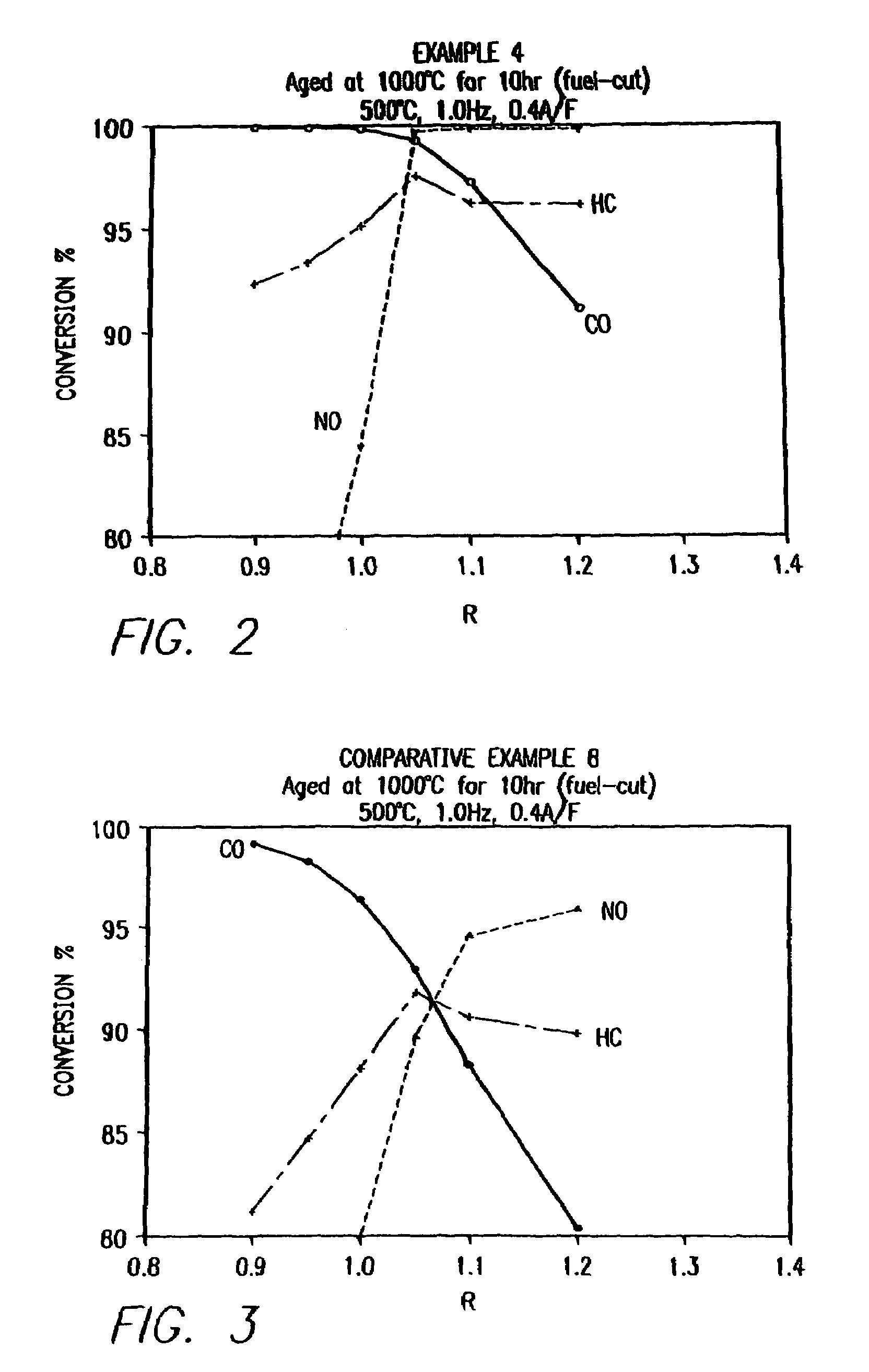
Mixed-phase ceramic oxide three-way catalyst formulations and methods for preparing the catalysts
InactiveUS7641875B1Improve efficiencyLess timeOrganic chemistryNitrogen compoundsLanthanidePt element
A multi-phase catalyst for the simultaneous conversion of oxides of nitrogen, carbon monoxide, and hydrocarbons is provided. A catalyst composition comprising the multi-phase catalyst and methods of making the catalyst composition are also provided. The multi-phase catalyst may be represented by the general formula of CeyLn1-xAx+sMOZ, wherein Ln is a mixture of elements originally in the form of single-phase mixed lanthanides collected from natural ores, a single lanthanide, or a mixture of lanthanides; A is an element selected from a group consisting of Mg, Ca, Sr, Ba, Li, Na, K, Cs, Rb, or any combination thereof; and M is an element selected from the group consisting of Fe, Mn, Cr, Ni, Co, Cu, V, Zr, Pt, Pd, Rh, Ru, Ag, Au, Al, Ga, Mo, W, Ti, or any combination thereof; x is a number defined by 0≦x<1.0; y is a number defined by 0≦y<10; s is a number defined by 0≦s<10; where s=0 only when y>0 and y=0 only when s>0. The multi-phase catalyst can have an overlayer of an oxide having the fluorite structure with a combination of platinum and / or rhodium.
Owner:CATALYTIC SOLUTIONS INC
Nanocarbon Composite Structure Having Ruthenium Oxide Trapped Therein
InactiveUS20080048153A1Increase capacitanceImprove electrochemical activityMaterial nanotechnologyCarbon compoundsReaction fieldNanoparticle
A novel nanocarbon composite structure is provided having trapped ruthenium oxide. By using Ketjen black, and through chemical-mechanical effect utilizing an ultracentrifugal reaction field, both the specific surface area of ruthenium oxide and space of electrode material are expanded to have nanoparticles of ruthenium oxide highly dispersed in a graphene layer. This nanocarbon composite structure having trapped ruthenium oxide exhibits high electrochemical activity and is suitable for use as an electrical energy storing device, such as a large-capacity capacitor.
Owner:NAT UNIV CORP TOKYO UNIV OF AGRI & TECH
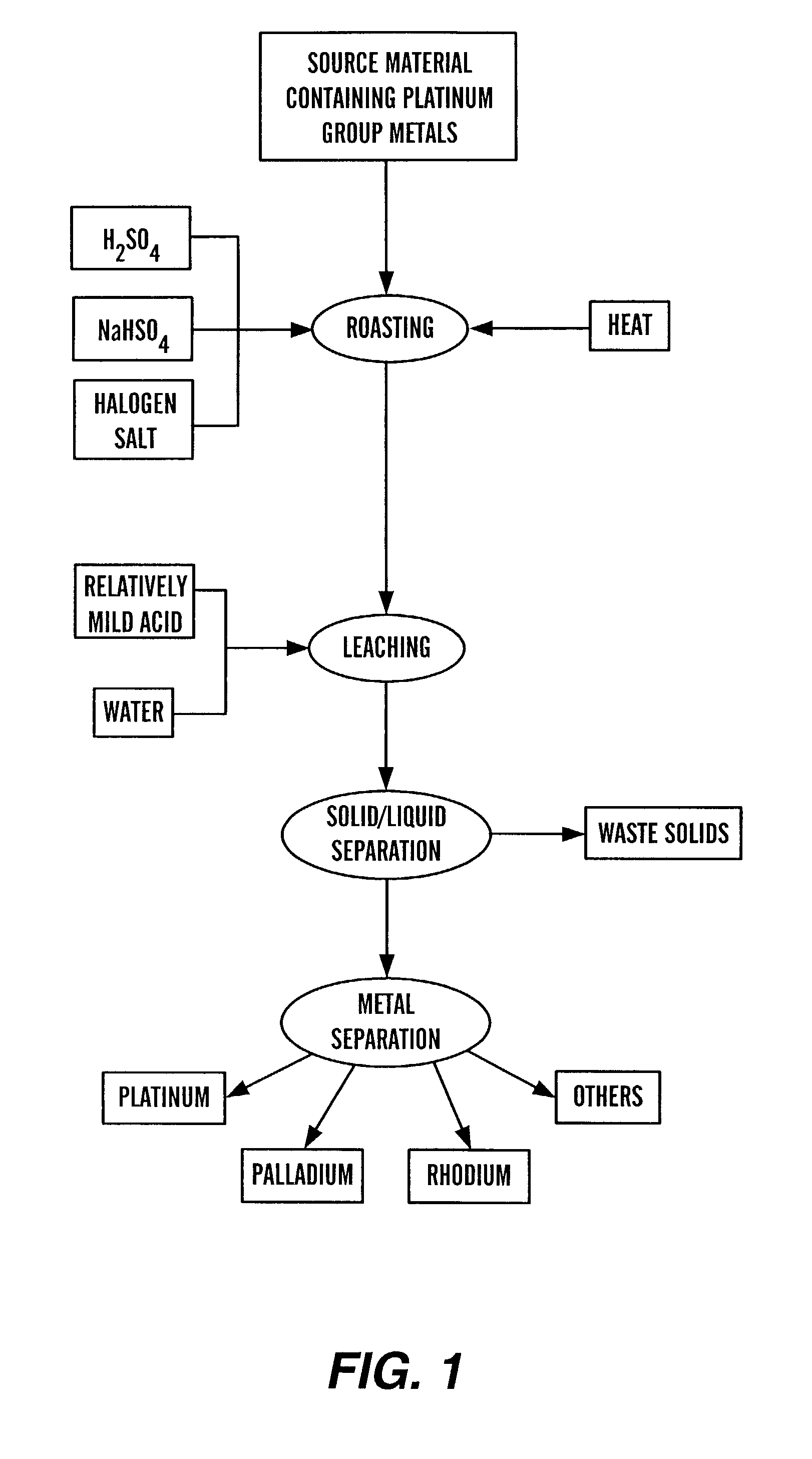
Recovery of platinum group metals
InactiveUS7067090B2Ruthenium/rhodium/palladium/osmium/iridium/platinum compoundsHalogenSource material
This invention relates to the recovery of platinum group metals and, more particularly, to the recovery of platinum group metals from various sources by roasting the source material with one or more of sulfuric acid, a sulfate and / or a bi-sulfate and with one or more halogen salt. The roasted product is put in contact with a leaching solution to dissolve at least a portion of the platinum group metals, which then may be separated and recovered.
Owner:SOUTH DAKOTA SCHOOL OF MINES & TECH DEPT OF MATERIALS & METALLURGIC ENG
Synthetic control of metal oxide nanocrystal sizes and shapes
A general, reproducible, and simple synthetic method that employs readily available chemicals permits control of the size, shape, and size distribution of metal oxide nanocrystals. The synthesis entails reacting a metal fatty acid salt, the corresponding fatty acid, and a hydrocarbon solvent, with the reaction product being pyrolyzed to the metal oxide. Nearly monodisperse oxide nanocrystals of Fe3O4, Cr2O3, MnO, Co3O4, NiO, ZnO, SnO2, and In2O3, in a large size range (3-50 nm), are described. Size and shape control of the nanocrystals is achieved by varying the reactivity and concentration of the precursors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
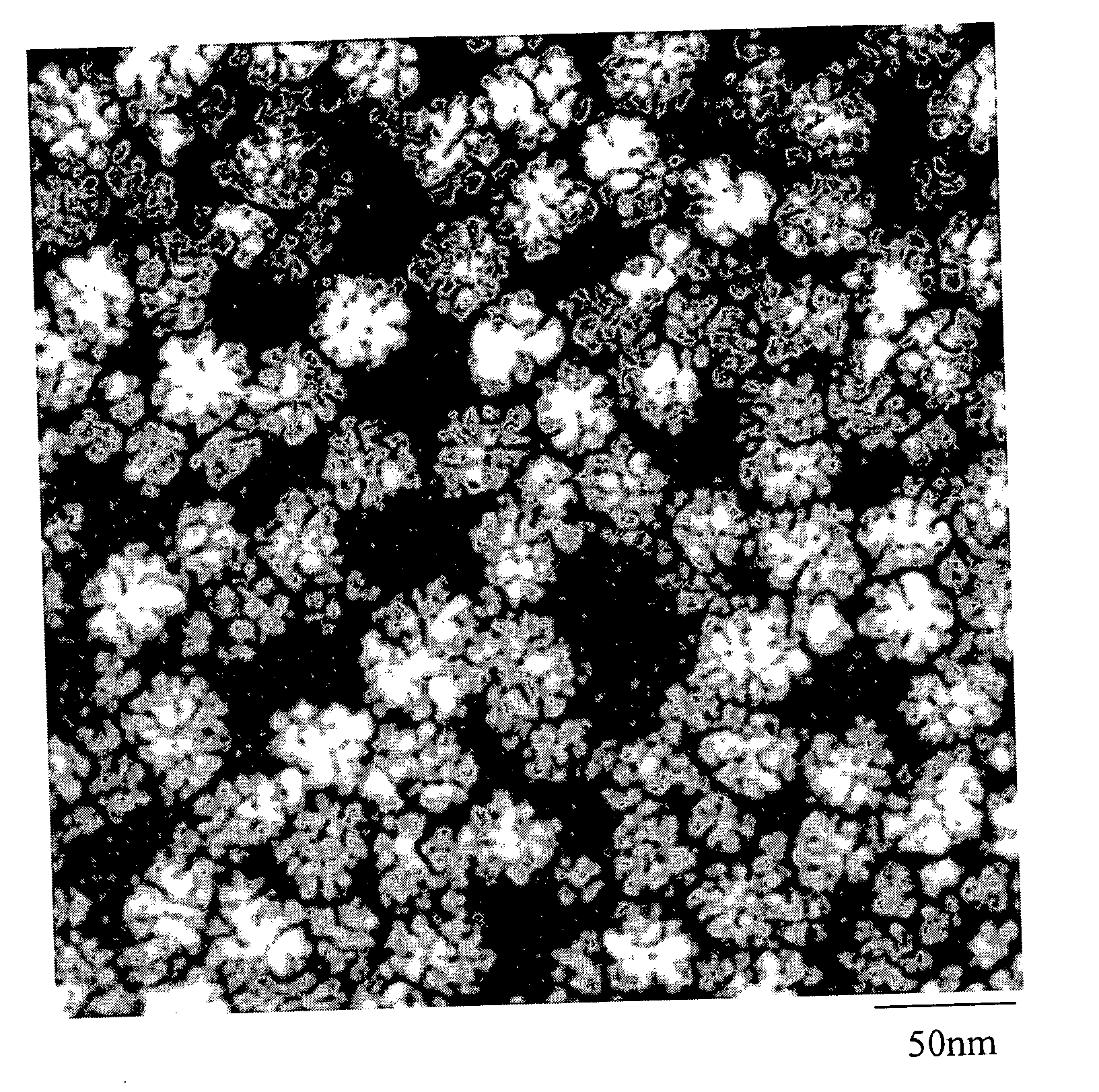
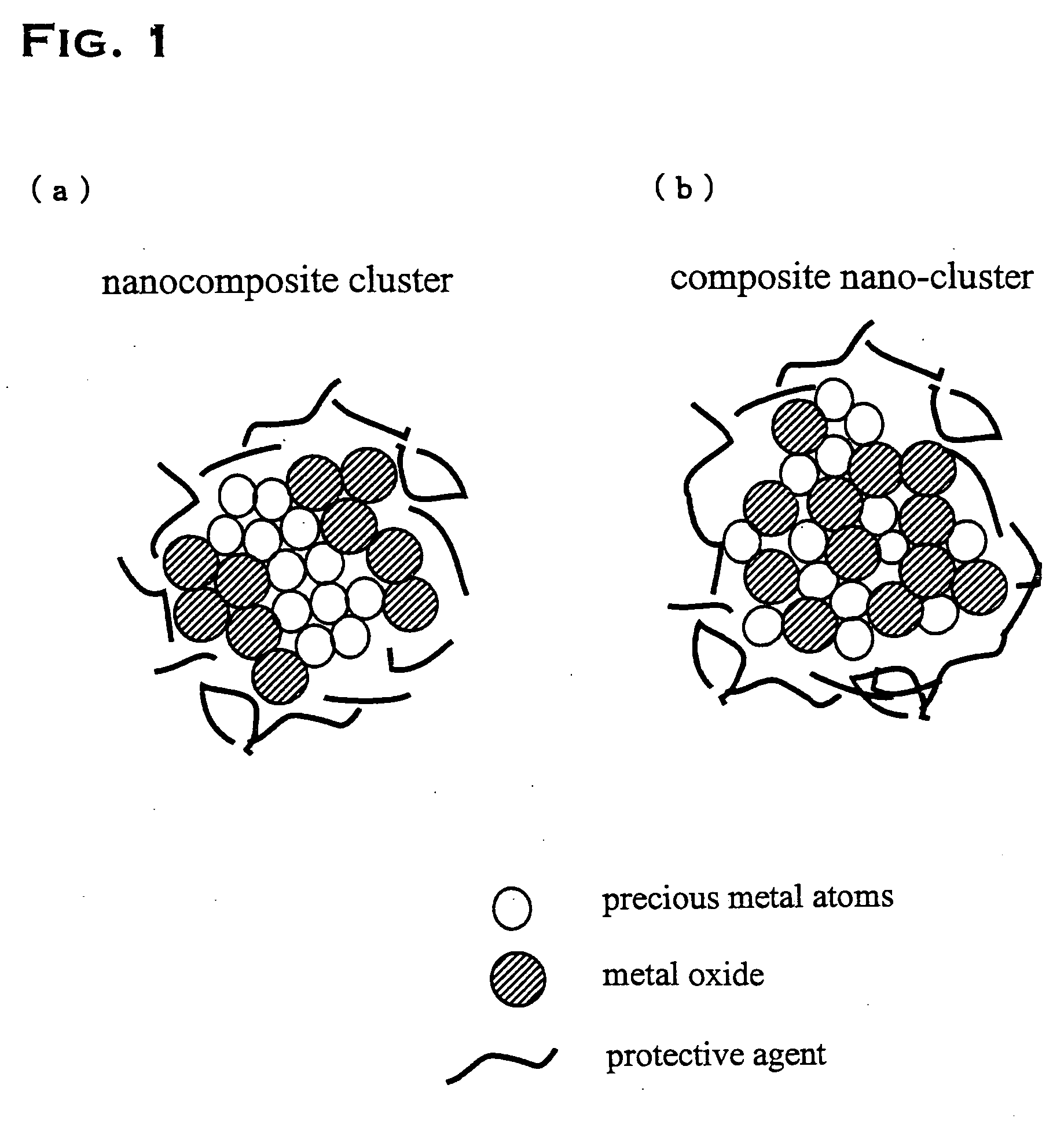
Precious metal - metal oxide composite cluster
InactiveUS20050065026A1Increase the effective surface areaSmall granularityMaterial nanotechnologyTransportation and packagingOxide compositeMaterials science
The present invention provides a precious metal—metal oxide composite cluster, wherein said cluster is formed as a single particle by combining a precious metal portion comprising a single atom or an aggregate of a plurality of atoms consisting of one or more precious metals, and a metal oxide portion comprising a single molecule or an aggregate of a plurality of molecules consisting of one or more metal oxides, and wherein said particle has a particle size between 1 and 100 nm.
Owner:TANAKA PRECIOUS METAL IND
Preparation method of quantum dot self-assembling nano structural material
The invention discloses a preparation method of a super nanostructure material formed by quantum dots self-assembly. That the alcohols are used as solvent to prepare nano-metal oxides and sulphides or metal oxides and sulphides are reduced to get nano-metal is a widely used method. The super nanostructure material formed by the quantum dots self-assembly draws much attention because of the superior comprehensive properties. The application prospect is wide enough. The invention adopts a method of using the alcohols as the solvent that a super nanostructure with different appearances and is formed by the quantum dots self-assembly which is obtained by changing the condition under the existence condition of surfactant. According to the invention, precursor, namely organic metal compound is dissolved in the alcohol solvent by ultrasonic, stirring and being laid down quietly. Under the effect of the surfactant, the precursor has a nucleation and grows into a plurality of quantum dots, the size of which is similar to nano. Then the dots form a super nanostructure which has a certain shape or space structure along the defined growing direction of the surfactant.
Owner:HUAZHONG NORMAL UNIV
NANO powder, NANO ink and micro rod, and the fabrication methods thereof
InactiveUS20100167078A1Easy to makeOvercome problemsAlkaline earth titanatesPigmenting treatmentFiberMicro rods
Disclosed are a method for fabricating nanopowders, nano ink containing the nanopowders and micro rods, and nanopowders containing nanoparticles, nano clusters or mixture thereof, milled from nano fiber composed of at least one kind of nanoparticles selected from a group consisting of metal, nonmetal, metal oxide, metal compound, nonmetal compound and composite metal oxide, nano ink containing the nanopowders and microrods, the method comprising spinning a spinning solution containing at least one kind of precursor capable of composing at least one kind selected from a group consisting of metal, nonmetal, metal oxide, metal compound, nonmetal compound and composite metal oxide, crystallizing or amorphizing the spun precursor to produce nano fiber containing at least one kind of nanoparticles selected from a group consisting of metal, nonmetal, metal oxide, metal compound, nonmetal compound and composite metal oxide, and milling the nano fiber to fabricate nanopowders containing nanoparticles, nano clusters or mixture thereof.
Owner:KOREA INST OF SCI & TECH
Selective recovery of aluminium, cobalt and platinum values from a spent catalyst composition
InactiveUS20040219082A1Ruthenium/rhodium/palladium/osmium/iridium/platinum compoundsProcess efficiency improvementFlocculationPlatinum
The invention provides a process for the selective recovery of aluminium, cobalt and platinum, and compounds thereof, from a catalyst composition including aluminium, cobalt and platinum, said proscess including the steps of treating the catalyst composition to selectively get ions of substantially only one of the aluminium. cobalt and platinum into solution, recovering, in separate process steps, the thus treated aluminium, cobalt, or platinum in salt or metal form, and repeating the treating and recovering steps for each of the aluminium, cobalt and platinum. The treating steps may include process steps such as leaching, washing, dissolving, stripping, and the like. The recovery steps may include filtration, precipitation, separation, flocculation, and the like.
Owner:SASOL TEKHNOLODZHI PROPRIEHJTEHRI LTD
Process for recovery of palladium from spent catalyst
InactiveUS20040241066A1High-temperature incinerationHigh purityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsProcess efficiency improvementOrganic chemistryPalladium
The present invention relates to an improved process for the recovery of palladium from spent catalyst. The present invention particularly relates to a suitable method of catalyst recovery of precious metals from spent catalysts or inorganic waste and more specifically the process concerns with the recovery of palladium that is anchored on carbon and is used as catalyst for the hydrogenation of nitro aromatics or as a catalyst for many other organic transformations.
Owner:COUNCIL OF SCI & IND RES
Rhodium electrocatalyst and method of preparation
InactiveUS6855660B2Lower and more consistent operating voltagesImprove performanceMachining electrodesCellsAqueous solutionRhodium salt
Owner:IND DE NORA SPA
Method for smelting noble metal
InactiveUS6126720AShorten the timeReduce impurityPhotography auxillary processesSolvent extractionDecompositionMaterials science
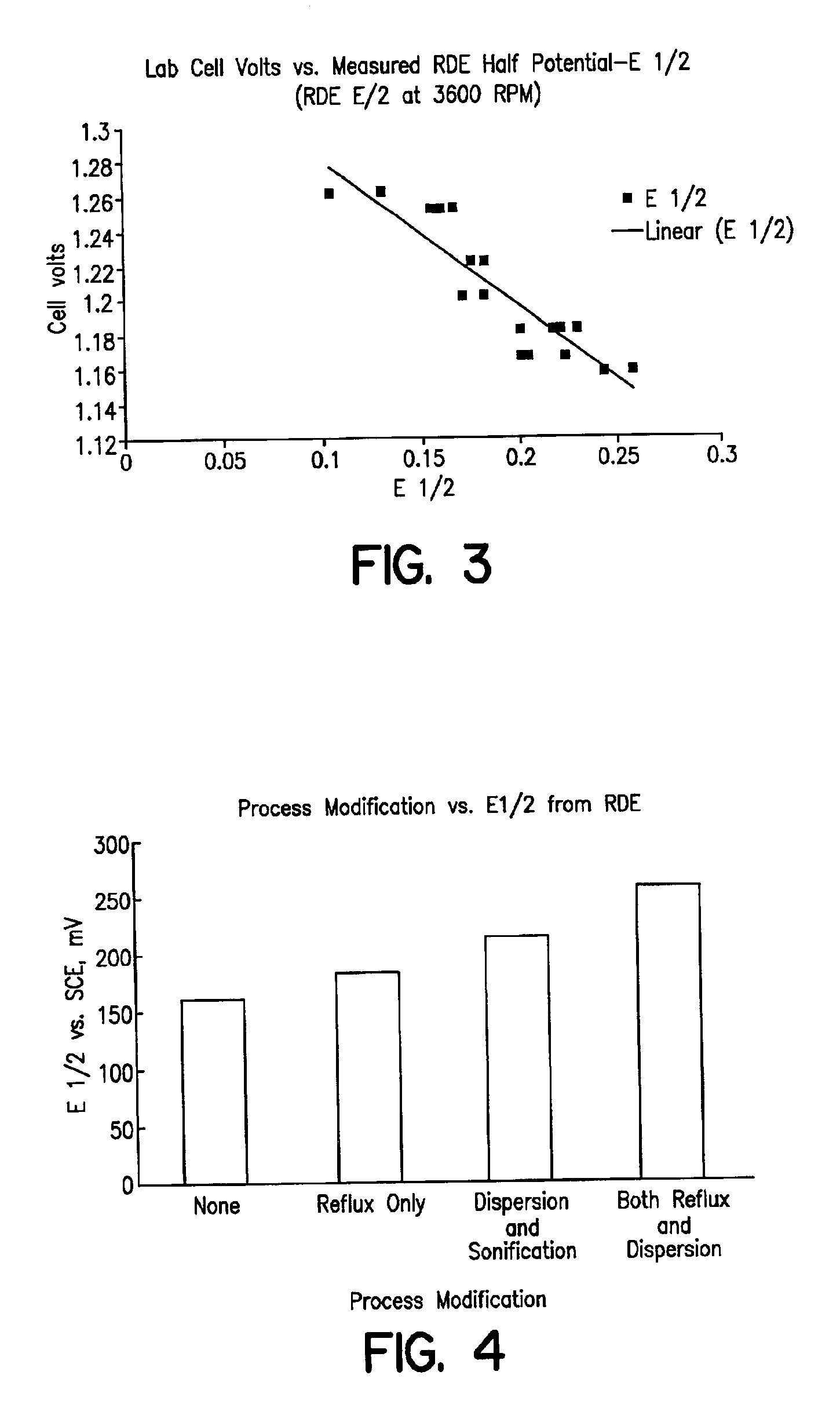
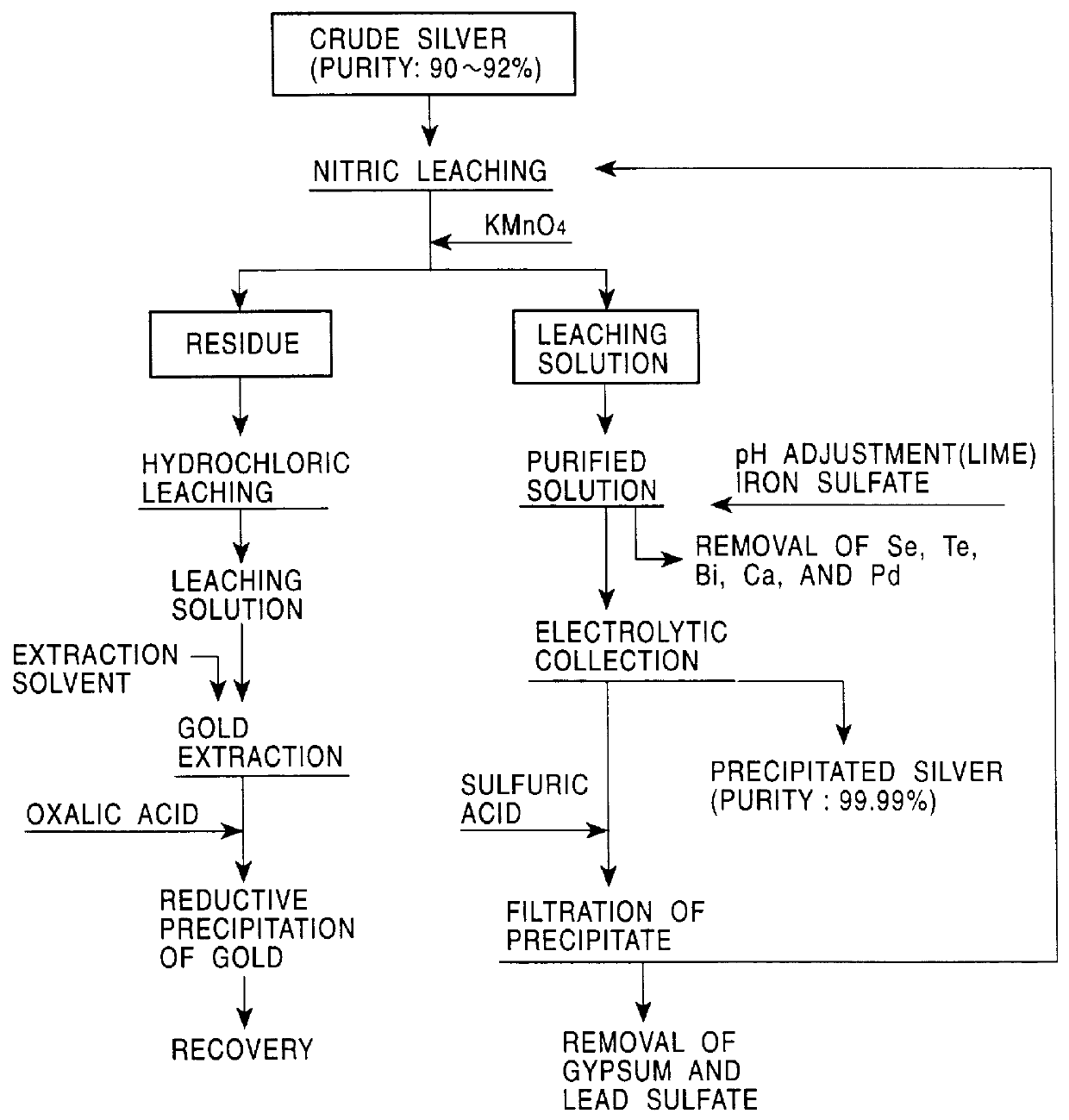
PCT No. PCT / JP98 / 02479 Sec. 371 Date Jan. 15, 1999 Sec. 102(e) Date Jan. 15, 1999 PCT Filed Jun. 4, 1998 PCT Pub. No. WO98 / 58089 PCT Pub. Date Dec. 23, 1998A method for refining noble metals has a silver treating process including a nitric acid leaching step of silver, a purification step of the leaching solution, an electrolytic decomposition step of silver, and a recycling step after the electrolytic decomposition, wherein in the purification step, lime is added in order to precipitate the metallic impurities, such as selenium, tellurium, bismuth, and copper, by neutralization of the leaching solution, and in the recycling step, sulfuric acid is added to the solution after electrolytic decomposition to regenerate nitric acid for recycling use by precipitation of calcium in the solution as gypsum. Preferably, the refining method has a gold recovery process, as well as the silver treating process, wherein the residue of the nitric leaching of the crude silver is dissolved by chlorination and gold is recovered from the leaching solution by solvent extraction or reductive precipitation. High purity gold and silver can be readily obtained, and the refining time for gold is significantly shorter than that in conventional methods.
Owner:MITSUBISHI MATERIALS CORP
Process for mutual separation of platinum group metals
ActiveUS20050066774A1Avoid high levels of impuritiesReduce impurityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsLiquid solutions solvent extractionIridiumDecomposition
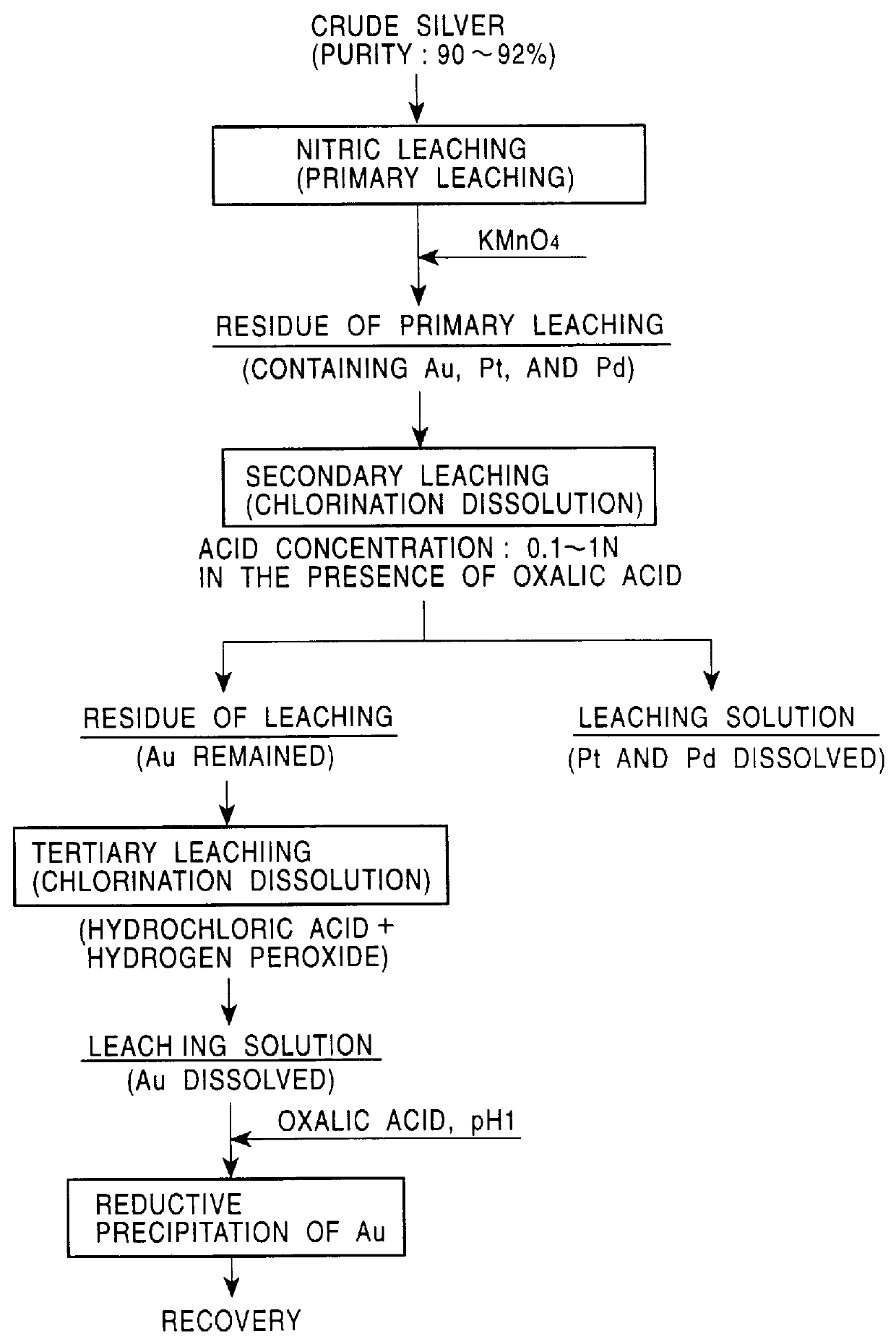
A process for mutual separation of platinum group metals (PGM), wherein highly stable compounds and steps are used to efficiently remove impurity elements while preventing increase of impurity content relative to that of the PGM in the mother liquor and also preventing decomposition of a chloro complex, and palladium, platinum, iridium, ruthenium and rhodium are separated mutually in such a way that each of the separated PGM has a sufficient purity to be a commercial product. A process for mutual separation of PGM, comprising the first step for leaching a raw material containing PGM and impurity elements, second step for removing the impurity elements from the leach liquor by solvent extraction, third step for recovering palladium from the raffinate, fourth step for removing cationic impurity elements from the raffinate by solvent extraction, fifth step for recovering platinum from the raffinate by hydrolysis, sixth step for recovering ruthenium from the precipitate by leaching, and seventh step for recovering iridium by solvent extraction to prepare the stripping liquor containing iridium and raffinate containing rhodium.
Owner:SUMITOMO METAL MINING CO LTD
Nanocarbon composite structure having ruthenium oxide trapped therein
InactiveUS7572542B2Increase capacitanceImprove electrochemical activityMaterial nanotechnologyCarbon compoundsReaction fieldNanoparticle
A novel nanocarbon composite structure is provided having trapped ruthenium oxide. By using Ketjen black, and through chemical-mechanical effect utilizing an ultracentrifugal reaction field, both the specific surface area of ruthenium oxide and space of electrode material are expanded to have nanoparticles of ruthenium oxide highly dispersed in a graphene layer. This nanocarbon composite structure having trapped ruthenium oxide exhibits high electrochemical activity and is suitable for use as an electrical energy storing device, such as a large-capacity capacitor.
Owner:NAT UNIV CORP TOKYO UNIV OF AGRI & TECH
Recovering metals from sulfidic materials
InactiveUS20070014709A1Facilitate precious metal dissolution precious metalFacilitate precious metal subsequent precious metal recoveryPhotography auxillary processesSolvent extractionOxidation stateSulfide
A process for recovering a precious metal from a sulfidic material comprises the steps of preparing an acidic aqueous halide solution having an oxidation potential sufficient to oxidise the sulfidic material and render the precious metal soluble in the solution, adding the material to the acidic aqueous halide solution so that the sulfidic material is oxidised and the precious metal is solubilised and separating the precious metal from the oxidised sulfidic material. In addition, a process for removing a contaminant from a contaminated sulfidic material comprises the steps of mixing the material in an aqueous solution wherein a multi-valent species of a relatively high oxidation state oxidises the contaminant to render it soluble in the solution, produces a contaminant refined material, and is reduced to a relatively lower oxidation state; and removing the contaminant from the solution whilst regenerating the multi-valent species to its relatively high oxidation state.
Owner:INTEC INT PROJECTS
Apparatus and method for preparing cerium oxide nanoparticles
InactiveUS7141227B2Quick responseMaterial nanotechnologyTransportation and packagingCerium nitrateHexamethylenetetramine
This invention provides a method for preparing cerium oxide nanoparticles with a narrow size distribution. The cerium oxide nanoparticles obtained by the method of the invention are nearly all crystalline. The method comprises providing a first aqueous solution comprising cerium nitrate and providing a second aqueous solution comprising hexamethylenetetramine. The first and second aqueous solutions are mixed to form a mixture, and the mixture is maintained at a temperature no higher than about 320° K to form nanoparticles. The nanoparticles that are formed are then separated from the mixture. A further aspect of the present invention is an apparatus for preparing cerium oxide nanoparticles. The apparatus comprises a mixing vessel having a first compartment for holding a first aqueous solution comprising cerium nitrate and a second compartment for holding a second aqueous solution comprising hexamethylenetetramine. The mixing vessel has a retractable partition separating the first and second compartments.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Method for recovering ruthenium catalyst carried by active carbon
InactiveCN1872418AReduce manufacturing costReduce consumptionRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsCatalyst regeneration/reactivationRecovery methodActivated carbon
A recovering process for the activated carbon carried Ru catalyst not containing the compound of alkali metal or alkali-earth metal includes such steps as calcining at 600-1000 deg.C for 2-20 hr to obtain gray mixture, mixing it with KOH and KNO3, holding the temp at 300-950 deg.C for 105 hr, cooling, dissolving in hot water at 50-90 deg.C to obtain K2RuO4 solution, adding sodium hypochlorite and concentrated sulfuric acid, distilling to generate RuO4 gas, absorbing it by strong acid solution, and distilling to obtain Ru salt.
Owner:ZHEJIANG UNIV OF TECH +1
Method for preparing high-purity ruthenium sputtering target and high-purity ruthenium sputtering target
InactiveUS6284013B1Minimize formation of particleSolve the large leakage currentTransportation and packagingRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsHydrogen atmosphereImpurity
There is provided a high-purity ruthenium sputtering target with a low impurity content, in particular producing extremely few particles, which is suitable for applications such as the formation of semiconductor thin films. The high-purity ruthenium sputtering target is manufactured by feeding crude ruthenium powder into a sodium hydroxide solution; blowing an ozone-containing gas while or after blowing chlorine gas into the solution to form ruthenium tetroxide; absorbing the ruthenium tetroxide in a hydrochloric acid solution or a mixed solution of hydrochloric acid and ammonium chloride, and evaporating the solution to dryness; sintering the resultant ruthenium salt in a hydrogen atmosphere to form high-purity ruthenium powder; and hot-pressing the ruthenium powder into a sputtering target.
Owner:NIKKO MATERIALS CO LTD
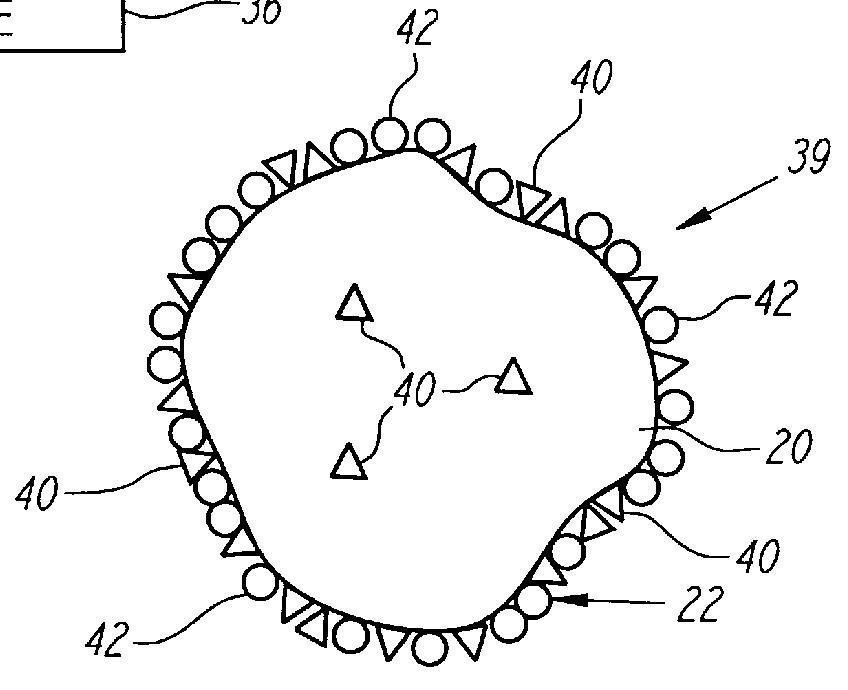
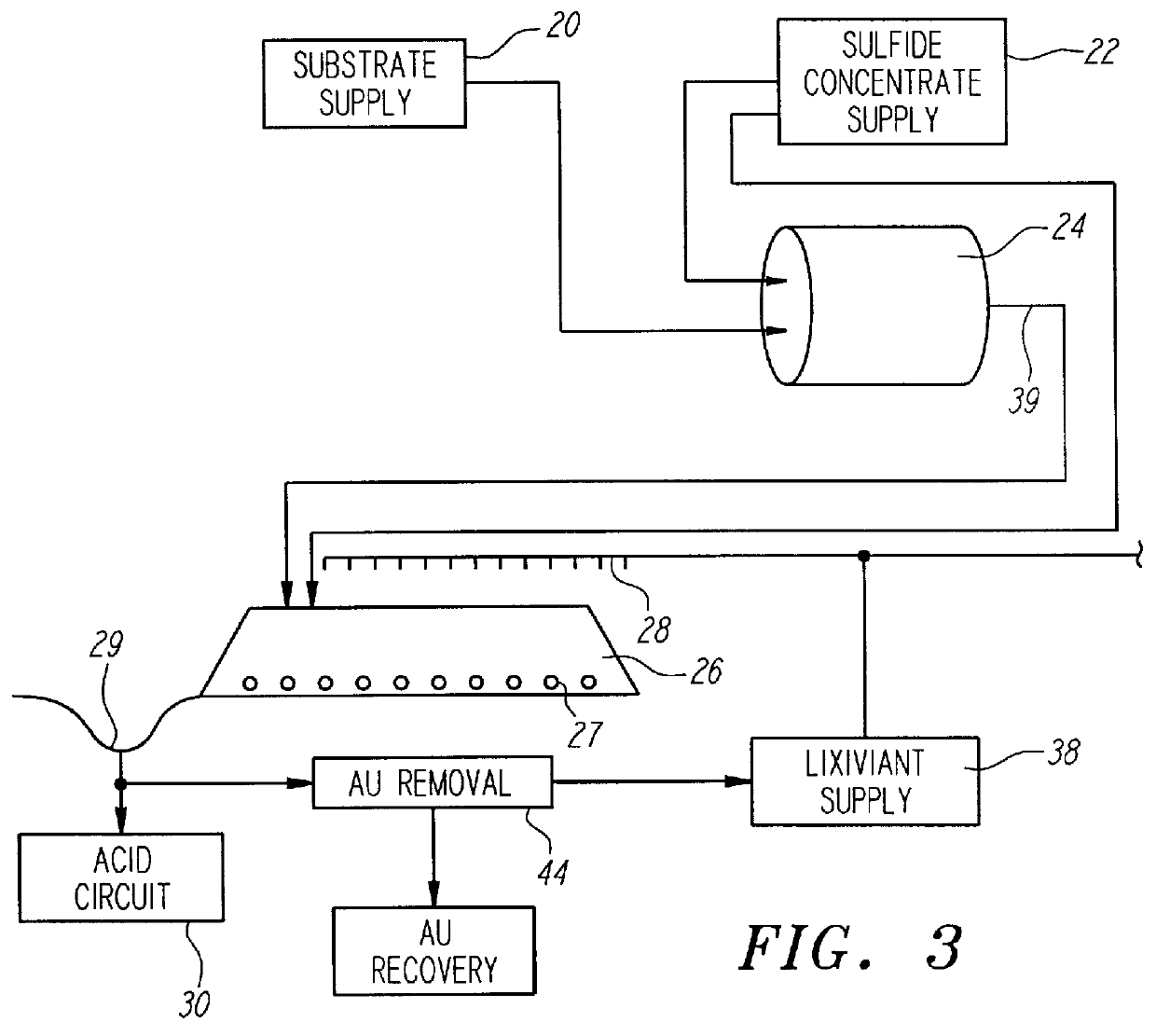
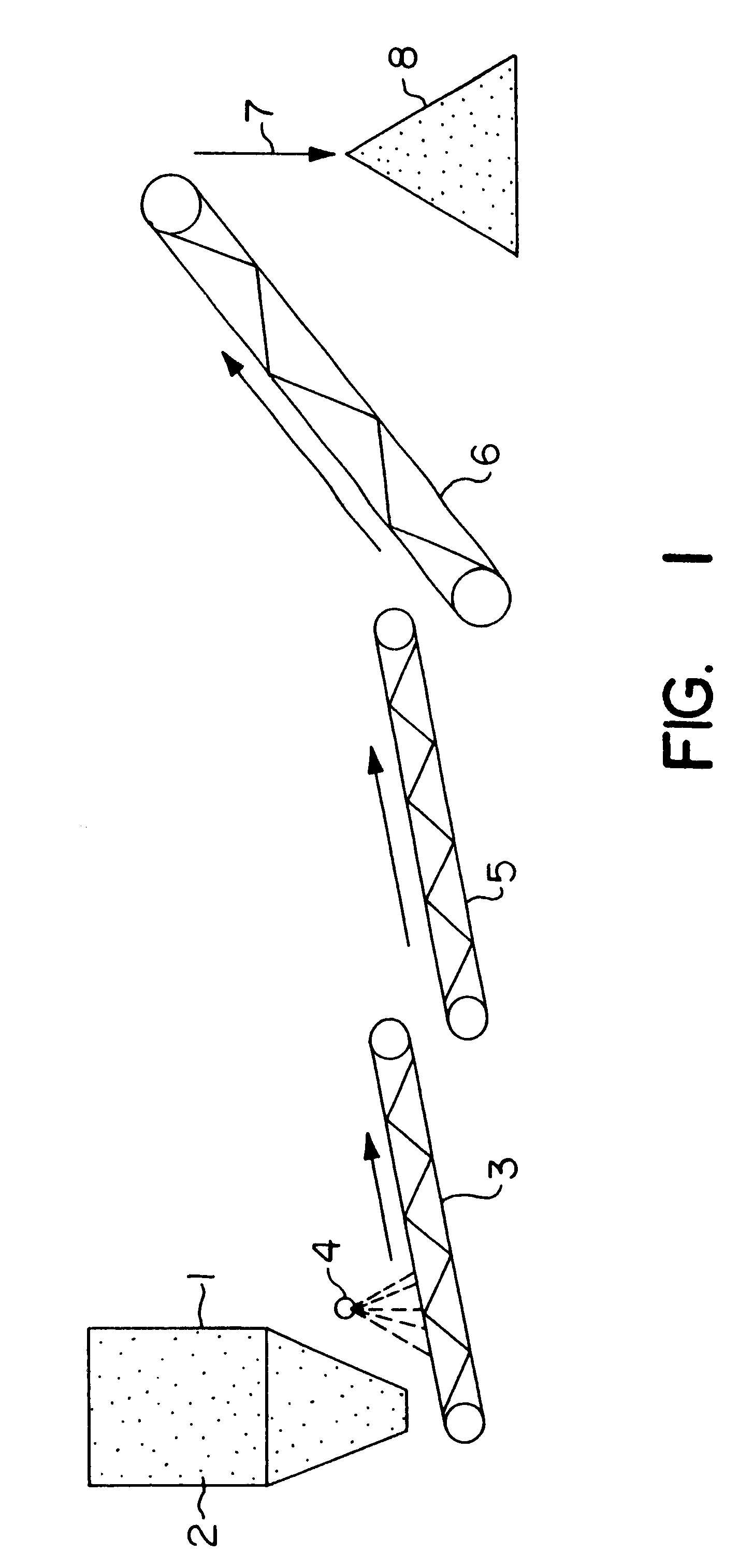
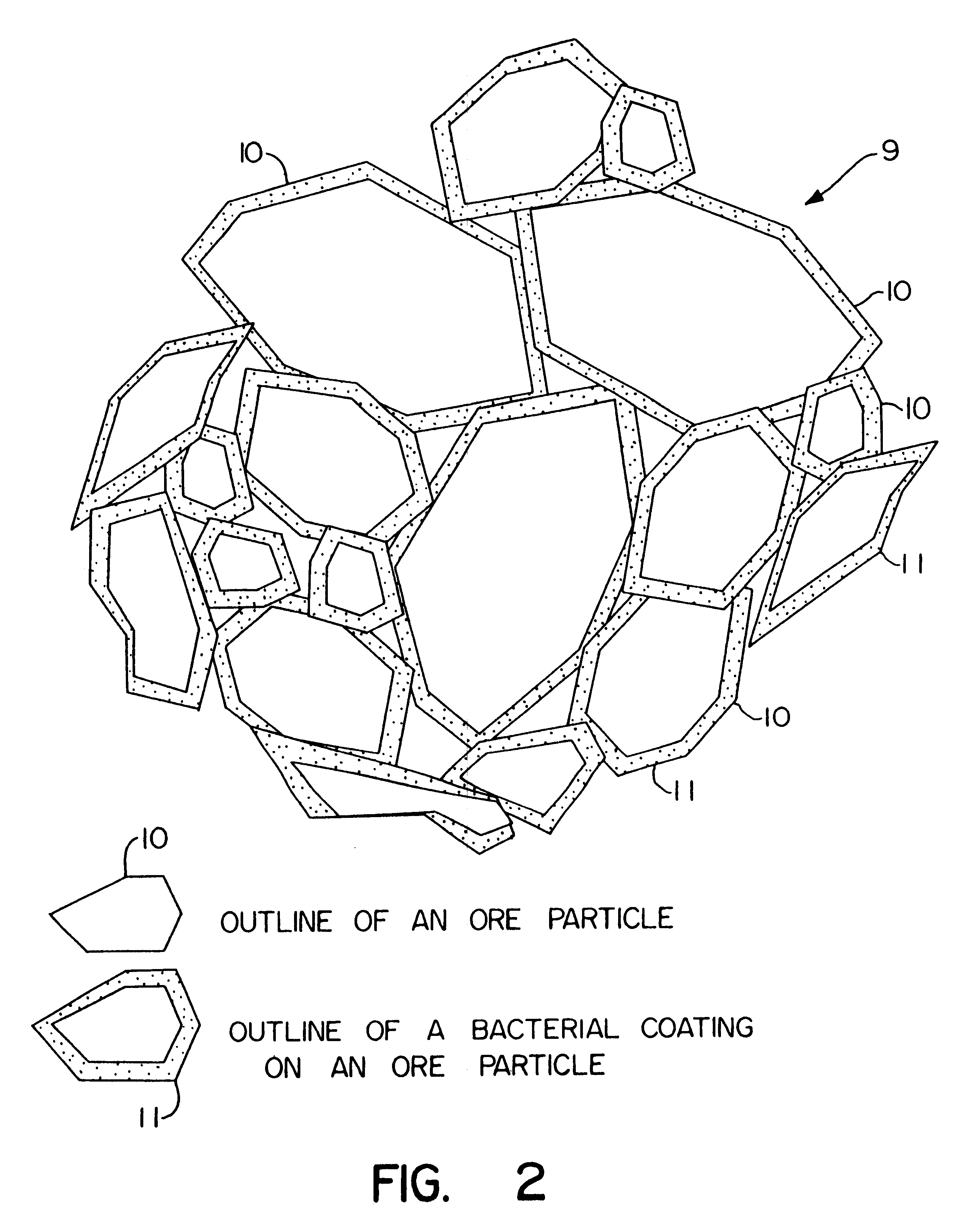
Nonstirred bioreactor for processing refractory sulfide concentrates and method for operating same
InactiveUS6083730AReduced pHIncrease equipment costSolvent extractionContaminated soil reclamationSulfide mineralsMetallic sulfide
A method of biooxidizing sulfide minerals in a nonstirred bioreactor is provided. According to the disclosed method, a concentrate of sulfide minerals is coated onto a substrate, such as coarse ore particles, lava rock, gravel or rock containing mineral carbonate as a source of CO2 for the biooxidizing bacteria. After the sulfide minerals are coated onto the substrate, a heap is formed with the coated substrates or the coated substrates are placed within a tank. The sulfide minerals are then biooxidized to liberate the metal value of interest. Depending on the particular ore deposit being mined, the sulfide mineral concentrates used in the process may comprise sulfide concentrates from precious metal bearing refractory sulfide ores or they may comprise sulfide concentrates from metal sulfide type ores, such as chalcopyrite, millerite or sphalorite. The distinction being that in the former, the metal of interest is a precious metal occluded within the sulfide minerals, whereas in the latter, the metal to be recovered is copper, nickel or zinc and is present as a metal sulfide in the sulfide concentrate.
Owner:GEOSYNFUELS LLC (US)
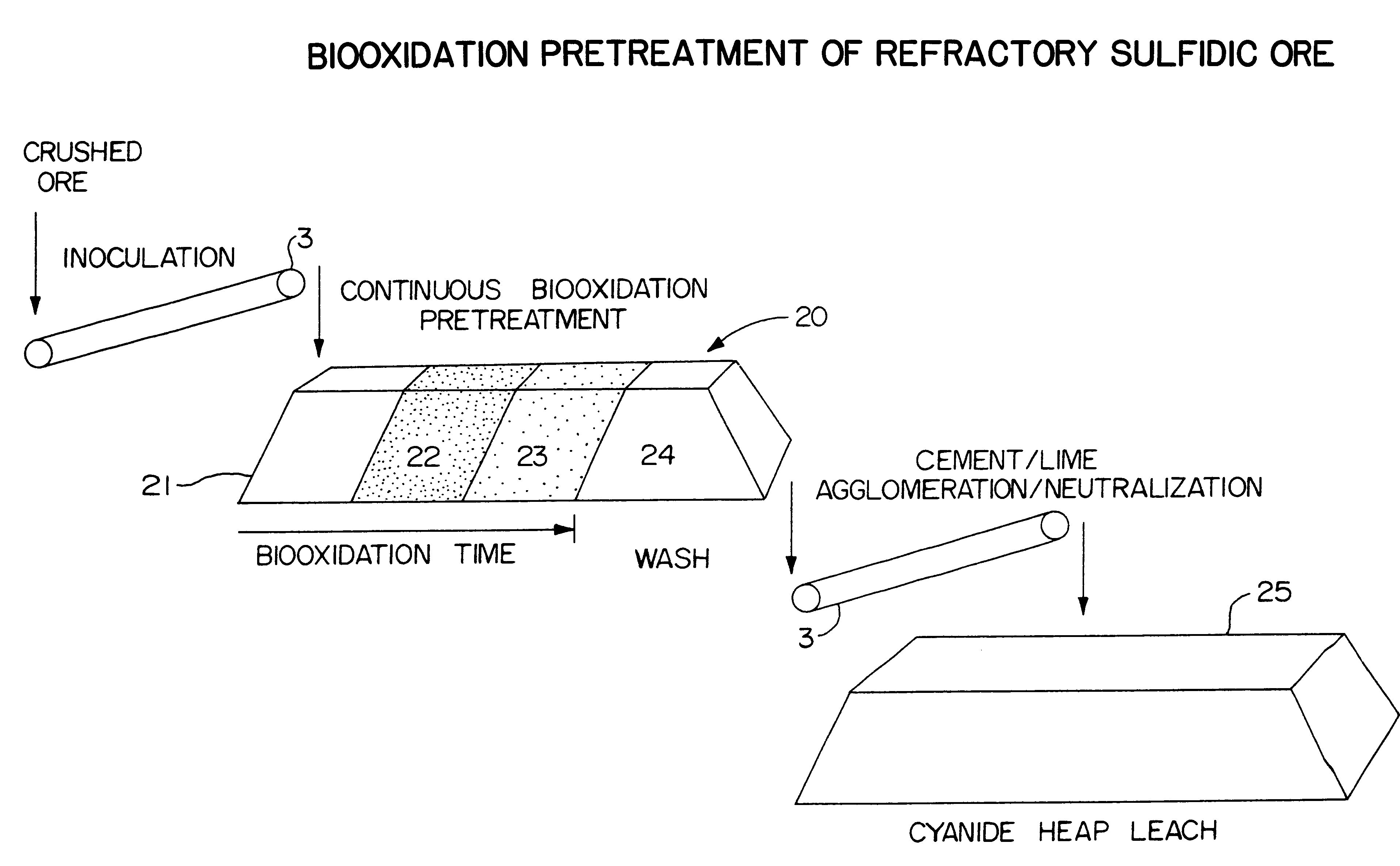
Biooxidation process for recovery of metal values from sulfur-containing ore materials
InactiveUS6383458B1Increase ratingsIncrease attractivenessTransuranic element compoundsSolvent extractionParticulatesPartial oxidation
A process for the recovery of one or more metal values from a metal ore material comprising those of one or more values and a matrix material having a sulfur content wherein the sulfur is present in an oxidation-reduction state of zero or less comprisinga. forming particulates from particles of said ore and an inoculate comprising bacteria capable of at least partially oxidizing the sulfur content;b. forming a heap of said particulates;c. biooxidizing the sulfur content andd. recovering those one or more metal values.
Owner:NEWMONT USA LTD
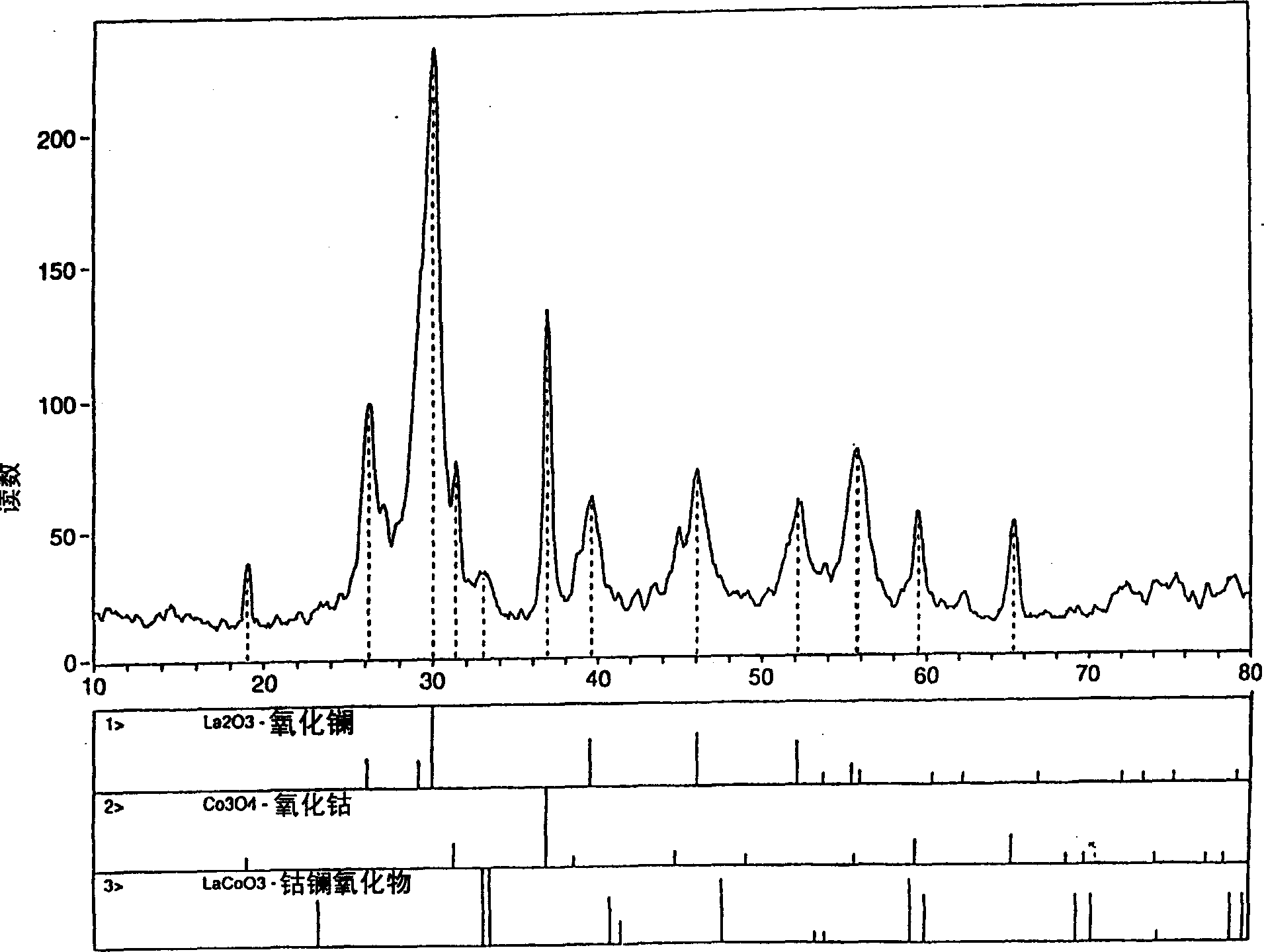
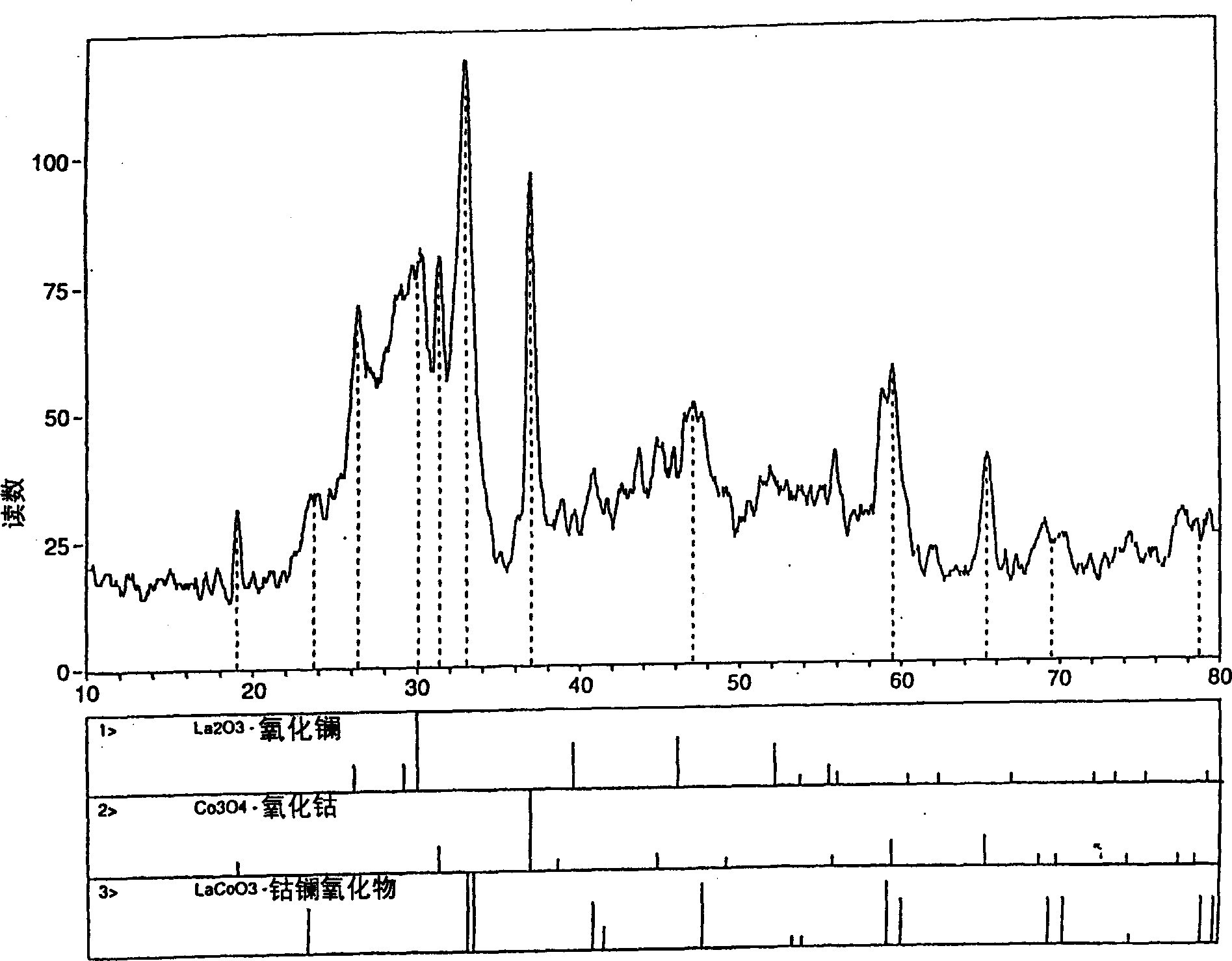
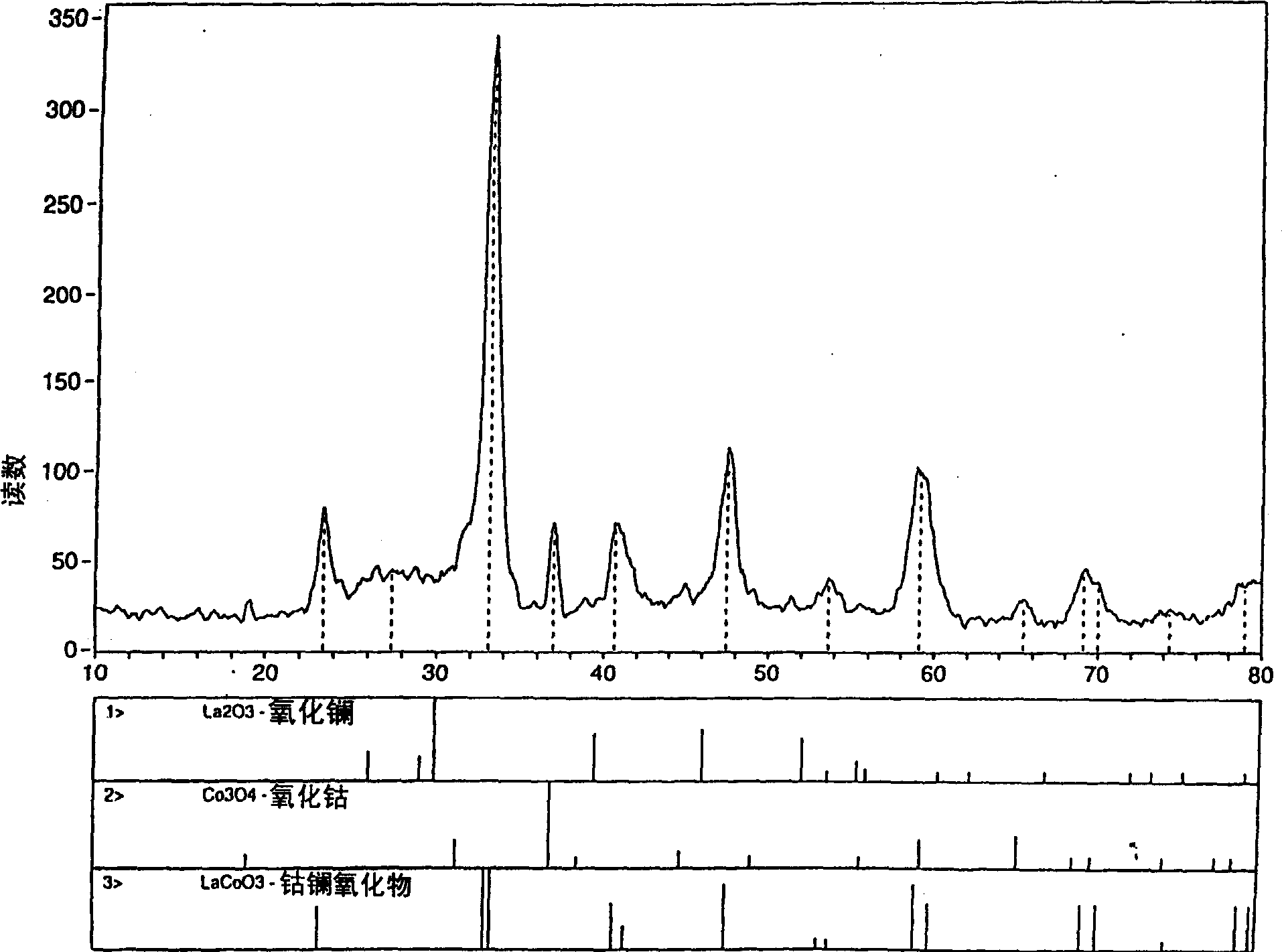
Process for synthesizing metal oxides and metal oxide having perovskite or perovskite-like crystal structure
InactiveCN1315920ALarge specific surface areaSimple methodNanotechOxide/hydroxide preparationHigh densityLattice defects
Perovskite-type structure compounds having the general empirical formula ABO3 are prepared by a process comprising subjecting a mixture of starting powders formulated to contain the components represented by A and B in the formula to a high energy milling sufficient to induce chemical reaction of the components and thereby synthesize a mechanically-alloyed powder comprising the perovskite in the form of nanostructural particles. The process according to the present invention is simple, efficient, not expensive and does not require any heating step for producing a perovskite that may easily show a very high specific surface area. Another advantage is that the perovskite obtained according to the present invention also has a high density of lattice defects thereby showing a higher catalytic activity, a characteristic which is highly desirable in their eventual application as catalysts and electronic conductors.
Owner:UNIV LAVAL
Popular searches
Indium organic compounds Group 5/15 element organic compounds Group 3/13 element organic compounds Group 6/16 element organic compounds Metallocenes Cadmium organic compounds Group 7/17 element organic compounds Vacuum evaporation coating Sputtering coating Semiconductor/solid-state device manufacturing
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com